Abstract
Neuropeptides are short peptides in the range of 3–40 residues that are secreted for cell-cell communication in neuroendocrine systems. In the nervous system, neuropeptides comprise the largest group of neurotransmitters. In the endocrine system, neuropeptides function as peptide hormones to coordinate intercellular signaling among target physiological systems. The diversity of neuropeptide functions is defined by their distinct primary sequences, peptide lengths, proteolytic processing of pro-neuropeptide precursors, and covalent modifications. Global, untargeted neuropeptidomics mass spectrometry is advantageous for defining the structural features of the thousands to tens of thousands of neuropeptides present in biological systems. Defining neuropeptide structures is the basis for defining the proteolytic processing pathways that convert pro-neuropeptides into active peptides. Neuropeptidomics has revealed that processing of pro-neuropeptides occurs at paired basic residues sites, and at non-basic residue sites. Processing results in neuropeptides with known functions, and generates novel peptides representing intervening peptide domains flanked by dibasic residue processing sites, identified by neuropeptidomics. While very short peptide products of 2–4 residues are predicted from pro-neuropeptide dibasic processing sites, such peptides have not been readily identified; therefore, it will be logical to utilize metabolomics to identify very short peptides with neuropeptidomics in future studies. Proteolytic processing is accompanied by covalent post-translational modifications (PTMs) of neuropeptides comprising C-terminal amidation, N-terminal pyroglutamate, disulfide bonds, phosphorylation, sulfation, acetylation, glycosylation, and others. Neuropeptidomics can define PTM features of neuropeptides. In summary, neuropeptidomics for untargeted, global analyses of neuropeptides is essential for elucidation of proteases that generate diverse neuropeptides for cell-cell signaling.
Keywords: neuropeptides, proteases, mass spectrometry, peptidomics, cathepsin, proprotein convertase, enkephalin, NPY, regulation, neurotransmission, nervous system, endocrine
Graphical Abstract
Introduction
Neuropeptides are utilized in the nervous and endocrine systems for cell-cell signaling to regulate cellular and physiological functions [1–3]. Neuropeptides of the nervous system function as the largest class of neurotransmitters, along with the smaller group of classical neurotransmitters (figure 1A) [2]. The brain central nervous system (CNS) utilizes neuropeptides in the control of behaviors and for central regulation of neuroendocrine systems. The peripheral sympathetic and parasympathetic nervous system utilizes neuropeptides for neural regulation of target physiological organ systems. Further, endocrine hormone systems utilize neuropeptides to coordinate physiological functions in homeostasis and in responses to the environment, utilizing feedback peptide hormone systems (figure 1B). The global participation of neuropeptides required for cell-cell communication among all organ systems in humans and vertebrate species, as well as in invertebrate species, underscores the critical importance to define neuropeptide structures and their corresponding biological activities for regulating target cell functions.
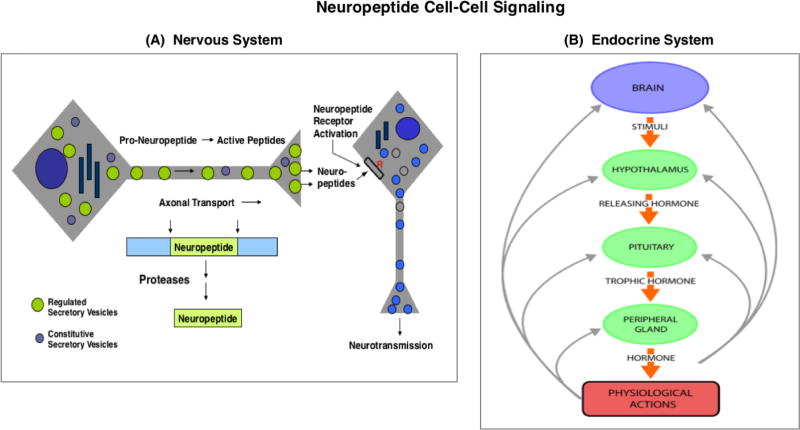
Figure 1. Neuropeptide cell-cell signaling in the nervous and endocrine systems.
A. Neuropeptides in the nervous system as peptide neurotransmitters. Neuropeptides are utilized as neurotransmitters for cell-cell communication in the brain and in the peripheral sympathetic and parasympathetic nervous system. Neuropeptides are synthesized within secretory vesicles that are transported from the neuronal cell body to nerve terminals. During axonal transport of such secretory vesicles, pro-neuropeptide precursors (or prohormone) are packaged into newly synthesized secretory vesicles with proteases for precursor processing into mature neuropeptides. Activity-dependent regulation of secretion releases neuropeptides at nerve terminals for neurotransmission.
B. Neuropeptides in the endocrine systems as peptide hormones. Neuropeptides function as peptide hormones to mediate cell-cell communication among brain and endocrine systems for regulation of physiological functions. Brain neuropeptides and endocrine peptide hormones are co-regulated in feedback systems for fine tuning of physiological regulation. For example, the hypothalamo-neurophypophyseal system regulates the pituitary-adrenal axis by secretion of CRF from the hypothalamus region of the brain to induce secretion of the ACTH peptide hormone from the pituitary. Released ACTH targets the adrenal cortex for stimulation of glucocorticoid production. Resultant increases in plasma glucocorticoid participate in feedback inhibition of CRF and ACTH to maintain constant levels of glucocorticoid. Numerous peptide hormones regulate physiological functions in stimulatory and feedback system.
The primary amino acid sequences of neuropeptides, without and with post-translational modifications, define their bioactivities. While numerous active neuropeptide structures with physiological functions are known, the discovery of novel, previously unknown peptides with potential for bioactivities is exploding due advances in peptidomics mass spectrometry. High throughput analyses of hundreds to thousands of peptides has been advanced by innovative LC-MS/MS tandem mass spectrometry technology combined with peptide bioinformatics [4–12].
This review illustrates how neuropeptidomics is essential for elucidating proteolytic processing mechanisms that convert pro-neuropeptides into small neuropeptides of diverse structures and biological functions. Further, neuropeptidomics fosters discovery of potentially novel bioactive peptides. The expanding repertoire of active neuropeptides increases the complexity of proteolytic mechanisms that generate neuropeptides for cell-cell communication networks among neuroendocrine and physiologic systems.
Global functions of neuropeptides in cell-cell signaling in brain regulation of behaviors and neuroendocrine hormone control of organ systems
Diverse neuropeptide bioactivities regulate brain and physiological organ functions (table 1). Active brain neuropeptides include the opioid peptides of enkephalin, dynorphin, and beta-endorphin that regulate analgesia and are important in chronic pain conditions [13–15]. Galanin regulates cognition in normal and neurodegeneration conditions [16, 17]. CCK (cholecystokinin) [18] and NPY (neuropeptide Y) [19] regulate feeding behavior and obesity. Calcitonin regulates calcium homeostasis [20] and migraine pain [21]. Substance P participates in the regulation of pain as w ell as memory [22, 23].
Table 1.
Neuropeptide Functions in the Nervous and Endocrine Systems
| Neuropeptides | Functions in Physiological Regulation |
|---|---|
| Brain Neuropeptides | |
| Enkephalin | Analgesia, pain relief |
| β-Endorphin | Analgesia, pain relief |
| Dynorphin | Chronic pain |
| Substance P | Pain neurotransmission |
| Galanin | Cognition |
| Cholecystokinin (CCK) | Appetite, learning, memory |
| Neuropeptide Y (NPY) | Feeding behavior |
| Calcitonin | Calcium regulation, migraine |
| Endocrine Neuropeptides | |
| Growth hormone releasing hormone (GHRH) | Growth and development |
| Thyrotropin releasing hormone (TRH) | Growth and development |
| ACTH (adrenocorticotropin hormone) | Steroid release and production |
| CRF (corticotropin releasing hormone) | Steroid production and stress |
| Insulin | Glucose metabolism |
| Glucagon | Glucose production and metabolism |
| Vasopressin | Water balance |
Neuropeptides function as brain neurotransmitters and as endocrine peptide hormones. Diverse regulatory functions of neuropeptides are illustrated by examples in this table.
In neuroendocrine hormone systems, neuropeptides function as peptide hormones that regulate essential physiological functions in development, growth, homeostasis, responses to environmental conditions, and related [24]. The peptide growth hormone releasing hormone (GHRH) produced in the hypothalamus is secreted to regulate growth hormone production and secretion from the pituitary gland in the regulation of developmental growth and metabolism. Thyrotropin releasing hormone (TRH), also produced in the hypothalamus, stimulates the release of thyrotropin stimulating hormone (TSH) from the pituitary to stimulate thyroxine release of the thyroid gland to control growth, development, and metabolism. The ACTH peptide hormone, released from the pituitary gland, stimulates glucocorticoid steroid hormone secretion from the adrenal cortex. Insulin is a key peptide hormone that regulates blood sugar levels, and is a key factor in diabetes disease. Vasopressin released from the posterior pituitary regulates water balance and kidney function; vasopressin is also known as antidiuretic hormone.
These examples illustrate the diverse actions of neuropeptides in the nervous and neuroendocrine systems.
Neuropeptide biosynthesis achieved by processing of pro-neuropeptide precursors by cysteine and serine protease pathways
Neuropeptides are synthesized as inactive precursors, pro-neuropeptides, that require proteolytic processing to generate the smaller, active neuropeptides of ~3–40 residues [3, 25, 26]. Genetic protease knockout studies in animal model studies have shown that the cysteine proteases cathepsin L and cathepsin V, and the serine subtilisin-like proteases PC1/3 and PC2 participate in processing pro-neuropeptides at paired basic residue cleavage sites (KR, RR, RK, and KK sites) into active neuropeptides (figure 2A) [3, 25, 26]. These two protease pathways include subsequent peptide processing steps by aminopeptidase B (AP-B) [27] and cathepsin H [28] exopeptidases, combined with carboxypeptidase E (CPE) [3, 29], respectively. Protease gene knockout studies combined with cellular studies in model systems show that these cysteine and serine protease pathway components participate in neuropeptide production [3, 25, 26)].
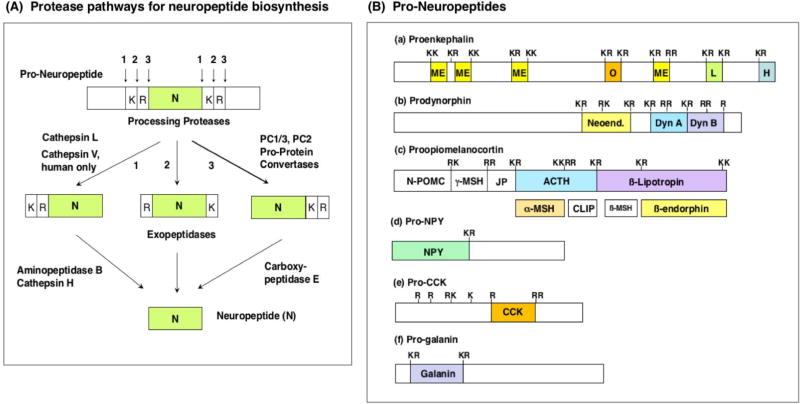
Figure 2. Protease Pathways to Convert Pro-Neuropeptides to Neuropeptides.
A. Protease pathways for neuropeptide biosynthesis. Pro-neuropeptides undergo proteolytic processing at dibasic residue cleavage sites by dual cysteine and serine subtilisin-like protease pathways. The cathepsin L cysteine protease cleavages pro-neuropeptides at paired basic residues. Resultant peptide intermediates require removal of N-terminal basic residues by aminopeptidase B or cathepsin H, and removal of C-terminal basic residues by carboxypeptidase E (CPE). The human-specific cathepsin V cysteine protease functions only in human cells for neuropeptide production, because the cathepsin V gene is present only in the human genome (and not in the genome of other species of organisms). The serine protease pathway consists of the pro-protein convertases (PC) PC1/3 and PC2 that cleave at paired basic residues. Resultant peptide intermediates then require removal of C-terminal basic residues by CPE.
B. Pro-neuropeptide structures. Schematic illustration of the opioid pro-neuropeptide precursors are shown for proenkephalin (PENK), prodynorphin (PDYN), and proopiomelanocortin (POMC), as well as the precursors of neuropeptide Y (NPY), cholecystokinin (CCK), and galanin (GAL). Mature neuropeptides are indicated in colored areas within the pro-neuropeptides. Neuropeptides within the precursors are typically flanked by paired basic residues (Lys-Arg (KR), Lys-Lys(KK), Arg-Lys (RK), and Arg-Arg (RR)), and occasionally at monobasic Arg sites, that are recognized and cleaved by processing proteases to generate mature neuropeptides.
Significantly, cathepsin V is a human-specific protease and, therefore, represents a unique human protease mechanism for production of human neuropeptides [30]. Cathepsin V in human neuroblastoma cells participates in the production of enkephalin and NPY neuropeptides, illustrated by gene silencing and expression of cathepsin V [30]. Cathepsin V and cathepsin L genes are both present in the human genome [30–33] as well as the primate genome [34], but the rodent genomes (mouse and rat, utilized for many neuropeptide studies) and those of several other mammalian genomes express only cathepsin L (not cathepsin V). Human cathepsin V and mouse cathepsin L share similar functions, as demonstrated by findings showing that expression of cathepsin V in the cathepsin L knockout mouse rescues defects in T cell function and keratinocyte proliferation [35, 36]. Human cathepsin V shares greater homology with mouse cathepsin L (74.6% identity in protein sequences) compared with its homology to human cathepsin L (71.5% identify in protein sequences). In contrast to the single mouse cathepsin L gene, the human genome utilizes the two closely related cathepsin V and cathepsin L genes for cathepsin L-like functions.
Neuropeptidomics mass spectrometry is essential for global analyses of neuropeptide structures
Global analyses of diverse neuropeptide structures by peptidomics tandem mass spectrometry (LC-MS/MS) is essential to define the primary peptide sequences combined with post-translational modifications that define distinct active neuropeptides. Notably, high throughput peptidomics LC-MS/MS analyses is necessary to investigate the multitude of diverse neuropeptide structures that are frequently composed of hundreds to thousands of peptides in biological samples. Recent achievements in peptidomics LC-MS/MS technology is rapidly expanding the repertoire of neuropeptides with known biological activities, and, importantly, is increasing discovery of novel neuropeptides that have not previously been identified. Several articles summarize neuropeptidomics research methodologies in humans, mammals, and invertebrates [4–12, 37–39] to advance knowledge of the structural features of neuropeptides.
This review focuses on neuropeptidomics efforts that define proteolytic processing mechanisms that convert pro-neuropeptides to known neuropeptides and previously unknown neuropeptides. Proteolytic processing is essential to transform inactive pro-neuropeptide precursors into active neuropeptide regulators of biological functions.
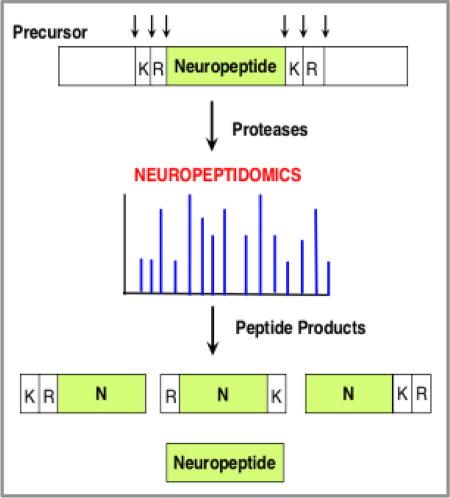
Neuropeptidomics reveals pro-neuropeptide processing at dibasic cleavage sites and non-dibasic cleavage sites
Pro-neuropeptide precursors contain active neuropeptides typically flanked by dibasic protease processing sites that are cleaved by proteases to generate neuropeptides. Within pro-neuropeptides, active neuropeptides are typically flanked by dibasic residues processing sites composed of KR, KK, RR, and RK processing sites, as well as monobasic Arg sites (figure 2B). Processing of pro-neuropeptides can generate multiple, related neuropeptides from a single precursor; for example, multiple enkephalin-related analgesic peptides are generated from proenkephalin (figure 2B.a), and several dynorphin-related peptides involved chronic pain are generated from prodynorphin (shown in figure 2B.b). Further, a pro-neuropeptide may be processed into several distinct neuropeptides of different functions (figure 2B.c); for example, POMC (proopiomelanocortin) is processed to ACTH that regulates steroid secretion, β-lipotropin that is an intermediate peptide that undergoes further processing to generate the β-endorphin analgesic neuropeptides, and α-MSH (α-melanocyte stimulating factor) that regulates skin pigmentation [24]. Some pro-neuropeptides are converted to single neuropeptide molecules, as for the production of NPY, CCK, and galanin (figure 2B.d.e.f).
Neuropeptidomics analyses have demonstrated proteolysis of dibasic processing sites to generate endogenous peptides (figure 3). Neuropeptidomics has analyzed endogenous peptides in secretory vesicles of that produce, store, and secrete neuropeptides [7, 8]. Neuropeptides of large dense core secretory vesicles, isolated from the human adrenal sympathetic nervous system, identified a multitude of peptides. Evaluation of the precursors from which such peptides were derived indicated proteolytic processing at dibasic cleavage sites (KR, KK, RK, and RR sites), illustrated by LOGO maps (figure 3A). These data represent prohormone cleavage sites of proenkephalin, pro-NPY, pro-SAAS, chromogranin A, chromogranin B, and secretoganin II (also known as chromogranin C) pro-neuropeptides [7].
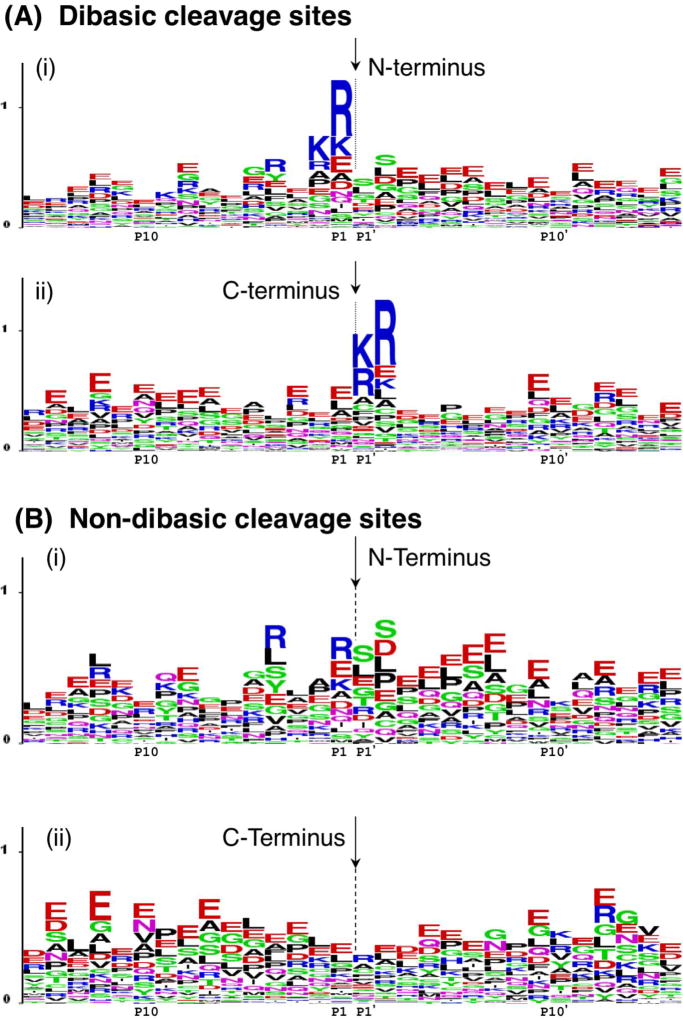
Figure 3. Pro-neuropeptide processing at dibasic and non-dibasic cleavage sites revealed by neuropeptidomics.
A. Dibasic residue processing sites. Dibasic residue sites are typical proteolytic cleavage sites of pro-neuropeptides in secretory vesicles. Sequence LOGO maps of the N- and C-termini of neuropeptides in secretory vesicles were generated by evaluation of adjacent residues to the identified peptides with their pro-neuropeptide precursors [7].
(i) N-terminal cleavage sites of identified neuropeptides. The N-termini of peptides are indicated by the arrow, with illustration of the peptide flanking amino acid sequences present within the pro-neuropeptides. The x-axis shows residues at the P1–P15 positions relative to the cleavage sites at P1-↓P1’ residues. The relative frequency of amino acids at each position is illustrated by the sequence LOGO maps.
(ii) C-terminal cleavage sites of identified neuropeptides. The C-termini of peptides are indicated by the arrow, with residues of respective pro-neuropeptides flanking the C-termini at P1’–P15’ positions.
B. Novel non-dibasic residue processing sites. Non-dibasic residue cleavage sites of pro-neuropeptides are illustrated after removal of the dibasic sites observed in the data of figure 5a.
(i) N-terminal cleavage sites. P1–P15 residues flanking the N-termini of identified peptides within their pro-neuropeptides are illustrated.
(ii) C-terminal cleavage sites. P1’–P15’ residues flanking the C-termini of identified peptides within their pro-neuropeptide precursors are illustrated.
Data for this figure was acquired as reported by Gupta et al., 2010 [7]. Briefly, dense core secretory vesicles from bovine adrenal medulla were isolated in the presence of protease inhibitors, and a low molecular weight peptide pool was prepared by millipore filtration. The sample was subjected to nano-LC-MS/MS peptidomics analyses. The identified peptides were mapped to respective pro-neuropeptide precursors to evaluate proteolytic cleavage sites. These cleavage sites were mapped by LOGO maps.
Novel cleavage sites of pro-neuropeptides were revealed by neuropeptidomics data, and illustrated by LOGO maps (figure 3B). Results showed abundant cleavages occurring at glutamate and leucine at the P1 position of putative cleavage sites (cleavage site is P1-↓P1'); proteases cleaving at glutamate or leucine residues for neuropeptide production have not yet been identified. Also, cleavages at P1' residues of serine, leucine, and glutamate were observed. These cleavage sites flank known and novel peptides within pro-neuropeptides.
In addition to direct identification of endogenous peptides to analyze cleavage sites of pro-neuropeptides, bioinformatics analyses of pro-neuropeptides can be used to predict proteolytic processing sites and potential neuropeptides. NeuroPred is a tool for prediction of neuropeptides derived by proteolytic cleavage of precursor proteins [40, 41]. Such searching algorithms have been developed that analyze genomes for structural hallmarks of neuropeptides comprised of dibasic processing sites of precursors, glycine as a potential amidation site, signal peptide, and neuropeptide motifs [42].
Neuropeptidomics results are necessary for understanding proteolytic processing mechanisms for neuropeptide production. Continued neuropeptidomics data will advance understanding of the proteolytic processing mechanisms for neuropeptide biosynthesis.
Discovery of potentially novel neuropeptides revealed by peptidomics mass spectrometry
Neuropeptidomics has identified known neuropeptides and previously unknown peptides derived from pro-neuropeptides. Mapping the identified peptides to their precursors illustrated the profile of endogenous peptides derived from proenkephalin, pro-NPY, pro-SAAS, as well as the granins of chromogranins A, B, and C [7].
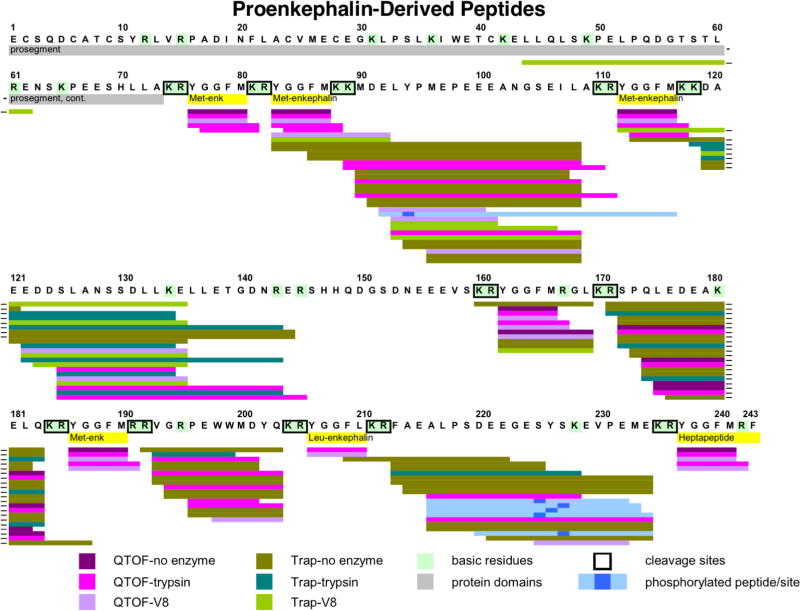
Proenkephalin-derived peptides, identified by neuropeptidomics, consisted of the known (Met)enkephalin pentapeptide (Tyr-Gly-Gly-Phe-Met) (ME), (Leu)enkephalin (Tyr-Gly-Gly-Phe-Leu), and the extended peptides ME-Arg-Gly-Leu and ME-Arg-Phe (figure 4). Further, intervening peptide sequences located between the enkephalin domains were identified, indicating discovery of new peptide products (figure 4). The presence of intervening peptides was enhanced by digestion with trypsin or V8 protease (figure 4).
Figure 4. Neuropeptidomics analyses of proenkephalin-derived peptides without and with trypsin or V8 protease digestion.
To identify peptides by neuropeptidomics, the soluble low molecular weight soluble fraction of human chromaffin granules was undigested, or digested with trypsin or V8 protease for nano-LC-MS/MS identification of peptides. Peptides derived from proenkephalin (PE) were mapped to PE, illustrated by colored lines: QTOF-no enzyme (purple), QTOF-trypsin (bright violet), QTOF-V8 (lavender), Trap-no enzyme (olive), Trap-trypsin (turquoise blue), Trap-V8 (bright green). Active enkephalin neuropeptides within PE are shown in yellow, and dibasic cleavage sites are highlighted by boxes. Phosphorylated peptides are indicated (light blue with phosphorylation site indicated by dark blue square). Hyphens at the end of some lines indicate peptides that were split between two lines in the figure.
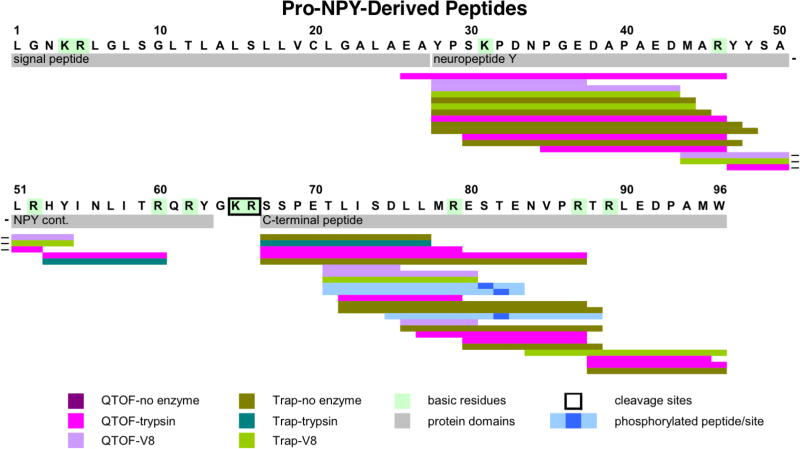
Peptides derived from pro-NPY identified by neuropeptidomics illustrated peptides spanning the NPY domain and the C-terminal domain (figure 5) [7]. Multiple peptides spanned the NPY of 36 residues. Peptides representing the C-terminal peptide domain were identified, which indicated cleavae at a monobasic Arg residue and several other cleavage sites. These data were obtained from undigested, trypsin-digested, and V8 protease-digested conditions.
Figure 5. Neuropeptidomics analyses of proNPY-derived peptides.
Peptides derived from human proNPY (NPY, neuropeptide Y) were analyzed by neuropeptidomics of the low molecular weight soluble pool of human chromaffin secretory vesicles without protease digestion, with trypsin, or with V8 protease digestion followed by nano-LC-MS/MS analyses. Peptides derived from proNPY are mapped, illustrated by colored lines: colored lines: QTOF-no enzyme (purple), QTOF-trypsin (bright violet), QTOF-V8 (lavender), Trap-no enzyme (olive), Trap-trypsin (turquoise blue), Trap-V8 (bright green). Phosphorylated peptides are indicated (light blue with phosphorylation site indicated by dark blue square). ProNPY domains of NPY neuropeptide and the C-terminal peptide are indicated. The signal sequence is also shown since one identified peptide apparently included several residues at the C-terminal end of the putative signal sequence.
The presence of pro-neuropeptide derived intervening peptides and active neuropeptides indicates the potential explosion of peptide discovery by neuropeptidomics. Future investigation of the newly discovered peptides for biological functions will expand the diversity of active neuropeptides.
Challenges in identification and quantitation of very short neuropeptides: future application of metabolomics with neuropeptidomics
A notable limitation of LC-MS/MS methods is that very short neuropeptides of 2–4 residues have not been readily identified, although many short peptide mass spectra are available in the NIST17 mass spectral library [43]. The presence of such very short neuropeptides in biological samples is expected, since very short peptide products of 2–4 residues are predicted from pro-neuropeptides processing at paired basic residue cleavage sites. Identification of very short peptides has encountered bioinformatics challenges since the probability of false positives increases as peptide sequence length decreases [44]. Therefore, very short peptides will be intrinsically more difficult to identify based on probability.
Significantly, metabolomics strategies may be ideally suited to identify and quantify very short peptides, based on their low molecular weight and more hydrophilic nature suitable for metabolomics. As example, TRH is an active neuropeptide composed of the tripeptide Gln-His-Pro that undergoes pyroglutamate and amidation modifications to generate active TRH of pGlu-His-Pro-amide [24]. TRH has been identified by GC-MS (gas chromatography mass spectrometry) [45], a mainstay methodology for metabolomics analyses of small molecules.
Bioinformatics and metabolomics analyses can be used for analyses of very short peptides derived from pro-neuropeptides. As example, bioinformatics analyses show that very short di-peptide, tri-peptide, and tetra-peptides, as well as longer peptides, are predicted to be generated from the NESP55 protein by processing at diba sic cleavage sites. Metabolomics analyses of these very short peptides should be compared to peptidomics LC-MS/MS analyses to advance identification and quantitation of short peptides. Such short peptides are predicted to possess biological activity since NESP55 regulates impulsive behaviors and genomic imprinting, demonstrated by NESP55 gene knockout studies [46]. Importantly, understanding the diversity of active neuropeptides will be enhanced by combining metabolomics and neuropeptidomics analyses with interrogation of biological functions mediated through receptor mechanisms.
Neuropeptidomics for analyses of post-translational modifications of neuropeptides
Neuropeptide primary sequence structures frequently undergo post-translational modifications (PTMS) that modify bioactivities of peptides. Peptide PTMs include C-terminal amidation, N-terminal pyroglutamate, disulfide bonds, phosphorylation, sulfation, acetylation, glycosylation, and others. The PTMs together with proteolytic processing of pro-neuropeptides generate bioactive peptides. C-terminal amidation and N-terminal pyroglutamate modifications occur after proteolytic processing of pro-neuropeptides [47, 48]. The disulfide peptide bonds of insulin occur at the proinsulin stage prior to proteolytic processing [49]. However, it is not yet entirely known whether the PTM events of phosphorylation, sulfation, acetylation, and glycosylation are present on the pro-neuropeptide, or if these modifications occur after proteolytic processing. Significantly, development of neuropeptidomics strategies to define both primary sequences and PTM features of neuropeptide structures will be necessary to understand mechanisms of proteolytic and covalent modifications of neuropeptides.
Several pro-neuropeptides that undergo PTM alterations for neuropeptide production are described here. The POMC precursor is a pro-neuropeptide that undergoes numerous post-translational modifications (PTMs) consisting of proteolysis, acetylation, amidation, phosphorylation, glycosylation, and disulfide linkage to generate mature peptides [50–53]. The TRH (thyrotropin releasing hormone) tripeptide is modified by N-pyroglutamate and C-terminal amidation [24]. Mature insulin contains disulfide linkage of its peptide domains; disulfide formation of proinsulin occurs prior to proteolytic processing [24]. Biological forms of CCK neuropeptides include sulfation [54]. Other types of peptide PTMs include palmitoylation [55] and sulfotyrosine modifications [56, 57], but these have been difficult to identify. Numerous neuropeptides with PTMs function as signaling molecules in cell-cell communication.
Coordinate regulation of families of related neuropeptide molecules in biological systems
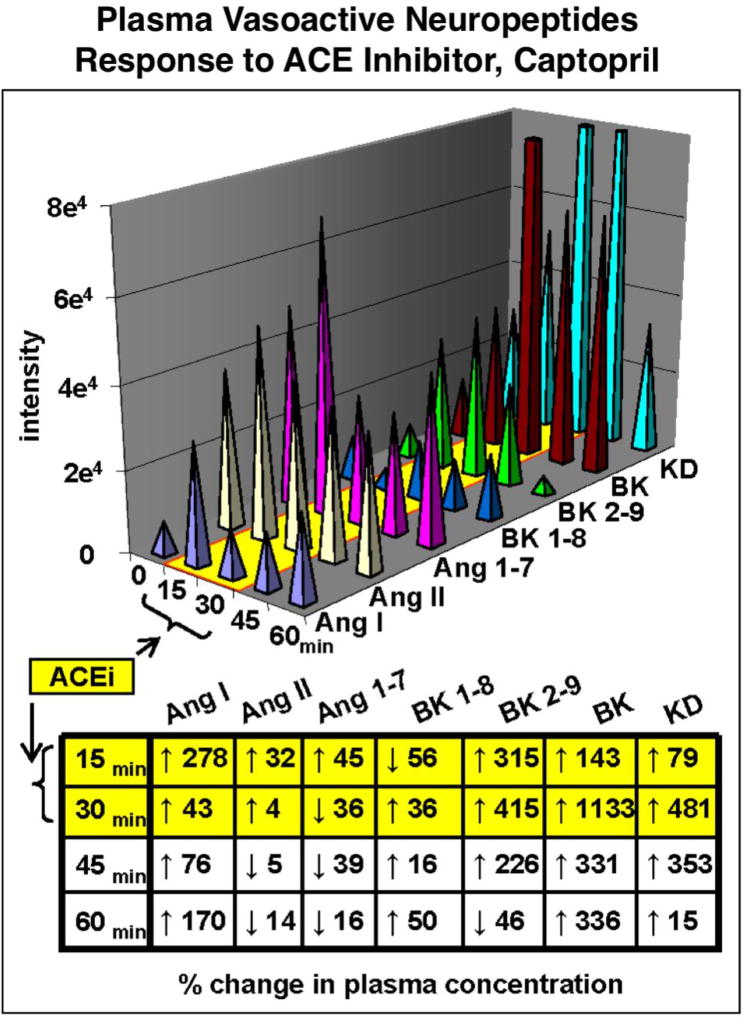
Proteolytic processing of pro-neuropeptides results in families of related neuropeptides that are often coordinately regulated in physiological functions. As example, regulation of blood pressure is achieved with a family of vasoactive neuropeptides that include angiotensin, bradykinin, vasopressin, and related peptides. Angiotensin I, angiotensin II, bradykinin, and kallidin neuropeptides undergo changes in plasma levels in response to administration (to rat) of an inhibitor of the angiotensin converting enzyme (ACE), known as captopril. ACE converts angiotensin I to angiotensin II which elevates blood pressure. Captopril blocks the formation of angiotensin II, and in parallel also regulates bradykinin and kallidin in a temporal fashion (figure 6) [9]. Neuropeptidomics can assess the concurrent changes of neuropeptide profiles in response to drug modulator and during different biological conditions. Neuropeptidomics analyses of human plasma will be valuable to define the changes occurring in neuropeptide profiles that participate in regulating neuroendocrine systems.
Figure 6. Regulation of vasoactive neuropeptide profiles by captopril inhibition of angiotensin converting enzyme (ACE).
Plasma neuropeptides in rat were analyzed by neuropeptidomics after administration of captopril, an anti-hypertensive drug inhibitor of the angiotensin converting enzyme (ACE). Neuropeptidomics was assessed in time-course studies by nano-LC-MS/MS with quantitation using stable isotope-labeled internal standards [9]. Separation of peptides by chromatography and multiple reaction monitoring (MRM) quantitated angiotensin I (Ang I), Ang II, Ang1–7, bradykinin 1–8 (BK 1–8), BK-2–9, and kallidin (KD) vasoactive neuropeptides involved in blood pressure regulation. The angiotensin peptides were significantly reduced by the ACE inhibitor, with parallel increases in bradykinins and kallidin (potent vasodilator). The percent change in plasma concentration at each time point after drug administration is shown. These results demonstrate simultaneous profiling of multiple plasma peptides by neuropeptidomics to assess drug-induced changes in vasoactive neuropeptides.
Defining neuropeptide diversity in the regulation of human health and disease
Neuropeptidomics will advance understanding of cell-cell signaling mechanisms, biomarkers of disease conditions, and neuropeptides as drug targets for discovery and development of new therapeutic agents. Investigation of human systems with model vertebrate and invertebrate organisms together will promote elucidation of human neuropeptide functions in neuroendocrine systems. Further, peptide signaling mechanisms in human cellular models of patient-derived induced pluripotent stem cells (iPSC) neurons and cell types [58] can be elucidated through neuropeptidomics. Future human neuropeptidomics research is expected to undergo accelerated expansion to understand human health.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) to V. Hook (R01 NS094597, R01NS094597-S1) and to O. Fiehn (U24DK097154). C. Lietz was supported by NIH T32MH019934 (awarded to Professor Dilip Jeste, Univ. of Calif., San Diego).
References
- 1.Kastin AJ. Handbook of biologically active peptides. Amsterdam: Elsevier; 2006. [Google Scholar]
- 2.Siegel GJ, Albers RW, Fisher SK, Uhler MD. Basic Neurochemistry. Lippincott Williams and Wilkins; Philadelphia: 1999. pp. 363–382. [Google Scholar]
- 3.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu. Rev. Pharmacol. Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin P, Hou X, Romanova EV, Sweedler JV. Neuropeptidomics: mass spectrometry-based qualitative and quantitative analysis. Methods Mol. Biol. 2011;789:223–236. doi: 10.1007/978-1-61779-310-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye H, Wang J, Tian Z, Ma F, Dowell JA, Bremer Q, Lu G, Baldo B, Li L. Quantitative mass spectrometry reveals food intake-induced neuropeptide level changes in rat brain: functional assessment of selected neuropeptides as feeding regulators. Mol. Cell Proteomics. doi: 10.1074/mcp.RA117.000057. pii: mcp.000057.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson A, Stroth N, Zhang X, Qi H, Fälth M, Sköld K, Hoyer D, Andrén PE, Svenningsson P. Neuropeptidomics of mouse hypothalamus after imipramine treatment reveal somatostatin as a potential mediator of antidepressant effects. Neuropharmacology. 2012;62:347–357. doi: 10.1016/j.neuropharm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Gupta N, Bark SJ, Lu WD, Taupenot L, O'Connor DT, Pevzner P, Hook V. Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing. J. Proteome Res. 2010;9:5065–5075. doi: 10.1021/pr100358b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hook V, Bark S, Gupta N, Lortie M, Lu WD, Bandeira N, Funkelstein L, Wegrzyn J, O'Connor DT, Pevzner P. Neuropeptidomic components generated by proteomic functions in secretory vesicles for cell-cell communication. AAPS J. 2010;12:635–645. doi: 10.1208/s12248-010-9223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lortie M, Bark S, Blantz R, Hook V. Detecting low-abundance vasoactive peptides in plasma: progress toward absolute quantitation using nano liquid chromatography-mass spectrometry. Anal. Biochem. 2009;394:164–170. doi: 10.1016/j.ab.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fricker LD. Neuropeptidomics to study peptide processing in animal models of obesity. Endocrinology. 2007;148:4185–4190. doi: 10.1210/en.2007-0123. [DOI] [PubMed] [Google Scholar]
- 11.Fälth M, Sköld K, Svensson M, Nilsson A, Fenyö D, Andren PE. Neuropeptidomics strategies for specific and sensitive identification of endogenous peptides. Mol. Cell Proteomics. 2007;6:1188–1197. doi: 10.1074/mcp.M700016-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Dowell JA, Heyden WV, Li L. Rat neuropeptidomics by LC-MS/MS and MALDI-FTMS: Enhanced dissection and extraction techniques coupled with 2D RP-RP HPLC. J. Proteome Res. 2006;5:3368–3375. doi: 10.1021/pr0603452. [DOI] [PubMed] [Google Scholar]
- 13.Inturrisi CE. Clinical pharmacology of opioids for pain. Clin. J. Pain. 2002;18:S3–13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76(Pt B):204–217. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podvin S, Yaksh T, Hook V. The Emerging Role of Spinal Dynorphin in Chronic Pain: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol. 2016;56:511–533. doi: 10.1146/annurev-pharmtox-010715-103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Juréus A, Clifton DK, Luo M, Gutshall M, Ma SY, Mufson EJ, Crawley JN. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Counts SE, Perez SE, Kahl U, Bartfai T, Bowser RP, Deecher DC, Mash DC, Crawley JN, Mufson EJ. Galanin: neurobiologic mechanisms and therapeutic potential for Alzheimer's disease. CNS Drug Rev. 2011;7:445–370. doi: 10.1111/j.1527-3458.2001.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degen L, Matzinger D, Drewe J, Beglinger C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides. 2001;22:1265–1269. doi: 10.1016/s0196-9781(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 19.Kageyama H, Takenoya F, Hirako S, Wada N, Kintaka Y, Inoue S, Ota E, Ogawa T, Shioda S. Neuronal circuits involving neuropeptide Y in hypothalamic arcuate nucleus-mediated feeding regulation. Neuropeptides. 2012;46:285–289. doi: 10.1016/j.npep.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Tordoff MG. Calcium: taste, intake, and appetite. Physiol. Rev. 2001;81:1567–1597. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- 21.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev. Mol. Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Avila ED, de Molon RS, de Godoi Gonçalves DA, Camparis CM. Relationship between levels of neuropeptide Substance P in periodontal disease and chronic pain: a literature review. J. Investig. Clin. Dent. 2014;5:91–97. doi: 10.1111/jicd.12087. [DOI] [PubMed] [Google Scholar]
- 23.Benoliel R, Eliav E, Mannes AJ, Caudle RM, Leeman S, Iadarola MJ. Actions of intrathecal diphtheria toxin-substance P fusion protein on models of persistent pain. Pain. 1999;79:243–253. doi: 10.1016/s0304-3959(98)00170-5. [DOI] [PubMed] [Google Scholar]
- 24.Felig P, Baxter JD, Frohman LA. Endocrinology and Metabolism. 3. McGraw Hill, Inc; New York: 1981. [Google Scholar]
- 25.Hook V, Bandeira N. Neuropeptidomics mass spectrometry reveals signaling networks generated by distinct protease pathways in human systems. J. Am. Soc. Mass Spectrom. 2015;26:1970–1980. doi: 10.1007/s13361-015-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 27.Hwang SR, O'Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J. Neurochem. 2007;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 28.Lu WD, Funkelstein L, Toneff T, Reinheckel T, Peters C, Hook V. Cathepsin H functions as an aminopeptidase in secretory vesicles for production of enkephalin and galanin peptide neurotransmitters. J. Neurochem. 2012;122:512–52. doi: 10.1111/j.1471-4159.2012.07788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fricker LD. Carboxypeptidase E. Annu. Rev. Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- 30.Funkelstein L, Lu WD, Koch B, Mosier C, Toneff T, Taupenot L, O'Connor DT, Reinheckel T, Peters C, Hook V. Human cathepsin V protease participates in production of enkephalin and NPY neuropeptide neurotransmitters. J. Biol. Chem. 2012;2287:15232–15241. doi: 10.1074/jbc.M111.310607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brömme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38:2377–2385. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 33.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Zhang YY, Li QY, Cai ZH. Evolutionary History of Cathepsin L (L-like) Family Genes in Vertebrates. Int J Biol Sci. 2015 Jul 14;11(9):1016–25. doi: 10.7150/ijbs.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sevenich L, Hagemann S, Stoeckle C, Tolosa E, Peters C, Reinheckel T. Expression of human cathepsin L or human cathepsin V in mouse thymus mediates positive selection of T helper cells in cathepsin L knock-out mice. Biochimie. 2010;92:1674–1680. doi: 10.1016/j.biochi.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Hagemann S, Günther T, Dennemärker J, Lohmüller T, Brömme D, Schüle R, Peters C, Reinheckel T. The human cysteine protease cathepsin V can compensate for murine cathepsin L in mouse epidermis and hair follicles. Eur. J. Cell Biol. 2004;83:775–780. doi: 10.1078/0171-9335-00404. [DOI] [PubMed] [Google Scholar]
- 37.Hook V, Bandeira N. Neuropeptidomics Mass Spectrometry Reveals Signaling Networks Generated by Distinct Protease Pathways in Human Systems. J. Am. Soc. Mass Spectrom. 2015;26:1970–1980. doi: 10.1007/s13361-015-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchberger A, Yu Q, Li L. Advances in Mass Spectrometric Tools for Probing Neuropeptides. Annu. Rev. Anal. Chem. 2015;8:485–509. doi: 10.1146/annurev-anchem-071114-040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svensson M, Sköld K, Nilsson A, Fälth M, Svenningsson P, Andrén PE. Neuropeptidomics: expanding proteomics downwards. Biochem. Soc. Trans. 2007;35:588–593. doi: 10.1042/BST0350588. [DOI] [PubMed] [Google Scholar]
- 40.Southey BR, Amare A, Zimmerman TA, Rodriguez-Zas SL, Sweedler JV. NeuroPred: a tool to predict cleavage sites in neuropeptide precursors and provide the masses of the resulting peptides. Nucleic Acids Res. 2006 Jul 1;34(Web Server issue):W267–72. doi: 10.1093/nar/gkl161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tegge AN, Southey BR, Sweedler JV, Rodriguez-Zas SL. Comparative analysis of neuropeptide cleavage sites in human, mouse, rat, and cattle. Mamm Genome. 2008 Feb;19(2):106–20. doi: 10.1007/s00335-007-9090-9. [DOI] [PubMed] [Google Scholar]
- 42.Clynen E, Liu F, Husson SJ, Landuyt B, Hayakawa E, Baggerman G, Wets G, Schoofs L. Bioinformatic approaches to the identification of novel neuropeptide precursors. Methods Mol Biol. 2010;615:357–74. doi: 10.1007/978-1-60761-535-4_25. [DOI] [PubMed] [Google Scholar]
- 43.Wallace WE, Ji W, Tchekhovskoi DV, Phinney KW, Stein SE. Mass spectral library quality assurance by inter-library comparison. J. American Society for Mass Spectrometry. 2017;28:733–738. doi: 10.1007/s13361-016-1589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann M, Wilm M. Error-tolerant identification of peptides in sequence databases by peptide sequence tags. Anal. Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- 45.Heki N, Noto M, Hosojima H. Analysis of thyrotropin releasing hormone (TRH) in serum and urine by mass fragmentography using GC-MS (author's transl) Nihon Naibunpi Gakkai Zasshi. 1977;53:690–702. doi: 10.1507/endocrine1927.53.5_690. [DOI] [PubMed] [Google Scholar]
- 46.Dent CL, Humby T, Lewis K, Plagge A, Fischer-Colbrie R, Wilkins JF, Wilkinson LS, Isles AR. Impulsive choices in mice lacking imprinted Nesp55. Genes Brain Behav. 2016;15:693–701. doi: 10.1111/gbb.12316. [DOI] [PubMed] [Google Scholar]
- 47.Eipper BA, Mains RE. Peptide alpha-amidation. Annu. Rev. Physiol. 1988;5:333–344. doi: 10.1146/annurev.ph.50.030188.002001. [DOI] [PubMed] [Google Scholar]
- 48.Schilling S, Wasternack C, Demuth HU. Glutaminyl cyclases from animals and plants: a case of functionally convergent protein evolution. Biol. Chem. 2008;389:983–991. doi: 10.1515/BC.2008.111. [DOI] [PubMed] [Google Scholar]
- 49.Rajpal G, Schuiki I, Liu M, Volchuk A, Arvan P. Action of protein disulfide isomerase on proinsulin exit from endoplasmic reticulum of pancreatic β-cells. J. Biol. Chem. 2012;287:43–47. doi: 10.1074/jbc.C111.279927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi A, Mizusawa K. Posttranslational modifications of proopiomelanocortin in vertebrates and their biological significance. Front. Endocrinol. (Lausanne) 2013;4:143. doi: 10.3389/fendo.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loh YP, Gainer H. Evidence that glycosylation of pro-opiocortin and ACTH influences their proteolysis by trypsin and blood proteases. Mol. Cell Endocrinol. 1980;20:35–44. doi: 10.1016/0303-7207(80)90092-1. [DOI] [PubMed] [Google Scholar]
- 52.Dores RM, Steveson TC, Price ML. A view of the N-acetylation of alpha-melanocyte-stimulating hormone and beta-endorphin from a phylogenetic perspective. Ann N Y Acad Sci. 1993;680:161–174. doi: 10.1111/j.1749-6632.1993.tb19682.x. [DOI] [PubMed] [Google Scholar]
- 53.Bleakman A, Bradbury AF, Darby NJ, Maruthainar K, Smyth DG. Processing reactions in the later stages of hormone activation. Biochimie. 1988;70:3–10. doi: 10.1016/0300-9084(88)90152-6. [DOI] [PubMed] [Google Scholar]
- 54.Vishnuvardhan D, Beinfeld MC. Role of tyrosine sulfation and serine phosphorylation in the processing of procholecystokinin to amidated cholecystokinin and its secretion in transfected AtT-20 cells. Biochemistry. 2000 Nov 14;39(45):13825–30. doi: 10.1021/bi0011072. [DOI] [PubMed] [Google Scholar]
- 55.Ji Y, Leymarie N, Haeussler DJ, Bachschmid MM, Costello CE, Lin C. Direct detection of S-palmitoylation by mass spectrometry. Anal. Chem. 2013;85:11952–11959. doi: 10.1021/ac402850s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G, Zhang Y, Trinidad JC, Dann C., 3rd Distinguishing Sulfotyrosine Containing Peptides from their Phosphotyrosine Counterparts Using Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2018 doi: 10.1007/s13361-017-1854-1. [DOI] [PubMed] [Google Scholar]
- 57.Hersberger KE, Håkansson K. Characterization of O-sulfopeptides by negative ion mode tandem mass spectrometry: superior performance of negative ion electron capture dissociation. Anal. Chem. 2012;84:6370–6377. doi: 10.1021/ac301536r. [DOI] [PubMed] [Google Scholar]
- 58.Hook V, Brennand KJ, Kim Y, Toneff T, Funkelstein L, Lee KC, Ziegler M, Gage FH. Human iPSC neurons display activity-dependent neurotransmitter secretion: aberrant catecholamine levels in schizophrenia neurons. Stem Cell Reports. 2014;3:531–538. doi: 10.1016/j.stemcr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]