Abstract
Sickle cell disease (SCD) is associated with intravascular hemolysis and oxidative inhibition of nitric oxide (NO) signaling. BAY 54-6544 is a small-molecule activator of oxidized soluble guanylate cyclase (sGC), which, unlike endogenous NO and the sGC stimulator, BAY 41-8543, preferentially binds and activates heme-free, NO-insensitive sGC to restore enzymatic cGMP production. We tested orally delivered sGC activator, BAY 54-6544 (17 mg/kg/d), sGC stimulator, BAY 41-8543, sildenafil, and placebo for 4–12 weeks in the Berkeley transgenic mouse model of SCD (BERK-SCD) and their hemizygous (Hemi) littermate controls (BERK-Hemi). Right ventricular (RV) maximum systolic pressure (RVmaxSP) was measured using micro right-heart catheterization. RV hypertrophy (RVH) was determined using Fulton’s index and RV corrected weight (ratio of RV to tibia). Pulmonary artery vasoreactivity was tested for endothelium-dependent and -independent vessel relaxation. Right-heart catheterization revealed higher RVmaxSP and RVH in BERK-SCD versus BERK-Hemi, which worsened with age. Treatment with the sGC activator more effectively lowered RVmaxSP and RVH, with 90-day treatment delivering superior results, when compared with other treatments and placebo groups. In myography experiments, acetylcholine-induced (endothelium-dependent) and sodium-nitroprusside–induced (endothelium-independent NO donor) relaxation of the pulmonary artery harvested from placebo-treated BERK-SCD was impaired relative to BERK-Hemi but improved after therapy with sGC activator. By contrast, no significant effect for sGC stimulator or sildenafil was observed in BERK-SCD. These findings suggest that sGC is oxidized in the pulmonary arteries of transgenic SCD mice, leading to blunted responses to NO, and that the sGC activator, BAY 54-6544, may represent a novel therapy for SCD-associated pulmonary arterial hypertension and cardiac remodeling.
Keywords: sickle cell disease, pulmonary hypertension, soluble guanylate cyclase
Clinical Relevance
The clinical and scientific importance of the findings reported in this manuscript are highly relevant to the pulmonary vasculopathy of sickle cell disease (SCD) and other group V hemolytic diseases, whereby chronic intravascular hemolysis promotes endothelial dysfunction and pulmonary vascular remodeling with progressive resistance to blood flow, facilitating the development of pulmonary hypertension. The submitted manuscript reports the first preclinical data for a direct comparison between soluble guanylate cyclase (sGC) activators versus sGC stimulators for the treatment of pulmonary vasculopathy in SCD. The study shows that bypassing the nitric oxide pathway and activating sGC directly to increase cGMP and induce vaso-relaxation may offer a much-needed therapeutic advantage for pulmonary arterial hypertension in patients with SCD.
Nitric oxide (NO) is an endothelial-derived mediator of vasodilation and vascular homeostasis, and has been proposed as a therapy for patients with sickle cell disease (SCD)–associated pulmonary arterial hypertension (PAH) (1–4). Under physiological conditions, NO activates soluble guanylate cyclase (sGC) by binding to the ferrous (Fe2+) heme active site in the β subunit of the sGC, which stimulates the enzyme to convert GTP to cGMP and promotes downstream vascular smooth muscle relaxation through protein kinase G (5–7). It is known that reactive oxygen species (ROS) can oxidize sGC heme iron from its ferrous to ferric (Fe3+) form. The consequence is a weak affinity for NO that also destabilizes the binding of the heme group and leads to heme-free sGC, which is insensitive to NO (8–10). Although we hypothesize that sGC heme oxidation, and subsequent heme loss, contribute to impaired NO signaling in SCD, this has not been assessed experimentally. The oxidative and proinflammatory stress that characterizes SCD (1, 11–14) can inactivate many proteins and enzymes involved in pathways that regulate vascular homeostasis, suggesting that similar effects on sGC are likely (15–18). Determining the redox state of the sGC heme in pulmonary hypertension (PH) is now possible with the development of experimental drugs that can specifically bind to and activate the heme-dependent and heme-independent forms of sGC (19). Indeed, the class of drug referred to as an sGC activator (BAY 54-6544 used in the current studies) can bind to the oxidized Fe3+ heme and heme-free sGC (apo sGC), activating sGC even in the absence of NO (20). A second class of sGC-targeting drug is referred to as an sGC stimulator (BAY 41-8543 in the current studies), which requires the Fe2+ heme iron of the nonoxidized sGC. It both stimulates the sGC independently of NO and amplifies cGMP signaling in the presence of NO (21, 22). Differential responses to the two drugs provide a molecular probe to assess sGC redox state.
Currently, hydroxyurea is the only U.S. Food and Drug Administration (FDA)–approved treatment for patients with SCD. Although highly effective, only about one-half of patients take the medication and responses can be incomplete (23). PDE5 inhibitors, such as sildenafil, have been effective on other forms of PAH (24); however, they require adequate NO bioavailability and sGC in its Fe2+ reduced form. Their failure to improve outcomes in patients with SCD has largely been associated with an apparent amplification of pain (25). Targeting vascular smooth muscle cell sGC directly may have important therapeutic potential in SCD, where NO bioavailability and synthesis is low, and oxidative burden is high, likely contributing to oxidation of sGC and formation of apo sGC (1, 10, 26). Indeed, a small molecule activator may be advantageous when compared with authentic endogenous or therapeutically applied NO, as the latter will react with ROS to form peroxynitrite and other potentially deleterious reactive nitrogen species.
Two phase II studies in patients with group I and group IV PH (NCT02562235, CT02191137) showed significant benefit for patients treated with the sGC stimulator, riociguat (27, 28). The stimulator achieved FDA approval in 2013 for the treatment of PAH and chronic thromboembolic pulmonary hypertension, and is currently distributed as Adempas (Bayer Healthcare Pharmaceuticals) (29, 30). In addition, the sGC stimulator, riociguat, was shown to improve cardiac output, stroke volume, and pulmonary vascular resistance (PVR) in patients with PH associated with systolic left ventricular (LV) dysfunction in a phase IIb double-blind, randomized, placebo-controlled study (31). Interestingly, in these studies, there was no evident limb or back pain, or muscle aches, as have been observed in clinical studies of PDE5 inhibitors. Taking into consideration that the sGC stimulators target the heme-containing enzyme, it might be of specific interest to study the sGC activators, which target the oxidized sGC form that may play an even more important role in SCD.
The present study compares oral treatment of sGC stimulator, BAY 41-8543, and the sGC activator, BAY 54-6544, in an animal model of SCD that has been shown to develop PAH spontaneously with advancing age (6–7 mo). Previous studies by our group using the Berkeley transgenic mouse model of SCD (BERK-SCD) mouse have shown that this mouse develops a hematologic, vascular, and pulmonary phenotype with age that is similar to the human phenotype (32, 33): severe hemolytic anemia, intravascular leukocytosis and congestion of pulmonary vessels, elevated PVR, decreased pulmonary and global NO bioavailability and responsiveness, spontaneous right ventricular (RV) hypertrophy and elevated pulmonary and RV pressures with right heart failure (1, 34). The current study reports superior effects for the sGC activator, BAY 54-6544, in SCD-associated PH, which suggests a major role for sGC oxidation and formation of apo sGC in the development of the disease.
Methods
SCD mice (BERK-SCD) and their littermate controls (BERK-hemizygous [Hemi]) with transgene insertions encoding for human α- and β-sickle hemoglobin used in studies were 2–4 months of age. Genomic DNA of BERK-SCD and BERK-Hemi mice was analyzed by PCR as previously described (33). Hemoglobin electrophoresis, performed with the Sebia Capillary System (Sebia), using the manufacturer’s reagents and guidelines, was used to confirm BERK-SCD and BERK-Hemi hemoglobin phenotypes.
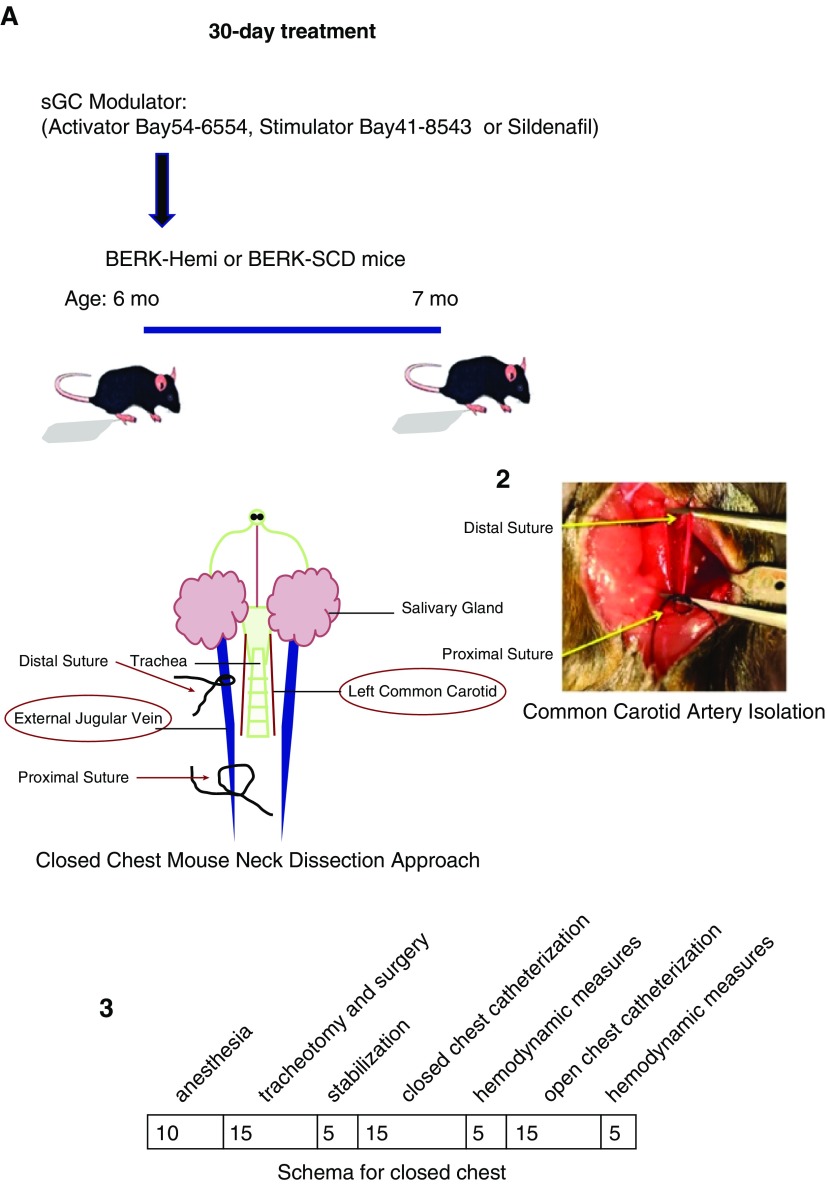
Mice were placed on the following experimental treatment diets for 5, 30, or 90 days: placebo; sGC stimulator (BAY 41-8543, 150 ppm); sGC activator (BAY 54-6544, 80 ppm) manufactured by Sniff Spezialdiäten GmbH (Soest) and provided by Bayer AG; or sildenafil (Pfizer) compounded (1,500 ppm) with Transgenic Dough diet chow (Bio-Serv). The effects of treatment diets on plasma drug levels and hemodynamic measurements were determined in plasma using high-performance liquid chromatography coupled to mass spectrometry and a radiotelemetry system (PA-C10; Data Science International), respectively. The circadian rhythm of mean arterial pressure and heart rate (HR) was monitored for 3–4 days in conscious, unrestrained mice, as described previously (35).
Microcatheterization studies of RV maximum systolic pressure (RVmaxSP) were performed using isoflurane for sedation and etomidate/urethane for anesthesia. Using a closed-chest approach, mice were catheterized via the external jugular vein, from which a micro pressure–volume catheter was advanced through the right atrium into the RV of the heart. The catheter was allowed to stabilize in the center of the RV for 2–5 minutes before recording pressure and volume for later offline analysis using IOX2 Software (EMKA Technologies).
Complete and cellular differential counts, which included data on hemoglobin, hematocrit, mean corpuscular volume (MCV), and red blood cell distribution width (RDW), were measured using the HemaVet HV950 (Drew Scientific Inc.) in EDTA-anticoagulated blood samples collected from animals after RVmaxSP recording. Reticulocyte counts were acquired using thiazole orange (Sigma) and flow cytometry, as described in a previously validated method (36).
Endothelium-dependent and -independent relaxation responses of second-order pulmonary arteries to cumulative doses of acetylcholine (Ach) and sodium nitroprusside (SNP) were evaluated using two-pin wire myography (Multiple Myograph Model 610 M; DMT).
Statistical Analysis
Results are presented as mean (±SEM). Only microcatheterization-derived data satisfying the following criteria was deemed suitable for inclusion in statistical analyses: artifact-free RV pressure waveform; HR of 400 bpm or greater; and RVmaxSP of 15 mm Hg or greater.
Statistical analysis of all data was performed offline with PRISM data analysis software (GraphPad 7.0a; GraphPad Software). A value of P less than 0.05 was considered statistically significant. Response data between groups were analyzed using Student’s unpaired t test.
Details on chemicals, mice, experimental treatments, genotyping, pharmacokinetics, anesthesia, radiotelemetry measurements of blood pressure, closed chest microcatheterization of the RV for RVmaxSP, hematological phenotyping, and wire myography are presented in the data supplement.
Results
Pharmacokinetic and Systemic Hemodynamic Effects of Orally Dosed sGC Modulators
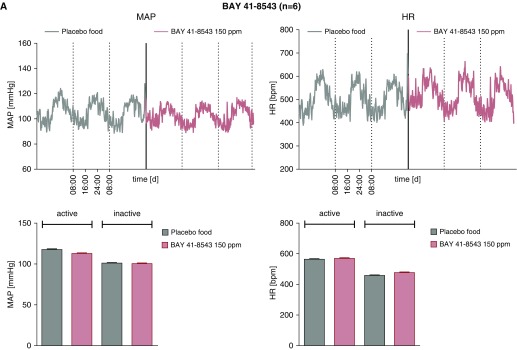
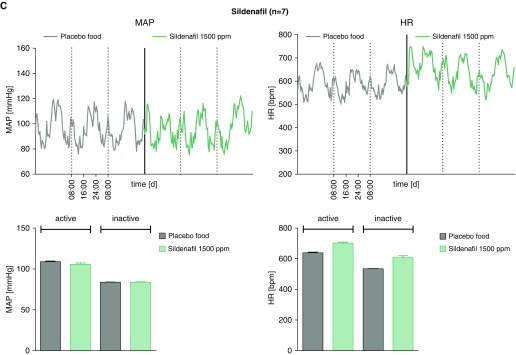
To establish oral dosing that did not change mean arterial blood pressures, C57B/l6 mice were treated with rodent chow compounded with placebo, sGC activator (BAY 54-6544, 17 mg/kg/d), sGC stimulator (BAY 41-8543; 30 mg/kg/d), or the PDE5 inhibitor, sildenafil, as previously described (37). Plasma drug levels were measured by using high-performance liquid chromatography coupled to mass spectrometry, as previously described (32). After 5 days of treatment, arterial blood pressures (systolic, diastolic, and mean) and HR measurements were made using surgically implanted radiotelemetry devices (Figures 1A–1C). Mice were maintained without anesthesia or restraint during the acquisition of blood pressure and HR data. No significant effect was seen for the experimental treatment protocols on BP and HR, whether measured during periods of activity (night) or inactivity (day). In a separate group of animals, BAY 54-6544 and BAY 41-8543 in chow were fed to BERK-SCD mice for 30 days. Average plasma drug levels for BAY 54-6544 and BAY 41-8543 were approximately 28.5 ug/L and approximately 17.5 ug/L, respectively (see Figure E1 in the data supplement). These pilot data informed the dose selection for subsequent studies using the sGC modulators or sildenafil for PAH therapy in BERK mice.
Figure 1.
Therapeutic drug levels of soluble guanylate cyclase (sGC) modulators, BAY 41-8543 and BAY 54-6544, do not affect systemic blood pressure and heart rate (HR) in wild-type C57/Bl6 mice. Mean arterial pressures (MAPs) and HR in mice treated for 5 days with placebo versus the sGC modulators, BAY 41-8543 (150 ppm, n = 4 [A]), BAY 54-6544 (80 ppm, n = 5 [B]), or the PDE5 inhibitor, sildenafil (1,500 ppm, n = 7 [C]), during active and inactive (rest) states.
Severe Hemolytic Anemia in BERK-SCD Mice Is Not Affected by 30-Day Treatment with sGC Modulators
To determine if there are systemic effects of the drugs that modulate the SCD phenotype, we evaluated hematological indices before and after therapy. We measured hemoglobin, hematocrit, MCV, RDW, and reticulocyte count in animals treated for 30 days with the sGC modulator or placebo chow, using same-age littermates for controls. Importantly, we observed no statistically significant difference in the groups treated with sGC activator, BAY 54-6544, or sGC stimulator, BAY 41-8543, when compared with the placebo group (Table 1).
Table 1.
Blood Cell Indices for Berkeley-Hemizygous (Control) Versus Berkeley–Sickle Cell Disease Mice with/without Treatments
| BERK-Hemi Placebo (n = 13–30) | BERK-SCD |
|||
|---|---|---|---|---|
| Placebo |
BAY 54-6544 |
BAY 41-8543 |
||
| (n = 10–17) | (n = 6–8) | (n = 7–8) | ||
| Hct, % | 36.7 ± 1.3 | 18.7 ± 0.8* | 17.3 ± 1.2* | 18.1 ± 2.1* |
| Hgb, g/dL | 10.7 ± 0.3 | 5.1 ± 0.2† | 4.8 ± 0.3† | 4.9 ± 0.5† |
| MCV, fL | 34.2 ± 1.7 | 39.6 ± 0.9* | 38.2 ± 0.4‡ | 37.7 ± 0.5‡ |
| RDW, % | 20.7 ± 0.3 | 28.0 ± 0.7* | 27.7 ± 0.6† | 26.9 ± 0.4‡ |
| Retic, 106 | 6.4 ± 1.9 | 46.9 ± 7.1* | 46.1 ± 0.9* | 39.6 ± 2.1‡ |
| WBC, 103 | 5.9 ± 0.9 | 22.9 ± 4.0† | 21.6 ± 4.7‡ | 16.9 ± 7.4§ |
| PLT, 103 | 566.1 ± 52.8 | 367.8 ± 40.8† | 257.3 ± 75.6‡ | 308.6 ± 53.7 |
Definition of abbreviations: BERK = Berkeley; Hct = hematocrit; hemi = hemizygous; Hgb = total hemoglobin; MCV = mean corpuscular volume; PLT = platelets; RDW = red blood cell distribution width; Retic = reticulocytes; SCD = sickle cell disease; WBC = leukocytes.
Values are ± SEM using unpaired Student’s t test. Red cell indices for control (BERK-Hemi) and SCD mice with/out soluble guanylate cyclase modulators.
P < 0.0001 versus control.
P < 0.0005 versus control.
P < 0.05 versus control.
P < 0.005 versus control.
Effects of 30-Day sGC Modulator Treatment on RVmaxSP and RV Remodeling in BERK-SCD Mice
The development of PAH by 6 months of age in the humanized, transgenic mouse model of SCD (referred to as BERK-SCD) used in our study was confirmed in a pilot study by measurements of RVmaxSP (Figure E2), corrected RV weight (RV weight adjusted for tibia length), and Fulton’s index (RV divided by the LV plus septal [S] weight; RV / LV + S) (data not shown).
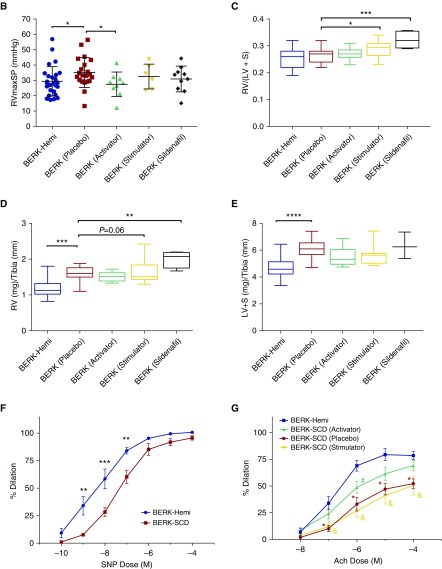
The activator, BAY 54-6544, and stimulator, BAY 41-8543, were subsequently examined for their effects on the PAH cardiophenotype. Additional age-matched groups of mice were treated with placebo or the PDE5 inhibitor, sildenafil, for comparison purposes. After 30 days of treatment in chow, mice were anesthetized with etomidate/urethane and closed-chest microcatheterization measurements of RVmaxSP were made. BERK-SCD mice (placebo) had significantly higher RVmaxSP in comparison to the BERK-Hemi group (P = 0.048 by Student’s t test). BAY 54-6544 decreased RVmaxSP in BERK-SCD mice when compared with the placebo group (P = 0.047; Figure 2B). By contrast, the BAY 41-8543 and sildenafil groups showed no treatment-associated change in RVmaxSP when compared with placebo. There was no effect of the sGC activator, BAY 54-6544, on measures of cardiac remodeling that often accompanies PAH (Figures 2C and 2D). Unexpectedly, the sGC stimulator, BAY 41-8543, enlarged the Fulton’s index (P = 0.048) and corrected RV weight (P = 0.064) in BERK-SCD mice when compared with their counterparts treated with placebo (Figures 2C and 2D). The PDE5 inhibitor, sildenafil, produced the greatest RV enlargement when measured by Fulton’s index (P = 0.0009) and corrected RV weight (P = 0.002) compared with placebo (Figures 2C and 2D). BERK-SCD mice exhibited left-heart remodeling, as measured by corrected LV weight (LV + S / tibia), when compared with BERK-Hemi controls (P < 0.0001), which none of the treatments was effective at normalizing.
Figure 2.
Right ventricular (RV) systolic pressure (RVmaxSP) in 6- and 7-month-old Berkeley (BERK)–sickle cell disease (SCD) mice is attenuated by 30 days of treatment with the sGC activator, BAY 54-6544. (A) Schematic of 30-day treatment protocol and closed-chest right-heart catheterization approach. (B) Right-heart catheterization measurements of RVmaxSP in BERK-hemizygous (Hemi; control, n = 25) and BERK-SCD mice treated with placebo (BERK-Placebo, n = 21), BAY 54-6544 (BERK-Activator, n = 9), BAY 41-8543 (BERK-Stimulator, n = 6), and Sildenafil (BERK-Sildenafil, n = 10). (C) Fulton’s index in BERK-Hemi (control, n = 26) and BERK-SCD mice treated with placebo (BERK-Placebo, n = 24), BAY 54-6544 (BERK-Activator, n = 14), BAY 41-8543 (BERK-Stimulator, n = 8), and Sildenafil (BERK-Sildenafil, n = 4). (D and E) Corrected weights for RV and left ventricle (LV) + septum (LV + S), determined by ratio of ventricle weight to tibia length, in BERK-Hemi (control, n = 28) and BERK-SCD mice treated with placebo (BERK-Placebo, n = 19), BAY 54-6544 (BERK-Activator, n = 10), BAY 41-8543 (BERK-Stimulator, n = 8), or Sildenafil (BERK-Sildenafil, n = 4) for 30 days. Myography studies indicate endothelial cell dysfunction in pulmonary arteries of 6-month-old BERK-SCD mice that is attenuated by acute treatment with the sGC activator BAY 54-6544. (F) Measurements of sodium nitroprusside (SNP)–stimulated dilation of pulmonary arteries from BERK-SCD mice relative to control mice (BERK-Hemi) (n = 5 per group). All statistical analyses performed using unpaired Student’s t test: *P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001. (G) Measurements of acetylcholine (Ach)-stimulated dilation of pulmonary arteries from control mice (BERK-Hemi, n = 5), BERK-SCD mice treated with placebo (BERK-Placebo, n = 3), BAY54-6544 (BERK-Activator, n = 7), or BAY 41-8543 (BERK-Stimulator, n = 4) for 30 days. *P < 0.05 for BERK-SCD (Placebo) versus BERK-Hemi; #P < 0.05 for BERK-SCD (BAY 54-6544) versus BERK-Hemi, and &P < 0.05 for BERK-SCD (BAY 41-8543) versus BERK-Hemi using unpaired Student’s t test.
Oral BAY 54-6544 Treatment Improves Pulmonary Endothelial Cell Function in SCD Mice
Experiments using wire myography were conducted to test the endothelial function of second-order pulmonary arteries in 6-month-old BERK-SCD mice. Consistent with the impaired NO responsiveness seen in patients with SCD (4), ex vivo relaxation responses of pulmonary arteries from the BERK-SCD group (n = 5) stimulated with the NO donor, SNP, were blunted when compared with the age-matched littermate BERK-Hemi control group (P < 0.05 at 10−9–10−7 M; Figure 2F). Relaxation responses of vessels from BERK-SCD mice were also significantly blunted to the endothelial cell-dependent vasodilator, Ach, when compared with vessels from the BERK-Hemi group (P < 0.05 at concentrations 10−7–10−4 M; Figure 2G, Table 2). Importantly, 30-day treatment with the sGC activator, BAY 54-6544, improved Ach-mediated (concentrations 10−7, 10−5–10−4 M) relaxation in pulmonary arteries from BERK-SCD mice relative to the BERK-Hemi group (Figure 2G, Table 2). By contrast, 30-day treatment with the sGC stimulator, BAY 41-8543, was largely without effect (Figure 2G, Table 2). These data are consistent with the endothelial cell dysfunction and NO resistance that has been described in the pulmonary vasculature of BERK-SCD mice and patients with SCD (4, 38), and apparent improvements after 30-day exposure to sGC activator (1, 17).
Table 2.
Ex vivo Pulmonary Artery Vasorelaxation Responses to Acetylcholine Dose Response
| Placebo | BAY 54-6544 | BAY 41-8543 | |
|---|---|---|---|
| Ach 10−8 | 0.301 | 0.622 | 0.458 |
| Ach 10−7 | 0.032 | 0.239 | 0.032 |
| Ach 10−6 | 0.005 | 0.027 | 0.001 |
| Ach 10−5 | 0.008 | 0.065 | 0.005 |
| Ach 10−4 | 0.006 | 0.199 | 0.017 |
Definition of abbreviation: Ach = acetylcholine.
P values for Berkeley–sickle cell disease with indicated treatment versus BERK-hemizygous. Data analyzed by unpaired Student’s t test.
Effects of 90-Day sGC Modulator Treatment on RVmaxSP and RV Remodeling in BERK-SCD Mice
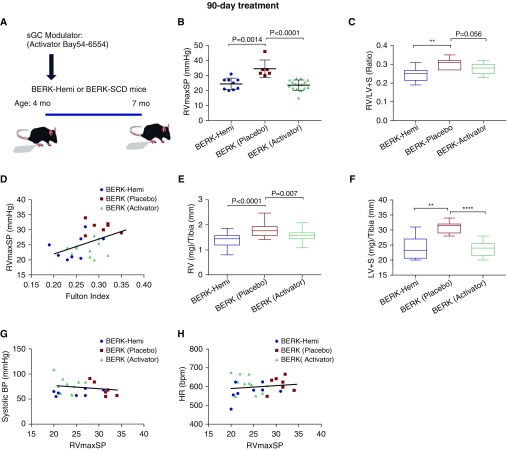
To determine whether the reduction in RVmaxSP by the 30-day sGC activator treatment could improve RV function in BERK-SCD mice with earlier and longer treatment, separate studies were conducted using 90-day feeding of the sGC activator, BAY 54-6544, initiated at 4 months of age and terminated 3 months later (Figure 3A). Plasma cGMP levels did not differ between groups (Figure E3), a likely consequence of their long half-life and regulation by the particulate, rather than soluble, form of guanylate cyclase (39–41). However, RVmaxSP in the BERK-SCD mice was significantly attenuated by the 90-day treatment with the sGC activator, BAY 54-6544 (n = 11; 23.7 ± 0.9; P = 0.0001), when compared with placebo (n = 7, 30.7 ± 2.4) (Figure 3B). RV remodeling, as measured by Fulton’s index (Figure 3C), tended to improve in the BERK-SCD mice treated with the activator, BAY 54-6544 (n = 11, 0.28 ± 0.009; P = 0.056) when compared with placebo (n = 7, 0.30 ± 0.006). RVmaxSP and Fulton’s index were significantly correlated (Figure 3D). The sGC activator treatment also improved the corrected RV weight (RV:tibia ratio, P = 0.007; Figure 3E) and decreased medial layer thickness (P = 0.057; Figure E4). The LV weight (LV + S:tibia ratio) was also increased in the SCD mice (BERK-Placebo versus BERK-Hemi, P = 0.001), and likewise improved with activator therapy (Figure 3F). Notably, effects for the sGC activator on RVmaxSP in the SCD mice (BERK-Activator) did not associate with changes to systemic blood pressure (Figure 3G) or HR (Figure 3H). The persistent insensitivity to NO signaling that was detected in isolated pulmonary arteries from the BAY 54-6544–treated animals, as measured by ex vivo myography experiments with SNP, is consistent with reported NO-independent beneficial effects for the sGC activator on vascular smooth muscle function (Figure E5).
Figure 3.
BAY 54-6544 treatment for 90 days lowers RVmaxSP and cardiac remodeling in 6- and 7-month-old BERK–SCD mice. (A) Schematic of 90-day treatment protocol in BERK-Hemi (control, n = 8) and BERK-SCD mice treated with placebo (BERK-Placebo, n = 7) or BAY 54-6544 (BERK-Activator, n = 11). (B and C) Measurements of RVmaxSP and Fulton’s index. Student’s t test used for statistical analysis of RVmaxSP data. (D) RVmaxSP versus Fulton’s index analyzed by Pearson’s correlation coefficient (R2 = 0.21 and P = 0.02). **P = 0.0057. (E and F) Corrected RV weight and LV weight. (G and H) Systemic blood pressure (BP) and HR versus RVmaxSP analyzed by Pearson’s correlation coefficient. **P = 0.0019, ****P < 0.0001.
Discussion
In SCD, chronic intravascular hemolysis promotes endothelial dysfunction and pulmonary vascular remodeling with progressive resistance to blood flow, facilitating the development of PH (17). There are currently no proven therapies to directly manage vasculopathy in SCD patients. A recent phase II trial of sildenafil for the treatment of PH in SCD (NCT00492531) was halted secondary to significant increases in the rate of hospitalization for pain, renewing the urgent need for novel drugs with effective and safe therapeutic profiles (25, 42). Several recent studies have demonstrated the effectiveness of the sGC stimulator, riociguat, for PAH and chronic thromboembolic pulmonary hypertension, two forms of PH that can affect patients with SCD (29, 30). However, the 12-week treatment period was well tolerated, with no increase in adverse clinical events, and the rate of mortality and heart failure hospitalization were lower when compared with placebo (43).
The sGC stimulators bind to the native reduced (Fe2+) form of the sGC enzyme, and stimulate the enzyme even when low levels of the NO ligand are available (26). By contrast, the sGC activators work on sGC enzymes with oxidized heme groups or the heme -free apo-enzyme, irrespective of NO availability. Consistent with this mechanism, studies uniformly identify a more potent response to the sGC activator, cinaciguat, in situations of high oxidative stress (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one or hyperoxia) (20). SCD drives significant intravascular oxidative stress, wherein ROS are generated by Fenton chemistry and peroxidase reactions of cell-free plasma hemoglobin and heme, up-regulated expression and activity of oxidative enzymes (nicotinamide adenine dinucleotide phosphate-oxidase and xanthine oxidase), and increase elaboration from mitochrondia (18, 44–46). This oxidative milieu impairs normal NO signaling mechanisms, including the NO→sGC→cGMP pathway. NO activation of sGC to produce cGMP requires the sGC enzyme’s heme to be reduced (Fe2+ form). NO-scavenging, ROS-driven oxidation of sGC and suppressed activity or expression of the sGC heme iron reductase, cytochrome b5 reductase 3, may combine to produce a relative NO-insensitive sGC in SCD. Indeed, a state of NO insensitivity has been linked to the development of PH in other models (1, 47, 48). Therefore, sGC activators may be superior to sGC stimulators for the treatment of vasculopathy in SCD, but preclinical data and a direct comparison are yet to be explored.
After 30 days of treatment, the sGC activator outperformed the sGC stimulator at lowering RVmaxSP in our murine model of SCD-associated PAH. After 90 days of treatment with the activator, less cardiac remodeling was observed, suggesting reverse remodeling with sustained reductions in PVR. PAH is typically associated with structural, hypertrophic change to the right heart due to elevated PVR, which is usually detected by comparing RV free wall mass to LV + S mass (Fulton’s index) (49). We also observed left-heart hypertrophy in these mice. The calculations of corrected RV and LV weights (RV / tibia and LV + S / tibia, respectively) that confirm a therapeutic effect for the sGC activator on heart remodeling in this study also indicate concomitant improvement in left-heart hypertrophy in the BERK-SCD mice. Importantly, the hemodynamic and cardiac remodeling effects of the sGC activator were independent of changes in systemic blood pressure and HR, which appeared unaffected using this oral dosing regimen.
Data from our ex vivo studies of pulmonary artery relaxation responses to the sGC modulators support our in vivo data. Our findings showing altered NO signaling in SNP- and Ach-stimulated pulmonary arteries from the BERK-SCD mice agree with a state of NO resistance in SCD. Blood flow responses to infusions of NO donor treatments in humans and mice with SCD have previously been shown to be similarly impaired (1–4, 50). The ability of the NO-independent activator, BAY 54-6544, to improve relaxation responses of the BERK-SCD vessels to Ach, which works via endothelial-dependent activity, indicates an important role for sGC in restoring normal vascular homeostasis. Although the mechanism for this improvement is not clear at this time, the restoration of the enzyme’s cGMP-producing activity effectively improves vasodilation (2, 51–54), which could be expected to lessen red blood cell activation and adhesion/occlusion events that promote endothelial cell injury (55, 56). Although we cannot rule out an effect for enhanced red blood cell sGC activity in the improved pulmonary hemodynamics achieved with the BAY 54-6544 activator therapy, our observation of similar reticulocyte counts, MCV, RDW, total hemoglobin, and hematocrit among the BERK-SCD groups, irrespective of treatment, suggest little direct effect for the BAY 54-6544 activator on red blood cell rheology. NO-independent cytoprotective effects for cGMP-sensitive endothelial HO-1 gene may also account for the observed effects (57). The ability of Ach to dilate arterioles by hyperpolarization via potassium channels is not entirely dependent on sGC (58), and may involve effects on calcium handling (59).
Bypassing the NO pathway and activating sGC directly to increase cGMP and induce vasorelaxation may offer a potential therapeutic advantage for PAH in hemolytic diseases such as SCD, wherein the oxidative stress in the vasculature may render much of the sGC oxidized and inactive. Raat and colleagues (41) recently demonstrated in rats that plasma cell-free hemoglobin causes vasoconstriction that is unresponsive to an NO donor or sildenafil treatment, but is responsive to sGC modulators. It is now appreciated that the pain episodes that brought the phase II trial of sildenafil to a halt are a class effect of the PDE5 inhibitor medications, which cause limb, back, and muscle aches and pains. The first two major FDA registration trials of riociguat were not accompanied by similar reports of pain, suggesting an improved safety profile for the sGC stimulator (29, 30, 60). These preclinical findings suggest promising therapeutic potential for the NO-independent sGC activator in this and other group 5 hemolytic disease states, in which pulmonary hypertension develops in the context of oxidative stress (61).
Cinaciguat was studied in randomized, double-blind, placebo-controlled studies in patients in acute decompensated heart failure with (COMPOSE 1 and 2) or without (COMPOSE EARLY) invasive hemodynamic monitoring. For cinaciguat, a large and sudden drop in systemic blood pressure was observed in some patients. The findings of the COMPOSE studies were the end of the development of the intravenous cinaciguat for acute decompensated heart failure (62, 63). However, it is important to recognize that these studies used the intravenous formulation of the drug; an oral sGC activator will have a different pharmacokinetic and clinical profile. The pharmacokinetics of the oral formulation are different, and do not produce the same acute changes in blood pressure that have been seen with IV dosing (64). On the beneficial side, cinaciguat produced improvements in the pulmonary capillary wedge pressures, indicating therapeutic effects for the sGC activator on LV end-diastolic volume overload (62, 63). Although the clinical data so far argue against the use of cinaciguat for therapeutic management of acute hemodynamic events, the preclinical data on hand suggest that sGC activators may offer protection against organ remodeling that occurs over the long term (65, 66). Indeed, our findings of a normalized RVmaxSP and Fulton’s index, independent of any effect on systemic blood pressure and HR, in adult SCD mice administered long-term (90-d) treatment with the sGC activator suggest possible benefit.
In conclusion, we have demonstrated in this study that chronic oral administration of these sGC modulators do not affect systemic blood pressure in mice in the doses used. We show that NO-independent sGC activator improved endothelial function and reversed pulmonary hypertension and cardiac remodeling in a mouse model of SCD. Although these studies show promise for use of an sGC activator, additional studies are needed to likewise discern the safety profile of the sGC activator and its potential for effectively treating PH in patients with SCD.
Acknowledgments
Acknowledgment
The authors thank the University of Pittsburgh Vascular Medicine Institute Phenotyping Core for technical assistance with acquisition of right ventricular maximum systolic pressure data.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health grants P01HL103455 (M.T.G.), R01 HL128304 and R01 HL133864 (A.C.S.), K12 K12HD052892 and L40 HL129422 (K.P.P.) and P01 HL103455-06 (A.L.M.), and by the Bayer AG Vascular Diseases Research Group in accordance with a cooperative agreement between the University of Pittsburgh and Bayer AG.
Author Contributions: Conception, design, and performance of experiments—K.P.P., M.T.G., J.J.B., M.B., S.A.H., B.S., M.W., J.S., I.M., and P.S.; data analysis and interpretation—K.P.P., K.C.W., M.B., S.A.H., R.R.V., T.B., J.S., I.M., P.S., A.L.M., A.C.S., and M.T.G.; manuscript draft and revisions—K.P.P., K.C.W., J.S., I.M., P.S., A.C.S., and M.T.G.; all authors approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0292OC on December 21, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide–mediated vasodilation in transgenic sickle mouse. 2000;278:H1799–H1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- 4.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 5.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ignarro LJ, Degnan JN, Baricos WH, Kadowitz PJ, Wolin MS. Activation of purified guanylate cyclase by nitric oxide requires heme: comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung. 1982;718:49–59. doi: 10.1016/0304-4165(82)90008-3. [DOI] [PubMed] [Google Scholar]
- 7.Stone JR, Marletta MA. Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- 8.Fritz BG, Hu X, Brailey JL, Berry RE, Walker FA, Montfort WR. Oxidation and loss of heme in soluble guanylyl cyclase from Manduca sexta. 2011;50:5813–5815. doi: 10.1021/bi200794c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surmeli NB, Marletta MA. Insight into the rescue of oxidized soluble guanylate cyclase by the activator cinaciguat. 2012;13:977–981. doi: 10.1002/cbic.201100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. 2011;123:2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanias T, Sinchar D, Osei-Hwedieh D, Baust JJ, Jordan A, Zimring JC, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. 2016;56:2571–2583. doi: 10.1111/trf.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris CR. New strategies for the treatment of pulmonary hypertension in sickle cell disease: the rationale for arginine therapy. 2006;5:31–45. doi: 10.2165/00151829-200605010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Minniti CP, Delaney KM, Gorbach AM, Xu D, Lee CC, Malik N, et al. Vasculopathy, inflammation, and blood flow in leg ulcers of patients with sickle cell anemia. 2014;89:1–6. doi: 10.1002/ajh.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. 2006;116:2330–2332. doi: 10.1172/JCI29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 16.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. 2012;307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. 2015;308:L314–L324. doi: 10.1152/ajplung.00252.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Martin E, Sharina I, Esposito I, Szabo C, Bucci M, et al. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. 2016;111:556–562. doi: 10.1016/j.phrs.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, et al. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. 2002;136:773–783. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker EM, Alonso-Alija C, Apeler H, Gerzer R, Minuth T, Pleiss U, et al. NO-independent regulatory site of direct sGC stimulators like YC-1 and BAY 41-2272. 2001;1:13. doi: 10.1186/1471-2210-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straub A, Stasch JP, Alonso-Alija C, Benet-Buchholz J, Ducke B, Feurer A, et al. NO-independent stimulators of soluble guanylate cyclase. 2001;11:781–784. doi: 10.1016/s0960-894x(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 23.Gordeuk VR, Castro OL, Machado RF. Pathophysiology and treatment of pulmonary hypertension in sickle cell disease. 2016;127:820–828. doi: 10.1182/blood-2015-08-618561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 25.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, et al. walk-PHaSST Investigators and Patients. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. 2011;118:855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain S, Khera R, Girotra S, Badesch D, Wang Z, Murad MH, et al. Comparative effectiveness of pharmacologic interventions for pulmonary arterial hypertension: a systematic review and network meta-analysis. 2017;151:90–105. doi: 10.1016/j.chest.2016.08.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoeper MM, Simonneau G, Corris PA, Ghofrani HA, Klinger JR, Langleben D, et al. RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. 2017;50:pii: 1602425. doi: 10.1183/13993003.02425-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 30.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, et al. PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 31.Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, et al. Left Ventricular Systolic Dysfunction Associated With Pulmonary Hypertension Riociguat Trial (LEPHT) Study Group. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. 2013;128:502–511. doi: 10.1161/CIRCULATIONAHA.113.001458. [DOI] [PubMed] [Google Scholar]
- 32.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. 2006;107:1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pászty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 34.Hsu L, Diwan B, Ward JM, Noguchi CT. Pathology of “Berkeley” sickle-cell mice includes gallstones and priapism. 2006;107:3414–3415. doi: 10.1182/blood-2005-11-4500. [DOI] [PubMed] [Google Scholar]
- 35.Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, et al. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. 2000;88:1537–1544. doi: 10.1152/jappl.2000.88.5.1537. [DOI] [PubMed] [Google Scholar]
- 36.Nobes PR, Carter AB. Reticulocyte counting using flow cytometry. 1990;43:675–678. doi: 10.1136/jcp.43.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clements D, Iqbal S, Mills C, Elias E. A new method of complete biliary retention. 1985;19:277–278. doi: 10.1258/002367785780887473. [DOI] [PubMed] [Google Scholar]
- 38.Detterich JA, Kato RM, Rabai M, Meiselman HJ, Coates TD, Wood JC. Chronic transfusion therapy improves but does not normalize systemic and pulmonary vasculopathy in sickle cell disease. 2015;126:703–710. doi: 10.1182/blood-2014-12-614370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heim JM, Gottmann K, Weil J, Haufe MC, Gerzer R. Is cyclic GMP a clinically useful marker for ANF action? 1988;77:41–46. [PubMed] [Google Scholar]
- 40.Stasch JP, Kazda S, Neuser D. Different effects of ANP and nitroprusside on cyclic GMP extrusion of isolated aorta. 1989;174:279–282. doi: 10.1016/0014-2999(89)90321-x. [DOI] [PubMed] [Google Scholar]
- 41.Raat NJ, Tabima DM, Specht PA, Tejero J, Champion HC, Kim-Shapiro DB, et al. Direct sGC activation bypasses NO scavenging reactions of intravascular free oxy-hemoglobin and limits vasoconstriction. 2013;19:2232–2243. doi: 10.1089/ars.2013.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, et al. SOCRATES-REDUCED Investigators and Coordinators. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED Randomized Trial. 2015;314:2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 44.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, et al. Oxygen radical inhibition of nitric oxide–dependent vascular function in sickle cell disease. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aslan M, Ryan TM, Townes TM, Coward L, Kirk MC, Barnes S, et al. Nitric oxide–dependent generation of reactive species in sickle cell disease: actin tyrosine induces defective cytoskeletal polymerization. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 46.Cardenes N, Corey C, Geary L, Jain S, Zharikov S, Barge S, et al. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. 2014;123:2864–2872. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chester M, Seedorf G, Tourneux P, Gien J, Tseng N, Grover T, et al. Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatal pulmonary hypertension. 2011;301:L755–L764. doi: 10.1152/ajplung.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahaman MM, Nguyen AT, Miller MP, Hahn SA, Sparacino-Watkins C, Jobbagy S, et al. Cytochrome b5 reductase 3 modulates soluble guanylate cyclase redox state and cGMP signaling. 2017;121:137–148. doi: 10.1161/CIRCRESAHA.117.310705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. 1952;14:413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nath KA, Shah V, Haggard JJ, Croatt AJ, Smith LA, Hebbel RP, et al. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. 2000;279:R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- 51.Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 52.Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, Archer DR, et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. 2013;123:4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahluwalia A, Foster P, Scotland RS, McLean PG, Mathur A, Perretti M, et al. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. 2004;101:1386–1391. doi: 10.1073/pnas.0304264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hobbs AJ, Moncada S. Antiplatelet properties of a novel, non–NO-based soluble guanylate cyclase activator, BAY 41-2272. 2003;40:149–154. doi: 10.1016/s1537-1891(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 57.Polte T, Abate A, Dennery PA, Schröder H. Heme oxygenase-1 is a cGMP-inducible endothelial protein and mediates the cytoprotective action of nitric oxide. 2000;20:1209–1215. doi: 10.1161/01.atv.20.5.1209. [DOI] [PubMed] [Google Scholar]
- 58.Jarrett C, Lekic M, Smith CL, Pusec CM, Sweazea KL. Mechanisms of acetylcholine-mediated vasodilation in systemic arteries from mourning doves (Zenaida macroura) 2013;183:959–967. doi: 10.1007/s00360-013-0757-0. [DOI] [PubMed] [Google Scholar]
- 59.Mieyal P, Fulton D, McGiff JC, Quilley J. NO-independent vasodilation to acetylcholine in the rat isolated kidney utilizes a charybdotoxin-sensitive, intermediate-conductance Ca(++)-activated K+ channel. 1998;285:659–664. [PubMed] [Google Scholar]
- 60.Humbert M, Lau EM, Montani D, Jaïs X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. 2014;130:2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 61.Saleemi S. Saudi guidelines on the diagnosis and treatment of pulmonary hypertension: pulmonary hypertension associated with hemolytic anemia. 2014;9:S67–S73. doi: 10.4103/1817-1737.134039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erdmann E, Semigran MJ, Nieminen MS, Gheorghiade M, Agrawal R, Mitrovic V, et al. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. 2013;34:57–67. doi: 10.1093/eurheartj/ehs196. [DOI] [PubMed] [Google Scholar]
- 63.Gheorghiade M, Greene SJ, Filippatos G, Erdmann E, Ferrari R, Levy PD, et al. COMPOSE Investigators and Coordinators. Cinaciguat, a soluble guanylate cyclase activator: results from the randomized, controlled, phase IIb COMPOSE programme in acute heart failure syndromes. 2012;14:1056–1066. doi: 10.1093/eurjhf/hfs093. [DOI] [PubMed] [Google Scholar]
- 64.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benz K, Orth SR, Simonaviciene A, Linz W, Schindler U, Rütten H, et al. Blood pressure–independent effect of long-term treatment with the soluble heme-independent guanylyl cyclase activator HMR1766 on progression in a model of noninflammatory chronic renal damage. 2007;30:224–233. doi: 10.1159/000104091. [DOI] [PubMed] [Google Scholar]
- 66.Jones ES, Kemp-Harper B, Stasch JP, Schmidt H, Widdop RE. Cardioprotective effects in aged spontaneously hypertensive rats due to chronic stimulation/activation of sGC without hypotension [abstract] 2009;9:A29. [Google Scholar]