Abstract
Study Objectives
The severity of obstructive sleep apnea (OSA) is known to vary according to sleep stage; however, the pathophysiology responsible for this robust observation is incompletely understood. The objective of the present work was to examine how ventilatory control system sensitivity (i.e. loop gain) varies during sleep in patients with OSA.
Methods
Loop gain was estimated using signals collected from standard diagnostic polysomnographic recordings performed in 44 patients with OSA. Loop gain measurements associated with nonrapid eye movement (NREM) stage 2 (N2), stage 3 (N3), and REM sleep were calculated and compared. The sleep period was also split into three equal duration tertiles to investigate how loop gain changes over the course of sleep.
Results
Loop gain was significantly lower (i.e. ventilatory control more stable) in REM (Mean ± SEM: 0.51 ± 0.04) compared with N2 sleep (0.63 ± 0.04; p = 0.001). Differences in loop gain between REM and N3 (p = 0.095), and N2 and N3 (p = 0.247) sleep were not significant. Furthermore, N2 loop gain was significantly lower in the first third (0.57 ± 0.03) of the sleep period compared with later second (0.64 ± 0.03, p = 0.012) and third (0.64 ± 0.03, p = 0.015) tertiles. REM loop gain also tended to increase across the night; however, this trend was not statistically significant [F(2, 12) = 3.49, p = 0.09].
Conclusions
These data suggest that loop gain varies between REM and NREM sleep and modestly increases over the course of sleep. Lower loop gain in REM is unlikely to contribute to the worsened OSA severity typically observed in REM sleep, but may explain the reduced propensity for central sleep apnea in this sleep stage.
Keywords: obstructive sleep apnea, loop gain, chemosensitivity, sleep stages, endotype
Statement of Significance
The severity of obstructive sleep apnea (OSA) is commonly observed to worsen during rapid eye movement (REM) sleep and to improve in NREM stage 3 sleep. Recent work has shown how key OSA pathophysiological factors such as airway collapsibility, muscle responsiveness, and arousal threshold vary during sleep; however, sleep stage–related changes in ventilatory control system stability (i.e. loop gain) have not been examined. This study demonstrates that in patients with OSA, loop gain decreases (indicating a more stable ventilatory control system) during REM sleep relative to NREM sleep and increases over the sleep period. Although these findings do not explain sleep stage variability in OSA severity, they do help explain the reduced propensity for central apnea events during REM sleep.
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder that has significant adverse effects on health, alertness, productivity, and safety. Although the severity of an individual’s OSA is known to be driven by a variety of factors, including sleeping position, it is well established that changes in sleep stage influence the predisposition to OSA within an individual [1]. Specifically, the severity of OSA in many patients tends to worsen during rapid eye movement (REM) sleep [2], whereas if patients can achieve slow-wave sleep (SWS) they can often exhibit periods of prolonged stable breathing (i.e. without obstruction).
Studies investigating the potential mechanisms driving the sleep-stage dependence of OSA severity have focused on how several of the key pathological traits (upper airway collapsibility and muscle responsiveness [3], arousal threshold [4–11]) known to cause OSA vary with sleep stage. Although an individual’s dynamic ventilatory control stability (i.e. loop gain) is also recognized as a key contributor to the pathophysiology of OSA, to date, there is a paucity of data examining how this trait is altered by sleep stage in patients with OSA. Loop gain characterizes the sensitivity of the negative feedback system controlling ventilation and is defined as the size of a “corrective” ventilatory response divided by the size of the ventilatory disturbance that elicits the correction; a large response to a small disturbance represents a system with a high loop gain [12]. A high loop gain indicates an unstable system prone to ventilatory oscillations (as seen in patients with OSA), and a low loop gain indicates a stable ventilatory control system.
Importantly, an individual’s loop gain is solely determined by the product of their controller (i.e. the sensitivity of the peripheral/central chemoreceptors to hypoxia and hypercapnia) and plant (i.e. how effectively and quickly ventilation is converted into changes in arterial blood gases and is a function of the mechanics of the respiratory control system) gains. The only previous investigations which directly examine how ventilatory control system sensitivity is altered by sleep stage have assessed one component (controller gain) in healthy individuals. These studies have typically shown that the responsiveness to both hypoxia and hypercapnia (i.e. controller gain) decreases from wake to NREM sleep and is at its lowest during REM sleep [13–15]. If controller gain was the only factor altered by sleep stage, then the overall loop gain would be expected to follow a similar trend. However, a potential rise in plant gain (e.g. as PCO2 rises and lung volume falls from wake to NREM to REM) [14] means that changes in loop gain may not occur in parallel with those reported in controller gain. To date, there has been no study that has assessed how an individual’s loop gain (the product of both controller and plant gain) is altered by the various stages of sleep. Additionally, several reports have demonstrated that controller gain increases from evening to morning [16, 17] and fluctuates according to circadian phase [18–20], which suggests that loop gain may also vary in a similar fashion over time.
Accordingly, we aimed to assess how loop gain varies by both (1) sleep stage and (2) time of night, in patients with OSA. We employed a published method to estimate loop gain from the ventilatory flow pattern in clinical sleep study [21]. We tested the hypothesis that loop gain would decrease from stage N2 to SWS with a further decrease in REM sleep.
Methods
Participants
Forty-four patients with OSA (defined by an apnea/hypopnea index [AHI] > 5 events/hr) who underwent diagnostic sleep studies performed at Monash Health, an academic sleep centre in Melbourne, Australia were included in the study. Participants were recruited from a presurgical population as part of a previously reported study [22]. The current aims, data, and analyses have not been reported previously. Ethics approval for this retrospective analysis was obtained from Monash Health Human Research Ethics Committee.
Polysomnographic recordings
A standard clinical recording montage was employed. This montage included the following: electroencephalogram (EEG); bilateral electrooculogram (EOG); mentalis/submentalis and anterior tibialis electromyogram (EMG); electrocardiogram (ECG); nasal pressure cannula; oronasal thermistor; thoracic and abdominal respiratory effort bands; and fingertip oximetry. Sleep studies were staged and scored according to the standard criteria [23]. Loop gain analysis was performed on signals obtained from the scored polysomnogram using our previously described and validated method [21] and is described briefly below.
Loop gain determined from polysomnogram
Dynamic loop gain was measured using the data extracted from the signals contained within the overnight diagnostic polysomnogram. The raw data were exported as a European Data Format file and imported into Matlab for evaluation. The data file was split into a series of overlapping 7 min windows, and all those that contained one or more scored obstructive apnea/hypopnea were identified for subsequent analysis. Nasal pressure was square-root transformed and taken as a surrogate of ventilatory flow and then integrated and normalized by the mean to provide a ventilation signal. A categorical breath-by-breath time series was created indicating which breaths were associated with EEG arousal and/or scored obstructive events (i.e. apneas and hypopneas). Using these data, a standard ventilatory control model was fit to determine the best set of system parameters (i.e. gain, time constant, and delay) for each 7 min window. Using the best set of parameters, the estimated ventilatory drive signal best matches the observed ventilation during unobstructed breaths (i.e. when ventilation reflects ventilatory drive). From these parameters, we can determine loop gain at specific frequencies of interest. For consistency with previous reports [21, 22, 24], the loop gain at 1 cycle/min for each 7 min epoch was determined. Further explanatory details are provided in Supplementary Figure S1.
Data and statistical analysis
To determine how loop gain is altered by sleep stage, a sleep-stage label for each 7 min window was determined using the dominant stage (>50% of the window). For example, loop gain measurements from each 7 min window in which greater than 50% was scored in NREM stage 2 sleep were binned as N2 loop gain measurements. Windows without a dominant stage were excluded from analysis. Loop gain measurements for each sleep stage were then averaged to provide a single loop gain value for each sleep stage. A minimum of three loop gain measurements were required for a given sleep stage for averaging for each patient. Any less and it was deemed that the participant had insufficient sleep in the sleep stage in question. Due to the transient nature of N1 sleep, only a small portion of patients (11/44) had sufficiently frequent or lengthy periods of N1 sleep such that loop gain could be determined for this sleep stage using this method. As such the present analysis was primarily focused assessing the differences between stages N2, N3, and REM sleep. Secondary sensitivity analyses were conducted to ensure that key findings were not dependent on the specific choice of the percentage of the window defined as a majority, or the minimum number of windows required in an individual. Furthermore, an alternative weighted average method for attributing loop gain measurements to each sleep stage was also performed. See Supplementary Material (Figures S2, S3, S4 and S5) for the details of these analyses.
An additional exploratory analysis was also conducted to assess whether loop gain is altered over the course of the sleep period (i.e. time of night effect). For this analysis, the total sleep period (i.e. defined as the total time from the first to the last epoch of sleep) for each patient was divided into three equal length tertiles. Within each tertile, multiple N2, N3, and REM loop gain measurements split into their groups by their dominant sleep state (described above) and were each averaged to provide N2, N3, and REM loop gain values for each tertile. Due to the early night preponderance of N3 sleep, we limited this analysis to N2 and REM sleep stages. To be included in this analysis, participants were required to have a minimum of three loop gain measurements of a given sleep stage in each tertile.
Loop gain values were compared between sleep stages (N2, N3, and REM), and between sleep period tertiles, using one-way within-participants analysis of variance (ANOVA). Post hoc comparisons were performed using Fisher’s Least Significant Difference (LSD). Due to individual differences in sleep architecture and OSA severity, in some participants loop gain estimates could not be obtained across all sleep stages. Thus, three supplementary pairwise comparisons (specifically: REM vs. N2, REM vs. N2, and N2 vs. N3) were also performed using within-participants t-tests in order to make comparison between individual sleep stages using all available data. Different sample sizes are anticipated in these pairwise comparisons due to differences in available periods between sleep stages. Bonferonni adjustment for multiple comparisons was performed. Additional exploratory comparisons were performed between N1 and stages N2, N3, and REM. Correlational analyses were performed to explore the relationship between AHI and loop gain. Analysis of covariance (ANCOVA) was also used to compare loop gains between sleep stages while accounting for individual differences AHITotal and proportionate differences between AHINREM and AHIREM. All statistically analyses were performed using SPSS version 22.
Results
Patient characteristics are shown in Table 1. An average of 110.1 ± 6.2 (Mean ± SEM) loop gain measurements were obtained in each patient, with 3.8 ± 1.5 in N1, 64.7 ± 5.2 in N2, 8.4 ± 1.5 in N3, and 17.6 ± 1.7 in REM (and 92.6 ± 5.8 in total in NREM). All participants had sufficient (i.e. greater than or equal to 3) loop gain measurements in combined NREM stages, and N2 sleep. However, only 24 and 39 participants had at least three measurements in N3 and REM, respectively, whereas 21 participants had sufficient loop gain measurements in each of stages N2, N3, and REM for a comparison between these sleep states.
Table 1.
Patient characteristics
| Characteristic | |
|---|---|
| Age (years) | 42.20 ± 14.09 |
| BMI (kg/m2) | 32.21 ± 6.57 |
| Sex | 33 males; 11 females |
| TIB (min) | 466.50 [87.63] |
| TST (min) | 379.90 ± 74.18 |
| SOL (min) | 18.50 [26.63] |
| WASO (min) | 51.75 [65.25] |
| N1 sleep (min) | 32.50 [39.75] |
| N2 sleep (min) | 218.51 ± 69.46 |
| N3 sleep (min) | 55.00 [52.38] |
| NREM sleep (min) | 319.32 ± 55.64 |
| REM sleep (min) | 57.59 ± 28.24 |
| AHITotal (events/hr) | 33.11 [38.87] |
| AHINREM (events/hr) | 30.01 [38.51] |
| AHIREM (events/hr) | 44.36 [55.67] |
| ODI3 (events/hr) | 25.21 [32.60] |
| ODI4 (events/hr) | 16.08 [26.06] |
| SpO2 sleep average (%) | 94.54 [2.30] |
| SpO2 nadir (%) | 86.24 [10.15] |
| Average event duration NREM (s) | 25.46 ± 10.15 |
| Average event duration REM(s) | 23.25 [14.63] |
Mean ± Standard Deviations or Median [Interquartile range] are shown.
BMI = body mass index; TIB = time in bed; TST = total sleep time; SOL = sleep onset latency; WASO = wake after sleep onset; AHI = apnea/hypopnea index; ODI = oxygen desaturation index.
Loop gain differs by sleep stage
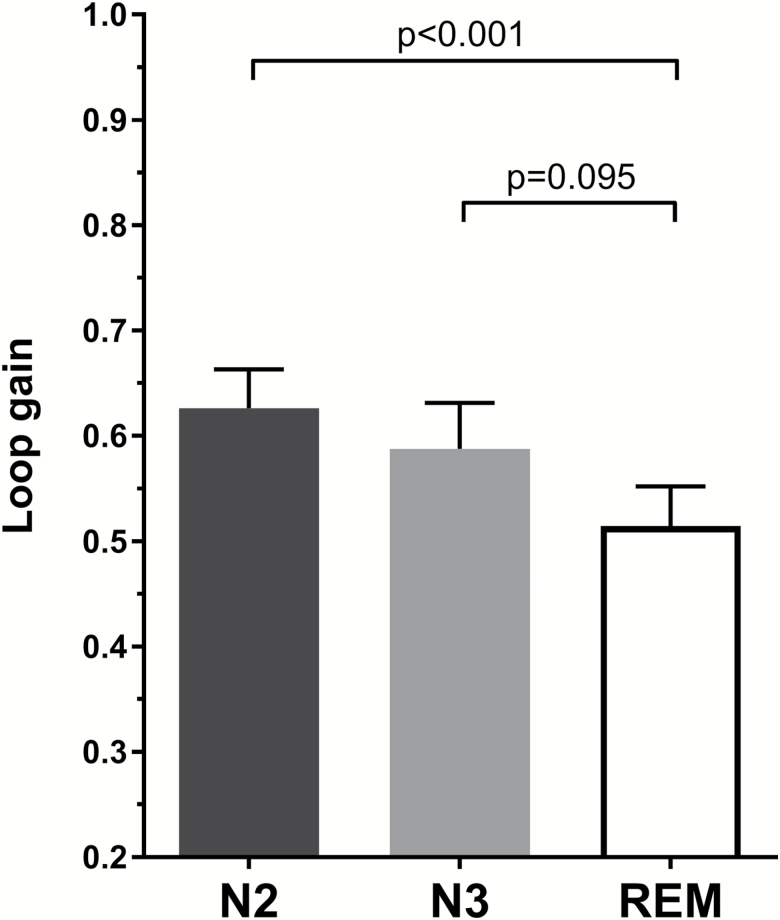
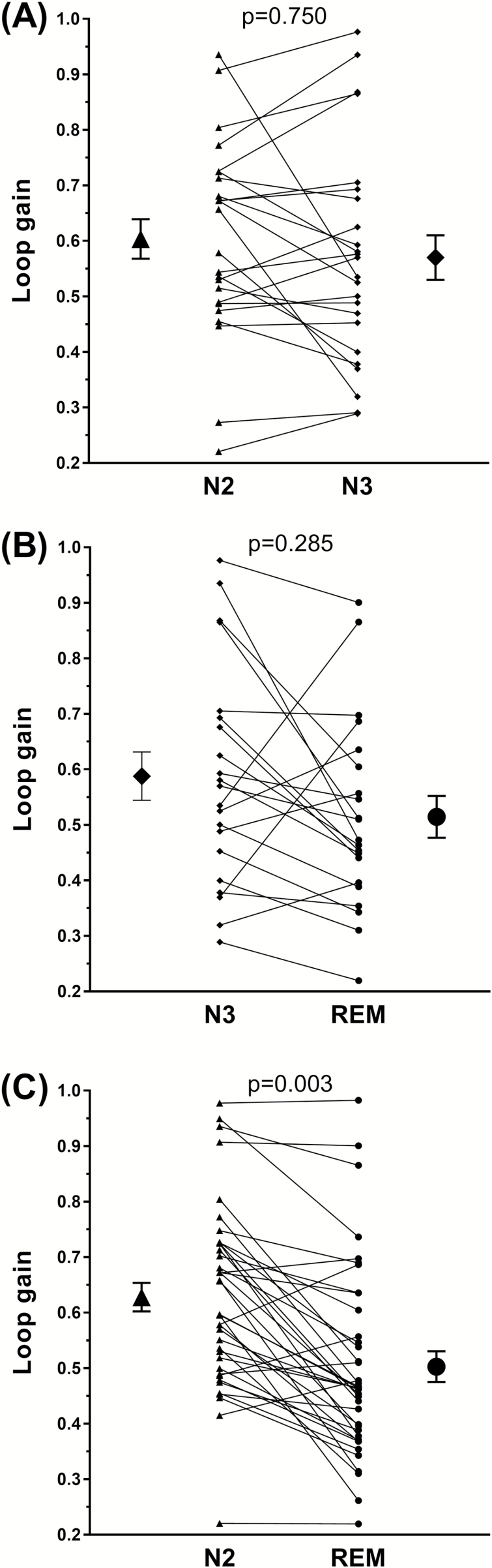
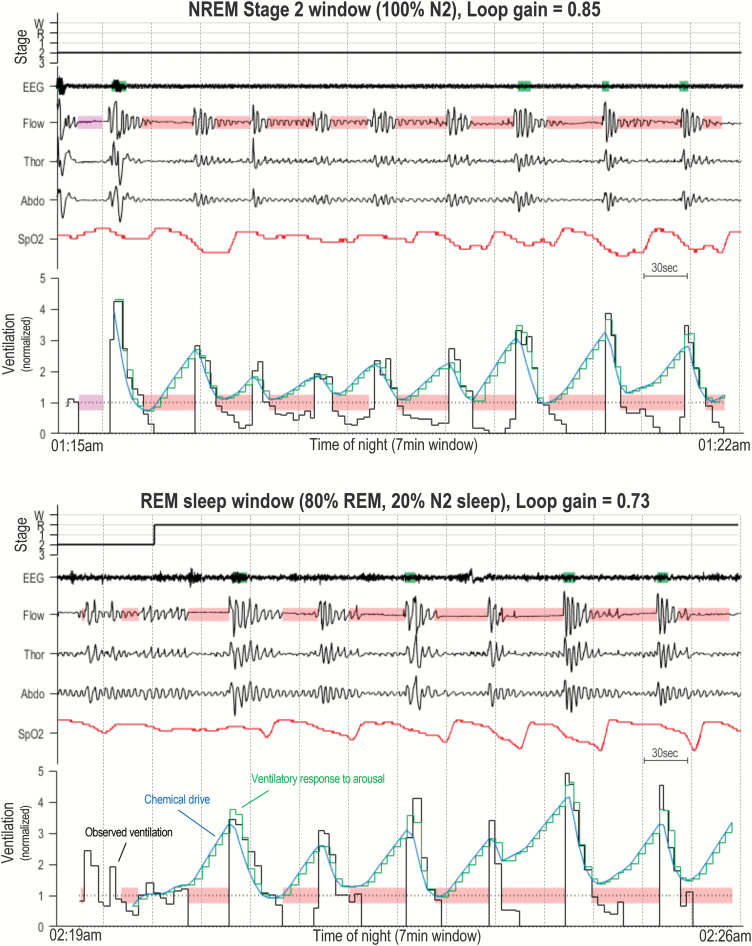
Figure 1 shows the mean loop gain for N2, N3, and REM sleep stages. A significant difference in loop gain by sleep stage was found [F(2, 40) = 5.44, n = 21, p = 0.008]. Specifically, loop gain was lower in REM compared with N2 sleep (0.51 ± 0.04 vs. 0.63 ± 0.04; p = 0.001). Loop gain in N3 sleep (0.59 ± 0.04) did not statistically differ from REM (p = 0.095) and N2 sleep (p = 0.247). These findings remained the same when the data were analyzed using pairwise within-participants t-tests between individual sleep stages [REM vs. N2, t(38) = 6.44, n = 39, p = 0.003; REM vs. N3, t(20) = 1.75, n = 21, p = 0.285; N2 vs. N3, t(23) = 1.18, n = 24, p = 0.750). Figure 2 shows individual participant data for each N2-N3, N3-REM, N2-REM comparison. Two example analysis windows from a single participant showing ventilation data and the fitting ventilatory control model for N2 and REM sleep periods are shown in Figure 3. Both intra-individual and inter-individual variability in loop gain estimates for each sleep stage are provided in the Supplementary Material (Table S3). When NREM stages were combined, an analysis that allowed sleep-stage comparisons between the majority of individuals in the sample (n = 40), loop gain remained significantly higher in NREM (0.61 ± 0.02) compared with REM sleep (0.50 ± 0.03; t(39) = 5.78, p < 0.001).
Figure 1.
Sleep state differences in loop gain. Bars represent mean loop gain values for N2 sleep (black), N3 sleep (grey), and REM sleep (white). Error bars represent standard error of the mean. In 21 individuals with sufficient measurements in each sleep stage, loop gain is significantly lower in REM sleep compared with N2 (p < 0.001), but not N3 sleep (p = 0.095). N2 and N3 loop gains did not significantly differ (p = 0.259).
Figure 2.
Individual differences in loop gain between sleep states. (A) shows individual participant differences between N2 and N3 (p = 0.750, n = 24), (B) N3 and REM (p = 0.25, n = 21), and (C) N2 and REM (p = 0.750, n = 39). Group means with error bars representing standard error of the mean are shown adjacent to the individual data columns. P-values reported correspond to the results of within-participants t-test.
Figure 3.
Example data showing NREM stage 2 and REM dominant analysis windows. Top window shows example data from a N2-dominant period which yields a loop gain estimate of 0.85. Bottom window shows a REM dominant period which yields a loop gain estimate of 0.73. Both windows are composed of the following traces: sleep stage hypnogram (W = Wake, R = REM, 1 = N1, 2 = N2, and 3 = N3), EEG (electroencephalograph), Flow, Thor (Thoracic excursions), Abdo (Abdominal excursions), SpO2, and Ventilation. Shaded green rectangles (superimposed on EEG trace) denote scored cortical arousals. The ventilation trace shows breath-by-breath ventilation measurements in black (Observed ventilation) which are normalized by the mean (1 = eupnea). The overlaying blue line depicts the estimated chemical drive and the green stair-case line shows the estimated ventilatory drive. The blue and green lines are equal during all nonarousal breaths, during arousal a “ventilatory response to arousal” is added to chemical drive. Shaded red rectangles denote obstructive events (apneas or hypopneas), shaded pink rectangles show central events. Each window is 7 min in duration, and 30 s sleep epochs are show by dotted lines.
Additional within-participants t-tests were performed to explore differences between loop gain in N1. No significant differences were found between N1 and stage N2 (0.63 ± 0.07 vs. 0.71 ± 0.05; t(10) = −1.78, p = 0.105), N3 (0.65 ± 0.10 vs. 0.66 ± 0.12; t(3) = −0.12, p = 0.909) or REM sleep (0.65 ± 0.08 vs. 0.55 ± 0.08; t(8) = 1.95, p = 0.086). Due to the transient nature of N1 sleep, loop gain measurements were rare in this sleep stage. Furthermore, participants who had greater numbers of N1 loop gain measurements also tended to have more fragmented sleep and thus had reduced N3 and REM sleep time. As a consequence, these N1 comparisons are based on a much smaller subset of participants and should be interpreted with caution.
A series of sensitivity analyses were performed (see Supplementary Material, Figures S2 and S3) to determine whether (1) the minimum number of loop gain measurements (e.g. ≥3) for a given sleep stage estimate or (2) the fraction a 7 min window (e.g. >50%) required to define a sleep stage loop gain estimate, affected our results. Importantly, sleep stage specific estimates of loop gain were largely unaffected by these data analysis steps; thus, we are confident that the observed sleep stage differences in loop gain are robust.
Sleep state differences in loop gain remain after controlling for AHI
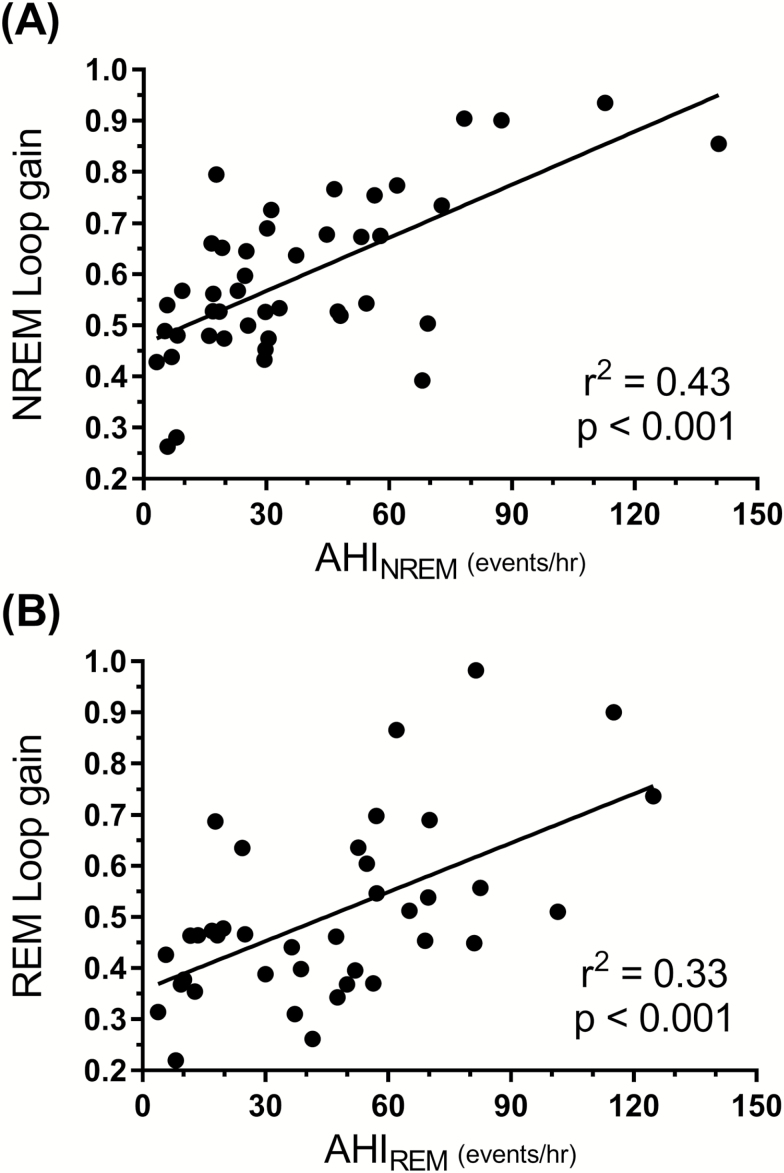
To investigate the possibility that differences in OSA severity may influence sleep-stage differences in loop gain, the following analyses were performed. First, we explored the association between loop gain and AHI. A significant positive correlation was found between loop gain in each sleep stage and the AHITotal, and similar associations were also found for AHINREM and AHIREM (all p < 0.05, see Supplementary Material , Table S1 for correlation matrix). Figure 4 shows the association between NREM sleep loop gain and the AHINREM (Figure 4A), which was noticeably stronger than the association between the REM sleep loop gain and the AHIREM (Figure 4B). Despite the concurrence between AHI and loop gain, sleep stage differences in loop gain were still evident after covarying for differences in the AHITotal [F(2, 38) = 3.36, p = 0.045]. Similarly, the REM loop gain was still significantly lower than the NREM loop gain after accounting for individual differences between AHINREM and AHIREM [F(1, 37) = 25.36, p < 0.001].
Figure 4.
Associations between loop gain and apnea–hypopnea index. (A) A significant positive association (r2 = 0.43, p < 0.001) is found between average NREM loop gain values and the NREM apnea–hypopnea index (AHINREM). (B) A similar, although weaker association (r2 = 0.33, p < 0.001) is found between REM sleep loop gain values and the REM apnea–hypopnea index (AHIREM).
A significant proportion of participants (20/44) in our sample had too few respiratory events in N3 sleep to yield accurate measurements of loop gain in this stage. To investigate whether the commonly observed rarity of N3 respiratory events (and hence our method’s inability to measure loop gain in N3 sleep) influenced the observed sleep stage differences in loop gain, we compared N2 and REM loop gains between those with and those without N3 loop gain measurements. Neither N2 loop gain (0.60 ± 0.03 vs. 0.62 ± 0.04; t(42) = 0.52, p = 0.61) nor REM loop gain (0.51 ± 0.04 vs. 0.51 ± 0.03; t(39) = −0.14, p = 0.89) differed between those with sufficient N3 loop gain measurements versus and those without.
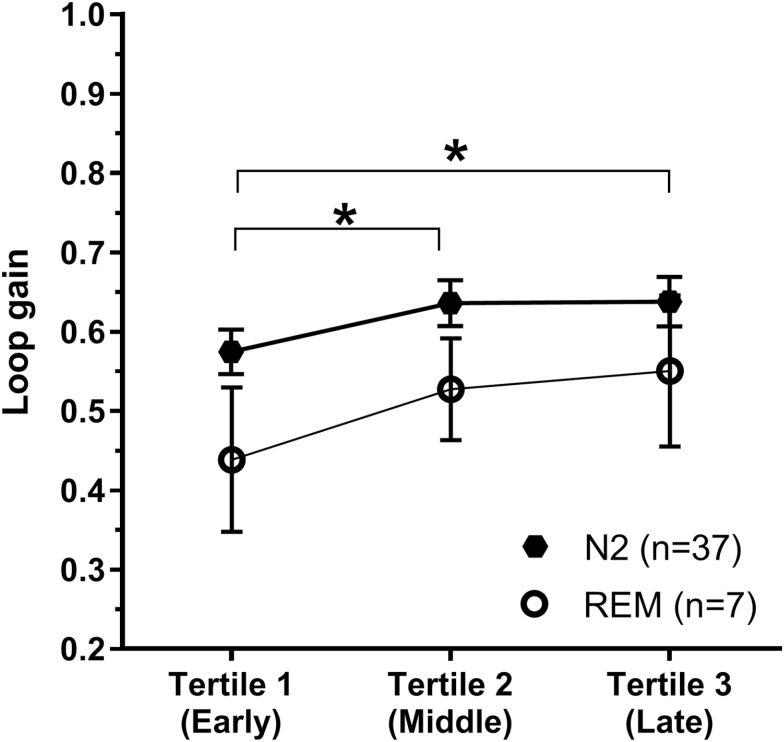
Loop gain changes across the sleep period
Data from 37 participants who had sufficient N2 loop gain measurements (i.e. ≥3 measurements in each of the sleep period tertiles) are presented in Figure 5. There was a significant difference in N2 loop gain between tertiles [F(2, 72) = 4.37, p = 0.016] with loop gain in first 3rd (0.57 ± 0.03) of the sleep period being significantly lower compared to later second (0.64 ± 0.03, p = 0.012) and third (0.64 ± 0.03, p = 0.015) tertiles. The percentage increase in N2 loop gain was not significantly associated with any measure of OSA severity including AHI, ODI, Nadir SpO2, or mean sleep SpO2 (Supplementary Material—Table S2). Furthermore, sleep-stage differences in loop gain remained significant even after covarying for this time of night increase in loop gain [F(2, 34) = 5.598, p = 0.008].
Figure 5.
Changes in loop gain across the sleep period. N2 loop gain values are represented by black hexagons and REM loop gain values by white circles. Averaged loop gain in the first sleep period tertile is found to be significantly lower than tertiles 2 (p = 0.012) and 3 (p = 0.012) for N2 sleep. A similar trend is found in REM sleep; however, this is not statistically significant (p = 0.09). Error bar represents standard error of the mean.
Only seven participants had sufficient REM loop gain measurements in each of tertiles 1, 2, and 3. As shown in Figure 5, similar to measurements in stage N2, REM loop gain tended to increase across the night; however, this trend was not statistically significant [F(2, 12) = 3.49, p = 0.09]. It was not possible to perform this analysis for N3 sleep, as only three participants had sufficient N3 loop gain measurements across each of the three tertiles for this sleep stage.
Discussion
The present findings show that ventilatory control stability (i.e. loop gain) changes during sleep in patients with OSA according to both sleep stage and time of night. Loop gain was found to be significantly lower during REM sleep compared with NREM sleep, particularly N2 sleep. Furthermore, while initially lower at the beginning of sleep, loop gain significantly increased during the middle third of the sleep period and remained relatively elevated into the morning. These findings extend our current understanding about how OSA pathophysiology and disease severity vary across sleep.
Sleep stage variation in loop gain
The current study is the first to examine whether loop gain changes across sleep stage in patients with OSA. Although several studies have examined sleep-stage difference in chemosensitivity [13, 14, 25–28], these studies were typically restricted to normal healthy participants and to measures that characterize controller sensitivity/gain (i.e. hypoxic ventilatory response [HVR] and hypercapnic ventilatory response [HCVR]) alone. Despite this, our findings are consistent with this previous work, which has shown HVR and HCVR responses to be lower in REM sleep compared with NREM sleep, with little difference between NREM stages N2 and N3. Although consistent in direction, there is a discrepancy in the magnitude of the NREM vs REM differences we found in loop gain versus those found previously in HVR and HCVR responses. By comparison, our data demonstrate a modest drop in loop gain of 16.4% in REM sleep compared with NREM, whereas previous studies investigating controller gain report larger NREM–REM differences, with REM chemosensitivity ranging between 20%–50% lower (20.7%–40.0% for HCVR slope [14, 26, 27], 27.7%–47.6% for HVR slope [13, 25, 28]) compared with NREM.
There are several potential reasons for the discrepancy in magnitude. Firstly, there are two important methodological differences between our study and previous work. In the present work, participants had OSA which may alter the relationship between loop gain/chemosensitivity and sleep stage. Furthermore, we estimated loop gain by modelling dynamic changes in ventilatory drive in response to dynamic disturbances in ventilation (due to spontaneous apnea/hypopnea events). Our measure of loop gain therefore represents a dynamic measure of loop gain that can be expressed as a function of frequency (note that we report loop gain determined at cycle period of 1/min). This contrasts with previous work which measured chemosensitivity via ventilatory responses to steady-state changes in inspired gases. It is possible that changes in sleep stage may have a larger effect on steady state ventilatory stability.
Despite the methodological differences between the present and previous work, there may be an alternative physiological explanation for the magnitude of sleep stage dependent differences in loop gain. If controller gain was the only factor that was altered by sleep state, then by definition, the change in loop gain would be expected to be proportional to the change in controller gain (as loop gain = controller × plant gain). However, it is possible that plant gain may differentially change according to sleep stage, which may act to attenuate the effect sleep state has on overall loop gain via reduced controller gain. Specifically, plant gain may increase during REM sleep relative to NREM. Plant gain is determined by the following three key factors: (1) the alveolar-inspired PCO2 gradient for gas exchange (PACO2–PICO2, where PICO2 is normally zero), (2) a complex timing factor (T) which is chiefly driven by circulatory delay, and (3) lung volume (Equation (1) [12]):
Alveolar PCO2 levels are known to increase from wakefulness to sleep [14, 29] and are higher in REM sleep compared with N2 sleep [14]. By contrast, although lung volume has been shown to fall from wakefulness levels during sleep, the NREM–REM difference has been reported to be negligible in healthy patients [30], suggesting that this is unlikely to alter plant gain. Similarly cardiac output, which is a key factor driving the circulatory delay, has been shown to decrease from wakefulness to sleep, but does not differ between sleep stages [31]. Taken together, the available evidence suggests that the increase in alveolar PCO2 would be the most likely explanation for any rise in plant gain and could in part explain why NREM–REM differences in loop gain observed in the current study are of smaller magnitude compared with previous work which have explored sleep stage–related differences in controller gain [13, 14, 25–28].
It is well documented that OSA is often less severe during N3 sleep relative to N2 sleep. Although our data suggest that ventilatory control is more stable (i.e. a lower loop gain) in N3 sleep compared with N2 sleep, this difference was small and not statistically different. Changes in loop gain are therefore unlikely to be the main driver of this robust clinical observation. Favorable changes in other OSA pathophysiology, including an increased respiratory arousal threshold concomitant with delta activity [11] and N3 sleep–related decreases in upper airway collapsibility due to increased genioglossus activity [3], are likely to explain the stable breathing and reduced severity associated with N3 sleep.
Our finding that loop gain is lower in REM compared with NREM does not explain the observation that the severity of OSA in many patients typically worsens during REM sleep, as a lower loop gain would tend to favor more stable breathing. Indeed approximately 40%–45% patients with OSA demonstrate respiratory events predominantly in REM sleep [32, 33]. In these patients, other physiological changes specific to REM sleep, including reduced responsiveness of the genioglossus to increases in negative pressure [3, 34] and an increased upper airway collapsibility [3], are therefore likely to be stronger determinants of respiratory events during REM sleep. Our findings provide some support for this notion, as the association between loop gain and AHI was noticeably weaker in REM sleep compared with NREM sleep (Figure 4). By contrast, available evidence suggests that NREM-predominant OSA is significantly influenced by an elevated loop gain [35]. In such patients, the improvement in the severity of OSA in REM is likely driven by the REM-related reduction in controller gain described above. Furthermore, in patients with central sleep apnea (a high loop gain disorder) [36], respiratory events are typically more common in NREM sleep and are often absent during REM sleep [37] supporting the concept that a REM-related reduction in loop gain may be the likely explanation.
Time of night variation in loop gain
In our data, we noted that loop gain modestly increased across the sleep period. Specifically, loop gain in N2 sleep was significantly lower in the first third of the sleep before increasing by 9.7% in the middle of the sleep episode, remaining stable thereafter. This time of night change in loop gain was significant for N2 only. Although time of night differences in the distribution of REM sleep limited the number of loop gain measurements available for analysis, a similar trend was evident for loop gain in REM sleep (Figure 5). Importantly, REM loop gains remained lower than N2 loop gains across the entire sleep period consistent with our main findings.
Several other studies, using a variety of different methodologies, have observed time of night variability in ventilatory control sensitivity [16, 18–20, 38]. It is unclear what mechanism is driving this variability; however, there are at least three physiological processes that may be responsible for this observation: (1) circadian oscillations in ventilatory control, (2) rostral fluid shift during sleep, and (3) hypoxia-induced respiratory plasticity (i.e. progressive augmentation of the hypoxic ventilatory response [39]).
Circadian variations to ventilation, as well as various measures of ventilatory control sensitivity (HCVR and HVR), have been demonstrated by several studies using constant routine protocols [17, 19, 20, 38]. The timing at which chemosensitivity peaks, however, has varied between studies and occurs either in the morning/early afternoon (10:00–14:00) [19] or late afternoon (~18:45) [38]. In each of these studies, however, HCVR or HVR sensitivity appears to remain relatively stable and lower than the 24 hr mean throughout a typical nocturnal sleep period and does not appear to increase until late morning. To date, only one study has measured chemosensitivity in sleeping OSA patients, while controlling for circadian phase. Using a variation of a constant routine protocol that included three scheduled sleep periods, El-Chami et al. [18] measured hypocapnic ventilatory responses during evening, morning, and afternoon sleep. Hypocapnic ventilatory response sensitivity was shown to vary by circadian phase and to be increased during the morning sleep (06:00–09:00) relative to afternoon (14:00–17:00) or evening (22:00–01:00) sleep periods. Importantly, given that participants were ventilated with BiPAP, this circadian change in controller gain occurred in the absence of any hypoxemia or uncontrolled sleep disordered breathing. Our data are generally consistent with that of El-Chami and colleagues [18]; however, we found an earlier rise in ventilatory control sensitivity occurring at approximately the middle of the nocturnal sleep period (this corresponded to 00:51–02:58 am on average). To summarize, while it has yet to be explicitly measured, it is possible that loop gain varies according to circadian phase, which may explain the time of night variability observed in the present study. It is important to note, however, that circadian phase was not measured in the present study, nor does our retrospective study design allow this to be accounted for. Thus, with the current data, we are unable to assess when the observed rise in ventilatory control sensitivity occurred relative to a patient’s endogenous circadian rhythm.
Rostral fluid shift may also account for some of the observed increase in loop gain over the sleep period. During waking, when upright or seated, an accumulation of fluid occurs in the legs. During recumbent sleep, a rostral redistribution of fluid occurs which can cause temporary edema around the airways and lungs [40]. This could work to reduce lung volume and subsequently increase plant gain; however, this mechanism had yet to be directly tested with our measurement of loop gain. The time course of fluid redistribution is reported to be relatively rapid, reaching a plateau within ~1–2 hr [41]. Although our time of night analysis is limited in its temporal resolution (we used sleep period tertiles of approximately ~2.5 hr duration), the relatively fast time scale and early plateauing profile associated with fluid shift are consistent with the time of night increase in loop gain measured in the present study.
Another potential mechanism by which loop gain may increase during sleep in patients with OSA is via hypoxia-induced respiratory plasticity [42, 43]. That is, exposure to nocturnal intermittent hypoxia induced by repetitive apneas/hypopneas could produce sustained changes in respiratory activity which can work to increase chemosensitivity and therefore overall loop gain [42]. In an effort to understand how obstructive respiratory events may destabilize ventilatory control, two studies have measured HVR and HCVR before and after sleep in patients with OSA compared with controls. Both studies found that results differed between OSA patients and controls, suggestive of increased ventilatory instability in the morning for patients with OSA, but little to no changes in control participants. Specifically, Fuse et al. [16] found that although HVR and HCVR sensitivity did not change after sleep in patients with OSA, ventilatory response thresholds decreased, the extent of which was correlated with the degree of hypoxemia occurring during the intervening sleep episode. By contrast, Mahamed and colleagues [17] found a 30% increase in HCVR sensitivity from evening to morning without any change in ventilatory response threshold. In the present data, we observed no significant associations between the increase in loop gain over the sleep period nor any direct or indirect measure of hypoxemia recorded within the overnight polysomnogram (i.e. AHI, ODI3, ODI4, nadir SpO2, and average sleep SpO2). Thus, although it is possible that respiratory plasticity may account for the time of night increases in loop gain, the current data do not appear to support this notion.
Methodological considerations
In the present study, we used our validated method to estimate variability in loop gain during sleep from routine polysomnography [21]. This technique has allowed us to investigate sleep stage and time of night variability in ventilatory control in the largest sample to date without using intrusive physiological instrumentation that may negatively affect sleep architecture. Moreover, by using spontaneous obstructive events to make these estimates, we yield a measure of ventilatory control sensitivity that is specifically relevant to OSA pathophysiology. Despite these strengths, there are several limitations of this methodology/analyses. Firstly, due to the need for respiratory perturbations, we were unable to measure loop gain in a sample of healthy controls or during wakefulness and hence are not able to make comparisons between either OSA and healthy (i.e. non-OSA) populations, or between wake and sleep. Secondly, due to the transient nature of N1 sleep, we were unable to robustly characterize loop gain in this sleep stage. Similarly, homeostatic and circadian variability in sleep-stage length limited our ability to measure time of night differences in REM loop gains and precluded these analyses for N3 sleep. Thirdly, our method for estimating loop gain from polysomnography makes two key assumptions that may affect its measurement: (1) it uses transformed nasal pressure signals as a substitute for gold standard measures of ventilation, and (2) it assumes that ventilatory drive is equal to observed ventilation for all breaths that are not part of an obstructive event, which may not always be the case (as subclinical periods of flow limitation can occur outside of scored respiratory events). Despite these assumptions, our method provides loop gain estimates that are strongly correlated with other more invasive methods for measuring loop gain [44] (which use gold standard measures of ventilation and harnesses CPAP to finely control airway obstruction) [21]. Fourthly, in these data, we were unable to determine the body position associated with each loop gain measurement; thus, sleeping position was not controlled for in our analyses. Previous work by Joosten et al. [24] has shown that there is a small but significant increase in loop gain in the supine relative to the lateral position. Although it is unlikely that the sleeping position would vary systematically by sleep stage or time of night, it is possible that postural effects on loop gain contributed to variability in our data. Finally, we were unable to measure the controller versus plant gain contributions to sleep stage variability in loop gain. Future work could incorporate a measurement of end tidal CO2 and simple gas exchange modeling [45] to further clarify how these individual loop gain components change during sleep.
Summary and Conclusions
In summary, our findings demonstrate that loop gain decreases during REM sleep compared with NREM, with no significant difference between NREM stages N2 and N3. Moreover, we found a small, but measurable, increase in loop gain over the course of the sleep period. These findings are likely to explain the NREM predominance of CSA and suggest that elevated loop gain may play an important pathophysiological role in NREM predominant OSA.
Supplementary Material
Supplementary material is available at SLEEP online.
Acknowledgments
The authors thank Professors Atul Malhotra and Sanjay Patel for their helpful suggestions regarding the data analysis.
Notes
Conflict of interest statement. A/Prof. Hamilton and Dr. Joosten have received equipment to support research from ResMed, Philips Respironics, and Air Liquide Healthcare. Dr. Sands consulted for Cambridge Sound Management and Nox Medical. All other authors have no conflicts to disclose and do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Dr. Landry is supported by NeuroSleep, a NHMRC Centre of Research Excellence (1060992) and the Monash University Faculty of Medicine Nursing and Health Sciences bridging postdoctoral fellowship. Dr. Sands was supported by the American Heart Association (15SDG25890059), American Thoracic Society (Unrestricted Grant), and the National Institute of Health (R01HL128658, R01HL102321, P01HL094307, R35HL135818). Dr. Edwards was supported by the National Health and Medical Research Council (NHMRC) of Australia’s CJ Martin Overseas Biomedical Fellowship (1035115) and is now supported by a Heart Foundation of Australia Future Leader Fellowship (101167). This work was supported by a National Health and Medical Research Council (NHMRC) of Australia project grant (1064163).
References
- 1. Subramanian S, et al. . Gender and age influence the effects of slow-wave sleep on respiration in patients with obstructive sleep apnea. Sleep Breath. 2013;17(1):51–56. [DOI] [PubMed] [Google Scholar]
- 2. Ratnavadivel R, et al. . Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5(6):519–524. [PMC free article] [PubMed] [Google Scholar]
- 3. Carberry JC, et al. . Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep. 2016;39(3):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards BA, et al. . Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boudewyns A, et al. . Respiratory effort during sleep apneas after interruption of long-term CPAP treatment in patients with obstructive sleep apnea. Chest. 1996;110(1):120–127. [DOI] [PubMed] [Google Scholar]
- 6. Eckert DJ, et al. . Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond). 2011;120(12):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith PL, et al. . The effects of oxygen in patients with sleep apnea. Am Rev Respir Dis. 1984;130(6):958–963. [DOI] [PubMed] [Google Scholar]
- 8. Pelin Z, et al. . The role of mean inspiratory effort on daytime sleepiness. Eur Respir J. 2003;21(4):688–694. [DOI] [PubMed] [Google Scholar]
- 9. Sforza E, et al. . Role of chemosensitivity in intrathoracic pressure changes during obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1741–1747. [DOI] [PubMed] [Google Scholar]
- 10. Wains SA, et al. . Impact of arousal threshold and respiratory effort on the duration of breathing events across sleep stage and time of night. Respir Physiol Neurobiol. 2017;237:35–41. [DOI] [PubMed] [Google Scholar]
- 11. Berry RB, et al. . Within-night variation in respiratory effort preceding apnea termination and EEG delta power in sleep apnea. J Appl Physiol (1985). 1998;85(4):1434–1441. [DOI] [PubMed] [Google Scholar]
- 12. Khoo MC, et al. . Factors inducing periodic breathing in humans: a general model. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(3):644–659. [DOI] [PubMed] [Google Scholar]
- 13. Douglas NJ, et al. . Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis. 1982;125(3):286–289. [DOI] [PubMed] [Google Scholar]
- 14. Douglas NJ, et al. . Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis. 1982;126(5):758–762. [DOI] [PubMed] [Google Scholar]
- 15. Krimsky WR, et al. . Physiology of breathing and respiratory control during sleep. Semin Respir Crit Care Med. 2005;26(1):5–12. [DOI] [PubMed] [Google Scholar]
- 16. Fuse K, et al. . Regulation of ventilation before and after sleep in patients with obstructive sleep apnoea. Respirology. 1999;4(2):125–130. [DOI] [PubMed] [Google Scholar]
- 17. Mahamed S, et al. . Overnight changes of chemoreflex control in obstructive sleep apnoea patients. Respir Physiol Neurobiol. 2005;146(2-3):279–290. [DOI] [PubMed] [Google Scholar]
- 18. El-Chami M, et al. . Time of day affects chemoreflex sensitivity and the carbon dioxide reserve during NREM sleep in participants with sleep apnea. J Appl Physiol (1985). 2014;117(10):1149–1156. [DOI] [PubMed] [Google Scholar]
- 19. Spengler CM, et al. . An endogenous circadian rhythm of respiratory control in humans. J Physiol. 2000;526 Pt 3:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stephenson R, et al. . Circadian rhythms in the chemoreflex control of breathing. Am J Physiol Regul Integr Comp Physiol. 2000;278(1):R282–R286. [DOI] [PubMed] [Google Scholar]
- 21. Terrill PI, et al. . Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45(2):408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joosten SA, et al. . Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnoea: ventilatory control abnormalities predict surgical responsiveness. Sleep. 2017;40. doi:10.1093/sleep/zsx094 [DOI] [PubMed] [Google Scholar]
- 23. Iber C, American Academy of Sleep Medicine.. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24. Joosten SA, et al. . Dynamic loop gain increases upon adopting the supine body position during sleep in patients with obstructive sleep apnoea. Respirology. 2017;22(8):1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White DP, et al. . Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis. 1982;126(3):530–533. [DOI] [PubMed] [Google Scholar]
- 26. White DP. Occlusion pressure and ventilation during sleep in normal humans. J Appl Physiol (1985). 1986;61(4):1279–1287. [DOI] [PubMed] [Google Scholar]
- 27. Berthon-Jones M, et al. . Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(1):59–67. [DOI] [PubMed] [Google Scholar]
- 28. Berthon-Jones M, et al. . Ventilatory and arousal responses to hypoxia in sleeping humans. Am Rev Respir Dis. 1982;125(6):632–639. [DOI] [PubMed] [Google Scholar]
- 29. Trinder J, et al. . Respiratory instability during sleep onset. J Appl Physiol (1985). 1992;73(6):2462–2469. [DOI] [PubMed] [Google Scholar]
- 30. Ballard RD, et al. . Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol (1985). 1990;68(5):2034–2041. [DOI] [PubMed] [Google Scholar]
- 31. Khatri IM, et al. . Hemodynamic changes during sleep in hypertensive patients. Circulation. 1969;39:785–790. [DOI] [PubMed] [Google Scholar]
- 32. Koo BB, et al. . Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134(6):1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joosten SA, et al. . Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17(1):99–107. [DOI] [PubMed] [Google Scholar]
- 34. Eckert DJ, et al. . The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135(4):957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas RJ, et al. . Obstructive sleep-disordered breathing with a dominant cyclic alternating pattern–a recognizable polysomnographic variant with practical clinical implications. Sleep. 2004;27(2):229–234. [DOI] [PubMed] [Google Scholar]
- 36. Orr JE, et al. . Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yumino D, et al. . Central sleep apnea and Cheyne-Stokes respiration. Proc Am Thorac Soc. 2008;5(2):226–236. [DOI] [PubMed] [Google Scholar]
- 38. Siekierka M, et al. . Low amplitude daily changes in reflex ventilatory response to progressive isocapnic hypoxia. J Physiol Pharmacol. 2007;58 Suppl 5(Pt 2):633–637. [PubMed] [Google Scholar]
- 39. Mateika JH, et al. . Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol. 2013;188(3):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White LH, et al. . Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591(5):1179–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berg HE, et al. . Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand. 1993;148(4):379–385. [DOI] [PubMed] [Google Scholar]
- 42. Deacon NL, et al. . The role of high loop gain induced by intermittent hypoxia in the pathophysiology of obstructive sleep apnoea. Sleep Med Rev. 2015;22:3–14. [DOI] [PubMed] [Google Scholar]
- 43. Mateika JH, et al. . Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea?Exp Physiol. 2009;94(3):279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wellman A, et al. . A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985). 2011;110(6):1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sands SA, et al. . Control theory prediction of resolved Cheyne-Stokes respiration in heart failure. Eur Respir J. 2016;48(5):1351–1359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.