Abstract
The purpose of this study was to determine the effects of glucocorticoid-induced metabolic dysfunction in the presence of diet-induced obesity. C57BL/6J adult male lean and diet-induced obese mice were given dexamethasone, and levels of hepatic steatosis, insulin resistance, and lipolysis were determined. Obese mice given dexamethasone had significant, synergistic effects on fasting glucose, insulin resistance, and markers of lipolysis, as well as hepatic steatosis. This was associated with synergistic transactivation of the lipolytic enzyme adipose triglyceride lipase. The combination of chronically elevated glucocorticoids and obesity leads to exacerbations in metabolic dysfunction. Our findings suggest lipolysis may be a key player in glucocorticoid-induced insulin resistance and fatty liver in individuals with obesity.
We evaluated lipolytic markers, insulin resistance, and hepatic steatosis in response to combined glucocorticoids and obesity in mice. All outcomes were exacerbated in comparison to lean counterparts.
Glucocorticoids are a class of steroid hormones that are important for proper glucose homeostasis during stress or fasting, but can lead to symptoms similar to those seen in metabolic syndrome if elevated for prolonged durations. Cushing syndrome encompasses a variety of conditions that manifest in response to chronically elevated levels of glucocorticoids, including exogenous corticosteroid treatment as well as endogenous overproduction of cortisol, and is often associated with changes in adipose mass and distribution, nonalcoholic fatty liver disease (NAFLD), and impaired glucose tolerance (1). Although endogenous forms of Cushing syndrome are rare, it is estimated that at any given time, 1% to 3% of the US, United Kingdom, and Danish populations are prescribed exogenous corticosteroids, which may increase their risk for developing the associated metabolic complications (2–5).
Similarly, obesity is accompanied by a multitude of metabolic disturbances, such as insulin resistance and NAFLD, and is a worldwide epidemic (6). Comparing the high rates of medically prescribed corticosteroids with the prevalence of overweight and obesity in developed countries, the combination of obesity and glucocorticoid excess may be present in many individuals. Given the similar comorbidities associated with obesity and chronically elevated glucocorticoids, we hypothesized that the combinations of these two conditions would lead to worse metabolic outcomes than either of them alone. This is supported by studies in rats showing that corticosterone and high-fat diets combine to cause worsened insulin resistance and NAFLD (7, 8).
There is an array of physiological changes that occur as a result of elevated glucocorticoids, including decreased lean mass (9–11), increased fat mass (10, 12, 13), NAFLD (8), and increased lipolysis (14–16), all of which have been associated with decreased insulin sensitivity (17–19). Recent tissue-specific knockouts of glucocorticoid-signaling mediators have implicated adipose tissue as a central node linking glucocorticoid action and lipolysis to systemic insulin resistance and NAFLD (20–23). In this study, we present the finding that chronically elevated glucocorticoids, via dexamethasone treatment, in the presence of diet-induced obesity have synergistic effects on lipolysis, insulin resistance, and fatty liver disease. Obese dexamethasone-treated mice have reduced fat mass compared with all other groups, yet have hyperglycemia and severe insulin resistance. Therefore, we speculate that glucocorticoid-induced adipocyte lipolysis drives insulin resistance in obese animals.
Methods
Animal procedures
C57BL/6J adult male mice were purchased from The Jackson Laboratory at 9 weeks of age (stock no. 000664). All animals were on a light/dark cycle of 12:12 hours and housed at 22°C. Following 1 week of acclimation, mice were placed on diets or treated with dexamethasone as described in the figure legends. Mice were treated with vehicle (water) or ∼1 mg/kg/d of water-soluble dexamethasone (catalog no. 2915; Sigma-Aldrich), a synthetic glucocorticoid, dissolved in their drinking water for 12 weeks, as described previously (10). Additional cohorts of mice used in these experiments either remained on a standard diet [normal chow diet (NCD); 13% fat, 57% carbohydrate, and 30% protein; 5L0D; LabDiet] or were provided a high-fat diet (HFD; 45% fat from lard, 35% carbohydrate mix of starch, maltodextrin, and sucrose, and 20% protein from casein; catalog no. D12451; Research Diets, Inc.) for either 8 or 12 weeks followed by dexamethasone treatment. Mice were group housed with four mice per cage, and food consumption was measured weekly by weight reductions per cage and calculated to reflect estimated intake of each mouse per day in a given cage. Mice remained on their respective diets for the duration of the study. All mice were provided with access to food and water ad libitum throughout the study, unless otherwise noted. Water intake was measured weekly to determine the concentrations of dexamethasone consumed per cage. Average concentration per mouse was estimated by accounting for number of mice in the cage. For the longer 6-week dexamethasone treatments, 16 HFD-fed, dexamethasone-treated mice appeared ill, were euthanized, and thus removed from all analyses once symptoms were noticed. Symptoms included lethargy, weight loss, and evidence of pancreatitis in some of the mice. Animal body weight and composition was determined weekly using a digital scale and EchoMRI 2100 (EchoMRI), respectively. We performed a Comprehensive Lab Animal Monitoring System (Columbus Instruments) experiment (data not shown) with the 12-week diet study prior to dexamethasone treatment in which mice were singly housed for ∼1 week, which led to fluctuations in body weight over the first week. Body weight quickly stabilized following removal from the Comprehensive Lab Animal Monitoring System in both groups. At the end of treatment, mice were fasted for 16 hours beginning at Zeitgeber time (ZT) 10 (dexamethasone water was not removed during this time) and euthanized by cervical dislocation after isoflurane anesthesia at ZT3 of the following day. Immediately following euthanasia, mice were dissected, and the right inguinal white adipose tissue (iWAT) and epididymal white adipose tissue (eWAT) depots were carefully removed and weighed. Adipose tissues, along with a section of the left lateral lobe of the liver, were snap frozen in liquid nitrogen for later analysis. Small pieces of tissues were fixed in 10% phosphate-buffered formalin for histology. Animal procedures were approved by the University of Tennessee Health Science Center and University of Michigan Institutional Animal Care and Use Committees.
Determination of serum dexamethasone
Serum from 16-hour–fasted lean and obese mice following 6 weeks of dexamethasone treatment was acquired prior to euthanizing at the end of the study and sent to the University of Michigan Pharmacokinetic and Mass Spectrometry Core for liquid chromatography–mass spectrometry analysis of dexamethasone concentration. Dexamethasone standard was used to make a calibration curve from 2.5 to 100 ng/mL. A separate weighing of dexamethasone was used to make quality control standards at 3 and 30 ng/mL. Quality control standards were run in triplicate before and during sample analysis. For each calibration standard and quality control standard, 10 µL of blank plasma, 10 µL of calibration or quality control standard, and 40 µL of internal standard were mixed in a 96-well plate. Each analytical sample was prepared by mixing 10 µL mouse plasma, 10 µL acetonitrile, and 40 µL internal standard into a well of a 96-well plate. Some samples were <10 µL in volume. In these cases, the volume collected was diluted to 10 µL and prepared in the same manner as the other samples. The plate was mixed at 1000 rpm for 5 minutes and then centrifuged at 3500 rpm for 10 minutes. Four microliters supernatant was injected for analysis onto a Xevo TQD triple quadrupole UPLC mass spectrometer (Waters) for analysis.
Insulin tolerance tests and hyperinsulinemic-euglycemic clamp experiments
Insulin responsiveness was assessed via an insulin tolerance test (ITT). Following a 6-hour fast beginning at ZT1, mice were given an intraperitoneal (IP) injection of insulin (Humulin R; Eli Lilly and Company) as described in the figure legends. Blood was collected from the tail, and glucose was determined using a OneTouch Ultra Glucometer (LifeScan). For the hyperinsulinemic-euglycemic clamp experiments, C57BL/6J adult (70 days) male mice were fed HFD for 8 weeks and treated with dexamethasone in their drinking water for 3 weeks or regular drinking water. Animals were anesthetized with an IP injection of sodium pentobarbital (50 to 60 mg/kg). Indwelling catheters were inserted into the right jugular vein and the right carotid artery, respectively. The free ends of catheters were tunneled subcutaneously and exteriorized at the back of the neck via a stainless-steel tubing connector (coated with medical silicone) that was fixed subcutaneously upon closure of the incision. Animals with healthy appearance, normal activity, and weight regain of ≥90% of their presurgery levels were used for the study. Experiments were carried out in conscious and unrestrained animals using techniques described previously (24–26). Briefly, the prime-continuous (1.0 μCi) infusion [0.05 μCi/min and increased to 0.1 µCi/min at time (t) = 0] of [3-3H] glucose (50 µCi/mL in saline) was started at t = −120 minutes. After a 5-hour fast, the insulin clamp was initiated at t = 0, with a prime-continuous infusion (40 mU/kg bolus, followed by 8.0 mU/kg/min) of human insulin (Novo Nordisk). Euglycemia (120 to 130 mg/dL) was maintained during the clamp by measuring blood glucose every 10 minutes and infusing 50% glucose at variable rates, accordingly. Blood samples were collected from the right carotid artery at t = 80, 90, 100, and 120 minutes for determination of glucose specific activity. Blood insulin concentrations were determined from samples taken at t = −10 and 120 minutes. A bolus injection of [1-14C]-2-deoxyglucose ([14C]2DG; 10 µCi; PerkinElmer) was given at t = 120 minutes. Blood samples were taken at 2, 5, 10, 15, and 25 minutes after the injection for determination of plasma [14C]2DG radioactivity. At the end of the experiment, animals were anesthetized with an intravenous injection of sodium pentobarbital, and tissues were collected and immediately frozen in liquid nitrogen for later analysis of tissue [14C]2DG phosphate radioactivity. Blood glucose was measured using an AccuChek glucometer (Roche). Plasma insulin was measured using rat/mouse insulin enzyme-linked immunosorbent assay kits (Linco Research). For determination of plasma radioactivity of [3-3H]glucose and [14C]2DG, plasma samples were deproteinized with ZnSO4 and Ba(OH)2 and counted using a Liquid Scintillation Counter (LS6500 Multipurpose Scintillation Counter; Beckman Coulter). Glucose turnover rate, hepatic glucose production, and tissue glucose uptake were calculated as described elsewhere (25–27).
Serum glycerol and fatty acid determination
Following 12 weeks of dexamethasone treatment, 22-week-old ad libitum chow-fed C57BL/6J male mice were anesthetized with isoflurane, and blood was collected into heparin-coated capillary tubes via retro-orbital bleed both prior to and 15 minutes following IP injection of 10 mg/kg isoproterenol (catalog no. I6504-1G; Sigma-Aldrich) in Dulbecco’s phosphate-buffered saline (catalog no. BW17512F1; Thermo Fisher Scientific). Serum from these mice, as well as from a cohort of 28-week-old mice on either HFD or chow, 6 weeks post–dexamethasone treatment, was collected following an overnight fast beginning at ZT10. Glycerol was assessed via the Serum Triglyceride Determination Kit (catalog no. TR0100-1KT; Sigma-Aldrich), and fatty acids were quantified using the HR Series NEFA-HR(2) kit (catalog no. 276-76491; Wako Diagnostics) in accordance with the manufacturer’s guidelines.
Cell culture
3T3-L1 fibroblasts (preadipocytes; American Type Culture Collection; authenticated via short tandem repeat analysis) were cultured in 10% newborn calf serum, Dulbecco’s modified Eagle medium (4.5 g/L d-glucose; catalog no. 11965118; Thermo Fisher Scientific) with penicillin, streptomycin, and glutamine until confluence. Cells were switched to a differentiation cocktail at 2 days postconfluence (250 nM dexamethasone, 500 μM 3-isobutyl-1-methylxanthine, and 1 μg/mL insulin in 10% fetal bovine serum, in 4.5g/L glucose Dulbecco’s modified Eagle medium with penicillin, streptomycin, and glutamine) for 4 days (28). Media was replaced with differentiation medium containing only insulin for an additional 3 days. For the following 3 days, cells remained in media with no additional treatment. Cells used for these experiments were not cultured beyond 22 passages. To assess markers of lipolysis, cells remained in media and were treated with ethanol (vehicle) or 250 nM dexamethasone for 5 days before lysing, with dexamethasone media being refreshed on day 3 and extracted on day 5.
Assessment of triglyceride content in cells and tissue
3T3-L1 cells were grown and treated as described above. At the end of the treatment period, cells were lysed in homogenization buffer (50 mM Tris, pH 8, 5 mM EDTA, 30 mM mannitol, and protease inhibitor) and subjected to three freeze-thaw cycles with liquid nitrogen thawed at room temperature. Frozen liver tissue was homogenized using a TissueLyser II (Qiagen). Lipids were extracted using KOH and a chloroform to methanol (2:1) extraction. Triglyceride content was assessed using the Serum Triglyceride Determination Kit (Sigma-Aldrich), and absorbance was detected as described in Lu et al. (29).
Histology
Tissues were fixed in 10% phosphate-buffered formalin for 24 hours and then stored in 70% ethanol until further processing. Tissues were dehydrated, embedded in paraffin, and sent to the University of Michigan Comprehensive Cancer Center Tissue Core, where they were processed and stained with hematoxylin and eosin (H&E) to assess cell morphology. Slides were imaged using the 10× objective of an iX18 inverted microscope (Olympus) and cellSense software.
Messenger RNA extraction and analysis
Cells and tissues were lysed in TRIzol using the TissueLyser II (Qiagen), as described previously, and RNA was extracted using a PureLink RNA kit (catalog no. 12183025; Life Technologies). Complementary DNA was synthesized from 0.5 to 1 μg of RNA using the High Capacity Reverse Transcription Kit (catalog no. 4368813; Life Technologies). Primers, complementary DNA, and Power SYBR Green PCR Master Mix (catalog no. 4368708; Life Technologies) were combined in accordance with the manufacturer’s guidelines, and quantitative real-time polymerase chain reaction (qPCR) was performed as previously described (29) using QuantStudio 5 (Thermo Fisher Scientific). Messenger RNA (mRNA) expression level was normalized to Actb and analyzed using the ΔΔ threshold cycle method after evaluation of several reference genes. qPCR primer sequences are listed in Table 1.
Table 1.
Primers Used for Reverse-Transcription qPCR
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Actb | ATGTGGATCAGCAAGCAGGA | AAGGGTGTAAAACGCAGCTCA |
| Fasn | GGAGGTGGTGATAGCCGGTAT | TGGGTAATCCATAGAGCCCAG |
| Pnpla2 | CCACTCACATCTACGGAGCC | GATGCAGAGGACCCAGGAAC |
| Srebf1 | AGGCCATCGACTACATCCG | TCCATAGACACATCTGTGCCTC |
Protein extraction and analysis
Cells and tissues were lysed in RIPA buffer [50 mM tris(hydroxymethyl)aminomethane, pH 7.4, 0.25% sodium deoxycholate, 1% Nonidet P-40, 150 mM sodium chloride, 1 mM EDTA, 100 μM sodium orthovanadate, 5 mM sodium fluoride, 10 mM sodium pyrophosphate, and 1× protease inhibitor] and centrifuged at 14,000 rpm for 10 minutes at 4°C. Lysates were heated with loading buffer at 85 to 95°C, and proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Life Technologies) and transferred onto nitrocellulose membranes overnight at room temperature. Membranes were blotted at room temperature using anti–adipose triglyceride lipase (ATGL) antibodies [molecular weight 54; catalog no. 30A4; Cell Signaling Technologies; Research Resource Identifier (RRID): AB_2167953] and antibodies against hormone-sensitive lipase (HSL; molecular weight 81; catalog no. 4107; Cell Signaling Technologies; RRID: AB_2296900) and its protein kinase A phosphorylation sites on serine 563 and 660 (catalog no. 4139, Cell Signaling Technologies, RRID: AB_2135495; and catalog no. 4126, Cell Signaling Technologies, RRID: AB_490997, respectively). Antibody complexes were detected by anti-mouse and anti-rabbit fluorescent-conjugated antibodies (Invitrogen) and visualized using an Odyssey CLx image scanner (Li-Cor Biosciences). Blots were quantified using Image Studio software version 5.2 (Li-Cor Biosciences) and normalized to Revert Total Protein Stain (catalog no. 926-11011; Li-Cor Biosciences).
Statistics
All data are presented as mean ± standard error of the mean. For animal studies, two-way analysis of variance was performed to test for significance of diet and dexamethasone treatment as well as their interaction. Pairwise comparisons, normality, and equal variance were tested using Shapiro-Wilk and Levene tests, respectively. Pending those results, a Mann-Whitney, Welch, or Student t test was used. P values <0.05 were considered significant. All statistical tests were performed using the R software package version 3.30. All raw data and analysis scripts are available at http://bridgeslab.github.io/CushingAcromegalyStudy/.
Results
Dexamethasone-induced insulin resistance is worsened in the presence of obesity
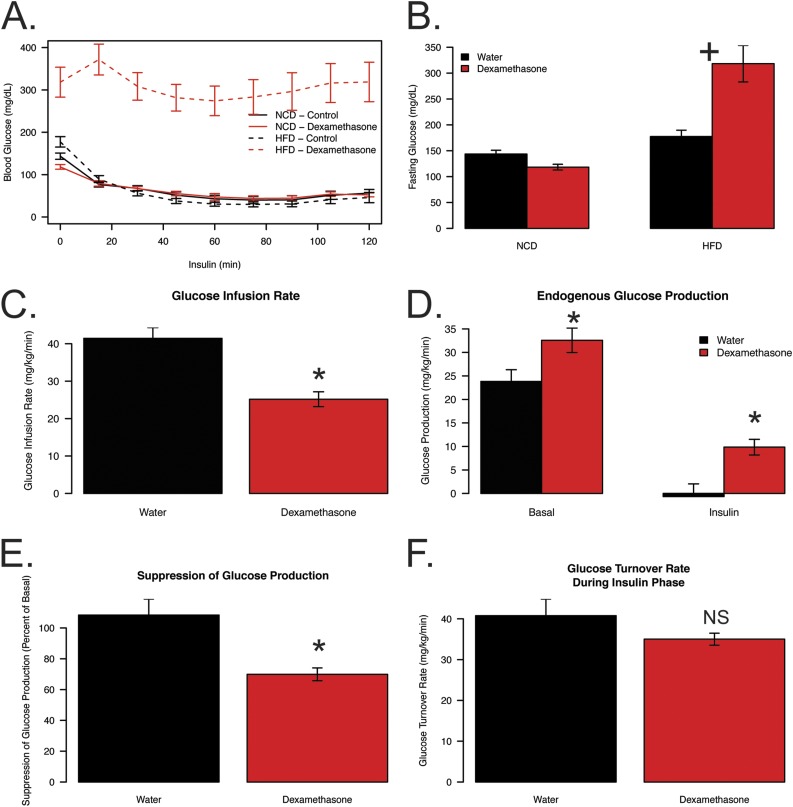
To investigate if obesity status influences insulin sensitivity in the presence of elevated glucocorticoids we performed an ITT on lean (NCD) and diet-induced obese (HFD) mice that were untreated (water) or treated with dexamethasone. HFD-fed, dexamethasone-treated mice were significantly more resistant to insulin-stimulated glucose disposal when compared with all other groups (Fig. 1A). When normalized to percent change from basal, dexamethasone treatment results in reduced glucose disposal when compared with water controls in lean and obese mice (Supplemental Fig. 1A). Additionally, HFD dexamethasone-treated mice exhibited dramatic fasting hyperglycemia, with a significant interaction between diet and drug (P = 0.00009; Fig. 1B). Although HFD animals had a 24% increase in fasting glucose when compared with NCD animals, in the presence of dexamethasone, HFD-fed animals had a 122% increase in fasting glucose relative to NCD controls not treated with dexamethasone. In the lean, NCD-fed animals, dexamethasone caused an 18% decrease in fasting glucose.
Figure 1.
Reductions in glucose handling are exacerbated when elevated glucocorticoids and obesity are combined. Mouse blood glucose levels during (A) ITT and (B) prior to insulin injection (basal). Insulin was given via IP injection at a concentration of 2.5 U/kg following 5 weeks of dexamethasone (NCD, n = 12; HFD, n = 12) or vehicle (NCD, n = 12; HFD, n = 12) treatment and 17 weeks of diet. Mouse (C) glucose infusion rate, (D) EGP, (E) insulin suppression of glucose production, and (F) glucose turnover rate during euglycemic clamp following 3 weeks of dexamethasone (n = 14) or vehicle (n = 11) treatment and 11 weeks of HFD. For clamp experiments, insulin was infused at 8 mU/kg/min following a prime continuous infusion of 40 mU/kg bolus. All mice were fasted for 5 to 6 hours prior to experiments. Plus symbols indicate a significant interaction between diet and treatment. Asterisks indicate a statistically significant treatment effect for the pairwise comparison. NS, not significant.
To evaluate glucose homeostasis in more detail, we performed hyperinsulinemic-euglycemic clamps in obese mice (11 weeks of HFD) treated with dexamethasone for the final 3 weeks. This shorter HFD/dexamethasone exposure still caused dramatic insulin resistance, hyperglycemia, and reductions in lean mass, but no differences in fat mass (Supplemental Fig. 1B–1E). Animals were clamped while conscious, and glucose levels during the clamp as well as insulin turnover rate were similar between groups (Supplemental Fig. 1F and 1G). During the hyperinsulinemic phase, the glucose infusion rate was 39% lower in obese dexamethasone-treated mice when compared with obese controls, indicating insulin resistance at euglycemia (Fig. 1C). Basal endogenous glucose production (EGP) was 37% higher in the dexamethasone-treated group (P = 0.026; Fig. 1D). Moreover, in the control group, EGP was reduced to near zero by a high dose of insulin but only reduced 70% in the dexamethasone group (P = 0.0091), resulting in glucose production being higher during the insulin phase in dexamethasone-treated mice (P = 0.014) when compared with controls (Fig. 1D and 1E). Glucose turnover was slightly decreased in the presence of insulin (P = 0.141; Fig. 1F). Despite these modest changes in glucose turnover, there were significant reductions in the obese, dexamethasone-treated animals in 2-deoxyglucose uptake in gastrocnemius (68% reduced; P = 0.00002) and heart tissues (34% reduced, P = 0.0003; Supplemental Fig. 1H and 1I). These data suggest that increased glucose production and its impaired suppression by insulin are the likely causes of poor glycemic control in obese, dexamethasone-treated animals.
HFD-induced liver steatosis in dexamethasone-treated mice
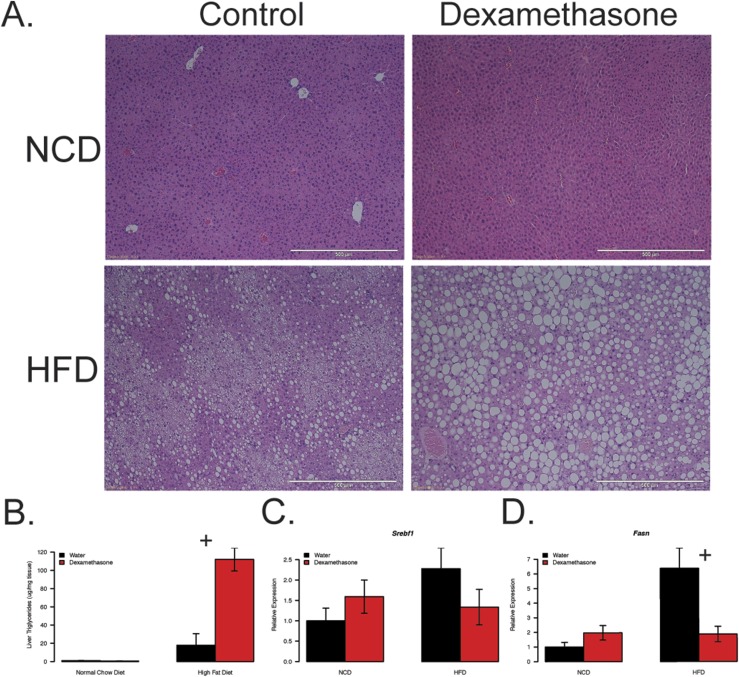
Obesity and chronic elevations in glucocorticoids are associated with NAFLD (30, 31). H&E staining of hepatic tissue clearly depicts exacerbated lipid levels in the obese, dexamethasone-treated group when compared with obese controls and lean groups (Fig. 2A). In support of this, we observe drastically elevated liver triglycerides when compared with all other groups with a significant interaction between drug and diet (P = 0.000068; Fig. 2B).
Figure 2.
Increased glucocorticoids lead to greater severity of hepatic steatosis in obese mice. Mouse (A) H&E-stained liver sections, (B) hepatic triglyceride levels, and (C and D) qPCR of hepatic de novo lipogenic transcripts. Mice were euthanized at 28 weeks of age following 6 weeks of dexamethasone (NCD, n = 7; HFD, n = 5) or vehicle (NCD, n = 6; HFD, n = 9) treatment and 18 weeks of diet. Liver stains are representative samples from each group. Plus symbols indicate a significant interaction between diet and treatment.
We used qPCR to measure the expression of genes involved in hepatic de novo lipogenesis, Srebf1 and Fasn, in liver lysates (Fig. 2C and 2D). We observed no synergism in expression levels between HFD and dexamethasone. This finding indicates that lipid accumulation in response to dexamethasone treatment is likely occurring via mechanisms other than accelerated glucocorticoid-dependent activation of de novo lipogenesis.
Dexamethasone causes decreased fat mass in obese mice
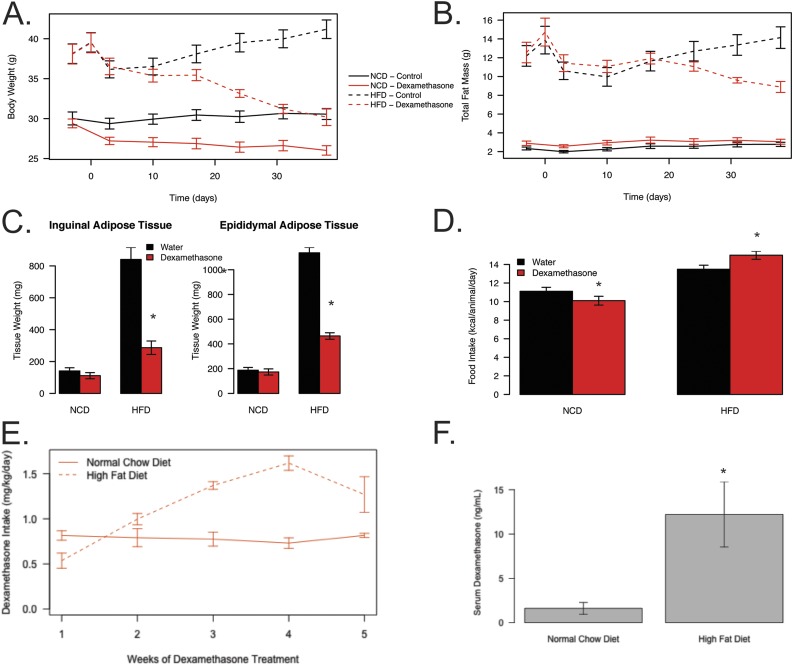
To understand the how dexamethasone effects body composition in these animals, we determined total fat mass. We observed reductions in body weight and fat mass in the HFD-fed dexamethasone-treated group (Fig. 3A and 3B). These reductions do not appear to be depot specific, as we observed reductions in both iWAT (65% reduced) and eWAT mass (59% reduced; Fig. 3C) in the obese, dexamethasone-treated mice. There were no marked differences in fat mass, either by magnetic resonance imaging or gross tissue weights of iWAT or eWAT depots in response to dexamethasone treatment in the chow-fed groups (Fig. 3B and 3C). To determine if changes in body composition could be explained by altered caloric consumption, we compared food intake among the groups (Fig. 3D). Lean dexamethasone-treated mice ate significantly less than lean controls (9% reduction; P = 0.006), as previously reported (32, 33). Surprisingly, we found that the obese dexamethasone-treated mice ate slightly more food (11% increase; P = 0.032), even though they lost both fat and fat-free mass. These data suggest that the weight loss in obese animals provided dexamethasone is not due to reductions in food intake.
Figure 3.
Dexamethasone treatment reduces fat mass in obese mice. Weekly total (A) body mass and (B) fat mass measures via EchoMRI in mice over the course of treatment (solid lines represent NCD mice, and dashed lines represent HFD mice). (C) Adipose tissue weights in 16-hour–fasted mice following euthanasia. Mice were euthanized at 28 weeks of age following 6 weeks of dexamethasone (NCD, n = 8; HFD, n = 12) or vehicle (NCD, n = 8; HFD, n = 22) treatment and 18 weeks of diet. (D) Food consumption measured weekly over the course of treatment. (E) Amount of dexamethasone consumed per mouse throughout the study normalized to body weight as determined by volume consumed per cage per week for NCD- (n = 12) and HFD-fed (n = 20) mice. (F) Concentration of dexamethasone in serum of NCD-fed (n = 8) and HFD-fed (n = 11) at the end of the study as determined by liquid chromatography–mass spectrometry. Asterisks indicate a statistically significant treatment or diet effect for the pairwise comparison.
Over the course of the experiment, obese dexamethasone-treated mice consumed more water, starting at a lower amount, which then increased over the duration of the experiment (Fig. 3E). Overall, this corresponded to a 47% increase when normalized to the animal’s body weight and a 92% increase when not normalized to body weight. By the end of the study, this increased intake resulted in a 7.6-fold increase in serum dexamethasone concentration in the obese dexamethasone-treated mice when compared with lean dexamethasone-treated mice (P = 0.031; Fig. 3F).
Dexamethasone treatment results in increased lipolysis
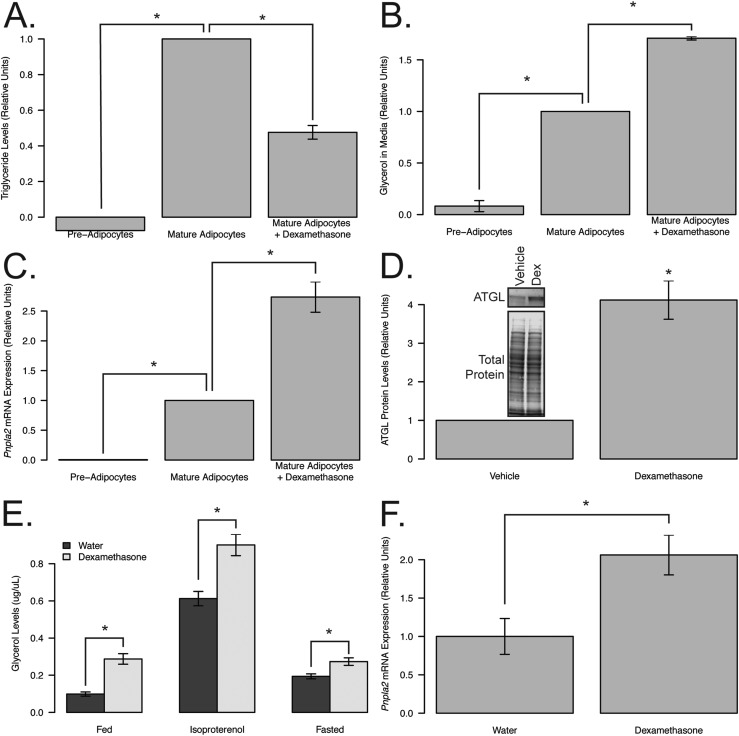
Lipolysis has previously been associated with insulin resistance (17, 34), is known to be elevated in patients with NAFLD (35), and has been shown to increase with high levels of glucocorticoids (10, 14–16). To assess whether dexamethasone was affecting the lipid content in adipose tissue, we measured markers of adipocyte lipolysis in cultured adipocytes. 3T3-L1 fibroblasts were undifferentiated (preadipocytes) or differentiated and treated with vehicle or dexamethasone following differentiation. Dexamethasone treatment following differentiation led to decreased lipid content (52.4% reduction; P = 0.005; Fig. 4A) and a 71% increase in the amount of glycerol in the media (P = 0.001; Fig. 4B), suggesting increased lipolysis. To identify a potential glucocorticoid receptor–dependent lipolytic target, we evaluated the levels of ATGL, the rate-limiting enzyme in lipolysis. Expression of ATGL (encoded by the Pnpla2 gene) was enhanced following dexamethasone treatment in 3T3-L1 cells at the transcript (2.7-fold; P = 0.002; Fig. 4C) and protein (4.2-fold; P = 0.025; Fig. 4D) levels. These data show that glucocorticoids elevate ATGL levels and metabolites of lipolysis in cultured adipocytes.
Figure 4.
Dexamethasone treatment induces lipolysis in vivo and in vitro. (A) Triglyceride levels, (B) glycerol released in media, (C) qPCR of Pnpla2 transcripts, and (D) representative western blot and quantification of ATGL from nondifferentiated (preadipocytes; n = 2) or differentiated 3T3-L1 mouse adipocytes (mature adipocytes) following 5 days of dexamethasone (n = 3) or vehicle treatment (n = 3). (E) Serum glycerol levels at basal (fed) and following stimulation (10 mg/kg isoproterenol or 16-hour fast) and (F) qPCR of iWAT lipolytic transcripts in 22-week-old, 12-week dexamethasone- (basal and isoproterenol, n = 7; fasted serum and qPCR, n = 4) or vehicle-treated (basal and isoproterenol, n = 12; fasted serum and qPCR, n = 11), chow-fed mice with the exception of isoproterenol-stimulated glycerol, which was performed 1 week prior to euthanasia. Asterisks indicate statistically significant treatment effect for the pairwise comparison. Dex, dexamethasone.
To measure the effects of glucocorticoid-induced lipolysis in vivo, we quantified glycerol levels in animals chronically exposed to dexamethasone in basal and stimulated conditions (Fig. 4E). Stimulation of lipolysis was achieved via isoproterenol, a β-adrenergic receptor agonist, or by a 16-hour fast. Dexamethasone treatment led to increases in glycerol in the fed (2.9-fold), fasted (1.5-fold), and isoproterenol-stimulated (1.4-fold) conditions (P < 0.05 for all pairwise comparisons), indicating that dexamethasone enhances basal and stimulated lipolysis in vivo in chow-fed mice. Consistent with these findings, mRNA analysis from iWAT of these mice showed an upregulation of Pnpla2 transcripts in the dexamethasone-treated mice compared with controls (2.1-fold, P = 0.016; Fig. 4F).
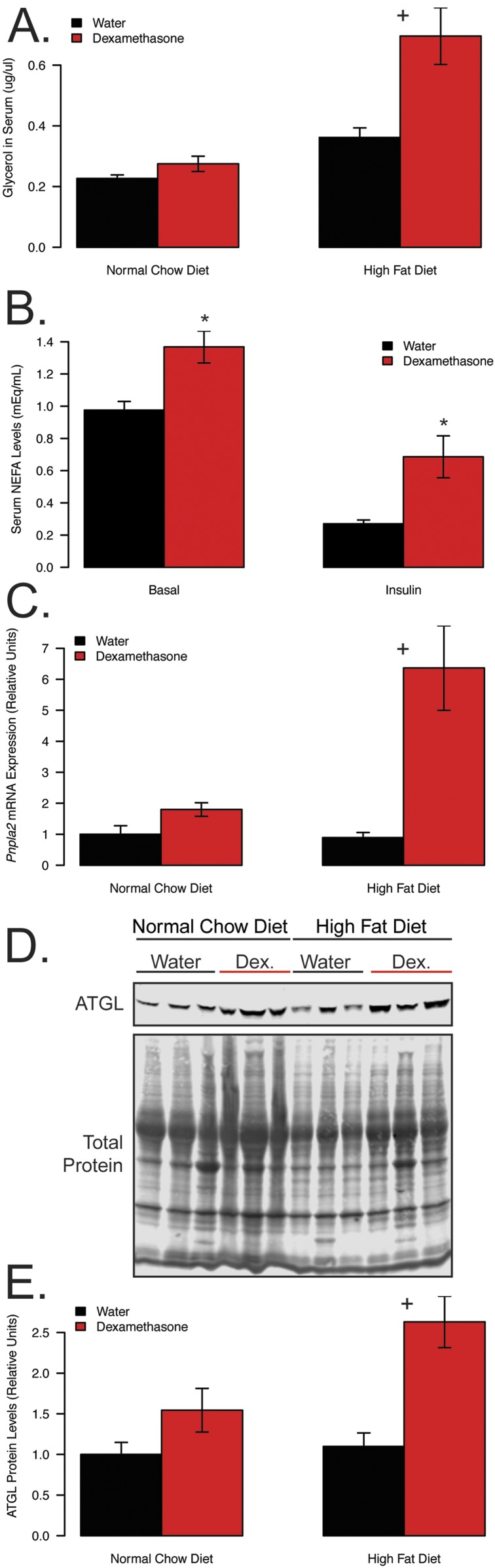
To understand how diet-induced obesity alters dexamethasone-induced lipolysis, we next quantified serum glycerol concentrations in our HFD/NCD-fed mice (Fig. 5A). We observed a nearly twofold increase in serum glycerol levels by dexamethasone treatment in the HFD-fed animals, compared with only an 18% increase in chow-fed mice (P = 0.017 for the interaction between diet and dexamethasone). For the hyperinsulinemic-euglycemic clamp in the obese mice, there was a 40% elevation in serum basal nonesterified fatty acids (NEFAs) in response to 3 weeks of dexamethasone treatment (P = 0.004; Fig. 5B). During the insulin phase, dexamethasone treatment attenuated the ability of insulin to suppress serum NEFA levels with insulin, leading to a 71% reduction in controls compared with only a 48% reduction in dexamethasone-treated mice (P = 0.058). These findings suggest that in the obese setting, dexamethasone elevates lipolysis to a greater extent and attenuates the effects of insulin.
Figure 5.
Obesity exacerbates dexamethasone-induced lipolysis. (A) Serum glycerol following 16-hour fast, (B) serum NEFA in obese dexamethasone-treated (n = 14) or control (n = 11) mice following a 5-hour fast, before and after insulin during hyperinsulinemic-euglycemic clamp, (C) qPCR of Pnpla2 transcripts from iWAT, (D) western blot image, and (E) quantification of ATGL protein from iWAT. Mice from (A), (C), (D), and (E) were euthanized at 28 weeks of age following 6 weeks of dexamethasone (NCD, n = 8; HFD, n = 10) or vehicle (NCD, n = 8; HFD, n = 10) treatment. Mice from (B) were fasted for 5 h prior to euglycemic clamp following 3 weeks of dexamethasone (n = 14) or vehicle (n = 11) treatment and 11 weeks of HFD. For clamp experiments, insulin was infused at 8 mU/kg/min following a prime continuous infusion of 40 mU/kg bolus. Plus symbols indicate a significant interaction between diet and treatment. Asterisks indicate a statistically significant treatment effect for the pairwise comparison. Dex, dexamethasone.
To investigate the molecular basis for this synergistic increase in lipolysis, we quantified mRNA and protein expression of ATGL in the iWAT of these mice (Fig. 5C–5E). Consistent with the hypothesis that ATGL activation could drive increased lipolysis in obese dexamethasone-treated mice, expression of ATGL was elevated in both dexamethasone-treated groups, with a significant synergistic effect of dexamethasone and obesity at the transcript (P = 0.02) and protein (P = 0.043) levels. There were no significant increases observed in HSL levels or phosphorylation that might explain enhanced lipolysis in the obese, dexamethasone-treated mice (Supplemental Fig. 2). In fact, phosphorylation of protein kinase A sites on HSL tended to be lower in obese mice when compared with lean, as has been reported previously (36). These data support the hypothesis that glucocorticoid-stimulated lipolysis is augmented in the context of obesity, potentially via increased transactivation of Pnpla2/ATGL.
Discussion
Chronic glucocorticoid elevations are associated with comorbidities such as increased fat mass (10, 12, 13), decreased muscle mass (9–11), insulin resistance, and NAFLD (1). Many of these adverse effects are similar to those seen in obesity; however, the combination of chronically elevated glucocorticoids in the context of pre-existing obesity has not been assessed. In this study, we show that the effects of glucocorticoid-induced insulin resistance and NAFLD are exacerbated when paired with obesity.
We appreciate that glucocorticoids directly affect many other tissues, such as muscle, liver, and pancreas, that may also influence insulin sensitivity. In support of a central role of adipocytes, several studies demonstrate that adipocyte-specific reductions in glucocorticoid signaling are associated with improved metabolic profiles (20–23). We hypothesize that adipose tissue lipolysis plays a major role in dexamethasone-induced insulin resistance and hepatic steatosis, especially in the case of obesity.
Excess adiposity, such is seen in obesity, has been associated with increased insulin resistance. Previous work from our laboratory shows increased fat mass following 12 weeks of dexamethasone treatment (10) in lean mice, in accordance with what others have reported (37), as well as reduced insulin sensitivity. However, to our surprise, the glucocorticoid treatment in obese mice led to a lipodystrophic phenotype, which indicates the disturbances in glucose homeostasis are not a result of increased fat mass. The loss in fat mass observed in the obese, dexamethasone-treated mice was not due to reduced food intake; in fact, these mice ate significantly more calories per day than obese controls. This suggests a potential increase in energy expenditure with the combination of obesity and dexamethasone treatment over time. This study evaluated glucocorticoid treatment in the context of diet-induced obesity; however, Riddell and colleagues have reported similar findings when providing HFD and glucocorticoids in concert to rats, prior to the onset of obesity (7, 8, 38). It is not clear whether diet and obesity status have similar mechanisms causing exacerbated metabolic risk, but these interactions deserve further evaluation.
Lipolysis has been linked to increased gluconeogenesis by several studies (39–43). Glucocorticoids are known to stimulate lipolysis (10, 14–16), possibly as a way to promote gluconeogenesis to maintain blood glucose levels. Lipolysis has also been implicated in insulin resistance (17, 34) and NAFLD (35). We found synergistic elevations in glycerol, indicative of enhanced lipolysis, as well as in hepatic fat accumulation in the HFD-fed, dexamethasone-treated mice, but no data supporting enhanced hepatic de novo lipogenesis. Therefore, we propose that the dexamethasone-induced increase in hepatic steatosis in the obese mice is primarily due to enhanced lipolysis observed in these animals.
There is some debate as to which genes glucocorticoids act on to promote lipolysis. Downregulation of Pde3b (44) and upregulation of β-adrenergic receptors (45) and ATGL transcripts (21, 46, 47) have been proposed as possible mechanisms. We found ATGL, the rate-limiting enzyme for adipose triglyceride lipolysis, to be synergistically activated by obesity and glucocorticoid treatment. These findings bear a resemblance to elevations in glycerol levels in obese, dexamethasone-treated mice when compared with diet or glucocorticoids alone. There were no significant differences in the effects of diet or treatment on HSL phosphorylation. Interestingly, obesity and dexamethasone treatment appeared to slightly decrease HSL phosphorylation, consistent with previous reports (36). Given these results, we attribute enhanced lipolysis seen in obese dexamethasone-treated mice in part to upregulated ATGL. The mechanisms by which obesity and glucocorticoids synergize to activate ATGL expression are not clear at this time, nor are the relative contributions of other glucocorticoid receptor–dependent targets.
The obese, dexamethasone-treated animals consumed increasing amounts of dexamethasone as the study progressed, resulting in increased serum dexamethasone at euthanization. This was unexpected and may be due to the increased urination and water requirement in severely diabetic animals, as has been documented previously (48). This is an important limitation to our study, although we note that several phenotypes, including fasting glucose, liver triglycerides, hepatic lipogenic gene expression, and adipose tissue mass, changed in different directions in lean and obese animals and therefore are unlikely to be due to an increased dose of dexamethasone. For example, dexamethasone reduced fasting blood glucose levels in lean mice, but led to hyperglycemia in obese mice. The dose of dexamethasone received was within the clinical range administered to human patients (49–51), corresponding to ∼5 mg/d in an average-sized human. Circulating concentrations of dexamethasone were similar to those observed following therapeutic doses of glucocorticoids (52–54) and in patients with Cushing syndrome (55, 56), even after accounting for dexamethasone’s higher potency and similar to other studies investigating glucocorticoid-induced metabolic effects in rodent models (7).
In summary, glucocorticoids are commonly prescribed drugs used to treat a multitude of health issues, but are known to induce a variety of adverse metabolic effects. Their actions in persons with obesity are not yet clear, even though there is a considerable number of individuals with obesity routinely taking prescription glucocorticoids. This paper shows that diet-induced obesity in mice exacerbates several comorbidities associated with chronically elevated glucocorticoids. These effects may be considered by physicians when determining glucocorticoid treatment options for patients with obesity.
Supplementary Material
Acknowledgments
We thank Lynne Geletka, Jennifer DelProposto, and Carey Lumeng (University of Michigan) for assistance with imaging liver sections and Melanie Schmitt for assistance with glucose clamp studies. We also thank the other members of the Bridges laboratory, Thurl Harris (University of Virginia), Christoph Buettner, and Eliza Geer (Icahn School of Medicine at Mount Sinai); Edwards Park (University of Tennessee Health Science Center); and Tobias Else (University of Michigan) for insights on this work.
Financial Support: This study was supported by funds from National Institutes of Health Grant R01-DK107535 (to D.B.) and a Pilot and Feasibility Grant from the Michigan Diabetes Research Center (P30-DK020572). This study also used the University of Michigan Metabolism, Bariatric Surgery and Behavior Core (U2C-DK110768), the Michigan Nutrition Obesity Research Center (P30-DK089503), and the University of Michigan Comprehensive Cancer Center Core (P30-CA046592). E.J.S. is partially supported by funding from Le Bonheur Children’s Hospital, the Children’s Foundation Research Institute, and the Le Bonheur Associate Board.
Author Contributions: I. Harvey performed all cell experiments and wrote the manuscript. I. Harvey, E.J.S., and J.R.R. performed mouse experiments. I. Harvey, I. Hochberg, and D.B. were responsible for conceptualizing the study. I. Harvey, N.Q., and D.B. designed the experiments. I. Harvey and D.B. edited and reviewed the manuscript. Q.T.T. and D.B. performed statistical analyses. D.B. acquired funding. All authors were involved in discussions and approved the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- [14C]2DG
[1-14C]-2-deoxyglucose
- ATGL
adipose triglyceride lipase
- EGP
endogenous glucose production
- eWAT
epididymal white adipose tissue
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- HSL
hormone-sensitive lipase
- IP
intraperitoneal
- ITT
insulin tolerance test
- iWAT
inguinal white adipose tissue
- mRNA
messenger RNA
- NAFLD
nonalcoholic fatty liver disease
- NCD
normal chow diet
- NEFA
nonesterified fatty acid
- qPCR
quantitative real-time polymerase chain reaction
- RRID
Research Resource Identifier
- t
time
- ZT
Zeitgeber time.
References
- 1. Paredes S, Ribeiro L. Cortisol: the villain in metabolic syndrome? Rev Assoc Med Bras (1992). 2014;60(1):84–92. [DOI] [PubMed] [Google Scholar]
- 2. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013;65(2):294–298. [DOI] [PubMed] [Google Scholar]
- 3. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford). 2011;50(11):1982–1990. [DOI] [PubMed] [Google Scholar]
- 4. Hsiao C, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report. 2007;2010(27):1–32. [PubMed] [Google Scholar]
- 5. Laugesen K, Jørgensen JOL, Sørensen HT, Petersen I. Systemic glucocorticoid use in Denmark: a population-based prevalence study. BMJ Open. 2017;7(5):e015237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beaudry JL, Anna MD, Teich T, Tsushima R, Riddell MC. Exogenous glucocorticoids and a high-fat diet cause severe hyperglycemia and hyperinsulinemia and limit glucose responsiveness in young male Sprague-Dawley rats. Endocinology. 2013;154(9):3197–3208. [DOI] [PubMed] [Google Scholar]
- 8. Shpilberg Y, Beaudry JL, D’Souza A, Campbell JE, Peckett A, Riddell MC. A rodent model of rapid-onset diabetes induced by glucocorticoids and high-fat feeding. Dis Model Mech. 2012;5(5):671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in aging. J Clin Invest. 1995;96(5):2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hochberg I, Harvey I, Tran QT, Stephenson EJ, Barkan AL, Saltiel AR, Chandler WF, Bridges D. Gene expression changes in subcutaneous adipose tissue due to Cushing’s disease. J Mol Endocrinol. 2015;55(2):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45(10):2163–2172. [DOI] [PubMed] [Google Scholar]
- 12. Abad V, Chrousos GP, Reynolds JC, Nieman LK, Hill SC, Weinstein RS, Leong GM. Glucocorticoid excess during adolescence leads to a major persistent deficit in bone mass and an increase in central body fat. J Bone Miner Res. 2001;16(10):1879–1885. [DOI] [PubMed] [Google Scholar]
- 13. Geer EB, Shen W, Gallagher D, Punyanitya M, Looker HC, Post KD, Freda PU. MRI assessment of lean and adipose tissue distribution in female patients with Cushing’s disease. Clin Endocrinol (Oxf). 2010;73(4):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djurhuus CB, Gravholt CH, Nielsen S, Pedersen SB, Møller N, Schmitz O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab. 2004;286(3):E488–E494. [DOI] [PubMed] [Google Scholar]
- 15. Kršek M, Rosická M, Nedvídková J, Kvasnicková H, Hána V, Marek J, Haluzík M, Lai EW, Pacák K. Increased lipolysis of subcutaneous abdominal adipose tissue and altered noradrenergic activity in patients with Cushing’s syndrome: an in-vivo microdialysis study. Physiol Res. 2006;55(4):421–428. [DOI] [PubMed] [Google Scholar]
- 16. Djurhuus CB, Gravholt CH, Nielsen S, Mengel A, Christiansen JS, Schmitz OE, Møller N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab. 2002;283(1):E172–E177. [DOI] [PubMed] [Google Scholar]
- 17. Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest. 1996;98(3):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang M, Hu T, Zhang S, Zhou L. Associations of different adipose tissue depots with insulin resistance: a systematic review and meta-analysis of observational studies. Sci Rep. 2015;5(1):18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJ. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. [DOI] [PubMed] [Google Scholar]
- 20. Mueller KM, Hartmann K, Kaltenecker D, Vettorazzi S, Bauer M, Mauser L, Amann S, Jall S, Fischer K, Esterbauer H, Müller TD, Tschöp MH, Magnes C, Haybaeck J, Scherer T, Bordag N, Tuckermann JP, Moriggl R. Adipocyte glucocorticoid receptor deficiency attenuates aging- and HFD-induced obesity and impairs the feeding-fasting transition [published correction appears in Diabetes. 2018;67(2):343-344] Diabetes. 2017;66(2):272–286. [DOI] [PubMed] [Google Scholar]
- 21. Shen Y, Roh HC, Kumari M, Rosen ED. Adipocyte glucocorticoid receptor is important in lipolysis and insulin resistance due to exogenous steroids, but not insulin resistance caused by high fat feeding. Mol Metab. 2017;6(10):1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgan SA, McCabe EL, Gathercole LL, Hassan-Smith ZK, Larner DP, Bujalska IJ, Stewart PM, Tomlinson JW, Lavery GG. 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc Natl Acad Sci USA. 2014;111(24):E2482–E2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Yan C, Liu L, Wang W, Du H, Fan W, Lutfy K, Jiang M, Friedman TC, Liu Y. 11β-Hydroxysteroid dehydrogenase type 1 shRNA ameliorates glucocorticoid-induced insulin resistance and lipolysis in mouse abdominal adipose tissue. Am J Physiol Endocrinol Metab. 2015;308(1):E84–E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009;297(4):E849–E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55(2):390–397. [DOI] [PubMed] [Google Scholar]
- 26. Halseth AE, Bracy DP, Wasserman DH.. Overexpression of hexokinase II increases insulinand exercise-stimulated muscle glucose uptake in vivo. Am J Physiol. 1999;276(1 Pt 1):E70–E77. [DOI] [PubMed] [Google Scholar]
- 27. Kraegen E, James D, Jenkins A, Chisholm D.. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248(3 Pt 1):E353–E362. [DOI] [PubMed] [Google Scholar]
- 28. Chiang SH, Chang L, Saltiel AR. TC10 and insulin-stimulated glucose transport. Methods Enzymol. 2006;406:701–714. [DOI] [PubMed] [Google Scholar]
- 29. Lu B, Bridges D, Yang Y, Fisher K, Cheng A, Chang L, Meng ZX, Lin JD, Downes M, Yu RT, Liddle C, Evans RM, Saltiel AR. Metabolic crosstalk: molecular links between glycogen and lipid metabolism in obesity. Diabetes. 2014;63(9):2935–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. Hepatic steatosis in Cushing’s syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. 2003;149(6):543–548. [DOI] [PubMed] [Google Scholar]
- 31. Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12(5):1106–1110. [DOI] [PubMed] [Google Scholar]
- 32. Haber RS, Weinstein SP. Role of glucose transporters in glucocorticoid-induced insulin resistance. GLUT4 isoform in rat skeletal muscle is not decreased by dexamethasone. Diabetes. 1992;41(6):728–735. [DOI] [PubMed] [Google Scholar]
- 33. Roussel D, Dumas JF, Augeraud A, Douay O, Foussard F, Malthiéry Y, Simard G, Ritz P. Dexamethasone treatment specifically increases the basal proton conductance of rat liver mitochondria. FEBS Lett. 2003;541(1-3):75–79. [DOI] [PubMed] [Google Scholar]
- 34. Edgerton DS, Kraft G, Smith M, Farmer B, Williams PE, Coate KC, Printz RL, O’Brien RM, Cherrington AD. Insulin’s direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2(6):e91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50(4):1087–1093. [DOI] [PubMed] [Google Scholar]
- 36. Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298(4):C961–C971. [DOI] [PubMed] [Google Scholar]
- 37. Burke SJ, Batdorf HM, Eder AE, Karlstad MD, Burk DH, Noland RC, Floyd ZE, Collier JJ. Oral corticosterone administration reduces insulitis but promotes insulin resistance and hyperglycemia in male nonobese diabetic mice. Am J Pathol. 2017;187(3):614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D’souza AM, Beaudry JL, Szigiato AA, Trumble SJ, Snook LA, Bonen A, Giacca A, Riddell MC. Consumption of a high-fat diet rapidly exacerbates the development of fatty liver disease that occurs with chronically elevated glucocorticoids. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):G850–G863. [DOI] [PubMed] [Google Scholar]
- 39. Nurjhan N, Consoli A, Gerich J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992;89(1):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986;35(12):1326–1331. [DOI] [PubMed] [Google Scholar]
- 41. Perry RJ, Peng L, Abulizi A, Kennedy L, Cline GW, Shulman GI. Mechanism for leptin’s acute insulin-independent effect to reverse diabetic ketoacidosis. J Clin Invest. 2017;127(2):657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci USA. 1966;56(1):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, Xu G. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol. 2009;23(8):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lacasa D, Agli B, Giudicelli Y. Permissive action of glucocorticoids on catecholamine-induced lipolysis: direct “in vitro” effects on the fat cell beta-adrenoreceptor-coupled-adenylate cyclase system. Biochem Biophys Res Commun. 1988;153(2):489–497. [DOI] [PubMed] [Google Scholar]
- 46. Campbell JE, Peckett AJ, D’souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol. 2011;300(1):C198–C209. [DOI] [PubMed] [Google Scholar]
- 47. Serr J, Suh Y, Oh SA, Shin S, Kim M, Latshaw JD, Lee K. Acute up-regulation of adipose triglyceride lipase and release of non-esterified fatty acids by dexamethasone in chicken adipose tissue. Lipids. 2011;46(9):813–820. [DOI] [PubMed] [Google Scholar]
- 48. Lee SM, Bressler R. Prevention of diabetic nephropathy by diet control in the db/db mouse. Diabetes. 1981;30(2):106–111. [DOI] [PubMed] [Google Scholar]
- 49. Tyrrell JB, Findling JW, Aron DC, Fitzgerald PA, Forsham PH. An overnight high-dose dexamethasone suppression test for rapid differential diagnosis of Cushing’s syndrome. Ann Intern Med. 1986;104(2):180–186. [DOI] [PubMed] [Google Scholar]
- 50. Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C; SEISMIC Study Investigators . Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97(6):2039–2049. [DOI] [PubMed] [Google Scholar]
- 51. Medscape. Decadron, dexamethasone intensol (dexamethasone) dosing, indications, interactions, adverse effects, and more. Available at: https://reference.medscape.com/drug/decadron-dexamethasone-intensol-dexamethasone-342741. Accessed 26 March 2018.
- 52. Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest. 1975;56(6):1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ballard PL, Ballard RA, Granberg JP, Sniderman S, Gluckman PD, Kaplan SL, Grumbach MM. Fetal sex and prenatal betamethasone therapy. J Pediatr. 1980;97(3):451–454. [DOI] [PubMed] [Google Scholar]
- 54. Weijtens O, Schoemaker RC, Cohen AF, Romijn FP, Lentjes EG, van Rooij J, van Meurs JC. Dexamethasone concentration in vitreous and serum after oral administration. Am J Ophthalmol. 1998;125(5):673–679. [DOI] [PubMed] [Google Scholar]
- 55. Martin NM, Dhillo WS, Banerjee A, Abdulali A, Jayasena CN, Donaldson M, Todd JF, Meeran K. Comparison of the dexamethasone-suppressed corticotropin-releasing hormone test and low-dose dexamethasone suppression test in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2006;91(7):2582–2586. [DOI] [PubMed] [Google Scholar]
- 56. Papanicolaou DA, Yanovski JA, Cutler GB, Chrousos GP, Nieman LK. A single midnight serum cortisol measurement istinguishes Cushing’s syndrome from pseudo-Cushing states. J Endocrinol Metab. 1998;83(4):1163–1167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.