Abstract
A hexanucleotide repeat expansion in the C9orf72 gene has been identified as the most common cause of amyotrophic lateral sclerosis and frontotemporal dementia. The expanded hexanucleotide repeat is translated by an unconventional mechanism to produce five species of dipeptide repeat (DPR) proteins, glycine-proline (GP), glycine-alanine (GA), glycine-arginine (GR), proline-alanine (PA) and proline-arginine (PR). Of these, the arginine-rich ones, PR and GR, are highly toxic in a variety of model systems, ranging from human cells, to Drosophila, to even the budding yeast, Saccharomyces cerevisiae. We recently performed a genetic screen in yeast for modifiers of PR toxicity and identified suppressors and enhancers, many of which function in nucleocytoplasmic transport. Whether or not GR toxicity involves similar mechanisms to PR is unresolved. Therefore, we performed a genetic screen in yeast to identify modifiers of GR toxicity and compared the results of the GR screen to results from our previous PR screen. Surprisingly, there was only a small degree of overlap between the two screens, suggesting potential for distinct toxicity mechanisms between PR and GR.
Keywords: ALS, C9orf72, yeast, dipeptide repeat protein, screen, GR
A genetic screen in yeast reveals insight into Lou Gehrig's disease mechanisms.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are devastating human neurodegenerative disorders (Swinnen and Robberecht 2014). The most common genetic cause of ALS and FTD is mutations in the C9orf72 gene (Renton, Chiò and Traynor 2014). C9orf72 mutations can cause either disease or sometimes both (Taylor, Brown and Cleveland 2016). The disease-causing mutation is a massive GGGGCC hexanucleotide repeat expansion in the first intron of the C9orf72 gene (DeJesus-Hernandez et al.2011; Renton et al.2011). Normally, the C9orf72 gene harbors between 2 and 25 repeats and repeat expansions from hundreds to thousands are considered pathogenic (DeJesus-Hernandez et al.2011; Renton et al.2011). Since C9orf72 mutations are the common cause of ALS and FTD, there is intense interest in defining the mechanisms by which they cause disease so that insight could be harnessed to develop therapeutic strategies.
Several potential mechanisms could explain how the C9orf72 repeat expansion causes disease. First, the large GGGGCC repeat in the regulatory regions of C9orf72 interferes with gene expression, resulting in reduced levels of C9orf72 transcript and protein—the loss of function could contribute to disease (DeJesus-Hernandez et al.2011; Waite et al.2014). Second, the expanded repeat region is bidirectionally transcribed to form distinct RNA secondary structures that could be toxic by sequestering RNA-binding proteins and splicing factors (DeJesus-Hernandez et al.2011; Gendron et al.2013; Haeusler et al. 2014). Third, the sense and anti-sense repeat-containing RNAs are translated in multiple frames, despite the absence of a start codon, by an unconventional form of translation, called RAN (repeat-associated non-AUG) translation (Zu et al.2011), to produce dipeptide repeat (DPR) proteins (Ash et al.2013; Mori et al.2013; Zu et al.2013). The sense transcript produces glycine-alanine (GA), glycine-arginine (GR) and glycine-proline (GP) DPRs, while the anti-sense transcript produces proline-alanine (PA), proline-arginine (PR) and GP DPRs. These DPRs are themselves aggregation prone and accumulate in the brain of C9orf72 mutation carriers and could thus contribute to disease by a toxic gain-of-function mechanism (Ash et al.2013; Mori et al.2013; Zu et al.2013). These three proposed mechanisms are not mutually exclusive and there is compelling evidence for and against each of them (Gitler and Tsuiji 2016).
Of the five distinct DPRs produced from the C9orf72 repeat (GA, PA, GP, GR and PR), the arginine-rich ones (GR and PR) are particularly toxic. They are potently toxic to human cells and cause neurodegeneration in Drosophila melanogaster and human motor neurons derived from induced pluripotent stem cells (iPSCs) (Kwon et al.2014; Mizielinska et al.2014; Wen et al.2014). These phenotypes do not depend on the repeat itself, because synthetic DPRs or use of constructs codon optimized to produce the DPRs without the GGGGCC or GGCCCC repeat sequence still cause cell death (Kwon et al.2014; Mizielinska et al.2014). This permits interrogation of DPR-specific toxicity pathways and their contributions to disease, without confounds of potential RNA-mediated toxicity.
Just like for human cells and Drosophila, GR and PR DPRs are also toxic in yeast cells. We recently used this toxicity as the basis for a genetic screen for modifiers of DPR toxicity (Jovičić et al.2015). We focused on PR and identified several yeast genes that suppressed and enhanced toxicity. These studies illuminated genes in the nucleocytoplasmic transport pathway as potent modifiers of PR toxicity in yeast (Jovičić et al.2015). Studies in other systems, including Drosophila and iPSC-derived neurons also provided evidence that C9orf72 mutations disrupt nucleocytoplasmic transport (Zhang et al.2015; Freibaum et al.2015; Boeynaems et al.2016). Since our previous study focused on PR, in this paper, we performed additional screens to identify modifiers of GR toxicity, to define the commonalities and differences between how GR and PR elicit toxicity.
MATERIALS AND METHODS
Yeast strains, media and plasmids
Yeast cells were grown in rich media or in synthetic media lacking uracil and containing 2% glucose (SD/-Ura), raffinose (SRaf/-Ura) or galactose (SGal/-Ura). To generate yeast expressing a GR dipeptide protein containing 100 repeats (GR100), we utilized a codon-optimized GR sequence, as described previously (Jovičić et al.2015). The ATG-DPR construct was synthesized by Genscript (Piscataway, USA) and was flanked by attB sites. Constructs were further subcloned into a pDONR221 plasmid and subsequently used in Gateway LR reactions with pAG416GAL-ccdB (Alberti, Gitler and Lindquist 2007) to produce yeast expression vectors. The resulting pAG416GAL-GR100 construct was transformed into Y7092 yeast using the PEG/lithium acetate method. Spotting assays verified GR100 toxicity in yeast.
Yeast transformation and spotting assays
Yeast procedures were performed according to the standard protocols. We used the PEG/lithium acetate method to transform yeast with plasmid DNA. For spotting assays, yeast cells were grown overnight at 30°C in liquid media containing SRaf/-Ura until they reached log or mid-log phase. Cultures were then normalized for OD600, serially diluted and spotted with a Frogger (V&P Scientific, San Diego, USA) onto synthetic solid media containing glucose (SD/-Ura) or galactose (SGal/-Ura) lacking uracil and were grown at 30°C for 2–3 days.
Yeast genetic screen
We used synthetic genetic array analysis (Tong and Boone 2006) to identify nonessential yeast deletions that modify C9orf72 GR100 toxicity. We performed this screen as described in Jovičić et al. (2015), using a Singer RoToR HAD (Singer Instruments, Emeryville, USA). We mated MATα strain expressing GR100 under galactose promoter control to the yeast haploid deletion collection of nonessential genes (MATa, each gene deleted with KanMX cassette conferring resistance to G418). Following diploid selection and sporulation, we selected haploids carrying both deletion and GR100 expression cassette. Colony sizes were measured using the ht-colony-measurer software (Collins et al.2006). We performed the entire screen for three independent times. Individual hits were validated by independent transformations and spotting assays.
RESULTS
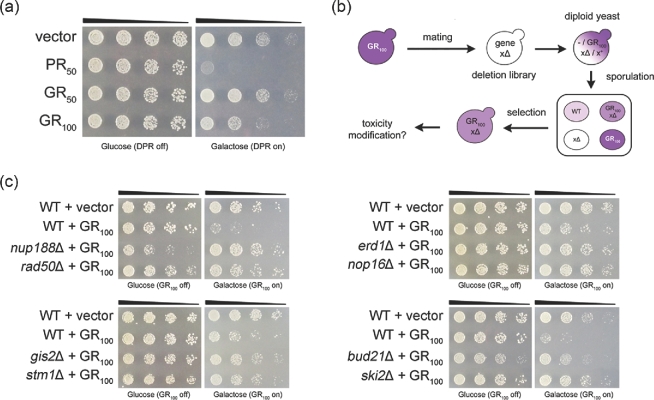
We screened a library of all 4850 nonessential yeast gene knockouts to identify deletions that could suppress GR100 toxicity (Fig. 1A and B). These types of genetic modifiers are an interesting class (gene deletions that suppress a phenotype) because they could represent potential drug targets. We identified 133 yeast deletions that suppressed GR100 toxicity (Table 1). We validated several modifiers from a variety of functional categories by individual transformations and spotting assays (Fig. 1C). Gene ontology analysis via YeastMine revealed an enrichment for cytoplasmic translation (P = 7.292e−7) and ribosomal small subunit biogenesis (P = 3.323e−4). The majority of the genes found in these categories encode ribosomal proteins and proteins involved in rRNA processing and ribosome synthesis in the nucleolus (Table 1). These ribosome-associated modifiers could act by reducing translation of the toxic GR100 protein. However, we did not identify these modifiers as suppressors of toxicity in deletion screens for other toxic proteins (PR50, FUS, and TDP-43) (Sun et al.2011; Armakola et al.2012; Jovičić et al.2015), suggesting that loss of these ribosomal proteins does not reduce expression of toxic proteins in general, but instead selectively affects GR100. Immunoblots to quantify GR100 were inconclusive (data not shown) and so the specific mechanism of action for these ribosomal hits remains to be determined.
Figure 1.
A yeast deletion screen reveals genetic suppressors of GR100 toxicity. (A) GR toxicity is length-dependent and less severe than PR toxicity in yeast. Five-fold serial dilutions of yeast cells were spotted onto glucose- or galactose-containing plates. Galactose induced expression of GR or PR in yeast, while glucose repressed DPR expression. (B) Schematic of the yeast deletion screen. (C) Example spotting assays validating specific hits from the deletion screen. Expression of GR is no longer toxic in strains lacking Nup188 (nuclear pore protein), Rad50 (double stranded break repair protein), Erd1 (ER protein), Nop16 (nucleolar protein), Gis2 (translational activator of specific mRNAs), Stm1 (ribosome preservation factor), Bud21 (ribosomal biogenesis protein) or Ski2 (RNA helicase).
Table 1.
List of yeast deletion strains that suppress GR100 toxicity.
| GR100 suppressors | Systematic name | PR50 suppressor | FUS suppressor | TDP-43 suppressor | Function | Human ortholog(s) |
|---|---|---|---|---|---|---|
| Ribosomal small subunit biogenesis (16/133, P = 3.323e–4) | ||||||
| rps0aΔ | YGR214W | ribosomal 40S subunit protein; rRNA maturation | RPSA | |||
| rps1bΔ | YML063W | ribosomal 40S subunit protein | RPS3A | |||
| rps6aΔ | YPL090C | rps6bΔ | ribosomal 40S subunit protein | RPS6 | ||
| rps8aΔ | YBL072C | yes | ribosomal 40S subunit protein | RPS8 | ||
| rps11aΔ | YDR025W | ribosomal 40S subunit protein | RPS11 | |||
| rps11bΔ | YBR048W | ribosomal 40S subunit protein | RPS11 | |||
| rps16bΔ | YDL083C | ribosomal 40S subunit protein | RPS16 | |||
| rps18aΔ | YDR450W | ribosomal 40S subunit protein | RPS18 | |||
| rps24aΔ | YER074W | ribosomal 40S subunit protein | RPS24 | |||
| sac3Δ | YDR159W | ribosome biogenesis; mRNA export | SAC3D1/MCM3AP | |||
| nsr1Δ | YGR159C | yes | yes | pre-rRNA processing; ribosome biogenesis | ||
| ltv1Δ | YKL143W | yes | Ribosomal small subunit export | LTV1 | ||
| hcr1Δ | YLR192C | pre-rRNA processing; translation initiation | EIF3J | |||
| tsr2Δ | YLR435W | yes | potential role in pre-rRNA processing | TSR2 | ||
| bud21Δ | YOR078W | part of the ribosomal small subunit processosome | ||||
| bud22Δ | YMR014W | rRNA maturation; ribosome biogenesis | SRFBP1 | |||
| Additional ribosomal proteins and ribosome-associated processes (23/133) | ||||||
| rpl12aΔ | YEL054C | ribosomal 60S subunit protein | RPL12 | |||
| rpl19bΔ | YBL027W | yes | ribosomal 60S subunit protein | RPL19 | ||
| rpl21bΔ | YPL079W | ribosomal 60S subunit protein | RPL21 | |||
| rpl34aΔ | YER056C-A | ribosomal 60S subunit protein | RPL34 | |||
| rpl37aΔ | YLR185W | ribosomal 60S subunit protein; pre-rRNA processing | RPL37 | |||
| rpl38Δ | YLR325C | ribosomal 60S subunit protein | RPL38 | |||
| rps29aΔ | YLR388W | ribosomal 40S subunit protein | RPS29 | |||
| rpp1bΔ | YDL130W | component of the ribosomal stalk | RPLP1 | |||
| rpp2bΔ | YDR382W | yes | component of the ribosomal stalk | RPLP2 | ||
| cgr1Δ | YGL029W | pre-rRNA processing; nucleolar integrity | CCDC86 | |||
| hpm1Δ | YIL110W | methyltransferase; modification of ribosomal protein | METTL18 | |||
| jjj1Δ | YNL227C | ribosome biogenesis | ||||
| kap120Δ | YPL125W | karyopherin; nuclear import of ribosomal maturation factor Rpf1p | IPO11 | |||
| kns1Δ | YLL019C | serine/threonine kinase; ribosome and tRNA biogenesis; rRNA transcription | CLK1-4 | |||
| nop12Δ | YOL041C | pre-rRNA processing; ribosome biogenesis | HNRNPD/DL/A0/AB | |||
| nop16Δ | YER002W | yes | ribosome biogenesis | NOP16 | ||
| rrp8Δ | YDR083W | methyltransferase; modification of ribosomal protein; pre-rRNA processing | RRP8 | |||
| stm1Δ | YLR150W | translation and ribosome preservation during nutrient stress; binds G-quadruplexes | SERBP1, HABP4 | |||
| tif4631Δ | YGR162W | ribosome biogenesis; translation initiation | EIF4G | |||
| syh1Δ | YPL105C | unknown function, but associates with nuclear pore and ribosomes | GIGYF1/2 | |||
| tma19Δ | YKL056C | associates with ribosomes | TPT1, 1P8 | |||
| ygl088wΔ | YGL088W | yes | unknown function, but partially overlaps with a snoRNA | |||
| yor309cΔ | YOR309C | yes | dubious open reading frame (ORF), but partially overlaps with NOP58 | |||
| RNA-related processes (15/133) | ||||||
| caf120Δ | YNL278W | part of a transcriptional regulatory complex; mRNA initiation, elongation, degradation | PAK2 | |||
| cgi121Δ | YML036W | yes | part of a tRNA modification complex | TPRKB | ||
| ebs1Δ | YDR206W | nonsense mediated decay; translation inhibition | SMG5/6/7 | |||
| gim3Δ | YNL153C | part of a prefoldin complex; transcriptional elongation | PFDN4 | |||
| gis2Δ | YNL255C | yes | activation of translation of IRES-containing mRNAs | |||
| lrp1Δ | YHR081W | RNA processing, degradation, export | C1D | |||
| nup188Δ | YML103C | part of nuclear pore complex, nucleocytoplasmic transport | NUP188 | |||
| nup84Δ | YDL116W | yes | part of nuclear pore complex, nucleocytoplasmic transport | NUP107 | ||
| she4Δ | YOR035C | regulation of myosin function; asymmetric mRNA localization | STIP1 | |||
| ski2Δ | YLR398C | RNA helicase; RNA degradation | ||||
| ski8Δ | YGL213C | yes | RNA helicase; RNA degradation | |||
| sky1Δ | YMR216C | serine/arginine kinase; regulation of proteins involved in mRNA metabolism | SRPK1/2/3 | |||
| stp1Δ | YDR463W | yes | transcription factor; potential role in tRNA processing | |||
| tex1Δ | YNL253W | mRNA export | THOC3 | |||
| tif1Δ | YKR059W | translation initiation; RNA helicase | EIF4A2 | |||
| Mitochondrial and NADPH-related metabolic pathways (12/133) | ||||||
| aco2Δ | YJL200C | mitochondrial aconitase isozyme | ||||
| flx1Δ | YIL134W | mitochondrial flavin adenine dinucleotide transporter | SLC25A32 | |||
| idh2Δ | YOR136W | mitochondrial NAD(+)-dependent isocitrate dehydrogenase | IDH3A | |||
| oxa1Δ | YER154W | mitochondrial inner membrane insertase | OXA1L | |||
| rcf2Δ | YNR018W | cytochrome c oxidase subunit | ||||
| zwf1Δ | YNL241C | glucose-6-phosphate dehydrogenase | H6PD, G6PD | |||
| gor1Δ | YNL274C | mitochondrial glyoxylate reductase | GRHPR | |||
| gpd2Δ | YOL059W | NAD-dependent glycerol 3-phosphate dehydrogenase | GPD1, 1L | |||
| gph1Δ | YPR160W | glycogen phosphorylase; mobilization of glycogen | PYGL/B/M | |||
| stb5Δ | YHR178W | transcription factor; oxidative stress, stress response | ||||
| nnr2Δ | YKL151C | yes | NADHX dehydratase | CARKD | ||
| ald6Δ | YPL061W | aldehyde dehydrogenase | ALDH1A1/A2/A3, ALDH2 | |||
| Nucleotide biosynthetic pathway (7/133, P = 3.488e–5) | ||||||
| ade1Δ | YAR015W | purine nucleotide biosynthesis | PAICS | |||
| ade2Δ | YOR128C | purine nucleotide biosynthesis | ||||
| ade4Δ | YMR300C | purine nucleotide biosynthesis | PPAT | |||
| ade5, 7Δ | YGL234W | purine nucleotide biosynthesis | ||||
| ade6Δ | S000003293 | purine nucleotide biosynthesis | PFAS | |||
| ade8Δ | YDR408C | purine nucleotide biosynthesis | ||||
| bas1Δ | YKR099W | purine nucleotide biosynthesis; transcription factor | ||||
| Amino acid and other molecular biosynthetic pathways (10/133) | ||||||
| alt1Δ | YLR089C | alanine transaminase; alanine amino acid synthesis and catabolism | CCBL1/2, GPT1/2 | |||
| aro1Δ | YDR127W | synthesis of chorismate, an amino acid precursor | ||||
| cho2Δ | YGR157W | methyltransferase; phosphatidylcholine biosynthesis | ||||
| dph6Δ | YLR143W | diphthamide biosynthesis | DPH6 | |||
| elo3Δ | YLR372W | fatty acid and sphingolipid biosynthesis | ||||
| ilv1Δ | YER086W | threonine deaminase; isoleucine biosynthesis | ||||
| ino1Δ | YJL153C | inositol, inositol-containing phospholipid biosynthesis | ISYNA1 | |||
| ipk1Δ | YDR315C | yes | synthesis of phytate | IPPK | ||
| met2Δ | YNL277W | methionine biosynthesis | ||||
| met22Δ | YOL064C | methionine biosynthesis | ||||
| ER-related processes (4/133) | ||||||
| erd1Δ | YDR414C | lumenal ER protein retention | ||||
| get1Δ | YGL020C | insertion of proteins into the ER membrane | WRB | |||
| lhs1Δ | YKL073W | chaperone of the ER lumen; protein translocation and folding | ||||
| sse1Δ | YPL106C | Yes | HSP90 chaperone complex; binds unfolded proteins | HSPA4/A4L/H1 | ||
| GTPase-related proteins (7/133) | ||||||
| aim44Δ | YPL158C | cytokinesis; regulates Rho1p | ||||
| tus1Δ | YLR425W | GEF for Rho1p activity | ||||
| lte1Δ | YAL024C | similar to GDP/GTP exchange factors | RASGEF1A-C | |||
| msb3Δ | YNL293W | Rab GTPase activation; endocytosis | TBC1D, SGSM3 | |||
| gtr1Δ | YML121W | yes | part of TORC1-stimulating GTPase complex | RRAGA/B | ||
| tco89Δ | YPL180W | TORC1 subunit | ||||
| tor1Δ | YJR066W | TORC1 subunit | MTOR | |||
| DNA repair (7/133) | ||||||
| asf1Δ | YJL115W | nucleosome assembly; recovery after double-stranded DNA break repair | ASF1A/B | |||
| rad50Δ | YNL250W | yes | processing double-stranded DNA breaks | RAD50 | ||
| rad51Δ | YER095W | double-stranded DNA break repair | RAD51 | |||
| rad52Δ | YML032C | double-stranded DNA break repair | RAD52 | |||
| vps75Δ | YNL246W | histone chaperone; double-stranded DNA break repair | SET/SIP, TSPYs, FAM197Y1 | |||
| mms22Δ | YLR320W | E3 ubiquitin ligase complex involved in replication repair | ||||
| slx5Δ | YDL013W | SUMO-targed ubiquitin ligase complex; DNA repair | ||||
| Serine/threonine and serine modifiers (8/133) | ||||||
| fus3Δ | YBL016W | mitogen-activated serine/threonine protein kinase | MAPK1,3,4,5,6 or NLK | |||
| ptk2Δ | YJR059W | serine/threonine protein kinase; regulation of ion transport | TSSKs | |||
| yck3Δ | YER123W | vacuolar membrane serine/threonine kinase; vacuole fusion | ||||
| pph21Δ | YDL134C | catalytic subunit of protein phosphatase 2a (serine/threonine phosphatase); mitosis | ||||
| ppm1Δ | YDR435C | Yes | methyltransferase; methylates the C terminus of Pph21p | LCMT1 | ||
| rts1Δ | YOR014W | regulatory subunit of protein phosphatase 2A | PPP2R5C/D | |||
| kex2Δ | YNL238W | calcium-dependent serine protease | ||||
| prb1Δ | YEL060C | vacuolar serine protease | ||||
| Acetyltransferases (3/133) | ||||||
| eaf6Δ | YJR082C | part of acetyltransferase complex; histone acetylation | MEAF6 | |||
| hpa3Δ | YEL066W | D-Amino acid N-acetyltransferase; histone acetylation | ||||
| mak10Δ | YEL053C | NatC N-terminal acetyltransferase | NAA35 | |||
| Other (8/133) | ||||||
| alf1Δ | YNL148C | yes | alpha-tubulin folding; microtubule maintenance | TBCB, CLIP3/4 | ||
| atx1Δ | YNL259C | cytosolic copper metallochaperone | ATOX1 | |||
| cdc50Δ | YCR094W | endosomal protein; involved with Golgi membrane trafficking | TMEM30A/B/C | |||
| clb2Δ | YPR119W | yes | cell cycle progression | CNTD2 | ||
| fcy22Δ | YER060W-A | purine-cytosine permease | ||||
| fen2Δ | YCR028C | H+-pantothenate symporterH | ||||
| sho1Δ | YER118C | transmembrane osmosensor for filamentous growth | ||||
| vps64Δ | YDR200C | yes | cytoplasm to vacuole targeting of proteins | TRAF3IP3, SLMAP, CEP170/B, CCDC136 | ||
| Uncharacterized proteins (13/133) | ||||||
| brp1Δ | YGL007W | protein of unknown function | ||||
| fyv1Δ | YDR024W | dubious ORF | ||||
| fyv6Δ | YNL133C | protein of unknown function | ||||
| gds1Δ | YOR355W | protein of unknown function | ||||
| hhy1Δ | YEL059W | dubious ORF | ||||
| irc14Δ | YOR135C | dubious ORF | ||||
| mtc7Δ | YEL033W | protein of unknown function | ||||
| rtc4Δ | YNL254C | protein of unknown function | ||||
| sdd1Δ | YEL057C | protein of unknown function | ||||
| ydr417cΔ | YDR417C | yes | dubious ORF | |||
| ygl165cΔ | YGL165C | yes | dubious ORF | |||
| ynl198cΔ | YNL198C | yes | dubious ORF | |||
| ynr005cΔ | YNR005C | yes | dubious ORF | |||
Though ribosomal genes were statistically enriched in the screen, additional functional clusters emerged (Table 1). One such cluster consisted of six ADE genes (P = 3.488e−5) and BAS1, all of which are involved in purine nucleotide biosynthesis (Cherry et al.2012). Similarly, DNA damage repair genes including RAD50, RAD51 and RAD52 were identified in the screen, and this specific pathway has been implicated in GR toxicity in iPSC-derived neurons (Cherry et al.2012; Lopez-Gonzalez et al.2016). We also identified numerous genes involved with various forms of RNA-interacting processes including nucleocytoplasmic transport, tRNA synthesis and the mRNA life cycle. Similar genes, or in the case of NUP107, identical genes, involved in nucleocytoplasmic transport and RNA export and degradation were also been identified in GGGGCC repeat and PR toxicity screens in Drosophila (Freibaum et al. 2015; Zhang et al.2015; Boeynaems et al.2016).
We next compared the hits from the GR100 to hits from other screens we have performed on ALS-related proteins, including PR50, FUS and TDP-43 (Table 1). Six of the hits from the GR screen were also hits in the PR screen. This number is small, in part because there were only 13 hits from the PR deletion screen (Jovičić et al.2015) and because some hits from the PR screen were identified in only two out of three rounds of the GR screen. Nevertheless, the overlapping hits are informative, pointing to a role for the shared arginine content in the way these genes interact with and modify these arginine-rich DPRs. Also, while the individual genes between the PR and GR yeast screens diverged, the classes of genetic modifiers that have emerged from this GR100 screen have been implicated in GR and PR biology in Drosophila and mammalian cell systems (Kwon et al.2014; Boeynaems et al.2016, 2017; Lee et al.2016). There was no overlap with the TDP-43 screen (Armakola et al.2012). Surprisingly, the biggest overlap of hits came from the GR100 and FUS screens, with 22 shared suppressors of toxicity (Table 1) (Sun et al.2011). This result could be due to the fact that the FUS protein contains several domains containing arginine/glycine/glycine (RGG) repetitive sequences (Boeynaems et al.2017; Ozdilek et al.2017) that may behave similarly to the repetitive GR100 sequence when overexpressed in yeast.
DISCUSSION
Here, we have used a yeast genetic screen to identify suppressors of C9orf72 GR100 toxicity, which provide clues into the potential mechanisms of GR toxicity. While recent studies have focused on the highly toxic PR species or grouped GR and PR together due to their shared arginine content, there has been little done to parse apart potential differences in GR and PR biology, even though such differences exist. From our screen, we have discovered that there is divergence in the genes that suppress GR and PR toxicity when deleted in yeast.
Several factors could contribute to this divergence. First, PR is more toxic than GR (Fig. 1A). This increased toxicity might contribute to the low number of genetic modifiers identified in the PR deletion screen (13) compared to the GR screen (133) (Jovičić et al.2015), since the threshold for suppressing PR toxicity is greater than for GR toxicity. In that case, we could be missing real commonalities between PR and GR, which may be detectable with a less-toxic PR species. And indeed, in other experimental systems, nucleolar and ribosomal proteins, which were modifiers of GR toxicity in yeast, can interact physically with PR (Lee et al.2016; Lin et al.2016; Boeynaems et al.2017). Both PR and GR have also been shown to disrupt the nucleolus and ribosome biogenesis (Kwon et al.2014). The positively charged arginines in both species most likely contribute to these interactions.
However, when we consider the biochemistry of these species, it is important to consider the glycines and prolines in addition to the arginines. Glycine, with a single hydrogen for a sidechain, is dramatically different from proline, which contains a large cyclic side chain that imparts a high degree of structural rigidity to proline-containing peptides. Understanding why the proline content appears to confer increased toxicity at shorter lengths will be an important next step in the field. Furthermore, the specific glycine content is also biologically relevant, as repetitive glycine/arginine rich (GAR) domains occur in numerous proteins and is in fact the second-most common RNA binding domain in the human genome (Ozdilek et al.2017).
The existence of GAR domains, as opposed to proline/arginine rich domains, provides an opportunity for the GR dipeptide species to have a unique impact on the cell. The GR repeats could mimic a protein's GAR domain, thereby wreaking havoc when inserted within specific GAR domain-mediated RNA/protein or protein/protein interactions within the cell. The results from our screen suggest that this is possible, given the large number of shared hits between screens for modifiers of FUS toxicity and GR100 toxicity (Table 1). It would be interesting to see whether ectopic expression of other proteins containing GAR domains would be toxic, and if so, whether they would share significant overlap in toxicity modifiers.
Furthermore, in yeast, the majority of GAR domain proteins are nucleolar proteins involved in ribosomal biogenesis (e.g. Gar1, Nsr1, Nop1, Nop3 and Ssb1) or proteins involved in mRNA handling (e.g. Scd6, Npl3, Gbp2, Nab2, Sbp1, etc.), two major groups identified in our screen for modifiers of GR toxicity (Girard et al.1992; Inoue et al.2000; McBride et al. 2009; Rajyaguru and Parker 2012). Nsr1, which contains GAR domains, was identified in both the PR and GR screens and GR-specific hits such as Rrp8 and EIF4G have been shown to directly interact with many of the GAR domain-containing proteins listed above (Bousquet-Antonelli et al.2000; Rajyaguru, She and Parker 2012), lending credence to the possibility that GR100 toxicity occurs by interfering with the activity of GAR domain-containing proteins.
Additional experiments to investigate exactly how GR100 impacts these pathways are required, but overall, this screen has given us a look into the ways through which GR DPRs produced by the C9orf72 repeat expansion might contribute to disease, and provide potential druggable targets to ameliorate DPR toxicity. The surprising lack of overlap between hits from our GR screen here and our previous PR screen (Jovičić et al. 2015) underscores the importance of considering GR and PR toxic mechanisms as distinct and in pursuing approaches to deal with them separately.
FUNDING
This work was supported by NIH grant R35NS097263 (A.D.G.), the Robert Packard Center for ALS Research at Johns Hopkins (A.D.G.), Target ALS (A.D.G.) and the Stanford Brain Rejuvenation Project of the Stanford Neurosciences Institute (A.D.G.).
Conflict of interest. None declared.
REFERENCES
- Alberti S, Gitler AD, Lindquist S. A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast 2007;24:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armakola M, Higgins MJ, Figley MD et al. . Inhibition of RNA lariat debranching enzyme suppresses TDP-43 toxicity in ALS disease models. Nat Genet 2012;44:1302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash PE, Bieniek KF, Gendron TF et al. . Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 2013;77:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Bogaert E, Kovacs D et al. . Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol Cell 2017;65:1044–55.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Bogaert E, Michiels E et al. . Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci Rep 2016;6:20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Antonelli C, Vanrobays E, Gélugne J-P et al. . Rrp8p is a yeast nucleolar protein functionally linked to Gar1p and involved in pre-rRNA cleavage at site A2. RNA 2000;6:826–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JM, Hong EL, Amundsen C et al. . Saccharomyces genome database: the genomics resource of budding yeast. Nucleic Acids Res 2012;40:D700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SR, Schuldiner M, Krogan NJ et al. . A strategy for extracting and analyzing large-scale quantitative epistatic interaction data. Genome Biol 2006;7:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF et al. . Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011;72:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Lu Y, Lopez-Gonzalez R et al. . GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 2015;525:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, Bieniek KF, Zhang YJ et al. . Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol 2013;126:829–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JP, Lehtonen H, Caizergues-Ferrer M et al. . GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J 1992;11:673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Tsuiji H. There has been an awakening: emerging mechanisms of C9orf72 mutations in FTD/ALS. Brain Res 2016;1647:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler AR, Donnelly CJ, Periz G et al. . C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 2014;507:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Mizuno T, Wada K et al. . Novel RING finger proteins, Air1p and Air2p, interact with Hmt1p and inhibit the arginine methylation of Npl3p. J Biol Chem 2000;275:32793–9. [DOI] [PubMed] [Google Scholar]
- Jovičić A, Mertens J, Boeynaems S et al. . Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci 2015;18:1226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Xiang S, Kato M et al. . Poly-dipeptides encoded by the C9orf72 repeats bind nucleoli, impede RNA biogenesis, and kill cells. Science 2014;345:1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Zhang P, Kim HJ et al. . C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles. Cell 2016;167:774–88.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Mori E, Kato M et al. . Toxic PR poly-dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell 2016;167:789–802.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gonzalez R, Lu Y, Gendron TF et al. . Poly(GR) in C9ORF72- related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-derived motor neurons. Neuron 2016;92:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AE, Conboy AK, Brown SP et al. . Specific sequences within arginine-glycine-rich domains affect mRNA-binding protein function. Nucleic Acids Res 2009;37:4322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizielinska S, Gronke S, Niccoli T et al. . C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science 2014;345:1192–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Arzberger T, Grasser FA et al. . Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol 2013;126:881–93. [DOI] [PubMed] [Google Scholar]
- Ozdilek BA, Thompson VF, Ahmed NS et al. . Intrinsically disordered RGG/RG domains mediate degenerate specificity in RNA binding. Nucleic Acids Res 2017;45:7984–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajyaguru P, Parker R. RGG motif proteins: modulators of mRNA functional states. Cell Cycle 2012;11:2594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajyaguru P, She M, Parker R. Scd6 targets eIF4G to repress translation: RGG motif proteins as a class of eIF4G-binding proteins. Mol Cell 2012;45:244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 2014;17:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A et al. . A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011;72:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Diaz Z, Fang X et al. . Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol 2011;9:e1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol 2014;10:661–70. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Nature 2016;539:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AH, Boone C. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol 2006;313:171–92. [DOI] [PubMed] [Google Scholar]
- Waite AJ, Baumer D, East S et al. . Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol Aging 2014;35:1779.e5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Tan W, Westergard T et al. . Antisense Proline-Arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron 2014;84:1213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Donnelly CJ, Haeusler AR et al. . The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 2015;525:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS et al. . Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci 2011;108:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Liu Y, Banez-Coronel M et al. . RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci 2013;110:E4968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]