Abstract
Background
Acne vulgaris is one of the main reasons for dermatological consultations. Severity and response to treatment may be impacted by various external factors or exposome.
Aim
To assess the impact of environmental factors on acne and to provide a comprehensive overview of the acne exposome.
Methods
Two consensus meetings of five European dermatologists and a comprehensive literature search on exposome factors triggering acne served as a basis for this review.
Results
Acne exposome was defined as the sum of all environmental factors influencing the occurrence, duration and severity of acne. Exposome factors impact on the response and the frequency of relapse to treatments by interacting with the skin barrier, sebaceous gland, innate immunity and cutaneous microbiota. They may be classified into the following six main categories: nutrition, psychological and lifestyle factors, occupational factors including cosmetics, as well as pollutants, medication and climatic factors. Moreover, practical considerations for the dermatologist's clinical practice are proposed.
Conclusion
Exposome factors including nutrition, medication, occupational factors, pollutants, climatic factors, and psychosocial and lifestyle factors may impact on the course and severity of acne and on treatment efficacy. Identifying and reducing the impact of exposome is important for an adequate acne disease management.
Introduction
The term ‘exposome’ was used for the first time by Wild in 2005 to describe the sum of environmental exposures to which an individual is subjected from conception to death.1 The definition of exposome varied over time (Table 1).1, 2, 3, 4, 5 In 2014, Miller and Jones refined this term as ‘the cumulative measure of environmental influences and the associated biological responses throughout the lifespan, including exposures from the environment, diet, behaviour, and endogenous processes’.2
Table 1.
Definitions of exposome
| Wild (2005)1 | Exposome is all life‐course environmental exposures (including lifestyle factors) from prenatal period onwards |
| Rappaport et al. (2010)4 | Exposome is the sum of all exposures throughout life, where the environment is the body's internal chemical environment and exposures are all biologically active chemicals in this internal environment |
| Buck Louis and Sundaram (2012)3 | Exposome is a mixture of environmental exposures, including man‐made and naturally occurring chemicals, physical agents (such as noise, vibration and temperature), macrolevel factors (including population density and sanitation) and lifestyle factors |
| National Research Council (2012)5 | ‘Eco‐exposome’ extends the concept from the point of contact between the stressor and the receptor, inwards into the organism and outwards to general environment |
| Miller and Jones (2014)2 | Exposome is the cumulative measure of environmental influences and associated biological responses throughout the lifespan including exposures from the environment, diet, behaviour and endogenous processes |
In 2016, the impact of exposome on chronic diseases was estimated at almost 80%; conversely, that of genomewide‐associated diseases did not exceed 20%.6 Exposome‐wide association studies may allow discovering factors that, over time, cause complex chronic diseases.
Skin is one of the major interfaces between the body and the external environment and one of the main routes of penetration of environmental factors and pathogens. It plays a protective role due to its barrier function and its microbiota.7 Until recently, the role of exposome in dermatology was not investigated. In 2017, Krutmann et al. studied the role of exposome in skin ageing and proposed that environmental factors belonging to the skin ageing exposome fall into the following categories: (i) sun radiations: ultraviolet radiation, visible light and infrared radiation; (ii) air pollution, (iii) tobacco smoke; (iv) nutrition; (v) a number of less well‐studied, miscellaneous factors; as well as (vi) cosmetic products.8
Acne is one of the main reasons for dermatologic consultation. It is an inflammatory disease of the pilosebaceous follicle occurring commonly in adolescents and some adults. It is associated with hyperseborrhoea altering the epithelium of the follicle with formation of comedones, a modification of the microbiote called dysbiosis targeting mainly Propionibacterium acnes (P. acnes) causing an activation of the innate immunity and thus inflammation.9 A family factor is frequently associated with acne severity.10 However, severity and response to treatment may also be impacted by different external or environmental factors.
With this article, we aimed at assessing the influence of environmental factors on acne and at providing a comprehensive overview of the acne exposome. Moreover, we propose practical considerations for the dermatologist's clinical practice.
Methodology
This article results from two consensus meetings of a board of European dermatologists held in July and October 2017.
Prior to the meetings, we conducted a comprehensive literature search through PubMed, combining the following key words: acne and exposome, sunlight, UV radiation, UVB radiation, UVA radiation, visible light, infrared radiation, air pollution, ozone, PM10, PM2.5, pollutants, tar, oil, nitrogen dioxide, tobacco, cannabis, smoking, stress, physical activity, occupation, nutrition, diet, alcohol, oil, whey proteins, hydrocarbons, cosmetics, skin care, make‐up, cosmetic procedures, heat, cold, climate, water, lack of sleep. All relevant articles including epidemiological, in vitro, ex vivo and clinical studies were selected.
During the meetings, we defined and analysed key elements of acne exposome factors based on published literature (Fig. 1).
Figure 1.
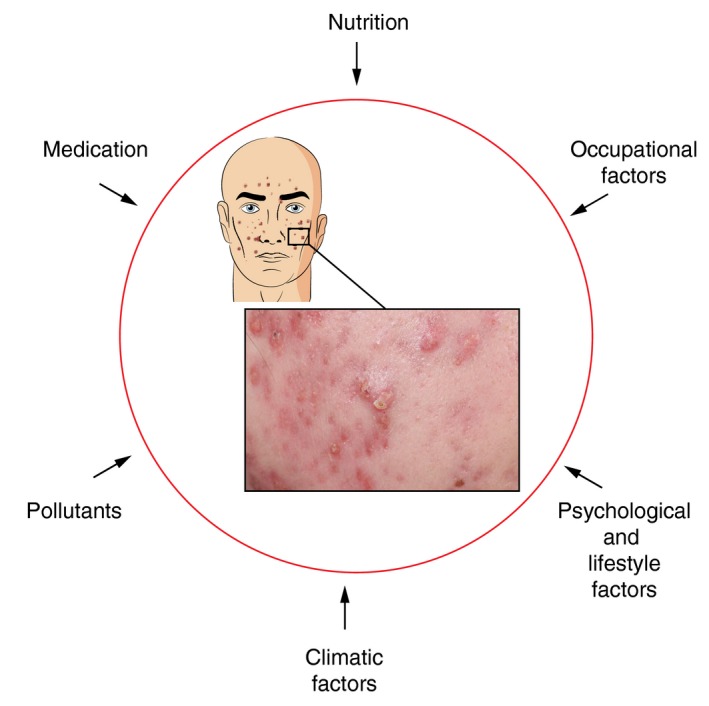
External exposome factors impacting on acne
Results
Definition of the acne exposome
We defined acne exposome as the sum of all environmental factors influencing the occurrence, duration and severity of acne. Exposome factors impact on the response and the frequency of relapse to treatments by interacting with the skin barrier, sebaceous gland, innate immunity and cutaneous microbiota.11, 12
They may be classified into the following six main categories: nutrition, medication, occupational factors including cosmetics, pollutants, climatic factors, and psychological and lifestyle factors.
Acne exposome factors
Nutrition
This first category is by far the most published acne exposome factor. In the past, nutrition was not a proven cause of acne. However, recent publications show a link between some dietary factors and acne.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Nowadays, main food classes considered triggering acne are dairy products (especially skim milk) and hyperglycaemic carbohydrates.10, 29, 30, 31, 32, 33, 34, 35, 36, 37
An average regimen of dairy products has been reported impacting on acne, and one paper indicated that cow milk impacts on acne after already two glasses per day.38, 39 Conversely, acne is not observed in native non‐Westernized populations consuming low glycaemic load diet and in populations not consuming refined sugars, grains, milk and dairy products.40 Moreover, individuals with congenital deficiencies of the insulin growth factor‐1 (IGF‐1) have been reported to present only rarely with acne.23 Recent papers provide additional arguments supporting the link at a transcriptional level between IGF‐1, leptin and liponectin and high glycaemic index through the activation of mTOR and FOXP1 pathways.13, 14, 28, 29
Although there is convincing evidence of a correlation between nutritional elements and acne, there are no interventional studies supporting that dietary regimes are of clinical relevance.37 Further research is required, in particular studies with larger sample sizes combining a low glycaemic index diet and restricted intake of cow milk products.
Nevertheless, we strongly support the idea of a subgroup of patients in whom acne severity relates to nutrition: these patients present with a moderate‐to‐severe acne with frequent relapses, an increased body mass index equal or superior to 25 and increased IGF‐1 and decreased leptin and liponectin levels.13
Nutritional supplements such as whey proteins containing leucine used by athletes may trigger or worsen acne.41, 42, 43 Leucine stimulates the production of IGF‐1.31, 41, 42, 43, 44
Very few controlled studies have investigated the benefit of oral probiotics in the treatment of acne. When compared to the placebo in 20 patients, the probiotic containing Lactobacillus rhamnosus SP1 provided a better clinical outcome according to the investigators.45 An adjunctive supplementation of lyophilized Lactobacillus acidophilus and Bifidobacterium bifidum adjunctively to standard antibiotics improved acne in 40 patients.46 Oral antibiotics and probiotics provided a synergistic benefit in inflammatory acne in decreasing both, inflammatory and non‐inflammatory lesions after 12 weeks compared to probiotics or oral antibiotics alone in three groups of 15 patients each.47
The capability of oral probiotics to reduce systemic oxidative stress, to regulate cytokines and to reduce inflammatory markers may contribute to its effects on acne.48 Despite encouraging results, further investigations are still necessary to confirm the clinical benefit of probiotics in acne.
Medications
Hormonal treatments
There is evidence that in first‐ and second‐generation oral contraceptives, progestin is changed in metabolites of testosterone that may exacerbate acne in adolescents and mainly in adult females.49, 50 The following androgenic progestins have been identified to cause or worsen acne: desogestrel and 3‐cetodesogestrel, levonorgestrel, lynestrenol, norgestrienone, norethisterone, norgestrel, gestodene, norgestimate and etonogestrel.
Conversely, chlormadinone acetate, dienogest, drospirenone and norgestimate oral contraceptive pills have been reported to be beneficial in the treatment of acne.49, 51, 52, 53
Cyproterone acetate present in combined hormonal antiacne treatments has also been reported to be efficient in acne.54
Anabolic steroids trigger acne through targeting androgen receptors on sebocytes and keratinocytes.55, 56, 57, 58
Other medical treatments
Corticosteroids, halogens, isoniazid, lithium, vitamin B12, immunosuppressants and certain anticancer agents and radiotherapy have been reported causing acneiform eruptions.59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 We consider these dermatoses as a differential diagnosis of acne.
Occupational factors
Cosmetics
The use of aggressive skin care regimens and inappropriate cosmetics may cause acne flare‐ups. These products modify the skin barrier and the skin microbiota balance especially in the sebaceous area, thus activating the innate immunity triggering inflammation.
Acne cosmetica was described for the first time by Kligman and Mills in 1972.71 It is characterized by small scattered comedones on the face with only very few inflammatory lesions such as papules, pustules. Acne flare‐up triggers in cosmetics include comedogenic ingredients, essential oils or too greasy or oily foundations, powder make‐up, aggressive skin cleansers and soaps with pH of 8.0.72, 73 Some hair care products, such as brilliantine, have been reported to cause comedones and cysts on the forehead and temple.74
Mechanical factors
Mechanical factors comprising rubbing, scrubbing, the use of home devices or medical devices such as sonic brushes, dermarollers or microneedling systems may trigger acne flare‐up.75 Dreno et al. reported that mechanical factors cause two types of mainly inflammatory cutaneous lesions: one ‘folliculitis mechanica’ presenting with inflammatory papules, open comedones or no comedonal lesions and the other one corresponding to a flare‐up of acne in areas prone to the condition. The authors also presented a clinical case of a patient using essential oils combined with a roller‐microdermabrasion device, diagnosed with severe inflammatory acne due a combination of a facial skin massage device and topical products.
Currently, the physiopathology of inflammatory cutaneous lesions caused by mechanical injury remains to be elucidated. Two different mechanisms may be involved: a first mechanism results in thickening of the epidermis leading to hyperkeratosis, with a modification of the stratum corneum, reduced water content, irritation and, finally, a disturbed skin barrier.75, 76 A second mechanism impacts on the microbiome and the innate immunity. Repeated pressure, friction and rubbing may cause both a modification of the lipid film at the surface of the corneocytes and of the cutaneous microbiome.75
Pollutants
Air pollutants
Air pollutants exert a harmful effect on the skin by increasing oxidative stress inducing severe alterations of the normal functions of lipids, deoxyribonucleic acid and/or proteins in the human skin.77 This phenomenon is more marked in acne patients as in this population the skin lipid film on the surface of the stratum corneum is altered through an increase in oxidized squalene and a decrease in linoleic acid.78 Two clinical studies comparing subjects in the Shanghai area and Mexico City, both highly polluted to less polluted areas, found skin quality changes with chronic exposure to ambient pollution.79, 80 Vitamin E and squalene levels, both signs of sebum oxidation, were reduced. Although the Mexican study did not measure clinical signs of acne, raised sebum levels were observed.79 Another Chinese study, conducted during 8 weeks on 64 acne patients, showed a relationship between exposure to environmental pollutants and increased sebum levels and a higher number of inflammatory and non‐inflammatory acne lesions.80
Even though there is growing evidence that air pollutants exert their harmful effects by means of reactive oxygen species and inflammation,77 more research is needed to better understand the link between air pollution and acne.
Industrial pollutants
Acne has been frequently observed in industry workers after prolonged exposure to certain organic molecules, such as coal tar or crude oil.81, 82, 83, 84, 85 Today, exposure to these molecules has become less common.86 Only sparse information about their impact on acne aetiology is available from the literature: it seems as if these insoluble products obstruct the sebaceous follicle by a mixture of coal tar or oil and keratin products.83, 86, 87, 88 Males are more commonly affected than females. This can be linked to the fact that males are more prone to acne or have been more frequently exposed.89
Chloracne, a chronic type of acneiform eruption, is induced by halogenated aromatic hydrocarbons and is a differential diagnosis with acne vulgaris.81, 83, 90, 91
Human‐dependent pollutants
Tobacco and cannabis consumption may be considered human‐dependent pollutants which may play a role in acne.
To date, the link between acne and tobacco is not confirmed. While Capitanio et al. reported that tobacco smoking may have an impact on acne, two recent online surveys reported contradictory information with a protective effect on acne.92, 93, 94, 95
Cigarette smoke (CS) is a highly complex aerosol composed of thousands of chemical substances, including nicotine, carbon monoxide, tar, formaldehyde, cyanhydric acid, ammonia, mercury, lead and cadmium.96, 97, 98 These chemical substances from CS increase transepidermal water loss, degeneration of connective tissue in the skin and upregulation of matrix metalloproteinases 1 and 3 which degrade collagen and elastic fibres, and lastly impact on the natural skin barrier.99, 100, 101
To date, it is still unclear how CS may trigger acne.102 Tobacco consumption may prompt acne by inducing interleukin‐1α and exacerbate comedogenesis and inflammatory changes in comedones that results in oxidative stress and the subsequent accumulation of lipid peroxide.94, 95, 103, 104 In keratinocytes and/or sebocytes cultures, CS exposure induced a scavenger receptor class B member 1 (SRB1 protein) loss. CS‐induced SRB1 protein loss caused an alteration of the sebocyte lipid content.105, 106
Regular use of cannabis was highly associated with acne (odds ratio of 2.88 (95% CI: 1.55–5.37)) according to a French survey in more than 10 000 subjects.92 No further data about acne and cannabis consumption has been published to date. However, our own clinical experience corroborates this observed relationship.
Climatic conditions
Climatic conditions and seasonal variations resulting in a combination of heat, humidity and intensive UVR may trigger inflammatory acne flare‐up, which has been called acne tropicana, acne majorca or tropical acne.107, 108, 109, 110, 111
One of the major environmental factors affecting the skin is ultraviolet radiation (UVR). Both UVB and UVA have been reported to cause hyperplasia of the sebaceous gland, thickening of the stratum corneum, increase in sebum secretion and in the number of comedones.112, 113, 114, 115, 116 By targeting the cells and molecules within the skin, UVR triggers the production and release of antimicrobial peptides, activates the innate immune system and ultimately suppresses the adaptive cellular immune response. As a consequence, skin microbiota may be altered and P. acnes may overcolonize the skin causing flares of acne. Alternately, or in concert with this, direct UVR‐induced DNA and membrane damage to the microbiome may cause pathogen‐associated molecular patterns that interfere with UVR‐induced immune suppression.117
Psychosocial and lifestyle factors
Clinical evidence about the impact of psychosocial and lifestyle factors on acne is sparse and clinical wisdom and experience, as well as anecdotal observations and uncontrolled case series support this situation.118, 119, 120, 121, 122, 123 Nevertheless, there is some evidence that psychosocial and lifestyle factors including stress, emotions, sleep deprivation and modern lifestyle impact on inflammatory skin diseases.119, 121, 122, 124, 125, 126, 127, 128, 129, 130 Cultural factors may influence the experience and presentation of acne.118, 131
Corticotropin‐releasing hormones and neuropeptides are present in the sebaceous glands, possibly activating pathways affecting immune and inflammatory processes leading to the development and stress‐ and neurologically induced exacerbation of acne.132, 133 Short wavelength visible light emitted from smartphones and tablets has been reported to increase the proliferation of Staphylococcus aureus thus unbalancing the skin microbiota and thus impacting on acne.123, 134 Therefore, modern lifestyle, defined as stressful situations including urban noises, socioeconomic pressures and light exposure, may play a role in acne.
Future research
Future research should be devoted to a better understanding of the interactions between the different exposome factors and between these factors and the skin barrier cells, innate immunity and skin microbiota.
Protecting the microbiota spectrum is crucial as it protects from activation of the innate immunity leading to inflammation. Moreover, besides exposome factors, genetic factors may also influence the reaction of the skin against different environmental factors. As a result, acquiring detailed knowledge about such gene/environment interactions may help identify different patient subgroups and thus allow the development of specific and efficient measures against the alteration of the microbiota and skin barrier.
Regarding the role of nutrition in acne, still many facets such as the skin–gut axis in patients with acne, the benefit of pre‐ and probiotics and specific regimens combining a low glycaemic index diet and restricted intake of cow milk remain to be investigated through randomized and prospective and large clinical studies. The development of validated specific nutrition recommendations will be a challenge in the future.
Regarding pollution, large clinical studies to assess its impact on acne still have to be conducted. The efficacy of topical or oral antioxidants to block the effect of pollution in acne patients remains to be demonstrated. Further improvement of cleansers and of antiparticle adhesion products could be of interest to protect the microbiota and skin barrier.
The impact of climatic factors, especially radiation other than UVR such as visible light and the role of psychosocial and modern lifestyle factors, should be more investigated through well‐designed studies.
Practical considerations
According to the above‐described exposome factors that can worsen acne, we propose the following practical considerations for managing acne patients:
Prior to any prescription, identify potential negative exposome factors, especially in patients with moderate‐to‐severe acne as these may impact on the treatment success. Table 2 lists factors to be checked together with the patient during the first visit.
The identified negative exposome impact should be limited as much as possible to allow the main exposome targets, the natural skin barrier and its microbiota to recover. Limitation of high glycaemic index food in predisposed patients and food supplements containing weigh proteins, decrease in smoking tobacco/cannabis, modifications of contraceptives and skin care regimens might be suggested.
The use of topical antibiotics in monotherapy is not recommended. This is to avoid increase in antibacterial resistance and modifications in the natural skin microbiome. Topical retinoids combined or not with benzoyl peroxide should be prescribed to be applied in the evening according to current guidelines to avoid irritation and damaging of skin barrier.135, 136, 137
Patients should be reminded to avoid harsh washing of their skin (avoid scrubs or exfoliating devices) as this may damage the natural skin barrier function. Cleansers with a pH of 5.5 (syndet) should be favoured over traditional detergents (i.e. soaps) allowing for a gentle cleansing of the skin and also for a reduction of the particle load on the skin in the evening. Optimal frequency of cleansing should be twice a day.138
Skin care products are important for the maintenance of the healthy skin microbiota, preventing the alteration of the skin barrier and thus its inflammation through the innate immunity. Moisturizers should be used in the morning to restore/improve the natural skin barrier function in order to reduce cutaneous pollutant penetration during the day and to limit irritation, frequently observed with topical retinoids, especially during the first few weeks of therapy.
Moreover patients should be advised to use non‐comedogenic make‐up and to apply sun‐protecting products with a sun‐protecting factor of at least 30 to prevent from postinflammatory hyperpigmentation as well as from phototoxicity reactions. Daily photoprotection is of importance as UV radiation may increase the impacts of pollutant particles on the skin.76
Table 2.
Exposome factors to be checked at the patient's 1st visit
| Nutrition |
| Skim milk, rapidly assimilated saccharides, nibbling |
| Nutritional supplements containing whey proteins/leucine 1 |
| Medication |
| Contraception: type of progestin used |
| Use of anabolic steroids, testosterone |
| Occupational factors |
| Cosmetics |
| Mechanical factors |
| Pollutants |
| Air and industrial pollutants |
| Tobacco and cannabis consumption |
| Climatic factors |
| Heat, humidity, UVR |
| Psychosocial factors modern lifestyle |
| Stress, emotions, sleep deprivation |
| Socioeconomic pressures |
| Excessive light exposure (tablets, smartphones, computers) |
Conclusion
Exposome factors including nutrition, medication occupational factors, pollutants, climatic factors and psychosocial and lifestyle factors may impact on the course and severity of acne and on the treatment efficacy. Exposome factors act on the natural skin barrier and on the skin microbiota, resulting in increased sebum production, hyperkeratinization, modification of the microbiote, activation of the innate immunity thus resulting in acne worsening.
Identifying the negative exposome factors and thus reducing their impact are mandatory for an adequate acne management.
Acknowledgements
The authors acknowledge Karl Patrick Göritz, SMWS, Scientific and Medical Writing Services, France, for writing and editing support.
Conflicts of interest
The authors have no conflict of interest to disclose. The authors received honoraria from Laboratoires Vichy International.
Funding sources
This Expert Board was organized and supported by Laboratoires Vichy International.
References
- 1. Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 2005; 14: 1847–1850. [DOI] [PubMed] [Google Scholar]
- 2. Miller GW, Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci 2014; 137: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buck Louis GM, Sundaram R. Exposome: time for transformative research. Stat Med 2012; 31: 2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rappaport SM, Smith MT. Epidemiology. Environment and disease risks. Science (New York, NY) 2010; 330: 460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Research Council . Exposure Science in the 21st Century: A Vision and a Strategy. The National Academies Press, Washington, DC, 2012. [PubMed] [Google Scholar]
- 6. Rappaport SM. Genetic factors are not the major causes of chronic diseases. PLoS ONE 2016; 11: e0154387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grice EA. The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin Cutan Med Surg 2014; 33: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci 2017; 85: 152–161. [DOI] [PubMed] [Google Scholar]
- 9. Dreno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol 2017; 31(Suppl 5): 8–12. [DOI] [PubMed] [Google Scholar]
- 10. Di Landro A, Cazzaniga S, Parazzini F, Ingordo V, Cusano F, Atzori L, et al Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol 2012; 67: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 11. Murillo N, Raoult D. Skin microbiota: overview and role in the skin diseases acne vulgaris and rosacea. Future Microbiol 2013; 8: 209–222. [DOI] [PubMed] [Google Scholar]
- 12. Wolf R, Parish LC. Effect of soaps and detergents on epidermal barrier function. Clin Dermatol 2012; 30: 297–300. [DOI] [PubMed] [Google Scholar]
- 13. Melnik BC, John SM, Plewig G. Acne: risk indicator for increased body mass index and insulin resistance. Acta Derm Venereol 2013; 93: 644–649. [DOI] [PubMed] [Google Scholar]
- 14. Cerman AA, Aktas E, Altunay IK, Arici JE, Tulunay A, Ozturk FY. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol 2016; 75: 155–162. [DOI] [PubMed] [Google Scholar]
- 15. Kucharska A, Szmurlo A, Sinska B. Significance of diet in treated and untreated acne vulgaris. Postepy Dermatol Alergol 2016; 33: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon HH, Yoon JY, Hong JS, Jung JY, Park MS, Suh DH. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol 2012; 92: 241–246. [DOI] [PubMed] [Google Scholar]
- 17. Smith RN, Braue A, Varigos GA, Mann NJ. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci 2008; 50: 41–52. [DOI] [PubMed] [Google Scholar]
- 18. Mahmood SN, Bowe WP. Diet and acne update: carbohydrates emerge as the main culprit. J Drugs Dermatol 2014; 13: 428–435. [PubMed] [Google Scholar]
- 19. Reynolds RC, Lee S, Choi JY, Atkinson FS, Stockmann KS, Petocz P, et al Effect of the glycemic index of carbohydrates on Acne vulgaris. Nutrients 2010; 2: 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fabbrocini G, Izzo R, Faggiano A, Del Prete M, Donnarumma M, Marasca C, et al Low glycaemic diet and metformin therapy: a new approach in male subjects with acne resistant to common treatments. Clin Exp Dermatol 2016; 41: 38–42. [DOI] [PubMed] [Google Scholar]
- 21. Smith R, Mann N, Makelainen H, Roper J, Braue A, Varigos G. A pilot study to determine the short‐term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res 2008; 52: 718–726. [DOI] [PubMed] [Google Scholar]
- 22. Kim H, Moon SY, Sohn MY, Lee WJ. Insulin‐like growth factor‐1 increases the expression of inflammatory biomarkers and sebum production in cultured sebocytes. Ann Dermatol 2017; 29: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ben‐Amitai D, Laron Z. Effect of insulin‐like growth factor‐1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol 2011; 25: 950–954. [DOI] [PubMed] [Google Scholar]
- 24. Ferdowsian HR, Levin S. Does diet really affect acne? Skin Therapy Lett 2010; 15: 1–2, 5. [PubMed] [Google Scholar]
- 25. Berra B, Rizzo AM. Glycemic index, glycemic load: new evidence for a link with acne. J Am Coll Nutr 2009; 28(Suppl): 450s–454s. [DOI] [PubMed] [Google Scholar]
- 26. Cemil BC, Ayvaz HH, Ozturk G, Ergin C, Akis HK, Gonul M, et al Effects of isotretinoin on body mass index, serum adiponectin, leptin, and ghrelin levels in acne vulgaris patients. Postepy Dermatol Alergol 2016; 33: 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abulnaja KO. Changes in the hormone and lipid profile of obese adolescent Saudi females with acne vulgaris. Braz J Med Biol Res 2009; 42: 501–505. [DOI] [PubMed] [Google Scholar]
- 28. Agamia NF, Abdallah DM, Sorour O, Mourad B, Younan DN. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin‐like growth factor‐1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol 2016; 174: 1299–1307. [DOI] [PubMed] [Google Scholar]
- 29. Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol 2015; 8: 371–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ismail NH, Manaf ZA, Azizan NZ. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol 2012; 12: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vongraviopap S, Asawanonda P. Dark chocolate exacerbates acne. Int J Dermatol 2016; 55: 587–591. [DOI] [PubMed] [Google Scholar]
- 32. Delost GR, Delost ME, Lloyd J. The impact of chocolate consumption on acne vulgaris in college students: a randomized crossover study. J Am Acad Dermatol 2016; 75: 220–222. [DOI] [PubMed] [Google Scholar]
- 33. Szabo K, Erdei L, Bolla BS, Tax G, Biro T, Kemeny L. Factors shaping the composition of the cutaneous microbiota. Br J Dermatol 2017; 176: 344–351. [DOI] [PubMed] [Google Scholar]
- 34. Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011; 9: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katta R, Desai SP. Diet and dermatology: the role of dietary intervention in skin disease. J Clin Aesthet Dermatol 2014; 7: 46–51. [PMC free article] [PubMed] [Google Scholar]
- 36. Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis 2011; 88: 84–91. [PubMed] [Google Scholar]
- 37. Norstedt S, Lindberg M. Dietary regimes for treatment of acne vulgaris: a critical review of published clinical trials. Acta Derm Venereol 2016; 96: 283–284. [DOI] [PubMed] [Google Scholar]
- 38. Adebamowo CA, Spiegelman D, Berkey CS, Danby FW, Rockett HH, Colditz GA, et al Milk consumption and acne in teenaged boys. J Am Acad Dermatol 2008; 58: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adebamowo CA, Spiegelman D, Danby FW, Frazier AL, Willett WC, Holmes MD. High school dietary dairy intake and teenage acne. J Am Acad Dermatol 2005; 52: 207–214. [DOI] [PubMed] [Google Scholar]
- 40. Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand‐Miller J. Acne vulgaris: a disease of Western civilization. Arch Dermatol 2002; 138: 1584–1590. [DOI] [PubMed] [Google Scholar]
- 41. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology 2012; 225: 256–258. [DOI] [PubMed] [Google Scholar]
- 42. Melnik BC. Evidence for acne‐promoting effects of milk and other insulinotropic dairy products. Nestle Nutr Workshop Ser Pediatr Program 2011; 67: 131–145. [DOI] [PubMed] [Google Scholar]
- 43. Cengiz FP, Cevirgen Cemil B, Emiroglu N, Gulsel Bahali A, Onsun N. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect 2017; 7: 106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis 2012; 90: 70–72. [PubMed] [Google Scholar]
- 45. Fabbrocini G, Bertona M, Picazo O, Pareja‐Galeano H, Monfrecola G, Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes 2016; 7: 625–630. [DOI] [PubMed] [Google Scholar]
- 46. Marchetti F, Capizzi R, Tulli A. [Efficacy of regulators of the intestinal bacterial flora in the therapy of acne vulgaris]. Clin Ter 1987; 122: 339–343. [PubMed] [Google Scholar]
- 47. Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open‐label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg 2013; 17: 114–122. [DOI] [PubMed] [Google Scholar]
- 48. Clark AK, Haas KN, Sivamani RK. Edible plants and their influence on the gut microbiome and acne. Int J Mol Sci 2017; 18. pii: E1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faure M, Drapier‐Faure E. [Acne and hormonal contraceptives]. Ann Dermatol Venereol 2010; 137: 746–749; quiz 5,50‐1. [DOI] [PubMed] [Google Scholar]
- 50. Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol 2013; 68: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 51. Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol 2016; 15: 670–674. [PubMed] [Google Scholar]
- 52. Ilse JR, Greenberg HL, Bennett DD. Levonorgestrel‐releasing intrauterine system and new‐onset acne. Cutis 2008; 82: 158. [PubMed] [Google Scholar]
- 53. Cohen EB, Rossen NN. [Acne vulgaris in connection with the use of progestagens in a hormonal IUD or a subcutaneous implant]. Ned Tijdschr Geneeskd 2003; 147: 2137–2139. [PubMed] [Google Scholar]
- 54. Palatsi R, Ylostalo P, Taipale A. Treatment of acne with cyproterone acetate and ethinyl estradiol. Acta Derm Venereol 1978; 58: 449–454. [PubMed] [Google Scholar]
- 55. Wierckx K, Van de Peer F, Verhaeghe E, Dedecker D, Van Caenegem E, Toye K, et al Short‐ and long‐term clinical skin effects of testosterone treatment in trans men. J Sex Med 2014; 11: 222–229. [DOI] [PubMed] [Google Scholar]
- 56. Walker J, Adams B. Cutaneous manifestations of anabolic‐androgenic steroid use in athletes. Int J Dermatol 2009; 48: 1044–1048; quiz 8. [DOI] [PubMed] [Google Scholar]
- 57. Kraus SL, Emmert S, Schon MP, Haenssle HA. The dark side of beauty: acne fulminans induced by anabolic steroids in a male bodybuilder. Arch Dermatol 2012; 148: 1210–1212. [DOI] [PubMed] [Google Scholar]
- 58. Perez M, Navajas‐Galimany L, Antunez‐Lay A, Hasson A. When strength turns into disease: acne fulminans in a bodybuilder. An Bras Dermatol 2016; 91: 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Monk B, Cunliffe WJ, Layton AM, Rhodes DJ. Acne induced by inhaled corticosteroids. Clin Exp Dermatol 1993; 18: 148–150. [DOI] [PubMed] [Google Scholar]
- 60. Kazandjieva J, Tsankov N. Drug‐induced acne. Clin Dermatol 2017; 35: 156–162. [DOI] [PubMed] [Google Scholar]
- 61. Moroni P, Pierini F, Zerboni R, Nava C, Pigatto P, Riboldi A, et al Skin pathology in industrial workers exposed to synthetic corticosteroids. Derm Beruf Umwelt 1985; 33: 220–222. [PubMed] [Google Scholar]
- 62. Mackesy MM, Selhorst C, Raza S. Immunosuppressant‐induced acneiform eruption of the breast. Breast J 2014; 20: 650–652. [DOI] [PubMed] [Google Scholar]
- 63. Balta I, Ozuguz P. Vitamin B12‐induced acneiform eruption. Cutan Ocul Toxicol 2014; 33: 94–95. [DOI] [PubMed] [Google Scholar]
- 64. Scarfi F, Arunachalam M. Lithium acne. CMAJ 2013; 185: 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thaler CJ. Folate metabolism and human reproduction. Geburtshilfe Frauenheilkd 2014; 74: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hubiche T, Sibaud V. Localized acne induced by radiation therapy. Dermatol Online J 2014; 20. pii: doj_21545. [PubMed] [Google Scholar]
- 67. Martin WM, Bardsley AF. The comedo skin reaction to radiotherapy. Br J Radiol 2002; 75: 478–481. [DOI] [PubMed] [Google Scholar]
- 68. Klemke CD, Nestoris S, Wolfer LU, Krengel S, Zouboulis CC, Tebbe B et al [Radiation‐induced acne]. Hautarzt 2000; 51: 187–191. [DOI] [PubMed] [Google Scholar]
- 69. Kang D, Shi B, Erfe MC, Craft N, Li H. Vitamin B12 modulates the transcriptome of the skin microbiota in acne pathogenesis. Sci Transl Med 2015; 7: 293ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kimyai‐Asadi A, Jih MH. Follicular toxic effects of chimeric anti‐epidermal growth factor receptor antibody cetuximab used to treat human solid tumors. Arch Dermatol 2002; 138: 129–131. [DOI] [PubMed] [Google Scholar]
- 71. Kligman AM, Mills OH Jr. Acne cosmetica. Arch Dermatol 1972; 106: 843–850. [PubMed] [Google Scholar]
- 72. Nguyen SH, Dang TP, Maibach HI. Comedogenicity in rabbit: some cosmetic ingredients/vehicles. Cutan Ocul Toxicol 2007; 26: 287–292. [DOI] [PubMed] [Google Scholar]
- 73. Tarun J, Susan J, Suria J, Susan VJ, Criton S. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J Dermatol 2014; 59: 442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol 1970; 101: 580–584. [PubMed] [Google Scholar]
- 75. Dreno B, Bettoli V, Perez M, Bouloc A, Ochsendorf F. Cutaneous lesions caused by mechanical injury. Eur J Dermatol 2015; 25: 114–121. [DOI] [PubMed] [Google Scholar]
- 76. Lebwohl M, Herrmann LG. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis 2005; 76(6 Suppl): 7–12. [PubMed] [Google Scholar]
- 77. Krutmann J, Moyal D, Liu W, Kandahari S, Lee GS, Nopadon N, et al Pollution and acne: is there a link? Clin Cosmet Investig Dermatol 2017; 10: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pham DM, Boussouira B, Moyal D, Nguyen QL. Oxidization of squalene, a human skin lipid: a new and reliable marker of environmental pollution studies. Int J Cosmet Sci 2015; 37: 357–365. [DOI] [PubMed] [Google Scholar]
- 79. Lefebvre MA, Pham DM, Boussouira B, Bernard D, Camus C, Nguyen QL. Evaluation of the impact of urban pollution on the quality of skin: a multicentre study in Mexico. Int J Cosmet Sci 2015; 37: 329–338. [DOI] [PubMed] [Google Scholar]
- 80. Lefebvre MA, Pham DM, Boussouira B, Qiu H, Ye C, Long X, et al Consequences of urban pollution upon skin status. A controlled study in Shanghai area. Int J Cosmet Sci 2016; 38: 217–223. [DOI] [PubMed] [Google Scholar]
- 81. Das M, Misra MP. Acne and folliculitis due to diesel oil. Contact Dermatitis 1988; 18: 120–121. [DOI] [PubMed] [Google Scholar]
- 82. Adams BB, Chetty VB, Mutasim DF. Periorbital comedones and their relationship to pitch tar: a cross‐sectional analysis and a review of the literature. J Am Acad Dermatol 2000; 42: 624–627. [PubMed] [Google Scholar]
- 83. Moustafa GA, Xanthopoulou E, Riza E, Linos A. Skin disease after occupational dermal exposure to coal tar: a review of the scientific literature. Int J Dermatol 2015; 54: 868–879. [DOI] [PubMed] [Google Scholar]
- 84. Wolf A, Gruber F, Jakac D. [Acneiform eruptions in female workers at a cable factory]. Berufsdermatosen 1977; 25: 229–236. [PubMed] [Google Scholar]
- 85. Fabbrocini G, Kaya G, Caseiro Silverio P, De Vita V, Kaya A, Fontao F, et al Aryl hydrocarbon receptor activation in acne vulgaris skin: a case series from the region of Naples, Italy. Dermatology 2015; 231: 334–338. [DOI] [PubMed] [Google Scholar]
- 86. Ancona AA. Occupational acne. Occup Med 1986; 1: 229–243. [PubMed] [Google Scholar]
- 87. Demir B, Cicek D. Occupational acne In Kartal SP, Gonul M, eds. Acne and Acneiform Eruptions. InTech, Rijeka, Croatia, 2017: 04. [Google Scholar]
- 88. Kokelj F. Occupational acne. Clin Dermatol 1992; 10: 213–217. [DOI] [PubMed] [Google Scholar]
- 89. Farkas J. Oil acne from mineral oil among workers making prefabricated concrete panels. Contact Dermatitis 1982; 8: 141. [DOI] [PubMed] [Google Scholar]
- 90. Ju Q, Zouboulis CC, Xia L. Environmental pollution and acne: chloracne. Dermatoendocrinology 2009; 1: 125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Caramaschi F, del Corno G, Favaretti C, Giambelluca SE, Montesarchio E, Fara GM. Chloracne following environmental contamination by TCDD in Seveso, Italy. Int J Epidemiol 1981; 10: 135–143. [DOI] [PubMed] [Google Scholar]
- 92. Wolkenstein P, Misery L, Amici JM, Maghia R, Branchoux S, Cazeau C, et al Smoking and dietary factors associated with moderate‐to‐severe acne in French adolescents and young adults: results of a survey using a representative sample. Dermatology 2015; 230: 34–39. [DOI] [PubMed] [Google Scholar]
- 93. Wolkenstein P, Machovcova A, Szepietowski JC, Tennstedt D, Veraldi S, Delarue A. Acne prevalence and associations with lifestyle: a cross‐sectional online survey of adolescents/young adults in 7 European countries. J Eur Acad Dermatol Venereol 2017. https://doi.org/10.1111/jdv.14475. [DOI] [PubMed] [Google Scholar]
- 94. Capitanio B, Sinagra JL, Ottaviani M, Bordignon V, Amantea A, Picardo M. Acne and smoking. Dermatoendocrinology 2009; 1: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Capitanio B, Sinagra JL, Bordignon V, Cordiali Fei P, Picardo M, Zouboulis CC. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol 2010; 632010: 782–788. [DOI] [PubMed] [Google Scholar]
- 96. Puri P, Nandar SK, Kathuria S, Ramesh V. Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol 2017; 83: 415–423. [DOI] [PubMed] [Google Scholar]
- 97. Belushkin M, Jaccard G, Kondylis A. Considerations for comparative tobacco product assessments based on smoke constituent yields. Regul Toxicol Pharmacol 2015; 73: 105–113. [DOI] [PubMed] [Google Scholar]
- 98. Calafat AM, Polzin GM, Saylor J, Richter P, Ashley DL, Watson CH. Determination of tar, nicotine, and carbon monoxide yields in the mainstream smoke of selected international cigarettes. Tob Control 2004; 13: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Valacchi G, Sticozzi C, Pecorelli A, Cervellati F, Cervellati C, Maioli E. Cutaneous responses to environmental stressors. Ann N Y Acad Sci 2012; 1271: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery 1998; 123: 450–455. [PubMed] [Google Scholar]
- 101. Just M, Ribera M, Monso E, Lorenzo JC, Ferrandiz C. Effect of smoking on skin elastic fibres: morphometric and immunohistochemical analysis. Br J Dermatol 2007; 156: 85–91. [DOI] [PubMed] [Google Scholar]
- 102. Patterson AT, Tian FT, Elston DM, Kaffenberger BH. Occluded cigarette smoke exposure causing localized chloracne‐like comedones. Dermatology 2015; 231: 322–325. [DOI] [PubMed] [Google Scholar]
- 103. Yang YS, Lim HK, Hong KK, Shin MK, Lee JW, Lee SW, et al Cigarette smoke‐induced interleukin‐1 alpha may be involved in the pathogenesis of adult acne. Ann Dermatol 2014; 26: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Klaz I, Kochba I, Shohat T, Zarka S, Brenner S. Severe acne vulgaris and tobacco smoking in young men. J Invest Dermatol 2006; 126: 1749–1752. [DOI] [PubMed] [Google Scholar]
- 105. Crivellari I, Sticozzi C, Belmonte G, Muresan XM, Cervellati F, Pecorelli A, et al SRB1 as a new redox target of cigarette smoke in human sebocytes. Free Radic Biol Med 2017; 102: 47–56. [DOI] [PubMed] [Google Scholar]
- 106. Sticozzi C, Belmonte G, Pecorelli A, Arezzini B, Gardi C, Maioli E, et al Cigarette smoke affects keratinocytes SRB1 expression and localization via H2O2 production and HNE protein adducts formation. PLoS ONE 2012; 7: e33592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tucker SB. Occupational tropical acne. Cutis 1983; 31: 79–81. [PubMed] [Google Scholar]
- 108. Plewig G, Kligman AM. Acne Tropicalis (Tropical Acne). ACNE and ROSACEA. Springer Verlag, Berlin Heidelberg, Germany, 2000. [Google Scholar]
- 109. Novy FG Jr. Tropical acne. Calif Med 1946; 65: 274–277. [PMC free article] [PubMed] [Google Scholar]
- 110. Sardana K, Sharma RC, Sarkar R. Seasonal variation in acne vulgaris–myth or reality. J Dermatol 2002; 29: 484–488. [DOI] [PubMed] [Google Scholar]
- 111. Gfesser M, Worret WI. Seasonal variations in the severity of acne vulgaris. Int J Dermatol 1996; 35: 116–117. [DOI] [PubMed] [Google Scholar]
- 112. Lee WJ, Park KH, Sohn MY, Lee WC, Lee SJ, Kim DW. Ultraviolet B irradiation increases the expression of inflammatory cytokines in cultured sebocytes. J Dermatol 2013; 40: 993–997. [DOI] [PubMed] [Google Scholar]
- 113. Lee WJ, Chae SY, Ryu HS, Jang YH, Lee SJ, Kim DW. Inflammatory cytokine expression and sebum production after exposure of cultured human sebocytes to ultraviolet A radiation and light at wavelengths of 650 nm and 830 nm. Ann Dermatol 2015; 27: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Plewig G, Kligman AM. Ultraviolet Radiation. ACNE and ROSACEA. Springer Verlag, Berlin Heidelberg, Germany, 2000. [Google Scholar]
- 115. Scott TL, Christian PA, Kesler MV, Donohue KM, Shelton B, Wakamatsu K, et al Pigment‐independent cAMP‐mediated epidermal thickening protects against cutaneous UV injury by keratinocyte proliferation. Exp Dermatol 2012; 21: 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Suh DH, Kwon TE, Youn JI. Changes of comedonal cytokines and sebum secretion after UV irradiation in acne patients. Eur J Dermatol 2002; 12: 139–144. [PubMed] [Google Scholar]
- 117. Patra V, Byrne SN, Wolf P. The skin microbiome: is it affected by UV‐induced Immune suppression? Front Microbiol 2016; 7: 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shenoi SD, Prabhu S. Role of cultural factors in the biopsychosocial model of psychosomatic skin diseases: an Indian perspective. Clin Dermatol 2013; 31: 62–65. [DOI] [PubMed] [Google Scholar]
- 119. Picardi A, Abeni D. Stressful life events and skin diseases: disentangling evidence from myth. Psychother Psychosom 2001; 70: 118–136. [DOI] [PubMed] [Google Scholar]
- 120. Welp K, Gieler U. [Acne vulgaris: morphologic, endocrinologic and psychosomatic aspects]. Z Hautkr 1990; 65: 1139–1145. [PubMed] [Google Scholar]
- 121. Lin TK, Man MQ, Santiago JL, Scharschmidt TC, Hupe M, Martin‐Ezquerra G, et al Paradoxical benefits of psychological stress in inflammatory dermatoses models are glucocorticoid mediated. J Invest Dermatol 2014; 134: 2890–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chiu A, Chon SY, Kimball AB. The response of skin disease to stress: changes in the severity of acne vulgaris as affected by examination stress. Arch Dermatol 2003; 139: 897–900. [DOI] [PubMed] [Google Scholar]
- 123. Albuquerque RG, Rocha MA, Bagatin E, Tufik S, Andersen ML. Could adult female acne be associated with modern life? Arch Dermatol Res 2014; 306: 683–688. [DOI] [PubMed] [Google Scholar]
- 124. Dangoisse C. Atopic dermatitis. Rev Med Brux 2011; 32: 230–234. [PubMed] [Google Scholar]
- 125. Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy 2015; 45: 566–574. [DOI] [PubMed] [Google Scholar]
- 126. Huynh M, Gupta R, Koo JY. Emotional stress as a trigger for inflammatory skin disorders. Semin Cutan Med Surg 2013; 32: 68–72. [DOI] [PubMed] [Google Scholar]
- 127. Heller MM, Lee ES, Koo JY. Stress as an influencing factor in psoriasis. Skin Therapy Lett 2011; 16: 1–4. [PubMed] [Google Scholar]
- 128. Rokowska‐Waluch A, Pawlaczyk M, Cybulski M, Zurawski J, Kaczmarek M, Michalak M, et al Stressful events and serum concentration of substance P in acne patients. Ann Dermatol 2016; 28: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Martin‐Ezquerra G, Man MQ, Hupe M, Rodriguez‐Martin M, Youm JK, Trullas C, et al Psychological stress regulates antimicrobial peptide expression by both glucocorticoid and beta‐adrenergic mechanisms. Eur J Dermatol 2011; 21(Suppl 2): 48–51. [DOI] [PubMed] [Google Scholar]
- 130. Campbell CE, Strassmann BI. The blemishes of modern society? Acne prevalence in the Dogon of Mali. Evol Med Public Health 2016; 2016: 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community‐based study. J Invest Dermatol 2009; 129: 2136–2141. [DOI] [PubMed] [Google Scholar]
- 132. Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin‐releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol 2009; 160: 345–352. [DOI] [PubMed] [Google Scholar]
- 133. Toyoda M, Nakamura M, Morohashi M. Neuropeptides and sebaceous glands. Eur J Dermatol 2002; 12: 422–427. [PubMed] [Google Scholar]
- 134. Taheri M, Darabyan M, Izadbakhsh E, Nouri F, Haghani M, Mortazavi SAR, et al Exposure to visible light emitted from smartphones and tablets increases the proliferation of Staphylococcus aureus: can this be linked to acne? J Biomed Phys Eng 2017; 7: 163–168. [PMC free article] [PubMed] [Google Scholar]
- 135. Nast A, Dreno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al European evidence‐based (S3) guideline for the treatment of acne – update 2016 – short version. J Eur Acad Dermatol Venereol 2016; 30: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 136. Gollnick HP, Bettoli V, Lambert J, Araviiskaia E, Binic I, Dessinioti C, et al A consensus‐based practical and daily guide for the treatment of acne patients. J Eur Acad Dermatol Venereol 2016; 30: 1480–1490. [DOI] [PubMed] [Google Scholar]
- 137. Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016; 74: 945–973.e33. [DOI] [PubMed] [Google Scholar]
- 138. Choi JM, Lew VK, Kimball AB. A single‐blinded, randomized, controlled clinical trial evaluating the effect of face washing on acne vulgaris. Pediatr Dermatol 2006; 23: 421–427. [DOI] [PubMed] [Google Scholar]


