Muscle strength may be a predictor of treatment toxicity and survival in older patients with cancer. This article reports the results of a study of the association of muscle mass, muscle radiodensity, and muscle strength with treatment toxicity and overall survival in a cohort of older patients with cancer.
Keywords: Muscle strength, Aging, Neoplasms, Sarcopenia, Muscle radiodensity, Survival, Body composition
Abstract
Background.
Identifying predictors of treatment toxicity and overall survival (OS) is important for selecting patients who will benefit from chemotherapy. In younger patients with cancer, muscle mass and radiodensity are associated with treatment toxicity and OS. In this study, we investigated whether muscle mass, radiodensity, and strength were associated with treatment toxicity and OS in patients with advanced cancer aged 60 years or older.
Materials and Methods.
Before starting palliative chemotherapy, muscle mass and radiodensity were assessed using computed tomography scans and muscle strength was assessed using a hydraulic hand grip dynamometer. Treatment toxicity was defined as any toxicity resulting in dose reduction and/or discontinuation of treatment. Multiple logistic and Cox regression analyses were performed to study potential associations of muscle mass, radiodensity, and strength with treatment toxicity and OS, respectively.
Results.
The participants were 103 patients, with a mean age of 70 years, with advanced colorectal, prostate, or breast cancer. Muscle parameters were not significantly associated with treatment toxicity. Higher muscle strength was associated with longer OS (hazard ratio 1.03; 95% confidence interval 1.00–1.05). Muscle mass and radiodensity were not significantly associated with OS.
Conclusion.
Higher muscle strength at the start of palliative chemotherapy is associated with significantly better OS in older patients with advanced cancer. None of the investigated muscle parameters were related to treatment toxicity. Future studies are needed to evaluate whether muscle strength can be used for treatment decisions in older patients with advanced cancer.
Implications for Practice.
This study in older patients with advanced cancer showed that adequate muscle strength is associated with longer overall survival. The results of this study imply that muscle strength might be helpful in estimating survival and therefore in identifying older patients who will benefit from anticancer treatment.
Introduction
Systemic treatment of older patients with cancer is challenging because of the heterogeneous conditions and comorbidity of this population and a lack of knowledge caused by underrepresentation of this group of patients in clinical studies [1]. The high prevalence of treatment toxicity and the higher mortality rate caused by higher disease‐specific mortality and competing comorbidity further complicate decision‐making in this population [2], [3], [4]. Knowledge of predictors for treatment toxicity and survival can contribute to the identification of patients who will benefit from treatment with chemotherapy. Low muscle mass and impaired muscle radiodensity have been found in adult patients with cancer and in healthy older adults [5], [6]. In adults with cancer, low muscle mass and increased fat infiltration of the muscle, reflecting lower muscle radiodensity (i.e., low muscle attenuation), have been observed in 15%–55% of patients and were found to be predictive for treatment toxicity and survival [5], [7], [8], [9], [10], [11]. Decrease in muscle mass and muscle radiodensity is associated with aging [6], [12]. Depending on the definition, the prevalence of low muscle mass in older adults is up to 50% [13], [14]. Muscle radiodensity is generally lower in older than in younger adults [6].
Because of the observed decrease in muscle mass and muscle radiodensity, both in patients with cancer and in patients at older age, it is likely that the combination of these two factors (cancer and old age) affects muscle parameters even more severely. Several studies have investigated muscle mass in patients with advanced cancer and reported age‐related differences, with conflicting results regarding prevalence of low muscle mass [5], [8], [10], [15], [16]. However, age‐related differences in prevalence of low muscle radiodensity have not been reported in patients with advanced cancer.
Next to muscle mass and muscle radiodensity, muscle strength is a third important determinant of muscle depletion [17]. In the general population, muscle strength contributes to the identification of both the most fit and the most vulnerable older patients [18], [19], [20]. Therefore, muscle strength may be a predictor of treatment toxicity and survival in older patients with cancer. In addition, muscle strength has been shown to be a predictor of poor clinical outcome in younger patients with advanced cancer [21].
In this study, we prospectively studied the association of muscle mass, muscle radiodensity, and muscle strength with both treatment toxicity and overall survival (OS) in a cohort of older patients with advanced cancer.
Materials and Methods
The present study was conducted as part of a large prospective study on nutritional status and muscle measures in patients with advanced cancer at the Vrije Universiteit Medical Center (VUmc), Amsterdam, The Netherlands. A first analysis of these data was published in 2014 [22], and changes in muscle mass during treatment for metastatic colorectal cancer (CRC) were reported in 2016 [23].
The research protocol was approved by the medical ethics committee of the VUmc, and the study was performed according to the 1964 Declaration of Helsinki. Written informed consent was obtained from all patients prior to participation.
Patients
In the large prospective study, eligible patients had advanced CRC or breast, prostate, or lung cancer and were scheduled to receive the first cycle of palliative chemotherapy or the first cycle of a new line of chemotherapy. Patients were excluded who had been treated with anticancer therapy within the last 30 days, who had ascites or serious pitting edema, or who were not able or willing to provide informed consent. Patients were recruited from the outpatient clinic and medical ward of the VUmc between October 2011 and July 2014.
For the current analysis we selected patients aged 60 years and older with CRC or breast or prostate cancer who had an evaluable computed tomography (CT) scan of the abdomen available within 40 days of the start of treatment. Because of absence of CT scans of the abdomen in the majority of patients with lung cancer, this group of patients was not included in this analysis.
Anthropometry
Body weight and height were assessed before the start of chemotherapy. Height was measured to the nearest cm using a stadiometer while the patient was standing barefoot. Weight was measured within 0.2 kg on a calibrated scale (seca type 888, seca GmbH, Hamburg, Germany). Body mass index (BMI) was calculated as the ratio of body weight to height squared (kg/m2).
Muscle Measures
Muscle Mass and Muscle Radiodensity.
All CT scans were obtained for clinical purposes. Muscle mass and muscle radiodensity were measured by analysis of electronically stored CT images. A certified investigator analyzed images using commercially available software (sliceOmatic version 5.0; TomoVision, Magog, Canada) on a single‐slice transverse CT image located at a standard vertebral landmark, L3 [24]. The software was used to specify the different tissues based on their anatomical features and pre‐established thresholds of Hounsfield units (HU). For skeletal muscle the range of HU is −29 to +150 HU, and for intermuscular adipose tissue, −190 to −30 HU [25].
Lumbar skeletal muscle measured at L3 is related to whole body muscle mass and is the sum of the paraspinal muscles (quadratus lumborum, erector spinae), psoas muscles, transversus abdominis, internal and external oblique, and rectus abdominis [24]. The software computed the surface area of lumbar skeletal muscle (e.g., cm2), and this value of muscle mass was normalized for stature using the lumbar skeletal muscle index (SMI, cm2/m2) [24]. Muscle radiodensity, also described as muscle attenuation, was measured using the muscle radiation attenuation rate (in HU).
Low muscle mass (low SMI) and low muscle radiodensity were defined according to the sex‐ and BMI‐specific threshold values associated with low survival by Martin et al [7].
Muscle Strength.
Hand grip strength is a valid indicator of general muscle strength and, being a bedside method, it is the most frequently used clinical tool to determine muscle strength [26]. Hand grip strength was measured using a hydraulic hand dynamometer (Baseline, Fabrication Enterprises, White Plains, NY). Patients performed the test while sitting with the shoulder adducted and neutrally rotated, elbow flexed at 90 degrees, and forearm and wrist in neutral position. Patients were instructed to perform one practice contraction. Subsequently, each patient performed two maximal isometric contractions with the left hand and two with the right hand. Maximal hand grip strength was defined as the maximum value of four contractions and was recorded to the nearest 0.5 kg. Low muscle strength was determined by sex‐specific cutoff values for maximal hand grip strength (<30.3 kg for men and <19.3 kg for women) [27].
Treatment Toxicity
Treatment toxicity was obtained from medical records and consisted of any toxicity leading to dose reduction or discontinuation of chemotherapy, such as (febrile) neutropenia, neurotoxicity, gastrointestinal symptoms, and fatigue. Treatment modifications due to other causes, such as progressive disease or vacation, were not taken into account. Treatment toxicity was dichotomized into present or absent. Furthermore, time until treatment toxicity and up‐front dose reductions were noted. Early treatment toxicity was defined as toxicity occurring within 42 days (two cycles of treatment).
Survival
OS data were obtained at least 1 year after inclusion of the last patient. The median length of follow‐up was 436 days (interquartile range 414). Patients who were still alive were censored on the date of last consultation. Six‐month OS was calculated.
Covariates
Demographic and clinical data were retrieved from medical records. Age, sex, cancer type, and World Health Organization and Eastern Cooperative Oncology Group performance statuses were recorded before the start of treatment with chemotherapy. Comorbidity was assessed with the Charlson Comorbidity Index [31]. A score of ≥2 indicates severe comorbidity. Polypharmacy was defined as the use of five or more medications. Treatment line was defined as consecutive chemotherapy line and dichotomized as first‐line or at least second‐line.
Statistical Analysis
All analyses were performed using SPSS for Windows, version 22.0 (IBM, Armonk, NY). Descriptive statistics were used to describe the basic features of the data. Continuous data were tested for normal distribution and presented as mean and standard deviation (SD). Pearson's chi‐squared test was applied to investigate statistically significant differences between groups when appropriate. Pearson correlation was used to investigate the correlation between SMI and hand grip strength. To investigate whether there was an association between muscle measures and treatment toxicity, univariable and multiple logistic regression analyses were applied. For this analysis, muscle measures were investigated as continuous variables. Odds ratios and corresponding 95% confidence intervals (CIs) were reported.
To investigate median OS per tumor type, the Kaplan‐Meier method was used.
Associations between muscle measures (continuous variables) and OS were investigated with univariable and multivariable Cox regression analyses. Hazard ratios and corresponding 95% CIs were reported.
To investigate the clinical value of muscle measures that were significantly associated with OS, we also studied the association of low versus normal muscle measures based on age‐ and gender‐based cutoff values, with OS using Cox regression analysis. Furthermore, the discriminative value of investigated cutoff values was examined by calculating sensitivity and specificity, as well as the area under the receiver operating characteristics (ROC) curve, with 0.70–0.80 representing fair and 0.80–1.00 representing good discrimination [28].
In the multivariable logistic and Cox regression analyses, we adjusted only for relevant confounders (defined as change of the regression coefficient of at least 10%) because of the sample size. Potential confounders included age, sex, comorbidity, cancer type, and treatment line. In the final analyses, age and comorbidity were not relevant and therefore not adjusted for.
A p value of ≤.05 was considered significant for all analyses.
Results
Of 364 patients included in the original study, 103 patients were eligible for the present analysis based on age, cancer type, and available CT scan. The mean age of patients was 70.0 years (SD 6.6), and 66% were men (Table 1). Approximately half of patients were diagnosed with CRC (52%), and 75% received first‐line cytotoxic treatment. Ten percent of patients had severe comorbidities, and polypharmacy was present in 39%.
Table 1. Patient characteristics (n = 103).
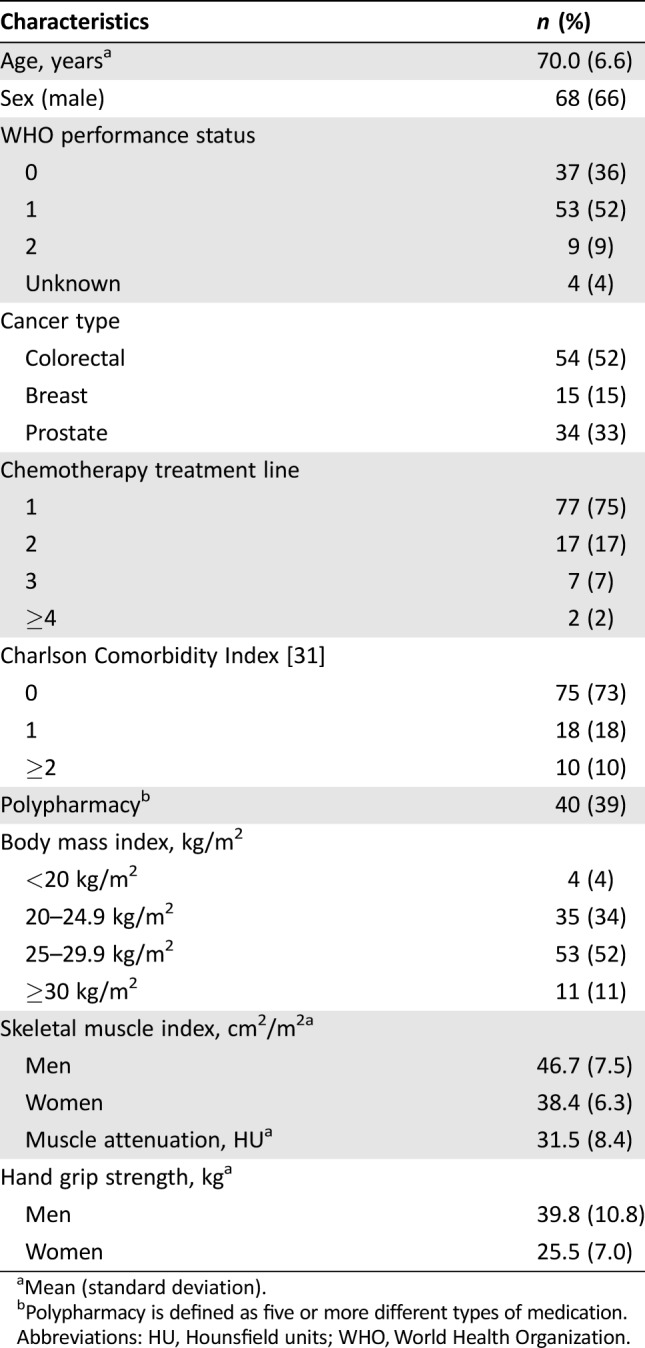
Mean (standard deviation).
Polypharmacy is defined as five or more different types of medication.
Abbreviations: HU, Hounsfield units; WHO, World Health Organization.
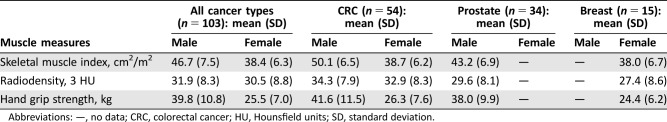
The mean SMI was 46.7 cm2/m2 (SD 7.5) in men and 38.4 cm2/m2 (SD 6.3) in women. Mean radiodensity was 31.9 HU (SD 8.3) in men and 30.5 HU (8.8) in women. Mean hand grip strength was 39.8 kg (SD 10.8) in men and 25.5 kg in women (SD 7.0). Table 2 shows the mean muscle measures for all patients and for each cancer type separately. Low muscle mass was present in 66% of patients, low muscle radiodensity in 88%, and low muscle strength in 21%. There was a correlation between SMI and hand grip strength only in male patients (male p = .003; female p = .137)
Table 2. Muscle measures.
Abbreviations: —, no data; CRC, colorectal cancer; HU, Hounsfield units; SD, standard deviation.
Treatment Toxicity
Treatment toxicity occurred in 46 patients (46%), resulting in dose reduction in 27 patients and discontinuation of treatment in 19 patients. In total, 19 patients (18%) started treatment with a reduced dose up front. The prevalence of treatment toxicity did not differ significantly between patients with and without baseline dose reductions (58% vs. 42%; p = .199.) Thirty‐seven percent of treatment toxicities were early treatment toxicities (within 42 days of treatment).
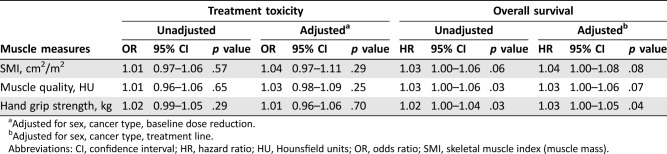
After adjusting for sex, cancer type, and up‐front dose reductions, we found no significant association of SMI (OR 1.04, 95% CI 0.97–1.11), muscle attenuation (OR 1.03, 95% CI 0.98–1.09), or muscle strength (OR 1.01, 95% CI 0.96–1.06) with treatment toxicity (Table 3). Adjusting for or limiting treatment toxicity to early treatment toxicity (within 42 days of treatment) did not change this.
Table 3. Associations between muscle measures and treatment toxicity and overall survival (n = 103).
Adjusted for sex, cancer type, baseline dose reduction.
Adjusted for sex, cancer type, treatment line.
Abbreviations: CI, confidence interval; HR, hazard ratio; HU, Hounsfield units; OR, odds ratio; SMI, skeletal muscle index (muscle mass).
Overall Survival
Median OS did not differ significantly per tumor type; it was 14.8 months (95% CI 10.56–19.08) for patients with CRC, 15.0 months (95% CI 4.46–25.50) for patients with breast cancer, and 17.7 months (95% CI 8.17–27.31) for patients with prostate cancer (p = .986).
After adjusting for sex, cancer type, and treatment line, higher muscle strength was significantly associated with longer survival (hazard ratio [HR] 1.03, 95% CI 1.00–1.05; Table 3). No significant associations were found between survival and muscle mass or muscle attenuation.
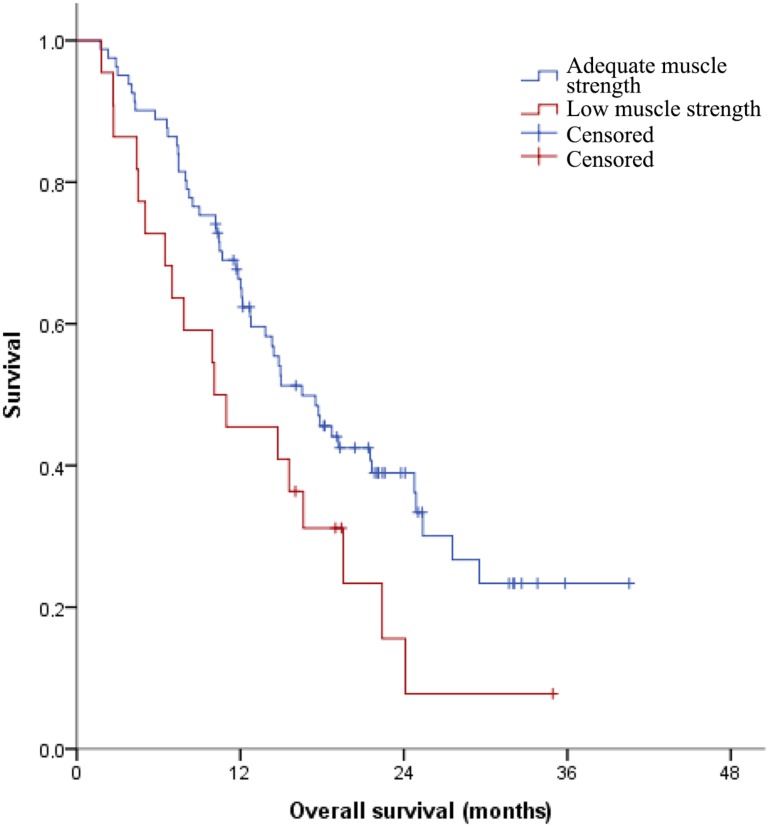
Furthermore, patients with normal muscle strength based on cutoff values had significantly longer OS than patients with low muscle strength (median OS 16.5 months vs. 10.1 months; HR 1.75, 95% CI 1.02–3.00; Fig. 1) [23].
Figure 1.
Kaplan‐Meier curve for the association of low hand grip strength, defined as <30.3 kg for men and <19.3 kg for women, and overall survival.
The sensitivity of low muscle strength in predicting six‐month OS was 40%, and specificity was 82%. The area under the ROC curve was 0.61.
Discussion
In this study, we evaluated whether muscle mass, muscle radiodensity, and muscle strength were predictive for treatment toxicity and survival in older patients treated with chemotherapy for advanced cancer. Patients with higher muscle strength had longer OS than patients with lower muscle strength. Muscle measures and treatment toxicity were not associated with OS. The significant association between muscle strength and OS supports findings from a previous study in patients with advanced non‐small cell lung and gastrointestinal cancer aged 18 years and older [21]. Muscle strength was also associated with OS in healthy older adults [19]. This implies that muscle strength, as a frailty marker, can potentially contribute to estimating survival and may be used as a factor to select older patients for anticancer treatment [29], [30]. However, although this study showed that Lauretani's cutoff values [27] for normal muscle strength can be used to predict OS, their discriminative value is low and may not be clinically useful [31]. This is mainly due to low sensitivity and poor positive predictive value. The low sensitivity found in this study might be caused by a relatively high percentage of patients with up‐front dose reductions who may have been fit to receive standard dosage [32].
In contrast with younger patients with advanced cancer [5], [7], [8], [9], [10], [11], muscle mass and muscle radiodensity were not significantly associated with OS in our group of older patients with advanced cancer. However, our finding that muscle strength is more important than muscle mass in estimating OS in older patients with advanced cancer supports the previous findings of a cohort study among 2,295 healthy participants aged 70 years and older [18]. A possible explanation for the discrepancy in findings between muscle mass and muscle radiodensity with OS in older patients is the relatively low muscle mass and low muscle radiodensity in our study compared with other studies in patients with cancer [7], [10], [11]. When cutoff values were used, the prevalence rates of low muscle mass and low muscle radiodensity in our study population were higher than those reported in younger patients with cancer [7]. This suggests that in older patients with cancer, both aging and cancer may contribute to loss of muscle mass and increase of fat accumulation in muscle. Therefore, age‐adjusted cutoff values for this group of patients are needed. Furthermore, the finding of lower muscle mass and lower muscle radiodensity in this population raises the question of whether improving muscle strength offers opportunities to improve OS, maintain functional independence, or improve quality of life. For older people living in the community, exercise‐based rehabilitation interventions or a resistance and aerobic exercise program have shown improvements in physical function and reduction in falls [33], [34]. Moreover, in younger patients with breast cancer, it was shown that a combined supervised resistance and aerobic exercise program during adjuvant chemotherapy improved physical function and muscle strength, and fewer dose adjustments were required [35]. Future trials should investigate whether improving muscle strength improves OS.
In contrast to findings from previous studies [5], [25], we found no significant association between muscle mass, muscle radiodensity, and treatment toxicity. There are several possible explanations for the lack of significant associations. First, we included patients with three different cancer types and multiple treatment lines; therefore, treatment regimens were heterogeneous. A previous study by Prado et al. and Barrett et al. [10], [36] included a more homogeneous group of relatively young patients with breast cancer (55 patients, mean age 55 years) or CRC (51 patients, median age 65 years) and one type of treatment. However, adjusting for cancer type did not change the association in this study. Second, treatment toxicity in younger patients may be due solely to pharmacokinetic effects caused by decreased muscle mass [10], whereas in older patients, pharmacokinetic effects are only partly responsible for causing treatment toxicity. Treatment toxicity in older patients may be multifactorial. Another possible cause is the aging of organ systems, such as the peripheral nervous system or bone marrow [37].
Strengths of this study are the simultaneous assessments of three important aspects of muscle (i.e., mass, radiodensity, and strength), the specific focus on older patients with advanced cancer, and the relatively large sample size. However, the inclusion of three cancer types may have resulted in heterogeneous treatment regimens. Although we did adjust for cancer type and treatment line, there may be some residual confounding affecting the association between muscle measures and treatment toxicity. Probably most importantly, patients starting treatment with chemotherapy were selected, which may have resulted in a selected population with relatively high muscle strength.
Conclusion
In older patients treated for advanced CRC or breast or prostate cancer, higher muscle strength is associated with longer OS. Therefore, future studies should evaluate the clinical use of muscle strength for judging whether or not a patient should receive palliative treatment to further optimize treatment outcome in this elderly population.
Acknowledgments
This study was funded by a grant from Fonds Nuts Ohra (grant number 1002‐039).
Author Contributions
Conception/design: Kathelijn Sophie Versteeg, Susanne Blauwhoff‐Buskermolen, Marian A.E. de van der Schueren, Jacqueline A.E. Langius, Henk M.W. Verheul, Inge R. Konings
Provision of study material or patients: Susanne Blauwhoff‐Buskermolen, Marian A.E. de van der Schueren, Henk M.W. Verheul
Collection and/or assembly of data: Kathelijn Sophie Versteeg, Susanne Blauwhoff‐Buskermolen, Henk M.W. Verheul
Data analysis and interpretation: Kathelijn Sophie Versteeg, Laurien M. Buffart, A.B. Maier, Inge R. Konings
Manuscript writing: Kathelijn Sophie Versteeg, Susanne Blauwhoff‐Buskermolen, Laurien M. Buffart, Marian A.E. de van der Schueren, Jacqueline A.E. Langius, Henk M.W. Verheul, A.B. Maier, Inge R. Konings
Final approval of manuscript: Kathelijn Sophie Versteeg, Susanne Blauwhoff‐Buskermolen, Laurien M. Buffart, Marian A.E. de van der Schueren, Jacqueline A.E. Langius, Henk M.W. Verheul, A.B. Maier, Inge R. Konings
Disclosures
The authors indicated no financial relationships.
References
- 1. Zullman DM, Sussman JB, Chen X et al. Examining the evidence: A systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med 2011;26:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Versteeg KS, Konings IR, Lagaay AM et al. Prediction of treatment‐related toxicity and outcome with geriatric assessment in elderly patients with solid malignancies treated with chemotherapy: A systematic review. Ann Oncol 2014;25:1914–1918. [DOI] [PubMed] [Google Scholar]
- 3. Mell LK, Jeong JH, Nichols MA et al. Predictors of competing mortality in early breast cancer. Cancer 2010;116:5365–5373. [DOI] [PubMed] [Google Scholar]
- 4. van de Water W, Markopoulos C, van de Velde CJ et al. Association between age at diagnosis and disease‐specific mortality among postmenopausal women with hormone receptor‐positive breast cancer. JAMA 2012;307:590–597. [DOI] [PubMed] [Google Scholar]
- 5. van Vledder MG, Levolger S, Ayez N et al: Body composition and outcome of patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550–557. [DOI] [PubMed] [Google Scholar]
- 6. Anderson DE, D'Agostino JM, Bruno AG et al. Variations of CT‐based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci 2013;68:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin L, Birdsell L, Macdonald N et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 8. Parsons HA, Baracos VE, Dhillon N et al: Body composition, symptoms, and survival in advanced cancer patients referred to a phase I service. PLoS One 2012;7:e29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prado CM, Lieffers JR, McCargar LJ et al: Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 10. Prado CM, Baracos VE, McCargar LJ et al.: Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 11. Antoun S, Baracos VE, Birdsell L et al: Low body mass index and sarcopenia associated with dose‐limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 2010;21:1594–1598. [DOI] [PubMed] [Google Scholar]
- 12. Buford TW, Anton SD, Judge AR et al. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age‐related muscle atrophy. Ageing Res Rev 2010;9:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bijlsma AY, Meskers CG, Ling CH et al. Defining sarcopenia: The impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging 2009;13:708–712. [DOI] [PubMed] [Google Scholar]
- 15. Peng PD, van Vledder MG, Tsai S et al.: Sarcopenia negatively impacts short‐term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lieffers JR, Bathe OF, Fassbender K et al: Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newman AB, Kupelian V, Visser M et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 19. Ling CH, Taekema D, de Craen AJ et al. Handgrip strength and mortality in the oldest old population: The Leiden 85‐plus study. CMAJ 2010;182:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taekema DG, Gussekloo J, Maier AB et al. Handgrip strength as a predictor of functional, psychological and social health. A prospective population‐based study among the oldest old. Age Ageing 2010;39:331–337. [DOI] [PubMed] [Google Scholar]
- 21. Kilgour RD, Vigano A, Trutschnigg B et al. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 2013;21:3261–3270. [DOI] [PubMed] [Google Scholar]
- 22. Blauwhoff‐Buskermolen S, de van der Schueren MA, Verheul HMW et al. “Pre‐cachexia”: A non‐existing phenomenon in cancer? Ann Oncol 2014;25:1668–1669. [DOI] [PubMed] [Google Scholar]
- 23. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MA et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 24. Shen W, Punyanitya M, Wang Z et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross‐sectional image. J Appl Physiol 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 25. Mitsiopoulos N, Baumgartner RN, Heymsfield SB et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 26. Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75‐year‐old men and women. Age Ageing 1994;23:132–137. [DOI] [PubMed] [Google Scholar]
- 27. Lauretani F, Russo CR, Bandinelli S et al. Age‐associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 28. Metz CE. Basic principles of ROC analysis. Sem Nucl Med 1978;8:283–298. [DOI] [PubMed] [Google Scholar]
- 29. Cooper R, Kuh D, Hardy R et al. Objectively measured physical capability levels and mortality: Systematic review and meta‐analysis. BMJ 2010;341:c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gale CR, Martyn CN, Cooper C et al. Grip strength, body composition, and mortality. Int J Epidemiol 2007;36:228–235. [DOI] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 32. Luciani A, Marussi D, Ascione G et al. Do elderly cancer patients achieve an adequate dose intensity in common clinical practice? Oncology 2006;71:382–387. [DOI] [PubMed] [Google Scholar]
- 33. Gillespie LD, Robertson MC, Gillespie WJ et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012:CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hars M, Herrmann FR, Fielding RA et al. Long‐term exercise in older adults: 4‐year outcomes of music‐based multitask training. Calcif Tissue Int 2014;95:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Waart H, Stuiver MM, van Harten WH et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J Clin Oncol 2015;33:1918–1927. [DOI] [PubMed] [Google Scholar]
- 36. Barret M, Antoun S, Dalban C et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66:583–589. [DOI] [PubMed] [Google Scholar]
- 37. Hurria A, Lichtman SM. Pharmacokinetics of chemotherapy in the older patient. Cancer Control 2007;14:32–43. [DOI] [PubMed] [Google Scholar]