Abstract
Background
Fine‐grained observational approaches to pain assessment (e.g. the Facial Action Coding System; FACS) are used to evaluate pain in individuals with and without dementia. These approaches are difficult to utilize in clinical settings as they require specialized training and equipment. Easy‐to‐use observational approaches (e.g. the Pain Assessment Checklist for Limited Ability to Communicate‐II; PACSLAC‐II) have been developed for clinical settings. Our goal was to compare a FACS‐based fine‐grained system to the PACSLAC‐II in differentiating painful from non‐painful states in older adults with and without dementia.
Method
We video‐recorded older long‐term care residents with dementia and older adult outpatients without dementia, during a quiet baseline condition and while they took part in a physiotherapy examination designed to identify painful areas. Videos were coded using pain‐related behaviours from the FACS and the PACSLAC‐II.
Results
Both tools differentiated between painful and non‐painful states, but the PACSLAC‐II accounted for more variance than the FACS‐based approach. Participants with dementia scored higher on the PACSLAC‐II than participants without dementia.
Conclusion
The results suggest that easy‐to‐use observational approaches for clinical settings are valid and that there may not be any clinically important advantages to using more resource‐intensive coding approaches based on FACS. We acknowledge, as a limitation of our study, that we used as baseline a quiet condition that did not involve significant patient movement. In contrast, our pain condition involved systematic patient movement. Future research should be aimed at replicating our results using a baseline condition that involves non‐painful movements.
Significance
Examining older adults with and without dementia, a brief observational clinical approach was found to be valid and accounted for more variance in differentiating pain‐related and non‐pain‐related states than did a detailed time‐consuming fine‐grained approach.
1. Introduction
Prevalence of pain in frail older adults with dementia is higher than as 75% (Williams et al., 2005). Pain in this population is undertreated (Kaasalainen et al., 1998; Jakobsson et al., 2004; Won et al., 2004; Martin et al., 2005; Reynolds et al., 2008) largely because communication impairments prevent the self‐report of pain, posing significant challenges in assessment and care. As a result, research has relied on non‐verbal pain expressions (Warden et al., 2003; Aubin et al., 2007; Sheu et al., 2011; Lints‐Martindale et al., 2012; Lukas et al., 2013; Hadjistavropoulos et al., 2014). Coding of non‐verbal pain behaviour in laboratory settings has been largely based on the Facial Action Coding System (FACS; Ekman and Friesen, 1978), an approach that requires special equipment (slow action and frame‐by‐frame coding of videos) and expertise (Hadjistavropoulos et al., 2002; Kunz et al., 2004, 2007). The FACS divides facial movements into 44 possible action units (AUs; e.g. tightening of the eye orbits, raising of the cheeks), each defined based on underlying musculature using explicit and rigorous criteria. Several investigations have confirmed consistency in pain expressions between individuals with and without dementia using FACS, although people with dementia may react with more vigour to painful stimulation (Hadjistavropoulos et al., 2000; Kunz et al., 2007).
Given the very time‐consuming nature of the system, Prkachin and Solomon (2008) reported on and validated a simplified FACS‐based coding approach, relying on four AUs consistently identified in studies of pain. Our first goal was to determine whether this simplified approach, validated since by other researchers in older adults (Gallant and Hadjistavropoulos, 2017), provides similarly valid results in older adults with dementia in terms of ability to differentiate painful from non‐painful states.
Given the technical requirements of the FACS, its utility has been limited to research settings, while a variety of clinically useful observational approaches focusing on gross behaviours (e.g. grimacing) have been developed and validated (Corbett et al., 2012; Herr et al., 2012). One of the approaches with the strongest validity evidence (Chan et al., 2014; Ammaturo et al., 2017), the Pain Assessment Checklist for Seniors with Limited Ability to Communicate‐II (PACSLAC‐II; Chan et al., 2014), was the focus of our study. Specifically, we examined the PACSLAC‐II in four ways: (1) its utility against the FACS‐based approach in differentiating painful from non‐painful states; (2) a comparison of pain behaviours identified with the PACSLAC‐II between older adults with and without dementia; (3) the relation of the PACSLAC‐II with self‐reported pain (in people who are capable of self‐report); and (4) the first‐time validation of the PACSLAC‐II using a movement exacerbated standardized pain protocol (Husebo et al., 2007, 2008, 2010) that was kept constant across participants (previous validation of the PACSLAC‐II did not involve a standardized movement protocol and the nature of the movement‐related situation varied somewhat across participants). Our primary hypothesis was that both the fine‐grained FACS assessment approach and the clinical PACSLAC‐II tool would effectively differentiate painful from non‐painful situations.
2. Methods
2.1. Participants
Participants (over 65 years of age) with severe dementia and severe limitations in ability to communicate (as determined by the nurse manager responsible for clinical care) were residents of long‐term care (LTC) facilities within a mid‐sized metropolitan area. Participants meeting inclusion criteria (age, diagnosis of dementia and severe limitations in ability to communicate) were identified by facility health care staff. Caregivers (i.e. family members and/or legal guardians) of eligible participants with dementia were informed by facility staff of the study and its voluntary nature and, if they expressed initial interest in the study, were presented with a consent form and information package. They were also given the opportunity to ask any questions about the study by contacting members of the research team and/or by meeting the research team just prior to the data collection. Participant assent was sought in all cases, and no potential participant took part if there were verbal or non‐verbal indications of unwillingness to participate. As a token of appreciation for participation, the research team set aside $20 for each participant. This amount was used to purchase items that each participant's family member deemed that participants would enjoy (e.g. flowers, music CDs, decorative items).
We also recruited a community sample of adults over 65 years of age without a diagnosis of dementia, living independently in the community and attending a physiotherapy clinic. The recruitment method was similar to the method used in the LTC facilities. Eligible participants were informed of the study by clinic staff and, if they expressed interest in participation, were given an information package including a consent form regarding the study. Each participant was paid $20 for their participation. All participants provided diagnostic and medication info. For individuals unable to communicate verbally, this information was provided by LTC facility staff.
Demographic information about the participants is presented in Table 1. It is noted that participants with dementia had an average score on the cognitive performance scale (Hartmaier et al., 1995; obtained from participants’ charts) placing them in the moderate to severe range.
Table 1.
Participant demographic information
| Participants with severe dementia (LTC sample; N = 48) | Participants without a dementia diagnosis (community sample; N = 52) | |
|---|---|---|
| Average age (SD) | 82.50 (9.25) | 75.46 (6.11) |
| Male | 13 | 23 |
| Female | 36 | 33 |
| Years of education (SD) | 13.5 (4.39) | 12.51 (2.79) |
| CPS score (SD) | 3.74 (1.72) | n/a |
CPS, Cognitive Performance Scale.
2.2. Measures
2.2.1. Facial Action Coding System
The FACS (Ekman and Friesen, 1978; Ekman et al., 2002) is an objective, fine‐grained measure of facial activity. The FACS evaluates 44 discrete muscle movements (or combinations of muscle movements) based on the functional anatomy of facial muscles (Ekman and Friesen, 1978). These discrete muscle movements, called action units (AUs; e.g. brow lowering, cheek raising, lip tightening, nose wrinkling), are coded for their frequency and intensity by trained coders (Ekman et al., 2002). The FACS has been found to be highly reliable and is well validated in investigations of non‐verbal pain behaviour in a wide variety of populations including older adults with dementia (Lints‐Martindale et al., 2007; Craig et al., 2011; Hadjistavropoulos et al., 2014). Previous investigations have shown specifically that the following AUs are related to pain most consistently (Prkachin, 1992; Prkachin and Solomon, 2008): AU4 (brow lowering); AU6 (cheek raising), AU7 (lid tightening), AU9 (nose wrinkling), AU10 (upper lip raising) and AU43 (eyes closed). These AUs were coded for the purposes of this study.
2.2.2. The Pain Assessment Checklist for Seniors with Limited Ability to Communicate‐II
The PACSLAC‐II (Chan et al., 2014) is an easy‐to‐use observational assessment tool that was developed for use in clinical settings. It consists of 31 pain behaviours (scored as present = 1 or absent = 0) that correspond to the non‐verbal pain assessment domains recommended by the American Geriatrics Society (AGS) Panel on Persistent Pain in Older Persons (2002) (i.e. facial expressions, verbalizations and vocalizations, body movements, changes in interpersonal interactions, changes in activity patterns or routines, and mental status changes). A total score is derived by summing the individual item scores. The tool has been shown to be valid and reliable and to account for a large portion of the variance in differentiating between painful and non‐painful situations (Chan et al., 2014; Ammaturo et al., 2017). In this study, internal consistency of the total PACSLAC‐II was satisfactory during the movement protocol period when used in a frame‐by‐frame coding approach (α = 0.80) and when used in a clinical manner (α = 0.81).
2.2.3. Numeric Rating Scale and Coloured Analogue Scale
For the community sample, we used a 0 (no pain) to 10 (extreme pain) to obtain self‐reported pain ratings where possible. The validity of this approach has been well documented both among younger and older adults (Hadjistavropoulos et al., 2007; Jensen and Karoly, 2011). For participants with dementia, we attempted to obtain self‐report (0–10) using a CAS (McGrath et al., 1996) which has been shown to be valid among some participants with dementia (Scherder and Bouma, 2000). The CAS is ruler‐like plastic scale that gradually changes in colour (from the bottom to the top of the scale) from light pink to darker red. The bottom of the scale is anchored by the words ‘no pain’, while the top is anchored with the words ‘most pain’. The participant moves a plastic glide to indicate his or her level of pain. At the back of the scale, numeric ratings (0–10) are used to provide a pain rating that corresponds to the participants’ placement of the plastic glide. A research assistant explained the CAS to the participants and asked comprehension questions about the scale such as ‘point to the scale’ and ‘where would you move the glide if you have no pain at all?’ (as described by Scherder and Bouma, 2000). If the participant responded correctly to the questions (e.g. what level the glide would be at for no pain and most severe pain) and understood the scale, they were asked to indicate where the glide should be to match their own pain level. Only one participant with dementia was able to respond correctly to the comprehension questions, and as such, useful pain ratings could not be obtained for our dementia sample.
2.2.4. Cognitive Performance Scale
The CPS is a reliable and valid index of cognitive functioning designed to evaluate memory, decision‐making ability, communications skills and eating impairments and is based on nurse opinions about patient functioning (Morris et al., 1994; Hartmaier et al., 1995; Paquay et al., 2007). It is scored on 0–6 scale with higher scores indicating more severe cognitive impairment. The tool has been validated against the Mini Mental State Examination (Folstein et al., 1975; Paquay et al., 2007). For the purposes of this study, scores were obtained from the patients’ charts and were never older than three months.
2.3. Procedure
First, we filmed the participants during a baseline state, while they were lying down on a bed or examination table for approximately 5 min. Following establishment of baseline, each participant took part in a standardized protocol of movements designed to identify painful areas. The protocol was also captured on video. This movement protocol was described by Husebo et al. (2007) and begins with the patient lying down on a bed or examination table. A qualified physiotherapist then proceeded to guide the patient to close both hands (one hand at a time), to raise both arms above the head (one arm at a time), to bring both knees towards the chest (one knee at a time), to extend both feet (one foot at a time) and to sit up on the bed/table (both sides). This protocol has been used successfully in previous investigations of movement exacerbated pain (Husebo et al., 2007, 2008, 2010). Following the protocol, participants who were able to respond to our self‐report pain assessment tool, were asked to indicate the amount of pain that they experienced during the examination procedure.
Following the data collection, baseline video segments were edited to be equal in length to their corresponding standardized movement segment and all videos (baseline and movement segments) were randomized and coded using a previously validated FACS‐based approach (Prkachin and Solomon, 2008; Gallant and Hadjistavropoulos, 2017), as well as the PASCLAC‐II. Trained coders completed the coding frame‐by‐frame. This frame‐by‐frame approach (resulting in an average of 3700 frames per baseline and movement segment) is far more rigorous than typically conducted in these types of investigation where video frames are not coded individually. Coding was performed using Noldus Observer XT software (Noldus Information Technology, 2015), which allows users to code observational data for each video frame.
As the PACSLAC‐II was designed as a gross observational tool to be completed once per interaction with patients, we also used the PACSLAC‐II in a manner representative of clinical settings. That is, an independent coder watched each video and completed the PACSLAC‐II the way it would be performed in a clinical setting.
We used a previously validated approach to FACS coding (Prkachin and Solomon, 2008; Gallant and Hadjistavropoulos, 2017), involving four categories of pain‐related facial actions: (1) brow lowering (i.e. AU4); (2) orbit tightening (i.e. AU6 and AU7); (3) levator tightening (i.e. AU9 and AU10); and (4) closing of the eye (i.e. AU43). For each video frame, we assigned a maximum intensity score for brow lowering, orbit tightening and levator tightening using a 0 (i.e. no facial action) to 5 (i.e. maximum facial action) scale. Closing of the eyes was scored as either 0 (not present) or 1 (present). The maximum intensity score for each category was summed to create a non‐verbal pain expression score (i.e. ranging from 0 to 16) for each video frame. This approach has the advantage that it focuses on well‐established pain‐related FACS AUs without the ‘noise’ created by AUs that have not been shown to have a consistent relationship to pain. It also relieves observer burden by reducing the number of decisions an observer must make in the course of measurement and correspondingly reduces the time necessary for coding.
Reliability of FACS‐based and PACSLAC‐II frame‐by‐frame coding was assessed by random selection of baseline and movement protocol coding from 21 participants (i.e. approximately 20% of the total sample). For each participant, two independent coders coded the same video. Interrater reliability was substantial for FACS‐based coding during both baseline (r = 0.72) and movement protocol (r = 0.89) segments, and for PACSLAC‐II coding during both baseline (r = 0.88) and movement protocol (r = 0.76) segments.
Reliability of the clinical PACSLAC‐II coding was also assessed. A second coder conducted clinical PACSLAC‐II coding for a random selection of 15% of the videos. Interrater reliability was outstanding for the movement protocol videos (r = 0.97) and acceptable for the baseline videos (r = 0.65). There was no significant difference between PACSLAC‐II scores completed frame‐by‐frame (averaged across each entire video segment) or in a clinical manner.
2.4. Analysis
We first compared the participants with dementia to our community sample with respect to age (independent samples t‐test) and gender (chi‐square). Any variable that showed a group difference was correlated with our dependent measures (self‐report and non‐verbal pain scores). A significant correlation would indicate the need to use that variable as a covariate.
To determine whether scores (i.e. total PACSLAC‐II score, self‐reported pain score and overall FACS‐based score) would be highest during a physiotherapy protocol designed to identify painful areas (as compared to a baseline condition) and to determine whether there were any group differences, we used mixed model (participants with vs. without dementia × baseline vs. physiotherapy examination) analyses of variance (ANOVA) with follow‐up comparisons where interaction effects were identified. In all analyses, we used the partial eta square statistic to determine effect sizes, allowing us to compare the ability of our various pain indices to differentiate between baseline and pain‐related distress.
3. Results
Table 2 shows the means and standard deviations of the participants on all measures of the study, and Table 3 shows means and standard deviations on specific items and item categories within the observational measures (PACSLAC‐II categories and FACS‐based individual movements). Table 4 summarizes our most central findings.
Table 2.
Descriptive statistics for all pain measures
| Dementia | Non‐dementia | All participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| NRS baseline | n/a | n/a | n/a | 52 | 1.83 | 2.25 | 52 | 1.83 | 2.25 |
| NRS movement protocol | n/a | n/a | n/a | 52 | 5.45 | 2.61 | 52 | 5.45 | 2.61 |
| PACSLAC‐II baseline | 49 | 3.59 | 2.04 | 52 | 2.67 | 1.56 | 102 | 3.12 | 1.85 |
| PACSLAC‐II movement protocol | 49 | 7.82 | 3.57 | 52 | 6.62 | 3.07 | 102 | 7.16 | 3.37 |
| FACS‐based baseline | 52 | 2.88 | 2.01 | 52 | 2.25 | 1.81 | 102 | 2.55 | 1.92 |
| FACS‐based score movement protocol | 52 | 4.39 | 2.31 | 52 | 4.28 | 2.30 | 102 | 4.31 | 2.29 |
NRS, Numeric Rating Scale; PACSLAC‐II, Pain Assessment Checklist for Seniors with Limited Ability to Communicate II; FACS, Facial Action Coding System.
Table 3.
Descriptive statistics for PACSLAC‐II categories and FACS‐based movements
| Dementia | Non‐dementia | All participants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | ||
| Baseline | PACSLAC‐II facial expressions | 48 | 3.67 | 1.99 | 52 | 2.67 | 1.56 | 101 | 3.15 | 1.83 |
| FACS‐based brow | 48 | 0.65 | 0.88 | 52 | 0.38 | 0.80 | 101 | 0.52 | 0.85 | |
| FACS‐based orbit | 48 | 0.77 | 0.81 | 52 | 0.71 | 0.85 | 101 | 0.74 | 0.83 | |
| FACS‐based levator | 48 | 0.65 | 0.64 | 52 | 0.41 | 0.69 | 101 | 0.52 | 0.67 | |
| FACS‐based eyes closed | 48 | 0.81 | 0.39 | 52 | 0.75 | 0.44 | 101 | 0.77 | 0.42 | |
| Movement protocol | PACSLAC‐II facial expressions | 49 | 6.76 | 3.14 | 52 | 6.42 | 2.93 | 102 | 6.55 | 3.03 |
| PACSLAC‐II verbalizations | 49 | 0.53 | 0.84 | 52 | 0.13 | 0.40 | 102 | 0.32 | 0.68 | |
| PACSLAC‐II body movements | 49 | 0.47 | 0.87 | 52 | 0.04 | 0.28 | 102 | 0.25 | 0.67 | |
| PACSLAC‐II interpersonal | 49 | 0.04 | 0.19 | 52 | 0.02 | 0.14 | 102 | 0.03 | 0.17 | |
| PACSLAC‐II activity | 49 | 0.02 | 0.14 | 52 | n/a | n/a | 102 | 0.01 | 0.10 | |
| FACS‐based brow | 49 | 1.31 | 0.93 | 52 | 1.37 | 1.01 | 102 | 1.34 | 0.96 | |
| FACS‐based orbit | 49 | 1.53 | 0.79 | 52 | 1.31 | 0.76 | 102 | 1.41 | 0.78 | |
| FACS‐based levator | 49 | 0.86 | 0.81 | 52 | 0.88 | 0.91 | 102 | 0.87 | 0.86 | |
| FACS‐based eyes closed | 49 | 0.69 | 0.47 | 52 | 0.71 | 0.46 | 102 | 0.70 | 0.46 | |
Due to lack of coded observations, the following PACSLAC‐II categories are not included: verbalizations (baseline), body movements (baseline), interpersonal changes (baseline), activity changes (baseline), mental changes (baseline and movement protocol).
Table 4.
Summary of main findings
|
|
|
|
|
|
|
NRS, Numeric Rating Scale; PACSLAC‐II, Pain Assessment Checklist for Seniors with Limited Ability to Communicate‐II; FACS, Facial Action Coding System.
Prior to conducting any comparisons to address our research questions, we compared the groups with respect to gender (chi square) and age (independent samples t‐test). There were no significant group differences with respect to gender. Nonetheless, the participants in the dementia group were significantly older than the community sample, t(98) = 4.522, p = 0.000. To determine whether it was necessary to use age as a covariate in our analysis, we examined correlations between age and our dependent measures (i.e. self‐reported pain and non‐verbal expression scores). As none of the correlations were significant, we deemed that there was no need to include age as a covariate in our main analyses.
3.1. Ability of pain measures to discriminate painful from non‐painful states
When we examined the self‐report scores of the participants without dementia, the results were consistent with our hypothesis. That is, the physiotherapy movement protocol was associated with higher NRS scores than the baseline (see Table 2 and Fig. 1), t(51) = −9.400, p < 0.001. The vast majority of this sample (98.1%) provided a self‐report NRS score above 0 in response to the movement protocol suggesting that pain was experienced by nearly all participants during the movements, while 84% of the participants self‐reported substantial pain (or a score >3) on the NRS, following the movement protocol.
Figure 1.
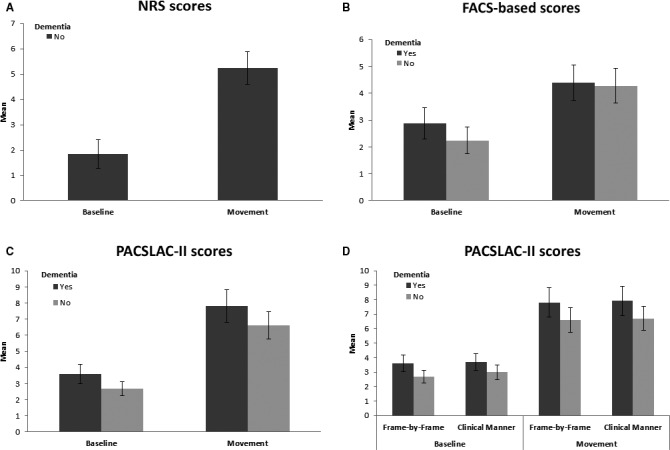
Scores on various pain indices by segment and dementia diagnosis. (A) Self‐reported pain during baseline and movement protocol, by individuals without dementia; (B) FACS‐based pain scores during baseline and movement protocol, individuals with and without dementia; (C) PACSLAC‐II scores during baseline and movement protocol, individuals with and without dementia; (D) PACSLAC‐II scores resulting from frame‐by‐frame judgements versus clinically administered during baseline and movement protocol, individuals with and without dementia. PACSLAC‐II, Pain Assessment Checklist for Seniors with Limited Ability to Communicate‐II; FACS, Facial Action Coding System; NRS, Numeric Rating Scale.
Examination of the distribution of the PACSLAC‐II and FACS scores indicated that parametric statistics would be appropriate for our analysis. To determine whether the PACSLAC‐II and FACS‐based scores would also discriminate between the discomforting physiotherapy protocol and the baseline condition, and to determine whether there were differences in pain expression between the dementia and community samples, we conducted two 2 between (LTC vs. community participants) × 2 within (baseline vs. pain) mixed model ANOVAs.
3.1.1. Total PACSLAC‐II scores (frame‐by‐frame coding)
The first mixed analysis involved the PACSLAC‐II score as dependent measure. As expected, there was a significant within‐subjects main effect for PACSLAC‐II responses, F(1, 99) = 123.450, p < 0.001, partial η2 = 0.555, indicating that participants scored higher during the movement protocol than the baseline conditions. There was also a significant between‐subjects effect of dementia on PACSLAC‐II scores, with participants with dementia scoring higher than community participants, F(1, 99) = 7.580, p = 0.007, partial η2 = 0.071. The interaction effect was not significant.
3.1.2. Specific categories of PACSLAC‐II behaviours (frame‐by‐frame coding)
To explore whether specific classes of behaviours (e.g. vocalizations, body movements) were useful in differentiating the baseline from the discomforting examination, we conducted a 2 within (baseline vs. physiotherapy movement protocol) × 2 between (group) mixed model multivariate analysis of variance on the PACSLAC‐II item groupings that contained items that occurred during the movement protocol (facial expressions, verbalizations and vocalizations, body movements). There was a significant multivariate within‐subjects effect, λ = 0.422, F(2, 94) = 25.724, p < 0.001, partial η2 = 0.578, a significant between‐subjects effect, λ = 0.801, F(2, 94) = 4.675, p < 0.001, partial η2 = 0.199, as well as a significant interaction effect, λ = 0.798, F(2, 94) = 4.772, p < 0.001, partial η2 = 0.202.
Follow‐up univariate analyses were conducted to determine the source of these multivariate effects. The first univariate analysis was focused on the facial expressions category of the PACSLAC‐II. There was a significant within‐subjects main effect, confirming increased pain‐related facial expressions during the physiotherapy movement protocol as compared to baseline, F(1, 98) = 99.511, p < 0.001, partial η2 = 0.504. There was also a significant between‐subjects effect, with participants with dementia scoring higher than community participants, F(1, 98) = 4.101, p = 0.046, partial η2 = 0.040. The interaction effect was not significant.
A second univariate analysis investigated the verbalizations category of the PACSLAC‐II. There was a significant within‐subjects main effect, with greater verbalization scores during the movement protocol versus baseline, F(1, 98) = 26.653, p < 0.001, partial η2 = 0.214. There was also a significant between‐subjects effect, with participants with dementia scoring higher than non‐dementia participants, F(1, 98) = 9.656, p = 0.002, partial η2 = 0.090. Finally, there was a significant interaction effect for baseline versus movement and dementia versus non‐dementia for the verbalization subscale, F(1, 98) = 0.9.656, p = 0.002, partial η2 = 0.090. Follow‐up analysis of this interaction indicated that there was a significant difference in verbalization/vocalization scores between individuals with and without dementia (individuals with dementia scored higher) during the physiotherapy examination but not during the baseline (Mean Diff = 0.407, SE = 0.131. p = 0.002).
The third univariate analysis revealed a significant within‐subjects main effect for the body movements category of the PACSLAC‐II, F(1, 98) = 16.425, p < 0.001, partial η2 = 0.144, indicating that individuals scored higher during the movement than during the baseline phase. There was also a significant between‐subjects effect on the body movements category, indicating that individuals with dementia scored higher than those without, F(1, 98) = 11.906, p < 0.001, partial η2 = 0.108. There was also a significant interaction effect for the body movements subscale, F(1, 98) = 0.11.906, p = 0.001, partial η2 = 0.108. Follow‐up analysis of this interaction indicated that there was a significant difference in body movements subscale score between individuals with and without dementia (individuals with dementia scored higher) during the physiotherapy protocol (Mean Diff = 0.441, SE = 0.128. p = 0.001), but not during baseline.
3.1.3. PACSLAC‐II scores obtained in a clinical manner
Gross PACSLAC‐II scores obtained in a clinical manner discriminated between baseline and physiotherapy movement conditions F(1, 99) = 124.720, p < 0.001, partial η2 = 0.557, indicating that participants scored higher during the movement protocol than the baseline conditions. There was also a significant between‐subjects effect of dementia on PACSLAC‐II scores, with participants with dementia scoring higher than community participants, F(1, 99) = 6.024, p = 0.016, partial η2 = 0.057. The interaction effect was not significant. Frame‐by‐frame PACSLAC‐II scores correlated with gross PACSLAC‐II scores for baseline (r = 0.917, p < 0.001) and movement (r = 0.987, p < 0.001) segments.
A paired samples t‐test confirmed no significant difference between scores obtained on the PACSLAC‐II using frame‐by‐frame (averaged across each entire video segment) versus gross application for the movement phase. Slightly higher scores were obtained on the PACSLAC‐II when applied as a gross measure, although this difference was significant only during the baseline phase, t(101) = −2.412, p = 0.018.
3.1.4. Overall FACS‐based pain score
A mixed model (2 groups × baseline vs. physiotherapy examination) analysis investigating the FACS‐based score revealed a significant within‐subjects main effect of video segment (baseline vs. physiotherapy examination) on FACS responses, indicating that participants scored higher during the physiotherapy examination than during baseline F(1, 98) = 38.616, p < 0.001, partial η2 = 0.283. This finding was consistent with our main hypotheses. The between‐subjects and interaction effects were not significant, suggesting that scores did not differ based on dementia diagnosis.
3.1.5. Specific FACS‐based movements
We conducted additional exploratory analysis to determine whether specific FACS‐based movement (brow, levator, orbit and eyes closing) scores were useful in discriminating between painful and non‐painful states; we conducted a 2 within (baseline vs. physiotherapy movements) × 2 between (group) mixed model multivariate analysis of variance on these FACS‐based movements. There was a significant multivariate within‐subjects effect, λ = 0.604, F(4, 95) = 15.551, p < 0.001, partial η2 = 0.396. The between‐subjects and interaction effects were not significant, suggesting that scores did not differ based on dementia diagnosis.
Follow‐up univariate analyses were conducted to delineate these multivariate effects. These analyses showed significant within‐subjects main effects confirming increased brow, F(1, 98) = 45.146, p < 0.001, partial η2 = 0.315, levator, F(1, 98) = 10.095, p = 0.002, partial η2 = 0.093 and orbit F(1, 98) = 39.386, p < 0.001, partial η2 = 0.287 movements (with higher scores for the physiotherapy condition) with no significant between‐subjects and interaction effects. No significant effects were found for eye closures.
3.2. Relationship of self‐report and non‐verbal measures of pain
To examine the relationship between self‐reported pain and our non‐verbal measure, we calculated Pearson correlation coefficients for the community sample. NRS score was correlated with overall PACSLAC‐II (r = 0.542, p < 0.01) and FACS‐based scores (r = 0.463, p < 0.01) during the movement protocol.
4. Discussion
Consistent with previous investigations (Warden et al., 2003; Pautex et al., 2007; Chan et al., 2014), our results support the use of non‐verbal pain assessment tools in both community‐dwelling seniors and seniors with limited ability to communicate due to dementia. Examination of the self‐report scores of community participants confirmed that more pain was experienced during the physiotherapy examination than during baseline. This supports the validity of our within‐subjects experimental manipulation. Considering the non‐verbal pain indices studied, the PACSLAC‐II had a higher correlation with self‐reported pain than did the FACS index (i.e. 0.54 vs. 0.46) although the difference between the two correlations was not statistically significant.
Our findings suggest that there may not be any clinical advantage to conducting fine‐grained behavioural coding compared to using an easy‐to‐use validated clinical scale such as the PACSLAC‐II or other similar scales that were not the focus of this investigation (Husebo et al., 2007; Pautex et al., 2007). That said, fine‐grained analysis of facial responses, as evaluated by FACS, would still be of great value for research. For example, fine‐grained analysis would allow for the evaluation of the interplay of facial expressions of pain and facial expressions of specific emotions (e.g. fear) that may manifest during pain (Gallant and Hadjistavropoulos, 2017).
In some ways, the finding concerning the relative utility of the PACSLAC‐II compared to the FACS‐based approach is not surprising because in addition to incorporating gross facial movements covered by FACS‐based methods (e.g. ‘lowering of the eyebrows or frowning’ corresponds to the FACS‐based ‘brow lower’; ‘grimacing’ on the PACSLAC‐II would also be related to FACS‐based facial movements), it contains additional categories of pain behaviours. Our exploratory analyses demonstrated that these additional categories of behaviours (i.e. body movements and vocalizations) are also capable of differentiating painful from non‐painful states. That said, it is clear that the grouping of facial behaviour PACSLAC‐II items accounted for the most variance, suggesting that, consistent with conclusions of other authors, the facial response to pain has more communicative value than other non‐verbal responses (e.g. Craig et al., 2011). In terms of specific pain‐related AUs, brow lowering and orbit tightening accounted for the most variance, supporting their robustness as indicators of pain. FACS‐based indices also correlated highly with self‐report. The high correlation with self‐report constitutes validity evidence for the two non‐verbal pain assessment methods.
Although we identified some differences between people with and without dementia on PACSLAC‐II behaviours, the effect size was small compared to the large within‐subjects effect. Nonetheless, this finding is consistent with previous research that has demonstrated that people with dementia tend to display increased non‐verbal pain behaviours compared to their cognitively intact counterparts (Hadjistavropoulos et al., 2000; Kunz et al., 2007). This difference may be reflective of increased pain‐related distress in light of participants with dementia having a more limited understanding of the painful situation.
A limitation in our study was that the pain situation involved movement, whereas the baseline period did not involve extensive movement (voluntary movement was permitted during the baseline). Although it could be argued that this makes it less clear as to whether our assessment tools differentiated pain behaviour from non‐pain behaviour as opposed to movement versus non‐movement, a number of factors mitigate this concern. In the first instance, the FACS AUs and AU combinations that we coded were specifically selected because they have been uniquely associated with pain (Prkachin, 1992; Prkachin and Solomon, 2008). Similarly, the PACSLAC‐II comprises of items that were selected with care to minimize the inclusion of behaviours that are not pain related (Chan et al., 2014). As such, it is reasonable to indicate that the baseline and the movement segments were differentiated primarily on the basis of absent versus present pain behaviours. Moreover, the large correlations between the observed behaviours (as assessed by the FACS and PACSLAC‐II systems) and self‐reported pain in the community sample (as well as examination of the self‐reported pain scores which were considerably lower during the baseline segment compared to the physical examination segment) support the conclusion that the observational systems, indeed, differentiated the baseline and physical examination conditions based on pain. Interestingly, the correlation of the PACSLAC‐II with self‐reported pain accounted for a greater portion of the variance than the corresponding correlation involving the FACS indices (although these two correlations were not significantly different from each other). It is also of interest that the PACSLAC‐II Facial Reactions subscale accounted for far more variance in differentiating painful movement from baseline (partial η2 = 0.504) than any other subscale of the checklist including the body movements section (partial η2 = 0.144), suggesting that body movements were much less important than the facial responses assessed by the PACSLAC‐II. Finally, the methodology that we used, involving comparisons of movement exacerbated pain with a quiet baseline period, has been used previously with success in the validation of a variety of successful non‐verbal pain assessment tools in populations with limited ability to communicate (Husebo et al., 2007; Horgas et al., 2009; Ersek et al., 2010; Lints‐Martindale et al., 2012). Despite the past support for our comparative methodology, the lack of significant patient movement during baseline represents a significant limitation of our study. That is, despite the mitigating factors mentioned above, the movement difference between baseline and pain condition underscores the need for caution when considering the conclusions of this investigation. As such, it would be important for future research to cross‐validate the PACSLAC‐II and the FACS in situations involving comparisons of painful versus non‐painful movements.
In this investigation, our goal was to compare independent community‐dwelling older adults to seniors with moderate/severe dementia residing in long‐term care facilities. Cognitive functioning was not assessed in the independently functioning community sample both due to practical constraints and because we were not intending to investigate any of our variables in relation to specific levels of cognitive functioning. Nonetheless, examination of specific levels of participant cognitive status, in relation to pain reactions, would be of interest to investigate in future research.
It is also important to note that some PACSLAC‐II behaviours were not observed in the brief acute‐phasic, movement exacerbated situation that we studied in this context. For example, it is not possible to observe changes in activity patterns such as sleep within our study protocol. Nonetheless, such reactions to pain are commonly observed, assessed and have clinical utility in long‐term care environments where patients are assessed over a wide range of situations and conditions as well as over time (Kaasalainen et al., 2013).
This is the first study that validated the PACSLAC‐II in a clinical situation involving a standardized protocol of movements that was kept constant across participants. Previous research involving this tool (Chan et al., 2014) involved study of pain‐related movements that were not identical or standardized across participants. The standardized approach (Husebo et al., 2007) is not only important because it provides a standardized situation in which to evaluate the tool but also preliminary normative information that can allow for clinical comparisons of a patient's score on the tool to those of a group of patients giving the clinician (who might use the same movement protocol) a new context in which to interpret the score. Of course, information from larger samples would be needed before firmer norms could be established. In future research, it might also be useful to compare clinically useful pain assessment tools other than the PACSLAC‐II to fine‐grained FACS‐based methods.
Author contributions
TH, BT, KP and AM prepared grant applications that funded the study. TH, BT, AM, KP and AA developed the clinical data collection protocol. TH oversaw the data collection protocol and played a major role in study conceptualization, interpretation and manuscript preparation. AA and BT oversaw technical aspects of data and video processing. MEB completed the data analysis, assisted with the supervision/training of research assistants and wrote portions of the manuscript. KP helped train research assistants with data coding, conducted reliability calculations and contributed to the conceptualization of the coding approach. All authors discussed the results, read, edited and approved the final manuscript.
Acknowledgements
We are grateful for the research assistance of Odell Tan, Amy Hampton, Delaine Ammaturo, Natasha Gallant and Brooke Hoffman. Portions of this paper were presented at the 2017 Annual Convention of the Canadian Pain Society, Halifax, Nova Scotia.
Funding sources
This study was supported by a grant from the Canadian Institutes of Health Research (CIHR) and a grant from the AGE‐WELL Network of National Centres of Excellence.
Conflicts of interest
TH is one of the copyright holders of the PACSLAC‐II (one of the tools considered in this investigation) but has no commercial interest in the PACSLAC‐II. There are no other conflicts to declare.
References
- AGS Panel on Persistent Pain in Older Persons (2002). The management of persistent pain in older persons. J Am Geriatr Soc 50, S205–S224. [DOI] [PubMed] [Google Scholar]
- Ammaturo, D.A. , Hadjistavropoulos, T. , Williams, J. (2017). Pain in dementia: Use of observational pain assessment tools by people who are not health professionals. Pain Med 18, 1895–1907. [DOI] [PubMed] [Google Scholar]
- Aubin, M. , Giguère, A. , Hadjistavropoulos, T. , Verreault, R. (2007). The systematic evaluation of instruments designed to assess pain in persons with limited ability to communicate. Pain Res Manag 12, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S. , Hadjistavropoulos, T. , Williams, J. , Lints‐Martindale, A. (2014). Evidence‐based development and initial validation of the pain assessment checklist for seniors with limited ability to communicate‐II (PACSLAC‐II). Clin J Pain 30, 816–824. [DOI] [PubMed] [Google Scholar]
- Corbett, A. , Husebo, B. , Malcangio, M. , Staniland, A. , Cohen‐Mansfield, J. , Aarsland, D. , Ballard, C. (2012). Assessment and treatment of pain in people with dementia. Nat Rev Neurol 8, 264–274. [DOI] [PubMed] [Google Scholar]
- Craig, K.D. , Prkachin, K.M. , Gruneau, R.E. (2011). In Turk D.C. and Melzack R., Handbook of pain assessment (3rd Edition), pp. 117–133. New York: Guilford Press. [Google Scholar]
- Ekman, P. , Friesen, W.V. (1978). Manual for the Facial Action Coding System (Palo Alto, CA: Consulting Psychologists Press; ). [Google Scholar]
- Ekman, P. , Friesen, W.V. , Hager, J.C. (2002). Facial Action Coding System (FACS). Salt Lake City, UT: Research Nexus. [Google Scholar]
- Ersek, M. , Herr, K. , Neradilek, M.B. , Buck, H.G. , Black, B. (2010). Comparing the psychometric properties of the Checklist of Nonverbal Pain Behaviors (CNPI) and the Pain Assessment in Advanced Dementia (PAIN‐AD) instruments. Pain Med 11, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein, M.F. , Folstein, S.E. , McHugh, P.R. (1975). “Mini‐mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gallant, N.L. , Hadjistavropoulos, T. (2017). Experiencing pain in the presence of others: A structured experimental investigation of older adults. J Pain 18, 456–467. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos, T. , LaChapelle, D.L. , MacLeod, F.K. , Snider, B. , Craig, K.D. (2000). Measuring movement‐exacerbated pain in cognitively impaired frail elders. Clin J Pain 16, 54–63. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos, T. , LaChapelle, D.L. , Hadjistavropoulos, H.D. , Green, S. , Asmundson, G.J. (2002). Using facial expressions to assess musculoskeletal pain in older persons. Eur J Pain 6, 179–187. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos, T. , Herr, K. , Turk, D.C. , Fine, P.G. , Dworkin, R.H. , Helme, R. , Jackson, K. , Parmelee, P.A. , Rudy, T.E. , Beattie, B.L. , Chibnal, J.T. , Craig, K.D. , Ferrell, B. , Fillingim, R.B. , Gagliese, L. , Gallagher, R. , Gibson, S.G. , Harisson, L. , Katz, B. , Keefe, F. , Lieber, S.J. , Lussier, D. , Schmader, K.E. , Tait, R.C. , Weiner, D.K. , Williams, J. (2007). An interdisciplinary expert consensus statement on assessment of pain in older persons. Clinical Journal of Pain 23, S1–S43. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos, T. , Herr, K. , Prkachin, K.M. , Craig, K.D. , Gibson, S.J. , Lukas, A. , Smith, J.H. (2014). Pain assessment in elderly adults with dementia. Lancet Neurol 13, 1216–1227. [DOI] [PubMed] [Google Scholar]
- Hartmaier, S.L. , Sloane, P.D. , Guess, H.A. , Koch, G.G. , Mitchell, C.M. , Phillips, C.D. (1995). Validation of the minimum data set cognitive performance scale: Agreement with the mini‐mental state examination. J Gerontol A Biol Sci Med Sci 50, M128–M133. [DOI] [PubMed] [Google Scholar]
- Herr, K. , Bursch, H. , Ersek, M. , Miller, L.L. , Swafford, K. (2012). Use of pain‐behavioral assessment tools in the nursing home: Expert consensus recommendations for practice. J Gerontol Nurs 36, 18–29. [DOI] [PubMed] [Google Scholar]
- Horgas, A.L. , Elliott, A.F. , Marsiske, M. (2009). Pain assessment in persons with dementia: Relationship between self‐report and behavioral observation. J Am Geriatr Soc 57, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebo, B.S. , Strand, L.I. , Moe‐Nilssen, R. , Husebo, S.B. , Snow, A.L. , Ljunggren, A.E. (2007). Mobilization‐Observation‐Behavior‐Intensity‐Dementia Pain Scale (MOBID): Development and validation of a nurse‐administered pain assessment tool for use in dementia. J Pain Symptom Manage 34, 67–80. [DOI] [PubMed] [Google Scholar]
- Husebo, B.S. , Strand, L.I. , Moe‐Nilssen, R. , Husebo, S.B. , Aarsland, D. , Ljunggren, A.E. (2008). Who suffers most? Dementia and pain in nursing home patients: A cross‐sectional study. J Am Med Dir Assoc 9, 427–433. [DOI] [PubMed] [Google Scholar]
- Husebo, B.S. , Strand, L.I. , Moe‐Nilssen, R. , Husebo, S.B. , Ljunggren, A.E. (2010). Pain in older persons with severe dementia. Psychometric properties of the Mobilization–Observation–Behaviour–Intensity–Dementia (MOBID‐2) Pain Scale in a clinical setting. Scand J Caring Sci 24, 380–391. [DOI] [PubMed] [Google Scholar]
- Jakobsson, U. , Hallberg, I.R. , Westergren, A. (2004). Pain management in elderly persons who require assistance with activities of daily living: A comparison of those living at home with those in special accommodations. Eur J Pain 8, 335–344. [DOI] [PubMed] [Google Scholar]
- Jensen, M.P. & Karoly, P. (2011). Self‐report scales: Procedures for assessing pain in adults. In Turk D.C. and Melzack R. Handbook of pain assessment (3rd Edition), pp. 19–44. New York: Guilford Press. [Google Scholar]
- Kaasalainen, S. , Middleton, J. , Knezacek, S. , Hartley, T. (1998). Pain and cognitive status in the institutionalized elderly. J Gerontol Nurs 24, 24–31. [DOI] [PubMed] [Google Scholar]
- Kaasalainen, S. , Akhtar‐Danesh, N. , Hadjistavropoulos, T. , Zwakhalen, S. , Verreault, R. (2013). A comparison between behavioral and verbal report pain assessment to tools for use with residents in long‐term care. Pain Manag Nurs 14, e106–e114. [DOI] [PubMed] [Google Scholar]
- Kunz, M. , Mylius, V. , Schepelmann, K. , Lautenbacher, S. (2004). On the relationship between self‐report and facial expression of pain. J Pain 5, 368–376. [DOI] [PubMed] [Google Scholar]
- Kunz, M. , Scharmann, S. , Hemmeter, U. , Schepelmann, K. , Lautenbacher, S. (2007). The facial expression of pain in patients with dementia. Pain 133, 221–228. [DOI] [PubMed] [Google Scholar]
- Lints‐Martindale, A.C. , Hadjistavropoulos, T. , Barber, B. , Gibson, S.J. (2007). A psychophysical investigation of the facial action coding system as an index of pain variability among older adults with and without Alzheimer's disease. Pain Med 8, 678–689. [DOI] [PubMed] [Google Scholar]
- Lints‐Martindale, A.C. , Hadjistavropoulos, T. , Lix, L.M. , Thorpe, L. (2012). A comparative investigation of observational pain assessment tools for older adults with dementia. Clin J Pain 28, 226–237. [DOI] [PubMed] [Google Scholar]
- Lukas, A. , Barber, J. , Johnson, P. , Gibson, S. (2013). Observer‐rated pain assessment instruments improve both the detection of pain and the evaluation of pain intensity in people with dementia. Eur J Pain 17, 1558–1568. [DOI] [PubMed] [Google Scholar]
- Martin, R. , Williams, J. , Hadjistavropoulos, T. , Hadjistavropoulos, H.D. , MacLean, M. (2005). A qualitative investigation of seniors’ and caregivers’ views on pain assessment and management. Can J Nurs Res 37, 142–165. [PubMed] [Google Scholar]
- McGrath, P.A. , Seifert, C.E. , Speechley, K.N. , Booth, J.C. , Stitt, L. , Gibson, M.C. (1996). A new analogue scale for assessing children's pain: An initial validation study. Pain 64, 435–443. [DOI] [PubMed] [Google Scholar]
- Morris, J.N. , Fries, B.E. , Mehr, D.R. , Hawes, C. , Phillips, C. , Mor, V. , Lipsitz, L.A. (1994). MDS cognitive performance scale. J Gerontol 49, M174–M182. [DOI] [PubMed] [Google Scholar]
- Noldus Information Technology (2015). Observer XT. Noldus Information Technology: Wegeningen, The Netherlands. [Google Scholar]
- Paquay, L. , Lepeleire, J.D. , Schoenmakers, B. , Ylieff, M. , Fontaine, O. , Buntinx, F. (2007). Comparison of the diagnostic accuracy of the Cognitive Performance Scale (Minimum Data Set) and the Mini‐Mental State Exam for the detection of cognitive impairment in nursing home residents. Int J Geriatr Psychiatry 22, 286–293. [DOI] [PubMed] [Google Scholar]
- Pautex, S. , Herrmann, F.R. , Michon, A. , Giannakopoulos, P. , Gold, G. (2007). Psychometric properties of the Doloplus‐2 observational pain assessment scale and comparison to self‐assessment in hospitalized elderly. Clin J Pain 23, 774–779. [DOI] [PubMed] [Google Scholar]
- Prkachin, K.M. (1992). The consistency of facial expressions of pain: A comparison across modalities. Pain 51, 297–306. [DOI] [PubMed] [Google Scholar]
- Prkachin, K.M. , Solomon, P.E. (2008). The structure, reliability and validity of pain expression: Evidence from patients with shoulder pain. Pain 139, 267–274. [DOI] [PubMed] [Google Scholar]
- Reynolds, K.S. , Hanson, L.C. , DeVellis, R.F. , Henderson, M. , Steinhauser, K.E. (2008). Disparities in pain management between cognitively intact and cognitively impaired nursing home residents. J Pain Symptom Manage 35, 388–396. [DOI] [PubMed] [Google Scholar]
- Scherder, E.J. , Bouma, A. (2000). Visual analogue scales for pain assessment in Alzheimer's disease. Gerontology 46, 47–53. [DOI] [PubMed] [Google Scholar]
- Sheu, E. , Versloot, J. , Nader, R. , Kerr, D. , Craig, K.D. (2011). Pain in the elderly: Validity of facial expression components of observational measures. Clin J Pain 27, 593–601. [DOI] [PubMed] [Google Scholar]
- Warden, V. , Hurley, A.C. , Volicer, L. (2003). Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc 4, 9–15. [DOI] [PubMed] [Google Scholar]
- Williams, C.S. , Zimmerman, S. , Sloane, P.D. , Reed, P.S. (2005). Characteristics associated with pain in long‐term care residents with dementia. Gerontologist 45, 68–73. [DOI] [PubMed] [Google Scholar]
- Won, A.B. , Lapane, K.L. , Vallow, S. , Schein, J. , Morris, J.N. , Lipsitz, L.A. (2004). Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc 52, 867–874. [DOI] [PubMed] [Google Scholar]


