Abstract
Background
Monoamine oxidases (MAOs) are outer mitochondrial membrane flavoenzymes. They catalyze the oxidative deamination of a variety of neurotransmitters. MAO-A and MAO-B may be considered as targets for inhibitors to treat neurodegenerative diseases and depression and for managing symptoms associated with Parkinson’s and Alzheimer’s diseases.
Purpose
The objective was to evaluate the inhibitory effect of Hypericum afrum and Cytisus villosus against MAO-A and B and to isolate the compounds responsible for the MAO-inhibitory activity.
Methods
The inhibitory effect of extracts and purified constituents of H. afrum and C. villosus were investigated in vitro using recombinant human MAO-A and B, and through bioassay-guided fractionation of ethyl acetate fractions of areal parts of the two plants collected in northeastern Algeria. In addition, computational protein-ligand docking and molecular dynamics simulations were carried out to explain the MAO binding at the molecular level.
Results
The ethyl acetate (EtOAc) fractions of H. afrum and C. villosus showed the highest MAO inhibition activity against MAO A and B with IC50 values of 3.37 μg/ml and 13.50 μg/ml as well as 5.62 and 1.87 μg/ml, respectively. Bioassay-guided fractionation of the EtOAc fractions resulted in the purification and identification of the known flavonoids quercetin, myricetin, genistein and chrysin as the principal MAO-inhibitory constituents. Their structures were established by extensive 1 and 2D NMR studies and mass spectrometry. Quercetin, myricetin and chrysin showed potent inhibitory activity towards MAO-A with IC50 values of 1.52, 9.93 and 0.25 μM, respectively, while genistein more efficiently inhibited MAO-B (IC50 value: 0.65 μM). The kinetics of the inhibition and the study of dialysis dissociation of the complex of quercetin and myricetin and the isoenzyme MAO-A showed competitive and mixed inhibition, respectively. Both compounds showed reversible binding. Molecular docking experiments and molecular dynamics simulations allowed to estimate the binding poses and to identify the most important residues involved in the selective recognition of molecules in the MAOs enzymatic clefts.
Conclusion
Quercetin and myricetin isolated from H. afrum together with genistein and chrysin isolated from C. villosus have been identified as potent MAO-A and -B inhibitors. H. afrum and C. villosus have properties indicative of potential neuroprotective ability and may be new candidates for selective MAO-A and B inhibitors.
Keywords: Antidepressant, Bioassay-guided fractionation, HR-ESI-MS, Molecular docking, Monoamine oxidase, NMR
Graphical Abstract
Introduction
Monoamine oxidases A and B (MAOs) are outer mitochondrial membrane enzyme catalyzing the oxidative deamination of a variety of neurotransmitters. Inside the human brain, there are two isoforms of MAO, i.e. MAO-A and MAO-B based on their sensitivity to particular substrates and modulators. MAO-A preferentially deaminates serotonin and norepinephrine, whereas MAO-B deaminates phenylethylamine and benzylamine. MAO-A is selectively inhibited by clorgyline (O’Brien et al., 1994), while MAO-B is preferentially inactivated by L-deprenil (Magyar and Szende, 2004) MAO-A and MAO-B have been considered as targets for neurodegenerative diseases, including Parkinson’s and Alzheimer’s diseases (Thomas, 2000; Yamada and Yasuhara, 2004) and for new antidepressants and anxiolytics.
Recent studies are focusing on new selective MAO-A or MAO-B inhibitors. Selective MAO-A inhibitors are useful in the management of depression and mood disorders (Marek and Seiden, 1988; Sacher et al., 2011), whereasMAO-B inhibitors are useful for the treatment of Alzheimer’s disease and Parkinson’s disease (Dézsi and Vécsei, 2017; Gökhan-Kelekçi et al., 2007; Rabey et al., 2000). Several medicinal plants used in folk medicine have been suggested as an important source for the treatment of depression, Parkinson’s disease and other neuropsychiatric as well as neurological disorders (Akhondzadeh et al., 2003; Saki et al., 2014). Consequently, the inhibitory effects of natural products, especially the phenolic compounds, on MAOs have attracted more interests in the last years. Flavonoids have been reported to be the responsible compounds for the antidepressant effects of herbal medicines (Butterweck et al., 2000). The inhibition of the MAOs activity of several medicinal plants may support their traditional use for the treatment of depression, neuropsychiatric and neurological disorders (Carradori et al., 2014; Fugh-Berman and Cott, 1999).
Hypericum belongs to the family of Hypericaceae, which is a widespread genus. A large number of Hypericum species have been used in folk medicine. Hypericum is a prolific source of various arrays of secondary metabolites with several biological activities (Nahrstedt and Butterweck, 1997; Patočka, 2003). Chemical and biological features of several species of Hypericum have been studied for their clinical efficacy in mild to moderate depression (Bladt and Wagner, 1994; Thiede and Walper, 1994). Flavonoids and other different compounds in Hypericum plants inhibited MAO (Demirkiran, 2012; Wang et al., 2010). H. afrum species is native to Algeria (Quézel et al., 1962). This plant grows as a shrub or herbaceous plant depending on its biological adaptation to the dampness of the environment. The plant reaches about 1.6 m high and blooms between June to July permitting the collection of samples in both two forms in the El Kala region. However, studying new Hypericum species is attractive to investigate new active secondary metabolites that may act as MAO inhibitors.
Cytisus genus belonging to the Fabaceae family, is traditionally used as diuretic and in the treatment of mild hypertension. A decoction of its leaves is used with lime for chest complaints (Iwu, 2014). The most known Cytisus scoparius, which has a very high medicinal value and is widely used in traditional Chinese medicine (TCM), is taken to nourish Yin and invigorate the heart and liver (Sundararajan and Koduru, 2014). According to previous studies, Cytisus genus contain a high content of polyphenol compounds, that can explain their bioactivities as antioxidant, cytoprotective, diuretic, hypnotic, anxiolytic, antiparasitic and antidiabetic (Pereira et al., 2013; Raja et al., 2007; Saraiva et al., 2013). C. villosus is a 1–2 m high shrub which spreads many twigs. The flowering takes place in April-May (Quézel et al., 1962). The plant frequently grows in Algeria, France, Italy, Spain, Portugal, and Tunisia. In Algeria, it is common in the region of the Tell Algéro-constantinois (Quézel et al., 1962). C. villosus was used by the rural population as effective remedy for wounds. However, to the best of our knowledge, there are no studies on the antidepressant activity and on the phytochemicals of C. villosus.
Therefore, we investigated the MAO inhibitory activity of H. afrum and C. villosus and identified the main active principles from these two species.
Material and methods
General experimental procedures
Melting points were determined on an Opti-Melt automated melting point system (Stanford Research Systems) and were uncorrected. IR spectra were recorded using an Agilent model Cary 630 FT-IR. Optical rotations were recorded using a Rudolph Research Analytical Autopol V Polarimeter. UV was obtained using a Perkin-Elmer Lambda 3B UV/vis-spectrophotomer. 1H and 13C NMR spectra were obtained on Bruker model AMX 500 NMR spectrometer with standard pulse sequences, operating at 400 MHz in 1H and 100 MHz in 13C. The chemical shift values were reported in parts per million units (ppm) from trimethylsilane (TMS) using known solvent chemical shifts. Coupling constants were recorded in Hertz (Hz). Standard pulse sequences were used for COSY, HMQC, HMBC, NOESY and DEPT. High-resolution mass spectra (HRMS) were measured on a Micromass Q-Tof Micro mass spectrometer with a lock spray source. Column chromatography was carried out on silica gel (70–230 mesh, Merck, Germany), C-18 SPE and SPE columns (500 mg Bed, Thermo Scientific, USA), Diaion HP-20 (Sorbetch Technologies, Norcross, USA) and Sorbadex 20-LH (SorbetchTechnologies). TLC (silica gel 60 F254) was used to monitor fractions from column chromatography. Preparative TLC was carried out on silica gel 60 PF254+366 plates (20 × 20 cm, 1 mm thick). Visualization of the TLC plates was achieved with a UV lamp (254 and 365 nm). Spots were visualized by spraying the TLC plates with 10% sulfuric acid in EtOH.
Plant material
The aerial parts of Hypericum afrum (Lam.) were collected from El Kala region, El Tarf, in the northeastern Algeria in July 2011. A voucher specimen (UM-10012014) has been deposited in the culture collection of the Department of BioMolecular Sciences, University of Mississippi. The aerial parts of Cytisus villosus (Pourr.) were collected from the Collo region, in northeastern Algeria in April 2010. A voucher specimen (UM-10232015) has been deposited in the culture collection of the Department of BioMolecular Sciences, University of Mississippi. The samples were identified by Dr. Djamila Belouahem-Abed from the National Institute of Forest Research (INRF).
Extraction and isolation
Dried powdered aerial parts (1000 g) of H. afrum were macerated three times at room temperature with ethanol-water (EtOH–H2O; 80:20, v/v) for 24 h. The obtained filtered crude extracts were combined and evaporated under vacuum at a temperature of 40 °C to give a residue (30 g). The obtained dry extract was suspended in water (800 ml) and successively partitioned with chloroform (CHCl3), ethyl acetate (EtOAc) and n-butanol (n-but), yielding 1 g (CHCl3), 7 g (EtOAc) and 12 g (n-but) fractions. Among all obtained fractions, the ethyl acetate (EtOAc) fraction showed the highest MAO inhibition activity against MAO A and B. The ethyl acetate (EtOAc) fraction showed good inhibition for MAO-A with IC50 value of 3.37 μg/ml and lower MAO inhibitory activity against MAO-B with IC50 value of 13.50 μg/ml. The EtOAc fraction (7 g) was fractionated on a silica gel column and eluted with CH2Cl2/MeOH solvent system of increasing polarity to yield 10 subfractions according to their TLC behavior. Subfractions F-1 to F-10 were tested in vitro against recombinant human MAO-A and MAO-B. F-1(100% CH2Cl2); F-2(9:1 CH2Cl2); F-3(8:2 CH2Cl2–MeOH); F-4(7:3 CH2Cl2-MeOH) were the most active subfractions on MAO-inhibitory bioassay with IC50 values of (16.56 μg/ml and 1.93 μg/ml), (6.18 and 3.05 μg/ml), (2.17 μg/ml and 4.12 μg/ml) and (9.49 μg/ml and 18.41 μg/ml) for MAO-A and MAO-B, respectively.
All active subfractions were further fractionated to isolate the bioactive pure compounds except of subfraction F-1 and F-2 due to a lack of sufficient amounts. The subfraction F-3 (115 mg) was re-chromatographed on column of sephadex LH-20 with CH2Cl2/MeOH (1:1) as eluent yielding compound 1 (16 mg) as a yellow precipitate. The subfraction F-4 (125 mg) was rechromatographed on sephadex LH-20 column with methanol and further purified by preparative TLC eluted with CHCl3/MeOH (10:1) to afford compound 2 (15 mg) as a yellow powder.
Dried powdered aerial parts (1000 g) of Cytisus villosus were macerated three times at room temperature with EtOH–H2O (80:20, v/v) for 24 h. The obtained filtered extracts were combined and evaporated under vacuum at a temperature of 40 °C to give a residue (25 g). The obtained dry crude extract was suspended in water (800 ml) and successively partitioned with chloroform (CHCl3), ethyl acetate (EtOAc) and n-butanol (n-but), yielding 500 mg (CHCl3), 5 g (EtOAc) and 10g (n-butanol) fractions. Among all obtained C. villosus fractions, the ethyl acetate extract fraction (5 g) showed good inhibition for MAO-A and MAO-B with IC50 values of 5.62 and 1.87 μg/ml, respectively. The ethyl acetate (EtOAc) fraction was slurried with an equal amount of celite, dried, powdered and subjected to silica gel which was eluted initially with CH2Cl2-MeOH (95:5), then subjected to gradient elution with CH2Cl2-MeOH (90:10), (85:15), (80:20), (50:50), (20:80) and finally with 100% MeOH. The subfractions Fr-1 to Fr-7 were evaluated in vitro against recombinant human MAO-A and MAO-B. Fr-1 (95:5 CH2Cl2-MeOH): Fr-2 (90:10 CH2Cl2-MeOH); Fr-3 (85:15 CH2Cl2 –MeOH) were the most active fractions on MAO-inhibitory bioassay. Subfraction Fr-1 showed the highest MAO-A and –B inhibition with IC50 values of 0.80 and 0.15 μg/ml, respectively. Subfraction Fr-2 showed good MAO-A and –B inhibition with IC50 values of 2.47 and 1.19 μg/ml, respectively. The subfraction Fr-3 showed MAO inhibitory activity with IC50 values of 7.22 μg/ml for MAO-A and 11.53 μg/ml for MAO-B. These active subfractions were further fractionated except of subfraction F-1 due to a lack of sufficient amounts.
The subfraction Fr-2 (170 mg) was subjected to Sephadex LH-20 using MeOH as an eluent to afford compound 3 (genistein, 5 mg). Subfraction Fr-3 (161 mg) was subjected to sephadex LH-20 using MeOH as an eluent to furnish compound 4 (4 mg).
Determination of MAOs inhibition effects
In vitro assays were performed to measure the inhibitory effects of C. villosus and H. afrum EtOAc fractions and its purified compounds on human recombinant MAO-A and -B activity. The ethyl acetate extract (0.001 to 100 μg/ml), purified compounds (10−9 to 10−2 M) and standard MAO inhibitors (phenelzine, clorgyline and deprenyl) (10−12 to 10−5 M) were tested on human MAO-A and B enzymes (Chaurasiya et al., 2016). The MAO-A and B activities were determined by fluorometric kynuramine deamination assay set up in clear 384 well plates (Parikh et al., 2002). The enzyme reactions were carried out in 0.1 M potassium phosphate buffer (pH-7.4). The reaction mixtures (total volume 75 μL) contained 5 μg/ml of MAO-A (18.75 μl in buffer) or 12.5 μg/ml of MAO-B (18.75 μl in buffer). The reaction mixtures with buffer/substrate/inhibitor were pre-incubated for 10 min at 37 °C, followed by the addition of MAO-A and B to initiate the reaction. The plates were incubated for 20 min at 37 °C and the enzymatic reaction was stopped by the addition of 28 μl of 2N NaOH to each well. The deaminated product of kynuramine, which spontaneously cyclizes to 4-hydroxyquinoline was, measured fluorometrically at 320 nm excitation and 380 nm emission wavelengths with a plate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA). The MAO-A and B inhibition activities were determined using fixed concentration of the substrate (MAO-A 80 μM and MAO-B 50 μM) and varying concentration of the test compounds (0.01 μM to 100 μM). The IC50 values were computed by XL-Fit® from the dose-response inhibition curves.
Enzyme kinetics and mechanism of inhibition of compounds 1 and 2 with MAO-A
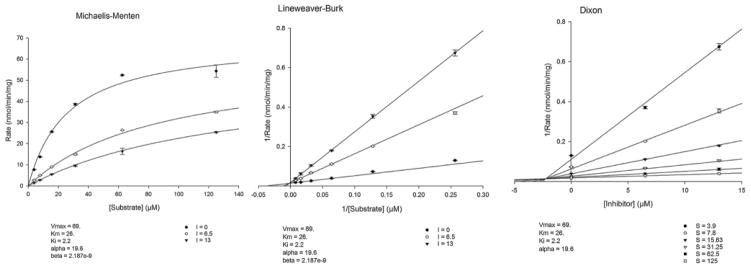
Quercetin (1) and myricetin (2) were selected for the inhibition kinetic studies with MAO-A and -B. For the enzyme kinetics analysis, the assays were performed at varying concentrations of kynuramine (1.90 μM to 500 μM) for the determination of the enzyme inhibition constants (Ki) for inhibition of MAO-A by quercetin and myricetin. In addition to controls without inhibitors, two concentrations (one below and one above the IC50 values) of the inhibitors [for MAO-A phenelzine (0.450 μM and 0.900 μM), quercetin and myricetin (0.125 μM and 0.250 μM) were tested. The results are presented as double reciprocal Lineweaver-Burk plots and the kinetic data namely KM, Vmax and Ki values were calculated by SigmaPlot 12.3 with Enzyme-Kinetics module using Michaelis-Menten equation. The results were also analyzed for the type of inhibition.
Equillibrium dialysis assay for analysis of binding of compounds 1 and 2 with MAO-A
Binding and inhibition of MAO-A with quercetin (1), and myricetin (2) were examined by incubating the enzyme with high concentrations of the inhibitor followed by an extensive dialysis of the enzyme-inhibitor complex and the recovery of enzyme activities. MAO-A (0.05 mg/ml protein) was incubated with quercetin and myricetin (20 μM and 100.0 μM) in an enzyme incubation mixture of 1 ml containing 100 mM potassium phosphate buffer (pH 7.4). Formation of enzyme-inhibitor complex was allowed by incucation of the reaction mixtures for 20 min at 37 °C. The enzyme-inhbitor mixtures after the incubation were chilled and dialyzed against 25 mM potassium phosphate buffer (pH 7.4) for 14–16 h at 4 °C (three times changing buffer). The enzyme catalytic activities were mesured before and after dialysis.
Time-dependent enzyme inhibition assay
MAO-A enzyme was pre-incubated for different time periods (0–15 min) with the inhibitor. The concentrations of the inhibitor tested for time-dependent inhibition with 20 μg/ml MAO-A was for quercetin and myricetin (7.50 μM), and phenelzine (0.600 μM). Controls without inhibitors were also run simultanoesly. The enzyme activities were deteremined as described above.
Molecular modeling study
Ligand preparation
The three dimensional (3D) structures of all ligands were created using LigPrep with OPLS2005 force field and charges. All possible ionization, tautomerization and protonation states were generated with Epik (Greenwood et al., 2010; Shelley et al., 2007) at target pH of 7.4. Stereochemical information were retained during ligand preparation because all stereogenic centers of the ligands are assigned. One low energy ring conformation was allowed per ligand. We kept the lowest energy conformer for each ligand.
Protein preparation
Protein structural files of the human MAO-A were acquired from the Protein Data Bank (PDB codes: 2BXR (De Colibus et al., 2005), 2BXS (De Colibus et al., 2005), 2Z5X (Son et al., 2008), 2Z5Y (Son et al., 2008)). The protein structures were prepared by operating the Protein Preparation Wizard (Sastry et al., 2013) (PrepWizard) of Schrödinger suite to assign bond orders, to add all missing hydrogen atoms, and to fill in missing side chains and loops with Prime (Jacobson et al., 2002; Jacobson et al., 2004). Those water molecules that are located within 5 Å from the native ligand and are forming at least two hydrogen bonds with non-waters were retained. The hydrogen bonding network was assigned and optimized by considering possible water orientations, minimizing the hydrogens of altered species and by sampling the flips for Asn, Gln, and His. The protein-ligand complexes were relaxed with Impref using OPLS2005 force field.
Receptor grid preparation
The receptor grids were generated using Glide version 6.9 (Friesner et al., 2004). The binding pocket was defined by the centroid of the cognate ligand. Ligand protein covalent bonds in crystal structures (PDB ID: 2BXR and 2BXS) were broken during protein preparation to allow for consistent receptor grid preparation.
Docking simulations
The ligands were docked into the generated receptor grids employing Glide with standard precision (SP) scoring option. Ensemble docking approach was applied utilizing four receptor grids. The docking poses were optimized and the best scoring pose was reserved.
Molecular dynamics simulations
DESMOND (Bowers et al., 2006; Shivakumar et al., 2010) software was used for the molecular dynamics (MD) simulations. The best scoring poses of quercetin (1) and myricetin (2) in complex with MAO-A (PDB code: 2Z5Y) were selected and solvated with a TIP4P water solvent model. We used an orthorhombic simulation box with dimensions of 90 Å × 90 Å × 90 Å. The appropriate numbers of solvent molecules were calculated as 19860 and 19861 for quercetin and myricetin, respectively. Sodium and chloride ions were added based on the total charge. The solvated complexes were energy-minimized with the DESMOND minimization algorithm for 5000 iterations considering convergence threshold of 1.0 kcal/mol/Å. Short MD simulations were performed before the production step to acquire further structural relaxation. The production step was executed for 40 ns using NPT ensemble, Nosé–Hoover chain thermostat, and Martyna–Tobias–Klein barostat (Bowers et al., 2006). A time step of 1 fs was used for the RESPA integrator. The short range Coulombic interactions cutoff was set to 9 Å and the Particle Mesh Ewald (PME) method (Darden et al., 1993) was used to treat the long-range electrostatics. The M_SHAKE algorithm was used to constrain the hydrogen bonds. The snapshots (frames) were saved at intervals of 4.8 ps.
Results
Bioassays guided-fractionation and MAOs Inhibitory Properties of H. afrum and C. villosus extracts, fractions and compounds 1–4
All obtained fractions of H. afrum and C. villosus were evaluated in vitro against recombinant human MAO-A and MAO-B, whereby the ethyl acetate (EtOAc) fractions of the two plants showed potent MAO-A and B inhibitory activities with IC50 values of 3.37 μg/ml and 5.62 μg/ml for MAO-A and IC50 values of 13.50 μg/ml and 1.87 μg/ml for MAO-B, respectively (Table 1). The inhibition of MAO-A by EtOAc fraction of H. afrum was 4-fold more potent (IC50: 3.37 μg/ml) as compared to the inhibition of MAO-B (IC50 value of 13.50 μg/ml), while the inhibition of MAO-B (IC50 1.87 μg/ml) by EtOAc fraction of C. villosus was 3-fold more potent as compared to the inhibition of MAO-A (IC50 value of 5.62 μg/ml) (Table 1).
Table 1.
Inhibition of recombinant human MAO-A and MAO-B by H. afrum and C. villosus fractions, subfractions and pure constituents
| Samples | MAO-A | MAO-B |
|---|---|---|
| IC50 value | IC50 value | |
| EtOAc fraction (H. afrum)# | 3.37 ± 0.03 | 13.50 ± 0.71 |
| EtOAc fraction (C. villosus)# | 5.62 ± 0.11 | 1.87 ± 0.03 |
| Subfraction F-1 (H. afrum)# | 16.56 ± 0.85 | 1.93 ± 0.21 |
| Subfraction F-2 (H. afrum)# | 6.18 ± 0.62 | 3.05 ± 0.15 |
| Subfraction F-3 (H. afrum)# | 2.17 ± 0.01 | 4.12 ± 0.59 |
| Subfraction F-4 (H. afrum)# | 9.49 ± 0.15 | 18.41 ± 0.33 |
| Subfraction Fr-1 (C. villosus)# | 0.80 ± 0.05 | 0.15 ± 0.014 |
| Subfraction Fr-2 (C. villosus)# | 2.47 ± 0.26 | 1.19 ± 0.03 |
| Subfraction Fr-3(C. villosus)# | 7.22 ± 1.44 | 11.53 ± 1.95 |
| Quercetin* | 1.52 ± 0.09 | 28.39 ± 5.41 |
| Myricetin* | 9.93 ± 0.63 | 59.34 ± 1.78 |
| Genistein* | 2.74 ± 0.01 | 0.65 ± 0.11 |
| Chrysin* | 0.25 ± 0.04 | 1.04 ± 0.17 |
| Phenelzine*a | 0.27 ± 0.03 | 0.14 ± 0.02 |
| Clorgyline*b | 0.007 ± 0.0005 | ---- |
| Deprenyl*c | ---- | 0.05 ± 0.01 |
Positive control for both MAO enzyme;
Positive control selective for MAO-A;
selective for MAO-B.
Shows inhibition = μM and
shows inhibition = (μg/ml)
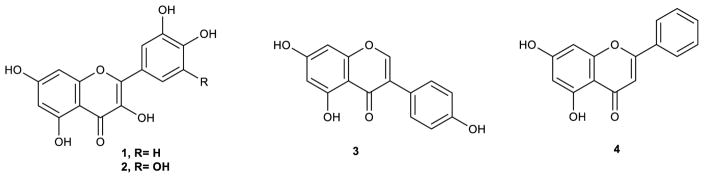
Bioassay-guided fractionation resulted in the isolation and identification of quercetin (1), myricetin (2), genistein (3) and chrysin (4) as the active constituents (Fig. 1). The structures of the compounds were confirmed by comparison of their 1H and 13C-NMR, and mass spectroscopic data with published data (Baoliang et al., 1993; Schmidt et al., 2008; Zdunić et al., 2011).
Fig. 1.
Structures of the identified compounds isolated from H. afrum and C. villosus
Quercetin (1) and myricetin (2) isolated from H. afrum were the most potent compounds with a selective inhibitory activity towards MAO-A. Quercetin demonstrated 18-fold preferential MAO-A inhibitory activity, with IC50 values of 1.52 μM against MAO-A and 28.39 μM against MAO-B. Myricetin showed 6-fold preferential MAO-A inhibitory activity with IC50 values of 9.93 μM and 59.34 μM against MAO-A and MAO-B respectively (Table 1, Fig. 1).
Genistein (3) and chrysin (4) isolated from C. villosus exhibited the highest MAO inhibitory activities. Genistein was shown to be more potent towards MAO-B than MAO-A with IC50 value of 0.65 μM against MAO-B and IC50 value of 2.74 μM against MAO-A. Chrysin was found to produce more pronounced inhibition of MAO-A than MAO-B with IC50 value of 0.25 μM against MAO-A and IC50 value of 1.04 μM against MAO-B (Table 1, Fig. 1).
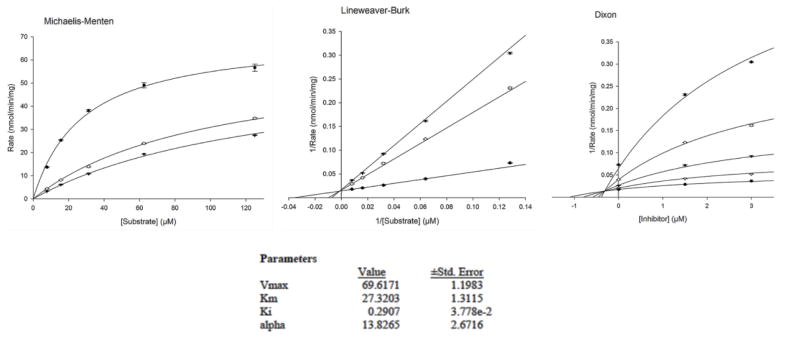
Enzyme Kinetics, Mechanism of Inhibition and Analysis of Time-Dependent Enzyme Inhibition of Compounds 1 and 2 with Monoamine oxidases isoenzymes A and B
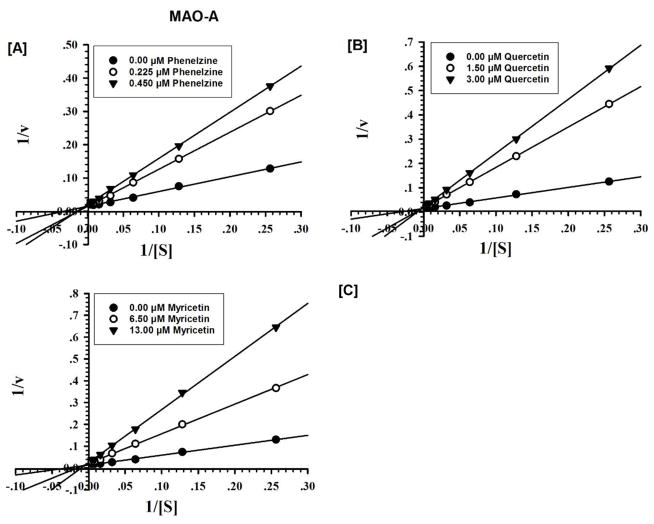
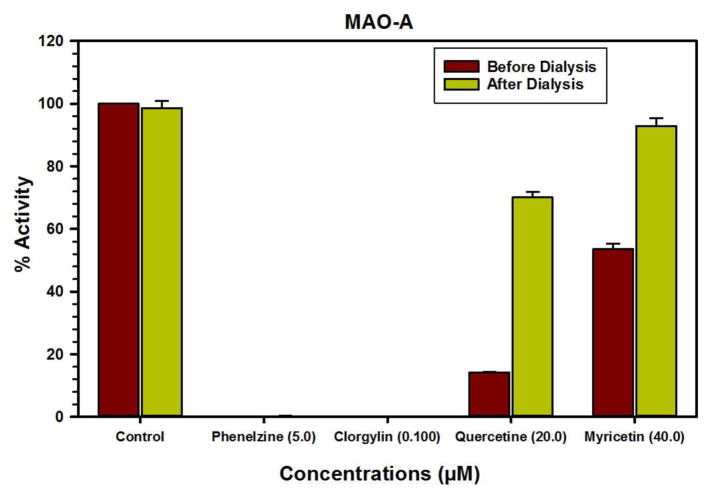
We further evaluated the kinetics and mechanism of inhibition of human MAO-A isoenzyme by quercetin and myricetin (Fig. 2 and 3). To comprehend the type of inhibition, we examined quercetin and myricetin against MAO-A at varying concentrations of kynuramine, a non-selective substrate. Two concentrations of quercetin and myricetin were preferred; one above and another one below the corresponding IC50 values. For each experiment, three sets of assays were done at variable concentrations of the substrate: two concentrations of the inhibitor/compound and one control without inhibitors. The results are expressed as double reciprocal Lineweaver-Burk plots and the kinetic data namely, KM, Vmax and Ki values were computed by SigmaPlot version 12.3 with Enzyme-Kinetics module using Michaelis-Menten equation (Table 2, Fig. 4 and 5). Quercetin bound as competitive inhibitor with human MAO-A. However, myricetin was a mixed-type MAO-A inhibitor (Fig. 3). The enzyme activities were examined before and after dialysis. Through overnight dialysis, the recombinant human MAO-A enzyme lost about 10–15% of activity. Incubation of MAO-A with 20.0 and 100 μM concentrations of quercetin and myricetin inhibited more than 65% of the enzyme activity (Fig. 6). These observations suggested reversible inhibition of MAO-A by both compounds due to the dissociable nature of their enzyme-inhibitor complexes.
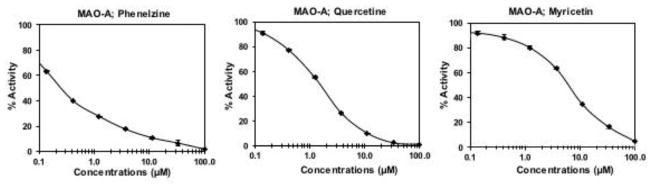
Fig. 2.
Inhibition dose response curves (IC50 values) of recombinant human monoamine amine oxidase-A by quercetin, myricetin and phenelzine (% activity vs. concentration).
Fig. 3.
Kinetic characteristics of inhibition of recombinant human MAO-A with [A] phenelzine [B] quercetin; [C] myricetin; (V = nmoles/min/mg protein and S = substrate kynuramine concentration (μM).
Table 2.
Inhibition/binding affinity constants (Ki) values for inhibition of recombinant human MAO-A by quercetin, myricetin and phenelzine.
| Compounds | Monoamine oxidase-A | |
|---|---|---|
|
| ||
| Ki (μM) | Type of Inhibition | |
| Quercetin | 0.29 ± 0.03 | Competitive/Reversible |
| Myricetin | 2.24 ± 0.25 | Mixed/Reversible |
| Clorgyline | 0.0018 ± 0.0002 | Mixed/Irreversible |
| Phenelzine | 0.121 ± 0.0061 | Mixed/Irreversible |
Values are mean ± S.D. of triplicate experiments.
Fig. 4.
The inhibition of recombinant human monoamine amine oxidase-A with Myricetin
Fig. 5.
The inhibition of recombinant human monoamine amine oxidase-A with Quercetin
Fig. 6.
Analysis of nature of binding of quercetin and myricetin with recombinant human MAO-A by recovery of catalytic activity of the enzyme after dialysis dissociation. Each bar shows mean ± S.D. of triplicate values.
Comparison of the IC50 values of isolated compounds indicated the structural requirements for MAO activity. The presence of an extra hydroxy group in ring B as in myricetin comparing to quercetin reduced the activity (Table 1).
In Silico based Analysis of Interactions of compounds 1–4 with MAO-A and MAO-B
Computational studies were carried out to substantiate the MAO-A inhibition of compounds 1 to 4. Docking experiments and molecular dynamics simulations were performed to identify the residues involved in ligand recognition of compounds.
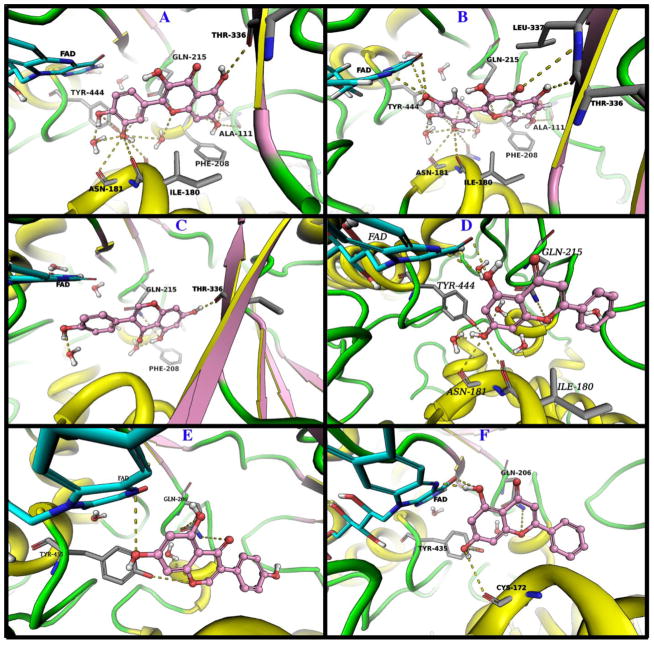
Quercetin (1) showed a favorable docking pose in MAO-A with a docking score of −11.3 kcal/mol. Quercetin interacts with the amino acid residues Ala111, Ile180, Asn181, Phe208, Gln215, Thr336, and Tyr444 and water molecules inside the ligand binding pocket (Fig. 7). It also forms π-π stacking with FAD. The polar groups of quercetin are in appropriate positions to create hydrogen bonds with several residues in the binding site.
Fig. 7.
The binding modes of quercetin (Panel A), myricetin (Panel B), genistein (Panel C) and chrysin (Panel D) are shown in MAO-A, and the binding modes of genistein (Panel E) and chrysin (Panel F) are displayed in MAO-B. Ligands are shown as pink balls and sticks. The interacting amino acids are shown as grey sticks. Protein is shown as cartoon with yellow helices, pink strands and green loops. All possible hydrogen bonds in the range of 3.5 Å are displayed as yellow dots.
Myricetin (2) has a docking score of −9.8 in MAO-A (Fig. 7). Myricetin forms strong hydrogen bonds with Ala111, Ile180, Asn181, and Thr336. Solvent molecules in the binding pocket play an important role in ligand stabilization as demonstrated by strong hydrogen bonds. Myricetin showed hydrogen bonds and π-π stacking with FAD. Energetically, the binding pose of quercetin is more favorable than that of myricetin, however, both compounds fitted well inside the ligand binding pocket of MAO-A.
The best docking pose of genistein (3) in MAO-A exhibited a score of −10.4 kcal/mol (Fig. 7). Several strong hydrogen bonds and π-π interactions are contributing to ligand binding. Genistein interacted with Phe208, Gln215 and Thr336. Chrysin (4) showed a docking score of −13.34 kcal/mol in MAO A (Fig. 7). The extra hydroxy group in genistein led to a considerable difference in the binding affinity against MAO-A. The 4-hydroxyphenyl of genistein could form hydrogen bonds with water molecules inside the binding pocket or with the surrounding amino acid residues. This increased polarity seemed not to be favorable for tight binding. In the case of chrysin, there was no 4-hydroxy group and the ligand oriented itself inside the binding pocket to have strong interactions with FAD, Ile180 and Asn181.
Genistein (3) and chrysin (4) displayed docking scores of −13.51 and −12.22 kcal/mol in MAO-B, respectively (Fig. 7). The binding pocket of MAO-B had different thermodynamics to allow for higher polarity on the phenyl group compared to that of MAO-A. Both compounds showed favorable interactions with the amino acid residues, water molecules and co-factor in the binding pocket.
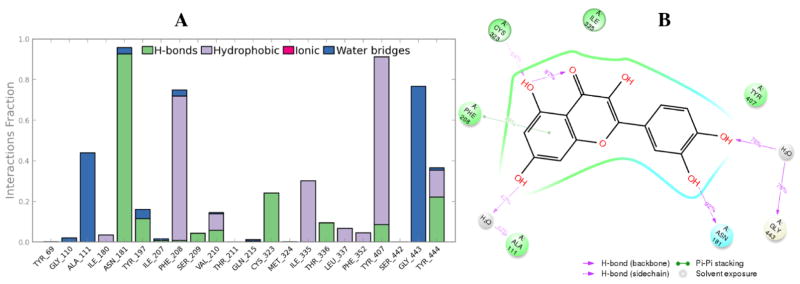
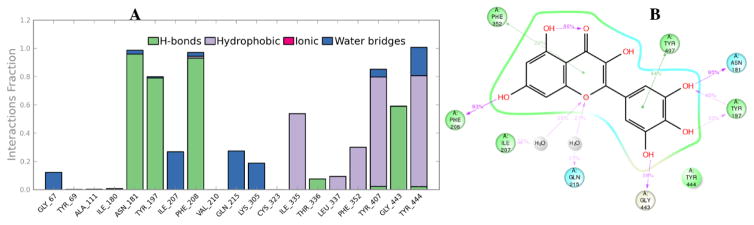
The binding modes and interaction profiles of quercetin (1) and myricetin (2), were further investigated by performing MD simulations for 40 ns. The binding pose of quercetin was stable during the course of MD as demonstrated by the fluctuations of the root mean square deviation (RMSD) of less than 1 Å. Several hydrogen bonds, hydrophobic contacts and interactions bridged by hydrogen bonded water molecules were found to be preserved during the MD simulation time (Fig. 8). An intramolecular hydrogen bond was traced for 87% of the simulation time. Three stable hydrogen bonds were observed with Ala111, Asn181and Gly443. In addition, a π-π interaction was observed with Phe208 for 29% of the simulation time. In the case of myricetin the fluctuations of RMSD through the simulation time was less than 1 Å indicating stability of the docking pose. Similar to quercetin, a long-lived intramolecular hydrogen bond was monitored. Some interactions were found to be well-kept (Fig. 9) such as hydrogen bonds with Asn181, Tyr197, Phe208 and Gly443. In addition, Phe352 and Tyr407 showed stable π-π contacts with myricetin.
Fig. 8.
Protein-ligand contacts of quercetin (Panel A). Four types of protein-ligand interactions were monitored throughout the simulation: hydrogen bond, hydrophobic, ionic and water bridges. 2D interaction diagram (Panel B) of the detailed ligand atom interactions of quercetin with the surrounding amino acid residues of MAO-A.
Fig. 9.
Protein-ligand contacts of myricetin (Panel A). Hydrogen bonds, hydrophobic, ionic and water bridges were monitored throughout the simulation. 2D interaction diagram of myricetin (Panel B) with the amino acid residues of binding site of MAO-A.
Discussion
Several flavonoids such as quercetin, kaempferol, luteolin, apigenin, naringenin, galangin and other flavanoids isolated from different natural resources inhibited MAO. Unfortunately, the IC50 values obtained from different studies cannot be always compared, as the assays have been performed under different conditions (Bandaruk et al., 2012; Lee et al., 2000; Olsen et al., 2008; Sloley et al., 2000). Quercetin, one of the major plant flavonoids, and structurally related flavonoids possess several biological and pharmacological activities, including anti-inflammatory, anti-oxidant effects and cytotoxic potentials (Choiprasert et al., 2010; Maciel et al., 2013). Quercetin exhibits neuroprotection properties (Zhang et al., 2011) and is a identified as potent MAO-A inhibitor (Bandaruk et al., 2014; Chimenti et al., 2006). Myricetin and its derivatives are very common in many plants, fruits and vegetables, and also in foods and beverages. It displays a range of biological activities, such as anti-oxidant, anti-cancer and anti-inflammatory activities (Dimas et al., 2000; Sun et al., 2012; Wu et al., 2016). Although myricetin was recently investigated for its protective effects on brain injury and neurological deficits (Domitrović et al., 2015), the MAO inhibitory effect of myricetin has not been investigated as yet. Genistein is an isoflavone isolated from soybean and several medicinal plants. Gensitein is largely studied because of its pharmacological activities (Suthar et al., 2001), it plays a role in breast cancer prevention (Lamartiniere, 2000). Chrysin is one of the important natural plant flavonoids with multiple biological activities, including antioxidant, anti-inflammatory, anti-aging and anticancer (Anand et al., 2012; Samarghandian et al., 2011). They are also potent MAO-A and B inhibitors (Jäger and Saaby, 2011; Zarmouh et al., 2016).
Conclusion
The inhibitory effect of H. afrum and C. villosus fractions and pure isolated compounds trough bio-guided fractionation on human monoamine oxidases was investigated. The results of our study revealed that both studied plants have properties indicative of potential neuroprotective ability. They may serve as new candidates for selective MAO-A and B inhibitors. The MAO-inhibiting activity of H. afrum and C. villosus fractions was primarily due to the presence of flavonoids such as quercetin, myricetin, genistein and chrysin. The computational docking and thermodynamic analysis of MAO-A and –B complexed with quercetin, myricetin, genistein and chrysin supported the experimental results regarding for inhibition of MAO-A and MAO-B.
Supplementary Material
Acknowledgments
The project described was partially supported by grant number P20GM104932 from the National Institute of General Medical Sciences (NIGMS) a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the NIH. Furthermore, this investigation was conducted in a facility constructed with support from research facilities improvement program C06RR14503 from the NIH National Center for Research Resources (NCRR).
Abbreviations
- BuOH
n-Butanol
- CC
Chromatographiccolumn
- CH2CL2
Dichloromethane
- CHCl3
Chloroform
- EtOAc
Ethyl acetate
- EtOH
Ethanol
- FAD
Flavin adenine dinucleotide
- HRSM
High-resolution mass spectroscopy
- MAO
Monoamine oxidase
- MAO-A
Recombinant human MAO-A inhibitor
- MAO-B
Recombinant human MAO-B inhibitor
- MeOH
Methanol
- TLC
thin layer chromatography
Footnotes
Conflict of interest
The authors declare there are no conflict of interest.
Chart for the fractionation workflow, 1D spectra and HRESIMS of quercetin, myricetin, genistein and chrysin.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Desmond Molecular Dynamics System, version 4.4. D. E. Shaw Research; New York, NY: 2015. [Google Scholar]
- Maestro-Desmond Interoperability Tools, version 4.4. Schrödinger; New York, NY: 2015. [Google Scholar]
- Epik, version 3.4. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- Glide, version 6.9. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- LigPrep, version 3.6. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- Prime, version 4.2. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- Schrödinger Suite 2015-4 Protein Preparation Wizard; Epik version 3.4. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]; Impact version 6.9. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]; Prime version 4.2. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi A, Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomised, placebo controlled trial. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:863–866. doi: 10.1136/jnnp.74.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KV, Jaabir M, Sultan M, Thomas PA, Geraldine P. Protective role of chrysin against oxidative stress in d-galactose-induced aging in an experimental rat model. Geriatrics & gerontology international. 2012;12:741–750. doi: 10.1111/j.1447-0594.2012.00843.x. [DOI] [PubMed] [Google Scholar]
- Bandaruk Y, Mukai R, Kawamura T, Nemoto H, Terao J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. Journal of agricultural and food chemistry. 2012;60:10270–10277. doi: 10.1021/jf303055b. [DOI] [PubMed] [Google Scholar]
- Bandaruk Y, Mukai R, Terao J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicology Reports. 2014;1:639–649. doi: 10.1016/j.toxrep.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baoliang C, Motoyuki N, Junei K. Chemical constituents of Astragali semen [J] Chem Pharm Bull. 1993;41:178–182. [Google Scholar]
- Bladt S, Wagner H. Inhibition of MAO by fractions and constituents of hypericum extract. Journal of geriatric psychiatry and neurology. 1994;7:S57–S59. doi: 10.1177/089198879400700115. [DOI] [PubMed] [Google Scholar]
- Bowers KJ, Chow E, Xu H, Dror RO, Eastwood MP, Gregersen BA, Klepeis JL, Kolossvary I, Moraes MA, Sacerdoti FD. Scalable algorithms for molecular dynamics simulations on commodity clusters. SC 2006 Conference, Proceedings of the ACM/IEEE; IEEE; 2006. pp. 43–43. [Google Scholar]
- Butterweck V, Jürgenliemk G, Nahrstedt A, Winterhoff H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta medica. 2000;66:3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- Carradori S, D’Ascenzio M, Chimenti P, Secci D, Bolasco A. Selective MAO-B inhibitors: a lesson from natural products. Molecular diversity. 2014;18:219–243. doi: 10.1007/s11030-013-9490-6. [DOI] [PubMed] [Google Scholar]
- Chaurasiya ND, Gogineni V, Elokely KM, León F, Núñez MJ, Klein ML, Walker LA, Cutler SJ, Tekwani BL. Isolation of Acacetin from Calea urticifolia with Inhibitory Properties against Human Monoamine Oxidase-A and-B. Journal of Natural Products. 2016;79:2538–2544. doi: 10.1021/acs.jnatprod.6b00440. [DOI] [PubMed] [Google Scholar]
- Chimenti F, Cottiglia F, Bonsignore L, Casu L, Casu M, Floris C, Secci D, Bolasco A, Chimenti P, Granese A. Quercetin as the Active Principle of Hypericum h ircinum Exerts a Selective Inhibitory Activity against MAO-A: Extraction, Biological Analysis, and Computational Study. Journal of natural products. 2006;69:945–949. doi: 10.1021/np060015w. [DOI] [PubMed] [Google Scholar]
- Choiprasert W, Dechsupa N, Kothan S, Garrigos M, Mankhetkorn S. Quercetin, quercetrin except rutin potentially increased pirarubicin cytotoxicity by non-competitively inhibiting the P-glycoprotein-and MRP1 function in living K562/adr and GLC4/adr cells. American Journal of Pharmacology and Toxicology. 2010;5:24–33. [Google Scholar]
- Darden T, York D, Pedersen L. Particle mesh Ewald: An N· log (N) method for Ewald sums in large systems. The Journal of chemical physics. 1993;98:10089–10092. [Google Scholar]
- De Colibus L, Li M, Binda C, Lustig A, Edmondson DE, Mattevi A. Three-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12684–12689. doi: 10.1073/pnas.0505975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkiran O. Three new benzophenone glycosides with MAO-A inhibitory activity from Hypericum thasium Griseb. Phytochemistry Letters. 2012;5:700–704. [Google Scholar]
- Dézsi L, Vécsei L. Monoamine oxidase B inhibitors in Parkinson’s disease. CNS & neurological disorders drug targets. 2017 doi: 10.2174/1871527316666170124165222. [DOI] [PubMed] [Google Scholar]
- Dimas K, Demetzos C, Angelopoulou D, Kolokouris A, Mavromoustakos T. Biological activity of myricetin and its derivatives against human leukemic cell lines in vitro. Pharmacological Research. 2000;42:475–478. doi: 10.1006/phrs.2000.0716. [DOI] [PubMed] [Google Scholar]
- Domitrović R, Rashed K, Cvijanović O, Vladimir-Knežević S, Škoda M, Višnić A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chemico-biological interactions. 2015;230:21–29. doi: 10.1016/j.cbi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. Journal of medicinal chemistry. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Fugh-Berman A, Cott JM. Dietary supplements and natural products as psychotherapeutic agents. Psychosomatic Medicine. 1999;61:712–728. doi: 10.1097/00006842-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Gökhan-Kelekçi N, Yabanoğlu S, Küpeli E, Salgın U, Özgen Ö, Uçar G, Yeşilada E, Kendi E, Yeşilada A, Bilgin AA. A new therapeutic approach in Alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorganic & medicinal chemistry. 2007;15:5775–5786. doi: 10.1016/j.bmc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Greenwood JR, Calkins D, Sullivan AP, Shelley JC. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. Journal of computer-aided molecular design. 2010;24:591–604. doi: 10.1007/s10822-010-9349-1. [DOI] [PubMed] [Google Scholar]
- Iwu MM. Handbook of African medicinal plants. CRC press; 2014. [Google Scholar]
- Jacobson MP, Friesner RA, Xiang Z, Honig B. On the role of the crystal environment in determining protein side-chain conformations. Journal of molecular biology. 2002;320:597–608. doi: 10.1016/s0022-2836(02)00470-9. [DOI] [PubMed] [Google Scholar]
- Jacobson MP, Pincus DL, Rapp CS, Day TJ, Honig B, Shaw DE, Friesner RA. A hierarchical approach to all-atom protein loop prediction. Proteins: Structure, Function, and Bioinformatics. 2004;55:351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- Jäger AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–1485. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. The American journal of clinical nutrition. 2000;71:1705s–1707s. doi: 10.1093/ajcn/71.6.1705S. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Chung HY, Lee IK, Oh SU, Yoo ID. Phenolics with inhibitory activity on mouse brain monoamine oxidase (MAO) from whole parts of Artemisia vulgaris L (Mugwort) Food Science and Biotechnology. 2000;9:179–182. [Google Scholar]
- Maciel R, Costa M, Martins D, Franca R, Schmatz R, Graca D, Duarte M, Danesi C, Mazzanti C, Schetinger M. Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Research in veterinary science. 2013;95:389–397. doi: 10.1016/j.rvsc.2013.04.028. [DOI] [PubMed] [Google Scholar]
- Magyar K, Szende B. (−)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology. 2004;25:233–242. doi: 10.1016/S0161-813X(03)00102-5. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Seiden LS. Selective inhibition of MAO-A, not MAO-B, results in antidepressant-like effects on DRL 72-s behavior. Psychopharmacology. 1988;96:153–160. doi: 10.1007/BF00177554. [DOI] [PubMed] [Google Scholar]
- Nahrstedt A, Butterweck V. Biologically active and other chemical constituents of the herb of Hypericum perforaturn L. Pharmacopsychiatry. 1997;30:129–134. doi: 10.1055/s-2007-979533. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Tipton K, Meroni M, Dostert P. Amine Oxidases: Function and Dysfunction. Springer; 1994. Inhibition of monoamine oxidase by clorgyline analogues; pp. 295–305. [DOI] [PubMed] [Google Scholar]
- Olsen HT, Stafford GI, van Staden J, Christensen SB, Jäger AK. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. Journal of ethnopharmacology. 2008;117:500–502. doi: 10.1016/j.jep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Parikh S, Hanscom S, Gagne P, Crespi C, Patten C. A fluorescent-based, high-throughput assay for detecting inhibitors of human Monoamine Oxidase A and B. BD Bioscience Discovery Labware. 2002 S02T081R082. [Google Scholar]
- Patočka J. The chemistry, pharmacology, and toxicology of the biologically active constituents of the herb Hypericum perforatum L. Journal of Applied Biomedicine. 2003;1:61–70. [Google Scholar]
- Pereira OR, Macias RI, Perez MJ, Marin JJ, Cardoso SM. Protective effects of phenolic constituents from Cytisus multiflorus, Lamium album L. and Thymus citriodorus on liver cells. Journal of Functional Foods. 2013;5:1170–1179. [Google Scholar]
- Quézel P, Santa S, Schotter O. Nouvelle flore de l’Algerie et des regions desertiques meridionales-v. 1962:1–2. [Google Scholar]
- Rabey J, Sagi I, Huberman M, Melamed E, Korczyn A, Giladi N, Inzelberg R, Djaldetti R, Klein C, Berecz G. Rasagiline mesylate, a new MAO-B inhibitor for the treatment of Parkinson’s disease: a double-blind study as adjunctive therapy to levodopa. Clinical neuropharmacology. 2000;23:324–330. doi: 10.1097/00002826-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Raja S, Ahamed KN, Kumar V, Mukherjee K, Bandyopadhyay A, Mukherjee PK. Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. Journal of ethnopharmacology. 2007;109:41–47. doi: 10.1016/j.jep.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sacher J, Houle S, Parkes J, Rusjan P, Sagrati S, Wilson AA, Meyer JH. Monoamine oxidase A inhibitor occupancy during treatment of major depressive episodes with moclobemide or St. John’s wort: an [11C]-harmine PET study. Journal of Psychiatry and Neuroscience. 2011;36:375. doi: 10.1503/jpn.100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki K, Bahmani M, Rafieian-Kopaei M, Hassanzadazar H, Dehghan K, Bahmani F, Asadzadeh J. The most common native medicinal plants used for psychiatric and neurological disorders in Urmia city, northwest of Iran. Asian Pacific Journal of Tropical Disease. 2014;4:S895–S901. [Google Scholar]
- Samarghandian S, Afshari JT, Davoodi S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics. 2011;66:1073–1079. doi: 10.1590/S1807-59322011000600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva SC, Pereira OR, Liberal J, Batista MT, Cruz MT, Cardoso SM. NO radical scavenging and iNOS expression inhibition by Cytisus multiflorus. 47th Annual Scientific Meeting of the European Society for Clinical Investigation; European Society for Clinical Investigation; 2013. [Google Scholar]
- Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. Journal of computer-aided molecular design. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Jaroszewski JW, Bro R, Witt M, Stærk D. Combining PARAFAC analysis of HPLC-PDA profiles and structural characterization using HPLC-PDA-SPE-NMR-MS experiments: Commercial preparations of St. John’s wort. Analytical chemistry. 2008;80:1978–1987. doi: 10.1021/ac702064p. [DOI] [PubMed] [Google Scholar]
- Shelley JC, Cholleti A, Frye LL, Greenwood JR, Timlin MR, Uchimaya M. Epik: a software program for pK a prediction and protonation state generation for drug-like molecules. Journal of computer-aided molecular design. 2007;21:681–691. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- Shivakumar D, Williams J, Wu Y, Damm W, Shelley J, Sherman W. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. Journal of chemical theory and computation. 2010;6:1509–1519. doi: 10.1021/ct900587b. [DOI] [PubMed] [Google Scholar]
- Sloley B, Urichuk L, Morley P, Durkin J, Shan J, Pang P, Coutts R. Identification of kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts of Ginkgo biloba leaves. Journal of pharmacy and pharmacology. 2000;52:451–459. doi: 10.1211/0022357001774075. [DOI] [PubMed] [Google Scholar]
- Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. Structure of human monoamine oxidase A at 2.2-Å resolution: the control of opening the entry for substrates/inhibitors. Proceedings of the National Academy of Sciences. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zheng XY, Ye J, Wu TT, Wang Jl, Chen W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutrition and cancer. 2012;64:599–606. doi: 10.1080/01635581.2012.665564. [DOI] [PubMed] [Google Scholar]
- Sundararajan R, Koduru R. Cytisus scoparius: A review of ethnomedical, phytochemical and pharmacological information. Indo American Journal of Pharmaceutical Research. 2014;4:2151–2169. [Google Scholar]
- Suthar A, Banavalikar M, Biyani M. Pharmacological activities of Genistein, an isoflavone from soy (Glycine max): Part II—Anti-cholesterol activity, effects on osteoporosis & menopausal symptoms. 2001 [PubMed] [Google Scholar]
- Thiede HM, Walper A. Inhibition of MAO and COMT by hypericum extracts and hypericin. Journal of geriatric psychiatry and neurology. 1994;7:S54–S56. doi: 10.1177/089198879400700114. [DOI] [PubMed] [Google Scholar]
- Thomas T. Monoamine oxidase-B inhibitors in the treatment of Alzheimers disease. Neurobiology of aging. 2000;21:343–348. doi: 10.1016/s0197-4580(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Wang D, Bai J, Sun F, Yang D. Chemical constituents and antidepressant activity of the new species Hypericum enshiense occurring in China. Phytomedicine. 2010;17:410–413. doi: 10.1016/j.phymed.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Wu S, Yue Y, Peng A, Zhang L, Xiang J, Cao X, Ding H, Yin S. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food & Function. 2016 doi: 10.1039/c6fo00419a. [DOI] [PubMed] [Google Scholar]
- Yamada M, Yasuhara H. Clinical pharmacology of MAO inhibitors: safety and future. Neurotoxicology. 2004;25:215–221. doi: 10.1016/S0161-813X(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Zarmouh NO, Messeha SS, Elshami FM, Soliman KF. Evaluation of the isoflavone genistein as reversible human monoamine oxidase-a and-b inhibitor. Evidence-Based Complementary and Alternative Medicine. 2016;2016 doi: 10.1155/2016/1423052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdunić G, Gođevac D, Šavikin K, Novaković M, Milosavljević S, Petrović S. Isolation and identification of phenolic compounds from Hypericum richeri Vill. and their antioxidant capacity. Natural product research. 2011;25:175–187. doi: 10.1080/14786410802401390. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Cheang LCV, Wang MW, Lee SMY. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. International journal of molecular medicine. 2011;27:195–203. doi: 10.3892/ijmm.2010.571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.