Abstract
Background
As part of a program to develop a novel estradiol-releasing contraceptive vaginal ring (CVR), we evaluated the pharmacokinetic (PK) profile of CVRs releasing segesterone acetate (Nestorone® (NES)) combined with one of three different estradiol (E2) doses.
Study design
A prospective, double-blind, randomized, multi-centered study to evaluate a 90-day CVR releasing NES [200μg/day] plus E2, either 10μg/day, 20μg/day, or 40μg/day in healthy reproductive-age women with regular cycles. Participants provided blood samples twice weekly for NES and E2 levels during the first 60 days (ring 1) and the last 30 days (ring 2) of use. A subset underwent formal PK assessments at ring initiation, ring exchange (limited PK), and study completion.
Results
The main study enrolled 197 women; 22 participated in the PK substudy. Baseline characteristics between the main and PK participants were comparable, with an average BMI of 25.8 kg/m2 (SD 4.3). In the PK substudy, all three rings showed similar NES PK: mean area under the curve (AUC(0-72)) 34,181 pg*day/mL; concentration maximum (Cmax) 918 pg/mL; time to maximum concentration (Tmax) 3.5 hours. For E2, the Cmax occurred at 2 hours, and was significantly higher with the 20 ug/day ring (mean 390 pg/mL); 10ug/day, 189 pg/mL, p = .003; 40 ug/day, 189 pg/mL, p < .001), and declined rapidly to ≤ 50 pg/mL for all doses by 24 hours. For all subjects, the median E2 levels remained under 35 pg/mL during treatment.
Conclusion
PK parameters of NES were not affected when paired with different doses of E2, but E2 levels from all three doses were lower than anticipated and no dose response was observed.
Implications
While these novel estradiol-releasing combination contraceptive vaginal rings provided sustained release of contraceptive levels of Nestorone over 90 days, the E2 levels achieved were not consistent with bone protection, and a dose-response was not observed.
Keywords: Contraceptive vaginal ring, Nestorone, estradiol, pharmacokinetics, contraception, continuous cycling
Introduction
Unintended pregnancies adversely impact a woman’s life, health, and wellbeing, and contribute to our world’s exponential population growth which is detrimental to the environment. Successful development of contraceptive methods that are highly effective, safe, easy to adhere to, and acceptable to women is essential to counteracting this public health crisis. Reversible methods with longer-acting duration, in contrast to the need for daily, weekly or monthly dosing, have grown in popularity, and their widespread use has been shown to directly decrease unplanned pregnancy and abortion rates within a population [1].
Long-acting reversible contraceptive (LARC) methods require a skilled health care provider for placement and removal. A woman-controlled long-term reversible method would present a novel delivery system. A vaginal ring delivery system releasing segesterone acetate (Nestorone® (NES)), a 19-norprogesterone derivative that binds selectively to progesterone receptors and not androgen receptors [2–4], combined with ethinyl estradiol (EE) designed for use over thirteen cycles (one year) has been tested for safety, efficacy, and bleeding profile [5–7]. This ring provided high efficacy and acceptability similar to existing shorter-acting combined hormonal methods but with the opportunity for longer-term dosing with a single product.
Although NES is not orally active, it is very potent transvaginally and is highly effective at suppressing ovulation [8, 9]. EE induces hepatic protein synthesis and clotting factors when given orally, transdermally, and transvaginally, therefore ring administration provides no safety advantage to existing products [10, 11]. In contrast, 17β-estradiol (E2) does not appear to increase the risk of thrombosis when delivered transdermally at physiologic doses, at least when studied in postmenopausal women [12]. Utilizing E2 rather than EE as the estrogen component in a CVR could improve the safety of combined hormonal contraception in regard to thrombosis risk. Continuous use of the ring may help improve adherence (missed or late next ring use) and thus increase effectiveness.
We present the pharmacokinetic (PK) parameters from a Phase IIa dose-finding study of a 90-day vaginal ring dosed continuously releasing 200μg/day NES and either 10, 20, 40μg/day of E2. These doses were specifically chosen based on previous studies supporting the ongoing CVR development program at the Population Council. Several doses of NES have been evaluated [13] with a dose of 150μg/d selected for development of the NES/ethinyl estradiol (EE) CVR [A New Drug Application for this CVR is currently under review by the U.S. Food and Drug Administration (press release, Population Council: http://www.popcouncil.org/news/population-councils-one-year-contraceptive-ring-advances-to-fda-review)]. Given that the estrogen component of a combined hormonal contraceptive contributes to ovarian suppression [14, 15], we assumed that higher levels of NES would be required for full ovarian suppression with an E2 CVR, due to the lower potency of E2 compared with EE. This led us to select the 200μg/d release for this NES/E2 vaginal ring. When selecting the dose of E2, previous studies demonstrated a less favorable bleeding profile when lower doses of NES, up to 100μg/d were used in combination with higher levels of E2 (>45 μg/day) in a CVR [unpublished data, Population Council]. Our goal was to achieve ovulation suppression with NES while preventing hypoestrogenism with E2.
Materials and Methods
We conducted a prospective multi-centered, double-blind, randomized, Phase IIa dose-finding study sponsored and developed jointly by the Population Council (NY, NY) and the Contraceptive Discovery and Development Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; Bethesda, MD). Eight NICHD Contraceptive Clinical Trials Network (CCTN) (Eastern Virginia Medical School, University of Pennsylvania, University of Pittsburgh, Johns Hopkins University, Oregon Health & Science University (OHSU), University of Cincinnati, New York University, and Columbia University) participated in the study from April 2012 to December 2013. Participants at the OHSU site only were offered the option of participation in a detailed PK substudy. The respective sites’ Institutional Review Boards approved the study protocol and all participants underwent written informed consent. The trial was registered with clinicaltrials.gov (NCT0158600).
Sites recruited healthy, reproductive-aged (18–39 years old) women not at risk for pregnancy (e.g. same-sex partner, heterosexually abstinent, use of a non-hormonal method of contraception or male partner with vasectomy if heterosexually active) and willing to abstain from non-water-based vaginal lubricants during the study. Main inclusion criteria included regular menstrual cycles, intact uterus and ovaries, BMI < 35 kg/m2, no recent use of hormonal contraception, no contraindications to combined hormonal contraception, no use of drugs known to interfere with the metabolism of sex steroids, and smoking <15 cigarettes/day if age <35 or no smoking if age 35 or greater. Once enrolled, women underwent a control cycle (Cycle 1), a treatment period equivalent to six 30-day cycles (Cycles 2–7) and then one post-treatment cycle (Cycle 8). Health Decisions (Research Triangle, NC) provided central randomization for all sites using a computer-generated randomization schedule with random permuted blocks. The OHSU Research Pharmacy provided a separate computer-generated randomization schedule for the PK substudy.
Subjects were randomized 1:1:1 to receive one of three CVRs developed to release NES 200 μg/day and either 10 μg/day, 20 μg/day, or 40 μg/day of E2. The Population Council formulated the silicone elastomer contraceptive vaginal rings (CVR) utilized in this study using a proprietary technology. The overall mean (56.4 mm) and cross-sectional (8.2 mm) diameter of the rings and color (white) did not differ with dose. Each study ring was used for a duration of 3 months (90 days); and subjects used 2 consecutive rings of the same dose over the course of the study (no hormone-free interval). Subjects self-inserted and removed rings during an in-person visit at the study site: Ring 1 between days one and five during Cycle 2, removed at day 90 and replaced by Ring 2, in turn removed on day 180. Subjects received instructions to not remove the ring during the study, including during sexual activity. Subjects recorded compliance with ring use and bleeding events using a paper daily diary; the study coordinator reviewed this at each visit. End of study participation was determined by a subject experiencing a second episode of spontaneous bleeding after ring removal.
All participants underwent twice weekly blood draws during the control cycle, the first 60 days of Ring 1, the week prior to the ring exchange, and the last 30 days of Ring 2 use. PK substudy subjects additionally completed full PK assessments at initiation of Ring 1 [accumulation PK; 0 (ring placement), 2, 4, 6, 8, 10, 12, 24, 48 and 72 hours] and removal of Ring 2 [elimination PK; 0 (ring removal), 2, 4, 6, 8, 10, 12, 24, 48 and 72 hours], and a limited PK evaluation during the exchange of Ring 1 to Ring 2 [0 (ring exchange), 2 and 24 hours]. Site staff allowed blood samples to clot, used a refrigerated centrifuge to separate serum, and stored serum aliquots at −80C prior to shipment to the central labs for analysis.
Assay characteristics
The Biomarkers Core Laboratory of Columbia University’s Irving Institute for Clinical and Translational Research (New York, NY) performed the E2 analysis following liquid-liquid extraction with deuterated internal standards using an ultra-performance Liquid Chromatography-tandem Mass Spectrometry (LC-MSMS) platform. The lower limit of quantification, defined as the level at which the residual of the calibration line is <20% of the expected concentration combined with a signal to noise ratio >10, was determined to be 2.5 pg/ml for E2 with intra-assay precision of 6.30% and inter-assay precision 8.93% (see Supplemental Methods for assay details). NES levels were quantified in serum specimens in the Population Council laboratory using a conventional extraction type radioimmunoassay developed and validated in their laboratory [14]. The lower limit of detection of this assay is 10 pg/mL with interassay and intraassay coefficient of variation less than 15% during validation and NES measurements for the current study.
Data Analysis
The planned sample size of approximately 189 subjects (63 subjects per group) was not based on formal power calculations, but considered appropriate for this descriptive study, assuming a discontinuation rate of 20%. We planned to enroll a minimum of 18 subjects (6 for each E2 dose) in the PK substudy.
We used SAS software version 9.4 for the PK analysis, and summarized the PK parameters (AUC, Cmax, Cmin, Tmax) for NES and E2 for all subjects contributing data for at least 1 PK parameter and included data in the analysis for any time point completed. We calculated the AUC over the full 72 hours of the initial detailed PK study (AUC(0-72)). Additionally, we summarized the half-life (t1/2), clearance and the volume of distribution (VD) for NES. As we expected E2 levels to rise upon discontinuation of follicle suppression by NES, we did not summarize PK elimination parameters for E2. We considered NES level of <40 pg/mL as evidence of noncompliance with ring use.
Results
We enrolled 197 (10μg: n=67, 20μg: n=66, 40μg: n=64) women in the main study; 139 women completed the study. In the PK substudy, 22 women enrolled (10μg: n=7, 20μg: n=7, 40μg: n=8) and 17 completed all study procedures (Figure 1). There were no notable differences in the baseline characteristics between the dosing groups for the main or PK substudy (Table 1). Of note, half of all subjects were either overweight (31%) or obese (19%).
Figure 1.
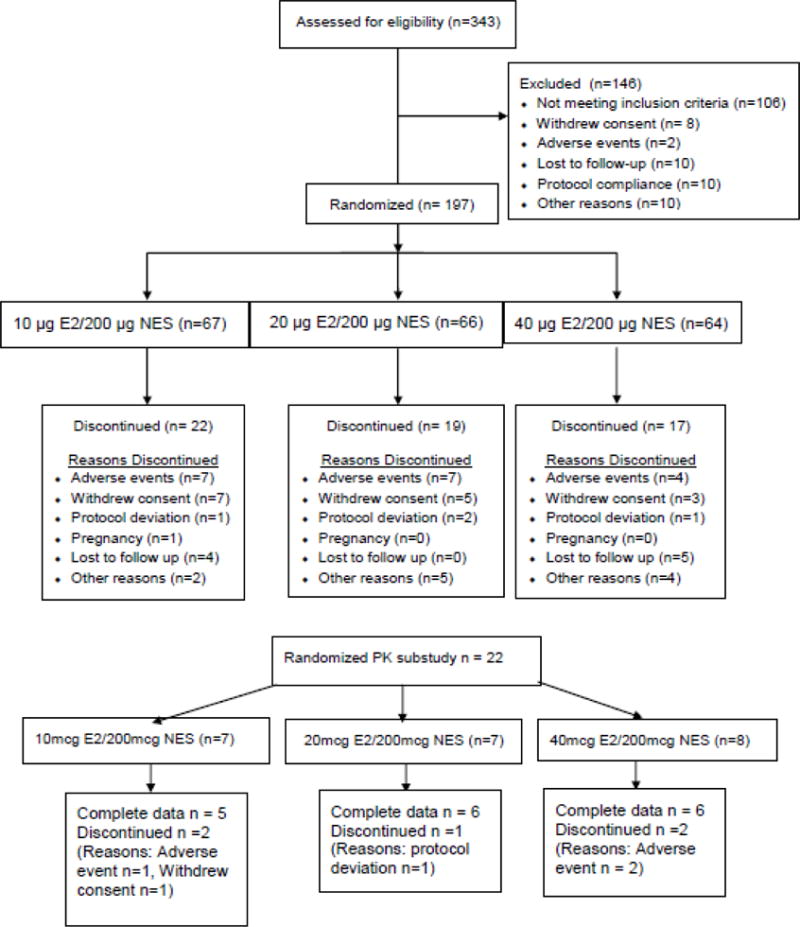
Subject participation in the main study (upper panel) and the PK substudy (lower panel). All PK subjects participated in the main study.
Table 1.
Demographics
| 200 μg/day NES + 10 μg/day E2 n=67 | 200 μg/day NES + 20 μg/day E2 n=66 | 200 μg/day NES + 40 μg/day E2 n=64 | Entire cohort N=197 | |
|---|---|---|---|---|
|
| ||||
| Age (years); mean (SD) | ||||
| All enrolled | 28.8 (5.6) | 28.2 (4.8) | 29.6 (5.8) | 28.8 (5.4) |
| PK substudy | 30.9 (4.3) | 31.9 (5.1) | 31.0 (5.8) | 31.2 (4.9) |
|
| ||||
| Ethnicity (Not Hispanic or Latina) | ||||
| All enrolled | 57 (85%) | 57 (86%) | 54 (84%) | 168 (85.3%) |
| PK substudy | 7 (100%) | 7 (100%) | 8 (100%) | 22 (100%) |
|
| ||||
| Weight (kg); mean (SD) | ||||
| All enrolled | 70.5 (12.4) | 68.4 (12.8) | 69.7 (12.2) | 69.5 (12.5) |
| PK substudy | 64.6 (5.3) | 68.9 (10.8) | 65.9 (7.4) | 66.3 (7.9) |
|
| ||||
| Body Mass Index (kg/m2); mean (SD) | ||||
| All enrolled | 26.4 (4.2) | 25.4 (4.4) | 25.7 (4.2) | 25.8 (4.3) |
| PK substudy | 24.8 (2.2) | 25.5 (4.1) | 25.2 (2.6) | 25.2 (2.9) |
Figure 2 presents the median NES and E2 concentrations for the main study subjects over the first 90 days of Ring 1 and the last 30 days of ring 2 use. NES levels peaked following initial ring placement, and gradually declined over the 90-day treatment intervals; Median NES levels remained stable and consistently above 150 pg/mL with no differences between the three CVRs. In contrast, we did not observe a sustained peak of E2 levels with initiation of any of the CVRs, and concentrations remained under 35 pg/mL throughout the treatment interval with no dose-response for E2. Of note, SHBG (mean for all subjects at baseline = 60.8 nmol/L) declined modestly with CVR use (end of treatment period 2, mean 51.6 nmol/L) with no difference between CVR dose groups.
Figure 2.

Median concentration of Nestorone (NES) and estradiol (E2) observed for the main study cohort using contraceptive vaginal rings releasing 200 mcg NES with either 10, 20, or 40 mcg/day. Sampling occured twice weekly over the first 60 days of ring 1 and the last 30 days of ring 2. Breaks in lines indicates intervals of no data collection (days 61 – 83 and days 91 – 152).
Pharmacokinetic Substudy
Figure 3 and Table 2 presents the detailed PK profile of the three NES/E2 CVRs. Upon initial ring placement, both steroids showed a rapid increase to maximum concentration (Cmax) within 2–4 hours, followed by a continuous decline over the next 24 hours, reaching steady state within 48–72 hours. Ring replacement resulted in a similar peak for NES, but an approximately 50% lower peak for E2. The three CVRs showed similar profiles for NES; pooled results showed an area under the curve over 72 hours (AUC(0-72)) of 34,181 pg*day/mL, concentration maximum (Cmax) 918 pg/mL, and time to maximum concentration (Tmax) of 3.5 hours.
Figure 3.
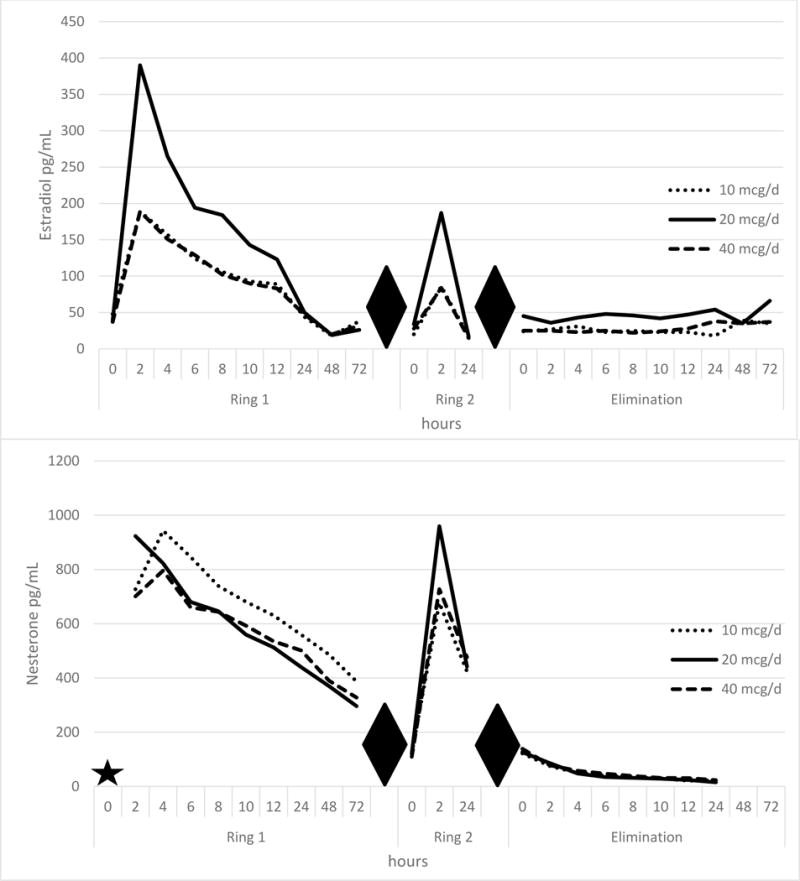
Concentration time curves (PK substudy) for 200 mcg NES with 10,20, or 40 mcg/day E2 rings at ring initiation (ring 1), ring exchange (ring 2), and at ring removal (elimination). Upper panel, estradiol (pg/mL) and lower panel, nestorone (pg/mL). Baseline values (T = 0) for Nestorone not run (indicated by star). Hours reflect specimen time collection from initial ring placement (ring 1), ring replacement (ring 2), and following ring removal (elimination). Diamonds indicate breaks between data collection intervals.
Table 2.
Pharmacokinetic (PK) profiles for estradiol (E2) and Nestorone (NES) from subjects using NES/E2 contraceptive vaginal rings in the PK substudy.
| E2 Mean (SD) | NES Mean (SD) | |||||
|---|---|---|---|---|---|---|
| 200 μg/day NES + 10 μg/day E2 | 200 μg/day NES + 20 μg/day E2 | 200 μg/day NES + 40 μg/day E2 | 200 μg/day NES + 10 μg/day E2 | 200 μg/day NES + 20 μg/day E2 | 200 μg/day NES + 40 μg/day E2 | |
| Cmax (pg/ml)* | 189.3 (71) | 390.4 (113) | 189.3 (45) | 942.9 (265) | 984.3 (287) | 837.5 (137) |
| Cmin (pg/ml)* | 17.6 (8.3) | 15.7 (3.3) | 19.5 (13.9) | 387.1 (121) | 292.9 (76) | 323.8 (117) |
| AUC(0-72) (pg*hour/ml)* | 3690.8 (1414) | 4910.3 (1068) | 3648.8 (1341) | 38685.7 (9770) | 31201.4 (4913) | 32845 (5371) |
| Tmax (hours)* | 2 (0) | 2 (0) | 2 (0) | 4.0 (0) | 2.9 (1) | 3.5 (0.9) |
| Thalf (hours) | 11.7 (9) | 15.6 (7.6) | 18.3 (20) | |||
Elimination profile of E2 not calculated.
AUC calculated for 72-hour interval of extended PK evaluation.
Values are derived from the PK studies at time of ring initiation, the remaining parameters are derived from ring removal.
Unexpectedly, we did not observe a relationship between the increasing E2 dose release of the three CVRs and the serum E2 Cmax or AUC(0-72). The highest values occurred in subjects using the 20 μg/day ring. (See Figure 3 and Table 2)
While serum NES levels declined rapidly following removal of Ring 2 (approaching the limit of detection by 24 hours) E2 levels increased during the elimination period, likely reflecting renewed follicle activity.
Discussion
In this study, we evaluated three release rates of estradiol in combination with Nestorone. Although a NES release rate of 150 μg/d results in adequate ovarian suppression in combination with EE/NES, we selected a NES release rate of 200 μg/d for the E2 CVR development as E2 does not suppress gonadotropins as well as EE (data on file, Population Council). Since this dose of NES results in significant suppression of ovarian E2 production, we evaluated a dose range of release rates of E2 to achieve physiologic replacement to offset effects of hypoestrogenism on key clinical endpoints like bleeding patterns and bone health, while avoiding stimulation of clotting pathways. Although we found that the varying release rates of E2 used in our study CVRs did not affect the NES release rate, the E2 serum levels achieved did not reach a threshold for clinical benefit. We considered an E2 target of ≥ 40 pg/mL as the minimum for bone protection.
We did not observe the expected relationship between the increase in E2 dose of the CVRs and serum E2 levels. This was surprising, given that we did observe the appropriate dose relationship with increasing E2 release during in vitro testing (see supplemental figure) suggesting that the CVR released the hormone as designed. Keeping in mind that the measured serum E2 reflects the sum of the administered dose and endogenous ovarian estradiol production, one explanation for the discrepancy in our steady-state PK results could be that the 200 μg/day NES in combination with 40 μg/day E2 ring dose resulted in greater suppression of the hypothalamic-pituitary-ovarian axis. The lower Cmax with the 40 μg/day ring likely reflects the shorter storage time and design differences compared to the lower dose CVRs that influenced the initial burst [16].
We had several other interesting PK findings. Typically, with contraceptive vaginal rings, subdermal implants, and intrauterine devices, a hormone “peak” or “burst” will be delivered following placement due to accumulation of hormones at the surface while in storage. While we observed a similar peak of NES with initial ring placement and ring replacement, exchange resulted in a significantly lower E2 peak. The significance of this effect is unclear given that we did not achieve our objective of early follicular phase levels of E2 over 12 weeks of ring use. Gabrielsson and colleagues reported a similar finding of a lower E2 peak following ring replacement in their pharmacokinetic evaluation of a very low dose (7.5 μg/d) estradiol-releasing vaginal ring developed for treatment of atrophic vaginitis [17]. Given the very low levels of E2 achieved and lack of first pass metabolism, it is not likely that this effect is due to changes in hormone binding proteins, especially since sex hormone binding globulin did not change significantly from baseline during our study. Prior studies have established that the vaginal epithelium efficiently absorbs estradiol when delivered locally [18]. Local changes in the vagina may have influenced estrogen absorption, but we did not specifically evaluate the vagina in this study.
Vaginal ring delivery of contraceptive steroid hormones offers women a user-controlled non-daily delivery system. Both the currently-approved etonogestrel CVR, and the investigational one-year Nestorone ring that has completed Phase III clinical trials, contain ethinyl estradiol. As EE results in substantial hepatic induction of clotting factors regardless of route of administration, vaginal delivery offers no safety advantage over oral therapy [11]. In contrast, E2 delivered transdermally at physiologic levels does not modify the clotting factors [13] and does not increase the risk of venous thrombosis in postmenopausal women [12]. We hypothesized that vaginal delivery of estradiol in a CVR could improve safety of combined hormonal contraception by reducing hepatic activation of clotting pathways and provide a safe, well-tolerated and highly effective contraceptive option for women at higher risk for thrombosis, such as obese women.
To summarize, we did not achieve our goal of maintaining adequate replacement levels of estradiol in the E2/NES CVRs evaluated in this study. While the release rate of estradiol in the CVRs evaluated in this study did not result in pharmacologic replacement of serum concentrations of E2 to levels that would be physiologic in the follicular phase of healthy premenopausal women, the concept of a novel vaginally-delivered hormonal contraceptive combining a metabolically neutral progestin, such as NES, and a physiological estrogen, such as 17-beta estradiol (E2), remains attractive. Additional studies evaluating CVRs releasing higher doses of E2 in combination with NES are underway.
Supplementary Material
Acknowledgments
The authors would like to thank the Women’s Health Research Unit at Oregon Health & Science University, Sarah Godfrey and Jim Higgins at Health Decisions, and Dr. Jason Woo at the National Institutes of Health.
Financial Support: Support for this research was from NICHD Contraception Clinical Trial: Network HHSN275200403378I (OHSU); HHSN 275201300019I (EVMS); HHSN 275201300020I (UPenn); HHSN27521100043U (UPitt); HHSN275201300002I (NYU); HHSN275201300004I (JHU); HHSN275201300010I (Columbia). The authors also acknowledge grant support from the National Institutes of Health for the OHSU Oregon Clinical & Translational Research Institute (NIH NCRR 1 UL1 RR024120), the Bioanalytical Shared Resource /Pharmacokinetics Core at OHSU, the Biomarkers Core Laboratory of Columbia University’s Irving Institute for Clinical and Translational Research (NIH UL1 TR000040), and the work conducted at the Population Council. The content of this publication is the sole responsibility of the authors and does not necessarily represent the official views of the NIH.
Authors Disclosures: Dr. Jensen has received payments for consulting and research support from Bayer Healthcare, Merck, Agile Therapeutics, Abbvie, HRA Pharma, Teva, and the Population Council, consulting only from MicroChips and Evofem, and research support only from Estetra SPRL and Medicines360. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. Dr. Edelman: consultant for World Health Organization, CDC, Gynuity Health Projects, Genzyme, and Agile Therapeutics. Nexplanon trainer for Merck. Author for UptoDate (Royalties received). Research funding from Merck and National Institutes of Health (NIH). Dr Sitruk-Ware is an employee of the Population Council, a not for profit organization, IND holder for Nestorone formulations, and developer of the vaginal ring described in this paper. Dr. Blithe is an employee of the NIH and involved in a CRADA with HRA Pharma. Dr. Chen has received research support through Agile Therapeutics, Bayer Healthcare, Medicines360, and Merck, all managed through the Magee-Womens Research Institute, and is on a Merck advisory board. Dr. Burke receives research support from Bayer and the OC OTC Working Group, through Johns Hopkins. Dr. Thomas has received research support through Agile Therapeutics, Bayer Healthcare, and Medicines 360, all managed through the University of Cincinnati College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. The New England journal of medicine. 2012;366(21):1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N, Fagart J, Liere P, Mitchell SJ, Knibb AR, Petit-Topin I, et al. Nestorone(R) as a Novel Progestin for Nonoral Contraception: Structure-Activity Relationships and Brain Metabolism Studies. Endocrinology. 2017;158(1):170–82. doi: 10.1210/en.2016-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47(4):277–83. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Sitruk-Ware R. New progestagens for contraceptive use. Human reproduction update. 2006;12(2):169–78. doi: 10.1093/humupd/dmi046. [DOI] [PubMed] [Google Scholar]
- 5.Sivin I, Mishell DR, Jr, Alvarez F, Brache V, Elomaa K, Lahteenmaki P, et al. Contraceptive vaginal rings releasing Nestorone and ethinylestradiol: a 1-year dose-finding trial. Contraception. 2005;71(2):122–9. doi: 10.1016/j.contraception.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Fraser IS, Weisberg E, Brache V, Alvarez F, Massai R, Mishell DR, Jr, et al. Serum Nestorone and ethinyl estradiol levels, and ovulation inhibition in women using three different dosage combinations of a Nestorone progestogen-ethinyl estradiol contraceptive vaginal ring on a bleeding-signaled regimen. Contraception. 2005;72(1):40–5. doi: 10.1016/j.contraception.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Weisberg E, Brache V, Alvarez F, Massai R, Mishell DR, Jr, Apter D, et al. Clinical performance and menstrual bleeding patterns with three dosage combinations of a Nestorone progestogen/ethinyl estradiol contraceptive vaginal ring used on a bleeding-signaled regimen. Contraception. 2005;72(1):46–52. doi: 10.1016/j.contraception.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000;65(10-11):629–36. doi: 10.1016/s0039-128x(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 9.Noe G, Salvatierra A, Heikinheimo O, Maturana X, Croxatto HB. Pharmacokinetics and bioavailability of ST 1435 administered by different routes. Contraception. 1993;48(6):548–56. doi: 10.1016/0010-7824(93)90117-p. [DOI] [PubMed] [Google Scholar]
- 10.Mashchak CA, Lobo RA, Dozono-Takano R, Eggena P, Nakamura RM, Brenner PF, et al. Comparison of pharmacodynamic properties of various estrogen formulations. American journal of obstetrics and gynecology. 1982;144(5):511–8. doi: 10.1016/0002-9378(82)90218-6. [DOI] [PubMed] [Google Scholar]
- 11.Sitruk-Ware R, Plu-Bureau G, Menard J, Conard J, Kumar S, Thalabard JC, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92(6):2074–9. doi: 10.1210/jc.2007-0026. [DOI] [PubMed] [Google Scholar]
- 12.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115(7):840–5. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 13.Brache V, Mishell DR, Lahteenmaki P, Alvarez F, Elomaa K, Jackanicz T, et al. Ovarian function during use of vaginal rings delivering three different doses of Nestorone. Contraception. 2001;63(5):257–61. doi: 10.1016/s0010-7824(01)00199-8. [DOI] [PubMed] [Google Scholar]
- 14.Bono Y, Kyo S, Kiyono T, Mizumoto Y, Nakamura M, Maida Y, et al. Concurrent estrogen action was essential for maximal progestin effect in oral contraceptives. Fertil Steril. 2014;101(5):1337–43. doi: 10.1016/j.fertnstert.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Vandever MA, Kuehl TJ, Sulak PJ, Witt I, Coffee A, Wincek TJ, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77(3):162–70. doi: 10.1016/j.contraception.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. Journal of controlled release : official journal of the Controlled Release Society. 2001;73(2-3):121–36. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 17.Gabrielsson J, Wallenbeck I, Birgerson L. Pharmacokinetic data on estradiol in light of the estring concept. Estradiol and estring pharmacokinetics. Acta obstetricia et gynecologica Scandinavica Supplement. 1996;163:26–31. discussion 2-4. [PubMed] [Google Scholar]
- 18.Martin PL, Greaney MO, Burnier AM, Brooks PM, Yen SS, Quigley ME. Estradiol, estrone, and gonadotropin levels after use of vaginal estradiol. Obstet Gynecol. 1984;63(4):441–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


