Abstract
Trypanosoma brucei possesses a streamlined secretory system that guarantees efficient delivery to the cell surface of the critical GPI-anchored virulence factors, variable surface glycoprotein (VSG) and transferrin receptor (TfR). Both are thought to be constitutively endocytosed and returned to the flagellar pocket via TbRab11+ recycling endosomes. We use conditional knockdown with established reporters to investigate the role of TbRab11 in specific endomembrane trafficking pathways in bloodstream trypanosomes. TbRab11 is essential. Ablation has a modest negative effect on general endocytosis, but does not affect turnover, steady state levels, or surface localization of TfR. Nor are biosynthetic delivery to the cell surface and recycling of VSG affected. TbRab11 depletion also causes increased shedding of VSG into the media by formation of extracellular vesicles. In contrast to GPI-anchored cargo, TbRab11 depletion reduces recycling of the trans-membrane invariant surface protein, ISG65, leading to increased lysosomal turnover. Thus, TbRab11 plays a critical role in recycling of transmembrane, but not GPI-anchored surface proteins. We proposed a two-step model for VSG turnover involving release of VSG containing vesicles followed by GPI-hydrolysis. Collectively, our results indicate a critical role of TbRab11 in the homeostatic maintenance of the secretory/endocytic system of bloodstream T. brucei.
Keywords: Trypanosome, glycosylphosphatidylinositol, transferrin receptor, variant surface glycoprotein, secretory trafficking, recycling, endocytosis
Graphical Abstract
INTRODUCTION
The kinetoplastid parasite Trypanosoma brucei is the causative agent of human African trypanosomiasis (Sleeping Sickness), a disease that affects sub-Saharan African populations, exposing 70 million people and causing thousands of infections annually. Domestic cattle are widely affected by trypanosomiasis on this continent as well, leading to extensive agricultural losses. The parasite has a complex digenetic life cycle involving an insect vector, the tsetse fly. One of the major virulence factors within vertebrate hosts is the presence of a surface coat that consists of Variant Surface Glycoprotein, VSG 1,2. Hundreds of variants of VSG exist in the genome, but only one is expressed at any given time. Periodic switches of VSG expression in bloodstream form (BSF) parasites allows for evasion of the immune system of the host – a process called antigenic variation 3,4.
The need for sustained production of an abundant VSG coat (approximately 107 molecules per cell per 6 hr cell cycle) is enabled by a highly efficient and streamlined secretory machinery 5,6. Initial synthesis of VSG occurs in the ER, where it is modified by addition of a glycosylphosphatidylinositol-anchor (GPI) and N-glycans. All secretory cargo, including VSG, leaves the ER from one of two ER exit sites, each with a closely apposed and dedicated Golgi. From there cargo is delivered to the cell surface via the flagellar pocket, a small invagination of the plasma membrane at the posterior end of the cell. The overall process is extremely rapid (t1/2 ~15 min) 7, but the precise route from the Golgi to the flagellar pocket is not known.
The flagellar pocket is also the site of all endocytosis in trypanosomes, both receptor-mediated and fluid phase 8,9. The rate of constitutive endocytosis is extremely high; it takes just 12 minutes to turnover an entire cell surface through the flagellar pocket, a domain that comprises ~5% of the total plasma membrane 10. This high rate is primarily for uptake of nutrients, but also for elimination of potentially lytic immune complexes 11–13. As a consequence of ongoing endocytosis VSG is constantly internalized such that ~10% resides in endosomal compartments at any given time 9,10 necessitating a machinery for efficient recycling to the surface.
Between the centrally located nucleus and the flagellar pocket are all the endosomal compartments typically found in most eukaryotes, marked by characteristic small GTPases called Rabs 14: early endosome, TbRab5A/B; recycling endosome, TbRab11; and late endosome, TbRab7. The terminal endocytic compartment is a vacuole-like lysosome marked by the type I transmembrane glycoprotein, p67, and the soluble cathepsin-L orthologue, TbCatL 15,16. Although the secretory route of newly synthesized VSG is poorly defined, the route of its internalization and recycling has been outlined in great detail [reviewed in 9]. VSG, as is all endocytic cargo, is internalized in clathrin-coated vesicles and then rapidly delivered to the early endosome. From here it is typically sorted to the recycling endosome for return to the flagellar pocket via TbRab11+ exocytic carriers. VSG can also traffic from the early endosome to the late endosome and then back to the recycling endosome for export. Collectively this recycling process is extremely efficient; VSG is normally never degraded in the lysosome, rather it is shed slowly from the cell surface by GPI hydrolysis 17–19.
Other surface proteins that are known to transit the TbRab11+ recycling endosome include the trypanosomal transferrin receptor (TfR) 20–22 and invariant surface glycoproteins (ISG) 23,24. TfR is a heterodimer of ESAG7 and GPI-anchored ESAG6 that cycles between the flagellar pocket and endosomal compartments bringing in host serum transferrin as a source of essential iron. TfR partially colocalizes with TbRab11 indicating that it traverses the recycling endosome during this process 25. Unlike VSG however, TfR is ultimately degraded in the lysosome as a consequence of having a single GPI anchor rather than two 26,27. ISG65 is a type I transmembrane glycoprotein of unknown function. It has distinct cell surface and internal pools that are regulated by ubiquitinylation of lysine residues in the C-terminal cytoplasmic domain 28,29. Like TfR, ISG65 partially colocalizes with TbRab11 in the recycling endosome and is ultimately delivered to and degraded in the lysosome 16,30.
In addition to the biosynthetic and endocytic/recycling pathways, we have demonstrated the existence in BSF trypanosomes of a post-Golgi default pathway to the lysosome that is evident when specific targeting signals are deleted from endogenous cargo proteins of the secretory pathway. For instance, GPI-minus VSG is redirected from the cell surface to the lysosome where it is rapidly degraded 17,31. Another example is p67, where deletion of the C-terminal cytoplasmic domain (p67ΔCD), containing di-leucine motifs that specify lysosomal targeting in procyclic stage trypanosomes, has no effect on lysosomal targeting in bloodstream form parasites 15. The precise itinerary of this pathway is unclear, but it is unaffected by RNAi silencing of several components of the endomembrane system including late endosomal TbRab7, the terminal ESCRT ATPase TbVps4; and the PI(3)P-5 kinase TbFab1, all of which have differential effects on the other trafficking pathways 16,32,33.
An earlier TbRab11 RNAi silencing study indicated that TbRab11 is essential, but found marginal effects on Tf uptake and VSG export 34. Since it is generally considered that all recycling VSG transits TbRab11+ endosomes/exocytic carriers 9, the later result was interpreted as meaning either that newly synthesized VSG also transits these compartments, but in a TbRab11 independent manner, or that another unidentified route from the Golgi to the flagellar pocket is operative. However, despite prominent colocalization of TfR and VSG with TbRab11, and other than an anecdotal account that TbRab11 silencing leads to internal accumulation of TfR 9, there is no direct evidence that TbRab11 actually mediates recycling of any endogenous proteins. In this work we reassess the role of TbRab11 in these various pathways using a toolkit of endogenous trypanosomal endolysosomal and secretory proteins. Other than minor differences in the behavior of TfR and lysosomal delivery of endocytic cargo, our results are generally consistent with those of Hall et. al 34. In addition, our results strongly indicate that TbRab11 does not affect recycling of GPI-anchored cargo, but does influence recycling of transmembrane cargo.
RESULTS
RNAi silencing of TbRab11
Previous work of Hall et al. used RNAi silencing to demonstrate the essentiality of TbRab11 34, and we have exploited the same approach here in order to further understand its role in protein trafficking within the endocytic/secretory pathways of bloodstream T. brucei. A cell line with an inducible stem-loop dsRNA vector targeting the endogenous TbRab11 gene was constructed. Induction of silencing resulted in cessation of growth by 24 hr, concurrent with an ~90% reduction in TbRab11 mRNA (Fig. 1A & B). Immunofluorescence microscopy localizing BiP and p67 showed no disruption of internal ER or lysosomal morphology respectively after TbRab11 depletion (Fig. 1C). Cells subsequently developed progressively abnormal morphology with complete death at ~60 hr post-induction. While our results confirm the essentiality of TbRab11, the kinetics of silencing differ from the findings of Hall et. al 34, who observed remarkably immediate growth arrest (essentially at T0) with onset of grossly distorted morphology as early at 5 hr post-silencing, all without a noticeable reduction in TbRab11. Conversely, our results are more in line with effects we typically see for silencing of essential genes of endomembrane systems in BSF trypanosomes, including late endosomal components TbRab7, TbVps4, and TbFab1 16,32,33. Therefore, we have performed all subsequent analyses at 22 hr of induction, a time point when TbRab11 depletion first impacts growth, but gross and internal morphology and motility are normal.
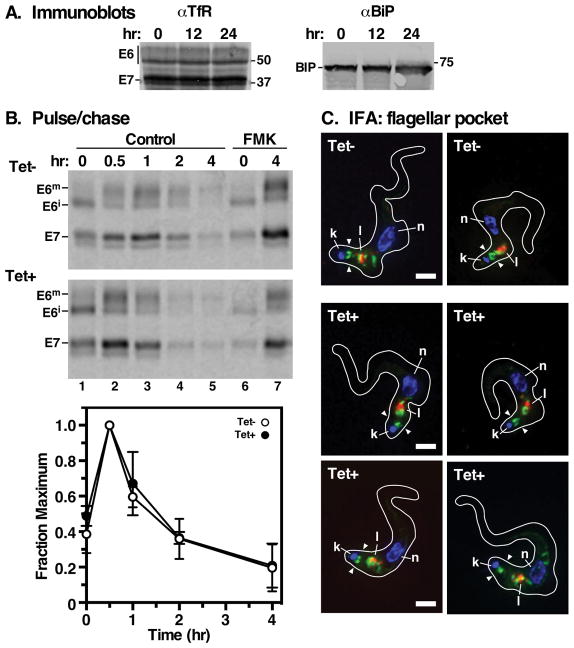
Figure 1. Rab11 is essential for proliferation of bloodstream T. brucei cells.
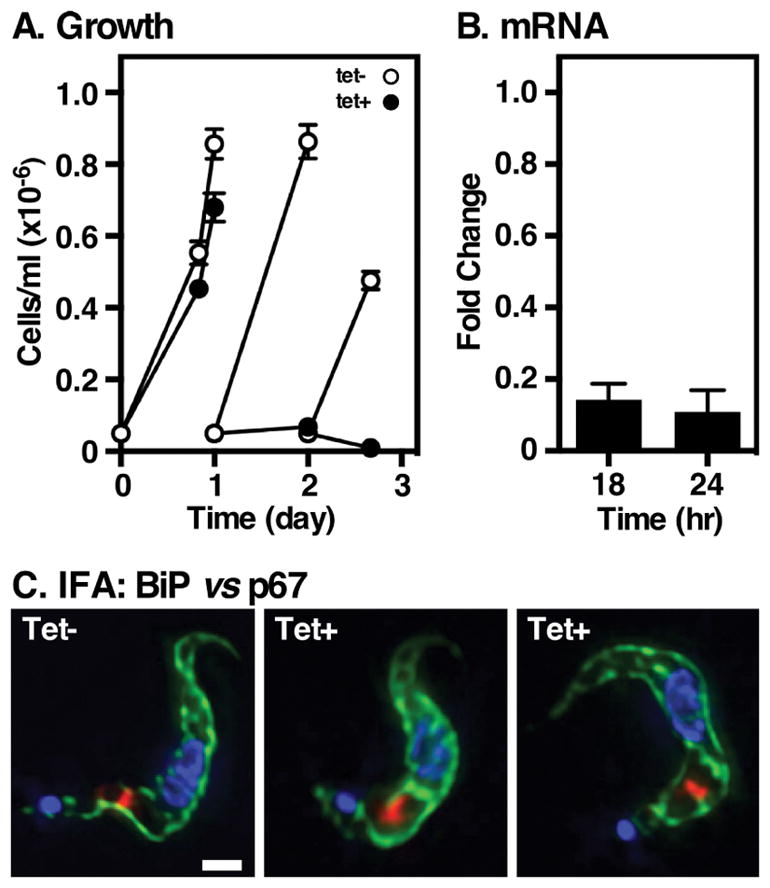
The TbRab11 RNAi cell line was grown in the presence of tetracycline (1 μg/ml) to induce specific knockdown. A. Cell density was measured and plotted against time (mean ± std. dev., n=3 biological replicates). Cultures were diluted to starting density every 24 hours. Empty circles, tet− control cells; closed circles, tet+ silenced cells. B. The extent of TbRab11 mRNA knockdown was assayed by real-time qRT-PCR at the indicated time points. Values are normalized to uninduced controls (mean ± std. dev., n=3 biological replicates). All subsequent experiments were performed at 22 hr of TbRab11 silencing. C. Immunofluorescence microscopy was performed with fixed permeabilized cells using anti-BiP (green) and anti-p67 (red) to localize the ER and lysosomes respectively. Nuclei and kinetoplasts were stained with DAPI (blue). Representative 3-channel images are presented. Bar indicates 2 μm.
Effects of TbRab11 depletion on endocytosis
We first assessed the effect of TbRab11 silencing on binding and uptake of receptor-mediated endocytic cargo, and subsequent delivery to the lysosome, using tomato lectin and transferrin in our standard repertoire of flow cytometry assays 16,32. Tomato lectin is a surrogate marker for receptor-mediated endocytosis; it binds to membrane glycoproteins in the flagellar pocket, and is then endocytosed and trafficked to the lysosome 15,35. Transferrin specifically binds to the transferrin receptor (TfR) and is likewise endocytosed and delivered to the lysosome. These markers were assayed under two different conditions: at 4°C binding of cargo to the flagellar pocket is permitted but no internalization occurs; at 37°C binding and continuous internalization can take place. Binding of tomato lectin was unimpaired, but internalization in the continuous uptake assay was reduced ~40% (Fig. 2A). This assay is not sensitive enough to detect binding of transferrin, however, uptake was impaired to an equivalent level as tomato lectin (~50%). The actual rate of endocytosis was determined using the pH-sensitive probe tomato lectin:FITC, which is quenched as it is taken up and delivered through endosomal compartments of increasing acidity to the terminal lysosome 36,37. Two observations are apparent (Fig. 2B), initial rate of uptake of this ligand is reduced in silenced cells, and the final fluorescent signal was elevated. This decrease in rate parallels the overall reduction in both TL and Tf uptake, and suggests a general defect in endocytosis upon TbRab11 ablation. The higher terminal pH in silenced cells suggests that TL is not being delivered to the lysosome. This would be consistent with the findings of Hall et. al, who showed that TbRab11 ablation disrupted delivery of ConA to the lysosome 34. Alternatively TbRab11 depletion may lead to dysregulation of the internal pH of endolysosomal compartments. To discriminate between these possibilities we imaged internalized tomato lectin by epifluorescence microscopy relative to the lysosomal marker protein p67. In control cells endocytosed probe co-localized prominently with p67 (Fig. 2C) indicating, as seen previously 15, delivery to the terminal lysosome. Depletion of TbRab11 had no effect on this localization. These results contradict those of Hall et. al 34, who saw disrupted lysosomal delivery of lectin, and suggest that TbRab11 silencing somehow affects regulation of lysosomal pH.
Figure 2. Effect of Rab11 depletion on endocytosis.
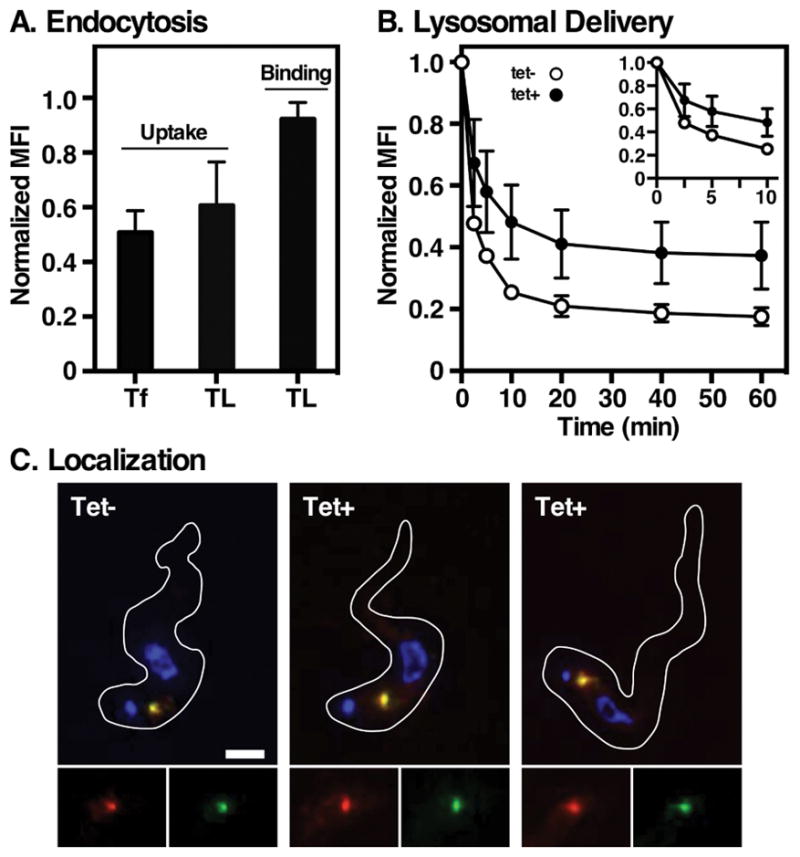
Control and TbRab11 silenced cells were used for ligand binding and uptake assays as described in Materials and Methods. A. Binding (TL:A488 only) and uptake (Tf:A488 and TL:A488) of receptor-mediated endocytic cargo were measured by flow cytometry. Mean fluorescence intensities (MFI) normalized to control un-silenced cells are presented (mean ± std. dev., n=3 biological replicates) B. The kinetics of endocytosis was determined by pulse-chase uptake of the pH-sensitive probe TL:FITC. Cell-associated fluorescence of live cells was measured over time by flow cytometry. Data are normalized to initial bound ligand and are presented as MFI (mean ± std. dev., n=3 biological replicates). Inset shows expanded graph at the earliest time points. C. Cells were pulsed with TL:Bio (30 min, 37°C), washed, and then reincubated (20 min, 37°C) to chase the lectin probe into terminal endocytic compartments. Imaging was performed on fixed/permeabilized cells stained with anti-p67 (red) and streptavidin:A488 (green). Representative 3-channel images are presented of control (tet−) and silenced cells (tet+). Inserts are corresponding single channel images of the lysosomal region. Cell outlines were captured from matched DIC images. Bar indicates 2 μm.
TbRab11 silencing does not affect TfR turnover
The observed decrease in Tf uptake under TbRab11 depletion could be explained most simply if there are fewer Tf receptors available in the flagellar pocket. We explored this possibility by first assessing total cellular transferrin receptor by immunoblotting and found that the steady state levels of TfR subunits were unaffected by 24 hours of TbRab11 silencing (Fig 3A). This result differs from that of Hall et. al 34, who observed a several fold increase in the ESAG7 subunit within 6 hour. We next examined turnover of the transferrin receptor by pulse/chase radiolabeling, in conjunction with pull down from cell extracts with Tf-beads. This assay allows direct assessment of turnover of newly synthesized functional receptor. In both control and TbRab11 depleted cells TfR is initially synthesized as a doublet of ESAG6 and ESAG7 (Fig. 3B, lane 1). At 30 minutes of chase ESAG6 has been mostly processed to a larger mature form, presumably by N-glycan and/or GPI processing (Fig. 3B, lane 2). Also at this time the total amount of TfR detected has doubled, a phenomenon we ascribe to ongoing folding and assembly of functional heterodimer. Thereafter, both subunits disappear coordinately with identical kinetics in both control and silenced cells (Fig. 3B, lane 3–5). Turnover is rescued by the thiol protease inhibitor FMK024 confirming turnover in the lysosome 27. Identical kinetics were obtained by anti-TfR immunoprecipitation with the exception that total TfR, both folded and unfolded, are detected at To (Fig S1). To determine if TfR still localizes to the flagellar pocket when TbRab11 is ablated we performed immunofluorescence imaging on control and silenced cells. As is typical in normal cells (Fig. 3C, tet−) TfR is seen throughout the post-nuclear endosomal compartments and in the flagellar pocket (arrowheads), closely apposed to and just anterior to the kinetoplast. A similar pattern of staining, with prominent flagellar pocket labeling, was typically seen in silenced cells (Fig. 3C, tet+). It is possible that the steady state level of TfR in the flagellar pocket is lower under TbRab11 knockdown, but no obvious differences was seen by visual observation, and since the level of staining is quite variable even in control cell no attempt was made to quantify such an effect by immunofluorescence.
Figure 3. Rab11 depletion does not affect the turnover of transferrin receptor.
A. TbRab11 silencing was induced for the indicated times and TfR levels were assessed by immunoblotting with anti-TfR (107 cell equivalents per lane), and with anti-BiP as a loading control. A representative LICOR image is presented. Mobilities of ESAG6 (E6), ESAG7 (E7) subunits, and BiP are indicated on the left; mobilities of molecular mass markers are indicated on the right (kDa). B. Control and TbRab11 silenced cells were used subjected to pulse/chase (15 min/4 hr) metabolic radiolabeling with [35S]Met/Cys. As indicated FMK024 (20 μM) was included to block lysosomal thiol proteases. At the indicted times cell extracts were prepared and functional transferrin receptor was pulled down using transferrin beads. Immunoprecipitates were analyzed by SDS-PAGE/phosphorimaging. Top. Representative phosphorimages for control (Tet−) and silenced (Tet+) cells are presented (107 cell equivalents per lane). ESAG7, E7; immature ESAG6 (E6i); mature ESAG6 (E6m). Bottom. ESAG7 signals were quantified and normalized to values at 0.5 hours (mean ± std. dev., n=4 biological replicates). C. TfR localization was determined in fixed permeabilized control and silenced cells by immunofluorescence with anti-TfR antibody (green). The lysosome (l) was visualized with mAb anti-p67 (red); nuclei and kinetoplasts (n, k) with DAPI. Representative deconvolved summed stack images are presented. The position of the flagellar pocket is indicated by opposing arrowheads. Scale bar: 2 μm.
Overall these results indicate that normal synthesis and turnover, and presumably route to the lysosome, are unimpaired under TbRab11 deficiency. Had TbRab11 silencing blocked TfR recycling via the recycling endosome one might expect turnover to be increased as receptor is diverted to late endolysosomal compartments. Thus, if internalized TfR does return to the cell surface via the recycling endosome this process must be independent of TbRab11 function. In this regard it is worth repeating that internal TfR has been shown to partially co-localize with TbRab11+ 25, and therefore likely transits this compartment en route to the cell surface.
Effect of Rab11 depletion on VSG trafficking
A subset of internal VSG also colocalizes with TbRab11 in immuno-EM 9,38, which is thought to be endocytosed VSG returning to the cell surface via the recycling endosome. However, it is not certain if the biosynthetic route of newly synthesized VSG to the surface also intersects with the recycling endosome. On the one hand Hall et. al demonstrated that transport of newly synthesized VSG is not affected by TbRab11 ablation 34. On the other, no obvious candidates for TbRab11 negative exocytic vesicles have been identified in trypanosomes. Consequently we have reinvestigated the role of TbRab11 in VSG trafficking and turnover.
We first confirmed the role of TbRab11 in the export of newly synthesized VSG using the hypotonic lysis assay in pulse/chase radiolabeling format 7. In this assay arrival at the cell surface is indicated by the onset of susceptibility to GPI hydrolysis and release by endogenous GPI-phospholipase C (GPI-PLC), which is activated during lysis. Newly synthesized VSG en route to the cell surface is sequestered from GPI-PLC and consequently is resistant to release. Using this assay we found no difference in the rate of VSG export in control vs. TbRab11 silenced cells (Fig. 4A), in agreement with the findings of Hall et. al 34.
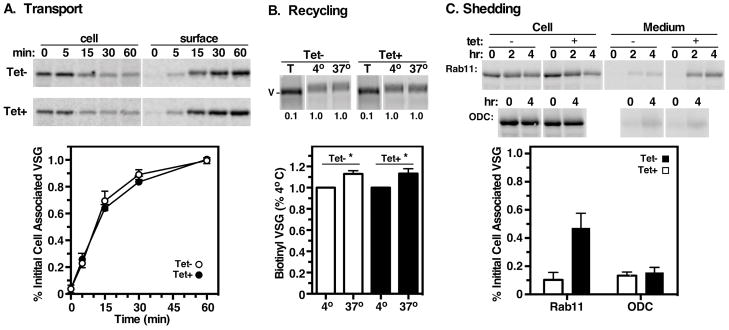
Figure 4. Effect of Rab11 knockdown on VSG transport, recycling and shedding.
The fate of endogenous VSG was assayed in control and TbRab11 silenced cells. A. Transport of newly synthesized VSG to the cell surface was assessed by the hypotonic release procedure. Cells were pulse/chase radiolabeled (2 min/60 min) and cell associated and released (cell surface) fractions were prepared at the indicated chase times. VSG221 polypeptides were specifically immunoprecipitated from fractions and analyzed by SDS-PAGE/phosphorimaging. Top. Representative images for control (Tet−) and silenced (Tet+) cells are presented (2×106 cell equivalents per lane). Bottom. Quantification of the released fraction indicating arrival at the cell surface (normalized to T60 total, mean ± std. dev., n=3 biological replicates). B. Recycling of VSG. Cells were cycloheximide ‘chased’ to flush the secretory pathway of nascent surface proteins (VSG) and reactive surface amines were blocked with NHS-Sulfo-Acetate. After incubation for 5 min at 4°C or 37°C to prevent or allow recycling of internal surface proteins, newly available amines on surface proteins were covalently labeled with NHS-Sulfo-Biotin. Top. VSG221 was specifically immunoprecipitated from cell lysates and blotted with streptavidin-IR dye. Representative Li-Cor images are presented. White stripes indicate irrelevant portions of the image that were digitally excised after image processing. T, total biotinylation of un-blocked cells (105 cell equivalents per lane, 0.1); 4°C and 37°C, biotinylation of blocked cells incubated at the indicated temperature (106 cell equivalents per lane, 1.0). Bottom. Biotinylated cells were stained with streptavidin-A488 and analyzed by flow cytometry as in Fig. 2. MFI is presented for each condition normalized to uninduced controls (mean ± std. dev., n=3 biological replicates). Asterisk indicates p<0.05 in paired Student’s T-test. C. Shedding of VSG. TbRab11 RNAi cells were pulse-chase radiolabeled (2 min/4 hr) and VSG221 polypeptides were immunoprecipitated from cell and media fractions at the indicated times and analyzed by SDS-PAGE/phosphorimaging. TbODC RNAi cells were used as a control for off-target effects. Top. Phosphorimages of representative gels (106 cell equivalents per lane). White stripes indicate empty lanes that were digitally excised after image processing. Bottom. VSG media signals were quantified and corrected (T4 – T0), and are presented as the fraction of T0 cell-associated VSG (mean ± std. dev., n=4 biological replicates).
We next developed an assay to measure recycling of internal VSG to the surface. Cells were preincubated with cycloheximide to flush nascent VSG from the exocytic pathway and then free surface amino groups were blocked by acetylation. Shifting to 37°C allows recycling of internal unblocked VSG, which is then susceptible to subsequent surface biotinylation. Residual unblocked VSG (~5% of total) was detected by specific immunoprecipitation and blotting with streptavidin (Fig. 4B, top), but this assay was not sensitive enough to detect an increased signal after incubation at 37°C. However, flow cytometry after staining with streptavin:A488 consistently revealed a significant increase (~10%) in surface biotinylation following temperature shift (Fig. 4B, bottom). Although this assay does not discriminate between VSG and other recycling cargo, it is the overwhelmingly dominant surface component and we infer that the increased signal represents recycling of VSG. These results agree with those of Engstler et. al who also observed a 10% recycling of VSG over a 5 minute incubation 10. Importantly, TbRab11 ablation had no effect on this process, suggesting that like TfR, Rab11 does not influence trafficking of endocytosed GPI-anchored VSG through the recycling endosome. This result also suggests that if TbRab11 mediates recycling of surface membrane proteins, it does so for a pool other than GPI-anchored proteins, e.g. transmembrane cargo, and that the large abundance of VSG may be masking its role in the recycling of other substrates in our assay.
Finally we investigated VSG turnover, which has been shown to involve slow release of VSG to the media by low level GPI hydrolysis (t1/2 ~30 hr) 18,19. We performed pulse/chase radiolabeling and immunoprecipitated labeled VSG from cell and media fractions at 0, 2, and 4 hours of chase (Fig. 4C). In control cells little VSG was detected in the media and this did not increase markedly (~10%) during the chase. In contrast, ablation of TbRab11 resulted in a dramatic increase in VSG shedding (~50%). This effect seems to be specific for disruption of the endomembrane system as silencing of the housekeeping enzyme ornithine decarboxylase, which has similar kinetics of silencing and lethality 39, did not affect VSG shedding. Collectively these results indicate that synthesis, transport and recycling of VSG are not regulated by TbRab11 function, and strongly suggest that loss of TbRab11 greatly accelerates VSG turnover via a shedding mechanism.
TbRab11 silencing increases membrane shedding
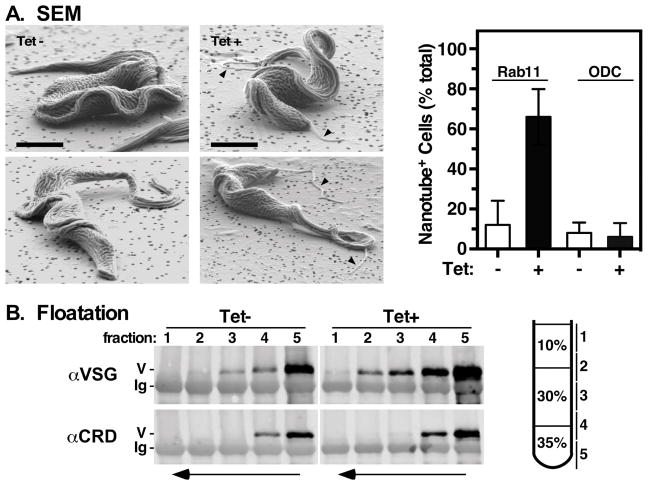
BSF trypanosomes actively shed surface membrane via structures called nanotubes, which then fragment into smaller exocyctic vesicles (EVs) 40. We performed SEM to investigate if increased shedding of VSG correlates with increased nanotube formation (Fig. 5A). Indeed, cells with obvious nanotubes segregating from either the anterior and/or posterior ends increased dramatically from ~10% to >60% under TbRab11 ablation. Silencing of ODC had no effect on nanotube production, indicating the phenomenon is not just a result of dying cells. We next assessed the degree to which the two modes of shedding, GPI hydrolysis vs. EV formation, contribute to overall VSG turnover. GPI hydrolysis removes dimyristoylglycerol and uncovers a cryptic immuno-epitope called the Cross Reacting Determinant (CRD) 41, providing a convenient assay for GPI integrity. Cells were incubated to allow VSG shedding and samples of the conditioned media were fractionated by density floatation. Fractions were then immuno-blotted with anti-VSG for total protein, or with anti-CRD to measure GPI hydrolysis (Fig. 5B). VSG with intact GPI-anchors should float with vesicles; hydrolyzed VSG should remain in the load volume (bottom). In both control and silenced cells the overwhelming majority of released VSG was found in the load and first interface (fractions 4 & 5), and this VSG was reactive with anti-CRD indicating GPI hydrolysis. However, in each case lesser amounts of non-reactive VSG was detected in floatation fractions 2 & 3, indicating inclusion in membranes via intact GPI anchors. In all cases considerably more VSG was detected in fractions from silenced cells, in good agreement with elevated biosynthetic release (Fig. 4C) and nanotube shedding (Fig. 5A). These results suggest that both modes of release are involved in VSG turnover, and that both are enhanced by TbRab11 ablation. The possibility of an ordered pathway for VSG turnover, EV formation followed by GPI hydrolysis, is discussed below.
Figure 5. TbRab11 silencing induces nanotubes.
A. Control (Tet−) and TbRab11 silenced (Tet+) cells were analyzed by scanning electron microscopy (SEM). Right. Representative images obtained at 2 kV using a lower detector at 70° tilt are presented. Tubular structures emerging from both anterior and posterior ends of cells are indicated by arrowheads. Bar = 2 μm. Left. Randomly selected cells (3 biological replicates, 100 cells each) were imaged with an in-lens detector (zero tilt) and quantified for the presence (one or more) or absence (zero) of nanotubes (mean ± std. dev.). Induced and uninduced ODC RNAi cells were used as controls for non-specific RNAi effects. B. Control (Tet−) and silenced (Tet+) cells harvested from late log phase cultures were incubated at 370C for 4 hr and conditioned supernatants were fractionated by density floatation. After immunoprecipitation with anti-VSG221 equivalent samples were immunoblotted with anti-VSG221 or anti-CRD. A representative experiment (1 of 4) is presented. For each blot the vertical stripe indicates an empty lane that was digitally excised following standard image processing. The mobilities of VSG (V) and immunoglobulin heavy chain (Ig) from the primary immunoprecipitation are indicated. Arrows indicate the direction of floatation. An approximate scale diagram of the density gradient and fractions collected is presented.
Effects of TbRab11 silencing on biosynthetic trafficking to the lysosome
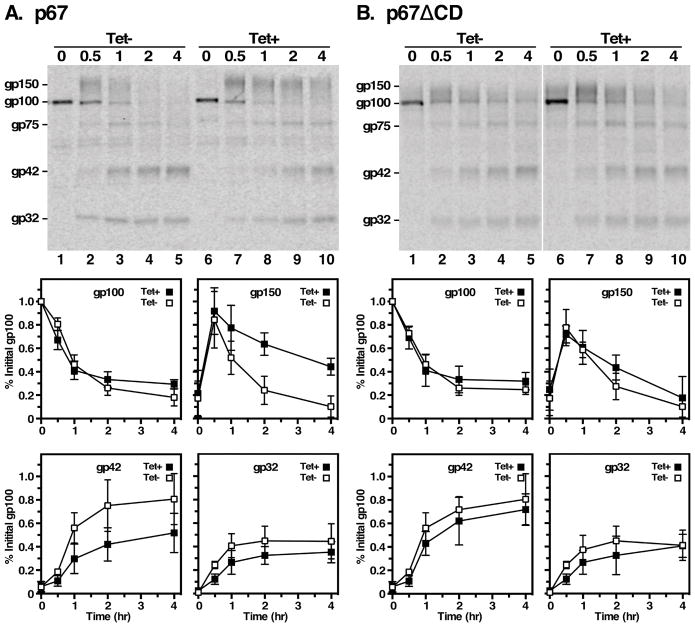
To investigate potential roles of TbRab11 in targeting newly synthesized proteins to the lysosome we followed the trafficking of two endogenous cargo proteins, the lysosomal cathepsin L orthologue TbCatL, and the type I transmembrane glycoprotein p67, in standard pulse/chase assays. TbCatL is synthesized initially as proproteins of 53 kDa (I) and 50 kDa (X), which upon arrival in the lysosome are converted to the mature catalytically active enzyme (M, 44 kDa) by removal of the N-terminal prodomain 42. Pulse/chase analyses indicate that this process is unaffected by depletion of TbRab11 (Fig. 6). We next assessed trafficking of the lysosomal type I transmembrane glycoprotein p67 15,43. As seen previously, p67 is initially synthesized in control cells as a 100 kDa ER glycoform (gp100) and is converted by N-glycan modification in the Golgi to a 150 kDa glycoform (gp150) (Fig 7A, lanes 1&2). Thereafter it is transported to the lysosome where proteolytic cleavage generates the quasi-stable gp42 and gp32 glycoforms (Fig 7A, lanes 3–5). TbRab11 silencing had no effect on transport of newly synthesized p67 to the Golgi as indicated by the timely loss of gp100 (Fig 7A, lanes 6–8), but subsequent transport to the lysosome was retarded as evidenced by marked delays in the kinetics and extent of both gp150 disappearance and gp42 appearance (Fig 7A, lanes 8–10). These results suggest that TbRab11 plays a role in normal biosynthetic trafficking of p67 to the lysosome. This could be due to non-specific secondary effects of TbRab11 silencing on general endomembrane pathways. However, the fact that trafficking of soluble TbCatL is unaffected by TbRab11 silencing suggests otherwise. Possible explanations for these phenomena are discussed below.
Figure 6. Rab11 depletion does not affect TbCatL trafficking.
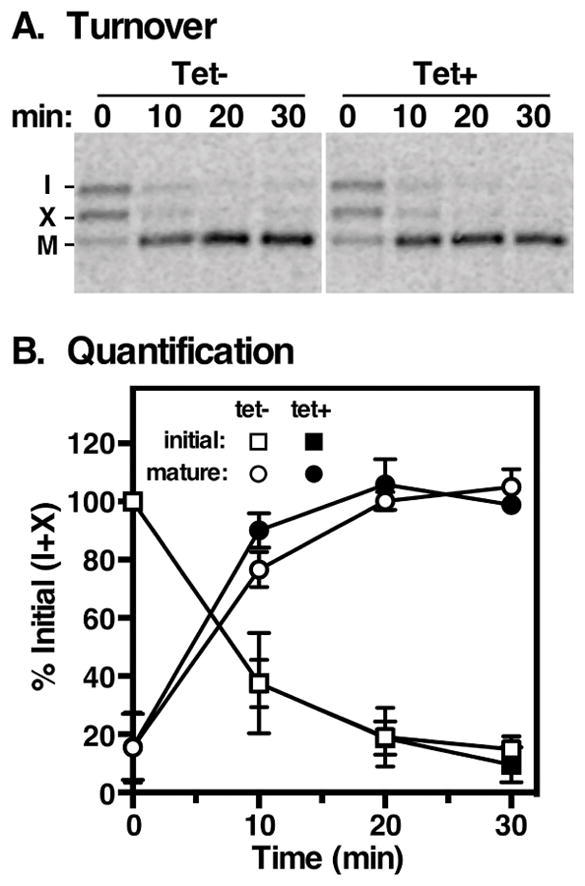
Transport of lysosomal TbCatL was assayed in control and TbRab11 silenced cells. A. Cells were pulse-chase (10 min/30 min) radiolabeled and at the indicated times TbCatL was immunoprecipitated from cell lysates and analyzed by SDS-PAGE/phosphorimaging. Top. A representative image is presented (107 cell equivalents per lane). Arrival in the lysosome is indicated by loss of precursor (I + X) and appearance of mature (M) forms. White stripes indicate irrelevant portions of the gel that were digitally excised after image processing. Bottom. Results were quantified and expressed as percentage of initial signal (I + X) at T0 (mean ± std. dev., n=3 biological replicates).
Figure 7. Effects of TbRab11 on p67 trafficking.
Transport of endogenous p67 and the default reporter p67ΔCD were analyzed in control and TbRab11 silenced cells. TbRab11 RNAi cells (A) or TbRab11 RNAi cells expressing p67ΔCD (B) were pulse/chase radiolabeled (15 min/4 hr) with [35S]Met/Cys and lysates were prepared. Endogenous p67 (A) or transgenic p67ΔCD reporter (B) were immunoprecipitated with anti-p67 and anti-HA, respectively and analyzed by SDS-PAGE/phosphorimaging. Top. Representative phosphorimages (107 cells equivalents per lane). In each case p67 glycoforms are: gp100, initial ER precursor; gp150, Golgi intermediate, gp75 lysosomal intermediate; gp43 and gp32, quasi-stable lysosomal fragments. Vertical white stripe indicates irrelevant lanes digitally excised following image processing. Bottom. Quantification of individual p67 glycoforms in control (open squares) and TbRab11 silenced (closed squares) cells. All data are normalized to initial gp100 (mean ± std. dev., n=4 biological replicates).
We have demonstrated the existence in bloodstream trypanosomes of a post-Golgi default pathway to the lysosome that is evident when specific targeting signals are deleted from endogenous cargo proteins of the secretory pathway. For instance, GPI-minus VSG is redirected from the cell surface to the lysosome where it is rapidly degraded 17,31. Another example is p67, where deletion of the C-terminal cytoplasmic domain, containing di-leucine motifs that specify lysosomal targeting in procyclic stage trypanosomes (p67ΔCD), has no effect on lysosomal targeting in bloodstream form parasites 15. To investigate this route we introduced the p67ΔCD reporter (with C-terminal 3× HA tag) 16,32 into the TbRab11 RNAi cell line for pulse/chase turnover analyses. As seen previously, despite the absence of the native C-terminal domain the reporter is processed identically and with similar kinetics as endogenous full-length p67 (Fig. 7B, lanes 1–5). However, unlike the native protein, depletion of TbRab11 has no effect on trafficking and processing of the reporter (Fig. 7B, lanes 6–10). These results indicate, as was the case with TbVps4 16 and TbRab7 32, that the default pathway to the lysosome is independent of TbRab11 function, and consequently must be distinct from the normal biosynthetic pathway.
Effects of TbRab11 depletion on turnover of ISG65
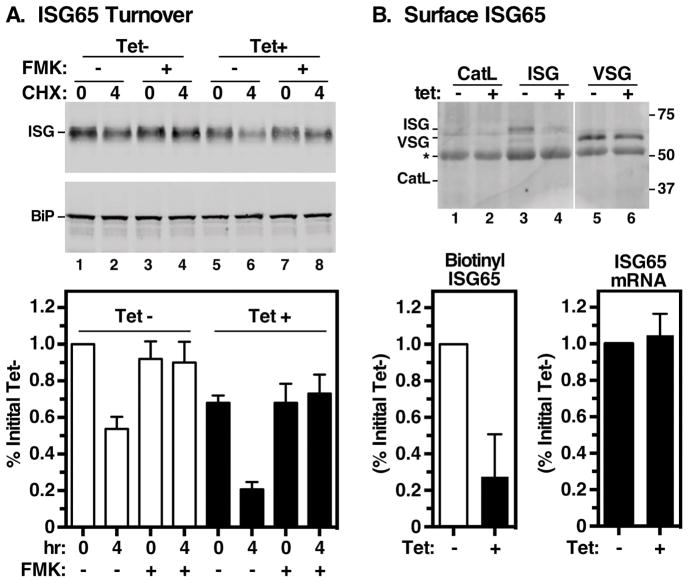
Lastly we assessed the role of TbRab11 in trafficking of a type I transmembrane surface glycoprotein that is known to recycle through the endocytic pathway, ISG65 (Invariant Surface Glycoprotein 65 kDa) 23,24,44. ISG65 is an invariant type I membrane glycoprotein that can be ubiquitinylated on conserved lysine residues in its cytoplasmic tail, internalized and ultimately degraded in the lysosome 16,28,29,45. We utilized a cycloheximide-chase protocol 16 to measure turnover of ISG65 in Rab11-depleted cells. Approximately 50% of initial ISG65 was degraded in the 4-hour chase period in control cells (Fig. 8A, lanes 1 vs. 2), consistent with our previous observations 16,33. The lysosomal thiol protease inhibitor FMK024 completely blocked this degradation, confirming lysosomal turnover (Fig. 8A, lanes 3 vs. 4). Depleting TbRab11 markedly reduced ISG65 levels relative to control cells (compare lanes 1 vs. 5), and the rate of ISG65 turnover increased (compare lanes 1 & 2 vs. 5 & 6). Again, ISG65 was restored to initial levels by FMK024 treatment (Fig 8A, lanes 7 & 8). Surface biotinylation assays were also performed to determine the relative levels of surface ISG65 in control and TbRab11 depleted cells (Fig. 8B). Biotinylation of VSG (lanes 5 & 6), but not lysosomal CatL (lanes 1 & 2), confirmed the specific labeling of surface proteins and plasma membrane integrity. Surface ISG65 was biotinylated in control cells, but the levels were greatly reduced (around 80%) in Rab11 depleted cells (lanes 3 & 4). Importantly for all these assays, ISG65 mRNA levels were unaffected by TbRab11 silencing (Fig. 8B). These observations are consistent with a model in which TbRab11 participates in the recycling of the internalized pool of ISG65 back to the plasma membrane. In the absence of this process, internalized ISG65 proceeds via the only other pathway available to it, i.e. towards the lysosome, leading to increased turnover and reduced steady state and surface levels.
Figure 8. Rab11 depletion accelerates ISG65 turnover.
Turnover of endogenous ISG65 was analyzed in control (Tet−) and TbRab11 silenced (Tet+) cells. A. Top. Protein synthesis was inhibited by treatment with cycloheximide and the steady state levels of ISG65 were assayed by immunoblotting at 0 and 4 hrs of culture. As indicated FMK024 was used to block degradation in the lysosome. Parallel blots were performed with anti-BiP as a loading control. Representative blots (4×106 cells equivalents per lane). Bottom. Quantification of ISG65 levels. ISG65 signals were normalized to BiP signals and corrected values were expressed as percentage of the initial signal for uninduced cells (mean ± SEM, n=3 biological replicates). Control cells, white bars; silenced cells black bars. B. Top. Intact control (Tet−) and TbRab11 silenced (Tet+) cells were surface biotinylated and immunoprecipitated with anti-ISG65, anti-TbCatL (negative control), and anti-VSG221 (positive control). After fractionation by SDS-PAGE (ISG65 & TbCatL, 107 cell equivalents/lane; VSG221, 5×104 cell equivalents/lane) blotting was performed with streptavidin-IR dye. Representative Li-Cor images are presented. Mobilities of TbCatL, ISG65 and VSG are indicated. Asterisk indicates background reaction IgG heavy chain from the immunoprecipitation. White stripe indicate irrelevant portions of the image that were digitally excised after image processing. Bottom Left. Biotinylation results were quantified and expressed as percentage of initial signal for uninduced cells (mean ± std. dev., n=3 biological replicates). Control cells, white bars; silenced cells black bars. Bottom Right. ISG65 mRNA levels were assessed by qRT-PCR at 0 (tet−) and 18 (tet+) hrs of TbRab11 silencing. Values are normalized to uninduced controls (mean ± std. dev., n=3 biological replicates)
DISCUSSION
Transit of membrane proteins and secretory cargo through the eukaryotic endomembrane system has been studied most comprehensively in yeast and mammalian cells. In each case secretory and endocytic cargo intersect to varying degrees in endosomal compartments, perhaps most notably in the Rab11+ recycling endosome 46–48. Such cargos typically include transmembrane proteins such as receptors and cell adhesion molecules. As a critical component of recycling endosomes, Rab11 has been shown to regulate cellular functions ranging from surface protein recycling to differentiation and polarization. Less is known about Rab11 function in exocytosis of GPI-anchored proteins. Bloodstream form African trypanosomes, with abundant GPI-anchored VSG cargo, a streamlined endomembrane system, and unusually high secretory and endocytic fluxes 2,6,9, provide an ideally suited model to explore questions related to recycling. TbRab11 has been generally considered to function in recycling of GPI-anchored proteins in trypanosomes, although no direct evidence, other than co-localization with TfR and VSG 25,38, has ever been presented. TbRab11 has been implicated in exocytosis from the lysosome of degradation fragments of both Tf and IgG 34, but this must be considered a distinct process from recycling of endogenous surface proteins. No other TbRab11 functions have been delineated. Here we exploit RNAi silencing to study the function of TbRab11 in protein trafficking with particular regard to GPI anchored proteins.
TbRab11 is an essential protein in T. brucei - knockdown leads to cell death following 24 hrs, setting the time frame for all our subsequent analyses. Given the lack of direct evidence for a role in recycling of GPI-anchored proteins, we first evaluated the effect of TbRab11 ablation on TfR and VSG trafficking. We found that TfR biosynthesis, processing, turnover and steady state levels were normal in the absence of TbRab11. There was a modest ~2-fold reduction in Tf uptake, but we ascribe this to secondary effects on clathrin-mediated endocytosis since a similar effect was seen with uptake of the non-specific ligand, tomato lectin. In support of this explanation we found no changes in levels of surface TfR following TbRab11 knockdown. All of these results suggest that normal trafficking of TfR, including recycling to the flagellar pocket after endocytosis, is not dependent on TbRab11 function. It is notable that while delivery of tomato lectin to the lysosome was unaffected by TbRab11 silencing, the actual pH of compartments along the way was higher than normal. Again we attribute this to secondary affects on general homeostasis of endosomal compartments since TbRab11 is not a component of late endosomal/lysosomal compartments. Similar to TfR, TbRab11 apparently has no role in normal trafficking of VSG. Under knockdown, VSG biosynthetic trafficking to the cell surface was unaffected, nor was recycling of internalized VSG.
Taken together, our results with TfR and VSG suggest that, while GPI-anchored cargos do pass through the recycling endosome 25,38, transit is not dependent on TbRab11 function as has long been assumed. This has implications for the route of trafficking of newly synthesized VSG (and TfR) from the Golgi to the flagellar pocket. Hall et. al 34 first observed the TbRab11 knockdown did not impair biosynthetic transport of VSG, and suggested two explanations: i) nascent VSG may join the pool of endocytic VSG in the recycling endosome and traffic to the flagellar pocket in a TbRab11-independent manner; or ii) there is a separate as of yet unidentified non-TbRab11 pathway specific for biosynthetic delivery to the flagellar pocket. Neither possibility can be ruled out from these data, but our finding that VSG recycling is TbRab11-independent strongly favors the former.
Surprisingly there is little evidence in the literature for a general role of Rab11 in recycling of GPI-anchored proteins. We could find only one instance in any system in which functional impairment of Rab11 (knockdown or mutagenesis) has been shown to impact trafficking of GPI anchored proteins – the related kinetoplastid parasite T. cruzi 49. TcRab11 localizes to the contractile vacuole, an organelle that is not present in T. brucei, but which may have an evolutionary relationship to recycling endosomes 50. The contractile vacuole is the site of export of several abundant classes of GPI-anchored proteins - trans-sialidases, TcTSSA and mucins. Overexpression of a dominant negative TcRab11 mutant curtailed exocytosis of trans-sialidase, but not the other GPI cargos. Thus it is not possible to assert a general role for Rab11 in GPI recycling in this system either.
TbRab11 knockdown did have one dramatic effect on VSG not seen with TfR – accelerated turnover. The rate of VSG shedding increased ~4–5 fold over a 4 hr period, and this was mirrored by an ~5 fold increase in nanotube bearing cells. The vast majority of shed VSG, in both control and knockdown cells, had hydrolyzed GPI anchors and consequently was not associated with shed membranes, but a small portion was consistently found to have intact anchors and to be associated with floatable membranes. VSG is normally shed (t1/2 ~30 hr) from the cell surface by the slow action of endogenous GPI-PLC 18,19, but how GPI-PLC, which is associated with the cytosolic face of internal membranes 51,52, gains access to surface VSG in intact dividing cells is unclear. More recently it has been shown that BSF trypanosomes constitutively shed membrane in the form of nanotubes that subsequently vesiculate into ~100 nm exocytic vesicles (EV) that contain both VSG and GPI-PLC 40. Our findings raise the intriguing possibility of a two-step process for VSG turnover - first shedding of nanotubes/EVs containing VSG and GPI-PLC, followed by GPI-PLC activation and release of soluble VSG. It is possible that VSG is also released directly from the cell surface by GPI hydrolysis, but our finding that TbRab11 ablation increases membrane shedding and VSG release strongly implies a role for nanotubes/EVs in normal VSG turnover.
We have also investigated the effects of TbRab11 ablation on biosynthetic trafficking to the lysosome. First, ablation had no affect on lysosomal delivery of the soluble hydrolase TbCatL. This was not unexpected since the pathway for this endogenous cargo is thought to be direct from the Golgi to the late endosome/lysosomal compartment. However, the lysosomal transmembrane glycoprotein p67 was affected in the absence of TbRab11. Transport from the ER to the Golgi was normal, but subsequent arrival in the lysosome was delayed. Available evidence indicates that post-Golgi sorting of p67 involves AP1 adaptin-dependent clathrin-mediated export from the Golgi 42 and transit of the late endosome 16. The affect of TbRab11 may again be due to secondary disruption of endosomal homeostasis. Alternatively, some portion of newly synthesized p67 may diverge from the Golgi to the recycling endosome, from which it is recovered by clathrin-mediated vesicular transport to the late endosome along with other endocytic cargo 9. TbRab11 could be required for this recovery process. Finally, we found that TbRab11 knockdown had no effect on the transport of the default lysosomal cargo p67ΔCD, in which the C-terminal targeting signals have been deleted. This adds TbRab11 to the growing list of secretory/endocytic machinery that are apparently uninvolved in the default lysosomal pathway, including TbRab7, the terminal ESCRT protein TbVps4, and the PI(3)P-5 kinase TbFab1s 16,32,33. Consequently the nature of the default pathway remains a mystery.
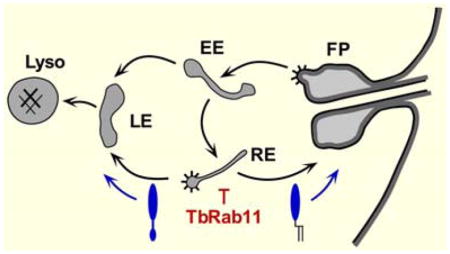
It is perhaps comforting, given its apparent non-involvement in recycling of VSG and TfR, that TbRab11 does play a role in the trafficking of another surface protein known to recycle from endosomal compartments, ISG65. Ablation led to increased lysosomal turnover and lower steady state levels of ISG65, and most importantly to reduced levels of surface ISG65, which we interpret as diversion of intracellular pools to the lysosome when recycling to the cell surface is impaired. A similar interpretation was made for increased turnover of ISGs with knockdown of TbRME-8, a clathrin regulatory protein with broad distribution throughout the endosomal compartment of trypanosomes 53. Our results allow us to elaborate on a previously proposed 16 roadmap for ISG65 trafficking (Fig. 9). ISG65 is a type I transmembrane protein that is ubiquitinylated on conserved lysine residues, endocytosed, and degraded in the lysosome 28,29,45. Knockdown of TbRab7 and the terminal ESCRT protein TbVps4, both components of the late endosome, results in enhanced lysosomal turnover of ISG65, suggesting that the primary pathway for this protein from the late endosome is retrieval to the recycling compartment for return to the cell surface 16. In contrast, knockdown of TbFab1, the kinase responsible for synthesis of the low abundance phosphoinositide PI(3,5)P2, completely blocks ISG65 turnover 33. There are two possible explanations for this finding: i) the resultant decrease in PI(3,5)P2 negatively impacts trafficking from the late endosome to the lysosome; ii) or conversely it enhances retrieval to the recycling compartment. Either is possible but we prefer the former since, while TbFab1 localizes to both the lysosome and late endosome, PI(3,5)P2 itself is located primarily in the lysosome 33. We can now add TbRab11 to the pathway with a clear role in recycling ISG65 to the cell surface. The point at which RME-8 knockdown impairs recycling of ISG65 to the surface is not clear, but a role in the recycling endosome seems likely 53.
Figure 9. Proposed ISG65 trafficking pathways.
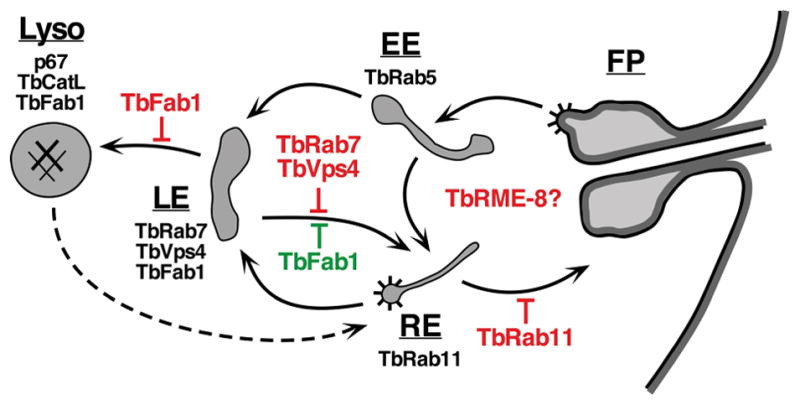
Schematic diagram of the endocytic pathways of trypanosomes. Black arrows indicate documented routes between the flagellar pocket (FP), early endosome (EE), recycling endosome (RE), late endosome (LE), and the terminal lysosome (Lyso). Dashed arrow indicates uncertain pathway for exocytosis of degraded endocytic cargo 34. Validated markers for each compartment are: early endosome, TbRab5 62; recycling endosome, TbRab11 63; late endosome, TbRab7 10,32, TbVps4 16; lysosome, p67 15, TbCatL 64. TbFab1 localizes to both late endosome and lysosome 33. Spiny coats indicate known sites of clathrin coated vesicle formation 38,65. Points at which RNAi silencing of a given component may block (red) or enhance (green) an ISG65 pathway are indicated. The point at which TbRME-8 silencing blocks recycling of ISGs is not clear 53.
In summary, we have provided a detailed analysis of the role of TbRab11 in secretory and endocytic trafficking in African trypanosomes. Unexpectedly, and contrary to long held assumptions, TbRab11 does not have an obvious role in normal trafficking of GPI anchored proteins, but it does strongly influence recycling of transmembrane proteins. While the role of Rab11 in recycling of membrane proteins is well established in other model systems, its function, if any, in GPI dependent trafficking is not well defined. Consequently our findings in this ancient eukaryotic lineage could set a paradigm for other systems. Finally, TbRab11 knockdown greatly altered normal cell surface dynamics leading to elevated shedding of membrane and VSG turnover. This cannot be due to general stress in dying cells since knockdown of TbODC, a housekeeping enzyme had no effect on these processes. Formation of EVs in Drosophila is known to be dependent on Rab1154,55, but this is the ‘classic’ pathway of internal vesiculation into multivesicular bodies, followed by fusion with the plasma membrane and direct release of EVs. Clearly this is a distinct process from the release of extended nanotubes followed by vesiculation first reported by Szempruch et al. 40 and confirmed here. More likely, normal flux through the endocytic pathway of trypanosomes is critical for endomembrane homeostasis. Consistent with this is the finding that TbRab7 knockdown has similar effect on VSG shedding (Fig. S2). In any case, our findings suggest a new two-step model for normal turnover of VSG that will be the target of future studies.
MATERIALS AND METHODS
Cell growth, transfections and cell lines
Bloodstream form T. brucei brucei single marker (SM) cell line which expresses both T7 RNA polymerase and tetracycline (Tet) repressor 56 was grown in HMI-9 media 57 supplemented with 10% fetal bovine serum (Tet free). All experiments were performed with cells harvested at mid-to-late log phase (0.5–1.0 × 106/ml). A stem-loop TbRab11 RNAi plasmid, pLew100v5x:pex11-Rab11, was constructed using a 403 bp region (nucleotides 68 to 470) of the TbRab11 coding sequence (TriTrypDB:Tb927.8.4330) as previously described for TbRab7 32. The construct was linearized by NotI and transfected into SM cells by electroporation 58 and clonal cell lines were selected with phleomycin. The ornithine decarboxylase RNAi cell line has been described previously 39. Both RNAi cell lines were induced with 1 μg/ml tetracycline for dsRNA expression. The default pathway trafficking reporter pXS6:p67ΔCD with in-frame C-terminal 3×HA tag is described elsewhere 32. This plasmid was linearized with NotI for transfection into the TbRab11 RNAi cells, and clonal cell lines were selected with puromycin.
qRT-PCR
Total RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA). RNA was treated with DNase (Qiagen) and cDNA was prepared using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) per the manufacturer’s instructions. Quantitative Reverse transcription-PCR (qRT-PCR) was performed using 15 ng cDNA and iTaq universal Syber Green supermix (BioRad) using primers specific for Rab11 (FP 5′ ACTATCGGCGTGGAGTTTATG 3′ and RP 5′ TGGTAAATCGAACGGGAGATG 3′) and ISG65 (FP 5′ CAGTCCTTGTACCTGCCATTAT 3′ and RP 5′ GTCCACATCCTTGGAGCTATT 3′). Starting quantities of cDNA were normalized to the quantity of TbZFP3. Real-time PCR was performed on three biological replicates and values are presented as means ± SEM.
Antibody, secondary and blotting reagents
Rabbit anti-VSG221, rabbit anti-VSG117, rabbit anti-TbCatL, rabbit anti-BiP, and mouse monoclonal anti-p67 were described previously 39,59. Rabbit anti-transferrin receptor (TfR) was generously gifted by Dr. Piet Borst and Dr. Henri van Luenen (The Netherlands Cancer Institute, Amsterdam). Rabbit anti-ISG65 was generously gifted by Professor Mark Carrington (Cambridge University, UK). Rabbit anti-HA tag was purchased from Sigma-Aldrich (St Louis, MO, USA). The ligand conjugates used for endocytosis assays include transferrin:Alexa Fluor 488 (Tf:A488, Molecular Probes, Eugene, OR); tomato lectin:biotin and tomato lectin:fluorescein (TL:Bio & TL:FITC, Vector Laboratories, Burlingame, CA); and tomato lectin:Alexa Fluor 488 [TL:A488, prepared as in 42]. Streptavidin:Alexa Fluor 488 (SA:A488, Molecular Probes) was used to detect TL:Bio.
Endocytosis assays
Endocytosis assays were performed as previously described 32. Briefly, trypanosomes were washed in Hepes-buffered saline (HBS; 50 mM HepesKOH, pH 7.5, 50 mM NaCl, 5 mM KCl, 70 mM glucose) and pre-incubated (106/ml) in serum-free HMI9 with BSA (0.5 mg/ml) for 10 minutes at 37°C followed by addition of tomato lectin (Tf:488, TL:488 or TL:FITC) for flow cytometry or TL:Bio for epifluorescence microscopy at a final concentrations of 5 μg/ml. For binding experiments (tomato lectin only) cells were incubated for 30 min at 5°C. For uptake experiments (tomato lectin and Tf) cells were incubated for 30 min at 37°C, washed and incubated in fresh media for 20 minutes to chase ligand into the terminal lysosome. In each case labeled cells were prepared and analyzed by flow cytometry or microscopy as described previously 32. In the case of TL:FITC pulse chase uptake assay, cells were labeled as above under binding conditions, then washed into fresh binding medium, and incubated at 37°C to internalize and chase bound ligand. Cells were processed for IFA or flow cytometry. TL:Bio was detected in fixed permeabilized cells using SA:488.
Pulse-chase transport analyses
We performed pulse-chase analyses using metabolic radiolabelling with [35S]methionine/cysteine (Perkin Elmer, Waltham, MA, USA) and subsequent immunoprecipitation of specific radiolabeled proteins from cell lysates as previously described 15,16. Pulse times were VSG (2 min), p67 and p67ΔCD (15 min), and TbCatL (10 min). Chase times are indicated in the relevant figures. Immunoprecipitated proteins were analyzed by SDS-PAGE and phosphorimaging using a Typhoon FLA 9000 with native ImageQuant Software (GE Healthcare, Piscataway, NJ, USA). VSG transport kinetics were assayed by the hypotonic release assay as previously established in our laboratory 7. In brief, aliquots of cells from the chase period were lysed in water to expose surface VSG to endogenous glycosylphosphatidylinositol-specific phospholipase C (GPI-PLC). This enzyme converts surface membrane form VSG to soluble VSG, which was then separated from cell-associated VSG by centrifugation. Shed VSG in the supernatant fraction was immunoprecipitated and analyzed by SDS-PAGE/phosphorimaging. Natural shedding of VSG from live cells was assayed by immunoprecipitation from cell and media fractions prepared at T0 and T4.
Recycling of surface VSG
This assay is a modification of that first developed by Engstler et. al 10. Mid-to-late log phase cells were cultured in the presence of cycloheximide (100 μg/ml, 1 hr, 37°C) to flush nascent VSG from the secretory pathway. Cells were then washed into PBSG (PBS, 10 mg/ml glucose, 106 cells/ml) and cell surface amino groups were blocked by treatment with membrane impermeant Sulfo-NHS-Acetate (Thermo Fisher Scientific, Waltham, MA; 30 min on ice, 10 mM final). After washing, cells were incubated in PBSG for 5 minutes at 4°C as a control, or at 37°C to allow recycling of unblocked VSG from internal endocytic compartments to the cell surface. Newly exposed surface amino groups were then labeled by addition of Sulfo-NHS-Biotin (Thermo Fisher Scientific; 1 mM, 15 minutes on ice). Biotinylation reactions were then quenched by addition of TrisHCl, pH 7.5 (25 mM for 5 min on ice) and cells were washed three times with PBSG. After labeling, cells were stained with streptavidin-A488 (Molecular Probes, Eugene, OR) and analyzed by flow cytometry using DAPI counter-staining (0.5 μg/ml) to exclude dead cells. Alternatively, cells were lysed in RIPA buffer and VSG was specifically immunoprecipitated with anti-VSG221 antibody covalently coupled to Protein A sepharose 60. Immunoprecipitates were fractionated by SDS-PAGE and transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA) using a Trans-Blot Turbo apparatus (BioRad, Hercules, California). Membranes were blocked and probed with streptavidin-IR dye 800 CW in Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln NE). All washes were performed with PBS, 0.5% Tween20. Quantitative fluorescent signals were scanned on an Odyssey CLx Imager (Li-Cor).
ISG65 turnover
ISG65 turnover was assayed by a cycloheximide ‘chase’ protocol 16. Protein synthesis was arrested by incubation with cycloheximide (100 μg/mL), and as indicated lysosomal thiol protease activity was inhibited by addition of FMK024 (morpholinourea- phenylalanine-homophenylalanine-fluoromethyl ketone; 20 μM; MP Biomedicals, Aurora, OH) prior to and during cycloheximide treatment. At the indicated times whole cell lysates were fractionated by SDS-PAGE and blotted as described above using primary anti-ISG65 antibody and secondary goat anti-rabbit IgG:IRDye800CW (Li-Cor).
Surface biotinylation
Biotinylation of surface proteins was performed as described previously 60. Cell surface amino groups were biotinylated in PBSG by treatment with membrane impermeant Sulfo-NHS-Biotin (Thermo Fisher Scientific; 30 min on ice, 1 mM final, 5×107 cells/ml). Biotinylation reactions were then quenched by addition of TrisHCl, pH 7.5 (25 mM for 5 min on ice). Cells were washed three times with PBSG and processed for immunoprecipitation followed by western analysis with streptavidin-IR dye 800 CW (Li-Cor) as described above.
Epifluorescence microscopy
Immunofluorescence (IFA) microscopy was performed as previously reported by our group 16,32. Cells were fixed with 2% formaldehyde, permeablized with 0.5% NP40 and stained with DAPI (0.5 μg ml−1) to reveal nuclei and kinetoplasts. Serial image stacks (0.2 micron Z-increment) were collected with capture times from 100–400 msec (100× PlanApo, oil immersion, 1.46 na) on a motorized Zeiss Axioimager M2 stand equipped with a rear-mounted excitation filter wheel, a triple pass (DAPI/FITC/Texas Red) emission cube, differential interference contrast (DIC) optics, and an Orca ER CCD camera (Hamamatsu, Bridgewater, NJ). All images were collected with Volocity 6.1 Acquisition Module (Improvision Inc., Lexington, MA) and individual channel stacks were deconvolved by a constrained iterative algorithm, pseudocolored, and merged using Volocity 6.1 Restoration Module. All images presented are summed stack projections of merged channels. We have previously validated the xyz pixel precision of this arrangement 5.
Scanning electron microscopy
Cells were fixed and dehydrated as previously described 61 with modifications established in 27. Briefly, cells were collected (8×105 per sample, and fixed in 2.5% EM grade glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) in complete HMI9 medium (2 hours at 4°C). Cells were then gently collected by syringe-passage onto 0.2 μm pore polycarbonate filters (Whatman Nucleopore, 25 mm dia., SIGMA-ALDRICH, St. Louis, MO) keeping fluid in the upper filter chamber (Whatman Swin-Lok Cartridge, 25 mm, SIGMA-ALDRICH) in all subsequent steps until final air-drying. Cells were washed and dehydrated through an ethanol series and finally dried with hexamethyldisilazane. Filters were removed, air-dried, and samples were coated with evaporated carbon at high vacuum (Denton 502 evaporator). Images were acquired on a Hitachi SU70 FESEM at 2.0 KeV using an in-lens secondary electron detector at zero tilt, or a lower detector at 70° tilt. Nanotubes were quantified from 100 randomly selected cells in zero tilt images.
Floatation analysis
Control and TbRab11 silenced (22 hr) cells were harvested from late log phase cultures, washed, resuspended (106 cells/ml) in complete HMI9 media, and incubated at 37°C for 4 hr. Conditioned supernatants were collected by spin filtration through SpinX centrifuge filters (0.45 μm, Corning Inc., Salt Lake City, UT). Cleared supernatant was adjusted to 35% Opti-Prep (SIGMA-Aldrich), and 350 μl was overlaid with 30% Opti-Prep/PBS (600 μl) and 10% Opti-Prep/PBS (450 μl) in thick wall polycarbonate TLS-55 tubes (Beckman Coulter Inc., Brea, CA). After centrifugation (55K rpm, 2 hr, 4°C) gradients were manually fractionated from the top (5× 280 μl fractions), adjusted to standard RIPA conditions and immunoprecipitated with anti-VSG221. Matched sets of immunoprecipitates were fractionated by SDS/PAGE and immunoblotted with anti-VSG221 for VSG or anti-VSG117 as a source of anti-Cross Reacting Determinant (anti-CRD) antibodies.
Data analyses
ImageJ (http://imagej.nih.gov/ij/) was used to quantify data from phosphorimager assays and fluorescence blot scans. For quantitative analysis of band intensities. signals from each lane were subtracted from the signal of the equivalent unlabeled areas of that lane. Data analyses were performed with Prism6 software (GraphPad Software, Inc, San Diego CA). Biological replicates (n) were obtained as indicated.
Supplementary Material
Following TbRab11 silencing (22 hr) cells were pulse-chase radiolabeled as in Fig. 3B and labeled polypeptides were immunoprecipitated with anti-TfR. Samples were fractionated by SDS-PAGE and representative phosphorimages for control (Tet−) and silenced (Tet+) cells are presented (107 cell equivalents per lane). ESAG7, E7; immature ESAG6 (E6i); mature ESAG6 (E6m).
Using a previously developed RNAi cell line 32 the effect of late endosomal TbRab7 silencing on VSG turnover was assessed as in Figs 4 & 5. Following specific dsRNA induction (20 hr) cells were analyzed in two ways. A. Control (tet−) and silenced (tet+) cells were pulse-chase radiolabeled and the presence of labeled VSG in cell and media fractions was determined by immunoprecipitation at 0 and 4 hrs of chase. A representative phosphorimage is presented. As was seen with TbRab11 silencing, increased VSG is found in the media fraction at the end of the chase (lane 5 vs. 6), and this shedding is greatly enhanced when TbRab7 is depleted (Lane 7 vs. 8). B. The presence of nanotubes was assessed by SEM. Cells with one or more nanotubes were scored positive. Data are presented as mean ± std. dev. (n=2 biological replicates, 100 cells each).
Synopsis.
The small GTPase TbRab11 is studied by RNAi silencing in trypanosomes. Knockdown has no effect on trafficking of the GPI-anchored virulence factors Transferrin Receptor and Variant Surface Glycoprotein. Knockdown greatly reduces recycling of transmembrane Invariant Surface Glycoproten 65 leading to enhanced lysosomal turnover. TbRab11 selectively regulates trafficking of parasite surface proteins.
Acknowledgments
The authors are grateful to Professor Mark Carrington (Cambridge University, UK) for anti-ISG65 antibody, and to Professors Piet Borst and Henri Luenen (Netherlands Cancer Institute, Amsterdam) for anti-TfR antibody. Appreciation to Brandon Waxman for assistance in developing the floatation assay, and to Dr. Jason Silverman for generating the TbRab11 RNAi cell line. This work was supported by United States Public Health Service Grants R01 AI056866 to JDB, and by support of the Jacobs School of Medicine and Biomedical Sciences.
References
- 1.Vickerman K, Luckins AG. Localization of variable antigens in the surface coat of Trypanosoma brucei using ferritin conjugated antibody. Nature. 1969;224(5224):1125–1126. doi: 10.1038/2241125a0. [DOI] [PubMed] [Google Scholar]
- 2.Cross GAM. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitol. 1975;71:393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- 3.Schwede A, Carrington M. Bloodstream form Trypanosome plasma membrane proteins: antigenic variation and invariant antigens. Parasitol. 2010;137(14):2029–2039. doi: 10.1017/S0031182009992034. [DOI] [PubMed] [Google Scholar]
- 4.Rudenko G. African trypanosomes: the genome and adaptations for immune evasion. Essays Biochem. 2011;51:47–62. doi: 10.1042/bse0510047. [DOI] [PubMed] [Google Scholar]
- 5.Sevova ES, Bangs JD. Streamlined architecture and glycosylphosphatidylinositol-dependent trafficking in the early secretory pathway of African trypanosomes. Mol Biol Cell. 2009;20(22):4739–4750. doi: 10.1091/mbc.E09-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman JS, Bangs JD. Form and function in the trypanosomal secretory pathway. Curr Opin Microbiol. 2012;15(4):463–468. doi: 10.1016/j.mib.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangs JD, Andrews NW, Hart GW, Englund PT. Posttranslational modification and intracellular transport of a trypanosome variant surface glycoprotein. J Cell Biol. 1986;103(1):255–263. doi: 10.1083/jcb.103.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langreth SG, Balber AE. Protein uptake and digestion in bloodstream and culture forms of Trypanosoma brucei. J Protozool. 1975;22:40–53. doi: 10.1111/j.1550-7408.1975.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 9.Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Micro. 2004;53:735–744. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]
- 10.Engstler M, Thilo L, Weise F, et al. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J Cell Sci. 2004;117:1105–1115. doi: 10.1242/jcs.00938. [DOI] [PubMed] [Google Scholar]
- 11.Balber AE, Bangs JD, Jones SM, Proia RL. Inactivation or elimination of potentially trypanolytic, complement-activating immune complexes by pathogenic trypanosomes. Infect Immun. 1979;24:617–627. doi: 10.1128/iai.24.3.617-627.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engstler M, Pfohl T, Herminghaus S, et al. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell. 2007;131:505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 13.Barry JD. Capping of variable antigen on Trypanosoma brucei, and its immunological and biological significance. J Cell Sci. 1979;37:287–302. doi: 10.1242/jcs.37.1.287. [DOI] [PubMed] [Google Scholar]
- 14.Ackers JP, Dhir V, Field MC. A bioinformatic analysis of the RAB genes of Trypanosoma brucei. Mol Biochem Parasitol. 2005;141(1):89–97. doi: 10.1016/j.molbiopara.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Alexander DL, Schwartz KJ, Balber AE, Bangs JD. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J Cell Sci. 2002;115(Pt 16):3253–3263. doi: 10.1242/jcs.115.16.3253. [DOI] [PubMed] [Google Scholar]
- 16.Silverman JS, Muratore KA, Bangs JD. Characterization of the late endosomal ESCRT machinery in Trypanosoma brucei. Traffic. 2013;14(10):1078–1090. doi: 10.1111/tra.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Triggs VP, Bangs JD. Glycosylphosphatidylinositol-dependent protein trafficking in bloodstream stage Trypanosoma brucei. Eukaryot Cell. 2003;2(1):76–83. doi: 10.1128/EC.2.1.76-83.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulow R, Nonnengasser C, Overath P. Release of the variant glycoprotein during differentiation of bloodstream to procyclic forms of Trypanosoma brucei. Mol Biochem Parasitol. 1989;32:85–92. doi: 10.1016/0166-6851(89)90132-1. [DOI] [PubMed] [Google Scholar]
- 19.Seyfang A, Mecke D, Duszenko M. Degradation, recycling, and shedding of Trypanosoma brucei variant surface glycoprotein. J Protozool. 1990;37(6):546–552. doi: 10.1111/j.1550-7408.1990.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 20.Ligtenberg MJL, Bitter W, Kieft R, et al. Reconstitution of a surface transferrin binding complex in insect form Trypanosoma brucei. Euro Mol Biol Org J. 1994;13:2565–2573. doi: 10.1002/j.1460-2075.1994.tb06546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steverding D, Y-D S, Fuchs H, Tauber R, Overath P. Transferrin-binding protein complex is the receptor for transferrin uptake in Trypanosoma brucei. J Cell Biol. 1995;131:1173–1182. doi: 10.1083/jcb.131.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salmon D, Geuskens M, Hanocq F, et al. A novel heterodimeric transferrin receptor encoded by a pair of VSG expression site-associated genes in T. brucei. Cell. 1994;78:75–86. doi: 10.1016/0092-8674(94)90574-6. [DOI] [PubMed] [Google Scholar]
- 23.Ziegelbauer K, Multhaup G, Overath P. Molecular characterization of two invariant surface glycoproteins specific for the bloodstream stage of Trypanosoma brucei. J Biol Chem. 1992;267:10797–10803. [PubMed] [Google Scholar]
- 24.Ziegelbauer K, Overath P. Identification of invariant surface glycoproteins in the bloodstream stage of Trypanosoma brucei. J Biol Chem. 1992;267(15):10791–10796. [PubMed] [Google Scholar]
- 25.Jefferies TR, Morgan GW, Field MC. A developmentally regulated rab11 homologue in Trypanosoma brucei is involved in recycling processes. J Biol Chem. 2001;114:2617–2626. doi: 10.1242/jcs.114.14.2617. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz KJ, Peck RF, Tazeh NN, Bangs JD. GPI valence and the fate of secretory membrane proteins in African trypanosomes. J Cell Sci. 2005;118(Pt 23):5499–5511. doi: 10.1242/jcs.02667. [DOI] [PubMed] [Google Scholar]
- 27.Tiengwe C, Bush PJ, Bangs JD. Controlling transferrin receptor trafficking with GPI-valence in bloodstream stage African trypanosomes. PLoS Pathog. 2017;13(5):e1006366. doi: 10.1371/journal.ppat.1006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung WL, Leung KF, Carrington M, Field MC. Ubiquitylation is required for degradation of transmembrane surface proteins in trypanosomes. Traffic. 2008;9(10):1681–1697. doi: 10.1111/j.1600-0854.2008.00785.x. [DOI] [PubMed] [Google Scholar]
- 29.Leung KF, Riley FS, Carrington M, Field MC. Ubiquitylation and developmental regulation of invariant surface protein expression in trypanosomes. Eukaryot Cell. 2011;10(7):916–931. doi: 10.1128/EC.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung WL, Carrington M, Field MC. Cytoplasmic targeting signals in transmembrane invariant surface glycoproteins of trypanosomes. J Biol Chem. 2004;279(52):54887–54895. doi: 10.1074/jbc.M409311200. [DOI] [PubMed] [Google Scholar]
- 31.Böhme U, Cross GAM. Mutational analysis of the variant surface glycoprotein GPI-anchor signal sequence in Trypanosoma brucei. J Cell Sci. 2002;115:805–816. doi: 10.1242/jcs.115.4.805. [DOI] [PubMed] [Google Scholar]
- 32.Silverman JS, Schwartz KJ, Hajduk SL, Bangs JD. Late endosomal Rab7 regulates lysosomal trafficking of endocytic but not biosynthetic cargo in Trypanosoma brucei. Mol Microbiol. 2011;82(3):664–678. doi: 10.1111/j.1365-2958.2011.07842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilden JK, Umaer K, Kruzel EK, et al. The role of the PI(3,5)P2 kinase TbFab1 in endo/lysosomal trafficking in Trypanosoma brucei. Mol Biochem Parasitol. 2017;214:52–61. doi: 10.1016/j.molbiopara.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall BS, Smith E, Langer W, Jacobs LA, Goulding D, Field MC. Developmental variation in Rab11-dependent trafficking in Trypanosoma brucei. Eukaryot Cell. 2005;4(5):971–980. doi: 10.1128/EC.4.5.971-980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolan DP, Geuskens M, Pays E. N-linked glycans containing linear poly-N-acetyllactosamine as sorting signals in endocytosis in Trypanosoma brucei. Curr Biol. 1999;9(20):1169–1172. doi: 10.1016/S0960-9822(00)80018-4. [DOI] [PubMed] [Google Scholar]
- 36.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCann AK, Schwartz KJ, Bangs JD. A determination of the steady state lysosomal pH of bloodstream stage African trypanosomes. Mol Biochem Parasitol. 2008;159(2):146–149. doi: 10.1016/j.molbiopara.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunfelder CG, Engstler M, Weise F, et al. Endocytosis of a glycosylphosphatidylinositol-anchored protein via clathrin-coated vesicles, sorting by default in endosomes, and exocytosis via RAB11-positive carriers. Mol Biol Cell. 2003;14(5):2029–2040. doi: 10.1091/mbc.E02-10-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peck RF, Shiflett AM, Schwartz KJ, McCann A, Hajduk SL, Bangs JD. The LAMP-like protein p67 plays an essential role in the lysosome of African trypanosomes. Mol Microbiol. 2008;68(4):933–946. doi: 10.1111/j.1365-2958.2008.06195.x. [DOI] [PubMed] [Google Scholar]
- 40.Szempruch AJ, Sykes SE, Kieft R, et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell. 2016;164(1–2):246–257. doi: 10.1016/j.cell.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbet AF, McGuire TC. Crossreacting determinants in variant-specific surface antigens of African trypanosomes. Proc Natl Acad Sci U S A. 1978;75(4):1989–1993. doi: 10.1073/pnas.75.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tazeh NN, Silverman JS, Schwartz KJ, Sevova ES, Sutterwala SS, Bangs JD. Role of AP-1 in developmentally regulated lysosomal trafficking in Trypanosoma brucei. Eukaryot Cell. 2009;8(9):1352–1361. doi: 10.1128/EC.00156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelley RJ, Alexander DL, Cowan C, Balber AE, Bangs JD. Molecular cloning of p67, a lysosomal membrane glycoprotein from Trypanosoma brucei. Mol Biochem Parasitol. 1999;98(1):17–28. doi: 10.1016/s0166-6851(98)00155-8. [DOI] [PubMed] [Google Scholar]
- 44.Overath P, Chaudhri M, Steverding D, Ziegelbauer K. Invariant surface proteins in bloodstream forms of Trypanosoma brucei. Parasitol Today. 1994;10(2):53–58. doi: 10.1016/0169-4758(94)90393-x. [DOI] [PubMed] [Google Scholar]
- 45.Chung WL, Carrington M, Field MC. Cytoplasmic targeting signals in transmembrane invariant surface glycoproteins of trypanosomes. J Biol Chem. 2004;279(52):54887–54895. doi: 10.1074/jbc.M409311200. [DOI] [PubMed] [Google Scholar]
- 46.Goldenring JR. Recycling endosomes. Curr Opin Cell Biol. 2015;35:117–122. doi: 10.1016/j.ceb.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 2014;24(7):407–415. doi: 10.1016/j.tcb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Niyogi S, Mucci J, Campetella O, Docampo R. Rab11 regulates trafficking of trans-sialidase to the plasma membrane through the contractile vacuole complex of Trypanosoma cruzi. PLoS Pathog. 2014;10(6):e1004224. doi: 10.1371/journal.ppat.1004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niyogi S, Docampo R. A novel role of Rab11 in trafficking GPI-anchored trans-sialidase to the plasma membrane of Trypanosoma cruzi. Small GTPases. 2015;6(1):8–10. doi: 10.4161/21541248.2014.978712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunter J, Webb H, Carrington M. Determinants of GPI-PLC localisation to the flagellum and access to GPI-anchored substrates in trypanosomes. PLoS Pathog. 2013;9(8):e1003566. doi: 10.1371/journal.ppat.1003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bulow R, Griffiths G, Webster P, Stierhof YD, Opperdoes FR, Overath P. Intracellular localization of the glycosyl-phosphatidylinositol-specific phospholipase C of Trypanosoma brucei. J Cell Sci. 1989;93(Pt 2):233–240. doi: 10.1242/jcs.93.2.233. [DOI] [PubMed] [Google Scholar]
- 53.Koumandou VL, Boehm C, Horder KA, Field MC. Evidence for recycling of invariant surface transmembrane domain proteins in African trypanosomes. Eukaryot Cell. 2013;12(2):330–342. doi: 10.1128/EC.00273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beckett K, Monier S, Palmer L, et al. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic. 2013;14(1):82–96. doi: 10.1111/tra.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koles K, Nunnari J, Korkut C, et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287(20):16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99(1):89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 57.Hirumi H, Hirumi K. Axenic culture of African trypanosome bloodstream forms. Parasitol Today. 1994;10(2):80–84. doi: 10.1016/0169-4758(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 58.Burkard G, Fragoso CM, Roditi I. Highly efficient stable transformation of bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 2007;153(2):220–223. doi: 10.1016/j.molbiopara.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 59.McDowell MA, Ransom DM, Bangs JD. Glycosylphosphatidylinositol-dependent secretory transport in Trypanosoma brucei. Biochem J. 1998;335(Pt 3):681–689. doi: 10.1042/bj3350681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gruszynski AE, DeMaster A, Hooper NM, Bangs JD. Surface coat remodeling during differentiation of Trypanosoma brucei. J Biol Chem. 2003;278(27):24665–24672. doi: 10.1074/jbc.M301497200. [DOI] [PubMed] [Google Scholar]
- 61.Gluenz E, Wheeler RJ, Hughes L, Vaughan S. Scanning and three-dimensional electron microscopy methods for the study of Trypanosoma brucei and Leishmania mexicana flagella. Methods Cell Biol. 2015;127:509–542. doi: 10.1016/bs.mcb.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pal A, Hall BS, Nesbeth DN, Field HI, Field MC. Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycosylphosphatidylinositol-specific endosomal pathway. J Biol Chem. 2002;277(11):9529–9539. doi: 10.1074/jbc.M110055200. [DOI] [PubMed] [Google Scholar]
- 63.Jeffries TR, Morgan GW, Field MC. A developmentally regulated rab11 homologue in Trypanosoma brucei is involved in recycling processes. J Cell Sci. 2001;114(Pt 14):2617–2626. doi: 10.1242/jcs.114.14.2617. [DOI] [PubMed] [Google Scholar]
- 64.Peck RF, Shiflett AM, Schwartz KJ, McCann A, Hajduk SL, Bangs JD. The LAMP-like protein p67 plays an essential role in the lysosome of African trypanosomes. Mol Microbiol. 2008;68(4):933–946. doi: 10.1111/j.1365-2958.2008.06195.x. [DOI] [PubMed] [Google Scholar]
- 65.Morgan GW, Allen CL, Jeffries TR, Hollinshead M, Field MC. Developmental and morphological regulation of clathrin-mediated endocytosis in Trypanosoma brucei. J Cell Sci. 2001;114(Pt 14):2605–2615. doi: 10.1242/jcs.114.14.2605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Following TbRab11 silencing (22 hr) cells were pulse-chase radiolabeled as in Fig. 3B and labeled polypeptides were immunoprecipitated with anti-TfR. Samples were fractionated by SDS-PAGE and representative phosphorimages for control (Tet−) and silenced (Tet+) cells are presented (107 cell equivalents per lane). ESAG7, E7; immature ESAG6 (E6i); mature ESAG6 (E6m).
Using a previously developed RNAi cell line 32 the effect of late endosomal TbRab7 silencing on VSG turnover was assessed as in Figs 4 & 5. Following specific dsRNA induction (20 hr) cells were analyzed in two ways. A. Control (tet−) and silenced (tet+) cells were pulse-chase radiolabeled and the presence of labeled VSG in cell and media fractions was determined by immunoprecipitation at 0 and 4 hrs of chase. A representative phosphorimage is presented. As was seen with TbRab11 silencing, increased VSG is found in the media fraction at the end of the chase (lane 5 vs. 6), and this shedding is greatly enhanced when TbRab7 is depleted (Lane 7 vs. 8). B. The presence of nanotubes was assessed by SEM. Cells with one or more nanotubes were scored positive. Data are presented as mean ± std. dev. (n=2 biological replicates, 100 cells each).