Abstract
In vitro erythroid differentiation from primary human cells is valuable to develop genetic strategies for hemoglobin disorders. However, current erythroid differentiation methods are encumbered by modest transduction rates and high baseline fetal hemoglobin production. In this study, we sought to improve both genetic modification and hemoglobin production among human erythroid cells in vitro. To model therapeutic strategies, we transduced human CD34+ cells and peripheral blood mononuclear cells (PBMCs) with lentiviral vectors and compared erythropoietin-based erythroid differentiation using fetal-bovine-serum-containing media and serum-free media. We observed more efficient transduction (85%–93%) in serum-free media than serum-containing media (20%–69%), whereas the addition of knockout serum replacement (KSR) was required for serum-free media to promote efficient erythroid differentiation (96%). High-level adult hemoglobin production detectable by electrophoresis was achieved using serum-free media similar to serum-containing media. Importantly, low fetal hemoglobin production was observed in the optimized serum-free media. Using KSR-containing, serum-free erythroid differentiation media, therapeutic adult hemoglobin production was detected at protein levels with β-globin lentiviral transduction in both CD34+ cells and PBMCs from sickle cell disease subjects. Our in vitro erythroid differentiation system provides a practical evaluation platform for adult hemoglobin production among human erythroid cells following genetic manipulation.
Keywords: lentiviral vector, hematopoietic stem cells, erythroid cells, erythroid differentiation, adult hemoglobin, gene therapy, genome editing
Introduction
In vitro erythroid differentiation from primary human cells is a valuable tool for the development of genetic strategies aimed at red blood cell (RBC) diseases. Genetic modification of hematopoietic stem cells (HSCs) is potentially curative for hemoglobin (Hb) disorders, including β-thalassemia and sickle cell disease (SCD). In current HSC-targeted gene therapy trials, a normal β-globin or anti-sickling βT87Q-globin gene is delivered with lentiviral transduction into HSCs in β-thalassemia and SCD patients. Therapeutic efficacy has proven more robust in β-thalassemia; however, further development of gene therapy strategies remains crucial to cure Hb disorders, especially for SCD.1, 2 The lentiviral delivery system for genetic modification is useful not only for addition of the β-globin (or γ-globin) gene but also an induction of Hb switching from adult Hb (or sickle Hb) to fetal Hb, which can be achieved by RNAi targeting BCL11A gene as well as through forced looping between the β-globin locus control region and the γ-globin promoter.3, 4 The recent development of robust genome-editing tools also allows for development of new genetic strategies to treat Hb disorders, including fetal Hb induction by DNA breakage of either the erythroid-specific BCL11A gene enhancer or the potential BCL11A binding site upstream of γ-globin promoter as well as gene correction of the SCD mutation through homology-directed repair in human CD34+ cells,5, 6, 7
To evaluate these genetic tools, in vitro human erythroid differentiation culture must be optimal, with high-level baseline adult Hb production as well as minimal fetal Hb. In addition, optimal in vitro erythroid differentiation methods could be useful as an alternative source of RBC transfusion, because RBC transfusion has potential risks of alloimmunization, transmitting infection, and transfusion reactions. For this purpose, erythroid cells are generated from human hematopoietic progenitor cells, including CD34+ cells and peripheral blood mononuclear cells (PBMCs), because predominant adult Hb production could result from these primary cells following erythroid differentiation.
Human erythropoietin is a key cytokine to induce erythroid differentiation from human progenitor cells, and several cytokines and metabolic hormones are added to support further differentiation and expansion.8 Fetal bovine serum (FBS) has also proven essential for erythroid cell generation in vitro, yet clinical application requires the development of serum-free erythroid differentiation methods. Furthermore, the elimination of FBS could prove valuable in reducing variability among serum lots while minimizing the risks of infection and immunoreaction in patients.8, 9
Current erythroid differentiation methods were developed for robust expansion of erythroid cells, but not for an optimal evaluation of Hb production following genetic modification, which requires not only efficient genetic modification but also high-level adult Hb production and minimal fetal Hb production. In this study, we sought to improve erythroid differentiation and Hb production following genetic modification of human CD34+ cells and PBMCs.
Results
Lentiviral Transduction of Human CD34+ Cells before and after Initiating Erythroid Differentiation
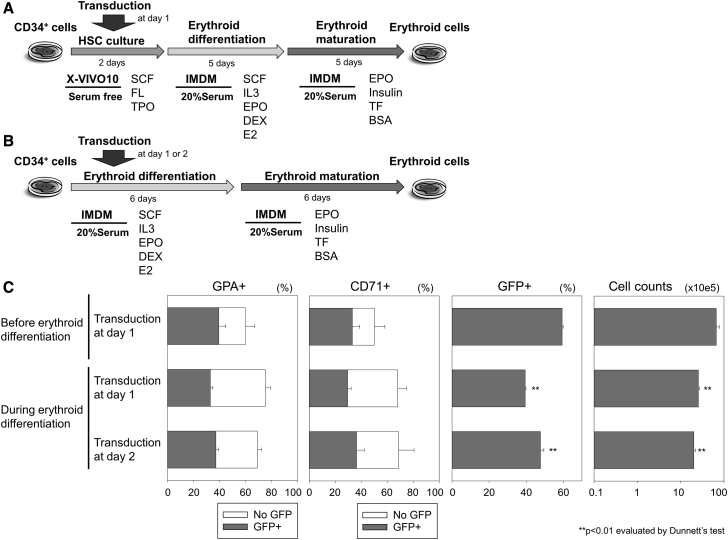
We transduced human CD34+ cells with an EGFP-expressing lentiviral vector in serum-free X-VIVO10 media, mimicking that utilized for clinical transplantation of HSCs with lentiviral transduction. These cells were differentiated into erythroid cells using Iscove’s modified Dulbecco’s medium (IMDM)-based erythroid differentiation media containing 20% FBS and 2 U/mL erythropoietin (EPO), including a differentiation phase with stem cell factor (SCF) and interleukin-3 (IL-3), followed by a maturation phase (Figure 1A).8 Two weeks later, we evaluated erythroid maturation (evidenced by high percentage of glycophorin A [%GPA] and low percentage of CD71 [%CD71]), transduction efficiency (GFP expression [%GFP] among GPA-positive erythroid cells), and total cell counts. To improve transduction efficiency specifically for the erythroid lineage, we transduced CD34+ cells at 1 or 2 days after initiating erythroid differentiation (Figure 1B) and compared to transduction in serum-free X-VIVO10 media at 1 day before erythroid differentiation.10 The 2-day expansion in X-VIVO10 culture before initiating erythroid differentiation allowed for 3-fold more proliferation of erythroid cells (p < 0.01; Figure 1C). We observed similar %GPA (60% ± 7% versus 69%–75%) between transduction before and after initiating erythroid differentiation, whereas unexpectedly, lower %GFP (44%–54%; p < 0.01) was observed when the transduction was performed during erythroid differentiation in serum-containing media, as compared to serum-free transduction before differentiation (66% ± 1%; Figures 1C and S1).
Figure 1.
Lentiviral Transduction of Human CD34+ Cells before and after Initiating Erythroid Differentiation
(A and B) We transduced CD34+ cells with an EGFP-encoding lentiviral vector at 1 or 2 days after initiating differentiation (B) and compared to transduction in X-VIVO10 media at 1 day before differentiation (A). (C) We evaluated erythroid differentiation (evidenced by high percentages of glycophorin A [GPA] and low percentages of CD71 expression), transduction efficiency (GFP positive), and cell counts 12 days after differentiation. DEX, dexamethasone; E2, estradiol; EPO, erythropoietin; FL, fms-related tyrosine kinase 3 ligand; IL-3: interleukin-3; IMDM, Iscove’s modified Dulbecco’s medium; SCF, stem cell factor; TF, transferrin; TPO, thrombopoietin; bars: mean ± SEM. All experiments were performed in triplicate.
More Efficient Transduction in Serum-free Erythroid Differentiation for Human CD34+ Cells and PBMCs
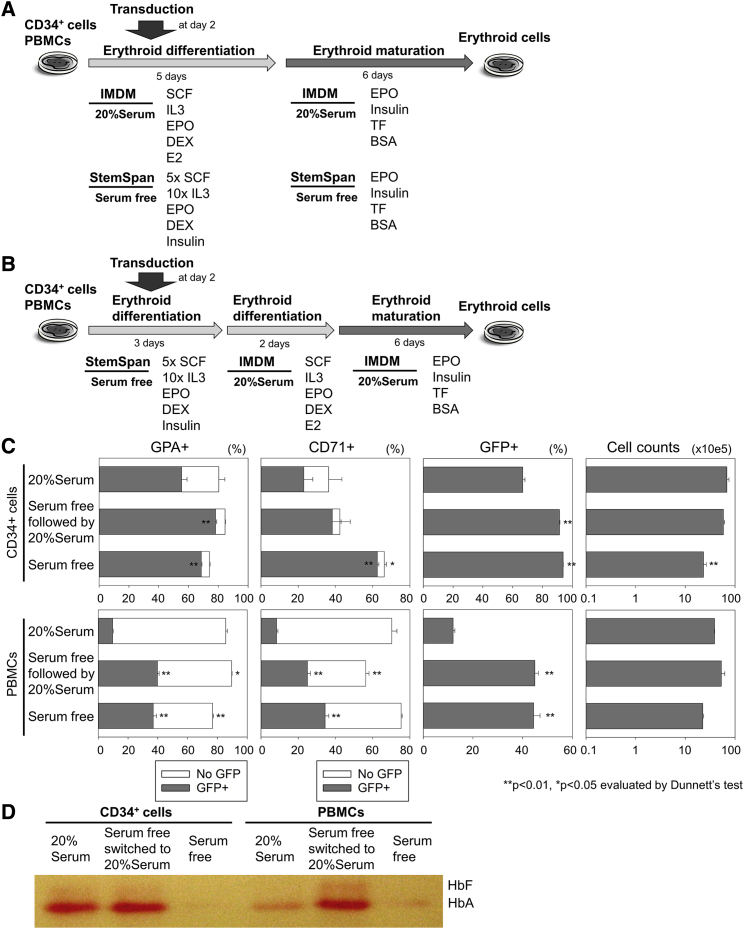
We hypothesized that lentiviral transduction is inhibited by FBS contained in differentiation media. Therefore, we evaluated StemSpan-based serum-free erythroid differentiation media, including the equivalent amounts of EPO (2 U/mL) and higher concentrations of cytokines (5-fold SCF [5×SCF] and 10-fold IL-3 [10×IL-3])11 with lentiviral transduction at 2 days after initiating differentiation from CD34+ cells, as compared to IMDM-based serum-containing differentiation media (20% FBS and EPO; Figure 2A). We selected StemSpan as a base of serum-free erythroid differentiation media, because it is produced from IMDM with several supplements. After a 2-week differentiation culture, we observed higher %GFP (93% ± 0%; p < 0.01) in the serum-free media than in the serum-containing media (69% ± 1%; Figures 2C and S2). However, the serum-free media resulted in insufficient erythroid differentiation with similar %GPA (74.1% ± 0% versus 80% ± 4%) and higher %CD71 (66% ± 1% versus 36% ± 7%; p < 0.05), 3-fold less erythroid cell expansion (p < 0.01; Figures 2C and S2), and undetectable Hb production by Hb electrophoresis (Figure 2D), as compared to serum-containing media.
Figure 2.
More Efficient Transduction in Serum-free Erythroid Differentiation for Human CD34+ Cells and PBMCs
(A) We used both CD34+ cells and peripheral blood mononuclear cells (PBMCs) to evaluate StemSpan-based serum-free differentiation media with lentiviral transduction at 2 days after initiating differentiation. (B) We performed transduction in StemSpan-based serum-free media followed by erythroid differentiation in serum-containing differentiation media. (C) We evaluated erythroid differentiation (GPA and CD71 expression), transduction (GFP positive), and cell counts 11 days after differentiation. (D) Hemoglobin (Hb) production was evaluated by Hb electrophoresis at day 11. HbA, adult hemoglobin; HbF, fetal hemoglobin; bars: mean ± SEM. All experiments except Hb electrophoresis (single run) were performed in triplicate.
To obtain both efficient transduction and high-level Hb production, we explored transduction in StemSpan-based serum-free differentiation media followed by erythroid differentiation in serum-containing media (Figure 2B), which resulted in higher %GFP (92% ± 1%; p < 0.01), efficient erythroid differentiation with similar %GPA (85% ± 0%) and %CD71 (42% ± 6%), similar erythroid cell expansion, and high-level adult Hb production, as compared to serum-containing differentiation media alone (Figures 2C, 2D, and S2). When we used human PBMCs for transduction in StemSpan-based serum-free media followed by erythroid differentiation in serum-containing media, we also obtained efficient erythroid differentiation with higher %GPA (90% ± 0% versus 86% ± 1%; p < 0.05) and lower %CD71 (56% ± 2% versus 70% ± 3%; p < 0.01) and sufficient Hb production with more efficient transduction (44% ± 1% versus 11% ± 1%; p < 0.01; Figures 2C, 2D, and S2), as compared to serum-containing differentiation media.
Improvement of Erythroid Differentiation with Efficient Lentiviral Transduction through Addition of Lipids or KSR
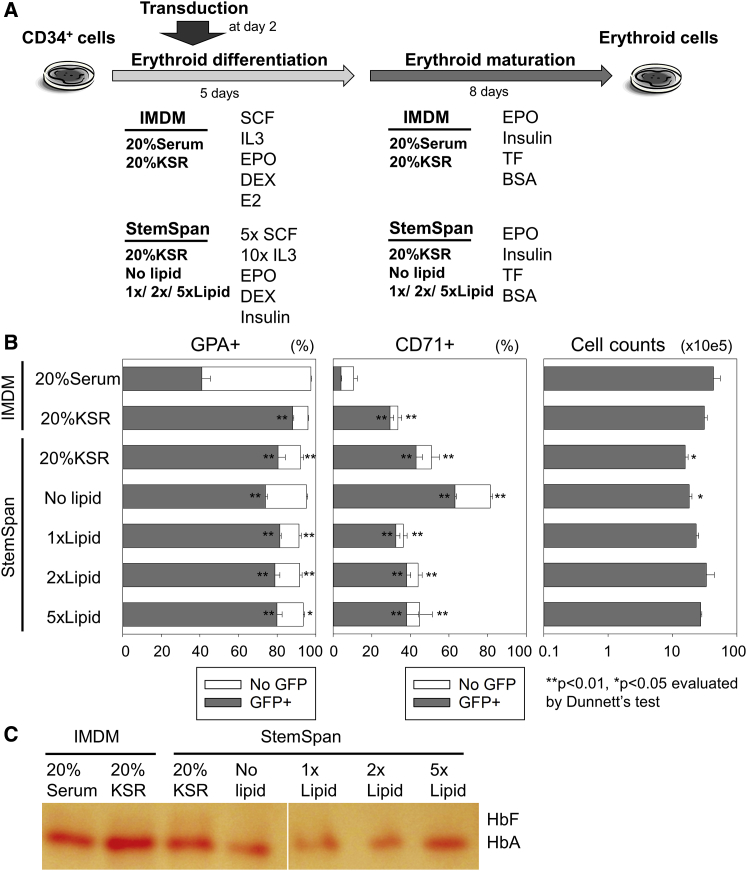
We further investigated serum-free erythroid differentiation with lentiviral transduction to eliminate FBS supplementation for the entire procedure, because FBS increases the variability observed among experiments.10 We hypothesized that lipid supplementation is essential for efficient erythroid differentiation in the absence of serum, because other supplements, including lipids, were described for optimal proliferation in erythroid cells as well as erythroid colonies, and lipids are contained in FBS.8, 12, 13 Therefore, we added a lipid mixture (1 μL/mL [1×], 2 μL/mL [2×], or 5 μL/mL [5×]) or 20% knockout serum replacement (KSR) (including lipid-rich albumin) into StemSpan-based serum-free erythroid differentiation media or simply replaced 20% FBS with 20% KSR in IMDM-based differentiation media (Figure 3A). Both lipid and KSR supplementation in serum-free differentiation media improved erythroid differentiation from CD34+ cells with efficient lentiviral transduction, resulting in higher %GFP (85%–92% versus 42% ± 4%; p < 0.01) and slightly less erythroid differentiation with comparable %GPA (91%–96% versus 97% ± 0%; p < 0.05 or not significant) and higher %CD71 (34%–51% versus 11% ± 2%; p < 0.01; Figure 3B) and robust Hb production (Figure 3C) as compared to serum-containing differentiation media. We observed nucleated cells with blue to pink cytoplasm (like erythroblasts) as well as enucleated cells with pink cytoplasm (like RBCs) in differentiated cells using either 20% serum or 20% KSR (Figure S3A). Even with less erythroid differentiation (higher %CD71), slightly higher or similar enucleation ratios were detected in 20% KSR serum-free differentiation media (15%–25%) as compared to 20% serum-containing differentiation media (13%–17%; Figure S3B). These data demonstrated that lipid (or KSR) supplementation is required for efficient erythroid differentiation with high-level transduction in serum-free differentiation media.
Figure 3.
Improvement of Erythroid Differentiation with Efficient Lentiviral Transduction by an Addition of Lipids or KSR
(A) We added a lipid mixture (1×, 2×, or 5×) or knockout serum replacement (KSR) (including lipid-rich albumin) into StemSpan-based serum-free erythroid differentiation media or replaced 20% fetal bovine serum with 20% KSR in IMDM-based differentiation media. (B) We evaluated erythroid differentiation (GPA and CD71 expression), transduction (GFP positive), and cell counts 13 days after differentiation. (C) Hb production was evaluated by Hb electrophoresis at day 13. The bars represent mean ± SEM. All experiments except Hb electrophoresis (single run) were performed in triplicate.
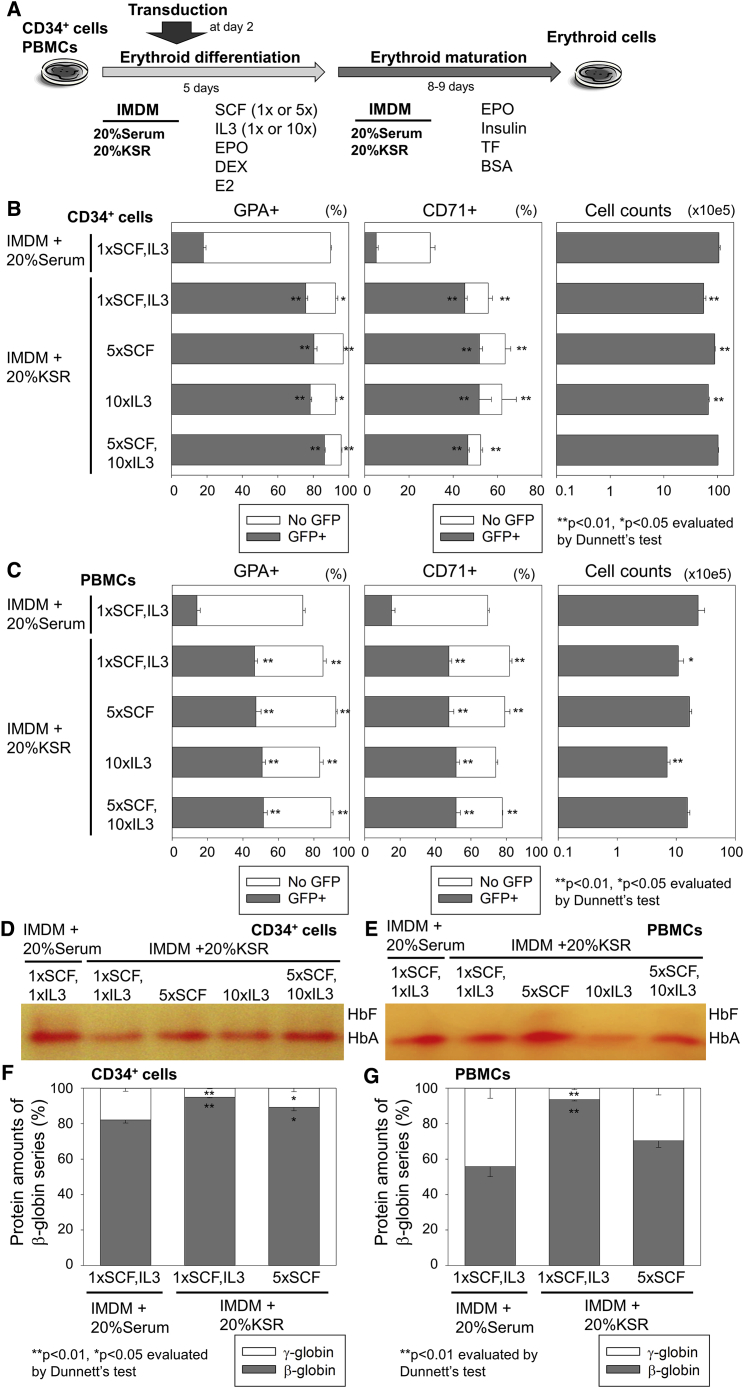
Additionally, we evaluated higher concentrations of SCF (5×SCF) and/or IL-3 (10×IL-3) in IMDM-based serum-free differentiation media with 20% KSR for CD34+ cells as well as PBMCs (Figure 4A). 5×SCF slightly increased erythroid cell expansion (1.5- to 1.6-fold in CD34+ cells and 1.5- to 2.2-fold in PBMCs), but not 10×IL-3, whereas neither transduction efficiency nor erythroid differentiation were strongly affected by 5×SCF and/or 10×IL-3 (Figures 4B and 4C).
Figure 4.
High-Level β-Globin Production with Efficient Lentiviral Transduction by Using IMDM-Based Serum-free Erythroid Differentiation Media with KSR Supplementation
(A) We evaluated higher concentration of SCF (5×) and/or IL-3 (10×) in IMDM-based serum-free differentiation media with 20% KSR in human CD34+ cells and PBMCs. (B and C) We evaluated erythroid differentiation (GPA and CD71 expression), transduction (GFP positive), and cell counts 13 and 14 days after differentiation of (B) CD34+ cells and (C) PBMCs, respectively. (D and E) Hb production was evaluated by Hb electrophoresis in (D) CD34+ cell- and (E) PBMC-derived erythroid cells. (F and G) Both β-globin (adult Hb) and γ-globin (fetal Hb) production was analyzed at the protein level by reverse-phase high-performance liquid chromatography (RP-HPLC). The globin amounts were quantified by areas of the peaks in RP-HPLC in (F) CD34+ cell- and (G) PBMC-derived erythroid cells. The bars represent mean ± SEM. All experiments except Hb electrophoresis (single run) were performed in triplicate.
Interestingly, lower levels of fetal Hb production were observed in IMDM-based serum-free differentiation media with 20% KSR for both CD34+ cells and PBMCs as compared to serum-containing differentiation media, whereas 5×SCF slightly increased fetal Hb production in serum-free differentiation media as compared to 1×SCF, analyzed by Hb electrophoresis (Figures 4D and 4E). Using reverse-phase high-performance liquid chromatography (RP-HPLC), higher levels of β-globin (adult Hb) production (p < 0.01) and lower levels of γ-globin (fetal Hb) production (p < 0.01) were confirmed at the protein level for both CD34+ cells and PBMCs in serum-free differentiation media, as compared to serum-containing differentiation media (Figures 4F, 4G, and S3C).
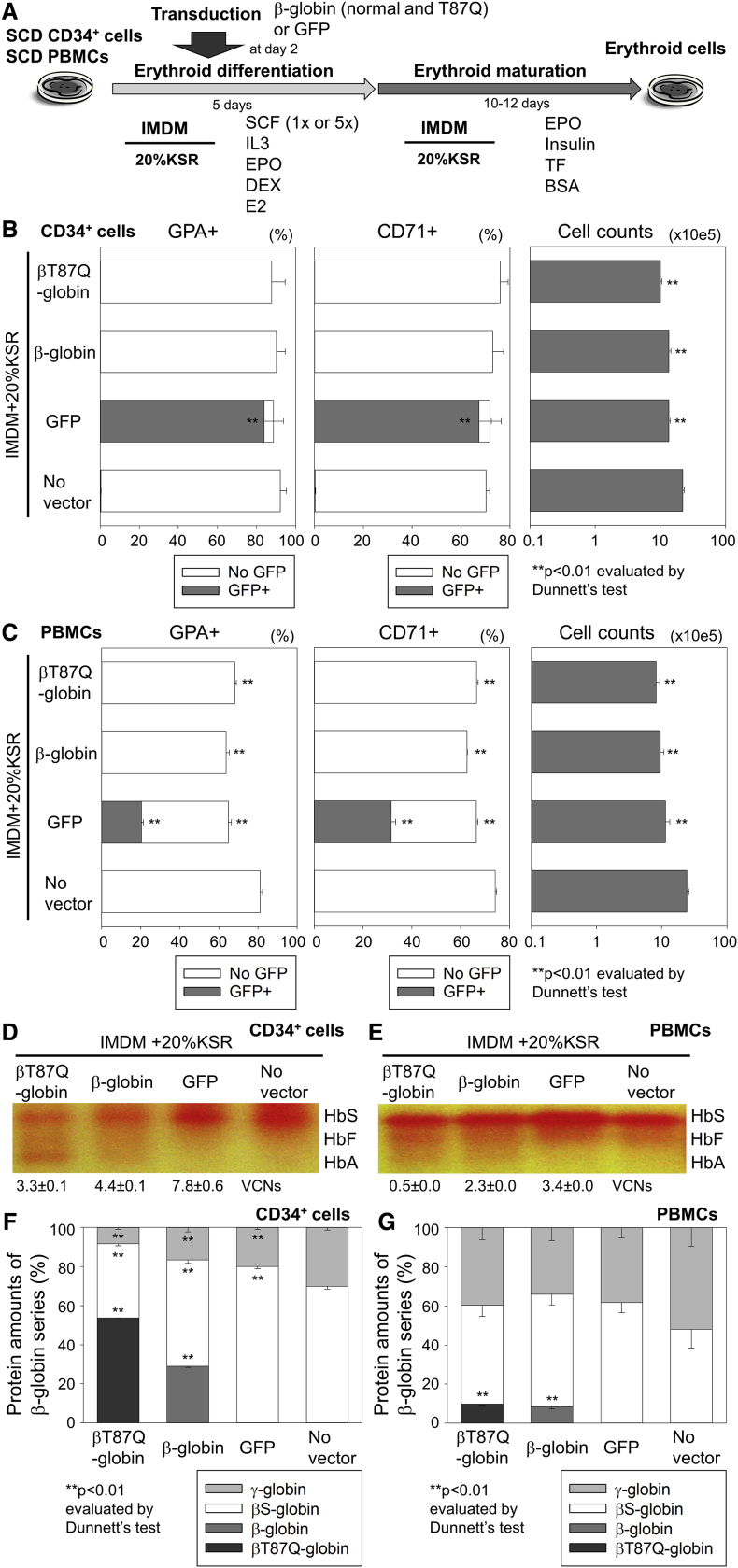
Detectable Adult Hb Production at the Protein Level with β-Globin Lentiviral Transduction in CD34+ Cells and PBMCs from SCD
We transduced CD34+ cells (1×SCF) and PBMCs (5×SCF) from SCD patients with lentiviral vectors encoding βT87Q-globin, β-globin, or GFP in the optimized IMDM-based serum-free differentiation media with 20% KSR (Figures 5A and S4A). After a 2-week culture, we observed efficient erythroid differentiation in all transduced cells with red color pellets, demonstrating hemoglobinization (Figure S4B) and similar or slightly lower %GPA and %CD71 as compared to no vector control (Figures 4B and 4C). Efficient transduction was observed for both SCD CD34+ cell-derived erythroid cells (vector copy number [VCN] 3.3–7.8; Figure 5D) and SCD PBMC-derived erythroid cells (VCN 0.5–3.4; Figure 5E). For both SCD CD34+ cells and SCD PBMCs (with pathogenic sickle Hb), therapeutic adult Hb production was observed in differentiated erythroid cells with βT87Q-globin and β-globin transduction, whereas we detected sickle Hb, but not adult Hb, in GFP transduction and no vector controls, analyzed by Hb electrophoresis (Figures 5D and 5E). βT87Q-globin or β-globin production was confirmed by RP-HPLC in SCD CD34+ cell- and PBMC-derived erythroid cells with each β-globin transduction (p < 0.01; Figures 5F, 5G, and S4C). Interestingly, βS-globin production was reduced in CD34+ cell-derived erythroid cells with βT87Q-globin or β-globin transduction, but not GFP transduction, as compared to no vector control (p < 0.01), suggesting that additional β-globin production might reduce the amounts of sickle Hb in SCD erythroid cells.
Figure 5.
Detectable Adult Hb Production at Protein Levels with β-Globin Lentiviral Transduction in CD34+ Cells and PBMCs from SCD
(A) We transduced CD34+ cells (1×SCF) and PBMCs (5×SCF) from sickle cell disease (SCD) patients with lentiviral vectors encoding βT87Q-globin, β-globin, and GFP in IMDM-based serum-free differentiation media with 20% KSR. (B and C) We evaluated erythroid differentiation (GPA and CD71 expression), transduction (GFP positive), and cell counts 17 and 15 days after differentiation of (B) CD34+ cells and (C) PBMCs, respectively. (D and E) We evaluated vector copy number per cell (VCN) for both (D) CD34+ cell-derived erythroid cells and (E) PBMC-derived erythroid cells. Therapeutic adult Hb production was evaluated by Hb electrophoresis in differentiated erythroid cells with βT87Q-globin and β-globin transduction. (F and G) The βT87Q-globin or β-globin (adult Hb) production was confirmed by RP-HPLC in SCD (F) CD34+ cell- and (G) PBMC-derived erythroid cells with each β-globin transduction (p < 0.01). HbS, sickle hemoglobin; bars: mean ± SEM. All experiments except Hb electrophoresis (single run) were performed in triplicate.
Discussion
We developed a serum-free in vitro erythroid differentiation system with efficient lentiviral transduction and high-level adult Hb production derived from human CD34+ cells as well as PBMCs. This serum-free culture system allowed high-efficiency production of human erythroid cells expressing around 90% GPA that were genetically modified without drug selection (Figures 2, 3, and 4). In addition, the high amount of Hb produced among the gene-modified erythroid cells allowed simple analysis by Hb electrophoresis (as well as RP-HPLC), and importantly in this study, mostly adult Hb production was observed among in vitro differentiated erythroid cells from both human CD34+ cells and PBMCs (Figures 2, 3, and 4). The high-level adult Hb production in our in vitro erythroid model allows not only for evaluation of additional globin production or Hb switching by genetic modification but also investigation of sickle Hb to develop new genetic strategies for SCD, including gene therapy as well as gene correction (Figure 5).
The level of adult Hb production obtained in both our serum-containing erythroid differentiation media as well as our serum-free differentiation media (Figures 2, 3, and 4) is sufficient to assay by Hb electrophoresis, a simple method to analyze Hb production and type that has been difficult to utilize from in vitro erythroid differentiation methods due to its low sensitivity. Our in vitro erythroid differentiation methods allowed us to detect Hb bands (mainly adult Hb) even by this low-sensitivity Hb electrophoresis, demonstrating high-level adult Hb production in differentiated erythroid cells.
We observed higher transduction efficiency for human erythroid cells in serum-free erythroid differentiation media; however, the serum-free condition resulted in less efficient erythroid differentiation and insufficient Hb production (undetectable in Hb electrophoresis; Figure 2). Initially, we simply circumvented this limitation by transduction in serum-free erythroid differentiation culture followed by robust erythroid differentiation with high-level adult Hb production in serum-containing media. Whereas switching to FBS-containing media during differentiation is practical to evaluate effects of genetic modification in human erythroid cells, we observed large variability among different FBS lots (20%–69% transduction efficiency and 80%–97% GPA in serum-containing erythroid media among several experiments in this manuscript). Therefore, we additionally developed a serum-free erythroid differentiation system to eliminate FBS in the entire procedure by adding 20% KSR (including lipid) to IMDM-based erythroid differentiation media, resulting in both efficient transduction and high-level adult Hb production among in vitro differentiated erythroid cells (Figures 3 and 4). Our findings are important for not only gene therapy research for Hb disorders but also in vitro erythroid cell generation for RBC transfusion, because our serum-free erythroid differentiation system represents a xeno-free erythroid differentiation method for a potential clinical usage by using commercially available xeno-free KSR and human albumin (instead of BSA). To our knowledge, this is the first report of high-level in vitro adult Hb production using a serum-free erythroid differentiation method. Human plasma and/or human serum were reported previously to allow the elimination of FBS from erythroid differentiation media.14, 15 An erythroid differentiation method was reported to use human serum albumin, animal-derived cholesterol, and soybean lecithin instead of serum (and much more materials); however, no globin expression data were included, and the protocol is impractical, due to very complicated components.8 Another serum-free method did not include lipid supplementation (and no globin protein data were reported),16 whereas a recent serum-free media contained a high concentration of SCF, resulting in a predominance of fetal Hb production from cord blood CD34+ cells.17 In addition, IMDM-based serum-free erythroid differentiation media with KSR is more cost effective, because IMDM and KSR are less expensive than StemSpan and FBS, respectively. Our serum-free erythroid differentiation method represents a practical and reliable platform with high-level adult Hb production (low-level fetal Hb) as well as efficient lentiviral transduction.
We also demonstrated more efficient lentiviral transduction for human erythroid cells in serum-free differentiation media (Figures 2, 3, and 4) as well as serum-free X-VIVO10 media (Figure 1), compared to serum-containing differentiation media. This is consistent with our previous data of higher transduction efficiency in human CD34+ cells in serum-free X-VIVO10 media than serum-containing media, suggesting that FBS inhibits lentiviral transduction for human hematopoietic progenitor cells.10 Lentiviral vectors with a vesicular stomatitis virus G protein (VSVG) envelope were previously shown to be inhibited by FBS, whereas FBS supplementation did not affect transduction with amphotropic pseudotyped retroviral vectors, suggesting that this inhibition may be related to VSVG envelope and/or its receptor.10, 18, 19 Importantly, lentiviral transduction during serum-free erythroid differentiation resulted in more efficient transduction among erythroid cells (∼90%; Figures 2, 3, and 4), as compared to serum-free transduction of CD34+ cells followed by erythroid differentiation (∼40%; Figure 1). Efficient transduction during erythroid differentiation is beneficial to evaluate the effects of genetic modification in erythroid cells, whereas CD34+ cell transduction in serum-free X-VIVO10 media (without erythroid differentiation) allows us to predict in vivo marking levels after transplantation.20 With this culture system, we also demonstrated therapeutic adult Hb production at protein levels derived from cells transduced using lentiviral vectors encoding β-globin or βT87Q-globin in both SCD CD34+ cells and SCD PBMCs when the transduction was performed using KSR-containing serum-free erythroid differentiation media (Figure 5). Indeed, transfer of a therapeutic adult Hb has recently been shown of benefit for SCD patients participating in ongoing gene therapy trials.1, 2 The serum-free differentiation media decreased fetal Hb production as compared to serum-containing differentiation media (Figure 4), which should allow for more clear separation of Hb signals in Hb electrophoresis as well as RP-HPLC to detect additional globin production with lentiviral transduction. Curiously, sickle Hb levels were decreased by therapeutic adult Hb (β-globin or βT87Q-globin) production in SCD CD34+ cell-derived erythroid cells (Figure 5), and this effect would be expected to translate in a further reduction of sickling in SCD RBCs by reducing the sickle Hb concentration.21 The α-like globin and β-like globin synthesis is balanced in erythropoiesis,22 and β-globin or βT87Q-globin production with lentiviral vectors might reduce endogenous βS-globin synthesis to match α-globin amounts in erythroid cells. The protein levels of βT87Q-globin were 1.8-fold higher than β-globin in SCD CD34+ cell-derived erythroid cells, whereas similar amounts of protein production were observed between βT87Q- and β-globins in SCD PBMC-derived erythroid cells (Figure 5). This small difference might be caused by a vector variability, because the difference between two vectors is only one codon within β-globin gene (T87Q). Our in vitro erythroid differentiation model should allow us to develop and refine therapeutic applications for Hb disorders, including β-thalassemia and SCD, utilizing gene transfer and genome editing as well as drug therapy.
Interestingly, serum-free differentiation media with 20% KSR resulted in higher adult Hb (β-globin) and lower fetal Hb (γ-globin) production in both CD34+ cells and PBMCs as compared to serum-containing media (Figure 4), which were evaluated by Hb electrophoresis and confirmed by RP-HPLC. This is particularly beneficial as an in vitro erythroid differentiation model because many efforts are now aimed at fetal Hb reactivation, which requires an in vitro culture system devoid of high-level baseline fetal Hb production. We demonstrated that higher SCF supplementation (5×SCF) during the differentiation phase (first half of the erythroid differentiation) increased erythroid cell expansion and fetal Hb (γ-globin) production (Figure 4), relatively similar to serum-containing differentiation media. Indeed, SCF supplementation was reported to induce fetal Hb production in differentiated erythroid cells from human primary cells.23 These data suggest that SCF (and FBS) can stimulate and expand erythroid progenitor cells, resulting in increased fetal Hb production.
Whereas we are able to collect mobilized peripheral blood stem cells on our own clinical trial evaluating plerixafor as a mobilization strategy to collect backup in patients undergoing haploidentical transplantation in this disorder, access to CD34+ cells from subjects with SCD is otherwise problematic. Hydroxyurea (HU) administration is a most common treatment to maintain SCD patients, because it induces fetal Hb expression.24 However, HU treatment must be stopped before collecting mobilized peripheral blood cells, because it inhibits cell growth in in vitro culture.25 Furthermore, bone marrow aspiration yields very few CD34+ cells for ex vivo culture. However, a large volume of peripheral blood can easily be obtained from SCD patients when RBC exchange is performed and allowed us to compare these two sources. In this study, CD34+ cells as well as PBMCs in SCD were used for serum-free erythroid differentiation with and without lentiviral transduction to analyze globin production at protein levels (Figure 5). Our results demonstrate that the serum-free erythroid differentiation method is practical to investigate SCD in in vitro culture even from the easy to obtain blood samples from patients with SCD.
In summary, we developed a robust method for human erythroid cell differentiation from both CD34+ cells and PBMCs using serum-free erythroid differentiation media. KSR supplementation allowed for efficient transduction, robust erythroid differentiation, and high-level adult Hb production sufficient for analysis by Hb electrophoresis as well as RP-HPLC. We demonstrated therapeutic adult Hb (β-globin or βT87Q-globin) production in transduced CD34+ cell- and PBMC-derived erythroid cells from SCD as a gene therapy model. Our in vitro erythroid differentiation system provides a practical evaluation platform for Hb production among human erythroid cells following genetic manipulation.
Materials and Methods
Erythroid Differentiation of Human CD34+ Cells and PBMCs with Lentiviral Transduction
Granulocyte-colony-stimulating-factor-mobilized CD34+ cells (from healthy donors), steady-state bone morrow CD34+ cells (from SCD patients), and PBMCs were collected from healthy donors and SCD patients under studies (08-H-0156 and 03-H-0015) that were approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK). All individuals gave written informed consent for the sample donation, and consent documents are maintained in the donor’s medical records. The consent document was approved by the Institutional Review Board prior to study initiation and is reviewed and updated yearly.
Human CD34+ cells (1 × 105) were cultured in fibronectin (RetroNectin; Takara, Shiga, Japan)-coated 12-well plates with serum-free X-VIVO10 media (Lonza, Basel, Switzerland) containing each 100 ng/mL of SCF (R&D Systems, Minneapolis, MN, USA), fms-related tyrosine kinase 3 ligand (FL) (R&D Systems), and thrombopoietin (TPO) (R&D Systems).10, 26 After overnight pre-stimulation, the cells were transduced with a self-inactivating HIV-type-1-based lentiviral vector encoding GFP under the control of a murine stem cell virus promoter (or encoding β-globin or βT87Q-globin gene derived from a β-globin promoter) with a VSVG envelope at MOI 50.27, 28 Next day, transduced cells were differentiated into erythroid cells using IMDM (Mediatech, Manassas, VA)-based erythroid differentiation media, which includes a differentiation phase for 5 or 6 days with 20% FBS (Mediatech), 2 U/mL EPO (AMGEN, Thousand Oaks, CA, USA), 10 ng/mL SCF, 1.0 ng/mL IL-3 (R&D Systems), 1.0 μM dexamethasone (DEX) (VETone, Boise, ID, USA), and 1.0 μM estradiol (Pfizer, NY, NY, USA) and a following maturation phase for 5–8 days with 20% FBS, 2 U/mL EPO, 10 ng/mL insulin (Lilly, Indianapolis, IN, USA), 0.5 mg/mL holo-transferrin (TF) (Sigma Aldrich, Saint Louis, MO, USA), and 2% BSA (Roche, Indianapolis, IN, USA; Figure 1A), which are slightly modified from human erythroid massive amplification culture.8 To improve transduction specifically for the erythroid lineage, we transduced CD34+ cells at 1 or 2 days after initiating erythroid differentiation (Figure 1B). For human PBMCs, 2 × 10e6 cells were used in 12-well plates with lentiviral transduction at MOI 2.5 for healthy donors’ PBMCs and MOI 5 for SCD PBMCs.
For serum-free erythroid differentiation, we used serum-free StemSpan (STEMCELL Technologies, Vancouver, BC, Canada)-based differentiation media, which includes a differentiation phase for 5 days with 2 U/mL EPO, 50 ng/mL SCF, 10 ng/mL IL-3, 1.0 μM DEX, and 10 ng/mL insulin and a following maturation phase for 6 days with 2 U/mL EPO, 10 ng/mL insulin, 0.5 mg/mL TF, and 2% BSA (Figure 2A).11, 29 For lipid supplementation, we added 1 μL/mL (1×), 2 μL/mL (2×), or 5 μL/mL (5×) of Lipid Mixture 1 (Sigma-Aldrich) or 20% KSR (Thermo Fisher Scientific, Waltham, MA, USA) into StemSpan-based serum-free erythroid differentiation media or simply replaced 20% FBS with 20% KSR in IMDM-based differentiation media (Figure 3A).
Evaluation of Lentiviral Transduction and Erythroid Differentiation
Following lentiviral transduction and erythroid differentiation, we performed flow cytometry (FACSCalibur, Becton Dickinson, East Rutherford, NJ, USA) to evaluate erythroid maturation using GPA antibody (clone GA-R2, Becton Dickinson) and CD71 antibody (clone M-A712, Becton Dickinson) and %GFP among GPA-positive erythroid cells for lentiviral transduction evaluation. The VCNs in transduced cells were evaluated by qPCR (QuantStudio 6 Flex Real-Time PCR System, Thermo Fisher Scientific) using self-inactivating long terminal repeat (SIN-LTR) probe and primers, as previously described.20 Total cell counts were evaluated by Countess Automated Cell Counter (Thermo Fisher Scientific). Cell morphology was evaluated by cytospin with Wright-Giemsa staining,29, 30 whereas enucleation was analyzed by flow cytometry with Hoechst 33342 staining (Thermo Fisher Scientific) according to the manufacturer’s instructions. In addition, we evaluated Hb production on the same starting cell numbers by Hb electrophoresis (HELENA LABORATORIES, Beaumount, TX, USA).29
RP-HPLC
We performed RP-HPLC for globin protein analysis, as previously described.29 Briefly, in vitro differentiated erythroid cells were washed 3 times with PBS (Corning, One Riverfront Plaza, NY, USA) and lysed by 100 μL HPLC-grade water (Sigma-Aldrich). The 90-μL lysates were mixed to 10 μL of 100 mM Tris (2-carboxyethyl) phosphine (Thermo Fisher Scientific) and then 85 μL solution containing 0.1% trifluoroacetic acid (TFA) (Thermo Fisher Scientific) and 32% acetonitrile (Honeywell Burdick and Jackson, Morris Plains, NJ, USA). The 10 μL supernatant was used in 0.8 mL/min flow for 45 min using the Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with a reverse-phase column, Aeris 3.6 μm Widepore C4 200 (250 × 4.6 mm; Phenomenex, Torrance, CA, USA) with two solvents: solvent A, 0.12% TFA in water, and solvent B, 0.08% TFA in acetonitrile. The globin types were detected at 215 nm.
Statistical Analysis
Statistical analyses were performed using the JMP 13 software (SAS Institute, Cary, NC, USA). Two averages were evaluated with Student’s t test. The averages in various conditions were evaluated by Dunnett’s test (one-way ANOVA for a control). A p value of <0.01 or 0.05 was deemed significant. SEM was shown as error bars in all figures. All experiments except Hb electrophoresis (single run) were performed in triplicate.
Author Contributions
N.U. designed the research, performed experiments, analyzed results, made the figures, and wrote the paper; S.D. designed the research and performed experiments; J.J.H.-M. designed the research and performed experiments; A.F. designed the research and performed experiments; L.N.R. performed experiments; M.M.H. performed experiments and wrote the paper; and J.F.T. designed the research and wrote the paper.
Conflicts of Interest
No competing financial interests exist.
Acknowledgments
This work was supported by the intramural research program (HL006009) of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) at the NIH. We thank Anna Shvygina and Luke P. Skala for helping out with experiments. We thank Dr. Duck-Yeon Lee from the NHLBI Biochemistry Core for RP-HPLC analysis help.
Footnotes
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.03.007.
Supplemental Information
References
- 1.Cavazzana M., Ribeil J.-A., Payen E., Suarez F., Beuzard Y., Touzot F., Cavallesco R., Lefrere F., Chretien S., Bourget P. Outcomes of gene therapy for severe sickle disease and beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral beta AT87Q-globin vector. Blood. 2015;126:202. [Google Scholar]
- 2.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sankaran V.G., Xu J., Ragoczy T., Ippolito G.C., Walkley C.R., Maika S.D., Fujiwara Y., Ito M., Groudine M., Bender M.A. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breda L., Motta I., Lourenco S., Gemmo C., Deng W., Rupon J.W., Abdulmalik O.Y., Manwani D., Blobel G.A., Rivella S. Forced chromatin looping raises fetal hemoglobin in adult sickle cells to higher levels than pharmacologic inducers. Blood. 2016;128:1139–1143. doi: 10.1182/blood-2016-01-691089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer D.E., Kamran S.C., Lessard S., Xu J., Fujiwara Y., Lin C., Shao Z., Canver M.C., Smith E.C., Pinello L. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. doi: 10.1126/science.1242088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traxler E.A., Yao Y., Wang Y.D., Woodard K.J., Kurita R., Nakamura Y., Hughes J.R., Hardison R.C., Blobel G.A., Li C., Weiss M.J. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 2016;22:987–990. doi: 10.1038/nm.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migliaccio G., Sanchez M., Masiello F., Tirelli V., Varricchio L., Whitsett C., Migliaccio A.R. Humanized culture medium for clinical expansion of human erythroblasts. Cell Transplant. 2010;19:453–469. doi: 10.3727/096368909X485049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heideveld E., Masiello F., Marra M., Esteghamat F., Yağcı N., von Lindern M., Migliaccio A.R., van den Akker E. CD14+ cells from peripheral blood positively regulate hematopoietic stem and progenitor cell survival resulting in increased erythroid yield. Haematologica. 2015;100:1396–1406. doi: 10.3324/haematol.2015.125492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida N., Hsieh M.M., Hayakawa J., Madison C., Washington K.N., Tisdale J.F. Optimal conditions for lentiviral transduction of engrafting human CD34+ cells. Gene Ther. 2011;18:1078–1086. doi: 10.1038/gt.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang W., Mills J.A., Sullivan S., Liu Y., French D.L., Gadue P. StemBook. Harvard Stem Cell Institute; 2012. iPSC reprogramming from human peripheral blood using sendai virus mediated gene transfer. [PubMed] [Google Scholar]
- 12.Eliason J.F., Odartchenko N. Colony formation by primitive hemopoietic progenitor cells in serum-free medium. Proc. Natl. Acad. Sci. USA. 1985;82:775–779. doi: 10.1073/pnas.82.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iscove N.N., Guilbert L.J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp. Cell Res. 1980;126:121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- 14.Giarratana M.C., Rouard H., Dumont A., Kiger L., Safeukui I., Le Pennec P.Y., François S., Trugnan G., Peyrard T., Marie T. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J., Liu J., Xue F., Halverson G., Reid M., Guo A., Chen L., Raza A., Galili N., Jaffray J. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121:3246–3253. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrnes C., Lee Y.T., Meier E.R., Rabel A., Sacks D.B., Miller J.L. Iron dose-dependent differentiation and enucleation of human erythroblasts in serum-free medium. J. Tissue Eng. Regen. Med. 2016;10:E84–E89. doi: 10.1002/term.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mettananda S., Clark K., Fisher C.A., Sloane-Stanley J.A., Gibbons R.J., Higgs D.R. Phenotypic and molecular characterization of a serum-free miniature erythroid differentiation system suitable for high-throughput screening and single-cell assays. Exp. Hematol. 2018;60:10–20. doi: 10.1016/j.exphem.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Millington M., Arndt A., Boyd M., Applegate T., Shen S. Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells. PLoS ONE. 2009;4:e6461. doi: 10.1371/journal.pone.0006461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluge K.A., Bonifacino A.C., Sellers S., Agricola B.A., Donahue R.E., Dunbar C.E. Retroviral transduction and engraftment ability of primate hematopoietic progenitor and stem cells transduced under serum-free versus serum-containing conditions. Mol. Ther. 2002;5:316–322. doi: 10.1006/mthe.2002.0544. [DOI] [PubMed] [Google Scholar]
- 20.Uchida N., Evans M.E., Hsieh M.M., Bonifacino A.C., Krouse A.E., Metzger M.E., Sellers S.E., Dunbar C.E., Donahue R.E., Tisdale J.F. Integration-specific in vitro evaluation of lentivirally transduced rhesus CD34(+) cells correlates with in vivo vector copy number. Mol. Ther. Nucleic Acids. 2013;2:e122. doi: 10.1038/mtna.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunshine H.R., Hofrichter J., Eaton W.A. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978;275:238–240. doi: 10.1038/275238a0. [DOI] [PubMed] [Google Scholar]
- 22.Higgs D.R., Engel J.D., Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379:373–383. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 23.Wojda U., Leigh K.R., Njoroge J.M., Jackson K.A., Natarajan B., Stitely M., Miller J.L. Fetal hemoglobin modulation during human erythropoiesis: stem cell factor has “late” effects related to the expression pattern of CD117. Blood. 2003;101:492–497. doi: 10.1182/blood-2002-03-0756. [DOI] [PubMed] [Google Scholar]
- 24.Charache S., Terrin M.L., Moore R.D., Dover G.J., Barton F.B., Eckert S.V., McMahon R.P., Bonds D.R., Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 25.Uchida N., Fujita A., Hsieh M.M., Bonifacino A.C., Krouse A.E., Metzger M.E., Donahue R.E., Tisdale J.F. Bone marrow as a hematopoietic stem cell source for gene therapy in sickle cell disease: evidence from rhesus and SCD patients. Hum. Gene Ther. Clin. Dev. 2017;28:136–144. doi: 10.1089/humc.2017.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida N., Washington K.N., Hayakawa J., Hsieh M.M., Bonifacino A.C., Krouse A.E., Metzger M.E., Donahue R.E., Tisdale J.F. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J. Virol. 2009;83:9854–9862. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanawa H., Kelly P.F., Nathwani A.C., Persons D.A., Vandergriff J.A., Hargrove P., Vanin E.F., Nienhuis A.W. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 28.Uchida N., Washington K.N., Lap C.J., Hsieh M.M., Tisdale J.F. Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol. Ther. 2011;19:133–139. doi: 10.1038/mt.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchida N., Haro-Mora J.J., Fujita A., Lee D.Y., Winkler T., Hsieh M.M., Tisdale J.F. Efficient generation of β-globin-expressing erythroid cells using stromal cell-derived induced pluripotent stem cells from patients with sickle cell disease. Stem Cells. 2017;35:586–596. doi: 10.1002/stem.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita A., Uchida N., Haro-Mora J.J., Winkler T., Tisdale J. β-globin-expressing definitive erythroid progenitor cells generated from embryonic and induced pluripotent stem cell-derived sacs. Stem Cells. 2016;34:1541–1552. doi: 10.1002/stem.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.