Significance
Tonic inhibition plays critical roles in cognitive functions under physiological and pathological conditions by controlling neuronal excitability. Although we have previously reported that cerebellar tonic inhibition is critically dependent on both the synthesis of GABA through monoamine oxidase B (MAOB) enzyme and the release via the bestrophin 1 (Best1) channel, the role of astrocytic GABA in cerebellar function in vivo has remained elusive. Here, we report that a reduction of tonic GABA release by genetic or pharmacological removal of Best1 or MAOB caused enhanced neuronal excitability, synaptic transmission, and motor performance on the rotarod test, whereas an augmentation of tonic GABA release by astrocyte-specific overexpression of MAOB caused opposite results. Our findings suggest the actions of astrocytic GABA in excitation/inhibition balance and motor coordination.
Keywords: tonic GABA, astrocyte, cerebellum, neuronal excitability, motor coordination
Abstract
Tonic inhibition in the brain is mediated through an activation of extrasynaptic GABAA receptors by the tonically released GABA, resulting in a persistent GABAergic inhibitory action. It is one of the key regulators for neuronal excitability, exerting a powerful action on excitation/inhibition balance. We have previously reported that astrocytic GABA, synthesized by monoamine oxidase B (MAOB), mediates tonic inhibition via GABA-permeable bestrophin 1 (Best1) channel in the cerebellum. However, the role of astrocytic GABA in regulating neuronal excitability, synaptic transmission, and cerebellar brain function has remained elusive. Here, we report that a reduction of tonic GABA release by genetic removal or pharmacological inhibition of Best1 or MAOB caused an enhanced neuronal excitability in cerebellar granule cells (GCs), synaptic transmission at the parallel fiber-Purkinje cell (PF-PC) synapses, and motor performance on the rotarod test, whereas an augmentation of tonic GABA release by astrocyte-specific overexpression of MAOB resulted in a reduced neuronal excitability, synaptic transmission, and motor performance. The bidirectional modulation of astrocytic GABA by genetic alteration of Best1 or MAOB was confirmed by immunostaining and in vivo microdialysis. These findings indicate that astrocytes are the key player in motor coordination through tonic GABA release by modulating neuronal excitability and could be a good therapeutic target for various movement and psychiatric disorders, which show a disturbed excitation/inhibition balance.
Proper brain function requires a balanced excitation and inhibition in synaptic transmission through regulation of neuronal excitability. Neuronal excitability is regulated by inhibitory synaptic transmission, which occurs primarily through GABAergic signaling. It has been reported that interactions between tonically released GABA and extrasynaptically localized high-affinity GABAA receptors (GABAARs) mediate tonic inhibition, which effectively inhibits neuronal excitability (1–5).
Tonic inhibition has been found in various brain regions, including cerebellum, hippocampus, and thalamus (3, 5, 6). Among these brain regions, the role of tonic inhibition in neuronal excitability, synaptic transmission, and brain function has been intensively studied in the cerebellum (3, 7–9). Tonic inhibition modulates the excitability of cerebellar granule cells (GCs), which have an exclusive expression of extrasynaptic GABAARs (10, 11), and subsequently influences synaptic transmission at parallel fiber (PF)-Purkinje cell (PC) synapses (3, 7). However, the relationship between tonic inhibition and motor performance has not been clearly demonstrated.
We have previously reported that cerebellar tonic inhibition is mediated by astrocytic GABA release through bestrophin 1 (Best1) from Bergmann glia and lamellar astrocytes (12). We have further reported that the astrocytic GABA is synthesized by the astrocytic mitochondrial enzyme monoamine oxidase B (MAOB) via the putrescine degradation pathway (13). However, in vivo function of the astrocytic GABA-mediated tonic inhibition has not been elucidated. Here, we investigated the modulation of neuronal excitability, synaptic transmission, and motor performance in the cerebellum by manipulating the level of astrocytic tonic GABA using various genetic and pharmacological tools.
Results
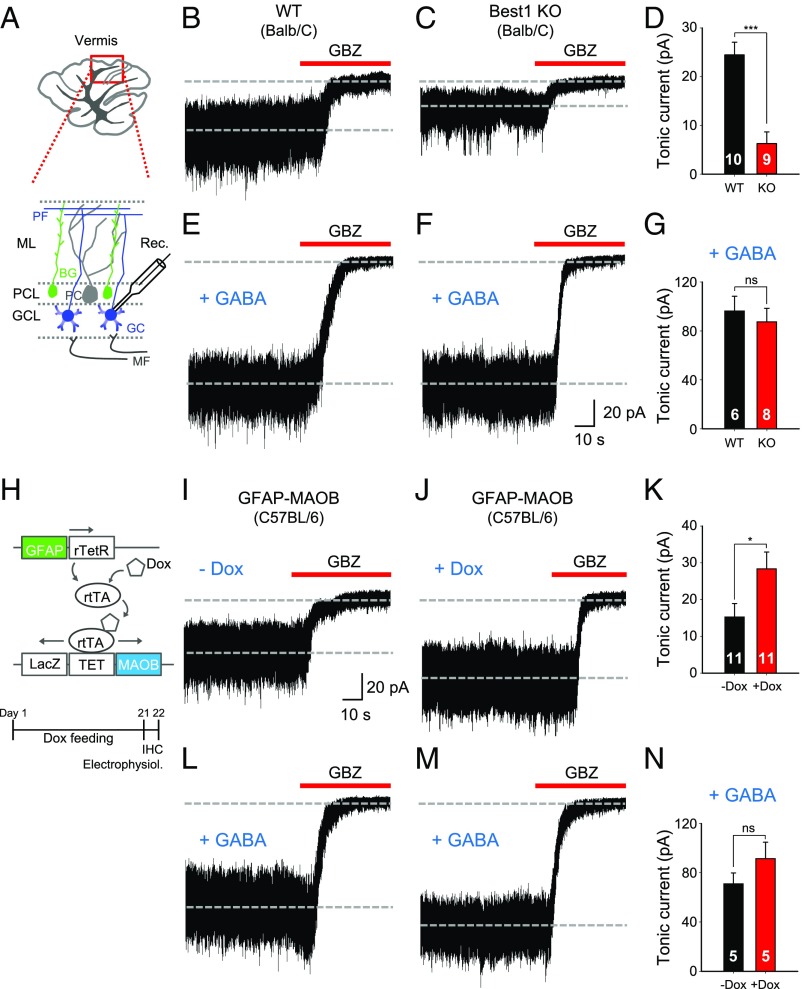
To test whether the genetic deletion of the GABA-releasing channel Best1 leads to an alteration of tonic inhibition, we measured the GABAzine-sensitive tonic current in cerebellar GCs from the acutely prepared cerebellar slices of Best1 knockout (KO) mice and compared with the wild-type (WT) mice as previously described (12, 13) (Fig. 1A). GCs, but not PCs, are known to express the high-affinity, nondesensitizing, extrasynaptic GABAARs composed of α6, β, and δ subunits in the cerebellum (10, 11, 14). The successful deletion of Best1 in Best1 KO mice was confirmed by a significant reduction of Best1 immunoreactivity in GFAP-positive lamellar and Bergmann glial cells (SI Appendix, Fig. S1). There was no significant difference in GFAP level between WT and Best1 KO mice (SI Appendix, Table S1). Best1 KO mice showed a significant reduction of tonic current by about 70% compared with WT mice in cerebellar GCs (Fig. 1 B–D). This was consistent with our previous findings based on the astrocyte-specific acute gene-silencing system using a Best1-specific shRNA (12). This reduction of tonic current was not due to changes of extrasynaptic GABAAR expression in Best1 KO mice, as evidenced by no significant difference in amplitude of the tonic current obtained in the presence of the saturating concentration of GABA at 5 μM (15) (Fig. 1 E–G) or 0.5 μM THIP [4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol], a specific agonist for a δ-subunit–containing GABAAR (16, 17) (SI Appendix, Fig. S2 B and C). There was no significant difference between WT and Best1 KO in the amplitude and frequency of spontaneous inhibitory synaptic currents, whose representative decay kinetics showed comparable values to those of previous studies utilizing similarly aged animals (10, 17, 18) (SI Appendix, Fig. S2 D–F and Table S2).
Fig. 1.
Suppression of tonic inhibition by genetic deletion of Best1 and enhancement by overexpression of MAOB. (A) Schematic illustration for tonic current recording in cerebellar GCs by whole-cell patch clamp. (B, C, E, and F) Representative traces of tonic current in cerebellar GCs from WT mice (B and E) and Best1 KO mice (Balb/C strain) (C and F). +GABA indicates the adding of 5 μM GABA in the recording solution. (D and G) The magnitude of 50-μM GABAzine-sensitive tonic current as indicated (unpaired t test). (H) Schematic showing doxycycline (Dox)-dependent MAOB overexpression in astrocytes. (I, J, L, and M) Representative traces of tonic current from GFAP-MAOB mice (C57BL/6 strain) in −Dox (I and L) and +Dox (J and M) conditions. +GABA indicates the adding of 5 μM GABA in the recording solution. (K and N) The magnitude of GABAzine-sensitive tonic current as indicated (unpaired t test). Error bars are SEM; *P < 0.05; ***P < 0.001; ns indicates P > 0.05. BG, Bergmann glia; GBZ, GABAzine; GCL, GC layer; IHC, immunohistochemistry; MF, mossy fiber; ML, molecular layer; PCL, PC layer; Rec., recording pipette; rtTA, reverse tetracycline responsive transactivator; TET, tetracycline-responsive promoter.
Recently, we reported that unlike the GABAergic neurons utilizing glutamic acid decarboxylase, the astrocytes in the cerebellum, as well as the reactive astrocytes in the diseased hippocampus, synthesize GABA using MAOB (1, 13, 19). To test whether increasing the MAOB expression level in astrocytes affects the degree of tonic inhibition, we utilized the doxycycline-dependent MAOB overexpression mouse system under the astrocyte-specific GFAP promoter (GFAP-MAOB; Fig. 1H) (20). Compared with the off-doxycycline condition (−Dox), astrocyte-specific overexpression of MAOB by doxycycline (+Dox) significantly enhanced the tonic current in cerebellar GCs by about 30% (Fig. 1 I–K). However, there was no difference in the 5-μM GABA-induced tonic current between +Dox and −Dox conditions (Fig. 1 L–N), indicating no difference in the extrasynaptic GABAARs. In addition, there was no significant change in synaptic responses between +Dox and −Dox conditions (SI Appendix, Table S2). The increased level of astrocytic GABA in +Dox mice was independently confirmed by immunohistochemistry using a GABA-specific antibody (SI Appendix, Fig. S3 A–C). There was no significant difference in GFAP level between +Dox and −Dox conditions (SI Appendix, Table S1). These results strongly support that the astrocytic GABA is synthesized by MAOB, and the cerebellar tonic inhibition is mediated by the tonic release of astrocytic GABA through Best1.
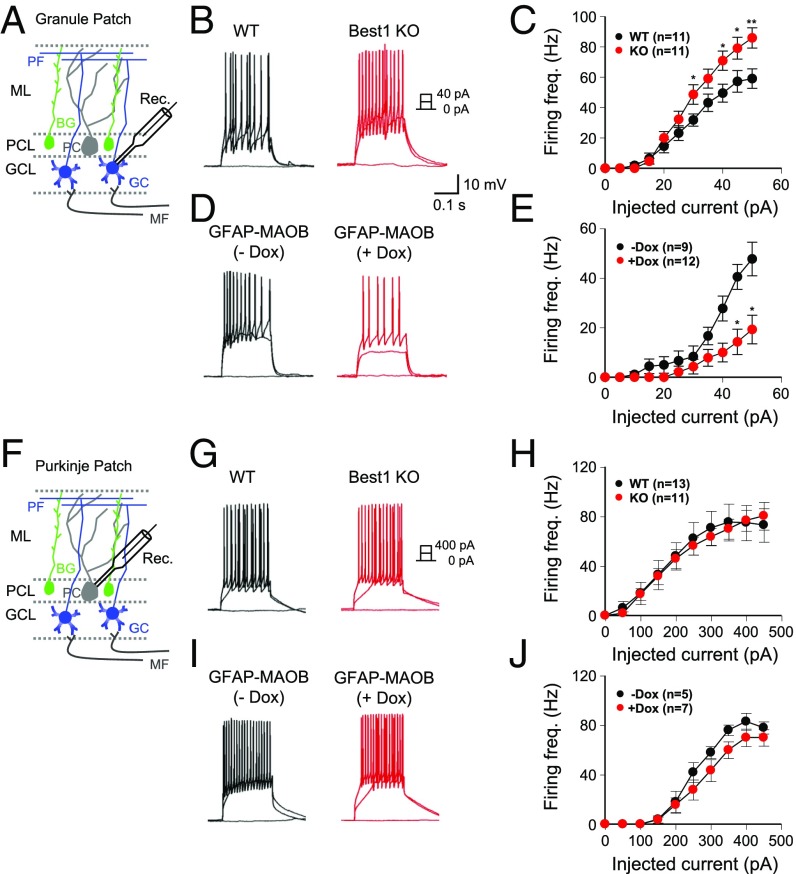
The tonically released astrocytic GABA should exert a strong inhibitory effect on the neighboring neurons, resulting in the change of neuronal excitability and synaptic transmission. To test this possibility, we first measured the intrinsic excitability of GCs and PCs in Best1 KO and GFAP-MAOB mice. We found that the frequency of the current injection-induced action potential firing (Fig. 2B) was steadily increased by increasing the amount of injected current (Fig. 2C). In Best1 KO mice, we found a significantly higher firing rate compared with WT littermates at high injected currents (Fig. 2C). These results are consistent with the previous report that pharmacologically inhibited tonic inhibition increased the excitability of GCs (3). In contrast, we found the opposite results in GFAP-MAOB mice, with a lower firing rate from +Dox condition compared with −Dox condition (Fig. 2 D and E). Interestingly, the intrinsic excitability of PCs was not different in both types of mice (Fig. 2 G–J), consistent with the fact that PCs do not have high-affinity, extrasynaptic GABAARs. There was no difference in the resting membrane potential of GCs in these mice [WT, −67.8 ± 1.93 mV; Best1 KO, −64.64 ± 2.18 mV (P = 0.29) and −Dox, −65 ± 2.32 mV; +Dox, −64 ± 1.91 mV (P = 0.24)]. These results demonstrate that the astrocytic GABA exerts a strong inhibitory effect on the intrinsic neuronal excitability of GCs.
Fig. 2.
Modulating tonic inhibition regulates excitability in GCs, but not in PCs. (A and F) Schematic illustration for measurement of excitability in GCs (A) and PCs (F) by current injection. BG, Bergmann glia; GCL, GC layer; MF, mossy fiber; ML, molecular layer; PCL, PC layer; Rec., recording pipette. (B, D, G, and I) Representative traces of excitatory postsynaptic potentials from WT and Best1 KO mice and GFAP-MAOB mice in −Dox and +Dox conditions. (C, E, H, and J) Summary data of firing frequency in GCs and PCs upon varying the injected current (unpaired t test). Error bars are SEM; *P < 0.05; **P < 0.01.
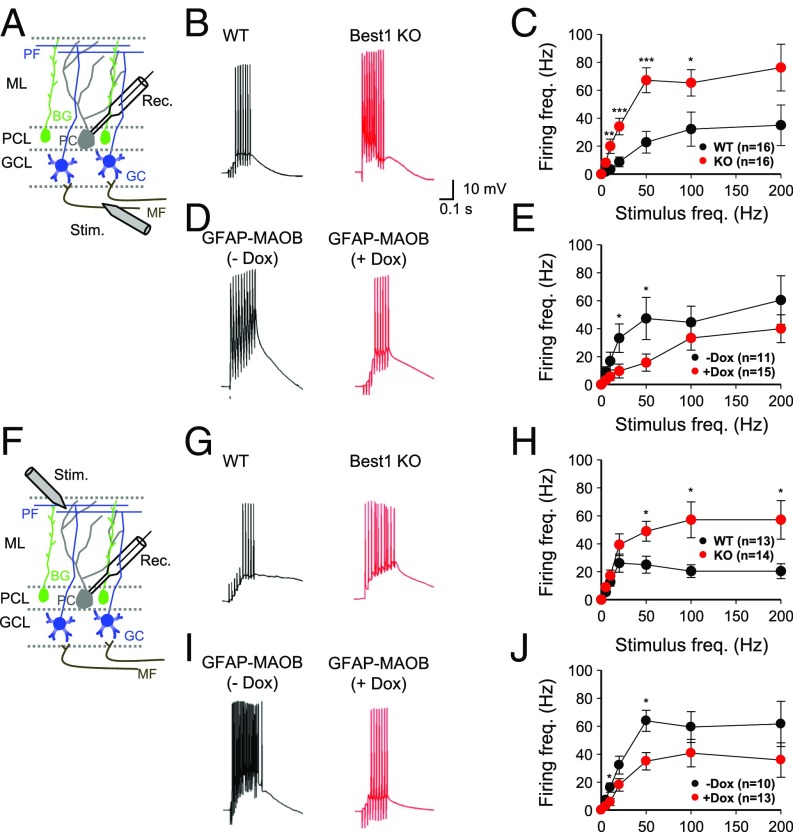
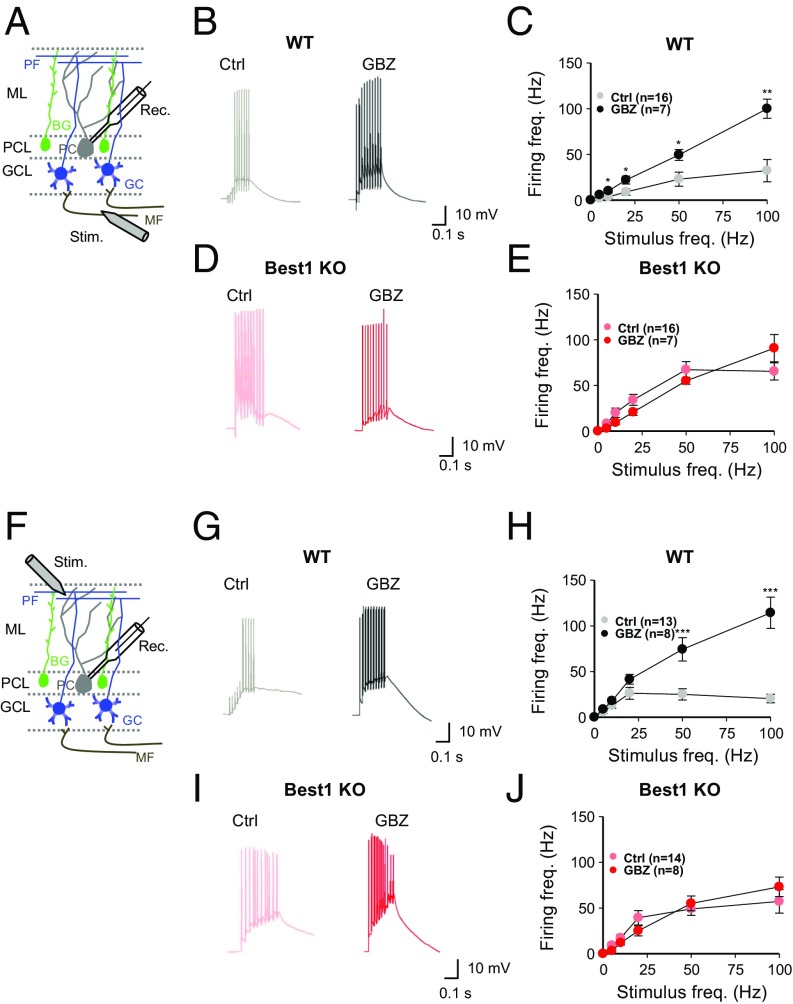
Next, we measured the frequency of synaptically induced action-potential firing in PCs—the sole output neuron of the cerebellar cortex and whose activity is controlled by GCs through the PF input (21)—upon electrical stimulation of the mossy fiber (MF) or PF at various stimulation frequencies, as previously described (3) (Fig. 3 A and F). In Best1 KO mice, we found a significantly higher firing rate over most of the stimulation frequencies compared with WT littermates (Fig. 2C). These results are consistent with the previous report that pharmacologically inhibited tonic inhibition increased the excitability of PCs (3). However, we found the opposite results in GFAP-MAOB mice, with a lower firing rate from +Dox condition compared with −Dox condition (Fig. 3E). There was no difference in the resting membrane potential of PCs in these mice [WT, −62 ± 1.41 mV; Best1 KO, −61 ± 1.45 mV (P = 0.47) and −Dox, −65 ± 2.32 mV; +Dox, −64 ± 1.91 mV (P = 0.75)]. We found very similar results with electrical stimulation of the PF (Fig. 3 G–J). To test if tonic inhibition is the cause of the difference in synaptically induced action-potential firing in PCs, we performed the same experiments in the presence of the GABAAR antagonist GABAzine (Fig. 4 A and F). We found that GABAzine enhanced PC firing induced by stimulation of MF or PF in WT mice, but not in Best1 KO mice (Fig. 4 E and J), indicating that tonic GABA causes a strong inhibitory action on PCs at the PF-PC synapse.
Fig. 3.
Modulating tonic inhibition regulates synaptic transmission at MF-PC and PF-PC synapses. (A and F) Schematic illustration for measurement of excitability in PCs by electrical stimulation (Stim.) of MF (A) and PF (F). BG, Bergmann glia; GCL, GC layer; ML, molecular layer; PCL, PC layer; Rec., recording pipette. (B, D, G, and I) Representative traces of excitatory postsynaptic potentials from WT and Best1 KO mice and GFAP-MAOB mice in −Dox and +Dox conditions (at 50-Hz stimulation). (C, E, H, and J) Summary data of firing frequency in PCs at various stimulation frequencies (unpaired t test). Error bars are SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 4.
Astrocytic GABA regulates synaptic transmission at MF-PC and PF-PC synapses. (A and F) Schematic illustration for measurement of excitability in PCs by electrical stimulation (Stim.) of MF (A) and PF (F). BG, Bergmann glia; GCL, GC layer; ML, molecular layer; PCL, PC layer; Rec., recording pipette. (B, D, G, and I) Representative traces of excitatory postsynaptic potentials from WT and Best1 KO mice with or without treatment of GABAzine (GBZ) (50 μM, at 50-Hz stimulation). Ctrl, control. (C, E, H, and J) Summary data of firing frequency in PCs at various stimulation frequencies (unpaired t test). Error bars are SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
In addition to action potential measurement, we also examined the paired-pulse ratio (PPR) in PCs by stimulating the PF. We found a lower PPR in Best1 KO mice (SI Appendix, Fig. S4 B and C), indicating that the presynaptic release probability at the PF-PC synapse in Best1 KO mice was higher than in WT due to a disinhibitory effect in Best1 KO by tonic GABA. We found that first excitatory postsynaptic potential amplitude was significantly enhanced in Best1 KO mice (SI Appendix, Fig. S4D), indicating that synaptic transmission is enhanced in Best1 KO mice and that tonic GABA is inhibitory at the PF-PC synapse. We measured the synaptic plasticity in terms of long-term potentiation (LTP) at the PF-PC synapse in WT and Best1 KO mice using the widely used stimulation protocol at this synapse (1 Hz, 5 min, 90 stimuli) (22). In WT mice, the LTP was not induced in the absence of inhibitors for GABARs. However, the same stimulation protocol induced a significant LTP in Best1 KO mice (SI Appendix, Fig. S4 F and G). These results support that the astrocytic GABA exerts a strong inhibitory effect on the synaptic transmission, as well as on synaptic plasticity at PF-PC synapses.
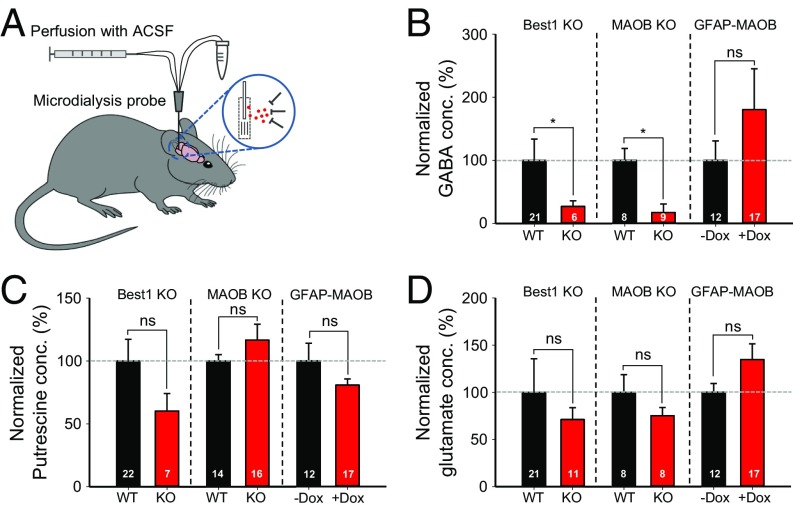
To determine the in vivo level of the astrocytic GABA, we directly measured the amount of extracellular GABA using in vivo microdialysis from the cerebellum of freely moving mice (Fig. 5A) and the subsequent analysis of the microdialysates by HPLC or mass spectrometry as previously described (1). We found that the extracellular GABA was significantly decreased in both Best1 KO and MAOB KO mice, but slightly increased in +Dox GFAP-MAOB mice compared with WT or −Dox GFAP-MAOB mice (Fig. 5B). In contrast, there was no difference in the level of putrescine [the substrate for GABA synthesis (13)], or glutamate (Fig. 5 C and D).
Fig. 5.
Extracellular GABA level is decreased by genetic deletion of Best1 or MAOB but increased by overexpression of MAOB. (A) Schematic illustration for microdialysis. ACSF, artificial cerebrospinal fluid. (B–D) Extracellular GABA (B), putrescine (C), and glutamate (D) concentration from Best1 KO, MAOB KO, and GFAP-MAOB mice (unpaired t test). Values were normalized to WT for Best1 KO and MAOB KO.
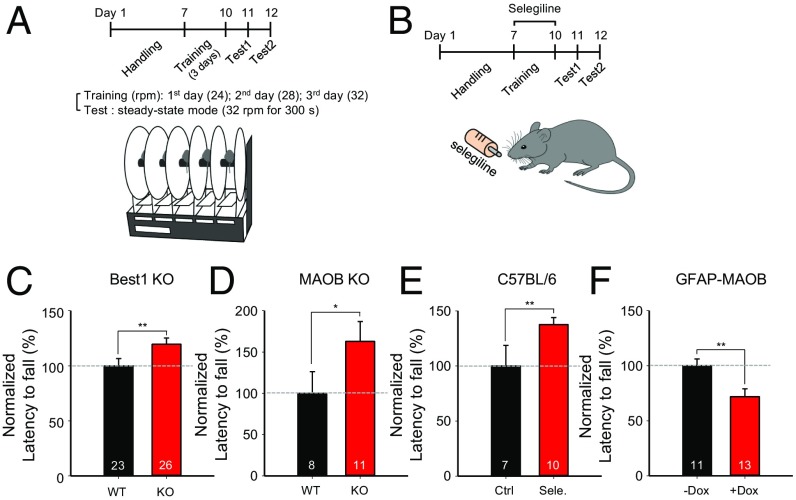
Lastly, we tested the in vivo function of the astrocytic GABA by performing the rotarod test (Fig. 6A), which is a well-known cerebellum-dependent behavior test for motor coordination (23–25). We found that the latency to fall was significantly higher in Best1 KO and MAOB KO mice compared with WT mice (Fig. 6 C and D). In addition, we utilized selegiline (Fig. 6B), which is a selective irreversible MAOB inhibitor and has been previously shown to effectively decrease both the tonic inhibition current in acutely prepared slices and the astrocytic GABA in histological sections (1). We found that the selegiline-treated mice similarly showed a significantly higher latency to fall compared with control mice (Fig. 6E). In contrast, +Dox GFAP-MAOB mice showed a significantly lower latency to fall compared with −Dox mice (Fig. 6F). These results indicate that the level of astrocytic GABA correlated inversely with the degree of motor coordination. Furthermore, compared with control mice, we found a tendency of increased latency to fall and maximum velocity to stay on the rotarod in both Best1 KO and MAOB KO mice, and a significantly decreased latency to fall and maximum velocity in +Dox GFAP-MAOB mice (SI Appendix, Fig. S5), consistent with the inverse correlation between tonic inhibition and motor performance.
Fig. 6.
Motor coordination is enhanced by genetic deletion of Best1 or MAOB but impaired by overexpression of MAOB. (A) Experimental timeline and schematic illustration for rotarod test. (B) Experimental timeline for selegiline treatment and rotarod test. (C–F) Summary graph showing latency to fall during test sessions. Error bars are SEM; *P < 0.05; **P < 0.01; ns indicates P > 0.05. Ctrl, control; Sele., selegiline.
Discussion
Here, we have demonstrated that the astrocytic tonic GABA release can control motor coordination by tonically inhibiting cerebellar neuronal excitability (SI Appendix, Fig. S6B). We found an inverse correlation between the amount of astrocytic GABA and motor performance. Our study raises a possibility of a dynamic control of motor coordination via selective modulation of the release, synthesis, and clearance of astrocytic GABA (SI Appendix, Fig. S6A).
In the cerebellar circuit, signals enter the cerebellum via MFs, which excite GCs and Golgi cells, which are the sole inhibitory interneuron in the input layer (21). GCs receive inhibitory signals (i) from the Golgi cells through the phasic GABA release, with an activation of synaptic GABAARs on GCs (3, 11, 26), and (ii) from the astrocytes through the tonic GABA release, with an activation of extrasynaptic GABAARs containing a δ-subunit on GCs (4, 11–13). GCs have been shown to have exclusive expressions of those extrasynaptic GABAARs (10, 11) along their soma, dendrites, and PFs (27). Those extrasynaptic GABAARs should be readily activated by GABA released from lamellar astrocytes in the GC layer and Bergmann glial cells along the PFs. In our study, we identified a role of astrocytic tonic GABA in the powerful control of GCs’ excitability, which synaptically influences the excitability of PCs, the sole source of the cerebellar output. Our results elucidate a mechanism of dynamic regulation of the cerebellar output by controlling the synaptic strength at the PF-PC synapses via tonic inhibition of GCs through the astrocytic tonic GABA release. One can consider the possibility that astrocytic tonic GABA is somehow transiently and locally suppressed, disinhibiting the local GCs in the vicinity and thus allowing particular PF-PC synapses to be activated. This exciting possibility needs future investigations.
We have confirmed that the majority of tonic GABA (70%) is derived from astrocytes through a series of studies using genetic, pharmacological, and molecular tools (12, 13). Nevertheless, there is an unaccounted, remaining portion of tonic GABA current of about 30%. In a very recent study, it has been reported that there is a direct inhibitory signal of PCs to GCs in the forms of both phasic and tonic inhibition modes of action (28). These lines of evidence suggest that PCs can also be a potential source of tonic GABA in GCs. Future studies are needed to test this possibility.
Contrary to our previous and current findings of the Best1-mediated tonic GABA release, Diaz et al. (29) reported that the Best1 channel does not mediate tonic GABAergic current in cerebellar GCs based on only pharmacological evidence using NPPB [5-nitro-2-(3-phenylpropylamino)benzoic acid] as a Best1 channel blocker. However, NPPB is not a specific blocker of Best1 and has many side effects such as inhibiting some potassium and calcium channels (30, 31). Using the cell type-specific gene-silencing method by lentiviral shRNA for Best1 (12) and Best1 KO mice, we unequivocally demonstrate the Best1-mediated tonic GABA release.
We have demonstrated that there was a positive correlation between tonic GABA detected by electrophysiology and extracellular GABA detected by microdialysis, whereas there was no correlation between synaptic GABA and extracellular GABA. These results imply that what we measure in in vivo microdialysis experiments is the level of extracellular tonic GABA rather than synaptically released GABA. Likewise, the extracellular glutamate detected by microdialysis is most likely the level of tonic glutamate, rather than synaptic glutamate, which should be taken up by glutamate transporters in adjacent astrocytes within a couple of seconds (32, 33). It has been reported that ambient glutamate is mediated mostly by the cystine/glutamate antiporter (34). This is probably why we did not detect a significant difference in the level of extracellular glutamate in Best1 KO via in vivo microdialysis. Based on our study, we propose that in vivo microdialysis could be a useful tool to measure the tonic level of extrasynaptic gliotransmitters such as GABA, glutamate, and d-serine.
Although it has been suggested that GCs’ excitability and the synaptic transmission between PF and PCs are strongly modulated by tonic inhibition (3, 7, 9), there has been controversy over the effect of GCs’ excitability and PF-PC synaptic transmission on cerebellar motor performance. For example, Egawa et al. (9) recently reported that decreased tonic inhibition in GCs resulting in an increase of GCs’ excitability caused a dysfunction of motor performance in a mouse model of Angelman syndrome. However, the authors did not measure PF-PC synaptic transmission and tested only the unidirectional manipulation of GCs’ excitability. On the other hand, Galliano et al. (8) demonstrated that silencing the majority of PF-PC synaptic transmission through the deletion of P/Q-type Ca2+ channels did not affect motor performance. Despite these lines of experimental evidence, the relationship among GCs’ excitability, PF-PC synaptic transmission, and motor performance has not been clearly demonstrated. In the current study, we show a clear positive relationship among motor performance, PF-PC synaptic transmission, and GCs’ excitability, which are inversely modulated by the astrocytic tonic GABA. We provide multiple lines of evidence from various genetic and pharmacologic mice models through the bidirectional manipulation of GCs’ excitability. We clearly demonstrate that less tonic inhibition by genetic removal or pharmacological inhibition of Best1 or MAOB causes an enhanced GC excitability, synaptic transmission in PF-PC synapse, and motor performance, whereas higher tonic inhibition by MAOB overexpression in astrocytes caused a reduced GC excitability, synaptic transmission, and motor performance. Our results are consistent with a previous report demonstrating the inverse correlation between tonic inhibition and sensory-evoked spike output in the cerebellum, although the authors did not show PF-PC synaptic transmission nor motor performance (35).
Many studies have focused on manipulating the extrasynaptic GABAAR, the molecular target for the tonically released GABA, to investigate the functional role of tonic inhibition in the brain (36–38). However, due to a variety of GABAAR subunits, consisting of 16 subunits and the pentameric composition plus each subunit’s differential regional expression pattern (4, 39), it has been extremely difficult to study the in vivo function of the tonic inhibition. Through a series of previous papers, we have demonstrated that the cerebellar tonic inhibition is mediated by the astrocytic GABA, synthesized by astrocyte-specific MAOB enzyme, and released via the GABA-permeable Best1 channel (1, 12, 13). The molecular, genetic, and pharmacological tools that we have characterized in this study should make it possible to selectively manipulate tonic inhibition without disturbing phasic inhibition. These tools will prove useful for studying the role of astrocytic GABA and tonic inhibition in physiological conditions as well as in pathophysiological conditions, including psychiatric disorders such as depression, seizure, stress, schizophrenia, and autism, in which the excitation/inhibition balance has been compromised.
Conclusions
We have reported that the cerebellar tonic inhibition is critically dependent on both MAOB-dependent GABA synthesis and Best1-mediated GABA release. By utilizing this mechanistic insight, we have demonstrated the role of astrocytic GABA in cerebellar motor function by modulating neuronal excitability and synaptic transmission (SI Appendix, Fig. S6B). We suggest that astrocytes are one of the key components for regulating the cerebellar circuit and output and motor coordination through the tonic release of GABA from astrocytes.
Methods
Animals.
Adult (∼8 to 10 wk) male and female WT of Best1 KO (Balb/C background), MAOB KO (129 background), and GFAP-MAOB (C57BL/6 background) mice (20) were used. All experimental procedures described below were performed in accordance with Korea Institute of Science and Technology (approval no. 2016-051) and Dankook University (approval no. DKU-17-022) Animal Experimentation Guidelines.
Slice Recording.
Cerebellar slicing and slice recording were performed as previously described (12, 13), within a normal artificial cerebrospinal fluid (ACSF) solution that contained 130 mM NaCl, 24 mM NaHCO3, 1.25 mM NaH2PO4, 3.5 mM KCl, 1.5 mM CaCl2, 1.5 mM MgCl2, and 10 mM d(+)‐glucose, pH 7.4.
Statistical Analysis.
The significance of data for comparison was assessed by Student’s two-tailed unpaired t test. Data are presented as mean ± SEM. Levels of statistical significance are indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
For full, detailed materials and methods, see SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by the Creative Research Initiative Program through the National Research Foundation (NRF) of Korea (Grant 2015R1A3A2066619); the Korea Institute of Science and Technology (KIST) Institutional Grant 2E26662; and the Korea University (KU)-KIST Graduate School of Science and Technology program (Grant R1435281) (to C.J.L.). This research was also supported by the Brain Research Program through the NRF of Korea, funded by the Ministry of Science, ICT, & Future Planning (Grant NRF-2016M3C7A1905074); and the Small Grant for Exploratory Research Program (Grant NRF-2016R1D1A1A02937398) (to B.-E.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721187115/-/DCSupplemental.
References
- 1.Jo S, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: Modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- 4.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 5.Jia F, et al. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lee V, Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits. 2014;8:3. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galliano E, et al. Silencing the majority of cerebellar granule cells uncovers their essential role in motor learning and consolidation. Cell Rep. 2013;3:1239–1251. doi: 10.1016/j.celrep.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Egawa K, et al. Decreased tonic inhibition in cerebellar granule cells causes motor dysfunction in a mouse model of Angelman syndrome. Sci Transl Med. 2012;4:163ra157. doi: 10.1126/scitranslmed.3004655. [DOI] [PubMed] [Google Scholar]
- 10.Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- 11.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, et al. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BE, et al. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 2014;592:4951–4968. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 15.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 16.Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan JS, Mohr C, Rossi DJ. Opposite actions of alcohol on tonic GABA(A) receptor currents mediated by nNOS and PKC activity. Nat Neurosci. 2013;16:1783–1793. doi: 10.1038/nn.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan JS, et al. Alcohol suppresses tonic GABAA receptor currents in cerebellar granule cells in the prairie vole: A neural signature of high-alcohol-consuming genotypes. Alcohol Clin Exp Res. 2016;40:1617–1626. doi: 10.1111/acer.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarishkin O, Lee J, Jo S, Hwang EM, Lee CJ. Disinhibitory action of astrocytic GABA at the perforant path to dentate gyrus granule neuron synapse reverses to inhibitory in Alzheimer’s disease model. Exp Neurobiol. 2015;24:211–218. doi: 10.5607/en.2015.24.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallajosyula JK, et al. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS One. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd GM. The Synaptic Organization of the Brain. 5th Ed Oxford Univ Press; Oxford: 2004. [Google Scholar]
- 22.Emi K, et al. Reevaluation of the role of parallel fiber synapses in delay eyeblink conditioning in mice using Cbln1 as a tool. Front Neural Circuits. 2013;7:180. doi: 10.3389/fncir.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones BJ, Roberts DJ. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J Pharm Pharmacol. 1968;20:302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 24.Caston J, Jones N, Stelz T. Role of preoperative and postoperative sensorimotor training on restoration of the equilibrium behavior in adult mice following cerebellectomy. Neurobiol Learn Mem. 1995;64:195–202. doi: 10.1006/nlme.1995.0002. [DOI] [PubMed] [Google Scholar]
- 25.Lalonde R, Bensoula AN, Filali M. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci Res. 1995;22:423–426. doi: 10.1016/0168-0102(95)00916-h. [DOI] [PubMed] [Google Scholar]
- 26.Farrant M, Brickley SG. Properties of GABA(A) receptor-mediated transmission at newly formed Golgi-granule cell synapses in the cerebellum. Neuropharmacology. 2003;44:181–189. doi: 10.1016/s0028-3908(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 27.Berglund K, Wen L, Dunbar RL, Feng G, Augustine GJ. Optogenetic visualization of presynaptic tonic inhibition of cerebellar parallel fibers. J Neurosci. 2016;36:5709–5723. doi: 10.1523/JNEUROSCI.4366-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo C, et al. Purkinje cells directly inhibit granule cells in specialized regions of the cerebellar cortex. Neuron. 2016;91:1330–1341. doi: 10.1016/j.neuron.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz MR, Wadleigh A, Hughes BA, Woodward JJ, Valenzuela CF. Bestrophin1 channels are insensitive to ethanol and do not mediate tonic GABAergic currents in cerebellar granule cells. Front Neurosci. 2012;5:148. doi: 10.3389/fnins.2011.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fioretti B, et al. NPPB block of the intermediate-conductance Ca2+-activated K+ channel. Eur J Pharmacol. 2004;497:1–6. doi: 10.1016/j.ejphar.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Reinach PS, Schoen HF. NPPB inhibits the basolateral membrane K+ conductance in the isolated bullfrog cornea. Biochim Biophys Acta. 1990;1026:13–20. doi: 10.1016/0005-2736(90)90326-j. [DOI] [PubMed] [Google Scholar]
- 32.Parsons MP, et al. Real-time imaging of glutamate clearance reveals normal striatal uptake in Huntington disease mouse models. Nat Commun. 2016;7:11251. doi: 10.1038/ncomms11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson CM, Swanson RA. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 34.Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci. 2007;27:111–123. doi: 10.1523/JNEUROSCI.4770-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duguid I, Branco T, London M, Chadderton P, Häusser M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci. 2012;32:11132–11143. doi: 10.1523/JNEUROSCI.0460-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: Their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Accardi MV, Brown PM, Miraucourt LS, Orser BA, Bowie D. α6-containing GABAA receptors are the principal mediators of inhibitory synapse strengthening by insulin in cerebellar granule cells. J Neurosci. 2015;35:9676–9688. doi: 10.1523/JNEUROSCI.0513-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engin E, et al. Alpha5-containing GABAA receptors in dentate gyrus enable cognitive flexibility. FASEB J. 2013;27(1 Suppl):661.7. [Google Scholar]
- 39.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.