Abstract
The development of the PAP smear test by Dr. George Nicholas Papanicolaou (1883–1962) is one of the most significant achievements in screening for disease and cancer prevention in history. The PAP smear has been used for screening of cervical cancer since the 1950s. The test is technically straightforward and practical and based on a simple scientific observation: malignant cells have an aberrant nuclear morphology that can be distinguished from benign cells.
Here, we review the scientific understanding that has been achieved and continues to be made on the causes and consequences of abnormal nuclear morphology, the basis of Dr. Papanicolaou’s invention. The deformed nuclear shape is caused by the loss of lamina and nuclear envelope structural proteins. The consequences of a nuclear envelope defect include chromosomal numerical instability, altered chromatin organization and gene expression, and increased cell mobility because of a malleable nuclear envelope.
HPV (Human Papilloma Virus) infection is recognized as the key etiology in the development of cervical cancer. Persistent HPV infection causes disruption of the nuclear lamina, which presents as a change in nuclear morphology detectable by a PAP smear. Thus, the causes and consequences of nuclear deformation are now linked to the mechanisms of viral carcinogenesis, and are still undergoing active investigation to reveal the details.
Recently a statue was installed in front of the Papanicolaou’s Cancer Research Building to honor the inventor. Remarkably, the invention nearly 60 years ago by Dr. Papanicolaou still exerts clinical impacts and inspires scientific inquiries.
Keywords: PAP smear, diagnosis, prevention, HPV, cervical cancer, chromosomal instability, nuclear envelope, nuclear lamina, nuclear deformation, nuclear morphology, nuclear budding, Lamin A/C, aneuploidy, polyploidy, carcinomas
A statue was recently constructed and placed in front of the Papanicolaou’s Cancer Research Building at the University of Miami Miller School of Medicine campus to commemorate “Dr. PAP” --- George Nicholas Papanicolaou (1883–1962), the inventor of the PAP smear test that was first used to screen for cervical and uterine cancer more than 60 years ago (1,2) (Figure 1). The cancer-screening test has been widely applied to the general population and is still in clinical use today. For the invention and its broad impact on public health, Dr. Papanicolaou received many awards and recognitions, including the Albert Lasker Award (1950) and the Medal of Honor from the American Cancer Society (1952).
Figure 1. A new statue of Dr. George Nicholas Papanicolaou (1883–1962).
In June 2017, a statue of Dr. George Nicholas Papanicolaou was placed in front of the Papanicolaou Cancer Research Building on the University of Miami Miller School of Medicine campus to commemorate the invention of PAP smear test.
Here in 1961, Dr. Papanicolaou became the head of the Cancer Institute of Miami, which was renamed the Papanicolaou Cancer Research Institute to honor Dr. Papanicolaou who died at 78 years of age, soon after his arrival in Miami.
Today, looking back on Dr. Papanicolaou’s achievement, the PAP smear test seems to base on an overtly simple idea: neoplastic cells are distinct from benign one and contain an aberrant, deformed nucleus. The PAP Smear test is still used in the clinical setting today, and aberrant nuclear morphology is still recognized as a universal indicator of malignancy. Over the decades, scientific research has brought new biological details and understanding to explain Dr. Papanicolaou’s concept of the deformed nuclear morphology of neoplastic cells. The cause of cervical cancer by persistent infection of Human Papilloma Virus (HPV) has been established and new screening methods and vaccines for prevention are available. Nevertheless, the concepts and effects on clinical practice of Dr. Papanicolaou’s discovery are still relevant today, and we will discuss the scientific issues pertinent to the concept that a deformed nuclear morphology can serve as a hallmark of malignant cells.
Deformed nuclear morphology and loss of nuclear structural proteins in malignant cells
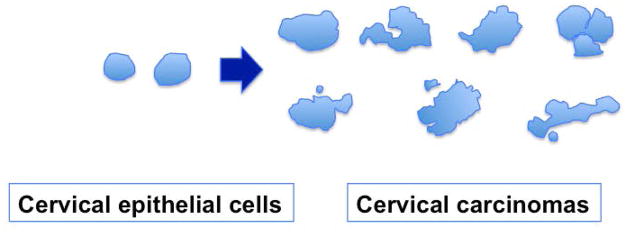
Normal cells usually have a smooth and oval shaped nucleus, and malignant cells present an aberrantly shaped/deformed, often enlarged nucleus (3), as shown by an illustration of nuclear shapes comparing normal cervical epithelial cells and neoplastic cells (Figure 2). Compared to largely round and oval shaped nucleus of normal cervical epithelial cells, malignant cervical cancer cells show a wide variety of nuclear shapes, which are often larger, irregular, multilobular, and contain micronuclei. An altered nuclear shape is the basis for the PAP smear in distinguishing malignant versus benign cells collected from scraping the cervix (1,2).
Figure 2. Nuclear shape changes in cervical cancer.
Outlines of nuclei were traced from images of sections of normal cervical epithelium and representative cervical carcinomas. Most nuclei in carcinoma specimens are larger and exhibit various irregular shapes. Additionally, micronuclei and nuclear fragments often associate with the cancer nuclei.
The molecular reasons underlying the deformed nuclear shape have not been clearly defined, likely because multiple mechanisms may cause the abnormalities (3). Early reports indicate that activation of oncogenes or inactivation of tumor suppressor genes leads to nuclear morphological changes (4–6). More recent studies suggest that loss or reduction of structural proteins of the nuclear envelope and lamina, such as Lamin A/C (7–9), Emerin (10), and the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex components such as nesprins (11), may explain the nuclear shape changes in cancer cells. In a study of ovarian cancer, it was estimated that 55% of ovarian carcinomas lack Lamin A/C expression, and about 30% of tumors show heterogeneous staining in the cancer cell population (7). From studies of the basic cell biology of these proteins, Lamin A/C, emerin, nesprins, etc, have been shown to function in maintaining a smooth and oval shaped nuclear envelope structure (12–14). However, no genetic deletion or gene mutations have been reported for these nuclear structural proteins in cancer (3). Additionally, the loss of Lamin A/C expression occurs at the protein but not the mRNA level (7), and protein degradation stimulated by phosphorylation during interphase may be a major cause of the loss/reduction of Lamin A/C (15,16). Furthermore, the loss of Lamin A/C appears to be heterozygous within a cancer cell population (7). These reasons may account for why the loss of reduced nuclear envelope and lamina structural proteins have not previously been exclusively and definitively linked to the misshaped nucleus of malignant cells.
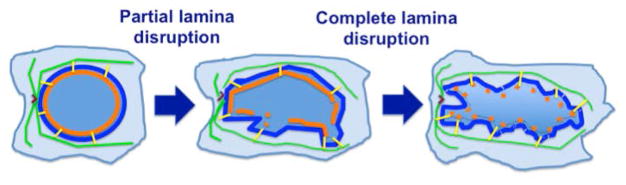
The collaboration between Lamin A and microtubules facilitates the nuclear shape (17), and likely the loss or reduction of nuclear lamina allows the nuclear envelope to be malleable and deformed from the force through the LINC bridges and the associated microtubule cytoskeleton (Figure 3). Partial disruption of nuclear lamina produces local perturbation of the nuclear envelope, and complete loss of nuclear lamina results in a malleable and deformed nuclear envelope from pulling by force from the microtubule cytoskeleton (Figure 3), producing the various nuclear morphology of carcinomas.
Figure 3. Illustration of nuclear envelope perturbation following lamina disruption.
The nuclear envelope (thin blue line) is supported by a layer of nuclear lamina (orange line). The nuclear envelope is anchored by binding to microtubules (green lines) through the Nesprin-SUN LINC bridge (yellow lines). Partial disruption of nuclear lamina produces local perturbation of the nuclear envelope. Complete loss of nuclear lamina results in a malleable nuclear envelope that deforms from pulling by force through the LINC bridges and the microtubule cytoskeleton that is organized by a centrosome (“V” like symbol).
Specifically, recent studies indicate that the loss of Lamin A/C proteins occurs in cervical cancer and may account for the nuclear shape changes (18). In a preliminary investigation of 50 archived human cervical carcinomas by immunohistochemistry, Lamin A/C was found lost in 70% of the cancer tissues, and abnormal distribution and patchy staining of Lamin A/C were seen in the other 30% (18). Furthermore, this preliminary report indicates the loss of Lamin A/C can be detected in PAP sampling (18). Thus, loss or greatly reduced nuclear envelope and lamina proteins likely account for significant nuclear deformation, the underlying principle for the PAP smear test (1,2).
Expression and roles of Lamin A/C in development and diseases
The biological functions of Lamin A/C may provide clues for why Lamin A/C expression is commonly lost/reduced in malignant cells including those of cervical cancer, leading to the aberration of nuclear morphology observed by Dr. Papanicolaou. Lamin A/C expression is low/absent in embryonic stem cells and early embryos, and is progressively expressed in nearly all tissues in later developmental stages (19,20). The initiation of Lamin A/C expression is associated with cell differentiation, suggesting that Lamin A/C expression may serve as a limit on the plasticity of cells for further developmental events (19–22). Additionally, the cell types that seem to lack Lamin A/C, such as embryonic carcinoma cells and some cells of the spleen, thymus, bone marrow and intestine in the adult mouse may fall into the “stem cell” category (19,20,22).
Lamin A/C mutations are the causes of several human diseases known as laminopathies, including muscular dystrophy, lipodystrophy, and progeria (23–25). Loss or reduction of Lamin A/C expression is often found in cancer cells (9), including breast (8), colon (26), gastric (27,28), leukemia (29,30), lung (31), prostate (5), and serous ovarian carcinomas (7). Additionally, emerin expression is lost in 38% of ovarian cancer (10).
The cell biology of the nuclear envelope and lamina has been well studied (32–35). Lamin A/C, but not Lamin B1/2, is critical for the maintenance of a smooth and oval shaped nucleus (14). Mutations or loss-of-function in several nuclear envelope structure proteins, including emerin and Man1, Baf, and Lamin in C. elegans, cause similar nuclear and mitotic phenotypes such as an enlarged and deformed nucleus, defective chromosome segregation, and the formation of chromatin bridges between divided nuclei, suggesting a critical role for the nuclear envelope and lamina in cytokinesis and mitosis (32,36–38).
Mutations in Lamin A/C gene (lmna) interfere with mitosis and cell cycle progression in mammalian cells (39,40), indicating to be an evolutionally conservative role. These findings are consistent with roles for these nuclear envelope proteins in both maintaining the nuclear structure and mediating cytokinesis/mitosis across species. Another major role of Lamin A/C is to bind heterochromatin and to influence gene expression (33,41–44). Because of their roles in chromatin organization and mitosis, defects of nuclear envelope proteins generate genomic instability due to aberrant gene expression and chromosomal numerical instability (45,46), leading to several human diseases such as muscular dystrophy and progeria (23–25). Lamin A/C null mice die at 4–6 weeks of age due to cardiac degeneration, a phenotype of muscular dystrophy in humans (24).
For relevance to cancer biology, we discuss below the effects of the loss of Lamin A/C and resulting nuclear envelope defects on genomic instability (specifically, chromosomal numerical instability), epigenetic dysregulation, cell malleability, and cancer metastasis.
Nuclear deformation and chromosomal numerical instability of carcinomas
Enlarged and deformed nuclei are characteristics of cancer cells, and the aberrant nuclear morphology correlates with malignancy and is a diagnostic and prognostic indicator, referred to as “nuclear grade” and is used universally for diagnostic and prognostic prediction of malignancies of tumor cells (3) such as in the PAP smear (1,2). In the last 5 decades, many investigators have attempted to decipher the reason behind the deformed and enlarged nuclei of cancer cells, but the molecular basis of nuclear deformation in malignant cells or its mechanistic link with malignancy are not well defined (3).
Another hallmark of cancer cells, first recognized over one hundred years ago by Boveri (47,48), is aneuploidy, or an abnormal and unbalanced number of chromosomes compared to normal cells. The majority of human ovarian and other cancer cells are aneuploid and possess a hyperdiploid (>46) to subtetraploid (< 96) chromosome number (49). Chromosome instability and aneuploidy may provide an unbalanced global expression profile of increases and decreases in gene dosages that create the cancer cell properties (50–52). Thus, aneuploidy or chromosomal numerical instability likely plays important roles in cancer initiation and progression, and possibly resistance to therapeutic drugs (53–55), but the causes and consequences in human cancer are not clear.
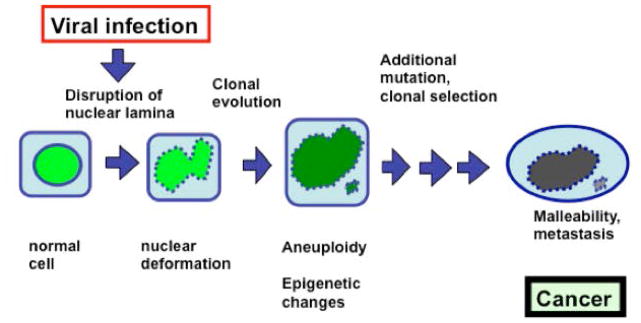
Aneuploidy in cancer is commonly thought to be caused by chromosome mis-segregation (nondisjunction) in mitosis (56–60). However, recent studies lead to a proposal that mitotic failure and breakage of a malleable nuclear envelope to form micronuclei is the key mechanism for chromosomal numerical instability and aneuploidy in ovarian carcinogenesis (7,8,61) (Figure 4). Early experimental results led to the conclusion that cells with lost or reduced Lamin A/C have frequent mitotic failure to form tetraploid cells, which then often undergo multipolar division to form aneuploid cells. Additionally, the Lamin A/C-deficient cells produce extensive nuclear buddings/protrusions, which often break off as micronuclei, leading to chromosome losses at non-mitotic cell cycle phases (61). Such frequent transient ruptures of nuclear envelope during interphase have been observed in cancer cells (62). Thus, one relevant consequence of nuclear deformation observed in PAP smear test is the development of aneuploidy, a potential driving force in carcinogenesis.
Figure 4. Consequences of viral disruption of nuclear envelope.
A cartoon illustrates our hypothesis on the consequences of viral infection and a nuclear envelope defect. We reason that loss of a nuclear envelope structural component such as Lamin A/C resulting from viral infection causes a misshapen nucleus. The Lamin A/C-deficient cells frequently fail to complete cytokinesis, and thus tetraploid and subsequently aneuploid cells are generated. Formation of micronuclei is another mechanism for the loss of individual chromosomes. Additional genetic and epigenetic changes may allow the aneuploid cells to survive and undergo clonal selection. Most aneuploid cells will die, but ultimately, a population of cells with a unique chromosomal composition is selected and expanded to form cancer.
Prior study suggests that a nuclear envelope defect caused by the loss of nuclear lamina structural protein such as Lamin A/C causes chromosomal numerical instability and aneuploidy in ovarian cancer (61). Here, we postulate a provocative hypothesis that loss of Lamin A/C proteins caused by persistent HPV infection and replication promotes cervical cancer by inducing aneuploidy and epigenetic alteration (since Lamin A/C have key roles in chromatin organization and impacts gene expression). Additionally, loss of lamina proteins allows cells to be malleable and harbor metastatic potential. Our hypothesis links the two hallmarks of cancer: nuclear envelope defects and chromosomal instability.
Roles of nuclear lamina in chromatin organization and epigenetic regulation
Another possible contributing factor for a deformed nuclear morphology is the impact on epigenetics: the role of lamina on chromatin organization and gene expression (33,41–44). Consequently, loss or reduction of Lamin A/C in cancer cells likely will contribute to deregulation of gene expression and deviant differentiation of a malignant phenotype.
A connection between nuclear morphological irregularities and nuclear DNA profiles in various disease states was recognized previously, dating back to the late 1960’s (63,64). An earlier notion is that the nuclear deformations are secondary to the changes in DNA and chromatins, and in neoplasia the portions of the genome are irregularly activated or suppressed, and the resulted genomic instability is proportional to the degree of nuclear deformation (63,64). However, the current hypothesis is that the changes in nuclear structural protein come first, causing alterations in both DNA contents (aneuploidy) (7,60) and conformation (epigenetics) (33,41–44).
One recent study used Lamin A/C and/or emerin deleted embryonic stem cells as a model to investigate the association between lamina and nuclear envelope structural proteins on nuclear shape and gene expression (65). The study found that the nuclear envelope structural proteins Lamin A/C and/or emerin mediate changes in nuclear size and shape. Additionally, the Lamin A/C and/or emerin deficient ES cells co-express both pluripotent and differentiation markers (such as Oct3/4 and Gata4, respectively), indicating an infidelity in the regulation of gene expression, and are compromised to undergo proper lineage differentiation (62). The effect of alterations in nuclear envelope proteins on gene regulation is thought to be mediated through the roles of nuclear lamina in binding and organizing chromatin (33,41–44). Although not yet thoroughly investigated in cancer cells, loss of nuclear envelope proteins in cancer cells presumably alters chromatin organization, the fidelity of gene regulation, and allows clonal selection of cells with a gene expression profile of malignant cells.
Nuclear deformation and metastatic potential of cancer cells
Furthermore, recent investigations suggest another mechanism for a deformed nuclear shape contributing to malignancy: a malleable nuclear envelope and increased potential for mobility and cancer metastasis (12,66).
Cell shape is determined by a network of cytoskeleton under plasma membrane. The nucleus serves as a central point to organize the actin, intermediate filament, and microtubule networks (67,68). These cytoskeleton networks are anchored on nuclear envelope and lamina through the LINC complexes composed of nesprins and SUN proteins (69). The loss or reduction of Lamin A/C, emerin, and/or nesprins likely would transform and corrupt the normally rigid cell cytoskeleton into a less organized and malleable shape. Additionally, the nuclei are deformable, which would allow cancer cells to penetrate narrower space and increase invasive and metastatic ability.
Chronic HPV infection in the disruption of nuclear lamina
Cervical cancer has an established viral etiology, and is caused by persistent infection of human papillomavirus (HPV) (70–74). The work linking cervical cancer to HPV infection was honored with a Nobel Prize in 2008 (75–78). High risk HPVs such as HPV-16 and HPV-18 transform cells following chronic infection, and the viral genes, E6 and E7, are known to be critical for transformation as the encoded proteins interfere with the functions of Rb and p53 and affect signaling pathways and the cell cycle (79,80). However, though essential, the infection by the virus is insufficient to induce malignant transformation, and our knowledge of viral oncogenesis remains incomplete (70,72,73,81). Chromosomal instability is thought to be important for progression to malignancy (82,83).
DNA virus including HPV replicates in nuclei and requires the disruption of nuclear lamina for egress of viral particles (84–91). However, the mechanism has not yet been established for HPV in the disruption of nuclear lamina and viral egress. A provocative hypothesis that viral disruption of the nuclear envelope is a mechanism contributing to chromosomal numerical instability and malignant transformation in viral carcinogenesis, especially for HPV-induced cervical cancer (Figure 4).
A hallmark of cervical carcinomas is the morphological deformation of the cancer nucleus, which is the basis of the PAP smear diagnostic test (3). In infections by many DNA viruses including HPV, the viral genome is replicated in the nucleus and the partially assembled capsids escape from the nucleus, in a process known as nuclear egress (92). In doing so, the nuclear lamina is disrupted following phosphorylation and degradation of Lamin A/C by viral or host kinases. Likely, the disruption of nuclear lamina by persistent HPV infection and replication accounts for the characteristic changes in nuclear morphology. Furthermore, we speculate that infection of cells by HPV may promote neoplastic transformation by the disruption of nuclear envelope and subsequent chromosomal numerical instability/aneuploidy and chromatin changes (Figure 4). Indeed, cytological distinction between high-risk and low-risk HPV infections is recognizable, and perturbation of nuclear lamina is associated with cell transformation by high-risk HPV (93).
The mechanisms in the disruption of nuclear lamina have been established in several oncogenic DNA virus such as KS-associated herpesvirus (KSHV) (also known as human herpesvirus-8 (HHV-8)) (91), herpes simplex virus (HSV) (84–90), Simian virus 40 (89), and Epstein–Barr virus (EBV) (86,89) (Figure 5). Disruption of nuclear envelope by these oncogenic viruses likely is an important mechanism to promote neoplastic transformation. However, extensive nuclear envelope disruption or distortion may prohibit efficient viral replication or may be an early event in establishing infection (92). Efficient virus replication found in high-risk HPV infection may be more efficient if nuclear envelope disruption occurs at a limited site (92).
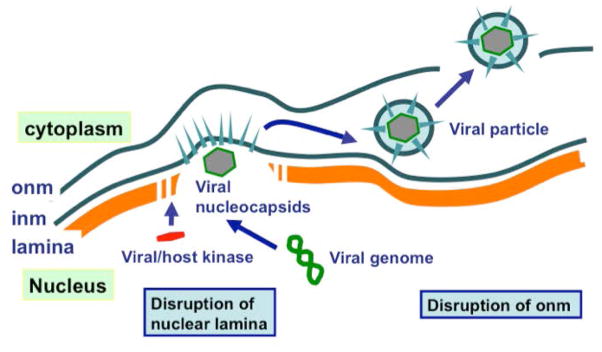
Figure 5. Disruption of nuclear envelope in viral infection and egress.
A cartoon illustrates the process of the escape of viral capsids from the nucleus of cells infected with DNA viruses such as HPV. Phosphorylation of the Lamin A/C proteins by viral or activated host kinase leads to the disassembly and degradation of the nuclear lamina, allowing the escape of viral capsids into the cytoplasm. Viral encoded kinases, BGLF4 in EBV and ORF36 in KSHV, function to disrupt the nuclear lamina by phosphorylation Lamin A/C. The viral coat proteins are produced and incorporated into the inner nuclear membrane (inm). The viral nucleocapsid uses the inm to produce a viral membrane, and breaks through the outer nuclear membrane (onm) into the cytoplasm.
For viruses such as KSHV (or, HHV-8), HSV, or EBV, following infection of cells, the viral genome is replicated in the nucleus and the partially assembled capsids escape by disrupting the nuclear envelope and the lamina lying underneath, and forming a viral envelope from the inner nuclear membrane (Figure 5) (70,88). The disruption of the nuclear lamina is accomplished by phosphorylation of the structural protein Lamin A/C, which leads to nuclear lamina disassembly and degradation as that occurs during mitosis. Viral encoded kinases, BGLF4 in EBV and ORF36 in KSHV, function to disrupt Lamin A/C (84–91). We speculate that infection of cells by KSHV or EBV may promote neoplastic transformation by the disruption of the nuclear envelope and subsequent aneuploidy and chromatin changes. HPV viral particles also need to disrupt nuclear lamina for nuclear egress to progress; however, the mechanism and host or viral gene(s) required to mediate nuclear lamina disruption has not yet been identified. The proposal that this disruption of the nuclear lamina following HPV infection contributes to cell transformation provides a plausible mechanism in cervical cancer development (Figure 4), which can be tested.
Therefore, a nuclear defect may cause chromosomal instability and aneuploidy, and also result in epigenetic changes, which promote the neoplastic transformation of cervical epithelial cells. Additionally, the malleable nuclear envelope allows the cancer cells to be invasive and metastatic. PAP test is able to identify the pre-neoplastic cells with a deformed nuclear morphology, and thus to predict cervical cancer risk.
Summary and Reflection
It is amazing that Dr. Pap’s discovery (1,2) of a simple method for cancer diagnosis more than half a century ago is still relevant today. Furthermore, the scientific community is still making discoveries to explain the causes and consequences of an altered nuclear shape characteristic of neoplastic cells, which the PAP test is based.
It seems to be apparent now that the loss or reduction of nuclear envelope and lamina structural proteins such as Lamin A/C, emerin, nesprins, etc, is the reason for an apparent changed nuclear shape of malignant cells, distinct from smooth and oval shape of benign cells. The causes may be phosphorylation and subsequent degradation of Lamin A/C or other nuclear envelope structural proteins persistent viral infection. The nuclear structural changes leading to a malleable nuclear envelope then promote malignancy by mechanisms of chromosomal instability, epigenetic dysregulation, and ability for cell mobility and metastasis. A big picture of the scientific connections underneath the principle of PAP smear test has emerged, but details warrant further investigation.
Currently, the PAP smear (94,95) and the detection of high risk HPV DNA (96–99) are common screening and diagnostic approaches. Special efforts have been targeted to the most at risk populations for education and screening (100–102). Vaccination to HPV has been developed and applied to prevention of HPV-induced cervical and other neoplasms (98). Although a large number of adults are infected with high-risk HPV in their lifetime, only a small fraction of women will develop cervical cancer following persistent chronic infection (70,73,81), and virus-induced cytological abnormality is a key predictor (81). Our study that defective nuclear lamina caused by HPV infection may provide a possible enhanced method than just morphology alone as used in the PAP smear. Potential method may be the analysis of nuclear lamina integrity using immunofluorescence microscopy to detect the loss or reduction of Lamin A/C, emerin, and additional nuclear envelope proteins, which may be more predictive for risk of cancer progression (17).
Dr. Papanicolaou never knew that his discovery, based on such a simple observation of a deformed nuclear shape present in malignant cells, would develop such long lasting clinical applications and inspired such wide spread scientific inquiries. The newly installed statue of Dr. Papanicolaou (Figure 1) in front of the Papanicolaou Cancer Research Building signifies our continual gratitude to his insight.
Acknowledgments
Our lab alumni and students, including Wensi Tao, Linlin Gao, Santas Rosario, Ziqi Wang, Justin Correa, Jessica Clark, and Anthony Guerrero, worked on this project and contributed to the basis of the current understanding. We acknowledge the excellent technical assistance from the Microscopy Facility, the Transgenic Mouse Facility, Flow Cytometry Core, and Cytogenetic Core at the University of Miami Miller School of Medicine.
Funding
The work from our lab cited in this article was partially supported by a Jay Weiss Disparity Research and Developmental Research grant from UM/Sylvester Comprehensive Cancer Center, and a pilot grant from the University of Miami cFAR grant P30AI073961 from NIH. Publications by the authors cited in this review were also supported by funds from concept awards BC097189 and BC076832 from Department of Defense (USA). Grants R01 CA095071, R01 CA099471, and CA79716 from NCI, NIH to X.X. Xu also contributed to the studies.
Abbreviations
- EBV
Epstein-Barr virus
- GFP
green fluorescence protein
- HHV-8
human herpesvirus-8
- HPV
human papillomavirus
- inm
inner nuclear membrane
- KSHV
KS-associated herpesvirus
- onm
outer nuclear membrane
Footnotes
Author Contributions:
XX initiated the concepts and the first draft of this review article. All authors were involved in discussing the content and questions to be addressed, and participated in editing and re-writing of the manuscript. XX is responsible for the overall content as guarantor.
Conflicts of Interest Statement:
All authors declare that they have no conflicts of interest.
References
- 1.Papanicolaou GN. A NEW PROCEDURE FOR STAINING VAGINAL SMEARS. Science. 1942;95:438–9. doi: 10.1126/science.95.2469.438. [DOI] [PubMed] [Google Scholar]
- 2.Diamantis A, Magiorkinis E, Koutselini H. 50 years after the death of George Nicholas Papanicolaou (1883–1962): evaluation of his scientific work. Acta Med Hist Adriat. 2014;12(1):181–8. [PubMed] [Google Scholar]
- 3.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–87. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 4.Boyd J, Pienta KJ, Getzenberg RH, Coffey DS, Barrett JC. Preneoplastic alterations in nuclear morphology that accompany loss of tumor suppressor phenotype. J Natl Cancer Inst. 1991;83:862–6. doi: 10.1093/jnci/83.12.862. [DOI] [PubMed] [Google Scholar]
- 5.Debes JD, Sebo TJ, Heemers HV, Kipp BR, Haugen DL, Lohse CM, Tindall DJ. p300 modulates nuclear morphology in prostate cancer. Cancer Res. 2005;65:708–12. [PubMed] [Google Scholar]
- 6.Fischer AH, Taysavang P, Jhiang SM. Nuclear envelope irregularity is induced by RET/PTC during interphase. Am J Pathol. 2003;163:1091–100. doi: 10.1016/S0002-9440(10)63468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capo-chichi CD, Cai KQ, Simpkins F, Ganjei-Azar P, Godwin AK, Xu XX. Nuclear envelope structural defects cause chromosomal numerical instability and aneuploidy in ovarian cancer. BMC Med. 2011;9:28. doi: 10.1186/1741-7015-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capo-chichi CD, Cai KQ, Smedberg J, Ganjei-Azar P, Godwin AK, Xu XX. Loss of A-type lamin expression compromises nuclear envelope integrity in breast cancer. Chin J Cancer. 2011;30:415–25. doi: 10.5732/cjc.010.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster CR, Przyborski SA, Wilson RG, Hutchison CJ. Lamins as cancer biomarkers. Biochem Soc Trans. 2010;38:297–300. doi: 10.1042/BST0380297. [DOI] [PubMed] [Google Scholar]
- 10.Capo-chichi CD, Cai KQ, Testa JR, Godwin AK, Xu XX. Loss of GATA6 leads to nuclear deformation and aneuploidy in ovarian cancer. Mol Cell Biol. 2009;29(17):4766–77. doi: 10.1128/MCB.00087-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto A, Hieda M, Yokoyama Y, Nishioka Y, Yoshidome K, Tsujimoto M, Matsuura N. Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med. 2015 Oct;4(10):1547–57. doi: 10.1002/cam4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell ES, Lammerding J. Causes and consequences of nuclear envelope alterations in tumour progression. Eur J Cell Biol. 2016 Nov;95(11):449–464. doi: 10.1016/j.ejcb.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–91. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–80. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 15.Bertacchini J, Beretti F, Cenni V, Guida M, Gibellini F, Mediani L, Marin O, Maraldi NM, de Pol A, Lattanzi G, Cocco L, Marmiroli S. The protein kinase Akt/PKB regulates both prelamin A degradation and Lmna gene expression. FASEB J. 2013 Jun;27(6):2145–55. doi: 10.1096/fj.12-218214. [DOI] [PubMed] [Google Scholar]
- 16.Kochin V, Shimi T, Torvaldson E, Adam SA, Goldman A, Pack CG, Melo-Cardenas J, Imanishi SY, Goldman RD, Eriksson JE. Interphase phosphorylation of lamin A. J Cell Sci. 2014;127(Pt 12):2683–96. doi: 10.1242/jcs.141820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tariq Z, Zhang H, Chia-Liu A, Shen Y, Gete Y, Xiong ZM, Tocheny C, Campanello L, Wu D, Losert W, Cao K. Lamin A and microtubules collaborate to maintain nuclear morphology. Nucleus. 2017 Jul 4;8(4):433–446. doi: 10.1080/19491034.2017.1320460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capo-Chichi CD, Aguida B, Chabi NW, Cai QK, Offrin G, Agossou VK, Sanni A, Xu XX. Lamin A/C deficiency is an independent risk factor for cervical cancer. Cell Oncol (Dordr) 2016a;39:59–68. doi: 10.1007/s13402-015-0252-6. [DOI] [PubMed] [Google Scholar]
- 19.Röber RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–78. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 20.Constantinescu SD, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 21.Lebel S, Lampron C, Royal A, Raymond Y. Lamins A and C appear during retinoic acid-induced differentiation of mouse embryonal carcinoma cells. J Cell Biol. 1987;105:1099–1104. doi: 10.1083/jcb.105.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013 Jan;14(1):13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 23.Bonne G, Di Barletta MR, Varnous S, Becane H-M, Hammouda E-H, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea J-A, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nature Genet. 1999;21:285–8. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–20. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson KL. The nuclear envelope, muscular dystrophy and gene expression. Trends Cell Biol. 2000;10:125–9. doi: 10.1016/s0962-8924(99)01708-0. [DOI] [PubMed] [Google Scholar]
- 26.Willis ND, Cox TR, Rahman-Casañs SF, Smits K, Przyborski SA, van den Brandt P, van Engeland M, Weijenberg M, Wilson RG, de Bruïne A, Hutchison CJ. Lamin A/C is a risk biomarker in colorectal cancer. PLoS One. 2008;3:e2988. doi: 10.1371/journal.pone.0002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss SF, Krivosheyev V, de Souza A, Chin K, Gaetz HP, Chaudhary N, Worman HJ, Holt PR. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut. 1999;45:723–9. doi: 10.1136/gut.45.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Wu L, Weng D, Xu D, Geng J, Zhao F. Reduced expression of lamin A/C correlates with poor histological differentiation and prognosis in primary gastric carcinoma. J Exp Clin Cancer Res. 2009;28:8. doi: 10.1186/1756-9966-28-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stadelmann B, Khandjian E, Hirt A, Lüthy A, Weil R, Wagner HP. Repression of nuclear lamin A and C gene expression in human acute lymphoblastic leukemia and non-Hodgkin’s lymphoma cells. Leuk Res. 1990;14:815–21. doi: 10.1016/0145-2126(90)90076-l. [DOI] [PubMed] [Google Scholar]
- 30.Agrelo R, Setien F, Espada J, Artiga MJ, Rodriguez M, Pérez-Rosado A, Sanchez-Aguilera A, Fraga MF, Piris MA, Esteller M. Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. J Clin Oncol. 2005;23:3940–7. doi: 10.1200/JCO.2005.11.650. [DOI] [PubMed] [Google Scholar]
- 31.Machiels BM, Broers JL, Raymond Y, de Ley L, Kuijpers HJ, Caberg NE, Ramaekers FC. Abnormal A-type lamin organization in a human lung carcinoma cell line. Eur J Cell Biol. 1995;67:328–35. [PubMed] [Google Scholar]
- 32.Gorjánácz M, Jaedicke A, Mattaj IW. What can Caenorhabditis elegans tell us about the nuclear envelope? FEBS Lett. 2007;581:2794–801. doi: 10.1016/j.febslet.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–53. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrés V, González JM. Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol. 2009 Dec 28;187(7):945–57. doi: 10.1083/jcb.200904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson KL, Berk JM. The nuclear envelope at a glance. J Cell Sci. 2010;123:1973–8. doi: 10.1242/jcs.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Rolef Ben-Shahar T, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000 Nov;11(11):3937–47. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Lee KK, Segura-Totten M, Neufeld E, Wilson KL, Gruenbaum Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4598–603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margalit A, Liu J, Fridkin A, Wilson KL, Gruenbaum Y. A lamin-dependent pathway that regulates nuclear organization, cell cycle progression and germ cell development. Novartis Found Symp. 2005;264:231–40. discussion 240–5. [PubMed] [Google Scholar]
- 39.Cao K, Capell BC, Erdos MR, Djabali K, Collins FS. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci U S A. 2007;104:4949–54. doi: 10.1073/pnas.0611640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dechat T, Shimi T, Adam SA, Rusinol AE, Andres DA, Spielmann HP, Sinensky MS, Goldman RD. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc Natl Acad Sci U S A. 2007;104:4955–60. doi: 10.1073/pnas.0700854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniura H, Glass C, Gerace L. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol. 1995 Oct;131(1):33–44. doi: 10.1083/jcb.131.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg M, Harel A, Gruenbaum Y. The nuclear lamina: molecular organization and interaction with chromatin. Crit Rev Eukaryot Gene Expr. 1999;9(3–4):285–93. doi: 10.1615/critreveukargeneexpr.v9.i3-4.130. [DOI] [PubMed] [Google Scholar]
- 43.Gotzmann J, Foisner R. Lamins and lamin-binding proteins in functional chromatin organization. Crit Rev Eukaryot Gene Expr. 1999;9(3–4):257–65. doi: 10.1615/critreveukargeneexpr.v9.i3-4.100. [DOI] [PubMed] [Google Scholar]
- 44.Harr JC, Luperchio TR, Wong X, Cohen E, Wheelan SJ, Reddy KL. Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol. 2015 Jan 5;208(1):33–52. doi: 10.1083/jcb.201405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capell BC, Collins FS. Human laminopathies: nuclie gone genetically awry. Nat Rev Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Suarez I, Gonzalo S. Nurturing the genome: A-type lamins preserve genomic stability. Nucleus. 2010 Mar-Apr;1(2):129–35. doi: 10.4161/nucl.1.2.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boveri T. Zur Frage der Enstehung maligner Tumoren. Gustav Fischer Verlag; Jena: 1914. [Google Scholar]
- 48.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–87. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roschke AV, Tonon G, Gehlhaus KS, McTyre N, Bussey KJ, Lababidi S, Scudiero DA, Weinstein JN, Kirsch IR. Karyotypic complexity of the NCI-60 drug-screening panel. Cancer Res. 2003;63:8634–47. [PubMed] [Google Scholar]
- 50.Pihan G, Doxsey SJ. Mutations and aneuploidy: co-conspirators in cancer? Cancer Cell. 2003;4:89–94. doi: 10.1016/s1535-6108(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 51.Duesberg P. Does aneuploidy or mutation start cancer? Science. 2005 Jan 7;307(5706):41. doi: 10.1126/science.307.5706.41d. [DOI] [PubMed] [Google Scholar]
- 52.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007 Apr;17(2):157–62. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 54.Michor F, Iwasa Y, Vogelstein B, Lengauer C, Nowak MA. Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol. 2005 Feb;15(1):43–9. doi: 10.1016/j.semcancer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006 Dec;18(6):658–67. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Draviam VM, Xie S, Sorger PK. Chromosome segregation and genomic stability. Curr Opin Genet Dev. 2004 Apr;14(2):120–5. doi: 10.1016/j.gde.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Jefford CE, Irminger-Finger I. Mechanisms of chromosome instability in cancers. Crit Rev Oncol Hematol. 2006;59:1–14. doi: 10.1016/j.critrevonc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Weaver BA, Silk AD, Cleveland DW. Cell biology: nondisjunction, aneuploidy and tetraploidy. Nature. 2006 Aug 17;442(7104):E9–10. doi: 10.1038/nature05139. [DOI] [PubMed] [Google Scholar]
- 59.Nicholson JM, Cimini D. How mitotic errors contribute to karyotypic diversity in cancer. Adv Cancer Res. 2011;112:43–75. doi: 10.1016/B978-0-12-387688-1.00003-X. [DOI] [PubMed] [Google Scholar]
- 60.Duijf PH, Benezra R. The cancer biology of whole-chromosome instability. Oncogene. 2013 Oct;32(40):4727–36. doi: 10.1038/onc.2012.616. [DOI] [PubMed] [Google Scholar]
- 61.Capo-Chichi CD, Yeasky TM, Smith ER, Xu XX. Nuclear envelope structural defect underlies the main cause of aneuploidy in ovarian carcinogenesis. BMC Cell Biol. 2016;17(1):37. doi: 10.1186/s12860-016-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 2012 Jan-Feb;3(1):88–100. doi: 10.4161/nucl.18954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frost JK. An evaluation of cellular morphologic expression of biologic behavior. Monogr Clin Cytol. 1969;2:1–142. [PubMed] [Google Scholar]
- 64.Frost JK. The cell in health and disease. An evaluation of cellular morphologic expression of biologic behavior. Monogr Clin Cytol. 1986;2:1–304. 2nd, revised edition. [PubMed] [Google Scholar]
- 65.Smith ER, Meng Y, Moore R, Tse JD, Xu AG, Xu XX. Nuclear envelope structural proteins facilitate nuclear shape changes accompanying embryonic differentiation and fidelity of gene expression. BMC Cell Biol. 2017;18:8. doi: 10.1186/s12860-017-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davidson PM, Denais C, Bakshi MC, Lammerding J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell Mol Bioeng. 2014 Sep 1;7(3):293–306. doi: 10.1007/s12195-014-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172(1):41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Curr Opin Cell Biol. 2011 Feb;23(1):47–54. doi: 10.1016/j.ceb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Shimi T, Butin-Israeli V, Goldman RD. The functions of the nuclear envelope in mediating the molecular crosstalk between the nucleus and the cytoplasm. Curr Opin Cell Biol. 2012 Feb;24(1):71–8. doi: 10.1016/j.ceb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laimins LA. The biology of human papillomaviruses: from warts to cancer. Infect Agents Dis. 1993;2:74–86. [PubMed] [Google Scholar]
- 71.Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117:S5–S10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 72.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012 Nov 20;30(Suppl 5):F55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 73.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015 Mar;25(Suppl 1):2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci. 2017;131:2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- 75.Cohen J, Enserink M. Nobel Prize in Physiology or Medicine. HIV, HPV researchers honored, but one scientist is left out. Science. 2008 Oct 10;322(5899):174–5. doi: 10.1126/science.322.5899.174. [DOI] [PubMed] [Google Scholar]
- 76.Hampton T. Nobel Prize honors HIV, HPV discoveries. JAMA. 2008;300(18):2109. doi: 10.1001/jama.2008.616. [DOI] [PubMed] [Google Scholar]
- 77.Watts G. Three Europeans win Nobel medicine prize for discovering HIV and HPV. BMJ. 2008;337:a2023. doi: 10.1136/bmj.a2023. [DOI] [PubMed] [Google Scholar]
- 78.zur Hausen H. The search for infectious causes of human cancers: where and why (Nobel lecture) Angew Chem Int Ed Engl. 2009;48(32):5798–808. doi: 10.1002/anie.200901917. [DOI] [PubMed] [Google Scholar]
- 79.Münger K1, Scheffner M, Huibregtse JM, Howley PM. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. [PubMed] [Google Scholar]
- 80.Wise-Draper TM, Wells SI. Papillomavirus E6 and E7 proteins and their cellular targets. Front Biosci. 2008 Jan 1;13:1003–17. doi: 10.2741/2739. [DOI] [PubMed] [Google Scholar]
- 81.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007 Jan;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 82.Duensing S, Münger K. Centrosomes, genomic instability, and cervical carcinogenesis. Crit Rev Eukaryot Gene Expr. 2003;13(1):9–23. doi: 10.1615/critreveukaryotgeneexpr.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- 83.Olaharski AJ, Sotelo R, Solorza-Luna G, Gonsebatt ME, Guzman P, Mohar A, Eastmond DA. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis. 2006 Feb;27(2):337–43. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- 84.Bjerke SL, Roller RJ. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology. 2006 Apr 10;347(2):261–76. doi: 10.1016/j.virol.2005.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mou F, Forest T, Baines JD. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol. 2007 Jun;81(12):6459–70. doi: 10.1128/JVI.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee CP, Huang YH, Lin SF, Chang Y, Chang YH, Takada K, Chen MR. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production. J Virol. 2008 Dec;82(23):11913–26. doi: 10.1128/JVI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cano-Monreal GL, Wylie KM, Cao F, Tavis JE, Morrison LA. Herpes simplex virus 2 UL13 protein kinase disrupts nuclear lamins. Virology. 2009;392:137–47. doi: 10.1016/j.virol.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee CP, Chen MR. Escape of herpesviruses from the nucleus. Rev Med Virol. 2010;20:214–30. doi: 10.1002/rmv.643. [DOI] [PubMed] [Google Scholar]
- 89.Meng Q, Hagemeier SR, Kuny CV, Kalejta RF, Kenney SC. Simian virus 40 T/t antigens and lamin A/C small interfering RNA rescue the phenotype of an Epstein-Barr virus protein kinase (BGLF4) mutant. J Virol. 2010 May;84(9):4524–33. doi: 10.1128/JVI.02456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morrison LA, DeLassus GS. Breach of the nuclear lamina during assembly of herpes simplex viruses. Nucleus. 2011 Jul-Aug;2(4):271–6. doi: 10.4161/nucl.2.4.16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim S, Cha S, Jang JH, Kim Y, Seo T. Kaposi’s sarcoma-associated herpesvirus ORF36 protein induces chromosome condensation and phosphorylation of histone H3. Acta Virol. 2013;57(1):75–9. doi: 10.4149/av_2013_01_75. [DOI] [PubMed] [Google Scholar]
- 92.Hennig T, O’Hare P. Viruses and the nuclear envelope. Curr Opin Cell Biol. 2015 Jun;34:113–21. doi: 10.1016/j.ceb.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 93.Wong NK, Ng FY, Leung G. Cytological distinction between high-risk and low-risk human papillomavirus infections in SurePath liquid-based cell preparations. J Clin Pathol. 2008 Dec;61(12):1317–22. doi: 10.1136/jcp.2008.059881. [DOI] [PubMed] [Google Scholar]
- 94.O’Meara AT. Present standards for cervical cancer screening. Curr Opin Oncol. 2002 Sep;14(5):505–11. doi: 10.1097/00001622-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Isehunwa OO, Carlton EL, Wang Y, Jiang Y, Kedia S, Chang CF, Fijabi D, Bhuyan SS. Access to Employee Wellness Programs and Use of Preventive Care Services Among U.S. Adults. Am J Prev Med. 2017 Dec;53(6):854–865. doi: 10.1016/j.amepre.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Twiggs LB, Hopkins M. High-risk HPV DNA testing and HPV-16/18 genotyping: what is the clinical application? J Low Genit Tract Dis. 2011;15(3):224–30. doi: 10.1097/LGT.0b013e3181fb46d8. [DOI] [PubMed] [Google Scholar]
- 97.Franco EL. Chapter 13: Primary screening of cervical cancer with human papillomavirus tests. J Natl Cancer Inst Monogr. 2003;(31):89–96. doi: 10.1093/oxfordjournals.jncimonographs.a003488. [DOI] [PubMed] [Google Scholar]
- 98.Crosbie EJ, Kitchener HC. Human papillomavirus in cervical screening and vaccination. Clin Sci (Lond) 2006 May;110(5):543–52. doi: 10.1042/CS20050230. [DOI] [PubMed] [Google Scholar]
- 99.Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Pract Res Clin Obstet Gynaecol. 2017 Aug 12; doi: 10.1016/j.bpobgyn.2017.08.002. pii: S1521-6934(17)30119-0. [DOI] [PubMed] [Google Scholar]
- 100.Kenya S, Carrasquillo O, Fatil M, Jones J, Jean C, Huff I, Kobetz E. Human Papilloma Virus and Cervical Cancer Education Needs among HIV-Positive Haitian Women in Miami. Womens Health Issues. 2015 May-Jun;25(3):262–6. doi: 10.1016/j.whi.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kobetz E, Kish JK, Campos NG, Koru-Sengul T, Bishop I, Lipshultz H, Barton B, Barbee L. Burden of Human Papillomavirus among Haitian Immigrants in Miami, Florida: Community-Based Participatory Research in Action. J Oncol. 2012;2012:728397. doi: 10.1155/2012/728397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kobetz E, Dunn Mendoza A, Menard J, Finney Rutten L, Diem J, Barton B, Kornfeld J, McKenzie N. One size does not fit all: differences in HPV knowledge between Haitian and African American women. Cancer Epidemiol Biomarkers Prev. 2010;19:366–70. doi: 10.1158/1055-9965.EPI-09-1180. [DOI] [PubMed] [Google Scholar]