Abstract
Purpose
This study aims to describe the experience and outcomes of in vitro maturation without ovarian stimulation (IVM-FP) and conventional in vitro fertilization after ovarian stimulation (IVF-FP) in a fertility preservation (FP) program for women with cancer.
Methods
Retrospective cohort study from 2003 to 2015 was conducted. The study population consisted of 353 women with cancer who underwent 394 FP cycles (187 IVF-FP cycles and 207 IVM-FP) for oocytes and/or embryos cryopreservation.
Result(s)
Comparatively with IVM-FP, IVF-FP had a higher median [25th–75th percentile] number of oocytes collected—12 [8–18] vs 7 [5–13]; oocytes cryopreserved—10 [6–15] vs 5 [2–8]; and, where applicable, embryos cryopreserved—5 [3–7] vs 3 [2–5] (p < 0.000001). Following FP treatment, 32 patients (9.0%) died, 18 patients (5.6%) conceived spontaneously, and 23 patients (6.5%) returned to attempt pregnancy with a median lapse of returning of 4.6 [3.1–6.1] years. Of these, cryopreserved oocytes or embryos were used in 33 cycles (19 after IVF-FP and 14 after IVM-FP). Overall, the cumulative pregnancy rate (CPR) was 47.6% (10/21) and the live birth rate (LBR) was 38.1% (8/21). Per cycle, CPR and LBR were 37 and 31% following IVF-FP and 14 and 7% following IVM-FP, although these differences did not reach statistical significance. We report the fourth live birth after IVM-FP in cancer, and the first one after IVM embryo warming resulting from in vivo oocyte retrieval and IVM procedure.
Conclusion(s)
Both IVF-FP and IVM-FP are possible options for FP women with cancer. Due to minimal data regarding ultimate outcomes, further follow-up is needed.
Keywords: Cancer survivor, Fertility preservation, In vitro maturation, In vitro fertilization, Cryopreservation
Introduction
Enhanced long-term survival rates of young women with cancer and advances in reproductive medicine and cryobiology have culminated in an increased interest in fertility preservation (FP) methods in reproductive-aged women with cancer, especially when gonadotoxic cancer therapy is anticipated [1]. Chemotherapy and radiation treatment for malignancies have resulted in improved survival rates but may lead to infertility [2].
To preserve reproductive function, cryopreservation of oocytes, embryos, or ovarian tissue has been proposed. Currently, cryopreservation of oocytes and/or embryos after controlled ovarian hyperstimulation (IVF-FP) represents the most established method for preserving adult female fertility [3]. Immature oocyte retrieval without ovarian stimulation (IVM-FP) is a possible alternative but is still considered as an experimental alternative by the American Society of Reproductive Medicine (ASRM) [3]. Nevertheless, IVM-FP represents an interesting alternative method when chemotherapy can not be delayed (as it is possible to perform IVM-FP oocyte retrieval almost immediately, flexibly in either follicular or luteal phase) or if ovarian stimulation is contraindicated [4–6].
Although a few studies have reported clinical outcomes between IVF and IVM cycles in polycystic ovary syndrome (PCOS) patients [7–10] and in normo-ovulatory patients [11], to date no literature has compared IVM-FP and IVF-FP in women with cancer.
Fertility preservation has been routinely used for relatively few years. As a consequence, little is known about the proportion of patients who come back to use their frozen material and about the efficacy of these techniques in terms of pregnancy and live birth rates [12]. Only a few cohort studies addressing this in female cancer survivors have been published following conventional IVF-FP. All report low use of frozen material (oocyte or embryos) and reasonable pregnancy rates after thawing of cryopreserved material IVF [13–22].
Although more than 5000 children have been born worldwide after fertilization of fresh in vitro maturation oocytes in PCOS or normo-ovulatory patients, very few livebirths have been reported with the use of in vitro maturation oocytes that have been cryopreserved and warmed [23]. Moreover, in cancer patients, only three separate case reports have been published, reporting three live births following IVM of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation [24–26].
The purpose of this study is to describe the fertility preservation outcomes in women with cancer who preserved embryos or oocytes over the past 13 years by IVF-FP and by IVM-FP and to describe the outcomes in those who have already returned to thaw their cryopreserved material after both IVF-FP and IVM-FP.
Materials and methods
Subjects
We retrospectively analyzed data from all women with a cancer diagnosis who underwent in vitro fertilization fertility preservation (IVF-FP) or in vitro maturation fertility preservation (IVM-FP) from January 2003 to December 2015 at an established university-hospital fertility preservation program.
Patients were offered oocyte or embryo cryopreservation according to their couple status and their wishes. All cancer types were included. The exclusion criteria were a previous history of chemotherapy or incomplete data.
Written informed consent was obtained from all the patients, as well as the approval of the oncologist to proceed with ovarian stimulation, when appropriate. This study was approved by the Institutional Review Board.
IVF-FP treatment
In all cases, the process, the timing, and the risks associated with both ovarian stimulation and with transvaginal oocyte retrieval were discussed with the referring oncologist as well as with the patient and her family. In non-hormone-dependent cancers where there was time to complete stimulation before starting chemotherapy, patients were treated under two different protocols: the long gonadotrophin-releasing hormone (GnRH) agonist protocol and the fixed GnRH antagonist protocol, as described by Oron et al. [27]. In “hormone-dependent” cancer patients (most usually breast cancer) where stimulation was approved, aromatase inhibitors were often administered during ovarian stimulation with GnRH antagonist protocol to reduce the risk of estrogen exposure [28, 29], particularly after 2007.
Final oocyte maturation was triggered with 10,000 IU hCG or 1 mg of Buserelin according to the protocol when the patient reached the usual goal for oocyte retrieval following ovarian stimulation. Ultrasound-guided retrieval was performed 36 h later under conscious sedation using a 17-gauge single lumen needle (K-OSN-1735-A-90-US, Cook Brisbane, Australia).
Initially, ovarian stimulation was started either after ovarian down-regulation in the GnRH-analog long protocol or at the start of the menstrual cycle (d2 or d3) in the antagonist protocol. After 2013, random start was used in the antagonist protocol.
IVM-FP treatment
IVM-FP was indicated when chemotherapy could not be delayed or if ovarian stimulation was contraindicated. IVM treatment was performed whatever the phase of the menstrual cycle. No stimulation was performed for IVM procedure.
IVM oocyte retrieval was performed 38 h after a subcutaneous administration of 10,000 IU hCG. Transvaginal ultrasound (TVUS)-guided retrieval of oocytes was performed using a 19-gauge single lumen needle (K-OPS-7035-RWH-ET, Cook, Australia) with a reduced aspiration pressure, under conscious sedation.
Immature oocytes (GV and MI) were then matured in vitro to the MII stage according to the technique described by Son et al. [30, 31].
Fertilization and embryo culture
Where applicable, in cases of fertilization and embryo cryopreservation, intracytoplasmic sperm injection (ICSI) was performed to inseminate the mature (MII) oocytes with the partner’s spermatozoa or donor sperm when appropriate in order to avoid the risk of fertilization failure. Fertilization was assessed 17–19 h after insemination for the appearance of two distinct pronuclei and two polar bodies. The zygotes were cultured in IVF culture media (Cook Medical, Bloomington, IN, USA). The embryos were vitrified either 2 or 3 days after ICSI.
Oocyte/embryo cryopreservation
Mature oocytes or embryos obtained from IVF or IVM cycles were cryopreserved using the vitrification method as described by Creux et al. [6].
Warming procedure
Patients rendered sterile as a result of their gonadotoxic treatments and who returned to the center to attempt pregnancy with the agreement of their oncologist underwent frozen-thaw cycles using their cryopreserved oocytes or embryos. The use of a gestational carrier was also possible.
Warming of oocytes and embryos was performed using a modified method from the one described by Chian et al. [32]. The Cryotop was directly inserted into warming medium containing 1.00 M Trehalose for 1 min at 37 °C. Warmed oocytes or embryos were transferred into diluent medium-I containing 0.50 M Trehalose for 3 min and then into diluent medium-II containing 0.25 M Trehalose for 3 min. Oocytes or embryos were washed twice in washing medium for 3 min each time. In cases of previous oocyte vitrification, all vitrified oocytes were warmed and the surviving oocytes were inseminated using ICSI 2 h after the warming process was completed. Embryos were selected for transfer strictly according to their morphological appearance.
All patients were prepared for embryo transfer (ET) with an artificial programmed cycle. 17β-Estradiol was started on day 2 or 3 of the menstrual cycle provided transvaginal ultrasound scan revealed quiescent ovaries and a thin endometrium (less than 5 mm) for at least 10 days. Once the endometrial thickness reached at least 8 mm (measured by TVUS), the luteal phase was created by the adjunction of daily vaginal or intra muscular progesterone. All ETs were routinely performed under TVUS guidance. A maximum of two thawed embryos or two embryos created from cryopreserved oocytes, taking into account maternal age, were transferred.
Clinical outcomes
Cycle outcomes were evaluated as clinical pregnancies and live births. Clinical pregnancy was defined as the presence of at least one gestational sac during the first ultrasound examination at 6 weeks’ gestation. Miscarriage was defined as the spontaneous loss of embryo before the 20th week of gestation. Implantation rate was defined as the percentage of embryos that successfully underwent implantation among those that were transferred.
Statistical analysis
Continuous data were expressed as median [25th–75th percentile], and compared using Wilcoxon rank-sum test. Categorical data were expressed as number (percentage), and compared using the Chi Squared test or Fisher’s exact test, as appropriate. Clinical and biological parameters related to the number of oocytes retrieved, such as age, antral follicle count (AFC), FSH level, and type of procedure (IVF or IVM) were included in a stepwise forward multivariate analysis. Results were considered significant at a p value of less than 0.05. All statistical analysis was performed using NCSS 10 (NCSS software, Kaysville, Utah).
Results
Patients’ characteristics
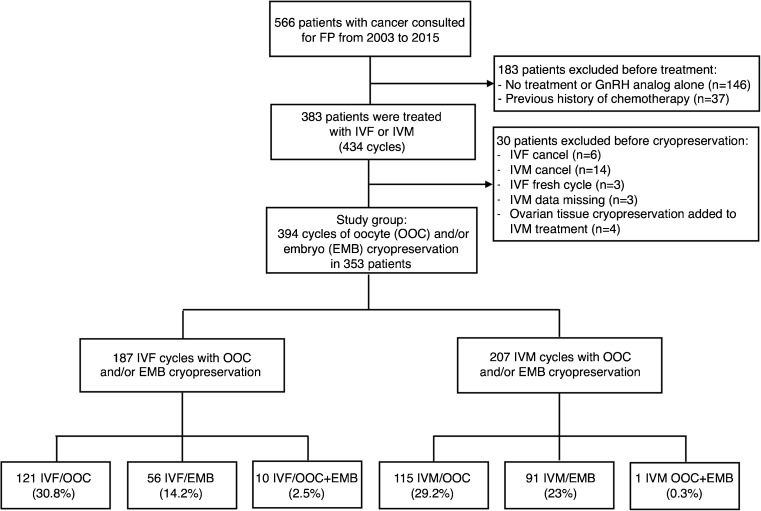
Among the 566 patients with cancer who consulted for fertility preservation during the study period, 37 were excluded as they had already started chemotherapy, 383 were treated with IVF-FP or IVM-FP, and 146 (27.6%) opted not to undergo any treatment for different reasons (for example: concerns regarding the efficacy of the FP treatments, lack of approval from the oncology team, wishing to avoid additional stress and anxiety associated with treatment, or ineligibility for medical reasons). Out of the 383 patients treated with IVF-FP or IVM-FP, 30 patients were excluded: 20 patients for cycle cancelations, 3 patients for IVF fresh cycles, 3 patients because of missing data, or 4 patients for ovarian tissue cryopreservation added to IVM-FP. Out of the 20 patients excluded for cycle cancelations, 14 resulted from IVM-FP and 6 from IVF-FP. The reasons for cancelation in IVM-FP cycles were the absence of oocytes retrieved for 8/14 (57.1%), psychological reasons for 2/14 (14.3%), and absence of maturation for 4/14 (28.6%). The reasons for cancelation in IVF-FP were a low ovarian response for 4/6 (66.7%), psychological reasons for 2/6 (33.3%). The study population consisted of 353 patients who underwent IVF-FP or IVM-FP treatment resulting in a cryopreservation of oocytes and/or embryos, with a total of 394 cycles. Figure 1 shows the study’s flow chart. Twenty-nine patients underwent more than one IVF-FP and/or one IVM-FP cycle. In total, 187 IVF-FP cycles (47.5%) and 207 IVM-FP cycles (52.5%) were performed. A total of 236 cycles (60%) consisted of oocyte cryopreservation, 147 cycles (37.2%) consisted of embryo cryopreservation and in 11 cycles (2.8%) both oocytes and embryos were cryopreserved. Among the 187 IVF-FP cycles, 178 cycles were performed during the follicular phase (95.2%) and 9 cycles during the luteal phase (4.8%). Among the 207 IVM-FP cycles, 166 cycles were performed during the follicular phase (80.2%) and 41 cycles during the luteal phase (19.8%).
Fig. 1.
Flow chart of in vitro fertilization and in vitro maturation procedures followed with oocytes or embryos cryopreservation in women with cancer. Abbreviations: IVF, in vitro fertilization; IVM, in vitro maturation; OOC, oocytes; EMB, embryos; IVF/OOC, in vitro fertilization with oocytes cryopreservation; IVF/EMB, in vitro fertilization with embryo cryopreservation; IVM/OOC, in vitro maturation with oocytes cryopreservation; IVM/EMB, in vitro maturation with embryo cryopreservation; IVM/OOC+EMB, in vitro maturation with oocyte and embryo cryopreservation; GnRH, gonadotrophin-releasing hormone; FP, fertility preservation
The proportion and numbers of IVF-FP or IVM-FP according to the type of cancer are detailed in Table 1. The most frequent indication for FP in our program was breast cancer (48.2%) followed by hematological cancer (27.2%). Except in cases of breast cancer, IVF-FP was the preferred treatment for other types of cancer, with a proportion from 61.1 to 100%. In breast cancer, IVM-FP was preferred (72.4%) especially before 2011 because of concerns regarding ovarian stimulation in women with hormone-dependent cancers. For a total of 47 IVF-FP treatments for breast cancer (27.6%), only 5 IVF-FP treatments were done before 2011. Among the 47 IVF-FP treatments for breast cancer, 39 used anti-aromatase therapy (letrozole) in addition to gonadotropins. No complication or severe side effects were noted in the overall study population.
Table 1.
Proportion of the study population according to the type of cancer and the type of procedure
| Type of cancer (N = 353) |
Patients n (%) |
Mean age yo ± SD |
IVF-FP n (%) |
IVM-FP n (%) |
|---|---|---|---|---|
| Total | 353 (100) | 29.6 ± 5.8 | 171 (100%) | 182 (100%) |
| Breast | 170 (48.2) | 32.1 ± 4.8 | 47 (27.6) | 123 (72.4) |
| Hematological | 96 (27.2) | 26.4 ± 5.3 | 64 (66.7) | 32 (33.3) |
| Gynecological | 23 (6.6) | 30.2 ± 5.9 | 15 (65.2) | 8 (34.8) |
| Digestive | 15 (4.2) | 30.3 ± 5.7 | 10 (66.7) | 5 (33.3) |
| Brain | 25 (7) | 26.4 ± 5.0 | 18 (72) | 7 (28) |
| Sarcoma | 18 (5.1) | 25.8 ± 5.0 | 11 (61.1) | 7 (38.9) |
| Others | 6 (1.7) | 30.8 ± 7.1 | 6 (100) | 0 (0) |
N = total number of the study population; n = number; others consisted of lung, thyroid, sinus, and pharynx cancers
Comparison of collection parameters between IVF-FP and IVM-FP
As shown in Table 2, the IVF-FP group consisted of 187 cycles for 171 patients and the IVM-FP group of 205 cycles for 182 patients. The two groups were comparable in terms of FSH level and antral follicle count (AFC), but patients were older in the IVM-FP group. In the IVM-FP group, the maturation rate after 48 h of culture was 58%.
Table 2.
Comparison between IVF-FP and IVM-FP collection parameters in women with cancer
| IVF-FP (187 cycles for 171 patients) | IVM-FP (205 cycles for 182 patients) | p value | |
|---|---|---|---|
| Age, years | 28.5 ± 5.6 | 30.6 ± 5.7 | 0.0007 |
| FSH, UI/l | 6.15 [4.2–8.4] | 6.2 [4.6–7.6] | 0.7 |
| Antral follicle count | 16 [10–24] | 17 [11.3–23] | 0.3 |
| Total oocytes on the collection day | 12 [8–18] | 7 [5–12.5] | < 0.000001 |
| Metaphase II oocytes on the collection day | 9 [5–12] | 2 [1–3] | < 0.000001 |
| Total metaphase II oocytes | 10 [7–15] | 5 [2–8] | < 0.000001 |
| Oocytes cryopreserved | 10 [6–15] | 5 [2–8] | < 0.000001 |
| Fertilization rate, % (WA) | 79 [67–88] | 75 [58–100] | 0.7 |
| Embryos cryopreserved (WA) | 5 [3–7] | 3 [2–5] | 0.007 |
Data are median [25th percentile-75th percentile], except the age that is mean ± SD
IVF in vitro fertilization, IVM in vitro maturation, FP fertility preservation, FSH follicle-stimulating hormone, WA when applicable
The number of oocytes collected, the total number of metaphase II (MII) oocytes, and the number of oocytes cryopreserved was significantly greater in the IVF-FP group. Similarly, the number of embryos cryopreserved, when applicable, was significantly greater in the IVF-FP group, whereas no significant difference was found in fertilization rates. Using multivariate analysis, independent parameters associated with the number of oocytes collected were as follows: AFC (R2 = 0.18), IVF procedure—either IVF-FP or IVM-FP—(R2 = 0.04), and age (R2 = 0.02).
Frozen-thawed cycles
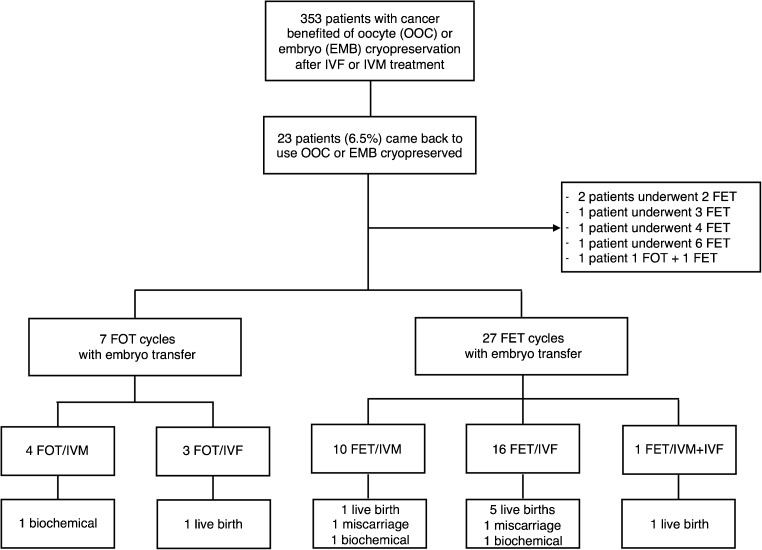
A total of 23 cancer survivors from the 353 FP patients (6.5%) returned to have their oocytes or embryos warmed. The median lapse of returning to attempt pregnancy was 4.6 [3.1–6.1] years. Twenty-one patients underwent an embryo transfer. As shown in Fig. 2, 33 cycles resulted in an embryo transfer, with 7 cycles using previously cryopreserved oocytes (3 cycles after IVF-FP and 4 cycles after IVM-FP) and 26 cycles using previously cryopreserved embryos (16 cycles after IVF-FP and 10 cycles after IVM-FP). In total, 19 frozen-thawed cycles followed IVF-FP and 14 frozen-thawed cycles followed IVM-FP. In cases of oocyte cryopreservation, all patients who returned were in a committed relationship and used their partner’s sperm. Two couples underwent an embryo transfer in a gestational carrier (9.5%). The primary disease from which cancer survivors had suffered was breast cancer (n = 9), hematological cancer (n = 9), digestive cancer (n = 2), gynecological cancer (n = 2), and sarcoma (n = 1).
Fig. 2.
Obstetrical outcomes of women with history of cancer who came back to use oocytes and/or embryos cryopreserved after IVF-FP or IVM-FP. Abbreviations: IVF, in vitro fertilization; IVM, in vitro maturation; OOC, oocytes; EMB, embryos; FET, frozen embryo transfer = embryo transfer resulted from a frozen embryo; FOT, frozen oocyte transfer = embryo transfer resulted from a frozen oocyte; FOT/IVM, embryo transfer resulted from a frozen oocyte obtained after an IVM cycle; FOT/IVF, embryo transfer resulted from a frozen oocyte obtained after an IVF cycle; FET/IVM, embryo transfer resulted from a frozen embryo obtained after an IVM cycle; FET/IVF, embryo transfer resulted from a frozen embryo obtained after an IVF cycle
Overall obstetrical outcome
Out of the 34 embryo transfers in 21 patients, 10 clinical pregnancies were achieved. The implantation rate was 14.2%. Two of the 10 clinical pregnancies ended in spontaneous abortions. No ectopic pregnancy was described. The clinical pregnancy rate per embryo transfer was 29.4%. The clinical pregnancy rate per patient was 47.6% (10/21). The clinical miscarriage rate was 20% (2/10). Out of the eight remaining ongoing pregnancies, nine healthy babies were born (one pregnancy was a twin pregnancy). The live birth rate per embryo transfer was 23.5% (8/34). The live birth rate per patient was 38.1% (8/21). Seven of the eight deliveries consisted of cesarean section (87.5%). Excepting the twin pregnancy, all babies were born at term with adequate weight for their gestational age. The mean gestational age was 38.9 gestational weeks and the mean fetal weight was 3280 g (1930–4385 g). The twin pregnancy delivered at 31 gestational weeks through cesarean section for premature labor. The twins’ fetal weights were 2150 and 1930 g. The cancer survivors who gave birth had suffered from breast cancer (n = 2), hematological cancers (n = 5), and sarcoma (n = 1). None had additional obstetric complication noted. One child was born with a tongue and lip tie that can be considered as a minor malformation. As shown in Fig. 2, among the eight live births, six followed IVF-FP treatment (five after embryo thawing and one after oocyte thawing), one followed IVM-FP treatment with embryo thawing, and one followed both IVF-FP and IVM-FP treatments. One further biochemical pregnancy was obtained after IVM-FP treatment with oocyte thawing and fertilization.
Two patients used a gestational carrier (one hematological patient because of an elapsed time of 1.1 years and one breast cancer 5 years after the cancer diagnosis); only one achieved a healthy live-born outcome. Out of the 23 patients who returned, 2 had one previous child (8.7%). Two other patients who became pregnant and delivered after the use of frozen material pursued a second treatment in order to have a second child after gonadotoxic therapy. Out of the two, only one succeeded and delivered a second healthy child.
Comparison between IVF-FP and IVM-FP pregnancy outcomes
The comparison of the fertility treatment outcomes between IVM-FP and IVF-FP procedures is shown in Table 3. No difference was found in terms of oocyte survival rates and embryo survival rates. Implantation rates were significantly lower in the IVM-FP group. The differences in clinical pregnancy rates per embryo transfer and live birth rates per embryo transfer did not reach statistical significance. No difference was shown in the miscarriage rate per pregnancy. We report the fourth live birth after IVM-FP for cancer, and the first one after embryo warming resulting from in vivo oocyte retrieval and IVM procedure.
Table 3.
Comparison between IVF-FP and IVM-FP obstetrical outcomes in women with cancer who came back to thaw oocytes or embryos cryopreserved
| IVF-FP (N = 19 cycles) | IVM-FP (N = 14 cycles) | p value | |
|---|---|---|---|
| Oocyte survival | 83 [67–86] | 75 [48–86] | 0.90 |
| Embryo survival | 67 [50–100] | 67 [67–71] | 0.91 |
| Implantation rate, % | 21.9 (7/32) | 3.7 (1/27) | 0.04 |
| Clinical pregnancies per embryo transfer, n (%) | 7/19 (36.8) | 2/14 (14.3) | 0.15 |
| Live births per embryo transfer, n (%) | 6/19 (31.6) | 1/14 (7.1) | 0.09 |
| Miscarriage per pregnancy, n (%) | 1/6 (16.7) | 1/2 (50) | 0.35 |
Data are median [25th percentile-75th percentile] or number (percentages)
Patients’ follow-up
Concerning the overall patient follow-up, complete data was only available from patients followed in our tertiary university hospital. As noted above, 23 patients returned for fertility treatment (6.5%). Thirty-two patients died (9.0%). Eighteen patients (5.6%) had healthy babies born following spontaneous conception (despite previous gonadotoxic therapy). Two patients divorced (one had embryos cryopreserved). Four patients gave their material frozen to research. Five patients moved towards a surrogate. Thirty-five patients were diagnosed with recurrence and were being treated by chemotherapy. Data from the remaining 264 patients is incomplete (68.9%).
Discussion
The present study shows that FP in women with cancer is a strategy which can result in pregnancies and in healthy babies being born, whether IVF-FP or IVM-FP and whether cryopreserved oocytes or embryos. The overall results in terms of clinical pregnancy rate and live birth rate are respectively 29.4 and 23.5% per embryo transfer and 47.6 and 38.1% per patient. Concerning the overall pregnancy and obstetric outcomes, these results are similar to previous publications, even if populations and fertility preservation techniques are heterogeneous between all previous studies [17, 20–22]. For example, Cardozo et al. [20] described a cumulative live birth rate per embryo transfer of 30% in women with cancer versus 32% in control patients. Oktay et al. [21] in the largest prospective study on women with breast cancer treated with Letrozole-FSH protocol reported a live birth rate per embryo transfer of 45% and concluded to success rate in terms of pregnancy and live birth at least similar to those achieved with standard protocols used in infertile women. Noyes et al. [17] reported a live birth rate per patient of 37.5% (3/8 patients), whereas in Alvarez et al.’s recent study [22], including all types of cancer, the cumulative pregnancy rate per patient was 54.5%, but the live birth per patient was only 22.72%. Alvarez et al. [22] reported a miscarriage rate per pregnancy of 57.1% following IVF treatment and embryo cryopreservation. They explained this fact with the previous exposure to chemotherapy. In our study, the miscarriage rate per pregnancy is much lower (20%). Another difference with previous studies is the lower rate of twin pregnancies (12.5%) compared with 38.8% in Oktay et al. study [21] and 44% in Cardozo et al. study [20]. This fact could be explained by a lower average number of embryos transferred (1.8) and a lower implantation rate (15%) in our study compared with 1.97 embryos transferred in average and implantation rate of 27.8% for Cardozo et al. [20], and 1.97 +/− 0.7 embryos transferred in average and implantation rate of 40.7% for Oktay et al. [21]. Moreover, the use of a gestational carrier is also lower in our study (9.5%), compared with Cardozo et al. (47.6%) and Oktay et al. (55%). This fact can be explained by a higher proportion of breast cancer who came back to attempt pregnancy in these two studies and a higher proportion of gynecological cancers in Cardozo et al. study [20].
Besides the long period of follow-up and the size of the population studied, the main strength of our study consists of the different techniques employed. Indeed, most of the publications consisted of series including only IVF-FP cycles followed by oocytes vitrification [18, 19] or embryo cryopreservation [16, 21] or both oocytes and embryo cryopreservation [17, 20, 22], or after ex vivo IVM procedure followed by embryo cryopreservation [24–26]. As a well-established technology, embryo cryopreservation has high pregnancy success rates [33]. However, outcomes in cancer patients are scarce. A similar live birth rate (LBR) per patient among women with cancer undergoing IVF and embryo cryopreservation, and cumulative live birth rate (CLBR) to that achieved with fresh embryos in non-cancer patients has been reported [34]. Success rates associated with oocyte vitrification are superior to slow freezing [35] and comparable to those achieved with fresh oocytes [35–37]. Outcomes after oocyte vitrification among female cancer patients are scarce. Nevertheless, Martinez et al. [18] reported fertilization rates up to 76.6%, and out of 11 women with cancer, 4 gave birth at term with no negative perinatal outcomes.
When we detail results according to the type of procedure, IVF-FP seems to be more effective than IVM-FP in women with cancer in terms of the number of oocytes retrieved, the total number of MII oocytes retrieved, and the number of oocytes or embryos cryopreserved. Similarly, in women returning to attempt pregnancy, IVF-FP is associated with higher implantation rates. Caution is needed when interpreting this data because of differences in the ages, diagnoses, and time-periods between the two groups. Even if IVM is still considered as an experimental technique in the field of fertility preservation for cancer [3], research focused on reduction of the efficiency gap between in vitro and in vivo oocyte maturation is in progress. The activity of factors from the TGF-béta family, cAMP modulators, and EGF-like factors has shown promising results [23]. It is also suspected that the fertilization potential of in vitro matured oocytes might be compromised by the cryopreservation process, therefore vitrification of mature oocytes is presently recommended over cryopreservation of immature oocytes [38, 39]. Moreover, during the past decade, vitrification has gradually replaced slow programmed freezing for the cryopreservation of embryos and oocytes. Vitrification can also appear as a promising technique in the field of the IVM procedure. Despite these concerns, IVM-FP is shown to be a possible FP treatment—as determined by the first reported post-cancer IVM-FP pregnancies and live births. IVM-FP remains an alternative when ovarian stimulation is contraindicated or in cases of urgent chemotherapy. Indeed, IVM can be performed at anytime during the menstrual cycle, there is no need for ovarian stimulation and IVM treatment usually takes no more than 48 h from decision to IVM oocyte retrieval combined with embryo or oocyte cryopreservation [4–6].
In our study, patients have been treated by IVF-FP and IVM-FP all along the menstrual cycle, but most of them were in the early follicular stage, especially in the IVF-FP group. We decided not to compare collection parameters according to the phase of the menstrual cycle for two reasons. The first reason was that our team has already published on random start in IVM procedure with a quite similar database in 2016 [6] showing similar results in terms of collection parameters, maturation rates after 48 h of culture, and fertilization rates, when compared early follicular, late follicular, and luteal phases. The second reason was because of small numbers in luteal phase in the IVF group (out of the 187 IVF cycles, only 9 cycles were done in the luteal phase). To compare IVF results according to the phase of the cycle would not have been strong enough in terms of statistical power. To date, literature is in favor of similar results in IVM-FP and IVF-FP all along the menstrual cycle, based on the concept of multiple follicular waves. There is increasing evidence to indicate that two or more cohorts of antral follicles are recruited during the human menstrual cycle, as it was previously documented in several animal species [40, 41]. In IVM-FP, the feasibility of IVM treatment in the luteal phase is consistent with animal models including cattles [42] and baboons [43], and also with the few previous publications in women with cancer [5, 6]. In IVF-FP, compared with conventional follicular phase ovarian stimulation, luteal phase stimulation resulted in comparable fertilization, implantation, and pregnancy rates [41]. However, a longer FSH treatment period and higher FSH doses have been required using luteal phase protocols [41]. Continued research is required to characterize the physiologic mechanisms underlying random-start IVF/IVM strategies and factors that may influence outcomes.
To date, our study is the first one to compare treatment and pregnancy outcomes in IVF-FP and IVM-FP in women with cancer. IVM has been compared with conventional IVF in several previous studies in infertile women. They have reported better pregnancy and live birth rates after ovarian controlled hyperstimulation when compared with IVM procedure in normo-ovulatory patients [11] and in PCOS patients ([7–10] excepted Shalom-Paz et al. [8] that reported comparable live birth rates (LBR) between IVF and IVM procedures despite higher mature oocytes in the IVM group). This would suggest that oocytes cryopreserved after ovarian stimulation may have a better developmental potential as compared with eggs matured in vitro, as recently evoked by the time lapse imaging. Roesner et al. reported differences in growth dynamics of embryos between IVM and IVF [44], whereas Walls et al. described a difference in the early stages of the first cell cycle in human oocytes after IVM compared with IVF [45].
In our study, the proportion of IVM-FP cycles is lightly higher than the proportion of IVF cycles (52.5 versus 47.5%). The high proportion of breast cancers and the routine use of IVM-FP prior to 2011 in these patients can explain this fact. Since the emergence of the Letrozole-FSH protocol in estrogen-dependant tumors [28, 29] and the demonstration of its safety and efficacy [46–49], IVF-FP for estrogen-sensitive breast cancer began to be used.
Few patients return to attempt pregnancy after undergoing FP. Although all patients are not routinely followed, our data shows that continuing cancer treatment, death, spontaneous pregnancy, and relationship breakdown account for some of these cases of non-return. The proportion of patients who came back to use their frozen material is relatively consistent with the few previous publications on the topic with similar period of follow-up: 6.5% in our study after 13 years of follow-up versus 7.2% in Alvarez et al. [22] after 14 years of follow-up and 5.6% in Noyes et al. [17] after 7 years of follow-up, whereas some authors reported higher use of frozen material as 36.8% according to Cardozo et al. [20] after 17 years of follow-up and 25.6% according to Oktay et al. [21] in breast cancer treated with IVF and Letrozole after 10 years of follow-up. In our study, proportionally fewer breast cancer survivors returned, in comparison with survivors from hematological cancers. The explanations could either be the older age of the breast cancer patients at diagnosis or conversely the lower gonadotoxicity of treatment in cases of breast cancer resulting in a higher spontaneous pregnancy rate.
The limitations of our study include its retrospective nature and the inherent confounding variables, that data from a single centre may not be more widely applicable, and a possible referral bias. Moreover, patients were included over 13 years and over that time technology and protocols have evolved. Due to the recent nature of FP techniques and the low use of the frozen material, the performance of these techniques needs further follow-up. More data combined with interdisciplinary communication are necessary to answer the questions about why so many women who undergo FP do not return. More data is also needed concerning pregnancy and perinatal outcomes.
In conclusion, this study demonstrates that oocyte or embryo cryopreservation after IVF-FP or IVM-FP in women with cancer can result in live births. IVF-FP appears superior in terms of collection parameters (such as the number of oocytes or embryos cryopreserved), although there are many potential confounding variables. The type of cancer, the age of the woman, and the time available until chemotherapy all need to be considered in determining which would be the most appropriate FP option.
Acknowledgments
The authors thank all physicians, embryologists, nurses, and administrative personal working for our Fertility Preservation Program in the Reproductive centre of McGill University Health Centre (MUHC), especially N. Sallman for her precious help in the constitution of the database, L. Jakubonis for her contribution in collecting patients’ follow-up data, and N. Lamothe for her assistance in administrative and computing issues. We also thank Dr. C. Cassinotto for his help in statistical analysis.
Compliance with ethical standards
Conflict of interest
None.
Contributor Information
Helene Creux, Phone: +33 556796033, Email: helenecreux@gmail.com.
Patricia Monnier, Email: patricia.monnier@muhc.mcgill.ca.
Weon-Young Son, Email: weon-young.son@muhc.mcgill.ca.
William Buckett, Email: william.buckett@muhc.mcgill.ca.
References
- 1.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–140. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luke B, Brown MB, Missmer SA, Spector LG, Leach RE, Williams M, et al. Assisted reproductive technology use and outcomes among women with a history of cancer. Hum Reprod. 2016;31:183–189. doi: 10.1093/humrep/dev288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berwanger AL, Finet A, El Hachem H, le Parco S, Hesters L, Grynberg M. New trends in female fertility preservation: in vitro maturation of oocytes. Future Oncol. 2012;8:1567–1573. doi: 10.2217/fon.12.144. [DOI] [PubMed] [Google Scholar]
- 5.Grynberg M, Poulain M, le Parco S, Sifer C, Fanchin R, Frydman N. Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients. Hum Reprod. 2016;31:623–629. doi: 10.1093/humrep/dev325. [DOI] [PubMed] [Google Scholar]
- 6.Creux H, Monnier P, Son W-Y, Tulandi T, Buckett W. Immature oocyte retrieval and in vitro oocyte maturation at different phases of the menstrual cycle in women with cancer who require urgent gonadotoxic treatment. Fertil Steril. 2017;107:198–204. doi: 10.1016/j.fertnstert.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Gremeau AS, Andreadis N, Fatum M, Craig J, Turner K, McVeigh E, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case-control study of 194 treatment cycles. Fertil Steril. 2012;98:355_60. doi: 10.1016/j.fertnstert.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Shalom-Paz E, Holzer H, Son W-Y, Levin I, Tan SL, Almog B. PCOS patients can benefit from in vitro maturation (IVM) of oocytes. Eur J Obstet Gynecol Reprod Biol. 2012;165:53–56. doi: 10.1016/j.ejogrb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Das M, Son W-Y, Buckett W, Tulandi T, Holzer H. In-vitro maturation versus IVF with GnRH antagonist for women with polycystic ovary syndrome: treatment outcome and rates of ovarian hyperstimulation syndrome. Reprod BioMed Online. 2014;29:545–551. doi: 10.1016/j.rbmo.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30:88–96. doi: 10.1093/humrep/deu248. [DOI] [PubMed] [Google Scholar]
- 11.Fadini R, Mignini Renzini M, Dal Canto M, Epis A, Crippa M, Caliari I, et al. Oocyte in vitro maturation in normo-ovulatiry women. Fertil Steril. 2013;99:1162–1169. doi: 10.1016/j.fertnstert.2013.01.138. [DOI] [PubMed] [Google Scholar]
- 12.Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010;116:1171–1183. doi: 10.1097/AOG.0b013e3181f87c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaan N, Ben-David G, Ben-Yosef D, Almog B, Many A, Pauzner D, et al. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol. 2010;149:175–177. doi: 10.1016/j.ejogrb.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 2011;95:588–591. doi: 10.1016/j.fertnstert.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Sabatini ME, Wolkovich AM, Macklin EA, Wright DL, Souter I, Toth TL. Pronuclear embryo cryopreservation experience: outcomes for reducing the risk of ovarian hyperstimulation syndrome and for fertility preservation in cancer patients. J Assist Reprod Genet. 2011;28:279–284. doi: 10.1007/s10815-010-9515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcroft J, Dayoub N, Thong KJ. Fifteen year follow-up of embryos cryopreserved in cancer patients for fertility preservation. J Assist Reprod Genet. 2013;30:1407–1413. doi: 10.1007/s10815-013-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noyes N, Melzer K, Druckenmiller S, Fino ME, Smith M, Knopman JM. Experiences in fertility preservation: lessons learned to ensure that fertility and reproductive autonomy remain options for cancer survivors. J Assist Reprod Genet. 2013;30:1263–1270. doi: 10.1007/s10815-013-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez M, Rabadan S, Domingo J, Cobo A, Pellicer A, Garcia-Velasco JA. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod BioMed Online. 2014;29:722–728. doi: 10.1016/j.rbmo.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril. 2013;99:1994–1999. doi: 10.1016/j.fertnstert.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet. 2015;32:587–596. doi: 10.1007/s10815-015-0428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015;33:2424–2429. doi: 10.1200/JCO.2014.59.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez RM, Ramanathan P. Fertility preservation in female oncology patients: the influence of the type of cancer on ovarian stimulation response. Hum Reprod. 2016. 10.1093/humrep/dew158. [DOI] [PubMed]
- 23.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasath EB, Chan MLH, Wong WHW, Lim CJW, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 25.Uzelac PS, Delaney AA, Christensen GL, Bohler HCL, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–1260. doi: 10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed] [Google Scholar]
- 26.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oron G, Son W-Y, Buckett W, Tulandi T, Holzer H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: a pilot study. Hum Reprod. 2014;29:1444–1451. doi: 10.1093/humrep/deu079. [DOI] [PubMed] [Google Scholar]
- 28.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol. 2005;23:3858–3859. doi: 10.1200/JCO.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Son W-Y, Chung J-T, Chian R-C, Herrero B, Demirtas E, Elizur S, et al. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23:2010–2016. doi: 10.1093/humrep/den210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son W-Y, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Hum Reprod Update. 2010;16:675–689. doi: 10.1093/humupd/dmq014. [DOI] [PubMed] [Google Scholar]
- 32.Chian R-C, Huang J-Y, Gilbert L, Son W-Y, Holzer H, Cui S-J, et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009;91:2391–2398. doi: 10.1016/j.fertnstert.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Bedoschi G, Oktay K. Current approach for fertility preservation by embryo cryopreservation. Fertil Steril. 2013;99:1496–1502. doi: 10.1016/j.fertnstert.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolmans MM, Hollanders de Ouderaen S, Demylle D, Pirard C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J Assist Reprod Genet. 2015;32:1233–1237. doi: 10.1007/s10815-015-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100:492–499. doi: 10.1016/j.fertnstert.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh versus vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010:66–73. [DOI] [PMC free article] [PubMed]
- 37.Solé M, Santalo J, Boada M, Clua E, Rodriguez I, Martinez F, et al. How does vitrification affect oocyte viability in oocyte donation cycles? A prospective study to compare outcomes achieved with fresh versus vitrified sibling oocytes. Hum Reprod. 2013;28:2087–2092. doi: 10.1093/humrep/det242. [DOI] [PubMed] [Google Scholar]
- 38.Brambillasca F, Guglielmo MC, Coticchio G, Mignini Renzini M, Dal Canto M, Fadini R. The current challenges to efficient immature oocyte cryopreservation. J Assist Reprod Genet. 2013;30:1531–1539. doi: 10.1007/s10815-013-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khalili MA, Shahedi A, Ashourzadeh S, Nottola SA, Macchiarelli G, Palmerini MG. Vitrification of human immature oocytes before and after in vitro maturation: a review. J Assist Reprod Genet. 2017;34:1413–1426. doi: 10.1007/s10815-017-1005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. 2003;69:1023–1031. doi: 10.1095/biolreprod.103.017772. [DOI] [PubMed] [Google Scholar]
- 41.Robertson DM, Gilchrist RB, Ledger WL, Baerwald A. Random start or emergency IVF/in vitro maturation: a new rapid approach to fertility preservation. Womens Health. 2016;12:339–349. doi: 10.2217/whe-2015-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chian R-C, Chung J-T, Downey BR, Tan SL. Maturational and developmental competence of immature oocytes retrieved from bovine ovaries at different phases of folliculogenesis. Reprod BioMed Online. 2002;4:127–132. doi: 10.1016/S1472-6483(10)61929-3. [DOI] [PubMed] [Google Scholar]
- 43.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roesner S, Dietrich JE, Weigert J, Montag M, Toth B, Strowitzki T. Time-lapse imaging reveals differences in growth dynamics of embryos after in vitro maturation compared with conventional stimulation. Fertil Steril. 2017;107:606–612. doi: 10.1016/j.fertnstert.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Walls ML, Hart R, Keelan JA, Ryan JP. Structural and morphologic differences in human oocytes after in vitro maturation compared with standard in vitro fertilization. Fertil Steril. 2016;106:1392–1398. doi: 10.1016/j.fertnstert.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 47.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 48.Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytes. Gynecol Endocrinol. 2016;26:1–4. doi: 10.1080/09513590.2016.1177013. [DOI] [PubMed] [Google Scholar]
- 49.Rodgers RJ, Reid GD, Koch J, Deans R, Ledger WL, Friedlander M, et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod. 2017;32:1033–45. doi: 10.1093/humrep/dex027. [DOI] [PubMed] [Google Scholar]