Abstract
It is now widely accepted that nutrition during critical periods in early development, both pre- and postnatal, may have lifetime consequences in determining health or onset of major diseases in the adult life. Dietary carotenoids have shown beneficial health effects throughout the life cycle due to their potential antioxidant properties, their ability to serves as precursors of vitamin A and to the emerging signaling functions of their metabolites. The non-provitamin A carotenoids lutein and zeaxanthin are emerging as important modulators of infant and child visual and cognitive development, as well as critical effectors in the prevention and treatment of morbidity associated with premature births. This review provides a general overview of lutein and zeaxanthin metabolism in mammalian tissues and highlights the major advancements and remaining gaps in knowledge in regards to their metabolism and health effects during pre- and early post-natal development. Furthering our knowledge in this area of research will impact dietary recommendation and supplementation strategies aimed at sustaining proper fetal and infant growth.
Keywords: lutein, zeaxanthin, mammalian development, placenta, transport, maternal milk
Introduction
In mammals, including humans, pre- and early post-natal development heavily depends on nutrients provided by the mother through the placenta (pre-birth) and during lactation (after-birth) (1–4). Carotenoids are examples of such nutrients that can be detected in the maternal circulation and milk (5). Carotenoids are C40 isoprenoid compounds synthesized by plants, algae, and bacteria. In plants, they support photosynthesis, function as precursors of various hormones, and enable critical functions, such as pollination and seed dispersion, by providing the characteristic yellow, red and orange color to many fruits and flowers (6–8). Mammals obtain dietary carotenoids predominantly through foods of plant origin. Even though hundreds of carotenoids exist in nature, only about 50 of them are commonly present in the human diet, and only about 10 of these can be detected in significant amounts in the human plasma. Examples include β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein, zeaxanthin, and β-canthaxanthin (9). Dietary carotenoids have shown beneficial health effects throughout the life cycle due to their potential antioxidant properties, their ability to serves as precursors of vitamin A and to the emerging signaling functions of their metabolites (10–12). Of note, evidence for their potential harmful activities also exists (13–16). The majority of the literature on the biological functions of carotenoids centers on their actions in adult tissues and organs. This review will focus on carotenoid metabolism and functions during pre- and early post-natal development. The most well-known contribution of these compounds to mammalian development is linked to the provitamin A activity of certain carotenoids, specifically β-carotene (17). The essential nutrient vitamin A indeed supports proper mammalian development by exerting critical transcriptional regulatory activities mediated by its active form retinoic acid (18, 19). The emphasis of this review, however, will be on the role of the non-provitamin A carotenoids lutein and zeaxanthin. These carotenoids, which are not only transferred from mother to fetus through the placenta, but are also abundant in the mother’s milk (5), are emerging as important modulators of infant and child visual and cognitive development, as well as critical effectors in the prevention and treatment of morbidity associated with premature births (5). We will provide a general overview of lutein and zeaxanthin metabolism in mammalian tissue. We will also highlight the major advancements and remaining gaps in knowledge in regards to their metabolism and health effects during pre- and early post-natal development.
Chemical structure, food sources and bioavailability
Based on their chemical structure, carotenoids can be classified as carotenes and xanthophylls. Carotenes (such as β-carotene, α-carotene, lycopene and β-cryptoxanthin) are non-oxycarotenoids that may be linear or possess cyclic hydrocarbons at one or both ends of the molecule. Xanthophylls (such as lutein, zeaxanthin, meso-zeaxanthin, astaxanthin and canthaxanthin) are oxygen-containing carotenoids (20). Lutein and zeaxanthin are also characterized by the presence of a hydroxyl group at both ends of the molecule, which sets them apart from other carotenoids (20). Lutein and zeaxanthin are isomers which differ in the location of a double bond unsaturation in the end ring; meso-zeaxanthin is a lutein derivative (Figure 1).
Figure 1. Chemical structures of lutein, zeaxanthin and meso-zeaxanthin.
Lutein is isomeric with zeaxanthin, differing only in the placement of one double bond in one of the end rings. The principal natural stereoisomer of lutein is (3R,3′R,6′R)-beta,epsilon-carotene-3,3′-diol. Zeaxanthin exists in three stereoisomeric forms that result from the configurations at its two chiral centers: the (3R,3′R), (3R,3′S) and (3S,3′S). The principal natural form of zeaxanthin is the (3R, 3′R). Lutein and zeaxanthin can be interconverted in the body through an intermediate called meso-zeaxanthin, which is the (3R,3′S) stereoisomer.
As for other carotenoids, mammals - including humans - obtain lutein and zeaxanthin through the diet, in particular from green leafy vegetables and orange and yellow fruits and vegetables (or from animals that have ingested them). These compounds are found in nature either esterified to fatty acids, or in an unesterified “free” form. The esterified forms are mainly found in flowers and fruits as they are more stable against oxidation and better suited as pigments (21). These forms are also readily absorbed by the body and found in the human bloodstream and organs (22). The unesterified forms work as accessory pigments in the photosynthetic apparatus to provide antioxidant photoprotection under conditions of high light intensity (6). The highest amount of lutein is found in cooked spinach (12.64 mg/100 g), cooked kale (8.88 mg/100 g), and cilantro (7.70 mg/100 g); other good sources are broccoli, peas, brussel sprouts and corn (23, 24). In chicken egg yolk the total xanthophyll content is ~1.2 mg/100 g and it is considered a better source of lutein and zeaxanthin compared to fruits and vegetables due to the high fat content of eggs, which increases their bioavailability (25–27). Despite not commonly found in food, mesozeaxanthin has been detected in shrimp carapace, fish skin, and turtle fat (28–30).
Bioavailability is the fraction of an ingested nutrient that is available for utilization or storage, thus indicating the fraction of the nutrient that reaches the systemic circulation (31). Before becoming bioavailable, nutrients or bioactive compounds must be released from the food matrix and modified in the gastrointestinal (GI) tract, a process that is generally referred to as bioaccessibility (the ratio of the specific nutrient solubilized in the mixed micelles to the total amount ingested) (31). In general, carotenoids’ bioavailability is affected by a number of factors such as food matrix, processing conditions, and fat content (32). Similar to what reported for other carotenoids (33), the bioavailability of lutein and zeaxanthin is greater in heat-processed than from unprocessed/raw vegetables (24). Lutein and zeaxanthin are also more (from 50% to 100%) bioaccessible from fruits (orange, kiwi, red grapefruit, and honeydew melon) than from dark green vegetables (spinach and broccoli; 19%–38%) (34). The co-presence of different types of carotenoids in the food matrix also affects bioavailability of lutein and zeaxanthin. For example, a large oral dose of β-carotene (12 or 30 mg) given daily for 6 weeks lowered serum lutein concentrations in men (35); whereas daily oral doses (10 mg) of lutein provided for three weeks to human subjects increased serum concentrations of zeaxanthin (36). This competitive inhibition of certain carotenoids towards the bioavailability of others may occur at various levels from micellar incorporation, to intestinal uptake, to lymphatic transport or at one of the later metabolic steps. The molecular mechanisms of such interactions, however, are still nor fully understood. In human serum the concentration of lutein ranges from 0.1–1.44 μmol/L, and from 0.07–0.17 μmol/L for zeaxanthin (37), the large variability being due to the wide difference in carotenoids intake among individuals.
Intestinal absorption and transport
In contrast to the intestinal uptake of β-carotene that has been shown to be mediated by the receptor SR-BI (38, 39), the details of the molecular mechanisms of lutein and zeaxanthin uptake by the enterocytes are still scarce (40, 41). It is generally assumed that, like other carotenoids, upon intestinal absorption lutein and zeaxanthin are incorporated in chylomicrons, together with other dietary lipids. Chylomicrons are rapidly remodeled by lipoprotein lipase in peripheral tissues and then enter as chylomicron remnants into the bloodstream (10). The resulting chylomicron remnants containing carotenoids are then transferred to the liver, where they can be either stored or re-secreted into the circulation in association with lipoproteins (10). In the fasting blood, lutein (> 50%) and zeaxanthin (> 40%) are predominantly transported by HDL (42, 43). However, in European populations, LDL levels of zeaxanthin/lutein appear to have a striking contribution to plasma levels of zeaxanthin/lutein (44).
Tissue uptake
The specific affinity of these xanthophylls to the HDL may control their post-hepatic tissue distribution via a preferential tissue uptake mediated by the receptor for HDL-lipoproteins and others key players involved in cholesterol and/or lipid transport. In humans, the highest levels of these pigments are reached in the macula of the eye where their concentrations range between 0.1 and 1 mM (27, 45, 46). Confirming earlier studies indicating that xanthophylls are preferentially taken up by retinal cells via a SR-B1-dependent mechanism (47), Shyam et al (48) have determined that all three human SR-B proteins and CD36 are capable of binding the macular xanthophyll carotenoids zeaxanthin, lutein, and its metabolite meso-zeaxanthin. They also showed in vitro that zeaxanthin and meso-zeaxanthin uptake was increased in the presence of HDL and mediated by SR-B1, SR-B2, and CD36, whereas lutein uptake is mediated by SR-B1 and CD36 and enhanced in the presence of LDL (48). A cross-sectional study from Renzi et al. (37) observed that serum lutein and zeaxanthin and lipoprotein concentrations are significantly related and changing lipoprotein levels may impact levels of these retinal xanthophylls. Recent findings also indicated accumulation of lutein and zeaxanthin in other region of the central nervous system (49). Interestingly, in this report it was shown that lutein concentrations are related to levels of StARD3 protein in human brain. Of note, this relationship was particularly strong in the pediatric brain (49). StARD3 belongs to the steroidogenic acute regulatory domain family of 15 soluble and membrane-associated proteins with high affinity for cholesterol and phosphatidylcholine. These proteins regulate cholesterol transfer within the mitochondria. They are primarily present in steroid-producing cells, including theca cells and luteal cells in the ovary, Leydig cells in the testis and in adrenal glomerulosa and faciculata (50). A role of StARD3 as a lutein-binding protein was first identified in the macula lutea of primates (51) together with glutathione S-transferase-1 (GSTP1) as a zeaxanthin-binding protein (52). StARD3 along GSTP1 provide numerous binding sites for lutein and zeaxanthin respectively, that account for the unique distribution and stability of carotenoids found in the primate macula lutea. It is possible that CD36 interacts with StARD3 as a surfaces receptor to mediate carotenoid uptake into the macula (51). These data provide evidence of the involvement of HDL transport and StARD3 in the uptake of lutein by the brain, and may partially explain the preferential accumulation of the xanthophylls in the central nervous system.
Metabolism
Another possible explanation for the selective accumulation of the xanthophylls in the macula of humans and primates is linked to the metabolism of these carotenoids in tissues. In mammals, two enzymes - β-carotene-15,15′-oxygenase (BCO1) and β-carotene-9′,10′-oxygenase (BCO2) - are responsible for mediating the cleavage of carotenoids at specific sites of the polyene chain (53). These two enzymes have different substrate specificity for various carotenoids, different cellular localization and likely perform very different functions within the cell (54). Specifically BCO2, which is localized in the mitochondria, has been proposed to prevent potentially toxic accumulation of these pigments in the tissues (53, 54). Xanthophylls can only serve as a substrate for BCO2 potentially giving rise to a number of 3-hydroxy metabolites depending on the side and number of cleavages (55, 56). Li et al. (57) reported that, unlike most other mammals, BCO2 expressed in the eye of humans is enzymatically inactive, thus resulting in the unique accumulation of these intact pigments in the macula. They demonstrated that BCO2 knockout mice accumulate zeaxanthin in their retinas and underlined the difference of enzymatic activities between human and mouse BCO2. One of the reasons that might explain why BCO2 lost its cleavage function was given through the examination of their primary structure. Human BCO2 has an insertion of GKAA that unfolds a short β-stranded loop into a larger unstructured loop, which might be key in preventing human BCO2 from cleaving xanthophylls. This structural difference might also explain why binding affinities between xanthophylls and mouse BCO2 are stronger than those associated with human BCO2, suggesting that weak binding of carotenoids may be the reason why human BCO2 has lost its cleavage function (57). However, these findings were not confirmed by later studies from the Von Lintig group (58) that showed that primate BCO2s are active enzymes, able to cleave all of the three major carotenoid components of the macula: lutein, zeaxanthin and meso-zeaxanthin.
Apart from the eye and brain, evidence of xanthophylls’ uptake in other mammalian tissues exists in the literature. Studies in sheep, chickens and bovines showed that mutations in BCO2 gene are associated with alterations of β-carotene and xanthophylls levels in tissues such as skin, adipose, blood and milk (59–61). Similarly, evidence showed that genetic disruption of BCO2 function in mice results in a significantly increased accumulation of xanthophylls’ metabolites in blood, liver, adipose and heart (62). Of note, accumulation of xanthophylls occurs also in human ovarian tissues, where lutein, lutein epoxide and violaxanthin were predominant among other carotenoids (63).
Lutein, zeaxanthin and pre-natal development
Evidence that lutein and zeaxanthin may play a role in pre-natal life comes from the presence of these carotenoids in the cord blood. Lutein is the most abundant carotenoid in cord plasma and its concentration was strongly correlated with maternal plasma lutein, suggesting a possible role in the neonatal period (64). In normal pregnancy, plasma levels of lutein/zeaxanthin are stable during the first trimester of gestation (0.46–0.48 μmol/L), increase steadily until the third semester (0.65 μmol/L) and remain elevated immediately after delivery, as opposed to β-carotene which has been reported to be about 20% lower in the third trimester compared to the first trimester (65, 66). Lutein and zeaxanthin levels in cord blood are lower than in maternal plasma (~0.13 μmol/L) (66); however, the changes in concentration follow a similar patter to the maternal blood. Specifically, it has been shown that lutein and 3′-oxo-lutein levels gradually increase, reaching a highest peak earlier in the third semester. From 37 weeks onwards, maternal lutein and 3-oxolutein levels progressively decreased to the lowest level at term, between 41 and 42 weeks of gestation. Notably, the increased levels of these xanthophylls between 33 and 36 weeks of gestation correspond to the stage of maximal whole fetal organs development, especially the central nervous system (67). Interestingly, it has been shown that lutein and zeaxanthin circulating concentrations in mothers of intrauterine growth restriction (IUGR) infants are depleted, indicating a possible increase in placental transfer of these carotenoids during gestation and the implication of a nutrient sparing mechanism in IUGR to preserve carotenoid levels in infants (68). In this respect, non-invasive measurements of carotenoid levels such optical quantitative measurements in human skin in vivo, i.e. Resonance Raman spectroscopy (RRS), would be useful to identify carotenoid status as a biomarker of dietary intake (69). Moreover, controlled trials on prenatal lutein and zeaxanthin supplementation, would be also instrumental to suggest a safe and beneficial consumption of these carotenoids during prenatal periods.
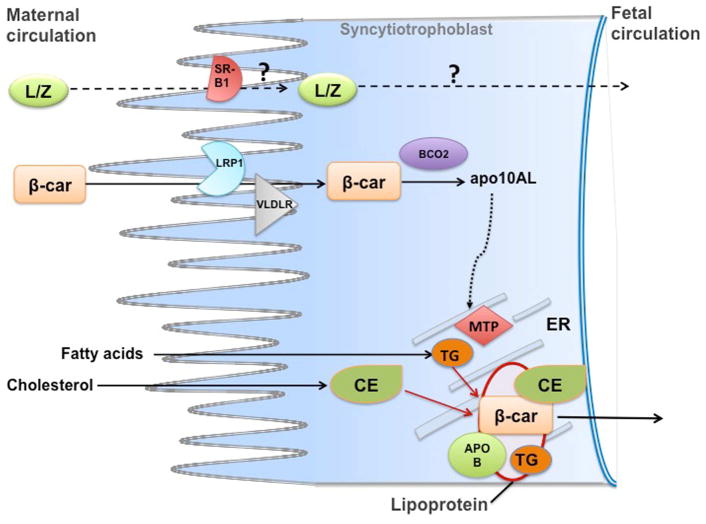
The current knowledge on molecular and physiological mechanisms that regulate transplacental transport of carotenoids is based mainly on investigations of maternal-fetal transfer of β-carotene in mouse models (70–72). Experiments conducted with wild-type dams maintained on a regular chow diet and supplemented with β-carotene, showed downregulation of LDL-receptor–related protein 1 (Lrp1) and VLDL receptor (Vldlr), but not lipoprotein lipase (Lpl), scavenger receptor B type 1 (Srb1) and LDL receptor (Ldlr) (71, 72). These lipoproteins receptors and remodeling enzyme play a critical role in the transport and metabolism of lipids (73). Although protein levels were not assessed in these studies, these findings suggest the existence of a potential feedback mechanism, mediated by key players of lipid metabolism, that attenuate placental β-carotene uptake when dietary vitamin A intake/status is sufficient. A role for LDLR in placental uptake of β-carotene, under vitamin A sufficiency, was also ruled out by studies on Ldlr knockout mice (74). Further studies in mice confirmed that the placental uptake and metabolism of β-carotene from the maternal circulation is dependent on the maternal status, being increased under maternal vitamin A deficiency (71), and attenuated when the mother were maintained on a high vitamin A diet (72). Finally, Costabile and colleagues (70) recently discovered a mechanism whereby, upon placental uptake, β-carotene is re-secreted towards the fetal circulation. This process is mediated by placental lipoprotein secretion, which in turn is regulated by β-apocarotenoids generated when β-carotene is asymmetrically cleaved by placental BCO2. This mechanism was proposed to fine-tune the flux of provitamin A carotenoids towards the fetus depending upon the maternal vitamin A status and the fetal demand for retinoids, ultimately protecting the embryo from the potentially harmful effects of vitamin A toxicity (70) (Figure 2). Data regards to placenta concentrations of xanthophylls in humans or animal models are scarce. Furthermore, the molecular mechanisms of transplacental transfer of xanthophylls have not been clarified. Based on the evidence discussed above in regards to xanthophylls’ uptake in adult tissues, it is reasonable to hypothesize that placental uptake of xanthophylls – circulating in the maternal bloodstream in association with HDL - may be also mediated by SR-B proteins and/or other key players of lipid transport. It is known that SR-B1, SR-B2 and CD36 proteins are highly expressed in placenta, where they play a crucial role in lipid transport to the fetus (75). Single nucleotide polymorphisms (SNPs) in the genes of BCO1, SR-B1 and CD36 have been associated with lower circulating levels of lutein (76, 77), but no association with plasma lutein and zeaxanthin levels was found in subjects bearing SNPs for apolipoprotein (apo) A-IV, apo B, apo E, lipoprotein lipase (41). In addition, SNPs in the genes of SR-B1 and ABC transporters have been associated with lower macular pigment density in women (78). However, evidence that SNPs in the above-mentioned genes may be associated with lutein/zeaxanthin’s levels in the cord blood is lacking. Overall, the molecular regulation of lutein, zeaxanthin and meso-zeaxanthin transport across the placenta needs to be explored in greater detail, in order to elucidate their biological functions in early life (Figure 2). Studies in mouse models would be desirable, given the ease to modify their genome to dissect molecular pathways.
Figure 2. Maternal-fetal transfer of carotenoids.
Proposed model for maternal fetal transfer of β-carotene (β-car): intact β-carotene from the maternal bloodstream is taken up by the syncytiotrophoblast cells of placenta, likely through the action of the lipoprotein receptors LRP1 and VLDLR, at least under a condition of vitamin A sufficiency. Placental β-carotene 9′,10′-oxygenase (BCO2) cleaves the provitamin A carotenoid asymmetrically to generate β-apo-10′carotenal (apo10AL). This metabolite then increases the transcription and activity of placental microsomal triglyceride transfer protein (MTP) which in turn stimulates lipoproteins biosynthesis. Thus, β-carotene is transported toward the fetal circulation in association with lipoproteins, assembled within and secreted from the placenta syncytiotrophoblast cells. Whether or not lutein and zeaxanthin (L/Z) are transferred across the maternal-fetal barrier with similar molecular mechanisms remains to be established. apoB, apolipoprotein B100; CE, cholesteryl esters; TG, triglycerides.
Growing evidence supports a role of lutein and zeaxanthin in visual and brain development in early life. In regard to visual development, it is known that the maturation of the eye structure requires the formation of oxygen-rich blood vessels as well as polyunsatured fatty acids to form biological membranes (79). This pro-oxidative environment could be detrimental for the eye and the presence of lutein and zeaxanthin in the developing macula might represent an important antioxidant barrier (80). Lutein and zeaxanthin have been detected in the region of incipient macula in 17–20 weeks fetuses in humans (81). A more recent study further explored carotenoids distribution in the prenatal development of the eye. Panova and colleagues (80) showed that only lutein and its oxidized forms, and not zeaxanthin, are present in the early fetal period of human development. Specifically, carotenoid analyses of the vitreous body of 16 to 28 weeks human fetuses clearly showed the presence of lutein, with maximum concentrations reached around 16–18 weeks followed by a sharp decrease between 24 and 26 weeks of gestation. After 28 weeks, lutein was no longer detected in the vitreous body, denoting that its presence is transient and suggesting a specific protective function against oxidative damage in early eye development (80). It has been proposed that the accumulation of these xanthophills in the eye during the intrauterine life may serve as a critical protective factor against oxidative stress in the perinatal period, due to the rapid exposure to the extrauterine environment with higher levels of oxygen compared to the relatively hypoxic uterine life. The free radical damage can lead to the development of several preterm newborn’s diseases, such as retinopathy of prematurity. Some evidence reported a protective role of an oral supplementation of lutein in term healthy newborns, by enhancing biological antioxidant potential and reducing lipid peroxidation (82). A cross-sectional study on interrelationships of maternal carotenoid status and newborn infant macular pigment levels and systemic carotenoid status revealed that zeaxanthin status may play a more important role than lutein status in macular pigment deposition in utero (68). Interestingly, in this study only infant serum zeaxanthin levels and not lutein correlated with macular pigment optical density (MPOD) measurements, indicating that physiologic relationship between zeaxanthin and macular development is present at birth. Maternal serum zeaxanthin levels also correlated with infant MPOD, suggesting an important link between maternal carotenoid status, especially zeaxanthin, and infant macular development (68). No causality was determined in this study; hence, controlled trials are warranted to explore in detail this relationship and the functions of zeaxanthin on macular physiology early in life. A possible novel neurotrophic role of lutein has been recently investigated by Picone and colleagues (67). These authors showed that lutein concentrations in the arterial cord blood of healthy preterm and term newborns were correlated with activin A, a well-established marker of central nervous system (CNS) development and damage. Moreover, lutein and activin A levels were higher during the pre-term period (33–36 weeks) and then declined overtime, suggesting that lutein may have a role in support of the development of the central nervous system, which is at its highest in terms of brain volume, weight and structure at this gestational stage (83).
Lutein, zeaxanthin and early post-natal development
Human milk is the only source of nutrients for the newborn thus enabling his/her optimal growth and development. Moreover, milk is the only dietary source of lutein and zeaxanthin before solid food is introduced (84). A 9-country survey conducted on breast milk carotenoid composition among 471 women determined that levels of lutein plus zeaxanthin in milk were ~ 0.043 μmol/L, but individual country means varied from a low of ~ 0.043 μmol/L in the U.S. to a high of ~ 0.077 μmol/L in Japan (85). In this study, the highest individual lutein concentration measured was ~ 0.040 μmol/L in China and the lowest was ~ 0.005 μmol/L in the U.K (85). A number of factors may affect milk concentrations of lutein and zeaxanthin, including stage of lactation, maternal nutritional and supplementation status (86–88). During the first month post-partum, lutein remains relatively elevated in the human milk (~ 0.03–0.04 μmol/L) underscoring its important role for the infant (87). A recent cross-sectional study explored carotenoids and tocopherols concentrations in human milk samples of healthy Chinese mothers and explored their association with lactation stage, region, socio-economic level, obstetric factors, and dietary patterns (89). This study showed that lutein was the most abundant carotenoid in mature human milk. Yet, the authors were unable to find significant associations between the concentrations of β-cryptoxanthin, lutein, or lycopene and the socio-economic traits of the cohort and its offspring, including maternal age, household income, or supplement intake. On the other hand, inverse correlations were found between human milk β-carotene and zeaxanthin concentrations and body mass index (BMI), suggesting that a maternal excess of body fat increases the utilization of several micronutrients, leading to lower carotenoids delivery via breast milk. In addition, mothers undergoing cesarean delivery exhibited lower zeaxanthin levels in their milk, thus lowering the potential antioxidant actions of human milk and suggesting a negative, albeit unexplored effect of cesarean delivery on maternal milk composition (89). Data on the effect of dietary lutein supplementation on breast milk lutein concentration are especially limited. The first longitudinal study to investigate the effect of lutein supplementation for 2–3 months postpartum evaluated 89 lactating women 4–6 weeks postpartum, randomly assigned to be administered either 0 (placebo), 6 (low-dose), or 12 mg/d (high-dose) of lutein (90). Significant correlations between breast milk concentrations and plasma lutein concentrations were reported and the correlation between breast milk and maternal plasma total lutein + zeaxanthin increased in magnitude over time with supplementation (90). In another study it was shown that upon maternal β-carotene supplementation during the first month postpartum, lutein levels in the milk where elevated and represented ~50% of total milk carotenoid. Moreover, lutein was the only carotenoid that remained elevated in the milk of these mothers for over 4 weeks, further supporting a potential beneficial role for the infant (87).
Recently, lutein has been reported to be the main carotenoid throughout human brain tissue not only in adult individuals, but also in infants (91–93), confirming its potential role in regulating cognition. It has been shown that the most relevant animal model to investigate lutein’s function in developing eyes and brain is represented by non-human primates, such as Resus Macaques (Macaca mulatta). This animal model, like humans and unlike most experimental animal models, absorbs and preferentially stores xanthophylls in the retina/macula and brain (94). A recent pilot study from Jeon et al. (95) investigated the effects of formula supplemented with lutein, zeaxanthin, β-carotene and lycopene on bioaccumulation in tissues of Rhesus Macaques. They found that increased early exposure to dietary lutein led to increased lutein tissue deposition. Specifically, lutein accumulated in highest amounts in occipital cortex, while zeaxanthin and lycopene were undetectable in any brain region. Some interesting data from the first metabolomic study in human infant brain showed that lutein levels were positively correlated with GABA and aspartate neurotransmitters involved in neuronal proliferation and maturation, neurite outgrowth and synapse formation (96). These novel findings may shed a light on important roles of lutein in the human brain during development or remodeling of neurons, but potential pathways for lutein’s activity and its molecular mechanisms of action still need further clarification.
Supplement manufacturers have begun adding lutein to infant formulas spearheading interest on the effects of such fortified formulas in early development. Prior the initiation of clinical trials, a few studies assessed safety, growth effect and tolerance of these new formulas (97, 98) with lutein concentration of 0.351 μmol/L. Overall, infants younger than 2 months fed lutein fortified formulas demonstrated appropriate growth and good intestinal tolerance, supporting the conclusion that fortification of an infant formula with lutein is safe for infant consumption. Additional studies with higher number of subjects are still needed to define the role and the physiological functions of lutein in the development of infants. In a randomized, prospective study (99) comparing serum lutein concentrations in healthy term infants consuming different concentrations of lutein in formula, it was shown that breastfed infants had higher serum lutein concentrations (0.142 μmol/L) than all formula-fed infants (0.022 μmol/L). Moreover, a four-fold higher lutein concentration was needed in formula compared to human milk to achieve similar lutein concentrations between formula-fed and breastfed infants. These data suggest that the bioavailability of lutein from formula was considerably lower than from human. Dietary intake of the mother, especially related to fat content of the diet (100) and interactions with other nutrients (101) may account for this difference in bioavailability; however additional studies are required. Overall, much still remains to be understood in regards to the role of lutein and zeaxanthin during development. Given that breast milk and or infant formula are the sole source of nutrition during early post-natal development, it is desirable that these studies would ultimately lead to establish specific dietary recommendation for lactating women, in order to facilitate a healthy growth and development of the infant.
Summary and conclusions
A wide-range of investigations have explored the health effects of lutein and zeaxanthin in the past decades. The majority of the studies focused on the functions of these xanthophylls on visual, neural, and cognitive health and often attributed their beneficial effects to their antioxidant and anti-inflammatory properties (21, 82, 102). A still limited but growing body of evidence has recently supported the role of lutein and zeaxanthin also in mammalian development, specifically in relationship to their potential protective activity on infant retinal and brain development and functions. It remains to be established whether or not these are the only body sites where accumulation of xanthophylls may be beneficial to the developing fetus and rapidly growing infant. Most of the human data derive from observational studies that are generally complicated by a number of variables, which have an impact on carotenoid and metabolites in serum and tissues. Examples of such variables include bioavailability from foods, lifestyle, and SNPs (103, 104). The major gaps and limitations of the available observational studies are the lack of information on dietary intake, including supplement use. This is especially important in pregnant women. Thus, studies with controlled supplementation of maternal diets are desirable. In general, there is an emerging interest for establishing intake recommendations for non-essential dietary bioactive molecules, such as lutein and zeaxanthin for prevention of chronic diseases and to maintain an optimal health status (105). According to the NHANES 2013–2014 survey, adults in the United States consume an average of 1.7 mg/day of lutein and zeaxanthin combined (106), which may be insufficient to attain the health benefits that epidemiological observations have shown. Currently, there are no established upper limits for lutein and zeaxanthin intake neither for adults nor for pregnant women. A systematic risk assessment of lutein supplements in placebo-controlled intervention trials indicate that lutein is safe up to 20 mg/day; however these levels are not sufficient to draw confident conclusions on long-term safety (107). Although some experimental safety data are available for lutein and zeaxanthin (107–109), further trials are warranted to evaluate the chronic use of high-dose supplementation, especially in specific populations such as pregnant women, infants and premature newborns. Further studies in experimental animal models are clearly needed to understand the molecular and physiological mechanisms of maternal-fetal transfer and milk incorporation of xanthophylls in relation to the maternal dietary intake of these carotenoids. For obvious ethical reasons and technical difficulties these investigations cannot be conducted in humans. However, it is undoubtedly challenging to identify the most appropriate animal/experimental model that could provide novel human-relevant knowledge in a relatively short time and without dramatically increasing the cost of research.
A role for apocarotenoids, especially from β-carotene and lycopene, generated upon enzymatic and not enzymatic cleavage in mammalian tissues or in the food, is clearly arising from the literature. Thus, suggesting that they may exert important biological activities as transcriptional regulators in vitro and in vivo. (70, 110–114). Hydroxyl derivatives of xanthophylls can be formed in mammalian tissues (62, 115) and whether their generation may be linked to the potential biological actions of their parent compounds remains to be established and certainly warrants future studies, especially in regards to mammalian development.
Supplementary Material
Highlights.
Lutein and zeaxanthin play an important role in visual and cognitive development
Maternal blood and milk provide lutein and zeaxanthin to the growing fetus/infant
Mechanisms of maternal-fetal transfer and milk incorporation are still unknown
Lutein and zeaxanthin status should be monitored in newborns and lactating mothers
Acknowledgments
The work form the author’s laboratory presented in this review was supported by grants R01HD057493, R01HD057493-02S1 and R01HD083331 from the U.S. National Institute of Health (NIH) and by NRI award #2006-35200-16580 from USDA-CSREES, Bioactive Food Component for Optimal Health (31.0).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kominiarek MA, Rajan P. Nutrition recommendations in pregnancy and lactation. Medical Clinics. 2016;100:1199–1215. doi: 10.1016/j.mcna.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papathakis PC, Singh LN, Manary MJ. How maternal malnutrition affects linear growth and development in the offspring. Mol Cell Endocrinol. 2016;435:40–47. doi: 10.1016/j.mce.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Verduci E, Martelli A, Miniello V, Landi M, Mariani B, Brambilla M, Diaferio L, Peroni D. Nutrition in the first 1000 days and respiratory health: A descriptive review of the last five years’ literature. Allergol Immunopathol. 2017;45:405–413. doi: 10.1016/j.aller.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Bravi F, Wiens F, Decarli A, Dal Pont A, Agostoni C, Ferraroni M. Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr. 2016;104:646–662. doi: 10.3945/ajcn.115.120881. [DOI] [PubMed] [Google Scholar]
- 5.Zielińska MA, Wesołowska A, Pawlus B, Hamułka J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients. 2017;9:838. doi: 10.3390/nu9080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto H, Uragami C, Cogdell RJ. Carotenoids and Photosynthesis. Subcell Biochem. 2016;79:111–139. doi: 10.1007/978-3-319-39126-7_4. [DOI] [PubMed] [Google Scholar]
- 7.Grotewold E. The genetics and biochemistry of floral pigments. Annu Rev Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- 8.Beltran JCM, Stange C. Apocarotenoids: A new carotenoid-derived pathway. Subcell Biochem. 2016;79:239–272. doi: 10.1007/978-3-319-39126-7_9. [DOI] [PubMed] [Google Scholar]
- 9.Von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Ann Rev Nutr. 2010;30:35–56. doi: 10.1146/annurev-nutr-080508-141027. [DOI] [PubMed] [Google Scholar]
- 10.Shete V, Quadro L. Mammalian metabolism of β-carotene: gaps in knowledge. Nutrients. 2013;5:4849–4868. doi: 10.3390/nu5124849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: Formation, occurrence, and function of apocarotenoids thematic review series: Fat-soluble vitamins: Vitamin A. J Lipid Res. 2013;54:1719–1730. doi: 10.1194/jlr.R039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xavier AAO, Pérez-Gálvez A. Carotenoids as a Source of Antioxidants in the Diet. Subcell Biochem. 2016;79:359–375. doi: 10.1007/978-3-319-39126-7_14. [DOI] [PubMed] [Google Scholar]
- 13.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Palozza P, Serini S, Di Nicuolo F, Piccioni E, Calviello G. Prooxidant effects of β-carotene in cultured cells. Mol Aspects Med. 2003;24:353–362. doi: 10.1016/s0098-2997(03)00031-1. [DOI] [PubMed] [Google Scholar]
- 15.Hurst JS, Saini MK, Jin G-F, Awasthi YC, van Kuijk FJ. Toxicity of oxidized β-carotene to cultured human cells. Exp Eye Res. 2005;81:239–243. doi: 10.1016/j.exer.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Burton GW, Ingold K. Beta-carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 17.Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. Maternal–fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta. 2012;1821:88–98. doi: 10.1016/j.bbalip.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin a and retinoid signaling: genomic and nongenomic effects thematic review series: Fat-soluble vitamins: Vitamin A. J Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison EH, Curley RW. Carotenoids and retinoids: nomenclature, chemistry, and analysis. Subcell Biochem. 2016;81:1–19. doi: 10.1007/978-94-024-0945-1_1. [DOI] [PubMed] [Google Scholar]
- 21.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Ann Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 22.Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. J Nutr. 2002;132:518S–524S. doi: 10.1093/jn/132.3.518S. [DOI] [PubMed] [Google Scholar]
- 23.Scripsema NK, Hu D-N, Rosen RB. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J Ophthalmol. 2015;2015:865179. doi: 10.1155/2015/865179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J Food Comp Anal. 2009;22:9–15. [Google Scholar]
- 25.Abdel-Aal ESM, Akhtar H, Zaheer K, Ali R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients. 2013;5:1169–1185. doi: 10.3390/nu5041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung H-Y, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr. 2004;134:1887–1893. doi: 10.1093/jn/134.8.1887. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maoka T, Arai A, Shimizu M, Matsuno T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp Biochem Physiol B. 1986;83:121–124. doi: 10.1016/0305-0491(86)90341-x. [DOI] [PubMed] [Google Scholar]
- 29.Nolan JM, Beatty S, Meagher KA, Howard AN, Kelly D, Thurnham DI. Verification of meso-zeaxanthin in fish. J Food Process Technol. 2014;5:335. doi: 10.4172/2157-7110.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurnham DI, Nolan JM, Howard AN, Beatty S. Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2015;253:1231–1243. doi: 10.1007/s00417-014-2811-3. [DOI] [PubMed] [Google Scholar]
- 31.Carbonell-Capella JM, Buniowska M, Barba FJ, Esteve MJ, Frígola A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr Rev Food Sci Food Saf. 2014;13:155–171. doi: 10.1111/1541-4337.12049. [DOI] [PubMed] [Google Scholar]
- 32.Kotake-Nara E, Nagao A. Absorption and metabolism of xanthophylls. Marine Drugs. 2011;9:1024–1037. doi: 10.3390/md9061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992;122:2161–2166. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- 34.O’Connell OF, Ryan L, O’Brien NM. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr Res. 2007;27:258–264. [Google Scholar]
- 35.Micozzi MS, Brown ED, Edwards BK, Bieri J, Taylor PR, Khachik F, Beecher GR, Smith J. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr. 1992;55:1120–1125. doi: 10.1093/ajcn/55.6.1120. [DOI] [PubMed] [Google Scholar]
- 36.Khachik F, Beecher GR, Smith JC. Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem. 1995;59:236–246. doi: 10.1002/jcb.240590830. [DOI] [PubMed] [Google Scholar]
- 37.Renzi LM, Hammond BR, Dengler M, Roberts R. The relation between serum lipids and lutein and zeaxanthin in the serum and retina: results from cross-sectional, case-control and case study designs. Lipids Health Dis. 2012;11:33. doi: 10.1186/1476-511X-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widjaja-Adhi MAK, Lobo GP, Golczak M, Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet. 2015;24:3206–3219. doi: 10.1093/hmg/ddv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, Von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β, β-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- 41.Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent-Baudry S, Maillot M, Gastaldi M, Darmon M, Portugal H. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr. 2007;137:2653–2659. doi: 10.1093/jn/137.12.2653. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr. 2007;85:762–769. doi: 10.1093/ajcn/85.3.762. [DOI] [PubMed] [Google Scholar]
- 43.Clevidence BA, Bieri JG. Association of carotenoids with human plasma lipoproteins. Methods Enzymol. 1993;214:33–46. doi: 10.1016/0076-6879(93)14051-j. [DOI] [PubMed] [Google Scholar]
- 44.Ziouzenkova O, Winklhofer-Roob BM, Puhl H, Roob JM, Esterbauer H. Lack of correlation between the alpha-tocopherol content of plasma and LDL, but high correlations for gamma-tocopherol and carotenoids. J Lipid Res. 1996;37:1936–46. [PubMed] [Google Scholar]
- 45.Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64:211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 46.Bone RA, Landrum JT, Dixon Z, Chen Y, Llerena CM. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res. 2000;71:239–245. doi: 10.1006/exer.2000.0870. [DOI] [PubMed] [Google Scholar]
- 47.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–1724. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shyam R, Vachali P, Gorusupudi A, Nelson K, Bernstein PS. All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch Biochem Biophys. 2017;634:21–28. doi: 10.1016/j.abb.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson EJ, Tanprasertsuk J, Li B, Bernstein PS, Vishwanathan R, Johnson MA, Poon LW. Relationship between concentrations of lutein and StARD3 among pediatric and geriatric human brain tissue. FASEB J. 2016;30:913.917–913.917. doi: 10.1371/journal.pone.0155488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kallen CB, Billheimer JT, Summers SA, Stayrook SE, Lewis M, Strauss JF., III Steroidogenic acute regulatory protein (StAR) is a sterol transfer protein. J Biol Chem. 1998;41:26285–8. doi: 10.1074/jbc.273.41.26285. [DOI] [PubMed] [Google Scholar]
- 51.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–2549. doi: 10.1021/bi101906y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhosale P, Li B, Sharifzadeh M, Gellermann W, Frederick JM, Tsuchida K, Bernstein PS. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;22:4798–807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 53.Von Lintig J. Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr. 2012;96:1234S–1244S. doi: 10.3945/ajcn.112.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palczewski G, Amengual J, Hoppel CL, Von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 2014;28:4457–4469. doi: 10.1096/fj.14-252411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lobo GP, Isken A, Hoff S, Babino D, von Lintig J. BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development. 2012;139:2966–2977. doi: 10.1242/dev.079632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.dela Seña C, Sun J, Narayanasamy S, Riedl KM, Yuan Y, Curley RW, Schwartz SJ, Harrison EH. Substrate Specificity of Purified Recombinant Chicken β-Carotene 9′, 10′-Oxygenase (BCO2) J Biol Chem. 2016;291:14609–14619. doi: 10.1074/jbc.M116.723684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W. Inactivity of human β, β-carotene-9′, 10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc Natl Acad Sci. 2014;111:10173–10178. doi: 10.1073/pnas.1402526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babino D, Palczewski G, Widjaja-Adhi MAK, Kiser PD, Golczak M, Von Lintig J. Characterization of the role of β-carotene 9, 10-dioxygenase in macular pigment metabolism. J Biol Chem. 2015;290:24844–24857. doi: 10.1074/jbc.M115.668822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eriksson J, Larson G, Gunnarsson U, Bed’hom B, Tixier-Boichard M, Strömstedt L, Wright D, Jungerius A, Vereijken A, Randi E. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genetics. 2008;4:e1000010. doi: 10.1371/journal.pgen.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy A, Oxley PE. Mutation in bovine β-carotene oxygenase 2 affects milk color. Genetics. 2009;182:923–926. doi: 10.1534/genetics.109.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Våge DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries) BMC genetics. 2010;11:10. doi: 10.1186/1471-2156-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, Von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czeczuga-Semeniuk E, Wolczynski S. Identification of carotenoids in ovarian tissue in women. Oncol Rep. 2005;14:1385–1392. [PubMed] [Google Scholar]
- 64.Yeum K-J, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr. 1998;17:442–447. doi: 10.1080/07315724.1998.10718791. [DOI] [PubMed] [Google Scholar]
- 65.Oostenbrug GS, Mensink RP, Al MD, van Houwelingen AC, Hornstra G. Maternal and neonatal plasma antioxidant levels in normal pregnancy, and the relationship with fatty acid unsaturation. Br J Nutr. 1998;80:67–73. doi: 10.1017/s0007114598001780. [DOI] [PubMed] [Google Scholar]
- 66.Kiely M, Cogan P, Kearney P, Morrissey P. Concentrations of tocopherols and carotenoids in maternal and cord blood plasma. Eur J Clin Nutr. 1999;53:711–715. doi: 10.1038/sj.ejcn.1600838. [DOI] [PubMed] [Google Scholar]
- 67.Picone S, Ritieni A, Fabiano A, Graziani G, Paolillo P, Livolti G, Galvano F, Gazzolo D. Lutein levels in arterial cord blood correlate with neuroprotein activin A in healthy preterm and term newborns: A trophic role for lutein? Clin Biochem. 2017;52:80–84. doi: 10.1016/j.clinbiochem.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Henriksen BS, Chan G, Hoffman RO, Sharifzadeh M, Ermakov IV, Gellermann W, Bernstein PS. Interrelationships Between Maternal Carotenoid Status and Newborn Infant Macular Pigment Optical Density and Carotenoid StatusMaternal/Infant Macular Carotenoid Interactions. Invest Ophthalmol Vis Sci. 2013;54:5568–5578. doi: 10.1167/iovs.13-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ermakov IV, Gellermann W. Optical detection methods for carotenoids in human skin. Arch Biochem Biophys. 2015;572:101–111. doi: 10.1016/j.abb.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 70.Costabile BK, Kim Y-K, Iqbal J, Zuccaro MV, Wassef L, Narayanasamy S, Curley RW, Harrison EH, Hussain MM, Quadro L. β-Apo-10′-carotenoids Modulate Placental Microsomal Triglyceride Transfer Protein Expression and Function to Optimize Transport of Intact β-Carotene to the Embryo. J Biol Chem. 2016;291:18525–18535. doi: 10.1074/jbc.M116.738336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wassef L, Shete V, Hong A, Spiegler E, Quadro L. β-Carotene Supplementation Decreases Placental Transcription of LDL Receptor-Related Protein 1 in Wild-Type Mice and Stimulates Placental β-Carotene Uptake in Marginally Vitamin A-Deficient Mice. J Nutr. 2012;142:1456–1462. doi: 10.3945/jn.112.162677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wassef L, Shete V, Costabile B, Rodas R, Quadro L. High Preformed Vitamin A Intake during Pregnancy Prevents Embryonic Accumulation of Intact β-Carotene from the Maternal Circulation in Mice. J Nutr. 2015;145:1408–1414. doi: 10.3945/jn.114.207043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khalil MF, Wagner WD, Goldberg IJ. Molecular interactions leading to lipoprotein retention and the initiation of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2211–2218. doi: 10.1161/01.ATV.0000147163.54024.70. [DOI] [PubMed] [Google Scholar]
- 74.Shete V, Costabile BK, Kim YK, Quadro L. Low-Density Lipoprotein Receptor Contributes to β-Carotene Uptake in the Maternal Liver. Nutrients. 2016;8:765. doi: 10.3390/nu8120765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woollett L, Heubi JE. Fetal and neonatal cholesterol metabolism. In: De Groot LJ, Chrouson G, Dungan K, Fenigold KR, Grossman A, Hershman A, Koch C, Korbonits C, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc; 2016. 2000. [Google Scholar]
- 76.Borel P, De Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand J-M, Meunier N, Drouault-Holowacz S, Bieuvelet S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med. 2011;43:47–59. doi: 10.3109/07853890.2010.531757. [DOI] [PubMed] [Google Scholar]
- 77.McKay GJ, Loane E, Nolan JM, Patterson CC, Meyers KJ, Mares JA, Yonova-Doing E, Hammond CJ, Beatty S, Silvestri G. Investigation of genetic variation in scavenger receptor class B, member 1 (SCARB1) and association with serum carotenoids. Ophthalmology. 2013;120:1632–1640. doi: 10.1016/j.ophtha.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP, Truitt B, Klein ML. Genetic Determinants of Macular Pigments in Women of the Carotenoids in Age-Related Eye Disease StudyGenetic Predictors of MPOD. Invest Ophthalmol Vis Sci. 2013;54:2333–2345. doi: 10.1167/iovs.12-10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mann I. The development of the human eye. 2. New York: Grune & Stratton; 1950. [Google Scholar]
- 80.Panova IG, Yakovleva MA, Tatikolov AS, Kononikhin A, Feldman TB, Poltavtseva RA, Nikolaev E, Sukhikh GT, Ostrovsky MA. Lutein and its oxidized forms in eye structures throughout prenatal human development. Exp Eye Res. 2017;160:31–37. doi: 10.1016/j.exer.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Bone R, Landrum J, Fernandez L, Tarsis S. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 82.Perrone S, Tei M, Longini M, Santacroce A, Turrisi G, Proietti F, Felici C, Picardi A, Bazzini F, Vasarri P. Lipid and protein oxidation in newborn infants after lutein administration. Oxid Med Cell Longev. 2014;2014:781454. doi: 10.1155/2014/781454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 84.Hammond BR., Jr Possible role for dietary lutein and zeaxanthin in visual development. Nutr Rev. 2008;66:695–702. doi: 10.1111/j.1753-4887.2008.00121.x. [DOI] [PubMed] [Google Scholar]
- 85.Canfield LM, Clandinin MT, Davies DP, Fernandez MC, Jackson J, Hawkes J, Goldman WJ, Pramuk K, Reyes H, Sablan B. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr. 2003;42:133–141. doi: 10.1007/s00394-003-0403-9. [DOI] [PubMed] [Google Scholar]
- 86.Macias C, Schweigert FJ. Changes in the concentration of carotenoids, vitamin A, alpha-tocopherol and total lipids in human milk throughout early lactation. Ann Nutr Metabol. 2001;45:82–85. doi: 10.1159/000046711. [DOI] [PubMed] [Google Scholar]
- 87.Gossage CP, Deyhim M, Yamini S, Douglass LW, Moser-Veillon PB. Carotenoid composition of human milk during the first month postpartum and the response to β-carotene supplementation. Am J Clin Nutr. 2002;76:193–197. doi: 10.1093/ajcn/76.1.193. [DOI] [PubMed] [Google Scholar]
- 88.Nagayama J, Noda K, Uchikawa T, Maruyama I, Shimomura H, Miyahara M. Effect of maternal Chlorella supplementation on carotenoid concentration in breast milk at early lactation. Int J Food Sci Nutr. 2014;65:573–576. doi: 10.3109/09637486.2014.898257. [DOI] [PubMed] [Google Scholar]
- 89.Xue Y, Campos-Giménez E, Redeuil KM, Lévèques A, Actis-Goretta L, Vinyes-Pares G, Zhang Y, Wang P, Thakkar SK. Concentrations of Carotenoids and Tocopherols in Breast Milk from Urban Chinese Mothers and Their Associations with Maternal Characteristics: A Cross-Sectional Study. Nutrients. 2017;9:1229. doi: 10.3390/nu9111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sherry CL, Oliver JS, Renzi LM, Marriage BJ. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. The Journal of nutrition. 2014;144:1256–1263. doi: 10.3945/jn.114.192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson EJ. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am J Clin Nutr. 2012;96:1161S–1165S. doi: 10.3945/ajcn.112.034611. [DOI] [PubMed] [Google Scholar]
- 92.Vishwanathan R, Schalch W, Johnson EJ. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr Neurosci. 2016;19:95–101. doi: 10.1179/1476830514Y.0000000141. [DOI] [PubMed] [Google Scholar]
- 93.Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72:605–612. doi: 10.1111/nure.12133. [DOI] [PubMed] [Google Scholar]
- 94.Erdman JW, Smith JW, Kuchan MJ, Mohn ES, Johnson EJ, Rubakhin SS, Wang L, Sweedler JV, Neuringer M. Lutein and brain function. Foods. 2015;4:547–564. doi: 10.3390/foods4040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeon S, Neuringer M, Johnson EE, Kuchan MJ, Pereira SL, Johnson EJ, Erdman JW. Effect of carotenoid supplemented formula on carotenoid bioaccumulation in tissues of infant rhesus macaques: A pilot study focused on lutein. Nutrients. 2017;9:51. doi: 10.3390/nu9010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lieblein-Boff JC, Johnson EJ, Kennedy AD, Lai CS, Kuchan MJ. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PloS One. 2015;10:e0136904. doi: 10.1371/journal.pone.0136904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kon IY, Gmoshinskaya MV, Safronova AI, Alarcon P, Vandenplas Y. Growth and tolerance assessment of a lutein-fortified infant formula. Pediatr Gastroenterol Hepatol Nutr. 2014;17:104–111. doi: 10.5223/pghn.2014.17.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Capeding R, Gepanayao CP, Calimon N, Lebumfacil J, Davis AM, Stouffer N, Harris BJ. Lutein-fortified infant formula fed to healthy term infants: evaluation of growth effects and safety. Nutr J. 2010;9:22. doi: 10.1186/1475-2891-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bettler J, Zimmer JP, Neuringer M, DeRusso PA. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur J Nutr. 2010;49:45–51. doi: 10.1007/s00394-009-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roodenburg AJ, Leenen R, van het Hof KH, Weststrate JA, Tijburg LB. Amount of fat in the diet affects bioavailability of lutein esters but not of α-carotene, β-carotene, and vitamin E in humans. Am J Clin Nutr. 2000;71:1187–1193. doi: 10.1093/ajcn/71.5.1187. [DOI] [PubMed] [Google Scholar]
- 101.Goñi I, Serrano J, Saura-Calixto F. Bioaccessibility of β-carotene, lutein, and lycopene from fruits and vegetables. J Agric Food Chem. 2006;54:5382–5387. doi: 10.1021/jf0609835. [DOI] [PubMed] [Google Scholar]
- 102.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 103.Zaripheh S, Erdman JW., Jr Factors that influence the bioavailability of xanthophylls. J Nutr. 2002;132:S531. doi: 10.1093/jn/132.3.531S. [DOI] [PubMed] [Google Scholar]
- 104.Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, Lesavre N. Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am J Clin Nutr. 2014;100:168–175. doi: 10.3945/ajcn.114.085720. [DOI] [PubMed] [Google Scholar]
- 105.Biesalski HK, Erdman JW, Hathcock J, Ellwood K, Beatty S, Johnson E, Marchioli R, Lauritzen L, Rice HB, Shao A. Nutrient reference values for bioactives: new approaches needed? A conference report. Eur J Nutr. 2013;52:1–9. doi: 10.1007/s00394-013-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.NHANES; U.S. Department of Agriculture, A. R. S, editor. Nutrient Intakes from Food and Beverages: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, 2013–2014. NHANES; 2016. [Google Scholar]
- 107.Shao A, Hathcock JN. Risk assessment for the carotenoids lutein and lycopene. Regul Toxicol Pharmacol. 2006;45:289–298. doi: 10.1016/j.yrtph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Ravikrishnan R, Rusia S, Ilamurugan G, Salunkhe U, Deshpande J, Shankaranarayanan J, Shankaranarayana M, Soni MG. Safety assessment of lutein and zeaxanthin (Lutemax™ 2020): Subchronic toxicity and mutagenicity studies. Food Chem Toxicol. 2011;49:2841–2848. doi: 10.1016/j.fct.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 109.Ravi K, Reddy KR, Shankaranarayanan J, Deshpande JV, Juturu V, Soni MG. Safety evaluation of zeaxanthin concentrate (OmniXan™): Acute, subchronic toxicity and mutagenicity studies. Food Chem Toxicol. 2014;72:30–39. doi: 10.1016/j.fct.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 110.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW, Harrison EH. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J Biol Chem. 2012;287:15886–15895. doi: 10.1074/jbc.M111.325142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun J, Narayanasamy S, Curley RW, Harrison EH. β-Apo-13-carotenone regulates retinoid X receptor transcriptional activity through tetramerization of the receptor. J Biol Chem. 2014;289:33118–33124. doi: 10.1074/jbc.M114.610501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eroglu A, Hruszkewycz DP, Curley RW, Jr, Harrison EH. The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch Biochem Biophys. 2010;504:11–16. doi: 10.1016/j.abb.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Asymmetric cleavage of β-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol. 2007;21:77–88. doi: 10.1210/me.2006-0225. [DOI] [PubMed] [Google Scholar]
- 114.Wang CX, Jiang H, Yuen JJ, Lee S-A, Narayanasamy S, Curley RW, Jr, Harrison EH, Blaner WS. Actions of β-apo-carotenoids in differentiating cells: differential effects in P19 cells and 3T3-L1 adipocytes. Arch Biochem Biophys. 2015;572:2–10. doi: 10.1016/j.abb.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang X-D. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and β-cryptoxanthin by ferret carotene-9′, 10′-monooxygenase. Arch Biochem Biophys. 2011;506:109–121. doi: 10.1016/j.abb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.