Abstract
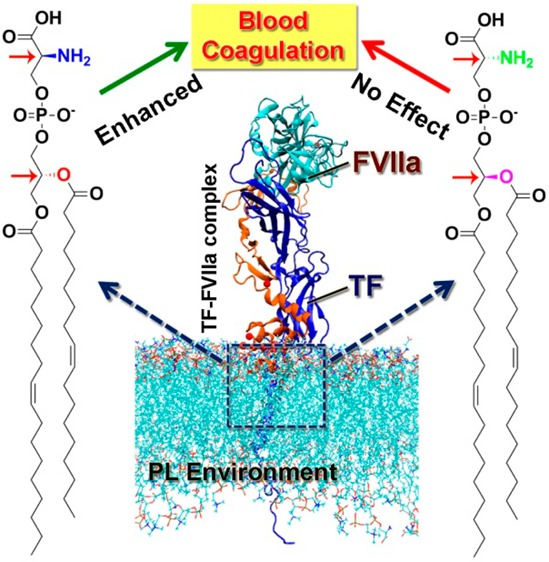
Natural phosphatidylserine (PS), which contains two chiral centers, enhances blood coagulation. However, the process by which PS enhanced blood coagulation is not completely understood. An efficient and flexible synthetic route has been developed to synthesize all of the possible stereoisomers of PS. In this study, we examined the role of PS chiral centers in modulating the activity of the tissue factor (TF)-factor VIIa coagulation initiation complex. Full length TF was relipidated with phosphatidylcholine, and the synthesized PS isomers were individually used to estimate the procoagulant activity of the TF-FVIIa complex via a FXa generation assay. The results revealed that the initiation complex activity was stereoselective and had increased sensitivity to the configuration of the PS glycerol backbone due to optimal protein–lipid interactions.
Keywords: Blood coagulation, tissue factor, factor VIIa, phospholipid, phosphatidylserine
Blood coagulation is a tightly regulated process.1−3 Any aberrations in its regulation may lead to pulmonary embolism, ischemic stroke, unstable angina, deep-vein thrombosis, acute myocardial infarction, and many other blood-related disorders.4−7 This process is initiated by the formation of a binary protein complex (TF-FVIIa) between a transmembrane protein, tissue factor (TF), and circulatory factor VIIa (FVIIa), followed by a series of protease reactions.1,8,9 Coagulation is primarily regulated by TF-FVIIa complex activity. It has been well documented that TF-FVIIa activity is highly modulated by the composition of the neighboring phospholipid (PL) environment.10−12 Incorporation of phosphatidylserine (PS) dramatically enhances TF-FVIIa complex activity.13−15 However, the detailed molecular level mechanism responsible for this is not fully understood. Structurally, PS consists of a polar headgroup comprising a serine and a phosphate group, a neutral glycerol backbone, and hydrophobic tails. From a stereochemical point of view, PS has two chiral centers (as shown in Figure 1), one in the headgroup serine moiety and the other in the glycerol backbone.16 Naturally occurring PS has the conformation of 1,2-diacyl-sn-glycero-3-phospho-L-serine. In the present work, we have provided a synthetic approach for synthesizing different PS stereoisomers and demonstrated how the configuration of the PS chiral centers regulates TF-FVIIa activity.
Figure 1.
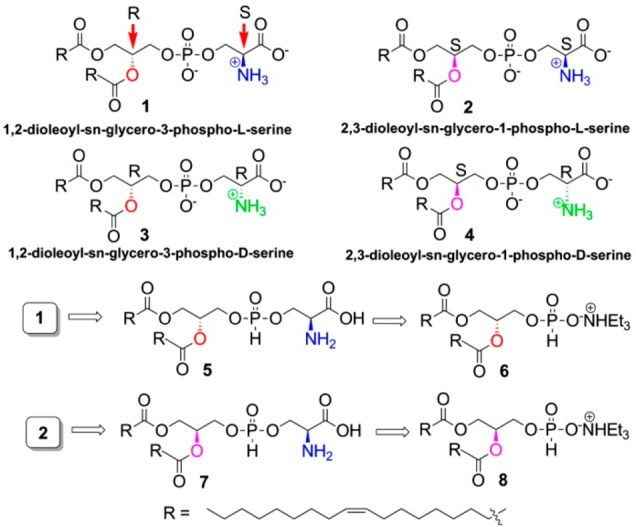
Chemical structure of different PS stereoisomers and their retrosynthetic analysis.
A number of approaches (phosphoramidite method, H-phosphonate method, phosphotrichloride method, alkyl or aryl dichlorophosphites, etc.)17−21 have been adopted to synthesize PL. In most of the cases, PLs have been synthesized using 2,3-isopropylidene-sn-glycerol22 or D-mannitol as the starting material.23 Our synthetic approach is quite different (Figure 1); we have planned to synthesize the target molecules (1, 2) from the corresponding triethylamine H-phosphonate salts (TEAHP 6 and 8) via H-phosphonates 5 and 7, respectively. Other targets (3, 4) could also be synthesized with a similar approach from intermediates 6 and 8 by coupling with D-serine. We have developed an efficient route for the preparation of the PL phosphodiester bond using diphenyl H-phosphonate instead of widely used PCl3/imidazole. The uniqueness of our synthesis is the easy separation of the highly stable intermediate (can be stored for several months) TEAHP 6/8.
In most earlier studies, PL amine and acid head groups were protected as Cbz and benzyl ester, respectively, which made it difficult to produce unsaturated fatty acid-containing PLs, as deprotection of these groups by hydrogenation would also lead to saturation of the fatty acid. We have protected the amine and acid functionalities of the headgroup as Boc and tert-butyl groups, respectively, which can be easily deprotected under mild acidic conditions without hampering the unsaturation in the fatty acid chains. This new synthesis route may provide a viable route for the synthesis of any type of PL (saturated, unsaturated, or mixed fatty acids) with high yield and excellent stereospecificity.
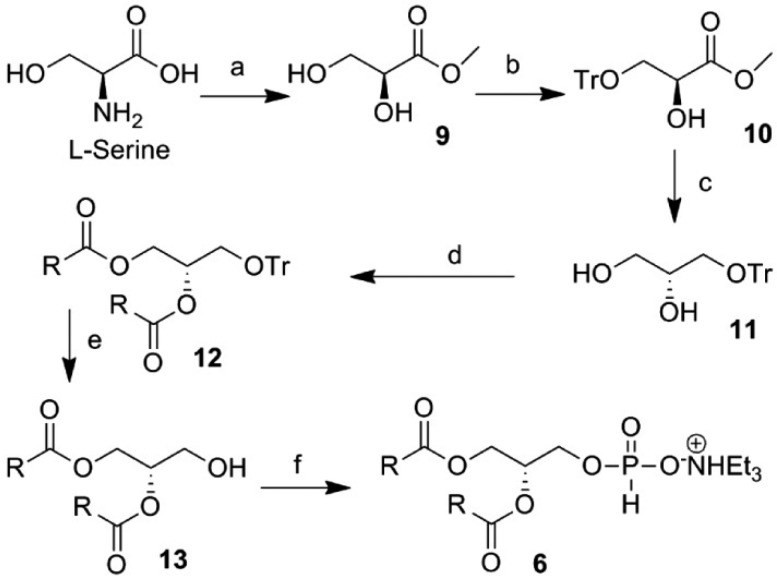
The synthesis of the TEAHP intermediate 6 is shown in Scheme 1. We initiated our synthesis from known compound 9 prepared from cheap and readily available L-serine following a literature procedure,24 which was subjected to react with TrCl/Et3N/TBAI to obtain compound 10. The ester functionality in compound 10 was reduced with LAH to obtain alcohol 11, which is a glycerol derivative with the same configuration as naturally occurring PS (1). Next, alcohol 11 was acetylated further with oleic acid in the presence of DCC to give compound 12 with a good yield. Compound 12 was then reacted with TFA to access trityl-deprotected compound 13, which was further treated sequentially with diphenyl phosphite/pyridine and Et3N–H2O to yield the TEAHP intermediate 6 with excellent yield (>90%).
Scheme 1. Synthesis of Intermediate TEAHP (6) with a Native Glycerol Backbone Conformation.
Reagents and conditions: (a) (i) H2SO4, H2O, and NaNO2 at 0 °C to rt for 48 h; (ii) MeOH and SOCl2 at 0 °C to rt, 78%; (b) TrCl, NEt3, TBAI, and CH2Cl2 at rt for 2 h, 86%; (c) LiAlH4 and Et2O at 0 °C, 94%; (d) oleic acid, DCC, DMAP, and CH2Cl2 at 0 °C to rt, 96%; (e) TFA and CH2Cl2 at 0 °C for 0.5 h, 98%; (f) diphenyl phosphite and pyridine for 0.5 h then NEt3/H2O (1:1) for 1 h at rt, 90%.
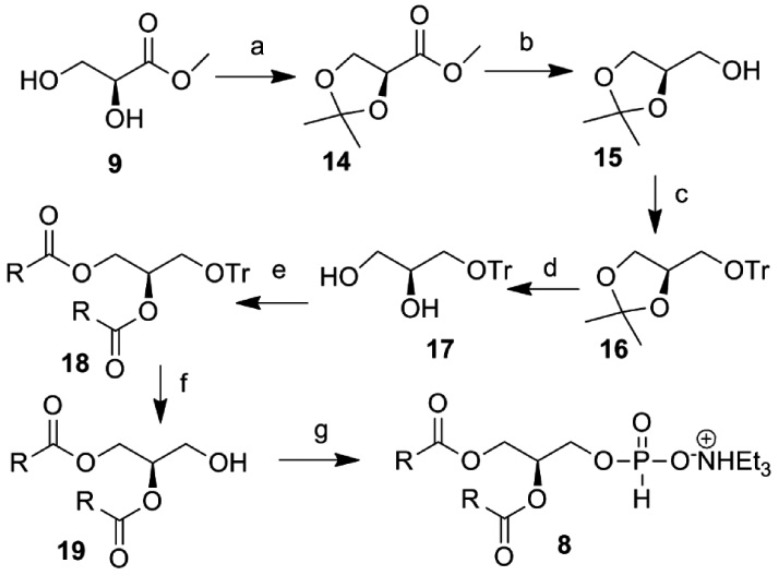
The synthesis of the other TEAHP intermediate 8, with an opposite configuration to compound 6, is described in Scheme 2. Our synthesis commenced with the easily available material 9, which was treated with 2,2-DMP in the presence of HClO4 to yield compound 14. The ester functionality of compound 14 was then reduced with LAH to obtain alcohol 15 and further reacted with TrCl/Et3N/TBAI to give compound 16. Next, the cyclic acetal in compound 16 was deprotected using SbCl3 in aqueous acetonitrile to obtain compound 17, which gets finally transformed into the required compound 8 via the intermediates 18 and 19 following similar chemistry developed earlier (Scheme 1).
Scheme 2. Synthesis of the Intermediate TEAHP (8) with the Opposite Conformation from the Native Glycerol Backbone.
Reagents and conditions: (a) 2,2-dimethoxypropane, HClO4 and acetone at rt for 2 h, 92%; (b) LiAlH4 and Et2O at 0 °C, 94%; (c) TrCl, NEt3, TBAI, and CH2Cl2 at rt for 2 h, 86%; (d) SbCl3, H2O, and CH3CN under reflux for 4 h, 53%; (e) oleic acid, DCC, DMAP, and CH2Cl2 at 0 °C to rt, 96%; (f) TFA and CH2Cl2 at 0 °C for 0.5 h, 98%; (g) diphenyl phosphite and pyridine for 0.5 h then NEt3/H2O (1:1) for 1 h at rt, 92%.
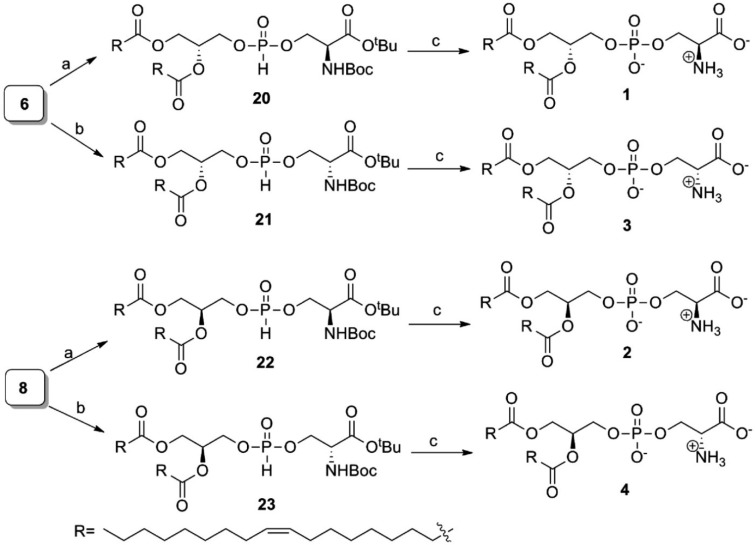
The completion of the synthesis of molecules 1–4 is depicted in Scheme 3. TEAHP 6 was subjected to react separately with acid- and amine-protected L- and D-serine in the presence of PivCl/pyridine to obtain H-phosphonates 20 and 21, respectively. Next, H-phosphonates 20 and 21 were oxidized separately to their corresponding phosphates using I2/pyridine25 and then subjected to global deprotection in the presence of TFA to obtain 1 and 3, respectively, with very good yields. These were purified easily using silica gel column chromatography. Similarly, 2 and 4 were also prepared in high yields from TEAHP 8 through intermediates 22 and 23, respectively. All the synthesized stereoisomers are >95% optically pure (details are provided in the Supporting Information).
Scheme 3. Syntheses of 1–4.
Reagents and conditions: (a) (S)-tert-butyl 2-((tert-butoxycarbonyl)amino)-3-hydroxypropanoate, PivCl and pyridine for 0.5 h at rt, 90–95%; (b) (R)-tert-butyl 2-(tert-butoxycarbonyl)amino)-3-hydroxypropanoate, PivCl and pyridine for 0.5 h at rt, 90–98%; (c) (i) I2 and 95% pyridine-H2O at rt; (ii) TFA and CH2Cl2 at 0 °C, 98%.
We adopted two different synthetic routes for the two enantiomers in Schemes 1 and 2 because we wanted to synthesize all the analogs starting from L-serine. Moreover, using L-serine as the starting material instead of D-serine is more cost-effective.
To investigate the role of these newly synthesized PSs and their stereoisomers in coagulation, we reconstituted full length TF (flTF) within liposomes containing 80% phosphatidylcholine (PC) and 20% phosphatidylserine 1 or its stereoisomers (2, 3, and 4). For flTF, we cloned human TF into the pET 28a vector with a His-tag, expressed it in E. coli (BL21 DE3 strain), and purified it with an established protocol.26 Liposomes were prepared using a known literature procedure.27 Their formation and stability were tested using dynamic light scattering measurements. With these liposomes, pro-coagulant activity was measured by a FXa generation assay as described earlier.28 In this assay, a saturated concentration of lipidated TF (1 nM) was incubated with a limited concentration of recombinant FVIIa (10 pM) to make sure that all the FVIIa remained in the TF-FVIIa complex form (detailed procedure is mentioned in the Supporting Information). The results show that maximal pro-coagulant activity was observed when native PS (1) was introduced in combination with PC. This activity is almost 6-fold over the activity shown by the only PC containing vesicles (Figure 2). The observed rate enhancement is consistent with previous observations.29 Changing the configuration of the headgroup in native PS from L to D (3) substantially decreases FXa generation in comparison with native PS (1), but the activity remained appreciably higher with respect to PC. In contrast, when the glycerol backbone configuration was inverted, keeping the headgroup L-serine intact (2), a further reduction in pro-coagulant activity was observed, and this reduction was significant with respect to both 1 and 3. The activity of 2 remained almost 2-fold compared to pure PC, but a complete attenuation of enhanced TF-FVIIa activity due to PS was observed when both the chiral centers were inverted (molecule 4). Thus, it is clearly demonstrated that the configuration of both the headgroup and the glycerol backbone are the important contributors for enhanced TF-FVIIa complex activity. These results demonstrate that the activity enhancement is more vulnerable to the configuration of the glycerol backbone than that of the headgroup.
Figure 2.
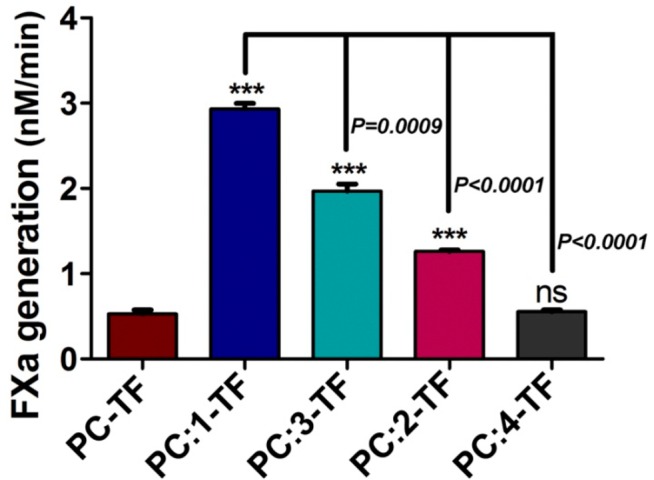
Comparison of the effect of different PSs on FX activation through the TF-FVIIa complex. The data presented here are the mean ± SEM (n = 3). Differences are statistically significant at p < 0.05 by the student’s t test. Phosphatidylserine lipids 1, 2, 3, and 4 are denoted by 1,2-dioleoyl-sn-glycero-3-phospho-L-serine; 2,3-dioleoyl-sn-glycero-1-phospho-L-serine; 1,2-dioleoyl-sn-glycero-3-phospho-D-serine; and 2,3-dioleoyl-sn-glycero-1-phospho-D-serine, respectively.
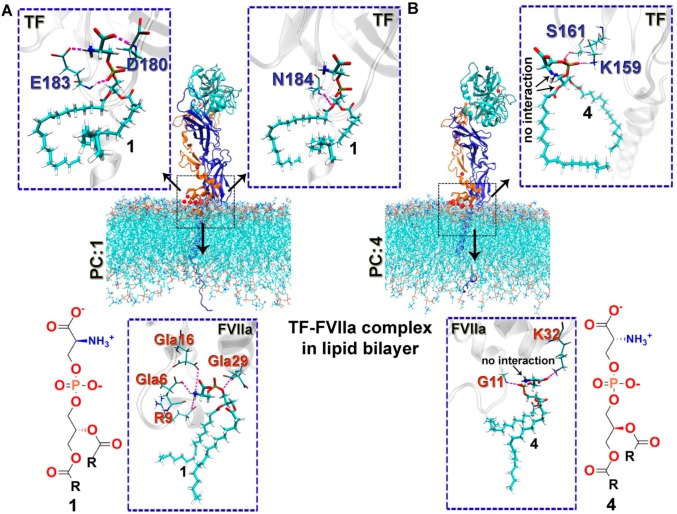
Next, to identify the probable reasons for the altered TF-FVIIa activity in the presence of phospholipids having different chirality at the atomic level, we adopted a molecular dynamics (MD) simulation approach. We modeled the flTF-FVIIa complex embedded in the PC:1 and PC:4 bilayers, as individually described in the Supporting Information. As depicted, the difference in the pro-coagulant activity of TF-FVIIa in the PC:1 and PC:4 phospholipid molecules was maximal; therefore, we have focused on these two stereoisomers (1 and 4) in the MD simulation study. We performed 40 ns simulations for these complexes after equilibration. From the trajectories, we mapped the protein–lipid interaction profiles of TF and FVIIa in the PC:1- and PC:4-containing lipid bilayer systems individually. Interestingly, we found a significant differential interaction profile for PC:1 and PC:4 with the TF-FVIIa binary complex (Table S2). PC:1 has a significantly higher degree of interactions with both TF and FVIIa in comparison with the PC:4 bilayer (Figure 3 and Table S2). More precisely, the amine, carboxylate, and phosphate groups from 1 interact with E183, D180, and E183 from TF, respectively, whereas the glycerol backbone interacts with the N184 residue. Changing the headgroup and backbone chirality completely attenuates the amine, carboxylate, and glycerol backbone interaction profile in 4; however, the interaction with the phosphate moiety remains intact. It is well-established that the FVIIa-GLA domain, comprising ten Gla residues (post-translationally modified glutamic acid, gamma-carboxyglutamic acid-rich), plays a critical role in TF-FVIIa proteolytic activity by interacting with PL.30−32 While analyzing the lipid interaction profile with FVIIa, we found that Gla6, R9, and Gla16 residues from the FVIIa-GLA domain interact with the amine moiety of 1, though these interactions are absent for 4.
Figure 3.
Protein–lipid interaction profile of the flTF-FVIIa complex with 1 and 4 containing a lipid environment. Snapshot of the TF and FVIIa H-bond-interacting residues with molecules (A) 1 and (B) 4 containing a lipid bilayer and obtained from MD simulation of the binary complex.
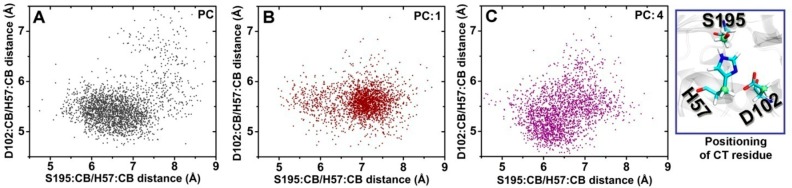
Among the several elements considered for efficient catalysis in serine proteases,33 the most important factor is the positioning of the catalytic triad (CT) residues (His57, Asp102, and Ser195) in the FVIIa protease domain. The distance between Asp102-His57 and Ser195-His57 should remain optimal for the efficient catalysis by the protease enzyme.34,35 The distances between the S195:CB-H57:CB and D102:CB-H57:CB residue pairs in FVIIa in the PC, PC:1 and PC:4 binary complexes were examined and plotted (Figure 4). Consistent with our earlier study,3 we found a highly dispersed pattern for the CT residue scattered profile when TF-FVIIa was relipidated in PC, which is considered as an almost inactive state for the binary complex. The TF-FVIIa complex activity showed a maximum when TF-FVIIa was relipidated in PC:1. We found that the distances between the complex CT residues in PC:1 were ordered and confined within the region. This result indicates that the CT of the binary complex in PC:1 remains in a relatively stabilized form, whereas the CT residues reside in a fluctuating state when the complex is formed with PC alone. We and other groups consider that stabilization of CT is the major guiding factor for showing the enhanced activity of the binary complex in PC:1.3,36 Interestingly, we found that when the binary complex is formed in PC:4, the CT residue distances remain scattered as in the binary complex embedded in PC. Overall, the data indicate that the chiral centers in PS play a vital role in guiding the activity of the TF-FVIIa complex by making proper interactions with TF and the FVIIa-GLA domain, which allosterically keep the CT residues stable in the FVIIa protease domain.
Figure 4.
Effect of the PC, PC:1, and PC:4 lipid bilayers on catalytic triad (CT) dynamics. Scattered plots for CT residues between the Ser195-His57 Cβ-atom and between the Asp102-His57 Cβ-atom obtained from a 40 ns simulation of flTF-FVIIa in the (A) PC-, (B) PC:1-, and (C) PC:4-containing phospholipid environments. The reference values for the CT residues are 6.5 and 5.5 Å for S195:Cβ/H57:Cβ and D102:Cβ/H57:Cβ, respectively. Localization of the CT residue is shown by the licorice representation (right panel). Lipids 1 and 4 denote 1,2-dioleoyl-sn-glycero-3-phospho-L-serine and 2,3-dioleoyl-sn-glycero-1-phospho-D-serine, respectively.
In summary, we have proposed a new synthetic route for PL that is advantageous in many aspects over previously mentioned procedures. Our synthetic method is efficient for synthesizing stereospecific PL with high yield. Adopting this procedure, we were able to synthesize four PS molecules with all the possible stereoisomers including the natural one. Here, we established that PS stereochemistry plays a vital role in regulating the activity of the coagulation initiation complex TF-FVIIa. Both the chiral centers have significant roles because alterations to either center abates TF-FVIIa activity. Among the two chiral centers, the center in the PS glycerol backbone has more impact on the activity, as inversion of this center reduces more activity than altering the other chiral center in the headgroup. Through our computational study, we provide a probable reason for this altered activity for TF-FVIIa in different PL environments. We suggest that alterations in the chiral centers reduce the interactions between the PS head groups and glycerol backbone with TF and FVIIa, which allosterically regulates the catalytic activity of the TF-FVIIa complex. However, there may be alternative explanations related to the ability of PS to promote factor X binding to the surface. Our findings provide new insights from the structural point of view for PL, which will certainly enrich the understanding of the structure–activity relationship (SAR) as well as protein–lipid interactions in coagulation at the molecular level.
Acknowledgments
The authors thank Dr. R. K. Goswami and Dr. A. Dey for insightful discussions and also for their technical assistance. S.M. and A.B. gratefully acknowledges the support of the SPM fellowship from the CSIR, Govt. of India. R.P. acknowledges the DST for the INSPIRE fellowship. This work was supported by project grants from the Department of Science and Technology (DST), Govt. of India (DST No-SR/SO/BB-0125/2012).
Glossary
ABBREVIATIONS
- TF
tissue factor
- FVIIa
activated factor VII
- FXa
activated factor X
- MD
molecular dynamics
- PL
phospholipids
- PS
phosphatidylserine
- PC
phosphatidylcholine
- TEAHP
triethylamine H-phosphonate salts
- PS
phosphatidylserine
- TrCl
triphenylmethyl chloride
- Boc
tert-butyl carbonate
- tBu
tertiary butyl
- DCC
N,N′-dicyclohexylcarbodiimide
- LAH
lithium aluminum hydride
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00008.
1H, 13C, and 31P NMR spectra of all new compounds, experimental procedures for their preparation, and computational methodology (PDF)
Author Contributions
P.S. designed the research. S.M. and R.P. performed the research and analyzed the data. A.B. performed the cloning and purification of TF. P.S., R.P., and S.M. wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Davie E. W.; Fujikawa K.; Kisiel W. The Coagulation Cascade: Initiation, Maintenance, and Regulation. Biochemistry 1991, 30 (43), 10363–10370. 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Butenas S.; Orfeo T.; Mann K. G. Tissue Factor in Coagulation: Which? Where? When?. Arterioscler., Thromb., Vasc. Biol. 2009, 29 (12), 1989–1996. 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R.; Sen P. Structural Modulation of Factor VIIa by Full-Length Tissue Factor (TF 1–263): Implication of Novel Interactions between EGF2 Domain and TF. J. Biomol. Struct. Dyn. 2018, 36, 621–633. 10.1080/07391102.2017.1289125. [DOI] [PubMed] [Google Scholar]
- Adams M. J.; Thom J.; Hankey G. J.; Baker R.; Gilmore G.; Staton J.; Eikelboom J. W. The Tissue Factor Pathway in Ischemic Stroke. Blood Coagulation Fibrinolysis 2006, 17 (7), 527–532. 10.1097/01.mbc.0000245294.41774.06. [DOI] [PubMed] [Google Scholar]
- Steffel J.; Lüscher T. F.; Tanner F. C. Tissue Factor in Cardiovascular Diseases: Molecular Mechanisms and Clinical Implications. Circulation 2006, 113 (5), 722–731. 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- Holy E. W.; Tanner F. C. Tissue Factor in Cardiovascular Disease Pathophysiology and Pharmacological Intervention. Adv. Pharmacol. 2010, 59 (59), 259–292. 10.1016/S1054-3589(10)59009-4. [DOI] [PubMed] [Google Scholar]
- Roy A.; Prasad R.; Bhattacharya A.; Das K.; Sen P.. Role of Tissue Factor-FVIIa Blood Coagulation Initiation Complex in Cancer. In Proteases in Physiology and Pathology; Springer Singapore: Singapore, 2017; pp 101–119. [Google Scholar]
- Butenas S.; Orfeo T.; Mann K. G. Tissue Factor in Coagulation: Which? Where? When?. Arterioscler., Thromb., Vasc. Biol. 2009, 29 (12), 1989–1996. 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. G. Biochemistry and Physiology of Blood Coagulation. Thromb. Haemostasis 1999, 82 (2), 165–174. 10.1055/s-0037-1615780. [DOI] [PubMed] [Google Scholar]
- Nemerson Y. The Phospholipid Requirement of Tissue Factor in Blood Coagulation. J. Clin. Invest. 1968, 47 (1), 72–80. 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H.; Neuenschwander P. F.; Huang Q.; McCallum C. D.; Su B.; Johnson A. E. Factor VIIa-Tissue Factor: Functional Importance of Protein-Membrane Interactions. Thromb Haemost 1997, 78 (1), 112–116. [PubMed] [Google Scholar]
- Zwaal R. F.; Comfurius P.; Bevers E. M. Lipid-Protein Interactions in Blood Coagulation. Biochim. Biophys. Acta, Rev. Biomembr. 1998, 1376 (3), 433–453. 10.1016/S0304-4157(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Rao L. V. M; Pendurthi U. R. Regulation of Tissue Factor Coagulant Activity on Cell Surfaces. J. Thromb. Haemostasis 2012, 10 (11), 2242–2253. 10.1111/jth.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y.; Pedersen A. H.; Kisiel W. Proteolytic Activation of Human Factors IX and X by Recombinant Human Factor VIIa: Effects of Calcium, Phospholipids, and Tissue Factor. Biochemistry 1990, 29 (40), 9418–9425. 10.1021/bi00492a016. [DOI] [PubMed] [Google Scholar]
- Bom V. J.; Bertina R. M. The Contributions of Ca2+, Phospholipids and Tissue-Factor Apoprotein to the Activation of Human Blood-Coagulation Factor X by Activated Factor VII. Biochem. J. 1990, 265 (2), 327–336. 10.1042/bj2650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. C. The Animal Phospholipids: Their Structure, Metabolism and Biological Significance. Biol. Rev. 1957, 32 (2), 188–229. 10.1111/j.1469-185X.1957.tb01562.x. [DOI] [Google Scholar]
- D’Arrigo P.; Servi S. Synthesis of Lysophospholipids. Molecules 2010, 15 (3), 1354–1377. 10.3390/molecules15031354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L.; Pascual J.; Borràs L.; Andreu J. A.; Fos E.; Mauleón D.; Carganico G.; Arcamonea F. Synthesis of New Ether Glycerophospholipids Structurally Related to Modulator. Tetrahedron 1991, 47 (48), 10023–10034. 10.1016/S0040-4020(01)96051-X. [DOI] [Google Scholar]
- Lindh I.; Stawinski J. A General Method for the Synthesis of Glycerophospholipids and Their Analogs via H-Phosphonate Intermediates. J. Org. Chem. 1989, 54 (6), 1338–1342. 10.1021/jo00267a020. [DOI] [Google Scholar]
- Iwashita M.; Makide K.; Nonomura T.; Misumi Y.; Otani Y.; Ishida M.; Taguchi R.; Tsujimoto M.; Aoki J.; Arai H.; Ohwada T. Synthesis and Evaluation of Lysophosphatidylserine Analogues as Inducers of Mast Cell Degranulation. Potent Activities of Lysophosphatidylthreonine and Its 2-Deoxy Derivative. J. Med. Chem. 2009, 52 (19), 5837–5863. 10.1021/jm900598m. [DOI] [PubMed] [Google Scholar]
- Lindberg J.; Ekeroth J.; Konradsson P. Efficient Synthesis of Phospholipids from Glycidyl Phosphates. J. Org. Chem. 2002, 67 (1), 194–199. 10.1021/jo010734+. [DOI] [PubMed] [Google Scholar]
- Martin S. F.; Josey J. A. A General Protocol for the Preparation of Phospholipids via Phosphite Coupling. Tetrahedron Lett. 1988, 29 (30), 3631–3634. 10.1016/S0040-4039(00)82140-1. [DOI] [Google Scholar]
- Xia J.; Hui Y.-Z. The Chemical Synthesis of a Series of Ether Phospholipids from D-Mannitol and Their Properties. Tetrahedron: Asymmetry 1997, 8 (18), 3131–3142. 10.1016/S0957-4166(97)00341-8. [DOI] [Google Scholar]
- Lok C. M.; Ward J. P.; van Dorp D. A. The Synthesis of Chiral Glycerides Starting from D- and L-Serine. Chem. Phys. Lipids 1976, 16 (2), 115–122. 10.1016/0009-3084(76)90003-7. [DOI] [PubMed] [Google Scholar]
- Ratajczak T.; Chmielewski M. K. Oxidation of H-Phosphonates with Iodine by Intramolecular Support of a 2-Pyridyl Thermolabile Protecting Group. J. Org. Chem. 2012, 77 (18), 7866–7872. 10.1021/jo300937k. [DOI] [PubMed] [Google Scholar]
- Sikarwar J.; Kaushik S.; Sinha M.; Kaur P.; Sharma S.; Singh T. P. Cloning, Expression, and Purification of Nucleoside Diphosphate Kinase from Acinetobacter Baumannii. Enzyme Res. 2013, 2013, 1–4. 10.1155/2013/597028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimms L. T.; Zampighi G.; Nozaki Y.; Tanford C.; Reynolds J. A. Phospholipid Vesicle Formation and Transmembrane Protein Incorporation Using Octyl Glucoside. Biochemistry 1981, 20 (4), 833–840. 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Sen P.; Komissarov A. A.; Florova G.; Idell S.; Pendurthi U. R.; Vijaya Mohan Rao L. Plasminogen Activator Inhibitor-1 Inhibits Factor VIIa Bound to Tissue Factor. J. Thromb. Haemostasis 2011, 9 (3), 531–539. 10.1111/j.1538-7836.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavoosi N.; Davis-Harrison R. L.; Pogorelov T. V.; Ohkubo Y. Z.; Arcario M. J.; Clay M. C.; Rienstra C. M.; Tajkhorshid E.; Morrissey J. H. Molecular Determinants of Phospholipid Synergy in Blood Clotting. J. Biol. Chem. 2011, 286 (26), 23247–23253. 10.1074/jbc.M111.251769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.; Rigby A. C.; Morelli X.; Grant M. A.; Huang G.; Furie B.; Seaton B.; Furie B. C. Structural Basis of Membrane Binding by Gla Domains of Vitamin K–dependent Proteins. Nat. Struct. Mol. Biol. 2003, 10 (9), 751–756. 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- Neuenschwander P. F.; Morrissey J. H. Roles of the Membrane-Interactive Regions of Factor VIIa and Tissue Factor. The Factor VIIa Gla Domain Is Dispensable for Binding to Tissue Factor but Important for Activation of Factor X. J. Biol. Chem. 1994, 269, 8007–8013. [PubMed] [Google Scholar]
- Stenflo J. Contributions of Gla and EGF-like Domains to the Function of Vitamin K-Dependent Coagulation Factors. Crit Rev. Eukaryot Gene Expr 1999, 9 (1), 59–88. [PubMed] [Google Scholar]
- Hedstrom L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102 (12), 4501–4524. 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- Warshel A.; Naray-Szabo G.; Sussman F.; Hwang J. K. How Do Serine Proteases Really Work?. Biochemistry 1989, 28 (9), 3629–3637. 10.1021/bi00435a001. [DOI] [PubMed] [Google Scholar]
- Frey P. A.; Whitt S. A.; Tobin J. B. A Low-Barrier Hydrogen Bond in the Catalytic Triad of Serine Proteases. Science 1994, 264 (5167), 1927–1930. 10.1126/science.7661899. [DOI] [PubMed] [Google Scholar]
- Madsen J. J.; Persson E.; Olsen O. H. Tissue Factor Activates Allosteric Networks in Factor VIIa through Structural and Dynamic Changes. J. Thromb. Haemostasis 2015, 13 (2), 262–267. 10.1111/jth.12791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.