Abstract
The inflammatory response is a major factor in stroke pathophysiology and contributes to secondary neuronal damage in both acute and chronic stages of the ischemic injury. Recent work in experimental cerebral ischemia has demonstrated the involvement of neurotransmitter signaling in the modulation of neuroinflammation. The present review discusses recent findings on the therapeutic potential and diagnostic perspectives of cholinergic, purinergic and glutamatergic receptors and transporters in experimental stroke. It provides evidence of the role of neurotransmission signaling as a promising inflammatory biomarker in stroke. Finally, recent molecular imaging studies using positron emission tomography of cholinergic receptors and glutamatergic transporters are outlined along with their potential as novel anti-inflammatory therapy to reduce the outcome of cerebral ischemia.
Keywords: cerebral ischemia, cholinergic, glutamatergic, inflammation, purinergic, stroke
Introduction
The ischemic injury is a complex system of pathological processes including excitotoxicity, oxidative stress, inhibition of protein synthesis, programed cell death and inflammation, among others, which occurs within minutes until hours and even days and months after the brain vessel occlusion.1 This pathological process is initiated as a result of the impairment of energy levels that maintain ionic gradients,2 inducing a loss of membrane potential in neurons and glia, a process known as anoxic depolarization (AD).3 AD spreads as a self-propagating wave-like depolarization across the susceptible brain parenchyma due to the release of K+, glutamate and adenosine triphosphate (ATP).4 As a result, increased entry of Ca2+ into the cell is thought to initiate a cascade of cytoplasmatic and nuclear events, including activation of proteolytic enzymes, production of reactive oxygen species (ROS), lipid peroxidation and membrane damage, the inhibition of protein synthesis, cerebral edema formation, cellular DNA fragmentation, and activation of apoptotic cell death which together lead to primary ischemic damage and the subsequent activation of inflammation.5 The inflammatory response plays a pivotal role in exerting both beneficial and detrimental effects on the acute ischemic injury and the functional recovery after stroke.6 In this review, we focus on the evidence regarding inflammatory biomarkers and cells that control the neuroinflammatory reaction and related mechanisms after stroke. In particular, the recent findings on the potential therapeutic and diagnostic perspectives of cholinergic, purinergic and glutamatergic biomarkers for the use in both therapy and diagnosis of stroke outcome will be also discussed.
Inflammation after brain ischemia
Inflammation is a complex response to necrotic cells and the subsequent generation of ROS during the secondary injury following stroke.7 Early after ischemia, the release of a repertoire of different proinflammatory prostaglandines, cytokines and chemokines results in the activation of microglia, the resident immune cell population of the brain.8 Once activated, microglia leads to the induction of adhesion molecules, integrins and selectines in both the brain vasculature and immune cells through the release of proinflammatory cytokines.9 Hence, the overexpression of all these molecules mediates leukocyte adhesion to the vascular endothelium leading to subsequent entry into the brain tissue.10 In addition, leukocyte infiltration is amplified by the disruption of the blood–brain barrier (BBB) through the release of cytotoxic agents such as metalloproteinases by microglia and other infiltrated leukocytes.7 Microglia and infiltrated macrophages can be classified into at least two subsets with distinct molecular phenotypes and functions depending on the activation pathway. The ‘classically activated’ proinflammatory microglial cells and macrophages play a central role in host defense against pathogens, but they can also damage healthy cells as neurons and glial cells. In contrast, anti-inflammatory phenotypes or ‘alternatively activated’ cells downregulate inflammation and promote tissue remodeling or repair and angiogenesis after stroke.11 Thus, a plethora of pathways and mediators might determine the final fate of these cells under a pathological situation in the brain.12 Both ex vivo and in vivo studies have suggested that many neurotransmitter receptors including cholinergic, purinergic and glutamatergic receptors or transporters are overexpressed in microglia under pathological situations, behaving as modulators of the inflammatory response12 (Table 1). Therefore, the precise management of neuroreceptors as inflammatory biomarkers may ultimately promote novel therapeutic and diagnostic strategies for treating ischemic damage.
Table 1.
Inflammatory biomarkers targeting cholinergic, purinergic and glutamatergic systems in stroke.
| Name of the target | Neurotransmitter system | Biological activity | References |
|---|---|---|---|
| α7 | Cholinergic | Neuroprotective and anti-inflammatory action | Duris et al.,13 Krafft et al.,14
Han et al.,15 Zou et al.,16 Han et al.,17 Guan et al.,18 Shimohama et al.,19 Kalappa et al.,20 Fujiki et al.,21 Colás et al.22 |
| α4β2 | Cholinergic | Inhibition of inflammation | Martin et al.23 |
| A1 | Purinergic | Modulation of interleukin release | Burnstock and Boeynaems24 |
| A2A | Purinergic | Control of immune cell infiltration after cerebral ischemia | Verkhratsky et al.,25 Puchalowicz et al.,26 Vuorimaa et al.27 |
| P2X7 | Purinergic | Neuroprotective activity and modulation of inflammation | Mayne et al.,28 Li et al.,29 Yu et al.,30 Dai and Zhou,31 Troadec et al.,32 Collo et al.,33 Skape et al.34 |
| P2X4 | Purinergic | Control of the inflammatory reaction | Suzuki et al.,35 Chu et al.,36 Monif et al.37 |
| P2Y12 | Purinergic | Modulation of the inflammatory reaction and platelet aggregation | Melani et al.,38 Vazquez-Villoldo et al.,39 Cavaliere et al.,40 Li et al.41 |
| System xc– | Glutamatergic | Control of oxidative stress underlying inflammation | Liu et al.,42 Patel et al.43 |
Cholinergic receptors
Cholinergic receptors show neuronal protective effects which have been related to the modulation of immune cells by the cholinergic anti-inflammatory pathway.44 The mechanism for inhibition of cytokine release is attributable to acetylcholine (Ach) through the inflammatory reflex of the vagus nerve.45–47 Macrophages and other immune cells express acetylcholine receptors (AChRs), which are able to transduce an intracellular signal that inhibits cytokine synthesis.48 To date, the most characterized cholinergic receptors as inflammatory modulators are those formed by the α7 subunit of the AChR. Studies in mice have shown that activation of these receptors is required for acetylcholine inhibition of macrophage tumor necrosis factor (TNF) release, becoming an essential key for the regulation of inflammation.48 In addition, pharmacological stimulation of α4β2 nicotinic receptors promotes inhibition of amyloid toxicity to cortical neurons and modulation of inflammation after cerebral ischemia in rats.23,49
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels composed of five subunits (α2–α10 and β2–β4) and the most abundant nAChRs in the mammalian brain are heteromeric receptors containing α4 and β2 subunits and homomeric α7.50 The expression of nAChRs has been described in a large variety of neural cells,51 and non-neuronal cells such as microglia, astrocytes, oligodendrocyte precursor cells and endothelial cells,52–56 where they can decrease the extent of cell death and enhance synaptic plasticity.57 Therefore, these receptors are potential therapeutic targets for several neurodegenerative disorders such as Parkinson’s disease, schizophrenia, depression and Alzheimer’s disease.58 nAChRs have been proposed as promising novel candidates for the treatment of neuroinflammation following stroke.57
As previously discussed, the cholinergic anti-inflammatory pathway is controlled by vagus nerve stimulation and particularly via the α7-nAChRs expressed on innate immune cells, evidencing the effect of the peripheral cholinergic system.48 Some studies have shown that stimulation of the vagus nerve attenuates cerebral ischemia injury and reperfusion.59,60 A brief stimulation of the vagus nerve after both permanent and cerebral ischemia displayed a reduction in the protein levels of α7 receptors followed by a reduction on inflammation, apoptosis and neuroprotection through the α7-nAChR/JAK2 anti-inflammatory pathway.59,60 Accordingly, the pharmacological activation of α7 receptors with selective agonists confirmed reduction of the brain injury and neuroprotection after intracerebral hemorrhage in rodent models through reduction of the inflammatory response.13,14 In the latter study, the use of methyllycaconitine as a potent and selective α7-nAChR antagonist reversed the antinflammatory potential of the agonist PHA-543613 after intracerebral hemorrhage in mice.14 Following cerebral ischemia, several preclinical studies have observed that both the local upregulation and the pharmacological activation of nicotinic receptors protects the brain against ischemic injury, suggesting the protective central cholinergic effect and the potential role of these receptors as promising inflammatory biomarkers.15–23
A novel approach proposed the use of positive allosteric modulators of α7 nAChRs that converts endogenous agonists of α7 nAChRs such as ACh into potent neuroprotective agents in postischemic neuronal injury in cortical and subcortical brain regions.20 In another study, the use of donepezil, an acetylcholinesterase inhibitor used for the treatment of Alzheimer’s disease, displayed upregulation of nAChRs that attenuated the cerebral brain infarction volume after cerebral ischemia in rats and mice.21,61 Moreover, the use of other cholinesterase inhibitors such as huperzine A and galantamine has shown their potential anti-inflammatory and neuroprotective effects after cerebral ischemia in rodents.62–64 The pharmacological use of nicotine after a rat model of global ischemia increased the neuronal survival of CA1 pyramidal neurons accompanied by a reduction of microglial cells, TNFα and interleukin (IL)-1β in the region of the infarction. In addition, pretreatment with α-bungarotoxin, a selective α7 nAChR antagonist, could prevent the inhibitory effects of nicotine on cultured microglial proliferation, suggesting the role of nicotine in microglial activation through the activation of nicotinic receptors.18 Therefore, these results suggest that cholinergic agonists may be of clinical relevance for the treatment of stroke.
The use of selective agonists of α7 nAChR such as PHA 568487 has also shown very promising results, reducing the ischemic brain injury and inflammatory response after experimental stroke in rodents.15,17,22 Following permanent cerebral ischemia in mice, treatment with PHA 568487 showed a reduction of functional deficits at the acute stage of cerebral ischemia.15 Furthermore, PHA treatment reduced lesion volume, decreased the number of CD68+ and M1 macrophages, and increased the number of M2 or anti-inflammatory microglia or macrophages at days 3 and 14 after permanent middle cerebral artery occlusion (MCAO) in mice.15 This study suggested that α7 receptors might decrease the inflammatory response through control over microglia or macrophage polarization after cerebral ischemia. Nevertheless, a recent study showed that the daily treatment of PHA 568487 during the first week after transient cerebral ischemia in rats displayed a nonsignificant decrease of the expression marker values for both proinflammatory and anti-inflammatory microglia markers.22 Moreover, this study observed fewer values of selectines, adhesion molecules and infiltrated T lymphocytes after treatment with PHA, suggesting a possible role of α7 nAChRs in the regulation of leukocyte infiltration into the ischemic tissue.22 Likewise, the infiltration of leukocytes after stroke can also be influenced by the disruption of the BBB; however, activation of α7 receptors showed similar levels of BBB disruption after MCAO in rats.22 In contrast, Zou and colleagues examined the effect of α7 nAChRs activation with PHA after cerebral ischemia in mice showing an improvement of the BBB integrity.16
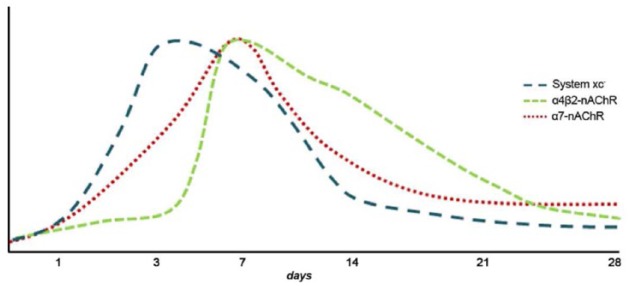
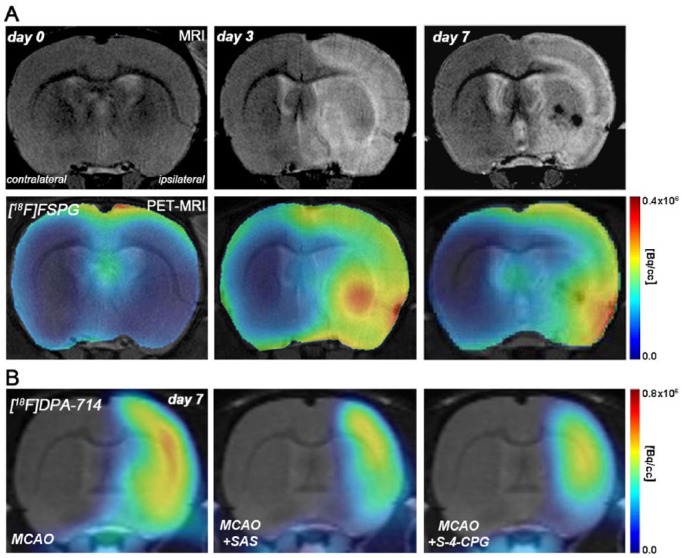
In spite of all these discrepancies, α7 nAChRs play a promising key role in the inflammatory reaction following cerebral ischemia in rodents. For this reason, in vivo imaging of these receptors with a positron emission tomography (PET) technique might be of great importance to further our understanding of the role of α7 receptors in brain diseases such as stroke. During the last few years, promising radiotracers for imaging these receptors have been synthesized;65–69 however, only a PET imaging study has been carried out to evaluate the role of α7 nAChRs in neuroinflammation. This study used the selective orthosteric α7 nAChR agonist PET radioligand, [11C]NS14492 to monitor the expression of α7 receptors during the following month after cerebral ischemia in rats. PET imaging with [11C]NS14492 described an overexpression of these receptors as a response to the ischemic injury that was identified in microglia and infiltrated macrophages at day 7 after ischemia (Figures 1 and 2). Finally, PET imaging of neuroinflammation with [18F]DPA-714, a specific radioligand for the translocator protein (18KDa) (TSPO),70 showed a reduction in the radioligand uptake as a result of treatment with PHA 568487 on day 7 after cerebral ischemia, supported by a reduction in activated microglia and macrophages22 (Figure 2). Hence, the anti-inflammatory activity exerted by the cholinergic system following stroke has been attributed to α7 nAChRs, although α4β2 nicotinic receptors have also been involved in the inflammatory reaction underlying cerebral ischemia in rats.23 The expression of α4β2 nicotinic receptors is increased after microglia or macrophage and astrocyte activation during cerebral ischemia evolution.23 In vivo PET imaging with 2[18F]-fluoro-A85380, a selective radiotracer for α4β2 nAChRs, showed a radioligand uptake increase at day 7 after ischemia followed by a progressive decrease later on (Figure 1). The α4β2 expression pattern is in accordance with the uptake increase of [11C]PK11195, a PET radiotracer for imaging inflammation.71 Furthermore, treatment with the α4β2 antagonist dihydro-β-erythroidine hydrobromide (DhβE) caused an increase in [11C]PK11195 binding after cerebral ischemia in rats, evidencing the potential role of α4β2 nAChRs in the regulation of the neuroinflammatory response after stroke23 (Figure 1).
Figure 1.
Temporal evolution of system xc−, α4β2 and α7 receptor expression after cerebral ischemia in rats. nAChR, nicotinic acetylcholine receptor.
Figure 2.
Magnetic resonance imaging (MRI) and positron emission tomography (PET) with [11C]NS14492 and [18F]DPA-714, selective radiotracers for α7 nicotinic acetylcholine receptors (nAChRs) and inflammation respectively. (A) [11C]NS14492-PET and MRI coregistered images at control (day 0), day 3 and day 7 after middle cerebral artery occlusion (MCAO). (B) MRI-T2W and PET images of [18F]DPA-714 before (day 1) and at day 7 after MCAO in controls and in rats treated with the α7 antagonist PHA.
Purinergic receptors
In the nervous system, ATP and its derivatives act as extracellular signaling molecules through a large variety of receptors known as purinergic receptors.12 The effects of purines and pyrimidines are mediated through an extended family of purinergic receptors, which are classified as metabotropic P1 adenosine receptors (A1, A2A, A2B and A3), metabotropic P2Y and ionotropic P2X purinoreceptors.72 Several investigations have reported the role of purinergic signaling following neurodegenerative diseases, epilepsy, neuropsychiatric disorders and stroke.73 Likewise, several studies have proposed ATP, adenosine and purinergic receptors as promising biomarkers for stroke therapy.74 Cerebral ischemia leads to the high increase of concentrations of both ATP and adenosine that can stimulate the purinergic receptors.75 Indeed, the effect of ATP and adenosine after cerebral ischemia may be due to the interaction of purinergic receptors expressed on neurons, glial cells and peripheral inflammatory cells such as lymphocytes and neutrophils.24–27,76
P1 receptors
Infusion of adenosine to the rat brain is protective against cerebral ischemia as it reduces the infarct volume and improves the neurological outcome77 due to mainly the response of the adenosine A1 receptors.74 Moreover, these receptors modulate the expression of IL-10 release by immune cells after neonatal hypoxic ischemic brain injury.78 Despite these findings, the role of A1 receptors on inflammatory reaction after stroke has been scarcely explored to date. However, the A2A adenosine receptors are expressed in innate (microglia, macrophages, mast cells and neutrophils) but also in adaptive immunity (lymphocytes) cells, supporting its control of the neuroinflammatory response.24 Following cerebral ischemia, activation of A2A receptors reduced processes related to the infiltration of peripheral inflammatory cells such as chemotaxis, rolling, adhesion and transmigration.79 Accordingly, low doses of the selective A2A agonist CGS21680 decreased the number of infiltrated granulocytes, microgliosis, astrogliosis and improved myelin organization in the injured lesion after cerebral ischemia in rats.80 In agreement with this effect, treatment with CGS21680 attenuated both the infiltration of neutrophils and TNFα production after intracerebral hemorrhage.28 Activation of these receptors with BAY 60-6583 inhibits tissue plasminogen activator (tPA)-induced hemorrhagic transformation, thus reducing brain swelling and lesion volume after experimental stroke.29 In addition, treatment with BAY 60-6583 induced a decrease in metalloproteinase-9 activation, which is mainly involved in the permeabilization of the BBB.29 Therefore, restoration of the BBB by A2A receptors can likely reduce the infiltration of leukocytes into the infarcted tissue after stroke. Conversely, selective inactivation of A2A receptors in bone marrow-derived cells (BMDCs) protected against brain injury by reducing the production of proinflammatory cytokines such as IL-1β, IL-6 and IL-12 in the brain.30 Hence, all these findings support the bidirectional modulation of inflammation by A2A receptors after cerebral ischemia.31 Finally, activation of adenosine A3 receptors displayed an anti-inflammatory effect and a depletion of the infiltration or migration of macrophages and microglia after cerebral ischemia in rats.81
P2 receptors
P2 receptors are classified in P2X ionotropic and P2Y metabotropic receptors. P2X receptors are a family of seven receptors (P2X1-7) permeable to cations (Na+, K+ and Ca2+) and expressed on the surface of cells. P2Y receptors are coupled to G protein and a total of nine subtypes P2Y1,2,4,6,11,12,13,14 have been cloned to date in mammalian species.72 An increase in the extracellular concentrations of ATP after brain injury results in direct damage to neurons and oligodendrocytes through Ca2+ intracellular loading, a result of P2X7 activation.82,83 Thus, P2X7 antagonists reduce neuronal damage and infarct size after transient focal ischemia32,83,84 and ameliorate oligodendroglial and axonal damage after white matter ischemia.82 Furthermore, ATP might also be crucial for the regulation of microglia after pathological situations.33 In the early stages of ischemia, low ATP levels can induce the recognition and migration of microglia to the lesion.34 In fact, activation of microglia from their resting state is marked by overexpression of P2X7 receptors in the peri-infarct region, which promotes the release of neurotrophic factors and the enhancement of neuronal survival.35,85 However, during the later stages of cerebral ischemia the ATP levels increase, promoting the proliferation of microglia and cell death.36 Under these conditions, microglia upregulate the expression of proinflammatory cytokines such as IL-1β, IL-6 and TNFα, enhancing the inflammatory response after cerebral ischemia.37 In addition, de novo expression of P2X7 receptors observed in both activated and reactive microglia suggests a differential role of these receptors in core and neighboring regions of the brain infarction.38 The P2 unselective antagonist Reactive Blue reduced ischemic brain damage by blocking activated microglia in the core of the lesion. Conversely, the same treatment increased the expression of P2X7 receptors in remote areas, promoting the restoration and defense of the tissue.38 Therefore, these temporal and spatial contrary processes should be taken into account in future therapeutic approaches targeting these receptors for treating stroke.
Microglial cells have also been characterized by the overexpression of P2X4 receptors that contribute to the control of the fate and the survival of the microglia.39 Following brain ischemia, P2X4 receptors are upregulated in microglia or infiltrated macrophages and P2 antagonists can decrease ischemic cell death, reducing P2X4 receptor expression.40 During hypoxic situations, expression of these receptors mediates activation of the amoeboid microglial cells and the release of proinflammatory cytokines induced by the increase in ATP levels.41 Therefore, P2X4 receptor expression on microglial cells is associated with the progression of neuroinflammation following cerebral ischemia, despite the underlying mechanisms not being clearly deciphered to date.86
P2Y12 receptors have also been shown to be expressed on microglial cells, suggesting their role in inflammation.87,88 The oral administration of the P2Y12 antagonist ticagrelor promotes protection against stroke damage through the inhibition of microglia activation, infiltration of blood-derived cells and the expression of proinflammatory mediators such as IL-1, monocyte chemoattractant protein 1 (MCP-1) and nitric oxide synthase (iNOS). This study also showed that ticagrelor inhibited adenosine diphosphate (ADP)-induced chemotaxis in primary cultured microglia.88 Furthermore, ticagrelor exerts antithrombotic actions and the P2Y12 receptor is also expressed on circulating platelets, mediating its aggregation and activation.89 Moreover, the use of clopidogrel, a P2Y12 receptor inhibitor with antiplatelet action, has been used in therapy for the secondary prevention of ischemic stroke.42 These findings suggest that P2Y12 antagonist can conduct anti-inflammatory, neuroprotective and antiplatelet activity after cerebral ischemia.74
Glutamate transporters
Although glutamate is the main excitatory neurotransmitter in the central nervous system (CNS), activation of glutamate receptors induces neuronal death after stroke.90 Under physiological situations glutamate is stored intracellularly, however under pathological conditions such as cerebral ischemia the levels of extracellular glutamate can dramatically be increased, promoting the excitotoxicity mechanism through the influx of calcium into the neurons.91 The clearance of extracellular glutamate levels through a family of transporter proteins called excitatory amino acid transporters (EAATs) has been mainly attributed to the glutamate-scavenging action of astrocytes.92 In the normal CNS, astrocytes express EAAT1, EAAT2 and glutamine synthetase (GS) that first transport glutamate into intracellular milieu, and second convert the glutamate into glutamine.93
Cystine glutamate antiporter
Another transporter involved in glutamate homeostasis is the cystine glutamate antiporter, also known as system xc−.94 It is a heterodimer composed of a heavy chain subunit (4F2hc) and a light chain subunit (xCT).95 System xc− mediates the cellular import of cysteine and the release of glutamate to the extracellular space in exchange. Cystine is intracellularly converted to cysteine, the rate-limiting substrate for gluthatione aproduction and oxidative protection. Thus, the expression and function of system xc− are modulated in different pathologies secondary to the induction of oxidative stress.96–98
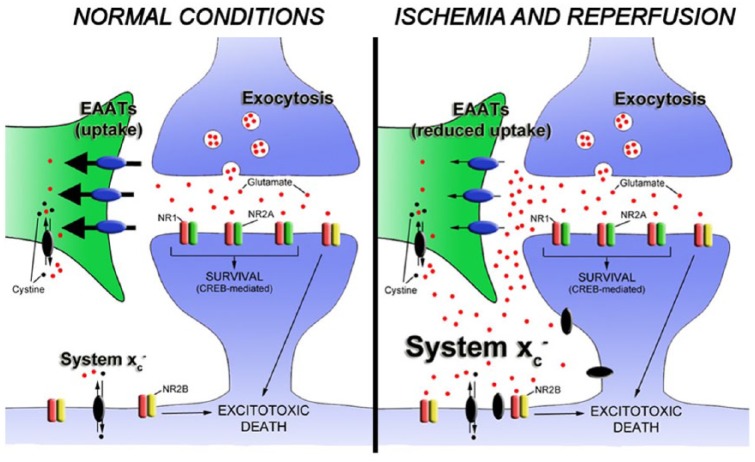
After cerebral ischemia, overexpression of the cystine glutamate antiporter contributes to the increase in the extracellular glutamate concentration that promotes ischemic neuronal death through activation of extrasynaptic N-methyl-D-aspartate (NMDA) receptors95 (Figure 3). This study shows that pharmacological inhibition of system xc− displays reduced neuronal death after in vitro ischemia.95 Furthermore, in vivo PET imaging of system xc− activity with the radiotracer [18F]FSPG showed an increase in the PET signal uptake from 5 min to 5 h after ischemia, showing the increase in the function of these transporters during the subacute stage of stroke.95 [18F]FSPG is a fluorine-18-labeled L-glutamate derivative taken by the system xc− due to the lack of discrimination between its natural substrate cystine and glutamate for the inward transport.43,99 Recently, a PET study with this radiotracer has demonstrated the overexpression of system xc− on microglial cells following experimental autoimmune encephalomyelitis (EAE) in rats.100 Additionally, the depletion of microglia with clodronate showed a reduction in the [18F]FSPG PET signal in the spinal cord, confirming the link between microglial activation and cysteine or glutamate antiporter activity in EAE rats.100 Another clinical study used PET with [18F]FSPG to detect inflammatory lesions provoked by the activation of macrophages in diseases such as sarcoidosis.101 Several studies support these findings showing that the expression of system xc− is particularly abundant in microglia, macrophages and other immune cells.102,103 In fact, the release of glutamate from both resting and activated microglia by system xc− can induce a regional increase in glutamate levels that leads to excitotoxic oligodendrocyte death contributing to the pathogenesis of white matter disorders.104
Figure 3.
The release of glutamate during brain ischemia triggers neuronal death by overactivation of NMDA receptors. Different mechanisms contribute to glutamate homeostasis alterations. Astrocytic glutamate transporters play a key role in maintaining synaptic glutamate levels. However, extrasynaptic glutamate is mainly regulated by the cystine glutamate antiporter, also known as system xc−.94 Because N-methyl-D-aspartate (NMDA) receptors involved in neuronal death are typically extrasynaptic, it has been proposed that glutamate release by the cysteine or glutamate antiporter activates extrasynaptic N-methyl-D-aspartate receptors (NMDARs).95 Cells involved in glutamate release by cystine glutamate antiporter during ischemic insults remain to be determined. EAAT, excitatory amino acid transporter.
Upregulation of system xc− occurs during the following week after ischemia reperfusion104 (Figures 1 and 4). This stands in accordance with the expression of TSPO, a marker or microglial activation, using the radiotracer [18F]DPA-714.105 Indeed, the expression of the cysteine/glutamate antiporter takes place in activated microglia and infiltrated macrophages during the first week, and marginally in astrocytes at 1 month after ischemia onset. Additionally, the inhibition of system xc− with sulfasalazine and S-4-CPG resulted in a decrease in inflammatory response through the inactivation of both microglia and infiltrated macrophages, a decrease in proinflammatory markers (CCL2, TNF and iNOS) and an increase in the anti-inflammatory marker arginase after experimental stroke in rats.105 These results showed that the blocking of the source of glutamate release on microglia and macrophages during cerebral ischemia evolution may be a relevant therapeutic intervention to halt progression of the brain damage after ischemia. Furthermore, these results fully support that system xc− might play a key role in the modulation of inflammatory response following stroke.
Figure 4.
Magnetic resonance imaging (MRI) (T2 weighting (T2W)) and positron emission tomography (PET) with [18F]FSPG and [18F]DPA-714, markers of cystine glutamate antiporter activity and inflammation respectively. (A) [18F]FSPG-PET and MRI coregistered images at control (day 0), day 3 and day 7 after middle cerebral artery occlusion (MCAO). (B) PET images of [18F]DPA-714 at day 7 after cerebral ischemia in vehicle (MCAO), SAS (MCAO+SAS) and S-4-CPG (MCAO+ S-4-CPG) treated rats. SAS and S-4-CPG are inhibitors of cystine glutamate antiporter.
Conclusion
Inflammation plays an important role at different stages of the cerebral postischemic injury. Consequently, the use of anti-inflammatory strategies in stroke therapy might offer a wider therapeutic window than current treatments. The inflammatory reaction following stroke involves a large variety of signaling pathways and mediators that can determine stroke outcome. During the last decade, both in vivo and ex vivo studies have described the role of neurotransmitter receptors and transporters, including cholinergic, purinergic and glutamatergic on the modulation of the inflammatory reaction after cerebral ischemia. It became evident that the expression of these receptors and transporters in neurons, glial cells and infiltrated immune cells in the ischemic brain promotes the release of a battery of signal molecules that enhance the inflammatory response after cerebral ischemia. Moreover, the activation or inhibition of these neuroreceptors and transporters has shown promising therapeutic responses in animal models of stroke. Recently, molecular imaging studies using PET of these neuroreceptors have described their link with the inflammatory reaction after ischemia and their potential role as novel imaging biomarkers of neuroinflammation. Therefore, the data reviewed here suggest that cholinergic, purinergic and glutamatergic agents may be useful both as biomarkers for neuroinflammation and as a treatment to attenuate the deleterious consequences of the inflammatory response after stroke. In our opinion, among the different candidates proposed here, the activation of nicotinic receptors might become a promising strategy for treating stroke in the near future. Despite this, future clinical studies are needed to support all these findings and their true potential to ameliorate the neurological care of stroke.
Footnotes
Funding: We acknowledge financial support by MINECO SAF2014-54070-JIN (A.M.) and SAF2016-75292-R (C.M.).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Abraham Martín, Experimental Molecular Imaging, Molecular Imaging Unit, CIC biomaGUNE, Pº Miramon 182, San Sebastian, Spain.
María Domercq, Department of Neurosciences, University of the Basque Country, Barrio Sarriena s/n, Leioa, Spain Achucarro Basque Center for Neuroscience-UPV/EHU, Zamudio, Spain Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Leioa, Spain.
Carlos Matute, Department of Neurosciences, University of the Basque Country, Barrio Sarriena s/n, Leioa, Spain Achucarro Basque Center for Neuroscience-UPV/EHU, Zamudio, Spain Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Leioa, Spain.
References
- 1. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22: 391–397. [DOI] [PubMed] [Google Scholar]
- 2. Martin RL, Lloyd HG, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci 1994; 17: 251–257. [DOI] [PubMed] [Google Scholar]
- 3. Katsura K, Kristian T, Siesjo BK. Energy metabolism, ion homeostasis, and cell damage in the brain. Biochem Soc Trans 1994; 22: 991–996. [DOI] [PubMed] [Google Scholar]
- 4. Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev 2001; 81: 1065–1096. [DOI] [PubMed] [Google Scholar]
- 5. Zhao SC, Ma LS, Chu ZH, et al. Regulation of microglial activation in stroke. Acta Pharmacol Sin 2017; 38: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonaventura A, Liberale L, Vecchie A, et al. Update on inflammatory biomarkers and treatments in ischemic stroke. Int J Mol Sci 2016; 17: pii: E1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawabori M, Yenari MA. Inflammatory responses in brain ischemia. Curr Med Chem 2015; 22: 1258–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19: 312–318. [DOI] [PubMed] [Google Scholar]
- 9. Becker KJ. Inflammation and acute stroke. Curr Opin Neurol 1998; 11: 45–49. [DOI] [PubMed] [Google Scholar]
- 10. Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl 1996; 66: 27–31. [DOI] [PubMed] [Google Scholar]
- 11. Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 2015; 11: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- 13. Duris K, Manaenko A, Suzuki H, et al. Alpha7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke 2011; 42: 3530–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krafft PR, McBride D, Rolland WB, et al. Alpha7 nicotinic acetylcholine receptor stimulation attenuates neuroinflammation through JAK2-STAT3 activation in murine models of intracerebral hemorrhage. Biomed Res Int 2017; 2017: 8134653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han Z, Shen F, He Y, et al. Activation of alpha-7 nicotinic acetylcholine receptor reduces ischemic stroke injury through reduction of pro-inflammatory macrophages and oxidative stress. PLoS One. 2014; 9: e105711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou D, Luo M, Han Z, et al. Activation of alpha-7 nicotinic acetylcholine receptor reduces brain edema in mice with ischemic stroke and bone fracture. Mol Neurobiol 2017; 54: 8278–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han Z, Li L, Wang L, et al. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J Neurochem 2014; 131: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan YZ, Jin XD, Guan LX, et al. Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol Neurobiol 2015; 51: 1480–1488. [DOI] [PubMed] [Google Scholar]
- 19. Shimohama S, Greenwald DL, Shafron DH, et al. Nicotinic alpha 7 receptors protect against glutamate neurotoxicity and neuronal ischemic damage. Brain Res 1998; 779: 359–363. [DOI] [PubMed] [Google Scholar]
- 20. Kalappa BI, Sun F, Johnson SR, et al. A positive allosteric modulator of alpha7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br J Pharmacol 2013; 169: 1862–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujiki M, Kobayashi H, Uchida S, et al. Neuroprotective effect of donepezil, a nicotinic acetylcholine-receptor activator, on cerebral infarction in rats. Brain Res 2005; 10: 1–2. [DOI] [PubMed] [Google Scholar]
- 22. Colás LDM, Ramos P, Palma A, et al. In vivo imaging of α7 nicotinic receptors as a novel method to monitor neuroinflammation after cerebral ischemia. Glia. Epub ahead of print 12 March 2018. DOI: 10.1002/glia.23326. [DOI] [PubMed] [Google Scholar]
- 23. Martin A, Szczupak B, Gomez-Vallejo V, et al. In vivo PET imaging of the alpha4beta2 nicotinic acetylcholine receptor as a marker for brain inflammation after cerebral ischemia. J Neurosci 2015; 35: 5998–6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal 2014; 10: 529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol 2009; 39: 190–208. [DOI] [PubMed] [Google Scholar]
- 26. Puchalowicz K, Baranowska-Bosiacka I, Dziedziejko V, et al. Purinergic signaling and the functioning of the nervous system cells. Cell Mol Biol Lett 2015; 20: 867–918. [DOI] [PubMed] [Google Scholar]
- 27. Vuorimaa A, Rissanen E, Airas L. In vivo PET imaging of adenosine 2A receptors in neuroinflammatory and neurodegenerative disease. Contrast Media Mol Imaging 2017; 2017: 6975841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayne M, Fotheringham J, Yan HJ, et al. Adenosine A2A receptor activation reduces proinflammatory events and decreases cell death following intracerebral hemorrhage. Ann Neurol 2001; 49: 727–735. [DOI] [PubMed] [Google Scholar]
- 29. Li Q, Han X, Lan X, et al. Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol Dis 2017; 108: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu L, Huang Z, Mariani J, et al. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med 2004; 10: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 31. Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Rev Neurosci 2011; 22: 231–239. [DOI] [PubMed] [Google Scholar]
- 32. Troadec JD, Thirion S, Petturiti D, et al. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes). Pflugers Arch 1999; 437: 745–753. [DOI] [PubMed] [Google Scholar]
- 33. Collo G, Neidhart S, Kawashima E, et al. Tissue distribution of the P2X7 receptor. Neuropharmacology 1997; 36: 1277–1283. [DOI] [PubMed] [Google Scholar]
- 34. Skaper SD, Facci L, Culbert AA, et al. P2X(7) receptors on microglial cells mediate injury to cortical neurons in vitro. Glia 2006; 54: 234–242. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki T, Hide I, Ido K, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci 2004; 24: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chu K, Yin B, Wang J, et al. Inhibition of P2X7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J Neuroinflammation 2012; 9: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol 2010; 42: 1753–1756. [DOI] [PubMed] [Google Scholar]
- 38. Melani A, Amadio S, Gianfriddo M, et al. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 2006; 26: 974–982. [DOI] [PubMed] [Google Scholar]
- 39. Vazquez-Villoldo N, Domercq M, Martin A, et al. P2X4 receptors control the fate and survival of activated microglia. Glia 2014; 62: 171–184. [DOI] [PubMed] [Google Scholar]
- 40. Cavaliere F, Florenzano F, Amadio S, et al. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience 2003; 120: 85–98. [DOI] [PubMed] [Google Scholar]
- 41. Li F, Wang L, Li JW, et al. Hypoxia induced amoeboid microglial cell activation in postnatal rat brain is mediated by ATP receptor P2X4. BMC Neurosci 2011; 12: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu F, Tantry US, Gurbel PA. P2Y12 receptor inhibitors for secondary prevention of ischemic stroke. Expert Opin Pharmacother 2015; 16: 1149–1165. [DOI] [PubMed] [Google Scholar]
- 43. Patel SA, Warren BA, Rhoderick JF, et al. Differentiation of substrate and non-substrate inhibitors of transport system xc(-): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology 2004; 46: 273–284. [DOI] [PubMed] [Google Scholar]
- 44. Pavlov VA, Wang H, Czura CJ, et al. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 2003; 9: 125–134. [PMC free article] [PubMed] [Google Scholar]
- 45. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007; 117: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tracey KJ. The inflammatory reflex. Nature 2002; 420: 853–859. [DOI] [PubMed] [Google Scholar]
- 47. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462. [DOI] [PubMed] [Google Scholar]
- 48. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421: 384–388. [DOI] [PubMed] [Google Scholar]
- 49. Ueno K, Togashi H, Matsumoto M, et al. Alpha4beta2 nicotinic acetylcholine receptor activation ameliorates impairment of spontaneous alternation behavior in stroke-prone spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder. J Pharmacol Exp Ther 2002; 302: 95–100. [DOI] [PubMed] [Google Scholar]
- 50. Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 2003; 147: 1–46. [DOI] [PubMed] [Google Scholar]
- 51. Murakami K, Ishikawa Y, Sato F. Localization of alpha7 nicotinic acetylcholine receptor immunoreactivity on GABAergic interneurons in layers I-III of the rat retrosplenial granular cortex. Neuroscience 2013; 252: 443–459. [DOI] [PubMed] [Google Scholar]
- 52. Rogers SW, Gregori NZ, Carlson N, et al. Neuronal nicotinic acetylcholine receptor expression by O2A/oligodendrocyte progenitor cells. Glia 2001; 33: 306–313. [PubMed] [Google Scholar]
- 53. Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A 2001; 98: 4148–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Simone R, Ajmone-Cat MA, Carnevale D, et al. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation 2005; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hawkins BT, Egleton RD, Davis TP. Modulation of cerebral microvascular permeability by endothelial nicotinic acetylcholine receptors. Am J Physiol Heart Circ Physiol 2005; 289: H212– H219. [DOI] [PubMed] [Google Scholar]
- 56. Sharma G, Vijayaraghavan S. Nicotinic receptor signaling in nonexcitable cells. J Neurobiol 2002; 53: 524–534. [DOI] [PubMed] [Google Scholar]
- 57. Neumann S, Shields NJ, Balle T, et al. Innate immunity and inflammation post-stroke: an alpha7-nicotinic agonist perspective. Int J Mol Sci 2015; 16: 29029–29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 2007; 151: 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang Y, Li L, Liu B, et al. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One 2014; 9: e102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lu XX, Hong ZQ, Tan Z, et al. Nicotinic acetylcholine receptor alpha7 subunit mediates vagus nerve stimulation-induced neuroprotection in acute permanent cerebral ischemia by a7nAchR/JAK2 pathway. Med Sci Monit 2017; 23: 6072–6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang T, Lv P, Jin W, et al. Protective effect of donepezil hydrochloride on cerebral ischemia/reperfusion injury in mice. Mol Med Rep 2014; 9: 509–514. [DOI] [PubMed] [Google Scholar]
- 62. Wang ZF, Wang J, Zhang HY, et al. Huperzine A exhibits anti-inflammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J Neurochem 2008; 106: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 63. Odorcyk FK, Nicola F, Duran-Carabali LE, et al. Galantamine administration reduces reactive astrogliosis and upregulates the anti-oxidant enzyme catalase in rats submitted to neonatal hypoxia ischemia. Int J Dev Neurosci 2017; 62: 15–24. [DOI] [PubMed] [Google Scholar]
- 64. Odorcyk FK, Sanches EF, Nicola FC, et al. Administration of huperzia quadrifariata extract, a cholinesterase inhibitory alkaloid mixture, has neuroprotective effects in a rat model of cerebral hypoxia-ischemia. Neurochem Res 2017; 42: 552–562. [DOI] [PubMed] [Google Scholar]
- 65. Hillmer AT, Li S, Zheng MQ, et al. PET imaging of alpha7 nicotinic acetylcholine receptors: a comparative study of [(18)F]ASEM and [(18)F]DBT-10 in nonhuman primates, and further evaluation of [(18)F]ASEM in humans. Eur J Nucl Med Mol Imaging 2017; 44: 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horti AG. Development of [(18)F]ASEM, a specific radiotracer for quantification of the alpha7-nAChR with positron-emission tomography. Biochem Pharmacol 2015; 97: 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hillmer AT, Zheng MQ, Li S, et al. PET imaging evaluation of [(18)F]DBT-10, a novel radioligand specific to alpha7 nicotinic acetylcholine receptors, in nonhuman primates. Eur J Nucl Med Mol Imaging 2016; 43: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rotering S, Deuther-Conrad W, Cumming P, et al. Imaging of alpha7 nicotinic acetylcholine receptors in brain and cerebral vasculature of juvenile pigs with [(18)F]NS14490. EJNMMI Res 2014; 4: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ettrup A, Mikkelsen JD, Lehel S, et al. 11C-NS14492 as a novel PET radioligand for imaging cerebral alpha7 nicotinic acetylcholine receptors: in vivo evaluation and drug occupancy measurements. J Nucl Med 2011; 52: 1449–1456. [DOI] [PubMed] [Google Scholar]
- 70. Martin A, Boisgard R, Theze B, et al. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab 2010; 30: 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rojas S, Martin A, Arranz MJ, et al. Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J Cereb Blood Flow Metab 2007; 27: 1975–1986. [DOI] [PubMed] [Google Scholar]
- 72. Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci 2007; 64: 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol 2017; 8: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pedata F, Dettori I, Coppi E, et al. Purinergic signalling in brain ischemia. Neuropharmacology 2016; 104: 105–130. [DOI] [PubMed] [Google Scholar]
- 75. Melani A, Corti F, Stephan H, et al. Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Exp Neurol 2012; 233: 193–204. [DOI] [PubMed] [Google Scholar]
- 76. Brodie C, Blumberg PM, Jacobson KA. Activation of the A2A adenosine receptor inhibits nitric oxide production in glial cells. FEBS Lett 1998; 429: 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kitagawa H, Mori A, Shimada J, et al. Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol Res 2002; 24: 317–323. [DOI] [PubMed] [Google Scholar]
- 78. Winerdal M, Winerdal ME, Wang YQ, et al. Adenosine A1 receptors contribute to immune regulation after neonatal hypoxic ischemic brain injury. Purinergic Signal 2016; 12: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hasko G, Linden J, Cronstein B, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 2008; 7: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Melani A, Corti F, Cellai L, et al. Low doses of the selective adenosine A2A receptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res 2014; 10: 59–72. [DOI] [PubMed] [Google Scholar]
- 81. Choi IY, Lee JC, Ju C, et al. A3 adenosine receptor agonist reduces brain ischemic injury and inhibits inflammatory cell migration in rats. Am J Pathol 2011; 179: 2042–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Domercq M, Perez-Samartin A, Aparicio D, et al. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 2010; 58: 730–740. [DOI] [PubMed] [Google Scholar]
- 83. Arbeloa J, Perez-Samartin A, Gottlieb M, et al. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis 2012; 45: 954–961. [DOI] [PubMed] [Google Scholar]
- 84. Amadio S, D’Ambrosi N, Cavaliere F, et al. P2 receptor modulation and cytotoxic function in cultured CNS neurons. Neuropharmacology 2002; 42: 489–501. [DOI] [PubMed] [Google Scholar]
- 85. Kharlamov A, Jones SC, Kim DK. Suramin reduces infarct volume in a model of focal brain ischemia in rats. Exp Brain Res 2002; 147: 353–359. [DOI] [PubMed] [Google Scholar]
- 86. Cheng RD, Ren JJ, Zhang YY, et al. P2X4 receptors expressed on microglial cells in post-ischemic inflammation of brain ischemic injury. Neurochem Int 2014; 67: 9–13. [DOI] [PubMed] [Google Scholar]
- 87. Amadio S, Parisi C, Montilli C, et al. P2Y(12) receptor on the verge of a neuroinflammatory breakdown. Mediators Inflamm 2014; 2014: 975849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gelosa P, Lecca D, Fumagalli M, et al. Microglia is a key player in the reduction of stroke damage promoted by the new antithrombotic agent ticagrelor. J Cereb Blood Flow Metab 2014; 34: 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kashiwazaki D, Kuwayama N, Akioka N, et al. The roles and issues of P2Y12 percent inhibition assessed by VerifyNow assay for patients undergoing neurointervention: a prospective study. J Stroke Cerebrovasc Dis 2014; 23: 1830–1836. [DOI] [PubMed] [Google Scholar]
- 90. Chamorro A, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 2016; 15: 869–881. [DOI] [PubMed] [Google Scholar]
- 91. Krzyzanowska W, Pomierny B, Filip M, et al. Glutamate transporters in brain ischemia: to modulate or not? Acta Pharmacol Sin 2014; 35: 444–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron 1994; 13: 713–725. [DOI] [PubMed] [Google Scholar]
- 93. Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977; 195: 1356–1358. [DOI] [PubMed] [Google Scholar]
- 94. Baker DA, Xi ZX, Shen H, et al. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 2002; 22: 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Soria FN, Perez-Samartin A, Martin A, et al. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J Clin Invest 2014; 124: 3645–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem 1980; 255: 2372–2376. [PubMed] [Google Scholar]
- 97. Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol 2008; 215: 593–602. [DOI] [PubMed] [Google Scholar]
- 98. Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (-): cystine supplier and beyond. Amino Acids 2012; 42: 231–246. [DOI] [PubMed] [Google Scholar]
- 99. Koglin N, Mueller A, Berndt M, et al. Specific PET imaging of xC- transporter activity using a (1)(8)F-labeled glutamate derivative reveals a dominant pathway in tumor metabolism. Clin Cancer Res 2011; 17: 6000–6011. [DOI] [PubMed] [Google Scholar]
- 100. Martin A, Vazquez-Villoldo N, Gomez-Vallejo V, et al. In vivo imaging of system xc- as a novel approach to monitor multiple sclerosis. Eur J Nucl Med Mol Imaging 2016; 43: 1124–1138. [DOI] [PubMed] [Google Scholar]
- 101. Chae SY, Choi CM, Shim TS, et al. Exploratory clinical investigation of (4S)-4-(3–18F-fluoropropyl)-L-glutamate PET of inflammatory and infectious lesions. J Nucl Med 2016; 57: 67–69. [DOI] [PubMed] [Google Scholar]
- 102. Sato H, Kuriyama-Matsumura K, Hashimoto T, et al. Effect of oxygen on induction of the cystine transporter by bacterial lipopolysaccharide in mouse peritoneal macrophages. J Biol Chem 2001; 276: 10407–10412. [DOI] [PubMed] [Google Scholar]
- 103. Qin S, Colin C, Hinners I, et al. System Xc- and apolipoprotein E expressed by microglia have opposite effects on the neurotoxicity of amyloid-beta peptide 1–40. J Neurosci 2006; 26: 3345–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Domercq M, Sanchez-Gomez MV, Sherwin C, et al. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol 2007; 178: 6549–6556. [DOI] [PubMed] [Google Scholar]
- 105. Domercq M, Szczupak B, Gejo J, et al. PET imaging with [(18)F]FSPG evidences the role of system xc(-) on brain inflammation following cerebral ischemia in rats. Theranostics 2016; 6: 1753–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]