Abstract
Light adaptation of photoreceptor cells is mediated by Ca2+-dependent mechanisms. In darkness, Ca2+ influx through cGMP-gated channels into the outer segment of photoreceptors is balanced by Ca2+ extrusion via Na+/Ca2+, K+ exchangers (NCKXs). Light activates a G protein signaling cascade, which closes cGMP-gated channels and decreases Ca2+ levels in photoreceptor outer segment because of continuing Ca2+ extrusion by NCKXs. Guanylate cyclase–activating proteins (GCAPs) then up-regulate cGMP synthesis by activating retinal membrane guanylate cyclases (RetGCs) in low Ca2+. This activation of RetGC accelerates photoresponse recovery and critically contributes to light adaptation of the nighttime rod and daytime cone photoreceptors. In mouse rod photoreceptors, GCAP1 and GCAP2 both contribute to the Ca2+-feedback mechanism. In contrast, only GCAP1 appears to modulate RetGC activity in mouse cones because evidence of GCAP2 expression in cones is lacking. Surprisingly, we found that GCAP2 is expressed in cones and can regulate light sensitivity and response kinetics as well as light adaptation of GCAP1-deficient mouse cones. Furthermore, we show that GCAP2 promotes cGMP synthesis and cGMP-gated channel opening in mouse cones exposed to low Ca2+. Our biochemical model and experiments indicate that GCAP2 significantly contributes to the activation of RetGC1 at low Ca2+ when GCAP1 is not present. Of note, in WT mouse cones, GCAP1 dominates the regulation of cGMP synthesis. We conclude that, under normal physiological conditions, GCAP1 dominates the regulation of cGMP synthesis in mouse cones, but if its function becomes compromised, GCAP2 contributes to the regulation of phototransduction and light adaptation of cones.
Keywords: photoreceptor, calcium, guanylate cyclase (guanylyl cyclase), retina, vision, calcium, cone photoreceptor, guanylate cyclase activating proteins, light adaptation, phototransduction, light activation, photoresponse, color vision
Introduction
Guanylate cyclase–activating proteins (GCAPs)3 are EF-hand proteins that regulate cGMP synthesis by retinal membrane guanylate (guanylyl) cyclases (RetGCs) in a Ca2+-dependent manner (1–6). In low Ca2+, when the active EF-hand sites of the GCAP protein are not occupied by Ca2+, GCAPs activate RetGCs and promote the synthesis of cGMP. High Ca2+ blocks the activation of GC by GCAPs, and only a low basal level of cGMP synthesis is maintained in the cells. The presence of several GCAP isoforms in photoreceptor cells has been well-established (7–10). The diversity of GCAPs is particularly apparent in fish photoreceptors where at least seven different GCAP genes are expressed (7). Human photoreceptors express GCAP1–3, whereas only GCAP1 and GCAP2 are present in mouse photoreceptor cells (8, 10). Several mutations in the GUCA1A gene encoding for GCAP1 cause severe hereditary blinding diseases, including Leber congenital amaurosis, macular dystrophy, and cone-rod dystrophies (11–18). Although significant advances have been made in understanding the etiology of these diseases, it is still not clear why mutations in GUCA1A preferentially lead to cone, rather than rod, dystrophies and loss of daytime vision.
GCAP-mediated regulation of cGMP synthesis in the photoreceptors has been shown to be the single most important Ca2+-mediated pathway of light adaptation (19, 20). In darkness, steady-state cGMP concentration in photoreceptor outer segments is maintained by a low basal synthesis of cGMP by RetGCs and its hydrolysis by phosphodiesterase 6 (PDE6). Light activates a G protein signaling cascade, leading to the increased hydrolysis rate of cGMP by PDE6 and a decline of the rod and cone outer segment cGMP concentration. Consequently, cGMP-gated channels in the outer segment plasma membrane close, leading to a decreased inflow of Na+ and Ca2+ into the outer segments (for a review, see Ref. 21). As Ca2+ ions are continuously extruded from outer segments by Na+/Ca2+, K+ exchangers (22–25), the Ca2+ level drops, and Mg2+ replaces Ca2+ in the Ca2+/Mg2+-binding sites of GCAPs (26). The Mg2+-GCAPs activate RetGCs to accelerate cGMP synthesis, promoting the recovery of the photoreceptor cell to its dark-adapted state after a transient light stimulus or preventing a closure of all cGMP-gated channels during continuous illumination.
It is believed that mouse rods express both GCAP1 and GCAP2, whereas mouse cones express only GCAP1 in their outer segments (9, 10). In rods, GCAP1 and GCAP2 regulate the cGMP synthesis in a relay fashion. Early in the photoresponse or at dim background light, when the Ca2+ level is only slightly lower than in darkness, GCAP1-mediated feedback dominates. Later in the photoresponse or at brighter background light, when Ca2+ drops to lower levels, GCAP2-mediated feedback is also engaged (19, 27). This model is consistent with the higher Ca2+ affinity of GCAP2 compared with GCAP1 (18, 28, 29). Although previous studies have suggested that GCAP2 may not be substantially present in normal or Nrl−/− mouse cone outer segments (10, 30, 31), direct genetic and functional approaches have not been used to test whether GCAP2 has any physiological role in mouse cones. Here, we aimed to determine the contribution of GCAP1 and GCAP2 in mouse cone phototransduction and light adaptation by using a comprehensive electrophysiology, genetic, biochemistry, and single-cell immunohistochemistry study.
Results
GCAP1 and GCAP2 are expressed in mouse cones
GCAP1 is expressed in outer segments of vertebrate rods and cones from zebrafish to human (8, 9). However, the expression pattern of GCAP2 varies among different species (9). Previous studies have shown contradicting results regarding its presence in mouse photoreceptors (9, 10, 32). Thus, we sought to determine the expression pattern of GCAP2 in mouse cones by single-cell immunohistochemistry in retinas from wildtype (WT) control, Gcap1−/−, Gcap2−/−, and GCAP1/2 double knockout (Gcaps−/−) mice. The top two panels of Fig. 1 demonstrate expression of GCAP2 in WT control and Gcap1−/− cones based on the colocalization of mouse cone arrestin (mCAR; green) and GCAP2 (red) antibodies. Additional GCAP2 signal around the cones is from rod photoreceptors that sometimes surrounded the cones even after the mechanical cell isolation (see “Experimental procedures”). We observed overlap between the cone arrestin and GCAP2 signals in both WT control and Gcap1−/− cones, suggesting that GCAP2 is expressed in mouse cones. As expected, the GCAP2 signal was not observed in Gcap2−/− or Gcaps−/− cones (Fig. 1, bottom two panels), thereby confirming the specificity of the GCAP2 antibody (33). Together, these results clearly demonstrate that GCAP2 is expressed in mouse cones.
Figure 1.
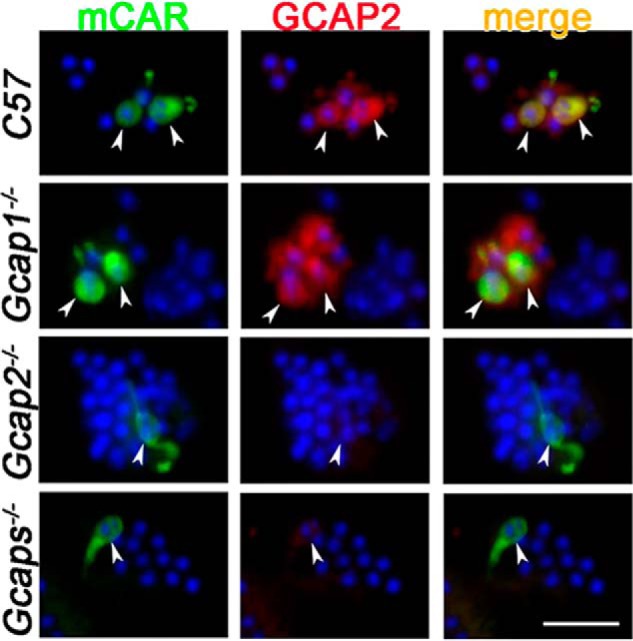
GCAP2 is expressed in the mouse cones. Dissociated retinal cells from mice of the indicated genotypes were incubated with the mCAR antibody (green) to label cones followed by incubation with GCAP2 antibody (red). Arrowheads point to the positions of the cones in each field. Nuclei were stained with DAPI (blue). Images shown are representative of 38 cones from 14 fields (C57), 26 cones from 10 fields (Gcap1−/−), 10 cones from seven fields (Gcap2−/−), and nine cones from three fields (Gcaps−/−). Scale bar, 20 μm.
GCAP1 and GCAP2 regulate the kinetics and sensitivity of mouse cone phototransduction
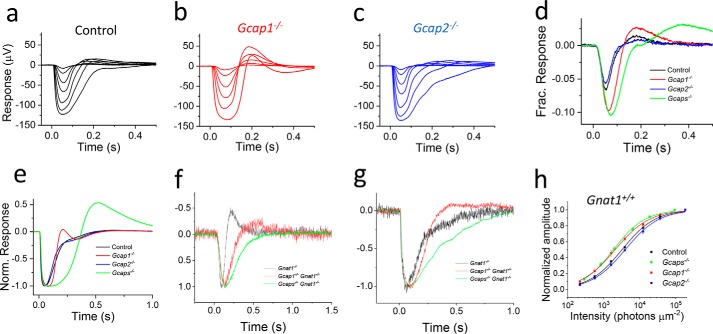
To determine the specific roles of GCAP2 and GCAP1 in mouse cone phototransduction, we compared light responses of dark-adapted cones from WT control, Gcap1−/−, Gcap2−/−, and Gcaps−/− mice using ex vivo electroretinography (ERG) recordings. To isolate the cone photoreceptor component of the ex vivo ERG signal, we used synaptic blockers and Ba2+ to remove b- and c-waves and rod-saturating background light. Key experiments and the light adaptation studies were also done in a Gnat1−/− genetic background to remove the rod component of the ERG signal. As has been shown previously (19), simultaneous deletion of GCAP1 and GCAP2 slowed down light response recovery and increased the sensitivity of cones to light flashes (see Fig. 2d and Table 1). Removal of GCAP1 alone increased time to peak (tp) of the responses elicited by dim light (Fig. 2, d and h, and Table 1) and increased the sensitivity of cones almost as much as the deletion of both GCAP1 and GCAP2 (Fig. 2d and Table 1). However, the recovery kinetics of the late tail phase of the responses in Gcap1−/− cones was not decelerated for both dim flashes (Fig. 2d) and bright saturating flashes (Fig. 2e). Afterdepolarization, or response recovery overshoot, which was often present both in control and GCAP-deficient cones, prevented us from fitting an exponential function to the late tail phase of the responses to estimate the response recovery time constant (τrec). However, the faster overall kinetics of Gcap1−/− cone dim flash responses as compared with that of Gcaps−/− cones was demonstrated by their shorter integration time when compared with Gcaps−/− mice (Table 1). Isolating the cone component of the response by using Gnat1−/− mice confirmed that the responses from GCAP1-deficient cones are still substantially faster than these of cones lacking both GCAP1 and GCAP2 (Fig. 2, f and g). These results suggest that GCAP2 can shape the light response kinetics specifically in brighter light, at least in the absence of GCAP1. In contrast, the sensitivity of dark-adapted cones to dim light flashes appears to be mediated mainly by GCAP1 (Fig. 2h and Table 1). We also recorded light responses from Gcap2−/− mice (Fig. 2c) but did not find any significant changes of response kinetics or light sensitivity of GCAP2-deficient cones compared with WT controls (Fig. 2, d, e, and h, and Table 1). Thus, we conclude that GCAP1 can support normal cone photoresponses in the absence of GCAP2.
Figure 2.
GCAP1 and GCAP2 regulate mouse cone phototransduction. a–c, responses of dark-adapted cones to 1-ms flashes of light with intensity, IF, ranging from 220 to 183,000 photons (530 nm) μm−2 in the presence of rod-saturating background light from isolated WT control (a), Gcap1−/− (b), and Gcap2−/− (c) mouse retinas. d, averaged responses of control (black), Gcap1−/− (red), Gcap2−/− (blue), and Gcaps−/− (green) mouse cones to a 220 photons μm−2 flash normalized with rmax. e, saturated responses of control (black), Gcap1−/− (red), Gcap2−/− (blue), and Gcaps−/− (green) mouse cones to the 183,000 photons μm−2 flash normalized (Norm.) with rmax. f and g, normalized dim flash (f) and saturated (g) light responses recorded from dark-adapted retinas of control (black), Gcap1−/− (red), and Gcaps−/− (green) mice that were bred on a Gnat1−/− background are shown. h, the smooth traces plot Equation 1 with I½ of 3,200, 1,900, 2,100, and 4,000 photons μm−2 fitted to the average response amplitude data (r/rmax as a function of IF) of each genotype. Error bars give S.E. n = 3 mice (six retinas) for each genotype.
Table 1.
Light (flash) response parameters from WT, Gcaps−/−, Gcap1−/−, and Gcap2−/− mouse cones
All recordings were from Gnat1+/+ mice except for the light adaptation parameters I0 and n, which were obtained from Gnat1−/− mice. Retinas were exposed to constant 70,000 photons (530 nm) μm−2 s−1 background light to suppress the rod component of the response except in the light adaptation experiments that were from Gnat1−/−mice. rmax, saturated photoresponse amplitude (IF = 183,000 photons μm−2 at 530 nm); tp, time to peak (IF = 220 photons μm−2 at 530 nm); ti, integration time defined as an area under a dim flash response divided by the amplitude of the response; I½, light flash intensity eliciting a response with peak amplitude r = 0.5rmax determined by fitting Equation 1 to the response amplitude data; I0, background light intensity at which cone sensitivity is 50% of that in darkness determined by fitting Equation 2 to the light adaptation data; n, steepness factor determined by fitting Equation 2 to the light adaptation data. * and † indicate statistically significant difference as compared with the WT control and Gcaps−/− mouse cones, respectively (p < 0.05, two-tailed Student's t test). NA, not available.
| Genotype | rmax | tp | ti | I½ | I0 | n |
|---|---|---|---|---|---|---|
| μV | ms | ms | photons μm−2 | photons μm−2 | ||
| WT | 144 ± 15 | 53 ± 1 | 51 ± 0.1 | 3,200 ± 130 | 14,500 ± 3,000 | 1.0 ± 0.1 |
| GCAPs−/− | 156 ± 26 | 76 ± 3* | 90 ± 9* | 1,900 ± 150* | 3,600 ± 800* | 1.5 ± 0.03* |
| GCAP1−/− | 135 ± 6 | 66 ± 3* | 64 ± 4*† | 2,100 ± 320* | 6,900 ± 900* | 1.0 ± 0.02† |
| GCAP2−/− | 125 ± 18 | 52 ± 2† | 49 ± 2† | 4,000 ± 450† | NA | NA |
GCAP2 promotes cGMP synthesis in low Ca2+ in mouse cones
Biochemical experiments have demonstrated that GCAP proteins activate cGMP synthesis of RetGCs in low Ca2+ (1, 6). Here, we asked whether GCAP2 could promote cGMP synthesis in intact mouse cones. To assess the Ca2+-mediated acceleration of cGMP synthesis in cones, we determined the change of the maximal saturated cone photoresponse amplitude (rmax) when the retinas were switched from normal perfusion solution with1.2 mm [Ca2+]o to low ∼30 nm [Ca2+]o in ex vivo ERG experiments. Such a treatment causes rapid reduction in the level of Ca2+ in photoreceptor outer segments and the subsequent GCAP-mediated up-regulation of cGMP synthesis (41). The rmax is proportional to the cGMP-gated channel current, and thus, increased cGMP concentration caused by accelerated cGMP synthesis rate is expected to increase rmax. We determined rmax from saturated cone responses elicited by periodic bright test flashes in dark-adapted mouse retinas before and after low-Ca2+ exposure. In control Gnat1−/− retinas, rmax increased about 4-fold after a low-Ca2+ exposure (Fig. 3, black squares), demonstrating the up-regulation of cGMP synthesis and subsequent opening of the cGMP-gated channels. However, the cells could not maintain such a high cGMP-gated (CNG) channel channel current for long, and eventually rmax declined under low Ca2+. When cones lacking both GCAP1 and GCAP2 (from Gcaps−/− Gnat1−/− retinas) were exposed to low Ca2+, a much more subtle increase of rmax was observed (Fig. 3, green squares), consistent with the lack of up-regulation of cGMP synthesis in low Ca2+ in the absence of both GCAPs. Notably, when we exposed Gcap1−/− Gnat1−/− retinas to low Ca2+, we observed substantial increase in rmax that was comparable with that in control Gnat1−/− mice (Fig. 3, red squares). These results demonstrate that Ca2+ feedback mediated by GCAP2 can promote acceleration of the cGMP synthesis in intact mouse cones in the absence of GCAP1.
Figure 3.
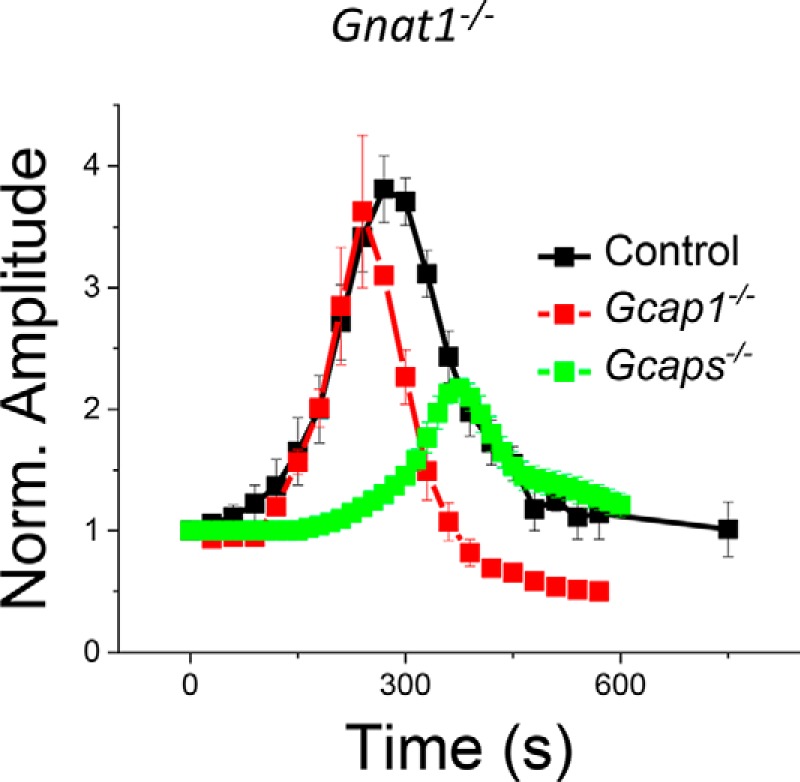
GCAP2 promotes CNG channel current in low Ca2+. Normalized (Norm.) rmax, the saturated photoresponse amplitude of dark-adapted cones, of control Gnat1−/− (black), Gcaps−/− Gnat1−/− (green), and Gcap1−/− Gnat1−/− (red) mice in normal Ca2+ (at t = 0 s) and during low-Ca2+ exposure (t > 0 s) is shown. The values for rmax were normalized to their respective value in normal Ca2+ just before the switch to low Ca2+ at t = 0 s. n = 3 mice (six retinas) for each genotype. Error bars give S.E.
GCAP2 contributes to mouse cone light adaptation in bright background light
GCAP-mediated Ca2+ feedback dominates the regulation of rod and cone photoreceptor sensitivity in response to fast increments or decrements of background light (19, 34). However, the distinct contributions of GCAP1 and GCAP2 to the light adaptation capacity of mouse cones is not known. To address this question, we determined how the sensitivity of cones is regulated by background light in isolated retinas from control mice expressing both GCAPs and from mice lacking either both GCAPs or only GCAP1. All mice were on a Gnat1−/− background to eliminate rod signaling and facilitate the quantification of cone light adaptation. When mouse cones are exposed to a step of light, they produce an initial hyperpolarizing response peak followed by partial relaxation to a plateau (Fig. 4, a–c). This relaxation was attenuated after removal of both GCAPs (Fig. 4b), consistent with the dominant role of the GCAP-mediated feedback in cone light adaptation. Notably, GCAP1-deficient cones exhibited prominent relaxation after the peak of the response comparable with that in control cones, indicative of efficient light adaptation. We quantified the relaxation magnitude and kinetics by fitting a sum of two exponential functions from the peak to the plateau of the step responses using Equation 3 (see Fig. 4, a–c). Although we used a two-exponential function, the relaxation was dominated by the exponential term with the faster of the two time constants (τ1). Thus, we used τ1 to assess the kinetics of relaxation and the amplitude from peak to the plateau (A) normalized by the peak amplitude (r0) to assess the magnitude of relaxation (see Equation 3). The A/r0 was similar between control (76 ± 1%) and Gcap1−/− (80 ± 1%) mice but significantly smaller in the absence of both GCAP1 and GCAP2 (27 ± 5%). This result demonstrates that the expression of GCAP2 in GCAP1-deficient cones was sufficient to promote robust light adaptation as demonstrated by the substantial relaxation of their response in steady background light. However, the kinetics of the relaxation was decelerated significantly by the deletion of GCAP1 alone (from 165 ± 30 ms in control to 495 ± 20 ms in Gcap1−/− mice), whereas the value for τ1 was not statistically significantly different in Gcaps−/− cones (423 ± 30 ms) as compared with that in Gcap1−/− cones. This result is consistent with the dominant role of GCAP1 in driving the rapid light adaptation of mouse cones.
Figure 4.
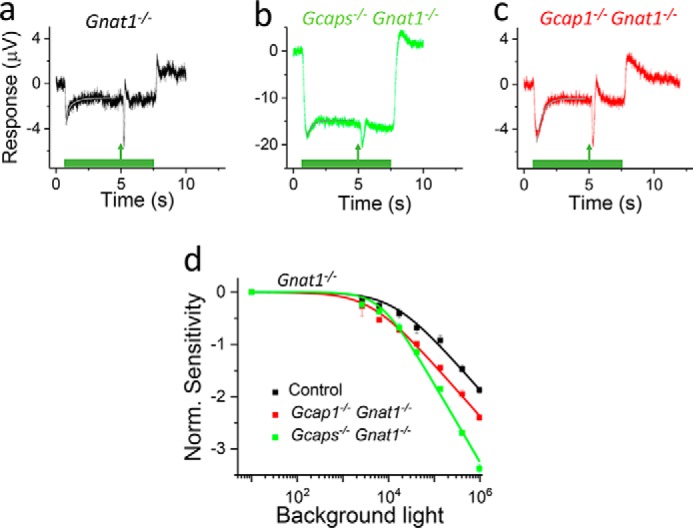
GCAP1 and GCAP2 contribute to the light adaptation capacity of cones. a–c, responses of cones to 7-s steps of light with a 1-ms flash delivered 4.5 s after the step onset from isolated retinas of control Gnat1−/− (a), Gcaps−/− Gnat1−/− (b), and Gcap1−/− Gnat1−/− (c) mice obtained using ex vivo ERG recordings. Smooth gray traces plot Equation 3 with best fitting parameters A1, τ1, and τ2. See “Results” for numerical values and statistical analysis. d, sensitivity (SF) normalized (Norm.) with the dark-adapted sensitivity (SF,D) of cones as a function of background light intensity (I) in control Gnat1−/− (black), Gcaps−/− Gnat1−/− (green), and Gcap1−/− Gnat1−/− (red). Smooth lines plot Equation 2 with I0 of 13,500 photons μm−2 s−1 (n = 1), 6,700 photons μm−2 s−1 (n = 1.4), and 4,100 photons μm−2 s−1 (n = 1) for control Gnat1−/− (black), Gcaps−/− Gnat1−/− (green), and Gcap1−/− Gnat1−/− (red). n = 3 mice (four retinas) for each genotype. Error bars give S.E.
To quantify the efficiency of light adaptation, we measured the sensitivity of cones to light flashes at 4.5 s after the step onset at different background light intensities. The sensitivity normalized to the sensitivity in darkness declined more steeply in Gcaps−/− cones than in control or Gcap1−/− cones (Fig. 4d). As expected, based on their higher sensitivity in darkness, the operating range of Gcap1−/− cones was shifted to dimmer background light intensities. However, the slope of the adaptation curve was not changed, and the adaptation capacity was clearly better in Gcap1−/− mice than in Gcaps−/− mice. These results indicate that GCAP2 can contribute to the light adaptation of mouse cones in the absence of GCAP1. We did not have Gcap2−/− Gnat1−/− mice. Thus, in an effort to investigate the role of GCAP2 in light adaptation, we compared light adaptation between Gcap2−/− and WT mice (on Gnat1+/+ background). In those experiments, we did not observe any change in light adaptation caused by the deletion of GCAP2 (data not shown), consistent with our flash response data showing only negligible phenotype in GCAP2-deficient cones (Fig. 2, c–e and h).
GCAP1 and GCAP2 compete for activation of RetGC1 in low Ca2+
Our results demonstrate that GCAP2 is expressed in mouse cones and that it can contribute to the Ca2+-dependent activation of RetGCs and phototransduction feedback in their outer segments. However, it remained unclear whether the expression level of GCAP2 in WT cones is sufficient to contribute to the overall Ca2+ feedback. A simple biochemical model (see “Experimental procedures” for details) predicts that a quite small concentration of GCAP2, ∼0.1–0.5 μm in the cone outer segment, could explain the ∼4-fold increase of rmax in low Ca2+ observed in Gcap1−/− cones (see Fig. 3). Based on the model prediction, we designed a biochemical experiment to assess the extent of activation of the native RetGC1 (the predominant guanylate cyclase isozyme expressed in the cones (30, 31, 35)) by recombinant GCAP1 and GCAP2. We used photoreceptor membranes from Gcaps−/− RetGC2−/− mouse retinas retaining only RetGC1 isozyme to measure cGMP synthesis by RetGC1 in low Ca2+ at normal physiological 0.9 mm Mg2+ (36). Consistent with our model, the low basal activity of RetGC1 was significantly increased by addition of either 0.5 μm GCAP2 (derived from the biochemical model) or 3 μm GCAP1 (the estimated GCAP1 concentration in mouse rods (29)) (Fig. 5). Next, we assessed whether GCAP2 can contribute to the regulation of RetGC1 activity in the presence of GCAP1. To test this, we used M26R GCAP1, a mutant form that can bind to RetGC1 like the WT GCAP1 but does not activate it (37, 38). In the presence of 3 μm GCAP1, addition of M26R GCAP1 started to decrease RetGC1 activity at ∼0.3 μm (Fig. 5, red circles), its near-physiological concentration (28, 29), and reached half-maximal inhibition at 1 μm. At the same concentration of M26R GCAP1, the activation of RetGC1 by 0.5 μm GCAP2, which in the absence of GCAP1 would be sufficient to effectively accelerate RetGC1 in vivo (Figs. 2 and 3), was almost completely suppressed (Fig. 5, black circles).
Figure 5.
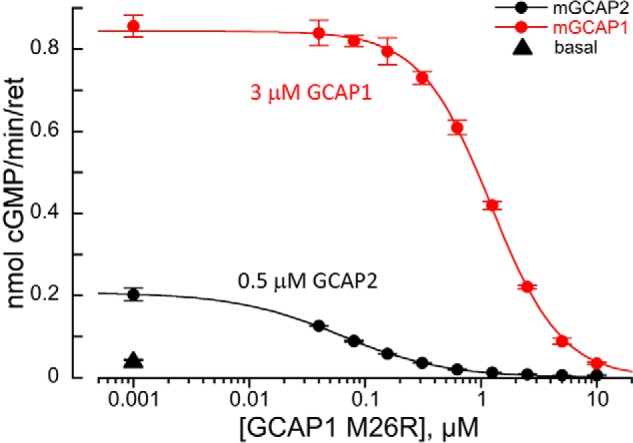
GCAP1 and GCAP2 compete for the activation of RetGC1. The native RetGC1 activity in Gcaps−/− RetGC2−/− mouse retinas was assayed as described under “Experimental procedures” in the absence of GCAPs (▴) or in the presence of 3 μm mouse GCAP1 (red circles) or 0.5 μm GCAP2 (black circles). Variable concentrations of the competing bovine M26R GCAP1 were added to the assay as indicated. The data, average ± S.D. (error bars) of two to four independent measurements, were fitted assuming a sigmoidal Hill function.
Discussion
Ca2+-dependent regulation of cGMP synthesis by GCAP2 in mouse cone photoreceptors
Our experiments clearly demonstrate that GCAP2 is expressed in mouse cones (Fig. 1). To address the possible functional role of GCAP2 in cones, we investigated its ability to up-regulate cGMP synthesis in low Ca2+ and to mediate light adaptation in cones lacking GCAP1. As previous studies have suggested that GCAP1 and RetGC1 dominate the synthesis of cGMP in the mouse cone outer segments, we expected that Gcap1−/− retinas would respond to low-Ca2+ exposure similarly to Gcaps−/− retinas (9, 10, 30, 31, 35). However, Gcap1−/− cones were able to boost their maximal response amplitude in low Ca2+ as much as control WT cones (Fig. 3). Based on our model presented under “Experimental procedures,” as low a concentration as 0.1 μm GCAP2 in the outer segments of Gcap1−/− cones could explain the ∼4-fold increase of their maximal response amplitude in low Ca2+. This concentration is more than 10-fold lower than the known GCAP1 or GCAP2 concentration in mouse rod outer segments (28, 29). The quantitative power of these experiments might be limited due to the cooperativity of the CNG channel for cGMP (39, 40) or the transient nature of the increase in photoreceptor response amplitude in low Ca2+ (41–45). However, despite the quantitative limitations of our study, our results clearly demonstrate that GCAP2 can activate RetGC in mouse cone photoreceptor cells when GCAP1 has been deleted.
Similarly, when we examined light adaptation in Gcap1−/− cones, we found that the slope of the light adaptation curve was comparable with that in control cones. In addition, the adaptation capacity of GCAP1-deficient cones was substantially better than that of Gcaps−/− cones (Fig. 4). Together, these results demonstrate that GCAP2 is able to up-regulate cGMP synthesis and to mediate light adaptation in cones in the absence of GCAP1.
The role of GCAP1 and GCAP2 in cone phototransduction and light adaptation
The relative contribution of GCAP1 and GCAP2 in rod physiology has been established in mouse rod photoreceptors (27). There, GCAP1 is more important in determining the peak amplitude of the dim flash response, whereas GCAP2 shapes the response recovery kinetics after the peak amplitude. These results are consistent with the known biochemical properties of GCAP1 and GCAP2. Namely, GCAP2 has a higher affinity to Ca2+ (KCa = 50 nm) as compared with GCAP1 (KCa = 130 nm) (18, 28, 29). In darkness, Ca2+ concentration in mouse rod outer segment is ∼250 nm, and it declines to ∼20–50 nm in bright light (46). Hence, after a dim flash, Ca2+ dissociates first from GCAP1, and the GCAP1-mediated feedback dominates over the GCAP2-mediated pathway. Later, when Ca2+ has dropped to a lower level, it can also dissociate from GCAP2, up-regulating the GCAP2-mediated feedback to contribute to the recovery phase kinetics of the dim flash response. Notably, the primary target for GCAP1 in mouse photoreceptors is RetGC1, whereas regulation of the ancillary isozyme RetGC2 is carried out mostly by GCAP2 (47). Hence, in mouse rods, activation of the cyclase after the flash of light occurs first as activation of RetGC1 by GCAP1 followed by additional activation of RetGC1 and RetGC2 by GCAP2 (27). Here, we compared dark-adapted cone flash responses from WT, Gcaps−/−, Gcap1−/−, and Gcap2−/− mice to understand the relative contributions of GCAP1 and GCAP2 in determining the sensitivity and response kinetics of mammalian cones (Fig. 2). We found that deletion of GCAP1 causes a comparable increase of the sensitivity and dim flash response amplitude as the deletion of both GCAP1 and GCAP2 (Fig. 2, d and h, and Table 1). Thus, just as in rods, GCAP1 seems to dominate the up-regulation of cGMP synthesis up to the peak of the dim flash response, and the sensitivity of cones is set almost completely by GCAP1.
Comparison of saturated bright flash responses from WT, Gcaps−/−, Gcap1−/−, and Gcap2−/− mice revealed that deletion of both GCAP1 and GCAP2 significantly delays the escape of cones from saturation, whereas the deletion of GCAP1 had a much less dramatic effect, and the deletion of GCAP2 had almost no effect at all on the recovery kinetics (Fig. 2e). Notably, the recovery kinetics of Gcap1−/− cones were not slower than those of WT cones so that cone responses from Gcap1−/− mice recovered to the baseline level at the same time as those of WT and Gcap2−/− mice (Fig. 2d). Thus, it appears that both GCAP1 and GCAP2 can compensate for the lack of the other isoform in accelerating the recovery of bright flash responses (Fig. 2e). These results are also consistent with the idea that a larger drop in Ca2+ caused by brighter light is required to activate the GCAP2 pathway. In support of this notion, we observed deviation between Gcap1−/− and Gcaps−/− mouse light adaptation only at brighter background light. This, again, suggests that GCAP2 is more important under brighter illumination and at lower Ca2+.
Although our data clearly show that GCAP2 contributes significantly to the physiology of mouse cones in Gcap1−/− mice, it is not clear whether GCAP2 plays a role in the phototransduction and/or light adaptation of healthy WT cones. Evidently, GCAP2 is present in native mouse and Nrl−/− cones at much lower levels than in rods, whereas GCAP1 expression in cones is very strong (5, 10, 31). However, our functional data from Gcap1−/− mice could be explained even by a rather low 0.1–0.5 μm GCAP2 concentration in the absence of GCAP1. In contrast, our biochemical experiments assessing the relative contribution of the two GCAP isoforms show that, even at equal concentrations of the two GCAP isoforms, GCAP1 effectively outcompetes GCAP2 from RetGC1 (see Fig. 5 and Refs. 28 and 38). Assuming further that GCAP2 expression in mouse cones is lower than that of GCAP1, we conclude that under normal physiological conditions GCAP1 would dominate the regulation of cGMP synthesis in mouse cones. However, if the function of GCAP1 becomes compromised, GCAP2 should be able to effectively regulate the phototransduction feedback and light adaptation of cones.
Experimental procedures
Ethical approval
All experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at Washington University in St. Louis, Salus University, and University of Southern California.
Animals
WT C57Bl/6J control and age-matched adult mice devoid of guanylate cyclase–activating protein 1 (Gcap1−/− (48)), 2 (Gcap2−/− (49)), or both (Gcaps−/− (19)) were used in this study. The mutant strains were bred to the control C57Bl/6J background for several generations but were not siblings of the control mice. For some electrophysiology experiments, Gcap1−/− and Gcaps−/− mice were bred into a Gnat1−/− background to remove the rod-driven light responses (50). Mice were kept under a 12/12-h light/dark cycle and had free access to regular mouse chow and clean water.
Single-cell immunohistochemistry
Freshly dissected retinas from C57, Gcap1−/−, Gcap2−/−, and Gcaps−/− mice were washed in Ames' medium, placed on an ice-cooled glass slide with a few drops of cold Ames' buffer, and chopped with razor blade. Dissociated cells and small cell clumps were collected into 8-chamber slides (Lab-Tek®, catalog number 177445) that were precoated with wheat germ agglutinin (100 μm wheat germ agglutinin was added to the wells and incubated for 1 h). After cells were collected into wells, equal volumes of formaldehyde (4% in PBS) were added. The slides were centrifuged for 10 min at 168 × g to attach the cells to the glass surface. Cells were washed in PBS; blocked with 5% goat serum, 0.1% Triton X-100 in PBS for 1 h; and incubated overnight with a rabbit polyclonal anti-mCAR antibody (51) (1:700 in blocking buffer). The next day, slides were washed in PBS and incubated with a secondary anti-rabbit antibody to visualize mCAR-labeled cones. Following PBS washes, cells were incubated with biotinylated anti-GCAP2 antibody (33) (1:300 of 1 mg/ml in blocking buffer). The GCAP2 signal was visualized by Texas Red-avidin (1:200; Vector Laboratories). The cells were mounted in Vectashield with DAPI (Vector Laboratories). Fluorescence images were acquired using a Zeiss Axio Scope microscope using the same settings and exposure times for the different genotypes.
Ex vivo electroretinography
We used ex vivo ERG to assess the function of mouse cone phototransduction and light adaptation (52). Either a background light of 70,000 photons (530 nm) μm−2 s−1 or Gnat1−/− genetic background (53) was used to remove the rod component of the ERG signal. The Gnat1−/− mouse rods do not respond to light but maintain normal morphology. The background light needed to fully saturate rods was surprisingly high and would have been expected to bleach a significant amount of pigments during our experiments. However, after about 10 min of exposure to the background light, the cone responses remained stable for up to at least 2 h (the longest experiment), potentially due to a balance between pigment bleaching and regeneration via the Müller cell (54) visual cycle pathway. Retinas were dissected from dark-adapted eyes under IR illumination and mounted to a custom-built ERG specimen holder described in Vinberg et al. (52). Flashes and steps of light were provided by green LEDs (530 nm; Luxeon Rebel LED SR-01-M0090) via an inverted microscope light path where the condenser was replaced by a 10× objective forming a homogenous 2.35-mm spot of light at the sample. The intensity of the light stimulus was calibrated at the level of the sample by a photometer (Model 211, UDT Instruments). Retinas were perfused at 1 ml/min at 37 °C with bicarbonate-buffered Locke's solution containing 112 mm NaCl, 3.6 mm KCl, 2.4 mm MgCl2, 1.2 mm CaCl2, 10 mm HEPES, 20 mm NaHCO3, 3 mm disodium succinate, 0.5 mm sodium glutamate, and 10 mm glucose. The solution was equilibrated with 95%O2 and 5%CO2 at 37 °C. Low-Ca2+ solution was prepared by using 0.1 mm CaCl2 instead of 1.2 mm and adding 0.4 mm EGTA. Addition of EGTA caused acidification of the medium, and we used NaOH to equalize the pH of our normal Locke's and low-Ca2+ media. We estimate that the free [Ca2+] of the low-Ca2+ medium is ∼30 nm in the presence of 2.4 mm Mg2+ (55).
A differential amplifier (DP-311, Warner Instruments) and Bessel filter (model 3382, Krohn-Hite Corp.) together with a DigiData 1440 digitizer and pCLAMP software (Axon Instruments) were used to acquire data at 10 kHz with a 300-Hz low-pass filter. Clampfit (Axon Instruments), Origin 9.0.0 (Originlab), and Excel (Microsoft) software were used to analyze and graph the data. A Naka-Rushton function was fitted to the response amplitude (r) data.
| (1) |
where rmax is the maximal saturated response amplitude, IF is flash intensity, and I½ is the light intensity (in photons μm−2) required to elicit a half-maximal response. A modified Weber-Fechner function was fitted to light adaptation data.
| (2) |
where SF is the sensitivity of cones to a flash of light (IF that elicits r < 0.2rmax) defined as r/IF, SF,D is the sensitivity in darkness, I is the background light intensity (in photons μm−2 s−1), I0 is the background light intensity in which SF = 0.5SF,D, and n is a factor determining the steepness of the adaptation curve.
A sum of two exponential functions was used to quantify the kinetics and magnitude of light response relaxation after the initial peak during light steps.
| (3) |
where r0 is peak amplitude measured at td, A is amplitude measured from the peak to the steady-state plateau of the step response, A1 is the fraction of recovery covered by the time constant τ1, and (A − A1) is the fraction of the recovery covered by the time constant τ2.
Biochemical model of RetGC1 activation by GCAP2
We used the following equations to model binding of Ca2+ to GCAP2 and binding/activation of RetGC1 by Ca2+-free GCAP2. The parameter values were taken from Peshenko et al. (28).
| (4) |
| (5) |
where KCa = 50 nm is the apparent dissociation constant of Ca2+ from GCAP2. We model the activation of RetGC1 by GCAP2 by assuming that only Ca2+-free GCAP2 can activate the RetGC1.
| (6) |
| (7) |
where KGC1 = 1.25 μm and GC1total = 3.2 μm. Cyclase activity (α; in μm s−1) can be calculated as follows.
| (8) |
if we assume that GTP (the substrate) ≫ Km(GTP-GC) (dissociation constant of the GTP from RetGC1). We assume that the basal RetGC1 activity kn1 = 2.6 s−1 and for the activated RetGC1 ks1 = 33 s−1 (28). Concentrations of GCAP2-free GC1 and GCAP2-bound GC1-GCAP2 in a specific [Ca2+] and [GCAP2] can be calculated from Equations 5, 6, and 7.
At steady state,
| (9) |
| (10) |
where β = 4.1 s−1 is the spontaneous cGMP hydrolysis activity of rod PDE in darkness (56). The CNG channel current (57) can be calculated as follows.
| (11) |
where Jmax is the CNG channel current at high [cGMP]. Assuming that [Ca2+] is 250 nm in a dark-adapted mouse cone outer segment under normal extracellular Ca2+ and declines to 25 nm during our low-Ca2+ exposure (see above), as low as a 0.1 μm total concentration of GCAP2 in the cone outer segment is predicted to cause a 4.4-fold increase of JcG when switched from normal (1.2 mm) Ca2+ to low Ca2+.
Expression and purification of GCAPs
We used recombinant mouse myristoylated GCAP1 (E6S) and GCAP2 expressed from pET11d vector (Novagen/Calbiochem) in BLR(DE3) Escherichia coli strain harboring yeast N-myristoyltransferase as described previously (28). GCAP2 was purified using urea extraction from the inclusion bodies and size-exclusion chromatography (26, 58). GCAP1 was purified using urea extraction and hydrophobic and size-exclusion column chromatography as described previously to reach a final protein of 95% purity by SDS-PAGE (28, 59). The M26R bovine GCAP1 mutant was produced and purified as described previously (37, 38).
RetGC assays
The native mouse RetGC1 activity was assayed under IR illumination in dark-adapted Gcaps−/− RetGC2−/− triple-knockout mouse retina homogenates isolated as described previously (28). Briefly, the assay mixture (25 μl) containing retinal homogenate, 30 mm MOPS-KOH (pH 7.2), 60 mm KCl, 4 mm NaCl, 1 mm DTT, 2 mm EGTA, 0.9 mm free Mg2+, 0.3 mm ATP, 4 mm cGMP, 1 mm GTP, 1 μCi of [α-32P]GTP, 100 μm zaprinast and dipyridamole, 10 mm creatine phosphate, and 0.5 unit of creatine phosphokinase was incubated at 30 °C for 8 min, and the reaction was stopped by heat inactivation at 95° for 2 min. The resultant [32P]cGMP product was separated by TLC using fluorescently backed polyethyleneimine cellulose plates (Merck) developed in 0.2 m LiCl and eluted with 2 m LiCl, and the radioactivity was counted using ScintiSafe liquid scintillation mixture (Thermo Fisher Scientific) with addition of 20% ethanol.
Author contributions
F. V., J. C., A. M. D., and V. J. K. conceptualization; F. V., I. V. P., and J. C. data curation; F. V. and I. V. P. formal analysis; F. V., J. C., A. M. D., and V. J. K. funding acquisition; F. V., J. C., A. M. D., and V. J. K. writing-original draft; F. V., I. V. P., J. C., A. M. D., and V. J. K. writing-review and editing; A. M. D. and V. J. K. project administration.
Acknowledgment
We thank Janis Lem from Tufts University for the Gnat1−/− animals.
This work was supported by National Institutes of Health Grants EY026651 (to F. V.), EY019312 (to V. J. K.), EY027387 (to V. J. K. and J. C.), EY012155 and EY027193 (to J. C.), EY011522 (to A. M. D.), and EY02687 (to Washington University, Department Ophthalmology); Research to Prevent Blindness; and the Ella and Georg Ehrnrooth Foundation (to F. V.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GCAP
- guanylate cyclase–activating protein
- RetGC
- retinal membrane guanylate (guanylyl) cyclase
- PDE
- phosphodiesterase
- mCAR
- mouse cone arrestin
- ERG
- electroretinography
- LED
- light-emitting diode.
References
- 1. Gorczyca W. A., Gray-Keller M. P., Detwiler P. B., and Palczewski K. (1994) Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc. Natl. Acad. Sci. U.S.A. 91, 4014–4018 10.1073/pnas.91.9.4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorczyca W. A., Polans A. S., Surgucheva I. G., Subbaraya I., Baehr W., and Palczewski K. (1995) Guanylyl cyclase activating protein. A calcium-sensitive regulator of phototransduction. J. Biol. Chem. 270, 22029–22036 10.1074/jbc.270.37.22029 [DOI] [PubMed] [Google Scholar]
- 3. Palczewski K., Subbaraya I., Gorczyca W. A., Helekar B. S., Ruiz C. C., Ohguro H., Huang J., Zhao X., Crabb J. W., Johnson R. S., Walsh K. A., Gray-Keller M. P., Detwiler P. B., and Baehr W. (1994) Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron 13, 395–404 10.1016/0896-6273(94)90355-7 [DOI] [PubMed] [Google Scholar]
- 4. Koch K. W., and Stryer L. (1988) Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature 334, 64–66 10.1038/334064a0 [DOI] [PubMed] [Google Scholar]
- 5. Dizhoor A. M., Olshevskaya E. V., Henzel W. J., Wong S. C., Stults J. T., Ankoudinova I., and Hurley J. B. (1995) Cloning, sequencing, and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J. Biol. Chem. 270, 25200–25206 10.1074/jbc.270.42.25200 [DOI] [PubMed] [Google Scholar]
- 6. Dizhoor A. M., Lowe D. G., Olshevskaya E. V., Laura R. P., and Hurley J. B. (1994) The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron 12, 1345–1352 10.1016/0896-6273(94)90449-9 [DOI] [PubMed] [Google Scholar]
- 7. Imanishi Y., Yang L., Sokal I., Filipek S., Palczewski K., and Baehr W. (2004) Diversity of guanylate cyclase-activating proteins (GCAPs) in teleost fish: characterization of three novel GCAPs (GCAP4, GCAP5, GCAP7) from zebrafish (Danio rerio) and prediction of eight GCAPs (GCAP1–8) in pufferfish (Fugu rubripes). J. Mol. Evol. 59, 204–217 10.1007/s00239-004-2614-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imanishi Y., Li N., Sokal I., Sowa M. E., Lichtarge O., Wensel T. G., Saperstein D. A., Baehr W., and Palczewski K. (2002) Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur. J. Neurosci. 15, 63–78 10.1046/j.0953-816x.2001.01835.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuenca N., Lopez S., Howes K., and Kolb H. (1998) The localization of guanylyl cyclase-activating proteins in the mammalian retina. Invest. Ophthalmol. Vis. Sci. 39, 1243–1250 [PubMed] [Google Scholar]
- 10. Howes K., Bronson J. D., Dang Y. L., Li N., Zhang K., Ruiz C., Helekar B., Lee M., Subbaraya I., Kolb H., Chen J., and Baehr W. (1998) Gene array and expression of mouse retina guanylate cyclase activating proteins 1 and 2. Invest. Ophthalmol. Vis. Sci. 39, 867–875 [PubMed] [Google Scholar]
- 11. Jiang L., Katz B. J., Yang Z., Zhao Y., Faulkner N., Hu J., Baird J., Baehr W., Creel D. J., and Zhang K. (2005) Autosomal dominant cone dystrophy caused by a novel mutation in the GCAP1 gene (GUCA1A). Mol. Vis. 11, 143–151 [PubMed] [Google Scholar]
- 12. Nishiguchi K. M., Sokal I., Yang L., Roychowdhury N., Palczewski K., Berson E. L., Dryja T. P., and Baehr W. (2004) A novel mutation (I143NT) in guanylate cyclase-activating protein 1 (GCAP1) associated with autosomal dominant cone degeneration. Invest. Ophthalmol. Vis. Sci. 45, 3863–3870 10.1167/iovs.04-0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sokal I., Dupps W. J., Grassi M. A., Brown J. Jr., Affatigato L. M., Roychowdhury N., Yang L., Filipek S., Palczewski K., Stone E. M., and Baehr W. (2005) A novel GCAP1 missense mutation (L151F) in a large family with autosomal dominant cone-rod dystrophy (adCORD). Invest. Ophthalmol. Vis. Sci. 46, 1124–1132 10.1167/iovs.04-1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payne A. M., Downes S. M., Bessant D. A., Taylor R., Holder G. E., Warren M. J., Bird A. C., and Bhattacharya S. S. (1998) A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum. Mol. Genet. 7, 273–277 10.1093/hmg/7.2.273 [DOI] [PubMed] [Google Scholar]
- 15. Downes S. M., Holder G. E., Fitzke F. W., Payne A. M., Warren M. J., Bhattacharya S. S., and Bird A. C. (2001) Autosomal dominant cone and cone-rod dystrophy with mutations in the guanylate cyclase activator 1A gene-encoding guanylate cyclase activating protein-1. Arch. Ophthalmol. 119, 96–105 [PubMed] [Google Scholar]
- 16. Wilkie S. E., Li Y., Deery E. C., Newbold R. J., Garibaldi D., Bateman J. B., Zhang H., Lin W., Zack D. J., Bhattacharya S. S., Warren M. J., Hunt D. M., and Zhang K. (2001) Identification and functional consequences of a new mutation (E155G) in the gene for GCAP1 that causes autosomal dominant cone dystrophy. Am. J. Hum. Genet. 69, 471–480 10.1086/323265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sokal I., Li N., Surgucheva I., Warren M. J., Payne A. M., Bhattacharya S. S., Baehr W., and Palczewski K. (1998) GCAP1 (Y99C) mutant is constitutively active in autosomal dominant cone dystrophy. Mol. Cell 2, 129–133 10.1016/S1097-2765(00)80121-5 [DOI] [PubMed] [Google Scholar]
- 18. Dizhoor A. M., Boikov S. G., and Olshevskaya E. V. (1998) Constitutive activation of photoreceptor guanylate cyclase by Y99C mutant of GCAP-1. Possible role in causing human autosomal dominant cone degeneration. J. Biol. Chem. 273, 17311–17314 10.1074/jbc.273.28.17311 [DOI] [PubMed] [Google Scholar]
- 19. Mendez A., Burns M. E., Sokal I., Dizhoor A. M., Baehr W., Palczewski K., Baylor D. A., and Chen J. (2001) Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 98, 9948–9953 10.1073/pnas.171308998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burns M. E., Mendez A., Chen J., and Baylor D. A. (2002) Dynamics of cyclic GMP synthesis in retinal rods. Neuron 36, 81–91 10.1016/S0896-6273(02)00911-X [DOI] [PubMed] [Google Scholar]
- 21. Fain G. L., Matthews H. R., Cornwall M. C., and Koutalos Y. (2001) Adaptation in vertebrate photoreceptors. Physiol. Rev. 81, 117–151 10.1152/physrev.2001.81.1.117 [DOI] [PubMed] [Google Scholar]
- 22. Yau K. W., and Nakatani K. (1984) Electrogenic Na-Ca exchange in retinal rod outer segment. Nature 311, 661–663 10.1038/311661a0 [DOI] [PubMed] [Google Scholar]
- 23. Vinberg F., Wang T., Molday R. S., Chen J., and Kefalov V. J. (2015) A new mouse model for stationary night blindness with mutant Slc24a1 explains the pathophysiology of the associated human disease. Hum. Mol. Genet. 24, 5915–5929 10.1093/hmg/ddv319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vinberg F., Wang T., De Maria A., Zhao H., Bassnett S., Chen J., and Kefalov V. J. (2017) The Na+/Ca2+, K+ exchanger NCKX4 is required for efficient cone-mediated vision. Elife 6, e24550 10.7554/eLife.24550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakurai K., Vinberg F., Wang T., Chen J., and Kefalov V. J. (2016) The Na+/Ca2+, K+ exchanger 2 modulates mammalian cone phototransduction. Sci. Rep. 6, 32521 10.1038/srep32521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peshenko I. V., and Dizhoor A. M. (2004) Guanylyl cyclase-activating proteins (GCAPs) are Ca2+/Mg2+ sensors: implications for photoreceptor guanylyl cyclase (RetGC) regulation in mammalian photoreceptors. J. Biol. Chem. 279, 16903–16906 10.1074/jbc.C400065200 [DOI] [PubMed] [Google Scholar]
- 27. Makino C. L., Wen X. H., Olshevskaya E. V., Peshenko I. V., Savchenko A. B., and Dizhoor A. M. (2012) Enzymatic relay mechanism stimulates cyclic GMP synthesis in rod photoresponse: biochemical and physiological study in guanylyl cyclase activating protein 1 knockout mice. PLoS One 7, e47637 10.1371/journal.pone.0047637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peshenko I. V., Olshevskaya E. V., Savchenko A. B., Karan S., Palczewski K., Baehr W., and Dizhoor A. M. (2011) Enzymatic properties and regulation of the native isozymes of retinal membrane guanylyl cyclase (RetGC) from mouse photoreceptors. Biochemistry 50, 5590–5600 10.1021/bi200491b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hwang J. Y., Lange C., Helten A., Höppner-Heitmann D., Duda T., Sharma R. K., and Koch K. W. (2003) Regulatory modes of rod outer segment membrane guanylate cyclase differ in catalytic efficiency and Ca2+-sensitivity. Eur. J. Biochem. 270, 3814–3821 10.1046/j.1432-1033.2003.03770.x [DOI] [PubMed] [Google Scholar]
- 30. Xu J., Morris L., Thapa A., Ma H., Michalakis S., Biel M., Baehr W., Peshenko I. V., Dizhoor A. M., and Ding X. Q. (2013) cGMP accumulation causes photoreceptor degeneration in CNG channel deficiency: evidence of cGMP cytotoxicity independently of enhanced CNG channel function. J. Neurosci. 33, 14939–14948 10.1523/JNEUROSCI.0909-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boye S. L., Peterson J. J., Choudhury S., Min S. H., Ruan Q., McCullough K. T., Zhang Z., Olshevskaya E. V., Peshenko I. V., Hauswirth W. W., Ding X. Q., Dizhoor A. M., and Boye S. E. (2015) Gene therapy fully restores vision to the all-cone Nrl(−/−) Gucy2e(−/−) mouse model of Leber congenital amaurosis-1. Hum. Gene Ther. 26, 575–592 10.1089/hum.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Otto-Bruc A., Fariss R. N., Haeseleer F., Huang J., Buczyłko J., Surgucheva I., Baehr W., Milam A. H., and Palczewski K. (1997) Localization of guanylate cyclase-activating protein 2 in mammalian retinas. Proc. Natl. Acad. Sci. U.S.A. 94, 4727–4732 10.1073/pnas.94.9.4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang T., and Chen J. (2014) Induction of the unfolded protein response by constitutive G-protein signaling in rod photoreceptor cells. J. Biol. Chem. 289, 29310–29321 10.1074/jbc.M114.595207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakurai K., Chen J., and Kefalov V. J. (2011) Role of guanylyl cyclase modulation in mouse cone phototransduction. J. Neurosci. 31, 7991–8000 10.1523/JNEUROSCI.6650-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang R. B., Robinson S. W., Xiong W. H., Yau K. W., Birch D. G., and Garbers D. L. (1999) Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J. Neurosci. 19, 5889–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen C., Nakatani K., and Koutalos Y. (2003) Free magnesium concentration in salamander photoreceptor outer segments. J. Physiol. 553, 125–135 10.1113/jphysiol.2003.053280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peshenko I. V., Olshevskaya E. V., Lim S., Ames J. B., and Dizhoor A. M. (2014) Identification of target binding site in photoreceptor guanylyl cyclase-activating protein 1 (GCAP1). J. Biol. Chem. 289, 10140–10154 10.1074/jbc.M113.540716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peshenko I. V., Olshevskaya E. V., and Dizhoor A. M. (2015) Evaluating the role of retinal membrane guanylyl cyclase 1 (RetGC1) domains in binding guanylyl cyclase-activating proteins (GCAPs). J. Biol. Chem. 290, 6913–6924 10.1074/jbc.M114.629642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fesenko E. E., Kolesnikov S. S., and Lyubarsky A. L. (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313, 310–313 10.1038/313310a0 [DOI] [PubMed] [Google Scholar]
- 40. Haynes L., and Yau K. W. (1985) Cyclic GMP-sensitive conductance in outer segment membrane of catfish cones. Nature 317, 61–64 10.1038/317061a0 [DOI] [PubMed] [Google Scholar]
- 41. Vinberg F., Turunen T. T., Heikkinen H., Pitkänen M., and Koskelainen A. (2015) A novel Ca2+-feedback mechanism extends the operating range of mammalian rods to brighter light. J. Gen. Physiol. 146, 307–321 10.1085/jgp.201511412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lipton S. A., Ostroy S. E., and Dowling J. E. (1977) Electrical and adaptive properties of rod photoreceptors in Bufo marinus. I. Effects of altered extracellular Ca2+ levels. J. Gen. Physiol. 70, 747–770 10.1085/jgp.70.6.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bastian B. L., and Fain G. L. (1982) The effects of low calcium and background light on the sensitivity of toad rods. J. Physiol. 330, 307–329 10.1113/jphysiol.1982.sp014343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matthews H. R. (1995) Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J. Physiol. 484, 267–286 10.1113/jphysiol.1995.sp020664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yau K. W., McNaughton P. A., and Hodgkin A. L. (1981) Effect of ions on the light-sensitive current in retinal rods. Nature 292, 502–505 10.1038/292502a0 [DOI] [PubMed] [Google Scholar]
- 46. Woodruff M. L., Sampath A. P., Matthews H. R., Krasnoperova N. V., Lem J., and Fain G. L. (2002) Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 542, 843–854 10.1113/jphysiol.2001.013987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olshevskaya E. V., Peshenko I. V., Savchenko A. B., and Dizhoor A. M. (2012) Retinal guanylyl cyclase isozyme 1 is the preferential in vivo target for constitutively active GCAP1 mutants causing congenital degeneration of photoreceptors. J. Neurosci. 32, 7208–7217 10.1523/JNEUROSCI.0976-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Makino C. L., Wen X. H., Michaud N. A., Covington H. I., DiBenedetto E., Hamm H. E., Lem J., and Caruso G. (2012) Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS One 7, e37832 10.1371/journal.pone.0037832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Makino C. L., Peshenko I. V., Wen X. H., Olshevskaya E. V., Barrett R., and Dizhoor A. M. (2008) A role for GCAP2 in regulating the photoresponse. Guanylyl cyclase activation and rod electrophysiology in GUCA1B knock-out mice. J. Biol. Chem. 283, 29135–29143 10.1074/jbc.M804445200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Calvert P. D., Krasnoperova N. V., Lyubarsky A. L., Isayama T., Nicoló M., Kosaras B., Wong G., Gannon K. S., Margolskee R. F., Sidman R. L., Pugh E. N. Jr., Makino C. L., and Lem J. (2000) Phototransduction in transgenic mice after targeted deletion of the rod transducin α-subunit. Proc. Natl. Acad. Sci. U.S.A. 97, 13913–13918 10.1073/pnas.250478897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu X., Li A., Brown B., Weiss E. R., Osawa S., and Craft C. M. (2002) Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Mol. Vis. 8, 462–471 [PubMed] [Google Scholar]
- 52. Vinberg F., Kolesnikov A. V., and Kefalov V. J. (2014) Ex vivo ERG analysis of photoreceptors using an in vivo ERG system. Vision Res. 101, 108–117 10.1016/j.visres.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lyubarsky A. L., Lem J., Chen J., Falsini B., Iannaccone A., and Pugh E. N. Jr. (2002) Functionally rodless mice: transgenic models for the investigation of cone function in retinal disease and therapy. Vision Res. 42, 401–415 10.1016/S0042-6989(01)00214-0 [DOI] [PubMed] [Google Scholar]
- 54. Wang J. S., Estevez M. E., Cornwall M. C., and Kefalov V. J. (2009) Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat. Neurosci. 12, 295–302 10.1038/nn.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vinberg F., and Koskelainen A. (2010) Calcium sets the physiological value of the dominant time constant of saturated mouse rod photoresponse recovery. PLoS One 5, e13025 10.1371/journal.pone.0013025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gross O. P., Pugh E. N. Jr., and Burns M. E. (2012) Spatiotemporal cGMP dynamics in living mouse rods. Biophys. J. 102, 1775–1784 10.1016/j.bpj.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lamb T. D., and Pugh E. N. Jr. (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449, 719–758 10.1113/jphysiol.1992.sp019111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Olshevskaya E. V., Hughes R. E., Hurley J. B., and Dizhoor A. M. (1997) Calcium binding, but not a calcium-myristoyl switch, controls the ability of guanylyl cyclase-activating protein GCAP-2 to regulate photoreceptor guanylyl cyclase. J. Biol. Chem. 272, 14327–14333 10.1074/jbc.272.22.14327 [DOI] [PubMed] [Google Scholar]
- 59. Peshenko I. V., and Dizhoor A. M. (2006) Ca2+ and Mg2+ binding properties of GCAP-1. Evidence that Mg2+-bound form is the physiological activator of photoreceptor guanylyl cyclase. J. Biol. Chem. 281, 23830–23841 10.1074/jbc.M600257200 [DOI] [PubMed] [Google Scholar]