Abstract
Meta‐analytic techniques for mining the neuroimaging literature continue to exert an impact on our conceptualization of functional brain networks contributing to human emotion and cognition. Traditional theories regarding the neurobiological substrates contributing to affective processing are shifting from regional‐ towards more network‐based heuristic frameworks. To elucidate differential brain network involvement linked to distinct aspects of emotion processing, we applied an emergent meta‐analytic clustering approach to the extensive body of affective neuroimaging results archived in the BrainMap database. Specifically, we performed hierarchical clustering on the modeled activation maps from 1,747 experiments in the affective processing domain, resulting in five meta‐analytic groupings of experiments demonstrating whole‐brain recruitment. Behavioral inference analyses conducted for each of these groupings suggested dissociable networks supporting: (1) visual perception within primary and associative visual cortices, (2) auditory perception within primary auditory cortices, (3) attention to emotionally salient information within insular, anterior cingulate, and subcortical regions, (4) appraisal and prediction of emotional events within medial prefrontal and posterior cingulate cortices, and (5) induction of emotional responses within amygdala and fusiform gyri. These meta‐analytic outcomes are consistent with a contemporary psychological model of affective processing in which emotionally salient information from perceived stimuli are integrated with previous experiences to engender a subjective affective response. This study highlights the utility of using emergent meta‐analytic methods to inform and extend psychological theories and suggests that emotions are manifest as the eventual consequence of interactions between large‐scale brain networks.
Keywords: affective processing, BrainMap, co‐activations, data mining, emotion, functional connectivity, functional magnetic resonance imaging, meta‐analysis, neuroinformatics
1. INTRODUCTION
Complex cognitive processes are often interrogated through functional neuroimaging techniques using heterogeneous task designs that can vary based on the given scientific question. The goal of coordinate‐based meta‐analyses is to provide quantitative reinforcement for consistent activation across similar studies. However, it has been demonstrated that the differentiating characteristics of task design have a neural basis (Barret & Satpute, 2013; Laird et al., 2015), and can provide insight regarding specialization of neural recruitment during the performance of a given task grouping. Advanced meta‐analytic methodologies have emerged to probe these cognitive processes, resulting in new information characterizing meta‐analytic networks and behavioral interpretations that provide support for complex psychological theories.
The research domains of affective and cognitive neuroscience have been influenced by the emergence of coordinate‐based meta‐analytic techniques that allow for statistically rigorous evaluation and interpretation of functional neuroimaging results across multiple studies (Fox, Lancaster, Laird, & Eickhoff, 2014). Initial coordinate‐based meta‐analytic approaches allowed for the identification of convergent activity modulations observed across a collection of studies utilizing similar experimental tasks. One method, termed Activation Likelihood Estimation (ALE; Turkeltaub, Eden, Jones, & Zeffiro, 2002; 2012; Eickhoff et al., 2009, 2012; 2016) offers a quantitative characterization (as opposed to the qualitative assessment of narrative reviews) of brain regions associated with psychological processes of interest. However, traditional neurocognitive views focusing on regional contributions have transitioned towards network‐level perspectives that may provide a more complete and coherent appreciation of the neural substrates linked to multifaceted psychological processes (Seeley et al., 2007; Bressler & Menon, 2010). As a result, meta‐analytic methodologies have likewise evolved with this network‐focused shift (Laird et al., 2013). Meta‐analytic connectivity modeling (MACM) has emerged as a useful tool for characterizing whole‐brain networks co‐activating with an individual brain region of interest (ROI) across various task domains (Laird et al., 2009a; Robinson, Laird, Glahn, Lovallo, & Fox, 2010; Eickhoff et al., 2010; Riedel et al., 2015). Extending this framework beyond a single isolated network co‐activating with an individual seed ROI, a recent meta‐analytic methodology leverages clustering techniques to characterize the recruitment of multiple, distinct networks across groups of studies (Laird et al., 2015). That previous work applied clustering techniques to modeled activation images associated with experiments within a heterogeneous task domain (i.e., facial processing; Laird et al., 2015), as opposed to clustering voxels within a user‐defined ROI (connectivity‐based parcellation; Neumann, von Cramon, & Lohmann, 2008; Cauda et al., 2012; Bzdok et al., 2015; Balsters, Mantini, Apps, Eickhoff, & Wenderoth, 2016).
Concurrent with the transition from regional to network‐based perspectives, the field of affective neuroscience has witnessed a similar shift toward understanding large‐scale network involvement in affective processing and the generation of emotions (Ochsner, Bunge, Gross, & Gabrieli, 2002; 2012; Barrett & Satpute, 2013; Pessoa, 2012; Touroutoglou, Lindquist, Dickerson, & Barrett, 2015). From a regional perspective, extensive evidence from both human and animal studies indicate a critical role of the amygdala in emotional processing (Ledoux et al., 1988; Pessoa, 2010; Phelps and Ledoux, 2005). Moving beyond the focus on the amygdala as a single node, resting‐state functional connectivity assessments have allowed for network‐level characterization of the regions that interact with the amygdala (Baur, Hänggi, Langer, & Jäncke, 2013; Roy et al., 2009; Bzdok, Laird, Zilles, Fox, & Eickhoff, 2013), such as selective activation of the insula during emotional awareness processing (Simmons et al., 2013) and the ventromedial and lateral prefrontal cortices implicated in emotional regulation (Jackson & Moghaddam, 2001). Additionally, multiple frontal and parietal regions are critically linked to emotion generation (Ramponi et al., 2011; Fruhholz & Grandjean, 2013; Otto et al., 2014), and interactions between limbic structures and cortical networks suggest that emotion and cognition are not easily separated, but rather, jointly contribute to behavior (Pessoa, 2008). Given the multifaceted nature of emotional processing, it is not surprising that multiple task paradigms have been employed to dissect the constituent processes. Previous emotion‐related meta‐analyses have utilized corpora specifically focused on emotional salience (Phan et al., 2004), emotional face processing (Fusar‐Poli et al., 2009), or studies resulting in the generation of discrete affective responses (Murphy, Nimmo‐Smith, & Lawrence, 2003; Kober et al., 2008; Vytal & Hamann, 2010; Lindquist, Wager, Kober, Bliss, & Barrett, 2012; Kirby & Robinson, 2015); however, the broader affective neuroimaging literature consists of complex experimental designs that often blur the boundaries between emotional, cognitive, and perceptual processes in the pursuit of this multidimensional construct (e.g., emotional Stroop, emotional n‐back). No meta‐analysis has yet utilized data‐driven methodologies and the full, complex range of affective neuroimaging results collectively complied over the last two decades to dissociate large‐scale network involvement independent of stimuli or tasks.
In the present study, we focused our attention on the wealth of neuroimaging results catalogued in the BrainMap database (http://www.brainmap.org) to characterize the brain networks and associated mental processes associated with various aspects of affective processing. In our investigation, any experiment in which an emotional stimulus was presented (e.g., faces, words) or an instruction was given to elicit an emotional response (e.g., recall an emotional memory) was included in the analyses. We performed data mining across this diverse range of experimental paradigms involving an affective component, representing the broadest inclusion criteria of any emotion‐related meta‐analysis to date. Our goals were to: (1) apply a recently developed clustering‐based meta‐analytic methodology to group the wide range of affective experiments according to similar brain activation architecture, thereby delineating multiple large‐scale brain networks associated with emotional processing; (2) perform post hoc analyses utilizing metadata terms from each individual experiment within emergent groups to highlight the functional differentiation of identified brain networks in the larger context of emotional processing; and (3) provide a quantitative meta‐analytic assessment to directly inform an existing psychological theory presented by Ochsner, Silvers, and Buhle, (2012), suggesting differential brain networks and linked mental operations that contribute to affective processing.
2. MATERIALS AND METHODS
2.1. Meta‐analytic data extraction and pre‐processing
The BrainMap database (Fox & Lancaster, 2002; Laird, Lancaster, & Fox, 2005; Laird et al., 2009b) is an online repository of over 15,000 published neuroimaging experiments (from over 3,000 journal articles) archived as three‐dimensional coordinates in stereotactic space (x,y,z). Each experiment is the result of a whole‐brain statistical analysis (i.e., no ROI analyses), and has been manually coded by expert annotators with metadata terms established by the Cognitive Paradigm Ontology (http://www.CogPO.org; Turner & Laird, 2012) describing the experimental design of the archived study. Experiments in BrainMap assessing the neural correlates of affective processing are classified under the behavioral domain of Emotion, or one of its associated subdomains, which include Anger, Anxiety, Disgust, Fear, Happiness, and Sadness. Furthermore, according to the BrainMap coding scheme (Fox et al., 2005), experiments may possibly be jointly classified under the additional behavioral domains of Action, Cognition, Interoception, or Perception. This feature of multi‐label classification in BrainMap, which is dependent on the unique behavioral conditions during which participants were scanned and the resultant choice of experimental contrasts yielding statistical parametric images, provides the basis for the present approach to studying a wide range of affective processing studies. We used the BrainMap search engine, Sleuth (http://www.brainmap.org/sleuth), to query the database for experiments classified with any of the above Emotion behavioral domains (and associated sub‐domains). The search results were filtered to identify only experiments reporting activations (not deactivations) from healthy adult participants; this strategy was intended to mitigate biases associated with age‐, treatment‐, or disorder‐related effects. In divergence from previous meta‐analyses utilizing only experimental contrasts resulting in an induced emotional response, the objective of the current study was to investigate all components of emotional processing. As such, no additional filtering of experimental contrasts was performed (i.e., the data set was not limited to emotional vs. neutral contrasts). We extracted activation foci (i.e., peak coordinates) from each identified BrainMap experiment and linearly transformed those coordinates reported in Talairach space (Talairach & Tournoux, 1988) into MNI space (Collins et al., 1994) using the Lancaster transform (Lancaster et al., 2007; Laird et al., 2010). Modeled activation (MA) maps were then generated in MNI space with 2 mm resolution by modeling foci as Gaussian probability distributions, thereby accounting for spatial uncertainty due to brain template and between‐subject variance (Eickhoff et al., 2009; Figure 1, Step 1).
Figure 1.
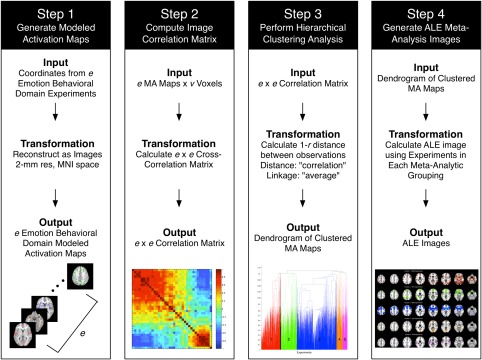
Data analysis pipeline. Using a data‐driven approach, we categorized affective processing experiments according to similar brain activation architecture. Step 1: Experiments in the BrainMap database catalogued under the behavioral domain of Emotion were identified, and the corresponding activation coordinates were extracted and blurred using a Gaussian filter to generate modeled activation (MA) maps. Step 2: The three‐dimensional MA map of each experimental contrast was reduced to one‐dimension, and concatenated to create an experiment‐by‐voxel matrix. A cross‐correlation matrix was calculated to quantify the pair‐wise correlations between each experimental contrast's one‐dimensional MA map. Step 3: Hierarchical clustering was performed on this correlation matrix using the “correlation distance” method and “average linkage” method to define meta‐analytic groupings (MAGs) of experiments. Step 4: After identifying the most suitable number of MAGs, Activation Likelihood Estimation (ALE) images were calculated utilizing the experiment foci assigned to each MAG [Color figure can be viewed at http://wileyonlinelibrary.com]
2.2. Correlation matrix based hierarchical clustering analysis
To interrogate the affective processing literature to reveal differential meta‐analytic network recruitment, we implemented a previously developed methodological approach (Laird et al., 2015), that was developed using the same techniques as in Co‐Activation Based Parcellation (Cauda et al., 2012; Bzdok et al., 2014; Balsters et al., 2016). The only differentiating characteristics in the current approach are the method of experimental contrasts selection inclusive in the meta‐analysis and the use of hierarchical (as opposed to k‐means) clustering. Each MA map was reduced to a one‐dimensional array and concatenated across all experiments to form an e x v matrix, where e is the number of experiments and v is the number of voxels in the MNI‐standardized brain (Collins et al., 1994; Figure 1, Step 2). An e × e symmetric cross‐correlation (CC) matrix was calculated representing the Pearson correlation coefficient (r) between each pair of MA maps. Hierarchical clustering analysis was then performed on the correlation matrix, in the MATLAB environment (version 2014b; Mathworks, Inc.) to parse experiments into meta‐analytic groupings (MAGs) exhibiting similar MA patterns (Figure 1, Step 3). First, the “correlation distance”, defined as 1 – r, was calculated between each experiment's distribution of correlation coefficients in the CC matrix to generate a vector of the e × e–1 pair‐wise distances. Essentially, higher distance values indicated greater dissimilarity between the pair‐wise correlation coefficient distributions of the respective experiments, inherently representative of the dissimilarity of each experiment's MA map. Then, the “average linkage” algorithm was employed to assemble experiments into MAGs by identifying the smallest distance (or dissimilarity) between experiments as defined in the previous step. Here, experiments were combined into a single MAG based on the smallest average of dissimilarity quantities between any constituent experiments of a MAG and any experiment not already assigned to a MAG. The “average” method was used in the present study to mitigate the problematic “chaining” effect in which increasing model order (i.e., number of clusters/MAGs) results in solutions differing only by the addition of one experiment. Solutions utilizing the correlation distance and average linkage parameterizations have been previously demonstrated with fMRI data (Liu, Zhu, Qiu, & Chen, 2012) and BrainMap‐based meta‐analytic maps (Laird et al., 2015).
The resultant dendrogram was assessed to create MAGs of experiments that clustered together, representing coherent groupings of similar activation patterns. To select a clustering solution yielding a suitable parcellation of BrainMap experiments, we assessed the comparative performance of multiple solutions using two metrics, “relative difference in cophenetic distance” and “experiment separation density” (Laird et al., 2015). Specifically, we evaluated clustering solutions with model orders of between 3 and 10 MAGs and identified those solutions yielding high relative difference in cophenetic distance values and low experiment separation density values. First, the relative difference in cophenetic distances between MAGs, dc, was used to characterize the extent to which increasing model order resulted in substantially different activation patterns respective to each resultant MAG:
| (1) |
The cophenetic distance represents the dissimilarity between two MAGs of experiments, and becomes increasingly larger as model order decreases. In an exemplar dendrogram (Supporting Information Figure S1), the cophenetic distance is represented on the y‐axis, where the distance along the axis corresponds to a junction of clusters with greater inherent dissimilarity. Thus, the union at the maximal height of the dendrogram represents the cophenetic distance between the two distinct MAGs. As one then progresses to the second highest junction, where one MAG is then fractionated to create a total of three MAGs, the cophenetic distance of that junction is evaluated, relative to the previous highest junction, to obtain a quantitative index for evaluating the dissimilarity for different clustering solutions. Here, the aim was to maximize the relative difference in cophenetic distances (c) between model orders (x and x + 1) thereby identifying clustering solutions yielding robustly different activation patterns. Reiterated, large values for this metric indicate that the higher model order resulted in MAGs consisting of experiments with substantially different MA maps.
Second, the experiment separation density, ds, was used to quantify the impact of increasing model order when parcellating a group of experiments of size n0 into sizes n1 and n2, defined as:
| (2) |
where n1 is greater than n2. MAGs are composed of sets of experiments, and as model order increases, all experiments in a single MAG are distributed to two independent MAGs based on the dissimilarity between experiments. In certain instances, a MAG may be decomposed into two MAGs, where one of the resulting MAGs consists of a single experiment. In such an extreme case, this model order would be ignored because the overall impact that a single experiment would have on the overall solution would be negligible. Here, the aim was to avoid a clustering solution yielding a high ds, which indicates that a resulting MAG was not substantially different from the parent MAG in terms of experiment contribution, leading to reduced differentiation between solutions.
2.3. ALE convergence of meta‐analytic groupings
After selecting a suitable clustering solution, we investigated convergent activation patterns within MAGs, thereby delineating meta‐analytic networks of activation across grouped experiments. Convergent activation patterns from sets of foci contributing to each MAG were produced using the Activation Likelihood Estimation (ALE) method (Turkeltaub et al., 2002) (Figure 1, Step 4) implemented in the MATLAB environment. The revised ALE algorithm was employed which accounts for between‐subject variability and between‐template variance due to differences in spatial normalization methods across publications (Eickhoff et al., 2009), as well as within‐experiment effects (Turkeltaub et al., 2012). This procedure was carried out to characterize convergent brain activity modulations across experiments within a single MAG. Voxel‐wise ALE scores were computed as the union of the MA maps, which provided a quantitative representation of convergent brain activation patterns; statistical significance was assessed by analytically deriving the null distribution of random spatial association between experiments (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012). According to recent guidelines based on massive ALE simulations (Eickhoff et al., 2016), ALE images for each MAG were differentially thresholded based on the number of experiments contributing to each MAG. Any MAG consisting of greater than 500 experiments was subjected to the more conservative voxel‐level FWE threshold (p voxel‐level < .05), while all other MAGs were thresholded at p cluster‐level < .05 (cluster‐level FWE corrected for multiple comparisons; cluster‐forming threshold: p voxel‐level < .001).
2.4. Functional decoding of meta‐analytic groupings
Once the convergent spatial activation patterns for each MAG were delineated, we examined the experimental design of the contributing experiments to identify which task‐related features (e.g., stimulus, response, or instructions) most likely led to these similar activation patterns. For example, for a dendrogram yielding n groupings of MA maps/experiments, we evaluated the behavioral tasks utilized in the experiments grouped together in MAG 1, the tasks in MAG 2, and so on for all n MAGs. Each experiment in BrainMap is annotated with metadata describing the behavioral domain, paradigm class, stimulus modality and type, response modality and type, and instructions utilized by the original neuroimaging study. We note that multiple metadata descriptors within the same class (e.g., behavioral domain) may be assigned to a single experiment (e.g., Emotion and Cognition), allowing BrainMap annotations to capture the complexity of the original study's experimental design (Laird et al., 2009a).
Forward and reverse inference analyses (Poldrack, 2006; Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011) were performed on the metadata terms associated with experiments to quantitatively assess the functional/behavioral properties of each MAG relative to the BrainMap database (Cieslik et al., 2013; Nickl‐Jockschat et al., 2015). Forward inference analyses were performed to determine the probability that brain activation among the experiments within an individual MAG was the result of a specific metadata term. Specifically, we used a binomial test to determine if the probability of activation given a metadata term P(activation | term) (Equation (1)) was significantly higher than the base‐rate probability of activating the MAG. The probability of activation given a metadata term was calculated (Equation (3)) using the number of experiments within a MAG j coded with the term i and the total number of foci reported active in all experiments in the BrainMap database coded with term i. If a binomial test between this quantity and the number of experiments in MAG j divided by the total number of coordinates in the BrainMap database (Equation (4)) resulted in a significant difference (p < .05, FDR‐corrected), then the likelihood ratio (Equation (5)) was determined using the ratio of the two quantities.
| (3) |
| (4) |
| (5) |
Reverse inference analyses were also performed to identify the likelihood that a specific metadata term resulted in brain activation among the experiments within a MAG. Specifically, a chi‐square test was employed to determine if the probability of a metadata term given activation in the MAG P(term|activation) was significantly (p < .05, FDR‐corrected) higher than the terms representation across the database.
| (6) |
| (7) |
The reverse inference probability (Equation (7)) was calculated using the probability of activation given a metadata term (Equation (3)), multiplied by the probability of a given metadata term occurring within the BrainMap database (Equation (6); where #i indicates the total number of metadata assignments within a field across experiments), divided by the probability of activation within a MAG (Equation (4)). The combined results of forward and reverse inference analyses were exported for visual assessment in the Cytoscape environment (Shannon et al., 2003).
To further enhance interpretation of each MAG's behavioral relevance, we also reviewed the prose descriptions of the constituent experiments, which provide a concise summary of the original study's purpose and experimental design. These short descriptions were manually coded by expert annotators associated with the BrainMap Project. Specifically, prose descriptions explicitly state the conditions (e.g., “subjects viewed a probe letter and recalled if that letter was a previously encoded letter”) and experimental contrasts (e.g., “Finger Tapping > Rest”) that resulted in the reported activation foci. Naming conventions for experimental contrasts are often adopted from the manuscript itself. Thus, these prose descriptions offered a more precise level of detail to appreciate the mental operations contributing to each MAG. To aid in summarizing these prose descriptions, simple term and phrase (consisting of no more than four sequential terms) frequencies were totaled for the assembly of prose descriptions associated with each MAG. Here, the prose descriptions associated with a single MAG were imported into the MATLAB environment and concatenated. All non‐alphanumeric characters (e.g., “& < >: , # .”) were removed to avoid any situation usage bias (e.g., a term appearing at the end of a sentence, contrast effects). Then, in an iterative fashion, terms and sets of contiguous terms or phrases, were assessed for frequency of occurrence within the concatenated text descriptions to determine how often each appeared across all prose descriptions for the experiments within a MAG. Assessments of term and phrase frequency were then utilized in the review process to enhance insight into the most consistent experimental strategies employed within each MAG.
3. RESULTS
From a potential 7,363 experiments archived in BrainMap reporting coordinates of activation in healthy participants, our search for experiments classified under the behavioral domain of Emotion (and associated sub‐domains) yielded 1,747 experimental contrasts from 905 papers, reporting 22,760 peak‐activation foci among 27,542 healthy participants. We generated MA maps for each of these 1,747 experiments and conducted a spatial correlation analysis to compare the topography of each pair of experiments.
3.1. Correlation matrix based hierarchical clustering analysis
We grouped experiments exhibiting the highest degree of similarity in MA topography using a hierarchical clustering analysis approach. To identify the most suitable number of MAGs (i.e., model order) for parcellating the 1,747 BrainMap experiments, we evaluated differences in cophenetic distance and experiment separation density values across increasing model order (i.e., from 3 to 10 MAGs; Supporting Information Figure S2). The difference in cophenetic distance values between the current solution and its predecessor was highest for solutions of 3, 4, and 5 MAGs. These high values indicated that the MA maps associated with those MAGs were substantially different in topographic distribution than those at the model order just below it. Regarding experiment separation density values, solutions of 4, 5, 7, and 10 exhibited smaller values than the immediately preceding lower model order. Here, the tendency for the experiment separation density to decrease indicated that increasing model order resulted in the formation of new MAGs composed of a substantial number of experiments, and were not merely the result of an arbitrary collection of potentially “noisy” experiments. Based on convergence of both metrics, 4 and 5 MAGs emerged as candidate solutions. Ultimately, we proceeded with the more discriminate 5‐MAG solution due to a continued decrease in experiment separation density and substantial divergence of neural systems (see Supporting Information Figure S3 for comparison of 4‐ and 5‐MAG solutions). The resulting 5‐MAG solution visualized in a dendrogram (Figure 2), depicts individual MAGs consisting of 123 (orange), 98 (purple), 808 (blue), 321 (green), and 397 (red) experiments, respectively (Supporting Information Table S1). To further highlight the degree of similarity of experimental contrast MA maps within each MAG and differentiation of MA maps between MAGs, the correlation coefficient between MA maps was calculated and averaged for each MAG and each pair‐wise comparison (Supporting Information Figure S4).
Figure 2.
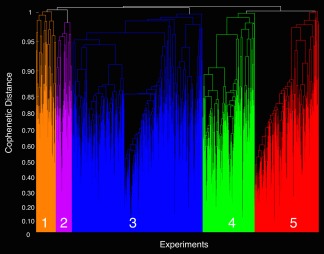
Hierarchical clustering of MA maps. Hierarchical clustering was performed to assemble emotional processing experiments into groupings with similar MA maps. Meta‐analytic groupings, distinguished by different colors in the dendrogram, were comprised of 123 (orange), 98 (purple), 808 (blue), 321 (green), and 397 (red) experiments [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. ALE convergence of meta‐analytic groupings
ALE maps were generated for each of the 5 MAGs using a MATLAB implementation of the ALE algorithm to identify regions of convergent activation (Figure 3, Table 1). The ALE map for MAG 1 (Figure 3; orange) exhibited convergent activations within the visual cortex (cuneus and lingual gyrus) and bilateral middle frontal gyri. The ALE map for MAG 2 (Figure 3; purple) exhibited convergent activations within the bilateral temporal gyri, and (mid)cingulate gyrus. Significant convergence was observed in MAG 3 (Figure 3; blue) within the bilateral insula, bilateral caudate, dorsal anterior cingulate cortex (ACC), and inferior frontal gyri. Significant convergence in MAG 4 (Figure 3; green) was observed in the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC). Lastly, MAG 5 (Figure 3; red) exhibited convergent activations within the bilateral amygdala, parahippocampal gyri, and bilateral fusiform gyri.
Figure 3.
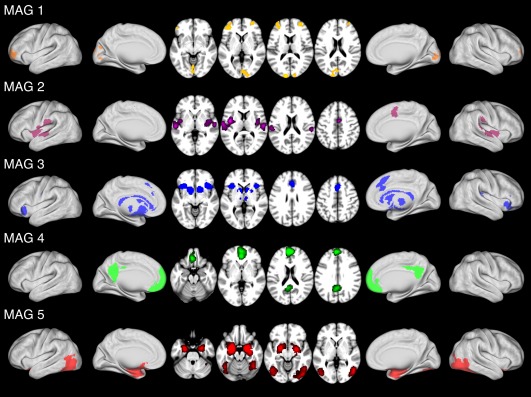
ALE images of meta‐analytic groupings of affective experiments. ALE images identified significant (MAGs 1, 2, 4, 5: p < .05, cluster‐level FWE; p < .001, cluster‐forming threshold; MAG 3: p < .05 voxel‐level FWE) convergence of activation in each meta‐analytic grouping (MAG) of experiments. Overlay colors reflect the corresponding MAGs displayed in Figure 2. See Supporting Information Table S1 for coordinates of each MAG's identified clusters of convergence [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
MNI coordinates of ALE‐derived meta‐analytic groupings
| Meta‐analytic grouping | Region | Volume | x | y | z | |
|---|---|---|---|---|---|---|
| 1 | Middle frontal gyrus (BA10) | B | 5448 | −37 | 47 | 5 |
| Lingual gyrus (BA17) | B | 5048 | 6 | −88 | 0 | |
| Middle frontal gyrus (BA10) | R | 3464 | 33 | 55 | 6 | |
| Cuneus (BA18) | L | 2760 | −10 | −93 | 16 | |
| 2 | Insula (BA13), Superior temporal gyrus (BA41) | L | 17400 | −50 | −14 | 8 |
| Superior temporal gyrus (BA41) | R | 14352 | 51 | −18 | 9 | |
| Cingulate gyrus (BA24) | R | 2960 | 3 | −3 | 46 | |
| 3 | Frontal gyrus (BA6/9/10/44/46), Insula, Thalamus | B | 134544 | 0 | 11 | 2 |
| Cingulate gyrus (BA32) | B | 30144 | 0 | 22 | 40 | |
| 4 | Medial frontal gyrus (BA9) | B | 41296 | −1 | 48 | 3 |
| Posterior cingulate (BA31) | L | 13184 | −3 | −50 | 26 | |
| 5 | Middle temporal gyrus | R | 22144 | 41 | −68 | −9 |
| Parahippocampal gyrus (Amygdala) | R | 19936 | 26 | −6 | −17 | |
| Parahippocampal gyrus (Amygdala) | L | 19712 | −22 | −4 | −17 | |
| Fusiform gyrus (BA37) | L | 16648 | −43 | −67 | −8 |
Note: MAGs 1, 2, 4, and 5 were subjected to cluster‐level FWE thresholding (p cluster‐level < .05 (FWE‐corrected); p voxel‐level < .001), while MAG 3 was subjected to voxel‐level FWE thresholding (p voxel‐level < .05) because of the extremely large experiment contribution (Supporting Information Table S1). The peak cluster coordinates associated with each thresholded (p cluster‐level < .05 [FWE‐corrected]; p voxel‐level < .001) ALE map corresponding to the meta‐analytic groupings. Data for each cluster include anatomical and functional (Brodmann area) labels (L: left hemisphere, R: right hemisphere, B: bilateral), spatial‐cluster volume, and the weighted center‐of‐mass reported in MNI coordinates.
3.3. Functional decoding of meta‐analytic groupings
The ALE analyses above delineated convergent regions of activation across experiments within MAGs, as well as differential spatial topographies across MAGs. To elucidate the functional/behavioral properties of these MAGs, we then characterized the metadata terms significantly associated with each MAG using forward and reverse inference analyses. Figure 4 illustrates the results of these analyses and represents significant BrainMap metadata terms for each MAG as a connection/line between the MAG number and the specific metadata term. Terms in which multiple lines project to different MAGs indicate those fields that were observed to be significant across multiple MAGs. All MAGs shared a number of significant metadata terms, located in the center of the groupings, which encompassed various emotional sub‐domains (e.g., sadness, disgust, happiness, fear, anger, anxiety), paradigm classes (e.g., emotional picture discrimination and face monitor/discrimination) and stimulus types (e.g., pictures and faces; for a list of all emotion‐related behavioral domains and paradigm classes represented in the database see Supporting Information Table S2). In addition, the forward and reverse inference analyses revealed a number of unique metadata terms associated with individual MAGs (Figure 4), which facilitated interpretation of the mental processes and tasks specifically related to each MAG. These unique metadata terms were found to be significantly represented in only a single MAG, and although those terms may be associated with experiments assigned to other MAGs, the cumulative amount did not reach the statistical threshold required for significant association. Beyond these unique metadata terms, we also reviewed the prose descriptions annotated with each experiment assigned to a given MAG, which provided a concise summary of the original study's purpose and experimental design. Joint evaluation of the metadata terms and prose descriptions allowed us to generate interpretations of the behavioral relevance of each MAG:
Figure 4.
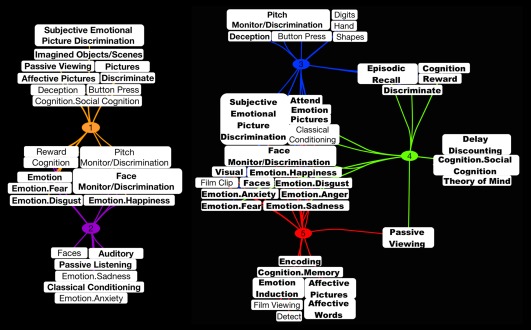
Functional decoding of meta‐analytic groupings of affective experiments. Forward and reverse inference analyses were performed across metadata fields to identify terms significantly associated with each meta‐analytic grouping (MAG) of experiments. Terms connected by multiple lines indicate significant associations with multiple MAGs. Bolded terms indicate those highlighted in the main text. The appearance of certain terms twice served only to distinguish MAGs 1 and 2 as separate from MAGs 3, 4, and 5 [Color figure can be viewed at http://wileyonlinelibrary.com]
MAG 1 consisted of experiments yielding convergent activation primarily in the visual cortex and bilateral medial frontal gyri. Forward and reverse inference analyses of metadata terms indicated significant within‐MAG representation of the paradigm classes of affective pictures, passive viewing, imagined objects/scenes, and subjective emotional picture discrimination, as well as the pictures stimulus modality. Examination of experimental design trends indicated an emphasis on participant instructions to discriminate or attend to emotional faces, words, or scenes. Together, the constituent experiments suggested this MAG was associated with Visual Perception.
MAG 2 consisted of experiments yielding convergent activation in the bilateral temporal gyri and (mid)cingulate cortex. Forward and reverse inference analyses of metadata indicated significant within‐MAG representation of the paradigm classes of passive listening and classical conditioning, as well as the auditory stimulus modality. Examination of experimental design trends indicated a focus on the identification or discrimination of vocal expressions based on tone or gender. Additional examples of experimental designs included passively listening to music and evaluating congruent or incongruent prosody of presented stimuli. Together, the constituent experiments suggested this MAG was associated with Auditory Perception.
MAG 3 consisted of experiments yielding convergent activation in regions comprising the salience network (Seeley et al., 2007), including the bilateral insula and dorsal ACC. Forward and reverse inference analyses of metadata indicated significant within‐MAG representation of the paradigm classes of pitch monitor/discrimination and deception. Examination of experimental design trends indicated a large representation of tasks requiring participants to intently focus on the presented stimulus, with instruction to either discriminate or respond to a secondary stimulus. Specifically, experiments took the form of gender, emotion, number, or orientation discrimination, and anticipation to respond to a forthcoming physical stimulus, or monetary gain or loss. Additional examples of experimental designs involved physical stimulation in the form of electrical shock, hand‐holding, or breath‐holding, as well as the shifting of attention toward external environmental factors. Together, the constituent experiments suggested this MAG was associated with Attending to Emotionally Salient Information.
MAG 4 consisted of experiments yielding convergent activation in regions comprising the default mode network (Raichle et al., 2001; Laird et al., 2009a), including the mPFC and PCC. Forward and reverse inference analyses of metadata indicated significant representation of the paradigm classes of theory of mind and delay discounting, as well as the behavioral domain Cognition.Social Cognition. Examination of experimental trends indicated a large representation of tasks requiring participants to imagine themselves experiencing emotions or events in different social situations, or to predict potential outcomes given different scenarios (e.g., predict expected behavior or whether a given outcome resulted in a reward). Additional examples of experimental designs included tasks associating an emotional response with familiar and unfamiliar visual stimuli, or the recognition of previously presented images. Together, the constituent experiments suggested this MAG was associated with the Appraisal and Prediction of Emotional Events.
MAG 5 consisted of experiments yielding convergent activation across the bilateral amygdala, parahippocampal gyri, and fusiform gyri. Forward and reverse inference analyses of metadata indicated significant representation of the paradigm classes of affective pictures, affective words, emotion induction, and encoding, as well as the behavioral domain Cognition.Memory. Examination of experimental design trends indicated a large representation of encoding and memory tasks coupled with various emotional terms. Participants in these experiments were asked to elicit memories associated with previously presented emotionally charged images. Additional examples of experimental designs involved attending to a spectrum of emotional categories presented as objects, scenes, or faces, or involved the identification of emotional states through the presentation of faces. Together, the constituent experiments suggested this MAG was associated with the Induction of Emotional Responses.
4. DISCUSSION
We delineated meta‐analytic groupings of neuroimaging experiments associated with convergent brain activations reported during affective processing via data mining of 1,747 experiments from 905 published papers archived in the BrainMap database. Operating under the premise that tasks resulting in similar brain activation patterns should be categorized as functionally similar, while tasks demonstrating differential activation patterns should be classified as functionally distinct, we grouped experiments together using a hierarchical clustering approach (Laird et al., 2015). The observed MAGs revealed affective processing to have an underlying architecture comprised of separate meta‐analytic networks associated with visual and auditory input as well as several other well‐known large‐scale functional brain networks, including the salience, default mode, and limbic networks. Furthermore, functional decoding of each MAG suggested five distinct roles for these networks in the context of affective experiments: (1) visual perception, (2) auditory perception, (3) attending to salient information, (4) appraisal and prediction of emotional events, and (5) induction of emotional responses.
4.1. Neural systems involved in emotional processing
Our findings describe the meta‐analytic architecture underlying affective processing via systematic and data‐driven classification of emotion‐related BrainMap experiments into separate MAGs according to similar activation patterns. Our results are in agreement with prior work suggesting that coherent interactions among an ensemble of large‐scale brain networks engender psychological processes and emotional states (Seeley et al., 2007; Smith et al., 2009; Spreng, Mar, & Kim, 2009; Lindquist et al., 2012). One contemporary psychological model of emotions (Ochsner et al., 2012) contends that emotional states are the result of self‐referential exposure (and subsequent response) to the emotional context of a perceived stimulus and are comprised of four stages. Accordingly, the first stage of emotion processing involves perceiving a stimulus in context, whether internally or externally driven. The stimulus is then attended to during the second stage to discriminate the aspects or features that are contextually most important from those that may be excluded from subsequent processing (Luo et al., 2014). The third stage involves the appraisal of the salient stimuli in terms of relevance to the individual's goals, wants, or needs, which under certain circumstances may include consequences of predicted affect in other individuals in social situations. According to appraisal theories of emotion, this phase corresponds to the mechanism that precedes positive vs. negative reactions or specific emotional responses (Scherer, Schorr, & Johnstone, 2001). The final stage of emotion generation translates the subjective appraisals into changes in experience, affective memory, emotionally‐expressive behavior, and/or autonomic physiology. The following discussion of the brain regions and associated behavioral inferences are presented in the context of the theory proposed by Ochsner et al., (2012).
Emotions emerge as contextual responses to external sensory inputs and/or internal stimuli, and MAGs 1 and 2 collectively encompass brain regions prioritized for perceptual processes. Convergent activation across MAG 1 was observed in the cuneus and lingual gyrus, and additionally in bilateral inferior and middle frontal gyri. This meta‐analytic network of regions has been implicated in basic visual processing, participating in visual memory (Todd, Won Han, Harrison, & Marois, 2011), as well as recognition and naming of words (Price et al., 1994; Bookheimer, Zeffiro, Blaxton, Gaillard, & Theodore, 1995; Mechelli, Humphreys, Mayall, Olson, & Price, 2000). The involvement of the visual perception network in emotion is well documented (Keightley et al., 2003; Kehoe, Toomey, Balsters, & Bokde, 2013; Isenberg et al., 1999), and has been discussed in several meta‐analyses (Kober et al., 2008; Fusar‐Poli et al., 2009; Vytal & Hamann 2010). Furthermore, neuroimaging evidence has suggested that activation in the visual cortices may be modulated by affective stimuli (Vuilleumier & Pourtois, 2007; Stolarova, Keil, & Moratti, 2006) or by differential eye fixation strategies when perceiving affect‐laden stimuli (van Reekum et al., 2007). Whereas MAG 1 was associated with visual input, experiments in MAG 2 were primarily associated with auditory processing, including regions consistently activated across the bilateral superior temporal gyri (Brunetti et al., 2005; Chang et al., 2010; Nourski et al., 2013; Mesgarani et al., 2014). Within the context of affective processing, the auditory network is consistently recruited across studies irrespective of specific emotional domains (Lindquist et al., 2012). More specifically, regions in this meta‐analytic network have demonstrated involvement in the recognition of emotional prosody and verbal components of spoken language (Buchanan et al., 2000), as well as participating in the judgment of emotional prosody when considering either words or music (Ethofer et al., 2006; Koelsch, Fritz, Cramon, Muller, & Friederici, 2006). Beyond simple acts of auditory perception, the role of this meta‐analytic network in the domain of affective processing may be one of interpreting emotional subjectivity of rhythm, intonation (Wildgruber et al., 2005), and/or pitch (Zarate, Wood, & Zatorre, 2010). Thus, we interpret MAGs 1 and 2 as meta‐analytic networks associated with perception of external stimuli, which in the current context were utilized to elicit emotional experiences.
In contrast, MAGs 3, 4, and 5 were associated with “higher‐order”, non‐perceptual functions associated with emotional processing. Experiments in MAG 3 revealed significant convergence of activation in regions associated with the salience network (Seeley et al., 2007), including the dorsal anterior cingulate and orbital frontoinsular cortices, regions also involved in the detection and evaluation of emotional pictures (Phan et al., 2004), facial stimuli (Britton, Taylor, Sudheimer, & Liberzon, 2006), and auditory pitch (Zarate et al., 2010). Here, assessing and evaluating relevant stimuli is thought to be the product of integrating processes associated with executive attention (Corbetta et al., 2002; Touroutoglou, Hollenbeck, Dickerson, & Barrett, 2012), interoception (Critchley et al., 2000; Critchley et al., 2004), and representations of affect, which in turn guide attention and behavior (Lindquist & Barrett 2008; Medford & Critchley, 2010). MAG 3 also included the inferior frontal and cingulate gyri, regions characterized by their collective involvement in attentional processes and response selection (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003, 2004; Badre, Poldrack, Paré‐Blagoev, Insler, & Wagner, 2005; Wager, Jonides, Smith, & Nichols, 2005; Wagner, Maril, Bjork, & Schacter, 2001). Supporting the integration of these regions into a unified network, recent meta‐analyses have recognized the functional coupling of these regions during affective processing (Lindquist et al., 2012; Ochsner et al., 2012), suggesting their role in extraction of emotionally salient information from perceived stimuli.
MAG 4 corresponded to experiments involving theory of mind tasks and social cognition, recruiting the medial prefrontal, posterior cingulate cortices, and precuneus (Spreng et al., 2009; Martinelli, Sperduti, & Piolino, 2013), collectively resembling constituents of the default‐mode network (Raichle et al., 2001; Laird et al., 2009a). Activation of regions within this meta‐analytic network has been associated with recognition of familiar faces (Leveroni et al., 2000; Gorno Tempini et al., 1998; Shah et al., 2001; Ramasubbu et al., 2007), internal meditation (Brewer et al., 2011), and prediction of rewarding or reinforcing outcomes (Knutson & Cooper, 2005; O'Doherty, 2004; Schultz, 2007; Bartra, McGuire, & Kable, 2013). Furthermore, this network has been linked to context‐sensitive predictions about others' thoughts and feelings (Saxe & Kanwisher, 2003), combining perceived information with personal experiences. The current results suggest that default‐mode network regions' involvement in affective processing may relate to internal stimuli involving reflection upon stored representations of previous emotional experiences to salient stimuli.
Convergence across the bilateral amygdala, bilateral fusiform gyri, and parahippocampal gyri observed in MAG 5 represents hallmark features of emotion generation (Ochsner et al., 2012). The regions comprising this meta‐analytic network have been implicated across a range of emotional domains (Neta & Whalen, 2011; Whalen et al., 2004; Vuilleumier & Pourtois, 2007), and demonstrate recruitment during perception of faces (Sergent, Ohta, & MacDonald, 1992; Haxby et al., 1994, 1999; Clark et al., 1996; Kanwisher, McDermott, & Chun, 1997; McCarthy, Puce, Gore, & Allison, 1997; Halgren et al., 1999; Ishai, Ungerleider, Martin, Schouten, & Haxby, 1999; Hoffman & Haxby, 2000), encoding of emotional stimuli (Davis & Whalen, 2001; Oschner et al., 2002; Davachi, 2006; Phelps, 2006; Cunningham et al., 2008, 2010; Hariri & Whalen, 2011), and associated with memory of previously encoded stimuli (Cahill et al., 1996; Hamann, Ely, Grafton, & Kilts, 1999; Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000). Furthermore, the involvement of regions in this meta‐analytic network in response generation is well documented, specifically as a function of stimulus evaluation in a context and goal‐dependent manner (Damasio, 1994; Oya et al., 2005; Hare, Camerer, & Rangel, 2009; Roy, Shohamy, & Wager, 2012;). Overall, we suggest this meta‐analytic network may represent regions participating in the generation of contextually relevant emotional responses based on the complex integration of salient features of external sensory input with internal stimuli and/or previous affective experiences.
4.2. A meta‐analytic model for emotional processes
Our meta‐analytic outcomes demonstrate correspondence with the contemporary model of affective processing (Ochsner et al., 2012) and provide support for intermediary functional participation of multiple networks during emotion regulation tasks. Specifically, we observed that MAGs 1 and 2 exhibited consistent activation across primary and associative visual and auditory cortices, respectively, implicating their roles in stimulus detection and/or perception (Figure 5, “Visual” and “Auditory”). MAG 1 was linked to identifying either internally‐ (e.g., imagined objects/scenes) or externally‐ (e.g., affective pictures) driven visual stimuli, whereas MAG 2 demonstrated involvement in audition and passive listening. In contrast, MAG 3 resembled the salience network and was associated with detection of relevant features of the stimuli (Figure 5, “Attention”) for assessment in subsequent stages of the model. MAG 3 included tasks requiring “discrimination”, requiring extraction of meaningful details of faces, shapes, and emotional pictures to elicit an emotional response. Following extraction of salient features, MAG 4 included canonical nodes of the default‐mode network and its functional decoding results were consistent with an appraisal process relating previous experiences and emotional consequences (Figure 5, “Appraisal”). Critical structures involved in such appraisal processes include the dorsomedial PFC, dorsolateral PFC, inferior parietal lobules, and ventrolateral PFC (Kohn et al., 2015). Accordingly, MAG 4 may operate during tasks (e.g., social cognition, theory of mind, and episodic recall) to coordinate the integration of subjective prior experience, empathetic gestures, or predictions of others' feelings in a social setting with the significant context of presented stimuli (Lamm, Decety, & Singer, 2011). MAG 5 was consistent with a final stage of the model involving the cumulative integration of contextual stimuli and previous knowledge into the generation of an appropriate emotional state (Figure 5, “Response”). Here, a core network of limbic regions, highlighted by the amygdala, execute the emotional response as a function of the sequential processing of stimuli, salient features, and individual appraisals. In the context of neurobiology, the extent of the emotional response in the amygdala and parahippocampal gyri may be dependent on memory encoding of previous responses.
Figure 5.
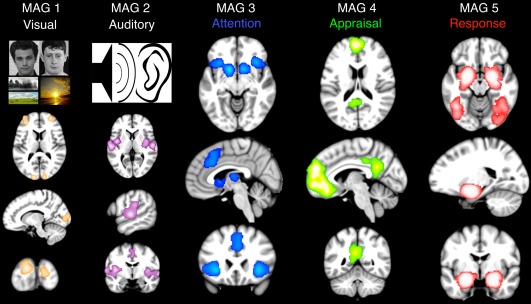
Meta‐analytic model of affective processing. Meta‐analytic networks recruited during emotional experiments demonstrated correspondence with a contemporary psychological model of affective processing (Ochsner et al., 2012). MAGs 1 and 2 correspond to “Visual” and “Auditory” perception, respectively, associated with sensory input. MAG 3 corresponds to the salience network associated with “Attention” and the detection/selection of stimulus features contextually most relevant for further processing. MAG 4 corresponds to the default‐mode network associated with subjective recall, evaluation, and integration of goals, wants, or needs during “Appraisal”. MAG 5 corresponds to the limbic network associated with the generation of an emotional “Response” based on context‐relevant interpretations [Color figure can be viewed at http://wileyonlinelibrary.com]
An additional feature of the model presented by Ochsner et al., (2012) is related to the regulation of emotions and more specifically, cognitive reappraisal. Here, cognitive reappraisal is understood as the reinterpretation of the meaning of a perceived stimulus by way of one's personal connection to it, thereby altering one's emotional response, whether consciously (Beauregard, Levesque, & Bourgouin, 2001; Beer, Heerey, & Keltner, 2003) or unconsciously (Williams, Bargh, Nocera, & Gray, 2009). Hypotheses of neural involvement in emotion regulation suggest dorsolateral and posterior prefrontal cortices and inferior parietal regions direct attention to reappraisal‐relevant stimulus features as well as the content of one's reappraisal (Miller, 2000; Wager & Smith, 2003; Wager, Jonides, & Reading, 2004). Additionally, dorsal anterior cingulate may monitor the extent to which one's current reappraisals are changing emotional responses in the intended way (Botvinick, Cohen, & Carter, 2004) and ventrolateral prefrontal cortex selects goal‐appropriate reappraisals of the re‐evaluated stimulus (Thompson‐Schill, Bedny, & Goldberg, 2005; Badre & Wagner, 2007). Indeed, previous meta‐analyses highlight these systems as contributing to emotion regulation processes (Frank et al., 2014; Kohn et al., 2015). Incidentally, the meta‐analytic network associated with MAG 3 is representative of some of those aforementioned regions, such as the ventrolateral and dorsomedial prefrontal cortices, while experiments within MAG 3 also focus on attention to secondary or forthcoming stimuli, requiring a cognitive component to recognize and assess the current situation while retaining information about a previously elected goal. Furthermore, Ochsner's model proposes that the ventromedial prefrontal cortex mediates relations between prefrontal regions and the amygdala (Urry, 2006; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007) while temporal gyri represent semantic and perceptual features. In this instance, it may be hypothesized that default‐mode regions provide valuation to the relevant stimuli during the process of reappraisal. As such, examining the associated functional interpretations of brain regions associated with MAG 4 also suggest its potential role as a potential intermediary system during the emotion regulation process. Despite the similarities between meta‐analytic networks in MAGs 3 and 4 with proposed regions involved in the cognitive reappraisal process (Ochsner et al., 2012), other studies specifically assessing the neural basis for cognitive reappraisal (Ochsner et al., 2012; Buhle et al., 2014; Kohn et al., 2015) also suggested or identified the involvement of the dorsolateral prefrontal cortex and superior parietal lobule. While the current study was not specifically intended to identify regions associated with the cognitive reappraisal process, the distribution of brain regions involved in such a system across multiple MAGs indicates a potential multiple‐network dynamic. This idea is currently being explored and will be presented in future work.
While our meta‐analytic findings are discussed in the context of an “evolving” model of the cognitive control of emotion outlined by Ochsner et al., (2012), the proposed model is itself an assembly of appraisal and emotion generation theories. Thus, to the extent that our findings demonstrate correspondence with the Ochsner et al. (2012) model, the outcomes are also broadly consistent with elements of related models. For instance, regarding emotion generation, current neuroscience literature suggests that there may not be specific neural systems for different discrete emotions (Kober et al., 2008; Wager et al., 2008), and our functional decoding results indicate that emotional domains are agnostic of meta‐analytic network involvement. Additionally, Ochsner's model may be extended to emotion regulation strategies beyond reappraisal, such as attentional deployment (Dolcos, Iordan, & Dolcos, 2011; Villemure & Bushnell, 2002), situation selection and modification in which prefrontal cortices and amygdala are recruited (Everitt et al., 1999; LeDoux & Gorman, 2001; Delgado, Jou, Ledoux, & Phelps, 2009), and response modulation, which relies on goal maintenance, response selection, and inhibition/suppression (Aron et al., 2004; Thompson‐Schill et al., 2005; Badre & Wagner, 2007; Goldin, McRae, Ramel, & Gross, 2008; Hayes et al., 2010). Finally, Ochsner et al. (2012) suggests this model may potentially explain other cognitive‐affective dynamics that rely on similar recruitment of neurological systems, such as affective/emotional learning, decision making, and expectancies.
In summary, our results highlight multiple meta‐analytic brain networks associated with emotional processing, and functional decoding of these observed MAGs provide objective delineation of their functions. These meta‐analytic networks may be recruited during emotion perception, detecting indicators of affect, appraisal of emotion relative to one's own memories and experiences, and the generation of an emotional response. Additionally, MAGs 3 and 4 may differentially participate in emotion regulation strategies whereby regions associated with cognitive control (MAG 3) maintain goal‐relevant information and monitor subsequent responses while emotionally‐relevant stimuli are reappraised according to one's own valuation (MAG 4) of internally or externally (social) motivated situations. We have demonstrated that large‐scale data mining of the BrainMap database provides a means to evaluate existing cognitive models of brain function, similar to our previous work (Laird et al., 2015) in confirming and extending a well‐known model of face perception (Gobbini & Haxby, 2007). Overall, a strength of this meta‐analytic approach is that it supports the idea that most complex, real‐world psychological constructs cannot be measured by a single task, but rather must be probed across a wide range of experimental designs.
4.3. Divergence from other meta‐analytic approaches
Throughout the analysis and interpretation stages of this work, we examined several existing meta‐analyses in the affective domain to not only verify our results were in‐line with previous findings, but also to ensure that our results offered a unique contribution to the field's understanding of emotional processing. One distinguishing characteristic of the current study from existing contributions is that our results serve as a qualifying demonstration that large‐scale meta‐analyses can provide support or even advance psychological models, whereas other investigations directly address the conversation of neurobiological substrates of affect. An initial observation when examining previous affective meta‐analyses was the clear delineation between studies attempting to localize discrete emotions to isolated brain regions (Haman et al., 2012; Kirby & Robinson, 2015) versus studies attempting to characterize large‐scale brain network contributions to emotions (Kober et al., 2008; Lindquist et al., 2012; Wager et al., 2015; Touroutoglou et al., 2015). These latter meta‐analyses specifically focused on emotion induction or experience paradigms (Kober et al., 2008; Lindquist et al., 2012) or the most commonly studied discrete emotional categories (Wager et al., 2015), and are better suited to address the neurobiological substrates associated with emotion generation. The current approach considers the diverse set of affective paradigms across the literature and thus emphasizes a more holistic interpretation of the coordinated involvement of multiple psychological constructs in emotional processing. In addition, a differentiating factor between the current study and the previously discussed meta‐analyses is that the behavioral inferences through metadata and term frequency analyses, in conjunction with the theoretical hypotheses proposed in Ochsner et al. (2012), serve to potentially inform the network dynamics involved in emotion regulation strategies. Buhle et al. (2014) and Kohn et al. (2015) presented meta‐analyses of emotion regulation studies, resulting in a large number of identified brain regions involved in cognitive reappraisal. Some of those regions were identified across multiple MAGs in the current study, and the suggested mental operations participating in emotion regulation, such as selection (discrimination) of appraisals and self‐reflective processes relevant to affective meaning (Buhle et al., 2014), are associated with MAGs 3, 4, and 5. Thus, a novel finding of the current study leverages metadata in a way not previously used to inform a theory on emotion regulation strategies.
In addition, the present results highlight the existing debate regarding the locationist and constructionist perspectives, where the former suggests emotional states contain independent neurobiological basis and the latter suggests that emotions are the eventual consequence of network interactions. While the objectives of the current study were not to address this debate, it may be worth noting that several emotional subdomains were found to be significantly associated with each MAG. This result may speak to the diverse accumulation of experimental contrasts that interrogate affective processes included in the analysis, and thus warrants further investigating and careful interpretation in the conversation of emotion generation.
4.4. Methodological issues and limitations
We observed convergence between our meta‐analytic findings, a contemporary psychological model, and emerging views of large‐scale brain network integration in affective neuroscience. Nevertheless, there are several caveats to consider when interpreting meta‐analytic outcomes. One limitation is that the use of the relative difference in cophenetic distances and experiment separation density as criteria for determining the parcellation solution is relatively simplistic, though they are not entirely different in interpretation from more advanced methods for separating variables into discrete MAGs using hierarchical clustering (Eickhoff, Thirion, Varoquaux, & Bzdok, 2015). Of the metrics suggested for thresholding a hierarchical assortment of variables, the inconsistency coefficient has received the most attention. Liu et al., (2012) demonstrated the most suitable clustering solution could be derived using a distribution of inconsistency coefficients, which necessitates the implementation of multiple dendrograms or sets of variables. Further complicating matters, the choice of the appropriate number of variables to include in a single inconsistency coefficient calculation is flexible as well, which itself requires further investigation.
A potential limitation of the current study is the strict assignment of each experiment to only one MAG. That is, some experiments assigned to a particular MAG may demonstrate spatial similarities to MA maps corresponding to experiments assigned to other MAGs. This occurs because of the hierarchical nature of the current approach, whereby one experiment is paired with another experiment or MAG based on the smallest dissimilarity of modeled activation patterns. If one were to consider the next smallest dissimilarity for a particular experiment's MA map, it is conceivable that in some instance, that may occur with an experiment assigned to a different MAG. These types of occurrences would typically be associated with complex experimental paradigms resulting in the activation of regions commonly identified in separate MAGs. Though beyond the scope of the current work, the possibility that such experiments could yield valuable information about interactions between large‐scale networks is certainly worth future exploration.
Another study limitation is the subjectivity associated with generalized interpretations of the prose descriptions of experiments based on term occurrence. While those interpretations enhanced our meta‐analytic results, we acknowledge that more statistically rigorous methods could provide a more principled approach for guiding those conclusions. To that end, we are currently developing meta‐analytic tools utilizing text‐mining algorithms for the Automated Text Harvesting and Exploration of Neuroimaging Annotations (ATHENA) project. These tools emphasize term frequencies appearing within and across neuroimaging articles, and will in the future provide a more informative ontology for describing and classifying the published literature.
With regards to the terminology used to describe the images resulting from ALE analyses of each cluster, it is worth noting the difference between “neural systems” obtained from analyses performed on raw functional imaging data and meta‐analytic networks. Neural systems, or functional brain networks, are brain regions demonstrating coherent temporal fluctuations in the BOLD signal, while the meta‐analytic networks described above consist of coordinates of activation that are consistently represented within a set of experiments. In the current usage, it would be inaccurate to state that two regions in a meta‐analysis are functionally connected or co‐activate with one another. However, meta‐analytic connectivity modeling (MACM; Laird et al., 2009a; Robinson et al., 2010; Eickhoff et al., 2010) and independent component analysis (ICA; Smith et al., 2009; Laird et al., 2011) performed on experiments archived in the BrainMap database provide supporting evidence that meta‐analytic networks indeed reflect functionally connected networks. Therefore, it is sufficient to state that the result of an ALE analysis constitutes a network of regions typifying the most commonly recruited brain areas across experiments.
With respect to meta‐analytic methodology, there are several limitations worth mentioning. First, bias is present in the BrainMap database toward experimental contrasts coded with specific metadata labels, such as the behavioral domain Cognition. The ALE algorithm does not take metadata labels into account and randomly selected gray matter coordinates are used for permutations in hypothesis testing, so this bias is not accounted for in the ALE calculation. Although a recently proposed method (Langner et al., 2014) for generating the null distribution attempts to control for the base rate of activation across the BrainMap database (which could inherently be associated with label representation), we highlight that (1) it is intended for task‐based co‐activation modeling, (2) cannot be subjected to cluster‐level multiple comparisons correction, and (3) in our experience results in more false‐positives than the previous null‐distribution approach. Conversely, other meta‐analytic algorithms, such as that used by Neurosynth (Yarkoni et al., 2011), provides both forward‐ and reverse‐inference results, accounting for biases present in the Neurosynth database. Second, Bayesian spatial point processing (Kang, Nichols, Wager, & Johnson, 2014) is another meta‐analytic algorithm that could potentially yield information regarding the inherent dissimilarity of a given construct. That is, the current study was motivated by differentiating the MA maps across MAGs; Bayesian spatial point processing could assess the degree of heterogeneity in the MA map dataset and may even be useful in assigning new studies to different categories based on coordinate‐based results.
4.5. Data sharing
In the spirit of transparency and reproducibility, we have created a GitHub repository where the scripts used to perform the above analyses as well as the thresholded and un‐thresholded meta‐analytic maps for each MAG are openly available (http://github.com/NBCLab/cmhc).
5. CONCLUSIONS
We assessed the neural activation patterns of emotional experiments archived in the BrainMap database utilizing a large‐scale data mining approach to investigate the neurobiological systems linked to affective processing. Meta‐analytic groupings of five co‐activation networks were identified demonstrating differential brain recruitment and functional properties in the context of affective experiments: (1) visual perception, (2) auditory perception, (3) attending to emotionally salient information, (4) appraisal and prediction of emotional events, and (5) induction of emotional responses. Our meta‐analytic results demonstrate correspondence with a well‐known model of affective processing, whereby emotions are instantiated as a mental state in response to personal experiences associated with the emotionally salient context of a perceived stimulus (Ochsner et al., 2012). As the field of neuroimaging continues to probe complex questions, we believe this work highlights the utility of large‐scale meta‐analytic techniques to develop, test, and refine psychological theories, providing a means to examine and emphasize neuroimaging reproducibility from a meta‐analytic perspective.
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
This study was supported by awards from the National Institute of Mental Health (R01‐MH084812, R01‐MH074457, and R56‐MH097870), the National Institute on Drug Abuse (K01‐DA037819, R01‐DA041353, U24‐DA039832, and U01‐DA041156), and the National Science Foundation (NSF 1631325).
Riedel MC, Yanes JA, Ray KL, et al. Dissociable meta‐analytic brain networks contribute to coordinated emotional processing. Hum Brain Mapp. 2018;39:2514–2531. 10.1002/hbm.24018
Funding information National Science Foundation, Grant/Award Number: 1631325; National Institute of Drug Abuse, Grant/Award Numbers: K01‐DA037819, R01‐DA041353, U01‐DA041156, and U24‐DA039832; National Institute of Mental Health, Grant/Award Numbers: R01‐MH‐74457, R01‐MH084812, and R56‐MH097870
REFERENCES
- Aron, A. R. , Fletcher, P. C. , Bullmore, E. T. , Sahakian, B. J. , & Robbins, T. W. (2003). Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, (2005). 6(2), 115–116. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8(4), 170–177. [DOI] [PubMed] [Google Scholar]
- Badre, D. , Poldrack, R. A. , Paré‐Blagoev, E. J. , Insler, R. , & Wagner, A. D. (2005). Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron, 47(6), 907–918. [DOI] [PubMed] [Google Scholar]
- Badre, D. , & Wagner, A. D. (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45(13), 2883–2901. [DOI] [PubMed] [Google Scholar]
- Balsters, J. H. , Mantini, D. , Apps, M. A. , Eickhoff, S. B. , & Wenderoth, N. (2016). Connectivity‐based parcellation increases network detection sensitivity in resting state fMRI: An investigation into the cingulate cortex in autism. Neuroimage: Clinical, 11, 494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, L. F. , & Satpute, A. J. (2013). Large‐scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Current Opinion in Neurobiology, 23, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra, O. , McGuire, J. T. , & Kable, J. W. (2013). The valuation system: A coordinate‐based meta‐analysis of BOLD fMRI experiments examining neural correlates of subject value. Neuroimage, 76, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur, V. , Hänggi, J. , Langer, N. , & Jäncke, L. (2013). Resting‐state functional and structural connectivity within an insula‐amygdala route specifically index state and trait anxiety. Biological Psychiatry, 73(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Beauregard, M. , Levesque, J. , & Bourgouin, P. (2001). Neural correlates of conscious self‐regulation of emotion. Journal of Neuroscience, 21, RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer, J. S. , Heerey, E. A. , & Keltner, D. (2003). The regulatory function of self‐conscious emotion: Insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology, 85, 594–604. [DOI] [PubMed] [Google Scholar]
- Bookheimer, S. Y. , Zeffiro, T. A. , Blaxton, T. , Gaillard, W. , & Theodore, W. (1995). Regional cerebral blood flow during object naming and word reading. Human Brain Mapping, 3(2), 93–106. [Google Scholar]
- Botvinick, M. M. , Cohen, J. D. , & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. [DOI] [PubMed] [Google Scholar]
- Bressler, S. L. , & Menon, V. (2010). Large‐scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. [DOI] [PubMed] [Google Scholar]
- Brewer, J. A. , Worhunsky, P. D. , Gray, J. R. , Tang, Y. Y. , Weber, J. , & Kober, H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. PNAS, 108(50), 20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton, J. C. , Taylor, S. F. , Sudheimer, K. D. , & Liberzon, I. (2006). Facial expressions and complex IAPS pictures: Common and differential networks. Neuroimage, 31(2), 906–919. [DOI] [PubMed] [Google Scholar]
- Brunetti, M. , Belardinelli, P. , Caulo, M. , Del Gratta, C. , Della Penna, S. , Ferretti, A. , … Romani, G. L. (2005). Human brain activation during passive listening to sounds from different locations: An fMRI and MEG study. Human Brain Mapping, 26(4), 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan, T. W. , Lutz, K. , Mirzazade, S. , Specht, K. , Shah, N. J. , Zilles, K. , & Jäncke, L. (2000). Recognition of emotional prosody and verbal components of spoken language: An fMRI study. Cognitive Brain Research, 9(3), 227–238. [DOI] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok, D. , Heeger, A. , Langner, R. , Laird, A. R. , Fox, P. T. , Palomero‐Gallagher, N. , … Eickhoff, S. B. (2015). Subspecialization in the human posterior medial cortex. Neuroimage, 106, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok, D. , Laird, A. R. , Zilles, K. , Fox, P. T. , & Eickhoff, S. B. (2013). An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping, 34(12), 3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, L. , Haier, R. J. , Fallon, J. , Alkire, M. T. , Tang, C. , Keator, D. , … McGaugh, J. L. (1996). Amygdala activity at encoding correlated with long‐term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America, 93(15), 8016–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli, T. , Zhao, Z. , Brewer, J. , Gabrieli, J. D. , & Cahill, L. (2000). Event‐related activation of the human amygdala associates with later memory for individual emotional experiences. Journal of Neuroscience, 20, RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda, F. , Costa, T. , Torta, D. M. E. , Sacco, K. , D'Agata, F. , Duca, S. , … Vercelli, A. (2012). Meta‐analytic clustering of the insular cortex: Characterizing the meta‐analytic connectivity of the insula when involved in active tasks. Neuroimage, 62(1), 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, E. F. , Rieger, J. W. , Johnson, K. , Berger, M. S. , Barbaro, N. M. , & Knight, R. T. (2010). Categorical speech representation in human superior temporal gyrus. Nature Neuroscience, 13(11), 1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik, E. C. , Zilles, K. , Caspers, S. , Roski, C. , Kellermann, T. S. , Jakobs, O. , … Eickhoff, S. B. (2013). Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation based parcellation. Cerebral Cortex, 23(1), 2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, V. P. , Keil, K. , Maisog, J. M. , Courtney, S. , Ungerleider, L. G. , & Haxby, J. V. (1996). Functional magnetic resonance imaging of human visual cortex during face matching: A comparison with positron emission tomography. Neuroimage, 4(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Collins, D. L. , Neelin, P. , Peters, T. M. , & Evans, A. C. (1994). Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography, 18(2), 192–205. [PubMed] [Google Scholar]
- Corbetta, M. , Kincade, J. M. , & Shulman, G. L. (2002). Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience, 14(3), 508–523. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Elliott, R. , Mathias, C. J. , & Dolan, R. J. (2000). Neural activity relating to generation and representation of galvanic skin conductance responses: A functional magnetic resonance imaging study. Journal of Neuroscience, 20(8), 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Ohman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. [DOI] [PubMed] [Google Scholar]
- Cunningham, W. A. , Van Bavel, J. J. , & Johnsen, I. R. (2008). Affective flexibility: Evaluative processing goals shape amygdala activity. Psychological Science, 19(2), 152–160. [DOI] [PubMed] [Google Scholar]
- Cunningham, W. A. , Arbuckle, N. L. , Jahn, A. , Mowrer, S. M. , & Abduljalil, A. M. (2010). Aspects of neuroticism and the amygdala: Chronic tuning from motivational styles. Neuropsychologia, 48(12), 3399–3404. [DOI] [PubMed] [Google Scholar]
- Damasio, A. (1994). Descartes' error: Emotion, reason, and the human brain. New York: G.P. Putnam. [Google Scholar]
- Davachi, L. (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16(6), 693–700. [DOI] [PubMed] [Google Scholar]
- Davis, M. , & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. [DOI] [PubMed] [Google Scholar]
- Delgado, M. R. , Jou, R. L. , Ledoux, J. E. , & Phelps, E. A. (2009). Avoiding negative outcomes: Tracking the mechanisms of avoidance learning in humans during fear conditioning. Frontiers in Behavioral Neuroscience, 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos, F. , Iordan, A. D. , & Dolcos, S. (2011). Neural correlates of emotion‐cognition interactions: A review of evidence from brain imaging investigations. Journal of Cognitive Psychology (Hove), 23(6), 669–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. , Jbabdi, S. , Caspers, S. , Laird, A. R. , Fox, P. T. , Zilles, K. , & Behrens, T. (2010). Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(18), 6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation revisited. NeuroImage, 59(3), 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Thirion, B. , Varoquaux, G. , & Bzdok, D. (2015). Connectivity‐based parcellation: Critique and implications. Human Brain Mapping, 36(12), 4771–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , & Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulations. Neuroimage, 137, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt, B. J. , Parkinson, J. A. , Olmstead, M. C. , Arroyo, M. , Robledo, P. , & Robbins, T. W. (1999). Associative processes in addiction and reward. The role of amygdala‐ventral strial subsystems. Annals of the New York Academy of Sciences, 877, 412–438. [DOI] [PubMed] [Google Scholar]
- Ethofer, T. , Anders, S. , Wiethoff, S. , Erb, M. , Herbert, C. , Saur, R. , … Wildgruber, D. (2006). Effects of prosodic emotional intensity on activation of associative auditory cortex. Neuroreport, 17(3), 249–253. [DOI] [PubMed] [Google Scholar]
- Fox, P. T. , Laird, A. R. , Fox, S. P. , Fox, P. M. , Uecker, A. M. , Crank, M. , … Lancaster, J. L. (2005). BrainMap taxonomy of experimental design: Description and evaluation. Human Brain Mapping, 25(1), 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, P. T. , & Lancaster, J. L. (2002). Mapping context and content: The BrainMap model. Nature Reviews. Neuroscience, 3(4), 319–321. [DOI] [PubMed] [Google Scholar]
- Fox, P. T. , Lancaster, J. L. , Laird, A. R. , & Eickhoff, S. B. (2014). Meta‐analysis in human neuroimaging: Computational modeling of large‐scale databases. Annual Review of Neuroscience, 37(1), 409–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, D. W. , Dewitt, M. , Hudgens‐Haney, M. , Schaeffer, D. J. , Ball, B. H. , Schwarz, N. F. , … Sabatinelli, D. (2014). Emotion regulation: Quantitative meta‐analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–211. [DOI] [PubMed] [Google Scholar]
- Fruhholz, S. , & Grandjean, D. (2013). Processing of emotional vocalizations in bilateral inferior frontal cortex. Neuroscience and Biobehavioral Reviews, 37(10), 2847–2855. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli, P. , Placentino, A. , Carletti, F. , Landi, P. , Allen, P. , Surguladze, S. , … Politi, P. (2009). Functional atlas of emotional faces processing: A voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience, 34, 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gobbini, M. I. , & Haxby, J. V. (2007). Neural systems for recognition of familiar faces. Neuropsychologia, 45(1), 37–41. [DOI] [PubMed] [Google Scholar]
- Goldin, P. R. , McRae, K. , Ramel, W. , & Gross, J. J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno Tempini, M. L. , Price, C. J. , Josephs, O. , Vandenberghe, R. , Cappa, S. F. , Kapur, N. , & Frackowiak, R. S. J. (1998). The neural systems sustaining face and proper‐name processing. Brain, 121(11), 2103–2118. [DOI] [PubMed] [Google Scholar]
- Halgren, E. , Dale, A. M. , Sereno, M. I. , Tootell, R. B. H. , Marinkovic, K. , & Rosen, B. R. (1999). Location of human face‐selective cortex with respect to retinotopic areas. Human Brain Mapping, 7(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann, S. B. , Ely, T. D. , Grafton, D. T. , & Kilts, C. D. (1999). Amygdala activity related to enhance memory for pleasant and aversive stimuli. Nature Neuroscience, 2(3), 289–293. [DOI] [PubMed] [Google Scholar]
- Hamann, S. (2012). Mapping discrete and dimensional emotions onto the brain: controversies and consensus. Trends in Cognitive Sciences, 16(9), 458–466. [DOI] [PubMed] [Google Scholar]
- Hare, T. A. , Camerer, C. F. , & Rangel, A. (2009). Self‐control in decision‐making involves modulation of the vmPFC valuation system. Science (New York, N.Y.), 324(5927), 646–648. [DOI] [PubMed] [Google Scholar]
- Hariri, A. R. , & Whalen, P. J. (2011). The amygdala: Inside and out. F1000 Biology Reports, 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby, J. V. , Horwitz, B. , Ungerleider, L. G. , Maisog, J. M. , Pietrini, P. , & Grady, C. L. (1994). The functional organization of human extrastriate cortex: A PET‐rCBF study of selective attention to faces and locations. Journal of Neuroscience, 14, 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby, J. V. , Ungerleider, L. G. , Clark, V. P. , Schouten, J. L. , Hoffman, E. A. , & Martin, A. (1999). The effect of face inversion on activity in human neural systems for face and object perception. Neuron, 22(1), 189–199. [DOI] [PubMed] [Google Scholar]
- Hayes, J. P. , Morey, R. A. , Petty, C. M. , Seth, S. , Smoski, M. J. , McCarthy, G. , & Labar, K. S. (2010). Staying cool when things get hot: Emotion regulation modulates neural mechanisms of memory encoding. Frontiers in Human Neuroscience, 4, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, E. A. , & Haxby, J. V. (2000). Distinct representations of eye gaze and neural identify in the distributed human neural system for face perception. Nature Neuroscience, 3(1), 80–84. [DOI] [PubMed] [Google Scholar]
- Isenberg, N. , Silbersweig, D. , Engelien, A. , Emmerich, S. , Malavade, K. , Beattie, B. , … Stern, E. (1999). Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences of the United States of America, 96(18), 10456–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai, A. , Ungerleider, L. G. , Martin, A. , Schouten, J. L. , & Haxby, J. V. (1999). Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences of the United States of America, 96(16), 9379–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, M. E. , & Moghaddam, B. (2001). Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. Journal of Neuroscience, 21(2), 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, T. , van Reekum, C. M. , Urry, H. L. , Kalin, N. H. , & Davidson, R. J. (2007). Failure to regulate: Counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. Journal of Neuroscience, 27(33), 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. , Nichols, T. E. , Wager, T. D. , & Johnson, T. D. (2014). A Bayesian hierarchical spatial point process model for multi‐type neuroimaging meta‐analysis. The Annals of Applied Statistics, 8(3), 1800–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher, N. , McDermott, J. , & Chun, M. M. (1997). The fusiform face area: A model in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe, E. G. , Toomey, J. M. , Balsters, J. H. , & Bokde, A. L. (2013). Healthy aging is associated with increased neural processing of positive valence but attenuated processing of emotional arousal: An fMRI study. Neurobiology of Aging, 34(3), 809–821. [DOI] [PubMed] [Google Scholar]
- Keightley, M. L. , Winocur, G. , Graham, S. J. , Mayberg, H. S. , Hevenor, S. J. , & Grady, C. L. (2003). An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia, 41(5), 585–596. [DOI] [PubMed] [Google Scholar]
- Kirby, L. A. J. , & Robinson, J. L. (2015). Affective mapping: An activation likelihood estimation (ALE) meta‐analysis. Brain and Cognition. In Press. [DOI] [PubMed] [Google Scholar]
- Knutson, B. , & Cooper, J. C. (2005). Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology, 18(4), 411–417. [DOI] [PubMed] [Google Scholar]
- Kober, H. , Barrett, L. F. , Joseph, J. , Bliss‐Moreau, E. , Lindquist, K. , & Wager, T. D. (2008). Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. Neuroimage, 42(2), 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch, S. , Fritz, T. , Cramon, D. Y. C. , Muller, K. , & Friederici, A. D. (2006). Investigating emotion with music: An fMRI study. Human Brain Mapping, 27(3), 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, N. , Eickhoff, S. B. , Scheller, M. , Laird, A. R. , Fox, P. T. , & Habel, U. (2015). Neural network of cognitive emotion regulation – An ALE meta‐analysis and MACM analysis. NeuroImage, 87, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, J. L. , Tordesillas‐Gutierrez, D. , Martinez, M. , Salinas, F. , Evans, A. , Zilles, K. , … Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapping, 28(11), 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Lancaster, J. L. , & Fox, P. T. (2005). BrainMap: The social evolution of a functional neuroimaging database. Neuroinformatics, 3(1), 065–078. [DOI] [PubMed] [Google Scholar]
- Laird, A. R. , Eickhoff, S. B. , Li, K. , Robin, D. A. , Glahn, D. C. , & Fox, P. T. (2009a). Investigating the functional heterogeneity of the default mode network using coordinate‐based meta‐analytic modeling. Journal of Neuroscience, 29, 14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Eickhoff, S. B. , Kurth, F. , Fox, P. M. , Uecker, A. M. , Turner, J. A. , … Fox, P. T. (2009b). ALE meta‐analysis workflows via the BrainMap database: Progress towards a functional brain atlas. Frontiers in Neuroinformatics, 3(23), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Robinson, J. L. , McMillan, K. M. , Tordesillas‐Gutierrez, D. , Moran, S. T. , Gonzales, S. M. , … Lancaster, J. L. (2010). Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. Neuroimage, 51(2), 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Fox, P. M. , Eickhoff, S. B. , Turner, J. A. , Ray, K. L. , McKay, D. R. , … Fox, P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Eickhoff, S. B. , Rottschy, C. , Bzdok, D. , Ray, K. L. , & Fox, P. T. (2013). Networks of task co‐activations. NeuroImage, 80, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Riedel, M. C. , Sutherland, M. T. , Eickhoff, S. B. , Ray, K. L. , Uecker, A. M. , … Fox, P. T. (2015). Neural architecture underlying classification of face perception. Neuroimage, 119, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm, C. , Decety, J. , & Singer, T. (2011). Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. [DOI] [PubMed] [Google Scholar]
- Langner, R. , Rottschy, C. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2014). Meta‐analytic connectivity modeling revisited: Controlling for activation base rates. Neuroimage, 99, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux, J. E. , & Gorman, J. M. (2001). A call to action: Overcoming anxiety through active coping. American Journal of Psychiatry, 158(12), 1953–1955. [DOI] [PubMed] [Google Scholar]
- LeDoux, J. E. , Iwata, J. , Cicchetti, P. , & Reis, D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience, 8(7), 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveroni, C. L. , Seidenberg, M. , Mayer, A. R. , Mead, L. A. , Binder, J. R. , & Rao, S. M. (2000). Neural systems underlying the recognition of familiar and newly learned faces. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(2), 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, K. , & Barrett, L. F. (2008). Constructing emotion: The experience of fear as. Psychological Science, 19(9), 898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, K. A. , Wager, T. D. , Kober, H. , Bliss, E. , & Barrett, L. F. (2012). The brain basis of emotion: A meta‐analytic review. Behavioral and Brain Sciences, 35(03), 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Zhu, X. H. , Qiu, P. , & Chen, W. (2012). A correlation‐matrix‐based hierarchical clustering method for functional connectivity analysis. Journal of Neuroscience Methods, 211(1), 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Y. , Qin, S. , Fernandez, G. , Zhang, Y. , Klumpers, F. , & Li, H. (2014). Emotion perception and executive control interact in the salience network during emotionally charged working memory processing. Human Brain Mapping, 35(11), 5606–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli, P. , Sperduti, M. , & Piolino, P. (2013). Neural substrates of the self‐memory system: New insights from a meta‐analysis. Human Brain Mapping, 34(7), 1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, G. , Puce, A. , Gore, J. C. , & Allison, T. (1997). Face‐specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 9(5), 605–610. [DOI] [PubMed] [Google Scholar]
- Mechelli, A. , Humphreys, G. W. , Mayall, K. , Olson, A. , & Price, C. J. (2000). Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proceedings. Biological Sciences, 267(1455), 1909–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford, N. , & Critchley, H. D. (2010). Conjoint activity of anterior insula and anterior cingulate cortex: Awareness and response. Brain Structure and Function, 214, 535–549 [Database]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesgarani, N. , Cheung, C. , Johnson, K. , & Chang, E. F. (2014). Phonetic feature encoding in human superior temporal gyrus. Science, 343(6174), 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. K. (2000). The prefrontal cortex and cognitive control. Nature Reviews. Neuroscience, 1(1), 59–65. [DOI] [PubMed] [Google Scholar]
- Murphy, F. C. , Nimmo‐Smith, I. , & Lawrence, A. D. (2003). Functional neuroanatomy of emotions: A meta‐analysis. Cognitive, Affective & Behavioral Neuroscience, 3(3), 207–233. [DOI] [PubMed] [Google Scholar]
- Neta, M. , & Whalen, P. J. (2011). The primacy of negative interpretations when resolving the valence of ambiguous facial expressions. Psychological Sciences, 21, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, J. , von Cramon, D. Y. , & Lohmann, G. (2008). Model‐based clustering of meta‐analytic functional imaging data. Human Brain Mapping, 29(2), 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl‐Jockschat, T. , Rottschy, C. , Thommes, J. , Schneider, F. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2015). Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Structure and Function, 220(4), 2355–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourski, K. V. , Brugge, J. F. , Reale, R. A. , Kovach, C. K. , Oya, H. , Kawasaki, H. , … Howard, M. A. (2013). Coding of repetitive transients by auditory cortex on posterolateral superior temporal gyrus in humans: An intracranial electrophysiology study. Journal of Neurophysiology, 109(5), 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Bunge, S. A. , Gross, J. J. , & Gabrieli, J. D. E. (2002). Rethinking feelings: An fMRI study of the cognitive regulations of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, J. P. (2004). Reward representations and reward related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology, 14(6), 769–776. [DOI] [PubMed] [Google Scholar]
- Otto, B. , Misra, S. , Prasad, A. , & McRae, K. (2014). Functional overlap of top‐down emotion regulation and generation: An fMRI study identifying common neural substrates between cognitive reappraisal and cognitively generated emotions. Cognitive, Affective, & Behavioral Neuroscience, 14(3), 923–938. [DOI] [PubMed] [Google Scholar]
- Oya, H. , Adolphs, R. , Kawasaki, H. , Bechara, A. , Damasio, A. , & Howard, M. A. (2005). Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 102(23), 8351–8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa, L. (2012). Beyond brain regions: Network perspective of cognition‐emotion interactions. Behavioral and Brain Sciences, 35(03), 158–159. [DOI] [PubMed] [Google Scholar]
- Pessoa, L. (2010). Emotion and cognition and the amygdala: From “what is it?” to "what's to be done? Neuropsychologia, 48(12), 3416–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa, L. (2008). On the relationships between emotion and cognition. Nature Reviews Neuroscience, 9(2), 148–158. [DOI] [PubMed] [Google Scholar]
- Phan, K. L. , Taylor, S. F. , Welsh, R. C. , Ho, S. H. , Britton, J. C. , & Liberzon, I. (2004). Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. NeuroImage, 21(2), 768–780. [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. (2006). Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology, 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. , & Ledoux, J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175–187. [DOI] [PubMed] [Google Scholar]
- Price, C. J. , Wise, R. J. , Watson, J. D. , Patterson, K. , Howard, D. , & Frackowiak, R. S. (1994). Brain activity during reading. The effects of exposure duration and task. Brain, 117(6), 1255–1269. [DOI] [PubMed] [Google Scholar]
- Poldrack, R. A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences, 10(2), 59–63. [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu, R. , Masalovich, S. , Peltier, S. , Holtzheimer, P. I. , Heim, C. , & Mayberg, H. S. (2007). Neural representation of maternal face processing: A functional magnetic resonance imaging study. The Canadian Journal of Psychiatry, 52(11), 726–734. [DOI] [PubMed] [Google Scholar]
- Ramponi, C. , Barnard, P. J. , Kherif, F. , & Henson, R. N. (2011). Voluntary explicit versus involuntary conceptual memory are associated with dissociable fMRI responses in hippocampus, amygdala, and parietal cortex for emotional and neutral word pairs. Journal of Cognitive Neuroscience, 23(8), 1935–1951. [DOI] [PubMed] [Google Scholar]
- Riedel, M. C. , Ray, K. L. , Dick, A. S. , Sutherland, M. T. , Hernandez, Z. , Fox, P. M. , … Laird, A. R. (2015). Meta‐analytic connectivity and behavioral parcellation of the human cerebellum. NeuroImage, 117, 327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. L. , Laird, A. R. , Glahn, D. C. , Lovallo, W. R. , & Fox, P. T. (2010). Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Human Brain Mapping, 31, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, A. K. , Shehzad, Z. , Margulies, D. S. , Kelly, A. M. C. , Uddin, L. Q. , Gotimer, K. , … Milham, M. P. (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage, 45(2), 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, M. , Shohamy, D. , & Wager, T. D. (2012). Ventromedial prefrontal‐subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe, R. , & Kanwisher, N. (2003). People thinking about people: The role of the temporo‐parietal junction in "theory of mind. NeuroImage, 19(4), 1835–1842. [DOI] [PubMed] [Google Scholar]
- Scherer, K. R. , Schorr, A. , & Johnstone, T. (2001). Appraisal processes in emotion: Theory, methods, research. New York: Oxford University Press. [Google Scholar]
- Schultz, W. (2007). Multiple dopamine functions at different time courses. Annual Review of Neuroscience, 30, 259–288. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent, J. , Ohta, S. , & MacDonald, B. (1992). Functional neuroanatomy of face and object processing: A positron emission tomography study. Brain, 115(1), 15–36. [DOI] [PubMed] [Google Scholar]
- Shah, N. J. , Marshall, J. C. , Zafiris, O. , Schwab, A. , Zilles, K. , Markowitsch, H. J. , & Fink, G. R. (2001). The neural correlates of person familiarity: A functional magnetic resonance imaging study with clinical implications. Brain: A Journal of Neurology, 124(Pt 4), 804–815. [DOI] [PubMed] [Google Scholar]
- Shannon, P. , Markiel, A. , Ozier, O. , Baliga, N. S. , Wang, J. T. , Ramage, D. , … Ideker, T. (2003). Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Research, 13(11), 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, W. K. , Avery, J. A. , Barcalow, J. C. , Bodurka, J. , Drevets, W. C. , & Bellgowan, P. (2013). Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human Brain Mapping, 34(11), 2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckman, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , Mar, R. A. , & Kim, A. S. N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta‐analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Stolarova, M. , Keil, A. , & Moratti, S. (2006). Modulation of the C1 visual event‐related component by conditioned stimuli: Evidence for sensory plasticity in early affective perception. Cerebral Cortex (New York, N.Y. : 1991), 16(6), 876–887. [DOI] [PubMed] [Google Scholar]
- Talairach, J. , & Tournoux, P. (1988). Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers. [Google Scholar]
- Thompson‐Schill, S. L. , Bedny, M. , & Goldberg, R. F. (2005). The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology, 15(2), 219–224. [DOI] [PubMed] [Google Scholar]
- Todd, J. J. , Won Han, S. , Harrison, S. , & Marois, R. (2011). The neural correlates of visual working memory encoding: A time‐resolved fMRI study. Neuropsychologia, 49(6), 1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou, A. , Hollenbeck, M. , Dickerson, B. C. , & Barrett, L. F. (2012). Dissociable large‐scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage, 60(4), 1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou, A. , Lindquist, K. A. , Dickerson, B. C. , & Barrett, L. F. (2015). Intrinsic connectivity in the human brain does not reveal networks for ‘basic’ emotions. Social Cognitive and Affective Neuroscience, 10(9), 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eden, G. F. , Jones, K. M. , & Zeffiro, T. A. (2002). Met‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage, 16(3 Pt 1), 765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, P. M. , Wiener, M. , & Fox, P. T. (2012). Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Human Brain Mapping, 33(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J. A. , & Laird, A. R. (2012). The cognitive paradigm ontology: Design and application. Neuroinformatics, 10(1), 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry, H. L. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26, 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reekum, C. M. , Johnstone, T. , Urry, H. L. , Thurow, M. E. , Schaefer, H. S. , Alexander, A. L. , & Davidson, R. J. (2007). Gaze fixations predict brain activation during the voluntary regulation of picture‐induced negative affect. NeuroImage, 36(3), 1041–1055. [DOI] [PubMed] [Google Scholar]
- Villemure, C. , & Bushnell, M. C. (2002). Cognitive modulation of pain: How do attention and emotion influence pain processing. Pain, 95(3), 195–199. [DOI] [PubMed] [Google Scholar]
- Vuilleumier, P. , & Pourtois, G. (2007). Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia, 45(1), 174–1794. [DOI] [PubMed] [Google Scholar]
- Vytal, K. , & Hamann, S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: A voxel‐based meta‐analysis. Journal of Cognitive Neuroscience, 22(12), 2864–2885. [DOI] [PubMed] [Google Scholar]
- Wager, T. D. , Jonides, J. , Smith, E. E. , & Nichols, T. E. (2005). Towards a taxonomy of attention‐shifting: Individual differences in fMRI during multiple shift types. Cognitive, Affective, and Behavioral Neuroscience, 5(2), 127–143. [DOI] [PubMed] [Google Scholar]
- Wager, T. D. , Jonides, J. , & Reading, S. (2004). Neuroimaging studies of shifting attention: A meta‐analysis. NeuroImage, 22(4), 1679–1693. [DOI] [PubMed] [Google Scholar]
- Wager, T. D. , & Smith, E. E. (2003). Neuroimaging studies of working memory: A meta‐analysis. Cognitive, Affective &Amp; Behavioral Neuroscience, 3(4), 255–274. [DOI] [PubMed] [Google Scholar]
- Wager, T. D. , Kang, J. , Johnson, T. D. , Nichols, T. E. , Satpute, A. B. , & Barrett, L. F. (2015). A Bayesian model of category‐specific emotional brain responses. PLoS Computational Biology, 11(4), e1004066–e1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Barrett, L. F. , Bliss‐Moreau, E. , Lindquist, K. , Duncan, S. , Kober, H. , … Mize, J. (2008). The Handbook of Emotion. In Lewis M., Haviland‐Jones J. M., & Barrett L. F. (Eds.), (pp. 249–271). Guilford, New York. [Google Scholar]
- Wagner, A. D. , Maril, A. , Bjork, R. A. , & Schacter, D. L. (2001). Prefrontal contribtions to executive control: fMRI evidence for functional distinction within lateral prefrontal cortex. NeuroImage, 14(6), 1337–1347. [DOI] [PubMed] [Google Scholar]
- Whalen, P. J. , Kagan, J. , Cook, R. G. , Davis, F. C. , Kim, H. , Polis, S. , … Johnstone, T. (2004). Human amygdala responsivity to masked fearful eye whites. Science, 306(5704), 2061. [DOI] [PubMed] [Google Scholar]
- Wildgruber, D. , Riecker, A. , Hertrick, I. , Erb, M. , Grodd, W. , Ethofer, T. , & Ackermann, H. (2005). Identification of emotional intonation evaluated by fMRI. NeuroImage, 24(4), 1233–1241. [DOI] [PubMed] [Google Scholar]
- Williams, L. E. , Bargh, J. A. , Nocera, C. C. , & Gray, J. R. (2009). The unconscious regulation of emotion: Nonconscious reappraisal goals modulate emotional reactivity. Emotion (Washington, D.C.), 9(6), 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Nichols, T. E. , Van Essen, D. C. , & Wager, T. D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate, J. M. , Wood, S. , & Zatorre, R. J. (2010). Neural networks involved in voluntary and involuntary vocal pitch regulation in experience singers. Neuropsychologia, 48(2), 607–618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


