Abstract
The TALLYHO (TH) mouse presents a metabolic syndrome of obesity, type 2 diabetes, and hyperlipidemia. Highly significant quantitative trait loci (QTLs) linked to adiposity (proximal) and hypercholesterolemia (distal) were previously identified on chromosome (Chr) 1 in a genome-wide scan of F2 mice from C57BL/6J (B6)×TH. In this study we generated congenic mouse strains that carry the Chr 1 QTLs derived from TH on a B6 background; B6.TH-Chr1-128Mb (128 Mb in size) and B6.TH-Chr1-92Mb (92 Mb in size, proximally overlapping). We characterized these congenic mice on chow and high fat (HF) diets. On chow, B6.TH-Chr1-128Mb congenic mice exhibited a slightly larger body fat mass compared with B6.TH-Chr1-92Mb congenic and B6 mice, while body fat mass between B6.TH-Chr1-92Mb congenic and B6 mice was comparable. Plasma total cholesterol levels were significantly higher in B6.TH-Chr1-128Mb congenics compared to B6.TH-Chr1-92Mb congenic and B6 mice. Again, there was no difference in plasma total cholesterol levels between B6.TH-Chr1-92Mb congenic and B6 mice. All animals gained more body fat and exhibited higher plasma total cholesterol levels when fed HF diets than fed chow, but these increases were greater in B6.TH-Chr1-128Mb congenics than in B6.TH-Chr1-92Mb congenic and B6 mice. These results confirmed the effect of the 128Mb TH segment from Chr 1 on body fat and plasma cholesterol values and showed that the distal segment of Chr 1 from TH is necessary to cause both phenotypes. Through bioinformatic approaches we generated a list of potential candidate genes within the distal region of Chr 1 and tested Ifi202b and Apoa2. We conclude that Chr 1 QTLs largely confer obesity and hypercholesterolemia in TH mice and can be promising targets for identifying susceptibility genes. Congenic mouse strains will be a valuable resource for gene identification.
Keywords: Obesity, Hypercholesterolemia, QTL, Congenic Mice, TALLYHO
Introduction
Obesity is associated with many comorbidities such as type 2 diabetes and coronary heart disease and affects 13% of the adult population worldwide, making it a major global health concern (Flynt and Daepp 2015). Hyperlipidemia characterized by increased plasma lipids, namely cholesterol and triglyceride, also signifies an increased risk of cardiovascular disease and often coexists with obesity (Erion and Corkey 2017). An important component in the development of obesity (Yazdi et al. 2015; Loos and Janssens 2017) and hyperlipidemia (Dron and Hegele 2016) is genetic factors. Although there are rare monogenic forms of obesity and hyperlipidemia, the genetic susceptibility to common forms is polygenic (Loos and Janssens 2017; Dron and Hegele 2016). Environmental factors are also a critical component in the development of obesity and hyperlipidemia, which is more likely the result of interplay between genes and environments with varying contributions in each individual (Doo and Kim 2015; Marais 2013). Among many environmental factors, nutrition is a strong connection between these interplays and especially, consuming food high in fat content is well known to promote obesity and hyperlipidemia (Doo and Kim 2015; Marais 2013).
The TALLYHO/Jng (TH) mouse is a polygenic model for human obesity and type 2 diabetes and characterized by increased adiposity, insulin resistance, hyperglycemia, and hyperlipidemia (Kim et al. 2001). In previous mapping study using F2 progeny of C57BL/6 (B6) and TH mice, we have identified two major quantitative trait loci (QTLs) on chromosome (Chr) 1; one linked to increased fat pad weight near D1Mit215 and the other linked to hypercholesterolemia near D1Mit113 (Stewart et al. 2010). Later, we named these QTLs; Tabw3 for the adiposity peak and Tachol1 for the hypercholesterolemia peak (Kim and Saxton 2012). To confirm the QTLs and develop a strategy for positional cloning of the responsible gene(s), in the present study, we generated and characterized congenic mice carrying these QTLs derived from the TH strain on a B6 genetic background.
Materials and Methods
Animals and diets
B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in our animal facility and TH mice used for construction of congenic strain were from our breeding colony. All mice had free access to food and water in a temperature and humidity controlled room with a 12-h light/dark cycle. At 4 weeks of age, mice were weaned onto standard rodent chow (14% kcal from fat, Purina 5001, PMI Nutrition, Brentwood, MO) or high fat (HF) diets (32% kcal from fat, 12266B, Research Diets; New Brunswick, NJ) and stayed on these diets throughout the study. Mice were euthanized by CO2 asphyxiation, and blood was collected by cardiac puncture and plasma obtained by centrifugation (1,200 g) at 4°C and stored at −20°C. Tissues were collected, frozen in liquid nitrogen and stored at −80°C. All animal studies were carried out with the approval of Marshall University Animal Care and Use Committee.
Construction of congenic mice
Mice congenic for the Chr 1 QTL genomic region were generated by marker-assisted backcrossing (Kim et al. 2005). Briefly, B6 female and TH male mice were crossed to yield F1 and then F1 was backcrossed with B6. The progeny were then genotyped with single sequence length polymorphism (SSLP) markers to select heterozygotes for the QTL. Selected heterozygotes were backcrossed again with B6 mice. This procedure was repeated 10 times, at which point female and male heterozygotes were crossed to obtain offspring homozygous for TH alleles. Homozygous mice were then interbred to maintain congenic lines. The congenic segment stretched from D1Mit213 to D1Mit113.
Genotyping by PCR
Genomic DNA was isolated from tail tips of mice using Gentra Puregene Mouse Tail Kit (Qiagen, Valencia, CA) for genotyping. SSLP primers synthesized (Sigma, St. Louis, MO) based on sequences from Mouse Genome Informatics (http://informatics.jax.org/) were assayed after PCR of genomic DNA by 3% agarose (Amresco, Solon, OH) gel electrophoresis and visualized with ethidium bromide (Thermo Fisher Scientific, Marietta, OH) staining.
Body composition
Quantitative magnetic resonance imaging was used to assess body composition including fat mass and lean mass in mice using EchoMRI-100 whole body composition analyzer (Echo Medical Systems, Houston, TX). A median of quintuple measurements for each animal was used (Parkman et al. 2016).
Indirect calorimetry, locomotor activity, and intake of food and water
An eight-chamber Comprehensive Laboratory Animal Monitoring System (CLAMS) (Columbus Instruments, Columbus, OH) was used to measure heat production, respiratory exchange ratio (RER), food intake, water intake, and locomotor activity (Stewart et al. 2012). All mice were acclimatized to monitoring cages for 24 hours prior to an additional 48 hours of recordings under the regular 12-hour light-dark cycle. In this system, heat production (kcal/hr) is calculated by multiplying the calorific value [CV = 3.815 + (1.232 × RER)] by the observed VO2 (Heat = CV × VO2). Heat production was then normalized by body weight to calculate energy expenditure (kcal/kg/hr) (Tschöp et al. 2011). RER is the ratio between the VCO2 and VO2 (RER = VCO2/VO2). Locomotor activity was determined a s ambulatory count, the number of times different infrared beams were broken in either the x- or y-axes during an interval.
Intraperitoneal glucose tolerance test
Mice were fasted overnight and injected with glucose in saline intraperitoneally (1mg/g body weight). Blood was collected via submandibular bleeding at 0, 15, 30, 60 and 120 minute after the injection. Blood glucose levels were measured and calculated using a One Touch Ultra2 Blood Glucose Monitoring System (Diagnostics Direct, Cape May Court House, NJ).
Non-fasting plasma insulin, triglyceride, and total cholesterol levels
Plasma insulin levels were determined using an ELISA kit (Crystal Chem, Downers Grove, IL). Plasma levels of total cholesterol (Thermo Electron, Louisville, CO) and free and total glycerol (Sigma) were determined using commercial colorimetric assays. Plasma true triglyceride concentrations were estimated by subtraction of free glycerol from total glycerol.
RNA isolation and real-time quantitative PCR (qRT-PCR)
Total RNA was isolated from epididymal adipose tissue and liver using RNeasy Plus Universal Midi Kit and RNeasy Midi Kit, respectively, according to the manufacturer’s instructions (Qiagen). Total RNA (2 µg) was reverse-transcribed with SUPERSCRIPT RT using oligo dT as primer to synthesize first-strand cDNA in 20-µl volume according to manufacturer’s instructions (Invitrogen, Waltham, MA). The primers used for the qRT-PCR for the interferon activated gene 202B (Ifi202b) gene were synthesized (Sigma) using sequences obtained from the published literature (forward: 5′-GGCAATGTCCAACCGTAACT-3′ and reverse: 5′-TAGGTCCAGGAGAGGCTTGA-3′) (Kimura et al. 2014) and the primers for apolipoprotein A135 II (Apoa2) gene were proprietary (PPM05347B, Qiagen). The real-time PCR reaction was carried out in a 25-µl volume in 1x SYBR Green PCR core reagents (Qiagen) containing 1 µl cDNA template diluate (1:5, v/v) and 6 pmol primers using the StepOne™ Real-Time PCR system (Thermo Fisher Scientific). For each sample, duplicate amplifications were performed and the average measurements used for data analysis. The 36B4 gene was used as a control to calculate the difference in average threshold cycle (ΔCt) values (Stewart et al. 2010).
RT-PCR and sequencing of the Ifi202b gene
The diluate cDNA described above was PCR amplified for Ifi202bNZO exon 1 and part of exon 2 using the Expand Long Template PCR system (Roche, Indianapolis, IN). The specific primers were forward (5′-CCCTCT TCCTTTACACCCAAC-3′) and reverse (5′-GCCTGGGACAGATGTCTC TT-3′) derived from Mus Musculus Ifi202b gene mRNA sequences (GenBank: JX945582.1) (Vogel et al. 2013). The PCR products were directly sequenced with primers originally used to amplify the PCR products. Sequencing was carried out automatically with fluorescent tags (Marshall University Genomics Core Facility).
Statistical analysis
Physiological data analysis
Variables were analyzed with analysis of variance, using a fixed effects factorial model of strain and diet and interaction. Least squares means were compared using Fisher's protected LSD at a 5% significance level.
Real-time qRT-PCR data analysis
Duplicate threshold cycle times were averaged for each mouse, and differences tested using a two-way ANOVA in SAS software (Cary, NC, USA), with an interaction contrast (housekeeping vs. gene interaction with strain or diet) used to estimate ΔΔCt. Data are presented as relative fold-change (Livak et al. 2001).
Bioinformatic analysis
We used a previously published catalog of sequence variants in the TH mouse (relative to the B6 strain) (Denvir et al. 2016) to list all variants lying between the markers D1Mit498 (position 135,687,493 bp on Chr 1 in the GRCm38 genome build) and D1Mit361 (186,261,999 bp on Chr 1). We restricted these to variants with the potential to alter the protein sequence: namely those with sequence ontology terms “frameshift_variant”, “inframe_deletion”, “inframe_insertion”, “missense_variant”, “stop_gained”, “stop_lost”, “initiator_codon_variant”, “splice_region_variant”, “splice_acceptor_variant”, or “splice_donor_variant”. We extracted molecular function and biological process terms for the genes containing each of these variants from the Gene Ontology (GO) database (Ashburner et al. 2000; The Gene Ontology Consortium 2015). We further restricted this collection of genes to contain only those that were associated to GO terms containing any of “cholesterol”, “fat”, “lipid”, “lipoprotein”, “lipase”, “glucose”, or “metabolic process”, to obtain a list of potential susceptibility genes.
Results
Generation of congenic mice for the obesity and hypercholesterolemia QTLs
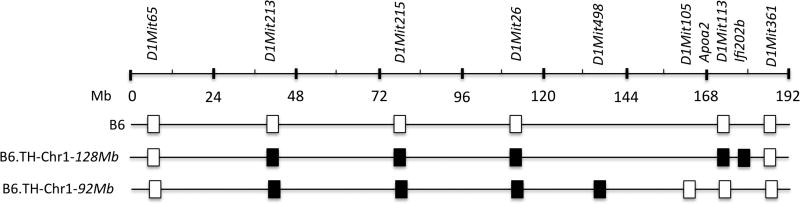
In previous mapping study, we have identified two major QTLs on Chr 1; one linked to fat pad weight (Tabw3) near D1Mit215 and the other linked to plasma cholesterol levels (Tachol1) near D1Mit113 (Stewart et al. 2010; Kim and Saxton 2012). We established a congenic line by introgressing a TH-derived genomic fragment containing both Tabw3 and Tachol1, defined by the flanking markers D1Mit213 and D1Mit113 (minimum of 128 Mb in size), onto B6 background for 10 generations. After the 10th backcross generation, the offspring were intercrossed to generate B6.TH-Chr1-128Mb congenic mice (Fig. 1). Using this congenic line we generated a second congenic line, B6.TH-Chr1-92Mb, carrying a smaller fragment of Chr 1 with only Tabw3, defined by the flanking markers D1Mit213 and D1Mit498 (minimum of 92 Mb in size) (Fig. 1).
Figure 1.
B6.TH-Chr1 congenic intervals on chromosome 1. Genetic markers shown at the top were used to allelotype the congenic interval. The open and filled boxes represent a C57BL/6 (B6) and TALLYHO (TH) allele, respectively. Mb: Megabase
Increased adiposity and plasma cholesterol levels in B6.TH-Chr1-128Mb, but not in B6.TH184 Chr1-92Mb, congenic mice
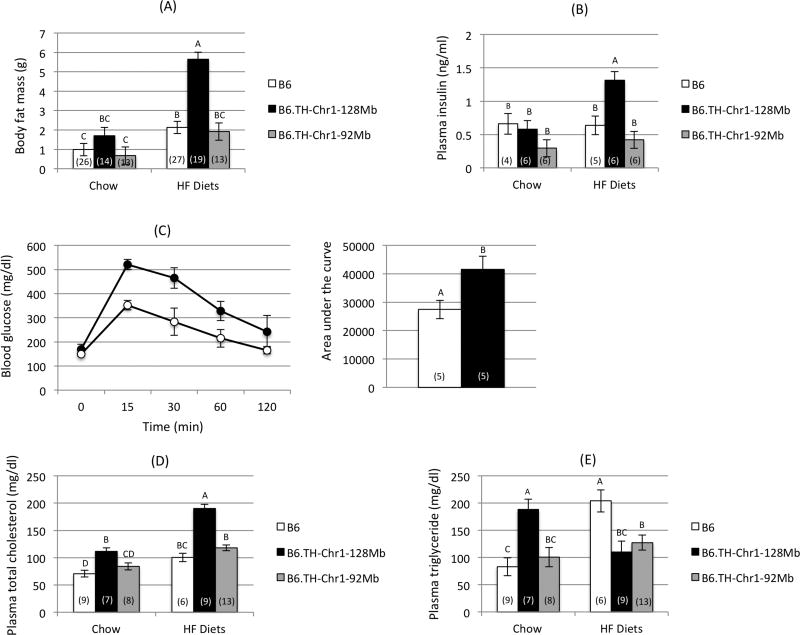
When animals were fed a standard rodent chow, B6.TH-Chr1-128Mb congenic mice exhibited a small increase in body fat mass compared with B6.TH-Chr1-92Mb congenic and B6 mice, while body fat mass between B6.TH-Chr1-92Mb congenic mice and B6 mice was comparable (Fig. 2A). When fed HF diets, all animals had more body fat than when fed chow, but the increase was greater in B6.TH-Chr1-128Mb congenic mice than either B6.TH-Chr1-92Mb congenic or B6 mice (Fig. 2A). Again, body fat mass between B6.TH-Chr1-92Mb congenic and B6 mice was comparable on HF diets (Fig. 2A). The increased adiposity in B6.TH-Chr1-128Mb congenic mice on HF diets was accompanied by moderate increases in plasma insulin levels (Fig. 2B) and slightly impaired glucose tolerance during IPGTT (Fig. 2C), indicating some degree of insulin resistance.
Figure 2.
(A) Body fat mass (14 wk), (B) plasma insulin levels (14–25 wk), (C) intraperitoneal glucose tolerance test (14–18 wk), and plasma (D) total cholesterol and (E) triglyceride levels (20–27 wk) in B6, B6.TH-Chr1-128Mb congenic, and B6.TH-Chr1-92Mb congenic mice fed chow and high fat (HF) diets (males). Data were reported as means ± SEM (N is indicated in each bar). Group means labeled with different letters are significantly different (P <0.05). wk: weeks of age
Plasma total cholesterol levels were significantly higher in B6.TH-Chr1-128Mb congenic mice compared to B6.TH-Chr1-92Mb congenic and B6 mice on chow (Fig. 2D), confirming the presence of the Tachol1 hypercholesterolemia QTL in the distal region of Chr 1. HF diets induced an increase in total plasma cholesterol in all three groups compared to chow, with the greatest degree in B6.TH-Chr1-128Mb congenic mice (Fig. 2D). Plasma triglyceride levels were also significantly higher in B6.TH-Chr1-128Mb congenic mice compared to B6.TH-Chr1-92Mb congenic and B6 mice on chow (Fig. 2E). While HF diets induced an increase in plasma triglyceride levels in B6 mice, no increases were shown in both congenic mice.
These results confirmed the effect of the 128Mb TH segment from Chr 1 on body fat and plasma cholesterol values and showed that the distal segment of Chr 1 from TH is necessary to cause both phenotypes.
Energy expenditure, locomotor activity, and food and water intakes
We measured energy expenditure and intake and locomotor activity of B6, B6.TH-Chr1-128Mb and B6.TH-Chr1-92Mb mice on chow and HF diets by CLAMS and summarized in Table 1. Overall, with increased body mass the heat production (kcal/hr) per animal increased with HF diet feeding compared to chow. However, when the heat production was normalized to body weight (kcal/kg/hr), B6.TH-Chr1-128Mb congenic mice exhibited lower values than B6.TH-92Mb congenic and B6 mice on both chow and HF diets.
Table 1.
Energy expenditure (heat production), respiratory exchange rate (RER), locomotor activity (ambulatory count), and food and water intakes in B6, B6.TH-Chr-128Mb congenic and B6.TH-Chr1-92Mb congenic mice on chow and HF diets over a 24-hour period (males, 19–23 weeks of age).
| Chow | HF diets | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| B6 | B6.TH-128Mb | B6.TH-92Mb | B6 | B6.TH-128Mb | B6.TH-92Mb | |
|
| ||||||
| N | 17 | 15 | 9 | 7 | 4 | 13 |
| BW (g) | 27.92±0.57C | 30.73±0.61B | 30.16±0.79B | 32.43±0.89B | 37.35±1.18A | 30.95±0.66B |
|
| ||||||
| Heat | ||||||
| kcal/hr | 0.45±0.01C | 0.44±0.01C | 0.46±0.01C | 0.56±0.01A | 0.55±0.01A | 0.51±0.01B |
| kcal/kg/hr | 15.97±0.26BC | 14.44±0.28D | 15.37±0.36C | 17.28±0.41A | 14.82±0.54CD | 16.68±0.30AB |
|
| ||||||
| RER | 0.92±0.01AB | 0.91±0.01BC | 0.93±0.01A | 0.89±0.01CD | 0.87±0.01D | 0.89±0.01CD |
|
| ||||||
| Food | ||||||
| G | 3.93±0.15AB | 3.90±0.16BC | 4.45±0.21A | 3.33±0.24CD | 3.17±0.32D | 3.31±0.18D |
| kcal | 13.20±0.57A | 13.11±0.60A | 14.95±0.78A | 14.68±0.89A | 13.98±1.17A | 14.61±0.65A |
|
| ||||||
| Drink (ml) | 3.54±0.17A | 3.22±0.18AB | 3.78±0.23A | 2.72±0.26BC | 2.40±0.34C | 2.87±0.19BC |
|
| ||||||
| Ambulatory count | 16210±1106AB | 13623±1178AB | 17395±1520A | 16163±1724AB | 11648±2280B | 16284±1265AB |
RER: respiratory exchange rate
ABCD means in a row with no common letter differ (P<0.05)
RER values were generally lower when animals were placed on HF diets compared to chow, demonstrating that whole body substrate metabolism was shifted towards fat oxidation. Additionally, compared to chow, in general animals consumed less food in grams on HF diets, balancing the total kcal intake. Water intake was also overall lower on HF diet. However, there was no genotype effect on RER, food intake, or water intake in either diet.
B6.TH-Chr1-128Mb congenic mice showed somewhat decreased locomotor activity compared to B6 and B6.TH-Chr1-92Mb mice on both diets, but it did not reach statistical significance.
The Ifi202b gene and Apoa2 gene in B6.TH-Chr-128Mb congenic mice
The Ifi202b gene maps to the distal region of mouse Chr 1, near the D1Mit113, Tachol1 QTL. It has been known that the gene expression levels of Ifi202b are closely associated with the obesity phenotype mediated by Nob3 obesity QTL identified from F2 population of NZO and B6 strains; mRNA of Ifi202b was generated by the NZO allele but not by the B6 allele (Vogel et al. 2012). The B6 allele of Ifi202b contains a deletion that comprised exon 1 and the 5′-flanking region of Ifi202b, causing the lack of mRNA (Vogel et al. 2012; Vogel et al. 2017).
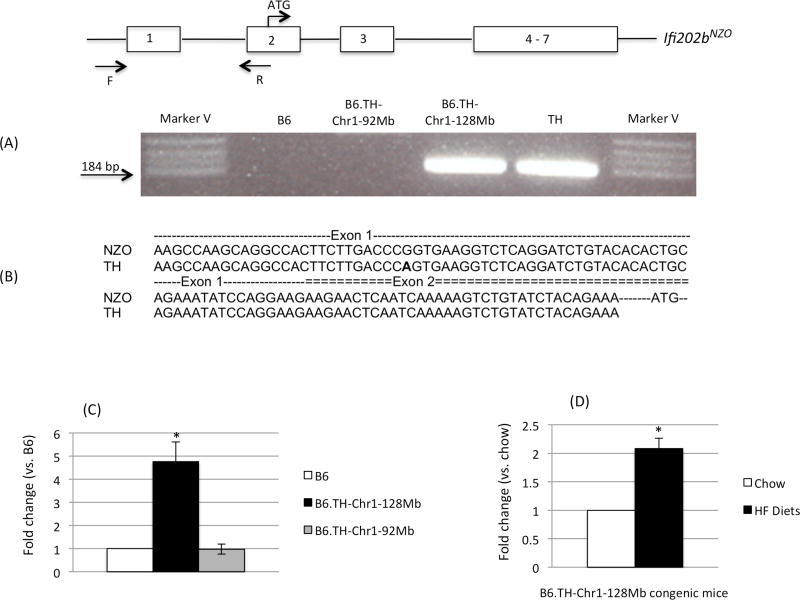
We performed PCR with primers corresponding to exon 1 and exon 2 of Ifif202b on the cDNA from adipose tissue in B6, congenic, and T H mice. B6.TH-Chr1-128Mb congenic and parental TH mice produced a PCR product, while B6 and B6.TH-Chr1-92Mb congenic mice did not as expected (Fig. 3A). We sequenced the PCR product of TH mice and found that they are the same as NZO except for one SNP in exon 1 (Fig. 3B). We then measured gene expression levels of Ifi202b in adipose tissue of B6 and congenic mice. Once the data were normalized to B6 mice having an expression value of one, B6.TH-Chr1-128Mb congenic mice had a relative expression value over 4 folds that of B6 mice, while B6.TH-Chr1-92Mb congenic mice had a comparable value to that of B6 mice on chow (Fig. 3C). The gene expression of Ifi202b was significantly up-regulated in adipose tissue of B6.TH-Chr1-128Mb congenic mice when fed HF diets compared to chow (Fig. 3D).
Figure 3.
Expression of Ifi202b in B6 and B6.TH-Chr1 congenic mice. (A) PCR amplification of exon 1 and part of exon 2 from cDNA (epididymal adipose tissue) of B6, B6.TH-Chr1-92Mb, B6.TH-Chr1-128Mb, and TH mice using a primer set of F and R as indicated in the scheme, (B) sequence comparison of the PCR product of TH to the sequence of NZO derived from GenBank accession number JX945582, and the mRNA levels of Ifi202b in epididymal adipose tissue from (C) B6, B6.TH-Chr1-128Mb, B6.TH-Chr1-92Mb mice on chow and (D) from B6.TH-Chr1-128Mb congenic mice on chow and HF diets. Data were reported as means ± SEM (N=3–4 each group, 17–27 weeks of age). * P <0.05.
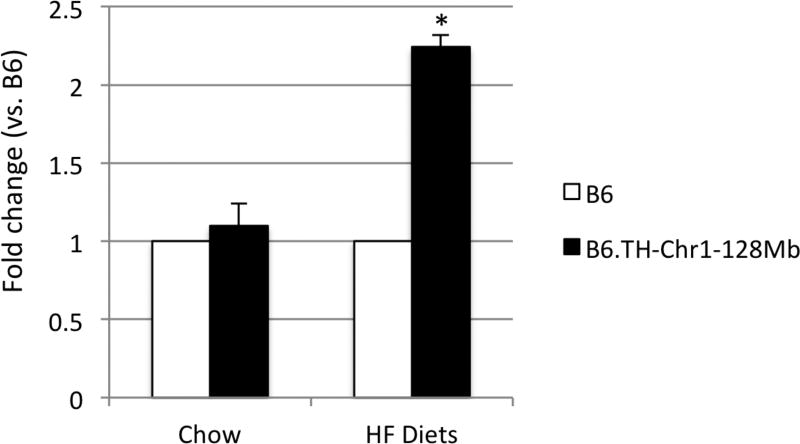
The Apoa2 gene is located near the Tachol1. An Apoa2b allele, characterized by Ala-to-Val substitution at amino acid residue 61, has been proposed to be hypermorphic in increasing plasma cholesterol levels in mice (Suto et al. 2004; Suto and Kojima 2017). The TH mouse carries the Apoa2b allele (Stewart et al. 2010 and Table 2). We examined the Apoa2 gene expression in liver from B6 and B6.TH-Chr1-128Mb congenic mice. While the Apoa2 gene expression levels were comparable between B6 and B6.TH-Chr1-128Mb congenic mice on chow, they were about 2-fold higher in B6.TH-Chr1-128Mb congenic than B6 mice on HF diets (Fig. 4).
Table 2.
Missense variants occurring in Apoa2 and Ifi202b.
| Nucleotide change (B6-TH) | Amino acid change (B6-TH) | Amino acid position | |
|---|---|---|---|
| Apoa2 | |||
| T-A | D-E | 43 | |
| A-G | M-V | 49 | |
| C-T | A-V | 61 | |
| Ifi202b | |||
| A-C | S-A | 432 | |
| T-A | T-S | 379 | |
| A-G | L-P | 350 | |
| A-T | F-I | 292 | |
| T-C | K-E | 204 | |
| G-A | T-I | 187 | |
| T-A | I-F | 142 | |
| A-C | I-M | 141 | |
| T-A | I-F | 127 | |
| T-C | E-G | 109 | |
Figure 4.
The mRNA levels of Apoa2 in liver from B6 and B6.TH-Chr1-128Mb congenic mice on chow and HF diets (males, 15–24 weeks of age). Changes in gene expression were expressed as fold change relative to mean values for B6 mice. Data were reported as means ± SEM (N=5–7 each group). * P <0.05.
Bioinformatic analysis of the distal region of Chr 1
We previously conducted a whole genome sequencing analysis for the TH mouse and generated a complete catalog of variants (relative to the B6 strain) (Denvir et al. 2016). We used this catalog to search potential candidates in the distal region of Chr 1. There were 167,833 SNPs and 36,284 indels in the region flanked by D1Mit498 and D1Mit361; of these, 934 SNPs and 89 indels had the potential to directly alter the protein product. These variants occurred in 252 unique genes, of which 223 had molecular function or biological process terms associated with them in the GO database (supplementary table S1). This list of genes included Apoa2 and Ifi202b. There were three missense variants in Apoa2 and 11 missense variants in Ifi202b (Table 2).
We further filtered the list of 223 genes to include only those whose associated GO terms included “cholesterol”, “fat”, “lipase”, “lipoprotein”, “metabolic process”, or “glucose”. This resulted in a list of 36 genes. These genes, the type of variant(s) that occur in them, and their relevant associated GO terms are shown in Table 3.
Table 3.
Genes in the region flanked by D1Mit498 and D1Mit361 with protein-altering variants that are associated with obesity-related Gene Ontology terms.
| Gene | Variant type(s) | Go Terms |
|---|---|---|
| Abl2 | missense_variant | positive regulation of phospholipase C activity |
| splice_donor_variant | ||
|
| ||
| Acbd3 | missense_variant | fatty-acyl-CoA binding; lipid metabolic process |
|
| ||
| Aldh9a1 | splice_region_variant | metabolic process; carnitine metabolic process; cellular aldehyde metabolic process |
|
| ||
| Apcs | splice_region_variant | negative regulation by host of viral glycoprotein metabolic process; negative regulation of glycoprotein metabolic process |
|
| ||
| Apoa2 | missense_variant | negative regulation of cholesterol import; high-density lipoprotein particle remodeling; cholesterol transporter activity; apolipoprotein receptor binding; high-density lipoprotein particle receptor binding; low-density lipoprotein particle remodeling; high-density lipoprotein particle clearance; negative regulation of cholesterol transport; cholesterol efflux; phospholipid efflux; phospholipid binding; fatty acid metabolic process; positive regulation of lipid catabolic process; phospholipid catabolic process; lipid transporter activity; triglyceride-rich lipoprotein particle remodeling; negative regulation of very-low-density lipoprotein particle remodeling; negative regulation of lipid catabolic process; lipoprotein metabolic process; cholesterol binding; lipid transport; cholesterol transport; positive regulation of cholesterol esterification; regulation of intestinal cholesterol absorption; reverse cholesterol transport; lipid binding; response to glucose; cholesterol metabolic process; negative regulation of lipase activity; high-density lipoprotein particle assembly; lipase inhibitor activity; high-density lipoprotein particle binding; negative regulation of cholesterol transporter activity; cholesterol homeostasis |
|
| ||
| Atp1a2 | missense_variant | ATP metabolic process |
| splice_region_variant | ||
|
| ||
| Bpnt1 | missense_variant | nucleobase-containing compound metabolic process |
| splice_region_variant | ||
|
| ||
| Crp | missense_variant | negative regulation of lipid storage; low-density lipoprotein particle receptor binding; regulation of low-density lipoprotein particle clearance; cholesterol binding; low-density lipoprotein particle binding |
|
| ||
| Ephx1 | missense_variant | cellular aromatic compound metabolic process |
| splice_region_variant | ||
|
| ||
| Eprs | missense_variant | metabolic process |
| splice_region_variant | ||
|
| ||
| Exo1 | splice_region_variant | nucleobase-containing compound metabolic process; DNA metabolic process |
|
| ||
| Fh1 | missense_variant | fumarate metabolic process; malate metabolic process |
| splice_region_variant | ||
|
| ||
| Hhipl2 | missense_variant | carbohydrate metabolic process |
| splice_acceptor_variant | ||
| splice_region_variant | ||
|
| ||
| Hsd17b7 | missense_variant | cholesterol biosynthetic process; lipid metabolic process |
|
| ||
| Itpkb | missense_variant | inositol trisphosphate metabolic process |
| splice_region_variant | ||
|
| ||
| Kmo | intron_variant | NAD metabolic process; kynurenine metabolic process |
| missense_variant | ||
| splice_region_variant | ||
|
| ||
| Lamc1 | missense_variant | glycosphingolipid binding |
| splice_region_variant | ||
|
| ||
| Marc1 | missense_variant | nitrate metabolic process |
| splice_region_variant | ||
|
| ||
| Marc2 | splice_region_variant | nitrate metabolic process |
|
| ||
| Mark1 | missense_variant | lipid binding |
| splice_region_variant | ||
|
| ||
| Mia3 | inframe_insertion | lipoprotein transporter activity; lipoprotein transport |
| missense_variant | ||
| splice_region_variant | ||
|
| ||
| Ncf2 | splice_region_variant | superoxide metabolic process; response to glucose |
|
| ||
| Ncstn | missense_variant | beta-amyloid metabolic process |
|
| ||
| Nit1 | missense_variant | nitrogen compound metabolic process |
| splice_region_variant | ||
|
| ||
| Npl | splice_region_variant | metabolic process; carbohydrate metabolic process |
|
| ||
| Parp1 | splice_region_variant | mitochondrial DNA metabolic process; DNA metabolic process |
|
| ||
| Pex19 | splice_region_variant | negative regulation of lipid binding |
|
| ||
| Pla2g4a | splice_region_variant | metabolic process; calcium-dependent phospholipase A2 activity; calcium-dependent phospholipid binding; phospholipase A2 activity; phospholipid catabolic process; lipid metabolic process; lysophospholipase activity; phospholipase activity; lipid catabolic process; arachidonic acid metabolicprocess |
|
| ||
| Ppox | splice_region_variant | protoporphyrinogen IX metabolic process |
|
| ||
| Psen2 | missense_variant | cellular protein metabolic process; beta-amyloid metabolic process |
| splice_region_variant | ||
|
| ||
| Rab3gap2 | missense_variant | positive regulation of protein lipidation |
| splice_region_variant | ||
|
| ||
| Rnasel | missense_variant | fat cell differentiation; positive regulation of glucose import in response toinsulin stimulus |
| splice_region_variant | ||
|
| ||
| Soat1 | missense_variant | cholesterol O-acyltransferase activity; cholesterol efflux; cholesterol binding; cholesterol metabolic process; cholesterol storage; very-low-density lipoprotein particle assembly; lipid metabolic process; steroid metabolic process; cholesterol homeostasis; cholesterol esterification |
| splice_region_variant | ||
|
| ||
| Tnr | missense_variant | sphingolipid binding |
|
| ||
| Uap1 | missense_variant | metabolic process; UDP-N-acetylglucosamine metabolic process |
| splice_region_variant | ||
|
| ||
| Usf1 | inframe_deletion | glucose metabolic process; positive regulation of transcription from RNA polymerase II promoter by glucose; lipid homeostasis |
Discussion
In this study, we generated congenic mice for previously mapped Chr 1 QTLs linked to adiposity (Tabw3) and hypercholesterolemia (Tachol1) derived from a TH fragment on a B6 background. On the B6 background, the TH derived Chr1 128Mb segment, containing the Tabw3 and Tachol1 QTLs, produced obesity and hypercholesterolemia in B6.TH-Chr1-128Mb congenic mice. However, contrary to our expectations, B6.TH-Chr1-92Mb congenic mice capturing the proximal segment corresponding with the Tabw3 QTL failed to develop obesity. This may suggest that the effect of Tabw3 alone may not be large enough or it may require interacting with other loci in the distal segment, potentially Tachol1 or other obesity QTLs undetected from our original genome-wide study (Stewart et al. 2010). In this context, the obesity in B6.TH-Chr1-128Mb congenic mice may reflect this complexity. Generating congenic mice containing only the distal segment of Chr 1 may allow us to test this complexity.
Daily food intake of B6.TH-Chr1-128Mb congenic mice was comparable with that of B6 mice on both chow and HF diets, suggesting greater energy storage efficiency in the congenic mice than B6. Energy expenditure determined by heat production normalized with body weight (kcal/kg/hr) was lower in B6.TH-Chr1-128Mb congenic mice than B6 mice. These findings are consistent with the characterization of parental TH strain (Parkman et al. 2016).
Based on the published knowledge, we tested Ifi202b and Apoa2 genes as potential candidate genes within the distal region of Chr 1. It has been reported that the gene expression levels of Ifi202b are positively associated with the development of obesity in mice (Vogel et al. 2012; Vogel et al. 2017). The Ifi202b (also called p202) is known to play a role in cell proliferation and differentiation including adipocyte differentiation (Li et al. 2014). The mRNA levels of Ifi202b were significantly higher in adipose tissue from B6.TH-Chr1-128Mb than B6 mice and they were significantly upregulated in response to HF diets, suggesting a potential gene-diet interaction.
Several studies have identified highly significant cholesterol QTLs in the Tachol1 interval and proposed the Apoa2 gene as a likely candidate (Machleder et al. 1997; Suto et al. 2004; Suto et al. 2017). APOA2, synthesized in liver, has an antagonist effect on cellular cholesterol efflux (Bandarian et al. 2012). Transgenic mice overexpressing Apoa2 develop hypercholesterolemia (Warden et al. 1993), while knockout of the gene in mice reduces plasma cholesterol levels (Weng and Breslow 1996). Further, transgenic mice overexpressing Apoa2 have larger fat mass than controls (Castellani et al. 2001). The TH mouse carries the APOA2-Val61 allele that is putatively hypermorphic in increasing cholesterol levels. This polymorphism, however, does not seem to directly alter the mRNA levels of Apoa2 as they were comparable between B6 and B6.TH-Chr1-128Mb congenic mice on chow. It was interesting to observe that the mRNA levels of Apoa2 were significantly higher in B6.TH-Chr1-128Mb congenic than B6 mice on HF diets.
Through bioinformatic approaches using a catalog of sequence variants between B6 and TH mice we generated a list of potential candidate genes (Table 3). Among these, genes that cause obesity in knockout mice include the ATPase, Na+/K+ transporting, alpha 2 polypeptide (Atp1a2) gene (Kawakami et al. 2005) and poly (ADP-ribose) polymerase family, member 1 (Parp1) gene (Devalaraja-Narashimha and Padanilam 2010). On the other hand, transgenic mice overexpressing the upstream transcription factor 1 (Usf1) gene exhibit lower adiposity and lower plasma cholesterol levels than wild type mice (Wu et al. 2010).
In addition to the genes for which obesity or cholesterol phenotype was found in knockout or transgenic mice, other genes are also noticeable in the list based on known function. The hydroxysteroid (17-beta) dehydrogenase 7 (Hsd17b7) gene encodes an enzyme required for the cholesterol biosynthesis in liver and is known to be downregulated in a hypocholesterolemic rodent model (Nemoto et al. 2013). The phospholipase A2, group IVA (cytosolic, calcium-dependent) (Pla2g4a) gene encodes a calcium activated enzyme that catalyzes the hydrolysis of membrane phospholipids to release arachidonic acid, mediating eicosanoid-driven inflammation; polymorphism in Pla2g4a was associated with obesity resistant phenotype in a primate model (Harris et al. 2016). The C-reactive protein (Crp) gene and serum amyloid P-component (Apcs, also called Sap) gene are located in close proximity on Chr 1. CRP and SAP are a superfamily of proteins that are characterized by pentraxin domain at the carboxyl-terminus and play key roles in innate immunity and inflammation (Garlanda et al. 2005). Human circulating CRP (Yudkin et al. 1997) and SAP (Jenny et al. 2007) concentrations were positively associated with BMI. Bochud et al. (2009) also suggested CRP as a causal factor for the obesity in human.
In conclusion, by analysis using congenic strains, we confirmed the effect of the 128Mb TH segment from Chr 1 in increasing body fat and plasma cholesterol values and demonstrated that the distal segment of Chr 1 from TH is necessary to cause both phenotypes. Our studies provided logical information regarding potential candidate genes for the Chr 1 QTL. The congenic strains will be a valuable resource for the identification of genes underlying these complex traits and their interactions with diets.
Supplementary Material
Acknowledgments
This work was supported in part by NIH/NIDDK Grant R01DK077202, American Heart Association Grant 0855300E, NIH/NIGMS P20GM103434 which funds the IDeA WV-INBRE program and supports the WV-INBRE Bioinformatics Core Facility, and institutional start-up funding from Marshall University to JHK.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandarian F, Daneshpour MS, Hedayati M, Naseri M, Azizi F. Identification of Sequence Variation in the Apolipoprotein A2 Gene and Their Relationship with Serum High-Density Lipoprotein Cholesterol Levels. Iran Biomed J. 2016;20(2):84–90. doi: 10.7508/ibj.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochud M, Marquant F, Marques-Vidal PM, Vollenweider P, Beckmann JS, Mooser V, Paccaud F, Rousson V. Association between C-reactive protein and adiposity in women. J Clin Endocrinol Metab. 2009;94(10):3969–3977. doi: 10.1210/jc.2008-2428. [DOI] [PubMed] [Google Scholar]
- Castellani LW, Goto AM, Lusis AJ. Studies with apolipoprotein A-II transgenic mice indicate a role for HDLs in adiposity and insulin resistance. Diabetes. 2001;50:643–651. doi: 10.2337/diabetes.50.3.643. [DOI] [PubMed] [Google Scholar]
- Devalaraja-Narashimha K, Padanilam BJ. PARP1 deficiency exacerbates diet-induced obesity in mice. J Endocrinol. 2010;205(3):243–252. doi: 10.1677/JOE-09-0402. [DOI] [PubMed] [Google Scholar]
- Denvir J, Boskovic G, Fan J, Primerano DA, Parkman JK, Kim JH. Whole genome sequence analysis of the TALLYHO/Jng mouse. BMC Genomics. 2016;17(1):907. doi: 10.1186/s12864-016-3245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doo M, Kim Y. Obesity: interactions of genome and nutrients intake. Prev Nutr Food Sci. 2015;20(1):1–7. doi: 10.3746/pnf.2015.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron JS, Hegele RA. Genetics of Lipid and Lipoprotein Disorders and Traits. Curr Genet Med Rep. 2016;4(3):130–141. doi: 10.1007/s40142-016-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion KA, Corkey BE. Hyperinsulinemia: a Cause of Obesity? Curr Obes Rep. 2017;6(2):178–186. doi: 10.1007/s13679-017-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynt A, Daepp MIG. Diet-related chronic disease in the northeastern United States: a model-based clustering approach. Int J Health Geogr. 2015;14:25. doi: 10.1186/s12942-015-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43(Database issue):D1049–D1056. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Alcott CE, Sullivan EL, Takahashi D, McCurdy CE, Comstock S, Baquero K, Blundell P, Frias AE, Kahr M, Suter M, Wesolowski S, Friedman JE, Grove KL, Aagaard KM. Genomic Variants Associated with Resistance to High Fat Diet Induced Obesity in a Primate Model. Sci Rep. 2016;6:36123. doi: 10.1038/srep36123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Ichii O, Nakamura T, Horino T, Otsuka S, Kon Y. BXSB-type genome causes murine autoimmune glomerulonephritis: pathological correlationbetwee n telomeric region of chromosome 1 and Yaa. Genes Immun. 2014;15(3):182–189. doi: 10.1038/gene.2014.4. [DOI] [PubMed] [Google Scholar]
- Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Serum amyloid P and cardiovascular disease in older men and women: results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2007;27(2):352–358. doi: 10.1161/01.ATV.0000254150.97741.fe. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Onaka T, Iwase M, Homma I, Ikeda K. Hyperphagia and obesity in Na,K-ATPase alpha2 subunit-defective mice. Obes Res. 2005;13(10):1661–1671. doi: 10.1038/oby.2005.204. [DOI] [PubMed] [Google Scholar]
- Kim JH, Sen S, Avery CS, Simpson E, Chandler P, Nishina PM, Churchill GA, Naggert JK. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics. 2001;74(3):273–286. doi: 10.1006/geno.2001.6569. [DOI] [PubMed] [Google Scholar]
- Kim JH, Stewart TP, Zhang W, Kim HY, Nishina PM, Naggert JK. Type 2 diabetes mouse model TallyHo carries an obesity gene on chromosome 6 that exaggerates dietary obesity. Physiol Genomics. 2005;22(2):171–181. doi: 10.1152/physiolgenomics.00197.2004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Saxton AM. The TALLYHO mouse as a model of human type 2 diabetes. Methods Mol Biol. 2012;933:75–87. doi: 10.1007/978-1-62703-068-7_6. [DOI] [PubMed] [Google Scholar]
- Li H, Liu F, Guo H, Zhu Z, Jiao Y. Role of interferon-inducible protein 202 (p202) in the regulation of adipogenesis in mouse adipose-derived stem cells. Mol Cell Endocrinol. 2014;382(2):814–24. doi: 10.1016/j.mce.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Janssens AC. Predicting Polygenic Obesity Using Genetic Information. Cell Metab. 2017;25(3):535–543. doi: 10.1016/j.cmet.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Machleder D, Ivandic B, Welch C, Castellani L, Reue K, Lusis AJ. Complex genetic control of HDL levels in mice in response to an atherogenic diet. Coordinate regulation of HDL levels and bile acid metabolism. J Clin Invest. 1997;99(6):1406–1419. doi: 10.1172/JCI119300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais AD. Dietary lipid modification for mild and severe dyslipidaemias. Proc Nutr Soc. 2013;72(3):337–341. doi: 10.1017/S0029665113001298. [DOI] [PubMed] [Google Scholar]
- Nemoto K, Ikeda A, Ito S, Miyata M, Yoshida C, Degawa M. Comparison of constitutive gene expression levels of hepatic cholesterol biosynthetic enzymes between Wistar-Kyoto and stroke-prone spontaneously hypertensive rats. Biol Pharm Bull. 2013;36(7):1216–1220. doi: 10.1248/bpb.b12-01030. [DOI] [PubMed] [Google Scholar]
- Parkman JK, Mao X, Dillon K, Gudivada A, Moustaid-Moussa N, Saxton AM, Kim JH. Genotype-dependent Metabolic Responses to Semi-Purified High-Sucrose High-Fat Diets in the TALLYHO/Jng vs. C57BL/6 Mouse during the Development of Obesity and Type 2 Diabetes. Exp Clin Endocrinol Diabetes. 2016;124(10):622–629. doi: 10.1055/s-0042-109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TP, Kim HY, Saxton AM, Kim JH. Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J × TALLYHO/JngJ) F2 mice. BMC Genomics. 2010;11:713. doi: 10.1186/1471-2164-11-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TP, Mao X, Aqqad MN, Uffort D, Dillon KD, Saxton AM, Kim JH. Subcongenic analysis of tabw2 obesity QTL on mouse chromosome 6. BMC Genet. 2012;13:81. doi: 10.1186/1471-2156-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto J, Takahashi Y, Sekikawa K. Quantitative trait locus analysis of plasma cholesterol and triglyceride levels in C57BL/6J × RR F2 mice. Biochem Genet. 2004;42(9–10):324–363. doi: 10.1023/b:bigi.0000039809.40228.d3. [DOI] [PubMed] [Google Scholar]
- Suto JI, Kojima M. Identification of Quantitative Trait Loci That Determine Plasma Total-Cholesterol and Triglyceride Concentrations in DDD/Sgn and C57BL/6J Inbred Mice. Cholesterol. 2017;2017:3178204. doi: 10.1155/2017/3178204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H, Scherneck S, Kanzleiter T, Benz V, Kluge R, Stadion M, Kryvych S, Blüher M, Klöting N, Joost HG, Schürmann A. Loss of function of Ifi202b by a microdeletion on chromosome 1 of C57BL/6J mice suppresses 11β-hydroxysteroid dehydrogenase type 1 expression and development of obesity. Hum Mol Genet. 2012;21(17):3845–3857. doi: 10.1093/hmg/dds213. [DOI] [PubMed] [Google Scholar]
- Vogel H, Montag D, Kanzleiter T, Jonas W, Matzke D, Scherneck S, Chadt A, Töle J, Kluge R, Joost HG, Schürmann A. An interval of the obesity QTL Nob3.38 within a QTL hotspot on chromosome 1 modulates behavioral phenotypes. PLoS One. 2013;8(1):e53025. doi: 10.1371/journal.pone.0053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H, Jähnert M, Stadion M, Matzke D, Scherneck S, Schürmann A. A vast genomic deletion in the C56BL/6 genome affects different genes within the Ifi200 cluster on chromosome 1 and mediates obesity and insulin resistance. BMC Genomics. 2017;18(1):172. doi: 10.1186/s12864-017-3552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden CH, Hedrick CC, Qiao JH, Castellani LW, Lusis AJ. Atherosclerosis in transgenic mice overexpressing apolipoprotein A-II. Science. 1993;261:469–472. doi: 10.1126/science.8332912. [DOI] [PubMed] [Google Scholar]
- Weng W, Breslow JL. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proc Natl Acad Sci U S A. 1996;93(25):14788–14794. doi: 10.1073/pnas.93.25.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Mar-Heyming R, Dugum EZ, Kolaitis NA, Qi H, Pajukanta P, Castellani LW, Lusis AJ, Drake TA. Upstream transcription factor 1 influences plasma lipid and metabolic traits in mice. Hum Mol Genet. 2010;19(4):597–608. doi: 10.1093/hmg/ddp526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi FT, Clee SM, Meyre D. Obesity genetics in mouse and human: back and forth, and back again. PeerJ. 2015;3:e856. doi: 10.7717/peerj.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.