Abstract
Population studies have linked insulin resistance to systemic low-grade chronic inflammation and have reported elevated levels of inflammatory cytokines such as TNFα, IL-1β and IL-6, individually or in certain combinations, in adipose tissues or in the serum. We undertook this comprehensive study to simultaneously evaluate the expression of several pro-inflammatory and anti-inflammatory cytokines in serum and in the visceral and subcutaneous adipose tissues from obese patients undergoing bariatric surgery. We observed that several inflammatory cytokines implicated in obesity-associated inflammation showed no significant difference in protein or gene expression between obese patients with or without diabetes and control groups. IL1B gene expression was significantly elevated in the visceral adipose tissues of obese patients, but did not correlate with their diabetes status. Despite the significant increase in IL1B expression in the obese group, a significant proportion of obese patients did not express TNFA, IL1B or IL6 in visceral adipose tissues. Certain inflammatory cytokines showed correlation with the chemokine CCL2 and VEGF-A in visceral adipose tissues. Our findings suggest that the inflammatory cytokine profile in metabolic syndrome is more complex than what is currently perceived and that chronic inflammation in obese patients likely results from incremental contribution from different cytokines and possibly other inflammatory mediators from within and outside the adipose tissues. It is possible that this obesity associated chronic inflammation is not predicted by a single mediator, but rather includes a large spectrum of possible profiles.
Keywords: Cytokine expression, Visceral adipose tissue, Subcutaneous adipose tissues, Bariatric surgery, Chronic inflammation
Abbreviations: AT, Adipose tissues; IL, interleukin; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; T2D, Type 2 diabetes
Highlights
-
•
Visceral and subcutaneous adipose tissues do not express similar pattern of cytokines.
-
•
VAT and SAT tissues from 30% of the obese patients do not express TNFA, IL6 or IL1B.
-
•
Protein levels and gene expression do not necessarily correlate in VAT or SAT.
-
•
The expression pattern of inflammatory mediators may present a larger spectrum than predicted from animal models.
Obesity, type 2 diabetes and cardiometabolic diseases are associated with a low-grade chronic inflammation. Various inflammatory mediators have been shown to mediate this inflammation. In this study we analyzed the expression of many of these inflammatory mediators in the visceral and subcutaneous adipose tissues obtained from patients undergoing bariatric surgery. Our results suggest that the profile of inflammatory mediators expressed in adipose tissue is diverse and varies from one patient to another.
1. Introduction
Excess visceral adiposity is associated with insulin resistance (IR), type 2 diabetes (T2D), atherosclerosis, hypertension and liver disorders (Despres and Lemieux, 2006). Adipose tissue, whose primarily function is to store energy in the form of triglycerides for release during starvation, exercise or infections, is also considered as an endocrine organ (Hotamisligil et al., 1993; Olefsky and Glass, 2010; Chawla et al., 2011). In the constant presence of excessive nutrient influx, storage of the excess fat leads to obesity followed by progressive development of insulin resistance. Many pathways have been proposed to explain the molecular mechanisms involved in the development of insulin resistance (Shoelson et al., 2006). In general, metabolic stress can activate JNK and NF-κB signaling pathways in adipose tissues, liver and muscles either directly or indirectly through inflammation (Hotamisligil, 2005). A role for inflammation in metabolic syndrome was revealed by the observation that TNFα promoted insulin resistance in adipose tissues and that its neutralization improved glucose sensitivity in fa/fa rats (Hotamisligil et al., 1994). Since then, population studies have linked insulin resistance to systemic low-grade inflammation, as evidenced by increased levels of circulating C-reactive protein (CRP, an acute phase protein synthesized by the liver) (Visser et al., 1999). Others have found that the circulating levels of IL-1β and IL-6, inducers of CRP, are also increased in diabetic patients (Ouchi et al., 2011). The close relationship between inflammation and obesity is further supported by the striking decrease in circulating pro-inflammatory markers following bariatric surgery that results in important weight loss and dramatic improvements in metabolic functions (Gumbs et al., 2005; Pardina et al., 2012; Bradley et al., 2012; Ouellet et al., 2012; Fruhbeck, 2015; Catalan et al., 2007).
While the role of inflammatory soluble mediators and cell types are relatively well characterized in pre-clinical models wherein various components of the inflammatory cytokine signaling pathways are genetically modified, the scenario in human adipose tissues is not at all clear (Lackey and Olefsky, 2016). Moreover, despite the strong correlation of TNFα with T2D as reported in earlier studies (Hotamisligil et al., 1994), various anti-TNFα therapies failed to show consistent improvement in insulin sensitivity in patients (Gisondi et al., 2008; Gonzalez-Gay et al., 2009; Peluso and Palmery, 2016; Saraceno et al., 2008). A large-scale prospective study of 27,000 individuals showed that plasma IL-6, in the presence of detectable levels of IL-1β was an independent predictor of T2D (Spranger et al., 2003), while the expression of TNFα or IL-1β was not predictive of T2D.
Given the different outcomes in the above studies, we hypothesized that human obesity and T2D will show correlation with some or all of the inflammatory cytokines that have been implicated in inflammation associated with obesity. We undertook this study to obtain an integrated gene and protein expression profile of the various inflammatory cytokines and certain chemokines in visceral and subcutaneous adipose tissues obtained from obese patients undergoing bariatric surgery. Our results show that the cytokine expression profile in the tissues is highly variable and that 30% of obese patients do not express most of the inflammatory cytokines shown to be associated with metabolic syndrome in the adipose tissues. Furthermore, while the range of expression of cytokines was generally increased in the VAT from the obese group (BMI > 30 kg/m2), it was not dependent on the diabetes status.
2. Materials and Methods
2.1. Study Subjects, Adipose Tissue Specimens and Sera
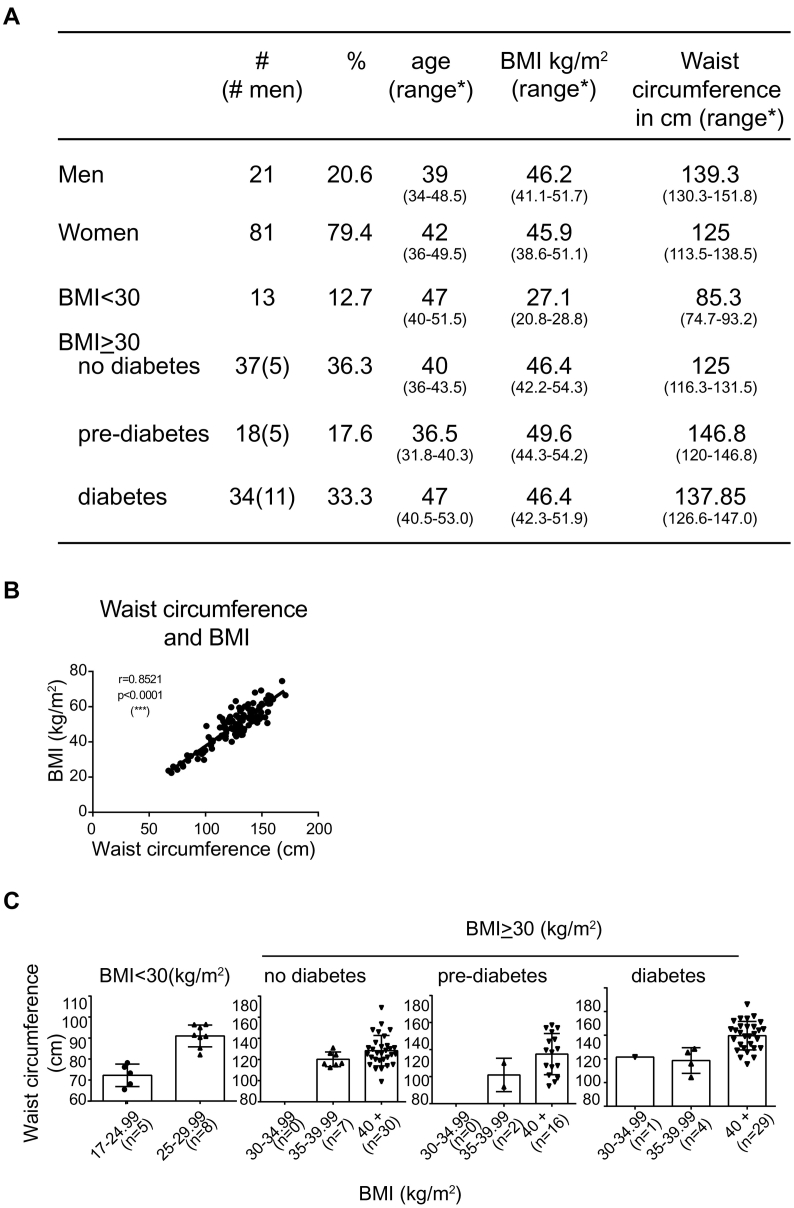
Samples of visceral (omental; VAT) and subcutaneous (SAT) adipose tissue and serum samples were obtained from 89 Caucasian men and women who underwent bariatric surgery, and 13 women with a BMI < 30 kg/m2 who underwent gynecologic surgery (control group), at Centre Hospitalier Universitaire de Sherbrooke through the adipose tissue bank established by Dr. MF Langlois (Fig. 1A). The SAT tissues were obtained at the site of incision in the abdomen. The sera were obtained 2 weeks (median 14 days) before the surgery when they were advised to follow a regimen of very low calorie diet before the bariatric surgery. We obtained the samples available in the BioBank without any specific cardiometabolic disease related exclusion criteria and the data available are derived from the medical chart except for height, weight and waist circumference that were measured by trained research personnel at the time serum was sampled. However, patients with cancer and other chronic inflammatory conditions were excluded. Most of the patients with diabetes (85%) were treated with metformin. Additionally some patients were treated with insulin (21%) or with other antidiabetic agents (50%). Approximately 75% of the obese diabetic patients were receiving treatment for hypertension. The control group consisted of patients who underwent non-inflammation-related gynecological surgery and whose BMI was <30 kg/m2. Data on the menopausal state were not part of the collection criteria. Eleven percent (9/81) were menopausal, while the menopausal status was not known for 18% (15/81). The study group with BMI ≥ 30 kg/m2 were further subdivided into those who showed no clinical manifestation of diabetes, were pre-diabetic or were diabetic based on a pre-existing clinical diagnosis, fasting blood glucose levels, oral glucose tolerance test and/or glycated haemoglobin according to the criteria established by the Canadian Diabetes Association (Canadian Diabetes Association Clinical Practice Guidelines Expert C, 2013). BMI showed highly significant correlation with waist circumference (Fig. 1B). The BMI values of most patients in the three study groups was >40 kg/m2 (Fig. 1C). Tissue specimens were immediately washed in saline buffer, snap frozen in liquid nitrogen and stored at −80 °C until use. The protocol was approved by the local ethics review board (Comité d'éthique de la recherche du CIUSSS de l'Estrie – CHUS) and all subjects gave informed consent.
Fig. 1.
Characteristics of the study population.
A) The distribution of the study population based on their diabetes status. # number of male samples in the group; * values given in brackets represent the range - 25th to 75th percentile. B) Correlation between body mass index (BMI- kg/m2) and waist circumference (in cm). C) Distribution of waist circumference in the study population that is grouped based on their BMI and diabetes status.
2.2. Analysis of Gene Expression
Total RNA from the adipose tissues samples was extracted using Trizol reagent (Invitrogen), following the manufacturer's instructions. cDNA was synthesized from 1 μg of RNA using QuantiTect Reverse Transcription Kit (Qiagen; Valencia, CA, USA). Quantitative RT-PCR amplification reactions were carried out using iCycler iQ™ (Bio-Rad, CA) PCR detection systems using iQ™ SYBR® Green Supermix kit (Bio-Rad). All reactions were run in duplicates along with no template controls for each primer sets. The list of primers used in this study is provided in Supplementary Table 1. All the tested primers showed 90–100% efficiency with single melting curve. Quantitative PCR data were analyzed by Ct method with a tolerated coefficient of variation of less than or equal to 10%. LPR10 and GADPH were used as housekeeping genes and their values were comparable (SFig. 1). Futher analyses were carried out with LRP10 as the housekeeping gene (Mehta et al., 2010). As the quantity of the tissue available from the control group (BMI < 30) was very low, it was not possible to analyze the expression of all the cytokine genes in these samples.
2.3. Protein Extraction
Proteins were extracted from frozen tissues by homogenizing in RIPA buffer containing protease inhibitors as described previously (Lacraz et al., 2016). Adipose tissue chemokines and cytokines protein were measured using multiplex assays (Multiplex Immunoassay analyzed with a Bioplex 200 using Millipore MILLIPLEX, EveTechnology, Alberta, Canada). As the lipid content can be different from one AT specimen to another, the cytokine proteins were expressed relative to the protein extracted, rather than in relation to the weight of the adipose tissue. This normalization method is similar to the analysis of cytokine gene expression which is normalized to housekeeping genes. In total we analyzed between 8 and 13 samples from each group for protein expression. Serum samples were analyzed for the presence of hsCRP using the kit from Origene Technology (Cederlane, Missisauga, Canada).
2.4. Statistical Analysis
The data obtained were expressed as mean or median with range values. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc.; San Diego, CA, USA). Data are analyzed by one-way ANOVA with Kruskal-Wallis test (significance p < 0.05) followed by Dunn's post test for multiple comparison. Correlation tests were performed by linear regression test and by non-parametric Spearman's rank correlation test where p < 0.05 was the threshold of significance. As the sample size was <20 in any given group, normality test was not applied (Ghasemi and Zahediasl, 2012; Yap and Sim, 2011).
3. Results
The cytokines and chemokines that were analyzed in this study were broadly categorized into cytokines associated with the innate immune system, adaptive immune system and cytokines known for their anti-inflammatory role in obesity and insulin resistance based on reported findings from animal models (Wensveen et al., 2015; Ota, 2013). We did not observe any significant difference in the levels of these proteins in the sera between controls and obese patients (SFig. 2). Similarly, the hsCRP levels were not significantly different between the control and obese groups (SFig. 3). Therefore we did not pursue the analysis of the sera in additional samples, and focused on adipose tissue specimens.
3.1. Inflammatory Cytokines in Adipose Tissues of Obese Patients
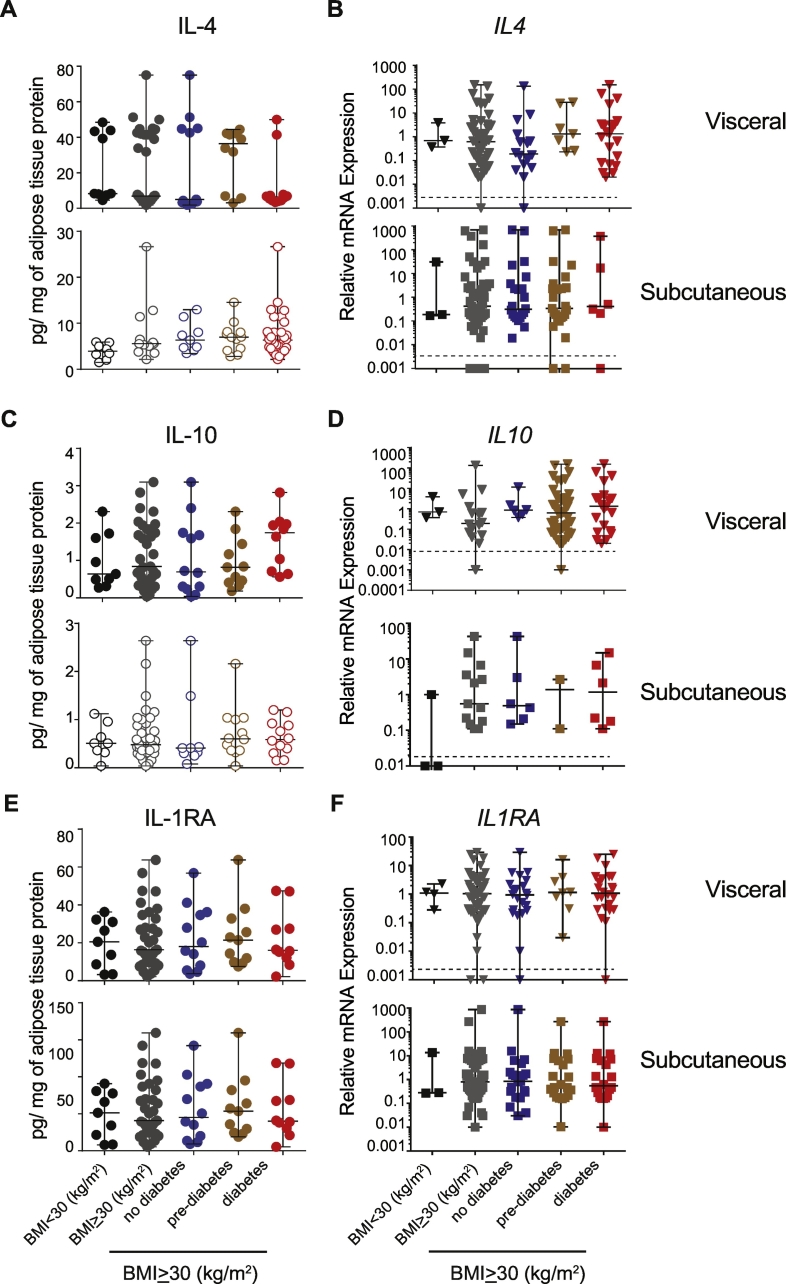
The expression of select pro-inflammatory cytokines, which have been shown to be associated with human obesity (Lackey and Olefsky, 2016), was analyzed at the gene and protein level in adipose tissues. Even though IL-6 protein showed elevated concentration in the VAT of obese patients (BMI ≥ 30 kg/m2), it was not significantly different from the BMI < 30 kg/m2 group, nor was it different among the non-diabetic, pre-diabetic and diabetic subgroups within the BMI ≥ 30 kg/m2 cohort (Fig. 2A). In contrast to VAT, SAT of the obese patients showed significantly diminished IL-6 compared to the values obtained for the control group. On the other hand, TNFα and IL-1β were barely detectable in the visceral and subcutaneous adipose tissue depots in both the control (BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2) groups (Fig. 2C and E). Apart from IL-6, we did not observe any significant difference in the concentration of other inflammatory cytokines such as IL-12, IL-15, IL-17, IL-1α and IFNγ between BMI < 30 kg/m2 and BMI ≥ 30 kg/m2 cohorts (SFig. 4). Again, there was no significant difference between controls and obese patients in the visceral or subcutaneous adipose tissues.
Fig. 2.
Expression of IL-6, TNFα and IL-1β in adipose tissues.
Protein and gene expression of IL-6, TNFα and IL-1β in the visceral and subcutaneous adipose tissues. Protein expression: n = 10 BMI < 30 kg/m2; n = 14 no diabetes BMI ≥ 30 kg/m2; n = 13 pre-diabetes BMI ≥ 30 kg/m2; n = 14 diabetes BMI ≥ 30 kg/m2. Gene expression: n = 10–11 BMI < 30 kg/m2; n = 30–35 no diabetes BMI ≥ 30 kg/m2; n = 13–14 pre-diabetes BMI ≥ 30 kg/m2; n = 30–40 diabetes BMI ≥ 30 kg/m2. * p < 0.05 Kruskal-Wallis test. BMI < 30 kg/m2 vs pooled BMI ≥ 30 kg/m2 and BMI ≥ 30 kg/m2 diabetes group. **p < 0.0001 Kruskal-Wallis test. BMI < 30 kg/m2 vs pooled BMI ≥ 30 kg/m2, BMI ≥ 30 kg/m2 no diabetes and BMI ≥ 30 kg/m2 diabetes group. Other than for the indicated groups, the differences were not significant.
To rule out the possibility that the expressed cytokines degraded to a variable extent in healthy and diseased adipose tissues or not adequately extracted, we analyzed their gene expression in VAT and SAT (Fig. 2B, D, F; SFig. 5). The expression of IL6, IFNG, IL15, TNFA and IL15RA did not show any statistically significant differences between the controls and obese patients. On the other hand, expression of the IL1B gene was significantly increased in the obese group compared to the controls in VAT, although only the non-diabetic and diabetic subgroups within the BMI ≥ 30 kg/m2 group showed elevated IL1B expression but not the pre-diabetic subgroup. SAT of obese patients did not show increased IL1B expression. Next we carried out correlation analysis between the protein and gene expression for each cytokine to determine whether the proteins were mostly produced in situ. The gene and protein expression for IL-6 showed a significant, but low positive correlation in the VAT and in the SAT (SFig. 6). The gene and protein expression for IL-15 showed low positive correlation in the SAT, while no such correlation was observed for TNFα or IL-1β in either of the AT depots.
3.2. Expression of Cytokines That Modulate Insulin Sensitivity
Pre-clinical murine models have established the paradigm that cytokines involved in a Th2-like response (IL-4, IL-13) and Tregs play a beneficial role in preventing the development of insulin resistance (Lee and Lee, 2014). In rheumatoid arthritis patients, inhibition of IL-1β signaling using IL-1RA, antibodies targeting IL-1 or its receptor have shown an improvement in the insulin resistance (Donath, 2014; Ballak et al., 2015). IL-4 and IL-1RA concentrations were not different between the different groups (Fig. 3A, C, E). The IL4, IL1RA and IL10 transcript levels indicate that these genes were expressed at levels comparable to that of the housekeeping gene in visceral and subcutaneous samples from most of the obese patients and did not show any significant differences between the groups (Fig. 3B, D, F). These observations indicate that ‘anti-inflammatory’ cytokines are expressed as much as ‘classical’ inflammatory cytokines in the VAT and SAT from obese individuals.
Fig. 3.
Expression of IL-4, IL-10 and IL-1RA in adipose tissues.
Protein and gene expression of IL-4, IL-10 and IL-1RA in the visceral and subcutaneous adipose tissues. Protein expression: (n = 9 and 8) BMI < 30 kg/m2; (n = 12 and 13) no diabetes BMI ≥ 30 kg/m2; (n = 11 and 9) pre-diabetes BMI ≥ 30 kg/m2; (n = 10 and 12) diabetes BMI ≥ 30 kg/m2. Gene expression: (n = 10and 11) BMI < 30 kg/m2; (n = 30 and 35) no diabetes BMI ≥ 30 kg/m2; (n = 13 and14) pre-diabetes BMI ≥ 30 kg/m2; (n = 30 and 40) diabetes BMI ≥ 30 kg/m2. (VAT and SAT). None of the data was statistically significant.
3.3. Expression Pattern of Cytokines in Adipose Tissues
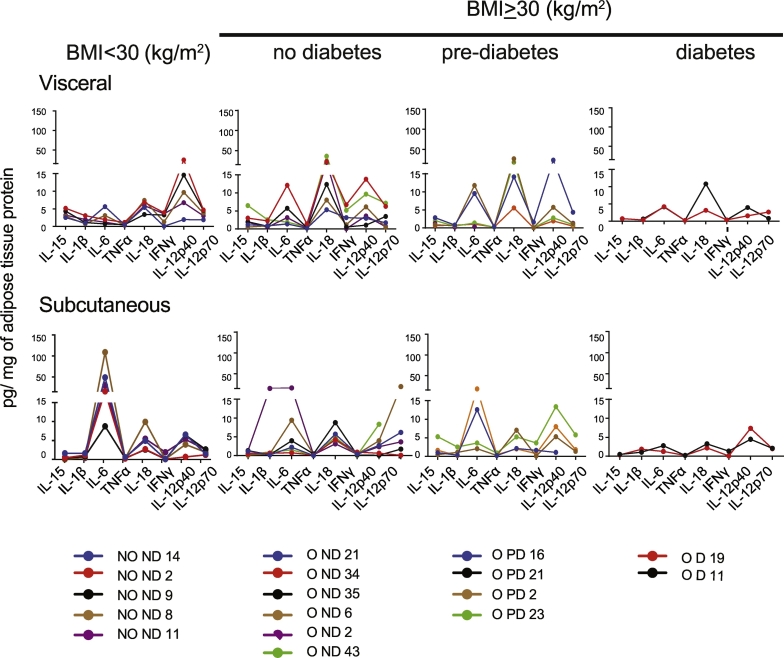
The data presented so far show that approximately one-third of the obese patients do not express or only express very low levels of protein or transcripts for any given inflammatory cytokine in VAT and SAT. As murine models have implicated numerous pro-inflammatory cytokines in promoting chronic inflammation in obesity, it is not unlikely that the expression profiles of these mediators could vary widely among patients, with the sum of their effects nonetheless leading to the same outcome. Hence, it is possible that the expression of any one of the pro-inflammatory cytokines could tip the balance towards the pro-inflammatory process. Therefore we analyzed some of these cytokines levels in the VAT and SAT of the same patient or control subject, which are indicated by the same color in Fig. 4. A continuous line joins the data obtained for a given patient to facilitate visualization of the expression pattern. Some of the salient features that are evident from this analysis are as follows: 1) the cytokine levels vary between the VAT and SAT of the same patient. 2) The quantity of a given cytokine in VAT or SAT is generally comparable among the samples within the group. 3) Inclusion of additional samples did not change the expression patterns in VAT or SAT (SFig. 7). However, we do not exclude the possibility that obese patients may express other inflammatory cytokines that were not included in our study.
Fig. 4.
Expression patterns of select cytokines in the visceral and subcutaneous adipose tissues.
Expression pattern of the indicated cytokines in the visceral and subcutaneous adipose tissues from a given patient. A line of a particular color in both the depots indicates data from each patient. The points have been joined for easy visualization.
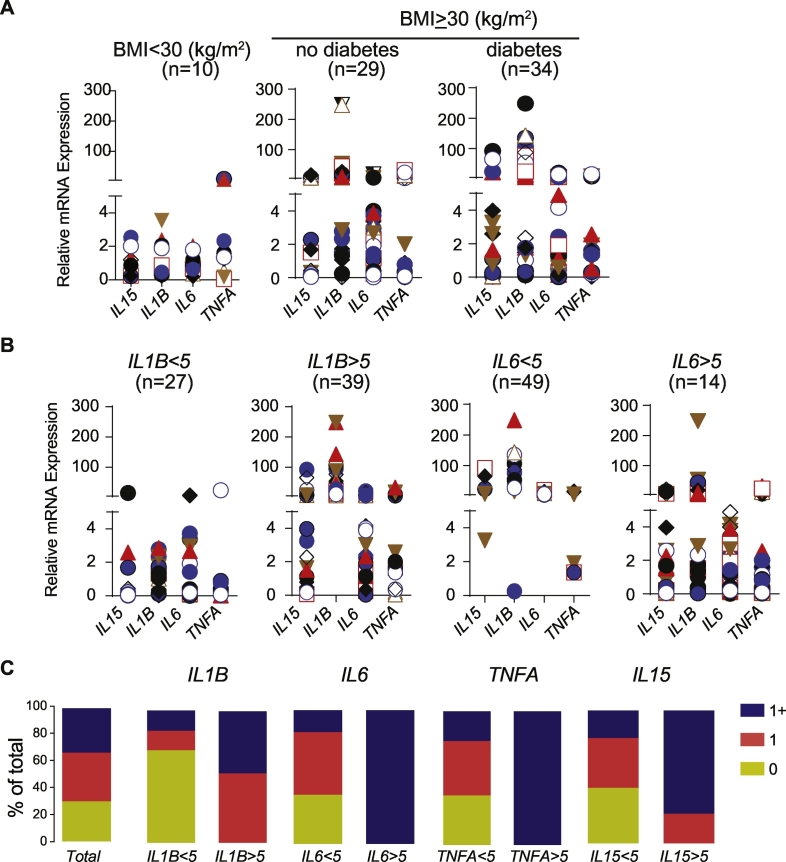
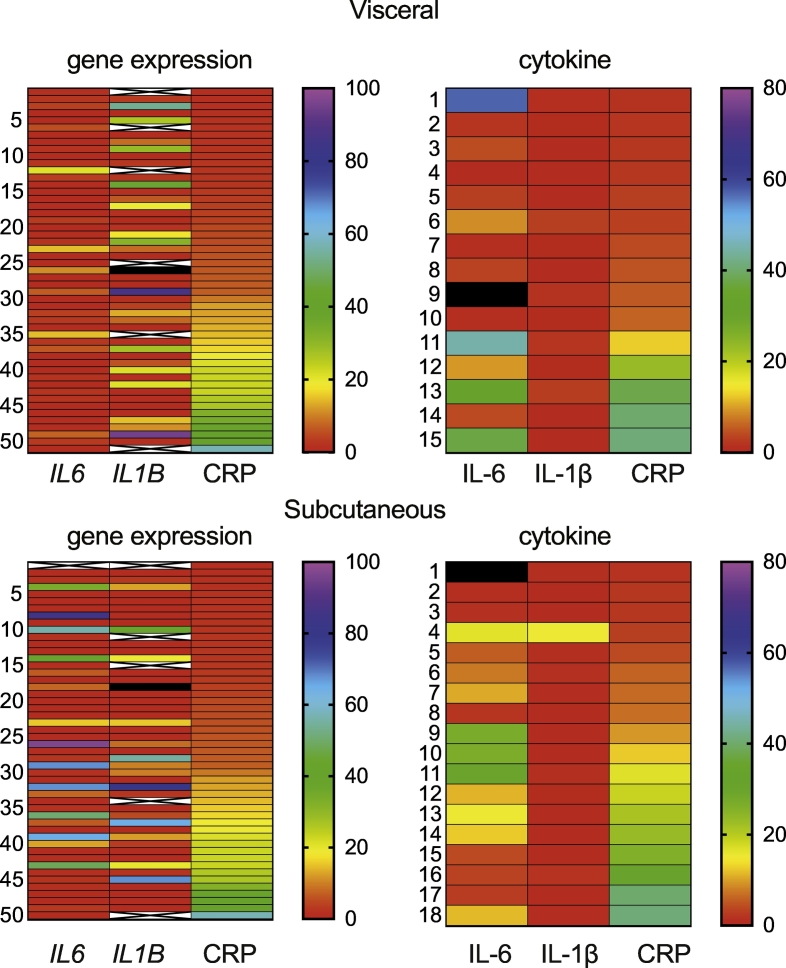
Similar patterns were observed for the mRNA levels of the cytokines studied (SFig. 8). A considerable proportion of obese patients expressed very low level of transcripts for IL1B, IL6 or TNFA, cytokines that have been generally associated with obesity-induced chronic inflammation. Assuming the mRNA expression levels observed in the adipose tissues of non-obese non-diabetic controls as ‘normal’, we assessed the proportion of obese patients who express the above cytokines at levels similar to that observed in the control group. As shown in Fig. 5A, the relative level of expression of IL1B, TNFA, IL6 and IL15 was less than ‘5’ in the visceral adipose tissues (except in 2 visceral adipose tissue samples for TNFA). Using the value ‘relative expression 5’ as an arbitrary cut off value, we determined the percentage of samples that had values greater than ‘5’ in the VAT of obese group. Also, we determined the proportion of obese samples where the expression of these cytokines was less than ‘5’ (designated as 0), expressed at least 1 (designated as 1) or >1 (designated as 1+) greater than ‘5’. Among the 4 cytokines included in the analysis, data obtained using IL1B as the indicator were more informative than that of IL6, TNFA or IL15 (Fig. 5B). In tissue samples where the relative expression of IL1B is <5, the expression levels of IL6, TNFA and IL15 were also less than ‘5’ in about 60% of the patients (Fig. 5C). In contrast, >60% of the patients express 1 or more inflammatory cytokines even if they express less than ‘5’ units of IL6, TNFA or IL15 (Fig. 5C). Approximately one-third of the obese patients expressed all the four cytokines analyzed at levels similar to those of controls in the VAT (relative expression < 5; Fig. 5C-Total). Another 30% of the samples showed values >5 for only one of the 4 cytokines analyzed. Expression levels of IL18, IL4 and IL10 did not contribute much to this classification (SFig. 8), and hence were not included in this analysis. These observations suggest that a significant number of patients do not express IL1B, IL6, TNFA or IL15 at the level of RNA. Furthermore, the presence of hsCRP in the serum does not reflect the status of inflammatory cytokines in the visceral adipose (Fig. 6).
Fig. 5.
Expression patterns of select cytokine genes in the visceral and subcutaneous adipose tissues.
A) Expression pattern of the indicated cytokine genes in the visceral adipose tissue. Each unique dot represents the same sample for the 4 genes in any given particular group. B) Data of pooled BMI ≥ 30 were grouped based on the relative expression of IL1B (<5 or >5) or IL6 (<5 or >5) and the number of samples is indicated. C) The proportion of patients who express none (0; yellow), one (1; orange) or more than one (1+; blue) of the 4 cytokines included in the analysis is represented. In addition to IL1B and IL6, data for IL15 and TNFA is presented. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Heatmap of the IL6 and IL1B expression in the tissues with hsCRP expression in the serum of obese patients.
Gene and protein expression of IL-6 and IL-1β in the visceral and subcutaneous adipose tissues were correlated with the presence of hsCRP in the serum in individual obese patient samples, denoted by the number in the left. ‘X’ refers to the absence of data. Black color denotes samples that were above the range used.
3.4. The Expression Pattern of Select Chemokines in Adipose Tissues
Obese individuals show increased presence of certain chemokines that are implicated in the recruitment of macrophages to the inflamed adipose tissues in circulation or in the visceral adipose tissue (Yao et al., 2014; Xu et al., 2015). We analyzed the expression of select chemokines that have been associated with obesity in humans or in mouse models. In general the expression of chemokines was higher in the VAT when compared to SAT (SFig. 9). However, the range of expression was comparable between the controls and obese patients and did not show any significant difference. Relative expression of CCL2, CCL5 and CXCL10 was not different between the groups (SFig. 10A). Curiously, the expression of CCL2 and IL6 showed positive correlation in the VAT at the level of gene expression (SFig. 10B). Similarly, CCL2 showed significant correlation with IL-6 and TNFα in the VAT. CXCL10, a chemokine that is induced by IFNγ in different cell types and is involved in recruiting immune cells showed significant correlation with IFNγ, IL-6 and TNFα in the VAT at the level of protein expression (SFig. 10C). During inflammation, CCL2 recruits macrophages to the site of inflammation. Thus it is possible that IL-6 and TNFα are produced by macrophages recruited in the visceral adipose tissues. Even though CD68 gene, a marker for macrophages showed significant positive correlation with IL6 in the VAT and SAT and with TNFA in the SAT (SFig. 11), the number of samples contributing to this correlation was not high. These observations suggest that cytokines and chemokines can be produced in situ or can be available from circulation in AT.
3.5. VEGF-A and PDGF-BB Expression Correlates With the Expression of Inflammatory Cytokines in VAT
In obese patients, the adipose tissue mass can lead to hypoxia as a consequence of insufficient vascularization. Vascular endothelial growth factor A (VEGF-A) produced by the endothelial cells can increase the vascularization of adipose tissues (Cao, 2007). Here we observed that the range of expression of VEGF-A and PDGF-BB was not significantly different between controls and the obese samples (SFig. 12). However, VEGF-A showed significant correlation in the VAT with IL-1β and IL-15, to a lesser extent with TNFα, but not with IL-6 (SFig. 13, Top Panel). On the other hand, PDGF-BB showed significant correlation with IL-6 and TNFα in the VAT. However, the pattern was completely different in the SAT (SFig. 13, Bottom Panel). VEGF-A, PDGF-BB and PDGF-AA showed significant negative correlation with IL-6, while TNFα and IL-1β showed positive correlation with VEGF-A. Thus the relationship between the growth factors and inflammatory mediators are different between the two adipose tissue depots.
4. Discussion
Despite the extensive characterization of mechanisms by which inflammatory cytokines promote insulin resistance and adipose tissue dysfunction, targeted immunotherapies have not yielded any positive outcome in human metabolic syndrome (Olefsky and Glass, 2010; Donath, 2014). One of the main reasons could be that we do not have a comprehensive understanding of the cytokine profile in the tissues of obese patients. To our knowledge, our study is the first report where many of the cytokines have been analyzed simultaneously in the VAT and SAT of obese patients for both gene expression and protein levels. We failed to identify cytokine profiles that specifically relate to obesity in adipose tissues obtained from patients undergoing bariatric surgery. In fact, the results presented in our study highlight the complexity of the cytokine profile in the AT of obese patients and suggest that our current views on the relationship between cytokines and obesity/metabolic syndrome are more complex than what we understand from murine models (Reilly and Saltiel, 2017). Our data suggests that the chronic inflammation associated with obesity cannot be attributed to any particular cytokine, but rather may vary from patient to patient. In general our data suggest that in obese patients the expression of cytokines is higher in the VAT when compared to SAT, but notably, is independent of the diabetes status of obese patients. Paradoxically, the classical pro-inflammatory cytokines such as IL-1β, TNFα and IFNγ were present at very low levels (0–10 pg/mg of tissue) largely comparable to the ‘classical anti-inflammatory’ cytokines such as IL-4, IL-10 or IL-1RA (Fig. 2, Fig. 3). As these ‘anti-inflammatory’ cytokines have also been shown to be associated with obesity and insulin resistance in humans (Juge-Aubry et al., 2004; Juge-Aubry et al., 2003; Surendar et al., 2011; Dandona et al., 2014), it is possible that they also contribute to the inflammation observed in the AT. The alternate explanation is that the presence of these cytokines in AT may contribute to certain degree of protection from insulin resistance by recruiting eosinophils and Th2-like cells as suggested in animal models (Lee and Lee, 2014).
The influence of weight loss on the expression of certain IL-1 family of genes (IL1B, IL1RA, IL37 and IL18 in subcutaneous fat pad and the liver) were compared before and 6 months after gastric banding surgery (Moschen et al., 2011). Transcripts for IL1B were decreased in the liver and SAT while IL18 was decreased only in the liver 6 months after surgery. IL1RA was decreased in liver and the expression of IL37 was increased in the SAT. However, the contribution of IL-1RA to the inflammatory process and insulin resistance is not clear as IL-1RA can, by itself promote insulin resistance (Perrier et al., 2006). These observations highlight the fact that expression of inflammatory markers does not follow a specific pattern between different organs associated with metabolism.
IL-6 appears to be produced in situ in the VAT, as evident from the significant correlation between IL-6 gene and protein expression. Adipose tissues are the major producers of IL-6 in the humans (Mohamed-Ali et al., 1997). In addition, IL-6 regulates central obesity by regulating energy expenditure (Stouthard et al., 1995). The significant correlation of CCL2 with TNFα and IL-6 at the level of transcripts and protein expression in the VAT suggests that the macrophages recruited to the VAT may be the source of some of these cytokines. Similarly the correlation between CXCL10 and IFNγ at the protein level suggest that the CXCL10 that is primarily induced by IFNγ can also be involved in the recruitment of macrophages (Griffith et al., 2014). However, the quantity of the tissue available precludes the isolation of macrophages to explore those possibilities. We cannot exclude the possibility that the cytokines can be available from local circulation as omental fat has lymphoid follicles (Meza-Perez and Randall, 2017) that can be a source of the cytokines detected in the VAT. It is not clear whether the slight increase in the tissue concentrations of certain inflammatory cytokines will contribute to their levels in circulation. In the samples used in this study, we have minimal evidence that the inflammatory cytokines are present in systemic circulation. In contrast to VAT, IL-6 levels were decreased in the SAT of patients with BMI > 30 and did not show any significant correlation with CCL2 or CD68. The significance of this observation is not clear at present, but again it is a clear illustration of the differential cytokine expression pattern between VAT and SAT. Recently VEGF-A has been shown to increase ‘browning’ in SAT (Park et al., 2017). Despite the induction of VEGF by hypoxia in VAT from obese patients, equivalent vascularization was not observed (Fusaru et al., 2012). In another study, analyses of a comparable panel of cytokines in the sera of patients with metabolic syndrome revealed significant differences for PDGF-BB only (Tisato et al., 2013). The correlation between VEGF-A, PDGF-BB and inflammatory cytokines in the VAT suggests that hypoxia induced inflammation may be present concomitantly with these growth factors. As mentioned above, it is equally possible that during the establishment of obesity the balance between the inflammatory mediators on one hand, and the anti-inflammatory mediators and these growth factors on the other, may determine whether chronic inflammation sets in or the adipose tissue expands with sufficient vascularization to result in ‘healthy obesity’ (Cao, 2007).
Unlike previous studies where only 1–3 cytokines were analyzed, recently 2 groups had analyzed the sera of obese patients for 12–24 analytes (Tisato et al., 2013; Azizian et al., 2016). Azizian et al. reported significant difference for only CCL2 while Tisato et al. observed an increase in CXCL10 and IL-6 and a decrease in PDGF-BB in the sera of patients with metabolic syndrome. These 3 studies (including this study) were carried out in 3 different geographical settings (Iran, Italy and French Canada). The ethnicity and the associated genetic profile, the dietary and cultural differences and the environmental differences due to the geographic location of the patients analyzed in these 3 studies can make the comparison between the studies difficult. Furthermore, our study shows that the two adipose tissue depots do not reflect the same trend in a given patient neither at the level of gene transcripts or the protein expression. The variations between these studies may be actually reflecting the existing reality which may be a complex mosaic of cytokine profiles in different populations. Thus the metabolic syndrome associated chronic inflammation observed in obese patients can be due to any one of the various inflammatory mediators, that may also evolve with the disease. The more important challenge will be to identify the inflammatory mediators in a given patient before it can be targeted. Thus even if chronic inflammation contributes to the development of obesity and the associated metabolic syndrome it is extremely difficult to pinpoint the source of inflammation and its progression over time. One of the salient observations in our study is that the ranges of transcript levels for IL1B, TNFA, IL6 and IL15 are similar to that of non-obese controls in 20–30% of the obese patients. While it is possible that other mediators may be implicated (Fruhbeck and Gomez-Ambrosi, 2001), it is equally possible that inflammation is not present at all times during progression and persistence of obesity. It is also possible that different subphenotypes with diverse degrees of metabolic derangements, that may or may not be associated with inflammation can be present (Naukkarinen et al., 2014). The major limitation of our study is that we used adipose tissues from patients undergoing bariatric surgery. Additionally, obese patients with type 2 diabetes were treated with metformin and other medications. It is possible that the level of inflammatory cytokines is reduced as a consequence of the medication in the obese diabetic patients (Cameron et al., 2016). In addition to inflammatory changes we cannot rule out the contribution of obesity associated changes in gene expression that directly affects lipid metabolism (Ortega et al., 2010). Further studies are needed to determine whether inflammation influences these changes. Nevertheless, comparable distribution of the cytokine profile in the three obese groups suggests that the lack of significance cannot be attributed to this variability. Analysis of the status of the inflammatory cytokines before and after surgery would have added value to the analysis as shown by others (Catalan et al., 2007).
As the patients undergoing bariatic surgery are advised to follow a low calorie diet regimen for 2 weeks to reduce their hepatic lipid content, it is not clear how this might influence the inflammation in AT. Two weeks of very low calorie diet regimen is sufficient to decrease inflammatory markers in SAT and diminish the levels of hsCRP in circulation (Mraz et al., 2011; Heilbronn and Clifton, 2002). However, certain reports have shown that the inflammation is not reduced in the adipose tissues following weight loss (Salas-Salvado et al., 2006). Reduction in TNFA transcripts in the adipose tissues was observed 6 weeks after calorie restriction (Hotamisligil et al., 1995). As the serum samples were collected at the start of the reduced diet regimen, our data suggests that the inflammation may not be uniform across patients. Various clinical trials that had targeted specific components of the inflammatory processes have not resulted in increasing insulin sensitivity as seen from HbA1c and blood glucose levels (Esser et al., 2015; Maiorino et al., 2017). Additional controlled studies are required to identify the cytokine that contributes to the chronic inflammation in a given patient and tailor the therapy accordingly.
Conflict of Interest
All the authors declare that they have no conflict of interest.
Author Contributions
VR, SR and MFL planned the study. VR, GL, MM carried out the experiments. VR, MM, AM, SI, SR and MFL analyzed the data. VR, AM, SI, SR and MFL interpreted the results and developed the discussion. MFL, JF, DR and SR were involved in establishing human protocols. MFL and CB are responsible for the adipose tissue bank. VR and SR wrote the first draft of the manuscript. All authors reviewed and corrected the manuscript.
Funding
This work was supported by CMDO-2013 (FRQS funded Research Network on Cardiometabolism, Diabetes and Obesity), CRCHUS-2016 and CIHR (MOP86530) to SR and MFL.
Acknowledgements
We thank Ms. Marie-Pierre Garant for help with statistical analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.03.004.
Appendix A. Supplementary data
Supplementary material
References
- Azizian M., Mahdipour E., Mirhafez S.R. Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender. Ann. Clin. Biochem. 2016;53(6):663–668. doi: 10.1177/0004563216629997. [DOI] [PubMed] [Google Scholar]
- Ballak D.B., Stienstra R., Tack C.J., Dinarello C.A., van Diepen J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75(2):280–290. doi: 10.1016/j.cyto.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D., Magkos F., Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143(4):897–912. doi: 10.1053/j.gastro.2012.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A.R., Morrison V.L., Levin D. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016;119(5):652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Diabetes Association Clinical Practice Guidelines Expert C, Goldenberg R., Punthakee Z. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabet. 2013;37(Suppl. 1):S8–11. doi: 10.1016/j.jcjd.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 2007;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan V., Gomez-Ambrosi J., Ramirez B. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes. Surg. 2007;17(11):1464–1474. doi: 10.1007/s11695-008-9424-z. [DOI] [PubMed] [Google Scholar]
- Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P., Ghanim H., Monte S.V. Increase in the mediators of asthma in obesity and obesity with type 2 diabetes: reduction with weight loss. Obesity (Silver Spring) 2014;22(2):356–362. doi: 10.1002/oby.20524. [DOI] [PubMed] [Google Scholar]
- Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Donath M.Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 2014;13(6):465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- Esser N., Paquot N., Scheen A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs. 2015;24(3):283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015;11(8):465–477. doi: 10.1038/nrendo.2015.84. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G., Gomez-Ambrosi J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001;15(11):1996–2006. doi: 10.1096/fj.00-0829hyp. [DOI] [PubMed] [Google Scholar]
- Fusaru A.M., Pisoschi C.G., Bold A. Hypoxia induced VEGF synthesis in visceral adipose depots of obese diabetic patients. Romanian J. Morphol. Embryol. 2012;53(4):903–909. [PubMed] [Google Scholar]
- Ghasemi A., Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486–489. doi: 10.5812/ijem.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisondi P., Cotena C., Tessari G., Girolomoni G. Anti-tumour necrosis factor-alpha therapy increases body weight in patients with chronic plaque psoriasis: a retrospective cohort study. J. Eur. Acad. Dermatol. Venereol. 2008;22(3):341–344. doi: 10.1111/j.1468-3083.2007.02429.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay M.A., Garcia-Unzueta M.T., Berja A. Anti-TNF-alpha therapy does not modulate leptin in patients with severe rheumatoid arthritis. Clin. Exp. Rheumatol. 2009;27(2):222–228. [PubMed] [Google Scholar]
- Griffith J.W., Sokol C.L., Luster A.D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- Gumbs A.A., Modlin I.M., Ballantyne G.H. Changes in insulin resistance following bariatric surgery: role of caloric restriction and weight loss. Obes. Surg. 2005;15(4):462–473. doi: 10.1381/0960892053723367. [DOI] [PubMed] [Google Scholar]
- Heilbronn L.K., Clifton P.M. C-reactive protein and coronary artery disease: influence of obesity, caloric restriction and weight loss. J. Nutr. Biochem. 2002;13(6):316–321. doi: 10.1016/s0955-2863(02)00187-0. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl. 2):S73–8. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York, NY) 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Budavari A., Murray D., Spiegelman B.M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Invest. 1994;94(4):1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L., Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge-Aubry C.E., Somm E., Giusti V. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes. 2003;52(5):1104–1110. doi: 10.2337/diabetes.52.5.1104. [DOI] [PubMed] [Google Scholar]
- Juge-Aubry C.E., Somm E., Chicheportiche R. Regulatory effects of interleukin (IL)-1, interferon-beta, and IL-4 on the production of IL-1 receptor antagonist by human adipose tissue. J. Clin. Endocrinol. Metab. 2004;89(6):2652–2658. doi: 10.1210/jc.2003-031219. [DOI] [PubMed] [Google Scholar]
- Lackey D.E., Olefsky J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- Lacraz G., Rakotoarivelo V., Labbe S.M. Deficiency of interleukin-15 confers resistance to obesity by diminishing inflammation and enhancing the thermogenic function of adipose tissues. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.C., Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta. 2014;1842(3):446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorino M.I., Bellastella G., Giugliano D., Esposito K. Cooling down inflammation in type 2 diabetes: how strong is the evidence for cardiometabolic benefit? Endocrine. 2017;55(2):360–365. doi: 10.1007/s12020-016-0993-7. [DOI] [PubMed] [Google Scholar]
- Mehta R., Birerdinc A., Hossain N. Validation of endogenous reference genes for qRT-PCR analysis of human visceral adipose samples. BMC Mol. Biol. 2010;11:39. doi: 10.1186/1471-2199-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Perez S., Randall T.D. Immunological functions of the omentum. Trends Immunol. 2017;38(7):526–536. doi: 10.1016/j.it.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V., Goodrick S., Rawesh A. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Moschen A.R., Molnar C., Enrich B., Geiger S., Ebenbichler C.F., Tilg H. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011;17(7–8):840–845. doi: 10.2119/molmed.2010.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz M., Lacinova Z., Drapalova J. The effect of very-low-calorie diet on mRNA expression of inflammation-related genes in subcutaneous adipose tissue and peripheral monocytes of obese patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2011;96(4):E606–13. doi: 10.1210/jc.2010-1858. [DOI] [PubMed] [Google Scholar]
- Naukkarinen J., Heinonen S., Hakkarainen A. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. 2014;57(1):167–176. doi: 10.1007/s00125-013-3066-y. [DOI] [PubMed] [Google Scholar]
- Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Ortega F.J., Mayas D., Moreno-Navarrete J.M. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 2010;18(1):13–20. doi: 10.1038/oby.2009.202. [DOI] [PubMed] [Google Scholar]
- Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab. J. 2013;37(3):165–172. doi: 10.4093/dmj.2013.37.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V., Labbe S.M., Blondin D.P. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 2012;122(2):545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardina E., Ferrer R., Baena-Fustegueras J.A. Only C-reactive protein, but not TNF-alpha or IL6, reflects the improvement in inflammation after bariatric surgery. Obes. Surg. 2012;22(1):131–139. doi: 10.1007/s11695-011-0546-3. [DOI] [PubMed] [Google Scholar]
- Park J., Kim M., Sun K., An Y.A., Gu X., Scherer P.E. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-independent metabolic improvements. Diabetes. 2017;66(6):1479–1490. doi: 10.2337/db16-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso I., Palmery M. The relationship between body weight and inflammation: lesson from anti-TNF-alpha antibody therapy. Hum. Immunol. 2016;77(1):47–53. doi: 10.1016/j.humimm.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Perrier S., Darakhshan F., Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006;580(27):6289–6294. doi: 10.1016/j.febslet.2006.10.061. [DOI] [PubMed] [Google Scholar]
- Reilly S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- Salas-Salvado J., Bullo M., Garcia-Lorda P. Subcutaneous adipose tissue cytokine production is not responsible for the restoration of systemic inflammation markers during weight loss. Int. J. Obes. 2006;30(12):1714–1720. doi: 10.1038/sj.ijo.0803348. [DOI] [PubMed] [Google Scholar]
- Saraceno R., Schipani C., Mazzotta A. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol. Res. 2008;57(4):290–295. doi: 10.1016/j.phrs.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J., Kroke A., Mohlig M. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- Stouthard J.M., Romijn J.A., Van der Poll T. Endocrinologic and metabolic effects of interleukin-6 in humans. Am. J. Phys. 1995;268(5 Pt 1):E813–9. doi: 10.1152/ajpendo.1995.268.5.E813. [DOI] [PubMed] [Google Scholar]
- Surendar J., Mohan V., Rao M.M., Babu S., Aravindhan V. Increased levels of both Th1 and Th2 cytokines in subjects with metabolic syndrome (CURES-103) Diabetes Technol. Ther. 2011;13(4):477–482. doi: 10.1089/dia.2010.0178. [DOI] [PubMed] [Google Scholar]
- Tisato V., Toffoli B., Monasta L. Patients affected by metabolic syndrome show decreased levels of circulating platelet derived growth factor (PDGF)-BB. Clin. Nutr. 2013;32(2):259–264. doi: 10.1016/j.clnu.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Wensveen F.M., Valentic S., Sestan M., Turk Wensveen T., Polic B. The “Big Bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 2015;45(9):2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- Xu L., Kitade H., Ni Y., Ota T. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomol. Ther. 2015;5(3):1563–1579. doi: 10.3390/biom5031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Herlea-Pana O., Heuser-Baker J., Chen Y., Barlic-Dicen J. Roles of the chemokine system in development of obesity, insulin resistance, and cardiovascular disease. J. Immunol. Res. 2014;2014:181450. doi: 10.1155/2014/181450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap B.W., Sim C.H. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011;81(12):2141–2155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material