Abstract
Purpose:
Continued smoking after a cancer diagnosis leads to poorer treatment outcomes, survival, and quality of life. We evaluated the perceptions of the effects of continued smoking on quality of life, survival, and fatigue among patients with cancer after a cancer diagnosis and the effects of these perceptions on smoking cessation.
Patients and Methods:
Patients with cancer from all disease subsites from Princess Margaret Cancer Centre (Toronto, Ontario) were surveyed between April 2014 and May 2016 for sociodemographic variables, smoking history, and perceptions of continued smoking on quality of life, survival, and fatigue. Multivariable regression models evaluated the association between patients’ perceptions and smoking cessation and the factors influencing patients’ perceptions of smoking.
Results:
Among 1,121 patients, 277 (23%) were smoking cigarettes up to 1 year before diagnosis, and 54% subsequently quit; 23% had lung cancer, and 27% had head and neck cancers. The majority felt that continued smoking after a cancer diagnosis negatively affected quality of life (83%), survival (86%), and fatigue (82%). Current smokers during the peridiagnosis period were less likely to perceive that continued smoking was harmful when compared with ex-smokers and never-smokers (P < .01). Among current smokers, perceiving that smoking negatively affected quality of life (adjusted odds ratio [aOR], 2.68 [95% CI, 1.26 to 5.72]; P = .011), survival (aOR, 5.00 [95% CI, 2.19 to 11.43]; P < .001), and fatigue (aOR, 3.57 [95% CI, 1.69 to 7.54]; P < .001) were each strongly associated with smoking cessation. Among all patients, those with a greater smoking history were less likely to believe that smoking was harmful in terms of quality of life (aOR, 0.98 [95% CI, 0.98 to 0.99]; P < .001), survival (aOR, 0.98 [95% CI, 0.98 to 0.99]; P < .001), and fatigue (aOR, 0.99 [95% CI, 0.98 to 0.99]; P < .001).
Conclusion:
The perceptions of continued smoking after a cancer diagnosis among patients with cancer are strongly associated with smoking cessation. Counseling about the harms of continued smoking in patients with cancer, and in particular among those who have lower risk perceptions, should be considered when developing a smoking cessation program.
INTRODUCTION
Cigarette smoking is a known risk factor for the development of head and neck cancers (HNC) and lung cancers, and also for other cancers (bladder, kidney, breast, ovarian, esophagus, stomach, pancreas, colon, cervix, blood) not traditionally perceived as being strongly related to smoking.1-10 Despite a decrease in the incidence and prevalence of smoking, one in five individuals still continues to smoke, sufficient to make tobacco use the leading cause of preventable death in the United States and Canada, with cancer responsible for 38% of all smoking-related mortality.11,12 Among all patients with cancer, approximately 20% smoke at the time of their diagnosis, and among patients with lung cancer and HNC, 45% to 75% smoke in the year leading up to their cancer diagnosis.13,14 Smoking cessation rates after a cancer diagnosis range from 42% to 86%, but these statistics are countered by smoking recidivism rates of up to 41% within 4 months among those who initially quit after diagnosis.14,15
Continued smoking after a cancer diagnosis is associated with worse short-term outcomes including reduced treatment efficacy and increased treatment-related toxicity and adverse effects.16-22 Long-term harms of continued smoking include an increased risk of cancer recurrence and the development of second primary malignancies.23-28 Patients with cancer who continue to smoke also experience greater fatigue and a reduced quality of life, possibly associated with depressed pulmonary and immune functions as a result of smoking.29,30 Other harms of continued smoking include worsening cardiovascular disease.1,29
Previous studies investigating the factors associated with smoking cessation have identified that marital status, income, cancer type, education level, and second-hand smoke all influence smoking cessation.31-34 Because risk perception has been deemed by the Theory of Reasoned Action (TRA)35 and the Health Belief Model (HBM)36to be an important precursor of health behavior change, a thorough understanding of behavior change in the form of smoking cessation among patients with cancer necessitates an evaluation of their perceptions related to smoking risk. In the TRA, a patient’s decision to perform a behavior is influenced by his or her behavioral intention, which comes from the belief that performing an action will lead to a specific outcome.35 In the HBM, the perceived benefits of and barriers to an action and a cue to action or trigger are thought to influence health behavior change.36 Despite the smoking cessation literature suggesting that smokers are more likely to attempt to quit if they acknowledge the personal health risks associated with smoking,37 studies have not explored directly the association between smoking risk perception and smoking cessation. Among the paucity of studies exploring smoking perceptions among cancer survivors, to our knowledge only one study, by our group, demonstrated an association between advancing age and more negative smoking risk perceptions in patients with cancer.38 The remainder of the smoking risk perception literature comes from noncancer populations, where smokers, particularly those of lower socioeconomic status, were found to hold optimistic beliefs and to underestimate their personal risk.39,40
In the HBM, for health behavior changes to be adopted, a patient must have sufficient motivation and must perceive a threat of sequelae from his or her behavior.36 With a cancer diagnosis providing the motivational impetus for smoking cessation, our overall objective was to assess the personal perceptions among patients with cancer of the effects of continued smoking on the previously established sequelae of survival, fatigue, and quality of life and to determine whether these perceptions are associated with smoking cessation. Our specific aims were (1) to evaluate among patients with cancer the perceptions of the effects of continued smoking after a cancer diagnosis; (2) to identify whether negative smoking risk perceptions were associated with smoking cessation after an established diagnosis of cancer, thereby lending support to the HBM and the TRA; and (3) to evaluate the factors associated with a negative smoking risk perception among current smokers.
PATIENTS AND METHODS
Patient Recruitment and Collection of Information
Between April 2014 and May 2016, patients with cancer in any disease site were recruited from ambulatory oncology clinics at a single comprehensive cancer center, Princess Margaret Cancer Centre, Toronto, Canada. The study was approved by the institutional research ethics board. Patients ≥ 18 years of age with a histologic diagnosis of a primary malignancy (hematologic or solid tumor) of any stage were included in the study. Patients with cognitive deficits or language barriers that limited their understanding of the study were excluded. Because our goal was to assess these perceptions in cancer survivors, patients diagnosed with cancer > 10 years before the date of recruitment were excluded.
After informed consent, patients completed a one-time self-administered questionnaire assessing sociodemographic factors, smoking history, and functional status at follow-up (as measured by the Eastern Cooperative Oncology Group performance score and a separate 5-point Likert scale of from poor to excellent). In addition, patient perceptions of the effects of continued smoking on quality of life, overall 5-year survival, and cancer-related fatigue in an individual patient with cancer were also assessed at follow-up. Clinicopathologic data (diagnosis date, site and stage of disease, treatments received, treatment intent, and validation of smoking history) up to the follow-up date were obtained through a review of each patient’s electronic medical record.
Given the diversity of cancer treatments from various sites, all forms of systemic therapy (hormonal, targeted, immunotherapy, chemotherapy, stem-cell transplant) and all forms of radiation therapy (external beam, brachytherapy, radioactive iodine) were grouped together.
Measurement of Smoking Variables
Cumulative cigarette smoking history was evaluated using pack-years (total number of years smoked multiplied by the average number of packs smoked daily, normalized to 20 cigarettes per pack). Patients smoking in excess of a total of 100 cigarettes in their lifetime were considered lifetime smokers, whereas the remaining patients were classified as never-smokers. Among lifetime smokers, those having quit at least 1 year before their diagnosis were classified as ex-smokers, whereas those smoking within the year of their diagnosis were classified as current smokers at baseline. Current smokers at baseline were defined as those smoking within the 1 year leading up to diagnosis, to avoid any confounding by the symptoms, investigations, and work-up in the peridiagnostic period that may have motivated behavior change in the form of smoking cessation, which was consistent with our prior studies.33,34,41 Subsequently, current smokers at baseline were then divided into patients who either quit smoking or continued to smoke.
Measurement of Perception Variables
Although patient perceptions regarding different outcomes can be evaluated, we focused on three outcomes covering different aspects of cancer survivorship: (1) quality of life, (2) 5-year overall survival, and (3) fatigue. Multiple validated instruments have been used to assess patient risk perceptions of smoking regarding different outcomes,42-44 but none have previously evaluated these three areas specifically, and using a similar scale. Therefore, we assessed patient perceptions of the harms of continued smoking after a cancer diagnosis to each of our three outcomes using a simple 5-point Likert scale (1 = make much worse, 3 = no effect, 5 = make much better). In addition, a cumulative perception index score (out of three) was calculated for each patient. Patients were given a single point for each perception variable they perceived to be worsened (1 or 2 on the Likert scale) by continued smoking.
Statistical Analysis
All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). Descriptive statistics provided the frequency of sociodemographic variables, clinicopathologic variables, and smoking history; comparisons were made using Pearson’s χ2 test or the Kruskal-Wallis test, where appropriate. Univariable logistic regression analysis was applied to assess the association between each perception variable or covariate and change in smoking status after diagnosis. Baseline multivariable logistic regression models were created using backward selection of all sociodemographic and clinicopathologic covariates found to be significantly associated with cessation (at P < .10). Each smoking risk perception variable was then added individually to the baseline multivariable model and was evaluated for significance using the Wald test. Adjusted odds ratios (aORs) and 95% CIs were then obtained.
As an additional exploratory analysis, multivariable models were used to identify the sociodemographic and clinicopathologic factors that were associated with patients who perceived continued smoking to being harmful to various health outcomes.
RESULTS
Patient Characteristics
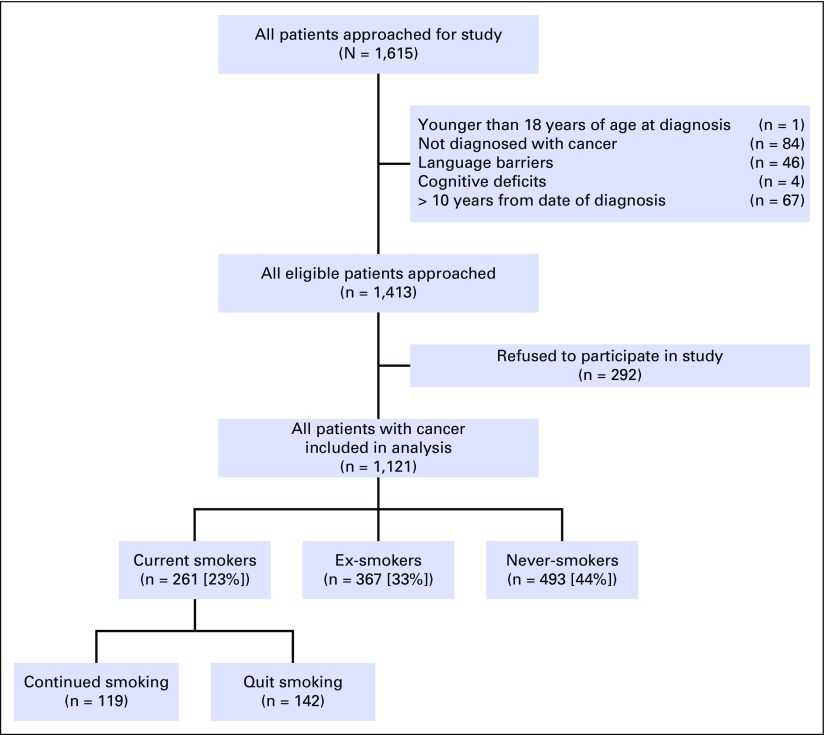
The overall study response rate was 79%. The distribution of patients on the basis of smoking status is presented in Figure 1. Among 1,121 patients recruited, 261 (23%) smoked in the year leading up to diagnosis (ie, current smokers), whereas 44% were never-smokers, and 33% were ex-smokers having quit at least 1 year before diagnosis. Of the 261 patients smoking in the year leading up to diagnosis, 142 (54%) had quit smoking at the time of study recruitment. The median time from diagnosis to study recruitment was 18.5 months (range, 0.0 to 119.3 months). Seventy-five percent were surveyed at least 6 months after diagnosis, 60% at least 1 year after diagnosis, and 41% at least 2 years after diagnosis.
Fig 1.
Summary of recruitment statistics for enrollment in this study and the distribution of current smokers, ex-smokers, and never-smokers at the time of diagnosis and at the time of follow-up.
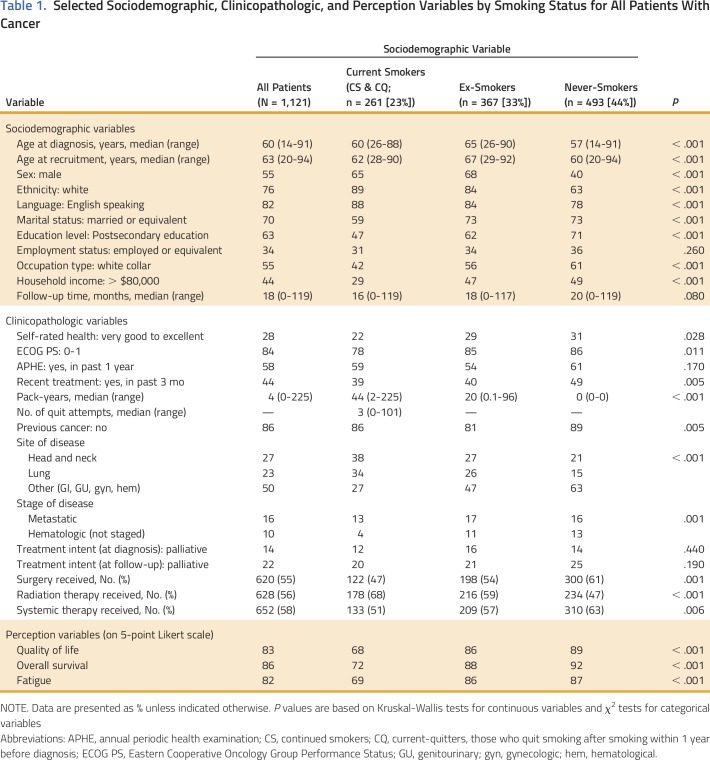
The sociodemographic and clinicopathologic characteristics of our patient population are listed in Appendix Table A1 (online only). Most patients were male (55%); had a mean age of 62 years at recruitment; were white (76%), English speaking (82%), married (70%); had a postsecondary education (63%); and were relatively asymptomatic at the time of their diagnosis (84%). In general, current smokers were more likely to be male (65%), white (89%), and English speaking (88%). They were less likely to be married (59%), have received any postsecondary education (47%), or a have high household income (29%).
With respect to disease site, most patients were diagnosed with a primary HNC (27%) or lung cancer (23%). Types of cancer among patients with non–tobacco-related cancers (50%) included breast, GI, genitourinary, gynecologic, and hematologic cancers.
Univariable and Multivariable Analysis of Factors Associated With Smoking Cessation
Univariable and multivariable analysis helped identify the sociodemographic and clinicopathologic covariates associated with smoking cessation among current smokers at 1 year before diagnosis. Multivariate factors found to be associated with a greater chance of smoking cessation among current smokers 1 year before diagnosis include smoking fewer pack-years (aOR, 0.98 [95% CI, 0.97 to 0.99]; P = .004), having recent oncologic treatment (aOR, 3.04 [95% CI, 1.49 to 6.22]; P = .002), and having received an annual periodic health examination with their family physician (aOR, 3.20 [95% CI, 1.62 to 6.31]; P < .001).
Effects of Perception of Harms of Continued Smoking on Smoking Cessation
Most patients believed that smoking after a diagnosis of cancer worsens quality of life (83%), overall survival (86%), and fatigue (82%). When comparing the perceptions of patients with different baseline smoking status, both ex-smokers and those smoking within the year of their diagnosis were less likely to perceive smoking as being harmful to quality of life, overall survival, and fatigue (P < .001 for each comparison), when compared with never-smokers; in addition, those smoking within the year of their diagnosis were less likely to perceive smoking as being harmful, when compared with ex-smokers (P < .001; Table 1).
Table 1.
Selected Sociodemographic, Clinicopathologic, and Perception Variables by Smoking Status for All Patients With Cancer
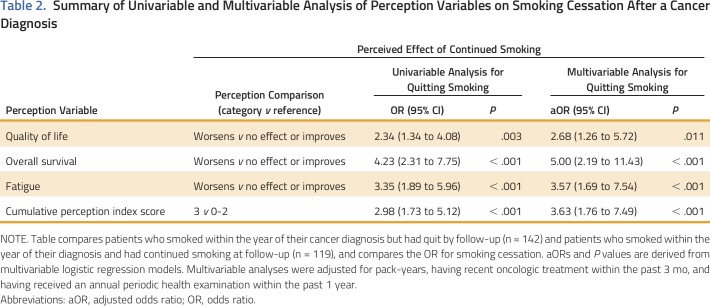
The association between these risk perceptions and smoking cessation among current smokers at 1 year before diagnosis is presented in Table 2. Perceiving that continued smoking after a cancer diagnosis worsens the quality of life (aOR, 2.68 [95% CI, 1.26 to 5.72]; P = .011), overall 5-year survival (aOR, 5.00 [95% CI, 2.19 to 11.43]; P < .001), or experiences of fatigue (aOR, 3.57 [95% CI, 1.69 to 7.54]; P < .001) of an individual patient with cancer were each found to increase the chance of quitting smoking. When analyzing multiple perception domains, those smokers who perceived smoking to be harmful in all three domains (index score of 3) were more than three times more likely to quit smoking when compared with those with negative perceptions in only zero to two domains (index score of 0 to 2; aOR, 3.63 [95% CI, 1.76 to 7.49]; P < .001).
Table 2.
Summary of Univariable and Multivariable Analysis of Perception Variables on Smoking Cessation After a Cancer Diagnosis
Exploratory subgroup analysis was conducted on patients with TRCs (n = 190) and those with non-TRCs (n = 71). Among patients with TRC, perceiving that continued smoking after a cancer diagnosis worsens the quality of life (aOR, 3.59 [95% CI, 1.40 to 9.16]; P = .008), overall 5-year survival (aOR, 5.90 [95% CI, 2.08 to 16.77]; P = .001), or experiences of fatigue (aOR, 4.10 [95% CI, 1.65 to 10.19]; P = .002) of an individual patient with cancer were each found to increase the chance of quitting smoking. Among those with non-TRCs, patients’ perceptions showed the same directionality as seen in TRCs but were lower in magnitude and were not found to be associated with tobacco cessation (P > .10), likely because of the smaller sample size.
Factors Associated With Worse Perceptions of Smoking Harms
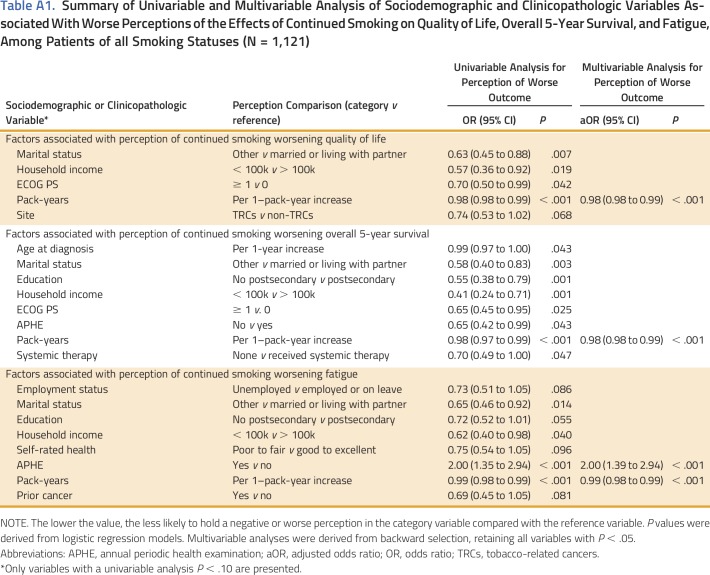
The sociodemographic and clinicopathologic factors associated with each risk perception variable among all patients, irrespective of smoking status, can be found in Appendix Table A1. In multivariable analysis, patients who received an annual periodic health examination from their family physician were more likely to perceive that smoking worsens fatigue (aOR, 0.50 [95% CI, 0.34 to 0.72]; P < .001). The number of pack-years smoked was the only factor that was found to be associated with all three of the perception variables: quality of life (aOR, 0.98 [95% CI, 0.98 to 0.99]; P < .001), survival (aOR, 0.98 [95% CI, 0.98 to 0.99]; P < .001), and fatigue (aOR, 0.99 [95% CI, 0.98 to 0.99]; P < .001). Subgroup analysis among those smoking 1 year before diagnosis (current smokers) identified that patients not having received an annual periodic health examination from their family physician within the past year were less likely to believe smoking was harmful in terms of fatigue (aOR, 0.50 [95% CI, 0.34 to 0.74]; P < .001). No other factors were associated with perceptions of survival or quality of life.
DISCUSSION
Continued smoking after a diagnosis of cancer is an important clinical concern because it is associated with poorer outcomes of survival, quality of life, and fatigue, in addition to other self-reported outcomes.28-30 In a large cohort of patients with cancer, we evaluated patient perceptions of the effect of continued smoking on various survivorship outcomes. We identified that most patients with cancer felt that continued smoking negatively affected quality of life, survival, and fatigue; those who were current smokers 1 year before diagnosis were less aware of these adverse outcomes. Furthermore, we have found that among patients with cancer who were smoking within the year leading up to diagnosis, perceiving smoking as being harmful was associated with a greater likelihood of quitting after diagnosis, particularly in those with TRCs. Of all the sociodemographic and clinicopathologic characteristics, only greater smoking history was found to be associated with perceiving smoking as being harmful. Taken together, these results suggest that cancer survivors who are smoking at diagnosis may benefit from counseling regarding the harms of continued smoking after a diagnosis of cancer, as one way to improve quit rates.
Previous studies have evaluated risk perceptions in noncancer populations and have found that smokers were more likely to underestimate the risks associated with continued smoking, which is consistent with our current findings.39,40 However, to our knowledge, no prior study has directly evaluated the effects of risk perceptions on smoking cessation in a cancer population. Several studies have examined the sociodemographic correlates of continued smoking versus smoking cessation among cancer survivors31-34; however, there is a paucity of information on the association between sociodemographic factors and smoking risk perception.
Our results speak to the need to address individuals’ perceptions relating to the harms of smoking when planning smoking cessation interventions for patients with cancer. Current practice guidelines for smoking cessation in patients with cancer focus mainly on pharmacotherapy; however, our study results lend support to having more educational interventions to help with changing perceptions, thereby potentially influencing tobacco use.45 The differences observed between patients with TRCs and those with non-TRCs may be a result of the fact that non-TRCs patients may be attributing their disease to another nonmodifiable cause, which may influence their perceptions.46-48 Demonstrating a consistent and significant link between greater smoking history and less accurate risk perceptions may help in the stratification and targeting of patients at a high risk of continued smoking, notably patients with greater pack-year smoking histories. Studies have shown that physicians often assess smoking cessation in patients with cancer at their initial visit, identifying cessation as being important for cancer care; however, physicians do not feel trained adequately in discussing smoking cessation and they perceive a lack of available resources.49 Our results will provide guidance on how clinicians should approach counseling patients about the importance of cessation to their cancer care.
Our results are underpinned by several behavioral change theories. The HBM implies that perception of risk is an important precursor to health behavior change.36 The results of our study support the application of the HBM in the smoking cessation setting of patients with cancer. The TRA is used to predict how individuals will behave on the basis of their attitudes and intentions; applied to our study, a decision by a patient with cancer to quit smoking is based on the outcome they perceive will occur as a result of quitting smoking (improved quality of life, survival, and fatigue).35 If patients with cancer perceive a negative outcome related to smoking continuation, they are more likely to acknowledge the need for smoking cessation and to engage in appropriate cessation behaviors.
The peridiagnostic period is a crucial time for behavior change, because the symptoms leading up to a diagnosis, and a cancer diagnosis itself, provide a strong impetus to adopt healthier lifestyle behaviors to improve survival and quality of life. Thus, in this critical time period, patients who recognize that smoking is a detrimental health behavior may be more likely to attempt to quit and potentially may be more likely to quit successfully. Timely screening of a patient’s perceptions of the harms of smoking informs the clinician early of those patients who are at greatest risk of smoking continuation and will allow subsequent evaluation of possible appropriate interventions.
Our study has some limitations. The use of a cross-sectional design with administration of a one-time questionnaire to patients at some point after their diagnosis did not allow us to collect baseline perception data and therefore did not allow us to assess for a change in perception of the harms of smoking after a cancer diagnosis. Therefore, although we can conclude that there is an association between perception of smoking harms and smoking cessation, the results of the study cannot conclude that a change in perception is associated with a change in smoking status or that a causal relationship exists between risk perception and quitting. Such inferences can be made only in future cohort studies. Second, although the average time from diagnosis to administration of the survey was 18.5 months, which is longer than in our previous studies, a longer follow-up could help establish whether smoking cessation is maintained long term in cancer survivors and whether patient perceptions influence long-term outcomes in terms of quality of life, survival, treatment response, and development of second primary malignancies. Third, patient perceptions were assessed using a nonvalidated single item consisting of a 5-point Likert scale. However, given that the responses were dichotomized into one group that perceived the negative harms of smoking and another group who did not, the psychometric soundness and internal validity of the scale is less relevant. Fourth, the self-administered questionnaire is prone to social desirability and recall biases, particularly with respect to smoking history. Given that our study focused only on the absolute end point of smoking cessation, not smoking reduction, an accurate smoking history should not affect the results substantially. Finally, our study did not assess the motivation for a patient to quit smoking, which would be an important factor to correlate to perception of smoking risk, to help aid in our understanding of behavioral change theories relating to smoking cessation.
In summary, to our knowledge, this study is the first to demonstrate that the likelihood of smoking cessation is influenced by the perception of whether continued smoking after a cancer diagnosis can negatively affect survival, quality of life, and fatigue. Educating patients to alter their perceptions of the harms of smoking may be an important component of a comprehensive cancer survivorship program. Our results have opened the door for additional research to determine whether a change in perception of smoking harms is associated with smoking cessation, whether perceptions regarding smoking can be altered, and, if so, the interventions that are best able to alter these perceptions.
ACKNOWLEDGMENT
D.A. and L.E. are co-first authors of this article. G.L. and M.E.G. are co-senior authors of this article.
Appendix
Table A1.
Summary of Univariable and Multivariable Analysis of Sociodemographic and Clinicopathologic Variables Associated With Worse Perceptions of the Effects of Continued Smoking on Quality of Life, Overall 5-Year Survival, and Fatigue, Among Patients of all Smoking Statuses (N = 1,121)
AUTHOR CONTRIBUTIONS
Conception and Design: Devon Alton, Lawson Eng, Christopher Harper, Robin Milne, Doris Howell, Jennifer M. Jones, Peter Selby, Wei Xu, David P. Goldstein, Geoffrey Liu, Meredith E. Giuliani
Financial Support: Geoffrey Liu
Administrative Support: Lawson Eng, Geoffrey Liu
Provision of Study Materials or Patients: Geoffrey Liu
Collection and Assembly of Data: Devon Alton, Lawson Eng, Delaram Farzanfar, Rahul Mohan, Olivia Krys, Katie Mattina, Christopher Harper, Sophia Liu, Tom Yoannidis, Robin Milne, M. Catherine Brown, Ashlee Vennettilli, Doris Howell, Geoffrey Liu, Meredith E. Giuliani
Data Analysis and Interpretation: Devon Alton, Lawson Eng, Lin Lu, Yuyao Song, Jie Su, Andrew J. Hope, Doris Howell, Peter Selby, Wei Xu, David P. Goldstein, Geoffrey Liu, Meredith E. Giuliani
Manuscript Writing: All authors
Final Approval of Manuscript: All authors
Accountable for All Aspects of the Work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Perceptions of Continued Smoking and Smoking Cessation Among Patients With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Devon Alton
No relationship to disclose
Lawson Eng
No relationship to disclose
Lin Lu
No relationship to disclose
Yuyao Song
No relationship to disclose
Jie Su
No relationship to disclose
Delaram Farzanfar
No relationship to disclose
Rahul Mohan
No relationship to disclose
Olivia Krys
No relationship to disclose
Katie Mattina
No relationship to disclose
Christopher Harper
No relationship to disclose
Sophia Liu
No relationship to disclose
Tom Yoannidis
No relationship to disclose
Robin Milne
No relationship to disclose
M. Catherine Brown
No relationship to disclose
Ashlee Vennettilli
No relationship to disclose
Andrew J. Hope
Travel, Accommodations, Expenses: Elekta
Doris Howell
No relationship to disclose
Jennifer M. Jones
No relationship to disclose
Peter Selby
Consulting or Advisory Role: Boehringer Ingelheim (I), Johnson & Johnson, Pfizer Canada, NVision Insight Group, Myelin & Associates
Research Funding: Pfizer, Bhasin Consulting Fund, Patient-Centered Outcomes Research Institute
Travel, Accommodations, Expenses: Johnson & Johnson, Pfizer Canada
Other Relationship: Johnson & Johnson, Novartis
Other Relationship: Pfizer, MedPlan Communications
Wei Xu
No relationship to disclose
David P. Goldstein
Stock and Other Ownership Interests: CVS (I), Johnson & Johnson (I), Merck (I), Pfizer (I)
Geoffrey Liu
Honoraria: Pfizer, AstraZeneca/MedImmune, Merck Serono, Takeda Pharmaceuticals, Novartis Canada, Roche Canada, AstraZeneca
Consulting or Advisory Role: AstraZeneca/MedImmune, Takeda Pharmaceuticals, Novartis, Abbvie
Speakers' Bureau: AstraZeneca
Meredith E. Giuliani
Travel, Accommodations, Expenses: Elekta
REFERENCES
- 1.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health : The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control; 2014 [PubMed] [Google Scholar]
- 2.Burger RA, Brady MF, Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Macleod LC, Hotaling JM, Wright JL, et al. : Risk factors for renal cell carcinoma in the VITAL study. J Urol 190:1657-1661, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaudet MM, Gapstur SM, Sun J, et al. : Active smoking and breast cancer risk: Original cohort data and meta-analysis. J Natl Cancer Inst 105:515-525, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Beral V, Gaitskell K, Hermon C, et al. : Ovarian cancer and smoking: Individual participant meta-analysis including 28,114 women with ovarian cancer from 51 epidemiological studies. Lancet Oncol 13:946-956, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohl H, Wrobel K, Bojarski C, et al. : Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 108:200-207, 2013 [DOI] [PubMed] [Google Scholar]
- 7.de Martel C, Forman D, Plummer M: Gastric cancer: Epidemiology and risk factors. Gastroenterol Clin North Am 42:219-240, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Bosetti C, Lucenteforte E, Silverman DT, et al. : Cigarette smoking and pancreatic cancer: An analysis from the international pancreatic cancer case-control consortium (Panc4). Ann Oncol 23:1880-1888, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roura E, Castellsagué X, Pawlita M, et al. : Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. Int J Cancer 135:453-466, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Musselman JR, Blair CK, Cerhan JR, et al. : Risk of adult acute and chronic myeloid leukemia with cigarette smoking and cessation. Cancer Epidemiol 37:410-416, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Statistics Canada: Health at a glance: Current smoking trends. http://www.statcan.gc.ca/pub/82-624-x/2012001/article/11676-eng.htm.
- 12.Jones A, Gulbis A, Baker EH: Differences in tobacco use between Canada and the United States. Int J Public Health 55:167-175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke L, Miller LA, Saad A, et al. : Smoking behaviors among cancer survivors: An observational clinical study. J Oncol Pract 5:6-9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooley ME, Lundin R, Murray L: Smoking cessation interventions in cancer care: Opportunities for oncology nurses and nurse scientists. Annu Rev Nurs Res 27:243-272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker MS, Vidrine DJ, Gritz ER, et al. : Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev 15:2370-2377, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Browman GP, Wong G, Hodson I, et al. : Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 328:159-163, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Des Rochers C, Dische S, Saunders MI: The problem of cigarette smoking in radiotherapy for cancer in the head and neck. Clin Oncol (R Coll Radiol) 4:214-216, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Peppone LJ, Mustian KM, Morrow GR, et al. : The effect of cigarette smoking on cancer treatment-related side effects. Oncologist 16:1784-1792, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chelghoum Y, Danaïla C, Belhabri A, et al. : Influence of cigarette smoking on the presentation and course of acute myeloid leukemia. Ann Oncol 13:1621-1627, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Karim AB, Snow GB, Siek HT, et al. : The quality of voice in patients irradiated for laryngeal carcinoma. Cancer 51:47-49, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Rugg T, Saunders MI, Dische S: Smoking and mucosal reactions to radiotherapy. Br J Radiol 63:554-556, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Lindström D, Sadr Azodi O, Wladis A, et al. : Effects of a perioperative smoking cessation intervention on postoperative complications: A randomized trial. Ann Surg 248:739-745, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Day GL, Blot WJ, Shore RE, et al. : Second cancers following oral and pharyngeal cancers: Role of tobacco and alcohol. J Natl Cancer Inst 86:131-137, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Do KA, Johnson MM, Doherty DA, et al. : Second primary tumors in patients with upper aerodigestive tract cancers: Joint effects of smoking and alcohol (United States). Cancer Causes Control 14:131-138, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Richardson GE, Tucker MA, Venzon DJ, et al. : Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann Intern Med 119:383-390, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Wynder EL, Stellman SD: Comparative epidemiology of tobacco-related cancers. Cancer Res 37:4608-4622, 1977 [PubMed] [Google Scholar]
- 27.Kaufman EL, Jacobson JS, Hershman DL, et al. : Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol 26:392-398, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Fentiman IS, Allen DS, Hamed H: Smoking and prognosis in women with breast cancer. Int J Clin Pract 59:1051-1054, 2005 [DOI] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services : The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Rockville, MD: United States Public Health Service, Office on Smoking and Health; 1990 [Google Scholar]
- 30.Cataldo JK, Dubey S, Prochaska JJ: Smoking cessation: An integral part of lung cancer treatment. Oncology 78:289-301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg CJ, Thomas AN, Mertens AC, et al. : Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psychooncology 22:799-806, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostroff JS, Jacobsen PB, Moadel AB, et al. : Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer 75:569-576, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Eng L, Su J, Qiu X, et al. : Second-hand smoke as a predictor of smoking cessation among lung cancer survivors. J Clin Oncol 32:564-570, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Kashigar A, Habbous S, Eng L, et al. : Social environment, secondary smoking exposure, and smoking cessation among head and neck cancer patients. Cancer 119:2701-2709, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Fishbein M Ajzen I: Belief, Attitude, Intention, and Behavior: An Introduction to Theory and Research. Reading, MA, Addison-Wesley, 1975. [Google Scholar]
- 36.Janz NK, Becker MH: The health belief model: A decade later. Health Educ Q 11:1-47, 1984 [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K, Sakurai M, Nishijo M, et al. : Characteristics of smoking cessation in former smokers in a rural area of Japan. Int J Prev Med 3:459-465, 2012 [PMC free article] [PubMed] [Google Scholar]
- 38.Niu C, Eng L, Qiu X, et al. : Lifestyle behaviors in elderly cancer survivors: A comparison with middle-age cancer survivors. J Oncol Pract 11:e450-e459, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Shiffman S, Pillitteri JL, Burton SL, et al. : Smoker and ex-smoker reactions to cigarettes claiming reduced risk. Tob Control 13:78-84, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peretti-Watel P, Constance J, Guilbert P, et al. : Smoking too few cigarettes to be at risk? Smokers’ perceptions of risk and risk denial, a French survey. Tob Control 16:351-356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eng L, Qiu X, Su J, et al. : The role of second-hand smoke exposure on smoking cessation in non-tobacco-related cancers. Cancer 121:2655-2663, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Schnoll RA, James C, Malstrom M, et al. : Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med 25:214-222, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Park ER, Ostroff JS, Rakowski W, et al. : Risk perceptions among participants undergoing lung cancer screening: Baseline results from the National Lung Screening Trial. Ann Behav Med 37:268-279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKee SA, O’Malley SS, Salovey P, et al. : Perceived risks and benefits of smoking cessation: Gender-specific predictors of motivation and treatment outcome. Addict Behav 30:423-435, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Rigotti NA: Clinical practice. Treatment of tobacco use and dependence. N Engl J Med 346:506-512, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Ferrucci LM, Cartmel B, Turkman YE, et al. : Causal attribution among cancer survivors of the 10 most common cancers. J Psychosoc Oncol 29:121-140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wold KS, Byers T, Crane LA, et al. : What do cancer survivors believe causes cancer? (United States). Cancer Causes Control 16:115-123, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Bassett JC, Gore JL, Kwan L, et al. : Knowledge of the harms of tobacco use among patients with bladder cancer. Cancer 120:3914-3922, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Warren GW, Dibaj S, Hutson A, et al. : Identifying targeted strategies to improve smoking cessation support for cancer patients. J Thorac Oncol 10:1532-1537, 2015 [DOI] [PubMed] [Google Scholar]