Abstract
The Dietary Reference Intakes set the protein RDA for persons >19 y of age at 0.8 g protein ⋅ kg body weight−1 ⋅ d−1. A growing body of evidence suggests, however, that the protein RDA may be inadequate for older individuals. The evidence for recommending a protein intake greater than the RDA comes from a variety of metabolic approaches. Methodologies centered on skeletal muscle are of paramount importance given the age-related decline in skeletal muscle mass and function (sarcopenia) and the degree to which dietary protein could mitigate these declines. In addition to evidence from short-term experimental trials, observational data show that higher protein intakes are associated with greater muscle mass and, more importantly, better muscle function with aging. We are in dire need of more evidence from longer-term intervention trials showing the efficacy of protein intakes that are higher than the RDA in older persons to support skeletal muscle health. We propose that it should be recommended that older individuals consume ≥1.2 g protein · kg−1 · d−1 and that there should be an emphasis on the intake of the amino acid leucine, which plays a central role in stimulating skeletal muscle anabolism. Critically, the often-cited potential negative effects of consuming higher protein intakes on renal and bone health are without a scientific foundation in humans.
Keywords: aging, sarcopenia, dynapenia, physical function, amino acid
Introduction
Aging is characterized by a decline in skeletal muscle mass and loss of muscle strength, collectively termed “sarcopenia.” Sarcopenia increases the risk of falls and fractures, dependent living, morbidity, and mortality (1, 2). In North America, it is projected that, by the year 2050, 20% of the US, 25% of the Canadian, and 28% of the Mexican population will be aged >65 y. It has been estimated that ∼5–13% of persons aged >60 y and ≤50% of persons aged >80 y are sarcopenic (3). The direct costs attributed to sarcopenia were ∼$18.5 billion in 2000, which represented 1.5% of the total US annual health care expenditure (4) and will likely increase with the increasing proportion of older individuals. To attenuate the direct and indirect impact of sarcopenia we need to define strategies that help to maintain or slow the loss of skeletal muscle mass and function. Such strategies would need to be affordable, scalable, and have a low off-target (side effect) burden.
The main anabolic stimuli for skeletal muscle are exercise and protein intake. Protein ingestion, and the ensuing hyperaminoacidemia, stimulates muscle protein synthesis (MPS) rates and suppresses, likely via insulin, muscle protein breakdown rates. Therefore, protein is a key component of the diet and should be present in sufficient quantities to preserve muscle mass. We have previously discussed how certain dietary and nutraceutical components interact with resistance exercise to promote muscle growth (5). In this review we present evidence that older individuals may have a greater requirement for protein and can benefit from protein intakes higher than the current protein RDA (6). We propose that this is a tenable thesis based primarily on the preservation of muscle mass with age. Our thesis rests on current and emerging evidence showing that older individuals are, to varying degrees, resistant to the anabolic properties of dietary protein, which compromises a “youthful” robust stimulation of MPS. A specific focus, beyond protein, is placed on the amino acid leucine, which plays a key role in activating MPS. The theme of this review is critically important in nutrition due to the global increase in the aging population and with the knowledge that with increasing age far fewer persons are consuming dietary protein at the level of the Estimated Average Requirement (EAR) and RDA, and in particular the protein intake level we are recommending here (or perhaps lower quality of dietary protein) (7, 8). In fact, intake data from persons aged >70 y show that 6% of older men and 12% of older women consume less protein than the EAR (Figure 1) (7). If, as we propose here, the current EAR and RDA are too low, then these estimates of insufficient protein intake are greater, which is a concern for the aging population.
FIGURE 1.
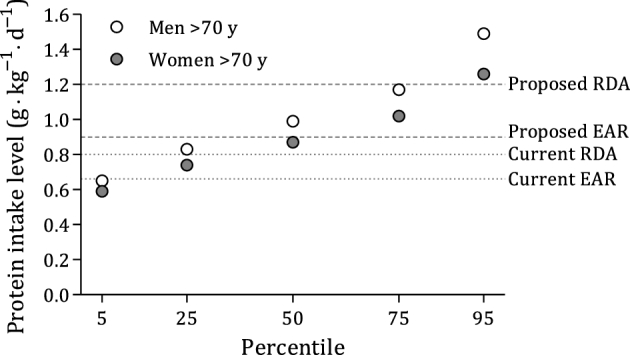
Mean amount of dietary protein expressed according to ideal body weight among men (n = 324) and women (n = 281) aged >70 y. Data were derived from NHANES 2005–2006, as published by Berner et al. (7). Horizontal dotted lines indicate the current EAR and RDA, and dashed lines indicate the proposed EAR (0.9 g · kg−1 · d−1) and RDA (1.2 g · kg−1 · d−1) based on values from Table 1. The data indicated that 6% of men aged >70 y and 12% of women aged >70 y consumed less protein than the current EAR (0.66 g · kg−1 · d−1). EAR, Estimated Average Requirement.
Current Status of Knowledge
The protein requirement for healthy adults is defined as the lowest amount of dietary protein intake that will balance the losses of nitrogen from the body, and thus maintain the body protein mass, in individuals at energy balance with modest levels of physical activity (9). The reference values for dietary protein for the United States and Canada appear in the report of the Food and Nutrition Board of the Institute of Medicine published in 2005 (6). The EAR for healthy adults is 0.66 g protein ⋅ kg body weight−1 ⋅ d−1. The safe level or RDA was identified as being 2 SDs beyond the EAR at 0.8 g · kg−1 · d−1. Thus, 0.8 g · kg−1 · d−1 would be expected to meet the requirements for most (97.5%) of the healthy adult population. These values are derived from a meta-analysis of all available nitrogen balance data obtained from adults (10). There is concern whether the current EAR and RDA for daily protein intake are accurate because of various well-recognized shortcomings of the nitrogen balance technique that critics have highlighted (10–13). It is acknowledged that no notable physiologic variable is known to be associated with being in nitrogen balance. In addition, very few nitrogen balance studies compared younger and older individuals, which precludes the ability to define specific requirements for older adults (10, 14). Finally, no recommendation exists on an “optimal” protein intake aimed to enhance healthy aging that requires more than the minimal dietary requirement (15, 16). The aim of the current perspective is to address evidence that indicates that older people have a greater requirement for protein than the RDA, and to provide evidence for dietary strategies that may help mitigate the loss of skeletal muscle mass.
Aging and Protein Requirements: Whole-Body Measures
The current protein RDA was determined with the use of the nitrogen balance technique. This technique is based on the balance between nitrogen intake and nitrogen losses through urine, feces, skin (primarily sweat), and miscellaneous means (e.g., hair, nails, and exhaled ammonia) (10). First, the study participants consume ≥3 different nitrogen intakes for a period of 10–14 d, with nitrogen balance being determined during the last 3–5 d. Next, linear regression is performed on these nitrogen intakes and zero nitrogen balance is then determined by interpolation to estimate individuals’ nitrogen requirement. To define the current protein requirements, Rand et al. (10) performed a meta-analysis including 19 nitrogen balance studies that reported individual data rather than grouped data. The overall median requirement of the meta-analysis was 105 mg N · kg−1 · d−1, which equates to 0.65 g protein · kg−1 · d−1. Comparing subsets of the data showed that the young individuals required 104 mg N · kg−1 · d−1, whereas older individuals required 131 mg N · kg−1 · d−1 (10). These values equate to 0.65 and 0.82 g protein · kg−1 · d−1 in young and old individuals, respectively, which translates to a substantial 25% greater protein requirement for older persons. This difference was not significant, and the authors provided 3 possible explanations for their finding (10). First, nitrogen balance data from older adults are somewhat contradictory and limited because only 1 of the 19 studies included older participants (10). In an effort to generate more nitrogen balance data in older individuals, Campbell et al. (14) assessed nitrogen balance in 23 younger and 19 older men and women and observed no difference in protein requirements between young and older individuals. Second, energy requirements generally decline with aging; therefore, the dietary protein-to-energy ratio would be higher for older people (10). Third, chronic diseases are more common in the elderly when compared with young individuals and tend to lower the efficiency of nitrogen utilization and retention (10). Decreases in nitrogen retention may result in loss of lean body mass that is often seen during hospitalization or other clinical settings, which could be detrimental for one's general health (17, 18). Recovery from such events is key for older people but often incomplete (19). To regain lean body mass, nitrogen balance needs to be positive during the recovery period, which can be supported by a higher protein intake. Re-examination of the data from Rand et al. (10) plus additional data at higher protein intakes with the use of biphasic linear regression yielded a higher EAR (0.91 g · kg−1 · d−1) and RDA (0.99 g · kg−1 · d−1) (20). In addition, with the use of the indicator amino acid oxidation (IAAO) approach, which is described below, Humayun et al. (20) estimated that, in young men, the EAR for protein was 0.93 g · kg−1 · d−1. These results are in line with the EAR obtained when biphasic linear regression was applied to the existing nitrogen balance data (0.91 g · kg−1 · d−1).
The IAAO method for determining protein requirements (20) is based on the concept that if dietary protein is below the requirement (i.e., limiting), then the labeled indicator amino acid, often l-[1-13C]phenylalanine, cannot be fully utilized for protein synthesis. The indicator amino acid will remain in the free amino acid pool where it will be oxidized, which can be measured as 13CO2 in breath (9). The indicator amino acid will be utilized for protein synthesis to an increasing extent with increasing intakes of dietary protein approaching the requirement. Therefore, oxidation rates will progressively decrease and reach a plateau at the point where the protein requirement is reached (9). Thus, the protein requirement is the lowest level (i.e., breakpoint) in the oxidation curve of the indicator amino acid against the amount of dietary protein (9). Studies that have applied the IAAO method observed a protein requirement and safe intake amount of 0.94 and 1.24 g · kg−1 · d−1 in older men (21), 0.96 and 1.29 g · kg−1 · d−1 in older women (22), and 0.85 and 1.15 g · kg−1 · d−1 in octogenarian women (23) (Table 1). The protein requirements derived from IAAO studies are substantially greater than the current RDA. Despite these recent findings, there is still an ongoing debate on whether or not to change the current protein recommendations (16, 24). Future studies need to obtain ample data in older men and women to refine the protein requirement for older adults. More importantly, it would be desirable to link the outcomes of such trials with measures of MPS and, ultimately, muscle function.
TABLE 1.
Dietary protein requirements and recommendations1
| Study (ref) and age | Sex | Subjects or studies, n | Method | EAR, g · kg−1 · d−1 | RDA, g · kg−1 · d−1 |
|---|---|---|---|---|---|
| Rand et al. (10) | |||||
| Y + O | M + F | 235 subjects | NB | 0.65 | 0.83 |
| Y | M + F | 221 subjects | NB | 0.65 | NR |
| O | M + F | 14 subjects | NB | 0.82 | NR |
| Humayun et al. (20) | |||||
| Y + O | M + F | 19 studies | NB (biphasic linear regression) | 0.91 | 0.99 |
| Y | M | 8 subjects | IAAO | 0.93 | 1.24 |
| Tang et al. (23) | |||||
| Age 80–87 y | F | 6 subjects | IAAO | 0.85 | 1.15 |
| Rafii et al. (22) | |||||
| Age 65–85 y | F | 12 subjects | IAAO | 0.96 | 1.29 |
| Rafii et al. (21) | |||||
| Age 66–79 y | M | 6 subjects | IAAO | 0.94 | 1.24 |
EAR, Estimated Average Requirement; IAAO, indicator amino acid oxidation; NB, nitrogen balance; NR, not reported; O, old; ref, reference; Y, young.
Aging and Protein Requirements: Muscle Measures
A substantive body of data obtained from stable isotope tracer studies that assessed postprandial MPS responses supports the notion to recommend protein intakes greater than the RDA for older people. Previous studies indicated that the postprandial increase in MPS rates is generally lower in older than in younger individuals (25, 26), which is known as age-related anabolic resistance. Wall et al. (27) retrospectively analyzed data from 6 studies conducted within the same laboratory with the use of a similar study design and showed a 16% lower MPS response to the ingestion of an equal dose of protein in older compared with younger men. In addition, Moore et al. (28) described, also based on a retrospective analysis, that the amount of dietary protein at which MPS reached a plateau was 67% higher in older compared with younger individuals when expressed relative to body weight. Although there is limited information on the existence of anabolic resistance in older women, current data indicate that older persons need more protein to achieve a robust stimulation of MPS.
Optimal Protein Intakes to Stimulate MPS
Mounting evidence suggests that age-related anabolic resistance to protein ingestion is ≥1 of the mechanisms underpinning sarcopenia. Protein dose-response studies have been conducted to assess how much dietary protein is required to maximally stimulate MPS. Cuthbertson et al. (29) assessed the MPS response to graded intakes of essential amino acids (EAAs) and observed in younger individuals that intakes ranging from 2.5 to 10 g EAAs (equivalent to ∼5–20 g whey protein) stimulated MPS in a dose-dependent manner. There was, however, no further increase in MPS after the ingestion of 20 g EAAs (29). The older subjects’ MPS rates were stimulated to the same extent as in younger individuals at doses of 2.5 and 5 g EAAs, but to a lesser extent at 10 and 20 g EAAs. Even a 40-g dose of EAAs did not stimulate MPS in the older men to the same level as observed in the young individuals (29). These data indicated that the muscle of older persons is equally sensitive to low doses of protein as in younger persons but lacks the capacity for greater doses of protein to stimulate MPS. This implies that greater doses of protein would not be useful to mitigate sarcopenia and that repeated small doses of protein may actually result in better maintenance of muscle mass with aging. However, both Mitchell et al. (30) and Agergaard et al. (31) reported no differences in MPS when protein was ingested as a single bolus compared with repeated small doses in older men.
Studies that assessed the MPS response to graded intakes of intact protein, as opposed to crystalline amino acids, did not directly compare younger with older individuals within one study, but similarities in study design allow us to make indirect comparison. Moore et al. (32) were the first, to our knowledge, to conduct a dose-response study in younger men after intense leg resistance exercise with postexercise consumption of intakes varying from 0 to 40 g egg protein. The results showed that maximal rates of MPS were achieved after the ingestion of 20 g protein with no significant difference between 20 and 40 g. These data also showed that an increasingly larger percentage of leucine, a key amino acid responsible for triggering MPS (33–36), was oxidized with increasing protein intakes. Witard et al. (37) confirmed the results of Moore et al. by using whey protein and a unilateral exercise model. Yang et al. (38) showed that, in older individuals, MPS was not significantly stimulated until older individuals had ingested 20 g protein. By using a unilateral exercise approach, Yang et al. also showed that whey protein further stimulated MPS in the exercised limb with double the dose (40 g) that maximally stimulated MPS in younger individuals (32). These data suggest that older individuals are less sensitive to lower protein and amino acid doses and yet do have the capacity to stimulate MPS to the same extent as young individuals, but they require a greater dose of protein. This finding is in contrast with Cuthbertson et al. (29), who noted a high sensitivity to low doses of EAAs but a reduced capacity to stimulate MPS after high intakes of EAAs.
Moore et al. (28) compiled data from 6 studies to obtain postprandial body weight–normalized MPS rates over a wide range of protein intakes in 65 younger and 43 older individuals. Biphasic linear regression was performed to determine the relative protein dose required to maximally stimulate MPS. The authors showed that young individuals required 0.24 g protein · kg−1 · meal−1, whereas older individuals required 0.40 g protein · kg−1 · meal−1. Importantly, the estimated maximal MPS rates at these respective doses were not statistically different between younger (0.058% ± 0.006%/h) and older (0.056% ± 0.007%/h) subjects. These results (28) led to the conclusion that aging is associated with a rightward shift in the dose-response curve for protein intake and MPS, and that higher doses are required to maximally stimulate MPS. An important point is that older persons are still able to mount a full MPS response to higher protein intakes that is not different from that seen in younger persons (38–41). Murphy et al. (42) recently assessed integrated MPS rates over a 3-d period during which older individuals consumed either a lower (0.8 g · kg−1 · d−1) or higher (1.2 g · kg−1 · d−1) protein intake diet. Surprisingly, higher protein intake did not result in greater integrated MPS rates than a lower protein intake (42). This suggests that older individuals may require protein intakes even greater than 1.2 g · kg−1 · d−1 to further elevate MPS rates. In agreement, Kim et al. (43) showed that 24-h MPS rates were higher when older individuals consumed 1.5 compared with 0.8 g protein · kg−1 · d−1. The higher-protein diet also resulted in greater whole-body protein synthesis rates without affecting protein breakdown rates, thereby further improving net protein balance in older men and women (43). This high-protein dosing strategy was twice the RDA and may not be feasible from an economic perspective. Nonetheless, twice the RDA has been shown to be effective in maintaining appendicular lean mass and improving physical function over a 10-wk period in older men (44).
The reduced sensitivity of muscle in response to meal-like amounts of protein in older persons may be caused by ≥1 of the following factors: reduced physical activity levels, impaired protein digestion and amino acid absorption, increased splanchnic amino acid retention leading to reduced aminoacidemia, impaired muscle perfusion reducing delivery of amino acids to the muscle, reduced uptake of amino acids by the muscle, and impaired intracellular anabolic signaling (45). Amino acid–mediated anabolic signaling in skeletal muscle is primarily activated by the EAA leucine (33). Leucine has been shown to bind to Sestrin2, which promotes translocation of mechanistic target of rapamycin complex 1 to the lysosomal membrane where it becomes activated, leading to activation of downstream signaling and subsequent initiation of MPS (46–48). A certain amount of leucine seems to be required to reach the threshold for activating the MPS machinery (49, 50). Impairments in ≥1 of the above-mentioned processes could compromise the postprandial elevation in intracellular leucine concentrations and subsequent activation of anabolic signaling. Ingesting a larger amount of protein could overcome this problem by providing more leucine. Nonetheless, higher protein intakes might not be feasible for older people because of reduced appetite, dislike of certain protein-dense foods, or the inability to masticate protein-containing foods such as meat. An alternative strategy for achieving a significant elevation in leucine and subsequent stimulation of MPS would be to supplement a lower-protein–containing diet with leucine or leucine-enriched proteins (51).
Leucine
The quantity of leucine on a per-meal basis required to maximally stimulate MPS is not known and likely differs between younger and older persons. Insofar as muscle is concerned, it is not protein per se that is needed to stimulate MPS but simply a greater quantity of EAAs (52). Of all of the EAAs, leucine appears to be the most potent in activating anabolic signaling, although all EAAs are required to support the leucine-driven increase in MPS (33, 53, 54). Katsanos et al. (34) showed that a mixture of all EEAs with leucine content at 1.7 g stimulated MPS in younger adults; however, this response was not observed in the older adults. When leucine content was increased to 2.8 g there was an observed increase in MPS rates in the older adults (34). In contrast to older men, Bukhari et al. (55) reported that a mixture of all EAAs with leucine content at only 1.2 g stimulated albumin protein synthesis and MPS in older women. Current research points to recommendations for older people to consume leucine-rich food products to robustly stimulate MPS and to promote muscle maintenance (34, 56–59). The administration of crystalline leucine might be one strategy to augment the postprandial increase in circulating leucine, because free leucine is rapidly absorbed by the gut and does not undergo high first-pass splanchnic extraction. We observed that, in young men, a small dose of whey protein (6.25 g) when coingested with free leucine (3 g leucine in total) was not as effective in stimulating MPS as was a higher dose of whey protein (25 g) (60, 61). However, when 4.25 g free leucine was added to the 6.25-g dose of whey protein (5 g leucine in total), the postprandial MPS response was not different from the 25-g dose of whey protein (60). We subsequently conducted an experiment in older men to examine whether integrated MPS rates changed with leucine supplementation over the course of 3 d. Our results indicated that coingestion of 5 g crystalline leucine with each of the main meals increased integrated rates of MPS over 3 d, and this effect of leucine coingestion was independent of whether older subjects consumed 0.8 or 1.2 g protein · kg−1 · d−1 (42). Thus, muscle maintains its capacity to respond to the anabolic effects of leucine across age, and a higher leucine content per meal appears to be an effective dietary strategy for enhancing leucinemia and stimulating MPS. This information is promising and warrants more long-term intervention studies on the effects of leucine-enriched products on muscle mass and function in older adults.
Few studies have examined the longer-term effects of leucine supplementation in older adults (62, 63). Verhoeven et al. (62) assessed muscle mass and strength before and after 3 mo of supplementing 7.5 g leucine/d (3 × 2.5 g/meal) in healthy older men. In contrast to the authors’ hypothesis, leucine supplementation did not increase muscle mass or strength (62). Leenders et al. (63) performed a follow-up study applying the same intervention over a 6-mo period in participants with type 2 diabetes, who generally show an accelerated loss of muscle mass (64, 65). Despite including more participants and an intervention period that was twice as long, there were no gains in muscle mass or strength in this more-compromised sample (63). It is possible that improvements in study outcomes would have been observed if a higher dose of leucine was administered to, for example, frail elderly persons with a suboptimal diet (42, 62, 63). In addition, when considering an estimated age-related loss of muscle mass of ∼0.8% annually (1), an 85-kg older man with ∼40–45 kg muscle (i.e., ∼60–62 kg lean body mass) would lose ∼160–180 g total muscle mass over a 6-mo period. Therefore, the supplementation period may have been too short to detect differences in body composition with the use of a DXA scan, and at best, leucine may only have counteracted the loss in muscle mass. Recent evidence indicated that twice-daily consumption of EAAs with a leucine content of ∼3 g for 12 wk increased lean body mass by 1.1%, whereas EAAs with a leucine content of ∼1.5 g or placebo did not significantly increase lean body mass in older people (66).
Leucine- and Vitamin D–Enriched Whey Protein Supplement
A body of research has investigated the effectiveness of a whey protein supplement enriched with leucine and vitamin D. In an acute setting, Kramer et al. (67, 68) showed that 21 g whey protein combined with 3 g leucine and 800 IU vitamin D increased MPS rates in both healthy and sarcopenic older men. Subsequently, Bauer et al. (69) showed that this supplement promoted a 170-g increase in appendicular muscle mass as well as increases in muscle function and quality-of-life scores in older men and women diagnosed with class I and II sarcopenia. The nutritional supplement was provided twice daily before breakfast and lunch for 13 wk in a large study population consisting of 184 and 196 participants in the active and control groups, respectively (69). In agreement, Verreijen et al. (59) performed a follow-up study with the use of the same supplement and reported that muscle mass was preserved during a weight-loss program in obese older adults. Furthermore, Chanet et al. (70) recently studied 24 healthy older (aged ∼71 y) men who consumed the supplement or a noncaloric placebo with breakfast for 6 wk. They observed that the experimental group gained more appendicular lean mass than the control group. This finding was supported by the greater MPS response after the ingestion of the supplemented breakfast compared with the control breakfast (70). Together, these data indicated that a leucine + vitamin D + whey protein formulation promotes maintenance of muscle mass in older adults.
Aging and Protein Requirements: Observational Data
Current protein intakes derived from the NHANES database indicate that most adults consume at least the RDA. Specifically, the average daily protein intake was 1.5 and 1.3 g · kg−1 · d−1 in young and middle-aged men and 1.2 and 1.1 g · kg−1 · d−1 in young and middle-aged women, respectively (8). In older men and women, the average protein intake was 1.0 g · kg−1 · d−1 (8), which is well above the RDA. However, ≥10% of older men and women consumed less than the RDA (8). Although few studies observed no relation between the amount of dietary protein and muscle mass (71–73), more recent observational evidence suggests that protein intakes above the RDA may be necessary to support healthy musculoskeletal aging (74–83). Observational studies indicate that higher protein intakes are associated with better preservation of muscle mass (Table 2) and physical function (Table 3). In addition, data in Table 2 indicate that the quality of dietary protein (animal compared with plant) may be an important modifiable risk factor for sarcopenia (8, 78, 81, 82, 85–87). Plant-based proteins are less anabolic than animal-derived proteins on a gram-for-gram basis due to lower digestibility and lower content of EAAs and leucine in particular (87, 88). However, with an adequate protein intake, the dietary protein source may not matter (78, 82, 85). More research in this field is required to determine whether a diet high in plant protein may allow for muscle mass maintenance with aging. A recent analysis of longitudinal data showed that higher dietary protein (1.25 g · kg−1 · d−1) accompanied by higher leucine (7.1 g/d) intakes were associated with greater lean mass retention in persons aged >65 y. More long-term longitudinal research is required to define requirements and optimal intakes of leucine to improve musculoskeletal health in older people (80). In general, these data suggest that increasing high-quality dietary protein intake is important for maintaining muscle mass and function with aging.
TABLE 2.
Studies assessing the relation between dietary protein intake and indexes of muscle mass1
| Study (ref) | Subjects, n | Age, y | Design | Dietary assessment | Body-composition measurement | Protein intake | Outcomes |
|---|---|---|---|---|---|---|---|
| Stookey et al. (83) | 298 M, 310 F | 50–69 | L (4 y) | 24-h recall | Arm circumference and skinfold thickness | In % of energy; T1: <10.4; T2: 10.4–12.1; T3: >12.1 | Higher protein intakes were associated with less loss of MAMA |
| Houston et al. (77) | 967 M, 1099 F | 70–79 | L (3 y) | FFQ | DXA | In % of energy (g · kg−1 · d−1); Q1: 10.9 (0.8); Q2: 12.7 (0.7); Q3: 14.2 (0.8); Q4: 15.9 (0.9); Q5: 18.6 (1.2) | Higher protein intake was associated with less LM and aLM loss over 3-y follow-up |
| Geirsdottir et al. (75) | 99 M, 138 F | 65–92 | CS | 3-d food record | DXA | In g · kg−1 · d−1; Q1: 0.63; Q2: 0.85; Q3: 1.01; Q4: 1.36 | Higher protein intake was associated with higher LBM; Q4 – Q1 = 2.3 kg LBM; Q4 – Q2 = 2.0 kg LBM |
| Morris and Jacques (81) | 1257 M, 1168 F | 50–85 | CS | 24-h recall, FFQ | DXA, upper arm, thigh, and calf circumferences, skinfold thickness | In g · kg−1 · d−1; T1: <0.8; T2: 0.8–1.0; T3: >1.0 | Higher protein intake was associated with higher SMMI in nonobese individuals who performed muscle strengthening, moderate aerobic exercise, or no exercise and in obese individuals who performed vigorous aerobic or muscle-strengthening exercise |
| Gregorio et al. (76) | 387 F | 60–90 | CS | 4-d food record | DXA | In g · kg−1 · d−1; LP: <0.8; HP: ≥0.8 | Fat-to-lean ratio was lower in the HP group |
| Isanejad et al. (78) | 554 F | 65–72 | L (3 y) | 3-d food record | DXA | In % of energy (g · kg−1 · d−1); Q1: 16.0 (0.79); Q2: 17.0 (0.90); Q3: 18.0 (0.96); Q4: 19.4 (1.18) | Higher total and animal (but not plant) protein intakes were associated with increased LM and aLM |
| Sahni et al. (82) | 1139 M, 1497 F | 29–86 | CS | FFQ | DXA | In g/d; Q1: M 64.9, F 57.8; Q2: M 70.8, F 63.1; Q3: M 79.2, F 73.5; Q4: M 101.1, F 93.4 | Higher total and animal (but not plant) protein intakes were associated with higher leg LM |
| Farsijani et al. (74) | 351 M, 361 F | 67–84 | L (2 y) | 24-h recall | DXA | In % of energy (g · kg−1 · d−1); Q1: M 11.9 (0.86), F 12.3 (0.8); Q2: M 14.2 (0.93), F 15.2 (1.0); Q3: M 15.9 (1.08), F 17.2 (1.1); Q4: M 19.1 (1.29), F 20.3 (1.3) | Greater protein intakes were associated with higher muscle mass |
| McDonald et al. (80) | 39 M, 40 F | >65 | L (6 y) | Dietary interview | Bioelectrical impedance | In g · kg−1 · d−1 (leucine in g/d); Q1: 0.61 (3.03); Q2: 0.92 (4.44); Q3: 0.97 (5.56); Q4: 1.26 (7.12) | Higher protein and leucine intakes were associated with preservation of LBM |
aLM, appendicular lean mass; CS, cross-sectional; HP, high protein; L, longitudinal; LBM, lean body mass; LM, lean mass; LP, low protein; MAMA, mid-arm muscle area; Q, quartile or quintile; ref, reference; SMMI, skeletal muscle mass index; T, tertile.
TABLE 3.
Studies assessing the relation between dietary protein intake and physical function1
| Study (ref) | Subjects, n | Age, y | Design | Dietary assessment | Physical function measurement | Protein intake | Outcomes |
|---|---|---|---|---|---|---|---|
| Gregorio et al. (76) | 387 F | 60–90 | CS | 4-d food record | PPT, SPPB | In g · kg−1 · d−1; LP: <0.8; HP: ≥0.8 | Upper and lower extremity function was impaired in those who consumed an LP diet |
| McLean et al. (84) | 759 M, 986 F | 29–85 | L (6 y) | FFQ | IHHD | In g/d; Q1: 63; Q2: 74; Q3: 82; Q4: 94 | Higher total and animal protein intakes preserved grip strength in adults ≥60 y |
| Sahni et al. (82) | 1160 M, 1496 F | 29–86 | CS | FFQ | IHHD | In g/d; Q1: M 64.2, F 56.9; Q2: M 70.2, F 63.1; Q3: M 78.9, F 73.4; Q4: M 101.6, F 93.6 | Higher plant (but not total and animal) protein intake was associated with greater quadriceps strength |
| Isanejad et al. (79) | 554 F | 65–72 | L (3 y) | 3-d food record | IHHD, SPPB | In % of energy (g · kg−1 · d−1); T1: 16.4 (≤0.8); T2: 17.4 (0.8–1.2); T3: 18.6 (≥1.2) | Higher protein intake is positively associated with muscle strength and physical function |
CS, cross-sectional; HP, high protein; IHHD, isometric hand-held dynamometer; L, longitudinal; LP, low protein; PPT, physical performance test; Q, quartile; ref, reference; SPPB, short physical performance battery; T, tertile.
Aging and Protein Requirements: Protein Distribution Recommendations
The distribution of dietary protein intake has recently received increasing interest. Many older people consume low, medium, and high amounts of protein with breakfast, lunch, and dinner, respectively. As the aging muscle becomes less sensitive to lower amounts of protein, breakfast and lunch may not provide sufficient protein to induce a robust stimulation of MPS, whereas dinner may provide more protein than required to maximally stimulate MPS. If the dose-response nature of MPS is correct, then a more even meal-to-meal protein distribution may stimulate MPS to a greater degree with each of the main meals and promote maintenance of muscle mass with aging. This concept has been tested in several intervention trials (89–96) and several observational studies (74, 97, 98) (Table 4). Figure 2 shows an example of a skewed and more even protein distribution at different protein intakes. Included is the optimal per-meal amount of dietary protein for older individuals to maximally stimulate MPS (0.40 g · kg−1 · meal−1; 95% CI: 0.21, 0.59 g · kg−1 · meal−1) derived from Moore et al. (28). At the current RDA, a skewed protein distribution may only reach the optimal amount of dietary protein 1 time/d with dinner. It is possible that an even protein distribution at the RDA (each meal would be ∼0.27 g/kg) may in fact be inadequate for the maintenance of muscle mass because none of the main meals would reach the optimal amount of dietary protein for maximally stimulating MPS. A skewed protein distribution at a higher protein intake (i.e., 1.2 g · kg−1 · d−1) would approach the optimal amount of dietary protein with lunch while providing more than required to maximally stimulate MPS with dinner. The amount of protein in excess would be better consumed with meals that are below the optimal amount of dietary protein for maximally stimulating MPS. Higher protein intake with an even protein distribution seems to be an optimal strategy to maximally stimulate MPS with ≥2 of the main meals consumed throughout the day. As such, it seems likely that an even distribution of protein over breakfast, lunch, and dinner increases the potential to mitigate sarcopenia, provided that sufficient amounts of protein are consumed with each meal (i.e., ∼0.4 g · kg−1 · meal−1 with a total of 1.2 g · kg−1 · d−1). In agreement, data from Kim et al. (43) suggested ∼30% greater MPS rates when consuming an even protein distribution at twice the RDA compared with an uneven protein distribution at the RDA in older adults. For the interested reader, we refer to a review article on this topic (99).
TABLE 4.
Studies assessing the relation between mealtime protein distribution and muscle mass and function1
| Study (ref) | Subjects, n | Age, y | Design | Dietary assessment | Body composition and physical function measurement | Protein intake | Outcomes |
|---|---|---|---|---|---|---|---|
| Farsijani et al. (74) | 351 M, 361 F | 67–84 | L (2 y) | 24-h recall | DXA | In % of energy (g · kg−1 · d−1); Q1 (most even): M 15.0 (1.05), F 16.4 (1.09); Q2: M 14.7 (0.99), F 16.2 (1.03); Q3: M 15.4 (1.10), F 16.0 (1.05); Q4 (most skewed): M 15.9 (1.02), F 16.5 (1.01) | More even protein distribution across meals is associated with higher muscle mass |
| Loenneke et al. (98) | 511 M, 570 F | 50–85 | CS | 24-h recall | DXA, isokinetic dynamometry | ≥30 g per meal; 0 times: 0.64 g · kg−1 · d−1; 1 time: 1.06 g · kg−1 · d−1; ≥2 times: 1.4 g · kg−1 · d−1 | More frequent consumption of meals containing 30–45 g protein/meal was associated with leg lean mass and strength |
| Farsijani et al. (97) | 827 M, 914 F | 67–84 | L (3 y) | 24-h recall | PPT | Ig ·kg−1 · d−1; M: 1.05; F: 1.04 | Even distribution of daily protein intake across meals is associated with greater muscle strength |
CS, cross-sectional; L, longitudinal; PPT, physical performance test; Q, quartile; ref, reference.
FIGURE 2.
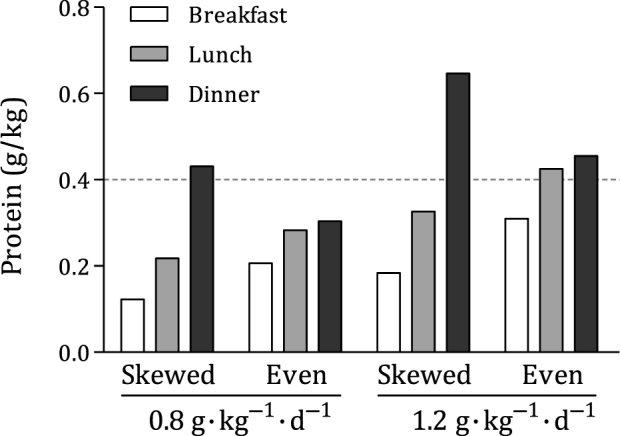
Mean amount of dietary protein during breakfast, lunch, and dinner at total protein intakes of 0.8 and 1.2 g · kg−1 · d−1 in a skewed and even protein intake distribution. The distribution of protein over the main meals is derived from Farsijani et al. (96), with the most skewed and most even distribution of protein intake that is typically seen in older men. The horizontal dashed line refers to the proposed optimal per-meal amount of dietary protein for maximal stimulation of MPS of 0.4 g · kg−1 · meal−1 (95% CI: 0.21, 0.59 g · kg−1 · meal−1) derived from Moore et al. (28). With the use of a skewed pattern at the RDA, only dinner reaches the optimal amount of dietary protein. We recommend protein intakes of 1.2 g · kg−1 · d−1. With the use of a skewed pattern at this higher amount of dietary protein, both lunch and dinner may provide ample protein for increasing MPS rates. Alternatively, more evenly distributing protein intake throughout the day at a higher amount of dietary protein may allow for a stimulation of MPS after all of the main meals, thereby supporting muscle mass maintenance with aging. MPS, muscle protein synthesis.
Protein: Potential Negative Outcomes
An often-proffered reason as to why higher protein intakes should not be recommended for older persons is the risk of renal disease. There is general agreement on the effect of dietary protein restriction on attenuating the decline in glomerular filtration rate (GFR) among most forms of renal injury in humans (100). Although there are potential injurious pathways that have been proposed whereby diets higher in protein could facilitate renal injury, the magnitude of the effect and whether it exists remain unknown. In fact, neither the Institute of Medicine (101) nor the WHO (9) acknowledges that protein intake contributes to functional declines in renal function with age. As stated, “Correlation of creatinine clearance with protein intake showed a positive linear relationship … suggesting that the low protein intake decreased renal function. These factors point to the conclusion that the protein content of the diet is not responsible for the progressive decline in kidney function” (101). Meta-analysis of intervention trials has shown that the GFR, as a working index of kidney function, does not decline when individuals have higher protein intakes, but rather increases (102). There is a belief that this renal hyperfiltration is a result of nephron loss; however, in humans, glomerular hyperfiltration occurs at the whole-kidney level as a result of increased kidney blood flow (103). Importantly, the increase in GFR, known as functional reserve, is a normal response to increased solute load and does not represent a risk factor for the development of chronic kidney disease (104). In addition, pregnancy and unilateral nephrectomy are characterized by significant renal hypertrophy and hyperfiltration with increases in GFR, whereas kidney function remains normal (105–107). In 1 of the longest dietary intervention trials that compared higher with lower protein intakes, it was reported that in otherwise healthy obese persons a low-carbohydrate, high-protein weight-loss diet over 2 y was not associated with detrimental effects on GFR or albuminuria (108). Caution is certainly warranted in older patients with type 2 diabetes; however, when following a protein-restricted diet at an intake less than the RDA, dietary proteins should be of the highest quality possible (i.e., higher in leucine) to prevent muscle loss.
An ostensibly negative aspect of recommending a higher protein intake is related to bone health and the potential dietary acid load associated with protein. The theory is that increased protein intake (and specifically sulfur-containing amino acids) results in metabolic acidosis, which is buffered through the release of alkalinizing compounds that are derived from bone, resulting in bone mineral loss. This so-called acid-ash hypothesis has been examined in meta-analyses and found to lack support (109, 110). In the most recent analysis conducted by the National Osteoporosis Foundation, the authors concluded that there were positive (not negative) trends in bone mineral density at most bone sites, and that only the lumbar spine showed moderate evidence to support benefits of higher protein intake (110). In congruence with this conclusion, it has been suggested that dietary protein is supportive of bone health, likely when calcium and vitamin D intakes are also adequate (111).
Conclusions
Age-related loss of muscle mass and function increases the morbidity and mortality risk of age-related chronic diseases and increases the risk of disability. These concerns are going to become an even greater issue given the global increases in the aging population. The etiology of sarcopenia is complex and multifaceted and there is no widely available pharmacologic intervention with an appropriately low side-effect profile to directly combat this condition. On the basis of a number of lines of evidence, we propose that the current protein RDA does not appear to meet the optimal protein requirements for older people to maintain skeletal muscle mass. Given that sarcopenia is, in part, underpinned by the reduced ability of dietary protein to stimulate MPS, increasing amounts of protein have been shown to result in better preservation of muscle mass. Data reviewed here show that protein-induced enhancements in skeletal muscle mass and physical function could be augmented by supplementation with leucine. In case higher protein intakes are not suitable or desirable, the quality of the protein source should be considered as an alternative variable to manipulate, and proteins of higher quality should be substituted for those of lower quality. In addition, observational data suggest that a more even protein distribution throughout the day to per-meal protein intakes within the range of the age-specific muscle anabolic threshold may increase the potential for improved long-term maintenance of muscle mass. As part of a multimodal intervention, increasing dietary protein intakes may support not only muscle mass maintenance but also bone health when calcium and vitamin D intakes are adequate. In summary, there is still an ongoing debate on changing the current protein recommendations; therefore, the question remains: What is the optimal protein intake in aging and are we ready to recommend more?
Acknowledgments
All authors read and approved the final manuscript.
Notes
Perspectives articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or points of view. Opinions expressed in Perspectives articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
There were no specific sources of funding for this article. SMP acknowledges the National Science and Engineering Research Council of Canada, the Canadian Institutes for Health Research, and the Canada Research Chairs Program for support.
Author disclosures: DAT and SHMG, no conflicts of interest. SMP reports having received competitive research funding, travel expenses, and honoraria from the US National Dairy Council (NDC). Some studies discussed in this article may have been funded by the NDC.
Abbreviations used:
- EAA
essential amino acid
- EAR
Estimated Average Requirement
- GFR
glomerular filtration rate
- IAAO
indicator amino acid oxidation
- MPS
muscle protein synthesis
References
- 1. Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab 2010;35(5):707–12. [DOI] [PubMed] [Google Scholar]
- 2. Rosenthal MD, Glew RH. Medical biochemistry. Hoboken (NJ): John Wiley & Sons, Inc.; 2009. [Google Scholar]
- 3. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010;1(2):129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52(1):80–5. [DOI] [PubMed] [Google Scholar]
- 5. Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr 2015;6(4):452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lupton JR, Brooks GA, Butte NF, Caballero B, Flatt JP, Fried SK, Garlick PJ, Grundy SM, Innis SM, Jenkins DJA. et al. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington (DC): National Academies Press; 2002/2005. [Google Scholar]
- 7. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet 2013;113(6):809–15. [DOI] [PubMed] [Google Scholar]
- 8. Fulgoni VL., III Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr 2008;87(Suppl):1554S–7S. [DOI] [PubMed] [Google Scholar]
- 9. Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser 2007;935:1–265. [PubMed] [Google Scholar]
- 10. Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr 2003;77(1):109–27. [DOI] [PubMed] [Google Scholar]
- 11. Hegsted DM. Minimum protein requirements of adults. Am J Clin Nutr 1968;21(5):352–7. [DOI] [PubMed] [Google Scholar]
- 12. Young VR. Nutritional balance studies: indicators of human requirements or of adaptive mechanisms? J Nutr 1986;116(4):700–3. [DOI] [PubMed] [Google Scholar]
- 13. Wolfe RR, Cifelli AM, Kostas G, Kim IY. Optimizing protein intake in adults: interpretation and application of the recommended dietary allowance compared with the acceptable macronutrient distribution range. Adv Nutr 2017;8(2):266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr 2008;88(5):1322–9. [DOI] [PubMed] [Google Scholar]
- 15. Phillips SM. Determining the protein needs of “older” persons one meal at a time. Am J Clin Nutr 2017;105(2):291–2. [DOI] [PubMed] [Google Scholar]
- 16. Phillips SM, Chevalier S, Leidy HJ. Protein “requirements” beyond the RDA: implications for optimizing health. Appl Physiol Nutr Metab 2016;41(5):565–72. [DOI] [PubMed] [Google Scholar]
- 17. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297(16):1772–4. [DOI] [PubMed] [Google Scholar]
- 18. Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL. et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 2015;593(18):4259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell KE, von Allmen MT, Devries MC, Phillips SM. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging 2016;5(1):33–41. [DOI] [PubMed] [Google Scholar]
- 20. Humayun MA, Elango R, Ball RO, Pencharz PB. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am J Clin Nutr 2007;86(4):995–1002. [DOI] [PubMed] [Google Scholar]
- 21. Rafii M, Chapman K, Elango R, Campbell WW, Ball RO, Pencharz PB, Courtney-Martin G. Dietary protein requirement of men >65 years old determined by the indicator amino acid oxidation technique is higher than the current estimated average requirement. J Nutr 2016;146:681–7. [DOI] [PubMed] [Google Scholar]
- 22. Rafii M, Chapman K, Owens J, Elango R, Campbell WW, Ball RO, Pencharz PB, Courtney-Martin G. Dietary protein requirement of female adults >65 years determined by the indicator amino acid oxidation technique is higher than current recommendations. J Nutr 2015;145(1):18–24. [DOI] [PubMed] [Google Scholar]
- 23. Tang M, McCabe GP, Elango R, Pencharz PB, Ball RO, Campbell WW. Assessment of protein requirement in octogenarian women with use of the indicator amino acid oxidation technique. Am J Clin Nutr 2014;99(4):891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci 2013;68(6):677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, Atherton PJ. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiol (Oxf) 2016;216(1):15–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shad BJ, Thompson JL, Breen L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab 2016;311(5):E803–E17. [DOI] [PubMed] [Google Scholar]
- 27. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJ. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One 2015;10(11):e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70(1):57–62. [DOI] [PubMed] [Google Scholar]
- 29. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19(3):422–4. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell WK, Phillips BE, Williams JP, Rankin D, Lund JN, Wilkinson DJ, Smith K, Atherton PJ. The impact of delivery profile of essential amino acids upon skeletal muscle protein synthesis in older men: clinical efficacy of pulse vs. bolus supply. Am J Physiol Endocrinol Metab 2015;309(5):E450–7. [DOI] [PubMed] [Google Scholar]
- 31. Agergaard J, Bulow J, Jensen JK, Reitelseder S, Drummond MJ, Schjerling P, Scheike T, Serena A, Holm L. Light-load resistance exercise increases muscle protein synthesis and hypertrophy signaling in elderly men. Am J Physiol Endocrinol Metab 2017;312(4):E326–E38. [DOI] [PubMed] [Google Scholar]
- 32. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 2009;89(1):161–8. [DOI] [PubMed] [Google Scholar]
- 33. Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010;38(5):1533–9. [DOI] [PubMed] [Google Scholar]
- 34. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291(2):E381–7. [DOI] [PubMed] [Google Scholar]
- 35. Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol 2006;575(Part 1):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BB, Senden JM, Gijsen AP, Verdijk LB, van Loon LJ. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr 2013;32(3):412–9. [DOI] [PubMed] [Google Scholar]
- 37. Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 2014;99(1):86–95. [DOI] [PubMed] [Google Scholar]
- 38. Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108(10):1780–8. [DOI] [PubMed] [Google Scholar]
- 39. Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 2012;302(8):E992–9. [DOI] [PubMed] [Google Scholar]
- 40. Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 2013;38(2):120–5. [DOI] [PubMed] [Google Scholar]
- 41. Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 2012;9(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr 2016;104(6):1594–606. [DOI] [PubMed] [Google Scholar]
- 43. Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, Wolfe RR, Ferrando AA. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab 2015;308(1):E21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, Sjodin A, Wagner KH. et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr 2017;106(6):1375–83. [DOI] [PubMed] [Google Scholar]
- 45. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 2013;41(3):169–73. [DOI] [PubMed] [Google Scholar]
- 46. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149(2):274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 2016;351(6268):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016;351(6268):43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med 2014;44(Suppl 1):S71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phillips SM. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr Metab (Lond) 2016;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Phillips SM. Current concepts and unresolved questions in dietary protein requirements and supplements in adults. Front Nutr 2017;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78(2):250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM. et al. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013;591(11):2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 2005;135(3):376–82. [DOI] [PubMed] [Google Scholar]
- 55. Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, Kobayashi H, Greenhaff PL, Smith K, Atherton PJ. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab 2015;308(12):E1056–65. [DOI] [PubMed] [Google Scholar]
- 56. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D. et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 57. Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev 2013;71(4):195–208. [DOI] [PubMed] [Google Scholar]
- 58. Luiking YC, Deutz NE, Memelink RG, Verlaan S, Wolfe RR. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: a randomized controlled trial. Nutr J 2014;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 2015;101(2):279–86. [DOI] [PubMed] [Google Scholar]
- 60. Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK. et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 2014;99(2):276–86. [DOI] [PubMed] [Google Scholar]
- 61. Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 2012;590(11):2751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 2009;89(5):1468–75. [DOI] [PubMed] [Google Scholar]
- 63. Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr 2011;141(6):1070–6. [DOI] [PubMed] [Google Scholar]
- 64. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M. et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32(11):1993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, van Loon LJ. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985) 2014;116(8):998–1005. [DOI] [PubMed] [Google Scholar]
- 66. Ispoglou T, White H, Preston T, McElhone S, McKenna J, Hind K. Double-blind, placebo-controlled pilot trial of L-leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65–75 years. Eur J Clin Nutr 2016;70(2):182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kramer IF, Verdijk LB, Hamer HM, Verlaan S, Luiking Y, Kouw IW, Senden JM, van Kranenburg J, Gijsen AP, Poeze M. et al. Impact of the macronutrient composition of a nutritional supplement on muscle protein synthesis rates in older men: a randomized, double blind, controlled trial. J Clin Endocrinol Metab 2015;100(11):4124–32. [DOI] [PubMed] [Google Scholar]
- 68. Kramer IF, Verdijk LB, Hamer HM, Verlaan S, Luiking YC, Kouw IW, Senden JM, van Kranenburg J, Gijsen AP, Bierau J. et al. Both basal and post-prandial muscle protein synthesis rates, following the ingestion of a leucine-enriched whey protein supplement, are not impaired in sarcopenic older males. Clin Nutr 2016;36(5):1440–9. [DOI] [PubMed] [Google Scholar]
- 69. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo ME, Mets T, Seal C, Wijers SL. et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2015;16(9):740–7. [DOI] [PubMed] [Google Scholar]
- 70. Chanet A, Verlaan S, Salles J, Giraudet C, Patrac V, Pidou V, Pouyet C, Hafnaoui N, Blot A, Cano N. et al. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr 2017;147(12):2262–71. [DOI] [PubMed] [Google Scholar]
- 71. Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 1999;107(2):123–36. [DOI] [PubMed] [Google Scholar]
- 72. Mitchell D, Haan MN, Steinberg FM, Visser M. Body composition in the elderly: the influence of nutritional factors and physical activity. J Nutr Health Aging 2003;7(3):130–9. [PubMed] [Google Scholar]
- 73. Starling RD, Ades PA, Poehlman ET. Physical activity, protein intake, and appendicular skeletal muscle mass in older men. Am J Clin Nutr 1999;70(1):91–6. [DOI] [PubMed] [Google Scholar]
- 74. Farsijani S, Morais JA, Payette H, Gaudreau P, Shatenstein B, Gray-Donald K, Chevalier S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am J Clin Nutr 2016;104(3):694–703. [DOI] [PubMed] [Google Scholar]
- 75. Geirsdottir OG, Arnarson A, Ramel A, Jonsson PV, Thorsdottir I. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr Res 2013;33(8):608–12. [DOI] [PubMed] [Google Scholar]
- 76. Gregorio L, Brindisi J, Kleppinger A, Sullivan R, Mangano KM, Bihuniak JD, Kenny AM, Kerstetter JE, Insogna KL. Adequate dietary protein is associated with better physical performance among post-menopausal women 60–90 years. J Nutr Health Aging 2014;18(2):155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87(1):150–5. [DOI] [PubMed] [Google Scholar]
- 78. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Association of protein intake with the change of lean mass among elderly women: the Osteoporosis Risk Factor and Prevention–Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci 2015;4:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr 2016;115(7):1281–91. [DOI] [PubMed] [Google Scholar]
- 80. McDonald CK, Ankarfeldt MZ, Capra S, Bauer J, Raymond K, Heitmann BL. Lean body mass change over 6 years is associated with dietary leucine intake in an older Danish population. Br J Nutr 2016;115(9):1556–62. [DOI] [PubMed] [Google Scholar]
- 81. Morris MS, Jacques PF. Total protein, animal protein and physical activity in relation to muscle mass in middle-aged and older Americans. Br J Nutr 2013;109(7):1294–303. [DOI] [PubMed] [Google Scholar]
- 82. Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr 2015;145(7):1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stookey JD, Adair LS, Popkin BM. Do protein and energy intakes explain long-term changes in body composition? J Nutr Health Aging 2005;9(1):5–17. [PubMed] [Google Scholar]
- 84. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham Offspring cohort. J Gerontol A Biol Sci Med Sci 2016;71(3):356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mangano KM, Sahni S, Kiel DP, Tucker KL, Dufour AB, Hannan MT. Dietary protein is associated with musculoskeletal health independently of dietary pattern: the Framingham Third Generation Study. Am J Clin Nutr 2017;105(3):714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gorissen SH, Horstman AM, Franssen R, Crombag JJ, Langer H, Bierau J, Respondek F, van Loon LJ. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J Nutr 2016;146(9):1651–9. [DOI] [PubMed] [Google Scholar]
- 87. van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr 2015;145(9):1981–91. [DOI] [PubMed] [Google Scholar]
- 88. Gorissen SHM, Witard OC. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc Nutr Soc 2017;77:20–31. [DOI] [PubMed] [Google Scholar]
- 89. Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM. et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 2013;591(Part 9):2319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Arnal MA, Mosoni L, Boirie Y, Gachon P, Genest M, Bayle G, Grizard J, Arnal M, Antoine JM, Beaufrere B. et al. Protein turnover modifications induced by the protein feeding pattern still persist after the end of the diets. Am J Physiol Endocrinol Metab 2000;278(5):E902–9. [DOI] [PubMed] [Google Scholar]
- 91. Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrere B. et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr 1999;69(6):1202–8. [DOI] [PubMed] [Google Scholar]
- 92. Bouillanne O, Curis E, Hamon-Vilcot B, Nicolis I, Chretien P, Schauer N, Vincent JP, Cynober L, Aussel C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: a randomized controlled trial. Clin Nutr 2013;32(2):186–92. [DOI] [PubMed] [Google Scholar]
- 93. Bouillanne O, Neveux N, Nicolis I, Curis E, Cynober L, Aussel C. Long-lasting improved amino acid bioavailability associated with protein pulse feeding in hospitalized elderly patients: a randomized controlled trial. Nutrition 2014;30(5):544–50. [DOI] [PubMed] [Google Scholar]
- 94. Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 2014;144(6):876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Moore DR, Areta J, Coffey VG, Stellingwerff T, Phillips SM, Burke LM, Cleroux M, Godin JP, Hawley JA. Daytime pattern of post-exercise protein intake affects whole-body protein turnover in resistance-trained males. Nutr Metab (Lond) 2012;9(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Murphy CH, Churchward-Venne TA, Mitchell CJ, Kolar NM, Kassis A, Karagounis LG, Burke LM, Hawley JA, Phillips SM. Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. Am J Physiol Endocrinol Metab 2015;308(9):E734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Farsijani S, Payette H, Morais JA, Shatenstein B, Gaudreau P, Chevalier S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: the Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am J Clin Nutr 2017;106(1):113–24. [DOI] [PubMed] [Google Scholar]
- 98. Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr 2016;35(6):1506–11. [DOI] [PubMed] [Google Scholar]
- 99. Murphy CH, Oikawa SY, Phillips SM. Dietary protein to maintain muscle mass in aging: a case for per-meal protein recommendations. J Frailty Aging 2016;5(1):49–58. [DOI] [PubMed] [Google Scholar]
- 100. Kaysen GA, Odabaei G. Dietary protein restriction and preservation of kidney function in chronic kidney disease. Blood Purif 2013;35(1–3):22–5. [DOI] [PubMed] [Google Scholar]
- 101. Trumbo P, Schlicker S, Yates AA, Poos M; Food, Nutrition Board of the Institute of Medicine The National Academies (TNA) Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002;102(11):1621–30. [DOI] [PubMed] [Google Scholar]
- 102. Schwingshackl L, Hoffmann G. Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: a systematic review and meta-analysis. PLoS One 2014;9(5):e97656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012;8(5):293–300. [DOI] [PubMed] [Google Scholar]
- 104. Thomas DM, Coles GA, Williams JD. What does the renal reserve mean? Kidney Int 1994;45(2):411–6. [DOI] [PubMed] [Google Scholar]
- 105. Calderon JL, Zadshir A, Norris K. A survey of kidney disease and risk-factor information on the World Wide Web. MedGenMed 2004;6(4):3. [PMC free article] [PubMed] [Google Scholar]
- 106. Higashihara E, Horie S, Takeuchi T, Nutahara K, Aso Y. Long-term consequence of nephrectomy. J Urol 1990;143(2):239–43. [DOI] [PubMed] [Google Scholar]
- 107. Regazzoni BM, Genton N, Pelet J, Drukker A, Guignard JP. Long-term followup of renal functional reserve capacity after unilateral nephrectomy in childhood. J Urol 1998;160(3 Part 1):844–8. [DOI] [PubMed] [Google Scholar]
- 108. Friedman AN, Ogden LG, Foster GD, Klein S, Stein R, Miller B, Hill JO, Brill C, Bailer B, Rosenbaum DR. et al. Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin J Am Soc Nephrol 2012;7(7):1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fenton TR, Tough SC, Lyon AW, Eliasziw M, Hanley DA. Causal assessment of dietary acid load and bone disease: a systematic review and meta-analysis applying Hill's epidemiologic criteria for causality. Nutr J 2011;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shams-White MM, Chung M, Du M, Fu Z, Insogna KL, Karlsen MC, LeBoff MS, Shapses SA, Sackey J, Wallace TC. et al. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr 2017;105(6):1528–43. [DOI] [PubMed] [Google Scholar]
- 111. Mangano KM, Sahni S, Kerstetter JE. Dietary protein is beneficial to bone health under conditions of adequate calcium intake: an update on clinical research. Curr Opin Clin Nutr Metab Care 2014;17(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


