Abstract
Objective
To prospectively examine how selected lifestyle factors and family history of Parkinson disease (PD) combine to determine overall PD risk.
Methods
We derived risk scores among 69,968 women in the Nurses' Health Study (NHS) (1984–2012) and 45,830 men in the Health Professionals Follow-up Study (HPFS) (1986–2012). Risk scores were computed for each individual based on the following factors previously associated with PD risk: total caffeine intake, smoking, physical activity, and family history of PD for the NHS, and additionally total flavonoid intake and dietary urate index for the HPFS. Hazard ratios were estimated using Cox proportional hazards models. In addition, we performed tests of interactions on both the multiplicative and additive scale between pairs of risk factors.
Results
We documented 1,117 incident PD cases during follow-up. The adjusted hazard ratios comparing individuals in the highest category of the reduced risk score to those in the lowest category were 0.33 (95% confidence interval: 0.21, 0.49; ptrend < 0.0001) in the NHS and 0.18 (95% confidence interval: 0.10, 0.32; ptrend < 0.0001) in the HPFS. Results were similar when applying the risk scores computed by summing the predictors weighted by the log of their individual effect sizes on PD risk in these cohorts. Additive interaction was present between no family history of PD and caffeine in men and between caffeine and physical activity in women.
Conclusions
Our results suggest that known protective factors for PD tend to have additive or superadditive effects, so that PD risk is very low in individuals with multiple protective risk factors.
While several genes modulate Parkinson disease (PD) risk,1–5 90% of PD cases have no discernible genetic cause,6,7 and there is strong evidence for a role of lifestyle factors.8
In particular, caffeine intake, smoking, and physical activity have been consistently associated with a lowered PD risk in both men and women. It remains uncertain, however, how these factors interact with each other and with family history of PD. Therefore, we generated risk scores and evaluated the association between these scores and long-term PD risk in 2 large prospective cohorts: the Nurses' Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). In addition, we assessed the presence of multiplicative and additive interactions between pairs of factors.
Methods
Study population
The participants of the current study comprised US women from the NHS and men from the HPFS. The NHS began in 1976 when 121,701 female registered nurses who were 30 to 55 years of age returned detailed mailed questionnaires regarding health-related factors and medical histories. The HPFS enrolled 51,529 male health professionals aged 40 to 75 years who returned similar questionnaires in 1986. Every 2 years, follow-up information on lifestyle practices, health-related factors, and incident diseases was collected from members of both cohorts. The present study was restricted to 69,968 women from the NHS and 45,830 men from the HPFS with no history of PD and complete and reliable dietary data in 1984 (NHS) or 1986 (HPFS) (figure e-1, links.lww.com/WNL/A418).
Standard protocol approvals, registrations, and patient consents
This study was approved by the Human Research Committees at the Brigham and Women's Hospital and the Harvard T.H. Chan School of Public Health.
Assessment of dietary components of the risk score
Nutritional information was ascertained every 4 years via validated food frequency questionnaires (FFQs). The study baseline was determined to be 1984 for the NHS and 1986 for the HPFS because dietary information was first comprehensively assessed in FFQs requested in those years. Participants were asked to self-report their average intake of approximately 130 foods or beverages over the past year using 9 possible multiple-choice responses provided for intake frequency for each item, ranging from “never or less than once per month” to “6 or more times per day.” Nutrient intake was then calculated using the quantity of nutrient in each item times the frequency of consumption. To account for correlation between an individual's daily nutrient intake with overall caloric intake, we adjusted nutrients for total energy intake using the residual method.9
Updated information on other factors regarding lifestyle characteristics were assessed biennially. The risk score was composed of factors that have been previously found to be associated with PD risk in each of these 2 cohorts. For NHS, these predictors included total caffeine intake, smoking, physical activity, and family history of PD (i.e., mother, father, sibling). Additional predictors for the HPFS included dietary urate index (comprising dairy protein, fructose, alcohol, and vitamin C intake) and total flavonoid intake.
Computation of the risk score
For each factor shown to be protective of PD, we ranked participants' levels into cohort-specific quintiles of cumulative averages up to the last questionnaire before the date of onset of PD symptoms, with the exception of smoking in pack-years for which we used the following categories: 0–9, 10–19, 20–49, ≥50 since almost 50% of participants had never smoked. We then assigned scores between 1 and 5—one point per increase in rank, with the lowest quintile or category being the reference. If the score could not be derived from a specific questionnaire because of missing values, we used the risk score derived from the previous questionnaire. All predictors are associated with a reduced risk of PD except for family history of PD, for which we assigned a score of 5 for absence of family history, and a score of 0 for presence of family history. The scores were summed to compute the overall score, which ranged from 3 to 20 for the NHS and 5 to 30 for the HPFS. Higher scores represent lifestyle and genetic characteristics associated with lower PD risk.
Ascertainment of PD cases
PD cases were identified via self-administered questionnaires, and incident diagnoses were biennially documented thereafter. We then requested that each patient's neurologist either return a self-administered questionnaire confirming the PD diagnosis or send a copy of the patient's medical records. Before 2003, PD cases were considered to be confirmed if the treating neurologist reported the diagnosis as definite or probable, or if the medical record either indicated a final diagnosis of PD made by a neurologist or the medical record indicated presence of at least 2 of 3 cardinal signs (i.e., rest tremor, rigidity, bradykinesia) in the absence of features suggesting other illness diagnoses. After 2003, PD cases were confirmed using a similar procedure with the exception that medical records were requested from all PD cases, which were then reviewed by a movement disorders specialist.
Statistical analysis
We conducted all analyses separately in each cohort using cohort-specific risk scores. Participants contributed person-time starting from the return date of the baseline questionnaire until the date of first PD symptoms, date of death, date of the latest completed questionnaire, or end of follow-up in 2012, whichever occurred first. Our analyses were stratified jointly by age in months at the start of follow-up, which was our time scale, and calendar year of the current questionnaire cycle.
Risk score analysis
We compared incident PD risk in quintiles of risk score in each cohort. We calculated hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) using time-dependent Cox proportional hazards models. Indicator variables were used to adjust for the number of missing FFQs.
To conduct tests of trend, midcategory scores (median lifestyle risk score value within each quintile) were modeled as a continuous variable. In addition to ranking the risk scores into quintiles, we compared PD risk in 5 categories of the risk score: 5–11 (reference), 12–16, 17–20, 21–25, 25–30 for the HPFS; 3–9 (reference), 10–12, 13–14, 15–16, and 17–20 for NHS. We also computed individuals' weighted risk score by summing the predictors weighted by the log-transformed effect size of each predictor and its association with PD risk in each cohort; thus, predictors with stronger HRs contributed more to the weighted risk score compared to a predictor with weaker HRs (table e-1, links.lww.com/WNL/A419). The weighted risk scores were then analyzed similarly, comparing the risk of PD between quintiles of weighted risk scores.
Interaction analysis
We used a 2 × 2 factorial design composed of 2 dichotomous risk factors with 4 corresponding possible exposure categories, among which the category with low/no exposure to either factor was the reference. In each cohort, predictors of PD were dichotomized at their respective median levels as follows: caffeine (high/low), physical activity (high/low), and smoking (ever/never). We dichotomized having no family history of PD (mother, father, or sibling) vs having any family history of PD. For men, dietary urate and total flavonoid intake were each dichotomized as high/low, and for women, postmenopausal hormone use was categorized as never/ever. Because the interpretations of the additive interaction indices are only appropriate for factors with harmful effects, we reversed the coding of all preventative factors before conducting tests of additive interaction.10
We compared the individual effects of exposures and their joint effect, each against the subgroup that is unexposed to either exposure to estimate 3 primary measures of additive interaction: the relative excess risk due to interaction (RERI), the attributable proportion due to interaction (AP), and the synergy index (S), where an RERI and AP of 0 and S of 1 indicate exact additivity and therefore no additive interaction. This allowed us to determine whether the joint effect of both exposures is superadditive (RERI > 0, AP > 0, S > 1) or subadditive (RERI < 0, AP < 0, S < 1) compared to the combined effect of each of the individual effects. To obtain the RERI and its 95% CI for the proportional hazards model, we followed the methods outlined by Li and Chambless.11 For analyses of additive interaction where caffeine is categorized into tertiles, we again computed the additive interaction measures for each tertile of caffeine intake compared to lowest tertile (reference) using methods described by Andersson et al.12
In addition, we performed tests of statistical interaction between predictors of the risk score on the association of PD risk on the multiplicative scale. We also conducted likelihood ratio tests comparing the model with interaction terms between caffeine intake (per 100 mg/d) and predictors (quintiles) to the model without the interaction terms. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Data availability
The datasets analyzed in the current study are not publicly available because of restricted access, but further information about the datasets is available from the corresponding author on reasonable request.
Results
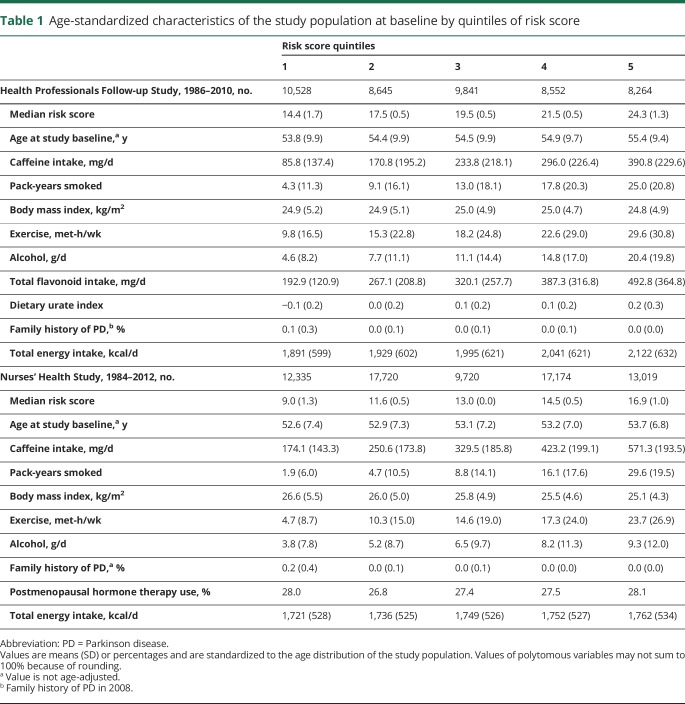
We documented 534 incident PD cases in women and 583 in men over 2,652,243 person-years of follow-up. The distribution of baseline characteristics across quintiles of the risk score for the NHS and HPFS are shown in table 1. Women generally had a higher caffeine intake, were less physically active, and tended to smoke less compared to men.
Table 1.
Age-standardized characteristics of the study population at baseline by quintiles of risk score
Interaction analyses
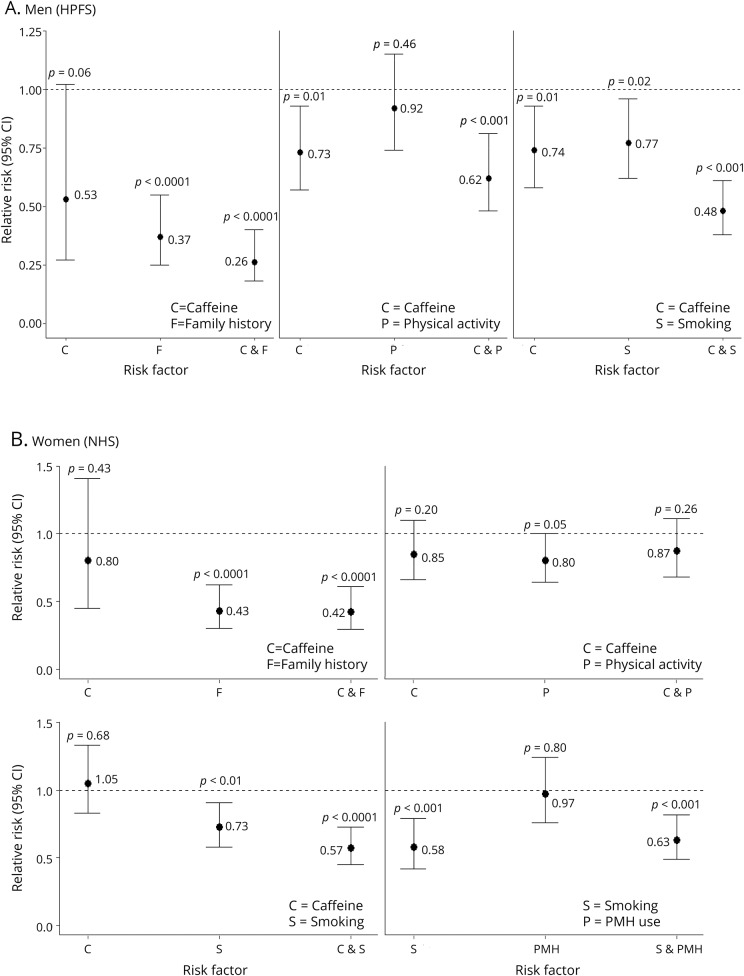
Figure 1, A and B, show the results of subgroup analyses between 2 dichotomized predictors of PD in the HPFS and NHS, respectively. Overall, among the 4 exposure categories, men and women who were in the high category for both predictors had the lowest PD risk compared to participants who were in the low category for both predictors. For the HPFS, ever smokers with high caffeine intake had a 52% decreased PD risk compared to the referent group of never smokers with low caffeine intake (HR = 0.48, 95% CI: 0.38, 0.61; p < 0.0001) (figure 1A). However, there was neither evidence of additive interaction nor effect modification on the multiplicative scale (pRERI = 0.35, pmulti = 0.18) (table e-2A, links.lww.com/WNL/A419). For the NHS, women who had ever smoked with high caffeine consumption had 0.57 times the PD risk compared to women who had never smoked with low caffeine intake (95% CI: 0.45, 0.73; p < 0.0001) (figure 1B). Sensitivity analyses in which smokers who had quit more than 20 years ago were grouped with never smokers gave virtually identical results.
Figure 1. Associations of individual and combined risk factors and Parkinson disease risk among (A) men (HPFS) and (B) women (NHS).
CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses' Health Study; PMH = postmenopausal hormone.
There was no evidence for multiplicative or additive interaction between caffeine and physical activity in the HPFS, but evidence for additive interaction between caffeine and having no family history was present (AP = 0.38, 95% CI: 0.04, 0.72; pAP = 0.03) (table e-2A, links.lww.com/WNL/A419). Furthermore, when caffeine intake was categorized into tertiles, additive interaction between men who were in the highest tertile of caffeine intake and family history was present (RERI = 2.01, pRERI < 0.05; AP = 0.48, pAP <0.01), but not for men who were in the middle tertile of caffeine intake (table e-3A). In women, additive interaction between caffeine and physical activity was significant (AP = 0.21, 95% CI: = 0.03, 0.39; pAP = 0.02) (table e-2B).
Risk score analyses
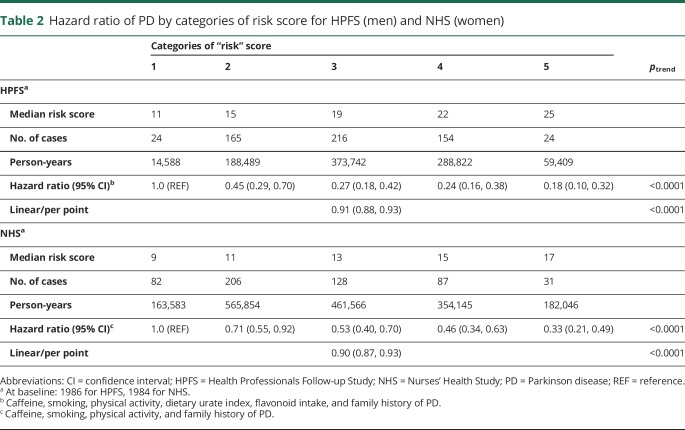
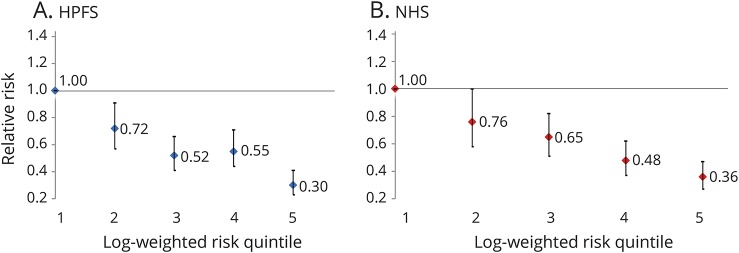
In both cohorts, a higher category of risk score was associated with a decreased risk of PD, as expected (ptrend < 0.0001) (table 2). Participants who were in the highest category compared to the lowest category of the score had an 82% decreased risk (HR = 0.18, 95% CI: 0.10, 0.32; p < 0.0001) in the HPFS and a 67% decreased risk in the NHS (HR = 0.33, 95% CI: 0.21, 0.49; p < 0.0001). A 1-point increase in the risk score was associated with a 9% decrease in risk in men (HR = 0.91, 95% CI: 0.88, 0.93; p < 0.0001) and a 10% decrease in risk in women (HR = 0.90, 95% CI: 0.87, 0.93; p < 0.0001). Similarly, a strong inverse association was observed when comparing the PD risk in the highest quintile to the lowest quintile of the log-weighted risk score for both men (figure 2A) and women (figure 2B). Conducting analyses using quintiles of the risk score did not change results (figure e-2, links.lww.com/WNL/A418). These results were robust in lag analyses (2, 4, 6, and 8 years), comparing deciles of the risk scores and using baseline risk scores. Further analyses adjusting for reported ibuprofen use, dairy intake, antioxidants (e.g., vitamin C and E), and head trauma injury (data only available in men) yielded consistent results. Finally, results remained unchanged when the components of dietary urate index and total flavonoid intake were excluded in the HPFS risk score to match that of NHS (table e-4, links.lww.com/WNL/A419), when smoking was excluded as a component of the risk score for both cohorts (table e-5), and when total anthocyanin intake was included in the NHS risk score while it replaced total flavonoid intake in the HPFS (table e-6).
Table 2.
Hazard ratio of PD by categories of risk score for HPFS (men) and NHS (women)
Figure 2. Relative risk of Parkinson disease for the (A) HPFS and (B) NHS according to quintiles of their respective log-weighted risk scores.
ptrend < 0.0001 for both. HPFS = Health Professionals Follow-up Study; NHS = Nurses' Health Study.
Using the population attributable risk proportion (PAR%), we estimated the proportion of the new PD cases that hypothetically could have been prevented if all participants in the highest quintile of the risk score had instead been in the lowest quintile of the risk score, assuming a causal relationship between the risk score and PD. For men, the PAR% was 80% for the original risk score, 50% for the risk score excluding family history of PD as a component, and 44% for the risk score excluding both family history of PD and smoking behavior. For women, the PAR% was 63%, 40%, and 15%, for the original risk score, risk score without family history of PD, and risk score without family history of PD and smoking behavior, respectively.
Discussion
Our risk score based on independent predictors supported in the literature that have previously been found to be associated with PD risk in our 2 large, prospective cohorts was associated with a decreased risk of PD.
Evidence for an inverse association between tobacco smoking and PD is robust and has been widely studied in many longitudinal studies.8,13–15 Similarly, caffeine is a well-established neuroprotective factor; the effect is stronger among men compared to women, most likely due to effect modification by postmenopausal hormone use.16,17 Physical activity is another factor that is suggested to be associated with a reduced PD risk in several longitudinal studies across different cohorts.18–22 In our health professional cohorts, total physical activity, as well as vigorous activity, was associated with a lower risk of PD in men even after lag analyses, suggesting evidence against reverse causation.23 Among women, physical activity was not associated with a reduced risk of PD, though women who reported strenuous exercise during early adulthood had a lower risk. In addition, because plasma urate24–26 and total flavonoid27 have been found to be protective factors of PD in men but not in women, they were both included as additional factors contributing to the risk score only for men. Alternatively, we performed sensitivity analyses using risk scores including anthocyanin, a subclass of flavonoids, because it was associated with reduced PD risk in both men and women. Finally, although family history of PD is not a modifiable risk factor, it was included as a hereditary component of the risk score because of its moderate genetic association with PD risk. In an alternate risk score, we removed smoking as a factor, as it would be immaterial from a public health perspective to recommend smoking, because of its adverse effects on respiratory, cardiovascular, and other health outcomes. However, if nicotine or other biological agents explained the protective effects of smoking, potential therapeutic interventions would be possible.8
In addition to applying risk scores to our cohorts, we also assessed effect modification by each predictor on the multiplicative and additive scale. Among the predictors of PD for the HPFS, there was evidence for additive interaction between total caffeine intake and family history of PD on incident PD risk, i.e., the increased PD risk associated with a positive family history plus caffeine abstinence is higher than the sum of the risks associated with each factor alone. Furthermore, additive interaction between total caffeine intake and physical activity was evident in women; 21% of PD among women who jointly had low caffeine intake and were physically inactive was due to the additive interaction (AP = 0.21, 95% CI: 0.03, 0.39; p = 0.02).
The main strengths of our study include large number of cases for greater power, a high active follow-up rate in both cohorts (approximately 94% in both cohorts), and minimized potential for recall bias due to a prospective collection of repeated detailed data on dietary intake and lifestyle factors. We also report 3 indices of additive interaction. While it is generally agreed that measuring interaction on the additive scale is particularly important for public health implications, many studies only report effect modification on the multiplicative scale.28–30 Knol et al.31 report that among a random sample of 138 studies assessing interaction, only 3 studies mentioned the use of additive interaction and none reported the RERI or AP. Assessing additive interaction would provide insight into possible biological mechanisms through which 2 factors can interact to have a greater effect than the combined effect of each individual factor alone.32
We recognize that there is potential for measurement error of lifestyle data from the FFQ. However, they have been validated in both cohorts,33–35 and any measurement error would be expected to bias our results toward the null since it is likely to be nondifferential regarding PD because of our prospective design. In addition, although we cannot disregard the possibility of unmeasured confounding, our results remained robust after conducting several sensitivity analyses adjusting for other potential confounders. Finally, because we dichotomized predictors to assess additive interaction for simplicity, we may have failed to detect potential additive interactions if the associations between predictors are not best captured by dichotomizing the predictors. However, in sensitivity analyses, categorizing caffeine into tertiles did not change results.
Future research should focus on the validation of risk scores, such as those presented here, in other populations. Since our risk score included nutrient data that are not easily measured, other modified risk scores could be developed for rapid use in a clinical setting.
Our results show that the risk score was associated with decreased risk of PD, and that the combination of family history and known lifestyle factors can explain 80% of cases of PD in men and 63% in women. Further analyses on additive interactions support evidence for protective synergism between caffeine intake and family history of PD in men and caffeine intake and physical activity in women.
Glossary
- AP
attributable proportion due to interaction
- CI
confidence interval
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- NHS
Nurses' Health Study
- PAR%
population attributable risk proportion
- PD
Parkinson disease
- RERI
relative excess risk due to interaction
- S
synergy index
Author contributions
Drafting/revising the manuscript: I.Y.K., É.J.O., K.C.H., X.G., M.A.S., M.T.H., R.A.B., A.A. Study concept/design: A.A., X.G., M.A.S. Analysis or interpretation of design: I.Y.K., É.J.O., K.C.H., X.G., M.A.S., M.T.H., R.A.B., A.A. Statistical analysis: I.Y.K. Acquisition of data: X.G., M.A.S., A.A. Study supervision: A.A. Obtaining funding: A.A.
Study funding
This study was supported by NIH grants UM1 CA186107, UM1 CA167552, and by Department of Defense grant W81XWH-14-0131.
Disclosure
I. Kim, É. O'Reilly, and K. Hughes report no disclosures relevant to the manuscript. X. Gao has served on a committee of the Parkinson Study Group and received funding from the NIH/National Institute of Neurological Disorders and Stroke. M. Schwarzschild receives research grants from the NIH, the Parkinson's Disease Foundation, Target ALS, and the Michael J. Fox Foundation for Parkinson's Research. M. Hannan and R. Betensky report no disclosures relevant to the manuscript. A. Ascherio receives research funding from the NIH, the Department of Defense, the National Multiple Sclerosis Society, and the ALS Association. Go to Neurology.org/N for full disclosures.
References
- 1.Valente EM, Salvi S, Ialongo T, et al. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol 2004;56:336–341. [DOI] [PubMed] [Google Scholar]
- 2.Davis AA, Andruska KM, Benitez BA, Racette BA, Perlmutter JS, Cruchaga C. Variants in GBA, SNCA, and MAPT influence Parkinson disease risk, age at onset, and progression. Neurobiol Aging 2016;37:209.e1–209.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas N, Lücking CB, Ricard S, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. Hum Mol Genet 1999;8:567–574. [DOI] [PubMed] [Google Scholar]
- 4.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004;44:601–607. [DOI] [PubMed] [Google Scholar]
- 5.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 1997;276:2045–2047. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Blomgren K. Mitochondrial cell death control in familial Parkinson disease. PLoS Biol 2007;5:e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda T, Mochizuki H. The regulatory role of α-synuclein and parkin in neuronal cell apoptosis: possible implications for the pathogenesis of Parkinson's disease. Apoptosis 2010;15:1312–1321. [DOI] [PubMed] [Google Scholar]
- 8.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15:1257–1272. [DOI] [PubMed] [Google Scholar]
- 9.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 10.Knol MJ, VanderWeele TJ, Groenwold RHH, Klungel OH, Rovers MM, Grobbee DE. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol 2011;26:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol 2007;17:227–236. [DOI] [PubMed] [Google Scholar]
- 12.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–579. [DOI] [PubMed] [Google Scholar]
- 13.Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002;52:276–284. [DOI] [PubMed] [Google Scholar]
- 14.Thacker EL, O’Reilly EJ, Weisskopf MG, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology 2007;68:764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology 2010;74:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE. Caffeine, postmenopausal estrogen, and risk of Parkinson’s disease. Neurology 2003;60:790–795. [DOI] [PubMed] [Google Scholar]
- 17.Palacios N, Gao X, McCullough ML, et al. Caffeine and risk of Parkinson's disease in a large cohort of men and women. Mov Disord 2012;27:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sääksjärvi K, Knekt P, Männistö S, et al. Reduced risk of Parkinson's disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol 2014;29:285–292. [DOI] [PubMed] [Google Scholar]
- 19.Logroscino G, Sesso HD, Paffenbarger RS, Lee IM. Physical activity and risk of Parkinson's disease: a prospective cohort study. J Neurol Neurosurg Psychiatry 2006;77:1318–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord 2008;23:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology 2010;75:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang F, Trolle Lagerros Y, Bellocco R, et al. Physical activity and risk of Parkinson's disease in the Swedish National March Cohort. Brain J Neurol 2015;138:269–275. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005;64:664–669. [DOI] [PubMed] [Google Scholar]
- 24.Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007;166:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X, O'Reilly ÉJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 2016;86:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Reilly EJ, Gao X, Weisskopf MG, et al. Plasma urate and Parkinson's disease in women. Am J Epidemiol 2010;172:666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012;78:1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol 1980;112:467–470. [DOI] [PubMed] [Google Scholar]
- 29.Blot WJ, Day NE. Synergism and interaction: are they equivalent? Am J Epidemiol 1979;110:99–100. [DOI] [PubMed] [Google Scholar]
- 30.Saracci R. Interaction and synergism. Am J Epidemiol 1980;112:465–466. [DOI] [PubMed] [Google Scholar]
- 31.Knol MJ, Egger M, Scott P, Geerlings MI, Vandenbroucke JP. When one depends on the other: reporting of interaction in case-control and cohort studies. Epidemiology 2009;20:161–166. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 33.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 34.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126. [DOI] [PubMed] [Google Scholar]
- 35.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are not publicly available because of restricted access, but further information about the datasets is available from the corresponding author on reasonable request.