INTRODUCTION
Pain is a highly complex and individual experience with biological, psychological and social (biopsychosocial) contributions. Pain is associated with activity within the nervous system – peripheral neurons and receptors, central spinal neurons, interneurons and receptors, as well as supraspinal components of the brainstem, midbrain, subcortical structures and cerebral cortex. The study of pain within the central nervous system (CNS) using neuroimaging is a large and growing area of research. By non-invasively studying the CNS using neuroimaging in healthy human volunteers and individuals with chronic pain, we now understand that pain processing involves an integrated network of regions and mechanisms throughout the body. The focus of this review is to provide an update on knowledge of pain that has been acquired by neuroimaging the CNS and to highlight important contributions to the field. It is not meant to be an exhaustive or systematic review, but rather, comprehensive enough to provide the practicing anesthesiologist and pain clinician a current human neuroimaging perspective of CNS processes related to acute and chronic pain. We also include a brief description of selected neuroimaging research of pharmacological and psychological modulation of pain; these studies are informative regarding how pain, and its CNS correlates, can be altered (for better or for worse) by both endogenous and exogenous influences. We discuss how knowledge from the field of pain neuroimaging is relevant for informing clinical practice and for providing a framework for understanding the complex contexts of the pain experience. Finally, we discuss future directions in the use of neuroimaging based treatments and how brain based biomarkers may help us achieve the goal of personalized pain management.
1. Current Neurophysiological Conceptual Framework of Pain
In the classic acute pain experience, noxious stimulation evokes nociceptive signals which are transmitted to the cerebral cortex via a series of complex multi-mechanistic pathways (for review:1). Receptors in the periphery (e.g., skin and body tissues) transduce mechanical, thermal and chemical stimulation from the environment into nociceptive signals which are relayed via peripheral primary afferents to the dorsal horn of the spinal cord. There, the primary afferents synapse onto and transmit their nociceptive signals to secondary spinal neurons, which in turn, transmit nociceptive information to supraspinal regions of cortical and subcortical structures. Nociceptive information is also relayed to brainstem, midbrain and medullary regions which can modulate (amplify or diminish) the perception and sensation of a noxious stimulus. The perception of pain occurs by the activation of a network of connections within the cerebral cortex. Because of this, nociceptive input is not required for the perception of pain, and a painful experience can be elicited by brain stimulation directly. Furthermore, within the brain, the experience of pain is created and shaped by past experiences, context, cognitive and emotional input.
The complex processes of the pain experience and modulation within the brain are of substantial interest to the field of pain neuroimaging. The brain and subcortical structures together serve as an organ system that can reasonably well be assessed using several non-invasive neuroimaging techniques, most commonly structural and functional magnetic resonance imaging (MRI). The importance of imaging and understanding these cortical and subcortical processes lies in that these structures are major sites for pain modulation via physiological (different brain regions involved in modulating pain perception), psychological, and pharmacological (both endogenous and exogenous) modalities.
While this review focuses on a selection of neuroimaging insights about pain, we first focus our attention on two major underlying principles of the nervous system and pain processing. First, the CNS (e.g., brain, brainstem and spinal cord) is highly plastic, meaning that it is often and easily altered by physiological and pharmacological processes, via both exogenous and endogenous effectors. Plasticity of the nervous system has been shown through a wide range of neuroimaging and non-neuroimaging research. For example, macroscopically, limb amputation causes regional changes in somatosensory cortex representation in such a way that suggests, “alterations of sensory processing are not hardwired, but are rather mediated by an extensive and interconnected neural network with fluctuating synaptic strengths”2. At the other end of the spectrum, microscopically, widespread changes in neural function are associated with pruning of the dendritic spines on individual neurons (e.g.,3) and pronounced dendritic branching occurs after sensorimotor training in rodents4. Another important principle is that pain processing is distributed, in that it engages multiple brain regions during the pain experience5. Individual brain regions and networks (i.e., multiple brain regions with concurrent and therefore presumed complementary function) have been demonstrated to contribute to certain aspects of pain processing (e.g., affective versus sensory components). However, our understanding of how pain is processed by and within the CNS is still a work in progress. Ultimately, the knowledge that 1) the brain is highly plastic, and 2) pain processing encompasses distributed regions of the brain with various functions that modulate the pain experience, supports the appropriateness of multi-modal, multi-interventional approaches for chronic pain prevention and ideal treatment.
2. Central Nervous System Processes of Acute and Chronic Pain
Introduction to Themes of “Normal” Pain Related Brain Activity
Before discussing evidence of altered brain processes in chronic pain, we need to first discuss evidence of normal pain processes in healthy states. Hallmark studies have indicated that multiple regions of the brain are involved in sensory, affective or emotional, and evaluative aspects of pain processing in healthy individuals. These studies have used noxious and innocuous stimuli including thermal6, mechanical (e.g., pin prick and pressure)7, chemical (e.g., capsaicin)8, electrical9, and incisional pain paradigms10 to model the pain response within the brain. While standard tonic stimuli have been most often used to study supraspinal responses to pain, several studies have employed dynamic stimuli that evoke pain phenomena such as offset analgesia11, conditioned pain modulation (formerly termed diffuse noxious inhibitory control)12, and temporal summation of pain13. As repeatedly shown across many studies, the key brain regions involved in pain processing include the primary somatosensory cortex, primary motor and supplementary motor cortices, secondary somatosensory cortex, insular cortex, anterior cingulate cortex, thalamus, as well as regions within the prefrontal and parietal cortices and regions of emotion, memory and fear processing in the amygdala, hippocampus and subcortical structures including the basal ganglia (Fig. 1)5,6,14–16. Individual neuroimaging investigations often indicate pain-related brain activity within a subset of these structures; the differences in brain activity across different investigations may result from the inclusion of different participant populations, different stimulation parameters and modalities, differences in analysis methods, differences in instructions for the participants, as well as differences in psychological states of the individual subjects. It is understood that the brain regions activated during the pain experience overlap with brain regions that are activated under multiple other highly salient sensory experiences such as the presentation of visual, auditory or innocuous somatosensory stimulation17. Similarly, viewing others in pain (i.e., empathy of pain) engages many of the same brain regions as the actual experience of physical pain18,19. Thus, it is important to keep this conceptual framework of salience processing in mind when evaluating the literature. However, many investigations have contributed elegant work to deduce the main brain regions activated during the pain experience and how activity within these regions contributes to the aspects of the pain experience, as described in more detail below.
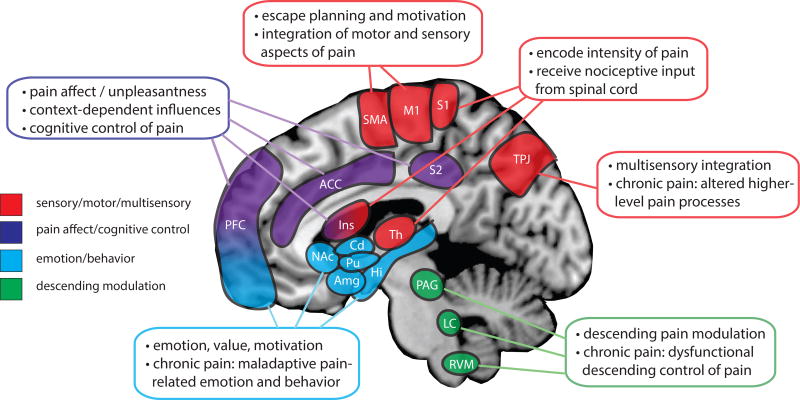
Figure 1.
Summary of the main supraspinal regions and their roles in pain processing. Multiple cortical and subcortical structures are involved in various primary roles and aspects of the pain experience (as color coded). Additional brain regions and networks not shown in the figure are involved in the pain experience - see text for details. Abbreviations: ACC - anterior cingulate cortex, Amg - amygdala, Cd - caudate, Hi - hippocampus, Ins - insular cortex, LC - locus coeruleus, M1 - primary motor cortex, NAc - nucleus accumbens, PAG - periacqueductal gray, PFC - prefrontal cortex, Pu - putamen, RVM - rostral ventral medulla, SMA - supplementary motor area, S1 - primary somatosensory cortex, S2 - secondary somatosensory cortex, Th - thalamus, TPJ – temporal-parietal junction.
Normal Pain Processes within the Brain
Brain regions receiving direct projections from spinal nociceptive neurons and processing the sensory-discriminative aspects of pain include the primary somatosensory cortex, posterior insular cortex and thalamus. Within the thalamus nociceptive inputs are modulated and then transmitted to cortical and subcortical structures20. The primary somatosensory cortex and posterior insular cortex are two brain regions that encode the intensity of painful stimuli, i.e., these regions increase activity in a graded fashion that corresponds to the intensity of the stimulus presented21. The affective dimension of pain is related to perceptive and context dependent pain processes within the brain. Classic brain regions associated with the affective dimension of pain processing include the secondary somatosensory cortex and anterior insular cortex. Activation measured within these regions corresponds to the levels of unpleasantness and context-dependent influences of the pain experience22. Cognitive modulation of the pain experience is thought to be driven largely by regions within the prefrontal cortex (e.g., anterior cingulate cortex, ventromedial prefrontal cortex, dorsolateral prefrontal cortex) as noted in many studies of placebo and nocebo effects23, controllable versus uncontrollable pain states24, reward induced by romantic love25, attentional distraction26, and real-time neuroimaging biofeedback27. The motor and supplementary motor cortices are involved in pain processing and may be related to the motivational or escape aspects related to the pain experience28. Regions involved in fear, anxiety29, and memory processing including the amygdala and hippocampus have also been noted to play a role in the pain experience30. Subcortical structures within the basal ganglia may be involved in the intensity discrimination, motor response, and motivational aspects of pain31. Brainstem, midbrain and medullary regions including the midbrain periaqueductal gray, locus coeruleus and rostral ventral medulla are involved in the descending modulation of pain, thus exerting both inhibitory and facilitatory effects on spinal circuits32,33.
Experimentally Induced Abnormal Pain Processes within the Brain
While brain processes involved in the pain experience can be studied under healthy states, experimentally induced perturbation of pain circuits can produce temporary hyperalgesia and allodynia, thus creating human models of the symptoms typically experienced in clinical pain states. The use of topical capsaicin creams, which contain the active ingredient in hot chili peppers, activates transient receptor potential cation channel subfamily V member 1 (i.e., “TrpV1”) receptors in the skin and can be used to induce a state of experimental or acute central sensitization. The classic capsaicin-heat sensitization model34 induces temporary experimental allodynia (i.e., perception of a normally innocuous stimulus as painful) and hyperalgesia (i.e., perception of a normally noxious stimulus as more painful than normal) that can be prolonged as necessary and then diminishes soon after the completion of the study procedures. Capsaicin-induced allodynia and hyperalgesia typically results in increased activation of pain-related brain regions including the somatosensory, prefrontal, insular and parietal cortices35. Other studies using capsaicin application in healthy volunteers have identified altered brainstem activation36 and changes distributed throughout the cortex that are consistent with increased pain and increased activation of countering endogenous analgesic circuit activation (e.g., descending control)37. Induction of hypersensitivity can also be used to contrast normal versus hypersensitive states of mechanical and thermal stimulation, indicating that each type of noxious stimulation produces a distinct signature of activations within the brain, and further distinct changes within these activations during induced hyperalgesic states8. Studies investigating altered brainstem activation after induction of experimental hyperalgesia36 and altered spinal cord activity by nocebo hyperalgesia effects38 further implicate the importance of potential non-cortical contributions to human chronic pain symptoms.
3. Central Nervous System Alterations in Chronic Pain
Neuroimaging of chronic pain relies on the same fundamental principles as neuroimaging of acute pain, however, clear differences exist (Box 1). In particular, the majority of neuroimaging investigations of chronic pain rely on group comparisons between patients with chronic pain and healthy volunteers. A large body of evidence of altered CNS structure and function in chronic pain now exists and below we summarize some of the most notable findings.
Box 1. Neuroimaging of Acute Versus Chronic Pain.
Acute
Healthy individuals without ongoing pain
Exogenously applied noxious stimulation (e.g., thermal, mechanical, dynamic)
Task-based design and analysis matched to stimulus (fMRI)
Stimulus versus baseline (no stimulus) comparison
Correlations with measures of intensity and unpleasantness ratings
Use of experimentally induced sensitization / altered pain states
Chronic Pain
Spontaneous or provoked chronic pain states, potentially with exogenously applied innocuous or noxious stimulation
Typical group comparison (versus healthy), or pre / post longitudinal design
Assess structural CNS group or longitudinal differences (gray matter density using MRI, axonal integrity using diffusion weighted MRI)
Assess functional group or longitudinal differences (e.g., task-based design, resting state fMRI, positron emission tomography, MR spectroscopy)
Correlations with measures of symptom and pain severity, anxiety, depression, other clinical and behavioral measures
Results may be partially influenced by concurrent medications taken by patients
Altered Brain Structure in Chronic Pain States
Considering that pain processing is distributed across multiple brain regions, and may be, in part, dependent on processes in the brainstem and spinal cord, it is not surprising that studies of CNS activity among individuals with chronic pain have identified alterations within multiple regions and levels of the nervous system. Structural brain differences in gray matter density, gray matter volume and cortical thickness between patients with chronic pain and healthy volunteers have been noted in many types of chronic pain including chronic low back pain39,40, fibromyalgia41, complex regional pain syndrome42, chronic pelvic pain syndromes43,44, and temporomandibular pain45,46, among others. Additionally, regional gray matter changes have been shown to reverse coinciding with effective treatment in patients with chronic low back pain47. These observations suggest that there is underlying structural plasticity and changes of the brain’s cellular composition in individuals who experience chronic pain.
The specific underlying physiological changes contributing to the observed differences in gray matter remain in question. Decreases in regional gray matter in chronic pain have been thought to suggest more rapid aging of the brain in chronic pain48,49, however, this explanation has been debated because regional gray matter increases have also been observed in chronic pain and do not follow logically with the aging explanation. Neuroimaging researchers have speculated that increases and decreases in gray matter may be due to changes in gray matter microstructure (e.g., changes in the number of dendritic spines and connections), and the prevalence of glial and other supporting and neuro-immune cells within brain regions, among other possible mechanisms (for review:50). A recent study in patients with fibromyalgia revealed evidence suggesting that regional gray matter decreases are due to decreased tissue water content and regional gray matter increases are due to increased neuronal matter and possibly inflammation51. However, these findings need to be further validated in other types of chronic pain and disease, and by additional complementary measures. Nonetheless, observed differences in brain structure in chronic pain implicate altered function within the CNS in patients, however, additional research investigating the underlying causes of observed differences in gray matter in chronic pain is needed.
Measurements of white matter, or axonal fiber pathways, within the brain have also provided evidence about how the brain structure and its underlying processes are altered in chronic pain. As compared with healthy states, chronic pain states of fibromyalgia52, chronic pelvic pain53, chronic low back pain54, complex regional pain syndrome55, and visceral pain syndromes56 have been identified as demonstrating differences in white matter integrity. The majority of these studies used a method of diffusion tensor imaging which uses properties of water molecule movement in axons to determine measure of integrity of the axon (i.e., more movement and coherence along the main direction of the axon equals better integrity of the axon fiber). Differences in the degree of fractional anisotropy, a measurement of coherence along a bundle of axon fibers, have been identified in regions of white matter indicating differences in structural connectivity between brain regions in individuals with chronic pain. For example, a study comparing white matter axonal integrity among patients with chronic pelvic pain, patients with irritable bowel syndrome, and healthy controls identified increased fractional anisotropy within the corticospinal tract in patients with chronic pelvic pain and decreased fractional anisotropy within the thalamic radiation in patients with irritable bowel syndrome57. These observations were correlated with symptoms of pain severity and therefore implicate changes in axonal microstructure that may be specific to these two types of chronic visceral pain. Patients with migraine, as compared with healthy controls, have been found to have lower fractional anisotropy in axon bundles within the corpus callosum suggesting regional degeneration of axonal connections in this population58. In these and other chronic pain conditions, the identification of regional increases and decreases in structural integrity of white matter tract connections suggests underlying enhancements and disruptions, respectively, of neural communication between the regions to which the tracts connect.
Altered Brain Function in Chronic Pain States
Neuroimaging techniques have been used to study brain functional differences in chronic pain versus healthy states. Neuroimaging studies have used fMRI extensively to study pain processing. FMRI relies on a correlate of activity due to differences in the magnetic properties of oxygenated and deoxygenated blood, known as the blood oxygenation dependent level (BOLD) signal. Several studies have investigated differences in perception of noxious stimulation in patients with chronic pain while undergoing fMRI scans. Examples of such studies include combining fMRI with the use of tests of temporal summation of heat pain in fibromyalgia59, tests of heat pain sensitivity in complex regional pain syndrome60 and chronic low back pain61, and tests of mechanical sensitivity in chronic low back pain62 and fibromyalgia63,64. Visceral pain studies have identified differences in brain activity associated with pain during bladder filling65, and in response to rectal distension in patients with irritable bowel syndrome66. Interesting neuroimaging observations of altered brain activity within the contralateral and ipsilateral regions of the brain in patients with neuropathic pain indicate that altered peripheral afferent (incoming) information produces dramatic shifts in representation within the brain67. Collectively, these investigations point towards heightened responsivity of the CNS to afferent noxious and innocuous stimuli in chronic pain.
With the emergence of resting state fMRI as a technique for studying non-evoked brain activity and functional connectivity, many investigations of chronic pain have used this technique to gain understanding of brain processes more generally, as opposed to specifically related to noxious stimuli, in chronic pain. Resting state activity is based on established principles68 identifying correlated low frequency (0.01 – 0.1 Hz) fluctuations of fMRI signals (BOLD activity) across brain regions. These principles allow for the parcellation, or subgrouping, of brain fMRI data into functional networks of regions that appear to “work together”, operating and contributing to related functions. Functional connectivity is the degree of correlated activity, based on the BOLD signal, among and between brain regions and networks. Based on these principles, several studies have used resting state fMRI to characterize differences in non-evoked (i.e., resting) brain activity among patients with chronic low back pain69, fibromyalgia70, chronic pelvic pain syndromes71, complex regional pain syndrome72 and others. Several resting state studies of chronic pain have identified alterations in default mode network connectivity, a network of brain regions including the precuneus, posterior cingulate cortex, medial prefrontal cortex and angular gyrus. The default mode network is more active at rest, suggesting that this network might be in a continuous hyperactive state in chronic pain73 and possibly that regions of this network are hyper-involved with pain-related processes ongoing in the brain71. Other studies have focused on more regional connectivity differences specifically within brain regions involved in motor and sensory processes65,74, emotion and fear processing75, reward and motivation76, and cognitive processes77, as well as alterations in brainstem functional connectivity78.
Additional neuroimaging techniques of nuclear magnetic resonance spectroscopy and positron emission tomography (PET) allow for identification of altered neurotransmitter/neuromodulator function within regions of the brain in individuals with chronic pain. Such studies have used PET imaging to detect decreased dopamine activity in fibromyalgia79,80 and decreased opioid receptor binding potential in fibromyalgia81. Widespread differences in measured metabolite and neurotransmitter function, including N-acetylaspartate and glutamate, have been identified in in chronic low back pain using proton magnetic resonance spectroscopy (for review:82). Similarly, reduced N-acetylaspartate levels in prefrontal cortex have been observed in patients with complex regional pain syndrome83. Altered N-acetylaspartate observed in regions such as the prefrontal cortex and somatosensory cortex of patients with chronic pain suggests possible degradation of receptors within these brain regions. In fibromyalgia patients, increased glutamate within the posterior insular cortex, a region implicated in the sensory-discriminative dimension of pain processing (e.g., pain intensity), is correlated with levels of pain sensitivity84, and levels of insular cortex glutamate co-vary with levels of pain measured pre and post effective treatment85. Collectively, such observed differences in metabolite and neurotransmitter function in patients with chronic pain complement structural and functional MRI evidence in chronic pain.
Combining Neuroimaging with Immunology and Genetics for the Study of Chronic Pain
Neuroimaging technology can now identify immune changes within the CNS (for review:86). PET imaging combined with a radioligand specific for detecting glial cell reactivity has identified increased glial reactivity within multiple brain regions, including somatosensory cortex and thalamus, in patients with chronic low back pain, as compared with healthy control subjects87. By combining neuroimaging and genetics data, subgroups of chronic pain states are starting to be identified based on their genetic and regional brain activity profiles. For example, in a study of women with primary dysmenorrhea, individuals with the G allele OPRM1 A118G polymorphism demonstrated decreased functional connectivity between the anterior cingulate cortex and periaqueductal gray, regions involved in descending modulation of pain88. As these types of studies continue to be performed, they will continue to advance our understanding of the links between neural processes, immune states, and genetic profiles.
Longitudinal and Multi-Modal Neuroimaging Investigations of the CNS in Chronic Pain
To date, the majority of chronic pain neuroimaging investigations are not longitudinal studies. Therefore, it cannot be determined whether observed differences in brain structure and function are pre-existing to the chronic pain condition, suggesting an underlying predisposition to acquire a chronic pain condition, or whether these differences were caused by or are the direct cause of the chronic pain condition itself. This issue represents one of the main limitations of neuroimaging, and a summary of additional limitations and benefits of neuroimaging methods for chronic pain are provided in Box 2. Nonetheless, a few longitudinal studies exist, and these studies suggest that the changes in brain structure and function are linked to presence and ongoing burden of the chronic pain condition and mirror the compounding effects of negative social and emotional factors over time89,90.
Box 2. Neuroimaging Benefits and Limitations.
Benefits and Uses
Non-invasive (MRI, fMRI, MR Spectroscopy) identification of brain structural and functional alterations
Variety of measurements and applications:
MRI: gray matter and white matter structure
fMRI: stimulus-induced and task-based activity and resting state functional connectivity (BOLD signal dependent), manipulation of cognitive and behavioral states
Positron emission tomography (PET): pharmacological based function (e.g., neurotransmitter estimates / receptor availability for endogenous opioids, dopamine and other metabolites)
MR Spectroscopy: neurotransmitter and metabolite concentrations (e.g., glutamate, N-acetylaspartate)
Limitations
Limited causal inference of observed group differences (improved interpretation with longitudinal and pre/post intervention designs)
Large immobile equipment required (MRI / PET scanner), expensive (average $500 per hour)
PET imaging is invasive, involving radiotracer injection and arterial blood sampling.
Potential artifacts in images (e.g., due to head motion, physiological noise from cardiac and respiration influences, magnetic field inhomogeneity at air/tissue interfaces)
Limited resolution (i.e., > 1mm) and fMRI signal based on correlates of blood flow, indirect measure of neural activity.
Ineligibility due to MRI contraindications (e.g., metallic implants, claustrophobia, pregnancy)
Requires post-processing of images prior to analysis and multi-step analysis using specialized software
Additional insightful research findings are now emerging from the use of multi-modal imaging. Multi-modal imaging combines the use of multiple types of neuroimaging data with the goal of identifying complementary structural and functional brain alterations. The degree of overlap between differences identified by each modality can enhance the certainty of validity and meaningfulness of the brain alterations identified. For example, complementary brain structural alterations of gray matter density and white matter axonal integrity have been identified in patients with complex regional pain syndrome within regions of the insular cortex, prefrontal cortex and basal ganglia55. Similar multi-modal studies have been conducted in fibromyalgia52,91 as well. As an example of complementary functional and structural brain alterations, differences in resting state functional connectivity and white matter axonal integrity, centered around regions within the insular and prefrontal cortex, appear to underlie disrupted cognitive processes in patients with chronic low back pain54. These findings demonstrate how complementary regional alterations in brain structure and function provide a more complete picture for understanding how the CNS is altered in chronic pain.
4. Neuroimaging Pharmacological and Psychological Modulation of Pain Processing
In addition to measuring brain structural and functional differences in patients with chronic pain, neuroimaging is a tool that can increase our understanding of how medications alter CNS activity in response to pain (acute or chronic). One goal of neuroimaging neuro-pharmacological research is to clarify how medications work to reduce the sensory and affective impact of chronic pain and comorbid symptoms including anxiety and depression. Currently, the global and regional impacts on CNS by medications used to treat chronic pain are generally unknown. Furthermore, medications are prescribed by clinicians for individual patients primarily in a trial-and-error fashion92. Pharmacological studies using neuroimaging can allow for elucidation of the mechanisms of action of medications that will guide development of future treatments. For example, reduced activity within the medial prefrontal cortex, a region involved in cognitive control of pain, has been shown to mediate decreases in mechanical pin-prick hyperalgesia after systemic administration of lidocaine in healthy individuals93. While our knowledge of CNS mechanisms of anti-nociception is still incomplete, advances in research combining clinical trials and neuroimaging may someday provide data to inform clinical practice. For example, neuroimaging may help to predict which patients may benefit more from a specific medication or therapy, thereby achieving the ultimate goal of personalized medicine. This has recently been demonstrated94 in the treatment of depression where amygdala activity combined with knowledge of early life stress was able to predict treatment response to an antidepressant with greater than 80% accuracy.
Recent neuroimaging research has shown that opioid medications produce widespread and rapid alterations in brain structure and function. In patients with chronic low back pain, structural brain changes occur rapidly, within one month of taking opioid medications. These changes include regional increases and decreases in gray matter density, which are slow to be reversed after discontinuing opioids (no changes observed at 6 months post-opioid cessation)95,96. Another study of pain-free individuals taking opioid medications demonstrated structural and functional differences across the brain as compared with pain-free individuals not taking opioids97. While the specific neurophysiological mechanisms underlying these opioid-induced brain changes are unknown, the consequences are of considerable interest and therefore call for further study.
Neural correlates of anti-nociception by antidepressants have been investigated using fMRI in patients with chronic pain. Analgesic effects of the serotonin norepinephrine reuptake inhibitor, milnacipran, in fibromyalgia may be due to reversal of altered default mode network activity as evidenced by increased posterior cingulate cortex (a core region of the default mode network) activation observed in patient responders98. Another study of milnacipran in fibromyalgia identified reduced functional connectivity between anti-nociceptive brain regions of the anterior cingulate cortex and periaqueductal gray, both regions of pain modulation, to the insular cortex, a region involved in both sensory and affective processes of pain99. Notably, decreases in the functional connectivity between these regions in patients with fibromyalgia were related to reductions in pain after administration of milnacipran. More recently a study using magnetic resonance spectroscopy identified ventricular lactate as a putative biomarker for milnacipran efficacy in fibromyalgia and thereby suggested that drug effects may occur by reducing glial reactivity and neuroinflammation within the CNS100. A study of brain activity changes associated with anti-nociceptive properties of the tricyclic antidepressant, amitriptyline, indicated that this treatment acts on reducing activity within the anterior cingulate cortex to reduce pain during a stressful experience of rectal distension in individuals with irritable bowel syndrome101.
The brain mechanisms by which anticonvulsants, such as gabapentin and pregabalin, may be beneficial for treatment of chronic pain have also been studied. Gabapentin has been shown to increase cortical neurotransmitter levels of gamma-aminobutyric acid, but not glutamate, within the brain’s occipital lobe after 1 month of treatment102. Research conducted in healthy volunteers has shown that a single oral dose of gabapentin, as compared with ibuprofen, reduced experimentally-induced secondary hypersensitivity (increased sensitivity to mechanical stimulation surrounding the directly sensitized skin) and partially reversed sensitization-induced increases in functional connectivity between pain-associated regions of the insular cortex, thalamus and somatosensory cortex103. In patients with fibromyalgia, pregabalin has been shown to reduce glutamatergic activity within the posterior insula, a key brain region involved in processes of pain104. Additionally, this study demonstrated that pregabalin administration reduced aberrant increased functional connectivity between the insula and default mode network brain regions. These changes were correlated with efficacy of pregabalin among the patients, and baseline neuroimaging measurements were predictive of responders and non-responders. Neuroimaging has even been used to identify changes in brain function pre and post treatment with ketamine for complex regional pain syndrome, further implicating the highly plastic nature of the brain in response to therapy105.
Neuroimaging additionally provides a method for investigating neural correlates of effective psychological therapies. Chronic pain often presents with patients having deficits in cognitive and emotional control particularly relating to their experience of pain (for review:106). Effective psychological therapies for pain strengthen practices of healthy psychology thereby reducing the negative impact of chronic pain. Such therapies include cognitive behavioral therapy, acceptance and commitment therapy, mindfulness based stress reduction, and mindfulness meditation. These types of treatments can benefit individuals suffering from chronic pain by reducing negative emotional, cognitive, and behavioral responses to pain and thereby improving pain-related outcomes107. Neurophysiologically, psychological-based therapies including cognitive behavioral therapy and acceptance and commitment therapy reduce an individual’s perceived severity of chronic pain via increased prefrontal cortex activity. Increased prefrontal cortex activity after cognitive behavioral therapy and acceptance and commitment therapy treatment implicate enhanced cognitive control of one’s psychology108 in agreement with earlier proposed neurological correlates of pain control109. Mindfulness based stress reduction and mindfulness meditation are somewhat similar in practice and may therefore exert positive effects on the chronic pain experience via similar neurophysiological processes. Meditation reduces pain and decreases activity within the prefrontal cortex110,111, and may assist in reducing the affective component of the pain experience by decreasing maladaptive cognitive processes of rumination and catastrophizing. Mindfulness meditation induces analgesia by recruiting increased activity within the anterior cingulate cortex and the anterior insula, additional regions involved in the cognitive regulation of pain112. Positive expectations and beliefs, underlying placebo effects, are powerful psychological modulators of the pain experience and involve similar brain regions involving cognitive control of pain23,113. Neuroimaging research in these areas continues to determine the how psychology interacts with the pain experience, and may in the future, aid in optimizing psychology-based therapies for individuals with chronic pain.
5. Clinical Relevance for Prevention and Treatment of Chronic Pain: Neuroimaging-based Implications and Therapies
Understanding how neurophysiology is altered in chronic pain and how it can be changed through effective treatment of pain, in itself, provides an intriguing set of questions. However, the main purpose for understanding these neurophysiological changes is to better treat patients suffering from chronic pain. Neuroimaging of acute and chronic pain has provided the field of pain research and clinicians with an important framework of understanding: that even though no bodily signs of injury or dysfunction may be observed in the patients, neurological differences have been observed in brain structure and function in patients across a wide variety of chronic pain conditions as compared with healthy individuals. These observations provided by neuroimaging research together provide strong evidence that the effective prevention and effective treatment of chronic pain must include considerations of cortical and subcortical CNS processes.
Just as pre- and perioperative best practices are suggested to reduce the instance of post-operative pain, for example local anesthetics prior to limb amputation114 or the use of gabapentin perioperatively115, clinical research aims to identify best practices for non-perioperative pain prevention and treatment. These best practices as informed by preclinical research, clinical research, clinical trials, and neuroimaging research include a multi-modal approach of prevention and therapy. Typical approaches to treating pain include pharmacologic, procedural, psychological, physical therapy, complementary and alternative medicine, and self-management. The comprehensive multi-modal approach utilizes 1) physiological treatment and positive stimulation of the peripheral nervous system and musculature, 2) psychological treatment to engage healthy cognitive and emotional processes in the presence of chronic pain, 3) pharmacological therapies to prevent and/or reverse aberrant activity within the CNS, and 4) alternative and complementary treatments and therapies as deemed beneficial for the patient. With even broader treatment implications, neuroimaging research of chronic pain has provided evidence for clinical treatment considerations of immunological dysfunction within the CNS, as described above87. Such neuroimaging-based evidence further encourages increased communication between pain clinicians and medical specialists from other fields, including psychology, immunology and nutrition. Ultimately, with this multi-modal treatment approach, it is important to consider the evidence of cortical and CNS alteration and dysfunction in treatment selection approaches.
Thus far, the primary influence of pain neuroimaging on clinical practice appears to be through changing the conversation regarding pain. Neuroimaging technologies have broadened the current understanding of acute and chronic pain processes in humans, and thus have allowed for new considerations and conversations between clinicians and their patients. Probably the most influential aspect of neuroimaging enhancing the conversation of pain, for patients and clinicians, is the now vast amount of neuroimaging evidence supporting structural and functional CNS alterations in patients with chronic pain.
While many people with chronic pain present with pain that is disproportionate to any physiological manifestation of injury in the body, neuroimaging evidence indicates that the CNS is altered in these patients. Clinicians can use neuroimaging evidence as a grounds for talking points with their patients in conversations that scientifically validate the patients’ pain as being real. Further, these types of conversations provide the patients with an, albeit still currently incomplete, understanding of why they may be experiencing chronic pain – because their CNS is altered such that it is creating an aberrant experience of pain within the brain and/or disproportionately responding to incoming sensory information. Thus, neuroimaging research in the field of pain has provided a context of brain and CNS physiology for understanding and discussing chronic pain symptoms in patients. In addition to providing talking points for clinicians to patients, neuroimaging evidence has provided grounds for education of medical professionals, patients, researchers, and the general population that chronic pain affects multiple systems including the CNS. Therefore, in light of this understanding, it follows that effective treatment strategies for chronic pain need to be comprehensive. Treatment should consist of therapeutic approaches targeted at multiple systems including psychological, physiological, and pharmacological therapies, combining therapeutics from conventional and alternative medicine.
Neuroimaging, of noxious stimulation in healthy volunteers and patients with chronic pain, has provided a helpful framework for thinking about chronic pain and talking about pain with patients, clinicians, neuroscientists and the public. Further, it has provided methods for increased understanding of drug mechanisms acting centrally, and the associated changes in brain structure and function that occur in response to pharmacological therapies. Neuroimaging studies have also provided evidence for how psychological and behavioral therapies are associated with changes in brain structure and function; this evidence aids us in understanding how these therapies proactively modulate the pain experience, and supports their usefulness and credibility as valuable complementary therapeutics.
Regarding actual changes in clinical practice via advanced therapeutics, neuroimaging-based therapies for pain are currently being used in clinical trials and clinical practice, as adjunct treatments where available. However, these therapies are still being used on a somewhat experimental basis. Neuroimaging-based therapies including transcranial direct current stimulation116,117, transcranial magnetic stimulation118,119, deep brain stimulation120, and real-time fMRI neurofeedback27,121 are under evaluation in ongoing clinical trials. Advancements of these technologies by supplementation of additional neuroimaging-based technologies, specification of ideal parameters, identification of novel and effective target brain regions, and individual patient predictors of success (such as differences in brain connectivity identified using neuroimaging) may lead to enhanced efficacy of neuroimaging-based therapies for individual patients in the not so distant future.
6. Current Prospects and Advancements in Neuroimaging of Pain
Newer areas of neuroimaging for chronic pain research involve identifying brain based biomarkers for prediction of who will develop pain after surgery, patient prognosis, and to aid in treatment selection for individual patients. Predictive signatures, or patterns of brain activity and structural differences, and clinical phenotypes of chronic pain are emerging through continued research in these areas. Eventually these brain signatures and clinical phenotypes may provide more specific predictive and prognostic measures to allow for improved risk assessment and advice (in particular for patients undergoing elective surgeries), as well as personalized treatment for unique chronic pain subgroups, possibly ultimately on an individual patient basis (for review:122). As one example of predictive research findings in progress, greater levels of correlated activity between the medial prefrontal cortex and the ventral striatum, a subcortical brain region involved in motivation and value processes, predicted worse outcomes (i.e., lower propensity for recovery) in patients with low back pain123. Thus, neuroimaging signatures may provide an additional data point to complement classic phenotypical predictors of outcome (e.g., age, sex, psychological status). A pain signature, distributed pattern of brain regions and specific activity levels within those regions, that is specific to acute heat pain sensitivity has been identified6,124. Such pain brain signatures may lead to enhanced predictive patterns of chronic pain patient subtypes in the future. In fact, researchers have recently identified a pain signature for pressure and multisensory sensitivity in fibromyalgia125. These studies and others43,126 have used multivariate methods for analyzing neuroimaging data. Multivariate methods allow for the identification of these preliminary signature patterns of brain structure and function in patients with chronic pain. Researchers are currently using multivariate methods operating on the premise that these methods are statistically better suited to identify patterns from neuroimaging data sets which contain approximately 200,000 voxels (3 dimensional pixels) per subject. Furthermore, multivariate methods provide output measures of classification accuracy, sensitivity, specificity, negative and positive predictive value. One major caveat, however, is that analyses using multivariate methods, similar to most neuroimaging analyses, still require groups of chronic pain patients and healthy volunteers for comparison. Further, acute pain and chronic pain signatures from these analyses are currently in the very preliminary research phase, are largely exploratory, and the signature patterns identified will require numerous iterations of improvement and validation prior to any glimpse of practical and reliable clinical application. Critically important medico-legal concerns surrounding the topic of brain biomarkers for chronic pain have been recently described in excellent detail in another review article127.
Other advancements in the field of pain neuroimaging include the use of combined neuroimaging modalities, large-scale multi-site investigations, meta-analytic research, and spinal cord fMRI. Neuroimaging researchers are now more frequently using analysis methods that combine neuroimaging modalities to understand chronic pain. One such investigation has recently used combined PET imaging and fMRI to identify reduced μ-opioid receptor availability and decreased pain-evoked BOLD activity in antinociceptive regions, such as the dorsolateral prefrontal cortex and anterior cingulate cortex, in individuals with fibromyalgia128. Neuroimaging of pain is also being included as a major component of large-scale multi-site investigations focused on understanding idiopathic chronic pain conditions such as urological chronic pelvic pain (i.e., interstitial cystitis, chronic prostatitis, bladder pain syndrome)129. Such neuroimaging investigations have identified changes among chronic pelvic pain patient data, collected and analyzed across multiple sites, that indicate dysfunctional resting state default mode connectivity71, increased gray matter density within the pelvic somatosensory cortex43,44, increased functional connectivity of the pelvic motor cortex to the posterior insula, a region involved in processing intensity or salience of pain74, and changes in white matter axon diffusivity57. These types of collaborative multi-site investigations are also allowing for longitudinal investigations that have shown changes in brain activity that track symptom profiles (improved and worsening symptoms) over time130. Meta-analyses of neuroimaging data are another useful tool becoming more prominently used to collectively re-analyze neuroimaging data from multiple previously published neuroimaging investigations (e.g.,131). Meta-analytic studies are beneficial for identifying the most reproducible, and therefore more reliable, results from neuroimaging analyses of chronic pain populations. For example, one meta-analysis has determined that brain regions, including dorsal anterior cingulate cortex, anterior insular cortex and dorsal medial thalamus, are commonly activated during tasks related to pain perception, innocuous stimulation, emotion, memory and introspection across multiple studies, suggesting that these brain regions are responsible for the processing and integration of salient information132. Spinal cord imaging is emerging, as field of neuroimaging research complimentary to brain and brainstem imaging, due to technological advancements in fMRI data collection and analysis133–135. Recent early investigations in spinal cord fMRI have provided additional evidence of descending modulatory control of pain136,137, as well as changes in spinal cord and brainstem activity in healthy humans after central sensitization36, and in individuals with chronic pain138. These findings are generally complementary to results from brain neuroimaging research, specifically, with greater stimulus-induced activation within the spinal cord being correlated with increased pain sensitivity. Ultimately, further advancements in these areas of promise, and others, will continue to contribute to the wealth of knowledge gained thus far by the neuroimaging of pain in healthy and clinical states.
SUMMARY
The field of neuroimaging of pain has grown tremendously over the last few decades. Numerous studies have now combined sensory and behavioral testing with fMRI technology. Together, these studies have greatly increased our understanding of how pain is processed within the human CNS. The advance of resting state fMRI technology has opened many opportunities to study functional brain activity and connectivity (i.e., the degree of correlated activity among brain regions) in patients with chronic pain. Neuroimaging has advanced the current understanding of the CNS’s role in chronic pain in humans, in particular, by demonstrating 1) that the CNS processes appear to be altered in patients with chronic pain, and 2) that there are multiple circuits involved in modulation of the pain experience and these circuits may also be altered (i.e., hyper- or hypoactive) in patients with chronic pain. Neuroimaging research has provided evidence for how modulation of the pain experience can occur by pharmacological, psychological, and physiological changes within the CNS and body via exogenous and endogenous processes. Importantly, although neuroimaging in humans is a convenient method of studying chronic pain and pain processing in general, it is limited in value in characterizing mechanisms on a microscopic scale such as with neurophysiological in vivo and in vitro preparations regarding pharmacological manipulations and intercellular recording. The marrying of neuroimaging discoveries and those from animal models will continue to provide a more complete picture of pain through translational research. While researchers are searching for useful neuroimaging based biomarkers for acute pain sensitivity and chronic pain predictors and prognostics, such biomarkers will need significant amounts of validation and tests for clinical utility prior to any potential use in clinical practice. Currently, neuroimaging-based methods of transcranial direct current stimulation, transcranial magnetic stimulation, deep brain stimulation, and real-time fMRI neurofeedback are being assessed for efficacy and optimization in clinical trials and may become a more prevalent modality of adjunct therapy for chronic pain in the near future. To date, however, the greatest influence of the research field of pain neuroimaging on clinical practice has been to validate the role of the CNS in chronic pain, thereby improving conversations between researchers, medical professionals, clinicians, patients, and society.
Summary Statement.
Neuroimaging has advanced our understanding of chronic pain and has collectively provided a framework for patient-clinician conversation regarding the complex, biopsychosocial aspect of chronic pain and the importance of multi-modal therapy for its alleviation.
Summary of Key Points.
Neuroimaging research has demonstrated definitive involvement of the central nervous system in the development, maintenance, and experience of chronic pain.
Structural and functional neuroimaging has helped elucidate central nervous system contributors to chronic pain in humans.
Neuroimaging of pain has provided a tool for increasing our understanding of how pharmacological and psychological therapies improve chronic pain.
To date, findings from neuroimaging pain research have benefitted clinical practice by providing clinicians with an educational framework to discuss the biopsychosocial nature of pain with patients.
Future advances in neuroimaging-based therapeutics (e.g. transcranial magnetic stimulation, real-time functional magnetic resonance imaging neurofeedback) may provide additional benefits for clinical practice.
In the future, with standardization and validation, brain imaging could provide objective biomarkers of chronic pain, and guide treatment for personalized pain management. Similarly, brain based biomarkers may provide an additional predictor of perioperative prognoses.
Acknowledgments
We thank Ms. Elizabeth “Lisa” Cha (undergraduate student at the University of Rochester, New York) who worked at Stanford University as a summer research assistant with Dr. Martucci for her assistance in the design, creation and editing of the figure in this review article.
Funding Statement: Drs. Katherine Martucci and Sean Mackey receive funding from the National Institutes of Health (NIH): National Institute on Drug Abuse (NIDA) K99 DA 040154 (KTM), K24 DA 029262 (SCM); National Center for Complementary and Integrative Health (NCCIH) P01 AT006651 (SCM).
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
References
- 1.Wall & Melzack's Textbook of Pain. 6. Philadelphia, PA: Saunders, an imprint of Elsevier Ltd; 2013. [Google Scholar]
- 2.Knecht S, Henningsen H, Hohling C, Elbert T, Flor H, Pantev C, Taub E. Plasticity of plasticity? Changes in the pattern of perceptual correlates of reorganization after amputation. Brain. 1998;121(Pt 4):717–24. doi: 10.1093/brain/121.4.717. [DOI] [PubMed] [Google Scholar]
- 3.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–60. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 4.Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–14. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- 5.Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JE, Chatterjee N, Younger J, Mackey S. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS One. 2011;6:e24124. doi: 10.1371/journal.pone.0024124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998;80:1533–46. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- 8.Maihofner C, Handwerker HO. Differential coding of hyperalgesia in the human brain: a functional MRI study. Neuroimage. 2005;28:996–1006. doi: 10.1016/j.neuroimage.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 9.Disbrow E, Buonocore M, Antognini J, Carstens E, Rowley HA. Somatosensory cortex: a comparison of the response to noxious thermal, mechanical, and electrical stimuli using functional magnetic resonance imaging. Hum Brain Mapp. 1998;6:150–9. doi: 10.1002/(SICI)1097-0193(1998)6:3<150::AID-HBM4>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogatzki-Zahn EM, Wagner C, Meinhardt-Renner A, Burgmer M, Beste C, Zahn PK, Pfleiderer B. Coding of incisional pain in the brain: a functional magnetic resonance imaging study in human volunteers. Anesthesiology. 2010;112:406–17. doi: 10.1097/ALN.0b013e3181ca4c82. [DOI] [PubMed] [Google Scholar]
- 11.Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009;29:10264–71. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain. 2014;155:2491–501. doi: 10.1016/j.pain.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–42. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100:8538–42. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–7. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 16.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–20. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 17.Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the "pain matrix". Neuroimage. 2011;54:2237–49. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 18.Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, Glover GH, Mackey SC. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3:144–60. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2:276–91. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willis WD., Jr Central nervous system mechanisms for pain modulation. Appl Neurophysiol. 1985;48:153–65. doi: 10.1159/000101121. [DOI] [PubMed] [Google Scholar]
- 21.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–43. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 22.Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz H-G, Rolke R, Treede R-D, Bartenstein P, Birklein F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64:1175–1183. doi: 10.1212/01.WNL.0000156353.17305.52. [DOI] [PubMed] [Google Scholar]
- 23.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Neural bases of conditioned placebo analgesia. PAIN®. 2010;151:816–824. doi: 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Brascher AK, Becker S, Hoeppli ME, Schweinhardt P. Different Brain Circuitries Mediating Controllable and Uncontrollable Pain. J Neurosci. 2016;36:5013–25. doi: 10.1523/JNEUROSCI.1954-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS One. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence JM, Hoeft F, Sheau KE, Mackey SC. Strategy-dependent dissociation of the neural correlates involved in pain modulation. Anesthesiology. 2011;115:844–51. doi: 10.1097/ALN.0b013e31822b79ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626–31. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomons TV, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Perceived helplessness is associated with individual differences in the central motor output system. Eur J Neurosci. 2012;35:1481–7. doi: 10.1111/j.1460-9568.2012.08048.x. [DOI] [PubMed] [Google Scholar]
- 29.Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120:69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt K, Forkmann K, Sinke C, Gratz M, Bitz A, Bingel U. The differential effect of trigeminal vs. peripheral pain stimulation on visual processing and memory encoding is influenced by pain-related fear. Neuroimage. 2016;134:386–95. doi: 10.1016/j.neuroimage.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev. 2002;40:29–44. doi: 10.1016/s0165-0173(02)00186-8. [DOI] [PubMed] [Google Scholar]
- 33.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KL, Rowbotham MC. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport. 1999;10:1511–6. doi: 10.1097/00001756-199905140-00022. [DOI] [PubMed] [Google Scholar]
- 35.Maihofner C, Schmelz M, Forster C, Neundorfer B, Handwerker HO. Neural activation during experimental allodynia: a functional magnetic resonance imaging study. Eur J Neurosci. 2004;19:3211–8. doi: 10.1111/j.1460-9568.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- 36.Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Lee MC, Zambreanu L, Menon DK, Tracey I. Identifying brain activity specifically related to the maintenance and perceptual consequence of central sensitization in humans. J Neurosci. 2008;28:11642–9. doi: 10.1523/JNEUROSCI.2638-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geuter S, Buchel C. Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci. 2013;33:13784–90. doi: 10.1523/JNEUROSCI.2191-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb Cortex. 2014;24:1037–44. doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. 2011;12:436–43. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barad MJ, Ueno T, Younger J, Chatterjee N, Mackey S. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J Pain. 2014;15:197–203. doi: 10.1016/j.jpain.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagarinao E, Johnson KA, Martucci KT, Ichesco E, Farmer MA, Labus J, Ness TJ, Harris R, Deutsch G, Apkarian AV, Mayer EA, Clauw DJ, Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain. 2014;155:2502–9. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol. 2015;193:131–7. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moayedi M, Weissman-Fogel I, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. Neuroimage. 2011;55:277–86. doi: 10.1016/j.neuroimage.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–8. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. Neuroimage Clin. 2013;3:249–60. doi: 10.1016/j.nicl.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–36. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pomares FB, Funck T, Feier NA, Roy S, Daigle-Martel A, Ceko M, Narayanan S, Araujo D, Thiel A, Stikov N, Fitzcharles MA, Schweinhardt P. Histological Underpinnings of Grey Matter Changes in Fibromyalgia Investigated Using Multimodal Brain Imaging. J Neurosci. 2017;37:1090–1101. doi: 10.1523/JNEUROSCI.2619-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–9. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- 53.Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117–24. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp. 2015;36:2075–92. doi: 10.1002/hbm.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, Tillisch K, Kutch JJ, Farmer MA, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Landis JR, Deutsch G, Harris RE, Clauw DJ, Mullins C, Ellingson BM, Network MR. Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. PLoS One. 2015;10:e0140250. doi: 10.1371/journal.pone.0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang L, Kutch JJ, Ellingson BM, Martucci KT, Harris RE, Clauw DJ, Mackey S, Mayer EA, Schaeffer AJ, Apkarian AV, Farmer MA. Brain white matter changes associated with urological chronic pelvic pain syndrome: multisite neuroimaging from a MAPP case-control study. Pain. 2016;157:2782–2791. doi: 10.1097/j.pain.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu D, Yuan K, Qin W, Zhao L, Dong M, Liu P, Yang X, Liu J, Sun J, Zhou G, von Deneen KM, Tian J. Axonal loss of white matter in migraine without aura: a tract-based spatial statistics study. Cephalalgia. 2013;33:34–42. doi: 10.1177/0333102412466964. [DOI] [PubMed] [Google Scholar]
- 59.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–89. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apkarian AV, Thomas PS, Krauss BR, Szeverenyi NM. Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neurosci Lett. 2001;311:193–7. doi: 10.1016/s0304-3940(01)02122-x. [DOI] [PubMed] [Google Scholar]
- 61.Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, Peterson AM, Firestone L. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16:158–68. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- 62.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–23. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 63.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 64.Pujol J, Lopez-Sola M, Ortiz H, Vilanova JC, Harrison BJ, Yucel M, Soriano-Mas C, Cardoner N, Deus J. Mapping brain response to pain in fibromyalgia patients using temporal analysis of FMRI. PLoS One. 2009;4:e5224. doi: 10.1371/journal.pone.0005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192:947–55. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–46. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- 68.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 69.Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 2014;6:100–8. doi: 10.1016/j.nicl.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, Martinez-Jauand M, Birbaumer N, Chialvo DR, Montoya P. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74:55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- 71.Martucci KT, Shirer WR, Bagarinao E, Johnson KA, Farmer MA, Labus JS, Apkarian AV, Deutsch G, Harris RE, Mayer EA, Clauw DJ, Greicius MD, Mackey SC. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP Research Network. Pain. 2015;156:1755–64. doi: 10.1097/j.pain.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolwerk A, Seifert F, Maihofner C. Altered resting-state functional connectivity in complex regional pain syndrome. J Pain. 2013;14:1107–1115. e8. doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34:3969–75. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Apkarian AV, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research Network Neuroimaging Study. Neuroimage Clin. 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang Y, Oathes D, Hush J, Darnall B, Charvat M, Mackey S, Etkin A. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain. 2016;157:1970–8. doi: 10.1097/j.pain.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berger SE, Baria AT, Baliki MN, Mansour A, Herrmann KM, Torbey S, Huang L, Parks EL, Schnizter TJ, Apkarian AV. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res Notes. 2014;7:739. doi: 10.1186/1756-0500-7-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weissman-Fogel I, Moayedi M, Tenenbaum H, Goldberg M, Freeman B, Davis K. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. PAIN®. 2011;152:384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 78.Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–41. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wood PB, Patterson JC, 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain. 2007;8:51–8. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 80.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–82. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 81.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao X, Xu M, Jorgenson K, Kong J. Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: A systematic review. Neuroimage Clin. 2017;13:33–38. doi: 10.1016/j.nicl.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grachev ID, Thomas PS, Ramachandran TS. Decreased levels of N-acetylaspartate in dorsolateral prefrontal cortex in a case of intractable severe sympathetically mediated chronic pain (complex regional pain syndrome, type I) Brain Cogn. 2002;49:102–13. doi: 10.1006/brcg.2001.1489. [DOI] [PubMed] [Google Scholar]
- 84.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, McLean SA, Gracely RH, Clauw DJ. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–7. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 86.Albrecht DS, Granziera C, Hooker JM, Loggia ML. In Vivo Imaging of Human Neuroinflammation. ACS Chem Neurosci. 2016;7:470–83. doi: 10.1021/acschemneuro.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–15. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei SY, Chen LF, Lin MW, Li WC, Low I, Yang CJ, Chao HT, Hsieh JC. The OPRM1 A118G polymorphism modulates the descending pain modulatory system for individual pain experience in young women with primary dysmenorrhea. Sci Rep. 2017;7:39906. doi: 10.1038/srep39906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–68. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282–97. doi: 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 91.Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131:3222–31. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 92.Borsook D. A future without chronic pain: neuroscience and clinical research. Cerebrum. 2012;2012:7. [PMC free article] [PubMed] [Google Scholar]
- 93.Seifert F, Bschorer K, De Col R, Filitz J, Peltz E, Koppert W, Maihofner C. Medial prefrontal cortex activity is predictive for hyperalgesia and pharmacological antihyperalgesia. J Neurosci. 2009;29:6167–75. doi: 10.1523/JNEUROSCI.4654-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goldstein-Piekarski AN, Korgaonkar MS, Green E, Suppes T, Schatzberg AF, Hastie T, Nemeroff CB, Williams LM. Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proc Natl Acad Sci U S A. 2016;113:11955–11960. doi: 10.1073/pnas.1606671113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Younger JW, Chu LF, D'Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–10. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin JC, Chu LF, Stringer EA, Baker KS, Sayyid ZN, Sun J, Campbell KA, Younger JW. One Month of Oral Morphine Decreases Gray Matter Volume in the Right Amygdala of Individuals with Low Back Pain: Confirmation of Previously Reported Magnetic Resonance Imaging Results. Pain Med. 2016;17:1497–504. doi: 10.1093/pm/pnv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jensen KB, Petzke F, Carville S, Choy E, Fransson P, Gracely RH, Vitton O, Marcus H, Williams SC, Ingvar M, Kosek E. Segregating the cerebral mechanisms of antidepressants and placebo in fibromyalgia. J Pain. 2014;15:1328–37. doi: 10.1016/j.jpain.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 99.Schmidt-Wilcke T, Ichesco E, Hampson JP, Kairys A, Peltier S, Harte S, Clauw DJ, Harris RE. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 2014;6:252–61. doi: 10.1016/j.nicl.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Natelson BH, Vu D, Mao X, Weiduschat N, Togo F, Lange G, Blate M, Kang G, Coplan JD, Shungu DC. Effect of Milnacipran Treatment on Ventricular Lactate in Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Trial. J Pain. 2015;16:1211–9. doi: 10.1016/j.jpain.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–7. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, Reddy R, Epperson CN. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T (1)H-MRS study. Neuropsychopharmacology. 2012;37:2764–71. doi: 10.1038/npp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wanigasekera V, Mezue M, Andersson J, Kong Y, Tracey I. Disambiguating Pharmacodynamic Efficacy from Behavior with Neuroimaging: Implications for Analgesic Drug Development. Anesthesiology. 2016;124:159–68. doi: 10.1097/ALN.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119:1453–64. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 105.Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, Pendse G, Morris S, Borsook D. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med. 2015;16:2368–85. doi: 10.1111/pme.12939. [DOI] [PubMed] [Google Scholar]
- 106.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–11. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KM. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45:5–31. doi: 10.1080/16506073.2015.1098724. [DOI] [PubMed] [Google Scholar]
- 108.Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153:1495–503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 109.Lorenz J, Minoshima S, Casey K. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 110.Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. PAIN®. 2011;152:150–156. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Gard T, Hölzel BK, Sack AT, Hempel H, Lazar SW, Vaitl D, Ott U. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cerebral Cortex. 2012;22:2692–2702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petersen GL, Finnerup NB, Grosen K, Pilegaard HK, Tracey I, Benedetti F, Price DD, Jensen TS, Vase L. Expectations and positive emotional feelings accompany reductions in ongoing and evoked neuropathic pain following placebo interventions. PAIN®. 2014;155:2687–2698. doi: 10.1016/j.pain.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 114.Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 115.Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. 2013;119:1215–21. doi: 10.1097/ALN.0b013e3182a9a896. [DOI] [PubMed] [Google Scholar]
- 116.Cummiford CM, Nascimento TD, Foerster BR, Clauw DJ, Zubieta JK, Harris RE, DaSilva AF. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther. 2016;18:40. doi: 10.1186/s13075-016-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci. 2013;33:11425–31. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain. 2006;123:187–92. doi: 10.1016/j.pain.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 119.Tzabazis A, Aparici CM, Rowbotham MC, Schneider MB, Etkin A, Yeomans DC. Shaped magnetic field pulses by multi-coil repetitive transcranial magnetic stimulation (rTMS) differentially modulate anterior cingulate cortex responses and pain in volunteers and fibromyalgia patients. Mol Pain. 2013;9:33. doi: 10.1186/1744-8069-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boccard SG, Fernandes HM, Jbabdi S, Van Hartevelt TJ, Kringelbach ML, Quaghebeur G, Moir L, Mancebo VP, Pereira EA, Fitzgerald JJ, Green AL, Stein J, Aziz TZ. Tractography Study of Deep Brain Stimulation of the Anterior Cingulate Cortex in Chronic Pain: Key to Improve the Targeting. World Neurosurg. 2016;86:361–70. e1–3. doi: 10.1016/j.wneu.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 121.Chapin H, Bagarinao E, Mackey S. Real-time fMRI applied to pain management. Neurosci Lett. 2012;520:174–81. doi: 10.1016/j.neulet.2012.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woo CW, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–9. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. An fMRI-based neurologic signature of physical pain. New England Journal of Medicine. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lopez-Sola M, Woo CW, Pujol J, Deus J, Harrison BJ, Monfort J, Wager TD. Towards a neurophysiological signature for fibromyalgia. Pain. 2017;158:34–47. doi: 10.1097/j.pain.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate Classification of Structural MRI Data Detects Chronic Low Back Pain. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis KD, Flor H, Greely HT, Iannetti GD, Mackey S, Ploner M, Pustilnik A, Tracey I, Treede RD, Wager TD. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol. 2017;13:624–638. doi: 10.1038/nrneurol.2017.122. [DOI] [PubMed] [Google Scholar]
- 128.Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta JK, Clauw DJ, Harris RE. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. 2016;157:2217–25. doi: 10.1097/j.pain.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alger JR, Ellingson BM, Ashe-McNalley C, Woodworth DC, Labus JS, Farmer M, Huang L, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Deutsch G, Harris RE, Clauw DJ, Glover GH, Parrish TB, Hollander J, Kusek JW, Mullins C, Mayer EA. Investigators MRN: Multisite, multimodal neuroimaging of chronic urological pelvic pain: Methodology of the MAPP Research Network. Neuroimage Clin. 2016;12:65–77. doi: 10.1016/j.nicl.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kutch JJ, Labus JS, Harris RE, Martucci KT, Farmer MA, Fenske S, Fling C, Ichesco E, Peltier S, Petre B, Guo W, Hou X, Stephens AJ, Mullins C, Clauw DJ, Mackey SC, Apkarian AV, Landis JR, Mayer EA, Network MR. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 2017;158:1069–1082. doi: 10.1097/j.pain.0000000000000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tanasescu R, Cottam WJ, Condon L, Tench CR, Auer DP. Functional reorganisation in chronic pain and neural correlates of pain sensitisation: A coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci Biobehav Rev. 2016;68:120–33. doi: 10.1016/j.neubiorev.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cauda F, Torta DM, Sacco K, Geda E, D'Agata F, Costa T, Duca S, Geminiani G, Amanzio M. Shared "core" areas between the pain and other task-related networks. PLoS One. 2012;7:e41929. doi: 10.1371/journal.pone.0041929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nash P, Wiley K, Brown J, Shinaman R, Ludlow D, Sawyer AM, Glover G, Mackey S. Functional magnetic resonance imaging identifies somatotopic organization of nociception in the human spinal cord. Pain. 2013;154:776–81. doi: 10.1016/j.pain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 134.Kong Y, Eippert F, Beckmann CF, Andersson J, Finsterbusch J, Buchel C, Tracey I, Brooks JC. Intrinsically organized resting state networks in the human spinal cord. Proc Natl Acad Sci U S A. 2014;111:18067–72. doi: 10.1073/pnas.1414293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cahill CM, Stroman PW. Mapping of neural activity produced by thermal pain in the healthy human spinal cord and brain stem: a functional magnetic resonance imaging study. Magn Reson Imaging. 2011;29:342–52. doi: 10.1016/j.mri.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 136.Stroman PW, Bosma RL, Cotoi AI, Leung RH, Kornelsen J, Lawrence-Dewar JM, Pukall CF, Staud R. Continuous Descending Modulation of the Spinal Cord Revealed by Functional MRI. PLoS One. 2016;11:e0167317. doi: 10.1371/journal.pone.0167317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sprenger C, Finsterbusch J, Buchel C. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical FMRI study. J Neurosci. 2015;35:4248–57. doi: 10.1523/JNEUROSCI.4897-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bosma RL, Mojarad EA, Leung L, Pukall C, Staud R, Stroman PW. FMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum Brain Mapp. 2016;37:1349–60. doi: 10.1002/hbm.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]