Abstract
BACKGROUND
Medication adherence is important to improve long-term outcomes after acute myocardial infarction (MI). We hypothesized that there is significant variation among United States (U.S.) hospitals in terms of post-MI medication adherence, and that patients treated at hospitals with higher post-MI medication adherence will have better long-term cardiovascular outcomes.
METHODS
We identified 19,704 Medicare patients discharged after acute MI from 347 U.S. hospitals participating in the Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines (ACTION Registry-GWTG) from 1/2/2007 to 10/1/2010. Using linked Medicare Part D prescription filling data, medication adherence was defined as proportion of days covered (PDC) >80% within 90 days post-discharge. Cox proportional hazards modeling was used to compare 2-year major adverse cardiovascular events (MACE) among hospitals with high, moderate, and low 90-day medication adherence.
RESULTS
By 90 days post-MI, overall rates of adherence to medications prescribed at discharge were 68% for beta-blockers, 63% for statins, 64% for angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), and 72% for thienopyridines. Adherence to these medications up to 90 days varied significantly among hospitals: beta-blockers (PDC >80%; 59–75%), statins (55–69%), thienopyridines (64–77%), and ACEIs/ARBs (57–69%). Compared with hospitals in the lowest quartile of 90-day composite medication adherence, hospitals with the highest adherence had lower unadjusted and adjusted 2-year MACE risk (27.5% vs. 35.3%, adjusted hazard ratio [HR] 0.88, 95% confidence interval [CI] 0.80–0.96). High adherence hospitals also had lower adjusted rates of death or readmission (HR 0.90, 95% CI 0.85–0.96), while there was no difference in mortality after adjustment.
CONCLUSIONS
Post-discharge use of secondary prevention medications vary significantly among U.S. hospitals and are inversely associated with two-year outcomes. Hospitals may improve post-discharge medication adherence and patient outcomes through better coordination of care between inpatient and outpatient settings.
Keywords: post-myocardial infarction medical adherence, patient outcomes
Cardiovascular therapies such as beta-blockers, statins, and antiplatelet agents have been shown to improve outcomes following acute myocardial infarction (MI).1 Performance measurement and quality improvement programs have traditionally focused on in-hospital use of these evidence-based therapies, leading to near ubiquitous prescription among eligible patients in the United States (U.S.).2 Despite their widespread prescription, several studies have demonstrated that downstream adherence to prescribed therapies is suboptimal.3,4 The reasons for low adherence rates are varied and involve complex relationships between the patient, provider, and society.5–7 Healthcare delivery continues to evolve with the influx of newly insured patients and the consolidation of health systems; consequently, measuring the quality of the care provided to patients has become quite common and is often linked to provider compensation and reimbursement.8,9 With these and other efforts to increase value in healthcare, medication adherence is a natural candidate for quality measurement since poor adherence has been associated with higher costs and worse patient outcomes.10–13
Multifaceted programs employed during patient admission have the potential to improve medication adherence.14–16 Recognizing this, several hospital-based initiatives have targeted the transition of care from the inpatient to outpatient setting with the goal of improving patient medication adherence and clinical outcomes after discharge.14 Health systems now variably employ discharge strategies that seek to identify barriers to medication adherence, provide patient education, and improve communication to outpatient providers. We hypothesize that transition of care interventions across hospital systems may contribute to variation in downstream medication adherence among patients.
The objectives of this study were twofold: 1) determine whether hospital-level variation in 90-day medication adherence exists among post-MI patients who are prescribed cardiac medications at index hospital discharge; and 2) compare 2-year outcomes among hospitals with high, moderate, or low post-discharge medication adherence, adjusting for differences in patient case-mix and hospital characteristics.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design and Population
The Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines (ACTION Registry-GWTG) is an ongoing, quality improvement registry of consecutive patients with a primary diagnosis of either ST-segment elevation MI (STEMI) or non–ST-segment elevation MI (NSTEMI) who are treated at participating hospitals across the U.S. The institutional review board of each reporting hospital approved participation in ACTION Registry-GWTG or determined a waiver of the need for review. Detailed clinical data are abstracted from the medical record, and include demographics, prior medical history, in-hospital procedures and therapies, clinical events before discharge, and medications prescribed at discharge (https://www.ncdr.com/webncdr/action/home/datacollection). The type and quality of data collected have been previously described.17–20 Since patient information was collected without unique patient identifiers in ACTION Registry-GWTG, we used five indirect identifiers in combination (date of birth, sex, hospital identification, date of admission, date of discharge) to link patients older than 65 years of age with Medicare claims data.20 This data linkage was supported by the Centers for Education and Research on Therapeutics grant (U19HS021092) from the Agency for Healthcare Research and Quality. The Duke University Medical Center Institutional Review Board granted a waiver of informed consent and authorization for this study, and all analyses were conducted by the Duke Clinical Research Institute (Durham, NC).
Study Population
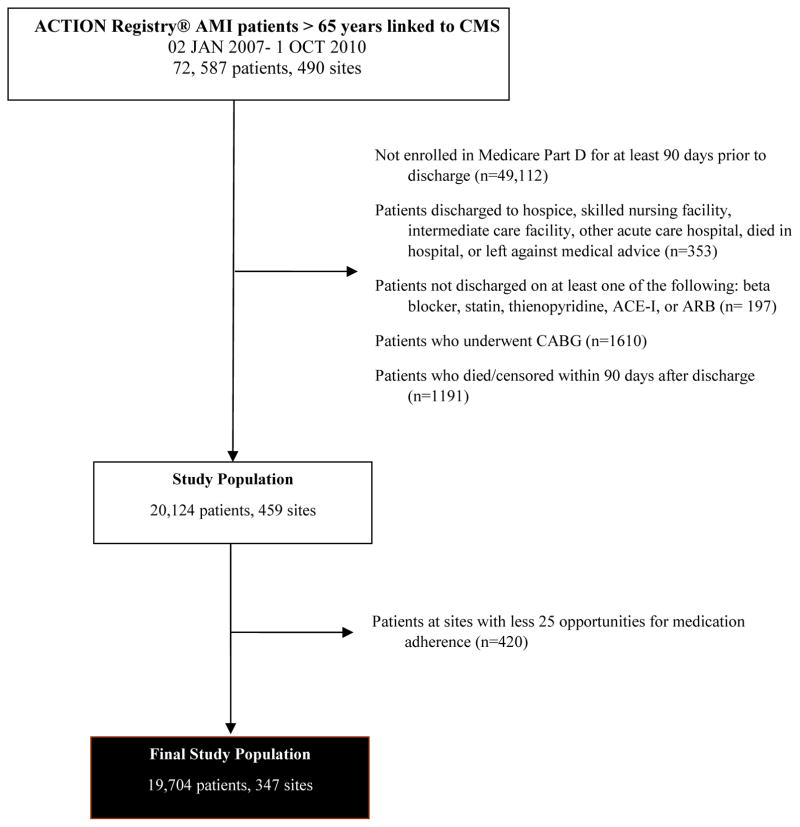
We included all patients with MI (STEMI or NSTEMI) in the ACTION Registry-GWTG database from January 2, 2007 to October 1, 2010 who were enrolled in the Centers for Medicare & Medicaid (CMS) Part D prescription coverage plan (n=23,475) (Figure 1). We excluded patients treated with coronary artery bypass graft (CABG) surgery (n=1,610), those not discharged to home (n=353), and those who did not survive to 90 days post-discharge (n=1,191). We also excluded patients who were discharged on none of the following cardiovascular medications: beta-blockers, statins, thienopyridines, and angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs) (n=197). In order to assess hospital variation in 90-day adherence, we examined hospitals with at least 25 opportunities for adherence (i.e., one opportunity for each evidence-based medication) among MI discharges. We did not include sites with less than 25 opportunities (112 sites) since we presumed that with such low volumes these sites were not routinely treating patients with acute MI (n=420). In addition, estimates for the smaller sites would be subject to larger sampling variation and noise. Our final study population was comprised of 19,704 patients at 347 hospitals.
Figure 1. Study Population.
This figure depicts the final study population, from the initial cohort, through exclusions.
ACEI indicates angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CMS, Centers for Medicare & Medicaid Services
Statistical Analysis
The primary outcome of interest was medication adherence at 90 days following discharge from a participating hospital post-acute MI. We defined adherence as >80% proportion of days covered (PDC) individually for beta-blockers, statins, ACEIs/ARBs, and thienopyridines; adherence to each medication was only assessed among patients who were prescribed this medication at hospital discharge. The use of a dichotomous variable of PDC >80% is consistent with previously used methods.21,22 We used logistic regression with random intercepts for hospitals, and Spearman correlations to assess if adherence to specific medications varied together according to a hospital and if 90-day medication adherence correlated with 1-year adherence. To assess for hospital-level composite adherence of the four cardiovascular medications, we included a fixed effect for medication type, a random effect for hospital, and estimated the hospital-specific odds ratio (OR) for composite adherence. We examined the distribution of hospital-specific OR and found the 25th percentile corresponded to a greater than 10% decrease in odds and the 75th percentile corresponded to a greater than 12% increase in odds for adherence. Since we felt these were meaningful cutoffs, we used percentiles of ORs to classify hospitals into high (>75th percentile), moderate (25th–75th percentile), and low (<25th percentile) adherence (Supplemental Figure 1). Patient and hospital characteristics were compared among the high, moderate, and low adherence hospitals using chi-square testing for categorical variables (if the sample size was not sufficient, exact test was used) and Wilcoxon rank-sum testing for continuous variables. We also assessed medication adherence across hospitals at 1 year post-discharge.
Next, we compared 2-year major adverse cardiovascular events (MACE) and death or all-cause readmission risk among hospitals with high, moderate, or low 90-day medication adherence. Long-term analysis of MACE and death or all-cause readmission included only those events that occurred after 90 days post-discharge since we felt that early events may affect 90-day adherence. MACE included death, as well as readmission for MI, stroke, or revascularization within 2 years post-discharge. Kaplan-Meier curves were generated to estimate the probability of each outcome by hospital adherence group and the log-rank test was used to assess whether the differences between the curves were statistically significant. Cox proportional hazards modeling was performed to examine the association between hospital adherence group and each outcome, using robust standard errors to account for clustering of patients within hospitals. The model was adjusted to account for differences in case-mix. Variables in the model included: demographics (age, gender, race, length of stay, and body mass index [BMI]), indicators of socioeconomic status23 (derived from patient ZIP Codes and U.S. Census data: percent of patients ≥25 years with ≥4 years of college, percent patients ≥25 years with high school diploma, percent white-collar workers, median estimate household income, median home value), additional insurance besides Medicare (private/health maintenance organization), comorbidities (tobacco use; hypertension; dyslipidemia; hemodialysis; diabetes; peripheral artery disease; and prior history of MI, heart failure [HF], percutaneous coronary intervention [PCI], CABG, and stroke), STEMI or STEMI equivalent on arrival, transfer-in status, cardiac catheterization within 24 hours of arrival, in-hospital PCI, left ventricular ejection fraction, end-stage renal disease, baseline hemoglobin, in-hospital HF complication, in-hospital major bleeding, and any blood transfusion. The following hospital characteristics were also included: geographic region, hospital type (catheterization lab only, PCI capability only, and CABG capability), teaching hospital (academic and non-academic), and hospital bed size.
All analyses were performed using the SAS software package version 9.4 (SAS Institute, Cary, NC).
RESULTS
Among patients discharged to home after an acute MI, use of evidence-based secondary prevention therapy prescription was high; with 96% discharged on beta-blockers, 89% on a statin, 84% on a thienopyridine, and 76% on ACEIs/ARBs among patients with no documented contraindications to therapy. Nonetheless, by 90 days post-discharge, only 68% of patients prescribed beta-blocker therapy at discharge remained adherent. Similarly, only 63%, 64%, and 72% of those discharged on statins, ACEIs/ARBs, and thienopyridines, respectively, remained adherent by 90 days. Importantly, there was significant inter-hospital variation in 90-day adherence to these therapies. As seen in Figure 2, the probability of adherence varied widely (p<0.001). Hospital-level adherence rates for various therapies was correlated; hospitals with high adherence to one medication were also likely to demonstrate high adherence to another medication (beta-blocker and statin correlation coefficient [r] 0.42, beta-blocker and ACEI/ARB r 0.35, beta-blocker and thienopyridine r 0.44, statin and ACEI/ARB r 0.41, statin and thienopyridine r 0.34, ACEI/ARB and thienopyridine r 0.29; Supplemental Figure 2). In addition, we found hospital adherence at 90 days was associated with higher hospital adherence at 1 year (Spearman’s rho=0.63). By 1 year, adherence had decreased approximately 8% across all medications: 61% of patients remained adherent to beta-blockers, 55% to statins and ACEIs/ARBs, and 64% to thienopyridines.
Figure 2. Hospital Variation in 90-day Adherence to Individual Medications.
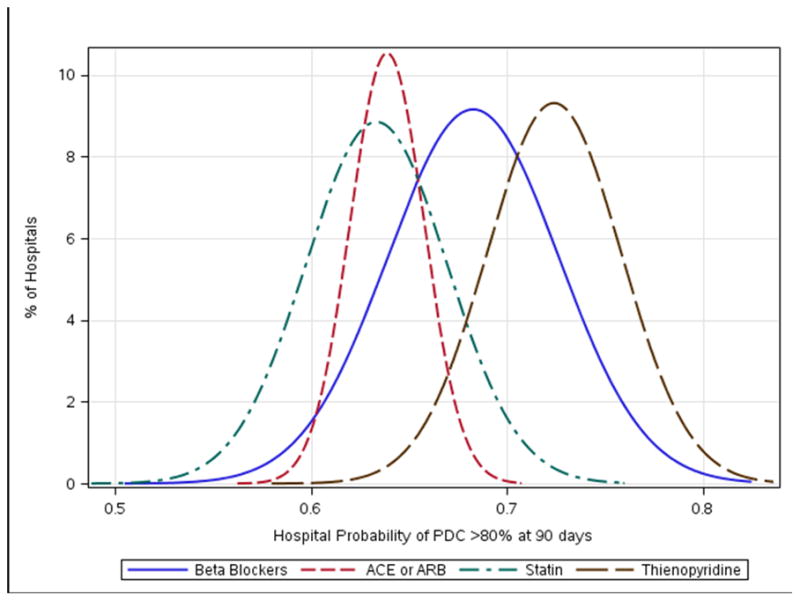
Unadjusted hospital variation in 90-day adherence (PDC >80% vs. ≤80%) to individual medications.
PDC indicates proportion of days covered
Hospitals were divided into those with high probability (OR 1.12 to 1.45, 86 hospitals, 6,570 patients), moderate probability (OR 0.90 to 1.12, 175 hospitals, 9,249 patients), or low probability (OR 0.57 to 0.90, 86 hospitals, 3,885 patients) of 90-day medication adherence. At 90 days post-discharge, adherence rates to secondary prevention medications for each group are shown in Table 1. We observed that hospitals with high 90-day medication adherence were less likely to be located in the Southern U.S. (25%) than hospitals with low and moderate adherence (51% and 58%, respectively). High adherence hospitals were more often academic centers (35%), compared with moderate (23%) and low adherence (25%) hospitals. High adherence hospitals were also substantially larger in terms of higher bed capacity and had approximately twice as many transfer-in patients than hospitals with low 90-day medication adherence (Table 1).
Table 1.
Medication Adherence and Hospital Characteristics by Hospital Composite Adherence Profiles
| Overall (n=19,704) | High Adherence (n=6,570) | Moderate Adherence (n=9,249) | Low Adherence (n=3,885) | |
|---|---|---|---|---|
| 90-day adherence | ||||
| All medications | 40.8% | 47.1% | 39.8% | 32.5% |
| Beta-blockers | 68.2% | 74.6% | 67.4% | 58.8% |
| ACEI/ARBs | 63.7% | 68.8% | 62.7% | 57.4% |
| Statins | 63.2% | 68.7% | 62.5% | 55.1% |
| Thienopyridines | 72.4% | 76.5% | 73.0% | 63.7% |
| Hospital characteristics* | ||||
| Region | ||||
| West | 11.5% | 12.0% | 11.2% | 11.2% |
| Northeast | 7.7% | 15.5% | 4.5% | 1.8% |
| Midwest | 35.3% | 47.4% | 26.5% | 35.6% |
| South | 45.6% | 25.0% | 57.8% | 51.5% |
| Urban | 90.7% | 89.4% | 94.3% | 84.2% |
| Academic | 27.3% | 35.2% | 22.6% | 24.8% |
| CABG capacity | 93.3% | 94.5% | 93.1% | 91.4% |
| Hospital beds† | 411 (283, 618) | 441 (332, 604) | 405 (279, 638) | 356 (249, 562) |
| Transfer-in | 35.1% | 42.3% | 35.4% | 22.4% |
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass surgery
p-values <0.0001 for all hospital characteristics
Reported as median (25th and 75th percentiles).
Table 2 compares patient case-mix and in-hospital treatment patterns among these hospital groups. Compared with other hospitals, those with low 90-day medication adherence had higher proportions of patients who were female, of non-white race, had less educational achievement, and lower household income. Patients treated at hospitals with low medication adherence were more likely to present with NSTEMI than STEMI, and to have a prior history of cardiovascular disease (prior MI, PCI, stroke, or peripheral artery disease) or cardiovascular risk factors (diabetes, hypertension, tobacco use) than patients treated at hospitals with moderate or high medication adherence. While rates of STEMI reperfusion were similar between hospitals, hospitals with low 90-day medication adherence were less likely to revascularize patients presenting with NSTEMI. The incidence of in-hospital HF and major bleeding was higher among patients treated in low adherence hospitals. The median length of stay was 4 days for low and moderate adherence hospitals, and 3 days for high adherence hospitals. High adherence hospitals more often referred patients for cardiac rehabilitation after discharge compared with moderate and low adherence hospitals: 78% (high adherence) vs. 75% (moderate adherence) vs. 64% (low adherence), respectively (p<0.001).
Table 2.
Patient Characteristics by Hospital Composite Adherence Profiles
| Overall (n=19,704) | High Adherence (n=6,570) | Moderate Adherence (n=9,249) | Low Adherence (n=3,885) | p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years)* | 75 (70, 82) | 75 (70, 82) | 75 (69, 81) | 75 (70, 82) | 0.07 |
| Female | 51.5% | 50.6% | 51.2% | 53.9% | 0.003 |
| Non-white race | 13.2% | 8.3% | 15.2% | 17.3% | <0.0001 |
| High school graduate† | 86.4 (82, 90) | 87.3 (84, 91) | 86.1 (81, 89) | 84.5 (79, 88) | <0.0001 |
| Median household income† | 45221 (40117, 52363) | 46595 (41592, 54806) | 44186 (39271, 51846) | 44231 (39580, 51457) | <0.0001 |
| Clinical features | |||||
| STEMI | 30.7% | 33.9% | 31.1% | 24.4% | <0.0001 |
| Prior MI | 30.0% | 27.9% | 29.8% | 33.9% | <0.0001 |
| Prior PCI | 17.3% | 14.0% | 17.4% | 22.5% | <0.0001 |
| Prior CABG | 22.3% | 22.3% | 22.5% | 24.1% | 0.08 |
| Prior stroke | 10.7% | 9.7% | 10.9% | 12.0% | 0.001 |
| Peripheral arterial disease | 14.4% | 13.1% | 14.8% | 15.7% | 0.001 |
| Diabetes | 35.1% | 33.1% | 35.3% | 38.2% | <0.0001 |
| Hypertension | 81.4% | 80.3% | 81.4% | 83.5% | 0.0004 |
| Current smoker | 17.5% | 16.2% | 18.0% | 18.3% | 0.005 |
| In-hospital characteristics | |||||
| Reperfusion (STEMI) | 94.1% | 93.2% | 94.1% | 94.9% | 0.50 |
| PCI (NSTEMI) | 49.8% | 54.1% | 49.5% | 44.3% | <0.0001 |
| Heart failure | 6.3% | 5.4% | 6.1% | 8.3% | <0.001 |
| Major bleeding | 10.1% | 9.5% | 10.0% | 11.3% | 0.02 |
| Length of stay* | 3 (2,5) | 3 (2,5) | 4 (3,5) | 4 (3,5) | <0.0001 |
| Cardiac rehabilitation referral | 73.6% | 77.7% | 74.8% | 63.6% | <0.0001 |
MI indicates myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; All other abbreviations can be found in Table 1.
Reported as median (25th and 75th percentiles).
Education and household income derived from patient residence zip code.
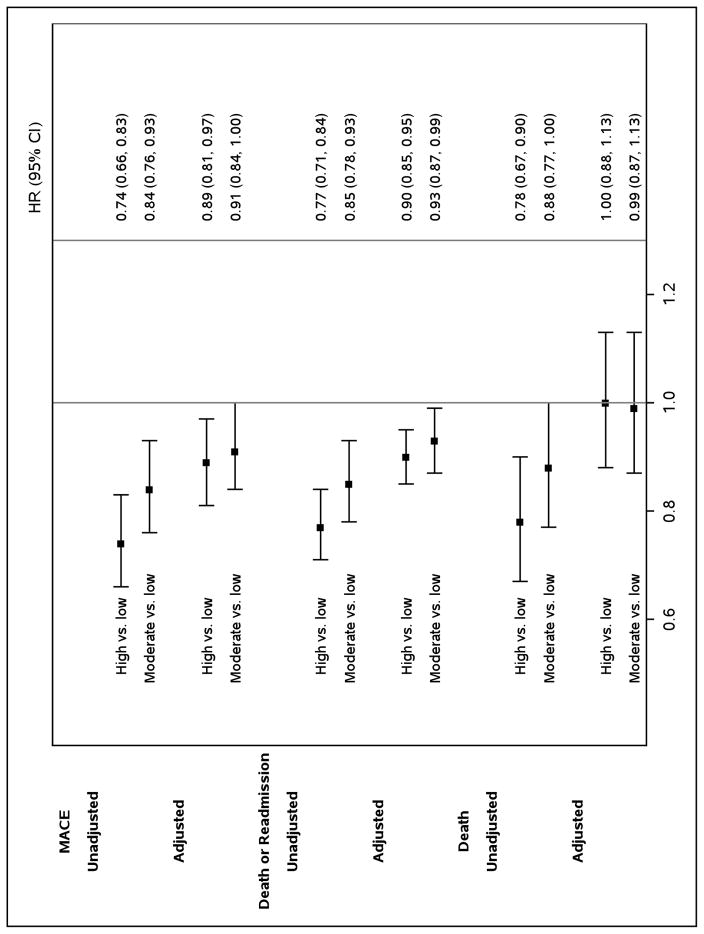
As seen in Figure 3, the unadjusted cumulative incidence of MACE at two years was lower among hospitals with higher 90-day medication adherence (27.5%) compared with moderate (31.0%) and low (35.3%) adherence hospitals (log-rank p<0.0001). After adjusting for differences in hospital characteristics and patient case-mix, patients at high adherence hospitals remained less likely to experience MACE (hazard ratio [HR] 0.88, 95% confidence interval [CI] 0.80–0.96); the difference in MACE between moderate and low adherence hospitals was attenuated, but remained significant (HR 0.91, 95% CI 0.83–1.0, Figure 4).
Figure 3. MACE by Hospital-level 90-day Composite Adherence Rates.
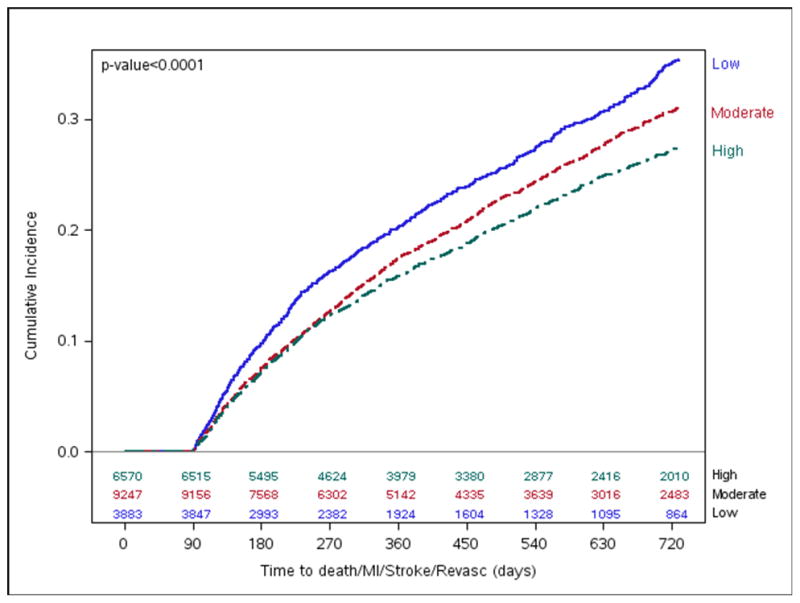
Long-term cumulative incidence Kaplan-Meier MACE estimates by hospital 90-day composite adherence groups.
Figure 4. Two-year Outcomes According to Hospital Adherence Classification.
A forest plot of unadjusted and adjusted MACE, mortality, and death or all-cause readmission by hospital-level 90-day composite adherence.
Two-year mortality rates differed among high (16.8%), moderate (19.1%), and low (21.1%) adherence groups (Figure 5), but there was no difference between the groups after multivariate adjustment (Figure 4). Nevertheless, we found lower rates of death or all-cause readmission among high adherence hospitals (56.0%) compared with hospitals that had moderate (59.4%) and low (64.1%) adherence (log-rank p-value <0.001, Figure 6) that persisted after multivariable adjustment (HR 0.90, 95% CI 0.85–0.96, Figure 4). Similarly, patients at moderate adherence hospitals were also less likely to experience these adverse outcomes compared with low adherence hospitals (HR 0.93, 95% CI 0.87–0.99).
Figure 5. Mortality by Hospital-level 90-day Composite Adherence Rates.
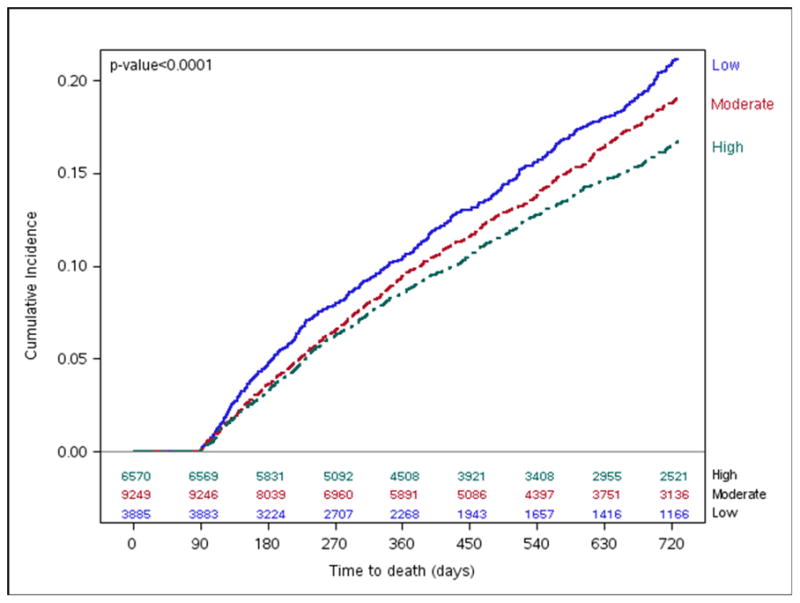
Long-term cumulative incidence Kaplan-Meier Mortality estimates by hospital 90-day composite adherence groups.
Figure 6. Mortality or All-cause Readmission by Hospital-level 90-day Composite Adherence Rates.
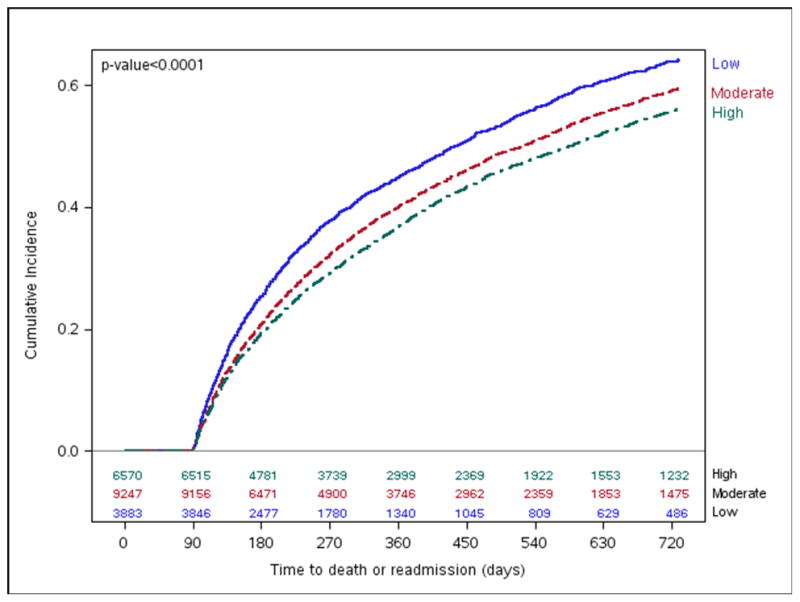
Long-term cumulative incidence Kaplan-Meier mortality or all-cause readmission by hospital-level 90-day composite adherence groups.
CI indicates confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular event
DISCUSSION
Our study found that adherence to prescribed evidence-based cardiovascular medications post-MI was suboptimal. By 90 days, adherence rates ranged from 63–72% for key secondary prevention medications, varying significantly across hospitals, and adherence rates further declined by 1 year (55–64%). Relative to patients treated at hospitals with low adherence, those treated at high and moderate 90-day adherence centers had lower observed MACE and death or readmission rates that persisted after adjustment for hospital characteristics and patient case-mix.
We observed a decline in overall medication adherence as early as 90 days after discharge for an MI, with marked variation in adherence rates across hospitals. Hospital adherence groups differed in several patient characteristics, as well as sociodemographic factors. Prior work has found factors such as non-white race and comorbidities to be independently associated with poorer adherence.3 In particular, patients who are non-adherent more often have lower health literacy, lower perceived quality of life, and higher rates of depression.4 Not surprisingly, we found these “high risk” characteristics of non-adherence to be more prevalent in low adherence hospitals; we would expect these differences in patient case-mix between hospital groups would contribute to adherence variation.
Importantly, after accounting for patient case-mix, hospital-level variation in medication adherence continues to persist in community practice. High adherence hospitals were typically larger and academic centers—two factors that may have increased the resources available to invest in the development of systematic and comprehensive inpatient and discharge practices that can potentially improve post-discharge adherence. For example, the use of a tailored medication management system by Veteran’s Administration hospitals led to improved patient adherence to anticoagulants (risk ratio [RR; 95% CI]: 1.31 [1.16–1.47]), with a positive correlation between duration of follow-up and longer-term adherence.22
Our findings demonstrate that hospitals with higher 90-day medication adherence were associated with improved MACE and readmission risk at two years. Mortality was not significantly different, suggesting that the difference in outcomes lies in preventing nonfatal events and unscheduled contact with the healthcare system. The relationship between post-discharge medication adherence and downstream outcomes is complex and may include several factors. For example, improvement in outcomes may be attributed to the benefits associated with the medication itself24–29; however, in addition to the cardio-protective benefits of the medications, other factors that contribute to downstream outcomes include patient burden of illness. When these differences were adjusted for via multivariate modeling, we found that the higher probability of cardiovascular events among low adherence patients was attenuated, but did not fully resolve, suggesting that factors beyond patient case-mix may contribute to outcomes at the hospital level; these factors may be on a provider, health system, or a societal level.
Unlike MACE and readmission risk, we did not find any association between hospital adherence and long-term mortality; the reasons for this lack of association are unclear, but may perhaps be explained by the age of our study population (median age 75 years). The increased baseline cardiovascular risk in an elderly population may mitigate the magnitude of association between adherence and survival noted in prior studies.24,25 Though medication adherence is especially impactful on higher risk patients, adherence remained suboptimal even among high adherence hospitals and may be below the level which would be needed to influence survival among this subgroup. Also of note, the relationship between adherence and long-term mortality has not been fully elucidated in real-world patient populations and may in some part be attributed to the healthy adherer bias.25
Higher adherence hospitals may be associated with the overall higher quality of services provided. One marker of high quality is a hospital’s adherence to evidence-based care processes that have been associated with improved clinical outcomes.30,31 We found that higher adherence hospitals performed better than low adherence hospitals on metrics such as time from arrival to electrocardiogram and time from arrival to cardiac catheterization. Hospitals that achieved high performance in both guideline adherence and issues of patient safety32 (i.e., reductions in excess dosing of anticoagulants) have been associated with a trend toward lower risk-adjusted mortality.33
Assessing differences in hospital quality has become more challenging since most hospitals now routinely achieve very high performance on traditional evidence-based process measures.2 As novel measures are considered, medication adherence on a hospital level may provide a new paradigm to assess quality. Though medication adherence has traditionally been considered a patient’s responsibility, our work suggests that hospitals have the capacity to impact downstream patient adherence. The transition from hospital to home has been identified as an important period that may directly influence patients’ risk of long-term cardiovascular events.34–36 Inter-hospital differences in discharge practices, such as early physician follow-up and cardiac rehabilitation referral, may contribute to variation in hospital-level medication adherence and subsequent differences in cardiovascular events noted in our study. For example, the routine use of a cardiovascular team-based model has been associated with a reduction in readmissions; this model includes pharmacists, advanced care practitioners, social workers, and the discharging physician.37–39 Similarly, transition of care models with early intense post-discharge monitoring have been associated with reduced all-cause hospitalization and 6-month mortality.40 Medication adherence has been associated with improved outcomes, so hospitals and health systems that enact policies promoting adherence may provide higher quality care. We believe that opportunities to assess hospital-level medication adherence through the use of prescription claims databases will continue to develop as administrative and clinical databases become more comprehensive and allow for appropriate adjustment of patient and hospital-level differences in patient mix and resource availability.
There is wide recognition that medication adherence is critical, but there can be a disconnect between provider and patient perceptions of factors contributing to (or impeding) adherence.41,42 The positive impact of high adherence on patient outcomes only reinforces the need for health systems and providers to remain proactive as advocates for their patients and promote adherence to prescribed therapies.
Future Steps
We found that health system factors may contribute to a patient’s likelihood of medication adherence and subsequent clinical outcomes. These factors may include providing patient education, behavioral support, provider communication, and access to care. Various interventions have had success with integrating evidence-based practices, such as screening for patient barriers to adherence and ensuring better continuity of care with outpatient physicians. For example, successful disease management programs are comprehensive and include components like telephone follow-up, self-management, dietary advice, exercise recommendations, medication review, and social support.40 Since there are numerous potential intervention targets, it is not surprising that there is wide variability across centers in the patient experience at discharge.
We propose a comprehensive assessment of current practices among a diverse group of hospitals. The next step should be to understand the impact these practices have on patient medication adherence and subsequent clinical outcomes, which is critical since resources are limited. Furthermore, the likelihood of sustaining an intervention is often related to the “return on investment” (e.g., improved outcomes and/or cost savings). Finally, those strategies that are most scalable across health systems should be identified and would provide the basis for the development of “best practices” that can be disseminated broadly across hospital networks. Policies are currently in place that penalize hospitals for “avoidable” readmissions.43 Though controversial, the intended purpose is to address hospital factors that can lead to poor outcomes. Conversely, hospitals should be recognized for creative and effective efforts that promote improved patient outcomes. Our work demonstrates that hospitals and health systems have the capacity to positively impact medication adherence, although the best way to accomplish this task remains unclear.
Limitations
Our study should be interpreted in light of several limitations. First, the use of PDC as a measure of adherence has been validated, but we cannot determine whether medication dispensing equated with patients actually taking the medication(s). Second, the number of hospital factors in our analysis were limited and we do not capture data on specific practices implemented at each hospital. We have attempted to account for these differences through multivariate modeling; however, as in any observational analysis, we are unable to account for unmeasured confounders that may influence outcomes. For example, resources available to address issues of mental illness, poverty, and poor social support vary across communities and likely influence a hospital’s effectiveness in achieving medication adherence. Third, we do not have data on adherence among patients not enrolled in Medicare Part D or on alternate prescription coverage among non-enrollees. Prior work has demonstrated that Part D enrollees are more often non-white and have a higher comorbidity burden compared to non-enrollees.44 The use of evidence-based medications on discharge has been shown to be similar between enrollees and non-enrollees, but the differences in patient characteristics may also influence long-term adherence and outcomes. Finally, we did not collect information on post-discharge processes that may influence medication adherence; therefore, we cannot account for unmeasured factors that may contribute to differences we observed in long-term outcomes.
Conclusions
Healthcare is facing challenges due to new regulatory requirements, an aging population requiring more complex care, and alternative payment models that emphasize value-based purchasing.45,46 We found that U.S. hospitals vary in downstream medication adherence to cardiac therapies after discharge for a myocardial infarction. Patient adherence is a critical component of an evolving healthcare system that is now prioritizing quality of care over traditional fee-for-service. We believe an understanding of factors associated with adherence on a hospital-level, in addition to those on a patient-level, provide a novel framework on which to further engage with the issues of poor medication adherence in healthcare. The transition of care from the inpatient setting to a patient’s home is a key opportunity for hospital systems and accountable care organizations to address (on a systems level) factors that impact adherence and subsequent outcomes.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
In this study of 19,704 elderly acute myocardial infarction patients across 347 hospitals, we used prescription drug coverage claims to assess medication adherence on a hospital level and found variation in 90-day adherence among hospitals: beta-blockers (59–75%), statins (55–69%), thienopyridines (64–77%), and ACEIs/ARBs (57–69%).
Hospitals with higher medication adherence were associated with lower downstream MACE (adjusted HR 0.88, 95% CI 0.80–0.96), as well as death or readmission (HR 0.90, 95% CI 0.85–0.96) after adjusting for differences in patient and hospital characteristics.
What are the clinical implications?
Hospitals have the capacity to influence a patient’s adherence to secondary prevention cardiac medications, thereby also potentially impacting long-term patient outcomes.
The transition from hospital to home represents a key opportunity to implement policies that address barriers to medication adherence.
Further work is needed to identify best hospital discharge practices that promote medication adherence and provide a uniform and seamless transition home.
Acknowledgments
The authors would like to thank Erin Campbell, MS for her editorial contributions to this manuscript. Ms. Campbell did not receive compensation for her assistance, apart from her employment at the institution where this study was conducted.
SOURCES OF FUNDING
Dr. Mathews is supported by grant number KM1CA156687 from the National Institute of Health/National Cancer Institute.
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
Footnotes
Author Contributions
All authors have been involved in the study design, analysis, and manuscript revision. All authors read and approved the final manuscript. Dr. Mathews is the guarantor who accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
R Mathews: Dr. Mathews had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Mathews contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
W Wang: Mr. Wang contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
LA Kaltenbach: Ms. Kaltenbach contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
L Thomas: Dr. Thomas contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
RU Shah: Dr. Shah contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
M Ali: Dr. Ali contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
ED Peterson: Dr. Peterson contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
TY Wang: Dr. Wang contributed to the conception and design of the study, the supervision, data acquisition, analysis and interpretation, the manuscript drafting, and the critical revision of the manuscript.
CONFLICT OF INTEREST DISCLOSURES
R Mathews: Dr. Mathews reports no relevant disclosures.
W Wang: Mr. Wang reports no relevant disclosures.
LA Kaltenbach: Ms. Kaltenbach reports no relevant disclosures.
L Thomas: Dr. Thomas reports no relevant disclosures.
RU Shah: Dr. Shah reports no relevant disclosures.
M Ali: Dr. Ali reports no relevant disclosures.
ED Peterson: Dr. Peterson reports grant support from American College of Cardiology, American Heart Association, Janssen; and consulting from Bayer, Boehringer Ingelheim, Merck, Valeant, Sanofi, Astra Zeneca, Janssen, Regeneron, Genentech.
TY Wang: Dr. Wang reports research funding from AstraZeneca, Gilead, Lilly, The Medicines Company, and Canyon Pharmaceuticals (all significant); educational activities or lectures (generates money for Duke) for AstraZeneca (modest); consulting (including CME) for Medco (modest) and American College of Cardiology (significant).
References
- 1.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 2.Hospital Compare. [Accessed April 17, 2017];Medicare.gov web site. http://www.medicare.gov/hospitalcompare/
- 3.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 4.Mathews R, Peterson ED, Honeycutt E, Chin CT, Effron MB, Zettler M, Fonarow GC, Henry TD, Wang TY. Early medication nonadherence after acute myocardial infarction: insights into actionable opportunities from the Treatment with ADP receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome (TRANSLATE) study. Circ Cardiovasc Qual Outcomes. 2015;8:347–356. doi: 10.1161/CIRCOUTCOMES.114.001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation. 2010;121:1455–1458. doi: 10.1161/CIRCULATIONAHA.109.904003. [DOI] [PubMed] [Google Scholar]
- 6.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Kronish IM, Ross JS, Zhao H, Muntner P. Impact of hospitalization for acute myocardial infarction on adherence to statins among older adults. Circ Cardiovasc Qual Outcomes. 2016;9:364–371. doi: 10.1161/CIRCOUTCOMES.115.002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seabury SA, Lakdawalla DN, Dougherty JS, Sullivan J, Goldman DP. Medication adherence and measures of health plan quality. Am J Manag Care. 2015;21:e379–389. [PubMed] [Google Scholar]
- 9.Jaskie S, Rodgers G. Current trends in U.S. cardiology practice. Trends Cardiovasc Med. 2014;24:350–359. doi: 10.1016/j.tcm.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Cherry SB, Benner JS, Hussein MA, Tang SS, Nichol MB. The clinical and economic burden of nonadherence with antihypertensive and lipid-lowering therapy in hypertensive patients. Value Health. 2009;12:489–497. doi: 10.1111/j.1524-4733.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 11.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 13.Miedema MD, Cohn JN, Garberich RF, Knickelbine T, Graham KJ, Henry TD. Underuse of cardiovascular preventive pharmacotherapy in patients presenting with ST-elevation myocardial infarction. Am Heart J. 2012;164:259–267. doi: 10.1016/j.ahj.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi C, Haynes RB. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;20:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger BB, Bosworth HB. Medication adherence: emerging use of technology. Curr Opin Cardiol. 2011;26:279–287. doi: 10.1097/HCO.0b013e328347c150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, Adams MB, Svetkey LP, Reed SD, Li Y, Dolor RJ, Oddone EZ. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med. 2009;151:687–695. doi: 10.1059/0003-4819-151-10-200911170-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson ED, Roe MT, Rumsfeld JS, Shaw RE, Brindis RG, Fonarow GC, Cannon CP. A call to ACTION (Acute Coronary Treatment and Intervention Outcomes Network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:491–499. doi: 10.1161/CIRCOUTCOMES.108.847145. [DOI] [PubMed] [Google Scholar]
- 18.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA NCDR Science and Quality Oversight Committee Data Quality Workgroup. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 19.National Cardiovascular Data Registry. [Accessed April 17, 2017];American College of Cardiology Quality Improvement for Institutions web site. https://www.ncdr.com/webncdr/action/
- 20.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 22.Shore S, Ho PM, Lambert-Kerzner A, Glorioso TJ, Carey EP, Cunningham F, Longo L, Jackevicius C, Rose A, Turakhia MP. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313:1443–1450. doi: 10.1001/jama.2015.2761. [DOI] [PubMed] [Google Scholar]
- 23.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 25.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155:772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Lincoff AM, Philippides GJ, Zidar JP American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e663–828. doi: 10.1161/CIR.0b013e31828478ac. [DOI] [PubMed] [Google Scholar]
- 29.Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach RG, Krumholz HM, Cohen DJ. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 30.Mehta RH, Montoye CK, Faul J, Nagle DJ, Kure J, Raj E, Fattal P, Sharrif S, Amlani M, Changezi HU, Skorcz S, Bailey N, Bourque T, LaTarte M, McLean D, Savoy S, Werner P, Baker PL, DeFranco A, Eagle KA American College of Cardiology Guidelines Applied in Practice Steering Committee. Enhancing quality of care for acute myocardial infarction: shifting the focus of improvement from key indicators to process of care and tool use: the American College of Cardiology Acute Myocardial Infarction Guidelines Applied in Practice Project in Michigan: Flint and Saginaw Expansion. J Am Coll Cardiol. 2004;43:2166–2173. doi: 10.1016/j.jacc.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Medicare & Medicaid Services (CMS) [Accessed April 17, 2017];CMS web site. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/index.html?redirect=/EHRIncentivePrograms. Updated November 22, 2016.
- 32.Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy Press; 2001. pp. 1–22. [PubMed] [Google Scholar]
- 33.Mehta RH, Chen AY, Alexander KP, Ohman EM, Roe MT, Peterson ED. Doing the right things and doing them the right way: association between hospital guideline adherence, dosing safety, and outcomes among patients with acute coronary syndrome. Circulation. 2015;131:980–987. doi: 10.1161/CIRCULATIONAHA.114.013451. [DOI] [PubMed] [Google Scholar]
- 34.Walker PC, Bernstein SJ, Jones JN, Piersma J, Kim HW, Regal RE, Kuhn L, Flanders SA. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009;169:2003–2010. doi: 10.1001/archinternmed.2009.398. [DOI] [PubMed] [Google Scholar]
- 35.van Veldhuisen DJ, Maass AH. Telemonitoring of outpatients with heart failure: a search for the holy grail? Circulation. 2012;125:2965–2967. doi: 10.1161/CIRCULATIONAHA.112.118141. [DOI] [PubMed] [Google Scholar]
- 36.Mathews R, Peterson ED, Honeycutt E, Chin CT, Effron MB, Zettler M, Fonarow GC, Henry TD, Wang TY. Early Medication Nonadherence After Acute Myocardial Infarction: Insights into Actionable Opportunities From the TReatment with ADP receptor iNhibitorS: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circ Cardiovasc Qual Outcomes. 2015;8:347–356. doi: 10.1161/CIRCOUTCOMES.114.001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley EH, Curry L, Horwitz LI, Sipsma H, Thompson JW, Elma M, Walsh MN, Krumholz HM. Contemporary evidence about hospital strategies for reducing 30-day readmissions: a national study. J Am Coll Cardiol. 2012;60:607–614. doi: 10.1016/j.jacc.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, Schwartz JS. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 39.Brush JE, Jr, Handberg EM, Biga C, Birtcher KK, Bove AA, Casale PN, Clark MG, Garson A, Jr, Hines JL, Linderbaum JA, Rodgers GP, Shor RA, Thourani VH, Wyman JF. 2015 ACC Health Policy Statement on Cardiovascular Team-Based Care and the Role of Advanced Practice Providers. J Am Coll Cardiol. 2015;65:2118–2136. doi: 10.1016/j.jacc.2015.03.550. [DOI] [PubMed] [Google Scholar]
- 40.Takeda A, Taylor SJ, Taylor RS, Khan F, Krum H, Underwood M. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;12:CD002752. doi: 10.1002/14651858.CD002752.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Längst G, Seidling HM, Stützle M, Ose D, Baudendistel I, Szecsenyi J, Wensing M, Mahler C. Factors associated with medication information in diabetes care: differences in perceptions between patients and health care professionals. Patient Pref Adherence. 2015;9:1431–1441. doi: 10.2147/PPA.S88357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linetzky B, Jiang D, Funnell MM, Curtis BH, Polonsky WH. Exploring the role of the patient-physician relationship on insulin adherence and clinical outcomes in type 2 diabetes: insights from the MOSAIc study. J Diabetes. 2017;9:596–605. doi: 10.1111/1753-0407.12443. [DOI] [PubMed] [Google Scholar]
- 43.Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, Krumholz HM, Horwitz LI. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316:2647–2656. doi: 10.1001/jama.2016.18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyal A, de Lemos JA, Peng SA, Thomas L, Amsterdam EA, Hockenberry JM, Peterson ED, Wang TY. Association of patient enrollment in Medicare Part D with outcomes after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2015;8:567–575. doi: 10.1161/CIRCOUTCOMES.115.001650. [DOI] [PubMed] [Google Scholar]
- 45.Maeng DD, Khan N, Tomcavage J, Graf TR, Davis DE, Steele GD. Reduced acute inpatient care was largest savings component of Geisinger Health System’s patient-centered medical home. Health Aff (Millwood) 2015;34:636–644. doi: 10.1377/hlthaff.2014.0855. [DOI] [PubMed] [Google Scholar]
- 46.Burwell SM. Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med. 2015;372:897–899. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.