Abstract
Background
Ongoing neuropathic pain is difficult to treat. We examined whether dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA), a peripherally acting mu-opioid receptor agonist, attenuates ongoing pain-associated manifestations after nerve injury in rats and mice.
Methods
Using conditioned place preference assay, we tested whether animals show a preference to the environment associated with drug treatment. Wide-dynamic range (WDR) and dorsal root ganglion (DRG) neuronal activities were measured by electrophysiology recording and calcium imaging.
Results
Nerve-injured animals stayed longer in DALDA-paired chamber after conditioning than during pre-conditioning (rats: 402.4 ± 61.3 versus 322.1 ± 45.0 s, 10 mg/kg, n=9, P = 0.009; mice: 437.8 ± 59.4 versus 351.3 ± 95.9 s, 2 mg/kg, n=8, P = 0.047). Topical ganglionic application of DALDA (5 μM, 1 μL, n=5) reduced the numbers of small-diameter DRG neurons that showed spontaneous activity (1.1 ± 0.4 versus 1.5 ± 0.3, P = 0.044) and that were activated by test stimulation (15.5 ± 5.5 versus 28.2 ± 8.2, P = 0.009) after injury. In neuropathic rats, DALDA (10 mg/kg, n=8) decreased spontaneous firing rates in WDR neurons to 53.2 ± 46.6% of pre-drug level, and methylnaltrexone (5 mg/kg, n=9) blocked DALDA-induced place preference and inhibition of WDR neurons. DALDA increased paw withdrawal threshold (17.5 ± 2.2 g) from baseline (3.5 ± 0.7 g, 10 mg/kg, n=8, P = 0.002) in nerve-injured rats, but the effect diminished after repeated administrations.
Conclusion
Peripherally acting mu-opioids may attenuate ongoing pain-related behavior and its neurophysiologic correlates. Yet, repeated administrations cause anti-allodynic tolerance.
Keywords: Ongoing pain, mu-opioid receptor, nerve injury, peripheral nervous system, dorsal root ganglion, spontaneous activity
Introduction
Approximately 25–30% of chronic pain in patients is neuropathic in nature, afflicting 7–8% of adults in the general population.1 These patients frequently experience ongoing pain and paroxysmal spontaneous pain, which are difficult conditions to treat. However, neuropathic pain-related behavior in animal models has commonly been inferred from observations of evoked reflex withdrawal responses to external stimuli. Clinically relevant behaviors suggestive of ongoing pain are more complex and have received less mechanistic investigation in preclinical studies. Because the underlying mechanisms and treatment strategies of ongoing pain may differ from those of evoked sensory hypersensitivity,2,3 animal behavioral assays that rely on reflex responses do not always correlate well with analgesic effectiveness in humans. Accordingly, new drug candidates for neuropathic pain should also be studied comprehensively in experimental paradigms that assess the efficacy, safety and mechanisms by which they inhibit ongoing pain.4–6
Improving opioid therapies for neuropathic pain remains a high research priority, largely because opioids are associated with severe central adverse effects that include sedation, addiction and abuse.7 Recent studies suggested that targeting mu-opioid receptors (MORs) in the peripheral nervous system (PNS) may alleviate pain hypersensitivity without causing central adverse effects.8,9 Dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA),10 which is derived from the natural heptapeptide MOR agonist dermorphin, is highly hydrophilic and exhibits a restrictive penetration into the central nervous system (CNS) after systemic administration.11,12 Our recent study showed that DALDA attenuated mechanical and heat hypersensitivities in nerve-injured rats.13 Primary afferent neurons may also be an attractive target for the development of novel therapies for ongoing pain. Afferent drive after injury including ectopic discharges, and spontaneous activity in dorsal root ganglion (DRG) and spinal wide-dynamic range (WDR) neurons are considered to be important neurophysiologic correlates of ongoing pain.14,15 One strategy would be to develop analgesics that act at spontaneously active sensory neurons and target “pain at its source” before the signals diverge over multiple pathways in the CNS.8,16,17 Yet, whether peripherally acting mu-opioids alleviate ongoing neuropathic pain and inhibit its neurophysiologic correlates remains unclear.
Developing novel analgesics based on preclinical data has been fraught with difficulties that are likely multifactorial. One factor may be the insufficient predictive ability of outcome measures used in animal models that are based solely on reflex responses. In this multidisciplinary study, we used non-reflex outcome measures including conditioned place preference (CPP), wheel running activity, and spontaneous activity in dorsal horn and DRG neurons, to assess the efficacy, safety, and cellular mechanisms by which DALDA inhibits ongoing pain-related manifestations in animal models of neuropathic injury. We further examined whether nerve-injured and naïve rats develop anti-allodynic tolerance and opioid-induced hyperalgesia (OIH) to DALDA after repeated systemic drug administrations. Our findings from complementary behavioral, electrophysiologic, and high-throughput in vivo calcium imaging studies suggest that acute systemic administration of DALDA, a peripherally acting MOR agonist, suppresses spontaneously active small-diameter DRG and dorsal horn WDR neurons, and induces peripheral inhibition of ongoing pain-related manifestations without adverse effects. However, long-term drug treatment may lead to the development of anti-allodynic tolerance and OIH.
Materials and Methods
Animals
The animal behavioral and electrophysiology studies were conducted in adult male Sprague-Dawley rats (250–300 g, Harlan Bioproducts for Science, Indianapolis, IN) and C57BL/6 mice (20–30 g, Jackson Laboratory, Bar Harbor, ME). For GCaMP calcium imaging, we utilized male and female pirt-GCaMP6 mice, which we generated by crossing Rosa26-loxP-STOP-loxP-GCaMP6 mice with pirt-cre mice. These mice exhibit GCaMP6 expression specifically in DRG and trigeminal ganglion neurons.18 Animals were housed under optimal laboratory conditions with a 12-h light/dark cycle and free access to food and water. Animals were acclimatized to laboratory conditions before the tests. All behavioral experiments were carried out between 9:00 a.m. and 5:00 p.m. by an investigator blinded to the drug assignment. The experimental protocols were approved by the Animal Care and Use Committee of Johns Hopkins University and complied with the National Institutes of Health Guide for the Use of Experimental Animals to ensure minimal animal use and discomfort.
Drugs
DALDA was purchased from US Biologicals (Salem, MA). Loperamide hydrochloride, methylnaltrexone bromide and 2-hydroxypropyl-beta-cyclodextrin (CDEX) were purchased from Sigma-Aldrich (St. Louis, MO). Loperamide was dissolved in 20% CDEX, made by diluting the 40% CDEX/water solution (isotonic) with saline. Other drugs were purchased from Tocris Bioscience (Bristol, UK). Stock solutions were freshly prepared as instructed by the manufacturer.
Neuropathic pain models
L5 spinal nerve ligation (SNL) surgery was used for the induction of neuropathic pain in rats. The procedure was a modification of that described in our previous studies.19,20 Briefly, rats were anesthetized with isoflurane (2%, Abbott Laboratories, North Chicago, IL) delivered through a nose cone. Under aseptic conditions, the skin was incised at the midline over the lumbar spine, and the L5, L6, and upper sacral vertebrae were exposed. The left transverse process of the L6 vertebra was removed, and the left L5 spinal nerve was exposed and dissected from the underlying tissue with fine forceps. The left L5 spinal nerve was then tightly ligated with a 6-0 silk suture and cut distally. The muscle layer was approximated with 4-0 silk suture and the skin closed with metal clips. After the surgery, the rats were returned to their cages, kept warm under a heat lamp, and monitored during recovery.
The sciatic chronic constriction injury (CCI) model was produced in mice as previously described.21,22 Briefly, mice were anesthetized with isoflurane (2%, Abbott Laboratories, North Chicago, IL) delivered through a nose cone. Under aseptic conditions, one side of the sciatic nerve was exposed at mid-thigh level by blunt dissection through the biceps femoris muscle. The sciatic nerve was further separated from the surrounding tissue and loosely tied with three nylon sutures (9-0 nonabsorbable monofilament, S&T AG, Neuhausen, Switzerland). The distance between two adjacent ligatures was approximately 0.5 mm. After hemostasis was confirmed, the muscle layer was closed with a 6-0 silk suture, and the skin was stapled.
Animal behavioral tests
CPP
Animals were habituated for 30 min per day in an automated 3-chamber box. During habituation, they had access to all chambers. The two larger chambers of this apparatus contained distinct visual (vertical stripes vs. triangular shapes) and tactile (smooth floor vs. grooved floor) cues. The third, smaller chamber was interposed between the other two and was devoid of overt spatial cues. On the pre-conditioning day, behavior was video recorded for 15 min while the animal was again free to explore all three chambers. The results were used to quantify any basal chamber preference or aversion in individual mice. In keeping with previous studies,2,21 animals that spent more than 80% (>720 s) or less than 20% (< 120 s) of the total time in any given chamber were eliminated from further testing. The next day, animals received vehicle treatment in the morning session without anesthesia 10 min before being placed in one of the conditioning chambers for 45 min. Four hours later, the same animals received drug treatment (e.g., subcutaneous [s.c.] DALDA: 5, 10 mg/kg; s.c loperamide: 5 mg/kg) and after 10 min were restricted to the opposite conditioning chamber for 45 min. On the post-conditioning test day, animals were placed in the same 3-chamber box with access to all chambers but received no injection. Their behavior was recorded for 15 min and used to examine chamber preference or aversion. Pairing of drug or vehicle with a given chamber was counterbalanced between groups. An increase in post-conditioning time spent in the drug-paired chamber, as compared with pre-conditioning time in the same chamber, indicated CPP. Difference scores were calculated by subtracting pre-conditioning time from post-conditioning time.
von Frey hair test in rats and mice
To assess mechanical hypersensitivity to punctuate mechanical stimuli in rats, we measured paw withdrawal threshold (PWT) to von Frey filaments (Stoelting Co., Wood Dale, IL, USA). Each filament (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, 13.1 g) was applied for 4 to 6 s to the test area between the footpads on the plantar surface of the hindpaw according to the up-down method as described previously.13,23 The 1.83-g stimulus was applied first. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next higher force was used. The test was continued until: (1) the responses to five stimuli were assessed after the first crossing of the withdrawal threshold, or (2) the upper/lower end of the von Frey hair set was reached before a positive/negative response had been obtained. Abrupt paw withdrawal, licking, and shaking were regarded as positive responses. The pattern of positive and negative responses was converted to a 50% threshold value using the formula provided by Dixon.24 To assess mechanical allodynia in mice, we applied calibrated von Frey monofilaments (0.1 and 0.45 g; Stoelting Co.) to the hind paw for approximately 1 s. Each stimulation was repeated 10 times to both hind paws, and paw withdrawal frequency (PWF) was determined as described previously.25
Hargreaves test
To test for signs of heat hypersensitivity, we used the Hargreaves test,26 which measures paw-withdrawal latency (PWL) to radiant heat stimuli. Animals were placed under individual plastic boxes on a heated glass floor (30°C) and allowed to habituate for at least 30 min before testing. Radiant heat was applied to the plantar surface of each hind paw three times at 3–5-min intervals with a plantar stimulator analgesia meter (IITC model 390, Woodland Hills, CA). PWLs were measured three times by an electronic timer, with at least 2 min between trials. A cut-off time of 20 s (rat) or 30 s (mouse) was used to avoid sensitization and damage to the skin. The average PWL of the three trials was used for data analysis.
Open field test
The open field test was used to assess the effect of systemic drug administration on spontaneous exploration and locomotor activity of the rats.13 Rats were placed in an open field chamber (73 × 45 cm rectangular plastic box with a wall height of 33 cm) for 10 min. We analyzed parameters such as total distance travelled; mean travel speed; and number of border periphery, internal periphery, and center crossings in video recordings using SMART 3 software (Panlab Harvard Apparatus, Barcelona, Spain).
Wheel running activity
Voluntary activity of the mice was measured inside their cages by using Spontaneous Activity Wheels from Bioseb (Pinellas Park, USA). These wheels have an LCD display and attach to a computer for data generation and analysis. Measurements included the distance run both ways; the number of wheel turns; average, minimum, and maximum speed; acceleration; and total time in the wheel as shown in a previous study.27
Induction of opioid tolerance
We used a modified paradigm in which repeated systemic drug injections were used to induce opioid tolerance in SNL rats.28 On days 5–9 post-SNL, we tested the rats to obtain baseline PWTs in the morning. Then we injected DALDA (10 mg/kg, s.c.) or saline (s.c.) and repeated the PWT tests after 30 min. In the afternoon of days 5–8 post-SNL, we administered the same dose of DALDA without conducting behavior tests.
Induction of OIH in naive rats
We used a modified paradigm to examine OIH in naïve rats.29 We treated rats with systemic, fixed-dose DALDA (10 mg/kg, s.c.) or saline once daily for 10 consecutive days. Each day, we measured thermal nociceptive threshold PWL and mechanical PWT before drug injection (baseline), and at 30 min and 60 min after injection, to evaluate OIH and tolerance.
Constipation assay
Rats were housed and habituated for 3 days in individual metabolic cages (Harvard Apparatus, Holliston, MA) with known amount of food and water. Each rat then received twice-daily subcutaneous injections of loperamide (5 mg/kg), DALDA (10 mg/kg) or vehicle (n=5/group) for 3 consecutive days. Metabolic cages allow a separation of feces and urine and measurement of food and water intake. We measured the daily number of stools produced, urine volume, and amount of food intake at baseline and on each day of drug treatment.
Colon motility assay
Rats received subcutaneous injections of loperamide (5 mg/kg), DALDA (10 mg/kg), or vehicle (n=5/group) twice-daily for 3 days. The distal colon motility assay was performed before drug treatment (baseline) and at 30–45 min after the first drug or vehicle injection on days 1 and 3. In each test, a 2-mm glass bead was gently inserted approximately 3 cm into the rectum of rats under brief and light anesthesia with isoflurane (1 %) to avoid stress and discomfort. The animal was then placed in a white box and allowed to wake up. We recorded the time required for the glass bead to be expelled after insertion (i.e., expulsion time).
Spinal dorsal horn recordings of WDR neurons
We conducted extracellular recordings of single dorsal horn neuronal activity as described in our previous studies.13,23 Briefly, a laminectomy was performed at vertebral levels T12–L1 corresponding to lumbar enlargements at spinal segments L3–S1. During neurophysiologic recording, animals were anesthetized with 1.5% isoflurane and paralyzed by an intraperitoneal (i.p.) injection of pancuronium bromide (0.15 mg/kg, Elkins-Sinn Inc., Cherry Hill, NJ) to facilitate controlled ventilation. WDR neurons with defined receptive fields in the plantar region of the hind paw were selected. WDR cells were identified by their characteristic responses to mechanical test stimulation at the skin receptive field,30,31 and spontaneous activity of the neurons was examined before and after drug treatment. Analog data were collected with a real-time, computer-based data acquisition and processing system (CED Spike 2, Cambridge, UK).
Calcium imaging
Adult male and female pirt-GCaMP6 mice were anesthetized with 1.5% isoflurane, and the lumber L4 DRG ipsilateral to the nerve injury was exposed as described in our previous study.18 Care was taken not to remove the epineurium. Animals were placed under a 5.0× long-working distance objective lens of a confocal microscope (Leica LSI, Wetzlar, Germany) for imaging. Time-lapse z-stacks of the intact DRG were acquired at 8 s/frame at 512 × 512 pixel resolution. Body temperature of the mice was maintained at 37.0°C for the duration of the imaging experiment. Electrical test stimulation (2 mA, 0.2 ms, 1 Hz) high enough to activate both A- and C-fibers was delivered through a pair of 30 gauge transdermal needles inserted into the glabrous skin of the hind paw. The train burst width was 8 s (time it takes to record one z-stack). Drug (DALDA, 5μM) or vehicle at a volume of 1 μL was applied directly to the DRG while recording was in progress. After 24 s of baseline imaging the test stimulus was applied to the hind paw for evoked response to electrical stimulation. For spontaneous activity recording, anesthesia level was reduced to 1% isoflurane and the DRG was imaged continuously for 5 min with no stimulation. Raw TIFFs were exported and analyzed with ImageJ (NIH, Bethesda, MD) as previously described.18 An experimenter manually traced activated cells and determined cell size and relative fluorescent intensity. Calcium signal amplitudes were expressed as a ratio of fluorescence difference to basal fluorescence (ΔF/Fo). Small, medium, and large diameter neurons were defined as having somal areas of <450 μm2, 450–700 μm2, and >700 μm2, respectively.
Statistical analysis
The number of animals used in each study was based on our experience with similar studies.13,21 We randomized animals to the different treatment groups and blinded the experimenter to drug treatment to reduce selection and observation bias. None of the variables had missing data. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was used to conduct all statistical analyses. Two-way repeated measures ANOVA was used for statistical comparison in the CPP test. Two-way mixed model ANOVA was used for statistical comparison in the wheel running activity, open field test, colon motility assay, electrophysiology recording of WDR neurons, and DALDA tolerance assay. One-way repeated measures ANOVA was used for statistical comparison in the GCaMP imaging and opioid-induced hyperalgesia studies. The specific method for statistical comparison in each study is also given in the figure legends. A Bonferroni post hoc test was used to compare specific data points. Data are expressed as mean ± SD. All tests were two-tailed and P < 0.05 was considered significant in all tests.
Results
Peripherally acting MOR agonists induce CPP in nerve-injured rats
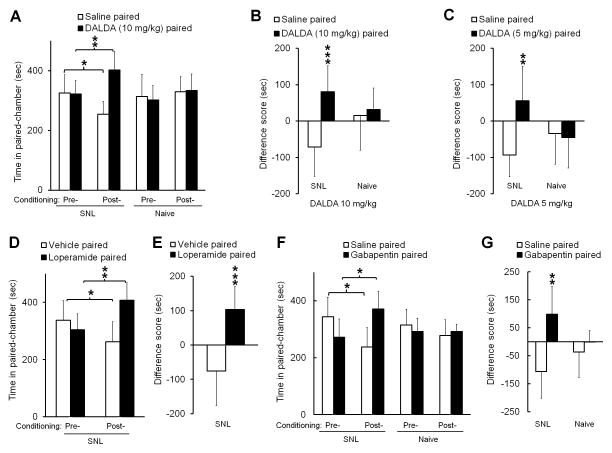
Relief of the aversive state associated with ongoing pain may be rewarding and motivate animals to seek a context associated with that relief (negative reinforcement), a behavior that can be assessed by CPP.6,32,33 Accordingly, we first examined whether subcutaneous injection of DALDA at 5 mg/kg and 10 mg/kg, doses known to alleviate neuropathic mechanical and heat hypersensitivity,13 induced CPP in rats at 2 week after an L5 SNL. This time point corresponds to the peak maintenance phase of neuropathic pain in this model.30 SNL rats spent more time in the DALDA-paired chamber in the post-conditioning period (402.4 ± 61.3 s, 10 mg/kg, n=9) than in the pre-conditioning period (322.1 ± 45.0 s, P = 0.009, Fig. 1A). In addition, SNL rats spent less time in the saline-paired chamber in the post-conditioning period (253.8 ± 42.3 s) than during pre-conditioning (325.5 ± 60.1 s, P = 0.027, Fig. 1A). At both 10 mg/kg and 5 mg/kg (n=8) doses, the difference scores for the DALDA-paired chambers were significantly greater than those for the saline-paired chambers (10 mg/kg: 80.41 ± 71.9 s versus −71.7 ± 79.8 s, P < 0.001; 5 mg/kg: 56.1 ± 94.3 s versus −93.5 ± 59.8 s, P = 0.002, Fig. 1B, C). Importantly, systemic DALDA (10 mg/kg) did not induce CPP in naive rats, which spent similar amount of time in the DALDA-paired chamber in the post- and pre-conditioning period (333.4 ± 55.6 s versus 301.9 ± 48.7 s, P = 0.176, n=8, Fig. 1A).
Figure 1. Systemic administration of peripherally acting mu-opioid receptor agonists induces conditioned place preference (CPP) in nerve-injured rats.
(A) SNL rats spent more time in a DALDA-paired chamber after drug conditioning (10 mg/kg, s.c., n=9) than during the preconditioning test period. However, DALDA did not induce CPP in naive rats (n=8). (B) In SNL rats, the difference score (post-conditioning time – pre-conditioning time) for the DALDA-paired chamber (n=9) was significantly greater than that for the saline-paired chamber. (C) A lower dose of DALDA (5 mg/kg, s.c., n=8) also significantly increased the difference score for the DALDA-paired chamber in SNL rats, but not naïve rats (n=8). (D) Loperamide (5 mg/kg, s.c., n=8), another peripherally acting opioid, induced CPP in SNL rats. (E) The difference score for the loperamide-paired chamber was significantly greater than that for the vehicle-paired chamber. (F) SNL rats spent more time in chambers paired with gabapentin (60 mg/kg, i.p., n=8) after drug conditioning than they did before conditioning. However, gabapentin did not induce CPP in naive rats (n=8). (G) The difference score for the gabapentin-paired chamber was significantly greater than that for the saline-paired chamber. A, D, F: Two-way repeated measures ANOVA with Bonferroni post hoc test, *P < 0.05, ** P < 0.01. B, C, E, G: Student’s t-test, **P < 0.01, ***P < 0.001 versus saline-paired. Data are expressed as mean ± SD. ANOVA, analysis of variance; DALDA, dermorphin [D-Arg2, Lys4] (1–4) amide; i.p., intraperitoneal; s.c., subcutaneous; SNL, spinal nerve ligation.
Another peripherally acting MOR-preferring agonist, loperamide (5 mg/kg, s.c., n=8),9,19 also induced CPP in SNL rats, as indicated by the significant increase in time spent in the drug-paired chamber after conditioning (407.5 ± 60.9 s), as compared to the pre-conditioning period (304.3 ± 55.7 s, P = 0.003, Fig. 1D). The difference score for the loperamide-paired chambers (103.2 ± 66.8 s) was also greater than that for the vehicle-paired chambers (−75.4 ± 100.4 s, P < 0.001, Fig. 1E). Gabapentin (60 mg/kg, i.p., n=8), which has been used clinically to attenuate neuropathic pain, was included as a positive drug control. SNL rats spent more time in the gabapentin-paired chamber during the post-conditioning period (370.8 ± 61.9 s) than during pre-conditioning period (272.1 ± 64.1 s, P = 0.026, Fig. 1F). In addition, they spent less time in the saline-paired chamber in the post-conditioning period (237.6 ± 68.2 s) than in the pre-conditioning period (344.1 ± 68.5 s, P = 0.016). The difference score for the gabapentin-paired chambers (98.9 ± 99.4 s) was significantly greater than that for the saline-paired chambers among SNL rats (−106.5 ± 95.3 s, P = 0.008, Fig. 1G). Gabapentin did not induce CPP in naive rats (n=8).
DALDA induces CPP and improves wheel running performance in nerve-injured mice and does not affect gastrointestinal motility or exploratory activity
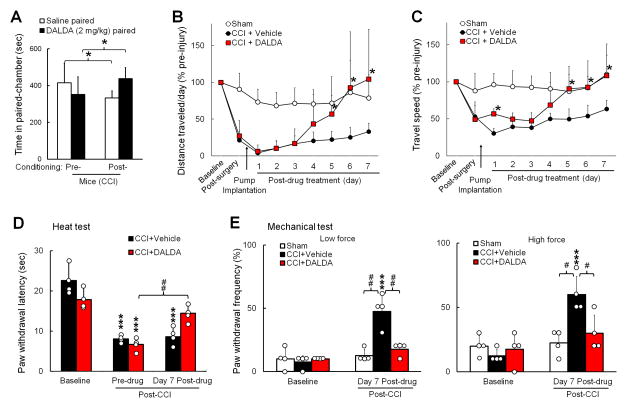
To extend our findings to other species, we conducted the CPP test in mice at 1 week after CCI of the sciatic nerve. Similar to SNL rats, CCI mice spent more time in the DALDA-paired chamber after conditioning with a bolus injection of DALDA (2 mg/kg, s.c.), as compared to that in the pre-conditioning period (437.8 ± 59.4 s versus 351.3 ± 95.9 s, n=8, P = 0.047, Fig. 2A). In addition, CCI mice spent less time in the saline-paired chamber in the post-conditioning period (332.3 ± 38.2 s) than in the pre-conditioning period (415.5 ± 102.7 s, P = 0.034, Fig. 2A).
Figure 2. Systemic administration of dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA) reduces neuropathic-pain related behavior and improves wheel running performance in nerve-injured mice.
(A) Mice that underwent chronic constriction injury (CCI) of the sciatic nerve spent more time in the DALDA-paired chamber after conditioning with DALDA (2 mg/kg, s.c., n=8). Two-way repeated measures ANOVA with Bonferroni post hoc test, *P < 0.05. (B) DALDA (3 mg/kg/day, s.c.) or vehicle (n=6/group) was delivered continuously to CCI mice through an implanted osmotic pump for 7 days. The distance traveled each day on the wheel decreased sharply after CCI, as compared to that after sham surgery (n=5). The distance traveled by CCI mice showed a greater increase from day 5 after DALDA than after vehicle treatment. (C) The average speed on the wheel after CCI and DALDA treatment. B–C: Two-way mixed model ANOVA with Bonferroni post hoc test, *P < 0.05 versus vehicle-treated CCI group. (D) A subgroup of animals was tested for mechanical and heat sensitivity. The decrease in paw withdrawal latency to heat stimulation after CCI was increased from pre-drug level on day 7 by DALDA, but not by vehicle (n=4/group). (E) The increased paw withdrawal frequency of CCI mice to mechanical stimulation (von Frey, 0.1 g, 0.47 g) was decreased by DALDA. D–E: Two-way mixed-model ANOVA with Bonferroni post hoc test, ***P < 0.001versus baseline, #P < 0.05, ##P < 0.01, versus the indicated group. Data are expressed as mean ± SD. ANOVA, analysis of variance; s.c., subcutaneous.
Changes in voluntary wheel running may constitute an indicator of daily wellbeing and an objective way to measure the overall impact of aversive state after injury in rodents.27 Continuous DALDA (3 mg/kg/day, s.c., n=6) or vehicle (n=6) was delivered to mice with an implanted osmotic pump for 7 consecutive days beginning on day 7 post-CCI. Wheel running activity (distance and speed of travel) was sharply decreased in mice after CCI, as compared to that after sham surgery (n=5). CCI mice that received DALDA ran longer distances and traveled at higher speeds on days 5–7 post-treatment than those that received vehicle (Fig. 2B, C). In a subgroup of mice that received sensory tests (n=4/group), the ipsilateral PWL to heat stimulation was significantly decreased from pre-injury baseline (vehicle: 22.5 ± 3.6 s; DALDA: 17.8 ± 2.4 s) on day 7 post-CCI (vehicle: 8.1 ± 0.9 s, P < 0.001; DALDA: 6.7 ± 1.6 s, P < 0.001, Fig. 2D). However, PWL in CCI mice after 7 days of DALDA treatment (14.4 ± 2.1 s) was significantly increased from pre-drug level (P = 0.008). In contrast, PWL in CCI mice after vehicle treatment (8.6 ± 2.2 s) remained significantly decreased from pre-injury baseline (P < 0.001). The ipsilateral PWF to mechanical stimuli in CCI mice at day 7 after receiving vehicle treatment (von Frey, 0.1 g: 47.5 ± 12.6%; 0.47 g: 60.0 ± 14.1%) was significantly increased from pre-injury baseline (0.1 g: 7.5 ± 5%, P < 0.001; 0.47 g: 12.5 ± 5.0%, P < 0.001, Fig. 2E). In contrast, on day 7 after DALDA treatment, the PWF of CCI mice (0.1 g: 17.5 ± 5.0%; 0.47 g: 30.0 ± 14.1%) was comparable to that at pre-injury baseline (0.1 g: 10.0 ± 0%, P = 1.0; 0.47 g: 17.5 ± 12.6%, P = 1.0, Fig. 2E) and in the sham-operated group (0.1 g: 12.5 ± 5.0%; 0.47 g: 22.5 ± 9.6%). PWF in CCI mice on day 7 after DALDA treatment was also significantly lower than that after vehicle treatment (0.1 g: P = 0.003; 0.47 g: P = 0.041).
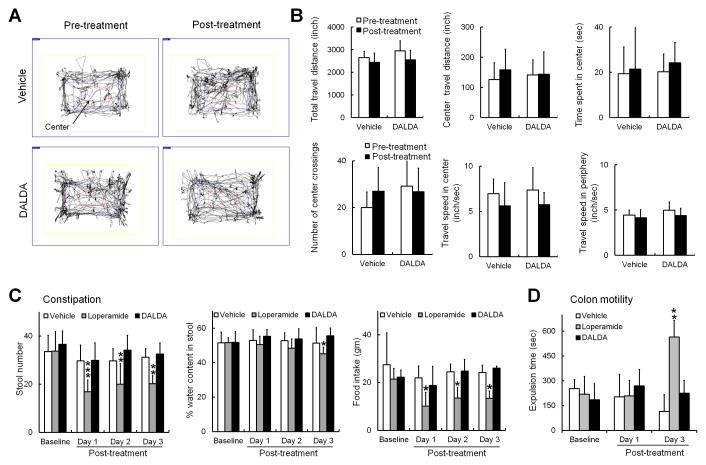
We further tested whether the dose of DALDA that induced CPP caused motor incoordination or constipation, two well-known mu-opioid-related side effects. Similar to our previous findings in the open field test, total distance traveled (in 10 min), center travel distance, time spent in the center, number of center crossings, and travel speed in the center and periphery were similar in SNL rats before and 45 min after one bolus injection of DALDA (10 mg/kg, s.c., n=6, Fig. 3A, B), and were comparable to those of the saline-treated group (n=6). We then examined whether repeated systemic injection of loperamide (5 mg/kg, b.i.d, s.c.), DALDA (10 mg/kg, b.i.d., s.c.) or vehicle (n=6/group) for 3 consecutive days induced constipation or impaired distal colon motility. Compared to pre-drug baseline, loperamide significantly decreased the daily number of fecal pellets (baseline: 33.8 ± 8.1; day 1: 16.8 ± 4.8, P < 0.001; day 2: 20 ± 8.5, P = 0.006; day 3: 20.2 ± 3.3, P = 0.007) and food intake (baseline: 21.5 ± 4.5g; day 1: 10.2 ± 5.8, P = 0.032; day 2: 13.5 ± 4.5g, P = 0.045; day 3: 13.4 ± 3.1 g, P = 0.029, Fig. 3C), suggesting the development of constipation. DALDA did not induce significant changes in these outcome measures from pre-drug baseline. We further examined distal colon motility after three twice-daily treatments with loperamide (5 mg/kg), DALDA (10 mg/kg), or vehicle (n=5/group). Loperamide significantly increased the expulsion time on day 3 (565.0 ± 78.3 s, P = 0.005, Fig. 3D), as compared to baseline (219.7 ± 97.1 s). In contrast, DALDA did not significantly change the time for bead expulsion on day 3 (225.0 ± 99.6 s), as compared to that on day 1 (185.7 ± 106.3 s, P = 1.0).
Figure 3. Systemic administration of dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA) does not reduce exploration activity or induce constipation.
(A) Examples of SNL rat exploration activity during a 10 min period in the open field test before and 45 min after administration of vehicle (saline) or DALDA (10 mg/kg, s.c.). (B) Neither DALDA (10 mg/kg, s.c., n=6) nor saline (n=6) significantly impaired the exploration activity of SNL rats. (C) Systemic administration of loperamide (5 mg/kg, b.i.d, s.c.), but not DALDA (10 mg/kg, b.i.d, s.c.) or vehicle (n=6/group), for 3 consecutive days significantly decreased the daily number of fecal pellets, water content of pellets (day 3), and food intake of rats. (D) Distal colon motility was examined after 3 days of treatment (b.i.d., s.c.) with loperamide (5 mg/kg), DALDA (10 mg/kg), or vehicle. The expulsion time of a glass bead was significantly increased on day 3 in the loperamide-treated group, but not in the DALDA-treated group (n=5/group). Two-way mixed model ANOVA with Bonferroni post hoc test, *P < 0.05, **P < 0.01, ***P < 0.001 versus baseline. Data are expressed as mean ± SD. ANOVA, analysis of variance; b.i.d., twice per day; s.c., subcutaneous; SNL, spinal nerve ligation.
DALDA inhibits spontaneous activity in small-diameter DRG neurons and in spinal WDR neurons after nerve injury
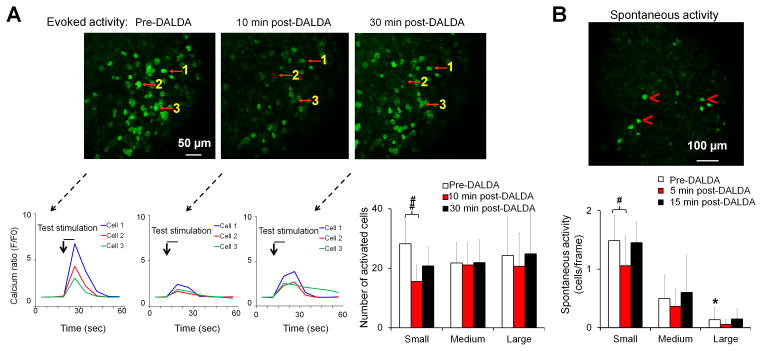
After nerve injury, a subpopulation of DRG neurons may develop spontaneous activity and ectopic discharge, the neurophysiologic correlates of spontaneous pain. We directly tested whether DALDA attenuates both spontaneous and evoked activities in primary sensory neurons in pirt-GCaMP6 mice after nerve injury.18 Electrical test stimulation (2 mA, 0.2 ms, 1 Hz, 8-s burst) was delivered to pirt-GCaMP6 mice through transdermal needles inserted into the glabrous skin of the hind paw, and the cell sizes of activated neurons were recorded. Small, medium, and large diameter neurons were defined as having somal areas of <450 μm2, 450–700 μm2, and >700 μm2, respectively. At 5–7 days after sciatic CCI in mice, topical application of DALDA (5 μM, 1 μL) to the lumbar L4 DRG reduced the evoked activities in small-diameter neurons at 10 min (15.5 ± 5.5), as compared to those at pre-drug baseline (28.2 ± 8.2, P = 0.009, n=5 experiments, Fig. 4A). DALDA did not significantly alter the evoked activities in medium- and large-diameter neurons (baseline: 24.3 ± 12.9, 10 min: 20.7 ± 11.2, P = 1.0). In CCI mice before drug treatment, the spontaneous activity (cells/frame) was greater in small-diameter (1.5 ± 0.3) than in large-diameter neurons (0.1 ± 0.2, P = 0.037, Fig. 4B). The small-diameter neurons showed a significant reduction in spontaneous activity at 5 min after DALDA treatment (1.1 ± 0.4, P = 0.044, Fig. 4B).
Figure 4. Topical application of dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA) attenuates both evoked and spontaneous activity in DRG neurons.
(A) Upper: GCaMP6 calcium imaging shows that topical application of DALDA (5 μM, 1 μL) to the lumbar DRG of pirt-GCaMP6 mice with chronic constriction injury (CCI) reduced the activity in small-diameter DRG neurons evoked by pinch stimulation at the ipsilateral hindpaw. Lower: Representative traces from three cells (red arrows) show that DALDA attenuated the calcium increase evoked by test pulses. Population data of evoked calcium activity in small-, medium-, and large-diameter neurons (n=5 experiments). (B) Upper: Topical application of DALDA (5 μM, 1 μL, n=5 experiments) to lumbar DRG also inhibited spontaneous activity mostly in small-diameter neurons (red arrowheads) of CCI mice. Lower: Population data of spontaneous activity in small-, medium-, and large-diameter DRG neurons. Data are shown as the number of activated neurons divided by the number of frames. One-way repeated measures ANOVA with Bonferroni post hoc test. #P < 0.05, ##P < 0.01 versus the indicated group, *P < 0.05 versus small-diameter neuron. Data are expressed as mean ± SD. ANOVA, analysis of variance; DRG, dorsal root ganglion.
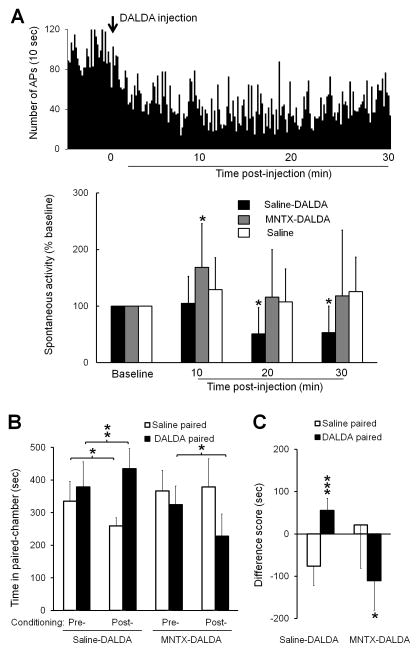
To further examine the site of action and cellular mechanisms by which systemic DALDA inhibits ongoing pain, we performed in vivo electrophysiologic studies to examine changes in spinal WDR neuronal response. WDR neurons receive converging afferent inputs in A-fibers and C-fibers and develop spontaneous activity after nerve injury. Systemic administration of saline (n=8) in SNL rats did not change spontaneous activity of WDR neurons. However, systemic administration of DALDA (10 mg/kg, s.c., n=8) significantly reduced the spontaneous firing rate of WDR neurons to 51.2 ± 46.4% (P = 0.039) and 53.2 ± 46.6% (P = 0.047) of pre-drug baseline at 20 min and 30 min post-treatment (Fig. 5A). Pretreatment with methylnaltrexone (5 mg/kg, n=9, i.p.), a peripherally acting MOR-preferring antagonist, 10 min before DALDA treatment, blocked the inhibitory effect of DALDA on spontaneous activity of WDR neurons in SNL rats (Fig. 5A).
Figure 5. Effects of methylnaltrexone on spontaneous activity of dorsal horn neurons and CPP to dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA) in nerve-injured rats.
(A) Upper: Peri-stimulus time histogram shows the spontaneous activity of a WDR neuron in an SNL rat before and 0–30 min after DALDA treatment (10 mg/kg, s.c.). Bin size: 10 s. Lower: The spontaneous activity rates of WDR neurons in SNL rats were significantly decreased at 20 and 30 min after treatment with DALDA (10 mg/kg, s.c., n=8), but not saline (n=8). The inhibitory effect of DALDA was blocked by methylnaltrexone (5 mg/kg, n=9, i.p., 10 min pretreatment). Two-way mixed-model ANOVA with Bonferroni post hoc test, *P < 0.05 versus baseline. (B) Pretreatment with methylnaltrexone (5 mg/kg, n=9, 10 min, i.p.), but not saline (n=7), blocked CPP to DALDA (10 mg/kg, s.c.) in SNL rats. (C) The difference scores of time spent in the DALDA-paired and saline-pared chambers. Two-way repeated measures ANOVA with Bonferroni post hoc test, *P < 0.05, **P < 0.01, ***P < 0.001 versus the indicated group or the saline-paired chamber. Data are expressed as mean ± SD. ANOVA, analysis of variance; APs, action potentials; CPP, conditioned place preference; i.p., intraperitoneal; MNTX, methylnaltrexone; s.c., subcutaneous; SNL, spinal nerve ligation; WDR, wide-dynamic range.
In behavioral tests, pretreatment of SNL rats with methylnaltrexone (5 mg/kg, 10 min, i.p.), but not saline, also blocked CPP to DALDA (10 mg/kg, s.c., Fig. 5B). In the saline-pretreated group, SNL rats spent more time in the DALDA-paired chamber after conditioning with DALDA (434.7 ± 61.6 s), as compared to that during pre-conditioning (379.0 ± 77.1 s, P = 0.005, n=7, Fig. 5B). These rats also spent less time in the saline-paired chamber in the post-conditioning period (259.1 ± 24.9 s) than during pre-conditioning (334.9 ± 60.9 s, P = 0.011). In addition, the difference score of time spent in the DALDA-paired chamber (55.7 ± 28.7 s) was significantly higher than that in saline-paired chambers (−75.9 ± 46.3 s, P < 0.001, Fig. 5C). In contrast, the difference score in the methylnaltrexone-pretreated group (−110.3 ± 69.9 s) was significantly lower than that in the saline-paired chambers (21.3 ± 102.2 s, n=9, P = 0.034). SNL rats pretreated with methylnaltrexone spent significant less time in the DALDA-paired chamber after conditioning (227.5 ± 68.2 s) than during pre-conditioning (324.2 ± 56.9 s, P = 0.011).
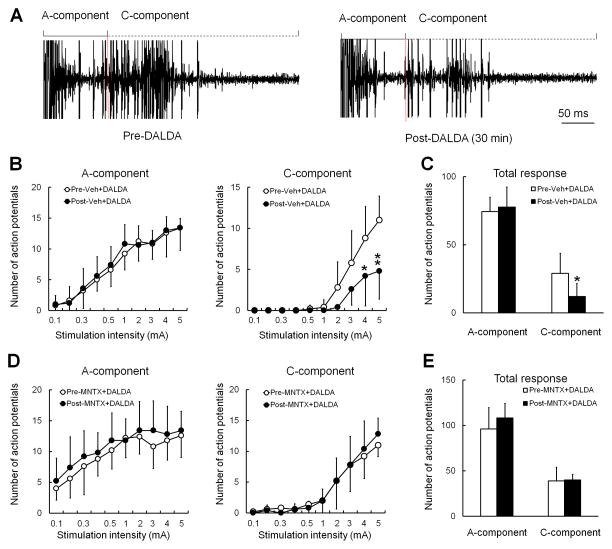
Unlike in nerve-injured rats, WDR neurons in naïve rats rarely showed spontaneous activity, similar to what we have reported previously.30 The evoked responses of WDR neurons to a supra-threshold electrical stimulus were separated into a short latency A-fiber component (0–100 ms) and a longer latency C-fiber component (100–500 ms, Fig. 6A). In naïve rats, DALDA (10 mg/kg, s.c., n=8) attenuated the stimulus-response function (Fig. 6B), and significantly decreased the total C-component [12.1 ± 8.4 action potentials (APs)] of WDR neurons to graded intracutaneous electrical stimuli (0.1–5 mA, 2 ms) from baseline (29.2 ± 13.1 APs, P = 0.024, Fig. 6C). The A-component was not changed by DALDA. Pretreatment with methylnaltrexone (5 mg/kg, i.p., n=6) 10 min before DALDA blocked DALDA-induced inhibition of the C-component (Fig. 6D,E).
Figure 6. Systemic dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA) reduces the evoked C-component of WDR neurons to electrical stimulation in naïve rats.
(A) An analog recording of WDR neuronal responses to an intracutaneous electrical stimulus (supra-C-fiber activation threshold, 2 ms) before and after systemic DALDA (10 mg/kg, i.p.). WDR neuronal responses display A- and C-components to the test stimulus. (B) The stimulus-response (S-R) functions of the A- and C-components of WDR neuronal response to graded intracutaneous electrical stimuli (0.1–5 mA, 2 ms) before and 30–45 min after systemic injection of vehicle (15-min pretreatment) with DALDA (10 mg/kg, n=6, i.p.). (C) The total A- and C-component to graded electrical stimuli before and after systemic injection of vehicle with DALDA. (D) The S-R functions of the A-component and C-component before and after systemic injection of methylnaltrexone (5 mg/kg, i.p.) with DALDA (10 mg/kg, n=6, i.p.). (E) The total A- and C-component to graded electrical stimuli before and after systemic injection of methylnaltrexone with DALDA. B,D: Two-way repeated measures ANOVA with Bonferroni post hoc test. C, E: paired t-test. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 versus pre-drug. ANOVA, analysis of variance; MNTX, methylnaltrexone.
Resiniferatoxin pretreatment reduces spontaneous activity of WDR neurons and precludes CPP to systemic DALDA in nerve-injured rats
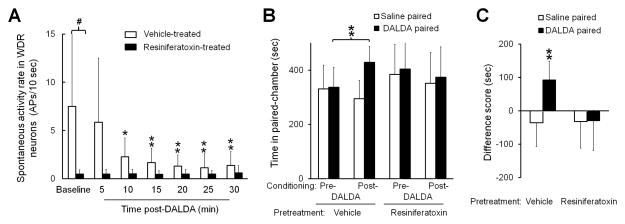
Recent studies suggested that activation of TRPV1-expressing DRG neurons may play an important role in spontaneous pain.2,34 To delineate the role of TRPV1-expressing neurons in DALDA-induced amelioration of ongoing pain in nerve-injured rats, we injected adult rats at day 7 post-SNL with vehicle (n=6) or resiniferatoxin (0.1 mg/kg, i.p., n=7). Resiniferatoxin is an ultra-potent and selective TRPV1 agonist that desensitizes TRPV1 receptors and decreases the excitability of TRPV1-expressing neurons.34 Our recent study showed that heat hypersensitivity was abolished in SNL rats 7–9 days after resiniferatoxin treatment.13 Like in the prior experiment (Fig. 5A), the spontaneous activity rates of WDR neurons gradually decreased from 10–30 min after DALDA (10 mg/kg, s.c.) in vehicle-pretreated SNL rats (Fig. 7A). Strikingly, the baseline spontaneous activity rates of WDR neurons were significantly lower in resiniferatoxin-pretreated SNL rats (0.5 ± 0.4 APs) than in vehicle-pretreated SNL rats (7.5 ± 8.7 APs, P = 0.039) and were not decreased after DALDA treatment (Fig. 7A).
Figure 7. Resiniferatoxin reduces the spontaneous activity of dorsal horn neurons and precludes CPP to dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA) in nerve-injured rats.
(A) The spontaneous activity rates of WDR neurons in vehicle-pretreated SNL rats were significantly decreased by DALDA (10 mg/kg, s.c.). The baseline spontaneous activity rates were significantly lower in SNL rats that received resiniferatoxin pretreatment (0.1 mg/kg, i.p, n=7) than in those that received vehicle (n=6). Two-way mixed-model ANOVA with Bonferroni post hoc test, *P < 0.05, **P < 0.01 versus baseline; #P < 0.05 versus the indicated group. (B) SNL rats pretreated with vehicle (n=7), but not resiniferatoxin (0.1 mg/kg, i.p, n=11), spent more time in the DALDA-paired chamber (10 mg/kg, s.c.) after conditioning, than they did during the preconditioning test period. (C) The difference scores. Two-way repeated measures ANOVA with Bonferroni post hoc test, **P < 0.01 versus the indicated group or the saline-paired chamber. Data are expressed as mean ± SD. ANOVA, analysis of variance; APs, action potentials; CPP, conditioned place preference; i.p., intraperitoneal; RTX, resiniferatoxin; s.c., subcutaneous; WDR, wide-dynamic range.
In a behavior study, vehicle-pretreated SNL rats spent more time in the DALDA-paired chamber after conditioning with systemic DALDA (10 mg/kg, s.c., 429.0 ± 58.7 s) than before conditioning (336.6 ± 74.4 s, n=7, P = 0.007, Fig. 7B). Additionally, the difference score for the DALDA-paired chamber (92.4 ± 56.3 s) was significantly greater than that for the saline-paired chamber (−36.1 ± 71.4 s, P = 0.005, Fig. 7C). In contrast to vehicle-pretreated SNL rats, those pretreated with resiniferatoxin (n=11) did not show preference for the DALDA-paired chamber after DALDA conditioning (Fig. 7B,C).
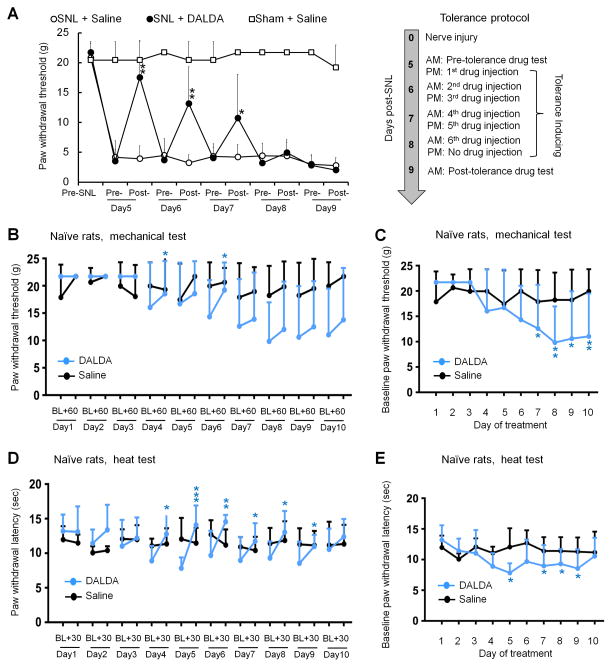
SNL rats develop tolerance to DALDA-induced inhibition of mechanical hypersensitivity after repeated systemic administration
On days 5–7 post-SNL, ipsilateral PWT was significantly increased from the pre-injection baseline (3.5 ± 1.9 g) at 30 min after systemic administration of DALDA (10 mg/kg, s.c., n=8, day5: 17.5 ± 6.2 g, P = 0.002; day6: 13.2 ± 6.1 g, P = 0.008; day7: 10.7 ± 7.3 g, P = 0.016).13 The drug effect gradually decreased after 5 days of repeated DALDA injections (n=8/group, Fig. 8A), and was mostly gone by 4–5 days of drug treatment (i.e., days 8–9 post-SNL), suggesting the development of anti-allodynic tolerance. Injection of saline did not significantly change PWT in SNL rats (n=8) or sham operated rats (n=8), as compared to pre-injection baseline on day 5 post-SNL.
Figure 8. Repeated drug administration induces tolerance to the anti-allodynic effect of dermorphin [D-Arg2, Lys4] (1–4) amide (DALDA) in nerve-injured rats and elicits hyperalgesia in naïve rats.
(A) Left: DALDA (10 mg/kg, s.c., n=8), but not vehicle (saline, n=8), reversed the decrease in paw withdrawal threshold (PWT) of the ipsilateral hind paw in rats on days 5–7 after SNL. Sham operated rats that received saline injection were included as controls (n=8). The inhibition of mechanical hypersensitivity by DALDA decreased after repeated treatments. PWT was measured before and at 30–60 min after injection. Right: The protocol to induce tolerance. Two-way mixed model ANOVA with Bonferroni post hoc test. *P < 0.05, **P < 0.01 versus pre-drug on day5. (B–E) Opioid-induced hyperalgesia was examined by injecting naïve rats once daily with DALDA (10 mg/kg, n=8, s.c.) or vehicle (saline, n=6) for 10 consecutive days. (B) PWT was measured before and 60 min after drug administration on each treatment day. (C) Time course of change in pre-drug PWTs on each treatment day. (D) Paw withdrawal latency (PWL) to heat stimulation was measured before and 30 min after drug administration. (E) Time course of change in pre-drug PWLs on each treatment day. B,D: paired t-test, *P < 0.05, **P < 0.01, ***P < 0.001 versus the pre-drug baseline. C,E: One-way repeated measures ANOVA with Bonferroni post hoc test, *P < 0.05, **P < 0.01 versus the pre-drug baseline of day 1. Data are expressed as mean ± SD. ANOVA, analysis of variance; SNL, spinal nerve ligation.
Naive rats develop OIH to repeated systemic administration of DALDA
Repeated systemic administration of fixed-dose DALDA (10 mg/kg, s.c., n=8) once daily for 10 consecutive days led to the development of mechanical (Fig. 8B,C) and heat (Fig. 8D,E) hypersensitivity in naïve rats. Pre-drug PWTs (baseline) on days 7–10 of DALDA treatment were significantly decreased from that measured on day 1 (Day7: P = 0.026; Day8: P = 0.003; Day9: P = 0.010; Day10: P = 0.007; Fig. 8C). Similarly, thermal nociceptive threshold (PWL) measured before drug injection on days 5 and 7–9 of DALDA treatment was significantly lower than that on day 1 (Day5: P = 0.011; Day7: P = 0.047; Day8: P = 0.042; Day9: P = 0.032, Fig. 8E). Mechanical PWT after DALDA treatment was significantly increased from pre-drug baseline on day 4 (P = 0.046) and day 6 (P = 0.013, Fig. 8B). On days 4–9 of DALDA treatment, PWLs at 30 min after DALDA injection (Day4: P = 0.034; Day5: P < 0.001; Day6: P = 0.006; Day7: P = 0.044; Day8: P = 0.017; Day9: P = 0.039) were significantly increased from the respective pre-drug baseline (Fig. 8D). Repeated saline injection in naïve rats for 10 days did not change PWL or PWT (Fig. 8B–E, n=6).
Discussion
Critical to the use of peripherally acting mu-opioids for neuropathic pain treatment is an understanding of their effects on ongoing pain, and knowledge of the underlying neurobiological mechanisms. By examining CPP and wheel running activity, which are operant behavioral outcome measures, we showed that DALDA treatment alleviates ongoing pain-associated behavior in nerve-injured animals through activation of MORs in the PNS, without adversely affecting gastrointestinal motility or exploratory activity. Furthermore, DALDA attenuated spontaneous neuronal activity after nerve injury. Yet, repeated DALDA treatments may induce anti-allodynic tolerance and OIH.
Nerve-injured rats and mice both developed CPP to DALDA. Loperamide, another peripherally acting MOR-preferring agonist,19 also induced CPP in nerve-injured rats. Importantly, DALDA did not induce CPP in naïve rats, suggesting that CPP in SNL rats results from the reward of pain relief rather than a direct activation of brain reward circuitry, which would indicate CNS opioid penetration and an abuse potential.35,36 The CPP to DALDA was blocked by methylnaltrexone, a peripherally acting MOR-preferring antagonist, further suggesting a peripheral site of drug action.
The decrease in voluntary wheel running activity after nerve injury may serve as another objective measure of ongoing pain-related behavior27 and might have multiple causes, including the aversive state associated with neuropathic pain, impaired locomotor function, and anhedonia. Travel distance decreased slightly from baseline in sham-operated mice, possibly because of incisional pain and decreased exploration after adaptation. Importantly, continuous subcutaneous infusion of DALDA gradually increased the speed and distance traveled by nerve-injured mice, suggesting improvements in daily wellbeing and functional recovery. At the end of drug treatment, evoked mechanical and heat hypersensitivities were also significantly decreased, further suggesting DALDA-induced pain relief.
In studies of pregnant sheep, DALDA did not cross the placental barrier to a significant extent.37,38 Additionally, DALDA (10 mg/kg, s.c.) did not disturb locomotor function or reduce exploration,13 suggesting minimal central side effects after systemic administration. Loperamide is a substrate extruded by P-glycoprotein transporter from the brain endothelial cells, and can be removed quickly from CNS.9,39–42 Nevertheless, CNS concentrations of loperamide and DALDA were not examined in the current experimental settings. Therefore, we cannot exclude the possibility that drug effects may partially involve central drug action. The pharmacokinetic and pharmacodynamic properties of loperamide and DALDA after subcutaneous administration warrant further investigation, especially after repetitive treatments.
Morphine and loperamide are known to decrease gastrointestinal secretion and motility.43 Yet, 3 consecutive days of DALDA administration did not reduce gastrointestinal motility. The reasons that DALDA and loperamide have differing effects on the gastrointestinal tract are not currently clear. We speculate that DALDA may be readily absorbed after subcutaneous administration and might not reach MORs in the gastrointestinal tract as effectively as loperamide.44,45 DALDA is a tetra-peptide and has several polar groups that facilitate its absorption by the enterocytes.11,37 In contrast, loperamide has three planar and non-polar phenyl rings and a tertiary alcohol group, which facilitate access to the gut wall. The distribution of DALDA (s.c.) in the gastrointestinal tract has not yet been determined and compared to that of other MOR agonists. Understanding the chemical properties that limit DALDA’s inhibition of gastrointestinal function will be beneficial to the development of opioid analgesics with minimal gastrointestinal adverse effects (e.g., constipation).
Several additional experiments were conducted in response to reviewer concerns. Similar to loperamide,28 repeated DALDA treatments induced anti-allodynic tolerance in SNL rats, and gradually increased pain sensitivity in naïve rats, indicating OIH. Recent findings suggested that MORs expressed on nociceptive afferent neurons initiate morphine analgesic tolerance and OIH.29 Yet, systemic morphine (3.0 mg/kg, s.c.) effectively inhibited neuropathic pain-related behavior in loperamide-tolerant SNL rats.28 This finding suggests that central MORs are functional under conditions of peripheral opioid tolerance. The apparent discrepancies may be due to differences in species, pain models, drug doses, and experimental approaches used in these studies. For example, in addition to losing MORs at peripheral axons and soma of nociceptive afferent neurons, conditional MOR knockout mice exhibit loss of MORs on their central terminals, which may play an important role in initiating morphine tolerance and OIH.29 In contrast, DALDA and loperamide (s.c.) may not directly affect MORs in the spinal cord. Rather, they may change the function but not ablate MORs in the PNS.28 Although beyond the scope of the current study, it is important to examine the mechanisms of tolerance and OIH to peripherally acting mu-opioids, and further delineate roles of MORs in different compartments of nociceptive afferent neurons.
We also sought to identify the cellular mechanisms by which DALDA inhibits ongoing pain. Both peripheral (e.g., ectopic discharge) and central mechanisms may contribute to ongoing neuropathic pain. After nerve injury, spinal WDR neurons showed increased spontaneous activity, which might partially underlie ongoing pain.30,46 Systemic DALDA inhibited the spontaneously active WDR neurons, an effect that might correlate with inhibition of ongoing pain. Importantly, this effect of DALDA was blocked by methylnaltrexone, suggesting that DALDA might inhibit ectopic peripheral inputs. Systemic DALDA at the doses tested did not induce heat antinociception.13 However, it reduced the C-component of WDR neurons in naïve rats, an effect that was blocked by methylnaltrexone. It is possible that multiple neuronal circuitries or compensatory mechanisms contribute to transmission of heat nociception,47,48 such as those mediated by nociceptive-specific dorsal horn neurons that are important to nociceptive pain.
Recent studies suggested that “heat receptor” TRPV1-expressing primary sensory neurons might contribute to ongoing pain.2,34 In line with this notion, DALDA-induced CPP was prevented by systemic pretreatment of SNL rats with resiniferatoxin. Resiniferatoxin desensitizes the TRPV1 receptor after strong and prolonged activation, and then selectively decreases the excitability of TRPV1-expressing neurons.2,34 Resiniferatoxin reversed heat hypersensitivity in SNL rats.13 Thus, it may ameliorate both heat hyperalgesia and ongoing pain by suppressing TRPV1-expressing DRG neurons, thereby preventing DALDA from inducing further pain inhibition. Our electrophysiologic finding that resiniferatoxin pretreatment decreased the spontaneous activity of WDR neurons in SNL rats supports the behavioral results. Because TRPV1 colocalizes with MORs in DRG neurons, DALDA might inhibit ongoing pain and spontaneous neuronal activity by inhibiting TRPV1-expressing neurons.
Recent microneurography studies indicated that spontaneous activity in peripheral C-nociceptors might be a biomarker for and underlie mechanisms of ongoing pain in patients with painful polyneuropathy and fibromyalgia.49,50 However, identifying spontaneously active DRG neurons in vivo has been challenging with conventional electrophysiologic recordings, as it requires sampling a large cell population. By generating pirt-GCaMP6 mice, we validated a high-throughput calcium imaging technique to examine DRG neuronal activity in vivo.18,51,52 GCaMP6 is a genetically encoded calcium indicator. The intensity of its green fluorescence increases robustly upon binding to intracellular calcium when the cell is active. Therefore, we used it to visualize neuronal activity.18,53 The pirt promoter is expressed in almost all primary sensory neurons, but not in the CNS, glia, or other peripheral tissue.18,54,55 The DRG is located outside of the blood-nerve barrier because the endothelium of vessels that supply DRG lacks tight junctions.56,57 Therefore, DRG neuron soma can be reached by peripherally acting opioids that are administered systemically. Topical application of DALDA to the ganglion attenuated both evoked and spontaneous excitation of DRG neurons. In line with findings from microneurography recording,49,50 the spontaneously active cells were mainly small-diameter neurons known to be important for pain signaling. Our study supports the premise that spontaneous activity in DRG neurons might represent a useful biomarker for ongoing pain in rodents that can be examined by high-throughput GCaMP6 imaging.
The lack of effective therapies for neuropathic pain and the increasing morbidity associated with the use of opioids highlights the need for new treatment strategies. The demonstration that peripheral opioid mechanisms may have a role in alleviating neuropathic pain is relatively recent. Nevertheless, the pharmaceutical industry (Nektar, NKTR-181) has begun to examine the clinical utility of a new class of opioids with low BBB permeability. Current behavioral and electrophysiologic findings offer evidence that systemic administration of DALDA attenuates ongoing neuropathic pain-related manifestations and spontaneous neuronal activity, by acting at a peripheral location. In light of gender-based differences in neuropathic pain,58–60 future studies in female rats are needed to examine potential sexual dimorphism in ongoing pain inhibition by DALDA.
Our integrated studies provide proof of concept for using peripherally acting mu-opioids to treat ongoing neuropathic pain, and begin to reveal the underlying neurophysiologic mechanisms. It remains to be determined whether DALDA is more effective than currently used non-opioid medications, such as gabapentin, for neuropathic pain treatment. Potential peripheral side effects and the mechanisms underlying tolerance and OIH after long term use of peripherally acting mu-opioids also need carefully investigation. Such studies will further establish the clinical translatability of peripherally acting mu-opioids. Intriguingly, a novel MOR agonist was recently developed by exploiting pathological conformation dynamics of MOR-ligand interactions. The study demonstrated the feasibility of preferentially targeting the pathologic conformation of MORs in the PNS and produced “injury-restricted inhibition” of inflammatory pain without eliciting respiratory depression, sedation, or constipation.61 The knowledge gained from these studies is critical to developing peripheral opioid analgesics for neuropathic pain treatment that have minimal adverse effects and limited capacity to induce tolerance and OIH after prolonged use.
Acknowledgments
Funding: This study was conducted at the Johns Hopkins University and was supported by grants from the National Institutes of Health (Bethesda, Maryland, USA): R01NS70814 (Y.G.), R01NS26363 (S.N.R.), R21NS99879 (Y.G.). This work was facilitated by the Pain Research Core funded by the Blaustein Fund and the Neurosurgery Pain Research Institute at the Johns Hopkins University. X.D. is a faculty of the Howard Hughes Medical Institute.
The authors thank Claire F. Levine, MS (scientific editor, Department of Anesthesiology/CCM, Johns Hopkins University) for editing the manuscript, and Qian Huang (post-doctoral fellow, Department of Anesthesiology/CCM, Johns Hopkins University) for technical support.
Footnotes
Competing interests: The authors declare no competing interests.
Authors’ contributions:
V.T., M.A. and F.Y. performed most of the experiments and were involved in writing a draft manuscript. V.T., Q.Z., S-Q.H, T.Z., B.S., X.C., S.G., K.S, and Z.C. performed or assisted with portions of the experiments and data analysis. X.D. was involved in discussion and interpretation. Y.G and S.N.R. designed and directed the project and wrote the final manuscript.
References
- 1.Torrance N, Lawson KD, Afolabi E, Bennett MI, Serpell MG, Dunn KM, Smith BH. Estimating the burden of disease in chronic pain with and without neuropathic characteristics: does the choice between the EQ-5D and SF-6D matter? Pain. 2014;155:1996–2004. doi: 10.1016/j.pain.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 4.Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur J Neurosci. 2014;39:1881–90. doi: 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- 5.Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans--how symptoms help disclose mechanisms. Nat Rev Neurol. 2013;9:572–82. doi: 10.1038/nrneurol.2013.180. [DOI] [PubMed] [Google Scholar]
- 6.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–6. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16:405–16. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag. 2011;7:55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
- 10.Shimoyama M, Shimoyama N, Zhao GM, Schiller PW, Szeto HH. Antinociceptive and respiratory effects of intrathecal H-Tyr-D-Arg-Phe-Lys-NH2 (DALDA) and [Dmt1] DALDA. J Pharmacol Exp Ther. 2001;297:364–71. [PubMed] [Google Scholar]
- 11.Schiller PW, Nguyen TM, Chung NN, Lemieux C. Dermorphin analogues carrying an increased positive net charge in their “message” domain display extremely high mu opioid receptor selectivity. J Med Chem. 1989;32:698–703. doi: 10.1021/jm00123a035. [DOI] [PubMed] [Google Scholar]
- 12.Scalia S, Salvadori S, Marastoni M, Bortolotti F, Tomatis R. Reversed-phase HPLC study on the in vitro enzymic degradation of dermorphin. Peptides. 1986;7:247–51. doi: 10.1016/0196-9781(86)90221-4. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari V, Yang F, He SQ, Shechter R, Zhang C, Shu B, Zhang T, Tiwari V, Wang Y, Dong X, Guan Y, Raja SN. Activation of Peripheral mu-opioid Receptors by Dermorphin [D-Arg2, Lys4] (1–4) Amide Leads to Modality-preferred Inhibition of Neuropathic Pain. Anesthesiology. 2016;124:706–20. doi: 10.1097/ALN.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–33. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160:847–57. doi: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci. 2014;17:153–63. doi: 10.1038/nn.3602. [DOI] [PubMed] [Google Scholar]
- 17.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source 49. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu, Young L, He S, Lavinka PC, Zhou F, Bergles D, Hanani M, Guan Y, Spray DC, Dong X. Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron. 2016;91:1085–96. doi: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–29. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F, Carteret AF, Wacnik PW, Chung CY, Xing L, Dong X, Meyer RA, Raja SN, Guan Y. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience. 2011;199:470–80. doi: 10.1016/j.neuroscience.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Tseng PY, Tiwari V, Xu Q, He SQ, Wang Y, Zheng Q, Han L, Wu Z, Blobaum AL, Cui Y, Tiwari V, Sun S, Cheng Y, Huang-Lionnet JH, Geng Y, Xiao B, Peng J, Hopkins C, Raja SN, Guan Y, Dong X. Targeting human Mas-related G protein-coupled receptor X1 to inhibit persistent pain. Proc Natl Acad Sci USA. 2017;114:E1996–E2005. doi: 10.1073/pnas.1615255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He SQ, Li Z, Chu YX, Han L, Xu Q, Li M, Yang F, Liu Q, Tang Z, Wang Y, Hin N, Tsukamoto T, Slusher B, Tiwari V, Shechter R, Wei F, Raja SN, Dong X, Guan Y. MrgC agonism at central terminals of primary sensory neurons inhibits neuropathic pain. Pain. 2014;155:534–44. doi: 10.1016/j.pain.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shechter R, Yang F, Xu Q, Cheong YK, He SQ, Sdrulla A, Carteret AF, Wacnik PW, Dong X, Meyer RA, Raja SN, Guan Y. Conventional and kilohertz-frequency spinal cord stimulation produces intensity- and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology. 2013;119:422–32. doi: 10.1097/ALN.0b013e31829bd9e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 25.Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci USA. 2010;107:15933–8. doi: 10.1073/pnas.1011221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 27.Liu MG, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: Challenges and perspectives. Prog Neurobiol. 2014;116:13–32. doi: 10.1016/j.pneurobio.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 28.He SQ, Yang F, Perez FM, Xu Q, Shechter R, Cheong YK, Carteret AF, Dong X, Sweitzer SM, Raja SN, Guan Y. Tolerance develops to the antiallodynic effects of the peripherally acting opioid loperamide hydrochloride in nerve-injured rats. Pain. 2013;154:2477–86. doi: 10.1016/j.pain.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G. Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med. 2017;23:164–73. doi: 10.1038/nm.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113:1392–405. doi: 10.1097/ALN.0b013e3181fcd95c. [DOI] [PubMed] [Google Scholar]
- 31.Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J Neurosci. 2006;26:4298–307. doi: 10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Percie du Sert N, Rice ASC. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 2014;171:2951–63. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King T, Porreca F. Preclinical assessment of pain: improving models in discovery research. Curr Top Behav Neurosci. 2014;20:101–20. doi: 10.1007/7854_2014_330. [DOI] [PubMed] [Google Scholar]
- 34.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Ann N Y Acad Sci. 2013;1282:1–11. doi: 10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. J Neurosci. 2015;35:13879–88. doi: 10.1523/JNEUROSCI.2711-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szeto HH, Clapp JF, Desiderio DM, Schiller PW, Grigoriants OO, Soong Y, Wu D, Olariu N, Tseng JL, Becklin R. In vivo disposition of dermorphin analog (DALDA) in nonpregnant and pregnant sheep. J Pharmacol Exp Ther. 1998;284:61–5. [PubMed] [Google Scholar]
- 38.Clapp JF, III, Kett A, Olariu N, Omoniyi AT, Wu D, Kim H, Szeto HH. Cardiovascular and metabolic responses to two receptor-selective opioid agonists in pregnant sheep. Am J Obstet Gynecol. 1998;178:397–401. doi: 10.1016/s0002-9378(98)80032-x. [DOI] [PubMed] [Google Scholar]
- 39.Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7(Suppl 3):S11–S18. [PubMed] [Google Scholar]
- 40.Nozaki-Taguchi N, Shutoh M, Shimoyama N. Potential utility of peripherally applied loperamide in oral chronic graft-versus-host disease related pain. Jpn J Clin Oncol. 2008;38:857–60. doi: 10.1093/jjco/hyn110. [DOI] [PubMed] [Google Scholar]
- 41.Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: assessment of unbound brain EC50,u and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther. 2007;323:346–55. doi: 10.1124/jpet.107.119560. [DOI] [PubMed] [Google Scholar]
- 42.Dehaven-Hudkins DL, Burgos LC, Cassel JA, Daubert JD, DeHaven RN, Mansson E, Nagasaka H, Yu G, Yaksh T. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J Pharmacol Exp Ther. 1999;289:494–502. [PubMed] [Google Scholar]
- 43.De LA, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103–15. doi: 10.1016/0163-7258(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 44.Baldi F, Bianco MA, Nardone G, Pilotto A, Zamparo E. Focus on acute diarrhoeal disease. World J Gastroenterol. 2009;15:3341–8. doi: 10.3748/wjg.15.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanauer SB. The role of loperamide in gastrointestinal disorders. Rev Gastroenterol Disord. 2008;8:15–20. [PubMed] [Google Scholar]
- 46.Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27:14059–68. doi: 10.1523/JNEUROSCI.3699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt SP, Mantyh PW. The molecular dynamics of pain control 196. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- 48.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleggetveit IP, Namer B, Schmidt R, Helas T, Ruckel M, Orstavik K, Schmelz M, Jorum E. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 2012;153:2040–7. doi: 10.1016/j.pain.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Serra J, Bostock H, Sola R, Aleu J, Garcia E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153:42–55. doi: 10.1016/j.pain.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Emery EC, Luiz AP, Sikandar S, Magnusdottir R, Dong X, Wood JN. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Sci Adv. 2016;2:e1600990. doi: 10.1126/sciadv.1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson M, Zheng Q, Dong X. Investigation of Pain Mechanisms by Calcium Imaging Approaches. Neurosci Bull. 2017 doi: 10.1007/s12264-017-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133:475–85. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YS, Chu Y, Han L, Li M, Li Z, Lavinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81:873–87. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;(Suppl 6):S27–S35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- 57.Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006;105:146–53. doi: 10.1097/00000542-200607000-00024. [DOI] [PubMed] [Google Scholar]
- 58.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–3. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 60.Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van d V, Laufer S, Ji RR. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spahn V, Del VG, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein C. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science. 2017;355:966–9. doi: 10.1126/science.aai8636. [DOI] [PubMed] [Google Scholar]