ABSTRACT
The lipopolysaccharide (LPS) is a major virulence factor of Brucella, a facultative intracellular pathogenic Gram-negative bacterium. Brucella LPS exhibits a low toxicity and its atypical structure was postulated to delay the host immune response, favouring the establishment of chronic disease. Here we carried out an in-depth in vitro and in vivo characterisation of the immunomodulatory effects of Brucella LPS on different dendritic cell (DC) subpopulations. By using LPSs from bacteria that share some of Brucella LPS structural features, we demonstrated that the core component of B. melitensis wild-type (Bm-wt) LPS accounts for the low activation potential of Brucella LPS in mouse GM-CSF-derived (GM-) DCs. Contrary to the accepted dogma considering Brucella LPS a poor TLR4 agonist and DC activator, Bm-wt LPS selectively induced expression of surface activation markers and cytokine secretion from Flt3-Ligand-derived (FL-) DCs in a TLR4-dependent manner. It also primed in vitro T cell proliferation by FL-DCs. In contrast, modified LPS with a defective core purified from Brucella carrying a mutated wadC gene (Bm-wadC), efficiently potentiated mouse and human DC activation and T cell proliferation in vitro. In vivo, Bm-wt LPS promoted scant activation of splenic DC subsets and limited recruitment of monocyte- DC like cells in the spleen, conversely to Bm-wadC LPS. Bm-wadC live bacteria drove high cytokine secretion levels in sera of infected mice. Altogether, these results illustrate the immunomodulatory properties of Brucella LPS and the enhanced DC activation ability of the wadC mutation with potential for vaccine development targeting Brucella core LPS structure.
KEYWORDS: Brucella, dendritic cell, intracellular bacteria, lipopolysaccharide, T cell proliferation, toll-like receptor, vaccine
Introduction
The Gram-negative bacteria of the genus Brucella are facultative intracellular pathogens that cause brucellosis, a worldwide zoonosis affecting wild life and livestocks with serious economic losses.1 This disease is transmitted to humans via contaminated food or aerosols and leads to chronic inflammation. Human brucellosis is difficult to treat with antibiotics and no effective vaccine exists yet to prevent it. Brucella is characterized by its ability to escape early detection by the innate immune system. An efficient strategy Brucella has developed to achieve such stealthy behaviour is to modulate pathogen-associated molecular pattern (PAMP) to avoid recognition by pathogen recognition receptors (PRR) on host cells like macrophages or dendritic cells (DCs).2–5
The most conspicuous PAMP bearing component of the surface of Gram-negative bacteria is the LPS. This molecule is composed of a hydrophobic lipid A, embedded in the outer membrane, linked to a charged oligosaccharide core associated with a hydrophilic O-polysaccharide chain (O-chain) that covers the bacterial surface.6 Canonical LPS, like Escherichia coli (E. coli) LPS, expresses a lipid A made of a glucosamine disaccharide linked predominantly to C12 to C14 acyl chains in ester, amide and acyl-oxyacyl bonds, which are recognised by the toll-like receptor 4 (TLR4)/MD-2 complex. In contrast, Brucella lipid A is a 2,3-diaminoglucose disaccharide substituted with C16, C18, C28 and other very long acyl chains. This peculiar structure is a poor agonist of TLR4/MD-2 and therefore a paradigm has emerged proposing Brucella LPS as a crucial virulence factor that hampers recognition by PRRs and plays essential roles during infection.2,4,5,7
DCs are the most potent antigen-presenting cells, equipped with a variety of PRRs, which detect bacterial PAMPs and trigger downstream signalling, resulting in antigen uptake and processing as well as cytokine secretion. These cells are regarded as ‘sentinels against pathogens’ for induction of T-dependent adaptive immunity.8 A variety of DC subsets exist in vivo as distinguished by specific cell surface markers and functions.9–11 Under steady-state conditions in mice, at least three splenic DC subsets have been identified: bone marrow stromal antigen-2 (Bst-2)+ plasmacytoid DCs (pDCs), CD8α+ and CD11b+ conventional DCs (cDCs).8,10 These subsets of DCs can display shared as well as distinct functions in controlling host immune responses and this is partly due to the expression of PRRs. As such, pDCs predominantly display TLR7 and 9, and sense viral and bacterial pathogens releasing high levels of Type I interferons (IFN-I).12 CD8α+cDCs express TLR3, 4 and 9, and play a critical role in the induction of cross-presentation in vivo.10,13 CD11b+ cDCs exhibit TLR4, 7 and 9, and present a weaker cross-priming ability. This latter subset, mainly localised in the marginal zone of the spleen at the steady state, migrates upon stimulation to the T cell areas and secretes cytokines.10,13 Upon microbial stimulation in vivo, monocyte-derived DC (MO-DC) are also differentiated and recruited from blood monocytes to immune T cell areas.14 mo-DCs also have the capacity to activate antigen-specific CD4+ T cell responses and to cross-prime CD8+ T cells, during viral, bacterial, and parasitic infections.15 They are distinguished from conventional DCs by the expression of CD64 and MAR-1 at least in the lung and mesenteric lymph nodes16,17 and DC-SIGN.18
In vitro generated mouse granulocyte macrophage-colony stimulated factor (GM-CSF)- or FMS-like tyrosine 3 ligand (Flt3L)-derived bone marrow DCs (GM-DCs and FL-DCs, respectively) are widely used to decipher the immunology and cell biology of DCs. Flt3L treatment of murine bone marrow progenitors generates three distinct DC subtypes including B220+pDCs, CD24+cDCs and CD11b+cDCs, which were proven to be equivalent of splenic pDCs, CD8α+cDCs and CD11b+cDCs, respectively.10,11 GM-DCs consist of a DC population suggested to be the equivalent to the mo-DCs that emerge during inflammation in vivo and of CD11c+MHCII+ macrophages.19–21
We previously demonstrated that LPSs with a partially defective core, which were purified from Brucella mutants with a mannosyltransferase (wadC) gene deletion, were much more potent than Brucella wild-type LPS at activating mouse BMDCs, as measured by assessing DC phenotype maturation and and secretion of pro-inflammatory cytokines such as IL6 and TNFα.22,23 However, these studies were based on the sole analysis of GM-DCs. Given that the magnitude and profile of DC activation triggered by Brucella infection in vitro vary according to the diversity of DC subtypes and functions,24 we carried out further characterisation of the immunomodulatory properties of B. melitensis LPS (Bm-wt and Bm-wadC LPS) in different DC subsets in vitro and in vivo. In vitro, we found that Bm-wt LPS activated FL-DCs unlike GM-DCs. By using LPSs from various sources that share some of Brucella structural features, we demonstrated that the core component of Bm-wt LPS was responsible for the low activation potential of Brucella LPS in mouse GM-DCs and human GM/IL-4 monocyte-derived DCs. Even though effective, Bm-wt LPS activation of FL-DCs was not powerful enough to lead to full T cell proliferation. In contrast, Bm-wadC LPS succeeded in activating both GM-DCs and FL-DCs, consequently accelerating CD4+ and CD8+ T cell proliferation. In vivo, the wadC mutation conferred an enhanced activation ability to Brucella LPS in splenic conventional DC subsets and elicited efficient mo-DC like cell mobilisation. This improved immunogenic ability of Bm-wadC LPS was reflected by potent secretion of Th1 cytokines in serum of mice infected by the Brucella wadC mutant.
Results
The core structure of Brucella LPS inhibits GM-DC activation
To unravel the molecular determinants accounting for the lack of GM-DC activation by Brucella wild-type (wt) LPSs,22,23 we first assessed the role of the different sugar moieties of Bm LPS on DC activation in vitro by comparing the effect of LPSs from various sources that share some of Brucella structural features. As mentioned above, Bm-wadC LPS has a partially defective oligosaccharide core but displays intact O-polysaccharide and lipid A compared to Bm-wt LPS.22,23,25 More precisely, the defect in the Bm-wadC LPS corresponds to a loss of an oligosaccharide branching connected to the core section linking lipid A and the O-polysaccharide.23,25 Yersinia enterocolitica O:9 LPS exhibits the same O-chain homopolymer as Bm-wt LPS but its lipid A is alike E. coli LPS.26,27 Ochrobactrum anthropi 3331 LPS carries a “Brucella-type” lipid A but different core and O-chain sugars.26–28 E. coli LPS differs by its overall structure from that of Bm-wt LPS, but as aforementioned has in common lipid A and core features with Y. enterocolitica O:9 LPS26,27 (Fig. S1). After 5 days of culture, murine bone marrow GM-DCs were stimulated for 24 h by this array of LPSs, all at 10 µg/mL but E. coli LPS at 100 ng/mL as described previously.22 Non-adherent cells were used for phenotypic analysis by flow cytometry and cell culture supernatants for cytokine measurement by ELISA. Confirming prior reports, Bm-wt LPS did not activate GM-DCs in terms of surface expression of activation markers (Fig. 1A) and secretion of inflammatory cytokines (Fig. 1B). In GM-DCs, all other LPSs drove induction of expression of surface activation markers, including MHCII, CD80, CD86 and CD40, at comparable levels (Fig. 1A). Similar cytokine (IL-12p40, IL-12p70, IFNγ, TNFα, IL-6, IL-1β) secretion patterns were also generated by all LPSs with the exception of Bm-wt LPS (Fig. 1B). Remarkably as regards IL-10, three sets of LPSs were distinguished: the inactive Bm-wt LPS, the Bm-wadC and O. anthropi 3331 LPSs, which share a “Brucella type”-lipid A and led to intermediate secretion levels and the E. coli LPS and Y. enterocolitica O:9 LPSs, which bear the same “E. coli type-lipid A” and elicited 5 times more secreted IL-10 levels. Altogether, the fact that similar DC activation patterns were achieved by a “Brucella-type” lipid A (Bm-wadC and O. anthropi 3331) or O-chain (Bm-wadC and Y. enterocolitica O:9) LPSs revealed that these molecular structures without the inhibitory effects of Brucella WT core component have the ability to trigger GM-DC activation. These results also indicated that the outer core deficiency by itself considerably increased DC activation in vitro.
Figure 1.
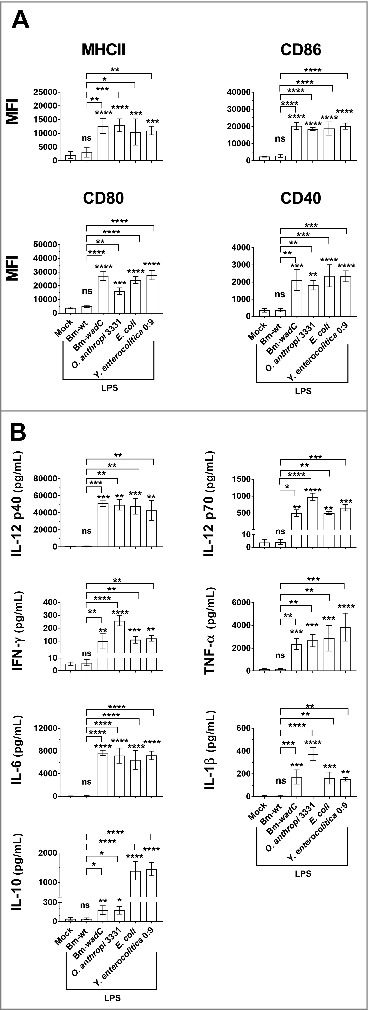
Comparison of several LPSs carrying different structure moieties in GM-DCs revealed a shield function of the Brucella core component. GM-DCs were non-treated (Mock) or stimulated with Bm-wt LPS, Bm-wadC LPS, Ochrobactrum anthropi 331 LPS, Yersinia enterocolitica O:9 LPS or E. coli LPS for 24 h. Brucella LPSs, Y. enterocolitica and O. anthropi LPS were used at the concentration of 10 μg/mL and E. coli LPS was at 100 ng/mL. (A) MHCII and co-stimulatory molecule levels of expression (MFI, Mean of Fluorescence Intensity) were measured by flow cytometry. (B) Cytokine secretion was determined in culture supernatants by ELISA. The graphs show combined data from at least three independent experiments. All error bars are standard deviations obtained from pooled data. Significant differences from mock or from Bm-wt LPS are shown. *, P < 0.05; **, P < 0.001; ***, P < 0.0001; ****, P < 0.00001. ns, non-significant.
WadC Brucella LPS activates human mo-DCs
We then determined whether Brucella LPSs would elicit activation of DCs from human origin in vitro. Human monocytes DCs (mo-DCs) were derived from PBMC from healthy blood donors in the presence of GM-CSF and IL-4 (GM/IL-4 mo-DCs). After 7 days of culture, these mo-DCs were stimulated for 72 h with Bm-wt or Bm-wadC LPS, both at 20 ng/mL. Cell culture supernatants were used for inflammatory cytokine measurement by ELISA. We found that Bm-wt LPS in human GM/IL-4 mo-DCs did not trigger any secretion of IL-6, IL-12p70, TNFα or IL-10. In contrast, Bm-wadC LPS prompted strong secretion of IL-12p70 and TNFα while IL-6 and IL-10 secreted levels reached higher values, comparable to those obtained with E. coli (Fig. 2). These findings reminiscent of those observed with the GM-DC model reveal that our murine GM-DC data can be translated to human GM/IL-4 mo-DCs.
Figure 2.
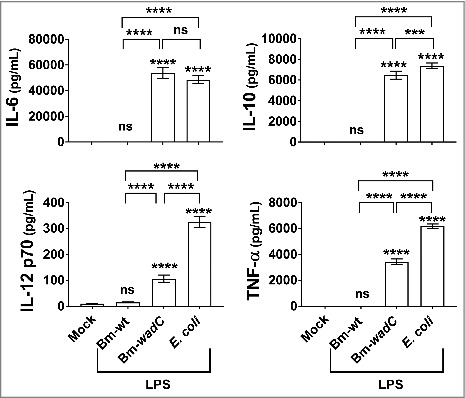
Unlike Bm-wt LPS, Bm-wadC LPS elicited cytokine secretion from human mo-DCs. Human GM-CSF and IL-4 derived mo-DCs were non-treated (Mock) or stimulated with Bm-wt LPS, Bm-wadC LPS or E. coli LPS for 72 h. All LPSs were used at a concentration of 20 ng/mL. Cytokine secretion was determined in culture supernatants by ELISA. The graphs show combined data from at least four independent experiments with n = 1 animal per condition. All error bars are standard deviations obtained from pooled data. Statistical analysis was performed with the parametric one-way ANOVA test, followed by variance analysis with the Tukey and Dunnett test. Significant differences from mock or from Bm-wt LPS were identical. ***, P < 0.0001; ****, P < 0.00001. ns, non-significant.
Brucella LPS activates FL-DCs
We next examined whether Brucella LPSs could activate FL-DCs. FL-DCs were generated from mouse bone marrow in the presence of the FLT3 Ligand (FL-DCs). After nine days of culture, these cells differentiated into heterogeneous DCs that could be split into 3 distinct populations, pDCs, CD24+ cDCs and CD11b+ cDCs (Fig. S2). Stimulations of FL-DC subsets were performed by culturing them for 24 h in the presence of Bm-wt or Bm-wadC LPS, O. anthropi 3331 LPS, Y. enterocolitica O:9 LPS (all at 10 µg/mL), or E. coli LPS at 100 ng/mL. Non-adherent cells were used for assessing surface phenotypes by flow cytometry and culture supernatants for inflammatory cytokine measurement by ELISA. Unlike GM-DCs (Fig. 1A), two of the FL-DC subsets (CD24+ cDCs and CD11b+ cDCs) upregulated MHC II, CD86, CD80, and CD40 expression on their surface in response to Bm-wt LPS. We further found that MHCII levels were alike for all LPSs, while expression of the other costimulatory molecules (CD86, CD80 and CD40) was enhanced but at lower levels than those driven by the other LPSs (Fig. 3A). Whereas pDCs were poorly activated by the different LPSs (Fig. 3A), Bm-wt LPS induced FL-derived CD24+ and CD11b+ cDCs to secrete significant amounts of certain cytokines, including TNF-α, IL-12p40, IL-6 and L-10. Others, such as IFN-γ, IL-12p70, IL-1β, were not significantly increased (Fig. 3B). In contrast, Bm-wadC LPS elicited a high activation profile, roughly comparable to that triggered by E. coli LPS in FL-DCs except for IL-10 and IL-1β (Fig. 3B). Interestingly, Y. enterocolitica O:9 LPS induced the greatest amount of IL-10 secretion from FL-DCs. In addition, O. anthropi 3331 was more potent than other LPSs at inducing IL-12p40, IL-12p70, TNF-α, IL-6, and IL-1β secretion from FL-DCs, revealing a specifc role for O. anthropi 3331 core and oligosaccharide moieties in these cells. Taken together, these findings disclosed that Brucella LPS exerted different modulatory properties depending on the targeted DC subtypes in vitro. They also unveil specific cytokine responses to the various LPS studied according to the DC model used.
Figure 3.
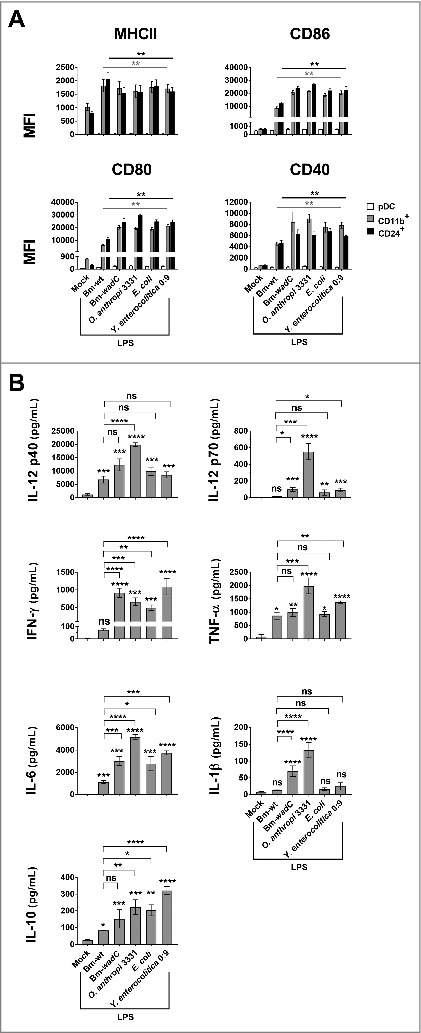
Bm-wt LPS preferentially triggered maturation of FL-DCs in vitro, while Bm-wadC LPS activated that of both GM-DC and FL-DC subsets. FL-DCs were non-treated (Mock) or stimulated for 24 h with Bm-wt LPS, Bm-wadC LPS, Ochrobactrum anthropi 331 LPS, Yersinia enterocolitica O:9 LPS or E. coli LPS. Brucella LPSs, Y. enterocolitica and O. anthropi LPS were used at the concentration of 10 μg/mL and E. coli LPS was at 100 ng/mL. (A) MHCII and co-stimulatory molecule expression levels (MFI, Mean of Fluorescence Intensity) were measured by flow cytometry. (B) Cytokine secretion was determined in whole FL-DC culture supernatants by ELISA. The graphs show combined data from at least three independent experiments. All error bars are standard deviations obtained from pooled data. Significant differences from mock only (A) or from mock or from Bm-wt LPS (B) are shown. *, P < 0.05; **, P < 0.001; ***, P < 0.0001; ****, P < 0.00001. ns, non-significant.
Bm-wadC LPS elicits T cell proliferation by both GM- & FL-DCs
We further investigated whether the observed phenotypic maturation and cytokine release triggered by Bm-wt LPS and Bm-wadC LPS led to the enhancement of antigen-specific T cell responses. For this purpose, we performed DC-T cell co-cultures using either GM-DCs or FL-DCs together with carboxyfluorescein succinimidyl ester (CFSE)-labelled T cells from transgenic mice that express either a TCR specific for MHC class-I restricted ovalbumin (OVA) (OT-I) or a TCR specific for MHC class-II restricted OVA (OT-II). DCs incubated with ovalbumin were activated by different LPSs (Bm-wt or Bm-wadC LPS, both at 10 µg/mL), or E. coli LPS at 100 ng/mL, as a positive control) and co-cultured with freshly isolated OT-I (CD8+) and OT-II (CD4+) T cells for 48 h. Proliferation of T cells was traced by measuring CFSE dilution with flow cytometry. BMDCs incubated with LPS alone without OVA did not induce T cell proliferation (data not shown). Data from one representative experiment are shown in Fig. 4A and statistical analyses from pooled data of at least 3 independent experiments are presented in Fig. 4B. Bm-wt LPS generated a slight but non-significant increase of CD4+ or CD8+ T cell proliferation in FL-DCs only, ranging from 11.9% in mock-treated cells to 16.5% and 20.8% to 35.2%, respectively (Fig. 4). The two DC models treated with Bm-wadC LPS were potent activators of both CD4+ and CD8+ T cell proliferation, as was E. coli LPS. These results indicated that wt Brucella LPS despite FL-DC activation capacity was unable to trigger full T cell activation in vitro, whereas the Bm-wadC LPS, was responsible for a strong T cell activation in both GM- and FL-DCs.
Figure 4.
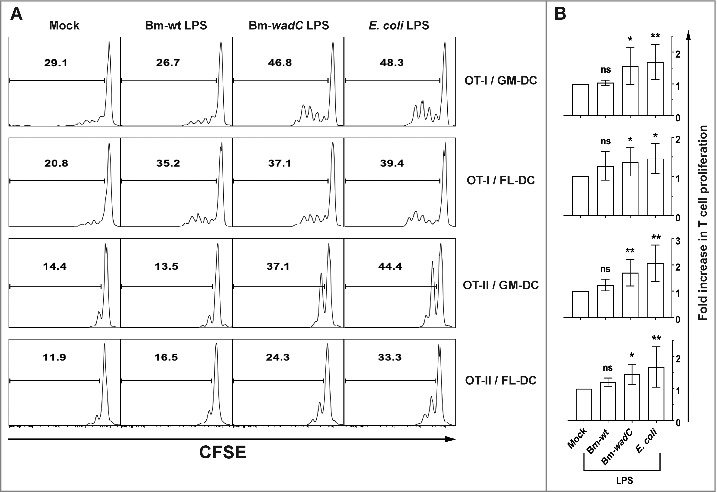
Bm-wt LPS-treated FL-DCs primed little T cell proliferation in vitro, while Bm-wadC LPS elicited significant T cell proliferation by both GM- & FL-DCs. GM- or FL-DCs were incubated for 16 h with OVA and were non-treated (Mock) or treated with different Bm LPSs (10 μg/mL) or E. coli LPS (100 ng/mL) for 8 h. Stimulated DCs were co-cultured with CFSE-labelled T cells from OT-I or OT-II mice. Proliferation of T cells was assessed after 2 days of co-culture by CFSE incorporation and flow cytometry. (A) One representative experiment is shown out of 3 independent experiments for OT-I and out of 5 for OT-II. (B) Pooled data are presented as mean ± standard deviation. Significant differences from mock. Significant differences from mock or from Bm-wt LPS were identical. *, P< 0.05; **, P < 0.001. ns, non-significant.
Emergence of a TLR4-mediated LPS-specific CD11cintCD11b+Bst-2hi mo-DC-like cell subset in vivo
To determine if Brucella LPS exhibited immunomodulatory effects on DC subsets in vivo, we injected intraperitoneally Bm-wt LPS, Bm-wadC LPS or E. coli LPS into mice. Single-cell suspensions were prepared after enzymatic digestion and gentle dissociation of the spleens. After excluding neutrophils, NK cells, B cells, and T cells, the remaining cells were analysed by flow cytometry for CD11c expression. DCs were divided into CD11chi DCs and CD11cint populations based on their CD11c expression level. CD11chi DCs were identified as conventional DCs (cDCs) due to the absence of BST-2hi population. Under steady-state conditions, CD11cint DCs were identified as pDCs (CD11bloBst-2hi) and cDCs (CD11blo to hi BST-2lo to int). Both cDC populations from CD11chi DCs and CD11cint DCs were subdivided into CD8α+ and CD11b+ subsets based on CD11b and CD8α expression (Fig. S3). Fig. 5A shows that, upon stimulation with all LPSs, an additional population displaying a CD11b+BST-2hi phenotype emerged within the CD11cint DC population compared to splenic cells from PBS-injected animals. Accumulation of such population exhibited an identical kinetics between Bm-wadC LPS and E. coli LPS, culminating at 24 h and vanishing at 48 h. Of note, Bm-wt LPS presented a weaker effect with lower cell numbers and a peak that started to diminish from 12 h on (Fig. 5B).
Figure 5.
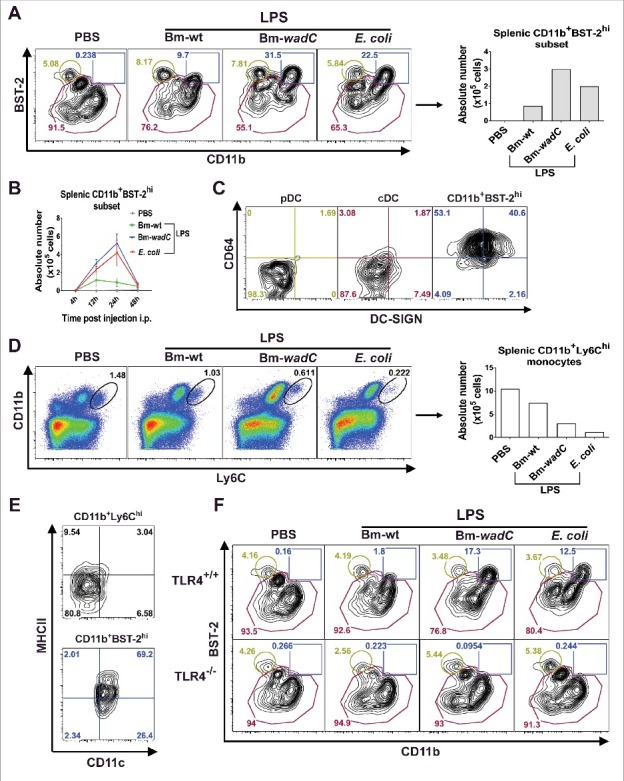
In vivo, LPSs favoured mobilisation of a mo-DC like cell type in a TLR4-dependent fashion. 6–8 weeks-old C57BL/6J mice were injected intraperitoneally with 1xPBS (PBS), Bm-wt LPS (30 μg/mouse), Bm-wadC LPS (20 μg/mouse) or E. coli LPS (10 μg/mouse). Quantities of injected LPS, calculated by KDO weight ratio, ensured equal molar weight for each of them. 4, 12, 24 and 48 h post-injection, mice were sacrificed, single-splenocyte suspensions were prepared and analysed by flow cytometry. (A) Dot plots of CD11b and BST-2 staining and absolute numbers of the CD11b+BST-2hi subset 12 h post-injection. (B) Absolute numbers of the CD11b+BST-2hi subset in spleen from injected mice at indicated time-points. (C) Dot plots of DC-SIGN and CD64 staining of the pDC, cDC and CD11b+BST-2hi subpopulations 12 h post-injection. (D) Dot plots of Ly6C and CD11b staining and absolute numbers of the CD11b+Ly6Chi monocytes 12 h post-injection. (E) Dot plots of CD11c and MHCII staining of the CD11b+Ly6Chi and CD11b+BST-2hi subpopulations 12 h post-injection. Data displayed from (A to E) are representative of 3 independent experiments, n = 3 mice per condition in each experiment. (F) 6–8 weeks-old TLR4+/+ or TLR4−/− mice were injected with PBS, Bm-wt LPS, Bm-wadC LPS or E. coli LPS as described above. 12 h later, mice were sacrificed, single-splenocyte suspensions were prepared and total CD11c+ cells were analysed by flow cytometry. Dot plots of BST-2 and CD11b staining is representative of 3 independent experiments, n = 3 mice per condition in each experiment.
Microbial stimulation has been shown to drive TLR4-mediated monocyte differentiation into mo-DC in immune T cell areas of lymph nodes.16,18 To determine if the CD11b+Bst-2hi subset herein identified corresponded to mo-DC-like cells, we examined the surface expression of CD64 and DC-SIGN and compared it with that of other cell types, including pDCs and cDCs. These molecules were indeed reported to mark the mo-DCs generated by TLR agonist in tissues during inflammation.16,18 We found that, more than 93% of the CD11b+Bst-2hi subset was CD64+, while more than 95% of cells remained CD64− in the cDC and pDC fractions. In total, DCs with CD64+DC-SIGNhi phenotype accounted for 40.6% of new emerging cells, and 1.87% and 1.69% of cDC and pDC, respectively (Fig. 5C). We further gated out splenic monocytes by CD11b and Ly6C expression, and found that reduction of CD11b+Ly6Chi monocytes upon stimulation occurred (Fig. 5D) simultaneously with the increase of the CD11b+BST-2hi population (Fig. 5A). Compared to steady-state monocytes, this population acquired DC morphology by elevating CD11c and MHCII expression levels (Fig. 5E). In TLR4−/− mice, mobilisation of CD11b+BST-2hi+ DCs by LPSs was completely abolished, demonstrating a critical dependence on the TLR4 signalling pathway (Fig. 5F). Altogether these findings suggested that LPS stimulation triggered a TLR4-mediated emergence of CD11b+BST-2hi DCs, recognised as mo-DC like cells, in the spleen. They also indicated that Bm-wt LPS played a limited but significant role in the mobilisation of such splenic mo-DC like cells while Bm-wadC LPS considerably favoured it.
Bm-wadC LPS, but not Bm-wt LPS, induces TLR4-mediated phenotypic activation of splenic cDC subsets
Finally, to characterise the maturation of splenic DC subsets upon Bm LPS stimulation in vivo, we examined the expression of a series of surface markers, indicators of activation (such as MHCII, CD86 or CD40) or inhibition (such as PDL-1). After 12 h of stimulation, Bm-wadC LPS dramatically induced the activation of cDC subsets (CD11b+ and CD8α+) from both CD11chi and CD11cint populations at comparable levels to those observed with E. coli LPS in terms of activation markers (MHCII, CD86, CD40) and PDL-1. pDCs displayed a low activation status with a slightly increased expression of maturation markers (Fig. 6 and S4). In comparison, Bm-wt LPS showed a poor ability to activate splenic DCs, with augmented CD86 expression in CD11chiCD8α+cDCs and PDL-1 in CD11chiCD11b+ and CD11cintCD11b+ cDCs only. In general, upon stimulation with Bm-wadC LPS or E. coli LPS, CD11c hi cDCs showed higher activation levels than CD11cint cDCs. Despite the absence of mo-DC-like cells in PBS-treated mice, we could evaluate the activation of this DC subset upon LPS treatments. Bm-wt LPS elicited the lowest activation levels of costimulatory molecule expression in the mo-DC-like subset. Contrasting with the mild expression of activation markers, PDL-1 expression on these cells displayed a greater response to Bm-wadC LPS and E. coli LPS. In TLR4−/− mice, splenic DCs failed to respond to any LPS leaving unchanged the basal levels of CD86 and CD40. These data demonstrated the absolute requirement of TLR4 for splenic DC maturation (Fig. 7).
Figure 6.
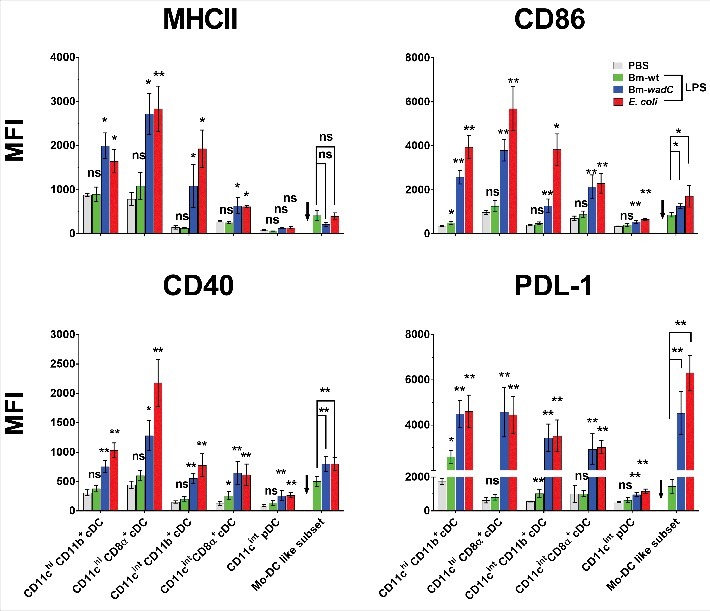
Bm-wt LPS poorly triggered phenotypic maturation of splenic DC subsets in vivo, in contrast to Bm-wadC LPS which promoted strong activation of DCs. 6–8 weeks-old C57BL/6J mice were injected intraperitoneally with 1xPBS (PBS), Bm-wt LPS (30 μg/mouse), Bm-wadC LPS (20 μg/mouse) or E. coli LPS (10 μg/mouse). 12 h later, mice were sacrificed, single-splenocyte suspensions were prepared and expression levels (MFI, Mean of Fluorescence Intensity) of MHCII, costimulatory molecules (CD86 and CD40) and inhibitory marker (PDL-1) on splenic DC subsets were determined by flow cytometry. Arrows point to the absence of data due to failure of mo-DC mobilisation by PBS. Data represent mean ± standard deviation of 3 independent experiments, each with n = 3 mice per condition. Statistical analysis was performed with the non-parametric one-way ANOVA test, followed by variance analysis with the Mann-Withney U test. Significant differences from PBS injected mice are shown; *, P < 0.05; **, P < 0.001. ns, non-significant.
Figure 7.
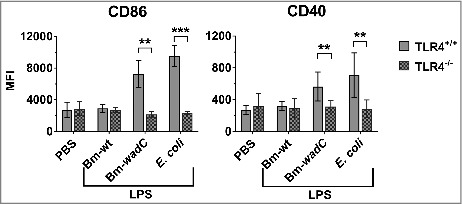
TLR4 was essential for LPS to drive maturation of splenic DCs. 6–8 weeks-old TLR4+/+ or TLR4−/- mice were intraperitoneally injected with 1xPBS (PBS), Bm-wt LPS (30 μg/mouse), Bm-wadC LPS (20 μg/mouse) or E. coli LPS (10 μg/mouse). 12 h later, mice were sacrificed, single-splenocyte suspensions were prepared and expression levels (MFI, Mean of Fluorescence Intensity) of costimulatory molecules (CD86 and CD40) on total splenic CD11c+ cells were determined by flow cytometry. Data obtained from 3 experiments, each with n = 3 animals per condition, are shown. All error bars are standard deviations obtained from pooled data. Statistical analysis was performed with the non-parametric one-way ANOVA test, followed by variance analysis with the Mann-Withney U test. Significant differences from TLR4+/+ injected mice are presented; **, P < 0.01; ***, P < 0.001.
B. melitensis mutant with a partially defective LPS core structure displays enhanced cytokine secretion levels in vivo
Our previous work demonstrated that the wadC mutants of both B. abortus22 and B. melitensis23 gave rise to an attenuated phenotype with lower CFUs in the spleen at 8 weeks post-infection in vivo as well as in GM-DCs 24 h post-infection in vitro compared to that observed with wild-type (WT) Brucella. In order to complete these studies and determine whether live wadC bacteria also impact the inflammatory response in vivo, we next investigated the effect of such wadC B. melitensis mutant on cytokine levels in the sera of infected mice. C57BL/6J mice were non-treated (Mock) or infected with Bm-wt or Bm-wadC strains for 8 days. Cytokine secretion was determined in sera by ELISA. Fig. 8 shows that wadC mutated Brucella triggered much higher levels of cytokine (IL-12p70, TNFα and IL-6) secretion in sera of mice at 8 days post-infection compared to those obtained with the WT strain. Altogether these data revealed that the wadC Brucella mutant drove a stronger inflammatory response than the WT strain.
Figure 8.
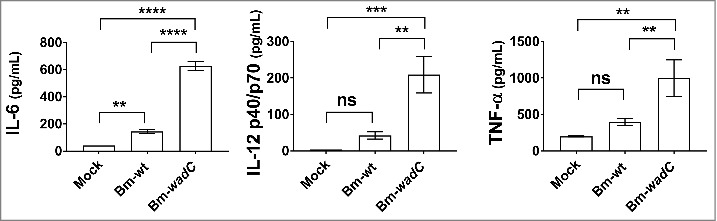
Bm-wadC mutant Brucella induced higher cytokine secretion than Bm-wt strain in infected mice. C57BL/6J mice were non-treated (Mock) or infected with B. melitensis 16M reference (Bm-wt) strain or Bm-wadC mutant strain for 8 days. Cytokine secretion was determined in sera by ELISA. Data obtained from 3 experiments, each with n = 3 animals per condition, are displayed. All error bars are standard deviations obtained from pooled data. Statistical analysis was performed with the parametric one-way ANOVA test, followed by variance analysis with the Tukey and Dunnett test. Significance was defined when P values were less than 0.05 (*, P < 0.05).
Discussion
Over the last decades Brucella LPS has been put forward as a poor immune stimulator that displayed less toxicity compared with other bacterial LPS, especially the E. coli one.7,29–31 Indeed, wild-type Brucella LPS did not induce inflammatory responses in macrophages and DCs, two of the most important sentinels of the immune system.2,4,5,7 This was attributed to its poor recognition by TLR4/MD-2, which is widely considered to be the major receptor complex for LPS binding and signalling.22 However, in recent years, a few reports demonstrated that Brucella LPS exhibited some adjuvant properties, which opened to debate its non-stimulatory immune properties.32–34 In addition, most of the findings at the cell level were obtained using macrophages and GM-DCs in vitro. In this context, further in-depth immunological characterisation of the effects of Brucella LPS in other cell models in vitro as well as in vivo appeared indispensable.
In this report, we first confirmed the inability of Bm-wt LPS to drive any activation of GM-DCs in terms of surface activation markers, inflammatory cytokine secretion, and T cell priming and proliferation in vitro. By comparing in this particular DC model the effects of a battery of LPSs that share some moeities with Bm-wt LPS, we found that LPSs made of a “Brucella-type” lipid A (O. anthropi 3331) or O-chain (Y. enterocolitica O:9) were in contrast able to drive strong GM-DC activation to levels comparable to those observed with E. coli LPS. Since the outer core deficiency (Bm-wadC LPS) by itself enhanced likewise GM-DC activation, including marked proliferation and activation of both CD4+ and CD8+ T cells, we identified this core component as the LPS molecular determinant responsible for Brucella poor immune cell activation ability. We propose that in Brucella wt LPS this oligosaccharide branching attached to the main oligosaccharide core section, which links lipid A and the O-polysaccharide, protrudes enough to mask lipid A determinants, hence hampering lipid A recognition by TLR4. Future crystallographic analyses of the Brucella wt LPS – TLR4 complex should determine the validity of this hypothesis. Collectively, these findings represent a substantial step in our understanding of the molecular mechanisms accounting for Brucella subversion of immune system recognition. Moreover, they define the LPS outer core deficiency as a key factor to be taken into consideration for the development of an efficient Brucella vaccine.
Towards this goal, we have previously published that both the wadC mutant of B. abortus25,35 and of B. melitensis23 give rise to an attenuated phenotype with reduced CFUs at 8 weeks post-infection compared to wild-type infected mice. We now show that compared to the wt strain, the wadC mutated Brucella from B. melitensis triggers much higher levels of pro-inflammatory cytokine secretion in sera of infected mice. Altogether these data indicate that the wadC Brucella mutant elicits a stronger inflammatory response than the wt strain resulting in an attenuated phenotype in vivo with a reduced bacterial burden. This enhanced immunoprotective ability opens up studies for assessing the potential of this mutant for vaccine development.
In addition, we reported that our murine GM-DC data could be translated to human GM/IL-4 mo-DCs, i.e. Bm-wadC LPS also stimulated cytokine secretion in human cells unlike Bm-wt LPS. These findings revealed that Brucella has developed some “universal” mechanisms of immune response subversion (including the inhibitory ability of its oligosaccharide LPS core) which operate both in human and mouse cells, although the latter animal recipient is not a natural Brucella host.
Our comparative approach also led us to disclose that Bm-wadC and O. anthropi 3331 LPSs, which share a “Brucella type”-lipid A, drove intermediate IL-10 secretion levels, whereas E. coli and Y. enterocolitica O:9 LPSs, which bear the same “E. coli type-lipid A” elicited 5 times more in GM-DCs. These results unravelled the “E. coli type”-lipid A as a critical feature for massive secretion of a key anti-inflammatory cytokine. As IL-10 has been shown to be one of the crucial cytokines involved in endotoxic shock driven by E. coli LPS,36 these data raise the question of the specific contribution of “E. coli type-lipid A” moiety in the etiology of this multiorgan failure syndrome upon infection. Concerning Brucella, further investigation will determine if its peculiar lipid A component is required to mediate mild IL-10 secretion in vivo. Moreover, assessing whether endotoxic shock might be generated by artificially augmented IL-10 to serum levels alike those produced by E. coli LPS in Bm-wt LPS injected mice, would formally shed light on one of the potential reasons explaining the low endotoxicity of Brucella LPS.
We extended our investigation of Brucella LPS immunomodulatory properties from GM-DCs to another DC system, the FL-DCs. We demonstrated that according to the DC model used, Bm-wt LPS differed in its ability to trigger immune cell activation. FL-DC pDCs displayed almost no activation status with only slightly increased expression of maturation markers, probably due to by-stander effects caused by the other DC subsets. The absence of pDC activation by any LPS, in FL-DCs in vitro or in splenic pDCs in vivo, most likely results from the low expression levels of TLR4 in these cells compared to that of other murine DC populations.37 In contrast, Bm-wt LPS efficiently promoted maturation of CD11b+ and CD24+ FL-cDC subsets by elevating MHCII, costimulatory molecules and by significantly releasing Th1 cytokines as well as low levels of IL-10. The differential response of the two DC models to a given microbial stimulation might result from their distinct intrinsic activation thresholds. In FL-DCs, the poor and non-significant secretion of IL-12p70 and IFN-γ by Bm-wt LPS may account for the partial activation (i.e. poor inducing T cell proliferation capacity) of these cells. The inability to stimulate secretion at high levels of such Th1 inducers by Bm-wt LPS in the FL-DCs in vitro seems to reflect the situation occuring in splenic DCs in vivo. Indeed, only the Bm-wadC LPS, but not the Bm-wt LPS, triggered significant maturation (i.e. up-regulation of co-stimulatory molecules) of splenic cDC subsets. Incomplete activation of DCs might contribute to the subversion of the immune system by Brucella LPS and participate in the establishment of Brucella chronicity, as IFN-γ and IL-12 have been shown to control Brucella infection.38–42 It remains that our work challenged the accepted paradigm by proving that in vitro Brucella LPS might exert some stimulatory effects on certain DC subsets. As in GM-DCs, the wadC defect relieved the inhibitory effect of Brucella LPS core structure on activation of FL-DC subsets, by further increasing maturation marker expression and cytokine release. These results therefore confirmed in another murine in vitro system the masking ability of Brucella core branching structure on lipidA immunogenicity. Y. enterolitica O:9 LPS-induced IL-10 levels were the highest in FL-DCs, but in the range of those engendered by the other LPS, reflecting a specific response of the FL-cDC subsets compared to that of GM-DCs. As regards Th1 cytokine secretion (IL-12p40, IL-12p70, TNF-α, IL-6, and IL-1β) in FL-DCs, O. anthropi 3331 was always the strongest inducer, revealing a peculiar role for O. anthropi 3331 core and oligosaccharide moieties.
The spleen is an ideal reservoir for a diversity of myeloid and dendritic cells distinct in antigen presenting functions.9 When examining the modulatory effects of Brucella LPS on DCs in vivo, we found that only the Bm-wadC LPS, but not the Bm-wt LPS, triggered significant maturation, i.e. notablly increased expression of surface activation markers, of splenic cDC subsets. The CD11chiCD11b+and CD11cintCD11b+ cDCs on the one hand and the CD11chiCD8α+ cDCs on the other hand were the sole splenic DC subsets which exhibited a faint up-regulation by Brucella wt-LPS of PDL-1 and CD40, respectively. CD8α+ cDCs are the predominant producers of IL-12, important for CD8+ T cell proliferation.43 CD8α− cDCs have a weaker cross-priming ability; upon LPS stimulation, they are relocalised from the marginal zone of spleen to T cell areas and secrete inflammatory chemokines. However, their primary role still remains unclear.44 The poor activation of these cDC subsets by Brucella wt-LPS in vivo most likely participates in the limited inflammatory response occurring during the course of Brucella infection.
We also observed that both Brucella LPS and E. coli LPS induced in a TLR4-dependent manner, concomitantly with the disappearance of splenic monocytes, the emergence of a cell type with mo-DC like phenotypic features. Mo-DCs are efficient antigen-presenting cells and good inducers of inflammatory responses.14,15,45 The weak, but significant, mobilisation of splenic mo-DC like cells elicited by Bm-wt LPS may, similarly to cDCs, restrict the in vivo inflammation during infection and promote setting up of Brucella replicative niche. This hypothesis is consistent with our previous in vitro report24 showing that higher DC activation correlates with an inefficient ER replicative niche targeting of intracellular bacteria.
Collectively, these findings illustrate how a poor activation of DC subsets in vivo may contribute to the immune system avoidance elicited by Brucella, ultimately causing establishment of chronic disease.
In conclusion, this study extensively documented both in vitro and in vivo Brucella LPS immunomodulatory abilities, shedding light on its effect on cDC subsets and mo-DC like cells in particular. The enhanced stimulatory property of Brucella LPS with a core structure defect pleads for the assessment of the benefits of this mutation for new vaccine strategies targeting bacterial LPS.
Materials and methods
Ethics statement
Animal experimentation was conducted in strict compliance with good animal practice as defined by the French animal welfare bodies (Law 87–848 dated 19 October 1987 modified by Decree 2001–464 and Decree 2001–131 relative to European Convention, EEC Directive 86/609). All animal work was approved by the Direction Départementale des Services Vétérinaires des Bouches du Rhône. Live Brucella infection protocol was approved by the ANSM. All efforts were made to minimise suffering during animal handling and experimentation. As regards human PBMCs, blood was taken from healthy volunteers acquired under protocols approved by the Institutional Review Board (IRB) of Baylor Research Institute (BRI).
Mice
6–8 week-old female C57BL/6J mice from Charles River, TLR4−/− mice46 on a C57BL/6J background and OT-I, OT-II TCR transgenic mice on a C57BL/6J background from the Jackson laboratory were used. Animals were housed in cages with water and food ad libitum in the CIML or CIPHE animal house facilities (for the latter, under biosafety containment conditions for 2 weeks before the start and all along infection with live bacteria).
Lipopolysaccharides
LPS from different Brucella strains (16M Biovar 1 reference strain, wt or wadC mutant23), E. coli ATCC 35218, Y. enterocolitica O:9 (MY79), O. anthropi (LMG 3331T) were extracted and purified as described previously.22,27 Briefly, Brucella and Y. enterocolitica LPSs were obtained from the phenol phase of a water-phenol extract. These crude LPS preparations were then purified by removing the free-lipids by solvent extraction, digestion with nucleases and proteinase K and ultracentrifugation.27 O. anthropi LPS (obtained from the water phase) was purified in the same way as Brucella LPS. E. coli LPS was obtained from the water phase of a similar phenol-water extract and then purified by the phenol-water deoxycholate method.47 A stock of 1 mg/mL of each LPS was prepared in pyrogen free sterile water, sonicated briefly and sterilized by autoclaving. Prior to use, the stock was heated at 56°C for 15 min and then cooled to room temperature. The molar mass ratio of different LPS (E. coli LPS: Bm-wt LPS: Bm-wadC = 1:3:2) was calculated based on the KDO content, as the use of this LPS core marker allows for correction of differences in dry weight that could result from different O-polysaccharide lenghts and lipid A molecular weights.48 To compare the immunomodulatory properties of different LPSs in vivo, equal molarity of LPSs were intraperitoneally injected in mice, with a dosage of 10 µg, 30 µg or 20 µg per mouse for E. coli LPS, Bm-wt LPS and Bm-wadC, respectively.
In vitro generation of BMDCs
BMDCs were prepared from 6–10 week-old C57BL/6J female femurs and tibias as previously described.24 Briefly, bone ends were cut off and bone marrow was flushed with RPMI medium (GIBCO, 21875–034) containing 5% FCS and 50 mM of 2-mercaptoethanol (GIBCO, 31350–010). Red blood cells were removed by 1 min exposure to 1xRBC lysis buffer solution (eBioscience, 00–4333–57). For GM-DCs, 3 × 106 cells were seeded onto 6-well plates in 5 mL medium containing 0.8 % supernatant of the J558L GM-CSF producing cell line. Medium was changed at day 2.5 and GM-DCs were ready to use at day 5. For FL-DCs, 8 × 106 cells were seeded onto 6-well plates in 4 mL medium containing 3% supernatant of the B16 FLT3 Ligand producing cell line. FL-DCs were ready to use at day 8.5–9 without any change of medium.
Stimulation of BMDCs
Cells were mock-treated or stimulated for 24h with Bm-wt LPS (10 µg/mL), Bm-wadC LPS (10 µg/mL), E. coli LPS (100 ng/mL). The dosage of LPS for stimulation was optimised previously22; in the GM-DC model, saturating concentration for Bm-wadC LPS was 10 μg/mL, while that of E. coli LPS was 100 ng/mL. Cells were collected for FACS analysis and supernatant was kept at −80°C. Cytokines were measured by ELISA kits for IL12p70 (88–7121-76), IL-12/IL-23 total p40 (88–7120-86), IL-6 (88–7064-88), IL-10 (88–7105-88), IFN-γ (88–7314-76), TNF-α (88–7324-88), IL-1β (88–7013-86).
Human mo-DCs
Human monocyte-derived DCs were generated from Ficoll-separated PBMC from healthy volunteers. Monocytes were enriched from the leukopheresis according to cellular density and elutriation following manufacturer's instructions. For DC generation, monocytes were resuspended in serum-free Cellgro DC culture supplemented with 1ng/mL GM-CSF and 0.1 ng/mL IL-4 and kept in culture for 7 days. The mo-DCs were then stimulated as previously described with each LPS at 20 ng/mL.49
Flow cytometry
Cells were harvested and stained for 20 min at 4°C with the antibody mix. After a wash in PBS with 2% of FCS, cells were stained with Fixable Viability Dye eFluor 506 (eBiosciences, 65–0866-14) for 10 min at room temperature. Cells were then fixed for 20 min in 3.2% PFA at room temperature. mAbs in the staining mix for BMDCs or splenocytes were the following: CD11c PeCy7 (N418), MHC II PE (M5/11.15.2), CD86 FITC (GL-1), CD86 PE (GL-1), CD80 PeCy5 (16–10A1), CD40 APC (HM40–3), CD24 FITC (M1/69), NK1.1 APC-Cy7 (PK136), PDL-1 BV605 (10F.9G2) from Biolegend; MHCII A700 (M5/11.15.2), CD11b eF450 (M1/70), Bst-2 EF610 (eBio927), DC-SIGN APC (eBio22D1) from eBiosciences; CD40 PeCy5 (3/23), B220 PETXR (RA3–6B2), CD3 APC-Cy7 (145–2C11), CD19 APC-Cy7 (1D3), CD64 PE (X54–5/7.1), CD8α BV711 (53–6.7) from BD Biosciences. Flow cytometry was performed using a FACS LSRII-UV (BD Biosciences) and data were analysed with the FlowJo_v9.9.4 software.
Murine splenocyte preparation
Mice were anesthetised by CO2 inhalation. Spleens were dissected from abdominal cavity, digested for 20 min at 37°C with type II collagenase (Worthington Biochemical Corporation, LS004174) and DNase I (Sigma, DN25–100MG) and then treated with 10 mM EDTA to stop digestion. Pieces of spleen cut by scissors were crushed with a syringe plunger in a 70-μm nylon strainer cell strainer and filtered in PEF buffer (1xPBS, 5 mM EDTA, 2% FCS). Red cell lysis buffer was used to remove red cells. Single splenic cell suspensions then proceeded to FACS analysis.
In vitro antigen presentation assays
GM- or FL-DCs (104 cells) were incubated for 16 h in 96-well culture plates with endotoxin-free OVA at 500 μg/mL (Hyglos, 321001). They were then stimulated or not (Mock) for 8 h with different LPSs as described above and washed. OT-I and OT-II T cells were isolated from spleens of OT-I or OT-II mice using a CD8+ or CD4+ T cell-negative isolation kit (Dynal, Invitrogen, 11348D and 11352D), respectively. Purity was determined by staining with CD4, CD8 and CD5. Purified OT-I and OT-II T cells were finally resuspended in 1xPBS containing 10 mM CFSE (Molecular Probes, C34554) for 10 min at 37°C. CFSE-labelled OT-II or OT-I cells (105 cells) were added to the BMDCs prepared as aforementioned. The proliferation of OT-I and OT-II T cells was assessed by measuring CFSE profiles by flow cytometry after 48 h of co-culture.
Splenic DC stimulation in vivo
4 h, 12 h, 24 h or 48 h after intraperitoneal injection of mice with 1xPBS or LPS, spleens were collected, single-cell suspensions were prepared by gentle dissociation and enzymatic digestion as described above and then analysed by flow cytometry.
In vivo Brucella infection
C57BL/6J mice were non-treated (Mock) or infected with B. melitensis 16M reference (Bm-wt) strain or Bm-wadC mutant strain. After 8 days, sera were collected and kept at −80°C until cytokine measurement.
Statistical analysis
All values are expressed as mean ± standard deviation. All experiments were performed at least three times in triplicate otherwise indicated. Statistical analyses were done using with the GraphPad Prism software. Non-parametric one-way ANOVA test followed by variance analysis with the Benjamin and Hochberg test were performed, otherwise indicated in the figure legend. Differences between values were considered significant at P < 0.05 (*, P < 0.05; **, P < 0.001; ***, P < 0.0001; ****, P < 0.00001).
Funding Statement
YZ was funded by the China Scholarship Council. This work was supported by Ministerio de Economía y Competitividad Grant AGL2014-58795-C4-1-R, by the Fondation de la Recherche Médicale Grant FRM-CS and by institutional grants from the Centre National de la Recherche Scientifique and the Institut national de la Santé et de la Recherche médicale.
Abbreviations
- Bm
Brucella melitensis
- DC
dendritic cells
- FL-DC
Flt3L-derived DCs
- Flt3L
Fms-like tyrosine kinase 3 Ligand
- GMCSF
Granulocyte macrophage-colony stimulating factor
- GM-DC
GM-CSF-derived dendritic cells
- IFN
interferon
- IL
interleukine
- LPS
Lipopolysaccharide
- mo
monocyte
- PBMC
peripheral blood mononuclear cells
- TLR
toll-like receptor
- TNF
Tumor necrosis factor
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowlegments
We thank A. Delgado-López for excellent technical assistance in the extraction and purification of LPS and CIML Animal house and Cytometry core facilities.We are also thankful to L. Spinelli, who helped with statistical analyses.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- [1].Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: A systematic review of disease frequency. PLoS Negl Trop Dis. 2012;6. doi: 10.1371/journal.pntd.0001865. PMID:23145195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gorvel JP, Moreno E. Brucella intracellular life: From invasion to intracellular replication. Vet Microbiol. 2002;90:281–97. doi: 10.1016/S0378-1135(02)00214-6. PMID:12414149 [DOI] [PubMed] [Google Scholar]
- [3].Gorvel JP. “If you bring an alarm, we will destroy it,” said Brucella to the host cell. Virulence. 2014;5:460–2. doi: 10.4161/viru.29092. PMID:24786767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzman-Verri C, Chacon-Diaz C, Rucavado A, Moriyón I, Moreno E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One. 2007;2. doi: 10.1371/journal.pone.0000631. PMID:17637846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Celli J. Surviving inside a macrophage: The many ways of Brucella. Res Microbiol. 2006;157:93–8. doi: 10.1016/j.resmic.2005.10.002. PMID:16364608 [DOI] [PubMed] [Google Scholar]
- [6].Erridge C, Bennett-Guerrero E, Poxton IR. Structure and function of lipopolysaccharides. Microbes Infect. 2002;4:837–51. doi: 10.1016/S1286-4579(02)01604-0. PMID:12270731 [DOI] [PubMed] [Google Scholar]
- [7].Cardoso PG, Macedo GC, Azevedo V, Oliveira SC. Brucella spp noncanonical LPS: Structure, biosynthesis, and interaction with host immune system. Microb Cell Fact. 2006;5:13. doi: 10.1186/1475-2859-5-S1-S13 . PMID:16556309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. PMID:11913066 [DOI] [PubMed] [Google Scholar]
- [9].Hey YY, O'Neill HC. Murine spleen contains a diversity of myeloid and dendritic cells distinct in antigen presenting function. J Cell Mol Med. 2012;16:2611–9. doi: 10.1111/j.1582-4934.2012.01608.x. PMID:22862733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen WF, et al.. Cutting edge: Generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–7. doi: 10.4049/jimmunol.174.11.6592. PMID:15905497 [DOI] [PubMed] [Google Scholar]
- [11].Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, et al.. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–26. doi: 10.1038/ni1522. PMID:17922015 [DOI] [PubMed] [Google Scholar]
- [12].Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–62. doi: 10.1111/j.0105-2896.2009.00881.x. PMID:20193017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Behboudi S, Moore A, Hill AVS. Splenic dendritic cell subsets prime and boost CD8 T cells and are involved in the generation of effector CD8 T cells. Cell Immunol. 2004;228:15–9. doi: 10.1016/j.cellimm.2004.03.010. PMID:15203315 [DOI] [PubMed] [Google Scholar]
- [14].Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol. 2008;86:320–4. doi: 10.1038/icb.2008.14. PMID:18362945 [DOI] [PubMed] [Google Scholar]
- [15].Qu CF, Brinck-Jensen NS, Zang MY, Chen K. Monocyte-derived dendritic cells: Targets as potent antigen-presenting cells for the design of vaccines against infectious diseases. Int J Infect Dis. 2014;19:1–5. doi: 10.1016/j.ijid.2013.09.023. PMID:24216295 [DOI] [PubMed] [Google Scholar]
- [16].Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Gregoire C, Malissen B, Guilliams M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188:1751–60. doi: 10.4049/jimmunol.1102744. PMID:22262658 [DOI] [PubMed] [Google Scholar]
- [17].Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, et al.. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–35. doi: 10.1016/j.immuni.2012.10.016. PMID:23352232 [DOI] [PubMed] [Google Scholar]
- [18].Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al.. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–29. doi: 10.1016/j.cell.2010.09.039. PMID:21029863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone-marrow cultures supplemented with granulocyte macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. PMID:1460426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weigel BJ, Nath N, Taylor PA, Panoskaltsis-Mortari A, Chen W, Krieg AM, Brasel K, Blazar BR. Comparative analysis of murine marrow-derived dendritic cells generated by Flt3L or GM-CSF/IL-4 and matured with immune stimulatory agents on the in vivo induction of antileukemia responses. Blood. 2002;100:4169–76. doi: 10.1182/blood-2002-04-1063. PMID:12393694 [DOI] [PubMed] [Google Scholar]
- [21].Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–84. doi: 10.4049/jimmunol.179.11.7577. PMID:18025203 [DOI] [PubMed] [Google Scholar]
- [22].Conde-Alvarez R, Arce-Gorvel V, Iriarte M, Mancek-Keber M, Barquero-Calvo E, Palacios-Chaves L, Chacón-Díaz C, Chaves-Olarte E, Martirosyan A, von Bargen K, et al.. The lipopolysaccharide core of brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog. 2012;8. doi: 10.1371/journal.ppat.1002675. PMID:22589715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fontana C, Conde-Alvarez R, Stahle J, Holst O, Iriarte M, Zhao Y, Arce-Gorvel V, Hanniffy S, Gorvel JP, Moriyón I, et al.. Structural studies of lipopolysaccharide-defective mutants from brucella melitensis identify a core oligosaccharide critical in virulence. J Biol Chem. 2016;291:7727–41. doi: 10.1074/jbc.M115.701540. PMID:26867577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Papadopoulos A, Gagnaire A, Degos C, de Chastellier C, Gorvel JP. Brucella discriminates between mouse dendritic cell subsets upon in vitro infection. Virulence. 2016;7:33–44. doi: 10.1080/21505594.2015.1108516. PMID:26606688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gil-Ramirez Y, Conde-Alvarez R, Palacios-Chaves L, Zuniga-Ripa A, Grillo MJ, Arce-Gorvel V, Hanniffy S, Moriyón I, Iriarte M. The identification of wadB, a new glycosyltransferase gene, confirms the branched structure and the role in virulence of the lipopolysaccharide core of Brucella abortus. Microb Pathog. 2014;73:53–9. doi: 10.1016/j.micpath.2014.06.002. PMID:24927935 [DOI] [PubMed] [Google Scholar]
- [26].Barquero-Calvo E, Conde-Alvarez R, Chacon-Diaz C, Quesada-Lobo L, Martirosyan A, Guzman-Verri C, Iriarte M, Mancek-Keber M, Jerala R, Gorvel JP, et al.. The differential interaction of Brucella and Ochrobactrum with innate immunity reveals traits related to the evolution of stealthy pathogens. PLoS One. 2009;4. doi: 10.1371/journal.pone.0005893. PMID:19529776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Velasco J, Bengoechea JA, Brandenburg K, Lindner B, Seydel U, Gonzalez D, Zähringer U, Moreno E, Moriyón I. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp., differ in outer membrane permeability and cationic peptide resistance. Infect Immun. 2000;68:3210–8. doi: 10.1128/IAI.68.6.3210-3218.2000. PMID:10816465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Velasco J, Moll H, Knirel YA, Sinnwell V, Moriyon I, Zahringer U. Structural studies on the lipopolysaccharide from a rough strain of Ochrobactrum anthropi containing a 2,3-diamino-2,3-dideoxy-D-glucose disaccharide lipid A backbone. Carbohydr Res. 1998;306:283–90. doi: 10.1016/S0008-6215(97)10029-5. PMID:9691452 [DOI] [PubMed] [Google Scholar]
- [29].Goldstein J, Hoffman T, Frasch C, Lizzio EF, Beining PR, Hochstein D, Lee YL, Angus RD, Golding B. Lipopolysaccharide (Lps) from Brucella-abortus is less toxic than that from Escherichia-coli, suggesting the possible use of B-abortus or Lps from B-abortus as a carrier in vaccines. Infect Immun. 1992;60:1385–9. PMID:1548064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lapaque N, Moriyon I, Moreno E, Gorvel JP. Brucella lipopolysaccharide acts as a virulence factor. Curr Opin Microbiol. 2005;8:60–6. doi: 10.1016/j.mib.2004.12.003. PMID:15694858 [DOI] [PubMed] [Google Scholar]
- [31].Martirosyan A, Moreno E, Gorvel JP. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol Rev. 2011;240:211–34. doi: 10.1111/j.1600-065X.2010.00982.x. PMID:21349096 [DOI] [PubMed] [Google Scholar]
- [32].Jamalan M, Ardestani SK, Zeinali M, Mosaveri N, Mohammad Taheri M. Effectiveness of Brucella abortus lipopolysaccharide as an adjuvant for tuberculin PPD. Biologicals. 2011;39:23–8. doi: 10.1016/j.biologicals.2010.08.005. PMID:20965746 [DOI] [PubMed] [Google Scholar]
- [33].Kianmehr Z, Soleimanjahi H, Ardestani SK, Fotouhi F, Abdoli A. Influence of Brucella abortus lipopolysaccharide as an adjuvant on the immunogenicity of HPV-16 L1VLP vaccine in mice. Med Microbiol Immunol. 2015;204:205–13. doi: 10.1007/s00430-014-0356-z. PMID:25187406 [DOI] [PubMed] [Google Scholar]
- [34].Moreno E, Kurtz RS, Berman DT. Induction of immune and adjuvant immunoglobulin G responses in mice by Brucella lipopolysaccharide. Infect Immun. 1984;46:74–80. PMID:6434430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Conde-Alvarez R, Arce-Gorvel V, Gil-Ramirez Y, Iriarte M, Grillo MJ, Gorvel JP, Moriyón I. Lipopolysaccharide as a target for brucellosis vaccine design. Microb Pathog. 2013;58:29–34. doi: 10.1016/j.micpath.2012.11.011. PMID:23219811 [DOI] [PubMed] [Google Scholar]
- [36].Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grünig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–47. doi: 10.1172/JCI118290. PMID:7593621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi: 10.3389/fimmu.2014.00316. PMID:25071777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ahmed K, Al-Matrouk KA, Martinez G, Oishi K, Rotimi VO, Nagatake T. Increased serum levels of interferon-gamma and interleukin-12 during human brucellosis. Am J Trop Med Hyg. 1999;61:425–7. doi: 10.4269/ajtmh.1999.61.425. PMID:10497984 [DOI] [PubMed] [Google Scholar]
- [39].Baldwin CL, Goenka R. Host immune responses to the intracellular bacteria Brucella: Does the bacteria instruct the host to facilitate chronic infection? Crit Rev Immunol. 2006;26:407–42. doi: 10.1615/CritRevImmunol.v26.i5.30. PMID:17341186 [DOI] [PubMed] [Google Scholar]
- [40].Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect Immun. 1995;63:1387–90. PMID:7890399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol. 2008;180:1080–7. doi: 10.4049/jimmunol.180.2.1080. PMID:18178848 [DOI] [PubMed] [Google Scholar]
- [42].Brandao AP, Oliveira FS, Carvalho NB, Vieira LQ, Azevedo V, Macedo GC, Oliveira SC. Host susceptibility to Brucella abortus infection is more pronounced in IFN-gamma knockout than IL-12/beta2-microglobulin double-deficient mice. Clin Dev Immunol. 2012;2012:589494. doi: 10.1155/2012/589494. PMID:22194770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. PMID:15233723 [DOI] [PubMed] [Google Scholar]
- [44].Naik SH. Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol. 2008;86:439–52. doi: 10.1038/icb.2008.28. PMID:18414430 [DOI] [PubMed] [Google Scholar]
- [45].Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582 . PMID:24445665 [DOI] [PubMed] [Google Scholar]
- [46].Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. PMID:10201887 [PubMed] [Google Scholar]
- [47].Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. PMID:10878331 [DOI] [PubMed] [Google Scholar]
- [48].Lapaque N, Takeuchi O, Corrales F, Akira S, Moriyon I, Howard JC, Gorvel JP. Differential inductions of TNF-alpha and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell Microbiol. 2006;8:401–13. doi: 10.1111/j.1462-5822.2005.00629.x. PMID:16469053 [DOI] [PubMed] [Google Scholar]
- [49].Martirosyan A, Ohne Y, Degos C, Gorvel L, Moriyon I, Oh S, Gorvel JP. Lipopolysaccharides with acylation defects potentiate TLR4 signaling and shape T cell responses. PLoS One. 2013;8:e55117. doi: 10.1371/journal.pone.0055117. PMID:23390517 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


