Abstract
Syndemic theory describes the clustering and synergistic interaction of disease driven by contextual and social factors, which worsen health outcomes for a population, and has been applied to men who have sex with men (MSM) and their risk for HIV and other sexually transmitted infections. Recent reviews, however, have critiqued prevailing approaches in syndemic studies that assess only additive associations without evaluation of synergy. Following these suggestions, we compared the traditional additive approach with a test for synergistic association of 5 syndemic conditions (alcohol dependence, illicit drug use, depression, intimate partner violence (IPV), and childhood sexual abuse (CSA)) with unprotected anal intercourse (UAI) and active syphilis infection among 11,771 MSM recruited through respondent driven sampling from 12 cities in India. UAI was assessed via self-report and active syphilis infection was diagnosed by RPR and THPA tests. An additive association was explored using a condition count (range 0–5), and synergy was tested using relative excess risk due to interactions (RERIs) calculated from all 2-way and common 3-way interactions between conditions in adjusted regression models. There was a significant dose response associated with the syndemic count and UAI, and a similar pattern for syphilis, though not statistically significant. RERIs showed synergy for only one pair of conditions for UAI and syphilis, respectively: IPV+depression and alcohol dependence+illicit drug use. In this study, we found an additive association between syndemic conditions and UAI with evidence of synergistic interaction between a pair of psychosocial conditions, and no significant additive association, but a synergistic interaction between another pair of psychosocial conditions for syphilis. Our results lend further support to a critical reassessment of syndemic analyses. Closer attention to the cumulative development, underlying causal pathways, and possible synergistic interaction of co-occurring epidemics through combined qualitative and quantitative methodologies may yield more effective interventions for vulnerable, marginalized populations.
Keywords: syndemic theory, psychosocial factors, HIV, men who have sex with men, unprotected anal intercourse, syphilis, India
INTRODUCTION
Syndemic theory has received significant attention in recent years as an approach for understanding and addressing key issues in public health. In a recent Lancet series Singer and colleagues revisited the concept, and defined syndemics as “Population-level clustering of social and health problems,” which fulfill three criteria: “(1) two (or more) diseases or health conditions cluster within a specific population; (2) contextual and social factors create the conditions in which two (or more) diseases or health conditions cluster; and (3) the clustering of diseases results in adverse disease interaction, either biological or social or behavioural, increasing the health burden of affected populations.” (Singer, Bulled et al. 2017). While syndemic theory has been applied to a wide range of contexts, it has gained particular prominence in conceptualizing the increased vulnerability of men who have sex with men (MSM) to sexually transmitted infections (STIs), especially HIV (Tsai and Burns 2015). Over the past 15 years, a substantial body of research has documented a clustering of psychosocial conditions experienced by MSM, which are associated with greater HIV risk behaviours (e.g., unprotected anal intercourse), often in a dose-response or additive manner (Mimiaga, O’Cleirigh et al. 2015). Recently, a study of 4295 men demonstrated that a greater number of syndemic conditions is also associated with greater HIV incidence (Mimiaga, O’Cleirigh et al. 2015). In these studies, the syndemic approach has been used to argue for developing integrated, multi-component psychosocial interventions for HIV-prevention among MSM.
In a recent systematic review of syndemic studies of psychosocial conditions and HIV risk, however, Tsai and Burns (2015) identified several key limitations to these studies. First, there is a dearth of research in low- and middle-income settings where the majority of people living with HIV (PLHIV) live; four-fifths (80%) of the reviewed studies were carried out in the U.S. Second, the majority of studies to date have not used analytical approaches that enable an examination of whether and how the co-occurring psychosocial conditions assert synergistic effects on HIV risk – a fundamental assumption of syndemic theory. The most common analytical approach is an additive (count or sum) approach, wherein the association of the cumulative number of conditions is explored with an HIV behavioral outcome (e.g., unprotected anal intercourse) or prevalent HIV or other sexually transmitted infection, essentially investigating the presence of a dose response. (This approach should not to be confused with an additive interaction, which evaluates whether the joint effect (e.g. risk) of factors differs from the sum of the individual effects.) In a recent update to this review for the Lancet syndemics series, Tsai et al. (2017) reported that while there are signs of greater attention to vulnerable populations both within and beyond the U.S., syndemic studies continue to use the language of synergistic interaction but fail to test for such a relationship in their methods.
Additionally, Tsai and Burns (2015) and Tsai and Venkataramani (2016) recommended alternative methodologies that assess disease interaction in order to investigate whether and how the conditions that are hypothesized to constitute a syndemic act together to worsen HIV risk beyond what would be expected by each of these factors alone (i.e., beyond additive). The authors in both papers also explored the implications of synergistic interaction for interventions. While most syndemic studies advocate for multi-component interventions, in low-resource settings with evidence of synergy, it may be more cost-effective to specifically target select conditions that amplify risk the most, rather than all of them simultaneously. In contrast, multi-component efforts that devote attention to all or most of the individual syndemic conditions in a single setting may be more effective in the absence of synergistic interaction.
Our paper takes up these calls for additional research on syndemics outside the U.S. that employ appropriate statistical methodologies for investigating the synergistic interaction of hypothesized syndemic conditions (Tsai and Burns 2015, Tsai and Venkataramani 2016, Tsai, Mendenhall et al. 2017). Specifically, our study draws on a large baseline data set for a cluster randomized trial for MSM in 12 sites across India as a “test case” for comparing the traditional count-based methodological approach with an alternative test for synergistic interaction. Our analyses also offer an opportunity to revisit the definition of syndemics and its application to quantitative studies of population health.
India presents an important setting for examining syndemic production of sexual risk among MSM outside the U.S. First, it has the third highest number of people living with HIV and MSM bear a disproportionately high burden of these infections, with a prevalence of 4.43% compared with 0.35% in the general population (Department of AIDS Control, 2013). A substantial literature documents how stigmatization and discrimination contributes to poor psychosocial health and limits access to HIV-related services (Go, Srikrishnan et al. 2004, Safren, Thomas et al. 2009, Mimiaga, Thomas et al. 2011, Sivasubramanian, Mimiaga et al. 2011, Thomas, Mimiaga et al. 2011, Solomon, Mehta et al. 2015, Tomori, McFall et al. 2016). Men are expected to conform to cultural expectations of masculinity in behavior and appearance, and to marry and have children, regardless of their preference (Setia, Sivasubramanian et al. 2010, Solomon, Mehta et al. 2010, Tomori, Srikrishnan et al. 2016). We have also previously identified high prevalence of a number of psychosocial conditions associated with syndemics in our study population, including alcohol use (Solomon, Mehta et al. 2015), depression and suicidal thoughts (Tomori, McFall et al. 2016), and childhood sexual abuse (Tomori, McFall et al. 2016).
Our study considers the impact of psychosocial conditions on two outcome measures: recent unprotected anal intercourse (UAI) and active syphilis infection. UAI is the most common behavioral sexual risk outcome used in the syndemic literature, while syphilis provides a biological outcome. Syphilis is currently on the rise among men who have sex with men globally (Abara, Hess et al. 2016, Fenton, Breban et al. 2008), with prevalence estimates of 3–6% of high-titre syphilis among MSM and hijras in southern Indian states (Brahman, Kodavalla et al. 2008) as compared to 0.5% among the general Indian population (Khan, Menezes, et al. 2014). Syphilis infection has further significance because of the biological mechanisms that facilitate HIV acquisition and transmission (Wang, Chen et al. 2014, Paz-Baily, Meyers et al. 2004).
METHODS
Study Design
We examined the relationship of syndemic conditions with UAI and syphilis infection using cross-sectional data from 11,771 MSM in India recruited using respondent-driven sampling, a chain-referral strategy for recruiting hard-to-reach participants whereby the resulting sample is considered representative of the target population (Volz and Heckathorn 2008, White, Lansky et al. 2012, Solomon, Lucas et al. 2013). Data were collected as the baseline assessment for a cluster-randomized HIV-prevention trial among MSM (ClinicalTrials.gov Identifier: NCT01686750) conducted in 12 cities from five states and one Union Territory of India (Solomon, Lucas et al. 2013, Solomon, Mehta et al. 2015) between October 2012 and June 2013.
Detailed study procedures for the baseline assessment have been published elsewhere (Solomon, Mehta et al. 2015). Briefly, recruitment in each city was initiated using two seeds (i.e., MSM who were well-connected in the community), with the exception of New Delhi where three seeds were used. Seeds and all other eligible enrolled participants were provided two recruitment coupons to give to other MSM they knew in the city. Recruitment continued until 1000 MSM were sampled in each city (median time to recruit: 98 days, range: 69–156 days). Eligibility criteria included: (1) age ≥18 years, (2) self-identify as male (hijra/transgender women were excluded), (3) report oral/anal sex with a man in the prior 12 months, (4) provision of oral informed consent, and (5) possession of a valid referral coupon (except for seeds). One study site was established in each city. Participants completed an interviewer-administered electronic survey, provided a blood sample, and underwent onsite rapid HIV testing with pre-and post-test counseling. The interviewer-administered survey included modules on socio-demographics, sexual risk behaviors and characteristics, substance use, and depression.
Outcome Measures
UAI in the prior 6 months was assessed via interview-administered survey using questions on condom use during anal intercourse with male sexual partners in the prior 6 months, both casual and regular partners. Active syphilis infection was diagnosed using a reflex testing algorithm on blood samples provided during the RDS study visit. An initial RPR (rapid plasma reagin) (Span Diagnostics Ltd., Surat, India) test was performed on all samples and samples testing positive were subject to a TPHA (Treponema pallidum haemagglutination) test, a treponemal antibody test (Omega Diagnostics, Scotland, United Kingdom). Active syphilis infection was defined as a positive RPR and TPHA test. All results with appropriate referrals for care were delivered to participants.
Syndemic Conditions
Five syndemic conditions were chosen based on their previous associations with HIV risk and their frequent use in the syndemic literature, including alcohol dependence, illicit drug use, depression, intimate partner violence (IPV), and childhood sexual abuse (CSA) (Tsai and Burns 2015). Syndemic conditions were ascertained by self-report using the interviewer-administered electronic survey. Alcohol use in the past year was assessed using the AUDIT instrument (Saunders, Aasland et al. 1993, Volk, Steinbauer et al. 1997), with a score of 15 or higher categorized as alcohol dependence. Recent illicit drug use was defined as reporting any injection or non-injection drug use (excluding marijuana and intoxicating tobacco) in the prior 6 months. The presence of depression was measured using a cut-off of 10 or higher on the Patient Health Questionnaire-9 (PHQ-9) (Kroenke, Spitzer et al. 2001), which has been validated and extensively used in India (Kochhar, Rajadhyaksha et al. 2007, Patel, Araya et al. 2008, Ganguly, Samanta et al. 2013). History of IPV was assessed by asking the participant whether he “ever had a sexual partner (including a current or former spouse, boy/girlfriend, or other sexual partner) who has hit, slapped, kicked, shoved, or otherwise hurt him.” History of CSA was assessed by asking the participant whether he “experienced any unwanted sexual experiences (i.e., sexual touching or sexual intercourse, either oral or anal) when he was growing up (before 16 years old).”
Statistical Methods
Population summary statistics were calculated using a composite weight that accounts for the relative size of the adult male population in each city using the 2011 Indian Census and the RDS-II weight (Volz and Heckathorn 2008), which weights for the inverse of an individual’s network size (i.e., the number of MSM in the city whom the participant saw in the prior 30 days). City-level UAI and syphilis prevalence estimates have been previously reported (Solomon, Mehta et al. 2015).
An additive association of syndemic conditions was explored using a cumulative condition count, summing all conditions endorsed or present for each individual, resulting in a range of 0 (no conditions) to 5 (all conditions). Since few men had 4 or 5 conditions (2.2% of the sample), the highest count category was re-defined as 3 or more conditions. Univariable and multivariable log poisson regression models were used to estimate the association (i.e., prevalence ratio) of the condition count as a categorical variable with recent UAI. Log poisson was used to estimate the prevalence ratio of UAI due to convergence problems with log binomial models. Log binomial models were used to estimate prevalence ratios of active syphilis infection.
Synergistic associations of syndemic conditions were tested using the relative excess risk due to interaction (RERI), which assesses departures of additivity from relative measures of association, such as relative risks and odds ratios (Rothman, Greenland et al. 2008). RERIs and 95% confidence intervals (VanderWeele and Knol 2014) for both UAI and syphilis were calculated for each 2-way condition interaction and the most common 3-condition patterns using separate multivariable log poisson/log binomial regressions. A positive RERI would suggest that the co-occurring conditions confer a greater than expected risk (i.e., prevalence ratio) of UAI/syphilis beyond the addition of the independent effects of the conditions.
Regression models for the count and synergistic approach included RDS-II weights as probability weights and multivariable analysis included adjustment for city, age, sexual identity, and educational attainment, a priori confounders of the syndemic-syphilis/UAI association. Due to the dependent nature of data collected using RDS, a clustered sandwich variance estimator was used to account for within seed-group correlations (i.e. clustering by seed chain, n=25) for all regression models (Rogers 1993). Unweighted analyses are presented in Supplementary Tables. Seeds were excluded from all analyses. Prevalence ratios and RERIs were considered statistically significant at p-value<0.05. Statistical analyses were performed using Stata version 13.0 (Stata Corp., College Station, Texas, USA)
RESULTS
Median age of the MSM was 25 years (interquartile range [IQR]: 21–32), 30.4% were currently married, and 20.9% had only a primary school education or less. Almost half (45.1%) of the MSM identified as panthi (more masculine appearance and predominantly engage in insertive anal sex), 18.1% were double deckers (either masculine or feminine appearance and engage in both insertive and receptive anal sex), 15.1% were bisexual, 14.0% were kothi (more feminine appearance and predominately engage in receptive anal sex), and 7.7% identified as gay or MSM.
Overall prevalence of UAI in the prior 6 months was 50.5%. City-level UAI prevalence ranged from 35.8% in Hyderabad (Andhra Pradesh) to 73.7% in Bhopal (Madhya Pradesh) (Solomon, Mehta et al. 2015). UAI was most common among double deckers (54.4%) but was reported by approximately half within all sexual identity groups (Table 1). Men who were previously married (58.1%) or single (52.2%) were more likely to report UAI than those currently married (46.0%). The median number of male sexual partners in the prior 6 months was higher among those reporting UAI (2 partners) than those not reporting UAI (1 partner). The number of recent female partners was similar across groups. UAI was more prevalent among those recently engaging in sex work (58.1%). UAI was slightly more common among those with an active syphilis infection (52.8%) compared to uninfected men (50.4%). HIV- and herpes simplex virus 2-(HSV-2) uninfected men were more likely to report UAI (51.5% and 51.4%, respectively) compared to infected men (41.6% and 46.6%, respectively).
Table 1.
Socio-demographic characteristics and sexual risk factors by syphilis infection and unprotected anal intercourse (UAI) in prior 6 months among men who have sex with men in India
| n (row %), median (IQR) | No UAI in prior 6 months (N=5,236 |
UAI in prior 6 months (N=6,533) |
No active syphilis infection (N=11,378) | Active syphilis infection (N=393) |
|---|---|---|---|---|
| Median age | 26 (21–33) | 24 (20–31) | 25 (21–32) | 28 (23–35) |
| Sexual identity | ||||
| Panthi | 1759 (50.0) | 2104 (50.0) | 3794 (97.9) | 69 (2.1) |
| Kothi | 1285 (50.1) | 1472 (49.9) | 2580 (94.7) | 179 (5.3) |
| Double deckers | 1126 (45.6) | 1623 (54.4) | 2649 (95.7) | 100 (4.3) |
| Gay | 93 (48.6) | 101 (51.4) | 190 (98.4) | 4 (1.6) |
| MSM | 277 (51.3) | 438 (48.7) | 706 (98.7) | 9 (1.3) |
| Bisexual | 696 (51.7) | 795 (48.3) | 1459 (97.5) | 32 (2.5) |
| Marital Status | ||||
| Single | 3238 (47.8) | 4359 (52.2) | 7386 (97.4) | 213 (2.6) |
| Married | 1885 (54.0) | 1990 (46.0) | 3713 (96.4) | 162 (3.6) |
| Widowed/divorced/separated | 113 (41.9) | 184 (58.1) | 279 (95.7) | 18 (4.3) |
| Education | ||||
| Primary school or less | 1131 (48.4) | 1478 (51.6) | 2487 (95.6) | 122 (4.4) |
| Secondary school | 2273 (48.1) | 2847 (51.9) | 4958 (97.4) | 163 (2.6) |
| High school and above | 1832 (52.1) | 2208 (47.9) | 3933 (97.5) | 108 (2.5) |
| Employment | ||||
| Monthly/weekly wages | 2716 (51.7) | 3071 (48.3) | 5574 (96.4) | 213 (3.6) |
| Daily/seasonal wages | 1801 (48.0) | 2356 (52.0) | 4001 (97.0) | 158 (3.0) |
| Unemployed | 177 (44.9) | 280 (55.1) | 445 (96.7) | 12 (3.3) |
| Other (student or retired) | 542 (46.7) | 826 (53.3) | 1358 (99.6) | 10 (0.4) |
| Unprotected anal intercourse in prior 6 months | ||||
| No | – | – | 5041 (97.2) | 195 (2.8) |
| Yes | 6335 (96.9) | 198 (3.1) | ||
| Median number of male sexual partners in prior 6 months | 1 (0–2) | 2 (1–4) | 2 (1–3) | 2 (1–5) |
| Median number of female sexual partners in prior 6 months | 1 (0–1) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Sex work in prior 6 months | ||||
| No | 4092 (51.1) | 4602 (48.9) | 8452 (97.2) | 242 (2.8) |
| Yes | 1135 (41.9) | 1909 (58.1) | 2895 (96.1) | 151 (3.9) |
| HIV status | ||||
| Negative | 4585 (48.9) | 6048 (51.1) | 10378 (97.7) | 256 (2.4) |
| Positive | 644 (58.4) | 481 (41.6) | 991 (89.5) | 135 (10.5) |
| Indeterminate | 7 (43.9) | 4 (56.1) | 9 (82.0) | 2 (18.0) |
| Herpes simplex virus 2 (HSV-2) status | ||||
| Negative | 3769 (48.6) | 5059 (51.4) | 8718 (98.3) | 111 (1.7) |
| Positive | 1327 (53.4) | 1304 (46.6) | 2369 (92.1) | 263 (7.9) |
| Indeterminate | 140 (49.4) | 170 (50.6) | 291 (95.5) | 19 (4.5) |
| Active syphilis infection | ||||
| Negative | 5041 (49.6) | 6335 (50.4) | – | – |
| Positive | 195 (47.2) | 198 (52.8) |
Percentages and medians (IQRs) are presented as RDS-II weighted.
Overall prevalence of active syphilis infection was 2.9%. City-level syphilis ranged from 0.8% in Belgaum (Karnataka) to 4.4% in Vijayawada (Andhra Pradesh) (Solomon, Mehta et al. 2015). Kothis had the highest prevalence at 5.3%, followed by double deckers at 4.3%, bisexuals at 3.5%, and panthis at 2.1% (Table 1). Men who were previously (4.3%) or currently married (3.6%) were more likely to have an active syphilis infection than men that had never been married (2.6%). Those with only a primary school education or less had higher syphilis prevalence (4.4%) as compared to those with secondary (2.6%) or at least a high school education (2.5%). Syphilis was slightly more common among men reporting recent UAI (3.1%). The median number of male and female sexual partners in the prior 6 months was the same for those with and without an active syphilis infection. Syphilis was more prevalent among those recently engaging in sex work (3.9%). Men with HIV and HSV-2 were more likely to have an active syphilis infection (10.5% and 7.9%, respectively) compared to men uninfected (2.4% and 1.7%, respectively).
The most prevalent syndemic condition was CSA at 22.4%, followed by alcohol dependence at 15.2%, depression at 10.9%, IPV at 10.0%, and recent illicit drug use at 2.5% (Table 2). In univariable analysis, all five syndemic conditions were associated with higher prevalence of UAI in the prior 6 months. In multivariable analysis after adjustment for city, age, sexual identity, and education, the associations remained significant except illicit drug use: alcohol dependence prevalence ratio (PR): 1.31, 95% confidence interval (CI): 1.19–1.45; illicit drug use PR: 1.11, 95% CI: 0.93–1.33; depression PR: 1.19, 95% CI: 1.05–1.35); CSA PR: 1.16, 95% CI: 1.10–1.23; and IPV PR: 1.39, 95% CI: 1.16–1.67). In univariable analysis, depression (PR: 1.59, 95% CI: 1.06–2.36) and IPV (PR: 1.73, 95% CI: 1.28–2.34) were associated with higher prevalence of active syphilis infection. Alcohol dependence, illicit drug use, and CSA were not significantly associated with active syphilis infection in univariable analysis. In multivariable analysis, none of the syndemic conditions were significantly associated with syphilis.
Table 2.
Prevalence of syndemic conditions and their association with unprotected anal intercourse (UAI) in prior 6 months among men who have sex with men in India
| Syndemic condition, n (column %) | Total | No UAI in prior 6 months (N=5,236) | UAI in prior 6 months (N=6,533) | Prevalence Ratioˆ (95% CI) | Adjusted† Prevalence Ratioˆ (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| Alcohol dependence1 | 2283 (15.2) | 756 (11.6) | 1527 (18.7) | 1.26 (1.14–1.41) | 1.31 (1.19–1.45) |
| Recent illicit drug use2 | 593 (2.5) | 188 (1.9) | 405 (3.0) | 1.22 (1.06–1.42) | 1.11 (0.93–1.33) |
| Depression3 | 1570 (10.9) | 610 (8.4) | 960 (13.4) | 1.19 (1.02–1.38) | 1.19 (1.05–1.35) |
| Childhood sexual abuse | 3782 (22.4) | 1510 (20.7) | 2272 (24.2) | 1.10 (1.01–1.20) | 1.16 (1.10–1.23) |
| History of intimate partner violence | 1898 (10.0) | 625 (7.0) | 1273 (12.9) | 1.35 (1.13–1.60) | 1.39 (1.16–1.67) |
|
| |||||
| Number of syndemic conditions | |||||
| 0 | 5671 (58.9) | 2792 (63.4) | 2879 (54.4) | REF | REF |
| 1 | 3342 (26.8) | 1507 (26.1) | 1835 (27.5) | 1.09 (0.96–1.23) | 1.13 (1.03–1.24) |
| 2 | 1777 (9.9) | 666 (8.2) | 1111 (11.5) | 1.28 (1.14–1.44) | 1.35 (1.21–1.51) |
| 3 or more | 979 (4.5) | 271 (2.3) | 708 (6.6) | 1.53 (1.22–1.92) | 1.67 (1.32–2.12) |
Measured using AUDIT (Saunders, JB et al. Development of the alcohol use disorders identification test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption-II. 1993 Addiction).
Injection or non-injection drug use in prior 6 months.
Depression defined as a score of 10 or more on the PHQ-9 (Kroenke K et al. The PHQ-9. Journal of General Internal Medicine. 2001).
Percentages are RDS-II weighted
Log poisson regression model with RDS-II weights as probability weights and clustered variance estimation;
Adjusted for city, sexual identity, age, and educational attainment
CI: confidence interval
Over half (58.9%) of the men had no syndemic conditions, 26.8% had 1, 9.9% had 2, and 4.5% had 3 or more (Table 1). In both univariable and multivariable analysis, there was a significant dose response pattern of the syndemic count with UAI (adjusted PR for 1 condition vs. none: 1.13, 95% CI: 1.03–1.24; 2 conditions: 1.35, 95% CI: 1.21–1.51; 3 or more conditions: 1.67, 95% CI: 1.32–2.12). In the univariable analysis of syphilis, there was a dose response pattern of the syndemic count, though no group reached statistical significance (Table 2). Multivariable analysis was similar to univariable; there was a dose response pattern of the syndemic count with syphilis (adjusted PR for 1 condition vs. none: 1.07, 95% CI: 0.69–1.67; 2 conditions: 1.19, 95% CI: 0.78–1.82; 3 or more conditions: 1.44, 95% CI: 0.78–2.68) but no group reached statistical significance.
RERIs of UAI for one pair of conditions showed synergy: IPV+depression (0.26, 95% CI: 0.05–0.47) (Figure 1). All other 2-way RERIs of UAI overlapped the null (zero) and were therefore not significantly different from zero. For co-occurring 3-condition patterns, IPV+CSA+depression (n=259), alcohol dependence+IPV+CSA (n=226), and alcohol dependence+CSA+depression (n=157) were most common. RERIs of UAI from these 3-way interaction models were all negative. RERIs from the 2-way syphilis interaction models were mostly negative or close to zero with the exception of three pairs with substance use: depression+drug use (1.17, 95% CI: −0.64 – 2.97), alcohol dependence+drug use (0.70, 95% CI: 0.09–1.30) and alcohol dependence+CSA (0.33, 95% CI: −0.56–1.23) (Figure 2). The RERI for alcohol dependence+drug use was the only pair of conditions that showed statistically significant synergy. RERIs of syphilis from the 3-way interaction models were all negative.
Figure 1.
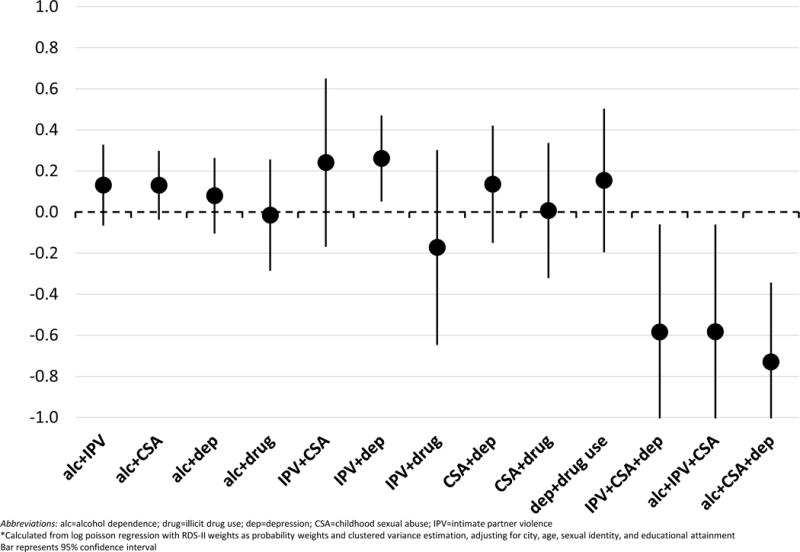
Relative excess risk due to interaction (RERI)* of unprotected anal intercourse (UAI) in prior 6 months for syndemic condition interactions among men who have sex with men in India
Abbreviations: alc=alcohol dependence; drug=illicit drug use; dep=depression; CSA=childhood sexual abuse; IPV=intimate partner violence
*Calculated from log poisson regression with RDS-II weights as probability weights and clustered variance estimation, adjusting for city, age, sexual identity, and educational attainment Bar represents 95% confidence interval
Figure 2.
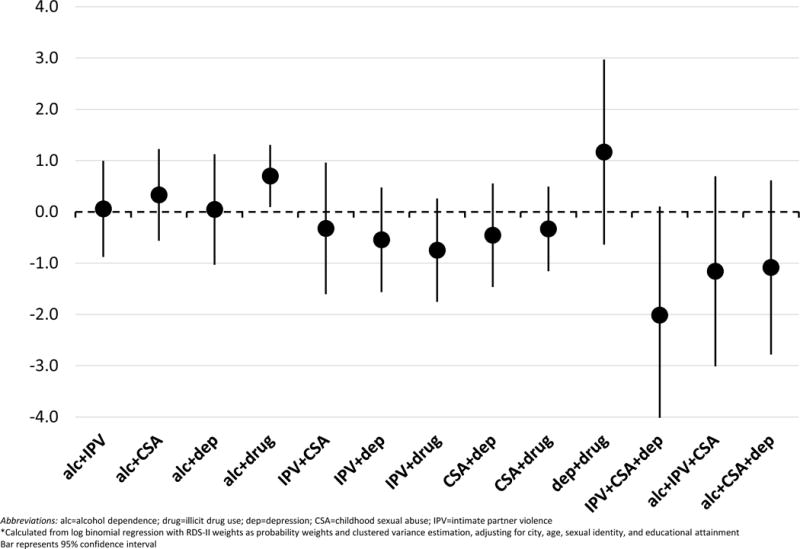
Relative excess risk due to interaction (RERI)* of active syphilis infection for syndemic condition interactions among men who have sex with men in India
Abbreviations: alc=alcohol dependence; drug=illicit drug use; dep=depression; CSA=childhood sexual abuse; IPV=intimate partner violence
*Calculated from log binomial regression with RDS-II weights as probability weights and clustered variance estimation, adjusting for city, age, sexual identity, and educational attainment Bar represents 95% confidence interval
DISCUSSION
In accordance with similar syndemic investigations, we found an additive relationship between psychosocial syndemic conditions and the behavioral outcome of UAI. The more syndemic conditions MSM in our study experienced, the greater likelihood that they reported UAI. A similar pattern emerged between the syndemic conditions and the biological outcome of syphilis, suggesting a dose-response relationship with more syndemic conditions and greater likelihood of syphilis, but this trend did not reach statistical significance. In previous studies, significant dose-response relationships led investigators to conclude that syndemic conditions acted in a synergistic manner to produce worse sexual behaviors and/or outcomes and ultimately greater likelihood of HIV. In a similar vein, for the biological outcome of syphilis, the trend towards dose-response with a lack of statistical significance in the findings would likely be interpreted as suggestive of a possible syndemic, with further data needed to ascertain its existence.
When we tested for synergistic relationships using RERI, however, a more complex portrait emerged. For the behavioral outcome of UAI, we found evidence of synergistic interaction in only one of the eight pairs of possible 2-way interactions (IPV+depression) and none of the 3-way combinations of syndemic conditions yielded significant positive findings. For the biological outcome of syphilis, we similarly found evidence of only one 2-way interaction with substance use (alcohol dependence+illicit drug use) and none of the 3-way combinations yielded significant positive findings. Notably, the condition pairs that showed significant synergy for UAI and syphilis were not the same. The RERIs imply that experiences of violence and depression interact to increase UAI, while substance use interacts to produce increased syphilis infection. Additionally, all 3-way RERIs were negative, with those for UAI all significantly less than zero. This suggests a lack of synergy when looking beyond pairs, which constitutes a distinct pattern in contrast to the additive (i.e. count) approach that tends to show a dose-response relationship with 3, 4, or more conditions. Thus, while there is indeed population-level clustering of these psychosocial conditions among MSM in India, only two pairs of the psychosocial conditions constituted a syndemic according to Singer et al.’s (2017) definition, with worse effects (i.e. engaging in UAI or acquiring syphilis) than expected for each condition individually. There was no evidence of the compounding of multiple psychosocial factors in a synergistic manner across multiple pairs or triplets, as some prior syndemic studies have suggested based on trends towards dose-response relationships in additive models.
Our “test case” comparison of two methodological approaches for assessing syndemics, based on a large sample of MSM in India, has several important implications. Foremost, our findings provide further support for Tsai and colleagues’ (Tsai and Burns 2015, Tsai and Venkataramani 2016, Tsai, Mendenhall et al. 2017) argument that synergy cannot be assumed based on a cumulative relationship of the number of syndemic conditions and sexual risk behaviors and that additional methods are necessary to formally evaluate the presence of synergistic interaction. The count approach for both of our outcomes yielded a linear dose-response relationship, which was statistically significant for UAI but not for syphilis. This pattern fits with Tsai and Burns’s (2015) observation that there is a pattern of dose-response relationships with syndemic conditions and sexual risk (or HIV seroconversion) in many syndemic investigations. This pattern is usually taken as evidence of synergy. For example, although Singer et al. (2017) acknowledge that further work is needed to demonstrate “the existence of a SAVA [substance use, alcohol, violence and AIDS] syndemic among men who have sex with men, consisting of interacting and mutually reinforcing health conditions,” they cite Stall and colleagues’ pioneering study (Stall, Mills et al. 2003), which found an additive pattern between syndemic conditions (multiple drug use, IPV, CSA, and depression) and risky sexual behaviors, as indicative of a likely syndemic. Similarly, a recent paper by Ferlatte and colleagues (2018) that investigated active syphilis infection among MSM in Canada found a strong dose-response with syndemic conditions and syphilis using the count approach. In contrast, however, Tsai and Burns (2015) and Tsai and Venkataramani (2016) point out that such a linear pattern suggests additive effects, but does not support substantial synergistic interactions.
The pervasive presence of a dose-response pattern in syndemic studies, including ours, poses a challenge for the third defining criteria of syndemics, which focuses on the mutually reinforcing interactions. If other investigators carry out similar tests for synergistic interaction as we did in our “test case”, it may be that some examples of the observed clustering of co-occurring conditions of psychosocial adversity and elevated sexual risk in sexual minority populations do not meet the stated criteria of syndemics or do so only in a limited manner, as in our example. Indeed, in our study, synergistic interactions were confined to one set of psychosocial conditions for UAI and a different pair of conditions for syphilis. Tsai and colleagues (2017) note that the first two criteria of syndemics about the clustering of epidemics due to “harmful social conditions” has been extensively discussed beyond the syndemic literature. It is the third criterion of synergistic interactions that distinguishes syndemic theory from other conceptualizations, while also making it problematic due to the lack of specificity about the nature of these interactions and the lack of adequate empirical support for such interactions. Importantly, relying solely on the count approach conceals where true synergies may lie among the specific psychosocial conditions and their magnitude, thus limiting the design and implementation of effective interventions for the target population.
Ferlatte et al.’s (2018) approach represents one step beyond previous approaches in syndemic studies by examining additive interactions using observed vs. expected prevalences in the absence of interaction as well as the RERI. However, both of these approaches used counts of syndemic conditions, rather than exploring interactions with specific conditions (e.g. substance use and depression). In contrast to our findings, this approach, which used a slightly different set of syndemic conditions (e.g. including suicidality but not CSA), yielded evidence of synergistic interaction with 3 or more conditions. Ferlatte et al’s findings may mean that synergistic interactions exist for syphilis in this different population of MSM in a high-income setting, but their methods assume all syndemic conditions have a similar effect on syphilis infection and do not enable us to identify which conditions are in a synergistic relationship with one another.
We suggest several possibilities for moving syndemic studies forward in light of our findings as well as our belief that current syndemic approaches, despite their limitations, have proven useful in drawing attention to the patterned clustering of adverse conditions and experiences in socially marginalized populations, such as sexual minorities. First, the third defining criterion of syndemic theory might be broadened to include cumulative (additive) adversity, rather than solely focusing on synergistic, mutually reinforcing interactions. If this modification was made, we could distinguish between different kinds of syndemics, and different kinds of “interactions.” For instance, we might distinguish between syndemics wherein there are well-documented mutual amplification of conditions (e.g., co-infections) and others, where there may be other causal pathways among the different syndemic conditions. Second, we recommend greater attention to the psychosocial conditions’ differential effects on sexual risk, their relationship to one another in causal pathways, and their development over time. These issues are currently overlooked in the count approach, yet the temporal, cumulative development of harmful psychosocial conditions over the life course is a key element of Stall and colleagues’ (Stall, Mills et al. 2003, Stall, Coulter et al. 2015) conceptualization of syndemics. Moreover, as is clear from our findings, there may be wide variation of the presence of syndemic conditions within vulnerable populations; over half of our participants, for example, had none of the five syndemic conditions, and only 4.5% had three or more. Since study populations, the selected syndemic conditions as well as their measures vary from study to study, there is no simple way to compare syndemics across studies. A better understanding of the above issues would have significant implications for interventions. For instance, early interventions focusing on temporally antecedent adverse conditions, such as CSA, may be particularly effective in preventing the cascade of adverse conditions that may amplify one another. Investigators may also wish to screen vulnerable populations for a broad set of these adverse conditions, examine who bear the greatest burdens of adverse conditions, and develop specific interventions for those who face the greatest risk. Finally, investigators may be able to better select the targets of interventions within contexts of limited resources so that they can have the most powerful effects on lowering sexual risk.
Both a broader conceptualization of syndemics and a greater focus on the life course could open the way for greater integration of quantitative and qualitative methodologies. For instance, ethnographers are well suited to examine the development and embodied experiences of multiple syndemic conditions as well as the social and political economic forces that make certain populations vulnerable to them. With limited funding opportunities for large cohort studies that follow participants for long periods of time, it is particularly important that quantitative and qualitative data on syndemics are brought together in projects and manuscripts so that they constitute an ongoing dialogue rather than discrete components. A holistic focus on understanding of the disease production and experience is consistent with the objectives of syndemic theory.
Our findings are limited by several factors. Given the cross-sectional study design, we cannot establish that syndemic conditions occurred or began prior to an individual’s outcome-UAI or syphilis acquisition. Relatedly, and as mentioned earlier, we are unable to investigate the temporal, causal relationships between the five syndemic conditions or potential antecedent factors. All syndemic conditions as well as UAI were self-reported on the survey and are subject to potential biases such as recall and social desirability bias. Seeking medical care for syphilis symptoms (i.e. sores and ulcer) was not associated with any of the syndemic conditions but we are unable to rule out bias if treatment for syphilis is associated with syndemic conditions. While we used RDS weights to account for the non-random sampling approach, we cannot confirm that the resulting analytical sample and effect estimates are reflective of the underlying population. Despite a large sample size, we may have been underpowered to detect significant synergy for 3-condition interactions. It is also possible, of course, that there are more substantial synergistic interactions among syndemic conditions that we did not test in the population, in other populations of MSM in India or elsewhere, or among other vulnerable groups.
In conclusion, our “test case” of a comparison of the traditional count approach and a test of synergistic interaction in a large dataset of Indian MSM offers further support for reevaluating current approaches in syndemic studies and for introducing novel methods to test for synergy as well as to better evaluate the development of co-occurring epidemics and their interactions over time. Our findings reinforce the challenge of translating the anthropological concept of syndemics to quantitative studies of population health and the need for the use of careful methodological approaches (and language) for undertaking syndemic investigations. Closer attention to the underlying dynamics in co-occurring epidemics through combined qualitative and quantitative methodologies may yield more effective interventions for vulnerable, marginalized populations.
Supplementary Material
Table 3.
Prevalence of syndemic conditions and their association with syphilis infection among men who have sex with men in India
| Syndemic condition, n (column %) | No active syphilis infection (N=11378) | Active syphilis infection (N=393) | Prevalence Ratioˆ (95% CI) | Adjusted† Prevalence Ratioˆ (95% CI) |
|---|---|---|---|---|
|
| ||||
| Alcohol dependence1 | 2217 (15.3) | 66 (12.4) | 0.79 (0.46–1.35) | 0.86 (0.45–1.63) |
| Recent illicit drug use2 | 581 (2.5) | 12 (1.7) | 0.55 (0.26–1.19) | 0.69 (0.32–1.51) |
| Depression3 | 1502 (10.7) | 68 (17.3) | 1.59 (1.06–2.36) | 1.41 (0.92–2.17) |
| Childhood sexual abuse | 3605 (22.2) | 178 (28.4) | 1.37 (0.91–2.06) | 1.15 (0.79–1.66) |
| History of intimate partner violence | 1798 (9.8) | 101 (15.7) | 1.73 (1.28–2.34) | 1.38 (0.99–1.91) |
|
| ||||
| Number of syndemic conditions | ||||
| 0 | 5521 (59.1) | 150 (51.1) | REF | REF |
| 1 | 3225 (26.7) | 119 (30.0) | 1.13 (0.70–1.82) | 1.07 (0.69–1.67) |
| 2 | 1699 (9.8) | 78 (12.4) | 1.35 (0.82–2.23) | 1.19 (0.78–1.82) |
| 3 or more | 933 (4.4) | 46 (6.5) | 1.69 (0.96–2.95) | 1.44 (0.78–2.68) |
Measured using AUDIT (Saunders, JB et al. Development of the alcohol use disorders identification test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption-II. 1993 Addiction).
Injection or non-injection drug use in prior 6 months.
Depression defined as a score of 10 or more on the PHQ-9 (Kroenke K et al. The PHQ-9. Journal of General Internal Medicine. 2001).
Percentages are RDS-II weighted
Log binomial regression model with RDS-II weights as probability weights and clustered variance estimation;
Adjusted for city, sexual identity, age, and educational attainment
CI: confidence interval
Syndemics theory addresses socially-driven disease clustering that worsens outcomes
Most syndemic investigations test for additive, but not synergistic associations
We compared additive and synergistic associations of syndemic factors and sexual risk
We found an additive association but only limited synergy among syndemic factors
Research is needed on how co-occurring epidemics develop and may interact over time
Acknowledgments
This study was supported by grants from the US National Institutes of Health (R01MH89266, R21MH101059 and T32AI102623) and the Johns Hopkins Center for AIDS Research (1P30AI094189). We thank the National AIDS Control Organization (NACO), India, all of our partner non-governmental organizations throughout India, and the countless participants, without whom this research would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abara WE, Hess KL, Neblett Fanfair R, Bernstein KT, Paz-Bailey G. Syphilis Trends among Men Who Have Sex with Men in the United States and Western Europe: A Systematic Review of Trend Studies Published between 2004 and 2015. PloS one. 2016;11:e0159309. doi: 10.1371/journal.pone.0159309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmam GN, Kodavalla V, Rajkumar H, Rachakulla HK, Kallam S, Myakala SP, et al. Sexual practices, HIV and sexually transmitted infections among self-identified men who have sex with men in four high HIV prevalence states of India. AIDS. 2008;22:S45–S57. doi: 10.1097/01.aids.0000343763.54831.15. [DOI] [PubMed] [Google Scholar]
- Chakrapani V, Newman PA, Shunmugam M, Logie CH, Samuel M. Syndemics of depression, alcohol use, and victimisation, and their association with HIV-related sexual risk among men who have sex with men and transgender women in India. Glob Public Health. 2017;12(2):250–265. doi: 10.1080/17441692.2015.1091024. [DOI] [PubMed] [Google Scholar]
- Fenton KA, Breban R, Vardavas R, Okano JT, Martin T, Aral S, et al. Infectious syphilis in high-income settings in the 21st century. The Lancet Infectious Diseases. 2008;8:244–253. doi: 10.1016/S1473-3099(08)70065-3. [DOI] [PubMed] [Google Scholar]
- Ferlatte O, Salway T, Samji H, Dove N, Gesink D, Gilbert M, et al. An Application of Syndemic Theory to Identify Drivers of the Syphilis Epidemic Among Gay, Bisexual, and Other Men Who Have Sex With Men. Sexually Transmitted Diseases. 2018;45:163–168. doi: 10.1097/OLQ.0000000000000713. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Samanta M, Roy P, Chatterjee S, Kaplan DW, Basu B. Patient health questionnaire-9 as an effective tool for screening of depression among Indian adolescents. J Adolesc Health. 2013;52(5):546–551. doi: 10.1016/j.jadohealth.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Go VF, Srikrishnan AK, Sivaram S, Murugavel GK, Galai N, Johnson SC, Sripaipan T, Solomon S, Celentano DD. High HIV prevalence and risk behaviors in men who have sex with men in Chennai, India. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004;35(3):314–319. doi: 10.1097/00126334-200403010-00014. [DOI] [PubMed] [Google Scholar]
- Khan S, Menezes GA, Dhodapkar R, Harish BN. Seroprevalence of syphilis in patients attending a tertiary care hospital in Southern India. Asian Pacific Journal of Tropical Biomedicine. 2014;4:995–997. [Google Scholar]
- Kochhar P, Rajadhyaksha S, Suvarna V. Translation and validation of brief patient health questionnaire against DSM IV as a tool to diagnose major depressive disorder in Indian patients. Journal of Postgraduate Medicine. 2007;53(2):102. doi: 10.4103/0022-3859.32209. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall E. Syndemics: a new path for global health research. Lancet. 2017;389(10072):889–891. doi: 10.1016/S0140-6736(17)30602-5. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, O’Cleirigh C, Biello KB, Robertson AM, Safren SA, Coates TJ, Koblin BA, Chesney MA, Donnell DJ, Stall RD. The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;68(3):329–336. doi: 10.1097/QAI.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, Thomas B, Mayer KH, Reisner SL, Menon S, Swaminathan S, Periyasamy M, Johnson CV, Safren SA. Alcohol use and HIV sexual risk among MSM in Chennai, India. International journal of STD & AIDS. 2011;22(3):121–125. doi: 10.1258/ijsa.2009.009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of AIDS Control, Indian Ministry of Health & Family Welfare. Annual Report 2013-14 2013 [Google Scholar]
- Patel V, Araya R, Chowdhary N, King M, Kirkwood B, Nayak S, Simon G, Weiss HA. Detecting common mental disorders in primary care in India: a comparison of five screening questionnaires. Psychol Med. 2008;38(2):221–228. doi: 10.1017/S0033291707002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Bailey G, Meyers A, Blank S, Brown J, Rubin S, Braxton J, et al. A Case– Control Study of Syphilis Among Men Who Have Sex With Men in New York City: Association With HIV Infection. Sexually Transmitted Diseases. 2004;31:581–587. doi: 10.1097/01.olq.0000140009.28121.0f. [DOI] [PubMed] [Google Scholar]
- Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global Epidemiology of HIV Infection and Related Syndemics Affecting Transgender People. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S210–219. doi: 10.1097/QAI.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers WH. Regression standard errors in clustered samples. Stata Technical Bulletin. 1993;13:19–23. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Safren SA, Thomas BE, Mimiaga MJ, Chandrasekaran V, Menon S, Swaminathan S, Mayer KH. Depressive symptoms and human immunodeficiency virus risk behavior among men who have sex with men in Chennai, India. Psychology, health & medicine. 2009;14(6):705–715. doi: 10.1080/13548500903334754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de LF, Jr, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Setia M, Sivasubramanian M, Anand V, Row-Kavi A, Jerajani H. Married men who have sex with men: the bridge to HIV prevention in Mumbai, India. International journal of public health. 2010;55(6):687–691. doi: 10.1007/s00038-010-0173-0. [DOI] [PubMed] [Google Scholar]
- Singer M. AIDS and the health crisis of the U.S. urban poor; the perspective of critical medical anthropology. Soc Sci Med. 1994;39(7):931–948. doi: 10.1016/0277-9536(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Singer M. A dose of drugs, a touch of violence, a case of AIDS: conceptualizing the SAVA syndemic. Free Inquiry in Creative Sociology. 1996;28(1):13–24. [Google Scholar]
- Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389(10072):941–950. doi: 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian M, Mimiaga MJ, Mayer KH, Anand VR, Johnson CV, Prabhugate P, Safren SA. Suicidality, clinical depression, and anxiety disorders are highly prevalent in men who have sex with men in Mumbai, India: findings from a community-recruited sample. Psychology, Health & Medicine. 2011;16(4):450–462. doi: 10.1080/13548506.2011.554645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Lucas GM, Celentano DD, Sifakis F, Mehta SH. Beyond surveillance: a role for respondent-driven sampling in implementation science. American Journal of Epidemiology. 2013;178(2):260–267. doi: 10.1093/aje/kws432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Mehta SH, Latimore A, Srikrishnan AK, Celentano DD. The impact of HIV and high-risk behaviours on the wives of married men who have sex with men and injection drug users: implications for HIV prevention. Journal of the International AIDS Society. 2010;13(Suppl 2):S7. doi: 10.1186/1758-2652-13-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Mehta SH, Srikrishnan AK, Vasudevan CK, Mcfall AM, Balakrishnan P, Anand S, Nandagopal P, Ogburn EL, Laeyendecker O. High HIV prevalence and incidence among MSM across 12 cities in India. AIDS. 2015;29(6):723–731. doi: 10.1097/QAD.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R, Coulter RW, Friedman MR, Plankey MW. Commentary on ”Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept“ by A. Tsai and B. Burns. Soc Sci Med. 2015;145:129–131. doi: 10.1016/j.socscimed.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Stall R, Mills TC, Williamson J, Hart T, Greenwood G, Paul J, Pollack L, Binson D, Osmond D, Catania JA. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. American Journal of Public Health. 2003;93(6):939–942. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Mimiaga MJ, Kumar S, Swaminathan S, Safren SA, Mayer KH. HIV in Indian MSM: reasons for a concentrated epidemic & strategies for prevention. Indian Journal of Medical Research. 2011;134(6):920–929. doi: 10.4103/0971-5916.92637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori C, McFall AM, Srikrishnan AK, Mehta SH, Nimmagadda N, Anand S, Vasudevan CK, Solomon S, Solomon SS, Celentano DD. The prevalence and impact of childhood sexual abuse on HIV-risk behaviors among men who have sex with men (MSM) in India. BMC Public Health. 2016;16:784. doi: 10.1186/s12889-016-3446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori C, McFall AM, Srikrishnan AK, Mehta SH, Solomon SS, Anand S, Vasudevan CK, Solomon S, Celentano DD. Diverse Rates of Depression Among Men Who Have Sex with Men (MSM) Across India: Insights from a Multi-site Mixed Method Study. AIDS Behav. 2016;20(2):304–316. doi: 10.1007/s10461-015-1201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori C, Srikrishnan A, Ridgeway K, Solomon S, Mehta S, Solomon S, Celentano D. Perspectives on Sexual Identity Formation, Identity Practices, and Identity Transitions among Men who have Sex with Men (MSM) in India. Archives of Sexual Behavior. 2016 doi: 10.1007/s10508-016-0775-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Burns BF. Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept. Soc Sci Med. 2015;139:26–35. doi: 10.1016/j.socscimed.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Mendenhall E, Trostle JA, Kawachi I. Co-occurring epidemics, syndemics, and population health. Lancet. 2017;389(10072):978–982. doi: 10.1016/S0140-6736(17)30403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Venkataramani AS. Syndemics and Health Disparities: A Methodological Note. AIDS Behav. 2016;20(2):423–430. doi: 10.1007/s10461-015-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiologic Methods. 2014;3:33–72. [Google Scholar]
- Volk RJ, Steinbauer JR, Cantor SB, Holzer CE., III The Alcohol Use Disorders Identification Test (AUDIT) as a screen for at-risk drinking in primary care patients of different racial/ethnic backgrounds. Addiction. 1997;92(2):197–206. [PubMed] [Google Scholar]
- Volz E, Heckathorn DD. Probability Based Estimation Theory for Respondent Driven Sampling. Journal of Official Statistics. 2008;24(1):79–97. [Google Scholar]
- Wang QQ, Chen XS, Yin YP, Liang GJ, Zhang RL, Jiang N, et al. HIV prevalence, incidence and risk behaviours among men who have sex with men in Yangzhou and Guangzhou, China: a cohort study. Journal of the International AIDS Society. 2014:17. doi: 10.7448/IAS.17.1.18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RG, Lansky A, Goel S, Wilson D, Hladik W, Hakim A, Frost SD. Respondent driven sampling–where we are and where should we be going? Sexually transmitted infections. 2012;88(6):397–399. doi: 10.1136/sextrans-2012-050703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


