ABSTRACT
Huanglongbing in citrus is caused by a phloem-limited, uncultivable, gram-negative α-proteobacterium, Candidatus Liberibacter asiaticus (CLas). CLas is transmitted by the phloem-sucking insect, Diaphorina citri (Hemiptera: Liviidae), in a persistent, circulative, and propagative manner. In this study, we investigated the metabolomic and respiration rates changes in D. citri upon infection with CLas using gas chromatography-mass spectrometry (GC-MS) and gas exchange analysis. The level of glycine, L-serine, L-threonine, and gamma-amino butyric acid were higher in CLas-infected D. citri, while L-proline, L-aspartic acid, and L-pyroglutamic acid were lower in CLas-infected D. citri compared with the control. Citric acid was increased in CLas-infected D. citri, whereas malic and succinic acids were reduced. Interestingly, most of the reduced metabolites such as malate, succinate, aspartate, and L-proline are required for the growth of CLas. The increase in citric acid, serine, and glycine indicated that CLas induced glycolysis and the tricarboxylic acid cycle (TCA) in its vector. In agreement with the GC-MS results, the gene expression results also indicated that glycolysis and TCA were induced in CLas-infected D. citri and this was accompanied with an increases in respiration rate. Phosphoric acid and most of the sugar alcohols were higher in CLas-infected D. citri, indicating a response to the biotic stress or cell damage. Only slight increases in the levels of few sugars were observed in CLas-infected D. citri, which indicated that sugars are tightly regulated by D. citri. Our results indicated that CLas induces nutrient and energetic stress in its host insect. This study may provide some insights into the mechanism of colonization of CLas in its vector.
KEYWORDS: Candidatus Liberibacter asiaticus, Diaphorina citri, GC-MS, Huanglongbing, tricarboxylic acid cycle
Introduction
Insect-transmitted plant pathogens have been intensely studied in recent years due to the large economic damage they cause. Insect vector-borne pathogens can be attributed to more than 700 plant diseases worldwide, which cause symptoms generally described as “scorch,” “blight,” “stunting,” and “yellows.”1 Specifically, insects of the order Hemiptera, such as psyllids, aphids, whiteflies, and leafhoppers, are largely responsible for transmitting many of these diseases.
In Citrus spp., Huanglongbing (HLB) also known as citrus greening, has rapidly dispersed across different geographical regions over the world and cost citrus growers billions of US dollars.1 HLB is caused by Candidatus Liberibacter spp, which are fastidious, phloem-limited, uncultivable, Gram-negative α-proteobacteria.2,3 Three Ca. Liberibacter species are associated with HLB; Ca. L. africanus (CLaf) in Africa, Ca. L. asiaticus (CLas) in Asia and Americas, and Ca. L. americanus (CLam) in Brazil.4 Although CLas can be transmitted by graft inoculation, it is mainly transmitted by psyllids.5 The two psyllids associated with HLB-transmission are the African citrus psyllid, Trioza erytrea (Hemiptera: Triozidae) which transmits CLaf; and the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), which transmits both CLas and Clam.5 CLas and the other Liberibacters are restricted to the phloem tissue of plants6 and therefore, the phloem sap must provide the required nutrients for the their multiplication.
Some hemipterans feed exclusively on plant phloem or xylem saps. The plant phloem sap is rich in sugars and organic acids, but usually low in essential amino acids.7 Usually, phloem-sucking insect consume large quantities of phloem sap and egest excess sugars in the form of honeydew.7 To complete the required balance of amino acids, most insects, including D. citri, harbor one or more bacterial endosymbionts which provide the missing nutrients.7,8
For insects to be able to host and transmit plant pathogens in persistent manner, the pathogen must be able to multiply within the insect hemocoel (propagative), circulate within the haemolymph and travel to the salivary glands, and finally be injected with saliva into a new plant host during feeding.9 In this type of transmission, the insect vector can transmit the pathogen for its whole life.9 D. citri transmits CLas in this manner.10 D. citri acquires CLas during feeding on the phloem sap of infected plants (acquisition), and the acquired pathogen is moved from the gut to haemolymph, then to the salivary glands and other tissues via haemolymph. Finally, CLas can be injected with the saliva into new tree phloem during subsequent feeding.10 CLas multiplies in both nymphs and adult psyllids11 indicating that the haemolymph of D. citri contains all the necessary nutrition needed for CLas propagation.10
Knowledge about pathogen-vector interactions is still limited, and is not without controversy. The specific interactions between plant bacterial pathogens and their hemipteran vectors remain poorly understood.12,13 These interactions may range from beneficial to harmful.13 Recently, Pelz-Stelinski and Killiny reported some mutually advantageous interactions in the case of the CLas-D. citri pathosystem. They found that CLas-infection increased the reproductive fitness of its vector.13 By contrast, in the same pathosystem, CLas-infection showed many harmful/negative effects on D. citri such as increasing the vector dispersal,14 susceptibility to insecticides,15 and decreasing its survival and life span.13 In addition, these interactions are difficult to study because many of the pathogenic bacteria have not been cultured yet.16
Metabolomics have become an important tool in the study of metabolites (small biological molecules) and has recently been applied to plants,17 insects,18,19 and bacteria.20,21 The non-targeted nature of metabolite profiling allows the analysis of the complete set of metabolites, commonly found in biologic fluids and tissues. The metabolomic profile of an organism can be affected by many factors including developmental stage, environmental factors, nutritional status, and biotic stresses.22 Previous studies showed that many metabolites were altered in the haemolymph of worker honeybees (Apis mellifera L.) upon their infection with the microsporidian Nosema ceranae.22 In addition, another metabolomic study revealed metabolic responses in silkworm after infection with the pathogenic fungus Beauveria bassiana.23 In the current study, we investigated the changes in D. citri metabolites upon infection with CLas using gas chromatography-mass spectrometry (GC-MS), and assessed the resulting impacts on insect respiration using gas exchange analysis. This study may provide insight into the mechanism of CLas colonization of its vector. In addition, the findings may give more information about how D. citri responds to and copes with CLas infection.
Results
Fifty-five metabolites were detected after TMS derivatization; 15 amino acids, 3 fatty acids, 7 organic acids, and 30 sugars and sugar derivatives (Table 1). A representative gas chromatography-mass spectrometry (GC-MS) chromatogram for the detected metabolites is shown in Fig. 1.
Table 1.
Concentrations of different metabolic compounds (mean ± std) detected in Asian citrus psyllid, D. citri body after the infection with CLas using GC-MS.
| Compound |
Control (n = 10) |
CLas-infected (n = 40) |
P-value |
Compound |
Control (n = 20) |
CLas-infected (n = 20) |
P-value |
|---|---|---|---|---|---|---|---|
| Amino acids (ng insect−1) | Sugars (ng insect−1) | ||||||
| L-Alanine | 13.517 ± 6.259 | 24.587 ± 7.540 | 0.0048 | Erthrulose | 0.128 ± 0.039 | 0.201 ± 0.069 | 0.0038 |
| L-Valine | 0.186 ± 0.071 | 0.184 ± 0.063 | 0.9498 | Lyxose | 0.754 ± 0.100 | 1.249 ± 0.212 | 0.0000 |
| Iso-Leucine | 0.487 ± 0.137 | 0.778 ± 0.173 | 0.0015 | Fructose | 4.228 ± 0.337 | 6.079 ± 0.773 | 0.0000 |
| L-Proline | 0.708 ± 0.179 | 0.334 ± 0.093 | 0.0030 | Mannose | 0.028 ± 0.004 | 0.050 ± 0.009 | 0.0000 |
| Glycine | 3.860 ± 1.150 | 5.280 ± 1.625 | 0.0317 | Glocose | 9.129 ± 0.406 | 8.857 ± 1.079 | 0.3341 |
| L-Serine | 1.451 ± 0.170 | 2.401 ± 0.773 | 0.0000 | β-Glucopyranose | 0.019 ± 0.002 | 0.021 ± 0.003 | 0.2237 |
| L-Threonine | 1.333 ± 0.513 | 1.933 ± 0.446 | 0.0340 | GlcNAc | 0.129 ± 0.077 | 0.202 ± 0.117 | 0.0889 |
| L-Aspartic acid | 0.357 ± 0.059 | 0.207 ± 0.042 | 0.0009 | Sucrose | 0.124 ± 0.028 | 0.115 ± 0.022 | 0.4772 |
| Glutamic acid | 1.135 ± 0.329 | 1.958 ± 0.564 | 0.0004 | Turanose | 0.094 ± 0.003 | 0.090 ± 0.002 | 0.0553 |
| L-Phenylalanine | 0.163 ± 0.061 | 0.231 ± 0.167 | 0.1207 | Trehalose | 0.091 ± 0.003 | 0.096 ± 0.006 | 0.0093 |
| L-Lysine | 0.673 ± 0.382 | 0.563 ± 0.264 | 0.5284 | Maltose | 0.093 ± 0.003 | 0.096 ± 0.008 | 0.1263 |
| Total | 23.871 ± 6.850 | 38.456 ± 9.327 | 0.0015 | Total | 14.818 ± 0.669 | 17.057 ± 1.853 | 0.0001 |
| Non-proteinogenic amino acids (ng insect−1) | Sugars alcohol (ng insect−1) | ||||||
| L-Homoserine | 0.047 ± 0.027 | 0.058 ± 0.029 | 0.4146 | Erythritol | 0.386 ± 0.017 | 2.060 ± 0.315 | 0.0000 |
| Pyroglutamic acid | 1.547 ± 0.288 | 0.399 ± 0.162 | 0.0001 | Xylitol | 0.305 ± 0.093 | 0.350 ± 0.065 | 0.3098 |
| GABA | 0.148 ± 0.022 | 0.402 ± 0.136 | 0.0000 | Glucitol | 0.254 ± 0.079 | 0.417 ± 0.199 | 0.0046 |
| Putrescine | 0.868 ± 0.261 | 0.864 ± 0.234 | 0.9766 | Mannitol | 0.524 ± 0.050 | 8.623 ± 0.711 | 0.0000 |
| Total | 2.610 ± 0.492 | 1.723 ± 0.273 | 0.0058 | Chiro-Inositol | 0.118 ± 0.017 | 0.352 ± 0.063 | 0.0000 |
| Organic acids (ng insect−1) | Scyllo-inositol | 0.241 ± 0.125 | 0.332 ± 0.107 | 0.1422 | |||
| Pyruvic acid | 0.006 ± 0.003 | 0.005 ± 0.002 | 0.2618 | Myo-Inositol | 3.380 ± 0.579 | 2.996 ± 0.920 | 0.2268 |
| Lactic acid | 0.011 ± 0.004 | 0.007 ± 0.003 | 0.0689 | SA1 | 0.180 ± 0.034 | 0.237 ± 0.078 | 0.0139 |
| Glycolic acid | 0.003 ± 0.001 | 0.003 ± 0.000 | 0.3060 | SA2 | 0.227 ± 0.074 | 0.300 ± 0.107 | 0.0793 |
| Succinic acid | 0.386 ± 0.080 | 0.185 ± 0.050 | 0.0011 | Total | 5.615 ± 0.654 | 15.668 ± 1.451 | 0.0000 |
| Malic acid | 0.910 ± 0.117 | 0.486 ± 0.132 | 0.0000 | Sugars acids (ng insect−1) | |||
| Threonic acid | 0.446 ± 0.102 | 0.477 ± 0.106 | 0.5206 | 2-Ketoglutonic acid | 0.103 ± 0.011 | 0.143 ± 0.043 | 0.0003 |
| Citric acid | 0.133 ± 0.035 | 2.633 ± 0.578 | 0.0000 | 2-Ketoglutaric acid | 0.089 ± 0.010 | 0.085 ± 0.011 | 0.4484 |
| Total | 1.895 ± 0.291 | 3.796 ± 0.556 | 0.0000 | Ribonic acid | 0.244 ± 0.166 | 0.319 ± 0.211 | 0.3740 |
| Fatty acids (ng insect−1) | Gluconic acid | 0.162 ± 0.051 | 0.152 ± 0.041 | 0.6722 | |||
| Palmitoleic acid | 0.271 ± 0.083 | 0.197 ± 0.090 | 0.0919 | Mannonic acid lactone | 0.108 ± 0.023 | 0.138 ± 0.062 | 0.0663 |
| Palmitic acid | 0.083 ± 0.032 | 0.082 ± 0.035 | 0.9536 | D-Glucuronic acid | 0.055 ± 0.006 | 0.057 ± 0.013 | 0.6598 |
| Oleic acid | 0.251 ± 0.197 | 0.253 ± 0.200 | 0.9793 | Total | 0.760 ± 0.176 | 0.894 ± 0.245 | 0.1554 |
| Total | 0.605 ± 0.283 | 0.532 ± 0.275 | 0.5889 | ||||
| Phospho-compounds (ng insect−1) | |||||||
| Erythrose-4- phosphate | 0.398 ± 0.111 | 0.378 ± 0.173 | 0.7318 | ||||
| Glucose-6-Phosphate | 0.0510 ± 0.0072 | 0.050 ± 0.010 | 0.8524 | ||||
| Phytic acid | 0.0514 ± 0.0065 | 0.046 ± 0.007 | 0.1327 | ||||
| Phosphoric acid | 17.913 ± 1.119 | 26.153 ± 1.827 | 0.0000 | ||||
| Total | 18.414 ± 1.217 | 26.628 ± 1.821 | 0.0000 | ||||
Figure 1.
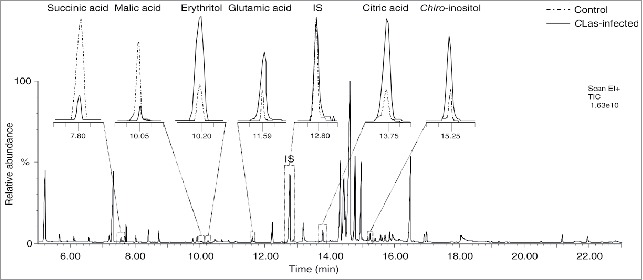
A representative gas chromatography-mass spectrometry (GC-MS) chromatogram of trimethylsilyl (TMS) derivatives detected in the extract of healthy and Candidatus Liberibacter asiaticus-infected Diaphorina citri, with a magnification of selected peaks.
Amino acids
Eleven proteinogenic amino acids were detected in the derivatized psyllid samples and L-alanine was the most abundant amino acid (Table 1). Five of these amino acids were significantly affected by CLas. Glycine, L-serine, L-glutamic acid, and L-threonine were higher in Clas-infected D. citri, whereas L-proline and L-aspartic acid were lower in Clas-infected D. citri compared with the control (Table 1). The total concentration of proteinogenic amino acids was also increased by CLas infection (Table 1).
Four non-proteinogenic amino acids (NPAAs) were detected in the derivatized samples. Gamma-amino butyric acid (GABA) in CLas-infected D. citri was higher than the controls (Table 1). Pyroglutamic acid, was lower in CLas-infected D. citri compared with the control (Table 1). The total concentration of NPAA acids in CLas-infected D. citri was reduced by CLas infection (Table 1).
Fatty and organic acids
Three fatty acids (palmitoleic acid, palmitic acid, and oleic acid) were detected in low amounts in the derivatized samples. None of these fatty acids was affected by CLas infection (Table 1). Seven organic acids were detected in the extract of D. citri (Table 1). Succinic and malic acid were lower in CLas-infected D. citri, whereas citric acid was higher (Table 1). As a result of the tremendous increase in citric acid, the total level of organic acids was increased in CLas-infected psyllids (Table 1).
Sugars, sugar alcohols, sugar acids, and phosphorous compounds
Eleven sugars were detected in the TMS-derivatized psyllid extracts (Table 1). Glucose was the most abundant sugar followed by fructose (Table 1). Erythrulose, lyxose, fructose, trehalose, and mannose were slightly higher than the control (Table 1). The disaccharide (turanose) in CLas-infected psyllids was lower than the controls (Table 1). The remaining sugars were not affected (Table 1). The total concentration of sugars was also slightly affected upon CLas infection (Table 1).
Nine sugar alcohols were detected in the TMS-derivatized samples and myo-inositol was the most abundant sugar alcohol in uninfected psyllids (Table 1). Erythritol, chiro-inositol and mannitol in CLas-infected psyllids were higher than the controls (Table 1). As a result of the increase in most of sugar alcohols, the total concentration of sugar alcohols was also increased in CLas-infected D. citri (Table 1).
None of the sugar acids was affected by CLas infection, except 2-ketoglutonic acid which was slightly increased (Table 1). Phosphoric acid was significantly higher in CLas-infected compared with the control (Table 1). As a result of the high increase in phosphoric acid, the total concentration of the phospho-compounds in CLas-infected psyllids was higher than the controls (Table 1).
Principal component analysis (PCA)
The PCA of CLas-infected and uninfected psyllids are shown in Fig. 2A-D. The PCA of CLas-infected and uninfected psyllids generated using the total concentration of the main groups is shown in Fig. 2A and B. Principal component 1 and 2 explained about 71.18% of the variation. As shown in the score plot (Fig. 2A), the uninfected psyllids were almost separated from the CLas-infected psyllids. The loading plot showed that the CLas-infected psyllids were higher in most of the metabolite groups, whereas the uninfected psyllids were higher in NPAA (Fig. 2B).
Figure 2.
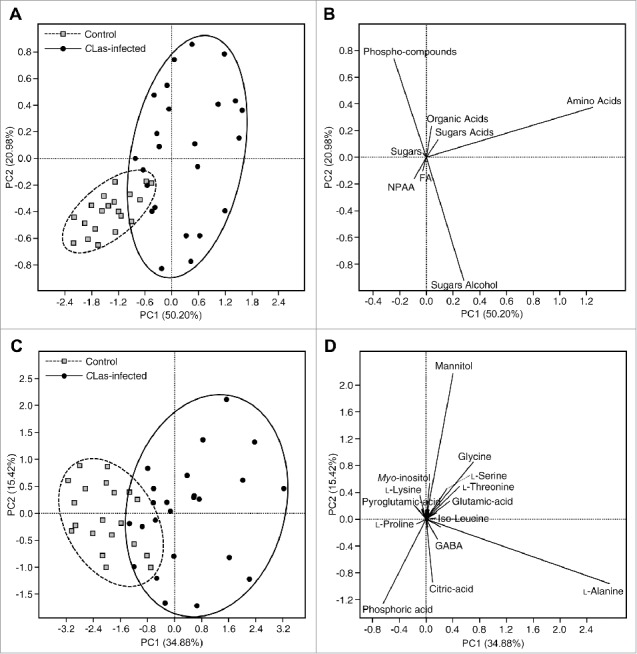
Principal components analysis (A) and its associated loading plot (B) showing the distribution of uninfected and Candidatus Liberibacter asiaticus-infected Diaphorina citri using the concentrations of the main metabolites groups. Principal components analysis (C) and its associated loading plot (D) showing the distribution of uninfected and Candidatus Liberibacter asiaticus- infected Diaphorina citri using the concentrations of individual metabolites groups (n = 20). In panel (D), Some of the detected compounds names have been deleted from the loading plot for better presentation.
The PCA of CLas-infected and uninfected psyllids generated using the concentration of all detected metabolites (Table 1) is shown in Fig. 2C and D. Principal component 1 and 2 explained about 50.30 % of the total variation. The uninfected psyllids were partially separated from the CLas-infected psyllids (Fig. 2C). These results indicated that the metabolite profile of CLas-infected psyllids was different from that of uninfected psyllids. The loading plot (Fig. 2D) visualized the distribution of the detected metabolites among the CLas-infected and uninfected psyllids. As shown in Fig. 2D, CLas-infected psyllids were higher in metabolites that appeared in the right of plot such as citric acid, erythritol, scyllo-inositol, mannitol, GABA, and phosphoric acid. On the other hand, uninfected psyllids were higher in metabolites that appeared in the left of the plot such as L-proline, pyroglutamic acid, succinic acid, lactic acid, and malic acid (Fig. 2D).
Effect of CLas on the genes expression of D. citri
The gene expression of citrate synthase (DcCS) and pyruvate carboxylase (DcPC), which are implicated in the synthesis of citric acid, were highly upregulated (5.1 ± 1.2 and 4.2 ± 0.95 fold change, respectively) in CLas-infected psyllids (Fig. 3). In addition, the expression of hexokinases (DcHK, DcHK-1, and DcHK-2), which catalyze the phosphorylation of hexoses, was upregulated in CLas-infected psyllids (Fig. 3). The gene expression of enzymes implicated in serine and glycine (DcPSPH and DcSHMT) biosynthesis were also upregulated (2–3-fold) in CLas-infected psyllids (Fig. 3). The level of the gene expression of mannose-6-phosphate isomerase (DcPMI) was increased in CLas-infected psyllids (Fig. 3). The gene expression of the enzymes implicated in erythrose biosynthesis (DcER and DcTKT) were slightly upregulated in CLas-infected psyllids. On the other hand, genes involved in trehalose metabolism such as α, α trehalose phosphate synthase (DcTPS) and trehalase (DcTREH), were expressed at a lower level (0.42 ± 0.02 and 0.47 ± 0.06, respectively) in infected-D. citri (Fig. 3).
Figure 3.
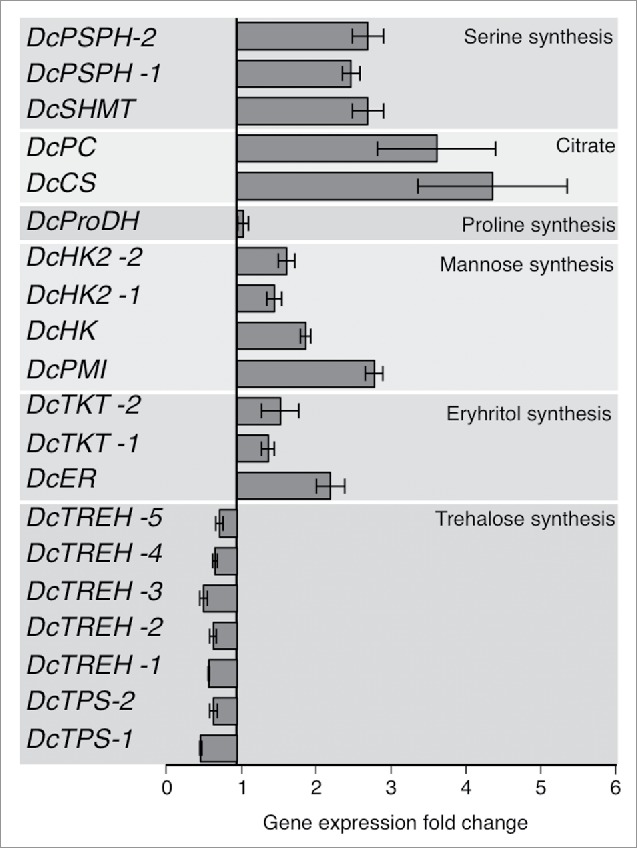
Differential gene expression patterns of expressed genes involved in TCA cycle of CLas-infected and uninfected D. citri. The complete list of expressed genes is available Table 2.
Respiration
Respiration rates of CLas-infected D. citri were higher than those of uninfected D. citri (F = 25.9, P = 0.007; Fig. 4), with the mean respiration rate of CLas-infected D. citri being 1.34 µg CO2 min−1 higher than that of uninfected D. citri (42% increase). The coefficient of variation was not affected.
Figure 4.
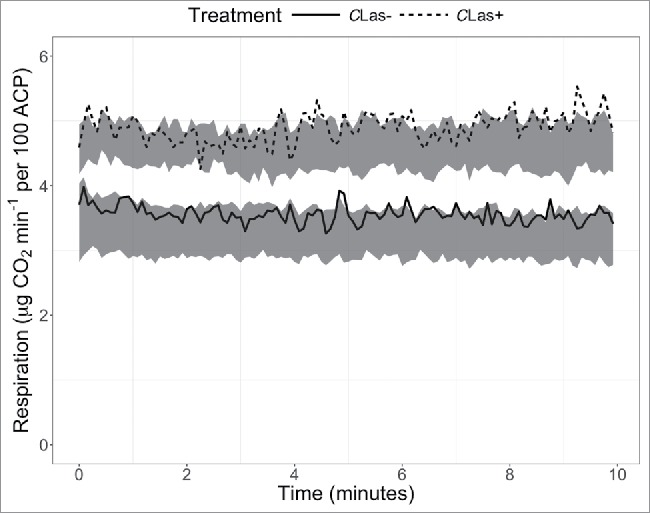
Respiration of CLas-infected and uninfected D. citri recorded over 10 min. Gray bands represent 95% confidence intervals (n = 6), and lines represent single representative samples.
Discussion
CLas-infection has tremendous effects on its host plants, resulting in mottled leaves, twig dieback and slow growth, lopsided and bitter or sour fruits, and finally results in tree decline and death.24 In addition, the CLas pathogen significantly affects the primary and secondary metabolites in its host plants.25-27 In this work, we focused on the effects of CLas on the D. citri metabolites.
The levels of many amino acids were altered in D. citri as a result of CLas infection. Our results showed that glycine, L-serine, and L-threonine were significantly higher in CLas-infected D. citri compared with the controls. In agreement with the GC-MS results, the gene expression of enzymes involved in glycine and L-serine were also upregulated in CLas-infected psyllids. Because glycine and serine can be converted to 3-phosphoglycerate and fed into the glycolysis pathway, the increase in these 2 amino acid indicated an induction in the glycolysis pathway in CLas-infected D. citri. The increase in glycolysis rate is also supported by the gene expression results which showed an increase in the gene expression of enzymes implicated in glycolysis such as hexokinase and pyruvate carboxylase. On the other hand, our current results also showed that L-aspartic acid was reduced in CLas-infected D. citri. The reduction in L-aspartic acid may also result from its oxidation to supplement the TCA cycle intermediates (fumarate and oxaloacetate) which would be under higher demand under the energetic stress caused by CLas infection. In addition, the reduction in L-aspartic acid could result from its consumption by CLas which cannot synthesize it and relies on exogenous L-aspartate.28
Our results also showed that the levels of proline in CLas-infected D. citri was lower than that of the controls. The reduction in proline may result from the reduction in its biosynthesis or the increase in its consumption or both. Proline biosynthesis in the haemolymph could be reduced as a result of high demand for Acetyl-CoA for the TCA cycle. Proline could also be catabolized to produce energy in CLas-infected psyllids and this suggestion is supported by the increase in respiration rate. Previous studies showed that proline was the major source of fuel for flight muscles in some insects.29 The decrease in proline in CLas-infected psyllids could result, at least in part, from its consumption by CLas and this suggestion is supported by the genome analysis which showed that CLas is incapable of producing proline.28
Our results also showed that GABA was higher in CLas-infected psyllids compared with the control. GABA is widely distributed in plant and animals30 and it was also detected in the citrus phloem sap and haemolymph of D. citri.31,32 The level of GABA in plants increases under various environmental stresses such as acidosis, mechanical damage, anoxia, heat and cold shock, drought, salt, and fungal, viral, and insect attack.30,33 The high level of GABA in CLas-infected D. citri could result from its feeding on infected host plants. However, it also possible that glutamic acid decarboxylase (GAD) enzymes is activated in CLas-infected D. citri to increase the production of GABA which can also be converted to succinate and fed in the TCA cycle. On the other hand, pyroglutamic acid was reduced in CLas-infested D. citri. The decrease in pyroglutamic acid could result from the activation of 5-oxoprolinase, which convert pyroglutamte to glutamate, as a result of high demand for glutamate to produce GABA. The increase in L-glutamic acid supports the previous assumption.
Our results showed that citric acid was higher in CLas-infected psyllids compared with the controls. The increase in citric acid level in in CLas-infected psyllids is in agreement with the gene expression results, which showed an increase in the expression of hexokinases, pyruvate decarboxylase, and citrate synthase, a regulatory enzyme in TCA cycle. In agreement with our results, a previous proteomic study showed that many enzymes involved in metabolism and cellular energy storage and utilization were induced at a higher rate in CLas-infected D. citri.34 These enzymes included several enzymes involved the citric acid cycle and such as succinate dehydrogenase, 2-oxoglutarate dehydrogenase (a regulatory enzyme in TCA cycle), and L-2-hydroxyglutarate dehydrogenase.34 Both Acetyl-CoA dehydrogenase and enoyl-CoA hydrolase proteins enzymes were also induced (2-fold and 12-fold, respectively) in CLas-infected D. citri.34 These 2 enzymes are involved in fatty acid β-oxidation and the production of Acetyl-CoA which feeds into the TCA cycle.34 Glycerol kinase enzyme which is required for triglyceride breakdown was also upregulated in CLas-infected D. citri.34 In addition, 2 glycolysis enzymes (aldose 1-epimerase and phosphoglycerate mutase) were induced in CLas-infected D. citri.34 Succinate semialdehyde dehydrogenase which converts succinate semialdehyde, produced from GABA, to succinate was also induced in CLas-infected D. citri.34 The metabolomics and the proteomic results together suggested that glycolysis and TCA cycle are enhanced in CLas-infected D. citri. In addition, a previous transcriptome study also showed that CLas infection alters the expression of many genes involved in nutrient reservoir activity in D. citri.35 Gene expression of CLas-infected D. citri suggested that CLas alters its host environment to import needed nutrients.35
Although the current gene expression and the previous proteomic analysis confirmed that most of the enzymes involved in the citric acid cycle were induced in CLas-infected psyllids,34 the level of malate and succinate in CLas-infected psyllids were lower than the control. This result indicated that CLas may acquire succinate and malate from D. citri. Since isocitrate lyase and malate synthase are absent in CLas genome, it is believed that CLas lack the glyoxylate bypass.28 Therefore, CLas depends on exogenous fumarate, malate, succinate, and aspartate as carbon substrates.28 These intermediates could also be exhausted by D. citri which is under the energetic stress due to CLas infection.
The activation of TCA cycle in CLas-infected psyllids indicated that infected psyllids are under nutrient and energy stress. Previous studies showed that CLas encodes an ATP/ADP translocase in its genome, indicating that CLas can import exogenous ATP.36,37 Recently, we studied the effect of CLas on the nucleotides profile of D. citri and found that ATP and many other nucleotides were increased upon CLas infection.38 The accumulation of ATP in CLas-infected D. citri psyllids increased the adenylated energy charge (AEC) and decreased the AMP/ATP and ADP/ATP ratios. In agreement with the increase in AEC, the survival and life span of CLas-infected psyllids were also lower than those of healthy psyllids.38 In addition, electropenetrography showed that CLas increased the feeding activity of D. citri indicating that CLas-induced energetic stress in its vector.
The increases in glycolysis and the TCA-cycle of CLas-infected D. citri likely account for increased respiration rates. Although there are potential biochemical pathways to decouple aging from respiratory rates,39 increased respiratory rates in other insect species are generally associated with reduced lifespan,,40,41 and increased respiratory rates are considered to be tradeoffs with other fitness characteristics.42 This is consistent with existing literature regarding the impact of CLas on D. citri that has found that CLas infection increases feeding activity, dispersal, and reproductive fitness, but reduced nymphal development rate and lifespan.13,14 Many of these changes may be influenced by increased respiration resulting from CLas manipulation of D. citri energetics metabolic pathways.
Although our results showed that the glycolysis and TCA pathways were induced in D. citri upon CLas infection, only a slight increase in trehalose, mannose, xylose, and fructose was observed in CLas-infected D. citri. This result indicated that the mechanism regulating sugar levels in the D. citri was not highly affected by CLas infection. Because carbohydrates are the main source of energy in insects, their levels must be maintained at a high level to support flight muscle and other activities.22 In general, sugars content is very high in the phloem sap, therefore phloem sap-feeding insects should be able to overcome the sugar barrier which can cause high osmotic pressure.7 A slight decrease in fructose and increase in glucose was observed in honeybee worker haemolymph infected with Nosema ceranae, whereas the level of trehalose was not affected.22 The previous results together with our result indicated that sugars are under tight regulation in insects. In fact, previous studies showed that trehalose synthesis is inhibited when trehalose concentration reaches a certain level and UDP-glucose is directed for glycogen synthesis.43 The increase in glycogenin protein in CLas-infected D. citri suggested that extra trehalose in CLas-infected D. citri is being converted to glycogen.34
Sugar alcohols (inositols) were previously reported in the citrus phloem sap44 and the haemolymph.32 Several roles for polyols in insects have been proposed including the regulation of osmotic pressure, protection against cold and heat stress, and meeting the nutritional requirements of insect symbionts like yeast.45 The increase in sugar alcohols in CLas-infected D. citri could be a response to the biotic stress caused by CLas infection. Phosphoric acid was also higher in CLas-infected D. citri. Phosphoric acid was also increased in honeybee worker (Apis mellifera L.) haemolymph upon infection with Nosema ceranae.22 Our current results together with the previous result indicated that the increase in phosphoric acid could be a general indicator of infection or cell damage.
In conclusion, our results showed that CLas alters the metabolomic profile of its vector and enhanced the glycolysis and TCA cycle. In agreement with the increase in the glycolysis and TCA cycle, the respiration rate of CLas-infected psyllids was induced. Our current results indicated that CLas-infected psyllids are under nutrients and energetic stress and this could explain their increased feeding and flight activity and reduced lifespan. Changes observed in this study could reveal insights into CLas pathogenicity.
Materials and methods
Asian citrus psyllid culture
Colonies of Asian citrus psyllid, Diaphorina citri, were reared on uninfected (control) or CLas-infected Valencia sweet orange (Citrus sinensis ‘Valencia’). Both colonies as well as the citrus plants were constantly monitored with PCR5 to confirm infection with CLas. Adult of healthy (control) and CLas-infected psyllids were sampled without age or gender discrimination from healthy and CLas-infected colonies. The collected CLas-infected psyllids were kept on uninfected plants for 2 weeks before metabolite extraction to avoid the possible effects of poor plant quality on D. citri metabolism. Colonies were kept in growth rooms which were set at 27–28°C, 60–65% relative humidity (RH), and a photoperiod of 14L:10D h.
Metabolite extraction
To improve the detection limits of the D. citri metabolites, we relied on whole-organism sample pooling. Groups of 50 D. citri adults were collected from CLas-infected (58 ± 6.0%) and healthy colonies (control). Ten replicates of 50 (500 total) insects from healthy D. citri adults (never been exposed to a CLas-infected plant) and 40 replicates of 50 (2000 total) insects from Clas-infected D. citri adults (reared on CLas-infected plants) were collected. The collected D. citri adults were placed in – 20°C for 2 h (cold anesthetized) to facilitate handling. Insects were transferred into 1.5 mL microcentrifuge tubes. To extract a broad range of metabolites, a 100 μL aliquot of the extraction solvent (8:1:11; methanol: chloroform: water) was added to each tube of insects. Insects were macerated by homogenizing with a motorized pestle in the extraction solution for 5 min. Tubes of macerated insects were placed on ice and a glass bead was added to each tube. Samples were then placed on an Ocelot platform mixer (Model 260300F, Boekel Scientific, Feasterville, PA) and rocked overnight at 4°C. After overnight extraction, the tubes of insect homogenate were vortexed briefly and centrifuged at 5°C for 5 min at 10, 000 rpm in a refrigerated centrifuge (Model 5430 R, Eppendorf, Hauppauge, NY) to remove solid debris. The supernatant was collected from each tube and placed into new 1.5 mL tubes.
Derivatization, GC-MS conditions and peak analysis
Ten μL aliquot of each D. citri supernatant was placed into a silanized GC vial with 200 μL fused-insert (MSCert4000–30LVW, National Scientific, Rockwood, TN) along with 10 μL of internal standard (1000 ppm ribitol in water, Sigma Aldrich, St. Louis, MO). This solution was dried under a nitrogen stream before adding the derivatization reagents. Methoxyamine hydrochloride solution (MOX) in pyridine (2%), and N-methyl-(N-trimethylsilyl) trifluoracetamide (MSTFA), were purchased from ThermoFisher Scientific (Waltham, MA). To the dried samples, 30 μL MOX was added and incubated at 60°C for 1 hour. Finally, 80 μL MSTFA was added and incubated an additional 1 h at 60°C. The derivatized samples were injected splitlessly into the GC injector (1 μL) for analysis using the same GC-MS column and conditions described by Killiny (2016).46 Total ion chromatograms were analyzed using Turbomass software and peaks of interest were identified based on the relative retention time and ion spectrum comparison of authentic standards to sample spectra and by using Wiley 9th ed. and NIST 2011 mass spectral libraries. Calibration curves for authentic standards were constructed from 4 classes of compounds, amino acids, organic acids, sugars and sugar alcohols derivatized in the same manner as the samples. For compounds in which a reference standard was not available, quantification was based on the standard curve of the reference compound most similar in chemical class and relative concentration. Compounds present in sufficient quantity were converted from peak area to μg g−1 FW insect concentration. Concentrations (ng/insect) reported are the mean of 10 biologic replicates.
Respiration
Uninfected (control) and CLas-infected (58 ± 6.0%) D. citri were collected from rearing cages in a contained growth chamber after at least 2-hours from the beginning of artificial daytime. Approximately 100 individuals at a time were placed in a respiration chamber. The chamber was allowed to warm to ambient temperature, approximately 24˚C, for 25 min before beginning measurements. The insect respiration was measured using an infrared gas analyzer (LI-6400; Licor Biosciences, Inc.; Lincoln, NE, USA) at a flow rate of 500 µmols min−1. Because insect respiration measurements depend on the dynamics of movement, measurements were recorded every 5 sec for 16.6 min for each sample.
Sampling was performed in replicate sets of CLas-infected and healthy psyllids. Two replicates each were tested each day for 3 d. After respiration measurements, the number of insects were counted, and insects were killed and fresh weight measured, followed by drying for 24 hours at 70˚C and recording of dry weights. Respiration was weighted by the number of insects, and analyzed as µg CO2 per 100 D. citri.
Gene expression analysis using quantitative real time PCR (RT-PCR)
The total RNA was extracted from 10 insects per replicate (3 replicates for each treatment), using TriZol® reagent (Ambion®, Life Technologies, NY, USA). Both quantity and quality of isolated RNA were determined using NanoDrop 2000 spectrophotometrer (Thermo Scientific, USA). For synthesising cDNA, SuperScript first-strand synthesis system (Invitrogen) with random hexamer primers was used as described by the manufacturer's instructions. Further, the qPCR was preformed using SYBER Green PCR master mix (Applied Biosystems), on an ABI 7500 Fast-Time PCR System (Applied Biosystems). Samples were analyzed in triplicate for each biologic replicate for each treatment. Primers for 29 selected genes, involved in the affected cycles of D. citri (increase or decrease in its compounds), were used to measure the gene expression (Table 2). The 2−ΔΔCT method was used to determine the relative expression of the consensus sequence among PCR products according to Livak and Schmittgen (2001).47 Gene expression data was normalized using α-Tubulin or actin as endogenous reference genes as described by Tiwari et al. (2011) and Killiny et al.(2014).48,49
Table 2.
Primers used in gene expression analysis of selected genes of D. citri by real time RT-PCRa.
| Gene | NCBI Reference Sequence | Primer (Forward and Reverse) | TM (˚C) | Product size (bp) | |
|---|---|---|---|---|---|
| DcTPS -1 | XM_017448460.1 | F | AGTCATTATCCAGGGCAACG | 59.96 | 198 |
| R | CAGAAGGTGCCATTACAGCA | 59.86 | |||
| DcTPS -2 | XM_008470475.1 | F | CCTACATCCGGATTGACCAG | 60.33 | 192 |
| R | CGTACAGTTGGCGAATTCCT | 60.13 | |||
| DcTREH -1 | XM_008470849.1 | F | GGTAATGGAGGCGAGTACGA | 60.10 | 190 |
| R | ACAGGAACAGGATGCCTCAC | 60.12 | |||
| DcTREH -2 | XM_008470850.2 | F | TCACCTCCAACCCGAGTATC | 59.93 | 191 |
| R | CAGTTCTCGAAACCGTCCTC | 59.84 | |||
| DcTREH -3 | XM_008476679.2 | F | CTGAAGTGTGTGCTCCGAAA | 60.02 | 197 |
| R | GAGTCGGGAAACAGTGAAGC | 59.85 | |||
| DcTREH -4 | XM_008476680.2 | F | TGCAGTTCAGATGGCTTCAC | 59.99 | 198 |
| R | GGCGACCAGTCTTCAAACTC | 59.85 | |||
| DcTREH -5 | XM_008476684.2 | F | GCAGTGGGACTACCCTAACG | 59.62 | 195 |
| R | CCGTGTCCTCCTGGTACTGT | 60.03 | |||
| DcER -1b | XM_008489926.2 | F | GAGAGGTGAAACAGGCGGTA | 60.25 | 200 |
| R | TGCAGGTTTCACGAGATCAG | 59.98 | |||
| DcTKT -1 | XM_008486923.2 | F | CCACGCACAGAGACATCATT | 59.71 | 201 |
| R | CGGTGGTCGGTACAGATTTT | 59.85 | |||
| DcTKT -2 | XM_008471702.2 | F | CCAGATCCGTATGGGAGCTA | 60.05 | 201 |
| R | CCTGGGATTGTACGGAACAT | 59.67 | |||
| DcMPI | XM_008474470.2 | F | CCAGAATTGGCCATAGCACT | 60.10 | 199 |
| R | GAGCTTGGGGAGCACTCATA | 60.36 | |||
| DcHK | XM_008477526.2 | F | AAGGATAGACCGGGATGACA | 59.36 | 199 |
| R | CTGGAAAGCGCCTAGTTTCA | 60.51 | |||
| DcHK2 -1 | XM_008480214.1 | F | GCGAGCTGGTTCGTGTAGTA | 59.10 | 200 |
| R | ATCCACCGCTATGGTGATGT | 60.23 | |||
| DcHK2 -2 | XM_017444207.1 | F | CTAGCCGGCCTAGGATTACC | 60.08 | 202 |
| R | TCATCAGGTCATGGAAGTGC | 59.64 | |||
| DcProDH | XM_017446986.1 | F | GAAGGTGACCAAGGATGCTC | 59.66 | 199 |
| R | TCGGACCAGGGTGTTTAGTC | 59.97 | |||
| DcCS | XM_008470046.1 | F | TGCCCGATGACTCCTTATTC | 60.04 | 190 |
| R | CCAAGAGCTCTGGACACTCC | 59.99 | |||
| DcPC | XM_008478621.1 | F | GAAGCTTTGGCTTCATTTGG | 59.82 | 201 |
| R | GTCGCGGACACTTACACTCA | 59.90 | |||
| DcSHMT | XM_008473310.2 | F | TCGAACCTCATGGGAGAATC | 60.01 | 197 |
| R | TCCTTTGTGAGTGGTGGTCA | 60.13 | |||
| DcPSPH-1 | XM_008483178.2 | F | GGCCAAAGTCATCGAGAGTC | 59.81 | 202 |
| R | GACCACGTTGCCTCCATAAC | 60.38 | |||
| DcPSPH-2 | XM_008472446.2 | F | TGAACCCGGAGCAGATATTC | 60.04 | 209 |
| R | ACTCAGGCTCGGCTTCATAA | 59.98 | |||
| α-Tubulinc | XM_008482608.2 | F | CTTTCCAACACCACCGCTAT | 59.99 | 196 |
| R | CTCCTTCTCCAGCCTCCTCT | 60.09 | |||
| Actinc | XM_008470468.2 | F | TATCCCAGCCCTGAGCTAGA | 59.93 | 204 |
| R | CACCATGACACCCTGATGAC | 59.80 |
The listed genes were selected depending on the affected cycles (increase or decrease in its compounds). Furthermore, these genes were assembled based on recent available data in national center for biotechnology information website (NCBI, http://www.ncbi.nlm.nih.gov/gene/).
DcER gene has been matched as Diaphorina citri aldose reductase-like (DcAR) using the protein-protein BLAST, based on recent available data in national center for biotechnology information website (NCBI, http://www.ncbi.nlm.nih.gov/gene/).
Genes have been used as a reference genes for data normalization according to Tiwari et al., 201148 and Killiny et al., 201449
Abbreviations:
DcTPS-1: Diaphorina citri-α, α Trehalose phosphate synthase;
DcTPS-2: Diaphorina citri-Trehalose phosphate synthase-like;
DcTREH: Diaphorina citri-Trehalase;
DcER: Diaphorina citri-Erythrose reductase;
DcTKT: Diaphorina citri-Transketolase-like;
DcMPI: Diaphorina citri-Mannose-6-phosphate isomerase;
DcHK: Diaphorina citri-Hexokinase-like;
DcHK2: Diaphorina citri-Hexokinase-2-like;
DcProDH: Diaphorina citri-proline dehydrogenase 1;
DcCS: Diaphorina citri-probable citrate synthase 2;
DcPC: Diaphorina citri-pyruvate carboxylase, mitochondrial-like;
DcSHMT: Diaphorina citri-serine hydroxymethyltransferase, cytosolic;
DcPSPH: Diaphorina citri-phosphoserine phosphatase-like
Statistical analysis
Data was manually aligned using retention time and mass values. The concentrations of each metabolites in CLas-infected D. citri were compared with the control using t-test. Principal component analysis (PCA) was performed on individual metabolite concentrations to discriminate healthy and CLas-infected psyllid using JMP version 9.0 (SAS Institute Inc.,). The PCA was also conducted using the total concentrations of the main groups of metabolites shown in Table 1. Analysis of variance was performed on the mean and coefficient of variation of each insect respiration sample (mean of 200 measurements each) for the purpose of testing overall differences in respiration rates (mean) and of testing the relative variation in rates (coefficient of variation). Analysis used a linear model in R statistics package (R Foundation, Vienna, Austria) with Treatment (CLas-infected or healthy D. citri) as a fixed effect and replicate as a random effect.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge our CREC colleagues for the helpful discussion. We thank Laina Lindsey and Floyd Butz for the technical assistance and maintaining the psyllid colonies, and Myrtho Pierre for assistance in insect respiration measurements.
Funding
This study was generously supported by the grant number 2016–70016–24824 received from NIFA-USDA.
References
- [1].Gottwald TR. Current epidemiological understanding of citrus Huanglongbing. Annu Rev Phytopathol 2010; 48:119-39; PMID:20415578; https://doi.org/ 10.1146/annurev-phyto-073009-114418 [DOI] [PubMed] [Google Scholar]
- [2].Jagoueix S, Bove JM, Garnier M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int J Syst Bacteriol 1994; 44:379-86; PMID:7520729; https://doi.org/ 10.1099/00207713-44-3-379 [DOI] [PubMed] [Google Scholar]
- [3].Bové JM. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 2006; 88:7-37; https://doi.org/ 10.4454/jpp.v88i1.828 [DOI] [Google Scholar]
- [4].Tatineni S, Sagaram US, Gowda S, Robertson CJ, Dawson WO, Iwanami T, Wang N. In planta distribution of “Candidatus Liberibacter asiaticus” as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathol 2008; 98:592-9; PMID:18943228; https://doi.org/ 10.1094/PHYTO-98-5-0592 [DOI] [PubMed] [Google Scholar]
- [5].Halbert SE, Manjunath KL. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Florida Entomol 2004; 87:330-53; https://doi.org/ 10.1653/0015-4040(2004)087[0330:ACPSPA]2.0.CO;2 [DOI] [Google Scholar]
- [6].Bové J, Garnier M. Phloem-and xylem-restricted plant pathogenic bacteria. Plant Sci 2003; 164:423-38; https://doi.org/ 10.1016/S0168-9452(03)00033-5 [DOI] [Google Scholar]
- [7].Douglas AE. Phloem-sap feeding by animals: problems and solutions. J Exp Bot 2006; 57:747-54; PMID:16449374; https://doi.org/ 10.1093/jxb/erj067 [DOI] [PubMed] [Google Scholar]
- [8].Meyer JM, Hoy MA. Molecular survey of endosymbionts in Florida populations of Diaphorina citri (Hemiptera: Psyllidae) and its parasitoids Tamarixia radiata (Hymenoptera: Eulophidae) and Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). Florida Entomol 2008; 91:294-304; https://doi.org/ 10.1653/0015-4040(2008)91[294:MSOEIF]2.0.CO;2 [DOI] [Google Scholar]
- [9].Fletcher J, Wayadande A, Melcher U, Ye F. The phytopathogenic mollicute-insect vector interface: A closer look. Phytopathol 1998; 88:1351-8; PMID:18944839; https://doi.org/ 10.1094/PHYTO.1998.88.12.1351 [DOI] [PubMed] [Google Scholar]
- [10].Hall DG, Richardson ML, Ammar E-D, Halbert SE. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol Exp Appl 2013; 146:207-23; https://doi.org/ 10.1111/eea.12025 [DOI] [Google Scholar]
- [11].Ammar E-D, Ramos JE, Hall DG, Dawson WO, Shatters RG. Acquisition, replication and inoculation of Candidatus Liberibacter asiaticus following various acquisition periods on Huanglongbing-infected citrus by nymphs and adults of the Asian citrus psyllid. PLoS One 2016; 11:e0159594; PMID:27441694; https://doi.org/ 10.1371/journal.pone.0159594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ammar ED, Shatters RG, Lynch C, Hall DG. Detection and relative titer of Candidatus Liberibacter asiaticus in the salivary glands and alimentary canal of Diaphorina citri (Hemiptera: Psyllidae) vector of citrus Huanglongbing disease. Ann Entomol Soc Am 2011; 104:526-33; https://doi.org/ 10.1603/AN10134 [DOI] [Google Scholar]
- [13].Pelz-Stelinski K, Killiny N. Better together: association with “Candidatus Liberibacter Asiaticus” increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae). Ann Entomol Soc Am 2016; 48:539-48; PMID:27418697; https://doi.org/ 10.1093/aesa/saw007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Martini X, Hoffmann M, Coy MR, Stelinski LL, Pelz-Stelinski KS. Infection of an insect vector with a bacterial plant pathogen increases its propensity for dispersal. PLoS One 2015; 10:e0129373; PMID:26083763; https://doi.org/ 10.1371/journal.pone.0129373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tiwari S, Pelz-Stelinski K, Stelinski LL. Effect of Candidatus Liberibacter asiaticus infection on susceptibility of Asian citrus psyllid, Diaphorina citri, to selected insecticides. Pest Manag Sci 2011; 67:94-9; PMID:20960471; https://doi.org/ 10.1002/ps.2038 [DOI] [PubMed] [Google Scholar]
- [16].Perilla-Henao LM, Casteel CL. Vector-borne bacterial plant pathogens: interactions with hemipteran insects and plants. Front Plant Sci 2016; 7:1163:1–15; PMID:27555855; https://doi.org/ 10.3389/fpls.2016.01163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J 2000; 23:131-42; PMID:10929108; https://doi.org/ 10.1046/j.1365-313x.2000.00774.x [DOI] [PubMed] [Google Scholar]
- [18].Hawes TC, Hines AC, Viant MR, Bale JS, Worland MR, Convey P. Metabolomic fingerprint of cryo-stress in a freeze tolerant insect. Cryo Letters 2008; 29:505-15; PMID:19280054 [PubMed] [Google Scholar]
- [19].Snart CJP, Hardy ICW, Barrett DA. Entometabolomics: applications of modern analytical techniques to insect studies. Entomol Exp Appl 2015; 155:1-17; PMID:27478203; https://doi.org/ 10.1111/eea.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cevallos-Cevallos JM, Danyluk MD, Reyes-De-Corcuera JI. GC-MS Based metabolomics for rapid simultaneous detection of Escherichia coli O157:H7, Salmonella Typhimurium, Salmonella Muenchen, and Salmonella Hartford in ground beef and chicken. J Food Sci 2011; 76:M238-46; PMID:22417363; https://doi.org/ 10.1111/j.1750-3841.2011.02132.x [DOI] [PubMed] [Google Scholar]
- [21].Birkenstock T, Liebeke M, Winstel V, Krismer B, Gekeler C, Niemiec MJ, Bisswanger H, Lalk M, Peschel A. Exometabolome analysis identifies pyruvate dehydrogenase as a target for the antibiotic triphenylbismuthdichloride in multiresistant bacterial pathogens. J Biol Chem 2012; 287:2887-95; PMID:22144679; https://doi.org/ 10.1074/jbc.M111.288894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aliferis KA, Copley T, Jabaji S. Gas chromatography–mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J Insect Physiol 2012; 58:1349-59; PMID:22841888; https://doi.org/ 10.1016/j.jinsphys.2012.07.010 [DOI] [PubMed] [Google Scholar]
- [23].Xu Y-J, Luo F, Gao Q, Shang Y, Wang C. Metabolomics reveals insect metabolic responses associated with fungal infection. Anal Bioanal Chem 2015; 407:4815-21; PMID:25895944; https://doi.org/ 10.1007/s00216-015-8648-8 [DOI] [PubMed] [Google Scholar]
- [24].Teixeira D do C, Danet JL, Eveillard S, Martins EC, Junior WC, de J, Yamamoto PT, Lopes SA, Bassanezi RB, Ayres AJ, Saillard C, et al.. Citrus Huanglongbing in São Paulo state, Brazil: PCR detection of the “Candidatus” Liberibacter species associated with the disease. Mol Cell Probes 2005; 19:173-9; https://doi.org/ 10.1016/j.mcp.2004.11.002 [DOI] [PubMed] [Google Scholar]
- [25].Cevallos-Cevallos JM, Futch DB, Shilts T, Folimonova SY, Reyes-De-Corcuera JI. GC–MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus Huanglongbing. Plant Physiol Biochem 2012; 53:69-76; PMID:22326359; https://doi.org/ 10.1016/j.plaphy.2012.01.010 [DOI] [PubMed] [Google Scholar]
- [26].Albrecht U, Fiehn O, Bowman KD. Metabolic variations in different citrus rootstock cultivars associated with different responses to Huanglongbing. Plant Physiol Biochem 2016; 107:33-44; PMID:27236226; https://doi.org/ 10.1016/j.plaphy.2016.05.030 [DOI] [PubMed] [Google Scholar]
- [27].Hijaz F, Manthey JA, Folimonova SY, Davis CL, Jones SE, Reyes-De-Corcuera JI. An HPLC-MS characterization of the changes in sweet orange leaf metabolite profile following infection by the bacterial pathogen Candidatus liberibacter asiaticus. PLoS One 2013; 8:1-15; PMID:24223954; https://doi.org/ 10.1371/journal.pone.0079485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang N, Trivedi P. Citrus Huanglongbing: a newly relevant disease presents unprecedented challenges. Phytopathol 2013; 103:652-65; PMID:23441969; https://doi.org/ 10.1094/PHYTO-12-12-0331-RVW [DOI] [PubMed] [Google Scholar]
- [29].Bursell E. The Role of Proline in Energy Metabolism In: Energy Metabolism in Insects. Boston, MA: Springer US; 1981. page 135-154; https://doi.org/ 10.1007/978-1-4615-9221-1_5 [DOI] [Google Scholar]
- [30].Ryu S-N, Ham T, Chu S, Han SJ. γ-Aminobutyric acid metabolism in plant under environmental stresses. Korean J Crop Sci 2012; 57:144-50; https://doi.org/https://doi.org/ 10.7740/kjcs.2012.57.2.144 [DOI] [Google Scholar]
- [31].Killiny N, Hijaz F. Amino acids implicated in plant defense are higher in Candidatus Liberibacter asiaticus-tolerant citrus varieties. Plant Signal Behav 2016; 11:e1171449; PMID:27057814; https://doi.org/ 10.1080/15592324.2016.1171449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Killiny N, Hijaz F, El-Shesheny I, Alfaress S, Jones SE, Rogers ME. Metabolomic analyses of the haemolymph of the Asian citrus psyllid Diaphorina citri, the vector of Huanglongbing. Physiol Entomol 2016; 42:134-45; https://doi.org/ 10.1111/phen.12183 [DOI] [Google Scholar]
- [33].Bown AW, MacGregor KB, Shelp BJ. Gamma-aminobutyrate: defense against invertebrate pests? Trends Plant Sci 2006; 11:424-7; PMID:16890474; https://doi.org/ 10.1016/j.tplants.2006.07.002 [DOI] [PubMed] [Google Scholar]
- [34].Ramsey JS, Johnson RS, Hoki JS, Kruse A, Mahoney J, Hilf ME, Hunter WB, Hall DG, Schroeder FC, MacCoss MJ, et al.. Metabolic interplay between the Asian citrus psyllid and its Profftella symbiont: An Achilles' heel of the citrus greening insect vector. PLoS One 2015; 10:e0140826; PMID:26580079; https://doi.org/ 10.1371/journal.pone.0140826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vyas M, Fisher TW, He R, Nelson W, Yin G, Cicero JM, Willer M, Kim R, Kramer R, May GA, et al.. Asian citrus psyllid expression profiles suggest Candidatus Liberibacter asiaticus-mediated alteration of adult nutrition and metabolism, and of nymphal development and immunity. PLoS One 2015; 10:e0130328; PMID:26091106; https://doi.org/ 10.1371/journal.pone.0130328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Duan Y, Zhou L, Hall DG, Li W, Doddapaneni H, Lin H, Liu L, Vahling CM, Gabriel DW, Williams KP, et al.. Complete genome sequence of citrus Huanglongbing bacterium, “Candidatus Liberibacter asiaticus” obtained through metagenomics. Mol Plant Microbe Interact 2009; 22:1011-20; PMID:19589076; https://doi.org/ 10.1094/MPMI-22-8-1011 [DOI] [PubMed] [Google Scholar]
- [37].Vahling CM, Duan Y, Lin H. Characterization of an ATP translocase identified in the destructive plant pathogen “Candidatus Liberibacter asiaticus”. J Bacteriol 2010; 192:834-40; PMID:19948801; https://doi.org/ 10.1128/JB.01279-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Killiny N, Hijaz F, Ebert TA, Rogers ME. Plant bacterial pathogen manipulates the energy metabolism of its insect vector. Appl Environ Microbiol 2016; e03005-16; AEM.03005-16; PMID:28039132; https://doi.org/ 10.1128/AEM.03005-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Copeland JM, Cho J, Lo T Jr, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol 2009; 19:1591-8; PMID:19747824; https://doi.org/ 10.1016/j.cub.2009.08.016 [DOI] [PubMed] [Google Scholar]
- [40].Rascón B, Harrison JF. Lifespan and oxidative stress show a non-linear response to atmospheric oxygen in Drosophila. J Exp Biol 2010; 213:3441-8; PMID:20889824; https://doi.org/ 10.1242/jeb.044867 [DOI] [PubMed] [Google Scholar]
- [41].Sohal RS. Oxygen consumption and life span in the adult male housefly, Musca domestica. Age (Omaha) 1982; 5:21-4; https://doi.org/ 10.1007/BF02431719 [DOI] [Google Scholar]
- [42].Crnokrak P, Roff DA. Trade-offs to flight capability in Gryllus firmus: the influence of whole-organism respiration rate on fitness. J Evol Biol 2002; 15:388-98; https://doi.org/ 10.1046/j.1420-9101.2002.00401.x [DOI] [Google Scholar]
- [43].Friedman S. Treholose regulation, one aspect of metabolic homeostasis. Annu Rev Entomol 1978; 23:389-407; https://doi.org/ 10.1146/annurev.en.23.010178.002133 [DOI] [Google Scholar]
- [44].Hijaz F, Killiny N. Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (Sweet Orange). PLoS One 2014; 9:e101830; PMID:25014027; https://doi.org/ 10.1371/journal.pone.0101830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Moriwaki N, Matsushita K, Nishina M, Matsuda K, Kono Y. High myo-inositol concentration in the hemolymph of planthoppers. Appl Entomol Zool 2003; 38:359-64; https://doi.org/ 10.1303/aez.2003.359 [DOI] [Google Scholar]
- [46].Killiny N. Generous hosts: What makes Madagascar periwinkle (Catharanthus roseus) the perfect experimental host plant for fastidious bacteria? Plant Physiol Biochem 2016; 109:28-35; PMID:27620272; https://doi.org/ 10.1016/j.plaphy.2016.09.002 [DOI] [PubMed] [Google Scholar]
- [47].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001; 25:402-8; https://doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- [48].Tiwari S, Gondhalekar AD, Mann RS, Scharf ME, Stelinski LL. Characterization of five CYP4 genes from Asian citrus psyllid and their expression levels in Candidatus Liberibacter asiaticus-infected and uninfected psyllids. Insect Mol Biol 2011; 20:733-44; PMID:21919983; https://doi.org/ 10.1111/j.1365-2583.2011.01103.x [DOI] [PubMed] [Google Scholar]
- [49].Killiny N, Hajeri S, Tiwari S, Gowda S, Stelinski LL. Double-stranded RNA uptake through topical application, mediates silencing of five CYP4 genes and suppresses insecticide resistance in Diaphorina citri. PLoS One 2014; 9:e110536; PMID:25330026; https://doi.org/ 10.1371/journal.pone.0110536 [DOI] [PMC free article] [PubMed] [Google Scholar]


