Introduction
Decades of research have demonstrated markedly elevated physical comorbidity and premature mortality in persons with schizophrenia, with a 15–20 year shorter life expectancy compared to the general population (1, 2). Much of this longevity gap has been attributed to natural causes of death, in particular, cardiovascular disease (3–5). Unhealthy lifestyles (e.g., smoking, poor diet, sedentary habits), suboptimal healthcare perpetuated by social stigma against mental illnesses, and biological factors (e.g., accelerated aging) contribute to increased mortality (4, 6–10). Recent individual studies have suggested an increasing mortality gap between persons with schizophrenia and the general population during the last 20–30 years (4, 9, 11–16). However, in contrast to regular epidemiologic examinations of changing mortality trends in serious physical illnesses such as cancer (17–19), there has been no systematic review of longitudinal trends in mortality among persons with schizophrenia.
Average life expectancy in developed countries (including Western Europe) increased from 72 years in 1970–1975 to 80 years in 2005–2010 (20). This increase is attributed mainly to medical advances and improved healthcare, turning illnesses that used to be almost inevitably fatal (e.g., myocardial infarctions and strokes) into chronic diseases. However, these benefits have not been universal, and have not had much impact on population subgroups such as ethnic/racial minorities and lower socioeconomic classes (20), likely due to discrimination, disparate use of healthcare services, race-specific risks for certain diseases, and lifestyle differences between low- and high-income areas. In the case of schizophrenia, the main changes in the mental healthcare system in the western world during the last century were the discovery and widespread use of antipsychotic medications in the 1950s and the subsequent community psychiatry movement that led to deinstitutionalization of persons with serious mental illnesses (SMI) from psychiatric hospitals. Psychiatric care was restructured from primary inpatient services to outpatient clinics, as most psychiatric hospitals closed in the 1960s and early 1970s throughout western European countries (21–23). In the United Kingdom, for instance, the number of psychiatric beds decreased from 150,000 in the 1950s to 27,000 in 2010, following sharp declines in the 1970s and 1980s (24). This change might have helped protect persons with SMI from the abuses of mental institutions, offered effective treatment of the symptoms for the first time, and promoted recovery within a community setting (5, 12). Initially, most people with SMI moved from institutions to live with their families; however, at least in the US, the proportion of SMI patients living with families has declined significantly over the past three decades, from 73% to 46% (25–27). At the same time, the mental health system has lacked adequate infrastructure for the necessary social support and healthcare for people with SMI, resulting in new challenges of homelessness and incarceration (23). In order to highlight an important area of policy reform and improved clinical practice, we sought to determine if the excess mortality rates in schizophrenia changed during the recent decades, explicitly focusing on the pre-1970 and post-1970 studies to assess possible associations of deinstitutionalization and changing mental healthcare system.
Methods
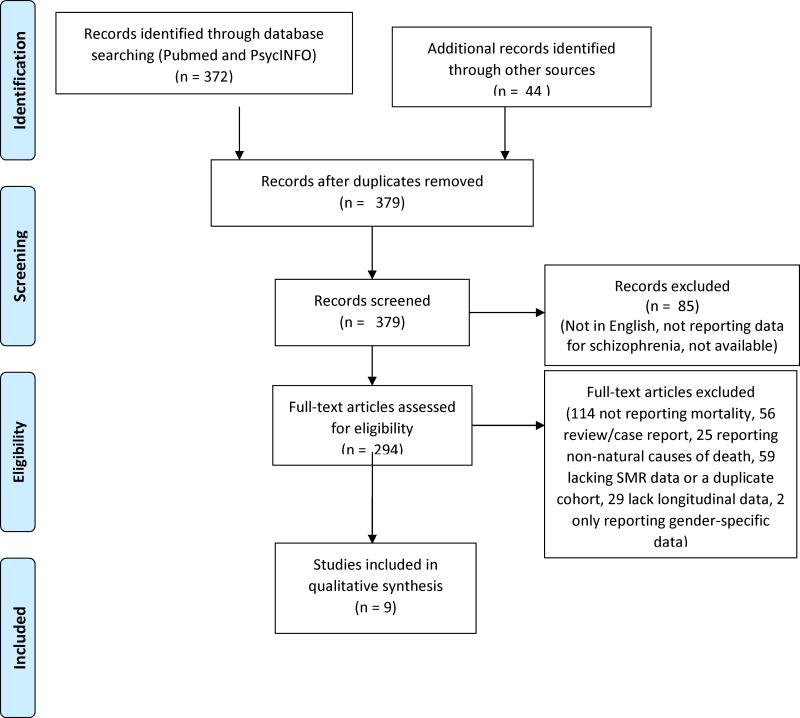
Using the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (28), we conducted a systematic literature search for longitudinal studies of mortality in schizophrenia, as outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram (Figure 1). Inclusion criteria were: (1) English-language reports published since 1950 as identified through a systematic search of two electronic databases (MEDLINE and PsycInfo) in March 2017 using the search terms/phrases for MEDLINE (“Mortality”[Mesh]) AND (“Schizophrenia Spectrum and Other Psychotic Disorders”[Mesh]) and PsycInfo, (2) reported mortality in patients with schizophrenia, schizoaffective disorder, and other psychotic disorders, (3) reported standardized mortality ratio (SMR) data or sufficient information to allow calculation of the SMR, (4) reported more than one time-point, and (5) reported mortality from all-cause, natural cause, or cardiovascular-related causes. SMR is a commonly used metric of the excess mortality in schizophrenia relative to the general population SMR data convey the rate of mortality in persons with schizophrenia compared to the general population, adjusted for age and gender. Review papers were excluded from this study; however, all original empirical reports identified through the above search terms were examined for possible inclusion.
Figure 1.
PRISMA flow diagram
Statistical Analyses
We calculated the annual rate of change in SMR within each study (difference in SMR divided by the original SMR and then by the number of years between the two SMR time-points) as well as the overall mean SMRs before and after the early 1970s. As the study populations varied from one another in several ways (Table 1) and our focus was on changes in SMR over time, we could not use formal statistical methods of meta-analysis such as a forest plot.
Table 1.
Summary characteristics of longitudinal studies included in analysis
| Authors | Country | Data source (pop.) | Study sample | Criteria | Years | SMR (all-cause) | % Annual change in SMR |
|---|---|---|---|---|---|---|---|
| Tsuang et al. 197729 | US | Citywide hospital cohort (27,000) | 200 patients with schizophrenia | Admission at a psychiatric hospital from 1935–1944. | 1935–44 1945–54 1955–64 1965–75 |
3.9 2.3 1.4 1.6 |
−4.2 −3.7 +1.0 |
| Saugstad et al. 19855 | Norway | Nationwide patient registry (3.7 million) | 9,616 inpatients with schizophrenia in psychiatric hospitals | All deaths during inpatient hospitalization between 1950 and 1974. Non-organic (functional) psychosis (ICD-8 295–298) | 1950–62 1963–74 |
2.2 1.9 |
−0.9 |
| Giel et al. 197810 & Brook et al. 19851 | Netherlands | Nationwide patient registry (8.3 million) | 8,142 and 5,226 long-stay hospitalized patients with schizophrenia | 20+ years of age. Long-stay psychiatric inpatients (hospitalized for ≥2 year continuously.) | 1970–71 1980–81 |
1.3 1.6 |
+2.6 |
| Osby et al. 200022 | Sweden | Countywide patient registry (1.8 million) | 5,802 patients with schizophrenia | First received psychiatric services for schizophrenia between 1976 and 1995. | 1976–80 1981–85 1986–90 1991–95 |
2.3 2.7 3.5 5.6 |
+3.1 +6.8 +11.7 |
| Lesage et al. 199021 | Italy | Citywide patient registry (75,000) | 129 patients with schizophrenia | Adults. Received psychiatric services in 1979 and 1982. Schizophrenia or paranoid states (ICD-9) | 1979 1982 |
4.9 5.2 |
+2.0 |
| Høye et al. 201111 | Norway | Countywide hospital registry (224,000) | 1,111 patients with schizophrenia | Admitted to psychiatric hospitals between 1980 and 2006. Schizophrenia (ICD-9: 295 and ICD-10: F20, F21, F25) | 1980–88 1989–97 1998–2006 |
2.4 3.4 3.3 |
+4.5 −0.3 |
| Brown et al. 20104 | UK | Citywide patient registry (2.2 million) | 363 community-dwelling individuals with schizophrenia. | 15+ years of age. Received psychiatric services. Schizophrenia (ICD-9 and ICD-10). Same cohort was followed throughout study. | 1981–86 1986–91 1991–96 1996–01 2001–06 |
3.8 2.6 2.7 2.8 2.9 |
−6.0 +0.1 +1.1 +0.9 |
| Hoang et al. 20119 | UK (England) | Nationwide patient registry (53 million) | 207,507 individuals with schizophrenia | <75 years old. Discharged from psychiatric hospital between 1999 and 2006. Schizophrenia (ICD-10: F20–F29) | 1999 2000 2001 2002 2003 2004 2005 2006 |
1.6 1.9 1.7 1.8 2.2 2.1 2.3 2.2 |
+18.8 −10.5 +5.9 +22.2 −4.5 +6.5 −4.3 |
SMR = Standardized Mortality Ratio.
Results
A total of eight longitudinal studies met our selection criteria and were included in this review – two initiated before and six initiated after 1970 (Table 1). The data on eight different populations were obtained from nine papers. Data regarding a population of Dutch patients were presented in two separate papers (1, 10). Aside from one pre-1970 study from the US (representing 50% of the data available for the pre-1970 estimates), all reports came from countries in western Europe. All these studies used patient registries to identify and track the population. The SMRs were computed using the general population of that locale/country as a reference population. With three exceptions (4, 21, 29), each study included more than 1,000 persons with schizophrenia. The studies differed in the length of follow-up and in the sizes of the patient registry (range 129 to 200,000 patients). Half of the studies examined outpatients, and others focused on inpatients. The subject inclusion criteria varied, e.g., age, admission/discharge from psychiatric hospital, receipt of psychiatric services, and the version of the diagnostic code (such as the ICD) employed. Studies did not report information that may impact mortality, e.g., individual patients’ family histories of diseases, smoking, and comorbid conditions.
Longitudinal SMR data by gender were provided in eight studies (1, 9–11, 21, 22, 29–31). All except Tsuang (1977) and Licht (1993) showed increasing SMRs over time in both genders. The Tsuang study (data over 1935–1975) showed decreasing SMRs over time in both genders; this study was the only one that reported on data prior to 1970. In the Lesage study (1979–1982), all-cause SMRs decreased in men, but increased in women. However, this study had the smallest number of participants (129 patients.) Most other investigations drew from county or national populations, with sample sizes of 1000+. Thus, overall there did not seem to be a gender difference in mortality rates.
Data on the SMRs for natural versus unnatural causes of death in longitudinal studies were available for only two studies (4, 9). Both investigations showed increasing SMRs for natural causes of death over time, while SMRs remained stable for unnatural causes of death over time.
Overall, the studies showed an increasing gap in mortality rates between persons with schizophrenia and the general population from all causes after the early 1970s. The SMR over the course of the studies showed an average rate of change of −1.6% per year, 95% CI [−10.5, 7.4] in the two pre-1970s studies, and an increase of 3.0%, 95% CI [0.1, 6.0] in the six post-1970s studies. The mean SMR was 2.2 in the pre-1970s studies and 3.0 in the post-1970s studies, representing an increase of 37%.
Discussion
Our findings suggest a widening longevity gap between persons with schizophrenia and general population since the early 1970s. Nearly all the post-1970 studies reported overall trends of increasing SMRs over time. Natural causes of death, often due to premature onset of serious medical diseases, are responsible for the increasing mortality gap in schizophrenia (9). Unnatural causes of death (suicides, accidents) have high SMRs, but account for <20% deaths in schizophrenia (2, 4, 32, 33), and the mortality gap due to unnatural causes has been stable over time (9, 34).
In contrast to schizophrenia, improving life expectancy and decreasing mortality rates have been observed in the general population for a number of serious medical conditions. For example, lung cancer one-year mortality rates in Denmark dropped 25% between 2000 and 2012, which the authors attribute to improved diagnostic procedures, accessibility to treatment, and efficacy of treatments (17). Similar mortality trends for breast cancer with a 16–29% decrease in deaths during 1986–2006 were reported in multiple European countries after implementation of mammography screening (35). Analogous changes have been observed in HIV/AIDS mortality, with all-cause SMRs dramatically decreasing from 126.8 in 1996 to 21.3 in 2011–2012 in HIV-positive individuals in Canada, following the initiation of combination antiretroviral therapy in 1996 (36). Western European countries observed 60–80% reductions in mortality from ischemic heart disease and acute myocardial infarctions over the past 30 years, with improved diagnostic and management of cardiovascular conditions (37, 38). Despite these trends in improved longevity for a number of different medical conditions, such improvements have not been observed in those with SMI, such as schizophrenia.
Our review has several limitations. The number of studies that met our inclusion criteria was small, especially before the early 1970s. The data from these studies were presented in different ways, with SMRs that included variable time periods (1 to 12 years.) Thus, it was difficult to determine if an SMR calculated for say 1935–1944 would best describe the 1930s or 1940s. The results may not be generalizable to non-western countries. The SMRs across different studies may not be consistent, due to differences in precision/reliability in measuring deaths, although three reports were based on nationwide registries with low lost-to-follow-up rates. Most studies used ICD-8, -9 or -10 as their diagnostic criteria for schizophrenia, though three studies did not outline which diagnostic guidelines were used. None of the reports used DSM criteria (nearly all studies came from Western European countries where ICD is more commonly used.) We did not use diagnostic criteria as an exclusion factor for the review as these changed over the years, sometimes even within a single study (e.g., from ICD-9 to ICD-10) (4, 11). Additionally, the registry studies tended to use chart diagnoses which may have entailed variable diagnostic criteria depending on when the patient was first evaluated and given a mental health diagnosis. Therefore, we focused on relative changes within each study. Finally, none of the studies was specifically aimed at determining possible causes for changes in SMRs over the years.
The reasons for our finding are not known. However, it is noteworthy that the early 1970s were a peak time of psychiatric inpatient bed closures (25). From the mid-1950s to the early 1970s, mortality seemed to have decreased in people with SMI, possibly due to receiving antipsychotics, leaving crowded psychiatric facilities with poor care, and moving in with families (5, 12, 26). However, subsequently the individuals with SMI encountered fragmented health services and inadequate housing resources (23, 25). Deinstitutionalization without adequate social support might have exposed people with SMI to the stressors of obtaining housing and food, and increased homelessness and incarceration rates (23). Additionally, persons with schizophrenia warrant comprehensive and regular physical monitoring by primary care services in order to prevent and treat chronic age-related medical conditions (39). Accelerated biological aging may also play a role in the premature morbidity and mortality observed in schizophrenia, with evidence of elevated inflammation and oxidative stress in persons with schizophrenia compared to healthy comparison subjects (7, 8, 40). Alternative explanations for the reported increase in SMR for schizophrenia over the past 5 decades might include methodological issues in computing SMR and adverse effects of chronic treatment with first and second generation antipsychotics (13). Despite the known metabolic and cardiac side effects, antipsychotic use in people with schizophrenia is associated with lower mortality, indicating that other factors (e.g., lifestyle habits) may have greater impact on mortality in this illness than antipsychotics (41–43).
These findings may be analogous to findings of longevity gaps in ethnic minorities (especially African-Americans) and people with lower incomes. African-Americans have a 17% increased mortality rate compared to Caucasians, controlling for education, home ownership, and family income, probably due to persistent disparities in healthcare usage, as well as increasing HIV and risk of death by homicide among African Americans during the 1980s (44). One may draw a parallel between socially or economically disadvantaged segments of the general population and people with schizophrenia. For instance, people in the bottom 1% of the income strata have a 10–15 year shorter life expectancy relative to the top 1% of the income strata (45). The individuals in the lowest socioeconomic strata face several challenges similar to those faced by people with schizophrenia, such as low level of education, poor diet, poverty, poor healthcare, and high levels of stress. Most strikingly, low-income earners have significantly improved life expectancies if they live in cities with highly educated and high-income populations and well-funded public services (45). These effects may be a result of local influences such as public policies that improve health (e.g., bans on smoking in public places), availability of social services, and positive health behaviors that may be ‘contagious’ between those with higher and lower incomes. Such findings may serve as guiding principles for reducing excess morbidity and mortality in schizophrenia.
It should be noted that community-based psychosocial interventions can result in substantially improved outcomes. In the Team for the Assessment of Psychiatric Services (TAPS) project led by Leff and colleagues (46) in London in the 1980s–90s, provision of necessary community services after deinstitutionalization, including meals and housing along with social supports, led to significant improvement in psychopathology including negative symptoms, and socialization at 5-year follow-up assessments. Such findings suggest a potential for improving prognosis and longevity in persons with chronic schizophrenia, by employing appropriate environmental and cultural interventions.
Even in developed nations, an important segment of the population - i.e., people with SMI - has not benefited from new scientific advances and lifestyle changes that have increased longevity of the general population. Over the past half-century, despite a growing body of empirical research on schizophrenia and the accompanied improvements in available treatments, the mortality gap between people with schizophrenia and the general population has widened, rather than narrowed. It is likely that these findings reflect on persistent and pervasive stigma against mental illness and societal neglect of this vital yet vulnerable segment of the population, which continues to be disenfranchised. Accordingly, work is urgently warranted to help reduce stigma, improve healthy lifestyles in a disseminable way, and tailor primary care services so that persons with SMI are not left behind in the longevity revolution.
Acknowledgments
We thank Paula Smith who helped with administrative preparation of this manuscript at UC San Diego.
Funding Support:
This study was supported, in part, by NIH R01MH094151-01 (PI: Dilip V. Jeste, MD), the NIMH T32 Geriatric Mental Health Program MH019934 (PI: Dilip V. Jeste, MD), and by the Stein Institute for Research on Aging at the University of California, San Diego.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no relevant conflicts of interest.
Contributors
Ellen E. Lee conducted literature reviews, data analyses, data interpretation, and manuscript preparation.
Jinyuan Liu was involved in data analyses, data interpretation and manuscript preparation.
Xin Tu was involved in data analyses, data interpretation and manuscript preparation. Barton W. Palmer was involved in data interpretation and manuscript preparation. Lisa T. Eyler was involved in data analyses, data interpretation and manuscript preparation.
Dilip V. Jeste was involved in data interpretation and manuscript preparation.
References
- 1.Brook OH. Mortality in the long-stay population of Dutch mental hospitals. Acta Psychiatr Scand. 1985;71:626–635. doi: 10.1111/j.1600-0447.1985.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 2.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 3.Olfson M, Gerhard T, Huang C, et al. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA Psychiatry. 2015;72:1172–1181. doi: 10.1001/jamapsychiatry.2015.1737. [DOI] [PubMed] [Google Scholar]
- 4.Brown S, Kim M, Mitchell C, et al. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196:116–121. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saugstad L, Odegard O. Recent rise in supposedly stress dependent causes of death in psychiatric hospitals in Norway indicating increased “stress” in hospitals? Acta Psychiatr Scand. 1985;71:402–409. doi: 10.1111/j.1600-0447.1985.tb02540.x. [DOI] [PubMed] [Google Scholar]
- 6.Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick B, Messias E, Harvey PD, et al. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37:451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoang U, Stewart R, Goldacre MJ. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999–2006. BMJ. 2011;343:d5422. doi: 10.1136/bmj.d5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giel R, Dijk S, van Weerden-Dijkstra JR. Mortality in the long-stay population of all Dutch mental hospitals. Acta Psychiatr Scand. 1978;57:361–368. doi: 10.1111/j.1600-0447.1978.tb06904.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoye A, Jacobsen BK, Hansen V. Increasing mortality in schizophrenia: are women at particular risk? A follow-up of 1111 patients admitted during 1980–2006 in Northern Norway. Schizophr Res. 2011;132:228–232. doi: 10.1016/j.schres.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Haugland G, Craig TJ, Goodman AB, et al. Mortality in the era of deinstitutionalization. Am J Psychiatry. 1983;140:848–852. doi: 10.1176/ajp.140.7.848. [DOI] [PubMed] [Google Scholar]
- 13.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 14.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25:83–88. doi: 10.1097/YCO.0b013e32835035ca. [DOI] [PubMed] [Google Scholar]
- 15.Capasso RM, Lineberry TW, Bostwick JM, et al. Mortality in schizophrenia and schizoaffective disorder: an Olmsted County, Minnesota cohort: 1950–2005. Schizophr Res. 2008;98:287–294. doi: 10.1016/j.schres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen RE, Uggerby AS, Jensen SO, et al. Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades–a Danish nationwide study from 1980 to 2010. Schizophr Res. 2013;146:22–27. doi: 10.1016/j.schres.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Jakobsen E, Rasmussen TR, Green A. Mortality and survival of lung cancer in Denmark: Results from the Danish Lung Cancer Group 2000–2012. Acta Oncol. 2016;55(Suppl 2):2–9. doi: 10.3109/0284186X.2016.1150608. [DOI] [PubMed] [Google Scholar]
- 18.Abdoli G, Bottai M, Sidorchuk A, et al. Trends in mortality after cancer diagnosis: A nationwide cohort study over 45 years of follow-up in Sweden by country of birth. Cancer Epidemiol. 2015;39:633–640. doi: 10.1016/j.canep.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 20.UN. Changing Levels and Trends in Mortality: the role of patterns of death by cause. United Nations, Department of Economic and Social Affairs, Population Division; 2012. [Google Scholar]
- 21.Lesage AD, Trapani V, Tansella M. Excess mortality by natural causes of Italian schizophrenic patients. Eur Arch Psychiatry Neurol Sci. 1990;239:361–365. doi: 10.1007/BF01734542. [DOI] [PubMed] [Google Scholar]
- 22.Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321:483–484. doi: 10.1136/bmj.321.7259.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb HR, Bachrach LL. Some perspectives on deinstitutionalization. Psychiatr Serv. 2001;52:1039–1045. doi: 10.1176/appi.ps.52.8.1039. [DOI] [PubMed] [Google Scholar]
- 24.Olson RP, editor. Mental Health Systems Compared: Great Britain, Norway, Canada and the United States. Springfield, IL: Charles C. Thomas Publisher; 2006. [Google Scholar]
- 25.Craig TJ, Lin SP. Death and deinstitutionalization. Am J Psychiatry. 1981;138:224–227. doi: 10.1176/ajp.138.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Tsai J, Stroup TS, Rosenheck R. Housing arrangements among a national sample of adults with chronic schizophrenia living in the United States: A descriptive study. Journal of Community Psychology. 2011:76–88. [Google Scholar]
- 27.Goldman HH. Mental illness and family burden: a public health perspective. Hosp Community Psychiatry. 1982;33:557–560. doi: 10.1176/ps.33.7.557. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Tsuang MT, Woolson RF. Mortality in patients with schizophrenia, mania, depression and surgical conditions. A comparison with general population mortality. Br J Psychiatry. 1977;130:162–166. doi: 10.1192/bjp.130.2.162. [DOI] [PubMed] [Google Scholar]
- 30.Munk-Jorgensen P, Mortensen PB. Incidence and other aspects of the epidemiology of schizophrenia in Denmark, 1971–87. Br J Psychiatry. 1992;161:489–495. doi: 10.1192/bjp.161.4.489. [DOI] [PubMed] [Google Scholar]
- 31.Licht RW, Mortensen PB, Gouliaev G, et al. Mortality in Danish psychiatric long-stay patients, 1972–1982. Acta Psychiatr Scand. 1993;87:336–341. doi: 10.1111/j.1600-0447.1993.tb03382.x. [DOI] [PubMed] [Google Scholar]
- 32.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen PB, Juel K. Mortality and causes of death in schizophrenic patients in Denmark. Acta Psychiatr Scand. 1990;81:372–377. doi: 10.1111/j.1600-0447.1990.tb05466.x. [DOI] [PubMed] [Google Scholar]
- 34.Laursen TM, Nordentoft M. Heart disease treatment and mortality in schizophrenia and bipolar disorder - changes in the Danish population between 1994 and 2006. J Psychiatr Res. 2011;45:29–35. doi: 10.1016/j.jpsychires.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Autier P, Boniol M, Gavin A, et al. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ. 2011;343:d4411. doi: 10.1136/bmj.d4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyawo O, Franco-Villalobos C, Hull MW, et al. Changes in mortality rates and causes of death in a population-based cohort of persons living with and without HIV from 1996 to 2012. BMC Infect Dis. 2017;17:174. doi: 10.1186/s12879-017-2254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartley A, Marshall DC, Salciccioli JD, et al. Trends in Mortality From Ischemic Heart Disease and Cerebrovascular Disease in Europe: 1980 to 2009. Circulation. 2016;133:1916–1926. doi: 10.1161/CIRCULATIONAHA.115.018931. [DOI] [PubMed] [Google Scholar]
- 38.Rahimi K, Duncan M, Pitcher A, et al. Mortality from heart failure, acute myocardial infarction and other ischaemic heart disease in England and Oxford: a trend study of multiple-cause-coded death certification. Journal of epidemiology and community health. 2015;69:1000–1005. doi: 10.1136/jech-2015-205689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- 40.Wolkowitz OM, Jeste DV, Martin AS, et al. Leukocyte telomere length: Effects of schizophrenia, age, and gender. J Psychiatr Res. 2017;85:42–48. doi: 10.1016/j.jpsychires.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41:656–663. doi: 10.1093/schbul/sbu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin H, Shih PA, Golshan S, et al. Comparison of longer-term safety and effectiveness of 4 atypical antipsychotics in patients over age 40: a trial using equipoise-stratified randomization. J Clin Psychiatry. 2013;74:10–18. doi: 10.4088/JCP.12m08001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer JM, Nasrallah HA, McEvoy JP, et al. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res. 2005;80:9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Sloan FA, Ayyagari P, Salm M, et al. The longevity gap between Black and White men in the United States at the beginning and end of the 20th century. Am J Public Health. 2010;100:357–363. doi: 10.2105/AJPH.2008.158188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chetty R, Stepner M, Abraham S, et al. The Association Between Income and Life Expectancy in the United States, 2001–2014. JAMA. 2016;315:1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leff J, Thornicroft G, Coxhead N, et al. The TAPS Project. 22: A five-year follow-up of long-stay psychiatric patients discharged to the community. Br J Psychiatry Suppl. 1994:13–17. [PubMed] [Google Scholar]