Abstract
Congenital nephrogenic diabetes insipidus (NDI) is characterized by defective urine concentrating ability. Symptomatic polyuria is present from birth, even with normal release of the antidiuretic hormone vasopressin by the pituitary. Over the last two decades, the aquaporin-2 (AQP2) gene has been cloned and the molecular mechanisms of urine concentration have been gradually elucidated. Vasopressin binds to the vasopressin type II receptor (V2R) in the renal collecting ducts and then activates AQP2 phosphorylation and trafficking to increase water reabsorption from urine. Most cases of congenital NDI are caused by loss-of-function mutations to V2R, resulting in unresponsiveness to vasopressin. In this article, we provide an overview of novel therapeutic molecules of congenital NDI that can activate AQP2 by bypassing defective V2R signaling with a particular focus on the activators of the calcium and cAMP signaling pathways.
Keywords: Congenital nephrogenic diabetes insipidus, AQP2, Calcium signaling, cAMP signaling, GPCRs agonists, PDE inhibitors
Introduction
Congenital nephrogenic diabetes insipidus (NDI) is characterized by the increased excretion of diluted urine in spite of appropriate secretion of the antidiuretic hormone vasopressin. In severe cases, patients excrete up to 10–20 L of urine per day [1]. Excessive urine output and drinking seriously reduce quality of life and the ability to participate in social activities. Polyuria also induces structural changes to the urinary tract, which lead to the onset of chronic kidney disease. Moreover, growth and mental retardation are often found on long-term follow-up despite adequate water balance control [2]. To prevent these complications of congenital NDI, further drug discovery is required.
Loss-of-function mutations to the vasopressin type 2 receptor (V2R) are found in approximately 90% of patients with congenital NDI [3]. V2R is encoded by the AVPR2 gene, which is located at chromosome Xq28. X-linked recessive NDI occurs in about one in 250,000 males. In Japan, an estimated 400 people have congenital NDI. In the other 10% of patients, congenital NDI has an autosomal recessive or autosomal dominant mode of inheritance with mutations to the aquaporin-2 (AQP2) gene [4, 5].
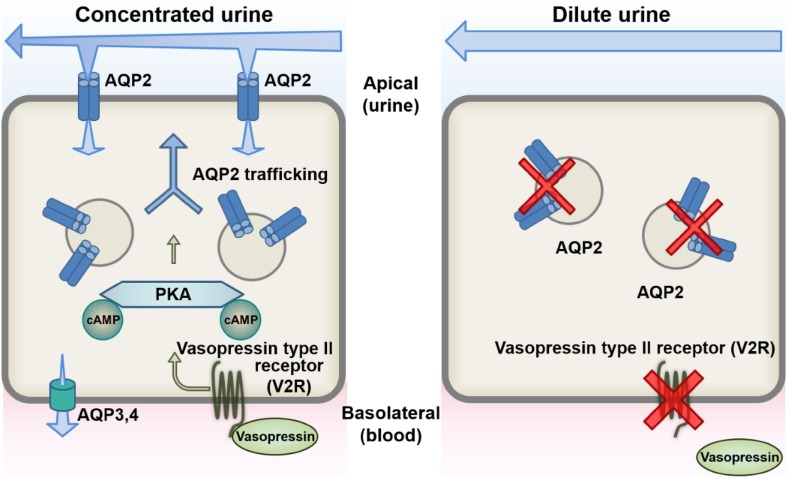
V2R and AQP2 are major regulators of urine concentration (Fig. 1). In response to dehydration, the antidiuretic hormone vasopressin is secreted from the posterior pituitary. Binding of vasopressin to its receptor V2R in the renal collecting ducts increases intracellular production of cyclic adenosine monophosphate (cAMP), which then activates cAMP-dependent protein kinase, PKA in a mechanism classically thought to be responsible for AQP2 phosphorylation [6]. Changes in AQP2 phosphorylation status promote AQP2 trafficking to the apical plasma membrane [7–9], which results in water reabsorption from urine through AQP2 water channels to improve the dehydrated states of the body. On the other hand, renal unresponsiveness to vasopressin or defective AQP2 function in patients with congenital NDI impairs AQP2 activity and water reabsorption, resulting in polyuria.
Fig. 1.
The mechanisms of urine concentration by vasopressin. (Left) Circulating vasopressin binds to V2R in the basolateral membrane of cells of the renal collecting ducts. Adenylyl cyclase is then activated and increases cAMP production and PKA activity, leading to AQP2 phosphorylation. Changes in AQP2 phosphorylation status leads to translocation of cytosolic AQP2 to the apical plasma membrane. Water is reabsorbed from urine through AQP2, AQP3, and AQP4, thereby concentrating the urine. (Right) V2R mutations account for 90% of all diagnoses of congenital NDI, while AQP2 mutations occur in the other 10%. Defective V2R or AQP2 function impairs water reabsorption, resulting in urine dilution
At present, only symptomatic treatment approaches are available for congenital NDI, such as a low sodium and low protein diet, as well as the use of thiazide diuretics and nonsteroidal anti-inflammatory drugs [10]. To develop curative therapies for congenital NDI caused by V2R mutations is a challenging research proposition which is a major driving force to elucidate various regulatory mechanisms of AQP2. Well-known therapeutic strategies for congenital NDI include the rescue of V2R mutants by chemical chaperones and bypassing defective V2R signaling. In this review, we focus on activators of calcium and cAMP signaling that can increase AQP2 activity in the absence of vasopressin.
Activators of calcium signaling
In the vasopressin signaling pathway, cAMP-induced PKA activation has been considered as a primary mechanism of AQP2 phosphorylation and trafficking [7, 11]. Recent studies have revealed that cAMP also induces intracellular calcium oscillation and both PKA and the calcium signaling pathway coordinately modulate AQP2 activity. Exchange protein directly activated by cAMP (Epac) is a key molecule that mediates cAMP and calcium signaling. Epac has two isoforms: Epac1 and Epac2. In the collecting ducts, Epac2 is mainly expressed in the apical region of all AQP2-positive cells [12]. Similar to PKA, Epac contains evolutionally conserved cAMP binding domains, which enhance calcium signaling in response to cAMP [13]. In fact, the exogenous cAMP analog 8-pCPT-2′-O-Me-cAMP, which selectively activates Epac, but not PKA, mimics the effects of vasopressin on calcium oscillation and promotes AQP2 trafficking toward the apical plasma membrane in isolated perfused inner medullary collecting duct (IMCD) [14, 15]. The mechanisms of Epac-mediated calcium oscillation are probably due to the release of calcium from the endoplasmic reticulum (ER) through the activation of calcium channels, such as inositol trisphosphate receptors and ryanodine receptors [16]. For this reason, calcium depletion of the ER by ryanodine or the calcium chelator BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) completely inhibits vasopressin-induced calcium oscillation [17]. Although ER calcium stores are important for the initial increase in intracellular calcium concentration, extracellular calcium influx through store-operated calcium entry is also required to sustain calcium oscillation [18]. Surprisingly, ryanodine and BAPTA suppress not only calcium oscillation, but also vasopressin-induced AQP2 activation, indicating that calcium signaling exerts a critical role in AQP2 regulation in an experimental model of isolated perfused IMCD.
The precise molecular mechanisms underlying AQP2 activation by vasopressin/Epac/calcium signaling pathway remains unknown. Generally, elevation of intracellular calcium serves as a second messenger in the activation of downstream signaling molecules. Calmodulin is a calcium-binding protein that regulates various target molecules, including calmodulin-dependent protein kinases and phosphatases [19]. In addition to calcium, calmodulin plays an important role in AQP2 activation in isolated perfused IMCD. The calmodulin inhibitors W7 and trifluoperazine significantly block AQP2 trafficking and transepithelial water transport by reducing vasopressin-induced cAMP production [15]. Calmodulin may be associated with the decrease in adenylyl cyclase activity [20]. The calmodulin-dependent serine/threonine phosphatase calcineurin has also been reported to regulate AQP2. Calcineurin and AQP2 are co-localized in the intracellular vesicles of renal collecting duct cells [21, 22]. The hypertonicity-induced calcium/calmodulin/calcineurin/nuclear factor of activated T cells, cytoplasmic (NFATc) signaling pathway was reported to increase AQP2 mRNA expression in the mouse cortical collecting duct mpkCCDcl4 cell line [23, 24]. Calcineurin dephosphorylates NFATc in the cytosol and NFATc is subsequently translocated to the nucleus where it binds to the promoter region of the AQP2 gene. In addition, calcineurin regulates AQP2-mediated water transport. The urine concentrating response to vasopressin is decreased in calcineurin Aα knockout mice and cyclosporine A (CyA)-treated mice [25]. CyA probably modulates AQP2 activity either directly or indirectly through impairment of the medullary osmotic gradient as a result of the inhibition of Na-K-2Cl cotransporters [26].
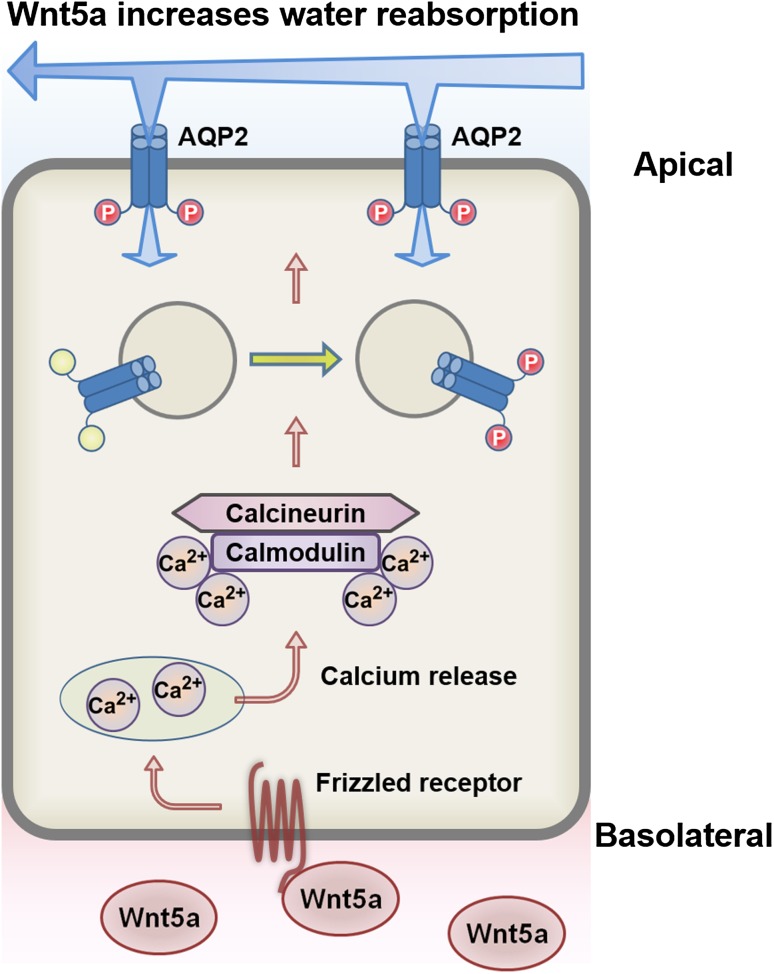
Previous studies have suggested that the calcium signaling pathway is a major target of AQP2 activation in the treatment of congenital NDI. Therefore, we focused on the classic calcium signal transducer Wnt5a, which is a ligand of frizzled (Fzd) receptors [27–30], and found that the Wnt5a/calcium/calmodulin/calcineurin signaling pathway induced phosphorylation, trafficking, and mRNA expression of AQP2 (Fig. 2) [31]. W7 and CyA were found to totally inhibit Wnt5a-induced AQP2 activation. These effects of Wnt5a on AQP2 were examined using mpkCCDCl4 cells, which are one of the most frequently used cell lines for the reliable analysis of AQP2 and exhibit endogenous expression of V2R and AQP2 [31–42]. Remarkably, contrary to the results of isolated perfused IMCD, W7 and CyA did not inhibit the effects of vasopressin on AQP2 phosphorylation in mpkCCD cells, indicating that calmodulin and calcineurin are not major regulators of vasopressin-induced AQP2 activation. The use of different experimental systems likely caused the high discrepancy in the effect of intracellular calcium on AQP2 [43]. Importantly, our results with mpkCCD cells are compatible with those obtained from clinical experience where CyA-induced NDI rarely occurred as a drug side effect. Although there are fewer effects of calcium signaling on AQP2 than those of vasopressin, Wnt5a is effective for AQP2 activation, especially in the absence of vasopressin. We demonstrated that Wnt5a increased osmotic water transport in isolated perfused cortical collecting duct (CCD) tubules of the mouse kidney and increased urine concentrating ability in a V2R-inhibited NDI mice model.
Fig. 2.
The mechanisms of urine concentration by Wnt5a. Wnt5a binds to Fzd receptors and increases intracellular calcium. The calcium-binding protein calmodulin stimulates calcineurin, which in turn, phosphorylates AQP2 and increases apical AQP2 expression. Water is then reabsorbed from urine. In addition, calcineurin increases AQP2 mRNA expression
The Wnt5a signaling pathway regulates AQP2 via different mechanisms of the vasopressin/cAMP signaling pathway. Analysis of Wnt5a suggested that calcineurin is a key molecule in the activation of AQP2. The importance of calcineurin was confirmed with its direct activator arachidonic acid, which possesses vasopressin-like effects in mpkCCD cells [31]. Hence, screening for calcineurin activators is a potential therapeutic strategy to develop novel drugs for the treatment of congenital NDI.
Elevation of cAMP concentration
cAMP is the most important key molecule in the regulation of AQP2. Significant AQP2 activation by cAMP is well established and direct cAMP activator forskolin is widely used as positive control in various assays of AQP2 activity. cAMP activation independent of defective V2R signaling is a promising therapeutic strategy for the treatment of congenital NDI and is largely divided into two methods: increased cAMP production and decreased cAMP degradation.
G protein-coupled receptors (GPCRs) agonists
The use of GPCRs to increase cAMP production in response to their ligands has been intensively studied as a treatment option for congenital NDI. Although each GPCR has different diverse biological functions, all GPCRs share common mechanisms of signal transduction. Similar to V2R, other GPCRs also potentially increase cAMP concentrations in renal collecting ducts and activate AQP2.
The results of a TaqMan mouse GPCR array analysis revealed that IMCD cells from C57BL/6 mice express many GPCRs, including V2R [44]. The calcitonin receptor is a GPCR in renal collecting ducts. Calcitonin increases intracellular cAMP levels and the membrane accumulation of AQP2 in LLC-PK1 cells [45]. Analysis of vasopressin-deficient Brattleboro rats showed that calcitonin reduced urine flow and increased urine osmolality two-fold during the first 12 h of treatment. Angiotensin II is a ligand of the AT1 receptor, which increases AQP2 expression in the apical plasma membrane of mpkCCD cells [38]. The effects of angiotensin II on AQP2 are mediated through cAMP and calcium signaling, and are inhibited by the PKA inhibitor H89 and the calmodulin inhibitor W7. Secretin, a ligand of the secretin receptor, also has a vasopressin-like effect. Secretin increases cAMP concentrations in isolated IMCD tubule suspensions from wild-type and tamoxifen-induced V2R knockout mice [46]. In addition, secretin receptor knockout mice exhibit mild polyuria, polydipsia, and reduced renal expression of AQP2 [47]. Although GPCR agonists certainly increase intracellular cAMP levels in renal collecting ducts and activate AQP2, their effects do not persist for very long, probably due to the downregulation or desensitization of receptors [45, 46].
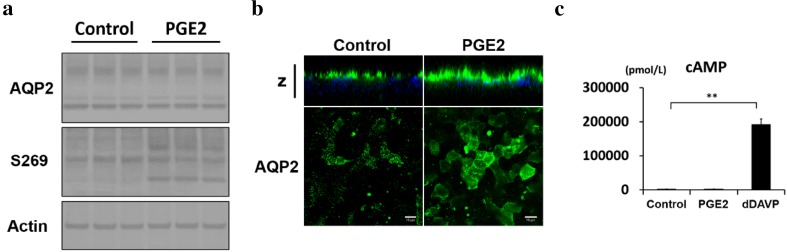
Prostaglandin E2 (PGE2) is a ligand of four different G protein-coupled E-prostanoid receptors: EP1–EP4. PGE2 and butaprost, selective agonists of EP2, both increased cAMP levels and AQP2 activity in MDCK cells [48]. In addition, butaprost increased urine concentrating ability in a V2R-inhibited NDI rat model [48], whereas EP4 activation by ONO-AE1-329 [44] or CAY10580 [49] increased cAMP levels and AQP2 activities in IMCD cells. The importance of EP4 in the regulation of AQP2 has also been clarified in vivo. Renal tubule-specific and collecting duct-specific EP4 knockout mice showed impaired urine concentrating abilities [49]. Moreover, subcutaneous injection of ONO-AE1-329 improved urine concentrating ability and other major manifestations, such as distension of the renal pelvis, in tamoxifen-inducible V2R knockout mice [44]. In contrast, EP4 possesses cAMP-independent effects on AQP2. In MDCK and mpkCCD cells, EP4 activation by CAY10580 increased AQP2 activity without elevating cAMP levels [50]. We also confirmed that PGE2 increased AQP2 phosphorylation and trafficking without the elevation of cAMP levels in mpkCCD cells, which endogenously express EP4, but not EP2 (Fig. 3a–c). However, the underlying mechanisms of EP4-induced AQP2 activation remains to be elucidated.
Fig. 3.
PGE2 activates AQP2 without an elevation in cAMP in mpkCCD cells. a PGE2-induced AQP2 phosphorylation at S269. PGE2 (10 nM) was added to the basolateral side of the mpkCCD cells for 1 h, as previously described [31]. b PGE2-induced AQP2 trafficking. mpkCCD cells were treated with PGE2 (10 nM) for 1 h, and the subcellular localization of AQP2 was then analyzed by immunofluorescence and confocal microscopy. The larger panels display confocal sections of the apical regions of the cells. Z-stack confocal images are shown at the top of each panel. Representative confocal images of three independent experiments are shown. Scale bars, 10 µm. c No significant elevation of cAMP concentration in response to PGE2. The mpkCCD cells were treated with PGE2 (10 nM) or [deamino-Cys1, d-Arg8]-vasopressin (dDAVP) (1 nM) for 1 h. Bars are mean values ± SD of three experiments. Asterisks indicate a significant difference as compared with the control. **p < 0.01
PGE2 is a lipid mediator that is not stored in cells, but rather is derived from arachidonic acid, which is released from phospholipids in the nuclear membranes of most cell types [51]. In the kidney, the conversion of arachidonic acid to PGE2 is catalyzed by the action of cyclooxygenase enzymes and two PGE synthases: cytosolic PGE synthase and microsomal PGE synthase type 1 [52]. As mentioned above, arachidonic acid directly activates calcineurin and then increases AQP2 phosphorylation, trafficking, and mRNA expression in mpkCCD cells [31]. In the arachidonic acid cascade, both arachidonic acid and PGE2 are responsible for AQP2 activity. Importantly, higher intake of omega-6 polyunsaturated fatty acids, such as arachidonic acid, appears to be safe and may reduce the risk of coronary heart disease, relative to a lower intake [53]. Hence, the arachidonic acid cascade is a potential therapeutic target for the treatment of congenital NDI.
Phosphodiesterase (PDE) inhibitors
cAMP-PDEs degrade cAMP to AMP and decrease intracellular cAMP levels. Interestingly, PDE activity was increased in a mouse model of NDI [54, 55]. Active PDEs and defective V2R coordinate to prevent cAMP elevation in response to vasopressin. As a result of PDE activation, the PDE3 inhibitor cilostamide and the PDE4 inhibitor rolipram were found to highly restore vasopressin-induced cAMP accumulation in IMCDs of NDI mice [56]. In addition, we previously evaluated the effects of PDE inhibitors in NDI mice. First, we identified three families with frameshift mutations in AQP2 that cause autosomal dominant NDI and then generated a disease model of knockin mice to verify the effects of PDE inhibitors [4]. Autosomal dominant NDI is characterized by a milder clinical presentation of symptoms because AQP2 tetramers composed of only wild-type AQP2 can be translocated to the cell surface, whereas those containing at least one AQP2 mutant are missorted to the basolateral membrane [5]. Rolipram increased cAMP content and promoted the translocation of wild-type AQP2 toward the apical plasma membrane. Conversely, the PDE3 inhibitor milrinone had no effect. These results suggest that rolipram is a potential therapeutic target for X-linked NDI caused by V2R mutations as well as autosomal dominant NDI. However, rolipram treatment failed to relieve the symptoms of two male patients with X-linked NDI [57]. For this reason, there are likely differences in AMP metabolism between mice and humans, thus alternative PDE4 inhibitors may be more suitable [58].
Conclusion
Over the last two decades, the mechanisms of AQP2 regulation have been gradually clarified and many target molecules for the treatment of congenital NDI have been proposed. Although these potential therapeutic candidates, including activators of calcium and cAMP signaling, induce AQP2 activation without vasopressin in vitro, they failed to sufficiently increase urine concentration in vivo. Therefore, no specific pharmacological drugs have yet reached clinical application. In the development of drugs for the treatment of congenital NDI caused by V2R mutations, it may be necessary to shift focus from conventional therapeutic approaches to novel strategies that can activate AQP2 more directly and potently. At the same time, it is also important for AQP2 activation to generate a medullary osmotic gradient in the kidney because AQP2 is not a transporter, but rather a water channel. Water reabsorption occurs only after a driving force of passive water movement is created. Further studies of AQP2-activating mechanism are required to design feasible drug candidates for improvement of the excessive urine output and quality of life of NDI patients.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Sands JM, Bichet DG, Physicians ACo. Society AP Nephrogenic diabetes insipidus. Ann Intern Med. 2006;144(3):186–194. doi: 10.7326/0003-4819-144-3-200602070-00007. [DOI] [PubMed] [Google Scholar]
- 2.Mishra G, Chandrashekhar SR. Management of diabetes insipidus in children. Indian J Endocrinol Metab. 2011;15(Suppl 3):S180–S187. doi: 10.4103/2230-8210.84858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bichet DG. V2R mutations and nephrogenic diabetes insipidus. Prog Mol Biol Transl Sci. 2009;89:15–29. doi: 10.1016/S1877-1173(09)89002-9. [DOI] [PubMed] [Google Scholar]
- 4.Kuwahara M, Iwai K, Ooeda T, Igarashi T, Ogawa E, Katsushima Y, et al. Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus. Am J Hum Genet. 2001;69(4):738–748. doi: 10.1086/323643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohara E, Rai T, Yang SS, Uchida K, Nitta K, Horita S, et al. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc Natl Acad Sci USA. 2006;103(38):14217–14222. doi: 10.1073/pnas.0602331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens. 2008;17(5):491–498. doi: 10.1097/MNH.0b013e3283094eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272(23):14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 8.Yui N, Ando F, Sasaki S, Uchida S. Ser-261 phospho-regulation is involved in pS256 and pS269-mediated aquaporin-2 apical translocation. Biochem Biophys Res Commun. 2017;490(3):1039–1044. doi: 10.1016/j.bbrc.2017.06.162. [DOI] [PubMed] [Google Scholar]
- 9.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, et al. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem. 2008;283(36):24617–24627. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bockenhauer D, Bichet DG. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol. 2015;11(10):576–88. doi: 10.1038/nrneph.2015.89. [DOI] [PubMed] [Google Scholar]
- 11.Noda Y, Horikawa S, Kanda E, Yamashita M, Meng H, Eto K, et al. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J Cell Biol. 2008;182(3):587–601. doi: 10.1083/jcb.200709177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Konings IB, Zhao J, Price LS, de Heer E, Deen PM. Renal expression of exchange protein directly activated by cAMP (Epac) 1 and 2. Am J Physiol Renal Physiol. 2008;295(2):F525–F533. doi: 10.1152/ajprenal.00448.2007. [DOI] [PubMed] [Google Scholar]
- 13.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40(7):651–62. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yip KP. Epac-mediated Ca(2+) mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(4):F882–F890. doi: 10.1152/ajprenal.00411.2005. [DOI] [PubMed] [Google Scholar]
- 15.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, et al. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem. 2000;275(47):36839–36846. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- 16.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577(Pt 1):5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip KP. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol. 2002;538(Pt 3):891–899. doi: 10.1113/jphysiol.2001.012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip KP, Sham JS. Mechanisms of vasopressin-induced intracellular Ca2 + oscillations in rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2011;300(2):F540–F548. doi: 10.1152/ajprenal.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10(8):322–328. doi: 10.1016/S0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 20.Hoffert JD, Chou CL, Fenton RA, Knepper MA. Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct. J Biol Chem. 2005;280(14):13624–13630. doi: 10.1074/jbc.M500040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo I, Ward DT, Baum MA, Scott JD, Coghlan VM, Hammond TG, et al. AQP2 is a substrate for endogenous PP2B activity within an inner medullary AKAP-signaling complex. Am J Physiol Renal Physiol. 2001;281(5):F958–F965. doi: 10.1152/ajprenal.2001.281.5.F958. [DOI] [PubMed] [Google Scholar]
- 22.Gooch JL, Pèrgola PE, Guler RL, Abboud HE, Barnes JL. Differential expression of calcineurin A isoforms in the diabetic kidney. J Am Soc Nephrol. 2004;15(6):1421–1429. doi: 10.1097/01.ASN.0000128076.91545.BB. [DOI] [PubMed] [Google Scholar]
- 23.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, et al. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol. 1999;10(5):923–934. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]
- 24.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, et al. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol. 2007;292(5):C1606–C1616. doi: 10.1152/ajpcell.00588.2005. [DOI] [PubMed] [Google Scholar]
- 25.Gooch JL, Guler RL, Barnes JL, Toro JJ. Loss of calcineurin Aalpha results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J Cell Sci. 2006;119(Pt 12):2468–2476. doi: 10.1242/jcs.02971. [DOI] [PubMed] [Google Scholar]
- 26.Lim SW, Ahn KO, Sheen MR, Jeon US, Kim J, Yang CW, et al. Downregulation of renal sodium transporters and tonicity-responsive enhancer binding protein by long-term treatment with cyclosporin A. J Am Soc Nephrol. 2007;18(2):421–429. doi: 10.1681/ASN.2006060664. [DOI] [PubMed] [Google Scholar]
- 27.Kremenevskaja N, von Wasielewski R, Rao AS, Schöfl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24(13):2144–2154. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 28.Dejmek J, Säfholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol. 2006;26(16):6024–6036. doi: 10.1128/MCB.02354-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+ pathway. J Biol Chem. 2006;281(41):30990–1001. doi: 10.1074/jbc.M603603200. [DOI] [PubMed] [Google Scholar]
- 30.Säfholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem. 2006;281(5):2740–2749. doi: 10.1074/jbc.M508386200. [DOI] [PubMed] [Google Scholar]
- 31.Ando F, Sohara E, Morimoto T, Yui N, Nomura N, Kikuchi E, et al. Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway. Nat Commun. 2016;7:13636. doi: 10.1038/ncomms13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, et al. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem. 2002;277(12):10379–10386. doi: 10.1074/jbc.M111880200. [DOI] [PubMed] [Google Scholar]
- 33.Hasler U, Mordasini D, Bianchi M, Vandewalle A, Féraille E, Martin PY. Dual influence of aldosterone on AQP2 expression in cultured renal collecting duct principal cells. J Biol Chem. 2003;278(24):21639–21648. doi: 10.1074/jbc.M212388200. [DOI] [PubMed] [Google Scholar]
- 34.Bustamante M, Hasler U, Kotova O, Chibalin AV, Mordasini D, Rousselot M, et al. Insulin potentiates AVP-induced AQP2 expression in cultured renal collecting duct principal cells. Am J Physiol Renal Physiol. 2005;288(2):F334–F344. doi: 10.1152/ajprenal.00180.2004. [DOI] [PubMed] [Google Scholar]
- 35.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Féraille E. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol. 2005;16(6):1571–1582. doi: 10.1681/ASN.2004110930. [DOI] [PubMed] [Google Scholar]
- 36.Umenishi F, Narikiyo T, Vandewalle A, Schrier RW. cAMP regulates vasopressin-induced AQP2 expression via protein kinase A-independent pathway. Biochim Biophys Acta. 2006;1758(8):1100–1105. doi: 10.1016/j.bbamem.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 37.van Balkom BW, Boone M, Hendriks G, Kamsteeg EJ, Robben JH, Stronks HC, et al. LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J Am Soc Nephrol. 2009;20(5):990–1001. doi: 10.1681/ASN.2008060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol. 2011;300(5):F1255–F1261. doi: 10.1152/ajprenal.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boone M, Kortenoeven ML, Robben JH, Tamma G, Deen PM. Counteracting vasopressin-mediated water reabsorption by ATP, dopamine, and phorbol esters: mechanisms of action. Am J Physiol Renal Physiol. 2011;300(3):F761–F771. doi: 10.1152/ajprenal.00247.2010. [DOI] [PubMed] [Google Scholar]
- 40.Kortenoeven ML, van den Brand M, Wetzels JF, Deen PM. Hypotonicity-induced reduction of aquaporin-2 transcription in mpkCCD cells is independent of the tonicity responsive element, vasopressin, and cAMP. J Biol Chem. 2011;286(15):13002–13010. doi: 10.1074/jbc.M110.207878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kortenoeven ML, Trimpert C, van den Brand M, Li Y, Wetzels JF, Deen PM. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am J Physiol Renal Physiol. 2012;302(11):F1395–F1401. doi: 10.1152/ajprenal.00376.2011. [DOI] [PubMed] [Google Scholar]
- 42.Kortenoeven ML, Schweer H, Cox R, Wetzels JF, Deen PM. Lithium reduces aquaporin-2 transcription independent of prostaglandins. Am J Physiol Cell Physiol. 2012;302(1):C131–C140. doi: 10.1152/ajpcell.00197.2011. [DOI] [PubMed] [Google Scholar]
- 43.Noda Y, Sasaki S. Regulation of aquaporin-2 trafficking and its binding protein complex. Biochim Biophys Acta. 2006;1758(8):1117–1125. doi: 10.1016/j.bbamem.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, et al. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest. 2009;119(10):3115–3126. doi: 10.1172/JCI39680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouley R, Lu HA, Nunes P, Da Silva N, McLaughlin M, Chen Y, et al. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol. 2011;22(1):59–72. doi: 10.1681/ASN.2009121267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Procino G, Milano S, Carmosino M, Barbieri C, Nicoletti MC, Li JH, et al. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int. 2014;86(1):127–138. doi: 10.1038/ki.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu JY, Chung SC, Lam AK, Tam S, Chung SK, Chow BK. Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol Cell Biol. 2007;27(7):2499–2511. doi: 10.1128/MCB.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olesen ET, Rützler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA. 2011;108(31):12949–12954. doi: 10.1073/pnas.1104691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao M, Cao R, Du S, Jia X, Zheng S, Huang S, et al. Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad Sci USA. 2015;112(27):8397–8402. doi: 10.1073/pnas.1509565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olesen ET, Moeller HB, Assentoft M, MacAulay N, Fenton RA. The vasopressin type 2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP-independent pathway. Am J Physiol Renal Physiol. 2016;311(5):F935–F944. doi: 10.1152/ajprenal.00559.2015. [DOI] [PubMed] [Google Scholar]
- 51.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119(3):229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Olesen ET, Fenton RA. Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol. 2013;24(2):169–178. doi: 10.1681/ASN.2012020217. [DOI] [PubMed] [Google Scholar]
- 53.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119(6):902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 54.Jackson BA, Edwards RM, Valtin H, Dousa TP. Cellular action of vasopressin in medullary tubules of mice with hereditary nephrogenic diabetes insipidus. J Clin Invest. 1980;66(1):110–122. doi: 10.1172/JCI109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kusano E, Yusufi AN, Murayama N, Braun-Werness J, Dousa TP. Dynamics of nucleotides in distal nephron of mice with nephrogenic diabetes insipidus. Am J Physiol. 1986;250(1 Pt 2):F151–F158. doi: 10.1152/ajprenal.1986.250.1.F151. [DOI] [PubMed] [Google Scholar]
- 56.Coffey AK, O’Sullivan DJ, Homma S, Dousa TP, Valtin H. Induction of intramembranous particle clusters in mice with nephrogenic diabetes insipidus. Am J Physiol. 1991;261(4 Pt 2):F640–F646. doi: 10.1152/ajprenal.1991.261.4.F640. [DOI] [PubMed] [Google Scholar]
- 57.Bichet DG, Ruel N, Arthus MF, Lonergan M. Rolipram, a phosphodiesterase inhibitor, in the treatment of two male patients with congenital nephrogenic diabetes insipidus. Nephron. 1990;56(4):449–450. doi: 10.1159/000186196. [DOI] [PubMed] [Google Scholar]
- 58.Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev. 2013;34(2):278–301. doi: 10.1210/er.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]