Abstract
Rationale: The availability of therapeutics to treat pregnancy complications is severely lacking, mainly due to the risk of harm to the fetus. In placental malaria, Plasmodium falciparum-infected erythrocytes (IEs) accumulate in the placenta by adhering to chondroitin sulfate A (CSA) on the surfaces of trophoblasts. Based on this principle, we have developed a method for targeted delivery of payloads to the placenta using a synthetic placental CSA-binding peptide (plCSA-BP) derived from VAR2CSA, a CSA-binding protein expressed on IEs.
Methods: A biotinylated plCSA-BP was used to examine the specificity of plCSA-BP binding to mouse and human placental tissue in tissue sections in vitro. Different nanoparticles, including plCSA-BP-conjugated nanoparticles loaded with indocyanine green (plCSA-INPs) or methotrexate (plCSA-MNPs), were administered intravenously to pregnant mice to test their efficiency at drug delivery to the placenta in vivo. The tissue distribution and localization of the plCSA-INPs were monitored in live animals using an IVIS imaging system. The effect of plCSA-MNPs on fetal and placental development and pregnancy outcome were examined using a small-animal high-frequency ultrasound (HFUS) imaging system, and the concentrations of methotrexate in fetal and placental tissues were measured using high-performance liquid chromatography (HPLC).
Results: plCSA-BP binds specifically to trophoblasts and not to other cell types in the placenta or to CSA-expressing cells in other tissues. Moreover, we found that intravenously administered plCSA-INPs accumulate in the mouse placenta, and ex vivo analysis of the fetuses and placentas confirmed placenta-specific delivery of these nanoparticles. We also demonstrate successful delivery of methotrexate specifically to placental cells by plCSA-BP-conjugated nanoparticles, resulting in dramatic impairment of placental and fetal development. Importantly, plCSA-MNPs treatment had no apparent adverse effects on maternal tissues.
Conclusion: These results demonstrate that plCSA-BP-guided nanoparticles could be used for the targeted delivery of payloads to the placenta and serve as a novel placenta-specific drug delivery option.
Keywords: trophoblast, chondroitin sulfate A, placental CSA binding peptide, nanoparticles
Introduction
Pregnancy complications, including pre-eclampsia, fetal growth restriction, preterm birth, placental abruption, and late pregnancy loss, are common, affecting more than 1 in 6 pregnancies, and lead to substantial maternal, fetal, and neonatal morbidity and mortality 1-3. The causes of these complications remain unclear, but it has been postulated that placental insufficiency plays a primary role in each case 1.
Currently, there are no effective preventive strategies for these disorders: only a few drugs have been licensed for pregnancy disorders over the last two decades 4, 5, and the efficacy of existing therapeutic agents is extremely limited. Moreover, these agents are small molecules with low molecular weights and readily cross the placenta from mother to fetus, often leading to serious fetal side effects 6. Minimizing fetal exposure to medication is a major challenge in developing safer medications to treat pregnancy complications.
Nanomedicine is a multidisciplinary field of medicine and nanotechnology that seeks to increase the safety and efficacy of drugs by directing them preferentially to specific tissues 7, and it has opened up opportunities for novel modalities for the delivery of drugs in pregnancy 8-10. Recently, we developed a well-defined lipid-polymer nanoparticle (NP) platform using a fast single-step sonication method that allows control of particle size and surface functionalities, and has demonstrated good monodispersity, high drug-loading capability, sustained drug release, and excellent in vitro and in vivo stability 11, 12. Importantly, toxicity studies in mice indicate that these nanoparticles are well tolerated, with no adverse side effects 11, 12.
Chondroitin sulfate A (CSA) is present on placental syncytiotrophoblasts and plays a key role in malarial pathogenesis. The malaria parasite Plasmodium falciparum has evolved a protein, VAR2CSA, that mediates the binding of infected erythrocytes (IEs) to a distinct type of CSA present on placental syncytiotrophoblasts 13, 14. The minimal CSA-binding region of VAR2CSA consists of the Duffy Binding Ligand-like (DBL) 2X domain along with flanking interdomain (ID) regions 15, 16. Phage screening identified a peptide from the DBL2X domain that selectively binds to CSA 17; we named this synthetic peptide placental CSA binding peptide (plCSA-BP).
Given this background, we were interested in whether synthetic plCSA-BP conjugation to our lipid-polymer nanoparticle could effectively target delivery of payloads to the placenta. Here, we describe the successful fabrication of a plCSA-NP that was able to deliver methotrexate (MTX), an antifolate agent used to treat ectopic pregnancy and choriocarcinoma 18-20, specifically to mouse placental trophoblasts in vitro, ex vivo, and in vivo. We demonstrate that plCSA-BP can be exploited for the targeted delivery of drugs to the placenta.
Methods
Materials
Soybean lecithin and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-maleimide (polyethylene glycol 2000) carboxylic acid (DSPE-PEG-COOH) were purchased from Avanti Polar Lipids (Alabama, USA). Poly (lactide-co-glycolide) (PLGA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), methotrexate (MTX) and indocyanine green (ICG) were obtained from Sigma-Aldrich (Missouri, USA). Unless stated otherwise, all other materials used were purchased from Sigma-Aldrich (Missouri, USA). Placental CSA-binding peptide (plCSA-BP, EDVKDINFDTKEKFLAGCLIVSFHEGKC) and N-terminal α-amino-biotinylated plCSA-BP (biotin-plCSA-BP) were purchased from ChinaPeptides Co.,Ltd. (Shanghai, China). The scrambled peptide (SCR, EVDNDKKLGLVFEKDKIFTEFACISHCG) and N-terminal α-amino-biotinylated SCR (biotin-SCR) were synthesized at Shanghai GL Biochem Co., Ltd. (Shanghai, China).
Synthesis of plCSA-BP-conjugated nanoparticles and SCR-conjugated nanoparticles
Lipid-polymer nanoparticles (NPs) loaded with MTX (MNPs) were synthesized from PLGA, soybean lecithin, MTX and DSPE-PEG-COOH using our previously published single-step sonication method 11. The peptides were conjugated to DSPE-PEG-COOH using EDC and NHS, as follows. First, 18 mg of the MNPs was suspended in 6mL of 0.1M 2-morpholinothanesulfonic acid (MES) buffer (pH 5.5). To pre-activate the carboxylic group, 15 mg of EDC and 5 mg of NHS were added, and the mixture was stirred for 15 min at room temperature. 3 mg of plCSA-BP or SCR was then dissolved in 500 μL of deionized water and the solution was added to the reaction mixture separately. Next, 350 μL of 20× phosphate-buffered saline (PBS) was added to buffer the reaction, which was maintained at pH 7.0-8.0. The reaction mixture was then stirred overnight at room temperature. Excess peptide and other impurities, such as EDC and NHS, were removed by triple filtration using AmiconUltra-4 centrifugal filters (MW10kD, Millipore, MA, USA) to obtain final plCSA-BP-conjugated nanoparticles loaded with MTX (plCSA-MNPs) and SCR-conjugated nanoparticles loaded with MTX (SCR-MNPs). The same procedures were used to prepare the plCSA-BP-conjugated nanoparticles loaded with indocyanine green (ICG, plCSA-INPs) and SCR-conjugated nanoparticles loaded with ICG (SCR-INPs).
Conjugation efficiencies were determined using a bicinchoninic acid (BCA) assay. Briefly, 200 μL of BCA assay reagent (50:1, BCA:CuSO4) was added to wells in a 96-well plate, followed by 25 μL of plCSA-NPs or SCR-NPs. The mixtures were further incubated for 30 min at 37°C, and fluorescence was measured at 562 nm with a microplate reader (Thermo Fisher, USA).
Characterization of the nanoparticles
The size, polydispersity, and surface charge of the nanoparticles were determined using a Beckman Coulter Delsa TM Nano C (Beckman, USA) at room temperature. Nanoparticle morphology and size were visualized using transmission electron microscopy (TEM, JEM-100CXII). Briefly, nanoparticles suspensions (0.1 mg/mL) were loaded onto carbon-coated copper grids for 5 min, and excess liquid was removed with filter paper. The grids were then stained with 2% (w/v) phosphotungstic acid for 1 min and dried at room temperature before image acquisition.
To determine encapsulation efficiency (EE) and drug loading efficiency (LE) for MTX in the nanoparticles, before ultrafiltration, newly synthesized nanoparticles were isolated from the aqueous suspensions using a Beckman Optima TM MAX-XP ultracentrifuge (34,000×g, 30 min) (Beckman, USA). The non-entrapped MTX in the supernatant was quantified using fluorescence spectroscopy (F900, Edinburgh Instruments Ltd., UK) with emission at 545 nm and excitation at 300 nm. The EE and LE were calculated as follows: EE=((weight of loaded MTX)/(weight of initially added MTX))×100%, and LE=((weight of loaded MTX)/(total weight of NPs))×100%.
A dialysis experiment was performed to measure MTX release from different MTX formulations, with PBS (pH 7.4) as the release medium. Different MTX formulations (100 μg MTX equivalent) dispersed in 1 mL of release medium were added to dialysis bags (MWCO, 3500 Da, Bestbio, Shanghai, China), and the bags were incubated in 100 mL of the same medium at 37°C on a rotator at 120 rpm. At predetermined time points, the dialysate was removed to measure drug release, and an equal volume of fresh PBS was added. MTX concentrations in the dialysates were measured by fluorescence spectroscopy with emission at 545 nm and excitation at 300 nm. The in vitro stabilities of different nanoparticles were investigated by dispersing the nanoparticles in 10% fetal bovine serum (FBS) and examining changes in size.
Animals
CD1 mice (6-8 weeks of age) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and caged in a specific pathogen-free animal room with a 14 h light/10 h dark cycle. Gestational ages were determined by monitoring formation of vaginal plugs (E0.5=vaginal plug day) and confirmed by measuring gestational sac lengths using an ultrasound imaging system. Pregnant mice were injected intravenously in the tail vein with free MTX, MNPs, SCR-MNPs, or plCSA-MNPs at an MTX equivalent dose of 1 mg/kg every second day starting at E6.5.
Immunofluorescence
Mice were anesthetized with 30% urethane. After cardiac perfusion with PBS, tissues were removed and fixed in 4% paraformaldehyde (PFA) overnight. After dehydration in 30% sucrose, tissues were embedded in OCT and frozen, and tissue blocks were stored at -80°C until sectioning. 10 μm serial sections were obtained for immunostaining. After blocking tissue sections with bovine serum albumin (BSA), sections were incubated with biotin-plCSA-BP (50 ng/mL), biotin-SCR (50 ng/mL) or primary antibodies overnight at 4°C as follows: rat monoclonal anti-CD31 (1:200; BD Pharmingen, CA, USA); rat monoclonal anti-cytokeratin 8 (CK8; 1:100; National Institutes of Health Developmental Studies Hybridoma Bank, Iowa, USA); or mouse monoclonal anti-C4S (2B6, 1:20; Amsbio, UK). Tissue sections were then washed 3 times (TBS, 5 min) and incubated at room temperature with Alexa 488-donkey anti-rat (1:200; Thermo Fisher Scientific, MA, USA), Alexa 594-donkey anti-rat (1:200; Thermo Fisher Scientific, MA, USA), or Alexa 594-goat anti-mouse (1:200, Thermo Fisher Scientific, MA, USA) secondary antibody or FITC-avidin (1:64; Boster, Wuhan, China) separately for 30 min. Stained sections were mounted using mounting medium containing DAPI (Vector Laboratories, CA, USA) and examined using fluorescence microscopy (Olympus, Japan) and confocal microscopy (Leica, TCS SP5, Germany).
Immunohistochemistry
Normal human placental villous tissues were collected after informed consent from elective terminations according to an IRB approved protocol at the Bao'an Maternal and Child Health Care Hospital of Shenzhen, described below. Immunohistochemistry (IHC) was performed according to our previously published procedures 21. Briefly, human and mouse tissue sections (5 μm) were deparaffinized and rehydrated. Slides were then microwaved in antigen retrieval buffer (0.01 M sodium citrate, pH 6.0). After cooling, slides were incubated with hydrogen peroxide (3% w/v) for 20 min to inactive endogenous peroxidases, washed in TBS, and incubated with BSA for 30 min. Sections were then incubated with biotin-plCSA-BP (50 ng/mL), biotin-SCR (50 ng/mL), mouse monoclonal anti-C4S antibody (2B6, 1:20; Amsbio, UK), or control IgG (Abcam, UK) overnight at 4°C. Tissue sections were then washed and incubated with HRP-donkey anti-mouse IgG (1:200; Thermo Fisher Scientific, USA) or HRP-streptavidin (1:200; Boster, Wuhan, China). Hematoxylin (blue) was used as a counter-stain. Staining was visualized on a light microscope (Olympus BX53, Japan).
H&E
Tissue collection and processing for histological analysis were performed following a standard protocol, as described previously by us and others 22, 23. Briefly, tissue samples from placentas, livers, and kidneys were collected and fixed in 4% PFA overnight at 4°C. Subsequently, the tissues were infiltrated with and embedded in paraffin, sectioned, stained with H&E, and examined on a light microscope (Olympus BX53, Japan).
Ultrasonographic measurements of embryo development
Mice were anesthetized with 5% isoflurane at 1 L of O2/min and maintained under 3% isoflurane in 1 L of O2/min for the duration of the scan. The mice were kept warm on a heated platform (37°C) connected to a temperature/physiology monitor. Embryo development was monitored using a dedicated small-animal high-frequency ultrasound (HFUS) imaging system (Vevo 2100; VisualSonics, Toronto, Canada) with ultrasound settings standardized as follows: frequency, 40 MHz; power, 100%; and gain, 30 dB. Images were acquired using a high-resolution transducer (MS550S). To ensure optimal image quality, fur from the abdominal region was removed using a commercial hair remover and pre-warmed ultrasound gel was applied to the skin. The B-mode was used to monitor gestational sac length, crown rump length, biparietal diameter, abdominal circumference, placental diameter, and placental thickness. Color Doppler mode was used to monitor fetal heart rate, and Power Doppler mode was used to monitor umbilical artery peak velocity. Images were saved as static images or cine loops. The imaging data sets for all mice were analyzed using Vevo 2100 built-in software.
TUNEL assay
Apoptotic cells were detected using the FragEL DNA Fragmentation Detection Kit (Calbiochem, Germany) according to the manufacturer′s instructions. Briefly, deparaffinized placental sections were incubated with terminal deoxynucleotidyl transferase (TdT) enzyme in TdT buffer in a humidified chamber at 37°C, and tagged nucleotides were detected using fluorescein-FragEL TdT conjugates. Sections were placed under a coverslip with a mounting medium containing DAPI and examined under a fluorescence microscope (Olympus BX53, Japan).
To quantify apoptotic cells, TUNEL-positive cells were counted using ImageJ (NIH) by examining ten random fields for each placenta per experimental condition. The apoptotic index was calculated as the percentage of nuclei exhibiting TUNEL-positive staining divided by the total number of DAPI-stained nuclei in each section.
IVIS in vivo imaging
To evaluate targeting by plCSA-INPs, pregnant mice at E12.5 or E15.5 were administered plCSA-INPs or SCR-INPs (5 mg/kg ICG equivalent) in tail veins. The mice were then scanned using an IVIS spectrum instrument (Perkin Elmer). Scans were taken at time intervals ranging from 10 min to 48 h. Data analysis was performed using Living Image Software (Caliper Life Sciences, CA).
High-performance liquid chromatography analysis
MTX concentrations in fetal and placental tissues were determined by high-performance liquid chromatography (HPLC). Mice were injected intravenously with PBS, free MTX, MNPs, SCR-MNPs, or plCSA-MNPs (1 mg/kg MTX equivalent). At predetermined time points, the mice were anesthetized with 30% urethane, and following cardiac perfusion with PBS to remove unbound nanoparticles, tissues were harvested and weighed. Tissues (~200 mg) were homogenized in a volume of PBS (pH 7.4) equivalent to twice the tissue weight, 200 μL 10% perchloric acid (v/v) was added, and the mixture was vortexed for 3 min and centrifuged at 14,000×g for 20 min to pellet proteins. The supernatants were then filtered through a Millipore Swineex syringe adapter (0.45 μm), and a total volume of 20 μL was injected into the HPLC system, which consisted of an LC20A VP solvent pump, an SPD-20A UV spectrophotometric detector (Shimadzu, Kyoto, Japan), and a 250×4.6 mm (i.d.) XTerra MS C18 column (5 μm particle size; Waters, MA, USA). A mixture of 40 mM potassium phosphate dibasic (pH 4.5) and acetonitrile (88:12, v/v) was used as the mobile phase, with a flow rate of 1.0 mL/min. The HPLC analysis was monitored by detecting ultraviolet absorbance at 313 nm 24.
Study approval
Pregnant women receiving care at the Bao'an Maternal and Child Health Care Hospital of Shenzhen were enrolled in this study after providing written informed consent according to a protocol approved by the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences Research Board. All mouse experiments were approved by the Animal Care and Use Committee of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences.
Statistics
All data are expressed as the mean±SD. Univariate analyses comparing measurements and embryonic days were performed using Spearman′s rank correlation (Rs). Continuous linear regression was adopted for multivariate analysis of significant parameters. All statistical tests were two-sided, and a p value <0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 6.01 (GraphPad Software Inc. USA).
Results
plCSA-BP specifically binds to a distinct placental-type CSA expressed on trophoblasts
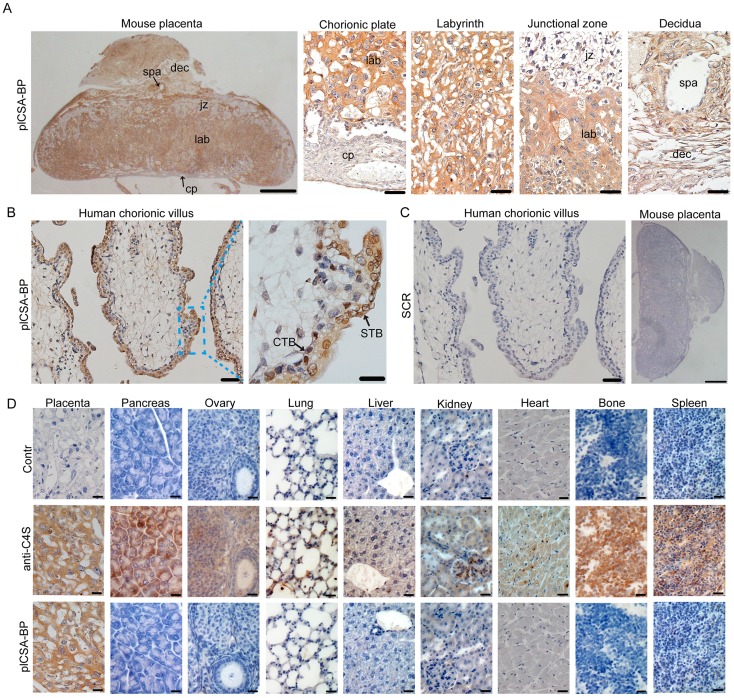
To examine whether synthetic plCSA-binding peptide (plCSA-BP) could be exploited for placental targeting, biotin-plCSA-BP and biotin-SCR were incubated with mouse and human placental tissue sections and detected by histochemical techniques. Biotin-plCSA-BP efficiently bound to the trophoblast cells in the labyrinth layer, the trophoblast lining of the remodeled spiral arteries, but not to the placental chorionic plate, junctional zone, or deciduas (Figure 1A). Similarly, in human placenta, biotin-plCSA-BP specifically bound to the syncytiotrophoblast (Figure 1B), which is in line with the fact that P.falciparum-infected VAR2CSA-expressing erythrocytes are sequestered to the placental syncytiotrophoblast 13, 14. Moreover, no specific binding of biotin-SCR to placental tissues was detected (Figure 1C).
Figure 1.
plCSA-BP specifically binds to placental tissues.(A) Images of full-thickness mouse placenta (left scale bar=1 mm) at E14.5 and magnified images of the chorionic plate (cp), labyrinth (lab), junctional zone (jz), decidua (dec) and spiral artery (spa) (right scale bar=50 μm). Placenta incubated with 50 ng/mL biotin-plCSA-BP and 1:200 HRP-streptavidin. (B) Human placenta chorionic villus stained as in (A) (left scale bar= 50 μm, right scale bar=20 μm). (C) Representative images of indicated tissue specimens incubated with 50 ng/mL biotin-SCR and detected by HRP-streptavidin (left scale bar= 50 μm, right scale bar=1 mm). (D) A selection of 9 normal mouse tissues stained for total CSA using enzymatic GAG end-processing and 1:20 anti-C4S (2B6) or for CS detected by biotin-plCSA-BP as in (A). Images representative of n=4. Scale bar=20 μm. CTB: cytotrophoblasts; STB: syncytiotrophoblasts. See also Figure S1.
Considering that chondroitin sulfate A (CSA) distribution is broad, with expression in several tissues 25, we also analyzed the interaction between plCSA-BP and other tissues. Staining with anti-C4S (2B6) revealed that CSA is abundant in most tissues, while none of the tissues analyzed (other than placental tissue) displayed plCSA-BP staining (Figure 1D). Moreover, plCSA-BP and CSA are co-localized in the mouse placental labyrinth and human placental syncytiotrophoblast (Figure S1). Thus, the data demonstrate that the plCSA-BP synthesized in this study binds with high affinity and specificity to a placental-type CSA (plCSA) expressed exclusively on trophoblasts.
plCSA-BP binds specifically to trophoblasts throughout gestation in the mouse
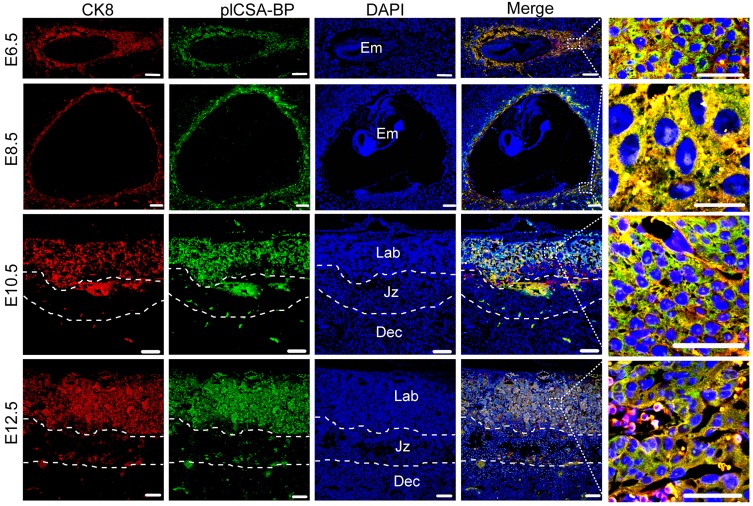
In order to test the ability of plCSA-BP to specifically bind to trophoblasts and not to other tissues throughout pregnancy, co-localization of the trophoblast marker cytokeratin 8 (CK8) and biotin-plCSA-BP was confirmed in mouse placenta at gestational stages E6.5, E8.5, E10.5, and E12.5 (Figure 2), and no plCSA-BP staining was detected in embryos, junctional zones, and deciduas. Along with our finding of placenta-specific plCSA-BP staining at E14.5 (Figure 1A), these results show that plCSA-BP specifically binds to trophoblast throughout gestation in the mouse.
Figure 2.
plCSA-BP specifically binds to trophoblasts during gestation in mice. Immunofluorescence staining of trophoblasts (CK8, red) and biotin-plCSA-BP (green) stained midline sections from mice at E6.5, E8.5, E10.5 and E12.5 showing that plCSA-BP is co-localized with trophoblasts in the placenta. Nuclei are counter-stained with DAPI, shown in blue. (n=4, scale bar=200 μm (50 μm in magnified pictures)). Dec: decidua; Em: embryo; Jz: junctional zone; Lab: labyrinth.
Synthesis and characterization of nanoparticles
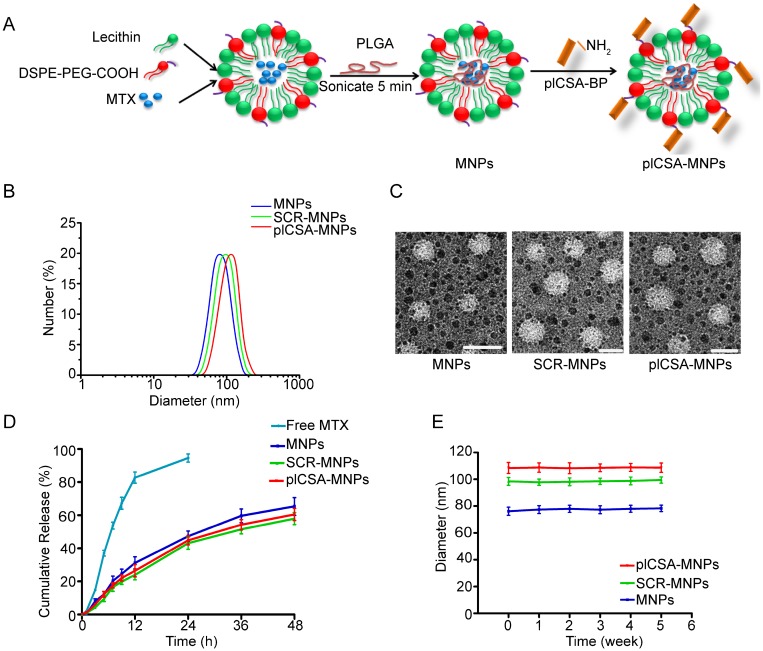
Next, we conjugated plCSA-BP to nanoparticles. MNPs and INPs were self-assembled from MTX or ICG with PLGA, soybean lecithin, and DSPE-PEG-COOH using a single-step sonication method 11. SCR and plCSA-BP were then conjugated to the nanoparticles by linking the amino-terminus of each peptide to carboxyl groups on the surfaces of the nanoparticles to produce SCR-MNPs/SCR-INPs and plCSA-MNPs/plCSA-INPs (Figure 3A).
Figure 3.
Synthesis and characterization of nanoparticles. (A) Schematic illustration of the single-step sonication process used to synthesize MNPs and the EDC/NHS reaction scheme used for conjugation with the plCSA-BP. (B) Diameter distributions of different nanoparticles measured by dynamic light scattering (DLS). (C) Representative TEM images of MNPs, SCR-MNPs, and plCSA-MNPs. Scale bars=100 nm. (D) Time course of MTX releasefrom MNPs, SCR-MNPs, and plCSA-MNPs in PBS (pH7.4) at 37°C. (E) Nanoparticle stability in serum (10% FBS) was evaluated by examining size changes. Data are shown as the mean±SD (n=4).
We next determined the physical properties of these nanoparticles. The mean diameters of the MNPs, SCR-MNPs, and plCSA-MNPs were approximately 76 nm, 98 nm, and 109 nm, respectively, with good polydispersity (PDI; a measure of the heterogeneity of sizes of molecules or particles in a mixture; for a uniform sample, PDI=1.0) (Figure 3B and Table 1). Conjugation with either plCSA-BP or SCR increased the MNP sizes. Transmission electron microscopy revealed that MNPs, SCR-MNPs, and plCSA-MNPs had spherical shapes and uniform size distributions (Figure 3C). Zeta potentials (the nature of the electrostatic potential near the surface of a particle; if it less than -15 mV, it usually represents the onset of agglomeration, and the higher the absolute zeta potential, the more stable the particle) were -21.3±2.65 mV, -24.6±2.48 mV, and -27.8±4.25 mV for MNPs, SCR-MNPs, and plCSA-MNPs, respectively. Hence, these nanoparticles are predicted to be highly stable. The efficiencies of plCSA-BP and SCR conjugation to MNPs were determined to be 52.3 ± 4.4% and 56.1 ± 3.7%, respectively (Table 1).
Table 1.
Characterization of the nanoparticles
| Diameter (nm) | Zeta potential (mV) | Polydispersity | EE of MTX (%) | LE of MTX (%) | Conjugation efficiency (%) | |
|---|---|---|---|---|---|---|
| MNPs | 76.2±5.3 | -21.3±2.65 | 0.102±0.023 | 37.2±1.54 | 5.5±0.36 | - |
| SCR-MNPs | 98.4±4.6** | -24.6±2.48 | 0.124±0.045 | - | - | 56.1±3.7 |
| plCSA-MNPs | 108.5±7.6** | -27.8±4.25 | 0.108±0.073 | - | - | 52.3±4.4 |
**p<0.01 compared with MNPs (n=3).
EE: drug encapsulation efficiency.
LE: drug loading efficiency.
Drug encapsulation efficiency, drug loading efficiency, and release ability of nanoparticles are crucial parameters for determining their clinical usefulness. The drug encapsulation efficiency and drug loading efficiency of the MNPs were 37.2±1.54% and 5.5±0.36%, respectively, indicating a high drug-loading capability. The in vitro release profiles of the MNPs, SCR-MNPs, and plCSA-MNPs revealed similar biphasic patterns (Figure 3D), characterized by a fast initial release within the first 24 h (~40% released) and a slower continuous release in the subsequent 48 h (~60%). Moreover, these results suggested that modifications with different peptides did not influence the in vitro release pattern of MTX from the lipid-polymer nanoparticles.
Next, the in vitro stabilities of MNPs, SCR-MNPs, and plCSA-MNPs were investigated by dispersing the nanoparticles in 10% fetal bovine serum (FBS) and examining changes in size (Figure 3E). The particle sizes of MNPs, SCR-MNPs, and plCSA-MNPs remained near the initial particle sizes without aggregation and precipitation over a one-month period, suggesting excellent stability for all three, which is suitable for in vivo experiments.
In vivo placenta targeting of plCSA-INPs
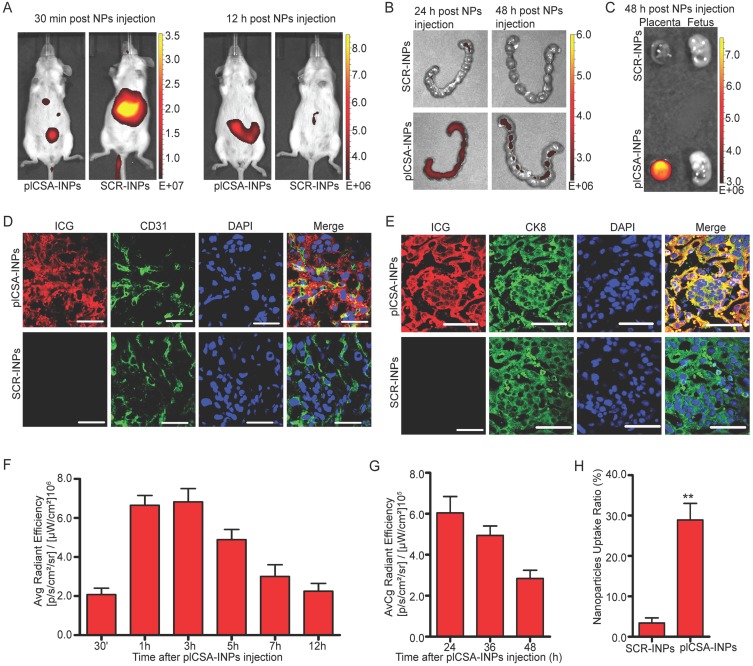
To investigate the placenta-targeting ability of plCSA-BP-conjugated nanoparticles in vivo, we synthesized SCR- and plCSA-BP-conjugated nanoparticles loaded with infrared fluorescent ICG (SCR-INPs and plCSA-INPs). SCR-INPs and plCSA-INPs were administered to pregnant mice by intravenous injection, and the tissue distributions and localization of the nanoparticles were monitored with the IVIS live animal imaging system. ICG signal from the uterus region was observed as early as 30 min after injection, reaching a peak at 3 h, and a strong signal was maintained upto 12 h (Figure 4A, F). The SCR-INPs also produced strong signals in the liver region after 30 min but were at very low levels at 12 h (Figure 4A). These results indicate that non-targeted nanoparticles are rapidly metabolized and eliminated by the liver after intravenous injection. Moreover, the signal from the plCSA-INPs could be detected in the uterus upto 48 h after injection based on ex vivo examination (Figure 4B, G). Notably, this signal was only found in placentas and not at all in fetuses (Figure 4C).
Figure 4.
In vivo placenta targeting by using plCSA-INPs.(A) Pregnant mice at E12.5 (n=5 each) were injected with SCR-INPs or plCSA-INPs (ICG, equivalent 5 mg/kg) via the tail vein and imaged with an IVIS spectrum optical imaging system. (B) Ex vivo detection of the ICG signal in uteri by IVIS. (C) Forty-eight hours after intravenous injection of SCR-INPs or plCSA-INPs at E15.5 (n=5 each). (D-E) Representative immunofluorescence images of placental middle sections. SCR-INPs or plCSA-INPs (ICG, red, equivalent 5mg/kg) were injected into the tail vein of pregnant mice. After 48 h, mice were subjected to cardiac perfusion to remove unbound nanoparticles. Placentas were collected, and serial sections were immunostained for CD31 (green) or CK8 (green) and examined by confocal microscopy. DAPI, blue. Scale bar=50 μm. Images representative of n=6. (F-G) Quantification of the IVIS signal from pregnant mice (E12.5) after injection with plCSA-INPs at different time points from 30 min to 48 h. (H) Quantitative analysis of cell-associated nanoparticles as in (E); ICG-positive cells were counted using ImageJin ten random fields per experimental condition. Values are expressed as the mean ±SD (n=6). **p< 0.01. See also Figure S2.
We next performed a detailed examination of placentas 48 h after a single plCSA-INP or SCR-INP injection by immunostaining for a vascular endothelial cell marker (CD31) and the trophoblast marker cytokeratin 8 (CK8) to visualize co-localization with ICG. ICG was mainly co-localized with the trophoblast, identified by CK8 staining (Figure 4E), with little or no staining in endothelial cells (Figure 4D) in the plCSA-INP group, and there was little or no ICG signal in the SCR-INP group (Figure 4D-E, H and Figure S2, showing a different serial section). These results indicate that plCSA-INPs target delivery of ICG to trophoblast. Together, these data suggest that plCSA-BP can be used as a targeting peptide for the efficient delivery of drug-loaded nanoparticles to the placenta in vivo.
Quantitative analysis of embryonic growth after the administration of plCSA-MNPs
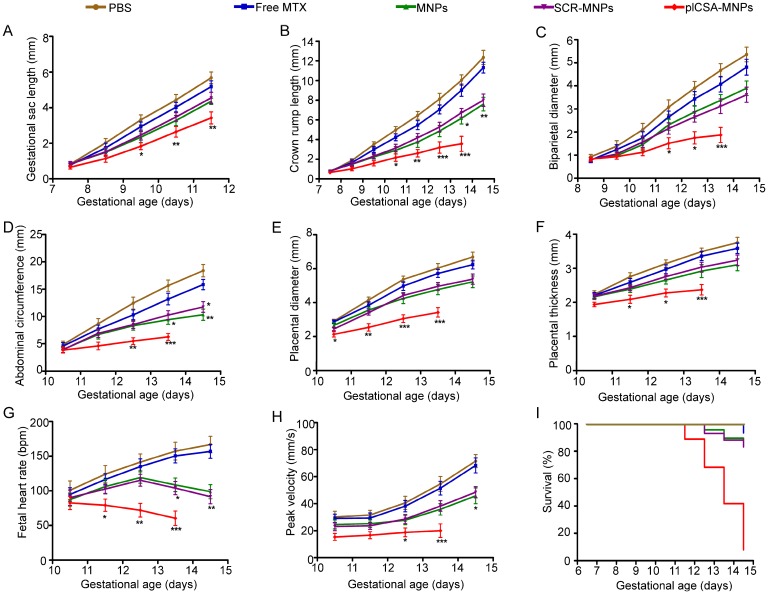
MTX, a drug used to treat ectopic pregnancy and choriocarcinoma, was selected to test the efficiency and effectiveness of plCSA-BP-conjugated nanoparticles as drug delivery vehicles to the trophoblast in the placenta. The effects of plCSA-MNPs were compared to those of free MTX, MNPs, and SCR-MNPs on placental and fetal development in pregnant mice from early to later pregnancy (E6.5-E14.5) and on pregnancy outcomes based on high-frequency ultrasound (HFUS). Treatment with plCSA-MNPs significantly reduced gestational sac length, crown rump length, biparietal diameter, abdominal circumference, placental diameter, and placental thickness (Figure 5A-F and Movies S1-2). Fetal heart rate and umbilical artery blood flow velocities also significantly decreased compared to those of the control groups (Figure 5G-H). The growth of both fetuses and placentas was slightly inhibited by free MTX, but the difference did not reach significance (Figure 5A-H). Pregnant mice treated with MNPs or SCR-MNPs showed impaired fetal development, and the survival rate of these two groups was approximately 83%. Remarkably, the plCSA-MNPs exerted a strong cytotoxic effect on both placental and fetal development, which decreased fetal survival rates to less than 10% at E14.5 (Figure 5I).
Figure 5.
Embryonic growth quantified using ultrasound parameters after various treatments. (A) Gestational sac length (n=10-30 embryos/day), (B) crown rump length (n=30-51 embryos/day), (C) biparietal diameter (n=30-51embryos/day), (D) abdominal circumference (n=30-51embryos/day), (E) placental diameter (30-51 embryos/day), (F) placental thickness (30-51 embryos/day), (G) fetal heart rate (n=20-33 embryos/day) and (H) umbilical artery peak velocity (n=12-36 embryos/day) measured non-invasively by ultrasound in vivo. (I) Fetal survival curves for different treatments (n=45-55 embryo). At pregnancy day 6.5, mice received intravenous injections of different nanoparticles; treatment was administered every 2 days. Values are expressed as the mean ±SD. *p<0.05, **p< 0.01, ***p<0.001 vs. the free MTX group.
HFUS evaluation of embryo morphology after the administration of plCSA-MNPs
Whole-embryo images at each stage from E6.5 to E14.5 were acquired by high-frequency ultrasound (HFUS) and used to assess physiological fetal and placental growth. On day E8.5, the S-shaped embryo starts to turn around its own axis, the allantois, which later gives rise to the umbilical vessels; this movement was visible in the PBS and free MTX groups (Figure 6A-B). Although the embryos were visible, compared to those in the PBS group, the embryonic cavities were smaller (Figure 6C-F) in the mice treated with MNPs, SCR-MNPs, and plCSA-MNPs.
Figure 6.
HFUS evaluation of embryo morphology after the administration of free MTX and different nanoparticles. At E6.5, mice (n=6 each) received intravenous injections of free MTX or MTX-containing different nanoparticles (1 mg/kg MTX equivalent); treatment was administered every 2 days. Embryonic growth was measured using a Vevo 2100 ultrasound imaging system. (A-B) The embryonic cavity and the embryo (Em) are visible. (C-F) Note the small embryonic cavity (arrows). (G-J) The embryonic heart (He) could be distinguished by ultrasound. (K)The placenta displays hyperechogenic calcification deposits (Ca, arrows) at the fetal-maternal boundary. (L) A resorption site with a small embryonic cavity (arrow). (M-P) An embryo enlarged in size. The placenta (Pl) could be differentiated by its pulsating blood vessels. (Q) An embryo exhibiting a reduced heart rate. Pericardial (Pc) effusion is evident. The placenta has a high echodensity spot and smaller size. (R) A dead embryo. (S-V) Progressive ossification is observed in the fetus. (W-X) Embryos with high echodensity spots are resorbed. Al: allantois; Am: amnion; UC: umbilical connection. Scale bars=1 mm. See also Figure S3.
On day E10.5, the embryos were considerably larger in size, and the hearts could be clearly distinguished by ultrasound. Although the placentas exhibited an echogenicity similar to that of the decidua basalis, they could be differentiated by their pulsating blood vessels and layers of higher echogenicity between the embryonic trophoblast and maternal decidua in the PBS and free MTX groups (Figure 6G-H). The fetuses had shorter crown rump lengths in the MNP and SCR-MNP groups (Figure 6I-J), while the placenta showed calcification in the plCSA-MNP group, as described by Akirav et al. 26. The concretions most likely originated from dystrophic calcification processes in dysfunctional cells, where active calcium transport is impaired. Growth-retarded embryos also exhibited bradycardia (Figure 6K-L and Movie S3).
On day E12.5, the umbilical cord that links the fetus to the placental chorionic plate was present in the PBS control group (Figure 6M). In contrast, the fetuses showed growth retardation in the free MTX group (Figure 6N) and were smaller in the MNP and SCR-MNP groups compared to the PBS group (Figure 6O-P). However, in the plCSA-MNP group, the fetuses showed pericardial edema, and placentas were smaller, with higher echogenicity, and approximately 50% of the fetuses were dead (Figures 6Q-R).
On day E14.5, the fetuses were considerably larger in the PBS group (Figure 6S). In contrast, in the free MTX group, the fetuses were smaller (Figure 6T). The placentas showed calcification, and approximately 30% of the fetuses were dead in the MNP and SCR-MNP groups; no significant difference (P>0.05) was found between these two groups (Figure 6U-V and Figure S3). However, in the plCSA-MNP group, more than 90% of the fetuses were dead and resorbed (Figure 6W-X).
plCSA-MNPs interfere with pregnancy outcome by targeting the placenta
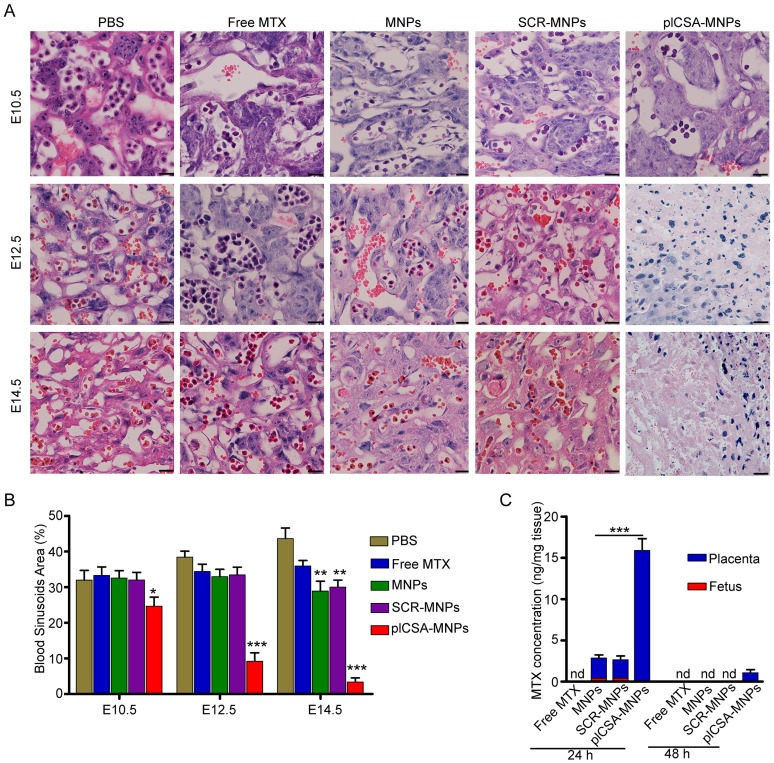
Next, we measured the cross-sectional areas of blood sinusoids in the placental labyrinthine region using computerized morphometry, which showed that the average blood sinusoid areas decreased markedly in the placentas of mice treated with plCSA-MNPs beginning on E10.5, and that the labyrinthine region was nearly gone at E14.5 (Figure 7A-B). Interestingly, the average blood sinusoid areas in the MNP and SCR-MNP groups were also substantially decreased at E14.5, indicating that nanoparticles might significantly improve the delivery of drug to the placenta via the enhanced permeability and retention (EPR) effect 27, 28.
Figure 7.
Methotrexate delivered by plCSA-MNPs significantly reduces placental vascular density. Pregnant mice (n=6 for each group) received intravenous injections of different nanoparticles on E6.5. (A) Histological cross sections of the labyrinth layers from placentas (n=10-14 each) of different groups stained with H&E. (B) The vascular areas in the labyrinthine regions were estimated from at least 3 non-consecutive sections from each placenta using ImageJ. Blood sinusoid cross-sectional areas were calculated as the ratio between the numbers of pixels within the area defined using the threshold function and the overall number of pixels in the image. Scale bar=20 μm. All data are expressed as the mean±SD. ***p< 0.001 vs. PBS group. (C) HPLC measurement of MTX concentrations in placentas (n=15 for each group) and fetuses (n=15 for each group) 24 h and 48 h after a single injection of free MTX or MTX-containing different nanoparticles (1 mg/kg MTX equivalent) in pregnant mice (n=5 for each group) at gestational stage E13.5. Values are expressed as the mean±SD. ***p< 0.001 vs. the MNPs group. nd: not detected. See also Figure S4.
To determine whether plCSA-MNPs can cross the placenta and reach the fetus, leading to serious fetal side effects, we measured MTX levels in placentas and fetuses by HPLC. Free MTX and MTX-containing nanoparticles were administered intravenously to pregnant mice. 24 h after injection, MTX was not detected in the placentas from the free MTX group, while it was detected at minimal levels in the MNP and SCR-MNP groups and at the highest levels in the plCSA-MNP group (Figure 7C). Furthermore, it was also detected in the fetuses, although at lower levels than in the placentas, in the MNP and SCR-MNP groups. This suggested that lipid-polymer nanoparticles slightly limit transfer of MTX across the placenta. In contrast, the plCSA-MNPs had efficiently accumulated in placentas after 24 h, and MTX was detectable in placentas 48 h after injection. Notably, MTX was not detectable in the fetuses at either time point in the plCSA-MNP group (Figures 7C and Figure S4).
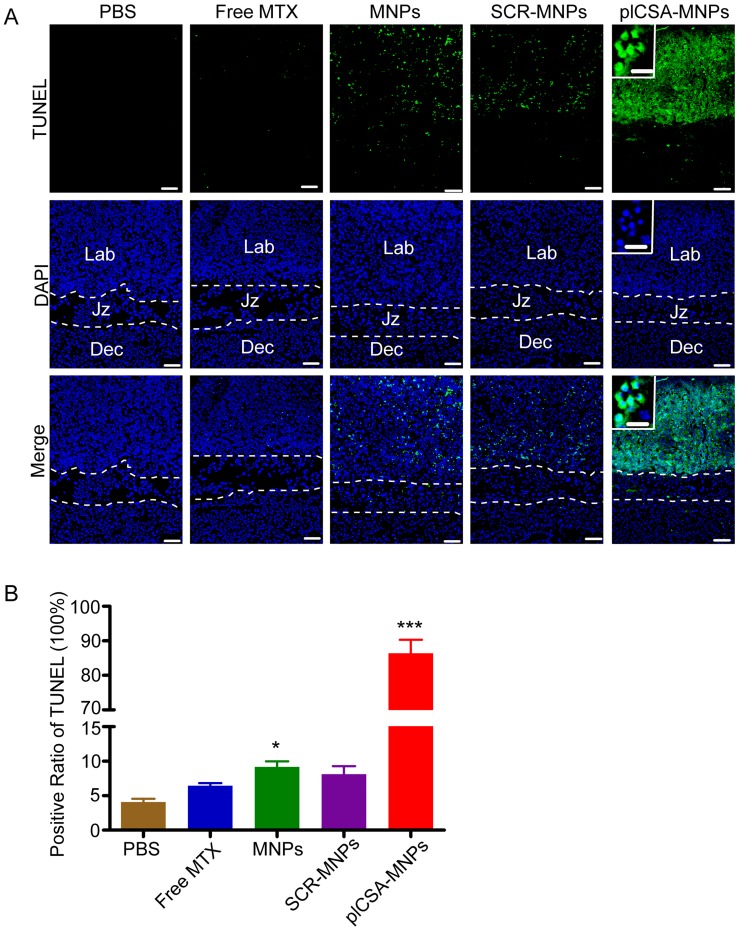
To confirm that plCSA-NPs delivered MTX specifically to placental trophoblasts, apoptosis was analyzed by TUNEL assay in placenta sections. After free MTX treatment, there was minimal apoptosis. As expected, MNPs and SCR-MNPs increased the levels of apoptosis in placental tissue. Notably, plCSA-MNPs vigorously increased apoptotic cell numbers. Specifically, TUNEL-positive cells were found mainly in the placental labyrinthine trophoblast (Figure 8A-B and Figure S5). Together, these data provide compelling evidence that plCSA-MNPs interfere with pregnancy outcomes by effectively targeting the placental trophoblast.
Figure 8.
Apoptosis is induced by plCSA-MNPs in the placenta, confirmed by TUNEL assay. (A) Middle sections viewed by fluorescence microscopy. TUNEL-positive cells are stained in green, whereas blue indicates DAPI-stained nuclei. (B) The percentages of TUNEL-positive cells from different treatment groups. Dec, decidua; Jz, junctional zone; Lab: labyrinth. Scale bar=100 μm and white boxes represent magnifications of the indicated areas (scale bar=20 μm). Data are presented as mean ±SD (n=6). *p< 0.05, ***p< 0.001 vs. the PBS group. See also Figure S5.
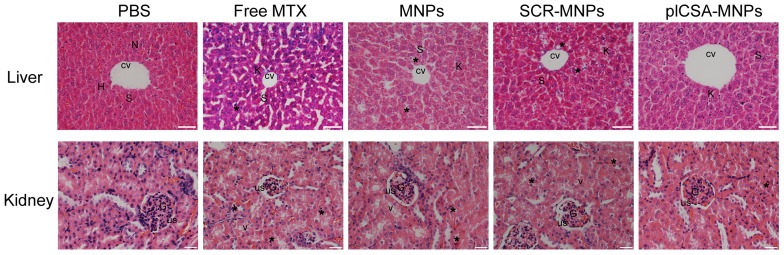
plCSA-MNPs exhibit minimal liver and kidney toxicity
The toxicities of each experimental treatment in maternal liver and kidney were evaluated by histological examination of tissue sections (Figure 9). Liver sections from the PBS group revealed normal liver structure with dilated blood sinusoids. In the free MTX group, liver sections revealed necrosis of the hepatocyte inflammatory cells around the central vein, dilated blood sinusoids, and activated Kupffer cells. In addition, distortions in cell arrangement around the central vein, pyknotic and apoptotic nuclei, and dilation of most sinusoids were observed. The liver sections in the MNP and SCR-MNP groups showed fewer abnormalities, with less hepatocyte necrosis, and fewer pyknotic nuclei, dilated blood sinusoids, and activated Kupffer cells compared to that in the free MTX group. However, liver sections from the plCSA-MNP group displayed a similar architecture similar to that in the PBS group, with intact hepatic cords, few inflammatory cells around the central vein, less hepatocyte necrosis and fewer activated Kupffer cells.
Figure 9.
Free MTX induces renal and hepatic toxicity but plCSA-MNPs do not. The livers from the PBS group had normal histology with the central vein (CV) and hepatic cords of hepatocytes (H) having prominent nuclei (N) separated by blood sinusoids (S). The free MTX group exhibited necrosis of hepatocytes (star), the most dilated blood sinusoids (S) and the highest rate of Kupffer cell activation (K). In addition, liver images from the free MTX group reveal distortion in the cell arrangement around the central vein. Livers from the MNP and SCR-MNP groups had pyknotic nuclei (star) and like the free MTX group had dilated blood sinusoids (S). These images demonstrate moderate improvement with little necrosis of hepatocytes, dilation of blood sinusoids, or activation of Kupffer cells. Mouse livers treated with plCSA-MNPs displayed normal hepatic cells with few Kupffer cells (scale bar=50 μm). Sections of normal kidney tissue from the PBS group verify normal architecture of glomeruli (G), urinary spaces (US), and renal tubules. In the free MTX group, the kidneys displayed vacuolation and pyknotic nuclei (star) in some tubules, glomerular atrophy and dilated urinary spaces. Kidneys from the MNP and SCR-MNP groups exhibited moderate vacuolation and pyknotic nuclei in renal epithelia, while kidneys from the plCSA-MNP group appeared nearly normal. Images representative of n=5. Scale bar= 20 μm
Examination of kidney sections revealed changes similar to those found in the liver sections from the different treatment groups. The kidney sections from the PBS group showed normal architecture, whereas vacuolation and pyknotic nuclei in some tubules and glomerular atrophy and dilated urinary spaces were observed in the free MTX group. In the MNP and SCR-MNP treatment groups, kidney sections showed moderate vacuolation and pyknotic nuclei in the renal epithelia. In contrast, the kidney sections from the plCSA-MNP group showed normal tissue architecture. Collectively, these results indicate that plCSA-NPs can facilitate delivery of MTX to the placenta in vivo with no detrimental effects to other tissues.
Discussion
No highly effective therapeutic agent is currently licensed to treat placental insufficiency and alleviate the resulting maternal and fetal complications. The risk of serious side effects in the fetus or mother is a major barrier to the development of medications in obstetrics. Inspired by the new insight that P. falciparum-infected erythrocytes bind to a distinct chondroitin sulfate (CSA) exclusively expressed in the intervillous spaces of human placentas through VAR2CSA protein 13, 14, here, we describe a novel nanoparticle drug delivery system that specifically targets trophoblasts and may be a highly effective and safe therapeutic approach to treat placenta-mediated pregnancy complications.
In this communication, we demonstrate that plCSA-BP, derived from VAR2CSA, specifically binds to human placental syncytiotrophoblasts and mouse trophoblasts throughout gestation. Furthermore, we show that nanoparticles conjugated to plCSA-BP efficiently bind to the murine placental labyrinth trophoblast cells in vivo and ex vivo, and nanoparticles decorated with plCSA-BP selectively deliver MTX to mouse placenta, producing highly specific effects not produced by untargeted MTX. Non-invasive and real-time high-frequency ultrasound imaging demonstrated that plCSA-MNPs significantly inhibited embryonic growth and increased fetal loss, unlike untargeted MTX, which had only minor effects on blood sinusoid volume. The effectiveness of plCSA-MNPs targeted to placental labyrinthine trophoblasts was evident by the marked increase in numbers of apoptotic trophoblast cells and the decreased blood sinusoid volume in the placental labyrinthine region. More importantly, plCSA-MNPs had no adverse effects on maternal tissues and did not detectably cross the placenta from mother to fetus, did not accumulate in fetal tissues, and did not cause any apparent fetal toxicity. Together, these findings demonstrate the utility of plCSA-BP-decorated nanoparticles as a novel carrier system that can specifically accomplish the targeted delivery of drugs to the placenta.
The concepts of nanomedicine have been applied clinically for more than two decades in the fields of oncology and infectious diseases 29-31, but their application to pregnancy disorders is in its infancy. Fortunately, from an obstetrical perspective, the placental transfer of nanoparticles has been evaluated using a number of experimental models. Muothet al. 32 reviewed past reports and showed that particle size, surface modifications and shape determine whether nanoparticles can cross the placenta barrier. These small and potentially toxic nanomaterials include quantum dots, polymeric nanoparticles, and those based on silica, gold, polystyrene, titanium, iron oxide, and silver, which readily cross the placental barrier and are detected within fetal circulation. Liposomes, phospholipid-based nanoparticles 7, are biodegradable and non-toxic and have been approved by the United States Food and Drug Administration. They are currently being used in clinical settings to enhance drug delivery to tumors and reduce the adverse effects of various drugs 30, 33, 34. Recently, liposomes as a nanoparticle vehicle were investigated in pregnancy and were revealed to lack transplacental uptake; they did not cross the placental barrier to the fetus in rodent and ex vivo models 8, 10, 35-37. However, despite the placenta's unrestricted exposure to drugs in the maternal blood stream, only a relatively small fraction of maternally administered non-targeted nanoparticles have been found to be taken up by the placenta.
In recent years, several research reports have focused on targeting nanoparticles to uteroplacental tissues for the therapy of pregnancy-related complications. In the first of these, Kaitu′u-Lino et al. developed a nanocell for the targeted delivery of doxorubicin into placental tissues to treat ectopic pregnancies and trophoblastic tumors via surface decoration with antibodies to target the epidermal growth factor receptor (EGFR). In normal tissues, EGFR levels are low, with the exception of placental trophoblasts, in which the levels are very high 38. In another novel study, King et al. showed that nanocarriers coated with the tumor-homing peptide CGKRK and iRGD efficiently deliver payloads to the mouse placenta and that these peptides bind selectively to the endothelium of established uterine spiral arteries and trophoblasts lining remodeled segments 39. Additionally, the placenta-homing peptide CCGKRK-micro RNA inhibitor conjugates, namely, peptide nucleic acid (PNA) conjugates, have been successfully exploited for the targeted delivery of micro RNAs to the placenta 40. Cureton et al. used the same phage screening approach that identified plCSA-BP to identify other novel placenta-targeted peptides that selectively bind to the endothelium of the uterine spiral arteries and the placental labyrinth in vivo 41. When the vasculature-targeting peptides were conjugated to liposomes, these liposomes selectively delivered payloads to the uteroplacental vasculature. Furthermore, as the levels of oxytocin receptor (OTR) are high in the pregnant uterus 42 and low on various tissues, including the brain and mammary tissue 43, liposomes conjugated to antibodies to the OTR have been created for the targeted delivery of payloads to the pregnant uterus 44, 45. Targeted nanomedicines represent a new frontier in obstetrics, and the clinical need for new approaches to deliver drugs in pregnancy is substantial 46. The plCSA-BP-guided nano-carrier described in this study is complementary to the current uteroplacental targeting delivery methods, as it targets the placental trophoblast throughout gestation, which is lacking in current applications.
VAR2CSA-expressing parasites only adhere to the placenta; they do not bind to CSA expressed elsewhere in the body 14, 47, 48. Placental CSA (plCSA) may therefore be a distinct CS subtype expressed exclusively in the placenta. This notion was supported by our experiments, in which we demonstrated that synthetic plCSA-BP specifically and efficiently binds to a distinct plCSA expressed exclusively in the placental trophoblast and not in other tissues in vitro and in vivo. The excellent characteristic of plCSA-BP selectively targeted to the placenta minimized the risk of off-target side effects of payloads. Minimizing fetal exposure and other maternal tissue/organ exposure to a drug is an important aspect of developing safer drug for use in pregnancy. The plCSA-BP-coated nanoparticles will make it possible to deliver some drugs that could improve placental vascular function but have side effects for the mother, for example, sildenafil citratein treatment of fetal growth restriction 49, 50. In the current study, we have shown that plCSA-BP-conjugated MNPs did not cross the placenta to accumulate in the fetus. More importantly, non-targeted lipid-polymer nanoparticles did cross the placental barrier. The nanoparticles changing from crossing the placenta to not crossing the placenta indicate that plCSA-BP efficiently binds to the placental tissue, minimizing the transport of associated nanoparticles across the placental barrier. Additionally, plCSA-NPs loaded with MTX gave no evidence of adverse effects on maternal tissues.
For targeted nanoparticles to be adopted and accepted in the clinic, further research and development will be required, including optimization of nanoparticles formulations, candidate payloads, drug encapsulation protocols, and dosing regimens to maximize drug delivery. In addition, toxicity testing in appropriate animal models and a comparison of how effectively they treat pregnancy complications against current therapies are necessary.
In summary, our technology facilitates the targeted delivery of drugs to the placenta and provides a novel platform for the development of placenta-specific therapeutics. We believe that the plCSA-BP is a highly effective tool that can be used to deliver large amounts of therapeutic drugs specifically to the placenta.
Acknowledgments
The authors would like to thank Fei Yan at the Paul C. Lauterbur Research Center for Biomedical Imaging of SIAT for the provision of ultrasound machines. We also thank Matt Petitt, PhD, for his comments and editorial assistance in preparation of the manuscript. This work was supported by grants from the National Key Research and Development Program of China (2016YFC1000402), the National Natural Sciences Foundation (81571445 and 81771617) and Natural Science Foundation of Guangdong Province (2016A030313178) to X.F.; Shenzhen Basic Research Fund (JCYJ20150521094519488) to X.F and (JCYJ20170413165233512) to J.Z. and X.F.; Public Welfare Project of Guangdong Province (2017A020211033) to J.Z, and US National Institute of Child Health and Human Development (HD088549-01) to N.N.; National Natural Science Foundation of China (31571013 and 81501580), Key International S&T Cooperation Project (2015DFH50230) to L.C.
Abbreviations
- AC
abdominal circumference
- BPD
biparietal diameter
- CK8
cytokeratin 8
- cp
placental chorionic plate
- CRL
crown-rump-length
- dec
decidua
- EE
drug encapsulation efficiency
- Em
embryo
- EPR
enhanced permeability and retention
- GSL
gestational sac length
- HFUS
high-frequency ultrasound
- HPLC
high-performance liquid chromatography
- HR
heart rate
- ICG
indocyanine green
- IEs
infected erythrocytes
- jz
junctional zone
- lab
labyrinth
- LE
drug loading efficiency
- MTX
methotrexate
- PD
placental diameter
- plCSA
placental-type chondroitin sulfate A
- PT
placental thickness
- spa
spiral arteries
- TEM
transmission electron microscopy
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick-end labeling
- UA
umbilical artery.
Supplementary Material
Movie S1.
Movie S2.
Movie S3.
Supplementary Figures.
Author contributions
X.F., B.Z., L.T., B.A., N.N., J.Z., and L.C. conceived the study. B.Z., X.F. and L.T. designed research studies. B.Z., L.T., Y.Y., B.W., J.H., J.C., Z.C., and M.L. conducted experiments. B.Z., X.F., L.T., T.X., and Q.Y. analyzed data. B.Z., B.A., N.N. and XF wrote the manuscript. All authors reviewed and proofed the manuscript.
References
- 1.Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. Jama. 2011;306:2459–68. doi: 10.1001/jama.2011.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg CJ, Atrash HK, Koonin LM, Tucker M. Pregnancy-related mortality in the United States, 1987-1990. Obstet Gynecol. 1996;88:161–7. doi: 10.1016/0029-7844(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 3.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D. et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisk NM, Atun R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008;5:e22. doi: 10.1371/journal.pmed.0050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisk NM, McKee M, Atun R. Relative and absolute addressability of global disease burden in maternal and perinatal health by investment in R&D. Trop Med Int Health. 2011;16:662–8. doi: 10.1111/j.1365-3156.2011.02778.x. [DOI] [PubMed] [Google Scholar]
- 6.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 7.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine—challenge and perspectives. Angew Chem Int Ed Engl. 2009;48:872–97. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajoria R, Sooranna S, Chatterjee R. Effect of lipid composition of cationic SUV liposomes on materno-fetal transfer of warfarin across the perfused human term placenta. Placenta. 2013;34:1216–22. doi: 10.1016/j.placenta.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Refuerzo JS, Godin B, Bishop K, Srinivasan S, Shah SK, Amra S. et al. Size of the nanovectors determines the transplacental passage in pregnancy: study in rats. Am J Obstet Gynecol. 2011;204:546.. doi: 10.1016/j.ajog.2011.02.033. e5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Refuerzo JS, Alexander JF, Leonard F, Leon M, Longo M, Godin B. Liposomes: a nanoscale drug carrying system to prevent indomethacin passage to the fetus in a pregnant mouse model. Am J Obstet Gynecol. 2015;212:508.. doi: 10.1016/j.ajog.2015.02.006. e1-7. [DOI] [PubMed] [Google Scholar]
- 11.Zheng M, Yue C, Ma Y, Gong P, Zhao P, Zheng C. et al. Single-step assembly of DOX/ICG loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS nano. 2013;7:2056–67. doi: 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- 12.Zhao P, Zheng M, Yue C, Luo Z, Gong P, Gao G. et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials. 2014;35:6037–46. doi: 10.1016/j.biomaterials.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa M, Arnot DE. et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–91. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 14.Salanti A, Dahlbäck M, Turner L, Nielsen MA, Barfod L, Magistrado P. et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clausen TM, Christoffersen S, Dahlbäck M, Langkilde AE, Jensen KE, Resende M. et al. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem. 2012;287:23332–45. doi: 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlbäck M, Jørgensen LM, Nielsen MA, Clausen TM, Ditlev SB, Resende M. et al. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem. 2011;286:15908–17. doi: 10.1074/jbc.M110.191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resende M, Nielsen MA, Dahlbäck M, Ditlev SB, Andersen P, Sander AF. et al. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar J. 2008;7:104. doi: 10.1186/1475-2875-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farley JH, Heathcock RB, Branch W, Larsen W, Homas D. Treatment of metastatic gestational choriocarcinoma with oral methotrexate in a combat environment. Obstet Gynecol. 2005;105:1250–4. doi: 10.1097/01.AOG.0000157761.18295.43. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JY, Chen L, Gumer AR, Tergas AI, Hou JY, Burke WM. et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49.e1–e10. doi: 10.1016/j.ajog.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamura K, Kawamura N, Kumazawa Y, Kumagai J, Fujimoto T, Tanaka T. Brain-derived neurotrophic factor/tyrosine kinase B signaling regulates human trophoblast growth in an in vivo animal model of ectopic pregnancy. Endocrinology. 2011;152:1090–100. doi: 10.1210/en.2010-1124. [DOI] [PubMed] [Google Scholar]
- 21.Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M. et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest. 2014;124:4941–52. doi: 10.1172/JCI76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML. et al. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008;22:3571–80. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard SE, Min J-Y, Merchan J, Lim K-H, Li J, Mondal S. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao K, Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int J Pharm. 2006;310:213–9. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 25.Campoli M, Ferrone S, Wang X. Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv Cancer Res. 2010;109:73–121. doi: 10.1016/B978-0-12-380890-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 26.Akirav C, Lu Y, Mu J, Qu D, Zhou Y, Slevin J. et al. Ultrasonic detection and developmental changes in calcification of the placenta during normal pregnancy in mice. Placenta. 2005;26:129–37. doi: 10.1016/j.placenta.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Lane LA, Qian X, Smith AM, Nie S. Physical chemistry of nanomedicine: understanding the complex behaviors of nanoparticles in vivo. Annu Rev Phys Chem. 2015;66:521–47. doi: 10.1146/annurev-physchem-040513-103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Aa EM, Peereboom-Stegeman JH, Noordhoek J, Gribnau FW, Russel FG. Mechanisms of drug transfer across the human placenta. Pharm World Sci. 1998;20:139–48. doi: 10.1023/a:1008656928861. [DOI] [PubMed] [Google Scholar]
- 29.Ragelle H, Danhier F, Préat V, Langer R, Anderson DG. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin Drug Deliv. 2017;14:851–64. doi: 10.1080/17425247.2016.1244187. [DOI] [PubMed] [Google Scholar]
- 30.Barenholz YC. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Takemoto K, Kanazawa K. AmBisome: relationship between the pharmacokinetic characteristics acquired by liposomal formulation and safety/efficacy. J Liposome Res. 2017;27:186–94. doi: 10.1080/08982104.2016.1205087. [DOI] [PubMed] [Google Scholar]
- 32.Muoth C, Aengenheister L, Kucki M, Wick P, Buerki-Thurnherr T. Nanoparticle transport across the placental barrier: pushing the field forward! Nanomedicine (Lond) 2016;11:941–57. doi: 10.2217/nnm-2015-0012. [DOI] [PubMed] [Google Scholar]
- 33.Lonner JH, Scuderi GR, Lieberman JR. Potential utility of liposome bupivacaine in orthopedic surgery. Am J Orthop (Belle Mead NJ) 2015;44:111–7. [PubMed] [Google Scholar]
- 34.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 35.Bajoria R, Contractor SF. Effect of surface charge of small unilamellar liposomes on uptake and transfer of carboxyfluorescein across the perfused human term placenta. Pediatr Res. 1997;42:520–7. doi: 10.1203/00006450-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Bajoria R, Contractor SF. Effect of the size of liposomes on the transfer and uptake of carboxyfluorescein by the perfused human term placenta. J Pharm Pharmacol. 1997;49:675–81. doi: 10.1111/j.2042-7158.1997.tb06091.x. [DOI] [PubMed] [Google Scholar]
- 37.Barzago MM, Bortolotti A, Stellari FF, Diomede L, Algeri M, Efrati S. et al. Placental transfer of valproic acid after liposome encapsulation during in vitro human placenta perfusion. J Pharmacol Exp Ther. 1996;277:79–86. [PubMed] [Google Scholar]
- 38.Kaitu'u-Lino TuJ, Pattison S, Ye L, Tuohey L, Sluka P, MacDiarmid J. et al. Targeted nanoparticle delivery of doxorubicin into placental tissues to treat ectopic pregnancies. Endocrinology. 2013;154:911–9. doi: 10.1210/en.2012-1832. [DOI] [PubMed] [Google Scholar]
- 39.King A, Ndifon C, Lui S, Widdows K, Kotamraju VR, Agemy L. et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci Adv. 2016;2:e1600349. doi: 10.1126/sciadv.1600349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beards F, Jones LE, Charnock J, Forbes K, Harris LK. Placental Homing Peptide-microRNA Inhibitor Conjugates for Targeted Enhancement of Intrinsic Placental Growth Signaling. Theranostics. 2017;7:2940–55. doi: 10.7150/thno.18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cureton N, Korotkova I, Baker B, Greenwood S, Wareing M, Kotamraju VR. et al. Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy. Theranostics. 2017;7:3715–31. doi: 10.7150/thno.19678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wathes DC, Borwick SC, Timmons PM, Leung ST, Thornton S. Oxytocin receptor expression in human term and preterm gestational tissues prior to and following the onset of labour. J Endocrinol. 1999;161:143–51. doi: 10.1677/joe.0.1610143. [DOI] [PubMed] [Google Scholar]
- 43.Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG. et al. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022–8. doi: 10.1210/endo.136.9.7649111. [DOI] [PubMed] [Google Scholar]
- 44.Paul JW, Hua S, Ilicic M, Tolosa JM, Butler T, Robertson S. et al. Drug delivery to the human and mouse uterus using immunoliposomes targeted to the oxytocin receptor. Am J Obstet Gynecol. 2017;216:283.e1–e14. doi: 10.1016/j.ajog.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Refuerzo JS, Leonard F, Bulayeva N, Gorenstein D, Chiossi G, Ontiveros A. et al. Uterus-targeted liposomes for preterm labor management: studies in pregnant mice. Sci Rep. 2016;6:34710. doi: 10.1038/srep34710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keelan JA, Leong JW, Ho D, Iyer KS. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine (Lond) 2015;10:2229–47. doi: 10.2217/nnm.15.48. [DOI] [PubMed] [Google Scholar]
- 47.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 48.Salanti A, Clausen TM, Agerbæk MØ, Al Nakouzi N, Dahlbäck M, Oo HZ. et al. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer cell. 2015;28:500–14. doi: 10.1016/j.ccell.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oyston C, Stanley JL, Oliver MH, Bloomfield FH, Baker PN. Maternal Administration of Sildenafil Citrate Alters Fetal and Placental Growth and Fetal-Placental Vascular Resistance in the Growth-Restricted Ovine Fetus. Hypertension. 2016;68:760–7. doi: 10.1161/HYPERTENSIONAHA.116.07662. [DOI] [PubMed] [Google Scholar]
- 50.von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B. et al. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG. 2011;118:624–8. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1.
Movie S2.
Movie S3.
Supplementary Figures.