Abstract
Spontaneous coronary artery dissection (SCAD) has emerged as an important cause of acute coronary syndrome, myocardial infarction, and sudden death, particularly among young women and individuals with few conventional atherosclerotic risk factors. Patient-initiated research has spurred increased awareness of SCAD, and improved diagnostic capabilities and findings from large case series have led to changes in approaches to initial and long-term management and increasing evidence that SCAD not only is more common than previously believed but also must be evaluated and treated differently from atherosclerotic myocardial infarction. High rates of recurrent SCAD; its association with female sex, pregnancy, and physical and emotional stress triggers; and concurrent systemic arteriopathies, particularly fibromuscular dysplasia, highlight the differences in clinical characteristics of SCAD compared with atherosclerotic disease. Recent insights into the causes of, clinical course of, treatment options for, outcomes of, and associated conditions of SCAD and the many persistent knowledge gaps are presented.
Keywords: AHA Scientific Statements, coronary artery dissection, spontaneous, fibromuscular dysplasia, myocardial infarction, women
Spontaneous coronary artery dissection (SCAD) is defined as an epicardial coronary artery dissection that is not associated with atherosclerosis or trauma and not iatrogenic. The predominant mechanism of myocardial injury occurring as a result of SCAD is coronary artery obstruction caused by formation of an intramural hematoma (IMH) or intimal disruption rather than atherosclerotic plaque rupture or intraluminal thrombus. Since the first description of SCAD by Pretty1 in 1931 at autopsy, our understanding of it has evolved tremendously during the past 8 decades, especially in the past 5 years. On the basis of isolated case reports and small series, SCAD was initially described as a rare and almost universally fatal cause of acute coronary syndrome (ACS), myocardial infarction (MI), and sudden cardiac death in peripartum women,2–4 but contemporary reports have refuted these misconceptions. Indeed, advances in our understanding of the epidemiology of SCAD, the availability of intravascular imaging techniques,5 the development of SCAD-specific angiographic classification,6 heightened awareness among providers, and parallel efforts by patients7 using social media to broadly disseminate information suggest that SCAD is far more common than previously thought, especially in young women. In addition, SCAD has unique risk factors and associated conditions and different diagnostic, therapeutic, and prognostic implications compared with atherosclerotic coronary disease.8–17
Despite these advances, dissemination of new knowledge has been slow, and SCAD continues to be misdiagnosed, underdiagnosed, and managed as atherosclerotic ACS, which may harm patients with SCAD. An emphasis on accurate diagnosis is key to not only providing early supportive care but also ensuring that an invasive strategy of percutaneous coronary intervention (PCI) be reserved for a select group of these patients because PCI for SCAD has been associated with lower technical success and higher complications than PCI for atherosclerotic disease.13,18–20 Increasing awareness of SCAD may overcome the limits of existing literature, which often excludes sex-specific information, a gap that may have clouded the understanding of this underrecognized condition.16,18,20–28
The authors of this statement hope to spur a change in the paradigm of care for women and men with SCAD. This American Heart Association scientific statement provides an overview of current evidence and expert consensus with the aims of improving the understanding and management of SCAD in the medical community and of enhancing future collaborations and efforts to elucidate this still relatively poorly understood disease.
EPIDEMIOLOGY
The true prevalence of SCAD remains uncertain, primarily because it is an underdiagnosed condition. Missed diagnoses are driven by a low suspicion of ACS in young women even in the presence of classic presenting symptoms, limitations of current coronary angiographic techniques, and lack of clinician familiarity with the condition. SCAD most commonly occurs in patients with few or no traditional cardiovascular risk factors.8–10,13,16,29 Recent series using careful diagnostic criteria that exclude iatrogenic, traumatic, and atherosclerotic dissection6 suggest that SCAD may be a cause of up to 1% to 4% of ACS cases overall8,30–32 (Table 1), occurs overwhelmingly in women,8,11,13,30,31,33 may be the cause of ACS in up to 35% of MIs in women ≤50 years of age,11,16,32 and is the most common cause of pregnancy-associated MI (43%)17 (Table 2). Pregnancy-associated SCAD appears to make up a smaller proportion of overall SCAD than early case series suggest,13,19,29,33,35 likely as a result of better detection of less severe SCAD among women who were not recently pregnant.
Table 1.
Angiographic Prevalence of SCAD in ACS Cohorts
| Reference | Year | Patients With SCAD, n | SCAD Prevalence as a Proportion of All ACS Cases, % | Women Among SCAD Cases, % | PA-SCAD, % | SCAD Prevalence in Subgroups With ACS | Methods, Population, Inclusion Criteria |
|---|---|---|---|---|---|---|---|
| Vanzetto et al31 | 2009 | 23 | 0.2 (0.6 women, 0.07 men) | 74 | 0 | 8.7% SCAD among ACS in women ≤50 y | Systematic retrospective review of 11 605
angiograms Included type 1 SCAD only Atherosclerosis-related coronary dissection not excluded |
| Mortensen et al30 | 2009 | 22 | 2.0 | 77 | 12 | NR | Retrospective search for coded diagnoses in database of 32 969 angiograms; reviewed only those with prior SCAD diagnosis |
| Alfonso and Bastante14 | 2014 | 27 | 0.16 | 85 | 3.7 | NR | Retrospective search for coded diagnoses among 16 813 first angiograms (2004–2010) |
| Saw et al15 | 2014 | 16 | NR | 100 | NR | 24.2% SCAD among ACS in women ≤50 y | Retrospective review of 177 angiograms in women ≤50 y representing 9% of angiograms (n=7605) performed during the study period (2009–2011) |
| Rashid et al16 | 2016 | 21 | 1.7 | 95.2 | NR | 22.5% SCAD among ACS in women ≤60 y | Retrospective search for coded diagnoses among 1332 angiograms (2012–2013) |
| Nakashima et al11 | 2016 | 63 | 0.31 | 94 | 8.1 | 35% SCAD among ACS in women ≤50 y | Retrospective review of 20 195 angiograms
(2000–2013) Excluded atherosclerosis-related coronary dissection Included type 2 SCAD Separate analysis for women ≤50 y with ACS (n=45) |
| Nishiguchi et al32 | 2016 | 13 | 4 | 53.8 | NR | NR | 326 Selected ACS patients undergoing OCT
(2008–2012) Atherosclerosis-related coronary dissection not excluded |
ACS indicates acute coronary syndrome; NR, not reported; OCT, optical coherence tomography; PA-SCAD, pregnancy-associated spontaneous coronary artery dissection; and SCAD, spontaneous coronary artery dissection.
Table 2.
Conditions and Factors Associated With SCAD
| Associated Condition or Factor | Reported Prevalence in Cohort Studies, % |
|---|---|
| Fibromuscular dysplasia | 25–8613,29,33,34 |
| Pregnancy | 2–88,9,13,33 |
| Multiparity (≥4 births) | 8.9–1013,33 |
| Inherited arteriopathy and connective tissue disorder (see Table 4) | 1.2–3.08,13 |
| Marfan syndrome, Loeys-Dietz syndrome, vascular Ehlers-Danlos syndrome, α1- antitrypsin deficiency, polycystic kidney disease | |
| Exogenous hormones | 10.7–12.68,13 |
| Oral contraceptives, postmenopausal therapy, infertility treatments, testosterone, corticosteroids | |
| Systemic inflammatory disease | <1–8.99,13 |
| Systemic lupus erythematosus, Crohn disease, ulcerative colitis, polyarteritis nodosa, sarcoidosis, Churg-Strauss syndrome, Wegener granulomatosis, rheumatoid arthritis, Kawasaki disease, celiac disease | |
| Migraine headache | NR |
| Coronary artery spasm | NR |
| Precipitating factors | >50% Patients recall a precipitating factor13 |
| Intense exercise (isometric or aerobic) | |
| Intense Valsalva | |
| Retching, vomiting, bowel movement, coughing, lifting heavy objects | |
| Intense emotional stress | |
| Labor and delivery | |
| Recreational drugs (cocaine, methamphetamines) | |
| Exogenous hormones/hormone modulators β-hCG injections, corticosteroid injections, clomiphene |
hCG indicates human chorionic gonadotropin; NR, not reported; and SCAD, spontaneous coronary artery dissection.
The average age of women with SCAD ranges from 45 to 53 years, but examples of patients presenting with SCAD in their second17 through eighth decades of life have been reported.6,8,11,13,30,31,33,36 One study reported that men presented with SCAD at a slightly younger age than women (mean age, 48.6 [SD, 9.8] years versus 52.3 [SD, 9.2] years; P=0.05).37,38 Although SCAD has been reported in all major racial and ethnic groups, the majority of patients are white, a finding that may reflect referral and sampling bias and the demography of patients cared for at reporting centers.8,13,15
The coronary distribution of SCAD has been well described. Although any artery can be affected, the left anterior descending artery is the most commonly affected (32%–46% of cases).8,10,13,16,19,33 In terms of territories, the left anterior descending and diagonal and septal branches are affected in 45% to 61% of cases; the circumflex and ramus and obtuse marginal branches in 15% to 45%; the right coronary artery and acute marginal, posterior descending, and posterolateral branches in 10% to 39%; and the left main artery in up to 4% of cases.10,11,13,16,19,33 In the majority of cases, the mid to distal segments of coronary arteries are affected; in only <10% of cases are the proximal left anterior descending or circumflex, right coronary, or left main arteries affected.13 Multivessel SCAD occurs in 9% to 23% of cases.8,10,11,13,16,19,33,35
HISTOPATHOLOGICAL CLASSIFICATION AND FINDINGS
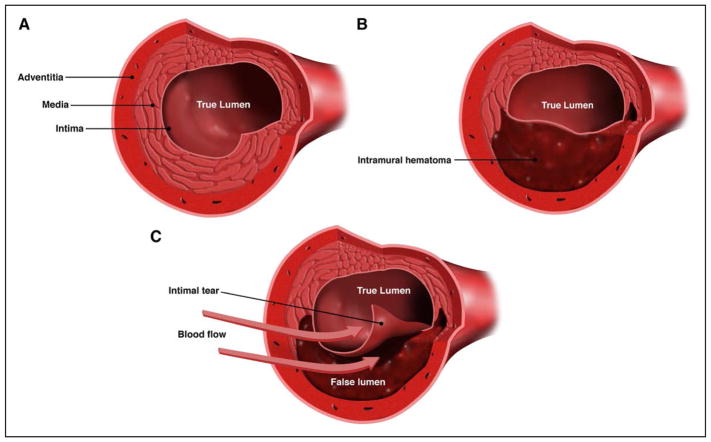
SCAD is characterized by the spontaneous formation of IMH within the wall of a coronary artery (Figure 1). This has been confirmed by both intracoronary imaging39–42 and histopathological case reports and series.43–55 More recent publications confirm historical reports3,56,57 that describe the separation occurring in the outer third of the tunica media and IMH occupying the dissection and compressing the true lumen, leading to coronary insufficiency and MI47,50 (Figure 2).
Figure 1. Cross-sectional views of the coronary artery.
A, Normal coronary artery. B, Coronary artery with intramural hematoma. C, Coronary artery with intimal tear. Spontaneous coronary artery dissection is characterized by the spontaneous formation of an intramural hematoma, which can lead to compression of the true lumen and myocardial infarction. An intimal tear may be present. Created by and used with permission from Dominic Doyle, MA.
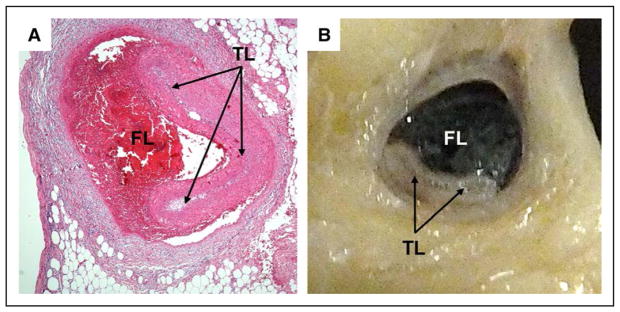
Figure 2. Pathological appearance of spontaneous coronary artery dissection.
Histological (A) and pathological (B) appearances show compression or obliteration of the true lumen (TL; arrows) by hematoma within the false lumen (FL). Used with permission from Mary N. Sheppard, Department of Cardiovascular Pathology, St. George Medical School, London, UK.
Two theories of how SCAD develops have been described.58 The first theory proposes that the primary pathological event is the development of a disruption in the vessel wall (intimal tear), which allows blood from the true lumen to enter and generate a false lumen. The second theory proposes that the primary event is a spontaneous hemorrhage arising from the vasa vasorum within the vessel wall.59 One group reporting an optical coherence tomography series from patients with SCAD noted that an intimal rupture site could not always be identified by this type of imaging, a finding supporting the second pathophysiological theory in at least a proportion of cases.42,60 For cases in which an intimal tear is identified, it remains unclear whether it is the initiating event or whether it is attributable to increasing pressure in the false lumen causing reverse intimal rupture into the true lumen or to the effect of coronary instrumentation during imaging.
There are several histopathological reports of a peri-adventitial mixed inflammatory infiltrate, often with a predominance of eosinophils.44–46,49,50,52,54,55,61 However, there is controversy about the pathophysiological significance of this eosinophilic infiltrate. Some authors consider it pathognomonic for SCAD,3,54,62 and others attribute it to a nonspecific response to vascular injury.50 The presence of an inflammatory cellular infiltrate surrounding the dissection may be useful in distinguishing SCAD from iatrogenic postmortem dissection, which can confound the diagnosis of true SCAD.50 Cystic medial necrosis was reported in a few older series,63–65 but it has either been reported to be absent44,49,53 or not commented on in reports since 2005. Fibromuscular dysplasia (FMD) involving coronary arteries in which SCAD occurred has been described in several histopathological case reports,53,66–68 an implication that coronary FMD was the cause of SCAD and in keeping with the recently observed strong association between SCAD and FMD.29,69
In addition, the postmortem diagnosis of SCAD may be challenging, and a strong suspicion coupled with careful coronary histopathological examination is required in cases of unexplained sudden cardiac death.47,50 When SCAD is suspected to be the cause, especially in young women, histopathological assessment of the coronary arteries should include distal segments of the epicardial arteries because of the predilection of SCAD for middistal coronary arteries.15
PATHOGENESIS OF SCAD AND SCAD-ASSOCIATED CONDITIONS
Because of the unique demographic and risk factor profile and the low prevalence of traditional cardiovascular risk factors of SCAD,6,8–10,13,16,29 its cause is hypothesized to be multifactorial with contributions from underlying arteriopathies, genetic factors, hormonal influences, inherited or acquired arteriopathies, or systemic inflammatory diseases, often compounded by environmental precipitants or stressors (Table 2).
FMD and Other Systemic Arteriopathies
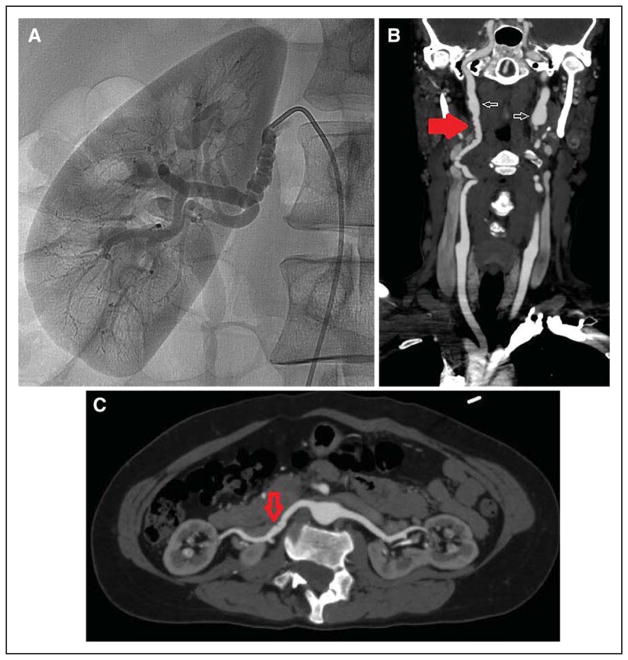
Of the described arteriopathies, the association of SCAD with multifocal FMD in extracoronary arteries has been the most commonly reported. FMD is a non-atherosclerotic, noninflammatory vascular disease that can affect nearly any arterial bed and can manifest as arterial stenosis, aneurysm, tortuosity, or dissection.70–72 Multifocal FMD is the most common type of FMD and is defined angiographically as areas of alternating stenosis and dilatation resulting in a string-of-beads pattern (Figure 3). Focal FMD appears angiographically as a single concentric or tubular narrowing; <10% of cases of FMD are of this type. FMD is not currently considered a connective tissue disorder, which for the purposes of this document refers to conditions in which arterial fragility is genetically mediated.
Figure 3. String-of-beads appearance of multifocal fibromuscular dysplasia (FMD).
Selective angiography of the right renal artery (A) and computed tomography angiography of the carotid (B) and renal (C) arteries. B and C are from the same patient. Findings of FMD in the right renal artery are more subtle (C, arrow) but suggest FMD given obvious beading elsewhere (B, red arrow). Also present in the bilateral internal carotid arteries is aneurysmal dilatation (aneurysm vs pseudoaneurysm; B, white arrows).
First described in 2005 and subsequently confirmed in several case series, the association of SCAD and FMD continues to be an area of considerable interest.69,73 Subsequent cohort studies have shown a varying prevalence of concomitant FMD in extracoronary vascular beds, ranging from 17% to 86%, depending on the patient population, number of imaged vascular beds, and type of imaging used for screening.6,8,13,29,33,34,74,75 There is also evidence that the prevalence of SCAD is not insignificant in patients with FMD. In the most recent report on dissection and aneurysm in patients with FMD in the US Registry for FMD, of the 237 of 921 patients (25.7%) who experienced ≥1 arterial dissections, 25 patients had SCAD, representing 10.5% of all dissections and an overall prevalence of coronary dissection of 2.7%.76 Case reports of typical histological and angiographic features of FMD in coronary arteries, including from patients with a prior SCAD event,53,58,66–68,77–81 have led some investigators to hypothesize that SCAD may be a manifestation of coronary FMD in at least a proportion of SCAD events.82 Although FMD is the most common extracoronary vascular abnormality identified among patients with SCAD, patients without imaging signs of FMD have been reported to have other arterial abnormalities, including dissections, aneurysms (including intracranial aneurysms in 14%–23%), or extracoronary and coronary arterial tortuosity (78%).6,8,13,29,33,74–76,83 Some of these patients may indeed have had FMD but it was not identified because of incomplete or insufficiently sensitive imaging.
Pregnancy and Female Sex Hormones
The term peripartum SCAD does not have a universal definition, and consistent with recent designations, we have elected to use the term pregnancy-associated SCAD.84 Although pregnancy-associated SCAD encompasses a relatively small proportion of SCAD cases,13,19,30,33,84–86 it is the most common cause of MI among patients who are pregnant or postpartum.17 A recent analysis of a US administrative database found a prevalence of 1.81 SCAD events per 100 000 pregnancies during pregnancy or in the 6-week postpartum period.87 The majority of pregnancy-associated SCAD events occur in the third trimester or early postpartum period, although SCAD has been reported as early as 5 weeks of gestation and up to several months to a year or more postpartum, particularly in women who are still lactating.35,85,86,88–90 The left main or left anterior descending artery has been described as the most commonly affected.17,35,85,86,89,91,92
The cause of pregnancy-associated SCAD is not fully understood. However, the hormonal changes of pregnancy may lead to alterations in the architecture of the arterial wall.17,84,91,93,94 One hypothesis suggests that the estrogen and progesterone receptors present in the coronary arteries may mediate changes, as they do in other connective tissues, that weaken the vessel wall, which culminates in arterial wall rupture, IMH, and onset of clinical symptoms.17 Accumulation of these changes over several pregnancies may explain the reported heightened risk of SCAD in multiparous women in some12,35,86,88 but not all87 series. Demographic and comorbid conditions reported with pregnancy-associated SCAD include black race, chronic hypertension, lipid abnormalities, chronic depression, migraines,86,87 advanced maternal age and age at first childbirth, and treatment for infertility. Gestational hypertension and preeclampsia have variably been associated.86,87 The average age at the time of pregnancy-associated SCAD has ranged from 33 to 36 years, a finding highlighting the potential impact of older age at time of pregnancy on cardiovascular complications such as SCAD.85,86,88,89,95 Because maternal age and multiparity are associated, the independent contribution of these factors to SCAD risk is uncertain.
Women with pregnancy-associated SCAD seem to have a poorer prognosis than women with SCAD unrelated to pregnancy.85,86,88,89 In a series of 23 women with SCAD, 7 of whom had pregnancy-associated SCAD, postpartum patients had larger infarcts, more proximal artery dissections (86% versus 19%; P<.004), and lower mean left ventricular (LV) ejection fraction (34% versus 49%; P<0.01).85 In the Mayo Clinic SCAD Registry, 54 women with pregnancy-associated SCAD were compared with 269 women with non–pregnancy-associated SCAD. Patients with pregnancy-associated SCAD were younger, were more likely to present with ST-segment–elevation MI (50% versus 36%; P=0.013) and left main and multivessel dissections, had poorer LV function immediately and at follow-up, and were less likely to have concurrent FMD.86 In a recent literature review of 120 pregnancy-associated SCAD cases by Havakuk et al,96 73% of women were postpartum and 76% presented with ST-segment–elevation MI. Maternal complications were common: cardiogenic shock in 24%, ventricular fibrillation requiring defibrillation in 16%, mechanical support in 28%, and an in-hospital mortality rate of 4%. It is unclear why pregnancy-associated SCAD is associated with more aggressive and extensive dissections than non–pregnancy-associated SCAD and why the majority of pregnant women are spared from pregnancy-associated SCAD.84
Inflammatory Conditions
SCAD is not commonly associated with systemic inflammatory disorders. Laboratory evaluation for inflammatory or autoimmune conditions is generally low yield but may be considered in the post-MI setting, particularly in patients who have symptoms of or physical examination findings of autoimmune or rheumatologic disease. SCAD has been described in case reports of patients with various systemic inflammatory diseases, including systemic lupus erythematosus,97–102 inflammatory bowel disease,103 polyarteritis nodosa,104 sarcoidosis,52 celiac disease,105 and cryoglobulinemia in the setting of hepatitis C.106 In these series, underlying coronary vasculitis100 or systemic inflammation100 may have contributed to the risk of SCAD, and larger cohort studies have shown that at least a small proportion of patients have either underlying inflammatory diseases or short-term increases in inflammatory markers.13,60 Taken together, these series indicate that underlying inflammatory conditions may be predisposing factors in the development of SCAD in a small proportion of patients. However, this possibility is based primarily on case reports or series of a small number of patients with inflammatory diseases that do not control for the prevalence of these conditions in the population reported and may represent coincidental association, especially because there is no published pathological proof of causation.
Inheritance and Genetics
Inherited arteriopathies and connective tissue disorders are infrequently reported as the underlying cause for SCAD (≈≤5%).8,13,107 Vascular Ehlers-Danlos syndrome (caused by mutations in COL3A1107–109), Marfan syndrome (caused by mutation in FBN1107–110), Loeys-Dietz syndrome (caused by mutation in genes encoding members of transforming growth factor-β signaling cascade such as TGFBRI, TGFBR2,111 and SMAD3112), and other mutations have been implicated and are known to be associated with arterial fragility and dissection, resulting in SCAD. Spontaneous arterial dissection without preceding aneurysm formation, as occurs in SCAD, is characteristic of vascular Ehlers-Danlos syndrome. SCAD has also been described in polycystic kidney disease.113–115
Outside the context of these known genetic conditions, however, SCAD does not seem to be strongly familial. In the largest series to investigate family history (421 subjects), Goel et al116 documented a family history of SCAD in a very low proportion of patients (1.2%). In that study, both dominant and recessive modes of inheritance were suggested, but most SCAD cases seem to be sporadic, and no genetic risk loci have yet been definitively associated with SCAD. This lack of a consistent inherited genetic pathway appears similar to FMD, for which, although heritability has been documented,70,117–120 genetic testing has been largely unfruitful because of the absence of an identified monogenetic cause.121,122
Barriers to gene discovery in SCAD are related mostly to the rarity of the condition and its predominantly sporadic nature. Sample collection is underway at multiple centers, and collaborative efforts will likely be needed to obtain sufficient statistical power to discover gene and gene-by-environment associations with SCAD.
Precipitants and Triggers
There appears to be a complex interplay between a vulnerable patient (ie, one with underlying arteriopathy) and potential triggers6,8,13,29,38,123–128 (Table 2) that initiate a spontaneous arterial tear or IMH. However, this connection is not the case for all patients, highlighting the incomplete understanding of the pathophysiology of this disease process. Furthermore, although the frequency of patient-reported precipitants of SCAD is relatively high compared with that reported in a large multinational cohort of patients with ACS, there may be recall bias in the reporting of events around a life-threatening event such as SCAD in typically young and previously healthy individuals. Thus, the potential for overreporting is important to consider.129
The most commonly reported precipitants are extreme physical or emotional stress. Among 168 patients at a single center, an extreme emotional (40%) or physical (24%) stressor was reported before their SCAD event.13 Emotional stressors have more often been reported as precipitants of SCAD in women than in men,38 whereas physical stressors such as intense isometric exercise and weight lifting have more often been reported among men with SCAD.8,38 Stress cat-echolamine surge during these events has been postulated to lead to coronary artery shear stress that, at least in part, contributes to the pathophysiology of SCAD. Although this hypothesis has not been specifically tested in patients with SCAD, a similar mechanism was proposed in other stress-induced cardiovascular conditions such as stress-induced cardiomyopathy (takotsubo syndrome).129,130
Unlike hormonal triggers related to pregnancy, other potential hormone-mediated SCAD triggers such as the perimenopausal state, use of oral contraceptives, postmenopausal hormone therapy (HT), infertility treatments, and high-dose corticosteroid administration have less supportive data, although associations have been reported.6,29,125–127,131 The biggest challenge for confirming any of these correlations is that the baseline prevalence of these conditions is either common in the general population or unknown.
CLINICAL PRESENTATION
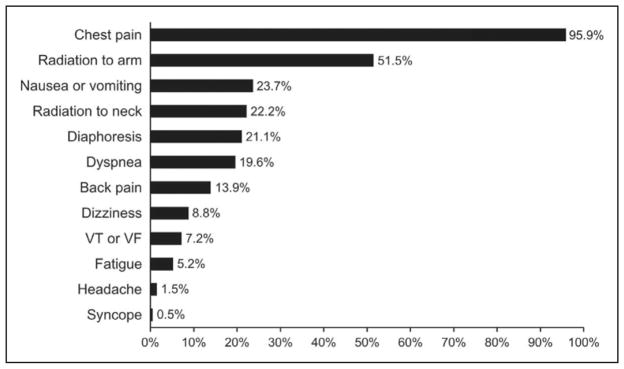
Although there are wide ranges of clinical presentations and severities of SCAD, patients who survive and present for initial evaluation almost universally experience ACS and increased levels of cardiac enzymes. As many as 2% to 5% of patients present in cardiogenic shock.10,11 Among available series of patients presenting for evaluation, 26% to 87% of patients with SCAD present with ST-segment–elevation MI, and 13% to 69% present with non–ST-segment–elevation MI.10,11,13,16,19,33 Presenting symptoms are consistent with atherosclerotic ACS, with chest pain being the most prevalent132 (Figure 4). Ventricular arrhythmias or sudden cardiac death account for SCAD presentation in 3% to 11% of reported series.8,10,11,13,19 These reports must be interpreted with care because individuals whose SCAD is not identified or is misdiagnosed, who do not survive to initial evaluation, or in whom coronary imaging or postmortem evaluation is not performed are not included in these studies.
Figure 4. Frequency of presenting symptoms of acute spontaneous coronary artery dissection.
VF indicates ventricular fibrillation; VT, ventricular tachycardia. Adapted from Luong et al132 with permission. Copyright © 2017, Wiley Periodicals, Inc.
Cardiac enzyme levels are almost invariably increased, although the initial troponin level in patients with SCAD presenting to the emergency department may be normal.19,133 In a cohort of 168 patients with SCAD from Vancouver, all patients had an increased troponin I level; the median increase was 6 μg/L (normal, <0.05 μg/L), and the interquartile range was broad (0.7–200 μg/L).13 In a Japanese cohort of 63 patients with SCAD, the mean peak creatine kinase level (normal, 25–250 IU/L) was lower in young women with SCAD than in patients without SCAD (1689 versus 2874 IU/L; P=0.025).11 LV wall motion abnormalities detected on echocardiography or early angiography were common, but overall LV ejection fraction was often preserved.19,134
DIAGNOSIS
Patients with SCAD are at risk of receiving alternative diagnoses and of being discharged after emergency department evaluation because their relatively young age and absence of atherosclerotic risk factors frequently do not fit the expected phenotype of an atherosclerotic patient with MI. Accurate diagnosis of SCAD in the early stages of ACS presentation is important because management and investigation are different from those for atherosclerotic forms of coronary artery disease.12,19 The suspicion for SCAD is typically instigated by clinical presenting features such as patient demographics, especially young age, female sex, and few or no conventional cardiovascular risk factors. Once SCAD is suspected, coronary angiography should be performed as early as feasible, especially in the setting of ST-segment–elevation MI. Despite the inherent limitations of conventional coronary angiography that reduce the diagnostic capability for SCAD, this technique remains the first-line diagnostic imaging method because it is widely available and recommended for early invasive management of ACS.135 The key limiting factor is that angiography is 2-dimensional “lumenography” and does not specifically image the arterial wall.
Dedicated intracoronary imaging methods, including intravascular ultrasonography and optical coherence tomography, provide detailed visualization of the arterial wall that aids the diagnosis of SCAD. However, these tools have additional risks and costs, and they are not readily available in all catheterization laboratories. Thus, availability, competence, and expertise can vary widely with these technologies. As a consequence, conventional coronary angiography remains instrumental in diagnosing SCAD, and cardiologists should become pro-ficient at recognizing its various angiographic patterns.
Coronary Angiography
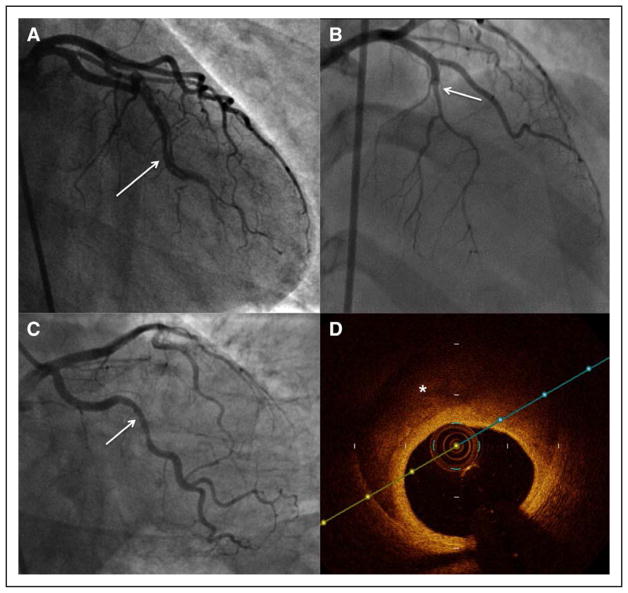
The traditional angiographic description of SCAD emphasized the presence of multiple radiolucent lumens and extraluminal contrast staining, which may have included spiral dissection or intraluminal filling defects.136 However, contemporary angiographic SCAD series have since shown that this “pathognomonic” appearance is present in only a small proportion of SCAD cases.6,41 Addressing the drawbacks of prior descriptions, a specific SCAD angiographic classification was proposed and now has been commonly adopted.6 In the Saw angiographic SCAD classification, type 1 refers to the classic appearance of multiple radiolucent lumens or arterial wall contrast staining (Figure 5A). Type 2 refers to the presence of diffuse stenosis that can be of varying severity and length (usually >20 mm; Figure 5B): Variant 2A is diffuse arterial narrowing bordered by normal segments proximal and distal to the IMH, and variant 2B is diffuse narrowing that extends to the distal tip of the artery.58 Type 3 is focal or tubular stenosis, usually <20 mm in length, that mimics atherosclerosis (Figure 5C); intracoronary imaging is required to confirm the presence of IMH and to diagnose SCAD (Figure 5D).
Figure 5. Angiographic features of spontaneous coronary artery dissection.
A, Type 1, multiple radiolucent lumens (arrow) or arterial wall contrast staining. B, Type 2, diffuse stenosis that can be of varying severity and length (dissection starting from arrow). C, Type 3: focal or tubular stenosis (arrow), usually <20 mm in length, that mimics atherosclerosis. Intracoronary imaging should be performed to confirm the presence of intramural hematoma or multiple lumens. D, Optical coherence tomography in type 3 (C) shows intramural hematoma (asterisk).
Several studies have reported that the diffuse smooth stenosis pattern (ie, type 2) is the most common angiographic manifestation of SCAD,11,13,16,41 occurring in up to 67.5% of dissected arteries, followed by type 1 in 29.1% and type 3 in 3.4%.13 Thus, reliance solely on the traditional multiple lumens or contrast staining of arterial walls to diagnose SCAD would result in missed diagnoses of >70% of SCAD cases. Therefore, familiarity with the diffuse narrowed-lumen appearance of IMH and use of intracoronary imaging are important steps to improve the diagnosis of SCAD. Care must be taken to ensure that SCAD is not misinterpreted as normal coronaries, atherosclerotic disease, coronary vasospasm, thromboembolism, or takotsubo syndrome.137,138
Although coronary angiography is the standard for the diagnosis of SCAD, this invasive procedure has intrinsic risk. Iatrogenic catheter-induced coronary artery dissection is reported to be increased in patients with SCAD; the reported overall prevalence is 3.4%,139,140 whereas it is <0.2% for standard coronary angiography.139,140 Underlying arterial fragility in patients with SCAD is believed to accentuate the risks for iatrogenic dissection.
Intracoronary Imaging
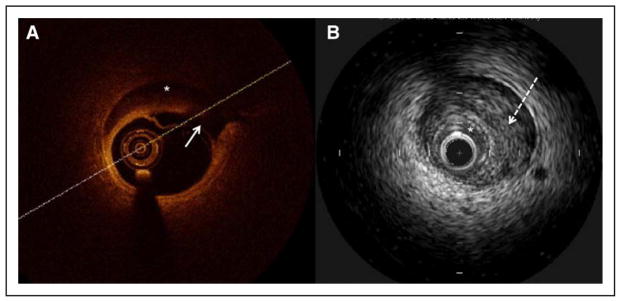
Intracoronary imaging has become instrumental to aid the diagnosis of SCAD in angiographically subtle or non-diagnostic cases. Fortunately, the need to use intracoronary imaging has diminished in recent years because of improved SCAD-pattern recognition on coronary angiography. Intravascular ultrasonography has been available for >20 years, and it offers good spatial resolution of ≈150 μm to image the coronary arterial wall. It can detect intimal tear, false lumen, IMH, and intraluminal thrombi, although its resolution may not be adequate to clearly identify all such abnormalities related to SCAD.42,141 It has the advantage of deep penetration to assess the depth and extent of IMH. Optical coherence tomography is a more advanced technology that uses light waves to image the arterial wall. This technology has revolutionized the diagnosis of SCAD since its first reported use in 2008.5,13,34,40,41,69 It has a spatial resolution of 10 to 20 μm, and thus, it is superior to intravascular ultrasonography for delineating the lumen-intimal interface and is better for visualizing intimal tears, false lumen, IMH, and intraluminal thrombi (Figure 6). This superior imaging improves the ease of detecting SCAD and is the preferred intracoronary imaging method when angiographic diagnosis is uncertain and when it is believed that intravascular imaging can be safely undertaken.12 Nevertheless, both optical coherence tomography and intravascular ultrasonography can provide complementary details for diagnosis of SCAD, which on intracoronary imaging requires the presence of IMH or double lumen.60 Because not all laboratories have both technologies accessible, angiographers should become familiar with interpreting features of SCAD with their available intracoronary imaging method.
Figure 6. Intracoronary imaging for spontaneous coronary artery dissection.
A, Optical coherence tomography shows intimal dissection (solid arrow) and intramural hematoma (asterisk). B, Intravascular ultrasonography shows intramural hematoma (dotted arrow) compressing the true lumen (asterisk).
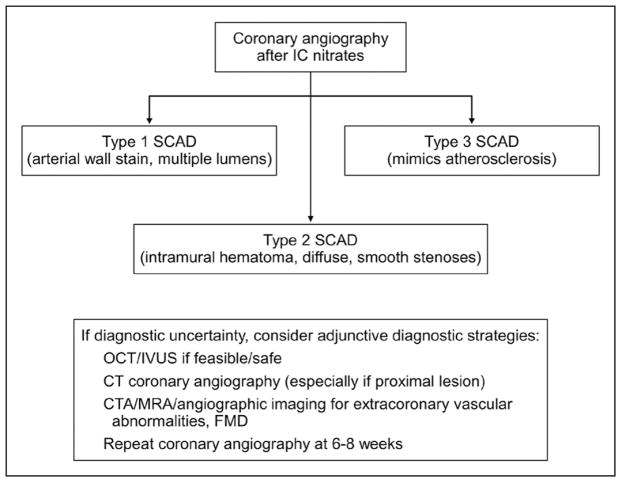
Despite the importance of intracoronary imaging for the diagnosis of SCAD, these tools have potential risks, including extending the coronary dissection with wire or imaging catheter, guide-catheter iatrogenic dissection,139 catheter-induced occlusion of true lumen, and hydraulic extension with contrast injection for optical coherence tomography. With weighing of the risks and benefits, intracoronary imaging should be pursued only when coronary angiographic diagnosis is uncertain (eg, type 3 or unclear lesions) and when the vessel diameter is large enough for intracoronary imaging. In addition, there is generally no clinical indication to evaluate the full depth and extent of IMH or false lumen because conservative management is typically preferred. Therefore, intracoronary imaging can be limited to the proximal segment of dissection without the need to image the entire dissected segment. An algorithm for the angiographic diagnosis of SCAD is presented in Figure 7.
Figure 7. Algorithm for diagnosis of spontaneous coronary artery dissection (SCAD) in the setting of acute coronary syndrome.
CT indicates computed tomography; CTA, computed tomography angiography; FMD, fibromuscular dysplasia; IC, intracoronary; IVUS, intravascular ultrasonography; MRA, magnetic resonance angiography; and OCT, optical coherence tomography.
Coronary Computed Tomography Angiography
Cardiac computed tomography angiography (CCTA) of the coronary arteries is emerging as an efficient tool for coronary assessment in low- and intermediate-risk patients presenting with ACS.142–146 Although the role of CCTA in patients with uncertain ACS diagnoses is promising, no dedicated study has addressed CCTA in the setting of acute SCAD. CCTA is generally contraindicated in patients presenting with high-risk ACS and is not recommended as the first-line investigation for suspected acute SCAD.143,147,148 Moreover, normal results on CCTA do not exclude SCAD. Coronary dissection seen on retrospective evaluation of initially negative CCTA images after SCAD was angiographically proved149 reinforces the need for CCTA or “triple rule-out” protocols that include CCTA to be interpreted with great caution when SCAD is in the differential diagnosis as a means to minimize false-negative results.147,148
Shortcomings of CCTA for SCAD include lower spatial and temporal resolution than coronary angiography,150 inclusion of patients with a low pretest probability for atherosclerotic coronary artery disease that thereby possibly affects the interpreters’ judgment, and a SCAD coronary appearance on CCTA that differs from that of typical atherosclerosis.149,151 Coronary atherosclerosis on CCTA commonly appears as discrete calcifications or focal dense, noncalcified plaques, whereas SCAD vessels may appear as having dual lumens on CCTA with contrast agent in the dissected plane. Most commonly, however, SCAD occurs without intimal disruption such that the contrast agent does not opacify the false lumen.58 Although in such cases the coronary artery narrowing and IMH are sometimes visualized on CCTA, these unique features can be challenging to recognize or are unidentifiable in small vessels.58,149,151 Poorly gated or nongated computed tomography angiography (such as computed tomography for pulmonary embolus) is inadequate for coronary artery assessment.53,149,152,153
CCTA may be useful for noninvasive follow-up of patients with SCAD, particularly in those with dissections in proximal or large-caliber coronary arteries.151,154,155 A study of 24 patients with SCAD who had follow-up CCTA found it to be a valuable, noninvasive study without complications and identified healing in most (83%).155 SCAD involvement of distal coronary arteries or side branches, or of vessel caliber <2.5 mm, generally is not well visualized on CCTA; therefore, this method is of limited value in many SCAD cases.6,13
INITIAL MANAGEMENT
The American College of Cardiology/American Heart Association and the European Society of Cardiology guidelines for the management of ACS advocate an early invasive strategy with revascularization of culprit lesions over conservative therapy alone.156,157 This stent-based lesion pacification reduces the risk of recurrent occlusion at the lesion site and associated adverse events in atherosclerotic MI, but there have been no randomized studies or subgroup analyses of treatment outcomes or comparisons between acute revascularization strategies for ACS caused by SCAD. These studies are critical because the mechanism of vessel obstruction, the acute vessel response to balloon dilation, and the natural history of conservatively managed lesions differ significantly in SCAD compared with atherosclerotic ACS.
Conservative Management
No comprehensive prospective studies have routinely performed angiographic restudy after SCAD, but observational data have indicated angiographic “healing” of SCAD lesions in the majority of patients (70%–97%) who were selectively restudied weeks to months after a conservatively managed index episode.6,8,16,19,33,41 A minority of patients had persistent dissection on angiography,8,13,19 and it is unclear why dissection persisted in these cases or whether very late healing may have occurred subsequently. The time course of healing remains uncertain, but it can be detected within days8,13,158 and is frequently observed by 1 month.8,13 In an observational study of 131 SCAD lesions that had repeat coronary angiography, spontaneous healing occurred in 88.5% of cases. For the cases in which repeat angiography was performed early (<35 days from index event), there were residual dissections. However, repeat angiography performed after 35 days showed angiographic healing in all cases.139 These results suggest a time dependency for healing to occur.
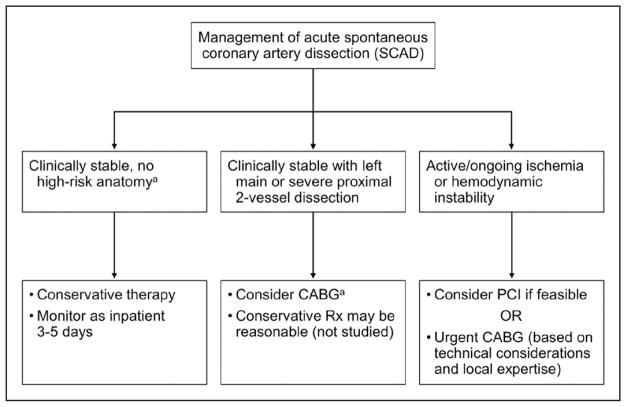
Of note, early complications of recurrent MI may develop in 5% to 10% of conservatively managed patients, mostly related to extension of dissection within the first 7 days after an acute episode.8,10,13 The majority of these patients require emergency revascularization, and no angiographic or clinical predictors of acute worsening have been identified. Because of these findings, inpatient monitoring for an extended period is typically recommended as part of a conservative strategy for SCAD management.12,19,159 Conservative therapy may not be appropriate in high-risk patients with ongoing ischemia, left main artery dissection, or hemodynamic instability. In such cases, it is the consensus of the working group that urgent intervention with PCI or coronary artery bypass grafting (CABG) should be considered, but such decisions should be individualized and contemplated in the context of the coronary anatomy and the expertise of the operators or centers. A proposed algorithm to guide conservative versus invasive management is outlined in Figure 8.
Figure 8. Algorithm for management of acute spontaneous coronary artery dissection.
CABG indicates coronary artery bypass grafting; PCI, percutaneous coronary intervention; and Rx, management. aLeft main or proximal 2-vessel coronary artery dissection.
Percutaneous Coronary Intervention
Observational studies have shown consistently that PCI for the treatment of SCAD is associated with an increased risk of complications8–11,13,16,19,33 and suboptimal outcomes. Affected coronary arteries may be inherently weak architecturally as a result of underlying arteriopathies, which can render them more susceptible to iatrogenic dissections and extension of dissections with PCI. Coronary guidewires may enter the false lumen and occlude the true lumen. Balloon dilation and stent placement can also confer risks of extending dissection of the intima or propagating IMH upstream and downstream from the vessel, causing worsening of vessel obstruction. Such extensions of dissection can be clinically significant, with some resulting in spiral dissections to the distal arterial segments or retrograde dissection into proximal arteries and adjacent branches, including the left main coronary artery. Given that dissection lengths are often extensive, complete PCI may require long coronary stents, which can increase the risk of subsequent instent restenosis and stent thrombosis. In addition, SCAD most frequently affects the distal coronary segments, which may be too small or too distal for stent implantation. Moreover, IMH naturally resorbs over time, which can result in subacute and late stent strut malapposition, potentially predisposing to future risk of stent thrombosis.158,159
These technical complications have been associated with adverse clinical outcomes in several series. The Mayo Clinic series of 189 patients showed PCI technical failure in 53% of the patients initially managed with PCI.19 Even when the percentage of residual stenosis was ignored as a parameter, technical failure occurred in 30%. Emergency CABG was required in 13% of those treated with primary PCI; emergency repeat PCI was required in 4%; and there was 1 death. Similar rates of adverse technical and clinical outcomes occurred whether there was impaired flow or normal or near-normal vessel flow at baseline.19 In the Vancouver cohort of 168 patients, technical failure occurred in 36%, stent thrombosis in 6%, and emergency CABG as a complication of PCI in 12%.13 Similarly, in a European study of 134 patients, 27% of PCIs resulted in technical failure, and 9% of patients required emergency CABG.10
Optimizing PCI Approaches
Improving the outcomes of PCI in SCAD requires meticulous angiographic and instrumentation techniques, including avoidance of deep catheter engagement, noncoaxial positioning of catheter tip, catheter dampening, and strong contrast agent injection. In addition, in potential SCAD cases, preferential use of femoral access or extra caution with radial catheterization should be considered in light of a retrospective series in which there was a 3-fold higher risk for catheter-induced iat-rogenic dissection in patients with SCAD undergoing coronary angiography when radial access was used as opposed to femoral access.13,139
Multiple interventional strategies have been described when PCI is pursued for SCAD lesions; however, no comparative studies have assessed the superiority of such techniques. Examples of approaches that have had successful outcomes include the following: (1) implantation of long drug-eluting stents covering in excess of 5 to 10 mm on both proximal and distal edges of the IMH so as to accommodate propagation of the IMH when compressed by the stent; (2) direct stenting without balloon predilation to avoid additional risks of extension of the IMH; (3) balloon angioplasty alone to restore coronary flow without stenting; (4) cutting balloon fenestration of the IMH to allow decompression of the false lumen blood pool into the true lumen160,161 with or without additional stenting (acknowledging the theoretical risk of coronary rupture, cutting balloon inflation should be performed cautiously with an undersized balloon); (5) multistent approach by sealing distal and proximal ends first with stents, before stenting the middle, to minimize IMH propagation162; and (6) use of bioresorbable stents to provide a temporary scaffold because complete resorption of struts occurs within 2 years.158,163 Finally, after successful PCI, dual-antiplatelet therapy should be administered according to the stents implanted.158,164,165
Coronary Artery Bypass Grafting
Published evidence on CABG after SCAD is limited to case reports, small case series, and retrospective observational studies with small sample size. CABG has been described as a treatment strategy for SCAD in patients with left main stem or proximal dissections, after technical failure of attempted PCI, when there is a complication of attempted PCI, and when ischemia is refractory despite attempted conservative therapy. Use of arterial and venous bypass conduits has been described, as have off-pump and robotic approaches.166–168 One study reported high rates of initial technical success and inhospital clinical outcomes with CABG as an index treatment strategy (n=20 patients, 32 conduits).19 Although these findings provide some reassurance for CABG as a viable revascularization option for acute SCAD, caution should be used in the overinterpretation of the data given the almost certain selection bias for patients deemed to have suitable anatomy. During long-term follow-up, 1 study reported a high rate of both venous and arterial conduit failure (11 of 16 graft failures from 11 of 20 patients undergoing follow-up angiography), almost certainly the result of subsequent healing of native SCAD vessels and competitive flow promoting graft occlusion. The study also showed that CABG was not protective against recurrent SCAD events.19 The use of vein grafts may be considered in light of frequent late graft failure and the opportunity to preserve arterial conduits for the future.
In summary, on the basis of the retrospective observational data cited above, conservative therapy is generally the preferred strategy in patients with SCAD who are clinically stable and without objective evidence of ongoing ischemia and has generally been associated with favorable outcomes.9,19 A conservative strategy is additionally appropriate in patients with occluded distal vessels or distal branches that would not routinely be amenable to PCI.
EARLY SCAD OUTCOMES: IMPLICATIONS FOR DURATION OF HOSPITALIZATION
Although rates of in-hospital mortality are low, up to 14% of patients require urgent in-hospital revascularization, usually because of extension of dissection. Recognized complications of SCAD depend on the initial treatment strategy (Table 3). Acknowledging the biases associated with selected retrospective series, among reports including >100 patients,10,13,19 49.7% to 89.7% received medical therapy as initial treatment, and 2.6% to 8.5% of conservatively treated patients eventually required revascularization during the index hospitalization. Revascularization with PCI was the initial treatment for SCAD in 16.7% to 47.1% of patients. Reported PCI success rates varied (36.4%–72.5%), most likely because of the wide variability in the definition of PCI success in SCAD, but all were substantially lower than success rates reported in control subjects with atherosclerotic ACS. Emergency CABG was required in 2.2% to 7.4% of patients who initially received medical therapy or PCI. An initial CABG revascularization approach was used in 0.6% to 3.7%, and initial success (87.5%–100%) was high in this small group of patients.
Table 3.
Acute Revascularization Outcomes in SCAD
| Reference | Year | Patients, n | Initial Medical Therapy, n (%) | Crossover to Revascularization, n (%) | PCI/CABG as Initial Therapy, n (%) | All In-Hospital Revascularizations, n (%) | All PCI Attempts, n. (% success)* | All CABG Procedures, n (% success) | In-Hospital MI, n (%) | In-Hospital Urgent Revascularizations, n (%) | In-Hospital Mortality, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unal et al169 | 2008 | 6 | 0 | NA | 1/5 (100) | 6 (100) | 1 (0) | 6 (83.3) | NR | 1 (16.7) | 1 (16.7)† |
| Vanzetto et al31 | 2009 | 23 | 10 (43.4) | 0 | 11/2 (56.5) | 13 (56.5) | 11 (72.7) | 5 (100) | 0 | 3 CABG (13) | 1 (4.3)‡ |
| Mortensen et al30 | 2009 | 22 | 7 (31.8) | NR | 13/2 (68.2) | NR | 13 (92.3) | 2 (NR) | NR | NR | 0 |
| Motreff et al170 | 2010 | 12 | 5 (41.7) | 3 (60) | 7/0 (58.3) | 10 (83.3) | 10 (NR) | NR | NR | NR | 1 (8.3)§ |
| Ito et al85 | 2011 | 23 | 18 (78.3) | 4 (22) | 3/3 (26.1) | 9 (39.1) | 5 (60) | 6 (NR) | 2 (8.7) | 4 (17.4) | 0 |
| Alfonso et al9 | 2012 | 45|| | 36 (80) | 7 (19.4) | 8/1 (20) | 16 (35.6) | 16 (81.3) | 2 (50)¶ | 1 (2.22)¶ | 7 (15.6) | 1 (2.22)¶ |
| Saw et al13 | 2014 | 168 | 139 (82.7) | 6 (4.3) | 28/1 (17.3) | 35 (20.8) | 33 (36.4) | 5 (100) | 8 (4.8) | 8 (4.8) | 0 |
| Tweet et al19 | 2014 | 189 | 94 (49.7) | 8 (8.5) | 89/6 (50.3) | 103 (54.4) | 97 (47.4) | 20 (94.1)# | NR | 26 (14) | 1 (0.53)** |
| Lettieri et al10 | 2015 | 134 | 78 (58.2) | 2 (2.6) | 51/5 (41.8) | 58 (43.3) | 55 (72.5) | 8 (87.5) | 7 (5.2) | 7 (5.2) | 3 (2.2)†† |
| Roura et al155 | 2016 | 34 | 26 (76.5) | 0 | 8/0 (23.5) | 8 (23.5) | 8 (75.0) | 0 | 0 | 0 | 0 |
| Rashid et al16 | 2016 | 21 | 17 (80.9) | 0 | 4/0 (17.4) | 4 (17.4) | 4 (100) | 0 | 0 | 0 | 0 |
| Nakashima et al11 | 2016 | 63 | 28 (44.4) | 0 | 34/1 (55.6) | NR | 34 (91.2) | 1 (NR) | NR | NR | NR |
| McGrath-Cadell et al171 | 2016 | 40 | 27 (67.5) | 0 | 12/1 (32.5) | 13 (32.5) | 12 (91.7) | 2 (100) | NR | 1 (2.5) | 0 |
| Cade et al89 | 2017 | 13‡‡ | 7 (53.8) | 2 (28.6) | 4/1 (38.4) | 7 (53.8) | NR | 1 | NR | 2 (15.4) | 1 (7.7) |
| Faden et al87 | 2016 | 79‡‡ | NR | NR | NR | 52 (65.8) | NR | 23 (NR) | NR | NR | 3 |
| Rogowski et al33 | 2017 | 64 | 56 (87.5) | 0 | 7/1 (12.5) | 8 (12.5) | 9 (66.7) | 1 (100) | 0 | 0 | 1 (1.6)§§ |
CABG indicates coronary artery bypass grafting; MI, myocardial infarction; NA, not applicable because all were treated with CABG; NR, not reported or able to be determined from data provided; PCI, percutaneous coronary intervention; and SCAD, spontaneous coronary artery dissection.
Success as defined per author or as patients not requiring second intervention for the same lesion.
Sepsis.
Cardiogenic shock.
Left main SCAD complicated by cardiogenic shock not improved by salvage left main PCI.
Eighteen patients in this cohort had concomitant atherosclerotic heart disease on angiography.
Perioperative MI and death in a patient with severe 3-vessel coronary disease and concomitant atherosclerotic disease.
Two of 34 intended bypass targets unable to be revascularized because of extent of dissection.
Multiorgan failure after bailout CABG for unsuccessful PCI.
One patient died of retrograde aortic dissection after PCI; 1 patient died after emergency CABG for multivessel SCAD with ST-segment–elevation MI and cardiogenic shock; and 1 patient died of out-of-hospital cardiac arrest and cardiogenic shock with coronary anatomy not suitable for revascularization.
All cases pregnancy-associated SCAD.
Left main artery SCAD in late pregnancy, cardiac arrest during emergency cesarean delivery, refractory cardiogenic and hemorrhagic shock.
In current clinical practice, the duration of hospitalization after ACS is therefore largely dictated by the management strategy for the patient and the ongoing symptoms.172 All patients with acute MI caused by SCAD are typically admitted for a minimum of 48 hours to allow adjustment of medications and in-hospital evaluation of physical activity tolerance before discharge.12 A prolonged period of in-hospital monitoring (3–5 days) is justified as part of a conservative strategy to observe for a small but important (5%–10%) early hazard of dissection extension or new recurrent SCAD.13,19 If clinical worsening occurs despite conservative management (worsening of symptoms together with evidence of ischemia by electrocardiography or significant arrhythmia), repeat angiography should be performed and emergency revascularization undertaken to relieve ischemia if feasible. Chest pain does not necessarily imply active myocardial ischemia. Chest pain may be a manifestation of vessel wall dissection itself. Thus, chest pain alone should not be an indication to pursue emergency revascularization, especially if there is no evidence of ischemia or if the vessel flow is normal. Patients in whom PCI is unsuccessful or complicated by extension of dissection, ongoing ischemia, ventricular arrhythmias, or heart failure often require longer hospitalizations, similar to conservatively treated patients.
OTHER SUPPORTIVE THERAPIES
Data on the use of therapies supportive of LV function or for persistent ischemia in SCAD are limited to single-patient case reports, each with a favorable outcome. Intra-aortic balloon pump counterpulsation has been used mostly to support surgical revascularization or sometimes to bridge pregnant patients through surgical delivery before CABG.173–181 LV assist devices or extracorporeal membrane oxygenation has been used as a bridge to transplantation or to support recovery after CABG.175,177,180,182–186 In 1 case, extracorporeal membrane oxygenation was used for cardiogenic shock after SCAD-associated cardiac arrest treated with PCI and was a successful bridge to myocardial recovery.180 The favorable outcomes in all reported cases likely represent a reporting bias given the high-risk scenarios described. The theoretical risks of percutaneous support devices in the setting of SCAD compared with atherosclerotic ACS include an increased propensity for iliac or femoral arterial dissection with the placement of large-bore catheters in the presence of FMD or other arteriopathies and hydraulic extension of intimal coronary dissection with increased diastolic coronary flow during intra-aortic balloon pump counterpulsation. Therapies supportive of LV function can be considered in cases of cardiogenic shock after SCAD as a bridge to recovery or transplantation. No data are available to support unique strategies in SCAD that differ from current consensus recommendations in this area.187
The role of implantable cardioverter-defibrillators or use of external defibrillator vests in patients with SCAD who present with sudden cardiac arrest temporally related to their ischemic event has not been studied. Patients with SCAD have been reported to receive early implantation of cardioverter-defibrillators or more aggressive management despite the absence of high-risk features such as recurrent arrhythmia, LV dysfunction, or persistent ischemia.188 There are potential harms with this unproven aggressive practice, and currently, there is insufficient evidence to suggest altering clinical decision making for implantation of cardioverter-defibrillators on the basis of the cause of the ischemic event.172,189
MEDICAL THERAPY
The ultimate goals of short- and long-term medical therapy of SCAD are to alleviate symptoms, to improve short- and long-term outcomes, and to prevent recurrent SCAD. Unfortunately, there is a substantial gap in evidence to guide clinicians in this regard because of the relatively recent recognition of SCAD as an important clinical entity and the absence of identified cellular and molecular targets or randomized controlled trials to support an evidence-based approach. Given the paucity of evidence currently available, the following approaches are based largely on expert opinions derived from the clinical experience of members of the writing group.
Anticoagulation and Antiplatelet Therapy
Because the pathophysiology, mechanisms of ischemia, PCI outcomes, and residua of SCAD are distinct from those associated with atherosclerotic ACS, many investigators have questioned the rationale and potential risks of using standard ACS therapies in patients with SCAD. For instance, early heparin use may provide benefit by reducing thrombus burden, but there are theoretical concerns about its use in the setting of acute SCAD presentation related to accentuating the risk of bleeding into the IMH or extension of dissection. Therefore, if systemic anticoagulation is started at hospital presentation, in the absence of other indications for systemic anticoagulation, consideration of discontinuation is appropriate once SCAD is diagnosed.190
Similarly, there are no data to guide the use of glycoprotein IIb/IIIa inhibitors in the emergency management of SCAD. Theoretical concerns about the extension of dissection, additional bleeding risk, and absence of evidence of benefit suggest a cautious approach to their use.190
Patients with SCAD who are undergoing coronary revascularization should receive the standard guideline-based antiplatelet therapy after PCI.156 Clear evidence supporting the use of dual-antiplatelet therapy in patients with SCAD who do not undergo coronary intervention is lacking. Although theoretical benefits of early dual-antiplatelet therapy in SCAD include protection from additional thrombosis in the prothrombotic environment caused by intimal dissection, many practitioners avoid its use in light of an increased bleeding risk and no evidence of benefit. No study has compared short- or long-term outcomes or bleeding risks related to the use of dual-antiplatelet therapy and aspirin alone in SCAD.
In adherence with current guideline-directed use of antiplatelet therapy after ACS, some experts recommend dual-antiplatelet therapy for at least 1 year after SCAD, regardless of initial management strategy, along with lifelong aspirin use.156 Others opt for no or limited use (1–3 months) followed by longer-term aspirin therapy. A retrospective Italian series examining in-hospital and long-term clinical outcomes among patients with SCAD found that dual-antiplatelet therapy with predominantly clopidogrel was administered to 82% of conservatively managed patients and 94% of patients who underwent PCI.10 This therapy was continued for a mean of 12.4 months (SD, 7.8 months) in patients who had stenting and for 10.9 months (SD, 2.9 months) in those treated conservatively. Bleeding complications were not reported, but long-term outcomes in both groups were similar.10
Most experts recommend aspirin use for at least 1 year and frequently indefinitely after SCAD in patients who receive medical treatment, in the absence of contraindications.10,31,190,191 In light of increased bleeding risks with antiplatelet agents, especially menorrhagia in premenopausal women, and uncertain benefits and risks, individual selection of suitability for dual-anti-platelet therapy and aspirin therapy in conservatively managed survivors of SCAD is indicated.
β-Adrenergic Blockers
β-Blockers should be considered in patients with SCAD who have LV dysfunction or arrhythmias and for management of hypertension. Some experts advocate for their routine use for SCAD12 on the basis of extrapolation from benefits in atherosclerotic MI135 or aortic dissection,192 whereas others recommend that they be used selectively out of concern for exacerbating vasospasm or symptomatic hypotension. In practice, β-blocker use is often limited by adverse effects, especially symptoms of fatigue and hypotension. However, in a recently reported 327-patient series from Vancouver with a median duration of follow-up of 3.1 years, the use of β-blockers was associated with a hazard ratio of 0.36 for recurrent SCAD in multivariable analysis,193 a finding strengthening the practice of β-blocker administration after SCAD.
Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
After SCAD, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers should be used when MI is complicated by LV systolic dysfunction, according to national guidelines for the management of patients after MI.135 These drugs are also a treatment option for concomitant hypertension. Female patients of reproductive age must be warned of the teratogenicity of renin-angiotensin system antagonists.
Statins
In a retrospective cohort of 87 patients, no relationship was found between post-SCAD medical management at discharge and SCAD recurrence, with the exception of statin use. Statin use was higher in patients who had a SCAD recurrence; however, this finding may have been affected by sample size, date of index event, and medication use that was started after discharge.8 In a more recent 327-patient SCAD cohort, no association was found between statin use and recurrent SCAD.193 Statin therapy is therefore not recommended routinely after SCAD but is reserved for patients meeting guideline-based indications for primary prevention of atherosclerosis194 and for the management of patients with established concomitant atherosclerotic disease or diabetes mellitus.194
Antianginal Therapy
The predominant role of antianginal therapies is for post-SCAD chest pain syndromes (see Evaluation and Management of Chest Pain Syndromes After SCAD). Inpatient management of chest pain may warrant antianginal therapy, but currently, there is not a role for the routine use of antianginal therapy for either the index SCAD hospitalization or long term. Chest discomfort is common in outpatients after SCAD, and for patients who are not candidates for revascularization or who have evidence suggesting coronary vasospasm or coronary microvascular dysfunction, relief of ischemia and symptoms may be achieved with nitrates, calcium channel blockers, or ranolazine. Their use, however, must be balanced with their adverse effect profile, most commonly symptomatic hypotension and headache (nitrates).
PREGNANCY-ASSOCIATED SCAD: DIAGNOSIS AND SHORT-TERM MANAGEMENT
The majority of pregnancy-associated SCADs occur in the first 4 weeks after delivery, but SCAD has been reported during virtually all stages of pregnancy.86,96 Management of SCAD in the pregnant or peripartum patient requires a multidisciplinary team approach195,196 from cardiology and obstetric services that incorporates management for the mother in combination with considerations for fetal well-being. Recommendations for pregnancy follow-up and delivery after SCAD have been reviewed elsewhere and depend on both maternal and fetal status with the goals of limiting maternal hemodynamic demand and close fetal monitoring.90 However, despite the special situation presented by pregnancy, the principles of SCAD management are largely the same as for non–pregnancy-associated SCAD, namely maintenance of a strong suspicion to ensure that the diagnosis is not missed, early and careful angiography to avoid iatrogenic dissection196,197 and to confirm the diagnosis (fetal radiation exposure is relatively low with current techniques90,198,199), and aiming for conservative management if there is no evidence of ongoing ischemia or infarction, hemodynamic instability, or particularly high-risk anatomy.17,200
Special consideration must be given to post-SCAD pharmacotherapy recommendations for women who are pregnant or lactating. Given the unclear usefulness of clopidogrel therapy in SCAD, its use during pregnancy by women who do not undergo PCI should be individualized.201 There are no clear safety data for clopidogrel use during pregnancy or breastfeeding, and although there are case reports of it being used safely,202–204 it is generally not recommended in women who are breastfeeding.205,206 Low-dose aspirin use is safe during pregnancy and breastfeeding.195
Although β-blockers are associated with fetal growth restriction, they are routinely used during pregnancy for the treatment of hypertension. Labetalol is the preferred agent, especially in early pregnancy, because both metoprolol and atenolol are more highly associated with lower placental and fetal weights at delivery.195,207 Atenolol has also been associated with bradycardia in breastfed infants; therefore, it should also be avoided during breastfeeding.206,208
CARDIAC REHABILITATION, PHYSICAL ACTIVITY, AND PSYCHOSOCIAL CONSIDERATIONS AFTER SCAD
Cardiac Rehabilitation
All patients who have MI caused by SCAD should be referred for cardiac rehabilitation209 consistent with the secondary prevention, risk reduction, and psychosocial support strategy recommendations.27,156,210,211 The program should be tailored and individualized, taking into account not only cardiopulmonary factors such as ejection fraction but also patient age, pre-SCAD physical activity level, and patient-centered recovery goals.211,212 Current evidence in the SCAD population suggests low rates of both referral to and participation in cardiac rehabilitation209,213,214 despite clear evidence of both safety and benefit, including improved measures of physical and psychosocial well-being, improved metabolic parameters, and lower symptom frequency.213–221 These low rates are compounded by the lower overall referral of women to cardiac rehabilitation,216,222 healthcare providers’ reluctance to refer patients with SCAD for cardiac rehabilitation because of fears that physical exertion will trigger recurrent SCAD, or the belief that relatively fit young patients without atherosclerotic heart disease may not benefit from cardiac rehabilitation.215 Patients with SCAD who enroll in cardiac rehabilitation often do not complete the program213,214 and may face barriers to participation unique to young women such as caregiving and work responsibilities, travel concerns, cost, and ongoing symptoms.214,222
Physical Activity During and After Cardiac Rehabilitation
In light of the association with physical activity as a trigger for SCAD, concern exists about the safety of various forms of physical activity and exercise after SCAD.13,223 As a result, and with the potential for physical and mental harm, some patients with SCAD continue to receive advice to severely restrict their activities such as to avoid lifting >10 lb or not to pursue activity beyond limited walking,220 which could place them at the risks associated with a sedentary lifestyle. Many patients had high levels of physical activity before their first SCAD event and express high levels of motivation to resume those activities.214 Therefore, providers are encouraged to prescribe a prudent approach to physical activity, balancing known benefits of exercise with the potential risks associated with high levels of exertion and strain. Referral to and participation in cardiac rehabilitation are paramount for addressing exercise-induced symptoms, providing immediate feedback on exercise hemodynamics, and individualizing fitness needs.
Although patients and providers want specific guidelines for or limits to physical activity, in the absence of evidence for benefit or harm, arbitrary heart rate and weight lifting limits can sometimes lead to frustration or fear with unclear benefit both during and after cardiac rehabilitation participation. Clinicians may want to consider conservative thresholds for a cardiac rehabilitation entrance exercise treadmill test that mirror those of the Vancouver General Hospital SCAD Program (blood pressure no higher than 130/80 mm Hg; heart rate 50% to 70% of heart rate reserve; free weights for resistance training of 2 to 12 lb, starting low and gradually increasing).214
Weight training with resistance up to 20 lb for women and 50 lb for men was shown to be safe in a small SCAD cardiac rehabilitation series.214,220 Strength training recommendations are typically to use low resistance and high repetitions designed to avoid strain or Valsalva maneuver. Cardiopulmonary stress testing after cardiac rehabilitation has been used by some groups as a way of developing more precise and individualized parameters based on level of fitness. This allows the development of parameters that avoid exercising near or above the anaerobic threshold and for detection of exercise-induced hypertension.192,220,224
In the absence of evidence for benefit or harm, such heart rate and weight lifting limits remain arbitrary, and larger prospective studies are needed to confirm comparison with standard cardiac rehabilitation programs or the safety of higher physical activity thresholds.209,225 Current practice is based largely on clinical experience214,220 and recommendations offered to patients with aortic dilatation or aortopathy,192,226 recognizing the substantial differences in exercise-induced hemodynamics within the aorta and coronary arteries. In addition to any symptom-limited physical activity guidelines, patients with SCAD should generally be advised to avoid prolonged high-intensity activities, highly competitive or contact sports, activities performed to exhaustion (racing, boot camp), abrupt increases in physical activity without a warm-up, exercise in extremes of temperature (hot yoga, cold weather) or terrain, or performance of Valsalva maneuver during lifting or exercise.
Psychosocial Considerations
Addressing mental health is a critical component of improving the short- and long-term health of patients with SCAD. The psychosocial effects of a diagnosis of heart disease are substantial, but patients with SCAD are a particularly high-risk population given that they are predominantly younger women who have received a diagnosis with substantial uncertainty in terms of management and prognosis. Anxiety and depression are common among survivors of SCAD, especially in those whose SCAD occurred during the peripartum period.225 In a recent cross-sectional study of 158 patients with SCAD, ≈40% reported a history of depression and anxiety at any point in their life, and about one third reported receiving medication or behavioral therapy for their anxiety and depression after their initial SCAD.225 Baseline characteristics of patients with SCAD who participated in cardiac rehabilitation showed that approximately one fifth214 to one third29 of patients reported a history of depression. Compared with patients with non-SCAD MI, patients with SCAD MI have higher psychological distress, depression and worry, anxiety, and tension scores on psychosocial questionnaires.221
Patients with SCAD are an already high-risk group for psychological morbidity. Among all patients with prior MI, younger women are at high risk for post-MI chest pain syndromes compared with men and older women. They experience short- and long-term outcomes20,227,228; angina229–231; psychosocial risk factors, including depression, anxiety, and posttraumatic stress disorder; increased prevalence of stress or depression at the time of MI230,232; more caregiving responsibilities233; lower health-related quality of life229,230; and reduced physical and mental functioning after discharge.20,227–231,234 Current guidelines recommend formal screening for depression and anxiety after MI,235 but younger and female patients often do not receive these services.222
Patients with SCAD also face unique issues. They are more often treated by a healthcare team that has little familiarity with their condition or who voice uncertainty about the diagnosis or treatment. They may be repeatedly advised to “change their lifestyle” as if they had atherosclerosis, despite the absence of modifiable cardiovascular disease risk factors. The lack of effective secondary prevention options can be anxiety provoking. Postpartum patients have the additional burden of caregiving, hormonal shifts, peripartum mood disorders, and disruption of expectations of early bonding with their infant or breastfeeding. Most survivors of SCAD are fearful of resuming physical activities in light of the possibility of triggering another dissection. The relatively uncommon nature of SCAD often results in a lack of peer support. In addition, the medical jargon inundating patients with newly diagnosed SCAD can often be intimidating; therefore, patient-focused medical literature such as a “patient page” describing SCAD in lay terms can be helpful.236
Online communities and virtual peer support are especially important because patients may be geographically separated from each other. The first SCAD-related international support community was hosted by WomenHeart: The National Coalition for Women With Heart Disease.237 Additional SCAD-specific patient support, advocacy, and nonprofit groups have formed around the world to raise awareness of SCAD, to connect patients, and to disseminate clinical practice and research developments. These organizations include SCAD Alliance,238 Beat SCAD,239 and SCAD Research, Inc.240 In addition, numerous patient-initiated online communities have emerged on social media platforms. These organizations and peer support groups have allowed geographically diverse survivors of SCAD to connect with, to provide support to, and to receive support from peers.
EVALUATION AND MANAGEMENT OF CHEST PAIN SYNDROMES AFTER SCAD
The collective experience suggests that chest pain is extremely common after SCAD, occurring in more than half of outpatients.86,214 It is unknown whether chest pain is more common in patients after SCAD than in sex- and age-matched control subjects after atherosclerotic MI. The symptoms can challenge both patient and clinician and often result in frequent evaluations, diagnostic testing, and substantial psychological burden. Post-SCAD symptoms range from acute chest pain that is reminiscent of the patient’s SCAD presenting symptoms, typical or atypical angina, to clearly noncardiac, but concerning, symptoms.
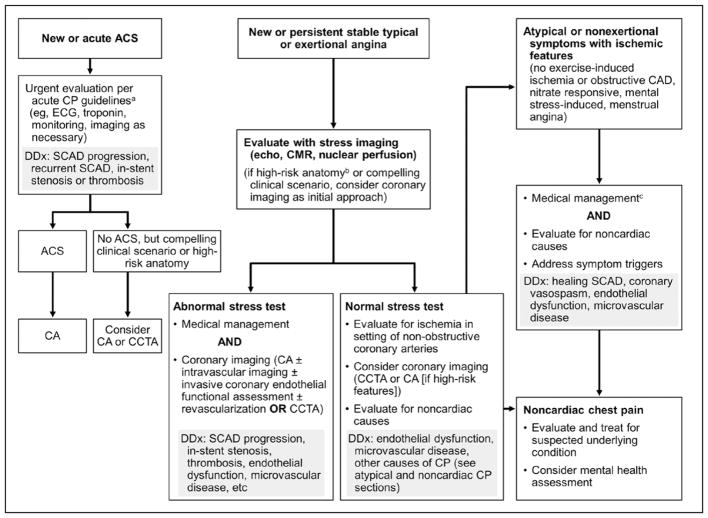
The differential diagnosis for patients who have chest pain syndrome after SCAD is similar to that in patients after ACS, with the additional consideration of extension or recurrent coronary dissection (Figure 9). An initial careful history and physical examination are critical. The clinician should be vigilant for signs and symptoms of instent restenosis or recurrent SCAD. The presence of noncardiac pain should be a diagnosis of exclusion, but differentiating chest wall and musculoskeletal discomfort on the basis of reproducibility on physical examination can be reassuring. If patients have more classic exertional symptoms, then diagnostic testing such as an exercise treadmill test, stress echocardiography, or perfusion imaging can be performed.25 If the study results are abnormal, coronary angiography, CCTA, and initial medical therapy are options, depending on the severity of symptoms and degree of ischemia. Alternatively, if symptoms are stable or there is evidence of a noncardiac cause of the discomfort, patients can receive close monitoring for cardiac symptoms, reassessment, and guidance on activities during cardiac rehabilitation.138,213
Figure 9. Evaluation and management of chest pain after spontaneous coronary artery dissection (SCAD).157,170.
aCurrent guidelines.135 bHigh-risk anatomy indicates SCAD affecting the left main or 2 proximal coronary arteries. cMedical management for post-SCAD chest pain (CP) without obstructive disease: long-acting nitrates, calcium channel blockers, or ranolazine. ACS indicates acute coronary syndrome; CA, coronary angiography; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance; DDx, differential diagnosis; and echo, echocardiography.
Frequently after SCAD, patients experience nitrate-responsive chest pain, often at rest or with mental stress, but have no inducible ischemia with even high levels of exercise. Premenopausal women may report chest pain at variable but predictable times of their menstrual cycle.241 One hypothesis is that this is a result of enhanced coronary vasomotion and abnormal endothelial function or persistent microvascular dysfunction. Clinically, some of these patients respond to empirical use of long-acting nitrates or calcium channel blockers, either daily or during days of the month when they are predictably symptomatic. Although the use of these agents may be limited by adverse effects of hypotension or headaches, empirical therapy may be considered if the patient’s symptoms are relieved with short-acting nitrates. The frequency and severity of chest pain have been observed to decrease over time, a result that may reflect vessel healing or resolution of stress, anxiety, and depression.
CORONARY ARTERY ASSESSMENT AFTER SCAD
After the acute presentation, diagnosis, and in-hospital management of SCAD, there may be reasons to reimage the dissected coronary arteries. Given the potential risks of iatrogenic dissections in patients with SCAD, repeat coronary angiography should be performed only when benefits may outweigh the potential risks, such as in patients with recurrent symptoms, abnormal functional tests, unclear cause of coronary stenosis (to assess for angiographic healing to help differentiate SCAD from other coronary disease), or high-risk anatomy (eg, left main, proximal, or multivessel dissection, in which residual dissections may benefit from revascularization for high ischemic burden). The role of CCTA for reassessment after SCAD was discussed previously. A summary of coronary imaging in the evaluation of post-SCAD chest pain in the nonacute setting is outlined in Figure 9.
PREGNANCY, CONTRACEPTION, AND HORMONE USE AFTER SCAD
Given the concerns for possible recurrent SCAD on re-exposure to the physical, hormonal, and potentially emotionally stressful conditions present during pregnancy and the postpartum period and the current lack of secondary prevention strategies, many clinicians recommend against subsequent pregnancy in women after SCAD, although there are few data to support this potentially life-changing recommendation. In the only published series to date examining subsequent pregnancy after a previous SCAD event, 8 patients were followed up for a median of 36 months (inter-quartile range, 24±70 months). One of the 8 patients experienced recurrent SCAD after delivery, presenting with ST-segment–elevation MI and ultimately requiring CABG because of unsuccessful PCI.242 Given the potential risk of recurrent SCAD in a subsequent pregnancy and the accumulating evidence that pregnancy-associated SCAD can result in worse outcomes,85,90,96 clinicians should carefully counsel any patient contemplating pregnancy of the potential risk. Included in the consideration are risks associated with medication use, functional status, and concomitant myocardial or valvular dysfunction.
Patients who elect to attempt pregnancy after SCAD should be referred to tertiary medical centers for preconception assessment and counseling, and those who become pregnant without such consultation should seek early postconception evaluation and close follow-up by a multidisciplinary team that includes maternal-fetal medicine specialists, cardiologists with experience managing SCAD, and obstetric anesthesiologists. Patients should be counseled on the potential increased risk of dissection and complications during the pregnancy. The management of these patients should include careful review of their medical regimen and adjustment or removal of medications that are unsafe or not recommended in pregnancy (angiotensin-converting enzyme inhibitors, atenolol, statins) and cardiac testing, including baseline electrocardiography and echocardiography.
Careful planning of the labor and delivery process and close postpartum care are essential.90,209 Mode of delivery should be individualized, taking into account LV function, vascular comorbidities such as FMD and vascular Ehlers-Danlos syndrome, and obstetric variables, with a goal of minimizing intrapartum cardiovascular stress. Considerations include early epidural placement, left lateral decubitus positioning, and treatment of hypertension. Although there is insufficient evidence to guide optimal mode of delivery in patients with SCAD, most cardiologists and maternal-fetal medicine specialists advocate for vaginal delivery, similar to recommendations for women with systemic vascular or connective tissue disorders. Other factors are the increased risk of hemorrhage, infection, thrombosis, and hemodynamic fluctuations with cesarean delivery in patients with cardiovascular disease.197,243,244 Minimizing maternal effort during vaginal delivery, with passive descent in the second stage of labor and delayed Valsalva effort, is advised; operative vaginal delivery or cesarean delivery is used secondarily.195,197 Because SCAD occurs primarily in women and is associated with pregnancy, there is a presumed pathophysiological association with female sex hormones, although the precise relationship remains unclear. Although there is no direct evidence linking hormonal contraception with recurrent SCAD, exogenous exposure to systemically absorbed hormones, including estrogen and progesterone for contraceptive and post-menopausal therapy, is usually avoided if possible, and they are used only if the nonhormonal options have been considered and the benefits of HT outweigh potential risks.8 Preferred methods of contraception include vasectomy for the male partners of patients with SCAD, tubal ligation (preferably performed once dual-antiplatelet therapy has been stopped), and intrauterine devices with local delivery of progestin (levonorgestrel 20 μg/d). Traditional or copper-containing intrauterine devices may be associated with increased cramping and menorrhagia, particularly in women receiving aspirin or dual-antiplatelet therapy.
Many female patients with SCAD are faced with decisions about management of menopause and post-menopausal HT. Compared with oral and transdermal hormonal contraceptives, postmenopausal HT formulations have substantially lower levels of estrogen and progesterone and have a lower risk of thrombosis, but concerns include the uncertain effects of HT on SCAD incidence and recurrence and the absence of safety data available to guide the initiation or continuation of HT specific to SCAD. Therefore, it is necessary to individualize recommendations through the use of relevant consensus statements, guidelines,245 and indications for use and to take into account patient preferences, known and perceived risks and benefits, and symptom severity while recognizing that the risks of HT differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progesterone is used.
Patients who experience SCAD while receiving HT should have their indications for HT reassessed, and unless there are compelling reasons to continue, HT should be discontinued. Indications for initiation of exogenous HT include premature and early surgical menopause, severe vasomotor symptoms that cannot be managed with lifestyle or nonhormonal treatments, and local treatment of genitourinary syndrome of menopause.245 If severe vasomotor symptoms or genitourinary syndrome of menopause develop at menopause or return on stopping the use of HT, consideration of the use of HT can be made in collaboration with cardiovascular and menopause specialists. The appropriate, often lowest, effective dose of systemic HT consistent with treatment goals that provides benefits and minimizes risks for the patient should be the therapeutic goal. Locally applied vaginal estrogen is generally thought to be safe because there is minimal systemic absorption. Patients with cardiovascular disease are typically advised to use a transdermal systemic agent to minimize activation of thrombotic factors and effects on lipids.246 The indications, benefits, and risks of continuing or discontinuing use of HT should be reviewed periodically.
MANAGEMENT OF ABNORMAL UTERINE BLEEDING AFTER SCAD
Antiplatelet therapy is frequently prescribed after SCAD, and many proposed therapeutic regimens call for the use of aspirin and short-term use of clopidogrel after PCI. In this setting, many women of reproductive age receiving dual-antiplatelet therapy report abnormal uterine bleeding. The most common type related to this therapy is heavy menstrual bleeding, defined as cyclic bleeding that is heavy for prolonged periods.247 Chronic or heavy bleeding can result in anemia and pose a substantial disruption to activities of daily living. An important first step is to reassess the indication for ongoing antiplatelet therapy and to discontinue its use if appropriate.
When chronic heavy menstrual bleeding develops in women receiving antiplatelet therapy after SCAD, especially if it is severe, they often require intervention to manage bleeding. Patients with hemodynamic instability or bleeding that saturates a large pad or tampon hourly for at least 4 hours usually warrant urgent evaluation, and all reproductive-age women with abnormal uterine bleeding should be tested for pregnancy. In women without cardiovascular disease, medical management with HT is usually sufficient and the first-line approach to control most heavy menstrual bleeding, but this approach is relatively contraindicated in SCAD, and careful clinical judgment is required. Other nonhormonal, noncontraceptive options for treating abnormal uterine bleeding such as nonsteroidal anti-inflammatory drugs and tranexamic acid should generally be avoided in women with SCAD given their association with MI and thrombosis.248–250
Among medical therapies for heavy menstrual bleeding, progestin-eluting intrauterine devices such as the levonorgestrel 20 μg/d–releasing device may be useful for controlling bleeding and protect against pregnancy.251,252 Systemic progesterone levels may increase minimally with these intrauterine devices, but the main effect is at the en-dometrial level. The levonorgestrel 20 μg/d–releasing device is the most effective approach (comparable to the efficacy of endometrial ablation), resulting in 71% to 95% reduction in menstrual blood loss, and is the only progestin intrauterine device that has been evaluated for treatment of abnormal uterine bleeding. In 2013, a lower-dose progestin device (releasing levonorgestrel 14 μg/d) became available253 and, if shown effective for management of abnormal uterine bleeding, may afford advantages for patients with SCAD in light of lower hormonal absorption. Although there are theoretical safety concerns, cyclic oral progestin treatment has been found to reduce bleeding by 87%252,254,255 and generally results in irregular bleeding and often reduction of menses to only light bleeding. In hemodynamically unstable women in whom bleeding is not controlled, high-dose oral or injectable progestin-only medications may be considered for the short term.
Because patients with SCAD are generally advised to avoid both pregnancy and exogenous hormones and because the long-term efficacy of conservative surgical treatment for heavy menstrual bleeding such as second-generation endometrial ablation techniques (thermal balloon, microwave, radiofrequency) is greater than that of oral medical treatment,256 these interventions may be considered in the management of heavy menstrual bleeding after SCAD. Advantages of these ablative procedures include effectiveness in managing bleeding, reduction of pregnancy risk, and the ability to perform the procedure on patients without discontinuation of antiplatelet or anticoagulation therapy. Other surgical options to control abnormal uterine bleeding are used primarily for women with structural abnormalities such as polyps and fibroids. Balloon tamponade (30-mL balloon catheter) has been described as an additional mechanical option to control acute bleeding in women who do not respond to HT.
SCREENING FOR SCAD-ASSOCIATED CONDITIONS
In the majority of patients, SCAD is not an isolated event but is reflective of an underlying vascular, genetic, or as-yet undetected condition. To help determine appropriate follow-up evaluations, practitioners caring for patients with SCAD should obtain a careful history, including a detailed review of systems and personal and family history of SCAD-associated symptoms and conditions. A list of screening questions to consider in the evaluation of the patient with SCAD is given in Table 4.
Table 4.
Screening Questions for SCAD-Associated Arteriopathies and Connective Tissue Disorders
| Personal history (Have you ever been diagnosed with or experienced any of the following?) |
| Early-onset hypertension |
| Stroke or transient ischemic attack |
| Pulsatile tinnitus |
| Migraine headaches |
| Renal infarction |
| Subarachnoid hemorrhage |
| Aneurysm (aortic, peripheral, brain) |
| Dissection (aortic, peripheral) |
| Rupture of hollow organs (intestinal, bladder, uterine) |
| Pneumothorax |
| Tendon or muscle rupture |
| Joint dislocation |
| Talipes equinovarus (clubfoot) |
| Umbilical or inguinal hernia |
| Scoliosis or pectus deformity |
| Pregnancy complications (cervical incompetence, hemorrhage, uterine prolapse, hypertension) |
| Poor wound healing |
| Ectopia lentis |
| Myopia |
| Detached retina, early glaucoma, or early cataracts |
| Tall stature |
| Abnormality of cardiac valve (bicuspid aortic valve, mitral valve prolapse) |
| Systemic inflammatory disease |
| Family history (Does anyone in your family have any of the following?) |
| Dissection (coronary, aortic, peripheral) |
| Inherited arteriopathy or connective tissue disorder (eg, vascular Ehlers- |
| Danlos syndrome, Marfan syndrome,
Loeys-Dietz syndrome) Fibromuscular dysplasia Aneurysm (aortic, peripheral, brain) |
| Early stroke, early myocardial infarction, sudden cardiac death |
| Review of systems (Are you currently experiencing any of the following?) |
| Headaches |
| Pulsatile tinnitus |
| Postprandial abdominal pain |
| Flank pain |
| Claudication |
| Easy bruising |
| Joint hypermobility or laxity |
SCAD indicates spontaneous coronary artery dissection.
DIAGNOSIS OF SCAD-ASSOCIATED EXTRACORONARY ARTERIOPATHIES
FMD and other extracoronary vascular abnormalities are strongly associated with SCAD13,34,257 and have the potential to affect management and inform prognosis. The rationale for extracoronary vascular screening is to diagnose underlying associated conditions such as FMD, which may point to a potential cause of SCAD, and to identify other vascular abnormalities such as aneurysms or dissections that may require additional evaluation, management, or longitudinal follow-up.75 Accordingly, all patients who have experienced SCAD should undergo a complete vascular physical examination with palpation and auscultation of the abdominal aorta, cervical carotid arteries, and peripheral arteries of the upper and lower extremities. A diminished pulse, side-to-side asymmetry, or bruit may be indicative of stenotic or dissected arteries, and widened or bounding pulse may be indicative of aneurysmal disease. In the US Registry for FMD, the presence of an epigastric or flank bruit had low sensitivity for the detection of renal FMD (24.0%); however, the specificity and positive predictive value were 93.3% and 92.6%, respectively.257 Thus, the absence of a bruit does not sufficiently rule out arterial disease, but its presence should heighten suspicion of an underlying arteriopathy.
Given the high coprevalence of FMD, aneurysm, and other extracoronary vascular abnormalities among patients with SCAD,13,34,70,76,258,259 vascular imaging from the brain to pelvis should be considered in all patients, similar to current practice for FMD.70,71 According to the US Registry for FMD,257 the most commonly involved vascular beds are the renal arteries (79.7%), followed by the extracranial carotid arteries (74.3%).70,257 Recent imaging series among patients with SCAD reported the presence of intracranial aneurysm in 14% to 23%.13,75 In addition, several conditions associated with an increased prevalence of unruptured intracranial aneurysms overlap with those associated with SCAD, including FMD, vascular Ehlers-Danlos syndrome, Loeys-Dietz syndrome, and polycystic kidney disease.257,260–264 The ultimate goal of vascular screening is the detection of high-risk aneurysms or extracoronary abnormalities requiring treatment in order to avoid aneurysm rupture and other complications associated with substantial morbidity and mortality.265–268 Screening for FMD and other extracoronary vascular abnormalities must include patient counseling for the possible outcomes, including a false-negative result (false reassurance), a false-positive result (and the risk of further testing), a true-positive result with advice for conservative management (and the potential for anxiety at the knowledge of harboring an aneurysm or dissection), and a true-positive result with advice for treatment. In the case of a true-positive result, there is a risk of treatment-associated harm (iatrogenic dissection, stroke, aneurysmal rupture, and adverse effects of contrast agent).
Long-term management of FMD and other extravascular abnormalities is beyond the scope of this statement, and we suggest using the approaches outlined in condition-specific American Heart Association guidelines and scientific statements.70,226,268
Angiography
The standard imaging method for diagnosing FMD is catheter-based angiography given its superior spatial resolution (<0.1 mm), and it is the definitive test to diagnose cases that are equivocal on noninvasive imaging.70 In patients undergoing coronary angiography to diagnose SCAD, additional nonselective or selective renal and iliac artery catheter-based angiography can be done in the same setting to assess for FMD. Similarly, for brain imaging, 2-dimensional digital subtraction angiography has historically been the reference standard for in vivo diagnosis of intracranial and cervical FMD and aneurysms, but the superior detection of small aneurysms or subtle FMD compared with noninvasive testing may not offer a clinically relevant advantage. Given this, as well as the low, but important, rate of procedural complications, digital subtraction angiography is not generally used as an initial method for cerebrovascular screening purposes but may be appropriate when the diagnosis is still uncertain after noninvasive imaging or when intervention is contemplated.70
Computed Tomography Angiography
In patients undergoing noninvasive imaging, the higher resolution of computed tomography compared with that of magnetic resonance imaging and ultrasonography and the ease of imaging across multiple vascular territories in a single scan may improve detection of the vascular abnormalities that are often found in SCAD.76 Notably, the FMD findings in patients with SCAD may be subtle and can be missed if not specifically sought. Regardless of the imaging method chosen, careful and focused image interpretation is critical. Multiple reconstruction formats, including multiplanar reformatted images, shaded surface display, and maximum-intensity projections, are necessary to adequately review for changes of FMD.70,269 For the detection of brain aneurysms, multirow detector computed tomography angiography is generally the preferred noninvasive imaging method because it provides detailed evaluation of the extracranial carotid, vertebral, and intracranial arteries for potential aneurysms.70,270,271 The sensitivity for aneurysms <3 mm is 82%.271 Advances in computed tomography technology such as third-generation dual-source systems continue to drive down radiation exposure and further shorten scanning time while preserving or improving image resolution. Novel protocols to image multiple vascular beds in a single diagnostic session can facilitate patient convenience and diagnostic accuracy over magnetic resonance angiography.75
Magnetic Resonance Angiography
Magnetic resonance angiography has lower spatial resolution than computed tomography angiography for FMD diagnosis,12,272 but newer magnetic resonance angiography can take advantage of volume rendering and strong magnets (3.0 T) that have increased the sensitivity for detection of aneurysms, despite the potential for false-negative and false-positive results.273 Benefits of magnetic resonance angiography include the absence of radiation, highly sensitive imaging of the brain parenchyma, and no requirement for contrast agent. The technique is limited by contraindications to magnetic resonance imaging (implanted metal, older pacemakers) and long scanning times that can lead to a higher likelihood of motion artifact degrading the image.
Duplex Ultrasonography
Duplex ultrasonography for the diagnosis of FMD is challenged by the need for highly skilled technicians and oversight by interpreting physicians.70 Given the lower sensitivity of duplex ultrasonography compared with that of computed tomography angiography or magnetic resonance angiography for FMD and other arteriopathies, this method has largely been replaced by computed tomography angiography or magnetic resonance angiography in most institutions for screening in the SCAD population.
Radiation Exposure: Special Considerations for Young and Female Patients
The disadvantage of angiographic or computed tomographic approaches to screening for extracoronary abnormalities and coronary imaging is the exposure to ionizing radiation. Given that the majority of SCAD occurs in young patients and that imaging plays a critical role in diagnosis and management, a discussion of radiation exposure is highly relevant. For women, the lifetime attributable risk of cancer is estimated at 2- to 4-fold greater for an exposure occurring at 20 versus 60 years of age compared with the risk in men.274 Because these young patients have a longer potential lifespan and are at risk of recurrent SCAD events and other conditions requiring imaging, there is an increased probability for multiple imaging tests, repeated procedures, and multiple healthcare providers being involved in their care. Cardiac imaging studies used in the evaluation of SCAD requiring ionizing radiation (invasive angiography and CCTA, stress myocardial perfusion imaging with positron emission tomography, and single-photon emission computed tomography) should be performed only after individualized assessment of risks and benefits. Both technological and operator radiation dose–reduction strategies should be used.275,276 Once systemic arteriopathy is diagnosed or an extracoronary dissection or aneurysm is identified that requires longitudinal follow-up, subsequent use of alternative imaging methods that do not expose the patient to ionizing radiation (eg, magnetic resonance angiography, duplex ultrasonography) should be considered.70,76 Particular efforts should be made to limit both patient and offspring radiation and contrast agent exposure in pregnant or lactating patients with SCAD, in whom non-emergency imaging should be deferred.199
ROLE OF GENETIC EVALUATION
The yield from routine genetic testing in patients with SCAD, especially among those without a suggestive personal or family history or physical examination of an inherited systemic arteriopathy or connective tissue disorder, has been shown to be low,107 and in the absence of a suggestive history or physical examination (Table 4), routine genetic testing after SCAD is not currently advised. Similarly, in patients with FMD, screening for variants in known arteriopathy-causing genes also seems to be of low yield.121,122 However, given the known association of SCAD with several inherited arteriopathies, referral to a specialist in medical genetics for further evaluation should be considered to aid in decision making for formal genetic assessment if an underlying systemic arteriopathy or connective tissue disorder is suspected in SCAD survivors. Patients with pregnancy-associated SCAD should also be considered for this evaluation because an underlying arteriopathy may be triggered by the hemodynamic and hormonal changes of pregnancy. Although the prevalence of these disorders is low, the diagnosis of a systemic arteriopathy may influence recommendations for frequency of vascular screening, timing of surgical intervention for aneurysms, or assessment of risk of pregnancy in the case of vascular Ehler-Danlos syndrome. This information can also guide screening in first-degree family members to determine their risk of aneurysm or dissection and need for clinical screening.
Formal medical genetics consultation, and testing if indicated, should be performed at a referral center with experience in genetically mediated arteriopathies and availability of medical genetics specialists.277 Given the complex financial, insurance, familial, and social issues surrounding genetic testing, genetic counseling is paramount before mutation screening.277 During the clinical evaluation for a genetically mediated arteriopathy, family history is assessed with a 3-generation pedigree, personal history, and examination for features characteristic of connective tissue disorders, including features of Marfan syndrome and other disorders caused by transforming growth factor-β pathway gene mutations, as reviewed in the Inheritance and Genetics section. Mar-fan syndrome and neurofibromatosis may be diagnosed clinically.278,279 Clinical findings of polycystic kidney disease should prompt an evaluation, which may include gene testing in some cases.
When findings suggestive of an inherited disorder are present, sequencing of a panel of genes inclusive of inherited arteriopathies (eg, TGFBR1, TGFBR2, COL3A1, FBN1, ACTA2, SMAD3, PLOD1, and COL5A1) should be considered an efficient approach to evaluate genetically mediated vascular disease. The history and physical examination may be unrevealing in vascular Ehlers-Danlos syndrome, and thus, the threshold for COL3A1 mutation testing may be lower.
Given the rapid expansion of identified genes for aortopathies, clinical genome sequencing (focused on whole genome or exome) may provide a single source of genetic data in which queries may be performed as new findings emerge. However, given the current lack of data showing defined, specific genetic causes for SCAD and the sporadic nature of the disease, routine whole-genome or exome sequencing is not currently recommended. In the absence of a confirmed diagnosis of a heritable arteriopathy or connective tissue disorder, clinical screening in first-degree family members of patients who experienced SCAD is not clearly indicated.
SCAD PROGNOSIS AND RECURRENCE RISK
The short- and long-term prognosis and risks of SCAD recurrence are highly relevant and of concern to both patients and their providers. After hospital dismissal, intermediate and long-term adverse cardiovascular events after SCAD are relatively common and include chest pain, depression, anxiety, and major adverse cardiac events such as recurrent MI, recurrent SCAD, unanticipated revascularization, congestive heart failure, stroke, and death. At an intermediate-term follow-up of 2 to 3 years, major adverse cardiac events were reported in 10% to 30% of cases, mostly caused by recurrent MI from recurrent SCAD, reported in 15% to 22% of patients.11,13,193 At longer-term follow-up, major adverse cardiac events were reported in 15% to 37% at 5 to 7 years, and the Kaplan-Meier–estimated rate of major adverse cardiac events is ≈50% at 10 years.8,10,13 These longer-term major adverse cardiac events are related predominantly to recurrent SCAD, which was estimated at up to 30% at 4 to 10 years of follow-up in different series.8,11,13,19
The reported rates of a recurrent episode of SCAD vary considerably in the literature (0%–37%),8–11,13,30,280 likely representing differences in study design, duration of follow-up, and definition of recurrence. Historically, the term recurrent SCAD has been used relatively interchangeably to describe 2 distinct forms of dissection: extension of the index dissection in the acute phase resulting from expansion of the IMH of the unhealed SCAD lesion and de novo dissection unrelated to the index dissection, affecting different coronary artery segments and occurring later (>30 days) and remote from the index event.8,11,13,19,281 Given the disparate features and pathophysiology of extension of dissection and de novo recurrent dissection, the term recurrent SCAD should be limited to describe subsequent de novo SCAD.
When reports of recurrent SCAD are limited to subsequent de novo dissections, incident recurrent SCAD rates are more consistent, and SCAD recurs in previously unaffected arteries 77% to 100% of the time.8,11,13,19,281 In the Mayo Clinic series, SCAD recurred in 17% of patients (15 of 87) at a median time to the second episode of 2.8 years, and the 10-year Kaplan-Meier–estimated risk of recurrence was 29.4%.8 In the updated series by Eleid et al83 with 246 patients, the reported rate was 16% over a median duration of 1.7 years. In the Japanese series, recurrent SCAD was reported in 19% of patients (14 of 63) at a median duration of 42 days.11 In the 168-patient Vancouver series, recurrent SCAD was reported in 13% of patients (22 of 168).6 In the updated 310-patient Vancouver cohort, recurrent SCAD occurred in 11% of patients (34 of 310); in all patients, new coronary artery segments were affected, and the recurrence was 30 days (median, 3.6 years) after the index SCAD.281
Identification of risk markers or risk factors for recurrent SCAD has been and remains an important clinical goal. Small sample size of studies has been a limitation, and to date, only severe coronary tortuosity has been identified as a risk factor for recurrence, with recurrence most likely to occur in a segment of tortuosity.83 Whether tortuosity itself confers a direct pathophysiological risk or is simply a marker of a high-risk patient phenotype remains uncertain. Coronary tortuosity was also shown to be a coronary manifestation of FMD12,82 and thus may be a confounder for the presence and strong association with FMD. Furthermore, the presence of FMD itself has not been identified as a predictor of recurrent SCAD.83,193
RESEARCH PRIORITIES AND CALL TO ACTION
SCAD is an important cause of ACS in otherwise healthy young and middle-aged individuals, particularly women without traditional cardiovascular risk factors. It is frequently underdiagnosed or misdiagnosed and can potentially result in substantial morbidity and mortality; a heightened suspicion for SCAD is required for accurate diagnosis and treatment. Despite increased recognition of the condition by the medical community and patients, large gaps in knowledge must be addressed to provide the best outcomes. It should be acknowledged that only recently have there been even limited prospective studies, and most available data are retrospective and observational. The findings are limited by referral bias and survivor bias, both of which may serve to overestimate recurrence risk. Conversely, the well-recognized sex bias in pursuing cardiac investigations in young women presenting with chest pain, together with known challenges in diagnosing SCAD even when investigations are undertaken, may serve to underestimate recurrence risk. Future larger-scale prospective and epidemiological studies will help further our understanding of the demographic, treatment, and anatomic factors associated with recurrence and allow more accurate prediction and ultimate prevention of recurrent SCAD.
The writing committee has classified research priorities into the areas of epidemiology, pathogenesis, diagnosis, and treatment (Table 5), each with key questions intended to spur collaborative research and advances in treatment for this disease. SCAD is an important cause of MI, particularly in young women. Although several key questions have yet to be answered, this writing group hopes that this document increases the awareness of SCAD among physicians and other healthcare providers and thus improves the recognition and management of SCAD. This awareness must then initiate collaborative research efforts to help understand the natural history of SCAD and to develop therapies to prevent SCAD complications and recurrence. We applaud the efforts of SCAD survivor–led initiatives, which have sparked collaboration and research thus far. We also call on the medical community to continue efforts to drive progress in this disease and to educate fellow physicians about SCAD. We hope that this inaugural American Heart Association scientific statement on SCAD becomes the catalyst for a call to action among the medical and patient communities.
Table 5.
Research Priorities and Key Questions in SCAD
| Epidemiology |
| What is the prevalence of SCAD in the general population? |
| Are there sex differences in the cause, presentation, diagnosis, and treatment of SCAD? |
| What is the recurrence rate of SCAD, and what are the determinants of recurrence? |
| Pathogenesis |
| Are there underlying genetic, hormonal, and environmental causes of SCAD, and what are the proportionate contributions of each? |
| What are the roles of physical and emotional stress and other potential triggers of SCAD? |
| What is the proportionate contribution of FMD and other systemic arteriopathies to the development and recurrence of SCAD? |
| Diagnosis |
| Under what circumstances and time frame and with what imaging method should follow-up coronary imaging be performed? |
| Treatment |
| What are the optimal approaches for initial revascularization? |
| What is the role of anticoagulation and antiplatelet therapy in SCAD immediately and after the event? |
| What are appropriate physical activity guidelines after SCAD? |
| What are the risks of exogenous hormone therapy and pregnancy after SCAD? |
FMD indicates fibromuscular dysplasia; and SCAD, spontaneous coronary artery dissection.
Acknowledgments
Sources of Funding
The authors gratefully acknowledge the family of Ann Fensterwald Eisenstein for their financial support for the creation of Figure 1.
Disclosures
Writing Group Disclosures
| Writing Group Member |
Employment | Research Grant | Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/ Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Sharonne N. Hayes | Mayo Clinic | None | None | None | None | None | None | None |
| Esther S.H. Kim | Vanderbilt University | None | None | None | None | None | None | None |
| Jacqueline Saw | Vancouver General Hospital Canada | Abbott Vascular (Canadian SCAD Study)†; Boston Scientific (Canadian Watchman Registry, ASAP- TOO study)† | None | Boston Scientific* | None | None | Abbott Vascular†; Boston Scientific† | None |
| David Adlam | University of Leicester, UK Glenfield Hospital United Kingdom | Abbott Vascular (support research relating to optical coherence tomography imaging)*; AstraZeneca (study of the role of antiplatelet agents for the prevention of cancer metastasis)*; BeatSCAD charity (spontaneous coronary artery dissection)*; British Heart Foundation (spontaneous coronary dissection)*; NIHR rare diseases TRC (rare coronary phenotypes including spontaneous coronary artery dissection)*; Leicester NIHR Biomedical Research Centre (spontaneous coronary artery dissection)* | None | None | None | None | None | None |
| Cynthia Arslanian-Engoren | University of Michigan School of Nursing | NIH/NINR: R21 (co-investigator [start date November 2017]; 5% for 1 year)† | None | None | None | None | None | University of Michigan School of Nursing† |
| Katherine E. Economy | Brigham and Women’s Hospital | None | None | None | None | None | None | None |
| Santhi K. Ganesh | University of Michigan | NIH†; Doris Duke Charitable Foundation† | None | None | None | None | None | None |
| Rajiv Gulati | Mayo Clinic | None | None | None | None | None | None | None |
| Mark E. Lindsay | Massachusetts General Hospital | None | None | None | None | None | None | None |
| Jennifer H. Mieres | Northwell Health | None | None | None | None | None | None | None |
| Sahar Naderi | Kaiser Permanente, Northern California | None | None | None | None | None | None | None |
| Svati Shah | Duke University Medicine DUMC | None | None | None | None | None | None | None |
| David E. Thaler | Tufts Medical Center | None | None | None | None | None | None | None |
| Marysia S. Tweet | Mayo Clinic | NIH (BIRCWH Scholar)† | None | None | None | None | None | None |
| Malissa J. Wood | Massachusetts General Hospital | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Heather L. Gornik | Cleveland Clinic | None | None | None | None | None | None | FMD Society of America (volunteer, Medical Advisory Board, FMD Society of America, a nonprofit organization)* |
| Roxana Mehran | Mount Sinai Medical Centre | None | None | None | None | None | None | None |
| Jeffrey W. Olin | Mount Sinai School of Medicine | Philanthropy (money donated by a patient for research on FMD; looking into genetic mechanisms of FMD)† | None | None | None | None | None | Fibromuscular Dysplasia Society of America (uncompensated)* |
| Jennifer Tremmel | Stanford University | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on December 1, 2017, and the American Heart Association Executive Committee on January 23, 2018. A copy of the document is available at http://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or kelle.ramsay@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e•••–e•••. DOI: 10.1161/CIR.0000000000000564.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.” Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at http://www.heart.org/HEARTORG/General/Copyright-Permission-Guidelines_UCM_300404_Article.jsp. A link to the “Copyright Permissions Request Form” appears on the right side of the page.
References
- 1.Pretty HC. Dissecting aneurysm of coronary artery in a woman aged rupture. Br Med J. 1931;1:667. [Google Scholar]
- 2.Bulkley BH, Roberts WC. Dissecting aneurysm (hematoma) limited to coronary artery: a clinicopathologic study of six patients. Am J Med. 1973;55:747–756. doi: 10.1016/0002-9343(73)90255-6. [DOI] [PubMed] [Google Scholar]
- 3.Robinowitz M, Virmani R, McAllister HA., Jr Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am J Med. 1982;72:923–928. doi: 10.1016/0002-9343(82)90853-1. [DOI] [PubMed] [Google Scholar]
- 4.DeMaio SJ, Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 1989;64:471–474. doi: 10.1016/0002-9149(89)90423-2. [DOI] [PubMed] [Google Scholar]
- 5.Alfonso F, Canales E, Aleong G. Spontaneous coronary artery dissection: diagnosis by optical coherence tomography. Eur Heart J. 2009;30:385. doi: 10.1093/eurheartj/ehn441. [DOI] [PubMed] [Google Scholar]
- 6.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84:1115–1122. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- 7.Tweet MS, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. Mayo Clin Proc. 2011;86:845–850. doi: 10.4065/mcp.2011.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jiménez-Quevedo P, Gon-zalo N, Escaned J, Bañuelos C, Pérez-Vizcayno MJ, Hernández R, Macaya C. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. 2012;5:1062–1070. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, Latib A, Ferlini M, Trabattoni D, Colombo P, Galli M, Tarantini G, Napodano M, Piccaluga E, Passamonti E, Sganzerla P, Ielasi A, Coccato M, Martinoni A, Musumeci G, Zanini R, Castiglioni B. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116:66–73. doi: 10.1016/j.amjcard.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, Taniguchi Y, Yamaguchi J, Tsuchihashi K, Seki A, Kawasaki T, Uchida T, Omura N, Kikuchi M, Kimura K, Ogawa H, Miyazaki S, Yasuda S. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol. 2016;207:341–348. doi: 10.1016/j.ijcard.2016.01.188. [DOI] [PubMed] [Google Scholar]
- 12.Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68:297–312. doi: 10.1016/j.jacc.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GB. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso F, Bastante T. Spontaneous coronary artery dissection: novel diagnostic insights from large series of patients. Circ Cardiovasc Interv. 2014;7:638–641. doi: 10.1161/CIRCINTERVENTIONS.114.001984. [DOI] [PubMed] [Google Scholar]
- 15.Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Non-atherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–819. doi: 10.1016/j.cjca.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, Gulati R, Malaipan Y, Meredith IT, Psaltis PJ. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome: a single-centre Australian experience. Int J Cardiol. 2016;202:336–338. doi: 10.1016/j.ijcard.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 17.Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, Roth A. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–1702. doi: 10.1161/CIRCULATIONAHA.113.002054. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D’Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, Holmes DR, Jr, Hayes SN, Gulati R. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 20.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 42: 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regitz-Zagrosek V. Sex and gender differences in symptoms of myocardial ischaemia. Eur Heart J. 2011;32:3064–3066. doi: 10.1093/eurheartj/ehr272. [DOI] [PubMed] [Google Scholar]
- 22.Lichtman JH, Leifheit-Limson EC, Watanabe E, Allen NB, Garavalia B, Garavalia LS, Spertus JA, Krumholz HM, Curry LA. Symptom recognition and healthcare experiences of young women with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2015;8(suppl 1):S31–S38. doi: 10.1161/CIRCOUTCOMES.114.001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction: National Registry of Myocardial Infarction 2 participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 24.Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–611. doi: 10.1161/CIRCULATIONAHA.111.086892. [DOI] [PubMed] [Google Scholar]
- 25.Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, Kramer CM, Min JK, Newby LK, Nixon JV, Srichai MB, Pellikka PA, Redberg RF, Wenger NK, Shaw LJ on behalf of the American Heart Association Cardiac Imaging Committee of the Council on Clinical Cardiology; Cardiovascular Imaging and Intervention Committee of the Council on Cardiovascular Radiology and Intervention. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association [published correction appears in Circulation. 2014;130:e86] Circulation. 2014;130:350–379. doi: 10.1161/CIR.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 26.Garcia M, Miller VM, Gulati M, Hayes SN, Manson JE, Wenger NK, Bairey Merz CN, Mankad R, Pollak AW, Mieres J, Kling J, Mulvagh SL. Focused cardiovascular care for women: the need and role in clinical practice. Mayo Clin Proc. 2016;91:226–240. doi: 10.1016/j.mayocp.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK on behalf of the American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang P, Wenger NK, Taylor D, Rich-Edwards JW, Steiner M, Shaw LJ, Berga SL, Miller VM, Merz NB. Strategies and methods to study female-specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ. 2016;7:19. doi: 10.1186/s13293-016-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibro-muscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6:44–52. doi: 10.1016/j.jcin.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009;74:710–717. doi: 10.1002/ccd.22115. [DOI] [PubMed] [Google Scholar]
- 31.Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, Blin D, Machecourt J. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250–254. doi: 10.1016/j.ejcts.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, Iwaguro T, Ueno S, Okumoto Y, Akasaka T. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5:263–270. doi: 10.1177/2048872613504310. [DOI] [PubMed] [Google Scholar]
- 33.Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, Joerg L, Rickli H. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv. 2017;89:59–68. doi: 10.1002/ccd.26383. [DOI] [PubMed] [Google Scholar]
- 34.Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, Gulati R, Vrtiska TJ. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672–1677. doi: 10.1016/j.amjcard.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation. 2014;130:1915–1920. doi: 10.1161/CIRCULATIONAHA.114.011422. [DOI] [PubMed] [Google Scholar]
- 36.Godinho AR, Vasconcelos M, Araújo V, Maciel MJ. Spontaneous coronary artery dissection in acute coronary syndrome: report of a series of cases with 17 patients. Arq Bras Cardiol. 2016;107:491–494. doi: 10.5935/abc.20160170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo-Rojas LM, Avedissian L, Pearse LA, Potter RN, Tremaine L, Gentlesk PJ, Huffer L, Reich SS, Stevenson WG Department of Defense Cardiovascular Death Registry Group. Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. doi: 10.1016/j.jacc.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Fahmy P, Prakash R, Starovoytov A, Boone R, Saw J. Pre-disposing and precipitating factors in men with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9:866–868. doi: 10.1016/j.jcin.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Arnold JR, West NE, van Gaal WJ, Karamitsos TD, Banning AP. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound. 2008;6:24. doi: 10.1186/1476-7120-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poon K, Bell B, Raffel OC, Walters DL, Jang IK. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ Cardiovasc Interv. 2011;4:e5–e7. doi: 10.1161/CIRCINTERVENTIONS.110.959593. [DOI] [PubMed] [Google Scholar]
- 41.Alfonso F, Paulo M, Dutary J. Endovascular imaging of angiographically invisible spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2012;5:452–453. doi: 10.1016/j.jcin.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, Gonzalo N, Jimenez-Quevedo P, Escaned J, Bañuelos C, Hernandez R, Macaya C, Alfonso F. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2013;6:830–832. doi: 10.1016/j.jcmg.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 43.De Giorgio F, Abbate A, Vetrugno G, Capelli A, Arena V. Non-atherosclerotic coronary pathology causing sudden death. J Clin Pathol. 2007;60:94–97. doi: 10.1136/jcp.2005.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lunebourg A, Letovanec I, Eggenberger P, Lehr HA. Images in cardiovascular medicine: sudden cardiac death due to triple vessel coronary dissection. Circulation. 2008;117:2038–2040. doi: 10.1161/CIRCULATIONAHA.107.729228. [DOI] [PubMed] [Google Scholar]
- 45.Wei JP, Kay D, Fishbein MC. Spontaneous dissection of the distal obtuse marginal coronary artery: a rare cause of sudden death. Am J Forensic Med Pathol. 2008;29:199–201. doi: 10.1097/PAF.0b013e318174f0fa. [DOI] [PubMed] [Google Scholar]
- 46.Stoukas V, Dragovic LJ. Sudden deaths from eosinophilic coronary monoarteritis: a subset of spontaneous coronary artery dissection. Am J Forensic Med Pathol. 2009;30:268–269. doi: 10.1097/PAF.0b013e31819d2158. [DOI] [PubMed] [Google Scholar]
- 47.Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart. 2010;96:1119–1125. doi: 10.1136/hrt.2009.185157. [DOI] [PubMed] [Google Scholar]
- 48.Pabla JS, John L, McCrea WA. Spontaneous coronary artery dissection as a cause of sudden cardiac death in the peripartum period. BMJ Case Rep. 2010;2010:bcr0520102994. doi: 10.1136/bcr.05.2010.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fengping Y, Jue H, Qingchun Y, Fangxing H. A case of sudden death due to spontaneous coronary artery dissection. Am J Forensic Med Pathol. 2011;32:312–313. doi: 10.1097/PAF.0b013e318219c8d3. [DOI] [PubMed] [Google Scholar]
- 50.Desai S, Sheppard MN. Sudden cardiac death: look closely at the coronaries for spontaneous dissection which can be missed: a study of 9 cases. Am J Forensic Med Pathol. 2012;33:26–29. doi: 10.1097/paf.0b013e3181e29598. [DOI] [PubMed] [Google Scholar]
- 51.D’Ovidio C, Sablone S, Carnevale A. Spontaneous coronary artery dissection: case report and literature review. J Forensic Sci. 2015;60:801–806. doi: 10.1111/1556-4029.12722. [DOI] [PubMed] [Google Scholar]
- 52.Kanaroglou S, Nair V, Fernandes JR. Sudden cardiac death due to coronary artery dissection as a complication of cardiac sarcoidosis. Cardiovasc Pathol. 2015;24:244–246. doi: 10.1016/j.carpath.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Makino Y, Inokuchi G, Yokota H, Hayakawa M, Yajima D, Motomura A, Chiba F, Torimitsu S, Nakatani Y, Iwase H. Sudden death due to coronary artery dissection associated with fibromuscular dysplasia revealed by postmortem selective computed tomography coronary angiography: a case report. Forensic Sci Int. 2015;253:e10–e15. doi: 10.1016/j.forsciint.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 54.Mandal R, Brooks EG, Corliss RF. Eosinophilic coronary periarteritis with arterial dissection: the mast cell hypothesis. J Forensic Sci. 2015;60:1088–1092. doi: 10.1111/1556-4029.12752. [DOI] [PubMed] [Google Scholar]
- 55.Melez İE, Arslan MN, Melez DO, Akçay A, Büyük Y, Avçar A, Kumral B, Şirin G, Karayel FA, Daş T, Dokudan YE, Şam B. Spontaneous coronary artery dissection: report of 3 cases and literature review hormonal, autoimmune, morphological factors. Am J Forensic Med Pathol. 2015;36:188–192. doi: 10.1097/PAF.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 56.Basso C, Morgagni GL, Thiene G. Spontaneous coronary artery dissection: a neglected cause of acute myocardial ischaemia and sudden death. Heart. 1996;75:451–454. doi: 10.1136/hrt.75.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lepper PM, Koenig W, Möller P, Perner S. A case of sudden cardiac death due to isolated eosinophilic coronary arteritis. Chest. 2005;128:1047–1050. doi: 10.1378/chest.128.2.1047. [DOI] [PubMed] [Google Scholar]
- 58.Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, Aymong E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87:E54–E61. doi: 10.1002/ccd.26022. [DOI] [PubMed] [Google Scholar]
- 59.Kwon TG, Gulati R, Matsuzawa Y, Aoki T, Guddeti RR, Herrmann J, Lennon RJ, Ritman EL, Lerman LO, Lerman A. Proliferation of coronary adventitial vasa vasorum in patients with spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2016;9:891–892. doi: 10.1016/j.jcmg.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 60.Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez-Quevedo P, Lennie V, Escaned J, Bañuelos C, Hernandez R, Macaya C. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073–1079. doi: 10.1016/j.jacc.2011.08.082. [DOI] [PubMed] [Google Scholar]
- 61.Siegel RJ, Koponen M. Spontaneous coronary artery dissection causing sudden death: mechanical arterial failure or primary vasculitis? Arch Pathol Lab Med. 1994;118:196–198. [PubMed] [Google Scholar]
- 62.Carreon CK, Esposito MJ. Eosinophilic coronary monoarteritis. Arch Pathol Lab Med. 2014;138:979–981. doi: 10.5858/arpa.2012-0610-RS. [DOI] [PubMed] [Google Scholar]
- 63.Asuncion CM, Hyun J. Dissecting intramural hematoma of the coronary artery in pregnancy and the puerperium. Obstet Gynecol. 1972;40:202–210. [PubMed] [Google Scholar]
- 64.Madu EC, Kosinski DJ, Wilson WR, Burket MW, Fraker TD, Jr, Ansel GM. Two-vessel coronary artery dissection in the peripartum period: case report and literature review. Angiology. 1994;45:809–816. doi: 10.1177/000331979404500909. [DOI] [PubMed] [Google Scholar]
- 65.Conraads VM, Vorlat A, Colpaert CG, Rodrigus IE, De Paep RJ, Moulijn AC, Vrints CJ. Spontaneous dissection of three major coronary arteries subsequent to cystic medial necrosis. Chest. 1999;116:1473–1475. doi: 10.1378/chest.116.5.1473. [DOI] [PubMed] [Google Scholar]
- 66.Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Hum Pathol. 1987;18:654–656. doi: 10.1016/s0046-8177(87)80368-4. [DOI] [PubMed] [Google Scholar]
- 67.Mather PJ, Hansen CL, Goldman B, Inniss S, Piña I, Norris R, Jeevanandam V, Bove AA. Postpartum multivessel coronary dissection. J Heart Lung Transplant. 1994;13:533–537. [PubMed] [Google Scholar]
- 68.Brodsky SV, Ramaswamy G, Chander P, Braun A. Ruptured cerebral aneurysm and acute coronary artery dissection in the setting of multivascular fibromuscular dysplasia: a case report. Angiology. 2007;58:764–767. doi: 10.1177/0003319707303645. [DOI] [PubMed] [Google Scholar]
- 69.Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv. 2012;5:134–137. doi: 10.1161/CIRCINTERVENTIONS.111.966630. [DOI] [PubMed] [Google Scholar]
- 70.Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, Gray WA, Gupta R, Hamburg NM, Katzen BT, Lookstein RA, Lumsden AB, Newburger JW, Rundek T, Sperati CJ, Stanley JC on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; Council for High Blood Pressure Research; Council on the Kidney in Cardiovascular Disease; Stroke Council. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129:1048–1078. doi: 10.1161/01.cir.0000442577.96802.8c. [DOI] [PubMed] [Google Scholar]
- 71.Persu A, Van der Niepen P, Touzé E, Gevaert S, Berra E, Mace P, Plouin PF, Jeunemaitre X on behalf of the Working Group “Hypertension and the Kidney” of the European Society of Hypertension and the European Fibromuscular Dysplasia Initiative. Revisiting fibromuscular dysplasia: rationale of the European Fibromuscular Dysplasia Initiative. Hypertension. 2016;68:832–839. doi: 10.1161/HYPERTENSIONAHA.116.07543. [DOI] [PubMed] [Google Scholar]
- 72.Olin JW. Expanding clinical phenotype of fibromuscular dysplasia. Hypertension. 2017;70:488–489. doi: 10.1161/HYPERTENSIONAHA.117.09646. [DOI] [PubMed] [Google Scholar]
- 73.Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv. 2005;64:138–145. doi: 10.1002/ccd.20246. [DOI] [PubMed] [Google Scholar]
- 74.Toggweiler S, Puck M, Thalhammer C, Manka R, Wyss M, Bilecen D, Corti R, Amann-Vesti BR, Lüscher TF, Wyss CA. Associated vascular lesions in patients with spontaneous coronary artery dissection. Swiss Med Wkly. 2012;142:w13538. doi: 10.4414/smw.2012.13538. [DOI] [PubMed] [Google Scholar]
- 75.Liang JJ, Prasad M, Tweet MS, Hayes SN, Gulati R, Breen JF, Leng S, Vrtiska TJ. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Comput Tomogr. 2014;8:189–197. doi: 10.1016/j.jcct.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW, Gray BH, Jaff MR, Kim ES, Mace P, Sharma A, Kline-Rogers E, White C, Olin JW. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the U.S. Registry for FMD. J Am Coll Cardiol. 2016;68:176–185. doi: 10.1016/j.jacc.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 77.Hill LD, Antonius JI. Arterial dysplasia: an important surgical lesion. Arch Surg. 1965;90:585–595. doi: 10.1001/archsurg.1965.01320100129020. [DOI] [PubMed] [Google Scholar]
- 78.Imamura M, Yokoyama S, Kikuchi K. Coronary fibromuscular dysplasia presenting as sudden infant death. Arch Pathol Lab Med. 1997;121:159–161. [PubMed] [Google Scholar]
- 79.Ropponen KM, Alafuzoff I. A case of sudden death caused by fibromuscular dysplasia. J Clin Pathol. 1999;52:541–542. doi: 10.1136/jcp.52.7.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawakami H, Matsuoka H, Koyama Y, Saeki H, Inoue K, Nishimura K, Itou T, Satou H, Tomino T. Isolated left coronary ostial stenosis as a result of fibromuscular dysplasia in a young man. Jpn Circ J. 2000;64:988–989. doi: 10.1253/jcj.64.988. [DOI] [PubMed] [Google Scholar]
- 81.Saw J, Bezerra H, Gornik HL, Machan L, Mancini GB. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation. 2016;133:1548–1559. doi: 10.1161/CIRCULATIONAHA.115.020282. [DOI] [PubMed] [Google Scholar]
- 82.Michelis KC, Olin JW, Kadian-Dodov D, d’Escamard V, Kovacic JC. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol. 2014;64:1033–1046. doi: 10.1016/j.jacc.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, Vrtiska TJ, Prasad M, Rihal CS, Hayes SN, Gulati R. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7:656–662. doi: 10.1161/CIRCINTERVENTIONS.114.001676. [DOI] [PubMed] [Google Scholar]
- 84.Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv. 2017;10:e005119. doi: 10.1161/CIRCINTERVENTIONS.117.005119. [DOI] [PubMed] [Google Scholar]
- 85.Ito H, Taylor L, Bowman M, Fry ET, Hermiller JB, Van Tassel JW. Presentation and therapy of spontaneous coronary artery dissection and comparisons of postpartum versus nonpostpartum cases. Am J Cardiol. 2011;107:1590–1596. doi: 10.1016/j.amjcard.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 86.Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–435. doi: 10.1016/j.jacc.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 87.Faden MS, Bottega N, Benjamin A, Brown RN. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart. 2016;102:1974–1979. doi: 10.1136/heartjnl-2016-309403. [DOI] [PubMed] [Google Scholar]
- 88.Koller PT, Cliffe CM, Ridley DJ. Immunosuppressive therapy for peripartum-type spontaneous coronary artery dissection: case report and review. Clin Cardiol. 1998;21:40–46. doi: 10.1002/clc.4960210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cade JR, Szarf G, de Siqueira ME, Chaves Á, Andréa JC, Figueira HR, Gomes MM, Jr, Freitas BP, Filgueiras Medeiros J, Dos Santos MR, Fiorotto WB, Daige A, Gonçalves R, Cantarelli M, Alves CM, Echenique L, de Brito FS, Jr, Perin MA, Born D, Hecht H, Caixeta A. Pregnancy-associated spontaneous coronary artery dissection: insights from a case series of 13 patients. Eur Heart J Cardiovasc Imaging. 2017;18:54–61. doi: 10.1093/ehjci/jew021. [DOI] [PubMed] [Google Scholar]
- 90.Codsi E, Tweet MS, Rose CH, Arendt KW, Best PJ, Hayes SN. Spontaneous coronary artery dissection in pregnancy: what every obstetrician should know. Obstet Gynecol. 2016;128:731–738. doi: 10.1097/AOG.0000000000001630. [DOI] [PubMed] [Google Scholar]
- 91.Bonnet J, Aumailley M, Thomas D, Grosgogeat Y, Broustet JP, Bricaud H. Spontaneous coronary artery dissection: case report and evidence for a defect in collagen metabolism. Eur Heart J. 1986;7:904–909. doi: 10.1093/oxfordjournals.eurheartj.a061979. [DOI] [PubMed] [Google Scholar]
- 92.Shamloo BK, Chintala RS, Nasur A, Ghazvini M, Shariat P, Diggs JA, Singh SN. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol. 2010;22:222–228. [PubMed] [Google Scholar]
- 93.Kamel H, Roman MJ, Pitcher A, Devereux RB. Pregnancy and the risk of aortic dissection or rupture: a cohort-crossover analysis. Circulation. 2016;134:527–533. doi: 10.1161/CIRCULATIONAHA.116.021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hartman JD, Eftychiadis AS. Medial smooth-muscle cell lesions and dissection of the aorta and muscular arteries. Arch Pathol Lab Med. 1990;114:50–61. [PubMed] [Google Scholar]
- 95.Sheikh AS, O’Sullivan M. Pregnancy-related spontaneous coronary artery dissection: two case reports and a comprehensive review of literature. Heart Views. 2012;13:53–65. doi: 10.4103/1995-705X.99229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Havakuk O, Goland S, Mehra A, Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv. 2017;10:e004941. doi: 10.1161/CIRCINTERVENTIONS.117.004941. [DOI] [PubMed] [Google Scholar]
- 97.Aldoboni AH, Hamza EA, Majdi K, Ngibzadhe M, Palasaidi S, Moayed DA. Spontaneous dissection of coronary artery treated by primary stenting as the first presentation of systemic lupus erythematosus. J Invasive Cardiol. 2002;14:694–696. [PubMed] [Google Scholar]
- 98.Sharma AK, Farb A, Maniar P, Ajani AE, Castagna M, Virmani R, Suddath W, Lindsay J. Spontaneous coronary artery dissection in a patient with systemic lupus erythematosis. Hawaii Med J. 2003;62:248–253. [PubMed] [Google Scholar]
- 99.Kothari D, Ruygrok P, Gentles T, Occleshaw C. Spontaneous coronary artery dissection in an adolescent man with systemic lupus erythematosus. Intern Med J. 2007;37:342–343. doi: 10.1111/j.1445-5994.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 100.Nisar MK, Mya T. Spontaneous coronary artery dissection in the context of positive anticardiolipin antibodies and clinically undiagnosed systemic lupus erythematosus. Lupus. 2011;20:1436–1438. doi: 10.1177/0961203311406765. [DOI] [PubMed] [Google Scholar]
- 101.Rekik S, Lanfranchi P, Jacq L, Bernasconi F. Spontaneous coronary artery dissection in a 35 year-old woman with systemic lupus erythematosus successfully treated by angioplasty. Heart Lung Circ. 2013;22:955–958. doi: 10.1016/j.hlc.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 102.Reddy S, Vaid T, Ganiga Sanjeeva NC, Shetty RK. Spontaneous coronary artery dissection as the first presentation of systemic lupus erythematosus. BMJ Case Rep. 2016;2016:bcr2016216344. doi: 10.1136/bcr-2016-216344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Srinivas M, Basumani P, Muthusamy R, Wheeldon N. Active inflammatory bowel disease and coronary artery dissection. Postgrad Med J. 2005;81:68–70. doi: 10.1136/pgmj.2004.018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu KH, Menapace FJ, Blankenship JC, Hausch R, Harrington T. Polyarteritis nodosa presenting as acute myocardial infarction with coronary dissection. Cathet Cardiovasc Diagn. 1998;44:320–324. doi: 10.1002/(sici)1097-0304(199807)44:3<320::aid-ccd16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 105.Bayar N, Çağırcı G, Üreyen ÇM, Kuş G, Küçükseymen S, Arslan Ş. The Relationship between spontaneous multi-vessel coronary artery dissection and celiac disease. Korean Circ J. 2015;45:242–244. doi: 10.4070/kcj.2015.45.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fernández-Gutiérrez B, Zamorano J, Batlle E, Alfonso F, Conde A, Sánchez-Harguindey L, Jover JA. Coronary dissection associated with hepatitis C virus-related cryoglobulinaemia. Rheumatology (Oxford) 1999;38:1299–1301. doi: 10.1093/rheumatology/38.12.1299. [DOI] [PubMed] [Google Scholar]
- 107.Henkin S, Negrotto SM, Tweet MS, Kirmani S, Deyle DR, Gulati R, Olson TM, Hayes SN. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. 2016;102:876–881. doi: 10.1136/heartjnl-2015-308645. [DOI] [PubMed] [Google Scholar]
- 108.Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J. 1995;74:112–116. doi: 10.1136/hrt.74.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hampole CV, Philip F, Shafii A, Pettersson G, Anesi GL, Patel JB, Menon V. Spontaneous coronary artery dissection in Ehlers-Danlos syndrome. Ann Thorac Surg. 2011;92:1883–1884. doi: 10.1016/j.athoracsur.2011.03.136. [DOI] [PubMed] [Google Scholar]
- 110.Bateman AC, Gallagher PJ, Vincenti AC. Sudden death from coronary artery dissection. J Clin Pathol. 1995;48:781–784. doi: 10.1136/jcp.48.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fattori R, Sangiorgio P, Mariucci E, Ritelli M, Wischmeijer A, Greco C, Colombi M. Spontaneous coronary artery dissection in a young woman with Loeys-Dietz syndrome. Am J Med Genet A. 2012;158A:1216–1218. doi: 10.1002/ajmg.a.35277. [DOI] [PubMed] [Google Scholar]
- 112.van der Linde D, van de Laar IM, Bertoli-Avella AM, Oldenburg RA, Bekkers JA, Mattace-Raso FU, van den Meiracker AH, Moelker A, van Kooten F, Frohn-Mulder IM, Timmermans J, Moltzer E, Cobben JM, van Laer L, Loeys B, De Backer J, Coucke PJ, De Paepe A, Hilhorst-Hofstee Y, Wessels MW, Roos-Hesselink JW. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60:397–403. doi: 10.1016/j.jacc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 113.Itty CT, Farshid A, Talaulikar G. Spontaneous coronary artery dissection in a woman with polycystic kidney disease. Am J Kidney Dis. 2009;53:518–521. doi: 10.1053/j.ajkd.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 114.Klingenberg-Salachova F, Limburg S, Boereboom F. Spontaneous coronary artery dissection in polycystic kidney disease. Clin Kidney J. 2012;5:44–46. doi: 10.1093/ndtplus/sfr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grover P, Fitzgibbons TP. Spontaneous coronary artery dissection in a patient with autosomal dominant polycystic kidney disease: a case report. J Med Case Rep. 2016;10:62. doi: 10.1186/s13256-016-0832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goel K, Tweet M, Olson TM, Maleszewski JJ, Gulati R, Hayes SN. Familial spontaneous coronary artery dissection: evidence for genetic susceptibility. JAMA Intern Med. 2015;175:821–826. doi: 10.1001/jamainternmed.2014.8307. [DOI] [PubMed] [Google Scholar]
- 117.Kiando SR, Tucker NR, Castro-Vega LJ, Katz A, D’Escamard V, Tréard C, Fraher D, Albuisson J, Kadian-Dodov D, Ye Z, Austin E, Yang ML, Hunker K, Barlassina C, Cusi D, Galan P, Empana JP, Jouven X, Gimenez-Roqueplo AP, Bruneval P, Hyun Kim ES, Olin JW, Gornik HL, Azizi M, Plouin PF, Ellinor PT, Kullo IJ, Milan DJ, Ganesh SK, Boutouyrie P, Kovacic JC, Jeunemaitre X, Bouatia-Naji N. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet. 2016;12:e1006367. doi: 10.1371/journal.pgen.1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Major P, Genest J, Cartier P, Kuchel O. Hereditary fibromuscular dysplasia with renovascular hypertension. Ann Intern Med. 1977;86:583. doi: 10.7326/0003-4819-86-5-583_1. [DOI] [PubMed] [Google Scholar]
- 119.Rushton AR. The genetics of fibromuscular dysplasia. Arch Intern Med. 1980;140:233–236. [PubMed] [Google Scholar]
- 120.Gladstien K, Rushton AR, Kidd KK. Penetrance estimates and recurrence risks for fibromuscular dysplasia. Clin Genet. 1980;17:115–116. doi: 10.1111/j.1399-0004.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 121.Poloskey SL, Kim ESh, Sanghani R, Al-Quthami AH, Arscott P, Moran R, Rigelsky CM, Gornik HL. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med. 2012;17:371–378. doi: 10.1177/1358863X12459650. [DOI] [PubMed] [Google Scholar]
- 122.Ganesh SK, Morissette R, Xu Z, Schoenhoff F, Griswold BF, Yang J, Tong L, Yang ML, Hunker K, Sloper L, Kuo S, Raza R, Milewicz DM, Francomano CA, Dietz HC, Van Eyk J, McDonnell NB. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features. FASEB J. 2014;28:3313–3324. doi: 10.1096/fj.14-251207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Velusamy M, Fisherkeller M, Keenan ME, Kiernan FJ, Fram DB. Spontaneous coronary artery dissection in a young woman precipitated by retching. J Invasive Cardiol. 2002;14:198–201. [PubMed] [Google Scholar]
- 124.Sivam S, Yozghatlian V, Dentice R, McGrady M, Moriarty C, Di Michiel J, Bye PT, Rees D. Spontaneous coronary artery dissection associated with coughing. J Cyst Fibros. 2014;13:235–237. doi: 10.1016/j.jcf.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 125.Hardegree EL, Tweet MS, Hayes SN, Gulati R, Kane GC. Multivessel spontaneous coronary artery dissection associated with hormonal infertility therapy in a 39-year-old female. J Cardiol Cases. 2012;5:e69–e72. doi: 10.1016/j.jccase.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lempereur M, Grewal J, Saw J. Spontaneous coronary artery dissection associated with β-HCG injections and fibromuscular dysplasia. Can J Cardiol. 2014;30:464.e1–464.e3. doi: 10.1016/j.cjca.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 127.Nakamoto K, Matsuda M, Kanno K, Segawa T, Nishimoto O, Nishiyama H, Tamura R, Kawamoto T. A case of a young, healthy woman with spontaneous coronary artery dissection associated with oral contraceptive use: long-term residual dissection of the coronary artery. J Cardiol Cases. 2013;8:179–182. doi: 10.1016/j.jccase.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steinhauer JR, Caulfield JB. Spontaneous coronary artery dissection associated with cocaine use: a case report and brief review. Cardiovasc Pathol. 2001;10:141–145. doi: 10.1016/s1054-8807(01)00074-6. [DOI] [PubMed] [Google Scholar]
- 129.Smyth A, O’Donnell M, Lamelas P, Teo K, Rangarajan S, Yusuf S on behalf of the INTERHEART Investigators. Physical activity and anger or emotional upset as triggers of acute myocardial infarction: the INTERHEART study. Circulation. 2016;134:1059–1067. doi: 10.1161/CIRCULATIONAHA.116.023142. [DOI] [PubMed] [Google Scholar]
- 130.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 131.Keir ML, Dehghani P. Corticosteroids and spontaneous coronary artery dissection: a new predisposing factor? Can J Cardiol. 2016;32:395.e7–395.e8. doi: 10.1016/j.cjca.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 132.Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, Saw J. Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2017;89:1149–1154. doi: 10.1002/ccd.26977. [DOI] [PubMed] [Google Scholar]
- 133.Lindor RA, Tweet MS, Goyal KA, Lohse CM, Gulati R, Hayes SN, Sadosty AT. Emergency department presentation of patients with spontaneous coronary artery dissection. J Emerg Med. 2017;52:286–291. doi: 10.1016/j.jemermed.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 134.Franco C, Starovoytov A, Heydari M, Mancini GB, Aymong E, Saw J. Changes in left ventricular function after spontaneous coronary artery dissection. Clin Cardiol. 2017;40:149–154. doi: 10.1002/clc.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 136.Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. 2004;16:493–499. [PubMed] [Google Scholar]
- 137.Hassen Y-S, Henareh L. Spontaneous coronary artery dissection triggered post-ischemic myocardial stunning and takotsubo syndrome: two different names for the same condition. Cardiovasc Revasc Med. 2013;14:109–112. doi: 10.1016/j.carrev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 138.Chou AY, Sedlak T, Aymong E, Sheth T, Starovoytov A, Humphries KH, Mancini GB, Saw J. Spontaneous coronary artery dissection mis-diagnosed as takotsubo cardiomyopathy: a case series. Can J Cardiol. 2015;31:1073.e5–1073.e8. doi: 10.1016/j.cjca.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 139.Prakash R, Starovoytov A, Heydari M, Mancini GB, Saw J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9:1851–1853. doi: 10.1016/j.jcin.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 140.Awadalla H, Sabet S, El Sebaie A, Rosales O, Smalling R. Catheter-induced left main dissection incidence, predisposition and therapeutic strategies experience from two sides of the hemisphere. J Invasive Cardiol. 2005;17:233–236. [PubMed] [Google Scholar]
- 141.Maehara A, Mintz GS, Castagna MT, Pichard AD, Satler LF, Waksman R, Suddath WO, Kent KM, Weissman NJ. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002;89:466–468. doi: 10.1016/s0002-9149(01)02272-x. [DOI] [PubMed] [Google Scholar]
- 142.Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547–564. doi: 10.1161/CIRCULATIONAHA.116.021886. [DOI] [PubMed] [Google Scholar]
- 143.Rybicki FJ, Udelson JE, Peacock WF, Goldhaber SZ, Isselbacher EM, Kazerooni E, Kontos MC, Litt H, Woodard PK. 2015 ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/SNMMI/STR/STS appropriate utilization of cardiovascular imaging in emergency department patients with chest pain: a joint document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force. J Am Coll Radiol. 2016;13:e1–e29. doi: 10.1016/j.jacr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 144.Takakuwa KM, Keith SW, Estepa AT, Shofer FS. A meta-analysis of 64-section coronary CT angiography findings for predicting 30-day major adverse cardiac events in patients presenting with symptoms suggestive of acute coronary syndrome. Acad Radiol. 2011;18:1522–1528. doi: 10.1016/j.acra.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 145.Achenbach S, Goroll T, Seltmann M, Pflederer T, Anders K, Ropers D, Daniel WG, Uder M, Lell M, Marwan M. Detection of coronary artery stenoses by low-dose, prospectively ECG-triggered, high-pitch spiral coronary CT angiography. JACC Cardiovasc Imaging. 2011;4:328–337. doi: 10.1016/j.jcmg.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 146.Yu L, Fletcher JG, Grant KL, Carter RE, Hough DM, Barlow JM, Vrtiska TJ, Williamson EE, Young PM, Goss BC, Shiung M, Leng S, Raupach R, Schmidt B, Flohr T, McCollough CH. Automatic selection of tube potential for radiation dose reduction in vascular and contrast-enhanced abdominopelvic CT. AJR Am J Roentgenol. 2013;201:W297–W306. doi: 10.2214/AJR.12.9610. [DOI] [PubMed] [Google Scholar]
- 147.Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, Hecht HS, Hlatky MA, Hodgson JM, Lauer MS, Miller JM, Morin RL, Mukherjee D, Poon M, Rubin GD, Schwartz RS. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2509–2543. doi: 10.1161/CIR.0b013e3181d4b618. [DOI] [PubMed] [Google Scholar]
- 148.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D ESC Committee for Practice Guidelines. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the Management of Acute Coronary Syndromes (ACS) in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 149.Eleid MF, Tweet MS, Young PM, Williamson E, Hayes SN, Gulati R. Spontaneous coronary artery dissection: challenges of coronary computed tomography angiography [published online ahead of print January 1, 2017] Eur Heart J Acute Cardiovasc Care. doi: 10.1177/2048872616687098. http://www.journals.sagepub.com/doi/abs/10.1177/2048872616687098?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed&. [DOI] [PubMed]
- 150.Stefanini GG, Windecker S. Can coronary computed tomography angiography replace invasive angiography? Coronary computed tomography angiography cannot replace invasive angiography. Circulation. 2015;131:418–425. doi: 10.1161/CIRCULATIONAHA.114.008148. [DOI] [PubMed] [Google Scholar]
- 151.Tweet MS, Gulati R, Williamson EE, Vrtiska TJ, Hayes SN. Multimodality imaging for spontaneous coronary artery dissection in women. JACC Cardiovasc Imaging. 2016;9:436–450. doi: 10.1016/j.jcmg.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 152.Ichihashi T, Ito T, Murai S, Ikehara N, Fujita H, Suda H, Ohte N. Acute myocardial infarction due to spontaneous, localized, acute dissection of the sinus of Valsalva detected by intravascular ultrasound and electrocardiogram-gated computed tomography. Heart Vessels. 2016;31:1570–1573. doi: 10.1007/s00380-015-0787-5. [DOI] [PubMed] [Google Scholar]
- 153.Alzand BS, Vanneste L, Fonck D, Van Mieghem C. Spontaneous coronary artery dissection undissolved using cardiac computed tomography. Int J Cardiol. 2016;222:1040–1041. doi: 10.1016/j.ijcard.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 154.Guo LQ, Wasfy MM, Hedgire S, Kalra M, Wood M, Prabhakar AM, Ghoshhajra BB. Multimodality imaging of spontaneous coronary artery dissection: case studies of the Massachusetts General Hospital. Coron Artery Dis. 2016;27:70–71. doi: 10.1097/MCA.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 155.Roura G, Ariza-Solé A, Rodriguez-Caballero IF, Gomez-Lara J, Ferreiro JL, Romaguera R, Teruel L, de Albert M, Gomez-Hospital JA, Cequier A. Noninvasive follow-up of patients with spontaneous coronary artery dissection with CT angiography. JACC Cardiovasc Imaging. 2016;9:896–897. doi: 10.1016/j.jcmg.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 156.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation 2014;130:e431–e432] Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 157.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 158.Lempereur M, Fung A, Saw J. Stent mal-apposition with resorption of intramural hematoma with spontaneous coronary artery dissection. Cardiovasc Diagn Ther. 2015;5:323–329. doi: 10.3978/j.issn.2223-3652.2015.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alfonso F, Bastante T, García-Guimaraes M, Pozo E, Cuesta J, Rivero F, Benedicto A, Antuña P, Alvarado T, Gulati R, Saw J. Spontaneous coronary artery dissection: new insights into diagnosis and treatment. Coron Artery Dis. 2016;27:696–706. doi: 10.1097/MCA.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 160.Yumoto K, Sasaki H, Aoki H, Kato K. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc Interv. 2014;7:817–819. doi: 10.1016/j.jcin.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 161.Alkhouli M, Cole M, Ling FS. Coronary artery fenestration prior to stenting in spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2016;88:E23–E27. doi: 10.1002/ccd.26161. [DOI] [PubMed] [Google Scholar]
- 162.Walsh SJ, Jokhi PP, Saw J. Successful percutaneous management of coronary dissection and extensive intramural haematoma associated with ST elevation MI. Acute Card Care. 2008;10:231–233. doi: 10.1080/17482940701802348. [DOI] [PubMed] [Google Scholar]
- 163.Panoulas VF, Ielasi A. Bioresorbable scaffolds and drug-eluting balloons for the management of spontaneous coronary artery dissections. J Thorac Dis. 2016;8:E1328–E1330. doi: 10.21037/jtd.2016.10.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions [published correction appears in Circulation. 2012;125:e412] Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 165.Cockburn J, Yan W, Bhindi R, Hansen P. Spontaneous coronary artery dissection treated with bioresorbable vascular scaffolds guided by optical coherence tomography. Can J Cardiol. 2014;30:1461.e1–1461.e3. doi: 10.1016/j.cjca.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 166.Patel KP, Sotolongo RP, Myrick TW. Off-pump coronary artery bypass grafting for spontaneous coronary dissection in a 26-year-old patient two weeks post-partum: a case report and review. J Extra Corpor Technol. 2008;40:127–129. [PMC free article] [PubMed] [Google Scholar]
- 167.Wehman B, Lehr EJ, Mukherjee R, Grigore A, Griffith B, Bonatti J. Robotic totally endoscopic coronary artery bypass grafting for spontaneous coronary artery dissection. Int J Med Robot. 2012;8:166–168. doi: 10.1002/rcs.437. [DOI] [PubMed] [Google Scholar]
- 168.Cetin E, Ozyuksel A. Beating heart myocardial revascularisation of a sudden cardiac death survivor with spontaneous coronary artery dissection: pitfalls from diagnosis to surgery. BMJ Case Rep. 2014;2014:bcr2014207188. doi: 10.1136/bcr-2014-207188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Unal M, Korkut AK, Kosem M, Ertunc V, Ozcan M, Caglar N. Surgical management of spontaneous coronary artery dissection. Tex Heart Inst J. 2008;35:402–405. [PMC free article] [PubMed] [Google Scholar]
- 170.Motreff P, Souteyrand G, Dauphin C, Eschalier R, Cassagnes J, Lusson JR. Management of spontaneous coronary artery dissection: review of the literature and discussion based on a series of 12 young women with acute coronary syndrome. Cardiology. 2010;115:10–18. doi: 10.1159/000244608. [DOI] [PubMed] [Google Scholar]
- 171.McGrath-Cadell L, McKenzie P, Emmanuel S, Muller DW, Graham RM, Holloway CJ. Outcomes of patients with spontaneous coronary artery dissection. Open Heart. 2016;3:e000491. doi: 10.1136/openhrt-2016-000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 173.Farhat F, Sassard T, Maghiar A, Jegaden O. Primary spontaneous coronary artery dissection complicated by iatrogenous aortic dissection: from David procedure to full arterial coronary revascularization. Interact Cardiovasc Thorac Surg. 2006;5:149–152. doi: 10.1510/icvts.2005.124065. [DOI] [PubMed] [Google Scholar]
- 174.Aliyary S, Mariani MA, Verhorst PM, Hartmann M, Stoel MG, von Birgelen C. Staged therapeutic approach in spontaneous coronary dissection. Ann Thorac Surg. 2007;83:1879–1881. doi: 10.1016/j.athoracsur.2006.11.085. [DOI] [PubMed] [Google Scholar]
- 175.Páez M, Buisán F, Herrero E. Spontaneous dissection of the left coronary artery trunk during the postpartum period treated with revascularization surgery, ventricular assistance and a successful heart transplant. Acta Anaesthesiol Scand. 2007;51:960–961. doi: 10.1111/j.1399-6576.2007.01359.x. [DOI] [PubMed] [Google Scholar]
- 176.Rahman S, Abdul-Waheed M, Helmy T, Huffman LC, Koshal V, Guitron J, Merrill WH, Lewis DF, Dunlap S, Shizukuda Y, Weintraub NL, Meyer C, Cilingiroglu M. Spontaneous left main coronary artery dissection complicated by pseudoaneurysm formation in pregnancy: role of CT coronary angiography. J Cardiothorac Surg. 2009;4:15. doi: 10.1186/1749-8090-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jacob JC, Kiernan FJ, Patel N, Rock J, Hammond J, Jr, Wencker D, Lasala AF. SCAD: a rare case of cardiac arrest in a young female. Conn Med. 2011;75:147–152. [PubMed] [Google Scholar]
- 178.Julià I, Tauron M, Muñoz-Guijosa C. Postpartum acute coronary syndrome due to intramural hematoma and coronary artery dissection. Thorac Cardiovasc Surg. 2013;61:85–87. doi: 10.1055/s-0032-1331152. [DOI] [PubMed] [Google Scholar]
- 179.Weinberg L, Ong M, Tan CO, McDonnell NJ, Lo C, Chiam E. Spontaneous coronary artery dissection in pregnancy requiring emergency caesarean delivery followed by coronary artery bypass grafting. Anaesth Intensive Care. 2013;41:251–255. doi: 10.1177/0310057X1304100215. [DOI] [PubMed] [Google Scholar]
- 180.Abu-Laban RB, Migneault D, Grant MR, Dhingra V, Fung A, Cook RC, Sweet D. Extracorporeal membrane oxygenation after protracted ventricular fibrillation cardiac arrest: case report and discussion. CJEM. 2015;17:210–216. doi: 10.2310/8000.2014.141439. [DOI] [PubMed] [Google Scholar]
- 181.Jorge-Pérez P, García-González MJ, Ávalos-Pinto RM, Cosio-Carmena G-MD, Renes-Carreño E, Delgado JF, Yanes-Bowden G, Ferrer-Hita JJ. Spontaneous coronary dissection and cardiogenic shock requiring mechanical circulatory support in a non-transplant center. Int J Cardiol. 2016;221:629–630. doi: 10.1016/j.ijcard.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 182.Patanè F, Boffini M, Sansone F, Campanella A, Rinaldi M. ECMO as a bridge to transplantation in biventricular dysfunction due to primary spontaneous coronary artery dissection. Transpl Int. 2009;22:500–502. doi: 10.1111/j.1432-2277.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- 183.Martins RP, Leurent G, Corbineau H, Fouquet O, Seconda S, Baruteau AE, Moreau O, Le Breton H, Bedossa M. Coronary angiography of pregnancy-associated coronary artery dissection: a high-risk procedure. Cardiovasc Revasc Med. 2010;11:182–185. doi: 10.1016/j.carrev.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 184.Bashir M, Mustafa H, Singh H, Bonser R. Cardiac transplantation for spontaneous coronary artery dissection. Interact Cardiovasc Thorac Surg. 2013;16:91–92. doi: 10.1093/icvts/ivs413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Evans R. Post-partum spontaneous coronary artery dissection and the use of veno-arterial extra-corporeal membrane oxygenation. Nurs Crit Care. 2014;19:304–309. doi: 10.1111/nicc.12085. [DOI] [PubMed] [Google Scholar]
- 186.Knapp KE, Weis RA, Cubillo EI, Chapital AB, Ramakrishna H. Spontaneous, Postpartum coronary artery dissection and cardiogenic shock with extracorporeal membrane oxygenation assisted recovery in a 30-year-old patient. Case Rep Cardiol. 2016;2016:1048708. doi: 10.1155/2016/1048708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society for Thoracic Surgeons (STS); American Heart Association (AHA); American College of Cardiology (ACC) 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care. J Card Fail. 2015;21:499–518. doi: 10.1016/j.cardfail.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 188.Sharma S, Rozen G, Duran J, Mela T, Wood MJ. Sudden cardiac death in patients with spontaneous coronary artery dissection. J Am Coll Cardiol. 2017;70:114–115. doi: 10.1016/j.jacc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 189.Kusumoto FM, Calkins H, Boehmer J, Buxton AE, Chung MK, Gold MR, Hohnloser SH, Indik J, Lee R, Mehra MR, Menon V, Page RL, Shen WK, Slotwiner DJ, Stevenson LW, Varosy PD, Welikovitch L. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94–125. doi: 10.1161/CIR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 190.Yip A, Saw J. Spontaneous coronary artery dissection: a review. Cardiovasc Diagn Ther. 2015;5:37–48. doi: 10.3978/j.issn.2223-3652.2015.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tweet MS, Gulati R, Hayes SN. What clinicians should know αbout spontaneous coronary artery dissection. Mayo Clin Proc. 2015;90:1125–1130. doi: 10.1016/j.mayocp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 192.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ ESC Committee for Practice Guidelines. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult: the Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 193.Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 194.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S46–S48 and Circulation. 2015;132:e396] Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853. [DOI] [PubMed] [Google Scholar]
- 195.Elkayam U, Goland S, Pieper PG, Silverside CK. High-risk cardiac disease in pregnancy, part I. J Am Coll Cardiol. 2016;68:396–410. doi: 10.1016/j.jacc.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 196.Jeejeebhoy FM, Zelop CM, Lipman S, Carvalho B, Joglar J, Mhyre JM, Katz VL, Lapinsky SE, Einav S, Warnes CA, Page RL, Griffin RE, Jain A, Dainty KN, Arafeh J, Windrim R, Koren G, Callaway CW on behalf of the American Heart Association Emergency Cardiovascular Care Committee, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Diseases in the Young, and Council on Clinical Cardiology. Cardiac arrest in pregnancy: a scientific statement from the American Heart Association. Circulation. 2015;132:1747–1773. doi: 10.1161/CIR.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 197.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L. ESC guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 198.Colletti PM, Lee KH, Elkayam U. Cardiovascular imaging of the pregnant patient. AJR Am J Roentgenol. 2013;200:515–521. doi: 10.2214/AJR.12.9864. [DOI] [PubMed] [Google Scholar]
- 199.American College of Obstetricians, Gynecologists’ Committee on Obstetric Practice. Committee opinion No. 656: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. 2016;127:e75–e80. doi: 10.1097/AOG.0000000000001316. [DOI] [PubMed] [Google Scholar]
- 200.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, Harper A, Hulbert D, Lucas S, McClure J, Millward-Sadler H, Neilson J, Nelson-Piercy C, Norman J, O’Herlihy C, Oates M, Shakespeare J, de Swiet M, Williamson C, Beale V, Knight M, Lennox C, Miller A, Parmar D, Rogers J, Springett A. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008: the eighth report of the Confidential Enquiries Into Maternal Deaths in the United Kingdom. BJOG. 2011;118(suppl):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 201.Myers GR, Hoffman MK, Marshall ES. Clopidogrel use throughout pregnancy in a patient with a drug-eluting coronary stent. Obstet Gynecol. 2011;118(pt 2):432–433. doi: 10.1097/AOG.0b013e318213d024. [DOI] [PubMed] [Google Scholar]
- 202.Boztosun B, Olcay A, Avci A, Kirma C. Treatment of acute myocardial infarction in pregnancy with coronary artery balloon angioplasty and stenting: use of tirofiban and clopidogrel. Int J Cardiol. 2008;127:413–416. doi: 10.1016/j.ijcard.2007.04.174. [DOI] [PubMed] [Google Scholar]
- 203.Babic Z, Gabric ID, Pintaric H. Successful primary percutaneous coronary intervention in the first trimester of pregnancy. Catheter Cardiovasc Interv. 2011;77:522–525. doi: 10.1002/ccd.22813. [DOI] [PubMed] [Google Scholar]
- 204.De Santis M, De Luca C, Mappa I, Cesari E, Mazza A, Quattrocchi T, Caruso A. Clopidogrel treatment during pregnancy: a case report and a review of literature. Intern Med. 2011;50:1769–1773. doi: 10.2169/internalmedicine.50.5294. [DOI] [PubMed] [Google Scholar]
- 205.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52:171–180. doi: 10.1016/j.jacc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 206.Briggs GG, Freeman RK. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 10. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 207.Peacock WF, 4th, Hilleman DE, Levy PD, Rhoney DH, Varon J. A systematic review of nicardipine vs labetalol for the management of hypertensive crises. Am J Emerg Med. 2012;30:981–993. doi: 10.1016/j.ajem.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 208.Lydakis C, Lip GY, Beevers M, Beevers DG. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541–547. doi: 10.1016/s0895-7061(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 209.Hayes SN. Spontaneous coronary artery dissection (SCAD): new insights into this not-so-rare condition. Tex Heart Inst J. 2014;41:295–298. doi: 10.14503/THIJ-14-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures for ST-Elevation and Non-ST-Elevation Myocardial Infarction) Circulation. 2008;118:2596–2648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 211.Thomas RJ, King M, Lui K, Oldridge N, Pina IL, Spertus J. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation) Circulation. 2010;122:1342–1350. doi: 10.1161/CIR.0b013e3181f5185b. [DOI] [PubMed] [Google Scholar]
- 212.Brubaker PH, Ross JH, Chan Joo K. Contemporary approaches to prescribing exercise in coronary artery disease patients [published online ahead of print January 19, 2016] Am J Lifestyle Med. doi: 10.1177/1559827615625482. https://doi.org/10.1177/1559827615625482. [DOI] [PMC free article] [PubMed]
- 213.Krittanawong C, Tweet MS, Hayes SE, Bowman MJ, Gulati R, Squires RW, Hayes SN. Usefulness of cardiac rehabilitation after spontaneous coronary artery dissection. Am J Cardiol. 2016;117:1604–1609. doi: 10.1016/j.amjcard.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 214.Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, Ignaszewski A, Chan S, Starovoytov A, Saw J. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 2016;32:554–560. doi: 10.1016/j.cjca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 215.Tweet MS, Gulati R, Hayes SN. Spontaneous coronary artery dissection. Curr Cardiol Rep. 2016;18:60. doi: 10.1007/s11886-016-0737-6. [DOI] [PubMed] [Google Scholar]
- 216.Kureshi F, Kennedy KF, Jones PG, Thomas RJ, Arnold SV, Sharma P, Fendler T, Buchanan DM, Qintar M, Ho PM, Nallamothu BK, Oldridge NB, Spertus JA. Association between cardiac rehabilitation participation and health status outcomes after acute myocardial infarction. JAMA Cardiol. 2016;1:980–988. doi: 10.1001/jamacardio.2016.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127:538–546. doi: 10.1016/j.amjmed.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Arena R, Williams M, Forman DE, Cahalin LP, Coke L, Myers J, Hamm L, Kris-Etherton P, Humphrey R, Bittner V, Lavie CJ on behalf of the American Heart Association Exercise, Cardiac Rehabilitation and Prevention Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity and Metabolism. Increasing referral and participation rates to outpatient cardiac rehabilitation: the valuable role of healthcare professionals in the inpatient and home health settings: a science advisory from the American Heart Association. Circulation. 2012;125:1321–1329. doi: 10.1161/CIR.0b013e318246b1e5. [DOI] [PubMed] [Google Scholar]
- 219.de Pinto MC, Camargo RC, Filho JC, Fregonesi CE, Gonçalves AC, de Abreu LC, Vanderlei LC, Lorençoni RM. Influence of cardiac rehabilitation in primigravida with spontaneous coronary artery dissection during postpartum. Int Arch Med. 2014;7:20. doi: 10.1186/1755-7682-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Silber TC, Tweet MS, Bowman MJ, Hayes SN, Squires RW. Cardiac rehabilitation after spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2015;35:328–333. doi: 10.1097/HCR.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 221.Saw JWL, Starovoytov A, Birnie T, Prakash R, Heydari-Kamjani M, Isserow S, Taylor C, Chan S, Ignaszewski A. Comparison of psychosocial questionnaires between spontaneous coronary artery dissection (SCAD) and non-SCAD populations undergoing cardiac rehabilitation program after myocardial infarction. J Am Coll Cardiol. 2016;67:1936. [Google Scholar]
- 222.Dunlay SM, Witt BJ, Allison TG, Hayes SN, Weston SA, Koepsell E, Roger VL. Barriers to participation in cardiac rehabilitation. Am Heart J. 2009;158:852–859. doi: 10.1016/j.ahj.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yiangou K, Papadopoulos K, Azina C. Heavy lifting causing spontaneous coronary artery dissection with anterior myocardial infarction in a 54-year-old woman. Tex Heart Inst J. 2016;43:189–191. doi: 10.14503/THIJ-15-5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Forman DE, Myers J, Lavie CJ, Guazzi M, Celli B, Arena R. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med. 2010;122:68–86. doi: 10.3810/pgm.2010.11.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liang JJ, Tweet MS, Hayes SE, Gulati R, Hayes SN. Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2014;34:138–142. doi: 10.1097/HCR.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 226.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine [published correction appears in Circulation. 2010;122:e410] Circulation. 2010;121:e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 227.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158:2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 228.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134:173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 229.Garavalia LS, Decker C, Reid KJ, Lichtman JH, Parashar S, Vaccarino V, Krumholz HM, Spertus JA. Does health status differ between men and women in early recovery after myocardial infarction? J Womens Health (Larchmt) 2007;16:93–101. doi: 10.1089/jwh.2006.M073. [DOI] [PubMed] [Google Scholar]
- 230.Dreyer RP, Wang Y, Strait KM, Lorenze NP, D’Onofrio G, Bueno H, Lichtman JH, Spertus JA, Krumholz HM. Gender differences in the trajectory of recovery in health status among young patients with acute myocardial infarction: results from the variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study. Circulation. 2015;131:1971–1980. doi: 10.1161/CIRCULATIONAHA.114.014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bucholz EM, Strait KM, Dreyer RP, Lindau ST, D’Onofrio G, Geda M, Spatz ES, Beltrame JF, Lichtman JH, Lorenze NP, Bueno H, Krumholz HM. Sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6:610–622. doi: 10.1177/2048872616661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Smolderen KG, Strait KM, Dreyer RP, D’Onofrio G, Zhou S, Lichtman JH, Geda M, Bueno H, Beltrame J, Safdar B, Krumholz HM, Spertus JA. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. J Am Heart Assoc. 2015;4:e001424. doi: 10.1161/JAHA.114.001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. Am J Prev Med. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 234.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V PREMIER Registry Investigators. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006;166:876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 235.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 236.Saw J, Sedlak T, Ganesh SK, Isserow S, Mancini GB. Cardiology patient page: spontaneous coronary artery dissection (SCAD) Circulation. 2015;131:e3–e5. doi: 10.1161/CIRCULATIONAHA.114.010773. [DOI] [PubMed] [Google Scholar]
- 237.WomenHeart. [Accessed February 8, 2018];The National Coalition for Women with Heart Disease website. http://www.womenheart.org.
- 238. [Accessed February 8, 2018];SCAD Alliance website. http://www.SCADAlliance.org.
- 239. [Accessed February 8, 2018];Beat SCAD UK website. http://www.beatscad.org.uk.
- 240.SCAD Research Inc. [Accessed February 8, 2018];Spontaneous coronary artery dissection. http://www.SCADResearch.org.
- 241.Tweet MS, Codsi E, Best PJM, Gulati R, Rose CH, Hayes SN. Menstrual chest pain in women with history of spontaneous coronary artery dissection. J Am Coll Cardiol. 2017;70:2308–2309. doi: 10.1016/j.jacc.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tweet MS, Hayes SN, Gulati R, Rose CH, Best PJ. Pregnancy after spontaneous coronary artery dissection: a case series. Ann Intern Med. 2015;162:598–600. doi: 10.7326/L14-0446. [DOI] [PubMed] [Google Scholar]
- 243.Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS Maternal Health Study Group of the Canadian Perinatal Surveillance System. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176:455–460. doi: 10.1503/cmaj.060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, Mital S, Rose C, Silversides C, Stout K on behalf of the American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e50–e87. doi: 10.1161/CIR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 245.The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017;24:728–753. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 246.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Lévesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY for the Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 247.Munro MG, Critchley HO, Broder MS, Fraser IS FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 248.Coxib and Traditional NSAID Trialists’ (CNT) Collaboration. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.US Food and Drug Administration. [Accessed October 12, 2017];FDA drug safety communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. 2015 Jul 9; last updated January 16, 2016. https://www.fda.gov/drugs/drugsafety/ucm451800.htm.
- 250.Garg J, Pinnamaneni S, Aronow WS, Ahmad H. ST elevation myocardial infarction after tranexamic acid: first reported case in the United States. Am J Ther. 2014;21:e221–e224. doi: 10.1097/MJT.0b013e31828fdb06. [DOI] [PubMed] [Google Scholar]
- 251.Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673–677. doi: 10.1016/j.fertnstert.2007.07.1315. [DOI] [PubMed] [Google Scholar]
- 252.Matteson KA, Rahn DD, Wheeler TL, 2nd, Casiano E, Siddiqui NY, Harvie HS, Mamik MM, Balk EM, Sung VW Society of Gynecologic Surgeons Systematic Review Group. Nonsurgical management of heavy menstrual bleeding: a systematic review. Obstet Gynecol. 2013;121:632–643. doi: 10.1097/AOG.0b013e3182839e0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, Gemzell-Danielsson K. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol. 2013;122:1205–1213. doi: 10.1097/AOG.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 254.Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104–1116. doi: 10.1097/AOG.0b013e3181a1d3ce. [DOI] [PubMed] [Google Scholar]
- 255.Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193–215. doi: 10.2165/11598960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 256.Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2016:CD003855. doi: 10.1002/14651858.CD003855. [DOI] [PubMed] [Google Scholar]
- 257.Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, Jaff MR, Kim ES, Mace P, Matsumoto AH, McBane RD, Kline-Rogers E, White CJ, Gornik HL. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 2012;125:3182–3190. doi: 10.1161/CIRCULATIONAHA.112.091223. [DOI] [PubMed] [Google Scholar]
- 258.Cloft HJ, Kallmes DF, Kallmes MH, Goldstein JH, Jensen ME, Dion JE. Prevalence of cerebral aneurysms in patients with fibromuscular dysplasia: a reassessment. J Neurosurg. 1998;88:436–440. doi: 10.3171/jns.1998.88.3.0436. [DOI] [PubMed] [Google Scholar]
- 259.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 260.Chapman AB, Rubinstein D, Hughes R, Stears JC, Earnest MP, Johnson AM, Gabow PA, Kaehny WD. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992;327:916–920. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 261.Germain DP. Clinical and genetic features of vascular Ehlers-Danlos syndrome. Ann Vasc Surg. 2002;16:391–397. doi: 10.1007/s10016-001-0229-y. [DOI] [PubMed] [Google Scholar]
- 262.Badani KK, Hemal AK, Menon M. Autosomal dominant polycystic kidney disease and pain: a review of the disease from aetiology, evaluation, past surgical treatment options to current practice. J Postgrad Med. 2004;50:222–226. [PubMed] [Google Scholar]
- 263.Brown RD. Unruptured intracranial aneurysms. Semin Neurol. 2010;30:537–544. doi: 10.1055/s-0030-1268858. [DOI] [PubMed] [Google Scholar]
- 264.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 265.Magnetic Resonance Angiography in Relatives of Patients with Subarachnoid Hemorrhage Study Group. Risks and benefits of screening for intracranial aneurysms in first-degree relatives of patients with sporadic subarachnoid hemorrhage. N Engl J Med. 1999;341:1344–1350. doi: 10.1056/NEJM199910283411803. [DOI] [PubMed] [Google Scholar]
- 266.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 267.Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 268.Thompson BG, Brown RD, Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, Jr, Duckwiler GR, Harris CC, Howard VJ, Johnston SC, Meyers PM, Molyneux A, Ogilvy CS, Ringer AJ, Torner J on behalf of the American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention; American Heart Association; American Stroke Association. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2368–2400. doi: 10.1161/STR.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 269.Bolen MA, Brinza E, Renapurkar RD, Kim ESH, Gornik HL. Screening CT angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC Cardiovasc Imaging. 2017;10:554–561. doi: 10.1016/j.jcmg.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 270.Persu A, Touzé E, Mousseaux E, Barral X, Joffre F, Plouin PF. Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest. 2012;42:338–347. doi: 10.1111/j.1365-2362.2011.02577.x. [DOI] [PubMed] [Google Scholar]
- 271.Wang H, Li W, He H, Luo L, Chen C, Guo Y. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15–e20. doi: 10.1016/j.crad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 272.Varennes L, Tahon F, Kastler A, Grand S, Thony F, Baguet JP, Detante O, Touzé E, Krainik A. Fibromuscular dysplasia: what the radiologist should know: a pictorial review. Insights Imaging. 2015;6:295–307. doi: 10.1007/s13244-015-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Li MH, Cheng YS, Li YD, Fang C, Chen SW, Wang W, Hu DJ, Xu HW. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke. 2009;40:3127–3129. doi: 10.1161/STROKEAHA.109.553800. [DOI] [PubMed] [Google Scholar]
- 274.National Research Council. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 275.Fazel R, Gerber TC, Balter S, Brenner DJ, Carr JJ, Cerqueira MD, Chen J, Einstein AJ, Krumholz HM, Mahesh M, McCollough CH, Min JK, Morin RL, Nallamothu BK, Nasir K, Redberg RF, Shaw LJ on behalf of the American Heart Association Council on Quality of Care and Outcomes Research, Council on Clinical Cardiology, and Council on Cardiovascular Radiology and Intervention. Approaches to enhancing radiation safety in cardiovascular imaging: a scientific statement from the American Heart Association [published correction appears in Circulation. 2014;130:e172] Circulation. 2014;130:1730–1748. doi: 10.1161/CIR.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 276.Hill KD, Einstein AJ. New approaches to reduce radiation exposure. Trends Cardiovasc Med. 2016;26:55–65. doi: 10.1016/j.tcm.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ashley EA, Hershberger RE, Caleshu C, Ellinor PT, Garcia JG, Herrington DM, Ho CY, Johnson JA, Kittner SJ, Macrae CA, Mudd-Martin G, Rader DJ, Roden DM, Scholes D, Sellke FW, Towbin JA, Van Eyk J, Worrall BB on behalf of the American Heart Association Advocacy Coordinating Committee. Genetics and cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2012;126:142–157. doi: 10.1161/CIR.0b013e31825b07f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, Rubenstein A, Viskochil D. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–57. [PubMed] [Google Scholar]
- 279.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 280.Sultan A, Kreutz RP. Variations in clinical presentation, risk factors, treatment, and prognosis of spontaneous coronary artery dissection. J Invasive Cardiol. 2015;27:363–369. [PubMed] [Google Scholar]
- 281.Main T, Prakash R, Starovoytov A, Sabbaghan A, Aymong E, Mancini G, Saw J. Characteristics of extension and de novo recurrent spontaneous coronary artery dissection. Eurointervention. 2017;13:e1454–e1459. doi: 10.4244/EIJ-D-17-00264. [DOI] [PubMed] [Google Scholar]