Abstract
The risk factors for developing alcohol addiction include impulsivity, high sensitivity to the rewarding action of ethanol, and low sensitivity to its sedative and intoxicating effects. Genetic variation in GABAA receptor subunits, including the ɣ2 subunit (Gabrg2), affects the risk for developing alcoholism. Alcohol directly potentiates GABAA receptors and activates the mesolimbic dopamine system. Here, we deleted Gabrg2 selectively in dopamine cells of adult mice. The deletion resulted in elevated firing of dopamine neurons and made them less sensitive to drugs acting at GABAA receptors. At the behavioral level, the deletion increased exploratory behavior and augmented both correct and incorrect responding in the go/no-go task, a test often used to assay the response inhibition component of impulsivity. In addition, conditioned place preference to alcohol, but not to cocaine or morphine, was increased. Ethanol-induced locomotor activation was enhanced in the mice lacking Gabrg2 on dopaminergic cells, whereas the sedative effect of alcohol was reduced. Finally, the alcohol drinking, but not the alcohol preference, at a high concentration was increased in the mutant mice. In summary, deletion of Gabrg2 on dopamine cells induced several behavioral traits associated with high risk of developing alcoholism. The findings suggest that mice lacking Gabrg2 on dopaminergic cells could be used as models for individuals at high risk for developing alcoholism and that GABAA receptors on dopamine cells are protective against the development of excessive alcohol drinking.
Introduction
Excessive intake of alcohol causes considerable morbidity and mortality [1]. Whereas many people drink alcohol occasionally, only a minority escalate their intake and develop alcohol addiction, here equated with alcoholism. The heritability of alcoholism is 50–70% [1]. This genetic susceptibility to a large extent operate through intermediate characteristics, such as impulsivity [2], high sensitivity to the rewarding effects of alcohol [3, 4], and low sensitivity to the sedative and intoxicating effects [5, 6].
Human gene association studies have identified strong linkage between variations in genes encoding GABAA receptor (GABAAR) subunits and alcoholism [7]. For example, variants of the α2 subunit (GABRA2) and the ɣ2 subunit (GABRG2) genes have been associated with increased risk of alcohol abuse [8, 9]. GABAARs are one of the best studied direct targets of ethanol. Many functional features of the GABAARs, including ethanol sensitivity, depend on their subunit composition [7, 10–12].
Ethanol induces dopamine release in the ventral striatum of both rodents [13] and humans [14, 15]. In rodents, it has been shown that alcohol activates dopaminergic cells both in vitro [16, 17] and in vivo [16] and that the activation of dopamine cells at least partially mediate the reinforcing effects of alcohol [1, 18]. Whereas the exact mechanisms behind the activation of dopamine cells are not clear, it most likely involves the modulation of GABAergic input onto dopaminergic cells [1, 19, 20]. Collectively, these findings suggest that the subunit composition of GABAARs on neurons of the dopaminergic reward system might be critical for shaping the response to alcohol and underpin the individual variability in the risk of developing alcoholism. Here, we generated mice selectively lacking Gabrg2 subunits on dopamine cells. We then examined their dopamine cells with emphasis on GABA responsiveness and their behavior with emphasis on phenotypes associated with risk for alcoholism.
Materials and methods
Animals
All experiments followed international and national guidelines and were approved by local animal care and use committees. The mouse line with floxed Gabrg2 was acquired from Jackson Laboratories (stock no: 016830). The DATCreER line has been described previously [21]. The animals were single-housed for a minimum of 24 h prior to the experiments and kept in a pathogen-free facility on a regular 12-h light/dark cycle. Only male mice were used. All mice were >10 weeks old at the onset of the behavioral or electrophysiological experiments, and the typical age was 10–20 weeks. Food and water were provided ad libitum, and all experiments were performed during the light phase, unless otherwise noted. The Cre-recombinase activity was induced by administering tamoxifen dissolved in sunflower seed oil/alcohol mixture 10:1. The mixture was intraperitoneally (i.p.) injected at 1 mg per mouse twice a day for 5 days to both Cre-positive and Cre-negative mice. Experiments were initiated at earliest 3 weeks after tamoxifen administration. The mice had a mixed 129x1/SvJ × C57BL/6J background (~50% of each). At the start of the behavioral experiments, there was no difference between the genotypes in body weight (Fig. S4A).
Electrophysiology
The electrophysiological experiments were performed using previously published protocols [22]. Briefly, mice were anesthetized with urethane and neuronal activity was extracellularly recorded. DA-like cells were identified based on electrophysiological criteria [23, 24] and localization in the ventral tegmental area and the substantia nigra (Figs. S1, S2). The responses of DA-like neurons to bicuculline and muscimol were tested by local, iontophoretic application of the drugs. All technical details are described in the Supplemental Methods.
Drugs
Ethanol was dissolved in 0.9% saline and injected i.p. in a volume of 0.1 ml per 10 g of body weight. Control mice received a similar volume of saline. Mice were injected with the dose of either 1 g/kg (for ethanol-induced locomotion), 2 g/kg (for conditioned place preference (CPP)), or 3.6 g/kg (for loss of righting reflex). Cocaine hydrochloride (APL, Kungens Kurva, Sweden) was dissolved in 0.9% saline and administered in a single i.p. injection at a dose of 15 mg/kg. Morphine hydrochloride (Biophausia AB, Stockholm, Sweden) was injected i.p. at 10 mg/kg.
Behavioral studies
Open-field test
Locomotor activity was assessed by placing individual mice in an open-field arena (45 × 45 cm), and recording their activity for 15 min with a video camera placed from the top of the arena. The software EthoVision (Noldus) was used for data collection and processing, which provided measures of the total distance traveled and the time mice spent in center of the arena.
Elevated plus maze test
The protocol used was based on previously published studies [25, 26]. The test was performed during the dark phase, ~1–2 h after lights were switched off. The apparatus consisted of two open (25 × 5 × 0.5 cm) and two closed arms (25 × 5 × 16 cm) that were perpendicularly connected with central platform (5 × 5 × 0.5 cm) [27]. The apparatus was elevated 50 cm above the ground. In order to decrease the number of animals falling down from the apparatus, open arms had a low raised lip (0.5 cm) around the edges. The test was performed in a brightly illuminated room. The mice were placed on the central platform facing the open arm and their behavior was recorded for 5 min. Video files were analyzed by EthoVision tracking system and the percentage of time mice spent in the open arm was quantified.
Forced swim test
The forced swim test [28] was conducted in glass cylindrical beakers (30 cm H × 20 cm diameter) with water temperature set at 25 ± 0.5 °C. The mice were placed in the beakers and videotaped by a camera set in front of beakers for 6 min. Subsequently, they were taken out and dried with paper towels before being placed back in their original cages. Water was replaced between each session and temperature adjusted. Recorded video files were analyzed by EthoVision software with immobility threshold set to 20%. Results were expressed as percentage of time mice spent immobile.
Reciprocal social interaction test
This test was used as an indicator of social exploration and the protocol was adopted from previous studies [29]. The mice were allowed to freely interact in novel arena and the time they spent interacting was measured. Interaction time for each mouse in the pair is directly impacted by the behavior of the partner animal. Prior to the experiment, mice were single-housed for at least 2 days. At the day of experiment, male mice of similar age and weight (one control and one transgenic) were placed in a larger cage that was brightly illuminated and unfamiliar to the subjects. A camera was mounted above the cage and interaction was videotaped for 10 min. Recorded video files were manually scored for the time mice individually spent in active social behavior, such as following, sniffing, and climbing on or under the other mouse. Aggressive behaviors were not considered as social behavior.
Sucrose preference test
Sucrose preference was assessed as described before [30]. Prior experiment, mice were single-housed for at least 3 days and habituated to the two bottles (50 ml Falcon tubes with nibbles) containing water for 2 days. In the next 2 days, both bottles were replaced with 1% (w/v) sucrose dissolved in water. On the fourth day, mice were offered one bottle of water and one of sucrose and preference was measured over the course of next 4 days. Bottles were switched daily to exclude possible side bias. Consumed fluid was measured daily. Preference for sucrose was calculated by the following formula: 100 × (volsucrose /(volwater + volsucrose)) and averaged across the last 4 days.
Go/no-go test
The go/no-go test was performed based on protocols previously used by us [31]. Details of the procedure can be found in the Supplemental Methods. Briefly, water-deprived (1.5 ml per day) mice were trained under continuous reinforcement schedule and were rewarded (10 μl water sweetened with 0.01% (w/v) saccharin) by poking their noses into the active port (with cue-light on). Animals were trained until they reached the criterion of 60 rewarded responses in 45 min. Subsequently, animals underwent two training phases during which they learned to respond to a “go” signal (cue-light in the nose-poke port). Beginning of each trial was signaled by a stimulus light located above the nose-poke port. At the same time, pre-cue period ranging from 9 to 24 s was initiated, after which the “go” signal was presented. Correct “go” response (a “hit”) resulted in delivery of 10 μl of saccharin solution. Trials in which animals failed to respond to a “go” signal (a “miss”) were punished by 5 s time-out period during which the house light was lit, and after which the 10 s inter-trial interval followed (with the house light off).
Next, animals had to discriminate between “go” (cue-light in the nose-poke port presented for 5 s) and “no-go” signals (cue-light and a continuous 65 dB 2.9 kHz tone presented for 5 s). Responding to the “go” signal and refraining from responding during “no-go” signal presentation (a “correct rejection”) was rewarded. Conversely, a nose-poke during the “no-go” signal (a “false alarm”) resulted in immediate trial termination and transition into 5 s time-out period, followed by 10 s ITI. Animals were tested for 10 consecutive sessions (each comprising 60 trials), during which “go” and “no-go” signals were presented randomly. A single session contained 30 “go” and 30 “no-go” trials.
Conditioned place preference test
We used a biased place conditioning procedure and 3-chambered Panlab Spatial Place Preference Boxes (Harvard Apparatus). On day 1, during a 15-min pretest, the individual mouse was allowed to move freely between the chambers of the box. Time spent in each compartment was recorded by camera mounted above the apparatus and analyzed by EthoVision software. Each mouse had to cross the corridor, entering the opposing chamber a minimum of five times to be included in the experiment. On day 2, in the morning session, mice were treated with saline (i.p.) and confined in the vehicle-assigned chamber for 15 min. Similarly, 4 h after the first injection, mice were injected i.p. with either ethanol, cocaine, or morphine and confined for 15 min in the compartment least preferred during the pretest. This training procedure was conducted for 4 consecutive days, until day 6, when the CPP was assessed by allowing the mice to freely explore all compartments of the box for 15 min. The preference score was calculated by subtracting the time the mouse spent in the drug-paired chamber during the post-test from that of the pretest.
Alcohol-induced locomotion
During 3 consecutive days, mice were placed in one of the chambers of a Panlab Spatial Place Preference Box (20 × 18 cm, Harvard Apparatus) for 15 min before receiving a saline injection (10 µl/g i.p.). After injection, they were put back in the chamber for another 15 min. On the fourth day, saline was replaced with an equal volume of ethanol (1 g/kg). Distance moved before and after injection was analyzed with EthoVision software. Difference in locomotion was calculated as distance moved after ethanol-injection day 4 minus distance moved after saline-injection day 3.
Loss of righting reflex
In order to assess the sedative effect of ethanol, mice were injected (i.p.) with 3.6 g/kg of ethanol (as 20% solution dissolved in saline). The time elapsed for mice to become ataxic was measured and then mice were turned on their back. Duration of LORR was measured in minutes. Mice were considered to regain their righting reflex if they were able to right themselves within 60 s [32].
Ethanol drinking
Mice were single-housed for 1 week before starting the experiment and offered two bottles of water in order to acclimatize. Ethanol preference was assessed by introducing one bottle filled with ethanol and another with water. Mice were offered escalating concentrations of ethanol (in tap water) ranging from 3 to 21%. Each ethanol concentration was offered vs. water for 2 days. Fluid intake was measured and positions of the bottles were switched every second day. Body weight was measured every 4 days. Ethanol consumption (g/kg × day) and total fluid consumption were measured [32].
Statistics
For the behavioral analysis, two-tailed Student’s t test was used when comparing one variable in two groups. For comparing multiple sessions in two groups, two-way repeated measures analysis of variance (ANOVA) was used. Analysis of ethanol consumption was done by two-way ANOVA followed by Sidak’s multiple comparisons test. For the electrophysiology, two-way ANOVA followed by Bonferroni multiple comparisons test was used.
Results
We generated mice lacking Gabrg2 in dopamine cells (Gabrg2DATCreER mice) by crossing mice in which critical parts of the gene encoding Gabrg2 were floxed [33], with mice carrying an inducible Cre under the control of the dopamine transporter promoter [21]. This Cre-line has been used in several previous studies and it has been shown to mediate efficient and selective recombination of dopaminergic cells using several different techniques [21, 34–36]. The mutations were induced by tamoxifen administration in mice of at least 7 weeks of age in order to minimize the risk for the development of compensatory mechanisms [21]. Mice with selective deletions induced by Cre were compared to littermates homozygous for the floxed Gabrg2 allele but without Cre (called WT (wild type) in the following text), which were also treated with tamoxifen.
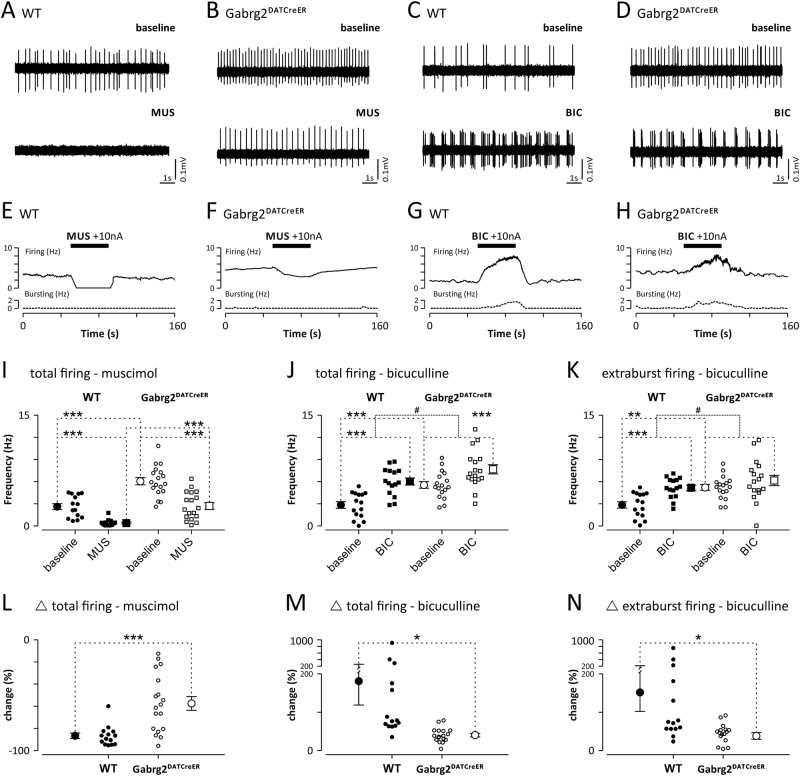
First, we used in vivo electrophysiology to investigate the consequences of the Gabrg2 deletion in the dopamine cells. We recorded spontaneous activity of 15 neurons with the electrophysiological characteristics of dopamine neurons (DA-like neurons) from four WT mice and 18 DA-like neurons from five Gabrg2DATCreER mice. The DA-like neurons in Gabrg2DATCreER mice had significantly higher total (5.6 ± 0.4 Hz vs. 2.9 ± 0.4 Hz; p < 0.001, unpaired Student’s t test) and extraburst (5.3 ± 0.4 Hz vs. 2.8 ± 0.5 Hz; p < 0.001, unpaired Student’s t test) firing rates compared to cells in the WT mice. Intensity of the baseline bursting and the burst parameters tended to be higher in the mutant animals but the differences did not reach statistical significance (Fig. S3).
A subset of the recorded neurons was subjected to iontophoretic application of the selective GABAAR agonist muscimol (WT, n = 14; Gabrg2DATCreER, n = 18) or the selective GABAAR antagonist bicuculline (WT, n = 15; Gabrg2DATCreER, n = 17). Both firing rates, at baseline and after muscimol application, were significantly higher in Gabrg2DATCreER mice (Fig. 1a, b, e, f, i; genotype × drug, F1,30 = 4.031, p = 0.06, genotype, F1,30 = 30.37, p < 0.0001, drug, F1,30 = 101.0, p < 0.0001) and significantly reduced effect of muscimol in the mutant mice was observed (Fig. 1l; −87 ± 2.5% vs. −58 ± 6.2%; p < 0.001, unpaired Student’s t test). In both groups of animals, iontophoretic application of bicuculline induced a significant increase in the total firing of DA-like neurons (Fig. 1c, d, g, h, j; genotype × drug, F1,30 = 6.94, p = 0.01; genotype, F1,30 = 9.71, p = 0.004; drug, F1,30 = 173.0, p < 0.0001), but the magnitude of the increase was significantly lower in Gabrg2DATCreER mice compared to WT mice (Fig. 1m; 181 ± 63.0% vs. 39 ± 4,6%; p < 0.05, unpaired Student’s t test). Similarly, in both groups, the firing frequency during extraburst firing (tonic activity) was increased by application of bicuculline (Fig. 1c, d, k; genotype × drug, F1,30 = 4.53, p = 0.04, genotype, F1,30 = 7.33, p = 0.011; drug, F1,30 = 25.45, p < 0.0001), but the increase was significantly impaired in mutants (Fig. 1n; 146 ± 54.9% vs. 21 ± 10.0%; p < 0.05, unpaired Student’s t test). Neither the amplitude of observed changes, nor the baseline burst parameters differed between the WT mice and Gabrg2DATCreER mice. The only exception was the intrabursts interspike interval, for which bicuculline induced opposite effects in mutants and controls (Fig. S3; genotype × drug, F1,15 = 6.938, p = 0.005, genotype, F1,15 = 0.84, p = 0.37, drug, F1,15 = 1.52, p = 0.24). In summary, the dopamine neurons of Gabrg2DATCreER mice displayed increased activity and reduced sensitivity to drugs acting at GABAA receptors.
Fig. 1.
DA-like neurons of Gabrg2DATCreER mice have impaired reactions to GABAA receptor agonist and antagonist. a–d show raw traces of extracellularly recorded firing of four representative DA-like neurons from WT a, c and Gabrg2DATCreER mice b, d. Top traces show firing during baseline conditions. Bottom traces show alternation of firing of the same neurons induced by application of the GABAA receptor agonist muscimol (MUS) or the GABAA receptor antagonist bicuculline (BIC). e–h show firing and bursting rate histograms of the example neurons shown on panels a–d, respectively. Time of drug application is marked with a black bar. Note that muscimol application induced a complete cessation of DA-like neuron firing in WT animals a, e and only partial reduction of DA-like neuron firing in Gabrg2DATCreER mice. Also increase of DA-like neuron firing induced by application of bicuculline was significantly larger in WT c, g comparing to Gabrg2DATCreER mice d, h animal. On i–k baseline and drug altered firing of all recorded DA-like neurons in WT (•—baseline, ■—drug) and Gabrg2DATCreER (○—baseline, □—drug) mice, are shown. Note that under baseline conditions, total and extraburst firing rates were significantly higher in mutant comparing to control animals. l–n show the relative change in firing induced by muscimol l, and bicuculline m, n. Vertical lines with whiskers indicate the mean value and SEM. * or # indicates Bonferroni corrected t tests that resulted in p < 0.05, ** indicates p < 0.01 and *** indicates p < 0.001
Before examining behaviors specifically related to alcohol, we subjected the mice to some basal behavioral tests measuring locomotor activity, exploratory behavior, and avoidance. In the open-field test, Gabrg2DATCreER mice displayed increased locomotor activity (Fig. 2a) and spent more time in the center of the arena compared to WT mice (Fig. 2b). Furthermore, Gabrg2DATCreER mice spent more time in the open arms of the elevated plus maze (Fig. 2c) without making significantly more entries (Fig. S4B). They also displayed a strongly increased social behavior in the reciprocal social interaction test (Fig. 2d). Gabrg2DATCreER mice displayed a marked reduction of the time spent immobile in the forced swim test (Fig. 2e) and they also showed an increased sucrose preference (Fig. 2f). Collectively, these findings indicate that the mutant mice had an increased exploratory drive that was not inhibited by aversive features of the environment.
Fig. 2.
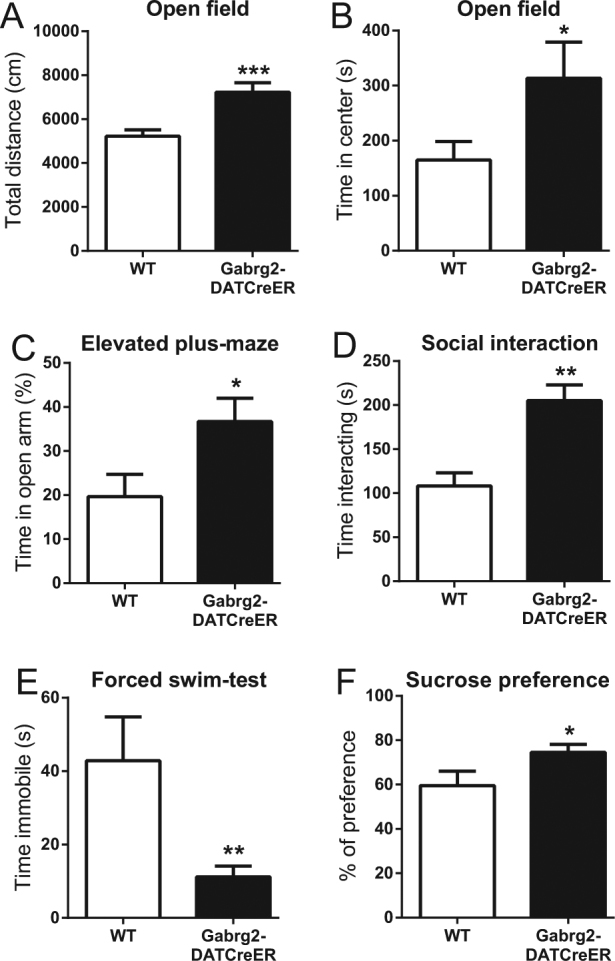
Basic behavioral testing in Gabrg2DATCreER mice. Behavior in the open-field test. a show total distance and b show the time spent in the center of the field (n = 21 and 16). Behavior in the elevated plus maze is shown in c (n = 13 and 15) and time spent interacting in the reciprocal social interaction test is shown in d (n = 6 and 6). e show the time spent immobile in the forced swim test (n = 6 and 13) and f show the sucrose preference in Gabrg2DATCreER and WT mice (n = 8 and 14). Error bars represent SEM. *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test
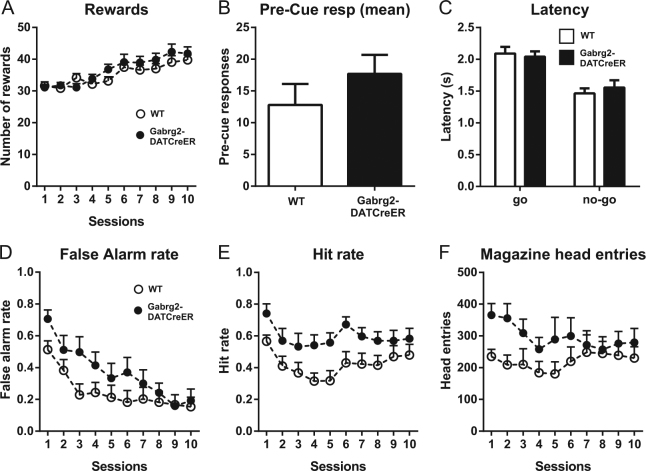
To test if the increased exploratory behavior was associated with impaired response inhibition, we used the go/no-go task, which measures the response inhibition component of impulsivity. In this task, mice should either respond or withhold a response dependent on which cue is shown. Both Gabrg2DATCreER and WT animals improved their performance over time and significantly increased the number of rewards obtained in subsequent sessions (Fig. 3a; session × genotype F9,207 = 1.02, p = 0.43, genotype F1,23 = 1.03, p = 0.32, session F9,207 = 14.22, p < 0.0001). The mutation did not significantly affect the rate of pre-cue responding (Fig. 3b) and had no effect on reaction times during “go” or “no-go” trials (Fig. 3c; trial type × genotype F1,46 = 0.54, p = 0.46, genotype F1,46 = 0.05, p = 0.82, trial type F1,46 = 33.46, p < 0.0001). Initially, Gabrg2DATCreER mice showed higher frequency of false alarms (responding to “no-go” signals), but they eventually reached the same level of performance as WT animals (Fig. 3d; session × genotype F9,207 = 2.12, p = 0.03, genotype F1,23 = 1.94, p = 0.18, session F9,207 = 23.00, p < 0.0001). Moreover, Gabrg2DATCreER mice were generally more likely to correctly respond to “go” signals, as indicated by a higher level of hits (Fig. 3e; session × genotype F9,207 = 0.62, p = 0.78, genotype F1,23 = 6.89, p = 0.02, session F9,207 = 4.25, p < 0.0001). In addition, we noted that the mutant mice initially made more head entries into the receptacle where the liquid rewards were delivered (Fig. 3f; session × genotype F9,207 = 2.05, p = 0.04, genotype F1,23 = 2.07, p = 0.16, session F9,207 = 1.91, p = 0.05). Taken together, the results in this test showed that Gabrg2DATCreER mice had heightened propensity to respond for both “go” and “no-go” signals and showed higher reward receptacle approach behavior.
Fig. 3.
Response inhibition testing of Gabrg2DATCreER mice with the go/no-go task. The graphs show mean numbers of a rewards obtained during each session, b responses preceding signal presentation, c response latency for “go” and “no-go” trials, d, e number of responses per session during “no-go” and “go” signal presentation, f head entries into water receptacle per session. Data in b and c are averaged across all sessions. n = 13 and 12. Error bars represent SEM
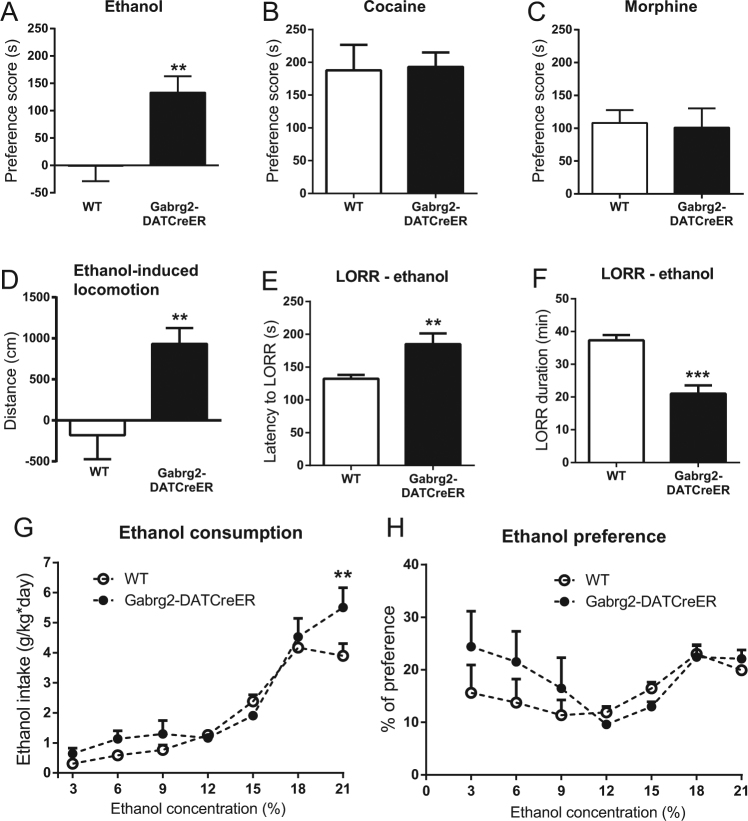
We next investigated if the rewarding effects of ethanol (2 g/kg) was affected in the mutant mice. WT littermates of the Gabrg2DATCreER mice displayed no CPP in response to ethanol (Fig. 4a). In contrast, Gabrg2DATCreER mice displayed a clear CPP (Fig. 4a). The fact that the WT mice from the Gabrg2-colony did not show a CPP to ethanol is in line with previous reports confirming weak or no CPP in some mouse strains [37]. However, since tamoxifen has been shown to affect certain responses to ethanol [38, 39], we investigated if the pre-treatment with tamoxifen or the strain was responsible for the lack of CPP in the WT mice. The WT mice from the Gabrg2-colony did not display CPP to ethanol even if they were not pre-treated with tamoxifen (Fig. S4C). In contrast, C57BL/6J mice displayed robust ethanol-induced CPPs both when they were pre-treated with tamoxifen and when they received no pre-treatment (Fig. S4C). Both WT and Gabrg2DATCreER mice developed robust preferences for cocaine (15 mg/kg; Fig. 4b) and morphine (10 mg/kg; Fig. 4c) without any obvious differences between genotypes. In order to determine if the stimulatory effects of ethanol were affected in Gabrg2DATCreER mice, we next monitored the locomotor response induced by ethanol. Whereas, ethanol (1 g/kg) had no locomotor stimulating effect in WT mice, it induced a robust increase in locomotor activity in Gabrg2DATCreER mice (Fig. 4d). To examine if the mutation affected the sedative effect of ethanol, we tested how rapidly a high dose of ethanol-induced loss of the righting reflex and how long such loss persisted. Latency to onset of the sedative effect was increased in Gabrg2DATCreER mice (Fig. 4e; t19 = 2.94, p = 0.008) and the duration of sedation was decreased (Fig. 4f; t17 = 5.30, p < 0.0001). When we investigated alcohol drinking in a two bottle-choice test with increasing concentrations of ethanol, Gabrg2DATCreER mice had a moderately increased ethanol consumption at a high concentration of ethanol (Fig. 4g; genotype × concentration, F6,84 = 2.73, p = 0.018, genotype, F1,14 = 1.78, p = 0.20; concentration, F6,84 = 78.06, p < 0.0001). Total fluid consumption was also increased by the mutation (genotype × concentration, F6,84 = 2.97, p = 0.011, genotype, F1,14 = 2.00, p = 0.18; concentration, F6,84 = 20.32, p < 0.0001) and consequently no genotype effect was seen on alcohol preference (Fig. 4h; genotype × concentration, F6,84 = 1.47, p = 0.20, genotype, F1,14 = 0.49, p = 0.50; concentration, F6,84 = 4.59, p < 0.0004).
Fig. 4.
Alcohol-related behaviors in Gabrg2DATCreER mice. Conditioned place preference to ethanol in Gabrg2DATCreER mice and their WT littermates (a; n = 7 and 12). Note that WT mice of this particular background did not form a place preference to ethanol. Place preference to cocaine (b; n = 11 and 8) and morphine (c; n = 17 and 14) in mutant and WT mice. Locomotor activity induced by ethanol (d; n = 6 and 7). Loss of righting reflex (LORR) in Gabrg2DATCreER mice e, f. Latency to LORR is shown in e (n = 10 and 11) and duration of the loss is shown in f (n = 9 and 10). Ethanol consumption g and alcohol preference h of Gabrg2DATCreER mice given increasing concentrations of ethanol in a two bottle-choice paradigm (n = 8 and 8). Preference scores were calculated by subtracting the time the mouse spent in the drug-paired chamber during the post-test from that of the pretest. Error bars represent SEM. *p < 0.05 **p < 0.01, ***p < 0.001. Student’s t test in a–e and two-way ANOVA followed by Sidak’s multiple comparisons test in f and g
Discussion
Previous studies indicate that the effect of Gabrg2 deletion on GABA responsiveness varies considerably dependent on the cell type targeted. Gabrg2 was reported to be necessary for maintaining GABAAR synapses in the hippocampus [33] and deletion of Gabrg2 reduces GABA responsiveness by 90% in hypothalamic neurons [40]. In contrast, deletion of Gabrg2 in the adult neocortex did not block GABAAR activity but only changed the characteristics of the GABA response [41]. Our findings indicate that Gabrg2 deletion in dopamine cells of adult mice leads to a substantial attenuation, but not a complete blockade, of GABAAR function in these cells. Thus, dopamine neurons from Gabrg2DATCreER mice displayed increased basal firing and an attenuated responsiveness to drugs acting at GABAARs. The residual GABA responsiveness observed in dopamine cells of the mutants could reflect activation of GABAARs not containing ɣ2 subunits and/or activation of GABAARs on excitatory afferents to the dopaminergic cells. We used urethane anesthesia for the electrophysiological experiments. One advantage with urethane anesthesia, as compared to isoflurane anesthesia, is that it mimics the awake brain in the sense that firing of dopamine cells is brain state-dependent [42–44]. On the other hand, brain state-dependent differences can lead to increased variability in the parameters monitored. Brain state-dependent modulation can be a particularly important factor when investigating the potency of GABAA receptor antagonists. It has been suggested that the heterogeneity of DA activity of neurons observed under anesthesia, at least partially, may be due to cyclic changes in activity of GABAergic inputs to the ventral tegmental area and the substantia nigra [43]. However, in our study, all recorded neurons increased their firing (at least 20%) after local blockade of GABAA receptors.
Many of the behavioral changes in Gabrg2DATCreER mice (summarized in Table 1) fit well with the finding that their dopamine cells had an increased basal activity. Optogenetic activation of midbrain dopamine cells has been reported to result in effects similar to those seen here in Gabrg2DATCreER mice in the forced swim test [45] and the social interaction test [46]. Furthermore, the deletion of NMDA receptors in dopaminergic cells induced changes in the opposite direction in many of the tests [22]. Even if results from basic behavioral tests such as the elevated plus maze and the forced swim test are difficult to interpret in general, our interpretation of the findings in this study is that that they, together with the go/no-go test, indicate that the Gabrg2DATCreER mice often display an increased activity and a high propensity to explore and interact. The mutant mice also seem to act and explore when the environment has aversive features or when withholding a response is rewarded. However, they eventually learned to withhold responses in the go/no-go task, so it is unclear if the phenotype in that task reflects heightened propensity for reward seeking, a deficit in certain aspects of behavioral inhibition or both.
Table 1.
Summary of phenotypes in Gabrg2-DATCreER mice
| Observed phenotype | Figure |
|---|---|
| Increased baseline firing frequency of dopamine (DA) cells | 1 |
| Attenuated decrease in firing of DA cells after a GABAA receptor agonist | 1 |
| Attenuated increase in firing of DA cells after a GABAA receptor antagonist | 1 |
| Increased locomotion in the open-field test | 2A |
| Increased time spent in the center in the open-field test | 2B |
| Increased time in the open arms in the elevated plus maze | 2C |
| Increased time interacting in the reciprocal social interaction test | 2D |
| Decreased time spent immobile in the forced swim test | 2E |
| Increased sucrose preference | 2F |
| Increased false alarm rate in the go/no-go task | 3D |
| Increased hit rate in the go/no-go task | 3E |
| More magazine head entries in the go/no-go task | 3F |
| Increased conditioned place preference to ethanol | 4A |
| Normal conditioned place preference to morphine and cocaine | 4B, 4C |
| Increased ethanol-induced locomotion | 4D |
| Attenuated loss of the righting reflex | 4E, 4F |
| Increased ethanol consumption at high concentrations | 4G |
| Normal alcohol preference | 4H |
We found that Gabrg2DATCreER mice displayed an increased CPP and an augmented locomotor activation in response to ethanol. In contrast, cocaine and morphine-induced CPP were unaltered in the mutant mice. The cellular and molecular underpinnings of the changes in alcohol-induced behaviors are difficult to pin-point in an exact way since the mechanisms by which alcohol induces reinforcement are far from clear [1, 47, 18]). Alcohol has direct activating effects on dopamine cells [16, 17] and also activates local inhibitory GABAergic neurons that inhibit the dopaminergic cells [48, 49]. In addition, alcohol acts in the nucleus accumbens to induce dopamine release [50, 18]). The dopamine-enhancing effect of accumbal ethanol can be blocked by manipulations in the ventral tegmental area indicating an indirect activation or a disinhibition of the dopaminergic cells [18]. Unfortunately, the respective contributions of these different modes of action to the different behavioral effects of alcohol have not been firmly established. When interpreting the behavioral findings of this study, it should also be taken into account that alcohol can affect GABA signaling onto dopamine cells both by affecting GABA release through the mechanisms discussed above and by direct potentiation of GABAA receptors on dopamine cells. Consequently, in addition to reducing the general sensitivity to GABA, the removal of Gabrg2 most likely induced a difference in the direct ethanol sensitivity of the remaining receptors since the subunit composition of GABAARs influence their responsiveness to ethanol. It is not clear which subunits that are most critical for the direct effect of ethanol [7, 51]. GABAA receptors containing ɣ2 subunits have been reported to be more sensitive to ethanol than those without [10, 52] but other studies indicate that extra-synaptic GABAARs lacking ɣ2 subunits are most alcohol responsive [11, 12]. In our mutant mice, we do not know how the expression of other GABAAR subunits was changed. The ɣ2 subunit can be replaced ɣ1, ɣ3, or δ subunits, but under normal conditions dopamine cells display no or very weak expression of these subunits [53, 54]. Finally, it should be noted that the Cre-line used also induces recombination in dopamine cells outside the ventral tegmental area. Thus, certain behavioral differences might be due to recombination in the substantia nigra or other structures with dopaminergic cells.
Irrespective of the exact mechanisms, our findings clearly demonstrate that the GABA-mediated inhibition of dopaminergic cells limits the reinforcing and stimulatory effects of alcohol. Removal of Gabrg2 on dopamine cells consequently exaggerates or unmasks ethanol-induced behaviors related to reinforcement and locomotor stimulation. Dopamine cells from mice selectively bred for high alcohol-induced locomotion and mice chronically treated with ethanol have dopaminergic cells that display reduced GABA responsiveness. Together with the findings presented here, this might indicate that different avenues leading to weakening of the inhibitory control of dopaminergic cells can increase the rewarding and stimulatory effects of alcohol and increase the risk of developing excessive alcohol intake.
In light of the strongly enhanced CPP in response to ethanol, it is perhaps surprising that the mice without Gabrg2 in the dopaminergic cells responded with normal CPPs to two different doses of morphine. This may suggest that GABAAR signaling onto dopaminergic cells is limiting ethanol-induced but not morphine-induced reinforcement or that the subunit composition after Gabrg2 deletion in some way selectively decreases the sensitivity of the receptors to ethanol. Morphine is assumed to activate dopamine cells by inhibition of GABAergic input [55, 56, 20]. Accordingly, one would predict that a complete deletion of GABA receptors on dopamine cells should abolish morphine CPP and a reduction in GABAAR function should attenuate the CPP. However, we saw no signs of an attenuation in our experiments.
At high doses, alcohol has sedative rather than stimulating effects. Converging lines of evidence also indicate that aspects of dopamine transmission are suppressed at high doses of alcohol [57–60]. We found that GABAARs on dopaminergic cells are powerful regulators of the sedative effect of alcohol. Together with studies showing that different substances increasing dopamine levels also reduce alcohol-induced sedation [59, 61, 62], this strongly suggests that activity of the dopamine system is a critical factor counteracting the sedative effects seen after alcohol drinking.
Our findings demonstrate that the deletion of Gabrg2 leads to several behavioral characteristics that, in humans, are known to be risk factors for developing alcoholism. These risk factors are to a large extent genetically determined. Whereas it is clear that variations in GABRG2 are associated with increased risk for alcoholism [8, 9], it has not been determined if these variations induce risk by operating through intermediate factors such as impulsivity or altered sensitivity to sedative or rewarding effects of ethanol. In studies on humans, it is notoriously difficult to pin-point the neuronal groups in which a given allele is mediating the effect on specific behaviors. Since our findings establish that the subunit composition of GABAARs on dopamine cells is critical for important responses to alcohol, they may suggest that genetic variability in GABAAR subunits promotes alcoholism and associated risk behaviors partially through effects on dopamine cells. Finally, since GABRG2 is a risk-associated gene and the deletion induces such a broad range of risk-associated traits, the Gabrg2DATCreER mice are an interesting model for the study of drinking in people at high risk for developing alcoholism.
Electronic supplementary material
Acknowledgements
We thank Maarit Jaarola for technical support. This work was supported by the European Research Council, the Swedish Medical Research Council, the Knut and Alice Wallenberg foundation, the Swedish Brain foundation, Parkinsonstiftelsen and Region Östergötland (to D.E.). P.E.C. and J.R.P. were supported by the grant HARMONIA 2012/06/M/NZ4/00143 from the Polish National Science Centre. M.W. was supported by the grant PRELUDIUM 2015/19/N/NZ4/00960 from the Polish National Science Centre.
Competing interests
The authors declare no competing interests.
Contributor Information
Tomasz Błasiak, Phone: +48 604404941, Email: tomasz.blasiak@uj.edu.pl.
David Engblom, Phone: +46 10-1038448, Email: david.engblom@liu.se.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41386-018-0022-z.
References
- 1.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 2.Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, et al. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–26. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–99. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D. A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry. 2016;79:489–98. doi: 10.1016/j.biopsych.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abus Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- 6.Schuckit MA, Smith TL, Heron J, Hickman M, Macleod J, Lewis G, et al. Testing a level of response to alcohol-based model of heavy drinking and alcohol problems in 1,905 17-year-olds. Alcohol Clin Exp Res. 2011;35:1897–904. doi: 10.1111/j.1530-0277.2011.01536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephens DN, King SL, Lambert JJ, Belelli D, Duka T. GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav. 2017;16:149–84. doi: 10.1111/gbb.12321. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Sulovari A, Cheng C, Zhao H, Kranzler HR, Gelernter J. Association of gamma-aminobutyric acid A receptor alpha2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology. 2014;39:907–18. doi: 10.1038/npp.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh EW, Higuchi S, Matsushita S, Murray R, Chen CK, Ball D. Association analysis of the GABA(A) receptor subunit genes cluster on 5q33-34 and alcohol dependence in a Japanese population. Mol Psychiatry. 2000;5:301–7. doi: 10.1038/sj.mp.4000719. [DOI] [PubMed] [Google Scholar]
- 10.Harris RA, Proctor WR, McQuilkin SJ, Klein RL, Mascia MP, Whatley V, et al. Ethanol increases GABAA responses in cells stably transfected with receptor subunits. Alcohol Clin Exp Res. 1995;19:226–32. doi: 10.1111/j.1530-0277.1995.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 11.Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, et al. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–2. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–23. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 15.Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–17. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–3. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 17.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–52. doi: 10.1111/j.1530-0277.1999.tb04082.x. [DOI] [PubMed] [Google Scholar]
- 18.Soderpalm B, Ericson M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci. 2013;13:127–61. doi: 10.1007/978-3-642-28720-6_170. [DOI] [PubMed] [Google Scholar]
- 19.Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology. 1998;139:79–85. doi: 10.1007/s002130050692. [DOI] [PubMed] [Google Scholar]
- 20.Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Jastrzebska K, Walczak M, Cieslak PE, Szumiec L, Turbasa M, Engblom D, et al. Loss of NMDA receptors in dopamine neurons leads to the development of affective disorder-like symptoms in mice. Sci Rep. 2016;6:37171. doi: 10.1038/srep37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–15. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 24.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–30. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;22:1088. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M. Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology. 2010;209:103–11. doi: 10.1007/s00213-010-1775-1. [DOI] [PubMed] [Google Scholar]
- 28.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 29.Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, et al. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012;107:649–62. doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cieslak PE, Llamosas N, Kos T, Ugedo L, Jastrzebska K, Torrecilla M, et al. The role of NMDA receptor-dependent activity of noradrenergic neurons in attention, impulsivity and exploratory behaviors. Genes Brain Behav. 2017;16:812–22. doi: 10.1111/gbb.12383. [DOI] [PubMed] [Google Scholar]
- 32.Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, et al. The role of clock in ethanol-related behaviors. Neuropsychopharmacology. 2013;38:2393–2400. doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, et al. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–50. doi: 10.1016/S1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 34.Fritz M, Klawonn AM, Nilsson A, Singh AK, Zajdel J, Wilhelms DB, et al. Prostaglandin-dependent modulation of dopaminergic neurotransmission elicits inflammation-induced aversion in mice. J Clin Invest. 2016;126:695–705. doi: 10.1172/JCI83844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–7. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 36.Rieker C, Engblom D, Kreiner G, Domanskyi A, Schober A, Stotz S, et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci. 2011;31:453–60. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–93. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- 38.Blednov YA, Black M, Benavidez JM, Stamatakis EE, Harris RA. PPAR agonists: I. Role of receptor subunits in alcohol consumption in male and female mice. Alcohol Clin Exp Res. 2016;40:553–62. doi: 10.1111/acer.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilakivi-Clarke L. Role of estradiol in alcohol intake and alcohol-related behaviors. J Stud Alcohol. 1996;57:162–70. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- 40.Lee K, Porteous R, Campbell RE, Luscher B, Herbison AE. Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology. 2010;151:4428–36. doi: 10.1210/en.2010-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerti-Szigeti K, Nusser Z, Eyre MD. Synaptic GABAA receptor clustering without the gamma2 subunit. J Neurosci. 2014;34:10219–33. doi: 10.1523/JNEUROSCI.1721-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown MT, Henny P, Bolam JP, Magill PJ. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci. 2009;29:2915–25. doi: 10.1523/JNEUROSCI.4423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walczak M, Blasiak T. Midbrain dopaminergic neuron activity across alternating brain states of urethane anaesthetized rat. Eur J Neurosci. 2017;45:1068–77. doi: 10.1111/ejn.13533. [DOI] [PubMed] [Google Scholar]
- 44.Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–41. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 45.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–41. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovinger DM, Alvarez VA. Alcohol and basal ganglia circuitry: animal models. Neuropharmacology. 2017;122:46–55. doi: 10.1016/j.neuropharm.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tateno T, Robinson HP. The mechanism of ethanol action on midbrain dopaminergic neuron firing: a dynamic-clamp study of the role of I(h) and GABAergic synaptic integration. J Neurophysiol. 2011;106:1901–22. doi: 10.1152/jn.00162.2011. [DOI] [PubMed] [Google Scholar]
- 49.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience. 2011;172:94–103. doi: 10.1016/j.neuroscience.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lof E, Ericson M, Stomberg R, Soderpalm B. Characterization of ethanol-induced dopamine elevation in the rat nucleus accumbens. Eur J Pharmacol. 2007;555:148–55. doi: 10.1016/j.ejphar.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 51.Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Sci Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wafford KA, Burnett DM, Leidenheimer NJ, Burt DR, Wang JB, Kofuji P, et al. Ethanol sensitivity of the GABAA receptor expressed in Xenopus oocytes requires 8 amino acids contained in the gamma 2L subunit. Neuron. 1991;7:27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- 53.Okada H, Matsushita N, Kobayashi K, Kobayashi K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem. 2004;89:7–14. doi: 10.1111/j.1471-4159.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- 54.Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, et al. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol. 2001;433:526–49. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- 55.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui Y, Ostlund SB, James AS, Park CS, Ge W, Roberts KW, et al. Targeted expression of mu-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat Neurosci. 2014;17:254–61. doi: 10.1038/nn.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res. 1984;292:63–9. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- 58.Schilaty ND, Hedges DM, Jang EY, Folsom RJ, Yorgason JT, McIntosh JM, et al. Acute ethanol inhibits dopamine release in the nucleus accumbens via α6 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2014;349:559–67. doi: 10.1124/jpet.113.211490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001;297:27–34. [PubMed] [Google Scholar]
- 60.Yorgason JT, Ferris MJ, Steffensen SC, Jones SR. Frequency-dependent effects of ethanol on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2014;38:438–47. doi: 10.1111/acer.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menon MK, Kodama CK, Cummins JT, Von Hungen K. Studies on the interaction between ethanol and amfonelic acid. Neuropharmacology. 1987;26:247–53. doi: 10.1016/0028-3908(87)90215-2. [DOI] [PubMed] [Google Scholar]
- 62.Todzy I, Coper H, Fernandes M. Interaction between d-amphetamine and ethanol with respect to locomotion, stereotypies, ethanol sleeping time, and the kinetics of drug elimination. Psychopharmacology. 1978;59:143–9. doi: 10.1007/BF00427748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.