Abstract
Objectives
We sought to evaluate the association between obesity and response to anti-tumor necrosis factor-α (TNF) agents, through a systematic review and meta-analysis.
Methods
Through a systematic search through January 24, 2017, we identified randomized controlled trials (RCTs) or observational studies in adults with select immune-mediated inflammatory diseases–inflammatory bowel diseases (IBD), rheumatoid arthritis (RA), spondyloarthropathies (SpA), psoriasis and psoriatic arthritis (PsA)–treated with anti-TNF agents, and reporting outcomes, stratified by body mass index (BMI) categories or weight. Primary outcome was failure to achieve clinical remission or response or treatment modification. We performed random effects meta-analysis and estimated odds ratios (OR) and 95% confidence interval (CI).
Results
Based on 54 cohorts including 19,372 patients (23% obese), patients with obesity had 60% higher odds of failing therapy (OR,1.60; 95% CI,1.39–1.83;I2 = 71%). Dose-response relationship was observed (obese vs. normal BMI: OR,1.87 [1.39–2.52]; overweight vs. normal BMI: OR,1.38 [1.11–1.74],p = 0.11); a 1kg/m2 increase in BMI was associated with 6.5% higher odds of failure (OR,1.065 [1.043–1.087]). These effects were observed across patients with rheumatic diseases, but not observed in patients with IBD. Effect was consistent based on dosing regimen/route, study design, exposure definition, and outcome measures. Less than 10% eligible RCTs reported outcomes stratified by BMI.
Conclusions
Obesity is an under-reported predictor of inferior response to anti-TNF agents in patients with select immune-mediated inflammatory diseases. A thorough evaluation of obesity as an effect modifier in clinical trials is warranted, and intentional weight loss may serve as adjunctive treatment in patients with obesity failing anti-TNF therapy.
Introduction
The global prevalence of obesity is rising, with one in 10 people across the world being classified as obese.[1, 2] In the United States, over 35% adults are obese, and increased healthcare spending on this population is estimated to account for nearly a third of the growth in healthcare expenditure.[3] Obesity may contribute to increased risk of developing select immune-mediated inflammatory diseases (IMIDs) such as rheumatoid arthritis (RA), psoriasis and Crohn’s disease (CD), and approximately 10–50% of patients with IMIDs are obese.[4–9] Obesity has been associated with more severe disease activity, inferior quality of life and higher burden of hospitalization in patients with these immune-mediated diseases.[5, 6, 10–14]
Targeted immunomodulators such as anti-tumor necrosis factor-α (TNF), are the mainstay of therapy for patients with select IMIDs with moderate-severe disease. Clinical response to these agents is seen in 40–80% patients with select IMIDs.[15–19] Population pharmacokinetic studies of different anti-TNF agents have consistently shown that high body weight is associated with accelerated clearance, resulting in lower trough concentrations.[20–22] Additionally, obesity, particularly visceral fat, independently contributes to higher systemic inflammatory burden.[23, 24] Several observational studies have shown that obesity may be a negative prognostic marker in patients with rheumatic diseases,[11, 25] and variably and inconsistently shown an inferior response to biologic agents in obese patients.[26–33]
Hence, we sought to systematically review the association between obesity and response to anti-TNF agents across selected IMIDs, and examine whether the effect varies across different diseases and between different anti-TNF agents based on route of administration (subcutaneous vs. intravenous) and dosing scheme (weight-based vs. fixed dosing).
Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was conducted following a priori established protocol (S1 Text).[34]
Selection criteria
We included phase 2 or phase 3 randomized controlled trials (RCT) or observational cohort studies, with at least 2 months follow-up, that met the following inclusion criteria: (1) patients with select IMIDs (IBD including CD or ulcerative colitis [UC], RA, psoriasis or psoriatic arthritis [PsA], spondyloarthropathy [SpA]), treated with US Food and Drug Administration (FDA)-approved anti-TNF agents (infliximab, adalimumab, certolizumab pegol, golimumab, etanercept), (b) outcome reported as failure to achieve clinical remission or response or need for treatment modification, and (c) outcomes stratified by baseline body mass index (BMI) or body weight. For our analysis, RCTs were considered as prospective cohort studies, and only patients receiving active intervention with anti-TNF agents were included in analysis; if two or more arms in the RCTs were receiving different active biologic therapy, then they were considered separate cohorts. We excluded cross-sectional studies or case-control studies, studies of non-TNF biologic agents, immunomodulators or small molecules, or studies in which outcomes were not stratified by baseline BMI or weight. We also excluded studies in which the exposure categories did not include any patients with overweight or obesity patients (for example, BMI <20 vs. ≥20kg/m2).[35] When there were multiple publications from the same cohort, we only included data from the most recent comprehensive report.
Data sources, search strategy and study selection
The search strategy was designed and conducted by an experienced medical librarian with input from study investigators, utilizing various databases from inception to January 24, 2017. The databases included Ovid Medline (January 1, 1946 to January 24, 2017), EMBASE (January 1, 1988 to January 24, 2017), Scopus (January 1, 2004 to January 24, 2017), Web of Science (January 1, 1990 to January 24, 2017), and Cochrane Central Register of Controlled Trials (January 1, 2005 to January 24, 2017). In addition, we searched clinical trial registries (www.clinicaltrials.gov and www.clinicaltrialsregister.eu), conference proceedings and published systematic reviews for additional studies. Controlled vocabulary supplemented with keywords was used to search for observational studies (S2 Text) and RCTs (S3 Text) reporting impact of obesity on response to anti-TNF therapy. Two authors independently reviewed the title and abstract of studies identified in the search to exclude studies that did not answer the research question of interest, based on pre-specified inclusion and exclusion criteria. The full text of the remaining articles was independently reviewed, to determine whether it contained relevant information. Next, we manually searched the bibliographies of the selected articles, as well as review articles on the topic for additional articles. We also performed a manual search of conference proceedings from major gastroenterology, rheumatology and dermatology meetings (Digestive Diseases Week, Annual Meeting of the American College of Rheumatology, Annual European Congress of Rheumatology, and Annual meeting of the American College of Dermatology from 2012 to 2016) for additional abstracts on the topic. Since RCTs may report results stratified by body weight or BMI in subgroup analyses in online supplements alone, we reviewed full-texts of all published phase 2 and 3 RCTs of candidate anti-TNF agents in detail, and on clinical trial registry websites (www.clinicaltrials.gov, www.clinicaltrialsregister.eu).
Data abstraction and risk of bias assessment
After study selection, two authors independently abstracted data on study and patient characteristics, exposure variables, outcomes, confounding variables and statistical analyses, using a standardized data abstraction form. Details of data abstraction are shown in the appendix (S4 Text).
Risk of bias was assessed by 2 investigators independently, using the Quality In Prognosis Studies tool, which evaluates validity and bias in studies of prognostic factors across six domains: participation, attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting.[36]
Outcomes assessed
The primary outcome of interest was failure of index biologic therapy. This was defined using a hierarchy of the following outcomes, i.e., if the first outcome was not reported, then the next reported outcome of interested was chosen: (1) failure to achieve clinical remission, (2) failure to achieve clinical response (based on validated disease activity indices or author-defined criteria); or (3) need for treatment modification (discontinuation or escalation of index therapy due to ineffectiveness and/or switching to alternative therapy, and/or need for surgery).
In order to evaluate stability of the association between obesity and response to anti-TNF therapy, and examine potential sources of heterogeneity, we performed several a priori subgroup analyses based on: type of diseases (IBD vs. RA vs. SpA vs. psoriasis-PsA), route of administration (subcutaneous vs. intravenous), dosing scheme (weight-based dosing vs. fixed dosing), exposure categories (based on BMI: obese vs. normal BMI, obese vs. non-obese [BMI≥30kg/m2 vs. BMI<30kg/m2], overweight vs. non-overweight [BMI≥25kg/m2 vs. BMI<25kg/m2]), outcome definition (based on disease activity indices vs. need for modification of therapy) and study design (RCTs vs. cohort). Sensitivity analysis restricting to studies that adjusted for key confounding variables was performed. We also performed meta-regression to assess whether effect estimates varied depending on prevalence of exposure (proportion of obese patients) or the prevalence of the outcome (proportion who failed index biologic therapy).
We examined dose-response relationship using two approaches. First, we limited analysis to studies reporting the association between 2 or more categories of obesity against a reference category, and compared as nominal groups. For example, for studies using WHO-defined categories, then comparing obese vs. normal BMI and overweight vs. normal BMI; for studies reporting tertiles of BMI or weight, then comparing 3rd tertile (T3) vs. 1st tertile (T1) and 2nd tertile (T2) vs. 1st tertile (T1). When studies reported multiple different exposure categories, then all categories beyond the bottom two were collapsed into a single category (labeled high BMI or weight) for consistent comparison. Second, studies that reported analyses per 1kg/m2 change in BMI were pooled separately.
Statistical analysis
We used the random-effects model described by DerSimonian and Laird to calculate summary OR and 95% confidence intervals (CI).[37] Maximally adjusted OR, where reported in studies, was used for analysis to account for confounding variables; where not reported, unadjusted OR were used or calculated based on event rates in different exposure categories. To estimate what proportion of total variation across studies was due to heterogeneity rather than chance, I2 statistic was calculated.[38] In this, a value of <30%, 30%-60%, 60%-75% and >75% were suggestive of low, moderate, substantial and considerable heterogeneity, respectively. Between-study sources of heterogeneity were investigated using subgroup analyses by stratifying original estimates according to study characteristics (as described above). In this analysis, a p-value for differences between subgroups of <0.10 was considered statistically significant. Publication bias was assessed qualitatively using funnel plots and quantitatively using Egger’s regression test.[39]
All analyses were performed using Comprehensive Meta-Analysis version 2.0 (Englewood, New Jersey). Since this was a meta-analysis of published studies, institutional review board approval was not required.
Results
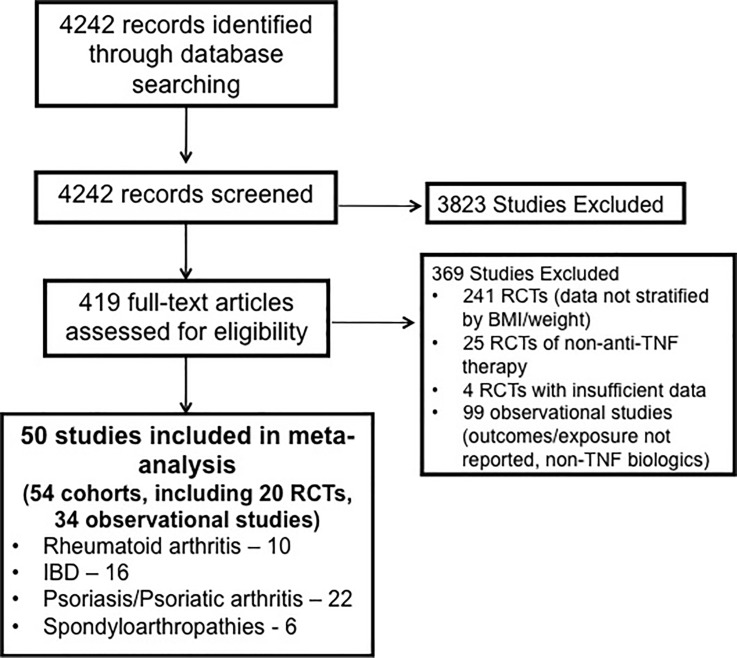
From 4242 unique studies identified based on systematic review, 3823 articles were excluded based on title and abstract review (since these were unrelated to biologic therapies or unrelated to diseases of interest). Overall, 419 articles were reviewed in full for eligibility. Of 241 eligible RCTs reviewed in full, 20 (8.3%) reported results stratified by BMI or weight and were included; the remainder did not provide data stratified by BMI or weight. Twenty five RCTs of non-TNF biologics were excluded, and 5 RCTs were excluded since the outcomes of interest were not reported. Of 149 cohort studies, 99 were excluded since they included either non-TNF biologics (n = 37), did not adequately stratify results by BMI or weight (n = 46) or were unrelated to diseases of interest (n = 16). We included 54 cohorts derived from 50 studies for categorical analysis,[14, 26–33, 40–80] and 9 cohorts from 8 studies for per-unit dose-response analysis.[32, 33, 42, 54, 81–84] These included 34 observational cohorts (n = 13,336) and 20 cohorts derived from RCTs (n = 6,036). Fig 1 shows the study selection flowchart.
Fig 1. Study selection flowsheet.
Characteristics of included studies
Tables 1 and 2 provide details of included studies and participants. Overall, these 54 cohorts reported on 19,372 patients; 10, 16, 6 and 22 studies included patients with RA, IBD, SpA and psoriasis-PsA, respectively. Median 23% (IQR, 16–31.5%) patients were classified as obese in these studies. Follow-up ranged from 2–36 months. Overall, the studies were deemed to be at moderate risk of bias, due to failure to adjust for confounding variables and non-standard exposure definition (S1 Table).
Table 1. Characteristics of included studies–RCTs.
| Author, Year of publication | Design; Intervention (N), Comparator (N) | BMI in biologic intervention arm; exposure categories | Outcome; timing of measurement; failure to achieve outcome (%) | Patient characteristics (Age [SD], % males) |
Concomitant therapy |
|---|---|---|---|---|---|
| RHEUMATOID ARTHRITIS | |||||
| Smolen, 2011 [75] (open-label enrollment into PRESERVE) [ABSTRACT] |
Open-label; ETN 50mg SQ + MTX qw; 36 weeks (N = 761); no comparator |
BMI<25–386 (50.7%); BMI 25–29–248 (32.6%) BMI≥30–127 (16.7%) |
Failure to achieve remission (DAS28<2.6), 36 weeks; 32.5% | Age: 48 (12) Sex: 17% |
CS– 59%; IM (MTX)– 100% |
| Kaeley, 2016[57] (MUSICA) [ABSTRACT] |
ADA 40mg SQ qow + MTX 20mg qw (N = 155) vs. ADA 40mQ SW qow + MTX 7.5mg qw (N = 154) | BMI<25–69 (22.3%); BMI 25–29–102 (33.0%) BMI≥30–137 (44.3%) |
Failure to achieve response (ACR50), 24 weeks; 65.2% | Age: 55 (12) Sex: 25% |
CS– 42%; IM (MTX)– 100% |
| Heimans, 2013[54] (BeSt study) |
4 arms (only arm 4 included for this analysis): Group 1. Sequential monotherapy starting with MTX (N = 126); Group 2: step-up combination therapy starting with MTX (N = 121), Group 3: initial combination therapy,with the COBRA scheme: MTX, sulfasalazine and tapered highdose prednisone (N = 133), vs. Group 4: a combination of MTX and IFX 3mg/kg IV every 8 weeks (N = 128) | Only group 4: Not reported Overall, BMI<25–216 (42.5%) BMI≥25–292 (57.5%) |
Failure to achieve remission (DAS28<2.6), 52 weeks; NR (Group 4: RR of failing to achieve remission in patients with BMI≥25 vs. BMI<25 = 2.20 [0.99–4.92] |
Age: 54 (14) Sex: 34% |
NR |
| Weinblatt, 2013[79] (GO-FURTHER) |
Golimumab 2mg/kg IV at w0 and w4, and then q8w (n = 395) + MTX vs. placebo + MTX | Weight categories: <59.6 kg: 95 59.6–69.6 kg: 100 69.6–81.15 kg: 101 >81.15 kg: 98 |
Failure to achieve response (ACR20), 14 weeks; 41% | Age: 52 (13) Sex: 18% |
CS–NR IM (MTX): 100% |
| INFLAMMATORY BOWEL DISEASES | |||||
| Kobayashi, 2016[58] | IFX 5mg/kg IV at weeks 0,2,6 and then q8w (N = 104) vs. placebo (N = 104) (Ulcerative colitis) |
Weight, mean (SD): 57.6 (12.7); Exposure categories: Weight <56kg vs. ≥56kg | Failure to achieve remission (CAI≤4), week 14; 44% | Age: 40 (13) Sex: 64% |
CS– 65% IM– 48% |
| Sandborn, 2012[73]; (ULTRA 2) | ADA 160/80, and then 40mg SQ qow (N = 248) vs. placebo (N = 246) (Ulcerative colitis) |
Weight, mean (SD): 75.3 (17.7); Exposure categories: Weight <70kg vs. ≥70kg | Failure to achieve remission (MCS<3), week 52; 82.7% | Age: 40 (12) Sex: 57% |
CS– 61% IM– 38% |
| Sandborn, 2011 [72] | CZP 400mg SQ at weeks 0,2,4 (N = 223) vs. Placebo (N = 215) (Crohn’s disease) |
BMI<25–124 (55.6%) BMI≥25–91 (44.4%) |
Failure to achieve remission (CDAI<150), week 6; 68% | Age: 36 (13) Sex: 47% |
CS– 44% IM– 35% |
| Reinisch, 2011[69] (ULTRA 1) | ADA 160/80, and then 40mg SQ qow (N = 130) vs. placebo (N = 130) (Ulcerative colitis) |
Weight, mean (SD): 75.5 (14.2); Exposure categories: Weight <70kg vs. 70–81kg vs. ≥82kg | Failure to achieve remission (MCS<3), week 8; 81.5% | Age: 36 (14) Sex: 64% |
CS– 37% IM– 22% |
| Colombel, 2010 [48] (SONIC) | IFX 5mg/kg IV at weeks 0,2,6 and then q8w (N = 169) vs. IFX + Azathioprine 2.5mg/kg po (N = 169) vs. Azathioprine (N = 170) (Crohn’s disease) |
Weight, median (Groups 1 and 2): 68.9kg, 69.6kg; Exposure categories: Weight <60kg vs. 60–74kg vs. ≥75kg | Failure to achieve remission (CDAI<150); week 26; 49.4% | Age, median: 35 Sex: 51% |
CS– 29% IM– 50% |
| Sandborn, 2009[71] (ACT-1 and ACT-2 trials) | IFX 5mg/kg or 10mg/kg IV at weeks 0,2,6, and then every 8 weeks (N = 448) vs. placebo (N = 244) (Ulcerative colitis) |
ACT1 Weight, mean (SD): IFX 5mg/kg: 80.0 (17.8), IFX 10mg/kg: 76.9 (17.1); ACT2 Weight, mean (SD): IFX 5mg/kg: 78.4 (17.8), IFX 10mg/kg: 79.6 (20.6); Exposure category: Weight <76kg vs. ≥76kg |
Colectomy, week 54; 10.3% (variables adjusted for age, sex, disease duration, disease extent, baseline disease severity, concomitant medications, CRP) | ACT1: Age: 42 (15) Sex: 62% ACT2: Age: 40 (13) Sex: 60% |
CS: 56% IM: 47% |
| PSORIASIS-PSORIATIC ARTHRITIS | |||||
| Cai, 2017[44] | ADA 80mg then 40mg SQ qow (N = 338) vs. Placebo (N = 87) (Psoriasis) | BMI<25–193 (51.4%); BMI 25–29–126 (37.3%); BMI≥30–19 (5.6%); Exposure categories: BMI <25 vs. ≥25 |
Failure to achieve response (PASI75); week 12; 22.2% | Age: 43 (12) Sex: 75% |
None |
| Prussick, 2015[67] (CHAMPION trial) | ADA 80mg then 40mg SQ qow (N = 99) vs. MTX (N = 94) vs. Placebo (N = 48) (Psoriasis) |
BMI<25–40 (40.4%); BMI 25–29–28 (28.3%) BMI≥30–31 (31.3%) |
Failure to achieve response (PASI75), 16 weeks; 22.2% | Normal vs. overweight vs. obese Age: 37 (11) vs. 47 (14) vs. 44 (12) Sex: 53% vs. 82% vs. 73% |
None |
| Poulin, 2014[66] (REACH trial) | ADA 80mg then 40mg SQ qow (N = 49) vs. Placebo (N = 23) (Psoriasis) | Weight, mean (SD): 90.4 (19.7); Exposure categories: Weight <88kg vs. ≥88kg | Failure to achieve ‘clear’ or ‘almost clear’ on hfPGA scale; week 16; 69.4% | Age: 49 (11) Sex: 43% |
None |
| Gottlieb, 2012[52] | ETN 50mg SQ twice/week x 12 weeks, then 50mg qw + MTX (N = 239) vs. ETN 50mg SQ twice/week x 12 weeks, then 50mg qw + placebo (N = 239) (Psoriasis) |
BMI, mean (SD): 32.3 (7.5); Exposure categories: BMI ≤35 vs. >35 | Failure to achieve response (PASI75); week 24; 31.2% | Age: 45 (13) Sex: 67% |
None |
| Bagel, 2012[41] | ETN 50mg SQ twice/week x 12 weeks, then 50mg qw + placebo qw x 12 weeks (N = 62) [Cohort 1] vs. Placebo x 12 weeks, then ETN 50mg SQ twice/week x 12 weeks (N = 62) [Cohort 2] (Psoriasis) |
BMI, median (range): 30.2 (18.2–44.2) [Cohort 1]; 30.1 (19.1–49.5); Exposure categories: BMI ≤35 vs. >35 | Failure to achieve response (PASI75); week 12 for cohort 1; week 24 for cohort 2; 40.3% | Cohort 1 Age: 39 (13) Sex: 53% Cohort 2 Age: 42 (13) Sex: 58% |
None |
| Paul, 2012[65](BELIEVE trial) | ADA 80mg then 40mg SQ qow + topical calcipotriol/betamethasone dipropionate vs. ADA+matching topical vehicle (N = 730) (Psoriasis and psoriatic arthritis) |
Weight, mean (SD): 84.6 (19); Exposure categories: Weight quartiles | Failure to achieve response (PASI75), 16 weeks; 32.9% | Age>40: 66.6% Sex: 69% |
None |
| Menter, 2010[60] (REVEAL trial) | ADA 80mg then 40mg SQ qow (N = 814) vs. Placebo (N = 398) (Psoriasis) |
BMI<25–144 (17.7%); BMI 25–29–273 (33.5%) BMI≥30–395 (48.5%) |
Failure to achieve response (PASI75), week 16; 29% | Age: 44 (13) Sex: 67% |
None |
| SPONDYLOARTHRITIS | |||||
| Huang, 2014[56] | ADA 40mg SQ qow (N = 229) vs. Placebo (N = 115) | Weight, mean (SD): 63.3 (12.4); Exposure categories: Weight <60kg vs. ≥60kg | Failure to achieve response (ASAS20), week 12; 32.8% | Age: 30 (9) Sex: 81% |
CS: 4% IM: 23% |
| Mease, 2014[59] (ABILITY-1 trial) | ADA 40mg SQ qow (N = 91) vs. Placebo (N = 94) | BMI<25–42 (46.2%) BMI 25–29–37 (40.6%) BMI≥30–12 (13.2%) |
Failure to achieve response (ASAS20), week 12; 48.3% | Age: 38 (11) Sex: 52% |
None |
ADA = Adalimumab; ASAS = Assessment of Spondyloarthritis International Society; BMI = Body mass index; CAI = Clinical activity index; CDAI = Crohn’s disease activity index; CS = corticosteroids; CZP = Certolizumab pegol; ETN = Etanercept; hfPGA = hand/feet physician global assessment; IFX = Infliximab; IM = Immunomodulators; MTX = Methotrexate; PASI = Psoriasis Area Severity Index; qw = every week; qow = every other week; SD = standard deviation
Table 2. Characteristics of included studies–observational studies.
| Author, Year of publication | Location, Time Period; Study Design | Medications | BMI (Mean, SD) and Exposure categories | Outcome; timing of measurement; failure to achieve outcome (%) | Patient characteristics (Age, % males, % concomitant steroids) |
|---|---|---|---|---|---|
| RHEUMATOID ARTHRITIS | |||||
| Iannone, 2015 [27] | Italy, 2003–14; Retrospective | ADA: 68; ETN: 147; IFX: 73; CZP: 4 |
BMI<25–117 (40.1%) BMI 25–29–109 (37.3%) BMI≥30–66 (22.6%) |
Failure to achieve remission (ESR-DAS28<2.6), 12m; NR | Normal vs. overweight vs. obese Age: 54 (20) vs. 61 (14) vs. 61 (14) Sex: 10% vs. 18% vs. 17% Steroids: 70% vs. 85% vs. 73% |
| Ottaviani, 2015[29] | France, 2005–12; Retrospective | IFX: 76 | BMI<25–25 (32.9%) BMI 25–29–29 (38.2%) BMI≥30–22 (28.9%); Exposure categories: BMI ≥30 vs. <30 |
Failure to achieve remission (DAS28<2.6), 6m; 46.1% (variables adjusted for: age, sex, disease duration, ESR, CRP, prior anti-TNF therapies, concomitant immunomodulator therapies, baseline disease activity, RF and CCP status) | Age, median (IQR): 49 (42–56) Sex: 17% Steroids: 93% |
| Rodrigues, 2014[70][Abstract] | Portugal, NR; Retrospective | Anti-TNF: 317 | BMI<30–244 (67%) BMI≥30–73 (23%) |
Failure to achieve remission (DAS28<2.6), 6m; NR (variables adjusted for: NR) | NR |
| Gremese, 2013[32] | Italy, NR; Retrospective | ADA: 260; ETN: 227; IFX: 154 |
BMI<25–368 (57.4%) BMI 25–29–207 (32.3%) BMI≥30–66 (10.3%) |
Failure to achieve remission (DAS28<2.6); 12m; 69.7%; adjusted OR (per unit BMI) for failure to achieve remission: 1.12 [1.01–1.24] (variables adjusted for: age, sex, disease duration, DAS28, ESR, VAS pain, global health, HAQ, steroids, RF, anti-CCP, drug) | Age: 52 (14) Sex: 19% Steroids: 38.9% |
| Klaasen, 2011[30] | Holland, NR; Retrospective | IFX: 89 | BMI<20–8 (9.0%) BMI 20–29–66 (74.2%) BMI≥30–15 (16.8%) |
Failure to achieve response (ΔDAS28<1.2), 16 weeks; 28% | BMI<20 vs. BMI 20–30 vs. BMI>30 Age: 50 (15) vs. 57 (11) vs. 53 (15) Sex: 25% vs. 29% vs. 13% Steroids: 25% vs. 33% vs. 20% |
| Abhishek, 2010[40] | UK, 2001–08; Retrospective | Anti-TNF: 395 | BMI, mean (SD)– 27.0 (6.4); Exposure categories: BMI tertiles, T1, ≤23.5 vs. T2, 23.6–28.6 vs. T3, >28.6 | Failure to achieve at least moderate EULAR response, 3m; 10.6% (variables adjusted for: age, sex, disease duration, prior DMARDs, DAS28, concurrent MTX or prednisone, smoking, RF, other comorbidities) | Age: 61 (12) Sex: 23% Steroids: 31.6% |
| INFLAMMATORY BOWEL DISEASES | |||||
| Guerbau, 2016[53] [Abstract] |
France, 2009–14; Retrospective | IFX: 140 (CD) | BMI<25–96 (68.6%) BMI 25–29–21 (15.0%) BMI≥30–23 (16.4%) |
Rate of optimization of infliximab therapy; 12m; median delay in infliximab dose escalation was shorter in obese and overweight patients vs. normal BMI patients: 8.5m vs. 8m vs. 17m (p = 0.03) | No significant differences in baseline demographic and clinical characteristics of patients in 3 BMI categories (disease location/behavior, disease activity, concomitant immunosuppressives, C-reactive protein) |
| Brown, 2016[43] | UK, 1999–2012; Retrospective | IFX: 388 (CD) | BMI<18.5–33 (10.4%) BMI 18.5–24.9–218 (56.2%) BMI 25–29–91 (23.5%) BMI≥30–46 (11.9%) BMI≥30 vs. BMI 25–30 vs. BMI 18.5–24.9 |
Loss of response (any of: dose escalation, switching to alternative agent, need for steroids, hospitalization related to CD, CD- related surgery), 12m; 41.6% (variables adjusted for: sex, disease localization, thiopurine or steroids use, perianal disease, age, duration of disease) | Age: 37 (14) Sex: 46% Steroids: 35.1% |
| Billiet, 2016[81] | Belgium, 1994–2016; Retrospective | IFX: 261 (CD) | BMI, median (IQR): 22.1 (19.4–24.7); Exposure categorized: per unit BMI | IFX failure free survival (loss of response, persistent and high-titer anti-drug antibodies, need for surgery); IFX-failure free survival at 12m, 93.7%; per unit BMI: HR, 1.06 [1.01–1.13], i.e., 6% higher risk of treatment failure per unit increase in BMI (only significant on univariate analysis) | Age: 31 (22–43) Sex: 47% Steroids: 21.5% |
| Harper, 2013[33] | USA, 2008–11; Retrospective | IFX: 123 (99 CD, 24 UC) | BMI, mean (SD): CD– 26.5 (6); UC– 26.4 (7.4); Exposure categories: BMI≥30 vs. BMI<30 | Time to clinical flare (any of: dose escalation, switching to alternative agent, need for steroids, hospitalization related to IBD, IBD- related surgery); HR for time to clinical flare per unit BMI: CD– 1.06 [1.01–1.11], UC– 1.30 [1.07–1.58] (variables adjusted for: prior surgery, steroid use, extraintestinal manifestations, age, disease duration) | Age: CD– 35 (13); UC– 35 (15) Sex: CD– 48%; UC– 53% Steroids: CD– 35.4%; UC– 66.7% |
| Bhalme, 2013[42] | UK, 2000–09; Retrospective | IFX: 76; ADA: 54 (CD) | BMI, mean (SD): IFX– 24.5 (6); ADA– 25.7 (5.8); BMI<30: IFX– 62; ADA– 46 BMI≥30: IFX– 14; ADA– 8 Exposure categories: BMI≥30 vs. BMI<30 |
Time to loss of response (need for escalation of therapy); 12m; Proportion of patients requiring escalation of therapy at 1 year: BMI 25 vs. 30 vs. 35: IFX– 19% vs. 16% vs. 15%; ADA– 20% vs. 29% vs. 40% | Age: IFX– 39 (15); ADA– 40 (16) Sex: IFX– 45%; ADA– 33% Steroids: NR |
| Rosen, 2012[84] [ABSTRACT] |
USA, 2002–11; Prospective | CZP: 74 (CD) | Exposure categories: per unit BMI | Need for surgery; 12m; adjusted OR for need for surgery per unit increase in BMI– 1.14 [1.01–1.30] (variables adjusted for: ESR) | Age: 36 (14) Sex: 28% Steroids: NR |
| Horst, 2012[55] [ABSTRACT] |
USA, 2008–11; Retrospective | CZP: 107 (CD) | BMI≤25–44 BMI>25–63 |
Discontinuation of therapy, NR (variables adjusted for: age, sex, smoking, disease type) | Age: 39 (14) Sex: 38% Steroids: NR |
| Click, 2012[47] [ABSTRACT] |
USA, 2006–11; Retrospective | ADA: 107; CZP: 28 (CD) |
BMI<30–100 BMI≥30–35 |
Failure to respond to induction therapy, NR; 29.6% | Age: 38 (14) Sex: 37% Steroids: NR |
| Bultman, 2012[82] | The Netherlands; 2007–10; Prospective | ADA: 122 (CD) |
BMI, median (range): 23 (15–42); Exposure categories: per unit BMI | Dose escalation; 38% required dose escalation; adjusted OR for need for dose escalation per unit increase in BMI: 1.11 [1.01–1.23] (variables adjusted for: sex, disease location, disease activity, perianal disease, prior anti-TNF, prior surgery, CRP) | Age: 35 (12) Sex: 44% Steroids: NR |
| Moore, 2011[62] [ABSTRACT] |
USA, 2009–10; Retrospective | ADA: 19; CZP: 22 (CD) |
BMI<25–21 (68.6%) BMI 25–29–20 (15.0%) BMI≥30–6 (16.4%) |
Failure to achieve clinical remission or response based on HBI, median follow-up: 8 weeks); 19.5% | Age: 31 (13) Sex: 42% Steroids: Normal BMI– 20%; Obese or overweight– 38% |
| Qumseya, 2009[68] [ABSTRACT] |
USA, NR; Retrospective | ADA: 118 (CD) |
BMI<25–63 (53.4%) BMI 25–29–27 (22.9%) BMI≥30–28 (23.7%) |
Failure of therapy (need for dose escalation or switching to alternative therapy), NR; 38.1% | Age: 24 (11) Sex: 36% Steroids: NR |
| PSORIASIS and/or PSORIATIC ARTHRITIS | |||||
| Zweegers, 2016[80] | The Netherlands, 2005–15; Prospective | ADA: 186; ETN: 238 (Psoriasis) |
BMI, mean (SD): 27.8 (6.6); Exposure categories: BMI≥30 vs. BMI<30 | Drug discontinuation due to ineffectiveness; 5-year discontinuation of ADA and ETN due to ineffectiveness, 46% and 55%, respectively (variables adjusted for: age, sex, trial eligible, family history for psoriasis, PsA, disease duration, baseline disease activity, prior therapy) | Age: ADA– 49 (13); ETN– 47 (13) Sex: ADA– 57%; ETN– 61% Steroids: NA |
| Villarasa, 2016[77] | Spain, 2007–13; Retrospective | ADA: 231; ETN: 248; IFX: 84 (Psoriasis) |
BMI, mean (SD): 28.6 (5.9) BMI<30–334 (59.4%) BMI≥30–229 (40.6%) |
Drug discontinuation due to primary non-response, loss of response or adverse events; 1-year drug discontinuation of ADA, ETN and IFX was 31%, 29.1% and 31.2%, respectively (variables adjusted for: drug use, baseline disease activity) | Age: 50 (13) Sex: 64% Steroids: NA |
| Ogdie, 2016[83] [ABSTRACT] |
CORRONA Registry, USA, 2005–13; Prospective | Anti-TNF: 725 | BMI, median (IQR): 30.9 (26.7–36.2); Exposure categories: per unit BMI | Failure to achieve remission (CDAI<2.8), 12m; 83%; each unit increase in BMI associated with 5% [2.0–8.4%] higher risk of failing to achieve remission (variables adjusted for: sex, prior therapy, baseline disease activity, work full time, pain) | Age, median (IQR): 52 (44–60) Sex: 44% Steroids: 14% |
| Menter, 2016[61] | PSOLAR, 2007–13; Prospective | ADA: 402; ETN: 289; IFX: 63 (Psoriasis) |
BMI, mean (SD): 30.4 (7.1); Exposure categories: BMI≥35 vs. BMI 25–35 vs. BMI<25 |
Drug discontinuation, reported as time to event; NR | Age: 47 (14) Sex: 57% Steroids: NA |
| Hojgaard, 2016[26] | Denmark (DANBIO)/Iceland (ICEBIO), NR; Prospective | ADA: 520; ETN: 287; IFX: 352; CZP: 27; GLM: 85 (Psoriatic arthritis) |
BMI<30–863 (68%) BMI≥30–408 (32%) |
Failure to respond to therapy (EULAR goof response); discontinuation of therapy due lack of efficacy higher in obese vs. non-obese patients (HR, 1.85 [1.38–2.48]) | Age: Obese– 49 (12); Non-obese– 47 (13) Sex: 45% Steroids: NR |
| Chiricozzi, 2016[46] | Italy, 2005–14; Retrospective | ADA: 316 (Psoriasis [n = 117] and psoriatic arthritis [n = 199]) |
BMI<25–123 (38.9%) BMI 25–29–138 (43.7%) BMI≥30–55 (17.4%) |
Discontinuation of therapy; 46.8% discontinued therapy during the observation period | Age: 48 (13) Sex: 76% Steroids: NR |
| Warren, 2015[78] | BADBIR Registry, UK, 2007–14; Prospective | ADA: 1879 ETN: 1098 IFX: 96 (Psoriasis) |
BMI<18.5–32 (0.9%) BMI 18.5–24.9–559 (16.1%) BMI 25–29–1024 (29.4%) BMI 30–34.9–788 (22.5%) BMI 35–40–464 (13.2%) BMI>40–346 (9.1%); Exposure categories: BMI≥30 vs. BMI 25–30 vs. BMI<25 |
Discontinuation of therapy due to ineffectiveness; 1-year drug discontinuation due to ineffectiveness rate, 13% (variables adjusted for age, sex, smoking status, comorbidities, disease duration, concomitant medications, biologic drug) | Age: 45 (13) Sex: 60% Steroids: NA |
| Di Lernia, 2014[50] | Italy; NR; Retrospective | Anti-TNF: 110 (Psoriasis) | BMI<25–123 (38.9%) BMI 25–29–138 (43.7%) BMI≥30–55 (17.4%) |
Failure to respond to therapy (PASI50) by week 12–16; 13 patients (11.8%) did not respond | Age: 51 (12) Sex: NR Steroids: NA |
| Costa, 2014[49] | Italy, 2008–11; Prospective | Anti-TNF: 330 (Psoriatic arthritis) | BMI, mean (SD): Metabolic syndrome– 28.5 (4.5); No metabolic syndrome– 26.4 (3.8); Exposure categories: BMI≥30 vs. BMI<30 | Failure to achieve minimal disease activity, 24 months; 52.2% failed to achieve minimal disease activity | Age: Metabolic syndrome– 47 (9); no metabolic syndrome– 47 (9) Sex: 45%; 46% Steroids: NR |
| Iannone, 2013[28] | Italy, 2006–11; Retrospective | ADA: 42; ETN: 48; IFX: 45 (Psoriatic arthritis) |
BMI<25–43 (31.9%) BMI 25–29–47 (34.8%) BMI≥30–45 (33.3%) |
Failure to achieve remission (DAS28<2.6), 36 months; 57.0% failed to achieve remission | BMI<25 vs. BMI 25–30 vs. BMI>30 Age: 51 (12) vs. 53 (11) vs. 56 (11) Sex: 47% vs. 57% vs. 47% Steroids: 83% vs. 50% vs. 38% |
| Di Minno, 2013[14] | Italy, 2007–10; Prospective | ADA: 80; ETN: 111; IFX: 79 (Psoriatic arthritis) |
BMI<30–135 (50%) BMI 30–35–100 (37%) BMI>35–35 (13%) |
Failure to achieve minimal disease activity, 12 months; 63.7% failed to achieve minimal disease activity (variables adjusted for: age, sex, disease duration, concomitant methotrexate, smoking, diabetes, hyperlipidemia, hypertension, ESR, CRP, baseline disease activity) | BMI<30 vs. BMI ≥30 Age: 51 (13) vs. 52 (10) Sex: 52% vs. 40% Steroids: NR |
| Di Renzo, 2012[51] | Italy, 2007–08; Prospective | ADA: 19; ETN: 28; IFX: 13 (Psoriasis [n = 36] and psoriatic arthritis [n = 24]) |
BMI<30–37 (46.3%) BMI≥30–43 (53.7%) [Only 60 patients completed study, and were reported] |
Failure to achieve response (PASI75), 24 weeks; 23% failed to achieve response | Age: 39 (9) Sex: NR Steroids: NR |
| Naldi, 2008[63] | PSOCARE, Italy, 2005–07; Prospective | ETN: 810; IFX: 256 |
BMI<30 –ETN: 526 (64.9%); IFX: 186 (72.7%); BMI≥30 –ETN: 231 (28.5%); IFX: 43 (25.8%) |
Failure to achieve response (PASI75), 8 weeks; 54.3% IFX-treated and 65.2% ETN-treated patients failed to achieve response | Age>40: 70.7% Sex: 66.6% Steroids: NA |
| Cassano, 2008[45] | APHRODITE, Italy, NR; Prospective | ADA: 144 (Psoriasis AND psoriatic arthritis) |
BMI<25–57 (39.6%) BMI 25–30–55 (38.2%) BMI>30–32 (22.2%); Exposure categories: BMI≥30 vs. BMI<30 |
Failure to achieve response (PASI50), 12 weeks; 22.9% failed to respond | Age: 49 (NR) Sex: 51% Steroids: NR |
| SPONDYLOARTHRITIS | |||||
| Vidal, 2016[76] [ABSTRACT] |
France, 2001–15; Retrospective | Anti-TNF: 168 (Axial SpA) |
BMI–NR Exposure categories: BMI ≥25 vs. <25 |
Failure to achieve response (BASDAI50), 3 months; NR | Age: 42 (11) Sex: 24% Steroids: NR |
| Simone, 2014[74] | Italy, NR; Retrospective | Anti-TNF: 153 (Axial SpA) | BMI–NR Exposure categories: BMI ≥25 vs. <25 |
Failure to achieve strong clinical response (BASDAI<1), 12 months; NR | Age: 40 (8) Sex: 50% Steroids: NR |
| Gremese, 2014[31] | Italy, NR; Retrospective | ADA: 35; ETN: 31: IFX: 104 (Axial SpA) |
BMI<25–92 (54.1%) BMI 25–30–55 (32.4%) BMI>30–23 (13.5%) |
Failure to achieve response (BASDAI50), 12 months; 38.8% | Age: 40 (12) Sex: 69% Steroids: NR |
| Ottaviani, 2012[64] | France, NR; Retrospective | IFX: 155 | BMI<25–63 (40.6%) BMI 25–30–54 (34.8%) BMI>30–38 (24.6%) |
Failure to achieve response (BASDAI50), 6 months; 44.6% | Age, median (IQR): 43 (35–52) Sex: 63% Steroids: NR |
ADA = Adalimumab; ASAS = Assessment of Spondyloarthritis International Society; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BMI = Body mass index; CAI = Clinical activity index; CDAI = Crohn’s disease activity index; CRP = C-reactive protein; CS = corticosteroids; CZP = Certolizumab pegol; ESR = Erythrocyte sedimentation rate; ETN = Etanercept; EULAR = European League Against Rheumatism; hfPGA = hand/feet physician global assessment; IFX = Infliximab; IM = Immunomodulators; MTX = Methotrexate; NR = Not reported; PASI = Psoriasis Area Severity Index; qw = every week; qow = every other week; SD = standard deviation; SpA = Spondyloarthropathy
Obesity and response to anti-TNF therapy
On meta-analysis, across included IMIDs and across all anti-TNF agents, obesity was associated with 60% higher odds of failure of index anti-TNF therapy (OR, 1.60; 95% CI, 1.39–1.83), with substantial heterogeneity (I2 = 71%).[14, 26–33, 40–80]
Dose-response relationship
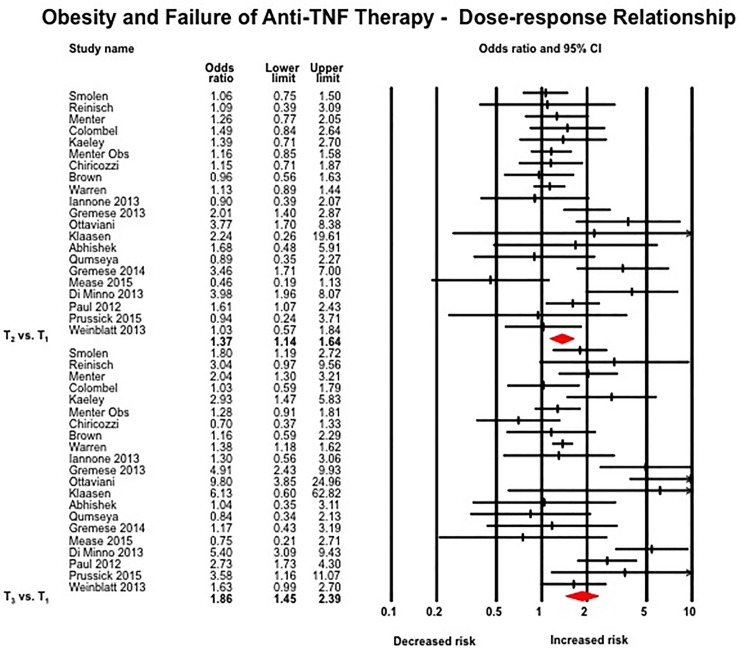
The odds of failing therapy were highest in the third tertile of BMI or weight as compared to patients in the second tertile (21 studies; T3 vs. T1: OR, 1.86 [1.45–2.39]; T2 vs. T1: OR, 1.37 [1.14–1.65], p-value comparing effect size for T3 and T2 = 0.05) (Fig 2).[14, 28–32, 40, 43, 46, 48, 57, 59–61, 65, 67–69, 75, 78, 79] On restricting analyses only to studies that reported dose-response in terms of WHO-defined categories (n = 17 studies), similar results were observed (obese vs. normal BMI: OR, 1.87 [1.39–2.52]; overweight vs. normal BMI: OR, 1.38 [1.11–1.73], p-value comparing effect size for obese and overweight = 0.11).[14, 28–32, 40, 43, 46, 57, 59–61, 67, 68, 75, 78] On analysis of studies reporting effect estimates per unit BMI, each 1 kg/m2 increase in BMI was associated with 6.5% higher odds of failing anti-TNF therapy (9 studies; OR, 1.065 [1.043–1.087], I2 = 5%) (S1 Fig).[32, 33, 42, 54, 81–84]
Fig 2. Association between obesity and response to anti-TNF therapy–dose-response relation, with comparison of patients in the 3rd tertile by weight or BMI with patients in the 1st tertile, and of patients in the 2nd tertile vs. the first tertile.
Subgroup analyses
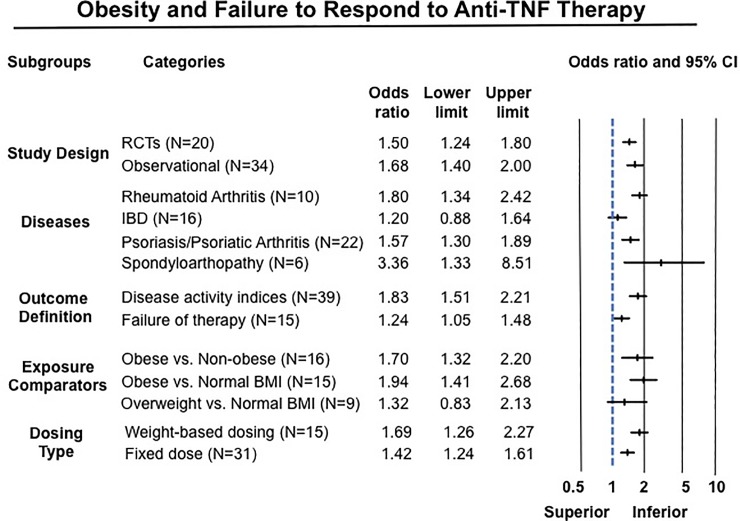
Results of subgroup analyses are summarized in Fig 3.
Fig 3. Summary results of subgroups analyses based on study design, disease type, outcome definition, exposure categories and drug dosing.
Types of diseases
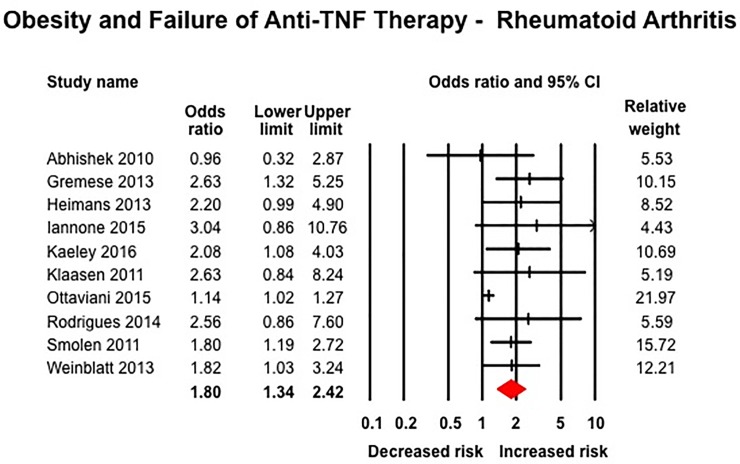
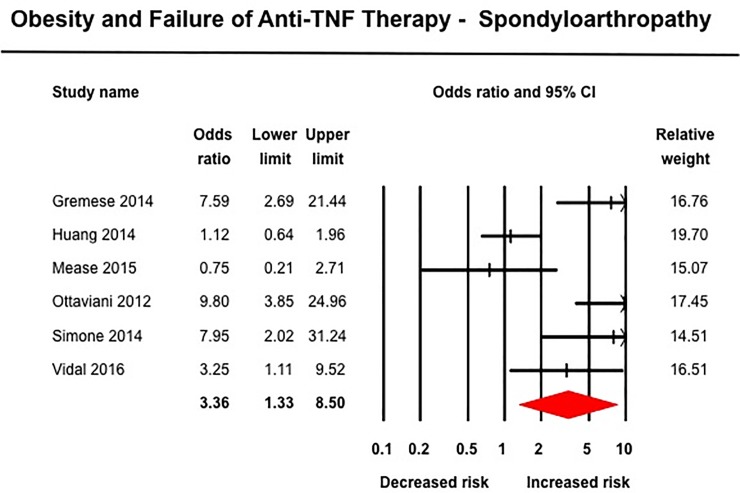
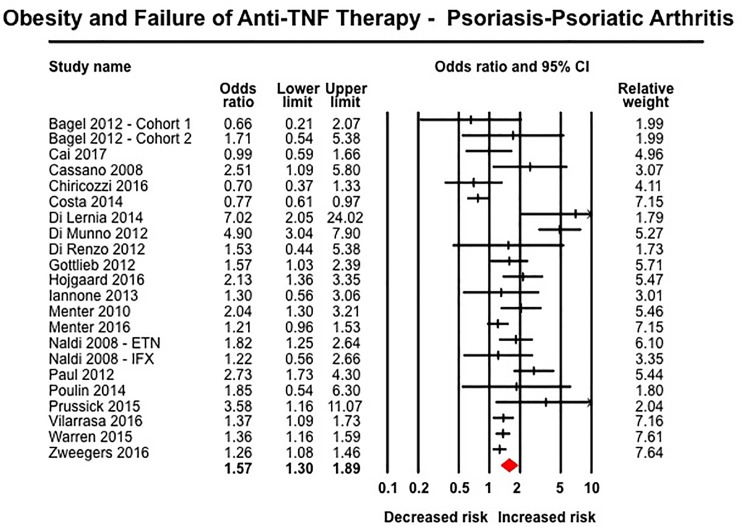
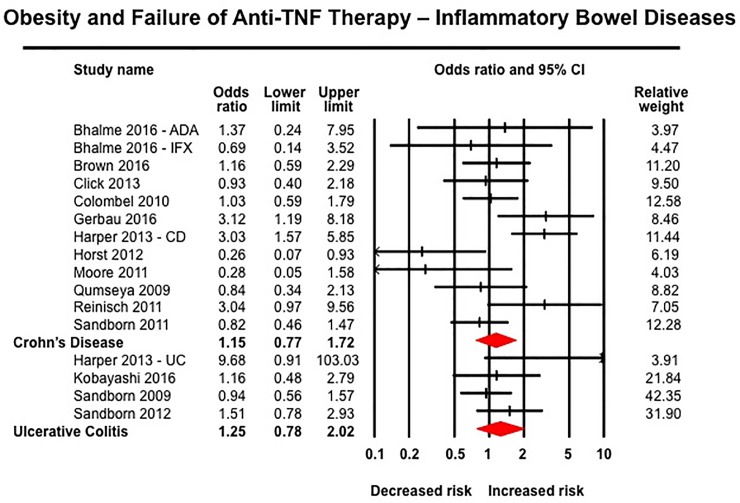
Obesity was associated with inferior response to anti-TNF therapy across all rheumatic diseases. In 10 studies in patients with RA including 3,403 patients (median [IQR], 23% [17–29%] obese), obese patients had 80% higher odds of failure of therapy (OR, 1.80 [1.34–2.42]; I2 = 58%) (Fig 4).[27, 29, 30, 32, 40, 54, 57, 70, 75, 79] Similarly, obese patients with SpA (6 studies, 966 patients, 14% obese) (OR, 3.36 [1.33–8.51], I2 = 81%) (Fig 5)[31, 56, 59, 64, 74, 76] and psoriasis-PsA (22 studies, 11,873 patients, median [IQR], 33% [22–50%]) (OR, 1.57 [1.30–1.89], I2 = 77%) had inferior response to anti-TNF therapy (Fig 6).[14, 26, 28, 41, 44–46, 49–52, 60, 61, 63, 65–67, 77, 78, 80] No significant association was observed between obesity and response to anti-TNF therapy in patients with IBD based on 16 studies, with 3,130 patients (median [IQR], 16.5% [16–24%] obese) (OR, 1.20 [0.88–1.64], I2 = 50%) (Fig 7), either in patients with CD (12 studies; OR, 1.15 [0.77–1.72]) or ulcerative colitis (4 studies; OR, 1.25 [0.78–2.02]).[33, 42, 43, 47, 48, 53, 55, 58, 62, 68, 69, 71–73] Of note, amongst IBD patients, 8/16 studies reported only dichotomous exposure assessment, generally categorized as above or below median weight and as overweight vs. non-overweight categories; more extreme weight categories were not reported.
Fig 4. Obesity and response to anti-TNF therapy in patients with rheumatoid arthritis.
Fig 5. Obesity and response to anti-TNF therapy in patients with spondyloarthritis.
Fig 6. Obesity and response to anti-TNF therapy in patients with psoriasis and psoriatic arthritis.
Fig 7. Obesity and response to anti-TNF therapy in patients with inflammatory bowel diseases.
Route of administration and dosing regimen
Infliximab is the only anti-TNF agent routinely administered intravenously in a weight-based dose; additionally, a form of golimumab (Simponi Aria®) is occasionally administered intravenously in a weight-based dose; all other agents are administered in a fixed dose independent of body weight, subcutaneously. Hence, route and dosing scheme could not be evaluated independently. Patients with obesity treated with both weight-based dosing regimens (16 studies; OR, 1.69 [1.26–2.27], I2 = 67%) (Panel A in S2 Fig)[26, 29, 30, 33, 42, 43, 48, 53, 54, 58, 63, 64, 71–73, 79] and fixed-dose regimens (31 studies; OR, 1.42 [1.24–1.61], I2 = 46%) (Panel B in S2 Fig)[26, 27, 32, 41, 42, 44–47, 52, 53, 55–57, 59–63, 65–69, 72, 73, 75–78, 80] had higher odds of failing therapy, without significant differences in the summary estimate (p-value comparing effect size for fixed-dose and weight-based dosing = 0.28).
Exposure categories and outcome definition
Studies comparing patients with obesity with either non-obese (16 studies, OR, 1.70 [1.32–2.20])[26, 29, 31–33, 42, 45, 47, 49–51, 63, 70, 77] or normal BMI (15 studies, OR, 1.94 [1.41–2.68])[14, 27, 28, 30, 43, 46, 53, 57, 59, 60, 64, 67, 68, 75, 78] patients consistently reported a negative effect of obesity on response to therapy; in contrast studies with exposure categories of only overweight vs. non-overweight (without more extreme BMI categories) did not observe a significant association (9 studies; OR, 1.32 [0.82–2.13]) (pinteraction = 0.42).[33, 44, 54, 55, 61, 62, 72, 74, 76] Based on outcome definition, the effect of obesity was more pronounced in studies that used validated definitions of clinical remission or response (based on disease activity indices; 39 studies) (failure to achieve remission/response: OR, 1.83 [1.51–2.21])[14, 26–32, 40, 41, 44, 45, 47–52, 54, 56–60, 63–67, 69, 70, 72–76, 79] as compared to studies that used pragmatic definition of treatment modification (15 studies; OR, 1.24 [1.05–1.48]) (pinteraction<0.01).[33, 42, 43, 46, 53, 55, 61, 62, 68, 71, 77, 78, 80]
Study design
Obesity was associated with inferior response to anti-TNF therapy in both RCTs (20 studies; OR, 1.50 [1.24–1.80], I2 = 40%) (Panel A in S3 Fig)[41, 44, 48, 52, 54, 56–60, 65–67, 69, 71–73, 75, 79] and observational studies (34 studies; OR, 1.68 [1.48–2.00], I2 = 78%) (Panel B in S3 Fig), without significant difference between subgroups (pinteraction = 0.39).[14, 26–33, 40, 42, 43, 45–47, 49–51, 53, 55, 61–64, 68, 70, 74, 76–78, 80]
On meta-regression, prevalence of obesity in included studies modestly but significantly affected summary estimate (p = 0.06), with smaller effect size observed in studies with lower prevalence of obesity (Panel A in S4 Fig); prevalence of outcome did not affect summary estimate (p = 0.54) (Panel B in S4 Fig).
Sensitivity analysis and publication bias
Overall results were similar when restricting to studies that reported analyses adjusted for key confounding variables (13 studies; OR, 1.52 [1.22–1.89], I2 = 79%),[14, 29, 32, 33, 40, 43, 55, 70, 71, 77, 78, 80] studies focusing only on failure to achieve remission based on standard DAI (22 studies; OR, 1.62 [1.26–2.08], I2 = 73%)[14, 27, 28, 32, 41, 44, 48, 49, 52, 57, 58, 60, 63, 66, 69, 70, 72, 73, 75, 76] and on limiting to studies published as full-texts (45 cohorts; OR, 1.61 [1.40–1.86], I2 = 73%).[14, 26–33, 40–46, 48–52, 54, 56, 58–61, 63–67, 69, 71–74, 77–80] There was no evidence of publication bias based on examination of funnel plot symmetry (S5 Fig) and quantitatively on Begg and Mazumdar’s test (p = 0.60).
Discussion
Principal findings
In this systematic review with meta-analysis of 54 cohorts with 19,372 patients with select IMIDs treated with anti-TNF agents, we made several key observations related to the influence of obesity on response to anti-TNF therapy. First, we observed that obesity was associated with a 60% higher odds of failing anti-TNF therapy as compared to non-obese and normal BMI counterparts, for most IMIDs, including RA, spondyloarthropathies and psoriasis and psoriatic arthritis, but not IBD. This association was stable across diverse subgroups including study design, exposure definition, outcome measurement, and was independent of other potential confounding variables. Second, a dose-dependent effect was seen, with each unit increase in BMI associated with 6.5% higher odds of failing therapy. Third, this effect was independent route of administration and dosing scheme. It is also important to note that <10% of eligible RCTs reported results stratified by baseline BMI. Our findings of negative impact of obesity on treatment response to anti-TNF agents suggests that obesity is a negative prognostic factor and treatment effect modifier that must be considered in clinical trial design and clinical practice.
Obesity is recognized as a perpetual state of chronic low-grade inflammation, through systemic and paracrine increase in levels of cytokines, chemokines and adipokines.[6] Besides its direct impact on inflammation, obesity can also modify pharmacokinetics of anti-TNF and other biologic agents. Population pharmacokinetic studies of all anti-TNF agents have identified high body weight as a risk factor associated with increased clearance of drug, resulting in shorter half-life and lower serum trough drug concentrations.[20–22] This effect might be related to rapid proteolysis and to a ‘TNF-sink’ phenomenon, wherein the clearance of a monoclonal antibody that binds to membrane antigen is faster at low doses as the unbound targets “sop up” antibody, serving as a sink.[85] This may explain why patients with obesity treated even weight-based regimens such as infliximab, had inferior response to therapy.
Comparison with other studies
Our findings expand on prior observations on the potentially negative impact of obesity on overall treatment response in patients with RA and psoriasis. However, these prior studies included all treatment categories, instead of focusing on anti-TNF therapy. Moreover, they were unable to simultaneously study differential impact across different diseases, and with different anti-TNF agent. Though we had hypothesized that obesity would negatively impact response to anti-TNF therapy across all selected diseases, we did not observe such an association in patients with IBD. This may be a true observation. Patients with IBD show a unique locally restrictive form of visceral adipose tissue—creeping fat—whereby mesenteric fat hyperplasia is limited to areas of inflamed bowel.[86, 87] It is likely that in IBD patients, this local mesenteric fat plays a more important role than systemic obesity. Additionally, dose of infliximab and adalimumab approved in patients with IBD is higher than that for other rheumatic diseases. Differential effect of confounding by disease severity in IBD and rheumatic diseases may also explain this finding–severe IBD is likely to result in weight loss and misclassification of obesity, whereas, severe rheumatic diseases would likely impact physical activity, promote sedentary lifestyle and contribute to obesity. Alternatively, it is possible that our observation in IBD patients is biased. Most studies in IBD patients presented dichotomous categories, usually as above or below median weight, and did not include patients at extreme categories of BMI, where the negative impact of obesity may be more pronounced. On meta-regression, effect estimates were smaller in studies with lower prevalence of obesity. Five studies in IBD patients were reported only as abstracts, putting them at high risk of bias (though excluding these studies did not modify the summary estimate). Further studies, of both fixed dose therapies and weight-based therapies, preferably using individual participant data from clinical trials are warranted to better understand this association.
Strengths and limitations
The strengths of this systematic review include: (a) comprehensive and systematic literature search with well-defined inclusion criteria, including both RCTs and observational studies as cohort studies, (b) a priori comprehensive sub-group and sensitivity analyses to evaluate the stability of findings and identify potential factors responsible for inconsistencies, (c) assessment of dose-response relationship using two approaches, and (d) simultaneous evaluation of unadjusted (based on raw numbers) and adjusted risk estimates, and hence, being able to evaluate the potential influence of measured confounders on the summary estimate.
There are several limitations in our study. First, the meta-analysis included only cohorts, derived from both RCTs and observational studies, but participants in RCTs were not stratified based on presence or absence of obesity. Most of the included studies did not account for several potential confounding factors including steroid use and baseline disease activity, both of which influence exposure and outcome. Additionally, factors that may be intrinsically related to obesity, such as depression, fibromyalgia, etc., may confound this association between obesity and inferior clinical response to therapy. While sensitivity analysis using the adjusted data had little effect on the summary OR, potentially suggesting that any difference attributable to using different confounders for adjustment is likely small, data regarding several confounders was inadequately reported. Second, substantial heterogeneity was observed in the overall analysis, which was partly explained by difference in outcome definition and prevalence of obesity in included cohorts, as well as different types of diseases. It is important to note that a stronger effect estimate was observed prospective studies that used validated disease activity indices, with fairly consistent outcome definitions, as compared to retrospective studies which used non-validated definitions such as need for escalation of index therapy, switching to alternative therapy or surgery, etc. Fourth, despite absence of small study effects, there was concern for reporting bias. Less than 10% of eligible RCTs results stratified by baseline BMI; when they did, exposure was frequently classified as dichotomous variable, above and below a median weight. Finally, we limited our analysis to only anti-TNF agents, instead of including other non-TNF-biologic agents and small molecules. This was done to minimize conceptual heterogeneity. Negative impact of obesity on response to other therapies has been observed, and merits detailed evaluation.
Implications for clinical practice
While the negative impact of obesity in patients with psoriasis is well-known, our observations that obesity uniformly results in inferior response to anti-TNF therapy across all rheumatic diseases that we studied has important implications for both clinical practice and clinical trial design. In clinical practice, physicians may consider aggressive treatment and close proactive monitoring in patients with obesity treated with anti-TNF agents such as empirically using higher dose of anti-TNF agent in obese patients, frequent therapeutic drug monitoring and/or use of combination therapy with immunomodulators to increase drug concentration and decrease risk of immunogenicity. Clinical trialists should consider obesity as a potential effect modifier and consider obesity as a stratification variable; at the very least, stratified analysis by baseline BMI should be performed and consistently reported. Finally, the presence of obesity may offer a potential therapeutic intervention, either through adjusting biologic dosing for body weight or through directly targeting the obesity itself for treatment in patients with select IMIDs with a multi-disciplinary approach. Small RCTs and cohort studies in patients with psoriasis, psoriatic arthritis and RA have suggested a beneficial effect of intentional weight loss on treatment response to anti-TNF agents.[88–90]
Conclusion
In conclusion, obesity is associated with inferior response to anti-TNF therapy in patients with rheumatic diseases, but not in patients with IBD. This effect is independent of route of administration, and is observed in patients treated with weight-based as well as fixed dose agents. However, current findings need to be interpreted with caution due to high heterogeneity and due to lack of adjustment for relevant confounding factors in included studies. Prospective cohort studies and post-hoc analyses of RCTs with individual participant level data are warranted to confirm this association. If this effect is consistent, interventional studies targeting obesity should be explored for difficult-to-treat obese patients with select IMIDs.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIFF)
Association between obesity and response to anti-TNF therapy–Subgroup analyses based on anti-TNF dosing regimen: (A) weight-based dosing, and (B) fixed dose therapies.
(TIFF)
Association between obesity and response to anti-TNF therapy–Subgroup analyses based on study design: (A) RCTs, and (B) observational studies.
(TIFF)
Meta-regression based on (A) prevalence of obesity, and (B) prevalence of outcome.
(TIFF)
(TIFF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Dr. Singh is supported by the American College of Gastroenterology and the Crohn’s and Colitis Foundation, and has received research support from Pfizer and AbbVie. Dr. Zarrinpar received support from NIH K08 DK102902, AASLD Liver Scholar Award, and American Heart Association Beginning Grant-in-Aid (16BGIA27760160), and has consulted for Takeda and Illumina. Dr. Curtis is supported by the Patient Centered Outcomes Research Institute (PCORI). Dr. Sandborn has served as a consultant and received research funding from Janssen, Abbvie, UCB Pharma, Takeda, and Pfizer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12(2):131–41. 10.1111/j.1467-789X.2009.00712.x . [DOI] [PubMed] [Google Scholar]
- 4.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17:86 10.1186/s13075-015-0601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremmer S, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG, Young M, et al. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;63(6):1058–69. 10.1016/j.jaad.2009.09.053 . [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14(2):110–21. 10.1038/nrgastro.2016.181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, et al. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21(2):361–8. 10.1097/MIB.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SF M.; Shaffer K.; Singh A.G.; Prokop L.J.; Grunvald E.; Zarrinpar A.; Sandborn W.J. Pre-Morbid Obesity is Associated with Increased Risk of Developing Immune-Mediated Inflammatory Diseases: a Systematic Review and Meta-Analysis. Gastroenterology. 2017;152:S976–S7. [Google Scholar]
- 9.Albrecht K, Richter A, Callhoff J, Huscher D, Schett G, Strangfeld A, et al. Body mass index distribution in rheumatoid arthritis: a collaborative analysis from three large German rheumatoid arthritis databases. Arthritis Res Ther. 2016;18:149 10.1186/s13075-016-1043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain AS S.; Martin C.; Sandler R.; Sandborn W.J.; Herfarth H.H.; Kappelman M.D.; Long M.D. Obesity is Associated with Worse Disease Activity in Patients with Inflammatory Bowel Diseases: An Internet Based Cohort Study. Gastroenterology. 2017;152:S973–S4. [Google Scholar]
- 11.Liu Y, Hazlewood GS, Kaplan GG, Eksteen B, Barnabe C. Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken). 2017;69(2):157–65. 10.1002/acr.22932 . [DOI] [PubMed] [Google Scholar]
- 12.Eder L, Thavaneswaran A, Chandran V, Cook RJ, Gladman DD. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis. 2015;74(5):813–7. 10.1136/annrheumdis-2013-204448 . [DOI] [PubMed] [Google Scholar]
- 13.Sandberg ME, Bengtsson C, Kallberg H, Wesley A, Klareskog L, Alfredsson L, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis. 2014;73(11):2029–33. 10.1136/annrheumdis-2013-205094 . [DOI] [PubMed] [Google Scholar]
- 14.di Minno MN, Peluso R, Iervolino S, Lupoli R, Russolillo A, Scarpa R, et al. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res (Hoboken). 2013;65(1):141–7. 10.1002/acr.21711 . [DOI] [PubMed] [Google Scholar]
- 15.Singh JA, Saag KG, Bridges SL Jr., Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis rheumatol. 2016;68(1):1–26. 10.1002/art.39480 . [DOI] [PubMed] [Google Scholar]
- 16.Ramiro S, Smolen JS, Landewe R, van der Heijde D, Dougados M, Emery P, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2016;75(3):490–8. 10.1136/annrheumdis-2015-208466 . [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Pardi DS. Update on anti-tumor necrosis factor agents in Crohn disease. Gastroenterol Clin North Am. 2014;43(3):457–78. 10.1016/j.gtc.2014.05.008 . [DOI] [PubMed] [Google Scholar]
- 18.Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369(8):754–62. 10.1056/NEJMct1209614 . [DOI] [PubMed] [Google Scholar]
- 19.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84. 10.1016/S0140-6736(16)31591-4 . [DOI] [PubMed] [Google Scholar]
- 20.Mostafa NM, Nader AM, Noertersheuser P, Okun M, Awni WM. Impact of immunogenicity on pharmacokinetics, efficacy and safety of adalimumab in adult patients with moderate to severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(3):490–7. 10.1111/jdv.13884 . [DOI] [PubMed] [Google Scholar]
- 21.Passot C, Mulleman D, Bejan-Angoulvant T, Aubourg A, Willot S, Lecomte T, et al. The underlying inflammatory chronic disease influences infliximab pharmacokinetics. MAbs. 2016;8(7):1407–16. 10.1080/19420862.2016.1216741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wade JR, Parker G, Kosutic G, Feagen BG, Sandborn WJ, Laveille C, et al. Population pharmacokinetic analysis of certolizumab pegol in patients with Crohn's disease. J Clin Pharmacol. 2015;55(8):866–74. 10.1002/jcph.491 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–39. 10.1016/j.mce.2009.08.018 . [DOI] [PubMed] [Google Scholar]
- 24.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–7. 10.1172/JCI57132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupoli R, Pizzicato P, Scalera A, Ambrosino P, Amato M, Peluso R, et al. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta-analysis. Arthritis Res Ther. 2016;18(1):297 10.1186/s13075-016-1194-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hojgaard P, Glintborg B, Kristensen LE, Gudbjornsson B, Love TJ, Dreyer L. The influence of obesity on response to tumour necrosis factor-alpha inhibitors in psoriatic arthritis: results from the DANBIO and ICEBIO registries. Rheumatology (Oxford). 2016;55(12):2191–9. 10.1093/rheumatology/kew326 . [DOI] [PubMed] [Google Scholar]
- 27.Iannone F, Fanizzi R, Notarnicola A, Scioscia C, Anelli MG, Lapadula G. Obesity reduces the drug survival of second line biological drugs following a first TNF-alpha inhibitor in rheumatoid arthritis patients. Joint Bone Spine. 2015;82(3):187–91. 10.1016/j.jbspin.2014.12.006. 10.1016/j.jbspin.2014.12.006 . [DOI] [PubMed] [Google Scholar]
- 28.Iannone F, Fanizzi R, Scioscia C, Anelli MG, Lapadula G. Body mass does not affect the remission of psoriatic arthritis patients on anti-TNF-alpha therapy. Scand J Rheumatol. 2013;42(1):41–4. 10.3109/03009742.2012.715186. 10.3109/03009742.2012.715186 . [DOI] [PubMed] [Google Scholar]
- 29.Ottaviani S, Gardette A, Tubach F, Roy C, Palazzo E, Gill G, et al. Body mass index and response to infliximab in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(4):478–83. . [PubMed] [Google Scholar]
- 30.Klaasen R, Wijbrandts CA, Gerlag DM, Tak PP. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum. 2011;63(2):359–64. 10.1002/art.30136. 10.1002/art.30136 . [DOI] [PubMed] [Google Scholar]
- 31.Gremese E, Bernardi S, Bonazza S, Nowik M, Peluso G, Massara A, et al. Body weight, gender and response to TNF-alpha blockers in axial spondyloarthritis. Rheumatology (United Kingdom). 2014;53(5):875–81. 10.1093/rheumatology/ket433. . [DOI] [PubMed] [Google Scholar]
- 32.Gremese E, Carletto A, Padovan M, Atzeni F, Raffeiner B, Giardina AR, et al. Obesity and reduction of the response rate to anti-tumor necrosis factor alpha in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res (Hoboken). 2013;65(1):94–100. 10.1002/acr.21768. 10.1002/acr.21768 . [DOI] [PubMed] [Google Scholar]
- 33.Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(10):2118–24. 10.1097/MIB.0b013e31829cf401. 10.1097/MIB.0b013e31829cf401 . [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64. . [DOI] [PubMed] [Google Scholar]
- 35.Choi CH, Song ID, Kim YH, Koo JS, Kim YS, Kim JS, et al. Efficacy and safety of infliximab therapy and predictors of response in korean patients with crohn's disease: A nationwide, multicenter study. Yonsei Medical Journal. 2016;57(6):1376–85. 10.3349/ymj.2016.57.6.1376. 10.3349/ymj.2016.57.6.1376 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. 10.7326/0003-4819-158-4-201302190-00009 . [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. . [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abhishek A, Butt S, Gadsby K, Zhang W, Deighton CM. Anti-TNF-alpha agents are less effective for the treatment of rheumatoid arthritis in current smokers. J. 2010;16(1):15–8. 10.1097/RHU.0b013e3181ca4a2a. 10.1097/RHU.0b013e3181ca4a2a . [DOI] [PubMed] [Google Scholar]
- 41.Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67(1):86–92. 10.1016/j.jaad.2011.07.034. 10.1016/j.jaad.2011.07.034 . [DOI] [PubMed] [Google Scholar]
- 42.Bhalme M, Sharma A, Keld R, Makin A, Willert R, Campbell S. Weight adjusted anti-TNF therapy favours obese patients with Crohn's disease. Gut. 2012;61:A228 10.1136/gutjnl-2012-302514c.107. . [DOI] [PubMed] [Google Scholar]
- 43.Brown P, Clark T, Dowson G, Warren L, Hamlin J, Hull M, et al. Relationship of Body Mass Index to Clinical Outcomes after Infliximab Therapy in Patients with Crohn's Disease. J Crohns Colitis. 2016;10(10):1144–50. 10.1093/ecco-jcc/jjw079. 10.1093/ecco-jcc/jjw079 . [DOI] [PubMed] [Google Scholar]
- 44.Cai L, Gu J, Zheng J, Zheng M, Wang G, Xi LY, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. Journal of the European Academy of Dermatology and Venereology. 2017;31(1):89–95. 10.1111/jdv.13746. 10.1111/jdv.13746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassano N, Loconsole F, Galluccio A, Miracapillo A, Pezza M, Vena GA. Once-weekly administration of high-dosage Etanercept in patients with plaque psoriasis: results of a pilot experience (power study). Int. 2006;19(1):225–9. . [PubMed] [Google Scholar]
- 46.Chiricozzi A, Zangrilli A, Bavetta M, Bianchi L, Chimenti S, Saraceno R. Real-life 9-year experience with adalimumab in psoriasis and psoriatic arthritis: results of a single-centre, retrospective study. J Eur Acad Dermatol Venereol. 2016;21:21 10.1111/jdv.13771. 10.1111/jdv.13771 . [DOI] [PubMed] [Google Scholar]
- 47.Click B, Tuskey AG, Behm BW. Does obesity impact treatment efficacy of adalimumab and certolizumab pegol in crohn's disease? Gastroenterology. 2012;1):S361 . [Google Scholar]
- 48.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. New England Journal of Medicine. 2010;362(15):1383–95. 10.1056/NEJMoa0904492. 10.1056/NEJMoa0904492 . [DOI] [PubMed] [Google Scholar]
- 49.Costa L, Caso F, Ramonda R, Del Puente A, Cantarini L, Darda MA, et al. Metabolic syndrome and its relationship with the achievement of minimal disease activity state in psoriatic arthritis patients: an observational study. Immunologic Research. 2014;61(1–2):147–53. 10.1007/s12026-014-8595-z. . [DOI] [PubMed] [Google Scholar]
- 50.Di Lernia V, Tasin L, Pellicano R, Zumiani G, Albertini G. Impact of body mass index on retention rates of anti-TNF-alfa drugs in daily practice for psoriasis. J Dermatolog Treat. 2012;23(6):404–9. 10.3109/09546634.2011.593489. 10.3109/09546634.2011.593489 . [DOI] [PubMed] [Google Scholar]
- 51.Di Renzo L, Bianchi A, Saraceno R, Calabrese V, Cornelius C, Iacopino L, et al. 174G/C IL-6 gene promoter polymorphism predicts therapeutic response to TNF-alpha blockers. Pharmacogenetics and Genomics. 2012;22(2):134–42. 10.1097/FPC.0b013e32834e5e7b. 10.1097/FPC.0b013e32834e5e7b . [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb AB, Langley RG, Strober BE, Papp KA, Klekotka P, Creamer K, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167(3):649–57. 10.1111/j.1365-2133.2012.11015.x. 10.1111/j.1365-2133.2012.11015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guerbau L, Gerard R, Duveau N, Desreumaux P, Boualit M, Branche J, et al. Crohn's disease patients with high body mass index present more frequent and rapid loss of response to infliximab. Gastroenterology. 2016;1):S417 . [DOI] [PubMed] [Google Scholar]
- 54.Heimans L, Wevers-de Boer KVC, Koudijs KKM, Visser K, Goekoop-Ruiterman YP, Harbers JB, et al. Health-related quality of life and functional ability in patients with early arthritis during remission steered treatment: results of the IMPROVED study. Arthritis Res Ther. 2013;15(5):R173 10.1186/ar4361. 10.1186/ar4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horst S, Abou-Ishmail A, Green M, Duley C, Wagnon J, Beaulieu D, et al. Efficacy of and factors contributing to dose adjustment in treatment with certolizumab for Crohn's disease. Am J Gastroenterol. 2012;107:S680–S1. 10.1038/ajg.2012.275. . [DOI] [Google Scholar]
- 56.Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis. 2014;73(3):587–94. 10.1136/annrheumdis-2012-202533. 10.1136/annrheumdis-2012-202533 . [DOI] [PubMed] [Google Scholar]
- 57.Kaeley GS, Evangelisto AM, Nishio MJ, Goss SL, Liu S, Kalabic J, et al. Methotrexate dosage reduction upon adalimumab initiation: Clinical and ultrasonographic outcomes from the randomized noninferiority MUSICA trial. J Rheumatol. 2016;43(8):1480–9. 10.3899/jrheum.151009. 10.3899/jrheum.151009 . [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi T, Suzuki Y, Motoya S, Hirai F, Ogata H, Ito H, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016;51(3):241–51. 10.1007/s00535-015-1102-z. 10.1007/s00535-015-1102-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mease P, Sieper J, Van den Bosch F, Rahman P, Karunaratne PM, Pangan AL. Randomized controlled trial of adalimumab in patients with nonpsoriatic peripheral spondyloarthritis. Arthritis rheumatol. 2015;67(4):914–23. 10.1002/art.39008. 10.1002/art.39008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menter A, Gordon KB, Leonardi CL, Gu Y, Goldblum OM. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. J Am Acad Dermatol. 2010;63(3):448–56. 10.1016/j.jaad.2009.09.040. 10.1016/j.jaad.2009.09.040 . [DOI] [PubMed] [Google Scholar]
- 61.Menter A, Papp KA, Gooderham M, Pariser DM, Augustin M, Kerdel FA, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). Journal of the European Academy of Dermatology and Venereology. 2016;30(7):1148–58. 10.1111/jdv.13611. 10.1111/jdv.13611 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore JM, Beaulieu DB, Horst SN, Armstrong S, Duncan PA, Wagnon JH, et al. Adalimumab and certolizumab pegol for the treatment of Crohn's disease: Does BMI make a difference? Gastroenterology. 2011;1):S588 10.1016/S0016-5085(11)62434-7. . [DOI] [Google Scholar]
- 63.Naldi L, Addis A, Chimenti S, Giannetti A, Picardo M, Tomino C, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis: Evidence from the psocare project. Dermatology. 2008;217(4):365–73. 10.1159/000156599. 10.1159/000156599 . [DOI] [PubMed] [Google Scholar]
- 64.Ottaviani S, Allanore Y, Tubach F, Forien M, Gardette A, Pasquet B, et al. Body mass index influences the response to infliximab in ankylosing spondylitis. Arthritis Res Ther. 2012;14(3):R115 10.1186/ar3841. 10.1186/ar3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paul C, van de Kerkhof P, Puig L, Unnebrink K, Goldblum O, Thaci D. Influence of psoriatic arthritis on the efficacy of adalimumab and on the treatment response of other markers of psoriasis burden: subanalysis of the BELIEVE study. Eur J Dermatol. 2012;22(6):762–9. 10.1684/ejd.2012.1863. 10.1684/ejd.2012.1863 . [DOI] [PubMed] [Google Scholar]
- 66.Poulin Y, Crowley JJ, Langley RG, Unnebrink K, Goldblum OM, Valdecantos WC. Efficacy of adalimumab across subgroups of patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet: post hoc analysis of REACH. J Eur Acad Dermatol Venereol. 2014;28(7):882–90. 10.1111/jdv.12198. 10.1111/jdv.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prussick R, Unnebrink K, Valdecantos WC. Efficacy of Adalimumab Compared With Methotrexate or Placebo Stratified by Baseline BMI in a Randomized Placebo-Controlled Trial in Patients With Psoriasis. J Drugs Dermatol. 2015;14(8):864–8. . [PubMed] [Google Scholar]
- 68.Qumseya B, Naik AS, Ananthakrishnan AN, Zadvornova Y, Skaros S, Johnson K, et al. Increased BMI is not associated with dose escalation or failure of adalimumab therapy in Crohn's disease. Gastroenterology. 2009;1):A655 10.1016/S0016-5085(09)63020-1. . [DOI] [Google Scholar]
- 69.Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7. 10.1136/gut.2010.221127. 10.1136/gut.2010.221127 . [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues AM, Reis JE, Santos C, Pereira MP, Loureiro C, Martins F, et al. Obesity is a risk factor for worse treatment response in rheumatoid arthritis patients-results from reuma.pt. Annals of the Rheumatic Disease Conference: European Workshop for Rheumatology Research. 2014;73(no pagination). 10.1136/annrheumdis-2013-205124.1. 71324888. [DOI]
- 71.Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137(4):1250–60; quiz 520. 10.1053/j.gastro.2009.06.061. 10.1053/j.gastro.2009.06.061 . [DOI] [PubMed] [Google Scholar]
- 72.Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, et al. Certolizumab pegol for active Crohn's disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011;9(8):670–8.e3. 10.1016/j.cgh.2011.04.031. 10.1016/j.cgh.2011.04.031 . [DOI] [PubMed] [Google Scholar]
- 73.Sandborn WJ, van Assche G, Reinisch W, Colombel J- F, D'Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257–65.e1-3. 10.1053/j.gastro.2011.10.032. 10.1053/j.gastro.2011.10.032 . [DOI] [PubMed] [Google Scholar]
- 74.Simone D, Canestri S, Nowik M, Messuti L, Miceli MC, Gremese E, et al. Genetic and clinical predictors of response to TNF blocker in an Italian axial-SPA cohort. Clinical and Experimental Rheumatology. 2014;32 (5):802 . [Google Scholar]
- 75.Smolen JS, Ilivanova E, Hall S, Irazoque-Palazuelos F, Park MC, Kotak S, et al. Low disease activity or remission induction with etanercept 50 mg and methotrexate in moderately active rheumatoid arthritis: Maintenance of response and safety of etanercept 50 mg, 25 mg, or placebo in combination with methotrexate in a randomized double-blind study. Arthritis and Rheumatism. 2011;63 (12):4041 10.1002/art.33477. . [DOI] [Google Scholar]
- 76.Vidal C, Lukas C, Combe B, Berenbaum F, Jorgensen C, Sellam J, et al. Efficacy of TNF inhibitors in axial spondyloarthritis according to the presence of objective signs of inflammation: A multicentric retrospective study. Arthritis and Rheumatology. 2016;68:2182–3. 10.1002/art.39977. . [DOI] [Google Scholar]
- 77.Vilarrasa E, Notario J, Bordas X, Lopez-Ferrer A, Gich IJ, Puig L. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): A retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74(6):1066–72. 10.1016/j.jaad.2016.01.037. 10.1016/j.jaad.2016.01.037 . [DOI] [PubMed] [Google Scholar]
- 78.Warren RB, Smith CH, Yiu ZZN, Ashcroft DM, Barker JNWN, Burden AD, et al. Differential Drug Survival of Biologic Therapies for the Treatment of Psoriasis: A Prospective Observational Cohort Study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–40. 10.1038/jid.2015.208. 10.1038/jid.2015.208 . [DOI] [PubMed] [Google Scholar]
- 79.Weinblatt ME, Bingham CO, 3rd, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis. 2013;72(3):381–9. 10.1136/annrheumdis-2012-201411. 10.1136/annrheumdis-2012-201411 . [DOI] [PubMed] [Google Scholar]
- 80.Zweegers J, van den Reek JMPA, van de Kerkhof PCM, Otero ME, Kuijpers ALA, Koetsier MIA, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long-term drug-survival study from the BioCAPTURE registry. Br J Dermatol. 2016;175(2):340–7. 10.1111/bjd.14552. 10.1111/bjd.14552 . [DOI] [PubMed] [Google Scholar]
- 81.Billiet T, Cleynen I, Ballet V, Ferrante M, Van Assche G, Gils A, et al. Prognostic factors for long-term infliximab treatment in Crohn's disease patients: a 20-year single centre experience. Alimentary Pharmacology and Therapeutics. 2016;44(7):673–83. 10.1111/apt.13754. 10.1111/apt.13754 . [DOI] [PubMed] [Google Scholar]
- 82.Bultman E, de Haar C, van Liere-Baron A, Verhoog H, West RL, Kuipers EJ, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn's disease patients. Aliment Pharmacol Ther. 2012;35(3):335–41. 10.1111/j.1365-2036.2011.04946.x. 10.1111/j.1365-2036.2011.04946.x . [DOI] [PubMed] [Google Scholar]
- 83.Ogdie A, Schwartzman S, Eder L, Maharaj AB, Zisman D, Raychaudhuri SP, et al. Comprehensive treatment of psoriatic arthritis: Managing comorbidities and extraarticular manifestations. J Rheumatol. 2014;41(11):2315–22. 10.3899/jrheum.140882. 10.3899/jrheum.140882 . [DOI] [PubMed] [Google Scholar]
- 84.Rosen MH, Scherl EJ, Schneider Y, Zhou XK, Taunk R, Lakehomer H, et al. Increased body mass index predicts need for surgery in crohn's disease patients treated with certolizumab. Gastroenterology. 2012;1):S349. . [Google Scholar]
- 85.Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507. 10.2165/11531280-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 86.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61(1):78–85. 10.1136/gutjnl-2011-300370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79(9):955–8. . [DOI] [PubMed] [Google Scholar]
- 88.Di Minno MND, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis. 2014;73(6):1157–62. 10.1136/annrheumdis-2012-202812. 10.1136/annrheumdis-2012-202812 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39(8):1197–202. 10.1038/ijo.2015.64 . [DOI] [PubMed] [Google Scholar]
- 90.Sparks JA, Halperin F, Karlson JC, Karlson EW, Bermas BL. Impact of Bariatric Surgery on Patients With Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2015;67(12):1619–26. 10.1002/acr.22629 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIFF)
Association between obesity and response to anti-TNF therapy–Subgroup analyses based on anti-TNF dosing regimen: (A) weight-based dosing, and (B) fixed dose therapies.
(TIFF)
Association between obesity and response to anti-TNF therapy–Subgroup analyses based on study design: (A) RCTs, and (B) observational studies.
(TIFF)
Meta-regression based on (A) prevalence of obesity, and (B) prevalence of outcome.
(TIFF)
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.