Abstract
Perlecan (HSPG2), a heparan sulfate proteoglycan, is a component of basement membranes and participates in a variety of biological activities. Here, we show physiological roles of perlecan in both obesity and the onset of metabolic syndrome. The perinatal lethality-rescued perlecan knockout (Hspg2−/−-Tg) mice showed a smaller mass and cell size of white adipose tissues than control (WT-Tg) mice. Abnormal lipid deposition, such as fatty liver, was not detected in the Hspg2−/−-Tg mice, and those mice also consumed more fat as an energy source, likely due to their activated fatty acid oxidation. In addition, the Hspg2−/−-Tg mice demonstrated increased insulin sensitivity. Molecular analysis revealed the significantly relatively increased amount of the muscle fiber type IIA (X) isoform and a larger quantity of mitochondria in the skeletal muscle of Hspg2−/−-Tg mice. Furthermore, the perlecan-deficient skeletal muscle also had elevated levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) protein. PGC1α expression is activated by exercise, and induces mitochondrial biosynthesis. Thus, perlecan may act as a mechano-regulator of catabolism of both lipids and glucose by shifting the muscle fiber composition to oxidative fibers. Our data suggest that downregulation of perlecan is a promising strategy to control metabolic syndrome.
Introduction
In recent years, human lifestyles have been largely changed, and metabolic syndrome is now threatening human health by enhancing the risks of cardiovascular disease and diabetes mellitus1–3. Metabolic syndrome is characterized by symptoms, such as visceral obesity, glucose intolerance, dyslipidemia, and hypertension, which are all related to each other and caused by metabolic disturbance. Adipose tissue and skeletal muscle are representative organs for the regulation of systemic metabolic dynamics, and both show dynamic changes in morphology and functions in response to environmental stimuli, such as diet and exercise conditions.
Adipose tissue acts as a fat storage depot in terms of energy sources, and it protects against abnormal lipid deposition in other organs4. Adipose tissue is also well recognized as the largest endocrine organ5; that is, adipocytes secrete a variety of adipocytokines. Smaller sized adipocytes contribute to the prevention of metabolic syndrome by secreting a unique cytokine, adiponectin, which elicits anti-inflammatory effects and enhances insulin sensitivity. By contrast, hypertrophic adipocytes degrade the metabolic status by the secretion of different type of cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and resistin6. The development of metabolic syndrome is therefore associated with dynamic remodeling of both adipose tissue and adipocytes7,8.
Skeletal muscle is also a dynamic metabolic organ, with four classifications based on the muscle fiber type. Types I, IIA, IIX, and IIB are distinguished by the maximum speed of contraction and the attributed energy metabolism9,10. Type I is a slow-twitch fiber and relies on oxidative phosphorylation. Type IIA is fast and oxidative while type IIX is fast, oxidative, and glycolytic. Type IIB is fast and glycolytic. The maximum speed of muscle contraction varies among the type II fibers with IIB as the fastest and IIX faster than IIA11,12. In response to exercise, the mass and composition of muscle fibers can change13.
Importantly, extracellular matrices (ECMs) modify dynamically their own structure and repertoires to affect metabolic environments14. Remodeling of ECMs is important for the reconstruction of tissue architecture during development, wound healing, pathological processes, etc15. ECMs regulate the functional as well as the structural microenvironment by interaction with various humoral ligands, such as growth factors16,17. The dynamics of ECMs are therefore associated with increased insulin resistance in skeletal muscle18, and with differentiation and remodeling of adipocytes in response to energy balance19,20.
Perlecan, a heparan sulfate proteoglycan, is one of components of ECMs. Perlecan contains 5 domains and can interact with basement membranes, growth factors, cell surface receptors, etc21. Perlecan surrounds individual adipocytes and skeletal muscle fibers, and therefore plays critical roles in the maintenance of the morphology and functions of adipose tissue and skeletal muscle. In addition, the heparan sulfate chains in domain I22 and domain II23 of perlecan bind to lipoproteins. Therefore, perlecan has been implicated in the regulation of the lipid dynamics of adipose tissue.
Our previous study showed that perlecan deficiency in plantaris muscle, a fast-twitch muscle, promotes hypertrophy under mechanical overload. This structural change is accompanied by reduced levels of myostatin, a negative regulator of muscle growth and differentiation, expression and signaling24. On the other hand, mechanical unloading on the soleus muscle, a slow-twitch muscle, induces a more substantial atrophy in perinatal lethality-rescued perlecan knockout (Hspg2−/−-Tg) mice than that in control mice, and this is accompanied by increased autophagic activity25. Consequently, perlecan may play a key role in the regulation of the dynamics of skeletal muscle in response to mechanical stimuli.
In the present study, we investigated the physiological roles of perlecan in both adipose tissue and skeletal muscle in terms of the regulation of metabolic status. Our results established the metabolic linkages between perlecan and both adipose tissue and skeletal muscle are discussed.
Results
Hspg2−/−-Tg mice are resistant to obesity
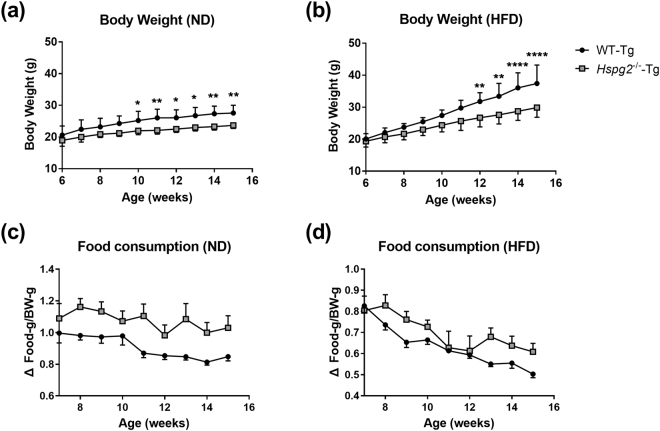
We investigated the physiological relationships between perlecan and obesity using Hspg2−/−-Tg mice. The mice were fed either a normal diet (ND) or a high fat diet (HFD) from 6 to 16 weeks of age. The relevant macroscopic features between Hspg2−/−-Tg and the background (WT-Tg) mice were evaluated. By 6 weeks of age, the body weight did not differ significantly between Hspg2−/−-Tg and WT-Tg mice. After 6 weeks of age, the age-dependent weight gain of the Hspg2−/−-Tg mice was lower than that of the WT-Tg mice in both nutritional conditions. These trends became significant after 10 weeks of age in the ND (p = 0.0420, at 10 weeks of age; p = 0.0068, at 15 weeks of age; Fig. 1a), and after 12 weeks of age in the HFD conditions (p = 0.0086, at 12 weeks of age; p < 0.0001, at 15 weeks of age; Fig. 1b). The Hspg2−/−-Tg mice did not show reduced levels of food consumption when compared to the WT-Tg mice in either nutritional condition (Fig. 1c,d).
Figure 1.
Hspg2−/−-Tg mice are resistant to obesity. (a,b) Body weight change of perinatal lethality-rescued perlecan knockout (Hspg2−/−-Tg, gray square) and control (WT-Tg, black circle) mice under (a) normal diet (ND) and (b) high fat diet (HFD) conditions. Body weight was monitored in the mice aged 6 to 16 weeks (mean ± S.D., n = 7). (c,d) Food consumption by WT-Tg and Hspg2−/−-Tg mice under (c) ND and (d) HFD conditions. The average consumption by 1 to 3 mice reared in the same animal cage represents food consumption per mouse (mean ± S.D., n = 4–6 cages). Data were analyzed by two-way ANOVA with Sidak’s multiple comparison. *p < 0.05, **p < 0.01, ****p < 0.0001.
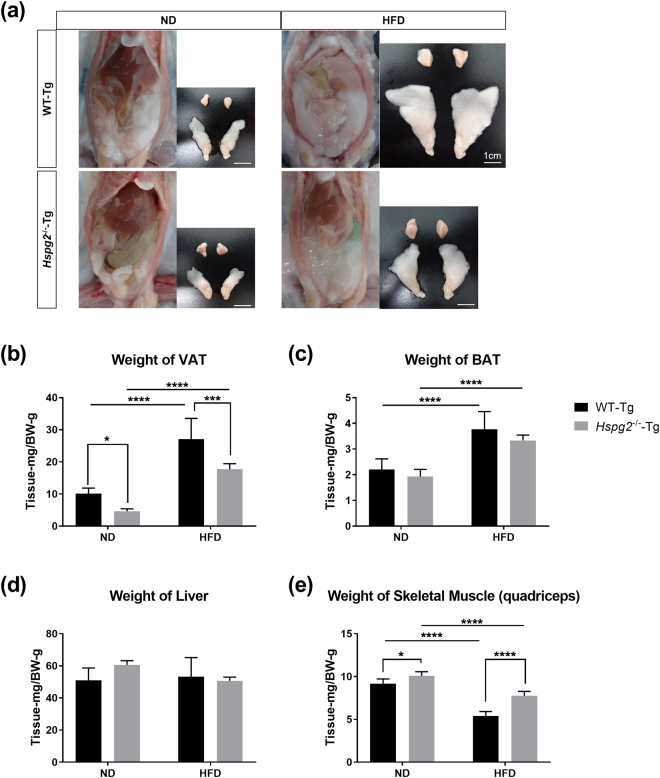
We also compared the mass of adipose tissue in WT-Tg and Hspg2−/−-Tg mice. Macroscopically, the mass of epididymal adipose tissue, a representative visceral white adipose tissue (VAT), was smaller in the Hspg2−/−-Tg mice than in the WT-Tg mice under the ND condition. The VAT became enlarged in both genotypes under the HFD condition; however, this enlargement was more prominent in the WT-Tg than in the Hspg2−/−-Tg mice (Fig. 2a). The relative weight of the VAT per body was significantly reduced in the Hspg2−/−-Tg mice, irrespective of the nutritional conditions (p = 0.0369, in ND; p = 0.0002, in HFD; Fig. 2b). By contrast, we did not observe such differences in brown adipose tissue (BAT) or in the liver between genotypes, whereas HFD promoted an increase in BAT mass in both animal types (Fig. 2c,d). In the skeletal muscle, the Hspg2−/−-Tg mice showed a significant increase in weight under both nutritional conditions (p = 0.0173, in ND; p < 0.0001, in HFD; Fig. 2e). These results suggest that perlecan deficiency may not lead to systemic hypoplasia, but instead may promote a reduction in the fat storage of white adipose tissue, and thereby prevent obesity.
Figure 2.
The mass of white adipose tissue is reduced in Hspg2−/−-Tg mice. (a) Macroscopic images of visceral fat deposition and adipose tissues of the WT-Tg (upper panels) and the Hspg2−/−-Tg (lower panels) mice at 16 weeks of age. The right-hand image of each panel represents brown adipose tissue (interscapular fats, upper row) and visceral adipose tissue (epididymal fats, bottom row). (b–e) Comparisons of tissue weight of (b) visceral adipose tissue (VAT), (c) brown adipose tissue (BAT), (d) liver, and (e) skeletal muscle (quadriceps) in mice at 16 weeks of age fed the different diets. Data points and error bars represent the mean ± S.D. (n = 7). Data were analyzed by two-way ANOVA with Tukey’s multiple comparison. *p < 0.05, ***p < 0.001, ****p < 0.0001. Scale bar, 1 cm.
Hspg2−/–-Tg mice show reduced size of white adipocytes
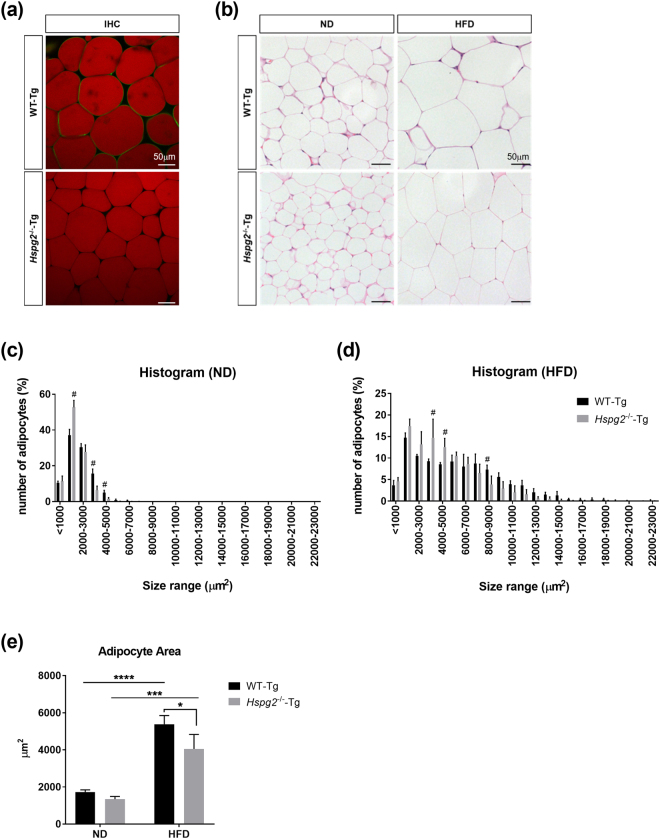
We performed a microscopy analysis to investigate the size of adipocytes in Hspg2−/−-Tg mice. We first confirmed the localized distribution of perlecan in adipose tissue. Immunofluorescence analysis revealed that perlecan surrounds each adipocyte in the VAT of WT-Tg mice, whereas perlecan was absent in the VAT of Hspg2−/−-Tg mice (Fig. 3a). Hematoxylin and eosin staining of the VAT demonstrated a reduced cell size of the white adipocytes in the Hspg2−/−-Tg mice under both nutritional conditions (Fig. 3b). Histograms of adipocyte size also revealed an increased number of smaller cells in the Hspg2−/−-Tg mice (p < 0.0001, in 1,000–2,000 μm2), associated with an decreased number of larger cells (p < 0.0001, in 3,000–4,000 μm2) in the ND condition (Fig. 3c). Such a trend was also observed in the HFD condition (p < 0.0001 in 3,000–4,000 μm2; p = 0.0345 in 8,000–9,000 μm2; Fig. 3d). Figure 3e shows the average size of the adipocytes; these cells were significantly smaller in the Hspg2−/−-Tg mice in the HFD condition (p = 0.0206), whereas no significant difference was observed in the ND condition.
Figure 3.
Perlecan deficiency leads to a reduction in adipocyte size. (a) Immunofluorescence analysis (IFA) of perlecan in visceral adipose tissue (VAT). Note that perlecan (green) surrounded each adipocyte (red) of the WT-Tg mice, whereas perlecan was absent in that of the Hspg2−/−-Tg mice. (b) The VAT of the WT-Tg (upper panel) and Hspg2−/−-Tg (lower panel) mice fed with either a normal diet (ND) or a high-fat diet (HFD). A section was stained with hematoxylin-eosin. (c,d) A histogram of the size (μm2) of the individual adipocytes under (c) ND and (d) HFD conditions. (e) The average size of adipocytes (μm2). (c–e) 1,000 adipocytes per mouse were evaluated. Data points and error bars represent the mean ± S.D. (n = 3–4). Data were analyzed by two-way ANOVA with Sidak’s multiple comparison (c,d) and Tukey’s multiple comparison (e). #p < 0.05 including p < 0.0001, *p < 0.05, ***p < 0.001, ****p < 0.0001. Scale bar, 50 μm.
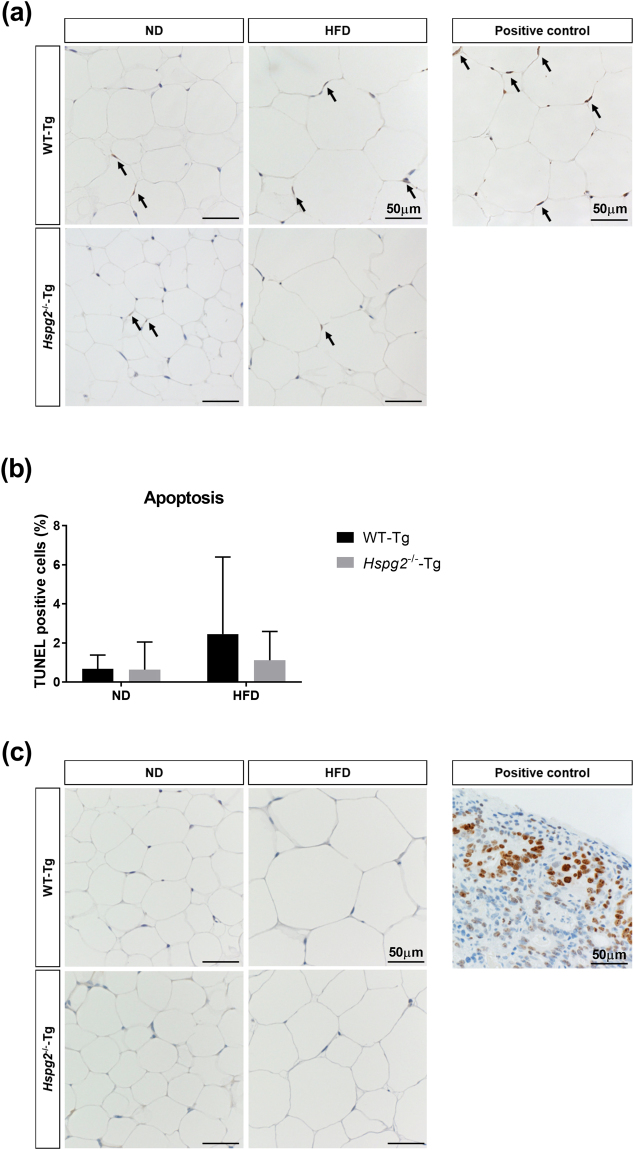
We also compared the turnover of adipocytes in the Hspg2−/−-Tg mice with that in the WT-Tg mice to investigate whether the smaller VAT mass in the perlecan deficient mice is due to either increased apoptosis or decreased proliferation of adipocytes. TUNEL assays of apoptotic cells demonstrated that the number of apoptotic adipocytes was not significantly higher in the Hspg2−/−-Tg mice than in the WT-Tg mice (Fig. 4a,b). We could not detect the protein expression of Ki67, a marker of cell proliferation, in the adipose tissue of either the Hspg2−/−-Tg or the WT-Tg mice (Fig. 4c).
Figure 4.
Perlecan deficiency does not affect cell turnover in white adipose tissue. (a) TUNEL staining in the VAT of the WT-Tg (upper panel) and the Hspg2−/−-Tg (lower panel) mice fed either ND or HFD. The areas containing positive nuclei of adipocytes are selected and shown. The positive control was made using DNase I. Note that TUNEL-positive nuclei are brown and negative nuclei are blue. (b) The percentage of TUNEL-positive nuclei of adipocytes. The nuclei from at least 100 adipocytes per mouse were evaluated. Data points and error bars represent the mean ± S.D. (n = 5). (c) Representative immunohistochemical staining of Ki67 in the VAT of the WT-Tg (upper panel) and the Hspg2−/−-Tg (lower panel) mice fed either ND or HFD. The tissue from human abdominal cancer was used as positive control. No nuclei were positive for Ki67 in any groups. Data were analyzed by two-way ANOVA with Tukey’s multiple comparison (b). Scale bar, 50 μm.
These observations suggest that the loss of perlecan inhibits lipid accumulation in individual adipocytes, but it does not affect cell turnover in white adipose tissue.
Perlecan deficiency does not induce abnormal lipid deposition
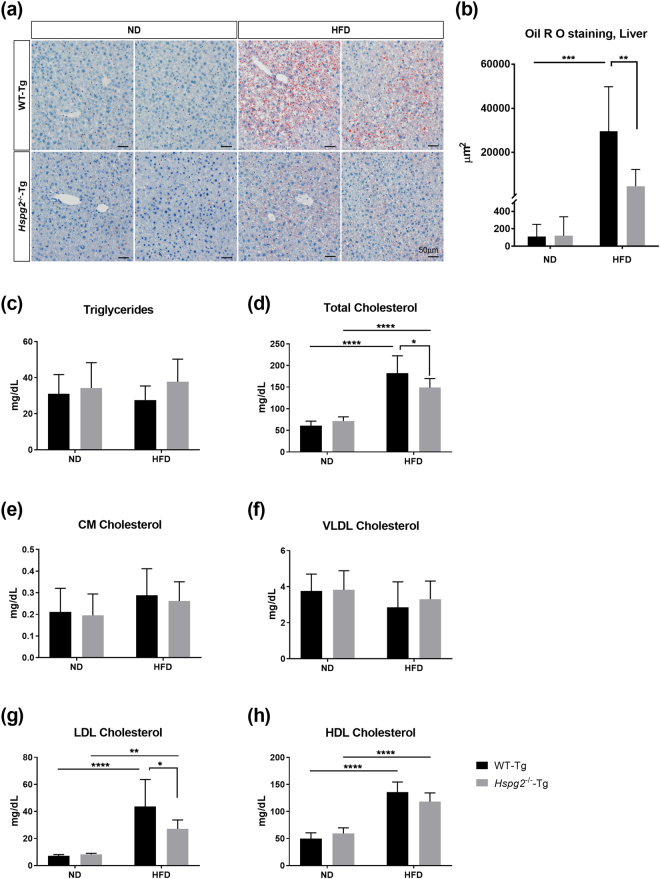
Adipose tissue plays an important role in the prevention of abnormal lipid deposition in other organs. Therefore, reduced fat storage in white adipose tissue could possibly reflect an enhancement of abnormal lipid deposition or dyslipidemia. We investigated whether abnormal lipid deposition is present in Hspg2−/−-Tg mice. Our histological analysis did not show any significant lipid deposition in the liver of Hspg2−/−-Tg mice in the ND condition, as in the liver of WT-Tg mice fed the ND. Even under the HFD condition, no significant fatty liver formation was observed in the Hspg2−/−-Tg mice, whereas the WT-Tg mice fed the HFD showed a high prominence of fatty liver (29,576 ± 18,714 μm2 in WT-Tg; 4,645 ± 7,033 μm2 in Hspg2−/−-Tg, p = 0.0013; Fig. 5a,b).
Figure 5.
Perlecan deficiency is resistant to abnormal lipid deposition. (a) Histological analysis of lipid deposition in the livers of the WT-Tg (upper panel) and Hspg2−/−-Tg (lower panel) mice fed either a normal diet (ND) or a high fat diet (HFD). A section was stained with Oil Red O for detection of lipids and with hematoxylin for counterstaining. Two representative images are shown for each experimental condition. (b) The differences in the lipid deposition between the WT-Tg and the Hspg2−/−-Tg mice fed the ND and HFD. Areas (μm2) of deposited lipids per image with same magnification were calculated using ImageJ software. Sixteen-week-old mice were used in the experiments. Data points and error bars represent the mean ± S.D. (n = 7). (c–h) Levels (mg/dL) of plasma (c) triglycerides, (d) total cholesterol, (e) chylomicron (CM) cholesterol, (f) very low density lipoprotein (VLDL) cholesterol, (g) low density lipoprotein (LDL) cholesterol, and (h) high density lipoprotein (HDL) cholesterol in the 16-week-old mice after fasting for 4 h (mean ± S.D., n = 8). Data were analyzed by two-way ANOVA with Tukey’s multiple comparison. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Scale bar, 50 μm.
The plasma levels of triglycerides were not significantly elevated in the Hspg2−/−-Tg mice, even in the HFD condition (Fig. 5c). Notably, the plasma levels of total cholesterol were significantly lower in the Hspg2−/−-Tg mice than in the WT-Tg mice under the HFD condition (p < 0.0001, Fig. 5d). We also examined cholesterol concentrations in four major lipoproteins: chylomicrons (CM), very low density lipoproteins (VLDL), low density lipoproteins (LDL), and high density lipoproteins (HDL). The plasma level of LDL cholesterol was significantly lower in the Hspg2−/−-Tg mice than in the WT-Tg mice under the HFD condition (p = 0.0198, Fig. 5g), whereas the CM cholesterol, VLDL cholesterol, and HDL cholesterol levels were not significantly different between the genotypes under either nutritional condition (Fig. 5e,f,h). The CM are synthesized in the intestine from dietary triglycerides, and VLDL are synthesized in the liver from endogenous triglycerides26. We conclude that an enhanced abnormal lipid deposition is unlikely, and that the fat storage alone is reduced in Hspg2−/−-Tg mice. Furthermore, this reduction is not caused by suppression of lipid synthesis in the intestine and liver.
Fatty acid oxidation is elevated in Hspg2−/−-Tg mice
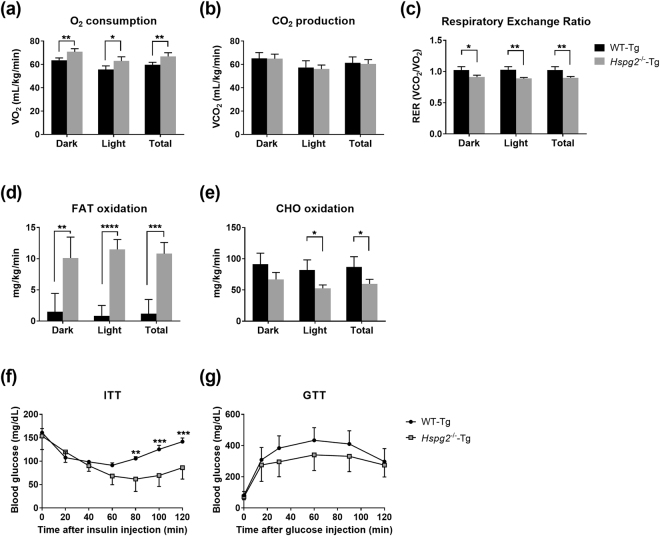
Lipid accumulation in the white adipose tissue and liver was suppressed in the Hspg2−/−-Tg mice, as was dyslipidemia. By contrast, neither food consumption nor lipid synthesis in the intestine and liver were suppressed in these mice. Our findings raised the possibility that the reduction in lipid storage in the Hspg2−/−-Tg mice was due to an acceleration of lipid catabolism. Therefore, we investigated lipid and glucose metabolism in the Hspg2−/−-Tg mice fed ND. We found a significantly increased whole-body oxygen (O2) consumption in the Hspg2−/−-Tg mice (dark period, p = 0.0047; light period, p = 0.0218; total period, p = 0.0092; Fig. 6a), whereas CO2 production did not differ significantly between the two genotypes (Fig. 6b). Colorimetric analysis further demonstrated a lower respiratory exchange ratio (RER) in the Hspg2−/−-Tg than in the WT-Tg mice (dark period, p = 0.0126; light period, p = 0.0015; total period, p = 0.0040; Fig. 6c), which indicated significantly higher levels of fat oxidation in the Hspg2−/−-Tg than in the WT-Tg mice (dark period, p = 0.0084; light period, p < 0.0001; total period, p = 0.0006; Fig. 6d). By contrast, carbohydrate (CHO) oxidation levels were significantly lower in the Hspg2−/−-Tg mice during the light and the total period (light period, p = 0.0146; total period, p = 0.0256; Fig. 6e). Notably, the insulin tolerance test (ITT) demonstrated a greater sensitivity to insulin in the Hspg2−/−-Tg than in the WT-Tg mice; that is, blood glucose levels were significantly lower in the Hspg2−/−-Tg mice (p = 0.0084, at 80 min after insulin injection, Fig. 6f), whereas the glucose tolerance test (GTT) did not show a significant difference between the genotypes (Fig. 6g). These data suggest that fats are the more preferred energy source in Hspg2−/−-Tg mice without induced insulin resistance. We conclude that the increased fat oxidation may contribute to the resistance to obesity in Hspg2−/−-Tg mice.
Figure 6.
Anti-obesity effects in Hspg2−/−-Tg mice depend on increased FAT oxidation. (a–e) Indirect calorimetry for (a) oxygen (O2) consumption, (b) CO2 production, (c) respiratory exchange ratio, (d) FAT oxidation, and (e) carbohydrate (CHO) oxidation. Data were collected every 2.5 min for 24 h and represent the mean ± S.D. (n = 4) during 6 h light/6 h dark periods. (f,g) Changes in plasma glucose levels by (f) the insulin tolerance test (ITT) and (g) the glucose tolerance test (GTT). Data points and error bars represent the mean ± S.D. (n = 4–7). Data were analyzed by the unpaired t-test (a–e) and two-way ANOVA with Sidak’s multiple comparison (f,g). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Loss of perlecan causes muscle fiber shift from glycolytic to oxidative fibers by activation of PGC1α
Skeletal muscle has a physiological role in regulating fatty acid oxidation and insulin sensitivity. Indeed, exercise enhances fatty acid oxidation and prevents insulin resistance in skeletal muscle27. We previously found that perlecan regulates skeletal muscle dynamics, so we hypothesized that alterations in skeletal muscle by perlecan deficiency would increase β-oxidation and insulin sensitivity. β-oxidation occurs more prominently in oxidative skeletal muscles, in which mitochondria are enriched. Therefore, we investigated the differences in muscle fiber composition between Hspg2−/−-Tg mice and WT-Tg mice. We used the quadriceps as a representative skeletal muscle, because it is the largest skeletal muscle in the mouse body28.
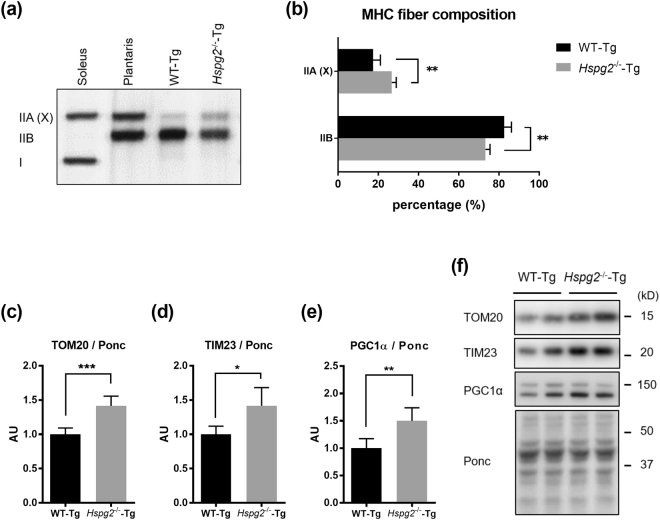
Myosin heavy chain (MHC) isoforms (type I, IIA, IIX, and IIB) can be distinguished by gel electrophoresis. The soleus and plantaris muscles are markers for type I and II fibers. In the soleus muscle, a slow-twitch muscle, type I fibers are dominant, whereas type II fibers predominate in the plantaris muscle. The MHC isoforms in the quadriceps showed a significant increase in the relative amounts of muscle fiber type IIA (X) in the Hspg2−/−-Tg mice (p = 0.0011; Fig. 7a, b), whereas the differences in type IIA from IIX fibers was variable as previously shown29. In addition, the levels of mitochondrial proteins, translocase of outer membrane 20 (TOM20) and translocase of inner membrane 23 (TIM23), were significantly increased in the perlecan-deficient muscle (p = 0.0003, in TOM20; Fig. 7c; p = 0.0104, in TIM23; Fig. 7d). These data indicated that perlecan-deficient muscle utilizes more oxygen than that of WT-Tg mice.
Figure 7.
Loss of perlecan modifies the composition of myosin heavy chains by activating PGC1α. Detection by (a) SDS-PAGE-coupled silver staining and (b) relative composition of myosin heavy chain isoforms in the quadriceps of the WT-Tg and Hspg2−/−-Tg mice. Soleus and plantaris represent markers for type I and II fibers, respectively. The relative intensity of the bands was quantified using ImageJ software. (c–e) Protein expression levels of (c) translocase of outer membrane 20 (TOM20), (d) translocase of inner membrane 23 (TIM23), and (e) peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) in the quadriceps of the WT-Tg and Hspg2−/−-Tg mice. (f) Representative images of proteins extracted from quadriceps and stained with Ponceau S (Ponc) after SDS-PAGE. The relative intensities of the respective bands detected by western blotting using the specific antibody to the Ponc-stained patterns were quantified using ImageJ software. Data points and error bars represent the mean ± S.D. (n = 5 in a and b; n = 5–6 in c–e). Data were analyzed by two-way ANOVA with Sidak’s multiple comparison (b) and unpaired t-test (c–e). *p < 0.05, **p < 0.01, ***p < 0.001.
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), a transcriptional coactivator involved in energy metabolism, induces mitochondrial biogenesis and promotes the switching of muscle fiber types from glycolytic to more oxidative30. Our investigation of the expression level of PGC1α, as expected, revealed increased protein levels of PGC1α in the Hspg2−/−-Tg mice (p = 0.0038; Fig. 7e). Thus, the loss of perlecan causes dynamic changes in lipid metabolism by shifting the muscle fiber composition to more oxidative fibers via PGC1α.
Discussion
Perlecan is a multi-functional heparan sulfate proteoglycan that interacts with a number of different molecules, including basement membrane components (e.g., laminin31, nidogen32, and collagen IV33), cell surface receptors (e.g., integrin34,35), growth factors (e.g., fibroblast growth factor (FGF) 2, FGF7, FGF18, FGF-BP, PDGF, and VEGF36–38), lipoproteins23, acetylcholinesterase39, and α-dystroglycan40.
Perlecan null mice are embryonic lethal due to chondrodysplasia; therefore, investigating the systemic physiological functions of perlecan is difficult. Several animal models have been created with mutations in Hspg221. The Hspg2Δ3/Δ3 mice lacking exon 3 of the perlecan gene, which encodes for two of the three heparan sulfate attachment sites41, are particularly useful for investigating the function of the heparan sulfate chains. The perinatal lethality-rescued perlecan knockout (Hspg2−/−-Tg) mice that we have established express perlecan only in the cartilage, making them useful for investigating the function of the whole perlecan protein. The Hspg2−/−-Tg mice and WT-Tg mice display similar life expectancies, thereby allowing the study of the role of perlecan role throughout adulthood and in the aged state.
The heparan sulfate chains of perlecan play significant roles in lipid uptake and in the proliferation and differentiation of muscle cells through the association of these chains with lipoproteins22,42 and FGFs43. The FGFs promote muscle cell proliferation and inhibit cell differentiation44. Notably, previous studies have reported the importance of the perlecan core protein itself. Domain II, which is similar to the cholesterol binding region of the LDL receptor23,45, has a pro-atherosclerosis effect, and the C-terminal domain V, endorepelin, is a key regulator of muscle development46. We therefore used the Hspg2−/−-Tg mice to investigate the physiological roles of perlecan, including the core protein, in metabolic dynamics.
In the present study, the Hspg2−/−-Tg mice showed a reduction in white adipose tissue weight. The cell proliferation and apoptosis in the adipose tissue of the Hspg2−/−-Tg mice were similar to that of the WT-Tg mice. Conversely, the size of the adipocytes was significantly smaller in the perlecan-deficient mice than in the control. Our findings indicated that a deficiency of perlecan does not affect cell turnover in adipose tissue, but it does decrease lipid accumulation. We also found that lipid accumulation in the liver was also suppressed in the Hspg2−/−-Tg mice under the HFD condition. Cell surface heparan sulfate proteoglycans (HSPGs), such as syndecans and glypicans, bind to both lipoproteins and lipoprotein lipase through the heparan sulfate chains47. Hence, HSPGs promote lipid uptake into both adipocytes and hepatocytes42,48. Because perlecan contains the multiple responsible sites for heparan sulfate side chains; 3 sites in domain I and one in domain V, perlecan can also play an important role in lipid accumulation. Indeed, previous reports proposed that the perlecan heparan sulfate in domain I has suggested a proatherogenic effect due to lipoprotein binding22. In addition, a recent study has shown that the perlecan domain II induced atherosclerosis23. Thus, the reduced lipid accumulation in the perlecan-deficient Hspg2−/−-Tg mice would seem to confirm these roles for perlecan.
Conversely, our observations that plasma levels of total cholesterol and triglycerides in the Hspg2−/−-Tg mice did not increase even under the HFD condition seem controversial to the aforementioned findings. The plasma levels of CM cholesterol and VLDL cholesterol were not suppressed in the Hspg2−/−-Tg mice, indicating that the loss of perlecan does not affect lipid synthesis in the intestine or the liver. A possible explanation is that Hspg2−/−-Tg mice have a greater lipid catabolism than WT-Tg mice, and this hypothesis is supported by our data indicating an activation of β-oxidation in the Hspg2−/−-Tg mice. We evaluated the skeletal muscle in the Hspg2−/−-Tg mice, because this tissue is responsible for activating β-oxidation. Skeletal muscles from the Hspg2−/−-Tg mice showed a significant relevant increase in the type IIA(X)/IIB ratio, suggesting an activation of β-oxidation. In mouse muscle fibers, the SDH activity is highest in type IIA and lowest in IIB fibers49. The quantity of lipid droplets in muscle fibers, which are often found adjacent to mitochondria and are used as an energy source during exercise, is higher in both type IIA and IIX fibers, and lowest in type IIB fibers50. The increase in the relative amount of type IIA (X) fibers in perlecan-deficient muscle indicates that the loss of perlecan enhances oxidative metabolism. The significantly increased levels of mitochondrial proteins also support this hypothesis.
The Hspg2−/−-Tg mice also showed increased PGC1α protein levels. PGC1α expression is activated by exercise, and it induces mitochondrial biogenesis and ultimately activated oxidative metabolism51–53. Muscle-specific PGC1α-transgenic mice showed increased expression of transcripts for mitochondrial enzymes, such as COX II and COX IV30. Conversely, PGC1α deficiency in muscle caused fiber type shifting from oxidative to glycolytic muscle fibers54. Hence, elevation of PGC1α expression in type II fiber-rich muscle promotes fiber-type switching from glycolytic toward more oxidative fibers. In our previous study, perlecan-deficient plantaris muscle shows decreased levels of myostatin and increased levels of insulin-like growth factor (IGF)-1 as compared to WT-Tg24. Myostatin55 and IGF-156–58 are important molecules in skeletal muscle function, and these expression levels are influenced by exercise, as well as PGC1α. Under mechanical loads, the skeletal muscles show decreased expression of myostatin59, but enhanced expression of IGF-160. Taken together with these findings, the enhanced expression of PGC1α in perlecan-deficient skeletal muscle indicates that perlecan may act as a mechano-regulator. Furthermore, the loss of perlecan promotes the catabolism of both lipids and glucose by shifting the muscle fiber composition to oxidative fibers via PGC1α signaling.
Methods
Animals
Perinatal lethality-rescued perlecan knockout (Hspg2−/−-Tg) mice and the WT-Tg mice (control) were used in the study. Perlecan null (Hspg2−/−) mice are embryonic lethal due to dyschondroplasia61,62. We rescued the premature cartilage development using a chondrocyte-specific Col2a1 collagen promoter and enhancer63 to create a perlecan transgenic mouse line (WT-Tg, Hspg2+/+; Col2a1-Hspg2Tg/−). We subsequently established the lethality-rescued mice (Hspg2−/−-Tg, Hspg2−/−; Col2a1-Hspg2Tg/−) by mating the transgenic mice with heterozygous Hspg2+/− mice24. We maintained these genetic backgrounds on C57BL/6 mice. Perlecan is absent from the skeletal muscle, liver, kidney, heart, and brain of the Hspg2−/−-Tg mice, whereas perlecan is expressed in all these tissues in the WT-Tg mice, and the recombinant perlecan is expressed in the cartilage of Hspg2−/−-Tg mice24,64. The mice were reared under a 12-h light-dark cycle at 23 ± 2 °C, housed in groups of 1–3 per cage, and fed a normal diet (CRF-1, ORIENTAL YEAST CO., LTD.). For the high fat diet, the animal food containing 60 kcal% fat (D12492, Research Diets, Inc.) was fed to the mice for 10 weeks when they were 6 to 16 weeks of age. Body weight and food consumption were monitored every week from 6 to 16 weeks of age. The average food consumption per cage containing 1–3 mice was used as food consumption per mouse (n = 4–6 cages). At 16 weeks old, the mice were sacrificed after fasting for 4 h and various metabolic tissues, including visceral white adipose tissue (VAT), brown adipose tissue (BAT), liver, and skeletal muscle were collected. The VAT and BAT were dissected from epididymal and scapular regions, respectively. The quadriceps was used as the skeletal muscle. All animal experiments of this study were carried out exclusively in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology (Notice No. 71, 2006), and approved by the Committee for Animal Experimentation of Juntendo University with the Approval No. 290207. The present study did not include research involving human participants, as well as non-human primates and unpublished de novo cell lines.
Immunofluorescence analysis (IFA)
Immunofluorescence analysis of perlecan and adipocytes was performed as described previously65. Briefly, the VAT was cut into small pieces (about 4 mm square) using scalpels. These pieces were washed in 1 × PBS for 10 min, fixed in 4% paraformaldehyde (PFA) for 45 min, and permeabilized with 1% Triton X-100 for 10 min. After washing with 1 × PBS for 10 min, the pieces were blocked with 1% bovine serum albumin (BSA) for 30 min and incubated overnight with rat anti-mouse HSPG monoclonal antibody (Clone A7L6, MAB1948P, Merck KGaA) at appropriate dilutions. The specimens were washed six times with 1 × PBS for 10 min, and then reacted with Alexa Fluor 546-labeled goat anti-rat IgG (A11081, Thermo Fisher Scientific Inc.). The whole mount was counterstained with 5 μM BODIPYTM 493/503 (D-3922, Thermo Fisher Scientific Inc.) for visualizing adipocytes. Microscopy observations were carried out using a confocal fluorescence microscope.
Evaluation of the adipocyte sizes in VAT
The adipocyte counts were evaluated by morphometric analysis using hematoxylin-eosin (HE) stained VAT sections. The VAT whole mount was fixed overnight with 4% PFA, embedded in paraffin, sectioned, and stained. The areas (μm2) of individual cells were measured automatically using the KS400 Image Analysis System (Carl Zeiss, Germany) and 1,000 adipocytes per animal were evaluated.
Apoptosis and proliferation assays
Cell turnover in adipose tissue due to apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assays. The paraffin-embedded VAT sections were analyzed with the In situ Apoptosis Detection Kit (MK500, TaKaRa). We used the protocol recommended by the manufacturer. A positive control for the TUNEL assay was made by incubating sections for 15 min at 37 °C with 1 μg/mL DNase I (D4263-1VL, SIGMA) in 50 mM Tris-HCl, pH 7.5, containing 10 mM MgCl2 and 1 mg/mL BSA. The cell proliferation by white adipocytes was detected by immunohistochemical staining for Ki67. The paraffin-embedded VAT sections were de-paraffinized and autoclaved at 121 °C for 15 min in 0.1 M citrate buffer, pH 6.0. The sections were then blocked for 30 min with 10% normal goat serum (S-1000, Vector) in 2% BSA/PBS and incubated overnight with anti-Ki67 primary mouse monoclonal antibody (clone MIB-1, M7240, Dako) diluted to 0.8 μg/mL. The slides were washed and then incubated at room temperature for 30 min with biotin-conjugated goat anti-mouse IgG antibody (E0433, Dako) at a dilution of 1:300. Endogenous peroxidase was blocked for 20 min with 0.3% hydrogen peroxide in methanol, and then the slides were washed. The sections were reacted at room temperature for 30 min with HRP-conjugated streptavidin (P0397, Dako) at a dilution of 1:300. The slides were washed and processed using the DAB reaction (D006, Dojindo). The nuclei were counterstained with hematoxylin.
Quantification of fatty liver formation
Lipid deposition was detected in whole liver fixed overnight in 4% PFA. The fixative was subsequently replaced with increasing concentrations of sucrose (10, 20, and 30% in PBS, for 24 h each). After sucrose replacement, the tissues were frozen-fixed in OCT mounting media in dry ice/acetone and cryosectioned. The sections were stained with Oil Red O and counterstained with hematoxylin. The areas (μm2) of lipid deposition per image at the same magnification were measured using ImageJ software (Rasband W; National Institutes of Health, USA).
Plasma assay
After fasting for 4 h, blood samples were collected from anesthetized mice using BD MicrotainerR PSTTM Tubes with lithium heparin-Amber (Becton, Dickinson and Company). Plasma were separated by centrifugation at 2,000 × g for 20 min at 4 °C and cryopreserved at −80 °C until use. The plasma levels of triglycerides, total cholesterol, CM cholesterol, VLDL cholesterol, LDL cholesterol, and HDL cholesterol were measured by LipoSEARCH (Skylight Biotech Inc., Akita, Japan).
Whole body consumption analysis: indirect calorimetry measurements
Indirect calorimetry, a noninvasive technique for measuring the mass of oxidation of carbohydrates (CHO) and lipids by analysis of the respiratory gas, was measured using a model ARCO 2000 mass spectrometer (Arco System, Chiba, Japan)66,67. Each mouse was caged in an individual metabolic chamber maintained at 24 °C in a 12-h light-dark cycle with ad libitum feeding. The metabolic chamber volume was 220 cm2 for the base and 11.5 cm in height. Briefly, room air was pumped through the acrylic metabolic chamber at a rate of 0.4 L/min, and the O2 consumption (mL/kg/min) and CO2 production (mL/kg/min) were measured every 2.5 min for 24 h. The respiratory exchange ratio, CHO oxidation (mg/kg/min), and fat oxidation (mg/kg/min) were calculated automatically from the O2 consumption and CO2 production, using the equation of Frayn68. These data were based on a 12 h measurement, which was divided into 6 h light/6 h dark periods.
Insulin and glucose tolerance tests
The insulin tolerance test (ITT) was performed in mice after a 4 h-fasting. Insulin at 0.75 U/kg body weight was injected intraperitoneally. The glucose tolerance test (GTT) was conducted after a 14 h-fasting, and then 2 g/kg body weight of glucose was injected intraperitoneally. After the injection, whole blood was collected from the tail vein at 20 min intervals for ITT and at 15, 30, 60, 90, and 120 min for GTT. The levels of glucose in the whole blood were measured using a ONETOUCH Ultra device (Johnson & Johnson K.K).
Myosin heavy chain (MHC) isoforms
Myosin heavy chain isoforms were analyzed by electrophoresis, as previously described69. Skeletal muscle was frozen in liquid nitrogen, powdered, and then homogenized in 10% SDS buffer (10% SDS; 40 mM dithiothreitol [DTT]; 5 mM EDTA; and 0.1 M Tris-HCl, pH 8.0) supplemented with a protease inhibitor cocktail (Complete Mini, EDTA-free, Roche Diagnostics). After heating at 90 °C for 3 min, the samples were diluted in 2 × sample buffer containing 4% SDS; 100 mM DTT; 0.16 M Tris-HCl, pH 6.8; 43% glycerol; and 0.2% bromophenol blue. The separating gel consisted of 35% glycerol; 8% acrylamide-N,N′-methylenebisacrylamide (Bis-acrylamide; 99:1); 0.2 M Tris-HCl, pH 8.8; 0.1 M glycine; 0.4% SDS; 0.1% ammonium persulfate (APS); and 0.05% N,N,N′,N′-tetramethylethylenediamine (TEMED). The stacking gel consisted of 30% glycerol; 4% bis-acrylamide (50:1); 70 mM Tris-HCl, pH 6.8; 4 mM EDTA; 0.4% SDS; 0.1% APS; and 0.05% TEMED. The lower running buffer consisted of 0.05 M Tris base; 75 mM glycine; and 0.05% SDS. The upper running buffer was 6-fold the concentration of the lower running buffer, and β-ME was added to 0.12%. A 50 ng sample of total protein was electrophoresed per lane. Electrophoresis was performed at 10 mA at 4 °C for 40 min and then at 140 V at 4 °C for 22 h. The gel was stained with a silver staining kit (Silver Stain KANTO III; Kanto Chemicals). The bands of interest were quantified by densitometry using ImageJ software (Rasband W; National Institutes of Health, USA).
Western blot analysis
Frozen skeletal muscle was powdered under liquid nitrogen and homogenized in ice-cold RIPA buffer containing 5 mM Tris-HCl, pH 7.4; 1% NP-40; 0.1% SDS; 1% sodium deoxycholate; 5 mM EDTA; 150 mM NaCl; protease inhibitor cocktail; and phosphatase inhibitor cocktail (PhosSTOP; Roche Diagnostics). The homogenates were centrifuged at 10,000 × g for 15 min at 4 °C. The resulting supernatant was mixed with 3 × sample buffer (Blue Loading Buffer; Cell Signaling Technology, Danvers, MA, USA) and heated at 95 °C for 5 min. Then, a 24 μg sample of total protein was electrophoresed on a polyacrylamide gel (4–12% gradient, NuPAGE® SDS-PAGE Gel system, Thermo Fisher Scientific Inc.) and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were stained with Ponceau S (Ponc) and Ponc-stained Images were used to verify equal loading in all lanes70. Membranes were then destained with Tris-buffered saline containing 0.1% Tween-20 (TBST) and blocked with 5% skim milk in TBST for 1 h at room temperature. Membranes were then washed and incubated overnight with primary antibodies at 4 °C. The primary antibodies used were: anti-TIM23 antibody (1:1,000 dilution, #611222, BD Biosciences), anti-TOM20 (FL-145) antibody (1:500, sc-11415, Santa Cruz Biotechnology), and anti-PGC1α antibody (1:1,000, ab54481, abcam). After reactions with the primary antibodies, the membranes were washed and incubated with either horseradish peroxidase-conjugated anti-rabbit antibodies or anti-mouse antibodies for 1 h at room temperature. After washing, chemiluminescence reagents (Luminata Forte; Millipore) were used to facilitate detection of the protein bands. Images were scanned with Amersham Imager 600 (GE Healthcare, Tokyo, Japan). Band intensities were measured on the captured images using ImageJ.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 7 software (San Diego, CA) using unpaired t tests, one-way ANOVA, and two-way ANOVA. P < 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
We thank Shinji Nakamura, who provided the programming for measuring the adipocyte area, and Aurelian Kelever for assistance with the confocal laser scanning microscope. We also thank Mika Kikkawa and Yoshiki Miura for assistance with MHC isoform analysis. We are grateful to Risa Nonaka and Yuka Hirasawa for the mice genotyping. We appreciate Takashi Ueno for giving technical advice for protein analysis. We thank Hynda Kleinman and Yoshihiko Yamada for critical reading and editing of the manuscript. This work was supported by Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT: 15K09326 to E.AH.), Japan Agency for Medical Research and Development (AMED: 17ek0109230s0101 to E.AH.), and Intramural Research Grant (29-4) for Neurological and Psychiatric Disorders of National Center of NCNP. Y.Y. is a member of the MEXT-Supported Program for “Fostering Global Physicians - Reform of Medical Education by Balancing Basic Research and Clinical Practice”. The authors have no conflict of interest directly relevant to the content of this article.
Author Contributions
Y.Y. and E.A.H. conceived and designed study; Y.Y., S.N., T.Y. and T.N. performed experiments; Y.Y., S.N., T.Y., T.N., N.F. and T.M. analyzed and interpreted data; Y.Y. wrote the manuscript; T.Y., T.N., T.M., N.H. and E.A.H. contributed to edit the manuscript; All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25635-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cornier MA, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Bays HE, et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:304–383. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 6.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy SM. Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest. 2015;45:1209–1217. doi: 10.1111/eci.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 10.Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 2010;199:451–463. doi: 10.1111/j.1748-1716.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 11.Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994;478(Pt 2):341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- 14.Huang G, Greenspan DS. ECM roles in the function of metabolic tissues. Trends Endocrinol Metab. 2012;23:16–22. doi: 10.1016/j.tem.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8:823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 16.Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang L, et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60:416–426. doi: 10.2337/db10-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Divoux A, Clement K. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev. 2011;12:e494–503. doi: 10.1111/j.1467-789X.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 21.Gubbiotti MA, Neill T, Iozzo RV. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol. 2017;57-58:285–298. doi: 10.1016/j.matbio.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran-Lundmark K, et al. Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res. 2008;103:43–52. doi: 10.1161/CIRCRESAHA.107.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu YX, et al. The glycosylation-dependent interaction of perlecan core protein with LDL: implications for atherosclerosis. J Lipid Res. 2015;56:266–276. doi: 10.1194/jlr.M053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, et al. Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition. Matrix Biol. 2010;29:461–470. doi: 10.1016/j.matbio.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ning L, et al. Perlecan inhibits autophagy to maintain muscle homeostasis in mouse soleus muscle. Matrix Biol. 2015;48:26–35. doi: 10.1016/j.matbio.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Ramasamy I. Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med. 2014;52:1695–1727. doi: 10.1515/cclm-2013-0358. [DOI] [PubMed] [Google Scholar]
- 27.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–1296. doi: 10.2522/ptj.20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- 29.Li F, et al. Nebulin deficiency in adult muscle causes sarcomere defects and muscle-type-dependent changes in trophicity: novel insights in nemaline myopathy. Hum Mol Genet. 2015;24:5219–5233. doi: 10.1093/hmg/ddv243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 31.Ettner N, Gohring W, Sasaki T, Mann K, Timpl R. The N-terminal globular domain of the laminin alpha1 chain binds to alpha1beta1 and alpha2beta1 integrins and to the heparan sulfate-containing domains of perlecan. FEBS Lett. 1998;430:217–221. doi: 10.1016/S0014-5793(98)00601-2. [DOI] [PubMed] [Google Scholar]
- 32.Reinhardt D, et al. Mapping of nidogen binding sites for collagen type IV, heparan sulfate proteoglycan, and zinc. J Biol Chem. 1993;268:10881–10887. [PubMed] [Google Scholar]
- 33.Villar MJ, Hassell JR, Brandan E. Interaction of skeletal muscle cells with collagen type IV is mediated by perlecan associated with the cell surface. J Cell Biochem. 1999;75:665–674. doi: 10.1002/(SICI)1097-4644(19991215)75:4<665::AID-JCB12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi K, Madri JA, Yurchenco PD. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: an adhesion modulated by glycosaminoglycan. J Cell Biol. 1992;119:945–959. doi: 10.1083/jcb.119.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakravarti S, Horchar T, Jefferson B, Laurie GW, Hassell JR. Recombinant domain III of perlecan promotes cell attachment through its RGDS sequence. J Biol Chem. 1995;270:404–409. doi: 10.1074/jbc.270.1.404. [DOI] [PubMed] [Google Scholar]
- 36.Ghiselli G, Eichstetter I, Iozzo RV. A role for the perlecan protein core in the activation of the keratinocyte growth factor receptor. Biochem J. 2001;359:153–163. doi: 10.1042/bj3590153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mongiat M, et al. Fibroblast growth factor-binding protein is a novel partner for perlecan protein core. J Biol Chem. 2001;276:10263–10271. doi: 10.1074/jbc.M011493200. [DOI] [PubMed] [Google Scholar]
- 38.Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci. 2002;5:119–123. doi: 10.1038/nn801. [DOI] [PubMed] [Google Scholar]
- 40.Peng HB, et al. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun. 1998;5:475–489. doi: 10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- 41.Rossi M, et al. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilsie LC, Chanchani S, Navaratna D, Orlando RA. Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 2005;4:2. doi: 10.1186/1476-511X-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-W. [DOI] [PubMed] [Google Scholar]
- 44.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 45.Noonan DM, et al. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266:22939–22947. [PubMed] [Google Scholar]
- 46.Zoeller JJ, McQuillan A, Whitelock J, Ho SY, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolset SO, Salmivirta M. Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell Mol Life Sci. 1999;56:857–870. doi: 10.1007/s000180050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 49.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One. 2012;7:e35273. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komiya, Y. et al. Mouse soleus (slow) muscle shows greater intramyocellular lipid droplet accumulation than EDL (fast) muscle: fiber type-specific analysis. J Muscle Res Cell Motil, 10.1007/s10974-017-9468-6 (2017). [DOI] [PubMed]
- 51.Wu Z, et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 52.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/MCB.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung S, Kim K. Exercise-induced PGC-1alpha transcriptional factors in skeletal muscle. Integr Med Res. 2014;3:155–160. doi: 10.1016/j.imr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Handschin C, et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 55.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 56.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol (1985) 1996;81:2509–2516. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- 57.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol (1985) 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 58.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 59.Hittel DS, et al. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc. 2010;42:2023–2029. doi: 10.1249/MSS.0b013e3181e0b9a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Machida S, Booth FW. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc Nutr Soc. 2004;63:337–340. doi: 10.1079/PNS2004354. [DOI] [PubMed] [Google Scholar]
- 61.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 62.Costell M, et al. Perlecan maintains the integrity of cartilage and some basement membranes. Journal of Cell Biology. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsumaki N, et al. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerever A, et al. Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Res. 2014;12:492–505. doi: 10.1016/j.scr.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishimura S, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pirnay F, et al. Fate of exogenous glucose during exercise of different intensities in humans. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:1620–1624. doi: 10.1152/jappl.1982.53.6.1620. [DOI] [PubMed] [Google Scholar]
- 67.Ishihara K, Oyaizu S, Onuki K, Lim K, Fushiki T. Chronic (-)-hydroxycitrate administration spares carbohydrate utilization and promotes lipid oxidation during exercise in mice. J Nutr. 2000;130:2990–2995. doi: 10.1093/jn/130.12.2990. [DOI] [PubMed] [Google Scholar]
- 68.Couture S, Massicotte D, Lavoie C, Hillaire-Marcel C, Peronnet F. Oral [(13)C]glucose and endogenous energy substrate oxidation during prolonged treadmill running. J Appl Physiol (1985) 2002;92:1255–1260. doi: 10.1152/japplphysiol.00437.2001. [DOI] [PubMed] [Google Scholar]
- 69.Mizunoya W, Wakamatsu J, Tatsumi R, Ikeuchi Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem. 2008;377:111–113. doi: 10.1016/j.ab.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 70.Romero-Calvo I, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.