Abstract
Background
Baicalein can suppress the growth of multiple tumors, including multiple myeloma (MM), but the exact mechanisms remains elusive. Here, we investigated the exact mechanisms of the anti-myeloma activity of baicalein.
Material/Methods
Proliferation and rates of apoptosis of myeloma U266 cells exposed to baicalein were detected. Microarray, polymerase chain reaction (PCR) assay, and Western blot analysis were applied to evaluate the mRNA and protein levels of associated molecules. Survival analysis of IKZF1 and IKZF3 was conducted as well.
Results
Baicalein suppressed the growth and stimulated apoptosis of myeloma U266 cells in a dose- and time-dependent way. Baicalein increased mRNA level of CRBN, and further studies suggested that baicalein downregulated IKZF1 and IKZF3 on a post-transcriptional level. Although the differences did not reach statistical significance, IKZF1 and IKZF3 were associated with poor overall survival.
Conclusions
Our results suggest that baicalein suppresses the growth and promotes apoptosis of myeloma U266 cells through downregulating IKZF1 and IKZF3. Baicalein increased the expression of CRBN, which might exert a reversion effect on resistance of IMiDs. MM patients in IKZF1 and IKZF3 low-expression groups had better overall survival than those in IKZF1 and IKZF3 high-expression groups. Thus, the present results indicate that baicalein might be a therapeutic choice for targeting IKZF1 and IKZF3.
MeSH Keywords: Cell Proliferation, Multiple Myeloma, Scutellaria baicalensis
Background
Multiple myeloma (MM) is the second most common hematological malignancy; it is characterized by the production of monoclonal M protein of malignant plasma cells in bone marrow [1]. The introduction of IMiDs such as thalidomide, lenalidomide, and pomalidomide has significantly improved the overall survival of MM patients [2–5]. However, the prognosis of MM patients is dismal due to recurrent disease and drug-resistance of IMiDs [6,7]. Cereblon (CRBN), an ubiquitously expressed protein, is a component of the cullin ring E3 ubiquitin ligase complex CUL4-RBX1-DDB1 (CRL4). Recent studies demonstrated that CRBN is the primary target of thalidomide and is required for the anti-myeloma activity and teratogenicity of IMiDs. The binding of IMiDs to cereblon inhibited the function of CRL4 and induced the CRBN-dependent proteasomal degradation of 2 lymphoid transcription factors: IKZF1 and IKZF3. The loss of these 2 proteins inhibited the expression of interferon regulatory factor 4 (IRF4) and Myc in MM cells and increased the production of interleukin-2 by T cells, leading to the anti-myeloma and immune modulation activity of IMiDs. Other studies suggested that high-level CRBN expression was associated with better clinical outcome of MM patients, and the resistance of IMiDs was accompanied by downregulation of CRBN [8–21].
Our previous studies demonstrated that baicalein, a component of the traditional Chinese medical formula Huang-Lian-Jie-Du-Tang (HLJDT), can suppress the growth of MM cells through downregulating the expression of IL-6 and XIAP [22–24]. Administration of thalidomide plus dexamethasone (TD) regimen with HLJDT as maintenance therapy improved the response rates and progression-free survival of patients with MM [25]. The exact mechanism of the synergistic effect of TD regimen and HLJDT (or baicalein) has not been clearly elucidated. In the present study, we prove that baicalein inhibits the growth and increases rates of apoptosis of myeloma cells through CRBN-dependent downregulation of IKZF1 and IKZF3.
Material and Methods
Baicalein and antibodies of CRBN, IKZF1, and IKZF3
Baicalein (purity, 98%) was purchased from Sigma-Aldrich (USA) and dissolved in dimethylsulphoxide (DMSO) (Shanghai Sidande Biotechnology Co. Shanghai, China). The final concentration of DMSO in all experiments was <0.1%. Monoclonal anti-CRBN, anti-IKZF1, and IKZF3 were purchased from Sigma-Aldrich (USA).
Cell culture
Human MM U266 cells were maintained at 37°C and 5% CO2 in RPMI-1640 plus 10% fetal bovine serum (FBS). The seeding medium was replaced with fresh medium every other day. Once 80% of the bottle wall was covered, myeloma cells were passed.
Cell proliferation assay
We used the Cell Counting Kit-8 (CCK-8), bought from Dojindo Laboratories Kumamoto, Japan), to assess the cell viability of U266 cells that were exposed to increasing concentrations of baicalein (0, 20, 40, 80 and 160 μmol/l) for set times (0, 6, 12, 24, and 48 h) in triplicate. About 5000 U266 cells were seeded in each 96-well plate. Groups exposed to dilution vehicle were regarded as controls. Once treatment ended, we added 10 μl CCK-8 reagents to each well. Then, myeloma cells were incubated for another 4 h and the value of optical density (OD) was measured at 450 nm using an enzyme-labeled instrument (Varian, Palo Alto, CA, USA). Relative cell viability (%)=[(As–Ab)/(Ac–Ab)], where As, Ab, and Ac denoted the absorbance of experimental, blank, and control groups, respectively.
Detection of cell apoptosis
Myeloma U266 cells were exposed to 40 μmol/l baicalein for 30 h, and then cell apoptosis was analyzed using the Annexin V–FITC/PI Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA). About 5×105/ml U266 cells were collected and then washed with cold PBS 2 times, after which U266 cells were resuspended in 500 μl 1×binding buffer. Subsequently, we mixed these cells with 5 μl annexin V – FITC and 5 μl of propidium iodide (PI) in the dark for 10 min at 32°C, then flow cytometry analysis was performed within 1 h to detect cell apoptosis (Annexin V-positive and PI-negative cells).
Microarray analysis
Total RNAs of myeloma U266 cells that were exposed to 70 μmol/l baicalein for 30 h in triplicate and normal control that were exposed to dilution vehicle were isolated using Trizol reagent (Invitrogen; Carlsbad, CA) and purified with the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA synthesis, biotin-labeled target synthesis, HTA_2.0 Gene Chip arrays (Santa Clara, CA) hybridization, staining, and scanning were performed following the manufacture’s recommendations. The expression value was processed using the Robust Multiarray Average (RMA) algorithm, which included background correction, normalization, and summarization of expression values [26]. We applied the Significant Analysis of Microarrays (SAM) [27] algorithm to identify genes with statistically significant changes in expression between the baicalein-treated group and control group, and differently expressed genes were determined based on the t test (p<0.05) and fold change (≥1.5).
Quantitative RT-PCR (quantitative reverse transcription-polymerase chain reaction)
We treated myeloma U266 cells with increasing concentrations of baicalein (0, 20, 40, 80, and 160 μmol/l) for set times (0, 6, 12, 24, and 48 h). Total RNA was exacted from U266 cells using Trizol reagent according to the instructions. We used the RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) to conduct reverse transcription. Then, we performed semi- RT-PCR using 1 μl cDNA in a 25 μl final reaction mixture (32–35 cycles for 4 min at 94°C, 30 s at 94°C, 30 s at 56°C, 25 s at 72°C, 30 cycles of 4 min of 72°C, and 4 min at 4°C) [28]), and the products of PCR were resolved using agarose gel electrophoresis. Real-time quantitative PCR was performed using SYBR Green PCR Master Mix (Thermo Fisher Scientific, Inc.) on the ABI 7900/Illumina Eco Fast Real-Time PCR system (Applied Biosystems) in accordance with the protocol of the manufacture. The following conditions were used for PCR amplification: 40 cycles of 50°C for 2 min, 95°C for 10 min, 95°C for 30 s, and 60 °C for 30 s. β-actin was regarded as the endogenous control and all experiments were performed in triplicate. ΔΔCt method was used for quantification. Specific primers used in the experiments were:
β-actin (forward: 5′-AGCGAGCATCCCCCAAAGTT-3′,
reverse: 5′-GGGCACGAAGGCTCATCATT-3′),
CRBN (forward: 5′-TCTGCCGACATCACATACATAC-3′,
reverse: 5′-AATTCCGCACCATACTGACTTCT-3′),
IKZF1 (forward: 5′-GACAGCAAAGCTCCAAGAGTGAC-3′,
reverse: 5′-GAATGCCTCCAACTCCCGACAAA-3′),
IKZF3 (forward: 5′-CCTCGGAGATGGTTCCAGTTAT-3′,
reverse: 5′-GCGTTCTTCATGGTTGCTGTC-3′).
Western blotting
The detailed steps of Western blotting were the same as previously reported [28]. In brief, total proteins of myeloma U266 cells were extracted and separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After being transferred to 0.45-m polyvinylidene difluoride (PVDF) membranes and blocked with non-fat milk, the proteins were incubated with anti-CRBN, anti-IKZF1, and anti-IKZF3 at 4°C overnight. After being washed with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies. Enhanced chemical luminescence method was performed to detect specific protein bands.
Prognosis analysis
The GEO dataset GSE2658 was downloaded from Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/), and expression values of IKZF1 and IKZF3 and survival data of 559 MM patients were extracted from the Series Matrix File of this dataset. The multivariate Cox proportional hazard model was used to evaluate the association between the expression of IKZF1 and IKZF3 and survival of MM patients.
Statistical analysis
SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) was used to conducted data analysis. All data are reported as mean ±SD (standard deviation). One-way analysis of variance was conducted to test differences between 2 groups. P<0.05 was considered to be statistically significant.
Results
Growth of myeloma U266 cells was suppressed by baicalein
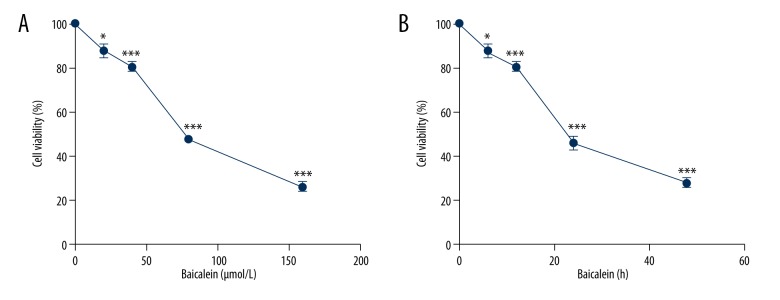
To examine the anti-myeloma activity of baicalein, we used the CCK-8 assay to measure the growth of myeloma cells exposed to increasing concentrations of baicalein for specific time periods. The growth rates of myeloma U266 cells exposed to 20, 40, 80, and 160 μM baicalein for 24 h were 87.7±3.11%, 80.22±2.23%, 47.74±1.52%, and 26.18±2.23%, respectively (Figure 1A). The proliferation of U266 cells exposed to 60 μM baicalein for 6, 12, 24, and 48 h were 87.8±3.21%, 80.9±2.53%, 46.4±2.6%, and 28.13±2.17%, respectively (Figure 1B). Our results indicate that the growth of myeloma was suppressed by baicalein in a dose- and time-dependent manner.
Figure 1.
Baicalein inhibits the growth of myeloma U266 cells in a time-dependent and dose-dependent manner. (A) U266 cells were treated with 0, 20, 40, 80, and 160 μmol/L baicalein for 24 h. (B) U266 cells were treated with 60 μmol/L baicalein for 0, 12, 24, and 48 h; Cell viability was measured using CCK-8 assay, data shown as mean ±SEM of 3 experiments and analyzed by two-sample t test, * P<0.05, ** P<0.01, *** P<0.001 relative to control group (Groups exposed to dilution vehicle were regarded as control); CCK-8 – cell counting kit-8; SEM – standard error of mean.
Apoptosis rates of myeloma U266 cells were increased under baicalein treatment
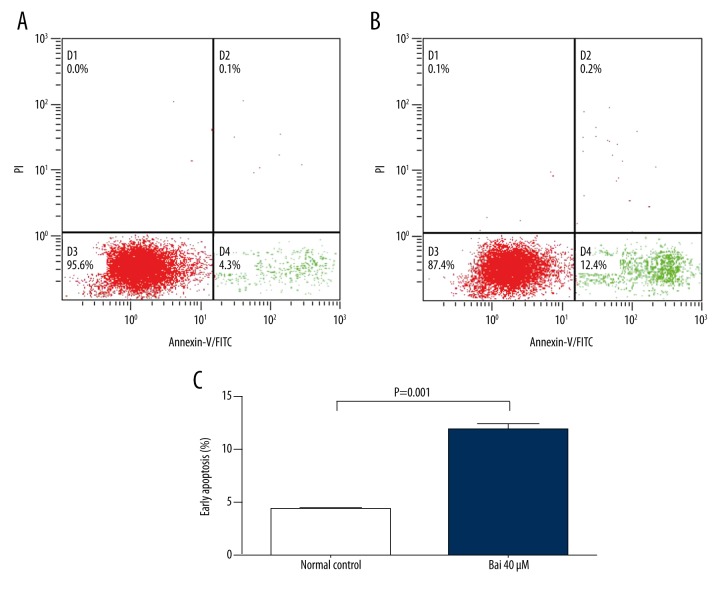
Next, we examined whether the treatment of baicalein was associated with the induction of apoptosis of U266 cells. The rates of apoptosis of U266 cells treated with 40 μM baicalein for 30 h were detected using flow cytometry to measure apoptosis (early apoptosis: annexin V-positive and PI-negative). Myeloma cells treated with 40 μmol/l baicalein for 30 h had significantly increased rates of early apoptosis, from 4.3±0.05% (normal control) to 11.9±0.4% (P=0.001) (Figure 2), suggesting that baicalein stimulates apoptosis of myeloma U266 cells.
Figure 2.
Baicalein stimulates the apoptosis of myeloma U266. (A) Flow cytometric analysis of apoptosis in U266 cells treated with DMSO (A) or 40 μmol/L baicalein (B) for 24 h. Percentage of apoptotic cells of U266 cells was analyzed by annexin V binding/PI staining using flow cytometry, data shown as mean ±SEM of 3 experiments and analyzed by two-sample t test (C).
Baicalein upregulated CRBN and downregulated IKZF1 and IKZF3
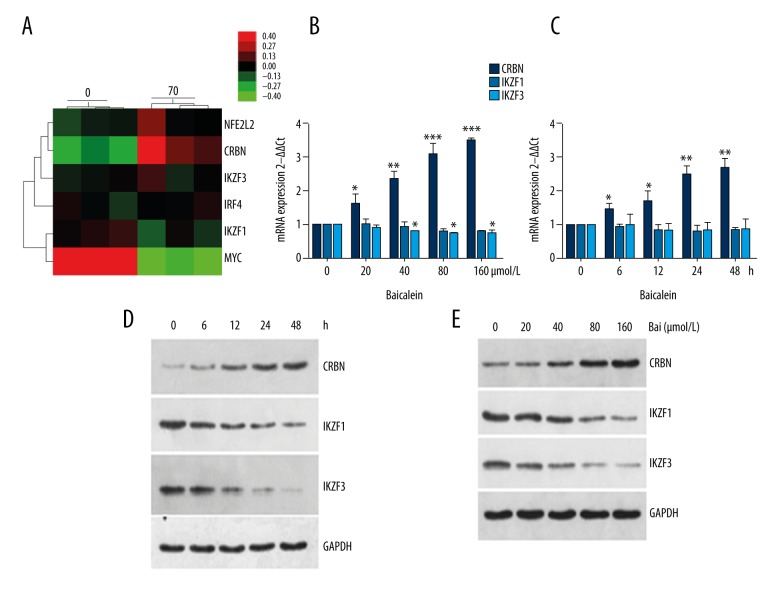
As mentioned above, the resistance of IMiDs was associated with downregulation of CRBN. Lee et al. demonstrated that Nrf2 stimulates CRBN gene transcription under hypoxia-reoxygenation (H/R) conditions or the production of reactive oxygen species (ROS) in neuronal cells [29]. Several studies demonstrated that baicalein is associated with the production of intracellular ROS [30–33]. Thus, we tested whether baicalein affects the expression of CRBN and its downstream targets. First, we applied microarray assay to study the expression of CRBN and its downstream targets. As shown in Figure 3A, the mRNA level of CRBN was increased when myeloma U266 cells were treated with 70 μM baicalein for 30 h, while no significant changes of the mRNA levels of IKZF1, IKZF3, and IRF4 were found. Our PCR results provided further confirmation that increasing concentrations of baicalein treatment for indicated time periods significantly upregulated the mRNA level of CRBN, but it did not significantly affect the mRNA levels of IKZF1 and IKZF3 (Figure 3B, 3C, and Supplementary Figure 1). Next, we investigated whether baicalein affects the protein levels of these 3 molecules. Our Western blotting results suggest that baicalein increased the expression of CRBN and decreased the expression of IKZF1 and IKZF3 in a dose- and time-dependent manner (Figure 3D, 3E). In summary, baicalein inhibits myeloma U266 cells through downregulating IKZF1 and IKZF3.
Figure 3.
Baicalein upregulates CRBN and downregulates IKZF1 and IKZF3. (A) Myeloma U266 cells were treated with 70 μmol/l baicalein for 30 h in triplicate, HTA_2.0 Gene Chip was used to investigate the difference in NFE2L2 (Nrf2), CRBN, IKZF3, IRF4, IKZF1, and Myc. Green corresponds to low expression, red corresponds to high expression. (B) U266 cells were treated 0, 20, 40, 80, and 160 μmol/L baicalein for 24 h. The mRNA levels of IKZF1, IKZF3, and CRBN were examined by RT-PCR, and β-actin was used as an internal control. Bars represent means and SEM of mRNA expression of our genes of interest. One-way ANOVA was used to compared experimental group and control group with * P<0.05, ** P<0.01, *** P<0.001. (C) U266 cells were treated 60 μmol/L baicalein for 0, 6, 12, 24, and 48 h. The mRNA levels of IKZF1, IKZF3, and CRBN were examined by RT-PCR, and β-actin was used as an internal control. Bars represent means and SEM of mRNA expression of our genes of interest. One-way ANOVA was used to compared the experimental group and control group with * P<0.05, ** P<0.01, *** P<0.001. (D) U266 cells were treated with 60 μmol/L baicalein for 0, 12, 24, and 48 h. The protein levels of CRBN, IKZF1, and IKZF3 were examined by Western blot, and their relative levels were normalized to GAPDH. (E) U266 cells were treated with 0, 20, 40, 80, and 160 μmol/L baicalein for 24 h. The protein levels of CRBN, IKZF1, and IKZF3 were examined by Western blot, and their relative levels were normalized to GAPDH. Bai – baicalein.
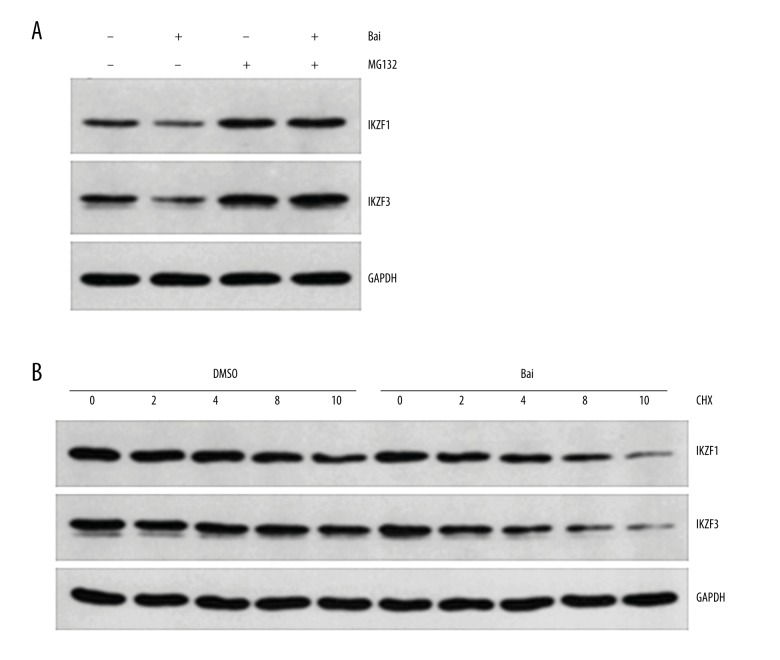
Baicalein downregulated IKZF1 and IKZF3 through proteasomal degradation
MG-132 is a peptide aldehyde (Z-Leu-Leu-Leu-al) that selectively blocks the proteolytic activity of the 26S proteasome; it is usually used as a tool for disrupting the proteasome-regulated degradation of intracellular proteins. Thus, to elucidate the difference in mRNA and protein levels of IKZF1 and IKZF3 when myeloma U266 cells were treated with baicalein, we first pretreated myeloma U266 cells with 10 μM MG132 for 3 h, and then co-treated these cells with 60 μM baicalein or DMSO for 24 h. As shown in Figure 4A, the presence of MG132 abrogated baicalein-stimulated reduction of IKZF1 and IKZF3. Then, we pretreated myeloma U266 cells with DMSO or 60 μM baicalein for 1 h prior to the addition of 100 μg/ml cycloheximide, a protein synthesis inhibitor, for set time periods. As shown in Figure 4B, the protein levels of IKZF1 and IKZF3 were degraded more rapidly in baicalein-treated U266 cells than in vehicle-treated controls. In summary, our data suggest that baicalein promotes proteasomal degradation of IKZF1 and IKZF3 in myeloma U266 cells.
Figure 4.
Baicalein downregulates IKZF1 and IKZF3 on a post-transcriptional level. (A) U266 cells were treated with DMSO or 60 μmol/L baicalein in the absence or presence of 10 μmol/L MG132 for 24 h, and the IKZF1 and IKZF3 levels were examined by Western blot. (B) U266 cells were pretreated with DMSO or 60 μmol/L baicalein. After 1 h, 100 μg/ml cycloheximide was added and protein lysates were obtained at indicated time points. IKZF1 and IKZF3 levels were examined by Western blot. GAPDH was used as an internal control. Bai – baicalein; CHX – cycloheximide.
Prognostic relevance of IKZF1 and IKZF3 expression in MM patients
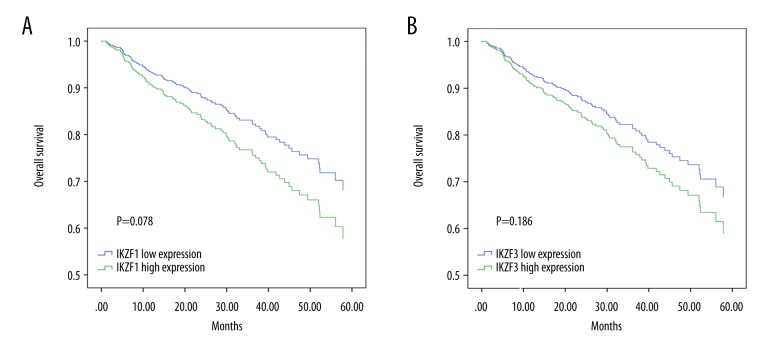
To investigate the prognostic relevance of IKZF1 and IKZF3 in MM patients, we reanalyzed the GEO dataset GSE2658, which contained pretreated samples of 559 MM patients whose clinical characteristics were well documented. We classified patients into IKZF1 and IKZF3 low-expression and high-expression groups based on the median expression of these 2 genes. As shown in Figure 5, although the difference did not reach statistical significance, patients in IKZF1 and IKZF3 low-expression groups had better overall survival compared with those in the IKZF1 and IKZF3 high-expression groups. These results suggest that baicalein may be a good therapeutic choice for MM patient through targeting IKZF1 andIKZF3.
Figure 5.
Overall survival of MM patients according to the expression of IKZF1 (A) and IKZF3 (B).
Discussion
The introduction of IMiDs has greatly improved the response rate and prognosis of patients with MM, but the disease remains incurable due to the inevitable disease relapse and drug resistance. Studies on the mechanism IMiDs are prompted by the remarkable clinical outcomes and drug resistance that develop from long-term administration of IMiDs. The anti-myeloma and teratogenic effects of IMiDs have traditionally been attributed to induction of oxidative stress, anti-angiogenesis, anti-inflammation, anti-proliferation, and multiple effects on the immune system [34]. In 2010, Ito et al. demonstrated that thalidomide exerts teratogenic effects by binding to CRBN and inhibiting the associated ubiquitin ligase activity [9]. Subsequent studies showed that CRBN is required for the anti-myeloma activity of IMiDs [10,11]. IMiDs target cereblon-E3 ubiquitin ligase CRL4 and promote proteasomal degradation of 2 transcription factors, IKZF1 and IKZF3, and the loss of these 2 transcription factors leads to downregulation of C-Myc and IRF4, which are essential for the proliferation of myeloma cells [12–21]. Our results demonstrate that baicalein, a Chinese herbal medicine found to have wide-ranging and seemingly disparate cellular actions (e.g., anti-inflammation, antioxidation, and antitumor) [36], can suppress the growth and increase apoptosis rates of myeloma U266 cells in a dose- and time-dependent manner and downregulate IKZF1 and IKZF3 on a post-transcriptional level, suggesting that baicalein inhibits the growth of myeloma U266 cells, at least in part through promoting degradation of IKZF1 and IKZF3. Our prognosis analysis of IKZF1 and IKZF3 indicated that lower expression of these 2 transcription factors predicted better overall survival in MM patients, suggesting that baicalein might exert an anti-myeloma activity in MM patients through targeting IKZF1 and IKZF3.
Myeloma patients with higher levels of CRBN have better clinical outcomes, and resistance of IMiDs is associated with downregulation of CRBN [36–43]. Lee et al. demonstrated that CRBN was upregulated by Nrf2 when neuroblastoma cells were exposed to hypoxia-reoxygenation, and a single Nrf2/ARE site in the upstream promoter region of mouse CRBN was responsible for most of the H/R-dependent increase in CRBN expression and overexpression of Nrf-2 upregulated CRBN [28]. Loboda et al. suggested that activation of the Nrf2/ARE system affects oxidative status of the cells and provides robust protection against oxidative challenge [44]. Several studies demonstrated that baicalein protects multiple types of cells from oxidative damage through regulating the Nrf2 signal pathway [45–49]. Our results suggest that baicalein upregulates the expression of CRBN, and baicalein treatment shows a trend of increasing Nrf2 expression (Figure 3A), indicating that baicalein exerts a reversion effect on resistance of IMiDs. Whether there was a similar mechanism by which baicalein upregulates CRBN through regulation of the Nrf2 signaling pathway remained to be elucidated.
Fischer et al. demonstrated that IMiDs block endogenous substrates of MEIS2 from binding to CRL4, leading to the degradation of IKZF1 and IKZF3 [20,21]. Our results showed that the treatment of baicalein upregulate the expression of CRBN, while downregulating its downstream targets IKZF1 and IKZF3. It is unclear whether the degradation of IKZF1 and IKZF3 by baicalein is CRBN-dependent or if other mechanisms are involved in the degradation of IKZF1 and IKZF3, such as lysosomal degradation pathway and apoptosis. Thus, further studies are required to elucidate the exact mechanisms by which the degradation of IKZF1 and IKZF3 occurs.
Conclusions
The present study suggests that baicalein suppresses the growth and promotes apoptosis of myeloma U266 cells via promoting proteasomal degradation of IKZF1 and IKZF3. Baicalein increased the expression of CRBN, which might exert a reversion effect on resistance of IMiDs. MM patients in IKZF1 and IKZF3 low-expression groups had better overall survival than those in IKZF1 and IKZF3 high-expression groups. Although further studies are required to elucidate the mechanism by which baicalein regulates CRBN, IKZF1, and IKZF3 in myeloma cells, the present results indicated baicalein might be a good therapeutic choice targeting IKZF1 and IKZF3.
Supplementary Figure
Regulation of CRBN, IKZF1, and IKZF3 by baicalein. (A) U266 cells were treated with 0, 20, 40, 80, and 160 μmol/L baicalein for 24 h. (B) U266 cells were treated 60 μmol/L baicalein for 0, 6, 12, 24, and 48 h). The mRNA levels of IKZF1, IKZF3, and CRBN were examined by general PCR, and β-actin was used as an internal control. Bai – baicalein.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Research Fund from the National Natural Science Foundation of China (No. 81272627 and No.81470007)
References
- 1.Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica. 2010;102(3):83–87. [PubMed] [Google Scholar]
- 2.Ria R, Reale A, Vacca A. Novel agents and new therapeutic approaches for treatment of multiple myeloma. World J Methodol. 2014;4(2):73–90. doi: 10.5662/wjm.v4.i2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantani L, Brioli A, Tacchetti P, et al. Current and emerging triplet combination therapies for relapsed and refractory multiple myeloma. Expert Rev Hematol. 2016;9(3):315–23. doi: 10.1586/17474086.2016.1127754. [DOI] [PubMed] [Google Scholar]
- 4.Fouquet G, Bories C, Guidez S, et al. Pomalidomide for multiple myeloma. Expert Rev Hematol. 2014;7(6):719–31. doi: 10.1586/17474086.2014.966074. [DOI] [PubMed] [Google Scholar]
- 5.Castelli R, Gualtierotti R, Orofino N, et al. Current and emerging treatment options for patients with relapsed myeloma. Clin Med Insights Oncol. 2013;7:209–19. doi: 10.4137/CMO.S8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreau P, Touzeau C. Multiple myeloma: from front-line to relapsed therapies. Am Soc Clin Oncol Educ Book. 2015:e504–11. doi: 10.14694/EdBook_AM.2015.35.e504. [DOI] [PubMed] [Google Scholar]
- 7.Nooka AK, Kastritis E, Dimopoulos MA, Lonial S. Treatment options for relapsed and refractory multiple myeloma. Blood. 2015;125(20):3085–99. doi: 10.1182/blood-2014-11-568923. [DOI] [PubMed] [Google Scholar]
- 8.Fionda C, Abruzzese MP, Zingoni A, et al. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget. 2015;6(27):23609–30. doi: 10.18632/oncotarget.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–50. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Girona A1, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–35. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu YX, Braggio E, Shi CX, Bruins LA, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–79. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–5. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu G, Middleton RE, Sun H, Naniong M, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–9. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjorklund CC, Lu L, Kang J, et al. Rate of CRL4 (CRBN) substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-Myc and IRF4. Blood Cancer J. 2015;5:e354. doi: 10.1038/bcj.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krönke J, Fink EC, Hollenbach PW, et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature. 2015;523(7559):183–88. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YA, Peng YJ, Hu MC, et al. The cullin 4A/B-DDB1-Cereblon E3 ubiquitin ligase complex mediates the degradation of CLC-1 chloride channels. Sci Rep. 2015;5:10667. doi: 10.1038/srep10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krönke J, Hurst SN, Ebert BL. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology. 2014;3(7):e941742. doi: 10.4161/21624011.2014.941742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu YX, Braggio E, Shi CX, et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood. 2014;124(4):536–45. doi: 10.1182/blood-2014-02-557819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.) Br J Haematol. 2014;164(6):811–21. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer ES, Böhm K, Lydeard JR, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512(7512):49–53. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamberlain PP, Lopez-Girona A, Miller K, et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol. 2014;21(9):803–9. doi: 10.1038/nsmb.2874. [DOI] [PubMed] [Google Scholar]
- 22.Ma Z, Otsuyama K, Liu S, et al. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105(8):3312–18. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Ma Z, Cai H, et al. Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur J Haematol. 2010;84(2):137–44. doi: 10.1111/j.1600-0609.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu CP, Cai HL, He L, et al. [Effect of baicalein on proliferation and migration in multiple myeloma cell lines RPMI 8226 and U266 cells]. Zhonghua Xue Ye Xue Za Zhi. 2012;33(11):938–43. [in Chinese] [PubMed] [Google Scholar]
- 25.Du G, Liu S, Xie X, et al. [The effect of Huang-Lian-Jie-Du-Tang combined with chemotherapy for multiple myeloma]. J Clin Hematology (China) 2012;25(5) [in Chinese] [Google Scholar]
- 26.Bolstad BM. Low-level analysis of high-density oligonucleotide array data: Background, normalization and summarization. University of California; Berkeley: 2004. [Google Scholar]
- 27.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Liu S, Chen J, et al. Baicalein suppresses the proliferation of acute T-lymphoblastic leukemia Jurkat cells by inhibiting the Wnt/β-catenin signaling. Ann Hematol. 2016;95(11):1787–93. doi: 10.1007/s00277-016-2766-z. [DOI] [PubMed] [Google Scholar]
- 29.Lee KJ, Lee KM, Jo S, et al. Induction of cereblon by NF-E2-related factor 2 in neuroblastoma cells exposed to hypoxia-reoxygenation. Biochem Biophys Res Commun. 2010;399(4):711–15. doi: 10.1016/j.bbrc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Chen YC, Chow JM, Lin CW, et al. Baicalein inhibition of oxidative-stress-induced apoptosis via modulation of ERKs activation and induction of HO-I1 gene expression in rat glioma cells C6. Toxicol Appl Pharmacol. 2006;216(2):263–73. doi: 10.1016/j.taap.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Kim KC, Lee IK, Kang KA, et al. Baicalein (5,6,7-trihydroflavone) reduces oxidative stress-induced DNA damage by upregulating the DNA repair system. Cell Biol Toxicol. 2012;28(6):421–33. doi: 10.1007/s10565-012-9233-y. [DOI] [PubMed] [Google Scholar]
- 32.Cui G, Luk SC, Li RA, et al. Cytoprotection of baicalein against oxidative stress-induced cardiomyocytes injury through the Nrf2/Keap1pathway. J Cardiovasc Pharmacol. 2015;65(1):39–46. doi: 10.1097/FJC.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 33.Michaelis M, Sithisarn P, Cinatl J., Jr Effects of flavonoid-induced oxidative stress on anti-H5N1 influenza a virus activity exerted by baicalein andbiochanin A. BMC Res Notes. 2014;7:384. doi: 10.1186/1756-0500-7-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Ando H, Handa H. Teratogenic effects of thalidomide: Molecular mechanisms. Cell Mol Life Sci. 2011;68(9):1569–79. doi: 10.1007/s00018-010-0619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Broyl A, Kuiper R, van Duin M, van der Holt B, et al. Dutch-Belgian HOVON group, German GMMG Group. High cereblon expression is assaciated with better survival in patients with newly diagnosed multiple myelomatreated with thalidomide maintenance. Blood. 2013;121(4):624–67. doi: 10.1182/blood-2012-06-438101. [DOI] [PubMed] [Google Scholar]
- 37.Yang DY, Ren JH, Guo XN, et al. Lenalidomide affect expression level of cereblon protein in multiple myeloma cell line RPMI8226. Genet Mol Res. 2015;14(4):13588–94. doi: 10.4238/2015.October.28.19. [DOI] [PubMed] [Google Scholar]
- 38.Díaz-Rodríguez E, Pandiella A. Modulation of cereblon levels by anti-myeloma agents. Leuk Lymphoma. 2016;57(1):167–76. doi: 10.3109/10428194.2015.1037752. [DOI] [PubMed] [Google Scholar]
- 39.Jonasova A, Bokorova R, Polak J, et al. High level of full-length cereblon mRNA in lower risk myelodysplastic syndrome with isolated 5q deletion is implicated in the efficacy of lenalidomide. Eur J Haematol. 2015;95(1):27–34. doi: 10.1111/ejh.12457. [DOI] [PubMed] [Google Scholar]
- 40.Jamroziak K, Szemraj J, Robak T, et al. Cereblon expression predicts clinical response in chronic lymphocytic leukemia treated with a thalidomide/fludarabine regimen. Leuk Lymphoma. 2015;56(3):808–10. doi: 10.3109/10428194.2014.933215. [DOI] [PubMed] [Google Scholar]
- 41.Huang SY, Lin CW, Lin HH, et al. Expression of cereblon protein assessed by immunohistochemical staining in myeloma cells is associated with superior response of thalidomide- and lenalidomide-based treatment, but not bortezomib-based treatment, in patients with multiple myeloma. Ann Hematol. 2014;93(8):1371–80. doi: 10.1007/s00277-014-2063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandhi AK, Mendy D, Waldman M, et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br J Haematol. 2014;164(2):233–44. doi: 10.1111/bjh.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuster SR, Kortuem KM, Zhu YX, et al. The clinical significance of cereblon expression in multiple myeloma. Leuk Res. 2014;38(1):23–28. doi: 10.1016/j.leukres.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loboda A, Damulewicz M, Pyza E, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–47. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin S, Deng F, Wu W, et al. Baicalein modulates Nrf2/Keap 1 system in both Keap1-dependent and Keap1-independent mechanisms. Arch Biochem Biophys. 2014;559:53–61. doi: 10.1016/j.abb.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Choi EO, Jeong JW, Park C, et al. Baicalein protects C6 glial cells against hydrogen peroxide-induced oxidative stress and apoptosis through regulation of the Nrf2 signaling pathway. Int J Mol Med. 2016;37(3):798–806. doi: 10.3892/ijmm.2016.2460. [DOI] [PubMed] [Google Scholar]
- 47.Sahu BD, Kumar JM, Kuncha M, et al. Baicalein alleviates doxorubicin -induced cardiotoxicity via suppression of myocardial oxidative stress andapoptosis in mice. Life Sci. 2016;144:8–18. doi: 10.1016/j.lfs.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Yeh CH, Ma KH, Liu PS, et al. Baicalein decreases hydrogen peroxide-induced damage to NG108-15 cells via upregulation of Nrf2. J Cell Physiol. 2015;230(8):1840–51. doi: 10.1002/jcp.24900. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Cui W, Li G, et al. Baicalein protects against 6-OHDA induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. J Agric Food Chem. 2012;60(33):8171–82. doi: 10.1021/jf301511m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regulation of CRBN, IKZF1, and IKZF3 by baicalein. (A) U266 cells were treated with 0, 20, 40, 80, and 160 μmol/L baicalein for 24 h. (B) U266 cells were treated 60 μmol/L baicalein for 0, 6, 12, 24, and 48 h). The mRNA levels of IKZF1, IKZF3, and CRBN were examined by general PCR, and β-actin was used as an internal control. Bai – baicalein.