Abstract
A remarkable, totally unexpected aspect of the bone-derived hormone osteocalcin is that it is necessary for both brain development and brain function in the mouse since its absence results in a profound deficit in spatial learning and memory and an exacerbation of anxiety-like behavior. The regulation of cognitive function by osteocalcin, together with the fact its circulating levels decrease in midlife compared to adolescence in all species tested raised the prospect that osteocalcin may be an anti-geronic hormone that could prevent age-related cognitive decline. As presented in this review, recent data indicate that this is indeed the case and that osteocalcin is necessary for the anti-geronic activity recently ascribed to the plasma of young WT mice. The diversity and amplitude of the functions of osteocalcin in the brain during development and postnatally had long called for the identification of its receptor in the brain, which was also recently achieved. This review presents our current understanding of osteocalcin's biology in the brain, highlighting the bony-vertebrate specificity of the regulation of cognitive function, and pointing toward where therapeutic opportunities may exist.
Introduction
The bone-derived hormone osteocalcin has become synonymous with the skeleton's regulation of energy metabolism, in part because this was the first endocrine function ascribed to this hormone 1. Although energy metabolism is certainly a critical aspect of osteocalcin biology, whereby it affects 2 in mice, rats and humans 3-12, functions of β-cells 13, myoblasts and likely other cell types, it by no means tells the entire story of osteocalcin. If anything, a hallmark of this hormone is that it regulates a large and continually growing number of physiological functions and developmental processes 14 (Figure 1). This review will focus exclusively on one aspect of osteocalcin biology - its regulation of brain development and functions, which is sometimes overlooked. Yet this function of osteocalcin is of fundamental importance to link this hormone, or its lack thereof, to the biology of aging. The first reason to write a review on this topic at the present time is that the biomedical importance of osteocalcin's regulation of several behaviors was recently underscored and linked to the aging of the brain 15. A second reason is that major progress has recently been made in the elucidation of the molecular basis of osteocalcin's functions in the brain. These advances illustrate the growing reach of this hormone as well as similarities and differences between osteocalcin's peripheral and central functions.
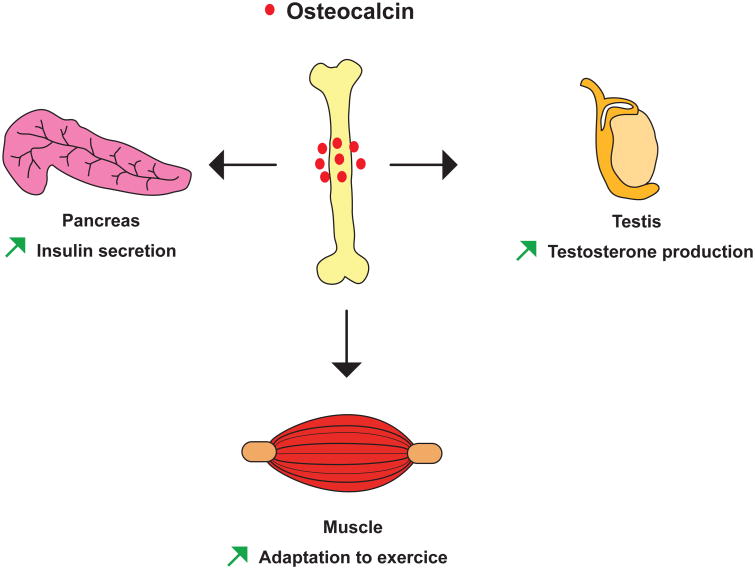
Figure 1. Osteocalcin functions in peripheral organs.
Schematic representation of the physiological functions regulated by osteocalcin.
The path to uncover osteocalcin's regulation of brain functions
Demonstrating that anxiety-like behavior and cognition are affected by a molecule made in a peripheral organ has never been an easy or straightforward task. What led to this suspicion in the case of osteocalcin is simply the fact that the extreme passivity of Osteocalcin-/- mice 16 could not be explained by any other means. What was already known about osteocalcin when this investigation started was that this molecule, when undercaboxylated, promotes insulin secretion and glucose homeostasis in animals fed a normal diet 1,13,17 and that it favors testosterone synthesis in Leydig cells of the testis but not in follicular cells of the ovary, because its receptor is expressed in the male but not in the female gonad 18-21. Although low circulating testosterone levels affect several behaviors in male mice 22,23, the poorly defined yet evident passivity was observed in both male and female Osteocalcin-/- mice. Hence, it ruled out that this phenotype was due to a defect in sex steroid hormone synthesis, since this process is not affected by osteocalcin in female mice. Likewise, it became clear very quickly that the passivity of Osteocalcin-/- mice was not secondary to their abnormal glucose metabolism either, since this passivity was not seen in mice lacking Gprc6a, the receptor used by osteocalcin to favor insulin synthesis in pancreatic β-cells 13,24-26. The fact that Gprc6a-/- mice had none of the behavioral abnormalities we will describe has been important throughout this investigation for several reasons. A first one is that it allowed us to uncover a decrease in muscle function during exercise caused by the absence of osteocalcin signaling through Gprc6a in myoblasts 2 and to distinguish this from the passivity caused by the absence of osteocalcin signaling through a different receptor present in the brain. This lack of an obvious explanation for their abnormal passivity led to the suspicion that these phenotypes of the Osteocalcin-/- mice might indicate that osteocalcin normally signals and functions in the brain. What added considerable credence to this notion is that osteocalcin had been shown in the original study of its central function to cross the blood brain barrier since peripheral delivery of uncarboxylated osteocalcin in Osteocalcin-/- mice resulted in an accumulation of osteocalcin in specific regions of the brain, mainly in the midbrain and brainstem 24. In contrast, when delivered peripherally, carboxylated osteocalcin crossed the blood brain barrier far less efficiently 24. This observation led us to a remarkable string of findings.
Osteocalcin's regulation of anxiety and cognition in adult mice
Moving from the entire animal to the molecular level, we observed that adult mice lacking Osteocalcin displayed a substantial increase in anxiety-like behavior compared to wild-type (WT) littermates from a young age (3-months-old), and had a major deficit in learning and memory. Written as such, this does not do justice to the severity of the phenotype observed in Osteocalcin-/- mice of both sexes. Indeed, in the Morris water maze test 27, Osteocalcin-/- mice were simply unable to learn the location of the platform and find it in a timely fashion. This finding identifies osteocalcin as a peripheral hormone exerting one of the strongest influences on spatial learning and memory.
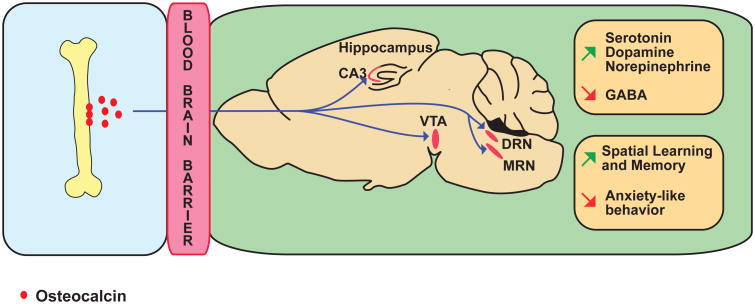
Anatomically, the brains of the Osteocalcin-/- mice are consistently smaller than those of their WT littermates 24. The most overt abnormality however, is seen in the hippocampal region where the area covered by the dentate gyrus is significantly 30% smaller in Osteocalcin-/- than in WT mice, and the corpus callosum, which serves as a bridge between the hippocampi of each hemisphere 28, is often missing in the Osteocalcin-/- mice 24. Biochemically, the content of all monoamine neurotransmitters 29, i.e, serotonin, dopamine and norepinephrine are significantly lower by 20-50% in the midbrain and brainstem of Osteocalcin-/- compared to WT mice. At the same time, the accumulation of GABA, an inhibiting neurotransmitter 30, in the same areas of the brain was increased by 15-30% in Osteocalcin-/- mice. These abnormalities are secondary, at least in part, to transcriptional events since the expression of the genes needed for the synthesis of monoamine neurotransmitters 31-33 is decreased by 15-50%, whereas the expression of two genes necessary for GABA synthesis, Gad1 and Gad2 34, is increased by almost 2-fold in the brainstem of Osteocalcin-/- mice. Even though the receptor for osteocalcin in these neuronal cells was not identified at the time these experiments were performed in 2013, we observed that osteocalcin binds to neurons of the CA3 region of the hippocampus, to neurons of the ventral tegmental area, a nucleus located in the midbrain which is enriched in dopaminergic neurons 35, and to the dorsal and medial raphe that contain serotonergic neurons 36 (Figure 2). Accordingly, in electrophysiological assays, osteocalcin could activate the action potential frequency of neurons by almost 2-fold in the brainstem and locus coeruleus 24.
Figure 2. Osteocalcin promotes spatial learning and memory and prevent anxiety-like behavior.
Osteocalcin is made in bone, uses the general circulation to cross the blood brain barrier (BBB) and then binds neurons from the CA3 region of the hippocampus, the ventral tegmental area (VTA) of the midbrain, and dorsal raphe (DRN) and medial raphe (MRN) nuclei of the brainstem. Osteocalcin acts on these neurons through transcriptional events to increase the synthesis of all monoamines neurotransmitters and decrease that of GABA. Osteocalcin promotes spatial learning and memory and prevents anxiety-like behavior.
Two experiments led to the conclusion that all these abnormalities reflect the absence of osteocalcin signaling in the brains of adult mice. The first one is that the delivery of osteocalcin in the brain through intracerebroventricular infusion, at a dose that does not result in any measurable leakage of osteocalcin in the general circulation, fully corrects anxiety-like behavior and memory phenotypes, as well as gene expression abnormalities. Second, an osteoblast-specific deletion of Osteocalcin performed in adult mice results in the same increased anxiety-like behavior phenotype and in a memory defect, albeit these are less severe than what is observed in Osteocalcin-/- mice. Neurotransmitter contents and gene expression were also significantly affected in mice harboring a post-natal and osteoblast-specific deletion of Osteocalcin. As expected, the only phenotype that could not be recapitulated in mice lacking Osteocalcin only postnatally was developmental, i.e. their hypomorphic hippocampi 15.
Maternal osteocalcin contributes to brain development and the acquisition of cognitive function in the offspring
The normal brain development observed in mice induced to lack Osteocalcin only in osteoblasts and when they reach adulthood, along with their more modest deficit in spatial learning and memory, were two reasons to suspect that osteocalcin could influence the brain during embryonic development. Since one cannot detect any osteocalcin expression in the mouse embryo before E17.5 37,38, this hypothesis implied that maternal osteocalcin might cross the placenta and the blood brain barrier to execute this putative function.
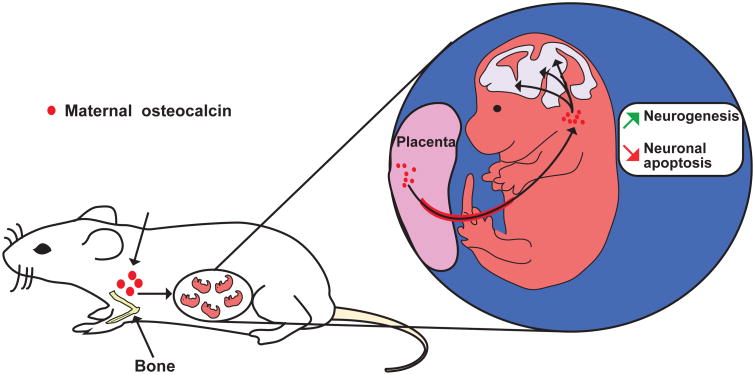
This hypothesis turned out to be correct. Maternal osteocalcin begins to cross the placenta between E14.5 and E15.5 24, at a time when development of structures such as the hippocampus is in full speed 39 (Figure 3). A complicated but efficient breeding strategy could establish unambiguously that virtually all the osteocalcin that one could find in an embryo before E18.5 is of maternal origin. Moreover, we could also show that maternally derived osteocalcin is needed to favor fetal neurogenesis and prevent neuronal apoptosis 24. Maternal osteocalcin is also necessary for the establishment of spatial learning and memory in adult mice. Indeed, delivering uncarboxylated osteocalcin or vehicle to Osteocalcin-/- mothers crossed with Osteocalcin-/- fathers throughout pregnancy, and testing the behavioral performance of their progeny at three months of age showed that maternal osteocalcin contributes to a substantial extent but does not completely explain the deficit in spatial learning and memory that is observed in adult Osteocalcin-/- mice 24.
Figure 3. Maternal osteocalcin acts directly in the offspring to regulate embryonic brain development.
Maternal osteocalcin crosses the placenta, promotes neurogenesis and prevents neuronal apoptosis in the hippocampal region of the offspring.
What did we learn, what did we not learn?
Taken together, this series of observations revealed several important and novel notions about brain physiology and brain development. Moreover, given where osteocalcin is synthesized, taken at face value, these observations also enriched bone physiology. If one looks at brain physiology, these findings showed unambiguously that peripheral hormones, and certainly one made in bone, osteocalcin, exert a rather powerful influence on neuronal activity, gene expression, neurotransmitter synthesis and ultimately behaviors such as anxiety-like behavior, spatial learning and memory. This notion is of fundamental importance, as too often we conceive and study functions of a given organ omitting the fact that this organ receives many, most of them still unknown, inputs from other organs. Underscoring the potential medical influence of this work, two retrospective studies performed in human subjects 40,41 after the initial paper of Oury et al., was published have suggested a correlation between decreased circulating osteocalcin levels and poor cognitive performance in healthy aged individuals. We are fully aware that these studies, because of their retrospective nature, can only be seen as encouraging, and that projective studies are now needed. In terms of brain development and physiology, this work showed that one molecular underpinning of the so-called Barker effect 42,43 (the maternal influence on neuropsychological functions of the offspring) may be a relative lack of maternal osteocalcin. This is important if we consider that the Barker effect also includes the maternal influence on energy metabolism in the offspring, as energy metabolism is one of the main physiological processes regulated by osteocalcin 44-49.
How does one place these data in a broader context of bony vertebrate-specific biology? Two aspects of osteocalcin biology are particularly helpful in that respect. The first one is that when to pre-pubertal mice and human beings, circulating levels of osteocalcin decrease steeply before 9 months of age in the mouse, and before age 30 and age 45 in women and men respectively, without reverting to levels observed during puberty 2. In other words, circulating osteocalcin levels are high during life when cognitive functions are at their peak as well. Importantly for our purpose, this is a specific feature of osteocalcin since other bone specific hormones like lipocalin 2 (data not shown) or sclerostin 50 circulating levels increase with age. A second feature of osteocalcin that is worth mentioning is that osteocalcin circulating levels surge during exercise, a biological event that is also known to improve cognitive function 2,51-55. These findings raise the following interconnected questions: (A) could the decrease in cognitive function that occurs during aging be due at least in part to the plummeting of circulating osteocalcin levels? And (B) could osteocalcin be sufficient to correct age-related cognitive defects?
Besides these biomedical questions, there is also a molecular question that needs to be answered - how does osteocalcin signal in the brain? Indeed, the fact that Gprc6a is not expressed anywhere osteocalcin signals in the brain, and does not mediate osteocalcin signal in the brain, has been initially a huge strength of this project as it allowed ruling out the possibility that central functions of osteocalcin were secondary, even in part, to peripheral ones. Once this point was made, the task remained to understand how osteocalcin signals in the brain, i.e., to identify its receptor in the brain. This could serve several purposes of varying nature. One purpose of course is to achieve a better understanding of how extracellular cues coming from the periphery can affect brain development and functions. A second one is, depending of the pattern of expression of this receptor, to possibly broaden the portfolio of central functions of osteocalcin. A third purpose, as important as the other two, dependent on the nature of this receptor and of its pattern of expression, could be to harness its biology for therapeutic purposes.
Osteocalcin is necessary and sufficient to correct age-related decline of cognitive function
The most fundamental question raised by this first study in the mouse, establishing bone as a regulator of brain development and functions via osteocalcin is to determine how useful these findings could be or could become from a medical point of view, especially in the aging population. This question stems from the severe behavioral phenotype of the Osteocalcin-/- mice and other types of observations. For instance, with the progressive aging of the general population 56-58 in developed countries but eventually all over the world, there is an urgent need to find ways to prevent age-related cognitive decline and other age-related diseases affecting brain functions 59-63. Moreover, it has been shown that for every function regulated by osteocalcin, delivery of exogenous uncarboxylated osteocalcin can improve the age-related decline of these functions 2,24. One reason for this could be that circulating levels of osteocalcin plummet beyond midlife in all species tested 2,64,65. Finally, the aforementioned clinical studies 40,41, which suggested an association between circulating osteocalcin levels and cognitive performance, were another reason to ask whether osteocalcin could be sufficient to restore cognitive functions in older mice. We have begun to address this question in animal models in several ways.
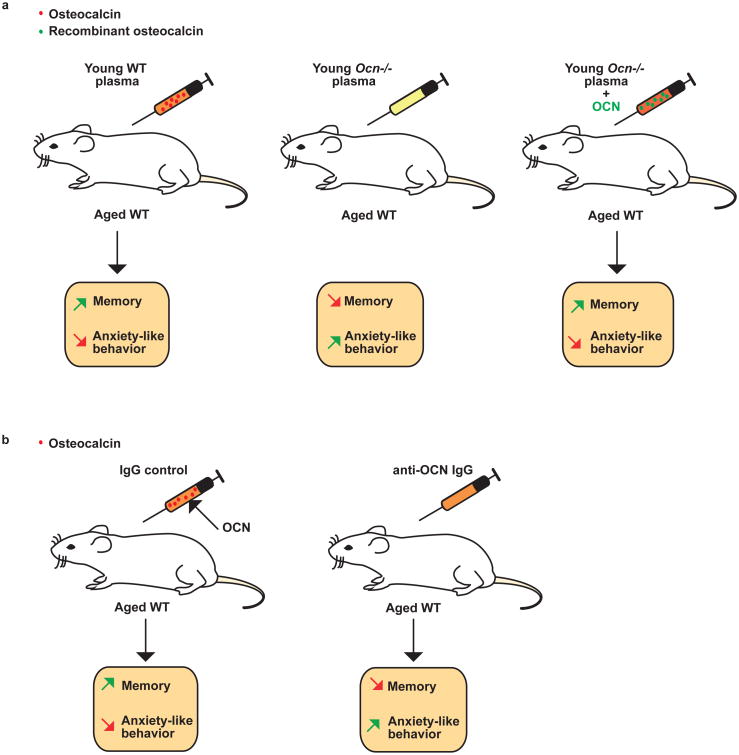
The first one was to use the assay popularized by Villeda et al 66 published in 2014, showing that repeated injections of plasma obtained from 3-month-old mice could improve cognitive function in older mice, i.e in 16-month-old mice. We need to add a precision here, we as well as many other laboratories are able to make mice live beyond 24 months of age in the confined environment of a modern animal facility, and this may be used as a definition of aging. However, in the wild, the definition of aging for a mouse is different as it is dubious that mice beyond 9- or 12-months would have the necessary fitness to survive in their somewhat hostile environment. In any case, after reproducing the results of Villeda et al., we showed that in contrast, injections of plasma from 3-month-old Osteocalcin-/- mice into 16-month-old WT mice could not improve cognitive function or anxiety-like behavior as plasma from 3-month-old WT mice did. There are many possible factors that could explain these results, yet they established on genetic grounds that osteocalcin is necessary for the beneficial effect of plasma from young mice on several brain functions in older mice. To rule out that this lack of efficacy of plasma from young Osteocalcin-/- mice in improving the behavioral performance of older mice represented a developmental effect, and to link osteocalcin itself to the beneficial effect of plasma from young WT mice, older mice were injected with plasma from young Osteocalcin-/- mice, which recombinant uncarboxylated osteocalcin had been added (“spiked plasma”) (Figure 4a). That this spiked plasma could improve cognitive function and decrease anxiety-like behavior in older WT mice to the same extend as plasma from young WT mice, was a strong indication that osteocalcin was not only necessary but also sufficient to improve cognitive function and to hamper anxiety-like behavior in adult mice. In a mirror image experiment, depleting every form of osteocalcin from the plasma of young WT mice though immunological means, removed from WT plasma its ability to improve cognitive functions and to decrease anxiety-like behavior 15 (Figure 4b).
Figure 4. Osteocalcin is sufficient to improve cognition in aged mice.
(a) Injections of plasma from young wild-type (WT) mice to aged WT mice improve memory performance and prevent anxiety-like behavior. This does not occur when plasma from young Osteocalcin-/- (Ocn-/-) mice is used instead of plasma from young WT mice. When Ocn-/- plasma is spiked with mouse recombinant osteocalcin, the ability of this plasma to improve cognitive functions is restored to same extend as when using plasma from young WT mice. (b) Plasma from young WT mice immunodepleted of osteocalcin cannot enhance cognition in aged mice.
The most direct evidence that osteocalcin is sufficient to rescue age-related cognitive decline and to decrease anxiety-like behavior, independently of any developmental effect, came from the following experiment. Since osteocalcin crosses the blood brain barrier 24 we implanted subcutaneously mini osmotic pumps delivering uncarboxylated osteocalcin or vehicle for 60 days in 10- or 14-month-old mice. Vehicle- and osteocalcin-treated were analyzed when they were 12-or 16-month-old. What was observed is that chronic delivery of uncarboxylated osteocalcin in 10-or 14-month-old mice fully rescued their deficit in cognitive function and decreased their anxiety-like behavior 15.
In exploring the molecular bases of this remarkable ability of osteocalcin to rescue age related cognitive decline, several lines of experimental evidence pointed toward brain-derived neurotrophic factor (BDNF) a molecule well known to favor hippocampal dependent memory 55,67-70, as a critical mediator of osteocalcin's regulation of cognitive function. The first was observed when monitoring multiple parameters of the transport of BDNF-containing dense-core vesicles in embryonic rat neurons in microchambers 71. In this setting, uncarboxylated osteocalcin was able to stimulate the dynamic of BDNF vesicle transport to synapse in rat neurons. This translated in an increase in velocity and in the number of BDNF vesicles moving to the synapses. In cell culture, osteocalcin treatment of primary hippocampal neurons with osteocalcin stimulated Bdnf expression. A second piece of evidence supported in vivo that BDNF is a mediator of osteocalcin in the brain. Stereotaxic injection of uncarboxylated osteocalcin in the anterior hippocampi could increase BDNF accumulation in the hippocampus. Lastly, peripheral injections of uncarboxylated osteocalcin increase Bdnf expression in hippocampi of 16-month-old mice. Similar to this, injection of plasma from 3-month-old mice to 16-month-old mice increased BDNF accumulation in the hippocampi of 16-month-old mice. This beneficial effect of young plasma on BDNF accumulation was abolished when plasma from 3-months-old Osteocalcin-/- mice was injected to 16-months-old WT mice 15.
Identification of a receptor mediating osteocalcin's regulation of cognitive function and anxiety-like behavior
The fact that osteocalcin is not only necessary but sufficient to improve cognitive decline at any age but even more in aged mice, and to reduce anxiety-like behavior, made it imperative to identify and characterize a receptor mediating the functions of this hormone in the brain. To tackle this problem, and in trying to avoid a long and often dangerous fishing expedition, three criteria were used to identify what could be a candidate receptor of osteocalcin in the brain. First, based on what has been observed in many fields, we posited that a new receptor for osteocalcin should be a G-coupled receptor (GPCR) that should belong to the same class as Gprc6a, the class C family 72,73. This criterion, because the class C orphan GPCR list is rather small 74, considerably reduced the size of the pools one had to explore. A second criterion was that this orphan GPCR should be expressed in an area where it had been previously shown that osteocalcin binds. The third criterion was that this orphan GPCR should not be expressed in any of the cell types where Gprc6a mediates osteocalcin's signaling 2,13,17,18. When these three criteria were used as a filter, only one orphan class C GPCR fulfilled all of them: Gpr158. It remained then to demonstrate that Gpr158 was the actual receptor of osteocalcin.
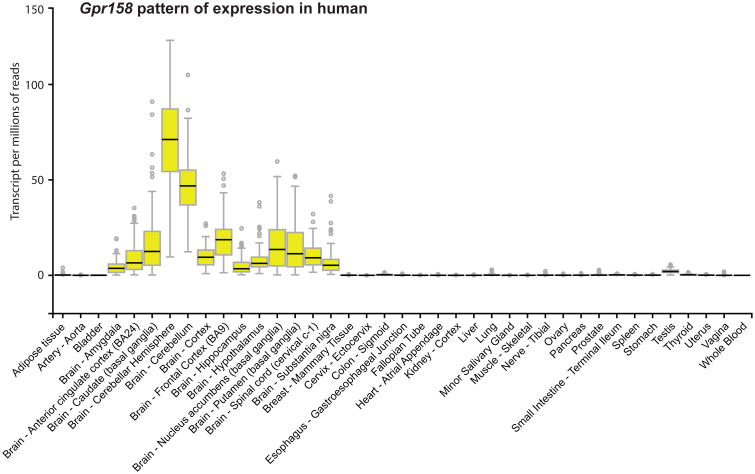
Gpr158's pattern of expression was certainly consistent with this notion since it is expressed in the CA3 region of the hippocampus where osteocalcin binds; in this area of the brain, Gpr158 is expressed in pyramidal neurons and not in astrocytes. The fact that Gpr158 was more abundant in Osteocalcin-/- than WT hippocampi was a further suggestion that it could be a receptor of osteocalcin in the hippocampus. Like Gprc6a, Gpr158 is expressed in humans in the same structure and regions where it is expressed in the mouse 75,76 (Figure 5). All the other evidences that were gathered in support of this hypothesis were of genetic nature and were obtained either ex vivo or in vivo 15.
Figure 5. Gpr158 pattern of expression in human tissues.
Gpr158's pattern of expression obtained from the GTEX database (www.gtexportal.org).
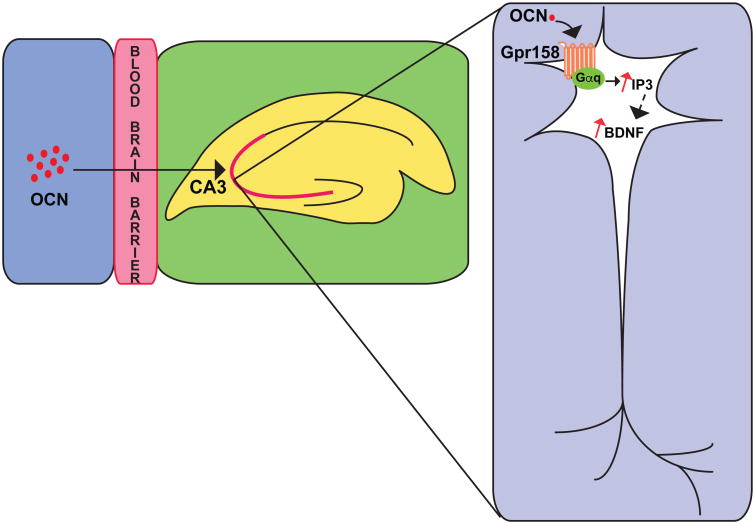
For instance, local delivery of osteocalcin in the anterior hippocampus induced c-Fos accumulation, a marker of neuronal activity 77,78, in neurons of the CA3 region in WT mice but not in Gpr158-/- mice. Osteocalcin could bind a complex containing Gpr158 and the Gαq protein in solubilized membranes obtained from Osteocalcin-/- hippocampi, whereas the quantity of pulled down by osteocalcin was considerably reduced when using solubilized membranes obtained from Gpr158-/- hippocampi. In agreement with this observation, osteocalcin increased the accumulation of IP3, a second messenger used by GPCR 79,80, in WT but not Gpr158-/- hippocampal neuron whereas it consistently failed to increase cAMP accumulation in hippocampal neuron. In electrophysiological assays, in whole-cell current clamp recording osteocalcin enhanced action potential frequency in pyramidal neurons of the CA3 region of the hippocampus of WT but not of Gpr158-/- hippocampi, this effect of osteocalcin was nearly abolished when using an IP3 receptor antagonist (Figure 6). An analysis of long term potentiation 81,82 (LTP) verified that osteocalcin signals in WT but not in Gpr158-/- hippocampal slices 15.
Figure 6. Gpr158 mediates osteocalcin's functions in the brain.
Osteocalcin binds Gpr158 in pyramidal neurons of the CA3 region of the hippocampus. This interaction promotes an increase in the accumulation of IP3 and BDNF in neurons.
In vivo, many arguments of genetic nature also indicated that Gpr158 is a receptor for osteocalcin in the brain. For instance, whether we analyzed mice lacking Gpr158 only in the forebrain or in all cells we observed the same severe deficit in hippocampal-dependent memory 83 in mice lacking this receptor 15. Likewise, Gpr158-/- mice manifested more severe anxiety-like behavior than WT littermates. That neither cognitive deficit nor anxiety-like behavior could be corrected by chronic delivery of osteocalcin in Gpr158-/- mice established that Gpr158 is necessary to mediate osteocalcin's regulation of cognitive function and anxiety-like behavior. Two additional lines of evidence, of genetic nature, demonstrated that it is indeed the case. The first was to show that stereotaxic injection of osteocalcin in the anterior hippocampi could improve hippocampal dependent memory in WT mice but not in mice in which Gpr158 accumulation has been decreased by a small hairpin RNA interference strategy 84. Second, compound heterozygous mice lacking one allele of Gpr158 and one allele of Osteocalcin (Gpr158+/-; Osteocalcin+/-) demonstrated the same cognitive deficit as that observed in Gpr158-/- or in Osteocalcin-/- mice whereas single heterozygous mice behaved like WT mice 15.
Independently of this body of work that identified Gpr158 as a receptor mediating osteocalcin regulation of cognitive function and anxiety-like behavior, several other important observations were made in the course of this investigation. One is that, as we had long surmised, osteocalcin function in the brain may not be limited to the hippocampus and the ventral tegmental area of the midbrain. Indeed, a systemic analysis of Gpr158 expression showed that it is also expressed in the somatosensory area, motor and auditory areas of the cortex, the piriform cortex and the retrosplenial area, where it was not known that osteocalcin binds. A second more surprising observation that needs to be interpreted cautiously, is that Gpr158 is apparently not expressed in serotonergic neurons of the dorsal and median raphe of the brainstem where osteocalcin binds 15. This suggests the possibility that a third receptor for osteocalcin may exist.
Conclusions
What was illustrated through this work is a classic and fundamental principle of physiology; no organ, as noble as it may be, is an island that develops and functions at the exclusion of other organs. In our current era of biology, we have developed the tools to explore molecular events so effectively, and yet mouse genetics has given us the power to uncover broader physiological phenomena that have remained hidden for so long. What we did not know and could not have anticipated a priori is that the skeleton, of all organs, would secrete a hormone that exerts such a powerful influence on the brain in the mouse and it seems from retrospective studies probably in humans. Here, one should focus first and foremost on the salient features of osteocalcin and how it could help medicine. it is impossible to address these questions without considering the natural history of osteocalcin, whose circulating levels decrease around mid-life in all species tested. This is a fundamental feature of osteocalcin in view of the fact that this hormone is necessary to maintain at an optimum level cognitive function and to decrease anxiety-like behavior. Maybe more importantly from a medical point of view is the notion that osteocalcin is necessary and sufficient to correct age-related cognitive defects and to hamper anxiety-like behavior. Together these features identify osteocalcin as an anti-geronic hormone that joins TIMP2 85 on the list of anti-geronic molecules. Having identified a receptor for this hormone in the brain now raises the prospect that this interaction between osteocalcin and Gpr158 could be used to treat various age-related degenerative conditions affecting the brain. This may even be more important going forward since the pattern of expression of Gpr158 suggests that we are not yet aware of all the functions of osteocalcin in the brain.
The physiology and aging of the brain is only one side of the coin. The other aspect one has to recognize is that osteocalcin is one of the first, if not the first, molecular substratum for the long-suspected maternal influence on brain development. How important the role of osteocalcin is in brain development is not yet fully known, in part because we do not know all the functions of osteocalcin in the brain, and in part because we also do not know the pattern of expression of Gpr158 in the developing brain. These notions, and the possibility that a third receptor for osteocalcin may exist, represent the most concrete challenges in front of us.
Acknowledgments
This work was supported by 2P01 AG032959-06A1 and the Columbia Aging Center (G.K.), a 5T32DK007328-38 Endocrinology Training Grant (L.K.) from the NIH, Fondation pour la Recherche Medicale grant AJE20130928594, the Human Frontier Scientific Program–Grant ATIP-AVENIR INSERM - R14080KS - RSE15007KSA Program–INSERM, Grant AGEMED-INSERM (F.O.) and the Philippe Foundation (A.O.).
References
- 1.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mera P, et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016;23:1078–1092. doi: 10.1016/j.cmet.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das SK, Sharma NK, Elbein SC. Analysis of osteocalcin as a candidate gene for type 2 diabetes (T2D) and intermediate traits in Caucasians and African Americans. Dis Markers. 2010;28:281–286. doi: 10.3233/DMA-2010-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Toni L, et al. Polymorphism rs2274911 of GPRC6A as a Novel Risk Factor for Testis Failure. J Clin Endocrinol Metab. 2016;101:953–961. doi: 10.1210/jc.2015-3967. [DOI] [PubMed] [Google Scholar]
- 5.De Toni L, et al. Osteocalcin and Sex Hormone Binding Globulin Compete on a Specific Binding Site of GPRC6A. Endocrinology. 2016;157:4473–4486. doi: 10.1210/en.2016-1312. [DOI] [PubMed] [Google Scholar]
- 6.Di Nisio A, et al. The rs2274911 polymorphism in GPRC6A gene is associated with insulin resistance in normal weight and obese subjects. Clin Endocrinol (Oxf) 2017;86:185–191. doi: 10.1111/cen.13248. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, et al. The PLC/PKC/Ras/MEK/Kv channel pathway is involved in uncarboxylated osteocalcin-regulated insulin secretion in rats. Peptides. 2016;86:72–79. doi: 10.1016/j.peptides.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, et al. Inhibition of voltage-gated potassium channels mediates uncarboxylated osteocalcin-regulated insulin secretion in rat pancreatic beta cells. Eur J Pharmacol. 2016;777:41–48. doi: 10.1016/j.ejphar.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 9.Korostishevsky M, et al. Significant association between body composition phenotypes and the osteocalcin genomic region in normative human population. Bone. 2012;51:688–694. doi: 10.1016/j.bone.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kover K, et al. Osteocalcin protects pancreatic beta cell function and survival under high glucose conditions. Biochem Biophys Res Commun. 2015;462:21–26. doi: 10.1016/j.bbrc.2015.04.095. [DOI] [PubMed] [Google Scholar]
- 11.Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol. 2015;30:599–614. doi: 10.1007/s10654-015-0058-x. [DOI] [PubMed] [Google Scholar]
- 12.Sabek OM, et al. Osteocalcin Effect on Human beta-Cells Mass and Function. Endocrinology. 2015;156:3137–3146. doi: 10.1210/EN.2015-1143. [DOI] [PubMed] [Google Scholar]
- 13.Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014;63:1021–1031. doi: 10.2337/db13-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karsenty G, Olson EN. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell. 2016;164:1248–1256. doi: 10.1016/j.cell.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khrimian L, Obri A, Ramos-Brossier M, Rousseaud A, Moriceau S, Nicot A, Mera P, Kosmidis S, Karnavas T, Saudou F, Gao XB, Oury F, Kandel E, Karsenty G. Gpr158 mediates osteocalcin's regulation of cognition. Journal of Experimental Medicine. 2017 doi: 10.1084/jem.20171320. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 17.Ferron M, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oury F, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamouni A, Oury F. Reciprocal interaction between bone and gonads. Arch Biochem Biophys. 2014;561:147–153. doi: 10.1016/j.abb.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Oury F, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123:2421–2433. doi: 10.1172/JCI65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikawa Y, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Miner Res. 2011;26:2012–2025. doi: 10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008;33:1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 24.Oury F, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pi M, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PloS one. 2008;3:e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–1683. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valenstein E, et al. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- 29.Törk I, Tracey DJ, Paxinos G, Stone J. Neurotransmitters in the human brain. Plenum Press; 1995. [Google Scholar]
- 30.Ende G. Proton Magnetic Resonance Spectroscopy: Relevance of Glutamate and GABA to Neuropsychology. Neuropsychol Rev. 2015;25:315–325. doi: 10.1007/s11065-015-9295-8. [DOI] [PubMed] [Google Scholar]
- 31.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Lasagna M, Daubner SC, Reinhart GD, Fitzpatrick PF. Fluorescence spectroscopy as a probe of the effect of phosphorylation at serine 40 of tyrosine hydroxylase on the conformation of its regulatory domain. Biochemistry. 2011;50:2364–2370. doi: 10.1021/bi101844p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 34.Tochitani S, Kondo S. Immunoreactivity for GABA, GAD65, GAD67 and Bestrophin-1 in the Meninges and the Choroid Plexus: Implications for Non-Neuronal Sources for GABA in the Developing Mouse Brain. PloS one. 2013;8:e56901. doi: 10.1371/journal.pone.0056901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- 36.Lira A, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biological psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 37.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 38.Wei J, et al. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell. 2015;161:1576–1591. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tole S, Christian C, Grove EA. Early specification and autonomous development of cortical fields in the mouse hippocampus. Development. 1997;124:4959–4970. doi: 10.1242/dev.124.24.4959. [DOI] [PubMed] [Google Scholar]
- 40.Bradburn S, et al. Association between osteocalcin and cognitive performance in healthy older adults. Age Ageing. 2016;45:844–849. doi: 10.1093/ageing/afw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puig J, et al. Lower serum osteocalcin concentrations are associated with brain microstructural changes and worse cognitive performance. Clin Endocrinol (Oxf) 2016;84:756–763. doi: 10.1111/cen.12954. [DOI] [PubMed] [Google Scholar]
- 42.Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 43.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 44.Broad KD, Keverne EB. Placental protection of the fetal brain during short-term food deprivation. Proc Natl Acad Sci U S A. 2011;108:15237–15241. doi: 10.1073/pnas.1106022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Challis JR. Endocrine disorders in pregnancy: Stress responses in children after maternal glucocorticoids. Nature reviews Endocrinology. 2012;8:629–630. doi: 10.1038/nrendo.2012.183. [DOI] [PubMed] [Google Scholar]
- 46.Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity--a determinant of perinatal and offspring outcomes? Nature reviews Endocrinology. 2012;8:679–688. doi: 10.1038/nrendo.2012.176. [DOI] [PubMed] [Google Scholar]
- 47.Osorio J. Endocrine disorders in pregnancy: Excessive maternal weight increases risk of infant overgrowth. Nature reviews Endocrinology. 2012;8:624. doi: 10.1038/nrendo.2012.169. [DOI] [PubMed] [Google Scholar]
- 48.Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Progress in brain research. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- 50.Modder UI, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 52.Levinger I, et al. The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22:1621–1626. doi: 10.1007/s00198-010-1370-7. [DOI] [PubMed] [Google Scholar]
- 53.Schwab P, Scalapino K. Exercise for bone health: rationale and prescription. Curr Opin Rheumatol. 2011;23:137–141. doi: 10.1097/BOR.0b013e3283434501. [DOI] [PubMed] [Google Scholar]
- 54.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005:25, 8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scahill RI, et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 57.Dall TM, et al. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff (Millwood) 2013;32:2013–2020. doi: 10.1377/hlthaff.2013.0714. [DOI] [PubMed] [Google Scholar]
- 58.Grady PA. Advancing the health of our aging population: a lead role for nursing science. Nurs Outlook. 2011;59:207–209. doi: 10.1016/j.outlook.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finch CE. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107 Suppl 1:1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramer AF, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 61.La Rue A. Memory loss and aging. Distinguishing dementia from benign senescent forgetfulness and depressive pseudodementia. Psychiatr Clin North Am. 1982;5:89–103. [PubMed] [Google Scholar]
- 62.Small SA. Age-related memory decline: current concepts and future directions. Arch Neurol. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- 63.Pavlopoulos E, et al. Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Sci Transl Med. 2013;5:200ra115. doi: 10.1126/scitranslmed.3006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiappe A, et al. Influence of age and sex in serum osteocalcin levels in thoroughbred horses. Arch Physiol Biochem. 1999;107:50–54. doi: 10.1076/apab.107.1.50.4357. [DOI] [PubMed] [Google Scholar]
- 65.Price PA, Nishimoto SK. Radioimmunoassay for the vitamin K-dependent protein of bone and its discovery in plasma. Proc Natl Acad Sci U S A. 1980;77:2234–2238. doi: 10.1073/pnas.77.4.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villeda SA, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 68.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 69.Dean C, et al. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci. 2009;12:767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao J, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zala D, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 72.Chun L, Zhang WH, Liu JF. Structure and ligand recognition of class C GPCRs. Acta Pharmacol Sin. 2012;33:312–323. doi: 10.1038/aps.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rondard P, Goudet C, Kniazeff J, Pin JP, Prezeau L. The complexity of their activation mechanism opens new possibilities for the modulation of mGlu and GABAB class C G protein-coupled receptors. Neuropharmacology. 2011;60:82–92. doi: 10.1016/j.neuropharm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 74.Brauner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8:169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- 75.Luo J, Liu Z, Liu J, Eugene CY. Distribution pattern of GPRC6A mRNA in mouse tissue by in situ hybridization. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:1–10. doi: 10.3969/j.issn.1672-7347.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Wellendorph P, Brauner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Dragunow M, Robertson HA. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987;329:441–442. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- 78.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 79.Burgess GM, et al. The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984;309:63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- 80.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 81.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 82.Wigstrom H, Gustafsson B. On long-lasting potentiation in the hippocampus: a proposed mechanism for its dependence on coincident pre- and postsynaptic activity. Acta Physiol Scand. 1985;123:519–522. doi: 10.1111/j.1748-1716.1985.tb07621.x. [DOI] [PubMed] [Google Scholar]
- 83.Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 84.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 85.Castellano JM, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544:488–492. doi: 10.1038/nature22067. [DOI] [PMC free article] [PubMed] [Google Scholar]