Supplemental digital content is available in the text.
Key words/Abbreviations: aging, childhood trauma, coronary artery disease, hair cortisol, perceived stress, sex differences, CAD = coronary artery disease, CTQ = Childhood Trauma Questionnaire, CVD = cardiovascular diseases, HCC = hair cortisol concentrations, HPA = hypothalamic-pituitary-adrenal, MHI = Montreal Heart Institute, PSQ = Perceived Stress Questionnaire
ABSTRACT
Objective
Childhood trauma has been associated with greater psychological and physical morbidity, including a greater risk of developing cardiovascular disease (CVD). This may partially reflect trauma-induced disturbances in how stress is later perceived and regulated. This study evaluated the associations of childhood trauma with perceived stress and hair cortisol concentrations (HCC) in a large sample of adults with coronary artery disease (CAD) and in non-CVD patients experiencing other nonfatal illnesses. Whether sex, age, or CVD status influenced these associations was also examined.
Methods
A total of 1124 men and women (aged 65.2 [6.9] years) recruited from a hospital cohort completed the Childhood Trauma and Perceived Stress Questionnaires, whereas hair samples were obtained from 598 participants. Health status was confirmed via medical records.
Results
Moderate to severe childhood trauma was experienced by 359 participants. Childhood trauma was associated with greater perceived stress levels for the past 2 years (r = .308, p = .01; β = 0.263, p < .001), but not 3-month cortisol secretion in hair. Perceived stress correlated negatively with age (r = −.241, p < .001). In secondary analyses, age moderated the relation between sexual abuse and perceived stress (β = −0.067, p = .016). Although sexual abuse was associated with greater levels of perceived stress among all participants, this relation was strongest in younger individuals.
Conclusions
Participants who experienced trauma in their youth reported greater levels of perceived stress, but not HCC, in late adulthood. Whether this suggests intact hypothalamic-pituitary-adrenal regulation in those exposed to childhood trauma or whether this reflects the characteristics of our sample requires further investigation.
INTRODUCTION
Stressful childhood experiences, including physical, emotional, and sexual abuse, as well as physical and emotional neglect (1,2), may have important implications for later health. Indeed, there is growing evidence that exposure to childhood trauma may contribute to psychiatric (e.g., mood disorders, anxiety disorders) and physical (e.g., autoimmune disorders, peptic ulcers) health problems in adulthood (3,4), including the development and progression of cardiovascular disease (CVD) (3,5–10). These effects may be mediated through trauma-induced influences on stress perception and regulation. Exposed children may develop difficulties in dealing with stressors later in adulthood (11), perhaps as a result of maladaptive coping strategies (12). Indeed, studies have confirmed high levels of perceived stress in adults who experienced trauma in childhood (11,13–15).
Childhood trauma may also influence brain development, particularly in the hippocampus and amygdala, known to play an important role in emotion regulation, memory, and stress response (16–19). For example, adults who experienced childhood trauma have been found to present smaller hippocampal and amygdala volumes (20,21). Alterations in neurobiological systems, such as the hypothalamic-pituitary-adrenal (HPA) axis, may ensue (22) and predispose to psychiatric vulnerability and health problems (16,23). However, studies on associations between childhood trauma and HPA-axis regulation present inconsistent findings (24). A few investigations have reported a flattened morning cortisol response in middle-aged or older adults who experienced trauma in childhood (25,26). However, Klaassens (27) reported no difference among a small sample of women. Stress-reactivity studies have similarly led to inconsistent findings. Whereas hyporeactivity of the cortisol response to stress has been observed in young to middle-aged adults who experienced childhood trauma (27,28), three other investigations have reported hyperreactivity in similarly aged individuals (29–31). DeSantis et al. (32) and Otte et al. (33), for their part, observed no relation between childhood trauma and cortisol response in young and middle-aged adults. Whether methodological differences in the assessment of childhood trauma, in participant characteristics, in the stress induction protocols, and in cortisol measurements (plasma, saliva), are responsible for these inconsistent findings is unclear (34,35).
Although interesting, studies on salivary or plasma cortisol reflect the present state rather than long-term regulation of the HPA-axis activity (36). Commonly assessed measures, such as the cortisol awakening response, are also sensitive to situational influences and/or state-related confounding that can cause spurious results (37), which may have contributed to the inconsistent findings mentioned previously.
Information on long-term cortisol regulation may provide greater explanatory power for the association between childhood experiences and disease development. According to the allostatic load model, for example, it is chronic or repeated activation of the HPA axis and other physiological systems that leads to biological damage and deleterious health outcomes (38,39). Seeman et al. (40) suggested that cumulative exposure to stressful situations (e.g., childhood traumas) may render the HPA axis less resilient to stimulation, which can result in prolonged activation (41). There is increasing support for the notion that hair cortisol concentrations (HCC) reflect long-term cumulative cortisol secretion for periods of several months (42,43) and provide a stable and robust biomarker in trauma and chronic stress-related research (41,44,45). To our knowledge, only three small studies have examined the relationship between childhood trauma and cumulative cortisol secretion in adulthood, and these have been performed in relatively young samples. Whereas the experience of childhood trauma was found to be associated with lower HCC in a sample of 84 middle-aged adults (46), the opposite pattern was found in a sample of 43 women (mean age = 35 years) (47). A third study by Steudte et al. (48) did not reveal a specific influence of childhood trauma on HCC in their adult sample of healthy individuals, traumatized healthy controls and patients with posttraumatic stress disorder (mean age = 39 years).
The purpose of this study was thus to evaluate the associations of childhood trauma with 3-month cumulative HCC and stress perceived for the last 2 years in older adults with coronary artery disease (CAD) and those with other (non-CVD) illnesses. This is the first study to provide information on the association of childhood traumatic experiences with both physiological and subjective stress. It was hypothesized that childhood trauma would be associated with greater perceived stress and attenuated HCC. Individual differences in relations between childhood trauma and stress as a function of age, sex, and CVD status were also explored. Stress and stress responses have been shown to contribute to CVD development and progression (49–52) and seem to differ as a function of sex (53–56) and age (55–57). Importantly, limited data suggest that the effect of trauma on stress outcome (cortisol levels) was amplified with advancing age (58) and observed mostly in men (26,29). Because previous studies exploring the effect of trauma on stress perception were generally small (11,14,15) or included only women (11,15), the possible influence of sex or age on this relationship is unknown. Given the paucity of data on individual differences in the relations between childhood trauma and stress, no specific hypotheses were emitted. Secondary analyses examined whether specific elements of childhood trauma (physical, sexual, and emotional abuse and/or neglect) seemed more deleterious to stress regulation.
METHODS AND MATERIALS
This study is part of an ongoing prospective research project (BEL-AGE) on pathological aging in adults with CAD and non-CVD patients experiencing other nonfatal illnesses. This study was approved by Le comité d'éthique de la recherche et du développement des nouvelles technologies de l'Institut de Cardiologie de Montréal. Recruitment for this study began September 2012 and finished in May 2016.
A total of 1124 participants (707 men, 65.23 [6.89] years) with and without CAD were recruited from the pool of individuals already participating in the André and France Desmarais Hospital Cohort of the Montreal Heart Institute (59,60). Any person working at or attending the Montreal Heart Institute (MHI) for any reason can be participants of the MHI Biobank. As such, they may include persons simply going for routine blood tests, patients with or at risk for heart disease, their family members, or employees of the MHI. Eligibility criteria for BEL-AGE were determined as follows: at entry in the MHI Cohort, (a) age between 30 and 70 years (for reasons of feasibility of recruitment and follow-up), (b) living in the greater Montreal area, (c) speaks French or English, (d) no previous or current diagnosis of major cognitive impairment or serious psychological disorders (e.g. bipolar disorder, schizophrenia, delirium, or dementia as reported by patient and/or medical files) with the potential to prevent understanding or participation in all aspects of the study, (e) no previous or current diagnosis of other major life-threatening diseases (e.g., Creutzfeldt-Jakob disease, amyotrophic lateral sclerosis, AIDS, cancer), and (f) women were not pregnant or breast feeding. Skin cancer (n = 29) was not excluded given its high prevalence and benign course when diagnosed early. Depression, anxiety, and endocrine disorders were not excluded.
Presence of CAD was defined by the experience of a previous myocardial infarction, coronary artery bypass, coronary angioplasty, or stenosis more than 50% on an angiography. Non-CVD status was defined by the absence of CAD, angina, arrhythmia, congenital heart disease, heart failure, cardiomyopathy, and stroke. The number and types of illnesses experienced by the non-CVD group were very diverse (e.g., arthritis, diabetes, high blood pressure, gastroesophageal reflux, asthma, eczema, etc.). Those few individuals in the overall sample who were very healthy (n = 54) were excluded from current analyses given vast differences across sociodemographic, psychological, and medical variables from both groups. Data for the article were obtained during the BEL-AGE evaluation performed 59.1 (8.99) months after participants' entry into the MHI cohort.
Eligible participants were contacted by telephone and scheduled for an appointment on a weekday between 8:00 am to 10:00 am at the MHI. They were asked to refrain from eating, drinking (except water), smoking, and exercising 12 hours before the appointment and to abstain from alcohol or recreational drugs for 24 hours. They were allowed to take their medication as prescribed. Once consent was obtained, a blood draw was performed, and waist circumference, weight, and height were measured. Participants completed demographic, life-style, medical, and psychological questionnaires.
Measures
Sociodemographic and Health Information
Data on sex, age, ethnicity, weight, height, waist circumference, years of schooling, marital status, personal and family income, and personal and family medical history were collected. Data on behavioral risk factors (tobacco, alcohol, caffeine consumption, diet, and physical activity) were obtained using the WHO STEPS Instrument (61).
Childhood trauma was assessed with the Childhood Trauma Questionnaire (CTQ) (2,62). This 25-item questionnaire evaluates childhood and adolescent trauma experiences using a five-point scale ranging from “never true” to “very often true.” The CTQ comprises the following subscales: physical, sexual, and emotional abuse and physical and emotional neglect. Participants are classified as positive for history of childhood trauma when they have scores within the moderate-to-severe range on at least one subscale: emotional abuse (≥13), physical abuse (≥10), sexual abuse (≥8), emotional neglect (≥15), and physical neglect (≥10). This instrument has acceptable to excellent internal consistency for the different subscales (0.68–0.94) and test-retest reliability coefficients that range from 0.79 to 0.86 for a 4-month period (62,63). In the current sample, internal consistency of the overall scale was α value of 0.91, whereas the internal consistency for the different subscales ranged from α value of 0.57 (physical neglect), 0.81 (physical abuse), and 0.83 (emotional abuse, sexual abuse, emotional neglect). The low internal consistency for the physical neglect subscale in the current study is consistent with that of previous studies (2,62,63).
Perceived stress was measured with the Levenstein Perceived Stress Questionnaire (PSQ) (64). This 30-item questionnaire is composed of the following seven dimensions: harassment, overload, irritability, lack of joy, fatigue, worries, and tension. Participants indicate on a four-point scale from “almost never” to “usually” how frequently they experienced each of the stress-related statements for the last 2 years. The sum of all items is added in a total PSQ index (raw score − 30/90), which represents the stress degree of each participant. The cut-off score for moderate level is between 0.45 and 0.60 and for high level more than 0.60. The PSQ has a test-retest reliability of 0.82 and an internal consistency of 0.90 (64,65). In the current sample, the internal consistency was α value of 0.94.
Cumulative HCCs
Because of insufficient hair length for some individuals, hair strands were obtained from only 598 participants. They were cut from the posterior vertex region of the head closest to the scalp and placed separately in aluminum foil with a mark indicating the scalp-near end. They were stored at room temperature in a dark place and later analyzed in the laboratory of TU Dresden, Germany (Prof. Clemens Kirschbaum). Analyses were performed on the proximal 3-cm hair segment that, based on hair growth rate of approximately 1 cm per month (66), is assumed to reflect cumulative cortisol secretion over the 3-month period before hair sampling. Wash and cortisol extraction procedures followed a protocol described elsewhere (67); study II), with 7.5 mg of whole, nonpulverized hair being washed with 2.5 ml of isopropanol and steroid extraction being conducted with 1800 μl of methanol for 18 hours at 45°C. Cortisol determination was performed in triplicate using a commercially available immunoassay with chemiluminescence detection (CLIA, IBL-Hamburg, Germany). The intra- and interassay coefficient of variance of this assay has been shown to be less than 8%. Differences across medical, sociodemographic, behavioral, and psychological variables were observed between those for whom we did and did not obtain samples (See Supplemental Digital Content 1, http://links.lww.com/PSYMED/A445).
Statistical Analyses
Preliminary Analyses
Twelve participants presented HCC greater than 3 SD from the mean. Although these HCC were accurate (as determined by redoing the assay), they were winsorized to 3 SD to reduce their impact in parametric statistical analyses. Natural logarithmic transformations were then applied to HCC and BMI data and square root transformation to childhood trauma data. Potential covariates were identified from the literature on perceived stress levels (14,68) and HCC (41,69,70). Pearson correlations and χ2 analyses were performed to finalize our list of demographic, medical, and health behavior covariates. Only those covariates that reached a p value of .10 with the dependent variables were retained, as they were most likely to confound results.
One-way analyses of variance were performed to examine whether childhood trauma, perceived stress level, and HCC differed significantly as a function of sex and health status. Pearson correlations between these variables and age were also performed.
Main Analyses
Associations of childhood trauma with perceived stress and HCC were examined using Pearson correlations and two hierarchical multiple regressions. Analyses were performed on continuous measures. Covariates were forced into block 1. Childhood trauma was forced into block 2 and its two- and three way interactions with age and sex were entered stepwise in block 3. The analyses were repeated for each childhood trauma subscale to determine whether specific types of trauma were more significantly associated with the outcomes of interest. When there were significant interaction effects, simple slopes analyses were performed with lower and higher estimates for age and childhood trauma based on values ± 1 SD from the mean (71). Statistical significance was set at a p value of less than .05.
RESULTS
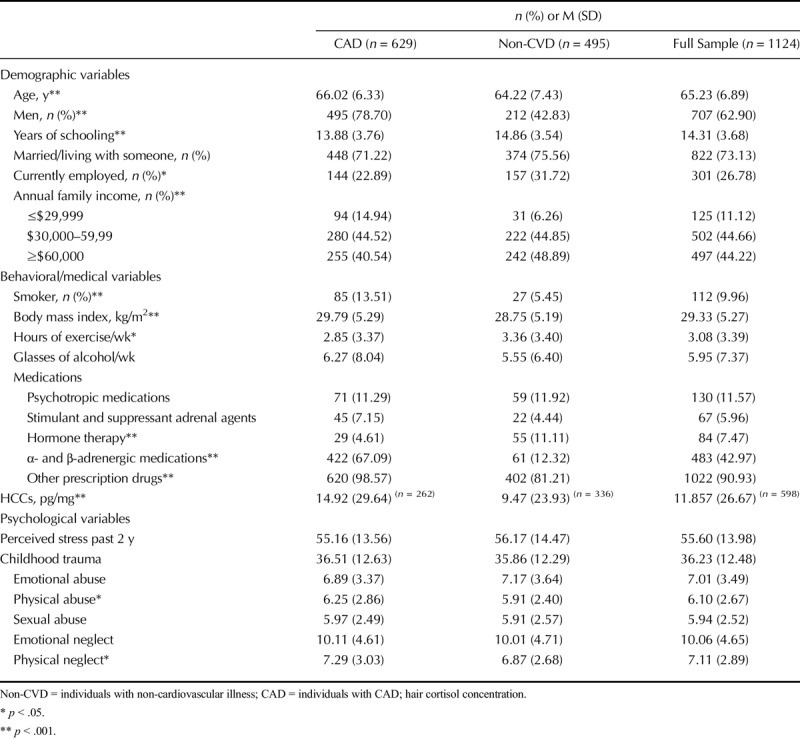
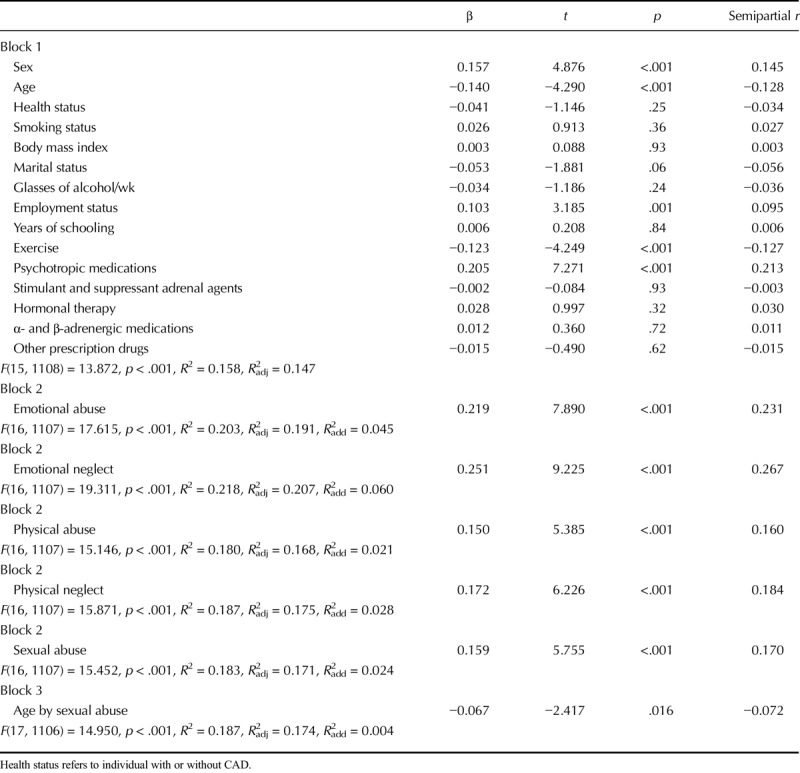
Table 1 presents the sample characteristics. The CAD participants were more likely to be male, slightly older, more overweight, and smokers compared with participants with non-CVD illness. Years of schooling, annual family income, and time spent exercising were somewhat greater in those with non-CAD illness. Whereas hormone therapy was more commonly prescribed in non-CAD patients, α- and β-adrenergic medications were more frequently prescribed in CAD patients, as would be expected. Although the groups did not differ with respect to overall childhood trauma experiences, CAD patients reported slightly more physical abuse and neglect compared with non-CAD participants.
TABLE 1.
Participant Characteristics
Individual Differences in Perceived Stress and HCCs
Table 1 indicates that CAD participants showed significantly higher-cortisol concentrations in hair compared with non-CVD patients experiencing other nonfatal illnesses (mean [SD] = 14.92 [29.64] versus 9.47 [23.93]; F(1, 597) = 22.834, p < .001). They did not, however, differ in terms of perceived stress. Women showed significantly lower cortisol concentrations in hair (mean [SD] = 10.06 [23.91] versus 14.16 [29.78]; F(1, 597) = 15.283, p < .001) compared with men. However, they reported significantly more perceived stress than men (mean [SD] = 58.92 [14.29] versus 53.65 [13.42]; F(1, 1123) = 38.605, p < .001). There was no significant correlation between HCC and age (r = .044, p = .281), although perceived stress correlated negatively with age (r = −.241, p < .001).
Associations Between Childhood Trauma, Perceived Stress, and Hair Cortisol, and Moderating Effects of Sex and Age
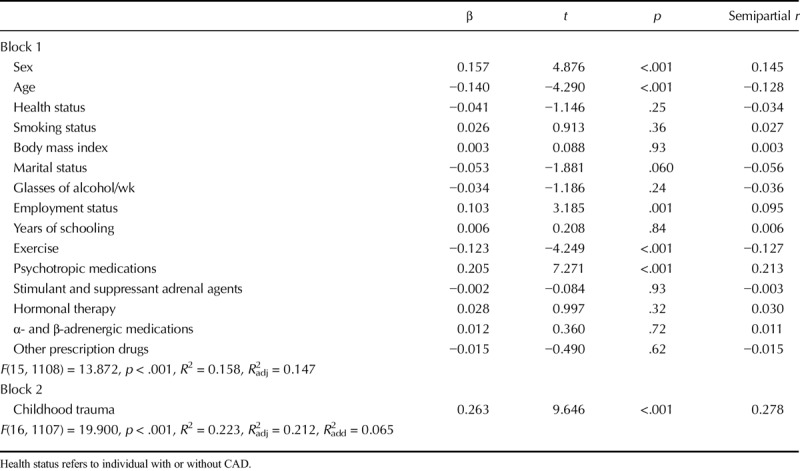
Table 2 presents bivariate correlations between the overall Childhood Trauma Questionnaire score (and subscales) with perceived stress and HCC conducted across groups of CAD and non-CVD patients. There was no association between HCC and the general or subscale CTQ scores. No significant association was noted when analyses were performed separately for men and women and for CAD and non-CVD groups. Perceived stress correlated significantly and positively with the overall CTQ score, as well as with the emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect subscales. Tables 3 and 4 provide results of the hierarchical multiple regression analyses. Childhood trauma was not associated with HCC but was associated with significantly greater perceived stress levels, and it explained 6.5% of the variance in perceived stress beyond that explained by the covariates and moderators. No interaction effects emerged.
TABLE 2.
Univariate Correlations Between Childhood Trauma, HCCs and Perceived Stress
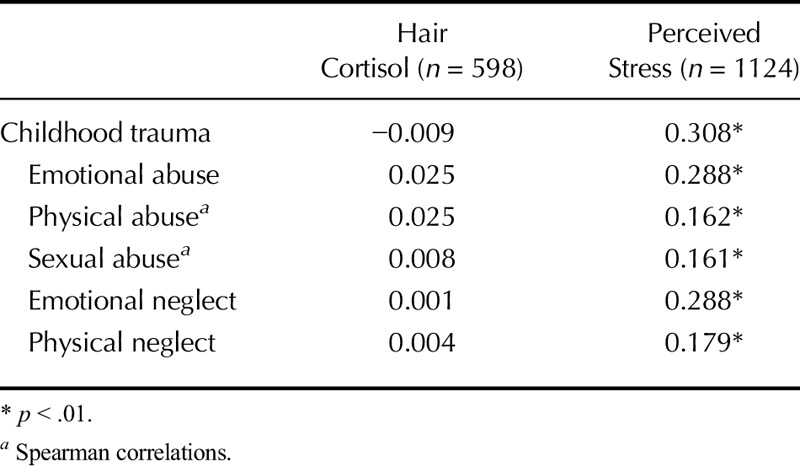
TABLE 3.
Hierarchical Regression Analysis Details for HCCs
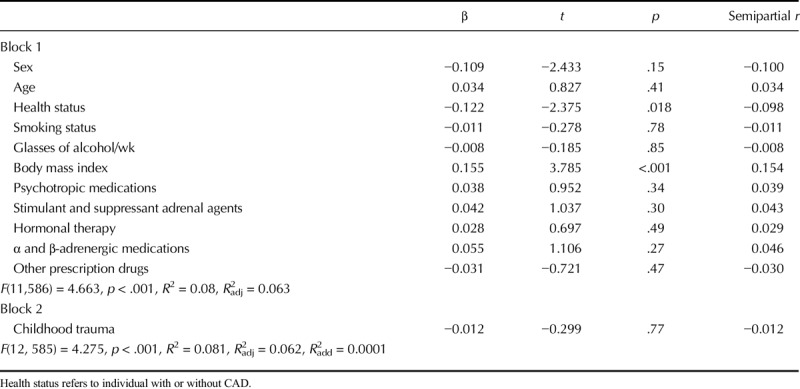
TABLE 4.
Hierarchical Regression Analysis Details for Perceived Stress
Secondary Analyses
None of the childhood trauma subscales were significantly associated with HCC. On the other hand, each CTQ subscale was significantly and independently associated with perceived stress level; emotional abuse and neglect explained respectively 4.5% and 6% of perceived stress level beyond that explained by the covariates. Physical abuse, sexual abuse, and physical neglect explained respectively 2.1%, 2.4%, and 2.8% of perceived stress level beyond that explained by covariates. Refer to Table 5 for details of the regression analyses.
TABLE 5.
Hierarchical Regression Analysis Details for Perceived Stress With Childhood Trauma Subscales
Only the age by sexual abuse interaction was significant (β = −0.067, t = −2.417, p = .016). Simple slopes analyses were performed based on age groups ± 1 SD from the mean. Analyses indicated that greater sexual abuse was associated with significantly greater perceived stress levels among all age groups but particularly among those that were somewhat younger: younger individuals (b = 28.70, t = 6.065, p < .001), intermediate-aged individuals (b = 20.21, t = 10.702, p < .001), and older individuals (b = 11.73, t = 2.302, p = .022). Refer to Figure 1.
FIGURE 1.
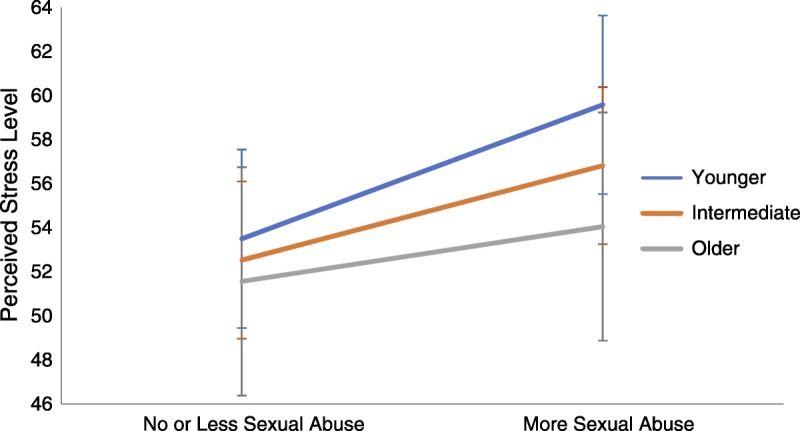
Association of sexual abuse with perceived stress differs by age. The association of sexual abuse with perceived stress differs as a function of age. The age by sexual abuse interaction was significant. More sexual abuse was associated with significantly greater levels of perceived stress among younger (b = 28.70, p < .001) compared with intermediate (b = 20.21, p < .001) and older (b = 11.73, p = .022) individuals. Color image is available only in online version (www.psychosomaticmedicine.org).
DISCUSSION
This research sought to confirm the hypotheses that trauma experienced in childhood would be associated with HCC for the past 3 months and the degree of stress perceived in the past 2 years among older adults with CAD or other non-CVD illness. It was expected that these associations might be influenced by age, sex, and cardiovascular status. Hypotheses were only partially corroborated: individuals who reported more childhood trauma also reported having experienced more stress in the past 2 years. However, no significant associations were found between childhood trauma and HCC. Furthermore, sex and coronary status were not found to moderate the associations between childhood trauma and our outcomes of interest. However, age moderated the association between sexual abuse and perceived stress.
The more traumas participants were exposed to in their childhood, the more they reported stress for the past 2 years. This effect was rather robust, with childhood trauma explaining 6.5 % of variance in perceived stress independently of that explained by other pertinent covariates. These results are consistent with those of previous investigations, despite very divergent sample types and measures of stress (11,13–15).
Although sample characteristics pertaining to age, sex, and cardiovascular status did not generally influence the associations observed between childhood trauma and our outcomes of interest, one interaction did emerge between sexual abuse and perceived stress. More specifically, sexual abuse experienced in childhood was more strongly associated with self-reported stress among those who were younger. To our knowledge, no previous studies have examined moderating effects of age on the relation between childhood trauma experiences and perceived stress in late adulthood. It has been suggested that as individuals grow older they tend to interpret situations as less stressful, focus less on negative emotions, and develop better coping strategies (55). Increased personal resources among our older participants may have contributed to them being less affected by earlier sexual abuse experiences compared with younger participants. This is consistent with the fact that older participants reported less stress overall compared with their younger counterparts in the current investigation. Given the number of tests in secondary analyses, this particular result may also reflect a spurious finding.
Childhood trauma was not associated with 3-month cumulative HCC in the current study nor in that by Steudte et al. (48). Hinkelmann et al. (46) and Schalinski et al. (47), for their part, observed significant associations between childhood trauma and HCC, albeit in opposite directions. A recent study investigated the impact of childhood maltreatment on HCC in a sizeable sample of children and adolescents (aged 3–16 years), who were recruited from child protection services, youth psychiatric services, and community (72). Childhood maltreatment was found to be associated with lower HCC from middle childhood on (age = 9–16 years), especially when maltreatment occurred in infancy or adolescence. It is still unclear what accounts for the differences between these findings and the present results. One factor is that the focused recruitment of maltreated youths in the latter study might have led to a more heavily traumatized sample and thus with more severe endocrine alterations as compared with the present study. In addition, the different assessment mode of childhood traumatic experiences might have affected results, i.e., the study by White et al. (72) used a multisource approach combining interview data with information from youths' child protection files. Conversely, it is also conceivable that childhood trauma-induced endocrine alterations might not be fully sustained into adulthood, as suggested by our results and those of Steudte et al. (48). Cortisol secretion changes considerably over time in childhood compared with adulthood, as brain structures continue to mature (73) and possibly as children develop the requisite coping skills to limit the impact of childhood experiences on the HPA axis.
Although women reported greater levels of perceived stress and lower HCC compared with men in the current study, sex did not influence the relation between childhood trauma and our outcome measures. Greater self-reported stress in women versus men is consistent with previous literature (55), as is lower HCC (45,69,74), free salivary cortisol, total plasma, and circadian cortisol secretion (53,75,76).
As expected, those with CAD reported greater HCC compared with non-CVD participants, indicating a potential role for cortisol in CVD development and progression. Indeed, higher cortisol levels have been associated with CVD risk factors, including atherosclerosis, hypertension, diabetes, obesity, and dyslipidemia (77–80).
Despite emerging data suggesting increased risk for CAD as a function of childhood trauma (3,5–8), only physical abuse and neglect were reported more frequently by CAD patients in the current investigation. Methodological differences related to age and timing of measure of childhood trauma may explain some of the differences. In previous studies, childhood trauma was measured at a younger age, which raises questions about memory bias. Alternatively, the increased risk associated with childhood trauma may not be limited to CAD specifically, as previous research has found that childhood trauma may increase risk for other physical or psychiatric diseases in adulthood (3,4).
Several factors may limit the conclusions that can be drawn from the current results. Hair characteristics (coloration, straightening, washing frequency, etc.) were not assessed and may have confounded the cortisol analyses (45,69,79,81). A large number of individuals (n = 526) had hair that was too short to provide a sample. These individuals differed significantly from those for whom we did obtain samples on numerous sociodemographic, medical, behavioral, and psychological variables (See Text, Supplemental Digital Content 1, which describes post-hoc analyses, http://links.lww.com/PSYMED/A445). These differences may have affected the cortisol outcomes. Childhood trauma history and perceived stress levels were self-reported and retrospective. Presence of memory bias is possible, particularly regarding childhood history of abuse and neglect (82). Moreover, the timing and the duration of the trauma experiences were not assessed, and these factors might have had different effects on HPA-axis activity (73). An interview about childhood abuse and neglect would have provided more comprehensive information. As can be seen from Table 1, our sample was generally older and sicker than those from previous studies (46–48). Furthermore, as CVD is a leading cause of death, participants still alive may represent individuals who were resilient to the effects of childhood trauma and other life stress. The cross-sectional, retrospective design of our study limits any conclusions regarding the causal relation between childhood trauma and stress experienced in later adulthood. Finally, analyses may have revealed limited evidence of moderation by age, sex, or CVD of the relations between childhood trauma experiences and stress measures in adulthood as a result of the low statistical power often associated with detecting significant interaction terms.
On the other hand, the current study has several strengths, including a large sample size and number of covariates assessed and included in analyses. Assessment of long-term cortisol secretion provides information on long-term cumulative cortisol secretion, which may inform on the sustained effect of childhood trauma on disease development and progression. The association of childhood trauma and hair cortisol had been previously assessed but three times in adult samples. Moreover, to our knowledge, this study is the first to simultaneously evaluate the impact of childhood traumatic experiences on perceived stress and HCC in adults, providing a fuller evaluation of the potential association or impact of childhood trauma on stress regulation, and compared these outcomes between CAD patients and non-CVD individuals.
In conclusion, our results suggest that childhood trauma is associated with greater reports of chronic stress among generally older individuals with health issues but may have no lasting effect on the activity of the HPA axis, at least as measured via HCC. Given evidence that greater levels of self-perceived stress, especially in those who have experienced childhood trauma, may increase risk for morbidity and mortality (68,83–87), prevention and intervention seem essential. Promoting the development of efficient coping skills from a young age, especially among more vulnerable populations, may help decrease vulnerability to stress in adulthood and prevent various stress-related health problems. The benefits are not limited to young or healthy individuals however. Gulliksson et al. (88) have found in a large sample of men and women experiencing CAD that stress management intervention lowered by 41% the rate of recurrent CVD events and by 28% all cause mortality during a mean 94 months of follow-ups.
Supplementary Material
Acknowledgments
We thank the André and France Desmarais Hospital Cohort of the Montreal Heart Institute, to all participants who voluntarily participated in this study, and to volunteers and research assistants for their work in participant recruitment, testing, and data entry.
Source of Funding and Conflicts of Interest: This research was supported by grants awarded to BDA by the Canadian Institutes of Health Research (CIHR; MOP # 111015). The authors report no conflicts of interest.
Footnotes
Supplemental Content
REFERENCES
- 1.Infurna FJ, Rivers CT, Reich J, Zautra AJ. Childhood trauma and personal mastery: their influence on emotional reactivity to everyday events in a community sample of middle-aged adults. PLoS One 2015;10:e0121840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein D, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994;151:1132–6. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34:509–20. [DOI] [PubMed] [Google Scholar]
- 4.Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis 2013;201:1007–20. [DOI] [PubMed] [Google Scholar]
- 5.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease adverse childhood experiences study. Circulation 2004;110:1761–6. [DOI] [PubMed] [Google Scholar]
- 6.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry 2004;65:249–54. [DOI] [PubMed] [Google Scholar]
- 7.Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood advers ities as predictors of incident coronary heart disease and cerebrovascular disease. Heart 2010;96:298–303. [DOI] [PubMed] [Google Scholar]
- 8.Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, Jun HJ, Wright RJ. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation 2012;126:920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleton AA, Holdsworth E, Ryan M, Tracy M. Measuring childhood adversity in life course cardiovascular research: a systematic review. Psychosom Med 2017;79:434–40. [DOI] [PubMed] [Google Scholar]
- 10.Winning A, Glymour MM, McCormick MC, Gilsanz P, Kubzansky LD. Childhood psychological distress as a mediator in the relationship between early-life social disadvantage and adult cardiometabolic risk: evidence from the 1958 British Birth Cohort. Psychosom Med 2016;78:1019–30. [DOI] [PubMed] [Google Scholar]
- 11.Han TJ, Felger JC, Lee A, Mister D, Miller AH, Torres MA. Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology 2016;25:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slavik S, Croake J. The individual psychology conception of depression as a stress-diathesis model. The J Individ Psychol 2006;62:417–28. [Google Scholar]
- 13.Mc Elroy S, Hevey D. Relationship between adverse early experiences, stressors, psychosocial resources and wellbeing. Child Abuse Negl 2014;38:65–75. [DOI] [PubMed] [Google Scholar]
- 14.Hyman SM, Paliwal P, Sinha R. Childhood maltreatment, perceived stress, and stress-related coping in recently abstinent cocaine dependent adults. Psychol Addict Behav 2007;21:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierrehumbert B, Torrisi R, Glatz N, Dimitrova N, Heinrichs M, Halfon O. The influence of attachment on perceived stress and cortisol response to acute stress in women sexually abused in childhood or adolescence. Psychoneuroendocrinology 2009;34:924–38. [DOI] [PubMed] [Google Scholar]
- 16.McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry 2011;2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry 2010;51:1079–95. [DOI] [PubMed] [Google Scholar]
- 18.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry 2006;163:630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron 2016;89:892–909. [DOI] [PubMed] [Google Scholar]
- 20.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus 2008;18:729–36. [DOI] [PubMed] [Google Scholar]
- 21.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 2003;27:33–4. [DOI] [PubMed] [Google Scholar]
- 22.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry 2004;65:18–28. [PubMed] [Google Scholar]
- 23.Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis—2012 Curt Richter Award Winner. Psychoneuroendocrinology 2013;38:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Doom JR, Gunnar MR. Stress physiology and developmental psychopathology: past, present, and future. Dev Psychopathol 2013;25:1359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerritsen L, Geerlings MI, Beekman AT, Deeg DJ, Penninx BW, Comijs HC. Early and late life events and salivary cortisol in older persons. Psychol Med 2010;40:1569–78. [DOI] [PubMed] [Google Scholar]
- 26.Power C, Thomas C, Li L, Hertzman C. Childhood psychosocial adversity and adult cortisol patterns. Br J Psychiatry 2012;201:199–206. [DOI] [PubMed] [Google Scholar]
- 27.Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:889–94. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry 2007;67:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, Van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events A study among healthy young subjects. Psychoneuroendocrinology 2008;33:227–37. [DOI] [PubMed] [Google Scholar]
- 30.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000;284:592–7. [DOI] [PubMed] [Google Scholar]
- 31.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry 2008;63:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeSantis SM, Baker NL, Back SE, Spratt E, Ciolino JD, Moran-Santa Maria M, Dipankar B, Brady KT. Gender differences in the effect of early life trauma on hypothalamic-pituitary-adrenal axis functioning. Depress Anxiety 2011;28:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, Yehuda R, Marmar CR. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry 2005;57:27–32. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214:367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder T. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology 2014;39:132–40. [DOI] [PubMed] [Google Scholar]
- 36.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology 2007;32:80–6. [DOI] [PubMed] [Google Scholar]
- 37.Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wust S, Dockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien SJ, Clow A. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology 2016;63:414–32. [DOI] [PubMed] [Google Scholar]
- 38.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171–9. [DOI] [PubMed] [Google Scholar]
- 39.Ronson A. Stress and allostatic load: perspectives in psycho-oncology [in French]. Bull Cancer 2006;93:289–95. [PubMed] [Google Scholar]
- 40.Seeman TE, Robbins RJ. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr Rev 1994;15:233–60. [DOI] [PubMed] [Google Scholar]
- 41.Steudte-Schmiedgen S, Kirschbaum C, Alexander N, Stalder T. An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: insight from recent hair cortisol findings. Neurosci Biobehav Rev 2016;69:124–35. [DOI] [PubMed] [Google Scholar]
- 42.D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav 2011;104:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, Wadhwa PD, Buss C. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology 2016;71:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 2012;37:589–601. [DOI] [PubMed] [Google Scholar]
- 45.Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, Kirschbaum C, Miller R. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 2017;77:261–74. [DOI] [PubMed] [Google Scholar]
- 46.Hinkelmann K, Muhtz C, Dettenborn L, Agorastos A, Wingenfeld K, Spitzer C, Gao W, Kirschbaum C, Wiedemann K, Otte C. Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biol Psychiatry 2013;74:e15–7. [DOI] [PubMed] [Google Scholar]
- 47.Schalinski I, Elbert T, Steudte-Schmiedgen S, Kirschbaum C. The cortisol paradox of trauma-related disorders: lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS One 2015;10:e0136921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steudte S, Kirschbaum C, Gao W, Alexander N, Schonfeld S, Hoyer J, Stalder T. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry 2013;74:639–46. [DOI] [PubMed] [Google Scholar]
- 49.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol 2008;51:1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am J Physiol Heart Circ Physiol 2015;308:H1476–98. [DOI] [PubMed] [Google Scholar]
- 51.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70. [DOI] [PubMed] [Google Scholar]
- 52.Krajnak KM. Potential contribution of work-related psychosocial stress to the development of cardiovascular disease and type II diabetes: a brief review. Environ Health Insights 2014;8:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol 2005;69:113–32. [DOI] [PubMed] [Google Scholar]
- 54.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31:151–78. [DOI] [PubMed] [Google Scholar]
- 55.Cohen S, Janicki-Deverts D. Who's stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol 2012;42:1320–34. [Google Scholar]
- 56.Xu X, Bao H, Strait K, Spertus JA, Lichtman JH, D'Onofrio G, Spatz E, Bucholz EM, Geda M, Lorenze NP, Bueno H, Beltrame JF, Krumholz HM. Sex differences in perceived stress and early recovery in young and middle-aged patients with acute myocardial infarction. Circulation 2015;131:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology 2001;26:225–40. [DOI] [PubMed] [Google Scholar]
- 58.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry 2009;66:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auer PL, Teumer A, Schick U, O'Shaughnessy A, Lo KS, Chami N, Carlson C, de Denus S, Dubé MP, Haessler J, Jackson RD, Kooperberg C, Perreault LP, Nauck M, Peters U, Rioux JD, Schmidt F, Turcot V, Völker U, Völzke H, Greinacher A, Hsu L, Tardif JC, Diaz GA, Reiner AP, Lettre G. Rare and low-frequency coding variants in CXCR2 and other genes are associated with hematological traits. Nat Genet 2014;46:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dube MP, Zetler R, Barhdadi A, Brown AM, Mongrain I, Normand V, Laplante N, Asselin G, Zada YF, Provost S, Bergeron J, Kouz S, Dufour R, Diaz A, de Denus S, Turgeon J, Rheaume E, Phillips MS, Tardif JC. CKM and LILRB5 are associated with serum levels of creatine kinase. Circ Cardiovasc Genet 2014;7:880–6. [DOI] [PubMed] [Google Scholar]
- 61.Bonita R, de Courten M, Dwyer T, Jamrozik K, Winkelmann R. Surveillance of risk factors for noncommunicable diseases: The WHO STEPwise approach. Summary. Geneva: World Health Organization; 2001. [Google Scholar]
- 62.Paquette D, Laporte L, Bigras M, Zoccolillo M. Validation of the French version of the CTQ and prevalence of the history of maltreatment [in French]. Sante Ment Que 2004;29:201–20. [DOI] [PubMed] [Google Scholar]
- 63.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-report Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 64.Levenstein S, Prantera C, Varvo V, Scribano ML, Berto E, Luzi C, Andreoli A. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res 1993;37:19–32. [DOI] [PubMed] [Google Scholar]
- 65.Lehman KA, Burns MN, Gagen EC, Mohr DC. Development of the brief inventory of perceived stress. J Clin Psychol 2012;68:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Int 2000;107:5–12. [DOI] [PubMed] [Google Scholar]
- 67.Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology 2012;37:602–10. [DOI] [PubMed] [Google Scholar]
- 68.Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol 2012;110:1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stalder T, Kirschbaum C. Analysis of cortisol in hair—state of the art and future directions. Brain Behav Immun 2012;26:1019–29. [DOI] [PubMed] [Google Scholar]
- 70.Dettenborn L, Tietze A, Kirschbaum C, Stalder T. The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 2012;15:578–88. [DOI] [PubMed] [Google Scholar]
- 71.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 2004;36:717–31. [DOI] [PubMed] [Google Scholar]
- 72.White LO, Ising M, von Klitzing K, Sierau S, Michel A, Klein AM, Andreas A, Keil J, Quintero L, Müller-Myhsok B, Uhr M, Gausche R, Manly JT, Crowley MJ, Kirschbaum C, Stalder T. Reduced hair cortisol after maltreatment mediates externalizing symptoms in middle childhood and adolescence. J Child Psychol Psychiatry 2017;58:998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents' cortisol stress response. The TRAILS study. Psychoneuroendocrinology 2012;37:1439–47. [DOI] [PubMed] [Google Scholar]
- 74.Wells S, Tremblay PF, Flynn A, Russell E, Kennedy J, Rehm J, Van Uum S, Koren G, Graham K. Associations of hair cortisol concentration with self-reported measures of stress and mental health-related factors in a pooled database of diverse community samples. Stress 2014;17:334–42. [DOI] [PubMed] [Google Scholar]
- 75.O'Donnell K, Badrick E, Kumari M, Steptoe A. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology 2008;33:601–11. [DOI] [PubMed] [Google Scholar]
- 76.Lederbogen F, Kuhner C, Kirschbaum C, Meisinger C, Lammich J, Holle R, Krumm B, von Lengerke T, Wichmann HE, Deuschle M, Ladwig KH. Salivary cortisol in a middle-aged community sample: results from 990 men and women of the KORA-F3 Augsburg study. Eur J Endocrinol 2010;163:443–51. [DOI] [PubMed] [Google Scholar]
- 77.Martocchia A, Stefanelli M, Falaschi GM, Toussan L, Ferri C, Falaschi P. Recent advances in the role of cortisol and metabolic syndrome in age-related degenerative diseases. Aging Clin Exp Res 2016;28:17–23. [DOI] [PubMed] [Google Scholar]
- 78.Fantidis P, Eladio S, Ibrahim T, Tomas P, Antonio CJ, Ramon GJ. Is there a role for cortisol in the accumulation of lipids in the intima a crucial step of atherogenesis? Curr Vasc Pharmacol 2015;13:587–93. [DOI] [PubMed] [Google Scholar]
- 79.Manenschijn L, Schaap L, van Schoor NM, van der Pas S, Peeters GM, Lips P, Koper JW, van Rossum EF. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab 2013;98:2078–83. [DOI] [PubMed] [Google Scholar]
- 80.Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, Stark S, Bosch JA, Fischer JE. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab 2013;98:2573–80. [DOI] [PubMed] [Google Scholar]
- 81.Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 2007;30:E183–91. [DOI] [PubMed] [Google Scholar]
- 82.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry 2004;45:260–73. [DOI] [PubMed] [Google Scholar]
- 83.Wiernik E, Lemogne C, Thomas F, Perier MC, Guibout C, Nabi H, Laurent S, Pannier B, Boutouyrie P, Jouven X, Empana JP. Perceived stress, common carotid intima media thickness and occupational status: The Paris Prospective Study III. Int J Cardiol 2016;221:1025–30. [DOI] [PubMed] [Google Scholar]
- 84.Ortega-Montiel J, Posadas-Romero C, Ocampo-Arcos W, Medina-Urrutia A, Cardoso-Saldaña G, Jorge-Galarza E, Posadas-Sánchez R. Self-perceived stress is associated with adiposity and atherosclerosis. The GEA Study. BMC Public Health 2015;15:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anda RF, Williamson DF, Escobedo LG, Remington PL, Mast EE, Madans JH. Self-perceived stress and the risk of peptic ulcer disease. A longitudinal study of US adults. Arch Intern Med 1992;152:829–33. [PubMed] [Google Scholar]
- 86.Novak M, Björck L, Giang KW, Heden-Ståhl C, Wilhelmsen L, Rosengren A. Perceived stress and incidence of Type 2 diabetes: a 35-year follow-up study of middle-aged Swedish men. Diabet Med 2013;30:e8–16. [DOI] [PubMed] [Google Scholar]
- 87.Prior A, Fenger-Gron M, Larsen KK, Larsen FB, Robinson KM, Nielsen MG, Christensen KS, Mercer SW, Vestergaard M. The association between perceived stress and mortality among people with multimorbidity: a prospective population-based cohort study. Am J Epidemiol 2016;184:199–210. [DOI] [PubMed] [Google Scholar]
- 88.Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, Svärdsudd K. Randomized controlled trial of cognitive behavioral therapy vs standard treatment to prevent recurrent cardiovascular events in patients with coronary heart disease: Secondary Prevention in Uppsala Primary Health Care project (SUPRIM). Arch Intern Med 2011;171:134–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


