Abstract
BACKGROUND
Recent evidence from health and demographic surveillance sites (HDSS) has shown that increasing access to antiretroviral therapy (ART) is reducing mortality rates in sub-Saharan Africa (SSA). However, due to limited vital statistics registration in many of the countries most affected by the HIV/AIDS epidemic, there is limited evidence of the magnitude of ART’s effect outside of specific HDSS sites. This paper leverages longitudinal household/family roster data from the Malawi Longitudinal Survey of Families and Health (MLSFH) to estimate the effect of ART availability in public clinics on population-level mortality based on a geographically dispersed sample of individuals in rural Malawi.
OBJECTIVE
We seek to provide evidence on the population-level magnitude of the ART-associated mortality decline in rural Malawi and confirm that this population is experiencing similar declines in mortality as those seen in HDSS sites.
METHODS
We analyze longitudinal household/family-roster data from four waves of the MLSFH to estimate mortality change after the introduction of ART to study areas. We analyze life expectancy using the Kaplan–Meier estimator and examine how the mortality hazard changed over time by individual characteristics with Cox regression.
RESULTS
In the four years following rollout of ART, life expectancy at age 15 increased by 3.1 years (95% CI 1.1, 5.1), and median length of life rose by over ten years.
CONTRIBUTION
Our observations show that the increased availability of ART resulted in a substantial and sustained reversal of mortality trends in SSA and assuage concerns that the post-ART reversals in mortality are not occurring at the same magnitude outside of specific HDSSs.
1. Background
While life expectancy in the rest of the world continued to increase from the late 1980s through the 2000s, much of southern and eastern sub-Saharan Africa (SSA) saw reductions in life expectancy associated with the HIV pandemic (WHO 2014). Beyond the simple loss of life, these declines in life expectancy have had negative impacts on child survival, household income, availability of skilled labor, and work efforts (Barnett and Whiteside 2006). The large-scale rollout of antiretroviral therapy (ART) in SSA has begun to reverse these trends (Joint United Nations Programme on HIV/AIDS (UNAIDS) 2013). ART works by enabling an individual’s immune system to recover to a functional state and can improve survival to levels almost on par with those of the non-HIV infected (Mills et al. 2011). Starting in the early 2000s, access to ART through public-sector programs was greatly improved in SSA. Recent studies have started to document the success of these programs in terms of population-level declines in mortality (both HIV-related and all-cause) in South Africa (Bor et al. 2013; Larson et al. 2014), Malawi (Floyd et al. 2010; Jahn et al. 2008; Price et al. 2016), and other countries in SSA (Floyd et al. 2012; Stoneburner et al. 2014).
An important aspect of the recent evidence on post-ART mortality change is that it is based largely on data from populations enrolled in health and demographic surveillance sites (HDSS), with the notable exception of a small group of studies based on aggregate-level data (Larson et al. 2014; Mwagomba et al. 2010; Stoneburner et al. 2014; Stover et al. 2008; Pillay-van Wyk et al. 2013). Though HDSSs are a valuable resource, the external validity of HDSS-based findings is potentially limited. Specifically, and directly relevant to this research question, the generalizability of the effects of ART introduction at HDSS sites may be limited by the additional services that some HDSSs provide their study populations. For example, the high rates of HIV testing coverage (Asiki et al. 2013; Kasamba et al. 2012; Odhiambo et al. 2012; Price et al. 2016; Tanser et al. 2008; Wambura et al. 2007) mean that HDSS populations may be more knowledgeable about their HIV status than non-HDSS populations. While this has not been a prominent concern in the recent literature about the mortality consequences of ART (possibly because very few non-HDSS mortality studies are available for comparison), it is nevertheless possible and plausible that the observed ART-related mortality reductions in HDSS sites represent a more ideal case than exists in much of SSA.
In this paper, we use an alternative approach and data source to estimate the effect of ART introduction on population-level mortality. Namely, we estimate the effect of ART availability in public clinics on population-level mortality based on a geographically dispersed, mostly rural sample of individuals from the Malawi Longitudinal Study of Families and Health (MLSFH; Kohler et al. 2015), which reflects substantial heterogeneity in ethnicity, religion, language, educational attainment, population density, and HIV prevalence.
Besides the comparison with related estimates from HDSS sites, our findings about pre- and post-ART mortality are key for documenting recent mortality levels and trends in the MLSFH. This study population is a publicly available, ongoing cohort study that has been used in more than 250 publications.3 Our finding that the mortality of the MLSFH study population is similar, in both level and trends, to that documented in other HDSS sites strengthens the assessment of data quality in the MLSFH, highlights an innovative new use of the MLSFH data based on linked household/family-roster data, and provides additional credence to the ongoing relevance of the MLSFH for studying contemporary trends in demographic, health, and social conditions in SSA.
2. Methods
2.1 Study design and participants
We estimate age-specific mortality among respondents and household/family members in the 2004–2012 MLSFH. The MLSFH is a longitudinal study monitoring social, economic, and health conditions in the rural population of Malawi, one of the world’s poorest nations. The study is based in three rural districts (Rumphi in the north, Mchinji in the center, and Balaka in the south; Figure A-1) that represent the substantial heterogeneity of Malawi in terms of HIV prevalence and ethnic/religious groups (Kohler et al. 2015). MLSFH respondents (N≈3,800) are evenly split among these three regions and are clustered in 121 villages. MLSFH sampling methods and related data collection procedures are described in a cohort profile (Kohler et al. 2015). Comparisons with the nationally representative Malawi Demographic and Health Surveys and the Malawi Third Integrated Household Survey show that basic demographic characteristics closely match the rural population of Malawi (Kohler et al. 2015; Payne, Mkandawire, and Kohler 2013).
At each wave, MLSFH respondents reported on the mortality of their resident and nonresident household/family members. The MLSFH offered HIV testing and counseling services to primary respondents and their spouses in 2004, 2006, and 2008, with referral to confirmatory testing at a local clinic for those with HIV+ results (Kohler et al. 2015). The survey team did not interact directly with other members of the household roster, who comprise about 70% of the individuals in this study. The MLSFH also had no part in the training, support, or management of ART provision, making the study population independent from the ART program that is being evaluated in terms of its effect on population-level mortality.
The Ministry of Health in Malawi began rolling out free ART to eligible individuals in urban areas in 2004, with rollout to smaller clinics beginning in 2006. In the MLSFH, median distance of respondents to the nearest ART clinic was 27 kilometers up until mid-2007, which made access to ART difficult given the limited means of transportation in this rural context (Baranov, Bennett, and Kohler 2015; Baranov and Kohler 2014). ART-providing clinics opened between August of 2007 and March of 2008 in each of the three study regions (shortly before data collection for the 2008 round of the MLSFH), reducing the median distance to the nearest ART clinic to 8.9 kilometers by the 2008 MLSFH (Baranov, Bennett, and Kohler 2015).
HIV prevalence among MLSFH respondents was 6.1% in 2010, with considerable variation across regions (Freeman and Anglewicz 2012). It was higher among men age 50–65 (8.9%) than women age 50–65 (5.4%), but lower among men age 15–49 (4.1%) than women age 15–49 (8.3%).
The study population consists of (a) MLSFH respondents and (b) individuals who were reported on the MLSFH household/family rosters by respondents in 2004, 2006, 2008, 2010, and 2012. However, the 2004 household roster questionnaire used a different inclusion criterion (only recording members who slept in the household the previous night), so our primary results will focus on the 2006–2012 MLSFH. In each wave, primary respondents completed a family and household roster listing the vital status of their family/household members independent of place of residence (more detail on the household/family rosters and the full set of questions asked in the roster module is included in Appendix Text 1).
Our analyses focus on individuals aged 15+ and compare all-cause mortality between the period directly before ART became widely available (2006–2008) with all-cause mortality after ART became available (2008–2012). By using all-cause mortality as our outcome, our analyses capture changes due directly to HIV+ individuals’ increased access to ART, as well as all possible spill-over effects of ART availability on the HIV-negative (Baranov, Bennett, and Kohler 2015).
Individuals reported on the MLSFH family/household roster were not previously linked across survey rounds to allow longitudinal analyses. We developed a probabilistic matching algorithm to link individuals across multiple MLSFH waves by name, age, sex, and relationship to primary respondent (see Appendix Text 2 for details on the matching process). Match rates between successive waves were 76–82% in 2006–2010 and 92% in 2012, with rates for close family members (parents, children, spouses) substantially higher. The higher match rate in 2012 resulted from the fact that household rosters were prepopulated with information from the 2010 survey. Failure to link is unlikely to introduce biases in our analyses (see Appendix Tables A-1 and A-2).
Our analysis population included all resident and nonresident family/household members aged 15 and older (including the respondent themselves) who were linked across at least two survey waves. To avoid duplicate records resulting from both husband and wife reporting on the same family/household members, we limited our analysis sample to individuals listed by female primary respondents and male respondents without a coresident spouse in the MLSFH sample. With these restrictions, our analyses sample consists of 9,586 individuals listed by 1,869 primary MLSFH respondents, contributing 33,103 person years of observation during the period 2006–2012. Appendix Table A-2 presents selected characteristics of the analytic sample. A total of 735 deaths were observed in the study population between 2006 and 2012.
2.2 Statistical analysis
Age-specific mortality rates are computed for pre-ART (2006–2008) and post-ART (2008–2010, 2010–2012) periods. Trends in pre-ART and post-ART survival curves with 95% confidence intervals are estimated using the Kaplan–Meier estimator (Kalbfleisch and Prentice 2002), and adult life expectancies were obtained as the area under the survival curve after age 15. Adult life expectancy measures the additional years a 15-year-old would expect to live if subjected to the prevailing pattern of mortality rates in the population. We also report the median length of life conditional on survival to age 15 as the age at which the survival curve for each period reached .5.
To increase analytic power, we compared data from 2006–2008 (pre-ART period) to combined 2008–2010 and 2010–2012 data (post-ART period). Analyses used 15-year age groups for the full sample, and the age groups 15–44, 45–59, and 60+ for estimates stratified by region. We estimated rate ratios using Poisson regression, with log-exposure time as the offset. Cox proportional hazards regression (using exact marginal methods for tied failure times) was used to compare the effect of several other individual-level variables on the hazard of mortality between the pre- and post-ART periods. These included: distance to nearest ART-providing clinic (under 8 kilometers vs. 8 or more kilometers), completed primary schooling (fewer than five years of completed schooling vs. five or more years completed schooling), and quartiles of household wealth (measured via an asset index and averaged over the period 2006–2012).
3. Results
3.1 Pre-ART/post-ART differences in overall life expectancy
Figure 1 shows that adult life expectancy (LE at age 15) before the introduction of ART (period 2006–2008) was approximately 42 years in the study population (95% CI 39.4, 44.6) – slightly below the 2010 GBD estimates for adult life expectancy in Malawi, 43.2 years (Salomon et al. 2012). Adult life expectancy increased to approximately 45 years (95% CI 42.5, 47.4) in the period directly after the introduction of ART (2008–2010). It was slightly higher, at 45.5 years (95% CI 41.9, 49.1), in the following period (2010–2012). The difference in life expectancy between the pre-ART and the post-ART period (2008–2012 combined, LE 45.1 years, 95% CI 43.1, 47.1) was 3.1 years (95% CI 1.1, 5.1). Life expectancies and 95% CIs for men and women separately are provided in Appendix Table A-3. Sensitivity analyses using alternative definitions of the study population led to similar results (see Appendix Table A-4). Appendix Figure A-2 replicates Figure 1, including the 2004 MLSFH data and using a restricted sample of individuals in the 2006–2012 surveys to correspond to the household listing instructions of the 2004 MLSFH, where only individuals who slept in the household the previous night were enumerated. Point estimates for LE show a declining trend between the 2004–2006 and 2006–2008 periods, with a turnaround after the introduction of ART to the study areas in 2008. However, the smaller sample sizes in this subsample lead to fairly wide confidence intervals around these estimates.
Figure 1. Life expectancy at age 15, 2006–2012.
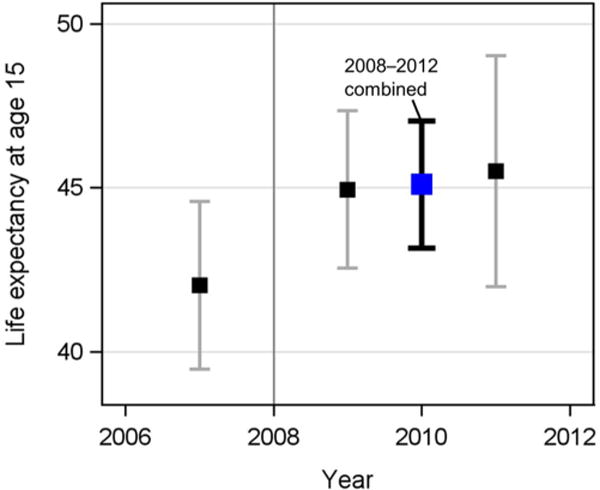
Notes: Life expectancy at age 15 is the mean length of remaining life for a 15-year-old if subjected to the prevailing pattern of age-specific mortality rates observed during a given period of time. Estimates of life expectancy for the periods 2006–2008, 2008–2010, and 2010–2012 are displayed as the squares, with 95% CIs. The timing of public-sector introduction of ART is shown using the gray vertical line.
Comparing the survival curves of the pre-ART and post-ART periods (Figure 2), the median length of life conditional on survival to age 15 rose by 11 years between 2006 and 2012, from 56 years (95% CI 51, 62) before the introduction of ART to 61 years (95% CI 59, 65) in the period directly after the introduction of ART (2008–2010) and to 67 years (95% CI 60, 70) in 2010–2012. For the post-ART period combined (2008–2012), median length of life conditional on survival to age 15 was 64 years (95% CI 60, 67). The survival curve moves outward after the introduction of ART, although the divergence in the slope of the curves only begins at around age 35 and continues until about age 55. After age 55, the survival curves run in near parallel, and they again converge in later life.
Figure 2. Survival curves pre-ART (2006–2008) and post-ART (2008–2010,2010–2012) for ages 15+.
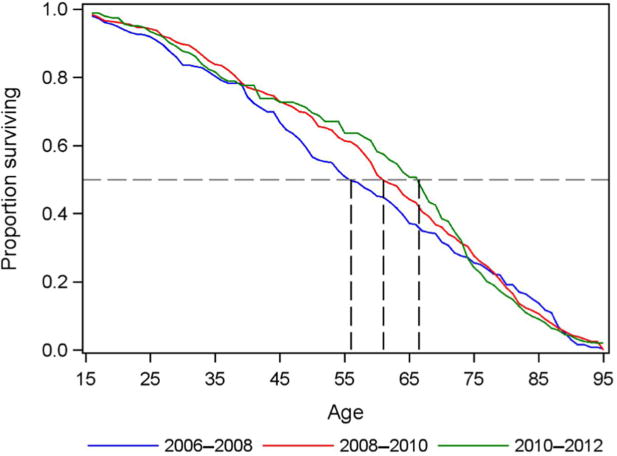
Notes: Survival curves for pre-ART (2006–2008, blue line) and post-ART (2008–2010, red line, and 2010–2012, green line) periods. Conditional on survival to age 15, median age at death was 56 years before the introduction of ART, rising after the introduction of ART to 61 years in 2008–2010 and 67 years in 2010–2012.
3.2 Pre-ART/post-ART differences in mortality
The all-cause mortality rate among those aged 15–59 declined by almost 30% between the pre-ART and post-ART periods (from 15.6 to 11.4 deaths per thousand, RR .73, 95% CI .59, .91), with the strongest declines in the 45–59 age group (Panel A of Table 1). At advanced ages (60+) these reductions subside.
Table 1.
Trends in mortality before and after introduction of ART to ruralMalawi
| Pre-ART (2006–2008) | Post-ART (2008–2012) | Rate Ratio: Post-/pre-ART | |||||
|---|---|---|---|---|---|---|---|
| Age | PY | Rate | PY | Rate | RR | 95% CI | |
|
|
|
|
|
||||
| A. Full sample | |||||||
| 15–29 | 4,362 | 10.1 | 7,288 | 7.7 | 0.76 | 0.51 | 1.13 |
| 30–44 | 2,902 | 13.1 | 4,614 | 12.1 | 0.93 | 0.61 | 1.40 |
| 45–59 | 2,697 | 27.1 | 4,011 | 17.2 | 0.64 | 0.46 | 0.88 |
| 60–74 | 1,941 | 35.5 | 2,978 | 41.6 | 1.17 | 0.87 | 1.57 |
| 75+ | 807 | 78.1 | 1,503 | 95.1 | 1.22 | 0.91 | 1.64 |
|
B. By region1
|
|
|
|
||||
| Mchinji | |||||||
| 15–44 | 2,138 | 8.4 | 3,093 | 9.4 | 1.11 | 0.60 | 2.13 |
| 45–59 | 785 | 29.3 | 1,021 | 14.7 | 0.50 | 0.24 | 1.00 |
| 60+ | 633 | 45.8 | 1,004 | 45.8 | 1.00 | 0.62 | 1.65 |
| Balaka | |||||||
| 15–44 | 1,955 | 18.9 | 2,956 | 15.6 | 0.82 | 0.52 | 1.30 |
| 45–59 | 792 | 32.8 | 1,221 | 23.8 | 0.72 | 0.41 | 1.28 |
| 60+ | 628 | 54.1 | 1,139 | 69.4 | 1.28 | 0.85 | 1.98 |
| Rumphi | |||||||
| 15–44 | 2,045 | 8.3 | 3,327 | 5.1 | 0.61 | 0.29 | 1.28 |
| 45–59 | 823 | 15.8 | 1,412 | 8.5 | 0.54 | 0.22 | 1.28 |
| 60+ | 981 | 43.8 | 1,540 | 57.1 | 1.30 | 0.90 | 1.92 |
Analyses by region use only individuals reported as primarily residing within that region.
Mortality rates were highest in Balaka, where HIV+ prevalence is highest, and lowest in Rumphi, where HIV+ prevalence is lowest, with Mchinji being in the middle (Panel B). Despite these level differences, mortality rates declined in all three MLSFH regions (Panel B), albeit statistical significance of the decline is only obtained in one of the three sites (Mchinji) due to the reduction in sample size in these regional analyses.
Results from a Cox proportional hazards model predicting time to death (Table 2) show that the hazard varied substantially by individual-level characteristics. The decline in the hazard of mortality was strongest among those living close to clinics – before the rollout of ART in 2008, there was little geographic variation in the mortality hazard. However, in the post-ART period, the mortality hazard for those living closer to ART clinics was 23% lower than those living far from these clinics. Males had a higher mortality hazard than females in both the pre- and post-ART periods, although this difference appears to have shrunk slightly in the post-ART period. Education was not significantly associated with mortality hazard. Increasing wealth was somewhat associated with decreased mortality hazard; however, this association does not appear to vary between the pre-ART and post-ART periods.
Table 2.
Cox hazard ratio estimates for distance from ART clinic, sex, education, and wealth pre-ART and post-ART
| Pre-ART (2006–2008) | Post-ART (2008–2012) | |||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
|
|
|
|||
| Distance to ART clinic | ||||
| 8 kilometers or more | ref | ref | ||
| fewer than 8 kilometers | 1.06 | [0.79, 1.41] | 0.77* | [0.62, 0.97] |
| Sex | ||||
| Female | ref | ref | ||
| Male | 1.74** | [1.34, 2.26] | 1.42** | [1.16, 1.73] |
| Education | ||||
| Fewer than five years of | ||||
| schooling | ref | ref | ||
| Five or more years of schooling | 0.97 | [0.72, 1.30] | 0.86 | [0.68, 1.10] |
| Wealth quantile | ||||
| 1st | ref | ref | ||
| 2nd | 0.70+ | [0.48, 1.03] | 0.78 | [0.58, 1.06] |
| 3rd | 0.76 | [0.52, 1.10] | 0.72* | [0.52, 0.98] |
| 4th | 0.77 | [0.52, 1.14] | 0.75+ | [0.54, 1.03] |
| 5th | 0.58* | [0.38, 0.90] | 0.75+ | [0.54, 1.05] |
Notes: Analyses additionally control for region and age, and they account for clustering within household.
p < 0.10
p < 0.05
p < 0.01
4. Discussion
We document gains of 3.1 years in adult life expectancy and 8 years of median length of life in the four years following the introduction of public-sector ART in rural Malawi. Our estimated mortality rates and the change in mortality rate post-ART are similar to published results from the Karonga HDSS in Malawi (Floyd et al. 2010; Larson et al. 2014; Price et al. 2016) and those of other HDSS sites in Southern and Eastern Africa (Floyd et al. 2012).
Our analyses illuminate some of the promises, and pitfalls, of using data from a socially focused panel study to conduct epidemiologic research. In studying a population that had little contact with the study team, we were not able to analyze biomedical information, including individual-level HIV status and CD4 count. Even though the majority of individuals in this study had not been tested for HIV by the study team, primary respondents and their spouses (representing about 30% of the sample) were offered testing, and thus our study population may have somewhat higher HIV status knowledge than the general population.
Although the increase in life expectancy coincided with the scale-up of ART (Figure 1 and Appendix Figure A-2), our estimates may also capture health and mortality trends not linked to the scale-up of ART. As there is no counterfactual group in our analyses, we cannot directly identify the causal mechanism behind the observed mortality declines. However, the coincidence of life expectancy increasing directly after the introduction of ART strongly suggests that the increased availability of ART was the primary driver. Few other health interventions addressing major causes of death occurred in rural Malawi during this time, and overall socioeconomic changes in Malawi were more likely to change mortality in the opposite direction, as the post-ART period coincided with substantial rises in food and oil prices, currency devaluation, political unrest, and reduced international aid (Ellis and Manda 2012; Wroe 2012).
Our analyses demonstrate the promise of using alternative data sources to add to the evidence base on the population-level effects of ART on mortality and life expectancy. Our results further support the assumption that the increased availability of ART resulted in a substantial and sustained reversal of mortality trends in rural Malawi and help assuage concerns that the post-ART reversals in mortality may not be occurring at the same magnitude outside of specific HDSSs. Future research will have to address the long-term consequences of ART on lifecycle behaviors among families affected by HIV and the possible economic benefits resulting from sustained health improvements through ART. Challenges in achieving high ART uptake and ART adherence among the rapidly growing population of older individuals with HIV will have to be addressed.
Acknowledgments
This work was supported by the National Institute for Child Health and Human Development (Grant no. R01HD053781) and the National Science Foundation Graduate Research Fellowship (Grant no. DGE 0822). The funder of the study had no role in study design, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Appendix Text 1: MLSFH household roster details
Beginning in 2006 and continuing in the 2008, 2010, and 2012 MLSFH waves, the MLSFH collected data on family structure. The MLSFH household and family roster included not only all individuals who currently live in the household (as frequently done in other studies), but it also asked for information about all parents, spouses (and the most recently deceased or divorced spouse for those not currently married), and children independent of their survival and resident status (Box 1).
Box 1. Individuals listed in MLSFH household/family roster.
| 1. | List the respondent |
| 2. | List name of spouse(s) of respondent. If respondent is not currently married, list name of most recently deceased or divorced spouse. For polygamous men: List all wives. If never married, proceed to instruction 3, below. |
| 3. | List name of respondents’ parents (list names even if parents are deceased). |
| 4. | [if R is married or widowed] List name of spouse’ s parents (list names even if parents are deceased). For polygamous men: List parents of all wives. |
| 5. | List the names of all children of the respondent (children ever born; include children who are no longer alive or do not live in respondent’s household). |
| 6. | List the names of any other children who usually live in this household (including nonbiological children, grandchildren, nieces and nephews). |
| 7. | List the names of all other persons who slept in this household last night. |
| 8. | List the names of all other persons who usually sleep in this household but did not last night. |
| 9. | List the names of all nonrelated children who are under your care but not living in the household (for example, anyone you have helped with school fees in the last five years). |
The questionnaire asked about each individual’s selected demographic, socioeconomic, and health characteristics and health as known to/perceived by the respondent (Box 2).
Box 2. Information about each person listed on the MLSFH household/family roster.
| Q2 | What is [name’s] relationship to you? |
| Q3 | Is [name] male or female? |
| Q4 | Is [name] alive? If [name] is dead, when did he/she die? (Note: Questions Q5 – 16 were not asked for persons who had died.) |
| Q5 | How old is [name]? Or in what year was [name] born? |
| Q6 | Where does [name] usually live? |
| Q7 | Did [name] sleep here last night? |
| Q8 | If a person does not regularly live here: W hen did [name] move to this place? |
| Q9 | Has [name] been ill in the past 12 months? If yes, for how long? |
| Q10 | How would you rate [name’s] health in general? |
| Q11 | How would you compare [name’s] health to other people in your village who are the same age and sex? |
| Q12 | What is [name’s] current marital status? |
| Q13– | What is the highest level of schooling [name] completed? How many grades |
| 14 | (in years) did [name] complete at that level? |
| Q15 | If age > 10: What is [name’s] main way of earning money? |
For persons who were reported as having died during the previous two years on the MLSFH household/family roster, the MLSFH also asked more detailed information about when the death occurred, how old the person was when he/she died, the level of schooling and the marital status of the diseased person, their health prior to dying, and the likelihood (as perceived by the respondent) that their death was due to AIDS.
Appendix Text 2: The matching process and diagnostics
We developed a probabilistic matching algorithm that generated longitudinal links between individuals on the household rosters across rounds of data collection. Individuals are matched based on name, age, sex, and familial relationship to respondent. To illustrate the matching process, we use two waves of data on a hypothetical household roster (the Salamu family) as an example.
Wave 1.
| First name | Last name | Age | Sex | Relationship to respondent |
|---|---|---|---|---|
| Rose | Salamu | 40 | Female | Respondent |
| Hastings | Salamu | 44 | Male | Husband/wife |
| Jaffali | Asamu | 22 | Male | Son/daughter |
Wave 2.
| First name | Last name | Age | Sex | Relationship to respondent |
|---|---|---|---|---|
| Rose | Sallumu | 42 | Female | Respondent |
| Hasing | Sallumu | 48 | Male | Husband/wife |
| Abiti | Sallumu | 72 | Female | Parent |
On the surface, we see that the first two individuals on each roster are a likely match, although there are some minor spelling differences in the names provided. The third individuals on each roster do not appear to be a strong match. Our algorithm initially sorts each dataset by primary respondent, and within each primary respondent by the listed members of the household. We then generate a dataset of all possible permutations of wave 1 to wave 2 matches within each primary respondent (that is, a file with one data line for each possible combination of individuals reported on the household roster within each primary respondent).
At this point, we generate a weighted match score based on first and last names (with gradations of match scores based on a generalization of the Levenshtein edit distance), age (with gradations of match scores based on proximity of ages between waves), sex, and relationship to respondent (respondent, spouse, child, parent, grandchild, grandparent, sibling, uncle/aunt, other related, or other nonrelated). Matching on name accounts for roughly half of the match score, while matching on age, sex, and relationship to respondent evenly account for the remainder of the match score. A match is counted as strong if the same individual is ranked first in each ranking (that is, the match has the highest score of all matches for each individual) and has a match score of over 65%. Lower-ranking matches (between 50% and 65%, or not ranking first across the same individual) are then set aside, cleaned of individuals with a strong match, and rematched using a higher threshold of 70%. This second match accounts for 1% or fewer of matched individuals. At this point, individuals with a match score of over 60%, or those who had a match score of over 50% but are missing information on one of the variables used in matching, are set aside for hand coding. These hand-coded matches also represent only about 1% of the final analysis sample and are generally cases where there were obvious mistakes in the data entry (such as an individual listed as the respondent’s grandparent being 77 years old in one wave, but listed as 7 years old two years later).
| Wave 1 | Wave 2 | Match | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| First name | Last name | Age | Sex | Relationship to respondent | First name | Last name | Age | Sex | Relationship to respondent | Match quality |
| Rose | Salamu | 40 | Female | Respondent | Rose | Sallumu | 42 | Female | Respondent | Strong |
| Rose | Salamu | 40 | Female | Respondent | Hasing | Sallumu | 48 | Male | Husband/wife | Poor |
| Rose | Salamu | 40 | Female | Respondent | Abiti | Asamu | 72 | Female | Parent | Poor |
| Hastings | Salamu | 44 | Male | Husband/wife | Hasing | Sallumu | 48 | Male | Husband/wife | Strong |
| Hastings | Salamu | 44 | Male | Husband/wife | Rose | Sallumu | 42 | Female | Respondent | Poor |
| Hastings | Salamu | 44 | Male | Husband/wife | Abiti | Asamu | 72 | Female | Parent | Poor |
| Jaffali | Salamu | 22 | Male | Son/daughter | Hasing | Sallumu | 48 | Male | Husband/wife | Poor |
| Jaffali | Salamu | 22 | Male | Son/daughter | Rose | Sallumu | 42 | Female | Respondent | Poor |
| Jaffali | Salamu | 22 | Male | Son/daughter | Abiti | Asamu | 72 | Female | Parent | Poor |
Table A-1 displays selected characteristics of the analysis sample. In the 2006–2012 rounds of data, few differences are evident on age composition, percent female, or education. The 2006–2010 waves of MLSFH data collection visited all available respondents from the MLSFH study population. In 2012, only primary respondents over the age of 45 were interviewed. This has little effect on the age composition of the sample (Panel A), although individuals on the household roster are more likely to be the children of respondents and somewhat less likely to be parents of the main respondent (Panel F). This higher proportion of children in the sample may also account for the slight increase in the proportion of listed individuals living within the same compound as the primary respondent and the commensurate decrease in the proportion living in the same household (Panel F). Percent female (Panel B), percent with 5+ years of education (Panel C), general health (Panel D), and method of matching (Panel H) show fairly small fluctuations across waves.
Figure A-1.
Study locations in Malawi
Table A-2 presents the match rates of individuals listed on the household rosters of the analysis sample by selected characteristics in 2006–2012. For the 2006–2010 rounds match rates varied between 76% (for 2006) and 84% (for 2010). In the 2012 round of survey collection, the household roster questionnaire was prefilled with names of spouses, children, and parents from the previous wave, which resulted in a higher overall match rate of over 92%. However, this increase in match rate does not appear to have affected sample characteristics (Table A-1). Match rates were similar in all age groups, although rates for the 60+ population in the 2006 wave lag behind the other waves somewhat. However, these ages are outside the prime ages where we would expect the effect of ART, and thus a differential in match rates is unlikely to bias our estimates. Match rates were generally higher for individuals who are closely related to the primary respondent (Panel B) and living geographically closer to the primary respondent (Panel C). Rates are comparable by sex (Panel D) and health status (Panel E). Overall, it does not appear that any systematic biases arise during the matching process, and the small variations in match rate that are observed occur in age ranges that are unlikely to affect our primary outcomes. Our findings on the increase in adult life expectancy post-ART are also robust to a number of alternative sample parameterizations (Table A-4).
Figure A-2.
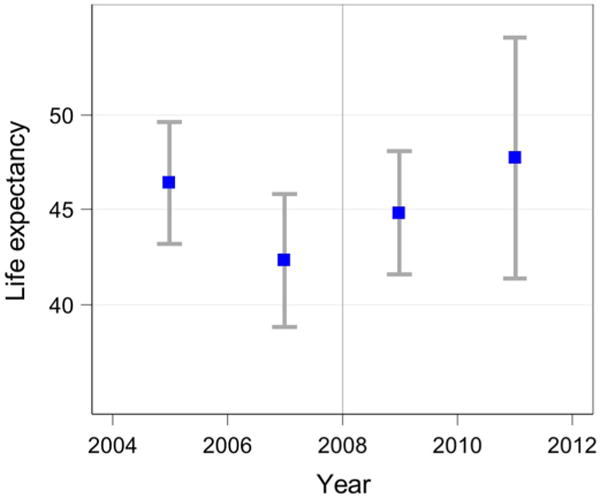
Life expectancy at age 15, 2004–2012 (including 2004–2006 sample)
Table A-1.
Selected characteristics of the analysis sample
| 2006 % |
2008 % |
2010 % |
2012 % |
||
|---|---|---|---|---|---|
|
A. Age
| |||||
| 15–29 | 39.1 | 36.7 | 37.5 | 42.4 | |
| 30–44 | 21.9 | 21.1 | 21.5 | 20.4 | |
| 45–59 | 20.5 | 20.8 | 18.7 | 17.0 | |
| 60–74 | 14.0 | 15.2 | 15.3 | 11.4 | |
| 75+ | 4.6 | 6.2 | 7.1 | 8.8 | |
| B. Percent female by age | |||||
|
| |||||
| 15–29 | 53.0 | 52.9 | 53.1 | 51.9 | |
| 30–44 | 57.0 | 55.5 | 55.3 | 50.9 | |
| 45–59 | 53.7 | 54.7 | 56.8 | 62.4 | |
| 60–74 | 50.0 | 49.7 | 50.5 | 51.2 | |
| 75+ | 42.6 | 42.4 | 46.7 | 54.5 | |
| C. Percent with five or more years education by age | |||||
|
| |||||
| 15–29 | 69.2 | 69.3 | 66.8 | 63.1 | |
| 30–44 | 57.0 | 56.8 | 57.6 | 60.9 | |
| 45–59 | 48.8 | 48.6 | 50.7 | 50.2 | |
| 60–74 | 34.8 | 36.1 | 37.5 | 32.2 | |
| 75+ | 28.2 | 27.6 | 27.9 | 20.9 | |
| D. General health | |||||
|
| |||||
| Excellent | 26.1 | 29.3 | 30.5 | 34.3 | |
| Very good | 37.2 | 36.2 | 36.1 | 34.3 | |
| Good | 29.0 | 29.4 | 29.1 | 26.8 | |
| Poor | 7.4 | 4.6 | 3.9 | 4.1 | |
| Very poor | 0.3 | 0.5 | 0.5 | 0.4 | |
|
E. Percent in poor/very poor health by age
| |||||
| 15–29 | 3.3 | 3.3 | 3.1 | 2.3 | |
| 30–44 | 5.5 | 5.7 | 5.1 | 5.5 | |
| 45–59 | 10.2 | 10.4 | 9.2 | 6.8 | |
| 60–74 | 22.7 | 18.7 | 17.8 | 20.2 | |
| 75+ | 35.9 | 35.9 | 31.2 | 41.8 | |
|
F. Relationship to primary respondent
| |||||
| Respondent | 24.4 | 20.5 | 19.7 | 14.4 | |
| Spouse | 17.5 | 16.4 | 15.2 | 10.2 | |
| Child/child-in-law | 26.8 | 29.2 | 36.2 | 62.8 | |
| Parent/parent-in-law | 29.1 | 32.4 | 27.8 | 11.4 | |
| Other | 2.2 | 1.5 | 1.1 | 1.2 | |
| G. Where individual usually lives | |||||
|
| |||||
| Same HH | 55.6 | 46.1 | 44.4 | 37.8 | |
| Same compound | 13.2 | 14.7 | 14.9 | 18.2 | |
| Same village | 5.1 | 6.2 | 7.3 | 5.1 | |
| Same TA | 9.7 | 12.5 | 13.1 | 13.6 | |
| Same district | 7.6 | 7.5 | 8.6 | 8.7 | |
| Elsewhere | 8.8 | 12.9 | 11.7 | 16.6 | |
|
H. How individual matched
| |||||
| First round | 98.8 | 97.8 | 98.7 | 99.1 | |
| Second round | 0.3 | 1.1 | 0.3 | 0.3 | |
| Hand matched | 0.9 | 1.1 | 1.0 | 0.6 | |
|
| |||||
| N observations | 7,481 | 8,883 | 7,913 | 3,733 | |
| N primary respondents | 1,822 | 1,821 | 1,557 | 526 | |
Table A-2.
Match rate for analysis sample by selected characteristics
| 2006 % |
2008 % |
2010 % |
2012 % |
||
|---|---|---|---|---|---|
| Overall match rate | 75.9 | 80.9 | 82.2 | 92.5 | |
| A. Age | |||||
|
| |||||
| 15–29 | 75.6 | 79.9 | 84.6 | 92.9 | |
| 30–44 | 83.1 | 85.6 | 89.0 | 97.3 | |
| 45–59 | 78.1 | 81.5 | 79.0 | 91.9 | |
| 60–74 | 67.9 | 80.1 | 74.4 | 89.3 | |
| 75+ | 62.4 | 77.4 | 75.8 | 85.0 | |
| B. Relationship to respondent | |||||
|
| |||||
| Respondent | 96.9 | 98.0 | 98.0 | 97.7 | |
| Spouse | 85.0 | 85.6 | 83.8 | 81.3 | |
| Child/child-in-law | 77.5 | 83.5 | 92.1 | 96.1 | |
| Parent/parent-in-law | 66.8 | 75.8 | 69.9 | 86.0 | |
| Other | 12.0 | 22.3 | 21.3 | 42.3 | |
| C. Where individual usually lives | |||||
|
| |||||
| Same household | 84.3 | 85.6 | 86.0 | 90.1 | |
| Same compound | 70.1 | 79.9 | 83.3 | 95.4 | |
| Same village | 65.1 | 78.4 | 76.0 | 93.2 | |
| Same traditional authority | 69.4 | 73.8 | 75.7 | 93.4 | |
| Same district | 65.0 | 80.0 | 80.9 | 95.0 | |
| Lilongwe | 60.6 | 78.1 | 83.3 | 92.4 | |
| Blantyre | 74.2 | 71.8 | 90.0 | 91.7 | |
| Elsewhere | 64.7 | 74.7 | 77.5 | 92.8 | |
|
D. Sex
| |||||
| Male | 75.2 | 80.3 | 80.1 | 90.5 | |
| Female | 76.5 | 81.4 | 84.2 | 94.5 | |
|
E. General health
| |||||
| Excellent | 80.3 | 80.7 | 84.4 | 93.4 | |
| Very good | 76.9 | 82.5 | 82.4 | 92.4 | |
| Good | 73.1 | 79.9 | 80.1 | 92.4 | |
| Poor | 71.2 | 81.6 | 83.0 | 92.1 | |
| Very poor | 72.0 | 86.0 | 84.2 | 82.4 | |
Table A-3.
Adult life expectancy and median length of life by sex
| A. Adult life expectancy | |||
|---|---|---|---|
| Period | Years | 95% CI | |
| Male | |||
| 2006–2008 | 40.3 | 36.4 | 44.1 |
|
| |||
| 2008–2010 | 43.0 | 39.6 | 46.5 |
| 2010–2012 | 45.2 | 40.7 | 49.7 |
| Post-ART (2008–2012) | 43.7 | 40.9 | 46.4 |
| Female | |||
| 2006–2008 | 47.2 | 43.6 | 50.9 |
|
| |||
| 2008–2010 | 47.0 | 43.4 | 50.5 |
| 2010–2012 | 47.6 | 41.9 | 53.3 |
| Post-ART (2008–2012) | 48.2 | 45.2 | 51.1 |
| B. Median length of life | |||
| Period | Years | 95% CI | |
|
| |||
| Male | |||
| 2006–2008 | 39 | 34 | 45 |
|
| |||
| 2008–2010 | 45 | 41 | 49 |
| 2010–2012 | 49 | 40 | 53 |
| Post-ART (2008–2012) | 45 | 42 | 49 |
| Female | |||
| 2006–2008 | 48 | 42 | 55 |
|
| |||
| 2008–2010 | 50 | 44 | 56 |
| 2010–2012 | 55 | 45 | 58 |
| Post-ART (2008–2012) | 50 | 46 | 56 |
Notes: Data was truncated at age 95 when dividing the sample by gender due to small samples at older ages. We close the survival function by exponentially extending the survival curve to zero.
Table A-4.
Adult life expectancy and median length of life for alternative definitions of the study population
| Years | 95% CI | |||
|---|---|---|---|---|
|
A. Coresident with primary respondent
| ||||
| Adult life expectancy | ||||
| 2006–2008 | 42.3 | 38.7 | 45.9 | |
| 2008–2010 | 44.8 | 41.5 | 48.1 | |
| 2010–2012 | 47.7 | 41.3 | 54.1 | |
| Post | 46.6 | 43.7 | 49.4 | |
| Median age at death | ||||
| 2006–2008 | 58 | 51 | 65 | |
| 2008–2010 | 67 | 59 | 69 | |
| 2010–2012 | 68 | 58 | 74 | |
| Post | 67 | 64 | 70 | |
|
B. All available observations
| ||||
| Adult life expectancy | ||||
| 2006–2008 | 42.7 | 40.7 | 44.7 | |
| 2008–2010 | 43.4 | 41.7 | 45.2 | |
| 2010–2012 | 45.2 | 43.0 | 47.5 | |
| Post | 44.1 | 42.7 | 45.5 | |
| Median age at death | ||||
| 2006–2008 | 58 | 55 | 62 | |
| 2008–2010 | 60 | 59 | 62 | |
| 2010–2012 | 65 | 60 | 68 | |
| Post | 61 | 60 | 64 | |
|
C. Matched in first round of matching
| ||||
| Adult life expectancy | ||||
| 2006–2008 | 45.3 | 42.6 | 48.0 | |
| 2008–2010 | 48.2 | 45.6 | 50.8 | |
| 2010–2012 | 47.5 | 43.6 | 51.3 | |
| Post | 47.9 | 45.7 | 50.0 | |
| Median age at death | ||||
| 2006–2008 | 60 | 55 | 65 | |
| 2008–2010 | 66 | 62 | 69 | |
| 2010–2012 | 68 | 63 | 73 | |
| Post | 67 | 64 | 69 | |
|
D. Respondent, spouse, children, and parents only
| ||||
| Adult life expectancy | ||||
| 2006–2008 | 43.0 | 40.4 | 45.6 | |
| 2008–2010 | 45.2 | 42.7 | 47.6 | |
| 2010–2012 | 45.9 | 42.2 | 49.5 | |
| Post | 45.1 | 43.1 | 47.1 | |
| Median age at death | ||||
| 2006–2008 | 58 | 54 | 63 | |
| 2008–2010 | 62 | 59 | 66 | |
| 2010–2012 | 67 | 60 | 70 | |
| Post | 64 | 60 | 67 | |
Notes: Panel A uses all individuals in the analysis sample who were listed as living in the same household or the same compound as the primary respondent. Panel B expands the analysis sample to all individuals listed on household rosters, including duplicate reports on the same household by spouses. Panel C uses only those individuals in the analysis sample who were matched in the first pass of the matching algorithm. Panel D limits the analysis sample to respondents and their and spouses, children, and parents.
Footnotes
See MLSFH Google Scholar Profile at https://scholar.google.com/citations?user=dNEAH3YAAAAJ.
References
- Asiki G, Murphy G, Nakiyingi-Miiro J, Seeley J, Nsubuga RN, Karabarinde A, GPC team The general population cohort in rural south-western Uganda: A platform for communicable and non-communicable disease studies. International Journal of Epidemiology. 2013;42(1):129–141. doi: 10.1093/ije/dys234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov V, Bennett D, Kohler HP. The indirect impact of antiretroviral therapy: Mortality risk, mental health, and HIV-negative labor supply. Journal of Health Economics. 2015;44:195–211. doi: 10.1016/j.jhealeco.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov V, Kohler HP. The impact of AIDS treatment on savings and human capital investment in Malawi. Philadelphia: University of Pennsylvania Libraries; 2014. (PSC Working Paper Series). http://repository.upenn.edu/psc_working_papers/55/ [Google Scholar]
- Barnett T, Whiteside A. AIDS in the twenty-first century: Disease and globalization. 2nd. Basingstoke: Palgrave Macmillan; 2006. [Google Scholar]
- Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: Valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis F, Manda E. Seasonal food crises and policy responses: A narrative account of three food security crises in Malawi. World Development. 2012;40(7):1407–1417. doi: 10.1016/j.worlddev.2012.03.005. [DOI] [Google Scholar]
- Floyd S, Marston M, Baisley K, Wringe A, Herbst K, Chihana M, Zaba B. The effect of antiretroviral therapy provision on all-cause, AIDS and non-AIDS mortality at the population level: A comparative analysis of data from four settings in Southern and East Africa. Tropical Medicine and International Health. 2012;17(8):e84–93. doi: 10.1111/j.1365-3156.2012.03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd S, Molesworth A, Dube A, Banda E, Jahn A, Mwafulirwa C, French N. Population-level reduction in adult mortality after extension of free anti-retroviral therapy provision into rural areas in Northern Malawi. PLoS One. 2010;5(10):e13499. doi: 10.1371/journal.pone.0013499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E, Anglewicz P. HIV prevalence and sexual behaviour at older ages in rural Malawi. International Journal of STD and AIDS. 2012;23(7):490–496. doi: 10.1258/ijsa.2011.011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, Munthali F, Glynn JR. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371(9624):1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd. Hoboken: Wiley-Interscience; 2002. [DOI] [Google Scholar]
- Kasamba I, Baisley K, Mayanja BN, Maher D, Grosskurth H. The impact of antiretroviral treatment on mortality trends of HIV-positive adults in rural Uganda: A longitudinal population-based study, 1999–2009. Tropical Medicine and International Health. 2012;17(8):e66–e73. doi: 10.1111/j.1365-3156.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler HP, Watkins SC, Behrman JR, Anglewicz P, Kohler IV, Thornton RL, Kalilani-Phiri L. Cohort profile: The Malawi Longitudinal Study of Families and Health (MLSFH) International Journal of Epidemiology. 2015;44(2):394–404. doi: 10.1093/ije/dyu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E, Bendavid E, Tuoane-Nkhasi M, Mbengashe T, Goldman T, Wilson M, Klausner JD. Population-level associations between antiretroviral therapy scale-up and all-cause mortality in South Africa. International Journal of STD and AIDS. 2014;25(9):636–642. doi: 10.1177/0956462413515639. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, Hogg RS. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: A cohort analysis from Uganda. Annals of Internal Medicine. 2011;155(4):209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- Mwagomba B, Zachariah R, Massaquoi M, Misindi D, Manzi M, Mandere BC, Harries AD. Mortality reduction associated with HIV/AIDS care and antiretroviral treatment in rural Malawi: Evidence from registers, coffin sales and funerals. PloS One. 2010;5(5):e10452. doi: 10.1371/journal.pone.0010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odhiambo FO, Laserson KF, Sewe M, Hamel MJ, Feikin DR, Adazu K, Vulule JM. Profile: The KEMRI/CDC Health and Demographic Surveillance System – Western Kenya. International Journal of Epidemiology. 2012;41(4):977–987. doi: 10.1093/ije/dys108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CF, Mkandawire J, Kohler HP. Disability transitions and health expectancies among adults 45 years and older in Malawi: A cohort-based model. PLOS Med. 2013;10(5):e1001435. doi: 10.1371/journal.pmed.1001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Matzopoulos R, Bradshaw D. Second National Burden of Disease Study South Africa: National and subnational mortality trends, 1997–2009. The Lancet. 2013;381(2):S113. doi: 10.1016/S0140-6736(13)61367-7. [DOI] [Google Scholar]
- Price AJ, Glynn J, Chihana M, Kayuni N, Floyd S, Slaymaker E, Crampin AC. Sustained 10-year gain in adult life expectancy following antiretroviral therapy roll-out in rural Malawi: July 2005 to June 2014. International Journal of Epidemiology. 2016 doi: 10.1093/ije/dyw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon JA, Wang H, Freeman MK, Vos T, Flaxman AD, Lopez AD, Murray CJL. Healthy life expectancy for 187 countries, 1990–2010: A systematic analysis for the Global Burden Disease Study 2010. Lancet. 2012;380(9859):2144–2162. doi: 10.1016/S0140-6736(12)61690-0. [DOI] [PubMed] [Google Scholar]
- Stoneburner R, Korenromp E, Lazenby M, Tassie JM, Letebele J, Motlapele D, Low-Beer D. Using health surveillance systems data to assess the impact of AIDS and antiretroviral treatment on adult morbidity and mortality in Botswana. PloS One. 2014;9(7):e100431. doi: 10.1371/journal.pone.0100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover J, Fidzani B, Molomo BC, Moeti T, Musuka G. Estimated HIV trends and program effects in Botswana. PloS One. 2008;3(11):e3729. doi: 10.1371/journal.pone.0003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanser F, Hosegood V, Bärnighausen T, Herbst K, Nyirenda M, Muhwava W, Newell ML. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. International Journal of Epidemiology. 2008;37(5):956–962. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambura M, Urassa M, Isingo R, Ndege M, Marston M, Slaymaker E, Zaba B. HIV prevalence and incidence in rural Tanzania: Results from 10 years of follow-up in an open-cohort study. Journal of Acquired Immune Deficiency Syndromes. 2007;46(5):616–623. doi: 10.1097/QAI.0b013e31815a571a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World health statistics 2014. Geneva: World Health Organization; 2014. http://public.eblib.com/choice/publicfullrecord.aspx?p=1741840. [Google Scholar]
- Wroe D. Donors, dependency, and political crisis in Malawi. African Affairs. 2012;111(442):135–144. doi: 10.1093/afraf/adr076. [DOI] [Google Scholar]


