Abstract
Zinc is emerging as a widely used and important biological regulatory signal. Cellular zinc levels are tightly regulated by a complex array of zinc importer and exporters to control processes such as apoptotic cell death. While caspase inhibition by zinc has been reported previously, the reported inhibition constants were too weak to suggest a critical biological role for zinc-mediated inhibition. In this work we have adopted a method of assessing available zinc. This allowed assessment of the accurate inhibition constants for apoptotic caspases, caspase-3, -6, -7 and -8. Each of these caspases are inhibited by zinc at intracellular levels, however, with widely differing inhibition constants and different zinc binding stoichiometries. Caspase -3, -6 and -8 appear to be constitutively inhibited by typical zinc levels and this inhibition must be lifted to allow activation. The inhibition constant for caspase-7 (76 nM) is much weaker than for the other apoptotic caspases (2.6–6.9 nM) suggesting that caspase-7 is not inactivated by normal zinc concentrations but can be inhibited under conditions of zinc stress. Caspase-3, -7, and -8 were found to bind three, one, and two zincs respectively. In each of these caspases, zinc was present in the active site, in contrast to caspase-6, which binds one zinc allosterically. The most notable new mechanism to emerge from this work is for zinc-mediated inhibition of caspase-8. Zinc binds caspase-8 directly at the active site and at a second site. Zinc binding inhibits formation of the caspase-8 dimer, the activated form of the enzyme. Together these findings suggest that zinc plays a critical role in regulation of apoptosis by direct inactivation of caspases, in a manner that is unique for each caspase.
Keywords: allosteric sites, exosites, zinc regulation, zinc gradients, zincon, protease, zinc buffering
Introduction
Until recently the magnitude of the impact of zinc on a wide array of biological processes has remained underappreciated. It has now become clear that zinc plays fundamental roles in growth, development, metabolism, gene transcription, and signaling. A large body of work has established both structural and catalytic roles for zinc in biology, and more recent investigations have observed zinc acting directly as a signal.1,2 In addition, sequence analysis suggests that up to 10% of the human proteome has potential zinc binding sites, leading to questions about the bioavailable levels of zinc in the cell, as well as the significance of zinc as a regulatory entity.3 The details of the function and consequence of zinc binding in relationship to the various biological roles it takes are still emerging. Nevertheless, it is becoming abundantly clear that zinc plays central roles in many processes.
The regulation of intracellular zinc levels and zinc localization is imperative for maintaining proper homeostasis, and the extent of zinc’s regulation is remarkable. Whereas levels of other metals, particularly metals that only play roles structurally or as cofactors, can be adequately regulated by a small number of transporters (e.g. one exporter for iron in humans)4,5, cellular levels of zinc are tightly controlled by no fewer than 14 zinc importers and 10 zinc exporters in humans, as well as metallothioneins as intracellular zinc buffering proteins (for review6). The complexity of the zinc regulatory machinery underscores the importance of tightly regulating and controlling zinc levels. It is estimated that total cellular zinc concentrations reach hundreds of micromolar, mostly bound to cellular proteins, but the “free” or “available” zinc pool is estimated to be in the high picomolar to low nanomolar range.7–10 This significant disparity between high total zinc levels and low available zinc levels further emphasizes importance of the tight regulation to maintain zinc at precisely the appropriate levels at various locations intra- and extracellularly. Small fluxes in the available pool of zinc can have dramatic consequences. Zinc sparks, where up to 1010 zinc ions are released during oocyte fertilization, lead to intense spikes in the local extracellular zinc.11 This stems from a coordinated release of zinc from vesicles averaging one million zinc atoms per vesicle, with some measuring 1.8 M zinc concentrations.12,13 In addition, zinc waves can constitute an intracellular signaling event.1 A zinc wave is mediated by zinc importers that allow influx of excess zinc into the cytosol, directly inactivating phosphatases,14,15 which as a result, also leads to activation of kinases.16 In addition to regulation of biological processes, control of intracellular zinc levels has been implicated in disease. For example, the zinc importer protein ZIP7, is overexpressed in breast cancer,17 suggesting that cancer progression may be aided by the presence of excess zinc.18 B-cells that are deficient in ZIP10 have been shown to have impaired maturation as well as increased activities of caspases-3, -8, -9, and -12 resulting in cell death, suggesting that zinc plays a key role in mediating cell survival.19 These are examples of how small changes in available zinc have substantial cellular implications and motivates our pursuit of detailed biochemical characterizations of zinc binding to important cellular targets.
Because zinc functions as a regulator and cellular signal, dysregulation of zinc homeostasis can have dramatic effects, and links to Alzheimer’s,20 cancer,21 and diabetes22,23 have been reported. One central theme interconnecting these multifactorial diseases is the apoptotic response; excessive and repressed cell death has been implicated in neurodegeneration and proliferation, respectively. Apoptosis is a biologically central pathway that is highly susceptible to regulation by zinc (for review see work by the Zalewski group24). It is known that zinc deficiency results in an increase in apoptosis, with some seminal experiments showing this correlation in adult animals.25 Furthermore, zinc has shown a protection effect by targeting apoptotic proteins, particularly the caspases, as well as other protective roles such as in oxidative stress.26
At the heart of apoptosis is a class of aspartate-directed cysteine proteases known as caspases, the activity of which is essential for programmed cell death. Apoptotic caspases are classified into two types, the upstream initiator caspases, and their substrates, the downstream executioner caspases. The executioner caspases -3, -6, and -7 exist as homodimers and require cleavage at their intersubunit linker to achieve full activity. In contrast, the activity of the initiator caspases -8, -9, and -10 is dependent on a trigger, such as dimerization or binding to an activating platform. Caspase-9 is activated on the heptameric apoptosome.27 In the case of caspase-8, a death inducing signaling complex (DISC) on the cytosolic face of the cell membrane recruits monomeric procaspase-8 via its death effector domain (DED) and is subsequently activated in an oligomeric dependent manner.28 Caspase-8 requires both dimerization and cleavage in order to be activated.29
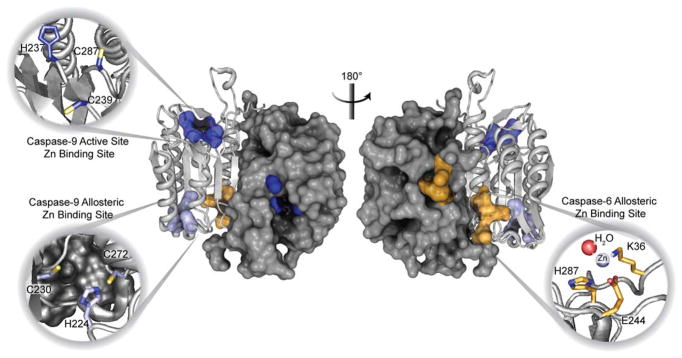
There is mounting evidence that zinc plays an inhibitory role by blocking caspase activity30–33 thus serving an anti-apoptotic function. Caspases use a cysteine-histidine catalytic dyad, both of which are necessary components for hydrolysis of protein substrates. This dyad is of interest in zinc regulation as the position of the cysteine and histidine in the catalytic dyad is ideal for zinc ligation. Thus, understanding the role of zinc-mediated regulation of the caspases has emerged as an important question. Previous studies have defined specific zinc regulatory sites on caspases-633 and -932, as well as mounting evidence for zinc-mediated inhibition of caspase-3.34–37 Caspase-9 is inhibited by zinc by binding at the active site cysteine. However, further analysis revealed a second zinc binding to caspase-9 at an exosite below the 210s helix (Figure 1).32 In addition, caspase-6 crystallographic studies established that zinc binds to an allosteric site composed of Lys38, Glu244 and His287 (Figure 1, Figure S1).33 In caspase-6 zinc does not bind to the catalytic dyad in the active site, because the enzyme rests in a helical conformation that keeps the dyad apart until substrate binds. Caspase-3 has been proposed to bind zinc via its catalytic histidine and a proximal methionine, rather than the reactive cysteine, yet this data still remains to be verified.36 However, it has been shown that caspase-3 is inhibited at extremely low concentrations down to 1.7 nM.34 These previous studies provide evidence that zinc inhibition may be nuanced for each caspase and may occur at biologically relevant, low levels of available zinc.
Figure 1.
Known zinc binding sites in caspases.
Previous investigations have found that caspase-9 (represented by PDB 1JXQ) binds zinc at the active site as well as at an allosteric at the bottom of the 210’s helix. Conversely, caspase-6 binds a single zinc at a defined allosteric site, but does not bind zinc at the active site (PDB 4FXO). Representative caspase structure modeled by PDB ID 1F1J.
Evidence suggests that direct zinc-mediated inhibition of caspases is important in regulation of apoptosis. PAC-1 was discovered as a proapoptotic small molecule that allows activation of procaspase-3.38 Later work showed that PAC-1 functions by sequestering inhibitory zinc bound to procaspase-3, allowing auto-activation in vitro and progression of apoptosis in cells.39 In addition, it was shown that zinc co-localizes with the apoptotic caspase-3 in airway epithelial cells.40 This finding highlights the importance of zinc-mediated regulation of caspase-3 in apoptotic control.
To truly appreciate the level of control zinc exerts in regulation of apoptosis it is essential to elucidate the details for zinc binding to all the caspases. In particular, to date, the details of zinc-mediated regulation of caspase-7 and -8 is entirely lacking. In this investigation, we have pursued detailed biochemical characterization of zinc inhibition for caspase-3, -6, -7, and -8. We have evidence that zinc selectively inhibits these caspases amongst a panel of relevant metals. Moreover, the zinc binding and inhibition of these caspases occurs at biologically relevant levels of available zinc, in the low nanomolar range. We also interrogated the mechanisms of inhibition and found zinc does indeed have an affinity for the catalytic dyad of caspase-7 and the catalytic cysteine for caspase-8. In addition, zinc has a destabilizing effect on caspase-8 and its oligomeric state by disrupting its ability to form the dimer necessary for activity. This provides key molecular details that establish a deeper understanding of zinc regulating caspases and apoptosis.
Results
Zinc Inhibits Caspases from Cleaving Peptide and Protein Substrates
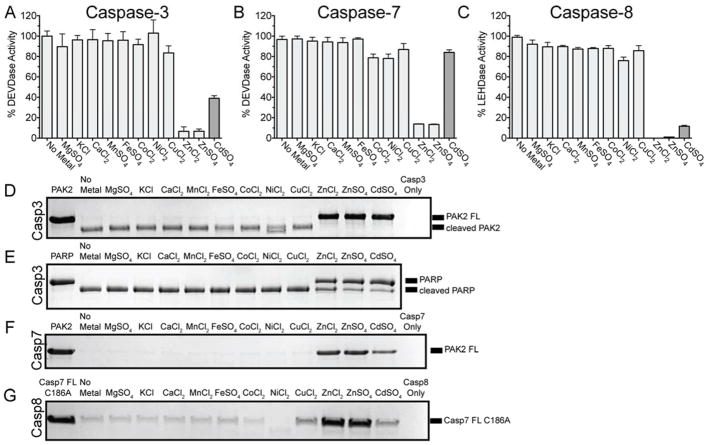
Each individual caspase has a variety of interactive surface elements as well as a reactive cysteine nucleophile in the active site, which could facilitate binding zinc or other biologically-relevant metals. Therefore, the effect of a panel of biologically relevant metals was tested on the two primary executioner caspases (caspase-3 and -7) as well as the initiator caspase-8, which had not been reported previously. Each of these apoptotic caspases were treated with a panel of metals and their activity was assessed by the ability to cleave a preferred fluorogenic tetrapeptide substrate (caspase-3, -7: DEVD-AMC; caspase-8: LEHD-AMC, Figure 2A–C). Among the biologically-relevant metals, zinc and only zinc inhibited all three caspases. Both ZnCl2 and ZnSO4 showed similar levels of inhibition suggesting that zinc and not the counter ion is responsible for the observed inhibition. Cadmium was tested for all three caspases because it shares similar properties to zinc: a group 12 metal with a d10 valence electron configuration. Cadmium had a marginal effect on the ability of caspase-7 to cleave a peptide substrate (84% activity remained), a more significant effect on caspase-3 (39% activity remained), and an almost complete inhibitory effect on caspase-8 (12% activity remained) (Figure 2A–C).
Figure 2.
Zinc inhibits caspase-3, -7, and -8 cleavage of peptide and protein substrates. A panel of biologically-relevant metals were independently incubated with (A) caspase-3 (B) caspase-7 or (C) caspase-8. The effect of each metal was assessed by monitoring cleavage of an appropriate fluorogenic tetrapeptide substrate. Data are shown as the mean ± SD for three separate experiments performed on three separate days. The effect of each metal was tested on individual caspases cleaving a protein substrate: (D) caspase-3 cleaving PAK2 (E) caspase-3 cleaving PARP (F) caspase-7 cleaving PAK2 and (G) caspase-8 cleaving the catalytically inactive caspase-7 full-length substrate.
Peptide substrates such as DEVD-AMC and LEHD-AMC occupy only the substrate-binding groove of the respective caspases, but for several caspases, zinc binding occurs outside the catalytic site, allosterically. Allosteric zinc binding may have a small impact on cleavage of peptide substrates, but a pronounced impact on protein substrates, which are larger and may engage exosites in binding and recognition. While hydrolyzing a protein substrate, the caspase is predicted to make contacts beyond the amino acids proximal to the active site, at exosites such as those that have been identified in the caspase-7 N-terminal region.41,42 Thus the effect of metal-mediated caspase inhibition on protein substrates may be a means to identify other caspase exosites. First, caspase-3 was incubated with each metal, and then allowed to cleave the protein substrate, PAK2 (Figure 2D). Both ZnCl2 and ZnSO4 completely inhibited substrate cleavage, with cadmium having a similar effect. Previous reports have suggested that other metals have an inhibitory effect on caspase-3, including copper and cobalt.35,39 We did not observe a copper or cobalt-mediated inhibition. To confirm this observation, the assay was repeated with another caspase-3 substrate, PARP (Figure 2E), as done in previous work.35 Similar to the PAK2 substrate, only zinc and cadmium had an inhibitory effect under these conditions. This result for cadmium is counter to what was observed with the peptide substrate (Figure 2A,D,E), suggesting that multiple metal sites, including exosites, may be relevant in caspase-3 function and inhibition.
Caspase-7 and caspase-8 were subjected to the metal panel in an identical fashion to caspase-3. Caspase-7 cleavage of the protein substrate PAK2 was inhibited by both ZnCl2 and ZnSO4, but no other biologically-relevant metal (Figure 2F); cadmium, however, had an inhibitory effect. This was a surprising result based on the previous observation that cadmium had only a slight effect on caspase-7 cleaving a peptide substrate. For caspase-8, procaspase-7 (C186A to prevent any self-processing) was used as a representative substrate (Figure 2G). Zinc and only zinc had a significant impact on cleavage of a protein substrate by caspase-8. Copper and cadmium had a minor impact on cleavage. These data underscore the nuanced nature of zinc binding to each caspase.
Biologically Relevant Zinc Concentrations Inhibit Caspase-3, -6, -7, and -8
While it is clear that zinc selectively inhibits caspase-3, -7, and -8 in standard caspase assay conditions (Figure 2) as well as caspase-6,33 the affinity can not be accurately measured unless the zinc-binding properties of every component of the solution are both understood and accounted for. In addition, zinc-mediated inhibition is only biologically relevant at certain affinities. While total intracellular zinc concentrations are estimated to be in the micromolar range,8 available zinc concentrations are tightly controlled at much lower levels, estimated to be high picomolar or low nanomolar.8 Therefore, it is imperative while making in vitro measurements at low zinc concentrations that the system be carefully managed so that the available zinc concentration can be known and accurately tested. To achieve such control, we used zinc-buffering systems15 and calculated the available zinc concentration utilizing the MaxChelator43 tool. By deliberately choosing each buffer component such that binding affinities for zinc ligation are accurately known, we are able to attain specific free zinc ion levels and prevent large fluctuations in free zinc concentration over the course of experimentation. HEPES buffer at pH 7.5 was used due its low binding capacity for zinc44 and TCEP as the reducing agent in order to avoid introducing excess thiols, which can coordinate heavy metals.45 In addition, the zinc buffering reagent was carefully chosen based on the buffering capacity and the concentration range of zinc under investigation. The experiments were also performed in the absence of a metal buffering agent to compare the effect of zinc buffering on the catalytic activity of these caspases.
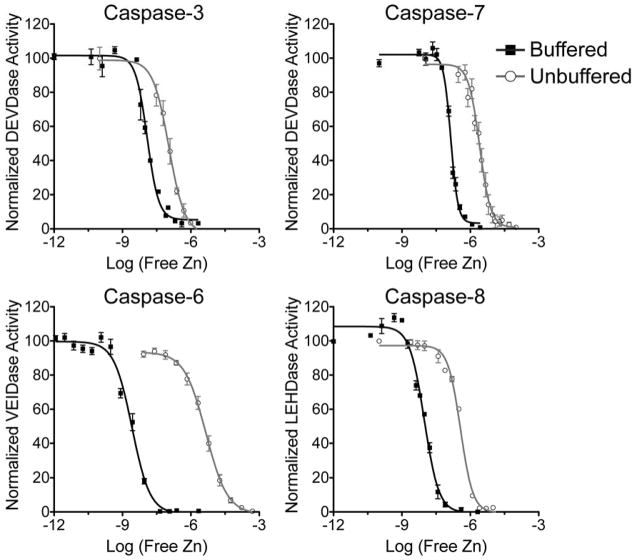
After careful consideration of the buffer, caspase-3, -6, -7, and -8 were subjected to a titration of increasing zinc concentrations and assayed for remaining activity (Figure 3). This was done in the absence of zinc buffering components, so only total zinc can be reported or in the presence of a zinc buffer system, which allows the available zinc concentration to be reported. Under zinc-buffering conditions there was a significant shift in IC50 values relative to the unbuffered conditions (Table 1). Caspase-6, which is known to bind zinc at an exosite, showed the largest shift in binding affinity (IC50 of 4.5 μM (unbuffered)33 to 2 nM (buffered)). Caspase-3, -7, and -8 were then assayed, under buffered conditions, at various concentrations of zinc to investigate the mechanism of inhibition (Figure S2). The curves for all three caspases best fit to a mixed model of inhibition, and Ki values were calculated based on a global fit. The inhibition constants for all caspases shifted into the biologically relevant range of zinc concentrations (Table 1) demonstrating the importance of assaying inhibition using zinc-buffering to accurately reflect cellular conditions.
Figure 3.
Zinc buffering conditions reveal zinc-mediated inhibition at biologically relevant concentrations. Dose response curves of caspase activity (10 nM) with increasing concentrations of zinc. A preferred tetrapeptide substrate was used for each caspase. Titrations were carried out under non-buffering conditions for zinc (gray lines) and repeated under zinc buffering conditions (black lines). Each titration was performed in duplicate on three separate days with values represented as means ± SEM and fit for the IC50 value.
Table 1. Parameters for Zinc Inhibition of Caspases under Metal Buffering Conditions and Unbuffered Conditions.
The zinc buffer utilized for caspase-3, -6, and -8 was NTA and citrate was used for caspase-7. All zinc calculations were done using MaxChelator. Inhibition constants were calculated assuming a mixed model of inhibition, with the exception of caspase-6 (*), which was assumed to be noncompetitive based on previous determination of allosteric binding of zinc.
| IC50 Values (nM) | Ki (nM) | ||
|---|---|---|---|
|
| |||
| Unbuffered | Buffered | ||
| Caspase-3 | 104 ± 26 | 12.5 ± 0.5 | 6.9 |
| Caspase-6 | 4530 ± 1100 | 2.61 ± 0.4 | 2.6* |
| Caspase-7 | 1580 ± 750 | 141 ± 2.1 | 76.1 |
| Caspase-8 | 367 ± 38 | 9.25 ± 1.2 | 4.3 |
Determining the Stoichiometry of Zinc Binding in Caspases
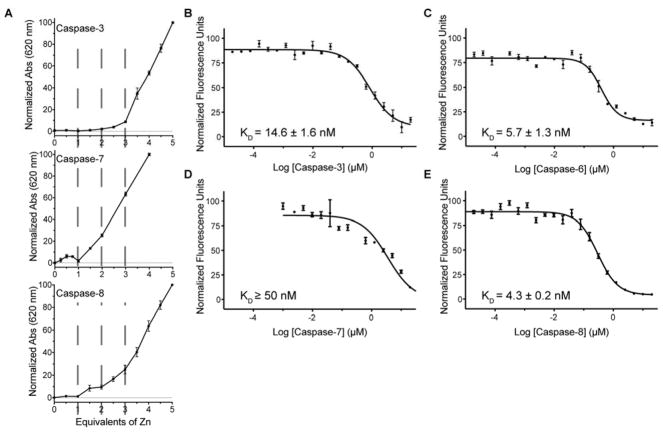
Given that inhibition was observed at biologically relevant concentrations, we next sought to determine the zinc binding stoichiometry for each caspase. Zincon, a colorometric zinc indicator, is a low affinity zinc chelator (KD= 12 μM)46,47 that undergoes a change in absorbance spectra upon zinc binding. Thus, zinc was titrated into a sample containing zincon and caspase at increasing molar equivalents to determine the zinc-binding stoichiometry for each caspase (Figure 4A). Once all caspase zinc-binding sites have been occupied, a colorimetric response is immediately produced and detected. Thus only caspase sites with a tighter binding affinity than zincon can be readily detected using this assay. The point where this colorimetric response occurs corresponds to the number of zincs binding per caspase monomer. Caspase-3, -7, and -8 were found to bind three, one, and two zincs respectively. Stoichiometries for caspase-3 and -7 agree with previously reported results obtained by ICP-OES.33 Caspase-8 was observed to bind two zincs, although the shape of the zincon response curve suggested that the two zincs bind with different affinities. A slight rise in the absorbance at two molar equivalents of zinc could indicate a competition with the zincon reporter. This suggests that the second binding site in caspase-8 and the zincon reporter bind the zinc with similar affinities, with estimations around 12 μM. Lastly, in the absence of caspase the indicator responded with an immediate increase in absorbance and in the presence of equimolar EDTA there was exactly one zinc binding site observed (Figure S3).
Figure 4.
Stoichiometry and affinity of zinc binding. (A) Zincon was used as a colorimetric zinc indicator to determine the stoichiometry of zinc binding for caspase-3, -7, and -8. Molar equivalents of zinc were added to a mixture of caspase and the zincon indicator. Absorption spectra recorded at 620 nm indicate a zincon response after three molar equivalents for caspase-3 (top), one equivalent for caspase-7 (middle), and two equivalents for caspase-8 (bottom). Each graph represents the mean ± SEM for three separate experiments on three separate days. The fluorescent zinc indicator Fluozin-3 was used to determine zinc affinity by titrating (B) caspase-3, (C) caspase-6, (D) caspase-7 and (E) caspase-8 against of a constant concentration of zinc. Each graph represents the mean ± SEM for three experiments on three separate days.
Caspases Exhibit Low Nanomolar Affinity for Zinc
After determining the stoichiometry of zinc binding to caspase-3, -7, and -8 using the low affinity indicator Zincon, we were motivated to assess zinc binding at biologically relevant zinc concentrations. Fluozin-3 is a zinc indicator that exhibits fluorescence when bound to zinc and has a KD for zinc equal to 15 nM.48 Due to the tight zinc binding of Fluozin-3 it is particularly useful when examining high affinity binding sites on proteins; only caspase binding sites that show a tighter affinity than Fluozin-3 will be accurately measured. Thus, a competition experiment was carried out wherein each caspase was titrated into a reaction with a constant amount of zinc and indicator. Each reaction was allowed to reach equilibrium and the fluorescence of Fluozin-3 was measured. The fluorescence signal was lost as increasing caspase concentrations competed for the zinc in solution. This loss in signal was fit to an IC50 curve (Figure 4B–E), and the KD for each caspase was determined by applying the Cheng-Prusoff equation.49 Caspase-3, -6, and -8 had a low nanomolar affinity site for zinc, which correlates well with the inhibition data previously determined (Figure 3, Table 1). In contrast, as predicted by the biochemical inhibition data (Figure 3, Table 1) caspase-7 had a weaker dissociation constant, which could not be accurately measured using Fluozin-3. Combined with previous zinc inhibition assays, this establishes that caspase-7 binds and is inhibited by zinc at higher concentrations than caspase-3, -6, and -8.
Caspase-7 Binds Zinc at the Catalytic Dyad
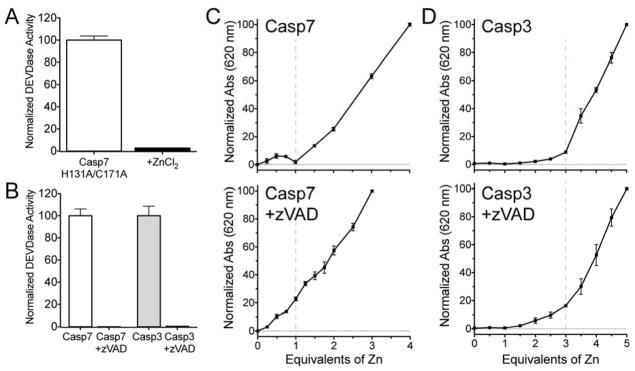
Caspase-9 was observed to bind two zincs, one at the active site and a second at an exosite at the base of the 210s helix (Figure 1).32 A sequence alignment of this caspase-9 exosite (His224 and Cys272 in caspase-9 numbering) with caspase-7 amino acids suggests caspase-7 lacks a critical cysteine necessary to bind zinc in the same manner as the caspase-9 exosite. However, an investigation of the caspase-7 structure reveals that there is indeed a cys-his cluster (His131 and Cys171) with potential for zinc binding (Figure S4). To determine if this site is important in caspase-7, both the histidine (His131 in caspase-7 numbering) and the cysteine (Cys171) were replaced with alanine in caspase-7. This variant (H31A/C171A) was then incubated in the presence and absence of ZnCl2 (Figure 5A). H31A/C171A was inhibited by zinc in a similar fashion to wild-type, suggesting this exosite has no inhibitory potential for caspase-7 and may be unique to caspase-9. As a result, we next interrogated the active site of caspase-7 as the binding site for the singular zinc.
Figure 5.
Substrate-mimic occupying the active site disrupts zinc binding to caspase-7 but not to caspase-3. (A)The activity of the caspase-7 variant H131A/C171A was tested in the absence and presence of ZnCl2. (B) Activity of caspase-7 and caspase-3 after a two hour incubation alone, or in the presence of an active site inhibitor zVAD-FMK. (C) Caspase-7 alone (top) and caspase-7 with its active site occupied by zVAD (bottom) were analyzed by increasing equivalents of zinc and stoichiometry of zinc binding was determined by measuring zincon absorbance at 620 nm (filled data points in line plot). (D) Zincon measurements were repeated for caspase-3 (top) and caspase-3 incubated with zVAD (bottom). Data represent the mean ± SEM for three separate experiments on three separate days.
The cysteine-histidine dyad comprising every caspase active site is also a potential zinc-binding site. However, when a caspase is bound to a peptide-based active-site inhibitor, the substrate binding groove is sterically blocked and the cysteine residue in the catalytic dyad is covalently blocked, and therefore unavailable to bind zinc. To determine if caspase-7 and caspase-3 bind zinc using active-site residues, we first incubated each caspase with a known covalent inhibitor, zVAD-FMK, which occupies the caspase active site by forming a covalent bond with the catalytic cysteine residue. A two-hour incubation zVAD-FMK completely inhibited both caspase-7 and caspase-3 (Figure 5B). After incubation with the active-site inhibitor zVAD-FMK, each caspase was subjected to increasing molar equivalents of zinc in the presence of zincon to assess zinc stoichiometry. Caspase-7 was unable to bind the single zinc that we observed in the absence of zVAD-FMK (Figure 5C), indicating zinc binds at the active site of caspase-7. However, caspase-3 showed only a modest alteration in the zincon response in the presence of zVAD-FMK (Figure 5D). This suggests that caspase-3 can bind three equivalents of zinc and at the same time interact with a covalent active site inhibitor. This result is consistent with a previous report that zinc and a fluorescently labeled caspase-3 active site inhibitor are able to simultaneously react with caspase-3.50 This prior analysis by Daniel et al.50 went on to suggest that the active site histidine was a zinc ligand but the active site cysteine was not involved in binding zinc. Our interpretation is that one zinc binds the caspase-3 catalytic histidine and resides at the active site while two other zinc bind outside the active site, potentially at exosites.
Zinc Binds to the Caspase-8 Catalytic Dyad and Impacts Oligomeric Structure
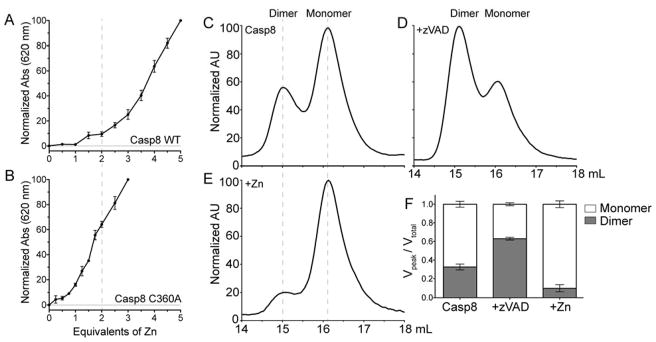
To test whether caspase-8 also binds zinc in its catalytic dyad the catalytic cysteine (C360) was replaced by alanine to ablate zinc-binding properties at this site. The stoichiometry of zinc binding for the caspase-8 wild-type (Figure 6A) and the C360A variant (Figure 6B) were assessed by the zincon assay. Replacement of the catalytic cysteine of caspase-8 with the non-zinc binding residue, alanine, significantly altered its ability to bind zinc. The stoichiometry shifted to fewer than two zincs (but greater than zero zincs) per monomer of caspase-8, suggesting that the catalytic dyad is responsible for binding one of the zincs.
Figure 6.
Zinc binds to the Active Site of Caspase-8 and Influences the Oligomeric State (A, B) Zinc binding to caspase-8 (A) and caspase-8 C360A (B) was measured by monitoring the absorbance at 620 nm (filled data points in line plot) in the presence of the colorimetric zinc indicator, zincon, and increasing concentrations of zinc. (C) The oligomeric state of caspase-8 was monitored by elution using size-exclusion chromatography (SEC), with two dominant peaks correlating to the caspase-8 dimeric or monomeric states. (D) Caspase-8 was incubated with an inhibitor, zVAD-FMK, which is known to increase the concentration of dimeric caspase-8. The distribution of monomer and dimer was monitored by SEC. (E) The distribution of caspase-8 monomeric and dimeric states in the presence of ZnCl2 assessed by SEC. (F) A graphical representation of quantified volumes under each curve representing the monomeric or dimeric states of caspase-8 alone, in the presence of zVAD, or in the presence of ZnCl2. These quantifications represent the mean ± SEM for three separate experiments on three separate days.
Size exclusion chromatography and analytical ultracentrifugation sedimentation velocity experiments have demonstrated that caspase-8 exists in an equilibrium between monomeric and dimeric states.51,52 At a concentration of 30 μM caspase-8 is approximately 69% monomer.51 In addition, dimerization is required for the protein to be active.29 Active-site-binding inhibitors, like substrate mimics, have been shown to strongly influence the equilibrium to favor the dimeric state of caspase-8.51 We used size exclusion chromatography to investigate whether zinc has any influence on the oligomeric state of the caspase-8. Caspase-8 alone eluted as 67% monomer and 33% dimer (Figure 6C). This observation agrees well with previous investigations of caspase-8 oligomeric state distributions.51 After incubation with the active site-binding inhibitor, zVAD-FMK, the ratio shifted as expected51 to 37% monomer and 63% dimer (Figure 6D). However, after incubation with zinc, the distribution heavily favored the monomeric state (Figure 6E). The caspase-8 monomer peak represented 90% of the total protein load, with only 10% eluting in the dimeric state (Figure 6E,F). This observation suggests that zinc inhibits caspase-8 by preventing the protein from reaching the dimeric state necessary to enable substrate cleavage.
Zinc Binding Destabilizes Caspase-8
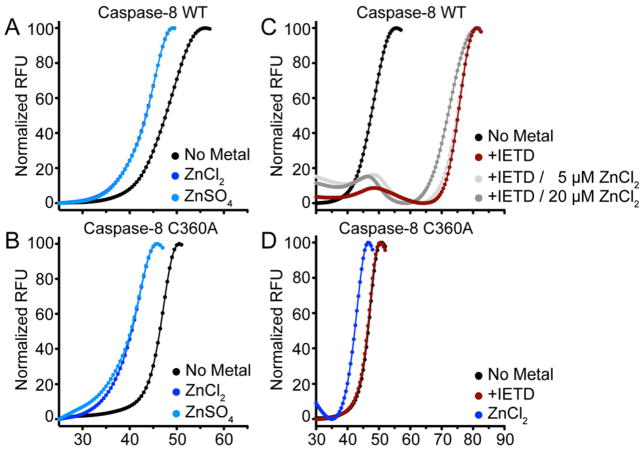
Due to the fact that zinc binding shifts the caspase-8 conformation to favor the monomeric state, we hypothesized that zinc destabilizes caspase-8. Thermal stability assays were conducted using differential scanning fluorimetry to assess caspase-8 stability in the presence or absence of zinc. Fluorescence of a protein-binding dye (SYPRO Orange), which increases when bound to hydrophobic residues exposed during denaturation, was monitored as a function of temperature. Wild-type caspase-8 was subjected to a melting curve in the absence of zinc or after incubation with ZnCl2 or ZnSO4 (Figure 7A). Both zinc salts destabilized caspase-8 by approximately 3°C (Table 2). Likewise, both zinc salts destabilized caspase-8 C360A by a similar amount (5°C, Figure 7B). Given that removing the active site cysteine (C360A) had a significant effect on the stoichiometry of zinc binding (Figure 7A,B), this suggests that the zinc binding site that leads to destabilization and loss of dimer, is not the catalytic dyad.
Figure 7.
Zinc Destabilizes Caspase-8.
The thermal stability of caspase-8 was interrogated by differential scanning fluorimetry using SYPRO Orange fluorescent dye. A melting curve was generated for (A) caspase-8 wild-type alone, after incubation with 50 μM ZnCl2 or ZnSO4 and repeated for (B) caspase-8 C360A, a catalytically inactive variant. (C) Caspase-8 thermal stability was then investigated after incubation with a known peptide substrate, IETD-CHO, which contained an aldehyde moiety that reversibly reacts with the active site cysteine of caspase-8. This caspase-8-IETD was then incubated with zinc. (D) Caspase-8 C360A thermal melts after incubation with the aldehyde IETD inhibitor.
Table 2.
Thermal Stability Tm Measurements
| TM Values (°C) | ||
|---|---|---|
|
| ||
| Wild Type | C360A | |
| Caspase-8 | 48.1 ± 0.5 | 46.7 ± 0.2 |
| + ZnCl2 | 44.5 ± 0.4 | 41.5 ± 0.9 |
| + ZnSO4 | 44.8 ± 0.3 | 41.6 ± 0.6 |
| + Ac-IETD-CHO | 75.5 ± 0.3 | 46.4 ± 0.2 |
In contrast to the impact of zinc, caspase-8 binding to the tetrapeptide substrate-like inhibitor IETD-CHO, has an extremely stabilizing effect, with an observed Tm increase of 27°C (Figure 7C). This increase in stability in the presence of substrate-like inhibitors has been observed previously as caspase-7 is stabilized by 17°C upon binding of DEVD-CHO.53 This is likely a result of stabilizing the dynamic active site loops upon formation of a caspase-8 dimer. After observing this extreme stabilization, we probed the effect of zinc on dimerization of caspase-8. After incubation with the inhibitor, the caspase-8 was exposed to increasing concentrations of zinc. Substrate-bound caspase-8 was destabilized by zinc treatment, and a slight increase in the monomer population was observed. Meanwhile, caspase-8 with the C360A substitution at the catalytic cysteine was unable to attain the stabilization shift from incubation with the substrate-like inhibitor IETD (Figure 7D) because it is unable to covalently bind in the absence of this critical cysteine. Nevertheless, we consistently observe that both wild-type and C360A caspase-8 are destabilized by zinc. This correlates with the ability of zinc to promote monomerization of caspase-8 and suggests that the zinc binding site that impacts dimerization does not include the catalytic cysteine, Cys360.
Due to the modest destabilizing effect in Tm upon zinc binding to the caspase-8 C360A variant, we aimed to further interrogate this shift by incubation with other metals (Figure S5). Thermal stability measurements after incubation with zinc continued to display a 5°C destabilizing effect, however, incubation with iron, calcium, and copper failed to significantly alter the Tm for caspase-8 C360A. This result confirmed the observation that the destabilizing effect shown by a shift in Tm is due to zinc binding and is not simply due to the presence of cations in solution.
Discussion
In this work, we have observed that while all of the major apoptotic caspases are inhibited by zinc, each of them use unique mechanisms. Given the mounting evidence that zinc is an effective signaling entity, it is possible that these different responses to zinc are not just vestigial to the sequence for each caspase, but have evolved to allow zinc to play unique functional roles in each member of the caspase family. We, and others, have demonstrated that zinc has a potent inhibitory effect on caspase-3.34,50 Our data revealed low nanomolar inhibition as well as low nanomolar binding, that is within the estimated range of free intracellular zinc. Interestingly, caspase-3 binds three zincs. The functional necessity for a caspase maintaining three zinc binding sites is both curious and intriguing. One possibility is that unique zinc binding sites could be playing a structural role regulating the various stages of caspase-3 maturation. For example, PAC-1, a procaspase-3 activator, shows strong zinc chelating capabilities. As a result, PAC-1 and its derivatives sequester free zinc from procaspase-3, allowing autoactivation.38,39,54 This suggests that one of the caspase-3 zinc binding sites could have evolved to prevent caspase-3 maturation from the zymogen to the active form. Another possible role for an additional zinc binding site could be to disrupt critical protein-protein interactions during substrate recognition. Caspases have numerous substrates and often rely on exosites to maintain specificity.41,42,55 It is possible that a zinc-binding site on caspase-3 could disrupt critical protein-protein interactions and limit substrate accessibility or even redirect hydrolysis towards particular proteins of interest.
In contrast to caspase-3, caspase-7 has only one zinc-binding site and this site shows a significantly weaker affinity for binding and inhibition. We hypothesize that under normal cell conditions caspase-3 activity is constitutively suppressed by intracellular free zinc. However, caspase-7 has an affinity for zinc that appears to reside at the upper limit of available zinc pools, suggesting caspase-7 may only be inactivated upon a slight elevation in zinc levels. Zinc concentrations have been shown to fluctuate during various biological events, and have been identified as zinc sparks or zinc waves.1,12 It is important to note that caspase-7 is more weakly apoptotic than caspase-3, and carries roles distinct from caspase-3.56,57 Perhaps caspase-7 has a weakened affinity for zinc in order to carry out pivotal non-apoptotic roles constitutively. For example, caspase-7 cleavage by caspase-1 in response to lipopolysaccharide prompts caspase-7 to cleave PARP. This results in a change in NF-κB signaling that alters gene transcription to aid in the inflammatory response.58,59 If caspase-7 were consistently inhibited by basal levels of zinc then it could not properly participate in this critical non-apoptotic response to infection, therefore justifying much weaker affinity for zinc in respect to the highly apoptotic caspase-3.
Zinc inhibition impacts both the initiator and executioner apoptotic caspases. Caspase-8 and caspase-9 are classified as initiators in the extrinsic and intrinsic apoptotic pathways (Figure 8), respectively, and rely on dimerization to achieve elevated activity. Caspase-8 and caspase-9 bind two zincs per monomer and suffer a loss in activity due to zinc blocking key active site residues. Interestingly, the mechanism for zinc inhibiting caspase-8 through disruption of oligomerization is distinct from that of caspase-9, in which zinc does not impact dimerization.32 Dimerization of caspase-8 is imperative for its activity to carry out both apoptotic and nonapoptotic roles.60,61 Thus, zinc disrupting caspase-8 dimerization could have dramatic implications in apoptotic initiation that are similar to the mechanism observed for FLIP-mediated inhibition of caspase-8, which also blocks homodimerization.60 We observe two zincs binding to caspase-8. The first clearly binds the active site cysteine and when bound should directly block substrate binding. However, the location of the second zinc-binding site remains unknown. We anticipate that this second zinc is likely to disrupt dimerization thus confining caspase-8 in the monomeric form. However, it is also possible that the zinc bound at the active site could be restricting loop conformations that are critical for dimerization.
Figure 8.
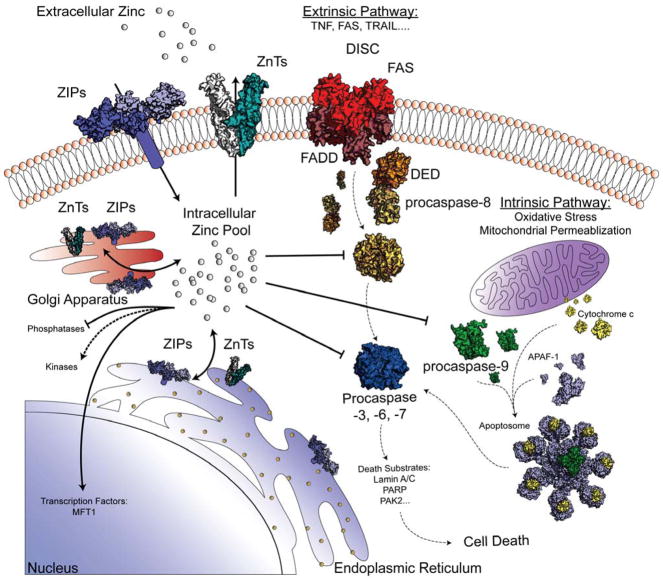
Schematic of the Influence of Zinc on Caspases and Apoptosis using cartoons and structures of relevant components. Levels of cellular zinc are tightly controlled by at least fourteen different Zinc Import Proteins (ZIPs; model based on Zip4 extracellular domain from Pteropus Alecto PDB ID 4X82) and ten different Zinc Transporters (ZnTs; model based on E. coli ZnT YIIP PDB ID 2QFI). These ZIPs and ZnTs respond to a huge number of different cellular cues to control the intracellular zinc pool. In addition to controlling cellular levels of zinc, ZIPs also form gradients at different extracellular locations and intracellular locations adjacent to the Golgi apparatus and the endoplasmic reticulum where ZIPs and ZnTs are abundant. In general, increasing zinc levels block phosphatase activity, which serves to activate kinases. Increased zinc also activates a number of transcription factors such as the metal regulatory transcription factor 1, MFT1, which regulates expression of metallothioneins. Zinc levels also significantly impact the function of apoptosis. Apoptosis can be trigged extrinsically by binding of ligands like TNF, FAS, or TRAIL to the Death Inducing Signaling Complex (DISC). The DISC recruits procaspase-8 through its Death Effector Domain (DED), which leads to the active conformation of caspase-8. Apoptosis can also be trigged intrinsically through oxidative or mitochondrial stresses. When this occurs cytochrome c is released from the mitochondria and binds, with dATP, to APAF-1, which forms a heptameric caspase-9 activation platform, the apoptosome. Activated caspase-8 or -9 cleave and activate the executioner procaspases caspase-3, -6 and -7. At available zinc concentrations of 1–10 nM, all of the apoptotic caspases except caspase-7 are inhibited by zinc binding. If available zinc levels exceed 100 nM, then caspase-7 is also inhibited. When zinc levels decrease, or if zinc is actively removed from binding sites on caspases through an as yet unknown mechanism, caspases are available to be activated by traditional routes. Active executioner caspases are then able to cleave cellular substrates leading to apoptotic cell death. Graphic generated using the following PDB IDs: 1QTN (caspase-8), 1F1J (caspase-7), 5JUY (apoptosome), 5L08 (caspase-8 +tDED), 30Q9 (FAS/FADD), 1Z6T (apaf-1), 2N9J (cytochrome-c)
Zinc signaling is emerging as being far more nuanced than originally anticipated, as it has numerous roles in the regulation of a myriad of pathways and targets. The fact that nearly 10% of the proteome has the potential to bind zinc, results in a very multi-factorial collection of ligands controlling the pool of available zinc at the low nanomolar to high picomolar range. A large number of Zinc Import Proteins (ZIPs) and Zinc Transporters (ZnTs) modulate the total levels of zinc (Figure 8), thus regulating biological functions based on environmental factors. The data presented here contribute to an understanding of how changes in zinc concentration directly affect the apoptotic caspases. The biologically relevant IC50 concentrations of zinc-mediated caspase inhibition and binding described in this work suggest that the molecular mechanisms of zinc uncovered here play a pivotal role in the regulation of apoptosis (Figure 8). Increase in zinc blocks the activation of caspases-8, -9 as well as the executioners (-3 and -6) and at even more elevated levels the activity of caspase-7 preventing the downstream cleavage of critical death substrates allowing for the propagation of cells (Figure 8). This is likely the system used during development, but is similarly susceptible to being hijacked in cancers to allow unregulated proliferation. For example, ZIP9 is expressed in various breast and prostate cancers increasing the endogenous levels of zinc promoting proliferation.62 In the converse situation, decreased zinc levels have been correlated to increased apoptosis.63 We hypothesize that as the intracellular levels of zinc fall below the IC50 values of zinc-mediated caspase inhibition, caspases are free to become active, in turn activating the apoptotic cascade, which may account for the toxicity observed in a zinc deficient system. Taken together, the data reported here (e.g. differing affinities of various caspases) suggest that regulation by zinc not only differs across the caspase family, but plays different roles under different conditions. The most compelling question remaining is whether activation of caspases during apoptosis is enabled simply by lowering available zinc concentrations or whether there is a direct mechanism for removal of zinc from binding sites on caspases, perhaps by metallochaperones, which enables progression of apoptosis.
Methods
A description of the methods used in this work is located in the Supporting Information.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM080532). DJM was supported in part by National Research Service Award T32 (GM08515) from the National Institute of Health. We thank L. Cunden at MIT from L. Nolans lab, for helpful discussions on zinc quantification by zincon.
Abbreviations
- DEVD-AMC
acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin, a caspase-3, -7 substrate
- LEHD-AMC
acetyl-Leu-Glu-His-Asp-aminomethylcoumarin, a caspase-8 substrate
- zVAD-FMK
carbobenzoxy-Val-Ala-Asp-fluoromethylketone, an active site inhibitor of all caspases
- IETD-CHO
acetyl-Ile-Glu-Thr-Asp-aldehyde, an active-site inhibitor of caspase-8
- DEVD-CHO
acetyl-Asp-Glu-Val-Asp-aldehyde, HEPES - 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- TCEP
tris(2-carboxyethyl) phosphine
- ZIPs
Zinc Import Proteins
- ZnTs
Zinc Transporters
- PARP
Poly [ADP-ribose] polymerase
- PAK2
p21 activated kinase 2
Footnotes
Associated Content
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Immunology, B. T.-A, editor. Roles of Zinc and Zinc Signaling in Immunity: Zinc as an Intracellular Signaling Molecule. Vol. 97. Academic Press; 2008. pp. 149–176. [DOI] [PubMed] [Google Scholar]
- 3.Andreini C, Banci L, Bertini I, Rosato A. Counting the Zinc-Proteins Encoded in the Human Genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 4.Ward D, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. 2013;1823:1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman BM. Cellular iron: Ferroportin is the only way out. 2005. pp. 155–157. [DOI] [PubMed] [Google Scholar]
- 6.Kimura T, Kambe T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int J Mol Sci. 2016;17:336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem Biol. 2006;1:103–111. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- 8.Krężel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. JBIC J Biol Inorg Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Maret W. Transient fluctuations of intracellular zinc ions in cell proliferation. Exp Cell Res. 2009;315:2463–2470. doi: 10.1016/j.yexcr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Suhy DA, Simon KD, Linzer DIH, O’Halloran TV. Metallothionein Is Part of a Zinc-scavenging Mechanism for Cell Survival under Conditions of Extreme Zinc Deprivation. J Biol Chem. 1999;274:9183–9192. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- 11.Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodru TK, Halloran TVO. Zinc Sparks Are Triggered by Fertilization and Facilitate Cell Cycle Resumption in Mammalian Eggs. ACS Chem Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep. 2016;6:24737. doi: 10.1038/srep24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, Woodruff TK, Halloran TVO. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem. 2014;7:130–139. doi: 10.1038/nchem.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haase H, Maret W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp Cell Res. 2003;291:289–298. doi: 10.1016/s0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 15.Wilson M, Hogstrand C, Maret W. Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase β activity. J Biol Chem. 2012;287:9322–6. doi: 10.1074/jbc.C111.320796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samet JM, Dewar BJ, Wu W, Graves LM. Mechanisms of Zn2+-induced signal initiation through the epidermal growth factor receptor. Toxicol Appl Pharmacol. 2003;191:86–93. doi: 10.1016/s0041-008x(03)00219-9. [DOI] [PubMed] [Google Scholar]
- 17.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JFR, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med. 2009;15:101–111. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Miyai T, Hojyo S, Ikawa T, Kawamura M, Irie T, Ogura H, Hijikata A, Bin BH, Yasuda T, Kitamura H, Nakayama M, Ohara O, Yoshida H, Koseki H, Mishima K, Fukada T. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc Natl Acad Sci. 2014;111:11780–11785. doi: 10.1073/pnas.1323549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, Bush AI. Elevated cortical zinc in Alzheimer disease. Neurology. 2006;67:69–75. doi: 10.1212/01.wnl.0000223644.08653.b5. [DOI] [PubMed] [Google Scholar]
- 21.Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 2012;4:875–903. doi: 10.3390/nu4080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Pnas. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chimienti F, Favier A, Seve M. ZnT-8, A Pancreatic Beta-Cell-Specific Zinc Transporter. BioMetals. 2005;18:313–317. doi: 10.1007/s10534-005-3687-9. [DOI] [PubMed] [Google Scholar]
- 24.Truong-Tran aQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14:315–30. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- 25.Zalewski PD, FI . Intracellular zinc and the regulation of apoptosis. In: Lavin M, Watters D, editors. Program Cell Death Cell Mol Biol Apoptosis. Melbourne: 1993. pp. 73–86. [Google Scholar]
- 26.do Marreiro DN, Cruz KJC, Morais JBS, Beserra JB, Severo JS, de OA. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants. 2017;6:24. doi: 10.3390/antiox6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou H, Li Y, Liu X, Wang X. An APAF-1/Cytochrome c Multimeric Complex Is a Functional Apoptosome That Activates Procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 28.Chang DW. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22:4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberst A, Pop C, Tremblay AG, Blais V, Denault JB, Salvesen GS, Green DR. Inducible Dimerization and Inducible Cleavage Reveal a Requirement for Both Processes in Caspase-8 Activation. J Biol Chem. 2010;285:16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stennicke HR, Salvesen GS. Biochemical Characteristics of Caspases-3 , -6 , -7 , and -8. October. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 31.Ho LH, Ratnaike RN, Zalewski PD. Involvement of Intracellular Labile Zinc in Suppression of DEVD-Caspase Activity in Human Neuroblastoma Cells. Biochem Biophys Res Commun. 2000;268:148–154. doi: 10.1006/bbrc.2000.2090. [DOI] [PubMed] [Google Scholar]
- 32.Huber KL, Hardy JA. Mechanism of zinc-mediated inhibition of caspase-9. Protein Sci. 2012;21:1056–1065. doi: 10.1002/pro.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velazquez-Delgado EM, Hardy JA. Zinc-mediated Allosteric Inhibition of Caspase-6. J Biol Chem. 2012;287:36000–36011. doi: 10.1074/jbc.M112.397752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maret W, Jacob C, Vallee BL, Fischer EH. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc Natl Acad Sci U S A. 1999;96:1936–40. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA. Zinc Is a Potent Inhibitor of the Apoptotic Protease, Caspase-3: A Novel Target for Zinc in the Inhibition of Apoptosis. J Biol Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 36.Daniel aG, Peterson EJ, Farrell NP. The Bioinorganic Chemistry of Apoptosis: Potential Inhibitory Zinc Binding Sites in Caspase-3. Angew Chemie. 2014;126:4182–4185. doi: 10.1002/anie.201311114. [DOI] [PubMed] [Google Scholar]
- 37.Schrantz N, Auffredou MT, Bourgeade MF, Besnault L, Leca G, Vazquez A. Zinc-mediated regulation of caspases activity: dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos) Cell Death Differ. 2001;8:152–161. doi: 10.1038/sj.cdd.4400772. [DOI] [PubMed] [Google Scholar]
- 38.Putt KS, Chen GW, Pearson JM, Sandhorst JS, Hoagland MS, Kwon J, Hwang S, Jin H, Churchwell MI, Cho M, Doerge DR, Helferich WG, Hergenrother PJ. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. 2006;2:543–550. doi: 10.1038/nchembio814. [DOI] [PubMed] [Google Scholar]
- 39.Peterson QP, Goode DR, West DC, Ramsey KN, Lee JJY, Hergenrother PJ. PAC-1 Activates Procaspase-3 in Vitro through Relief of Zinc-Mediated Inhibition. J Mol Biol. 2009;388:144–158. doi: 10.1016/j.jmb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter JE, Truong-Tran AQ, Grosser D, Ho L, Ruffin RE, Zalewski PD. Involvement of redox events in caspase activation in zinc-depleted airway epithelial cells. Biochem Biophys Res Commun. 2002;297:1062–1070. doi: 10.1016/s0006-291x(02)02292-1. [DOI] [PubMed] [Google Scholar]
- 41.Eron SJ, Raghupathi K, Hardy JA. Dual Site Phosphorylation of Caspase-7 by PAK2 Blocks Apoptotic Activity by Two Distinct Mechanisms. Structure. 2016 doi: 10.1016/j.str.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boucher D, Blais V, Denault J-B. Caspase-7 uses an exosite to promote poly(ADP ribose) polymerase 1 proteolysis. Proc Natl Acad Sci. 2012:4–9. doi: 10.1073/pnas.1200934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bers DM, Patton CW, Nuccitelli R. Biology, M. W. B. T.-M. in C, editor. Calcium in Living Cells. Vol. 99. Academic Press; 2010. Chapter 1 - A Practical Guide to the Preparation of Ca2+ Buffers; pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 44.Anwar ZM. Complexation Equilibria of Zn(II), Pb(II) and Cd(II) with Reduced Glutathione (GSH) and Biologically Important Zwitterionic Buffers. J Chinese Chem Soc. 2005;52:863–871. [Google Scholar]
- 45.Kr zel a, Lesniak W, Jezowska-Bojczuk M, Mlynarz P, Brasuñ J, Kozlowski H, Bal W. Coordination of heavy metals by dithiothreitol, a commonly used thiol group protectant. J Inorg Biochem. 2001;84:77–88. doi: 10.1016/s0162-0134(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 46.Shaw CF, Laib JE, Savas MM, Petering DH. Biphasic kinetics of aurothionein formation from gold sodium thiomalate: a novel metallochromic technique to probe zinc(2+) and cadmium(2+) displacement from metallothionein. Inorg Chem. 1990;29:403–408. [Google Scholar]
- 47.Sadek FS, Schmid RW, Reilley CN. Visual egta titration of calcium in the presence of magnesium. Talanta. 1959;2:38–51. [Google Scholar]
- 48.Probes M, Pitchford AV, Uni V, Gee KR, Zhou Z, Qian W, Kennedy R. Detection and Imaging of Zinc Secretion from Pancreatic -Cells Using a New Fluorescent Zinc Indicator. JACS. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 49.PRUSOFF Y, CHENG WH. RELATIONSHIP BETWEEN THE INHIBITION CONSTANT (&) AND THE CONCENTRATION OF INHIBITOR WHICH CAUSES 50 PER CENT INHIBITION (I50) OF AN ENZYMATIC REACTION*. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 50.Daniel AG, Peterson EJ, Farrell NP. The Bioinorganic Chemistry of Apoptosis: Potential Inhibitory Zinc Binding Sites in Caspase-3. Angew Chemie Int Ed. 2014;53:4098–4101. doi: 10.1002/anie.201311114. [DOI] [PubMed] [Google Scholar]
- 51.Donepudi M, Sweeney A, Mac, Briand C, Grütter MG. Insights into the Regulatory Mechanism for Caspase-8 Activation. Mol Cell. 2003;11:543–549. doi: 10.1016/s1097-2765(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 52.Keller N, Mares J, Zerbe O, Grütter MG. Structural and biochemical studies on procaspase-8: new insights on initiator caspase activation. Structure. 2009;17:438–48. doi: 10.1016/j.str.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 53.Witkowski WA, Hardy JA. L2′ loop is critical for caspase-7 active site formation. Protein Sci. 2009;18:1459–1468. doi: 10.1002/pro.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F, Wang L, Zhao Y, Li Y, Ping G, Xiao S, Chen K, Zhu W, Gong P, Yang J, Wu C. A novel small-molecule activator of procaspase-3 induces apoptosis in cancer cells and reduces tumor growth in human breast, liver and gallbladder cancer xenografts. Mol Oncol. 2014;8:1640–1652. doi: 10.1016/j.molonc.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill ME, MacPherson DJ, Wu P, Julien O, Wells JA, Hardy JA. Reprogramming Caspase-7 Specificity by Regio-Specific Mutations and Selection Provides Alternate Solutions for Substrate Recognition. ACS Chem Biol. 2016 doi: 10.1021/acschembio.5b00971. acschembio.5b00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slee EA, Adrain C, Martin SJ. Executioner Caspase-3, -6, and -7 Perform Distinct, Non-redundant Roles during the Demolition Phase of Apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 57.Walsh JG, Cullen SP, Sheridan C, Alexander UL, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. 2008;105:12815–12819. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, Marsh CB, Wewers MD, Tridandapani S, Kanneganti TD, Amer AO. Caspase-7 Activation by the Nlrc4/Ipaf Inflammasome Restricts Legionella pneumophila Infection. PLoS Pathog. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erener S, Pétrilli V, Kassner I, Minotti R, Castillo R, Santoro R, Hassa PO, Tschopp J, Hottiger MO. Inflammasome-Activated Caspase 7 Cleaves PARP1 to Enhance the Expression of a Subset of NF-κB Target Genes. Mol Cell. 2012;46:200–211. doi: 10.1016/j.molcel.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Maelfait J, Beyaert R. Non-apoptotic functions of caspase-8. Biochem Pharmacol. 2008;76:1365–1373. doi: 10.1016/j.bcp.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 62.Thomas P, Pang Y, Dong J, Berg AH. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology. 2014;155:4250–4265. doi: 10.1210/en.2014-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clegg M, Hanna L, Niles B, Momma T, Keen C. Zinc deficiency-induced cell death. IUBMB Life (International Union Biochem Mol Biol Life) 2005;57:661–669. doi: 10.1080/15216540500264554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.