Concern about health risks associated with rising obesity has become nearly universal, with the mean body mass index (BMI) and the prevalence of obese and overweight individuals increasing substantially worldwide during the previous three decades. Unfortunately, prevention and treatment of obesity and related complications have proven complex, and successful strategies to tackle this pathology remain limited. Epidemiological studies have highlighted potential environmental exposures, including diet, energy expenditure, early life influences, sleep deprivation, endocrine disruptors, chronic inflammation, and microbiome status, contributing to higher risk of obesity (Franks and McCarthy, 2016). Among these, the microbiome has received extensive attention during the previous decade.
Variation in gut microorganisms might play an important role in the pathogenesis of obesity. Although the composition of intestinal microbiota is highly diverse in healthy individuals, those exhibiting overall adiposity, insulin resistance and dyslipidemia are characterized by low bacterial richness (Le Chatelier et al., 2013). Moreover, composition of gut microbiota in obesity individuals differs from that in lean individuals, although inconsistent changes have been reported. Bacteroidetes prevalence is lower in obese people, with this proportion increasing along with weight loss based on a low-calorie diet (Ley et al., 2006a). Lactobacillus and Clostridium species are associated with insulin resistance, with Lactobacillus positively correlated with fasting glucose and HbA1c levels, whereas Clostridium showed a negative correlation with these parameters (Karlsson et al., 2013). These data suggest that specific bacterial phyla, class, or species or bacterial metabolic activities could be beneficial or detrimental to the onset of obesity. Therefore, the gut microbiome has been suggested as a driving force in the pathogenesis of obesity.
Causal evidence linking intestinal microbiota to obesity mostly originates from animal studies. Germ free (GF) mice are resistant to high-fat diet (HFD)-induced obesity, despite a higher food intake. Interestingly, administration of subtherapeutic antibiotic therapy increased adiposity and metabolism-related hormone levels in young mice, with these changes altering the copies of key genes involved in the metabolism of carbohydrates to short-chain fatty acids (SCFAs) and the regulation of hepatic metabolism of lipids and cholesterol (Cho et al., 2012). Furthermore, colonization of GF mice with “obese microbiota” resulted in a significantly greater increase in total body fat than colonization with “lean microbiota” (Turnbaugh et al., 2006). Notably, GF mice that received fecal microbiota transplantation (FMT) from an obese donor gained more weight as compared with those receiving it from a lean donor (Ridaura et al., 2013), with this result further accelerating the establishment of the causal role of gut microbiota in the development of obesity.
Mechanisms by which gut microbiota promote metabolic disturbances are not well understood. To date, leading theories about the mechanisms include changes in molecular signaling chemicals released by bacteria in contact with local tissue or distant organs (Schroeder and Backhed, 2016; Meijnikman et al., 2017) (Fig. 1).
Figure 1.
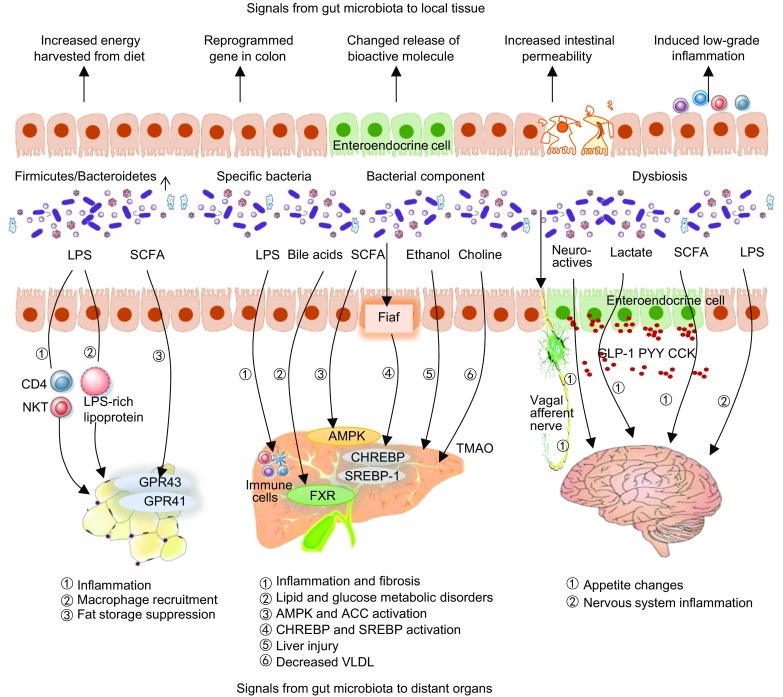
Impact of gut microbiota on local and distant organs contributes to obesity development and progression. In local tissues, obesity-associated gut microbiota have an increased capacity to harvest energy from the diet, stimulate gene reprogramming in the colon, change polypeptide hormones and other bioactive molecules released by EC cells, decrease the intestinal barrier, and disturb immune homeostasis. Gut microbiota also communicate with host adipose tissue and the liver and brain. Microbiota-fat-signaling axis. Gut microbiota participates in the regulation of adipogenesis through distinct mechanisms. LPS triggers an immune response along with inflammation and immune-cell infiltration. SCFAs also participate in insulin-mediated fat accumulation in adipocytes via activation their receptors GPR43 and GPR41, which inhibits lipolysis and encourages adipocyte differentiation. Gut-liver axis. The presence of a dysbiotic microbiome causes subsequent increases in gut permeability to bacteria-derived pathogens, including LPS and ethanol. In the liver, LPS causes inflammation by stimulating immune cells. Certain metabolites, such as bile acids, SCFAs, and TMAO, also play a role in NAFLD pathophysiology. Microbiota-brain-gut axis. Gut afferent neuron and gut hormones are key signaling molecules involved in gut-brain communication and host metabolism. Bioactive molecules involved in this process include LPS, gut peptides, SCFAs and lactate
Changes in gut microbiota perturb homeostatic interaction between microbiota and the intestine and might contribute to metabolic disorders. Local contacts between microbiota and intestine cells determine which signals are sensed and presented and which reactions are subsequently initiated. Increased energy harvesting by obesity associated gut microbiota is another possible explanation for obesity. The obese microbiome is typified by a reduced presence of taxa belonging to the Bacteroidetes phylum and a proportional increase in members of the Firmicutes phylum, revealing an association with a higher presence of enzymes for complex carbohydrate degradation and fermentation (Ley et al., 2006b), which are related to elevated levels of energy harvesting from the diet (Jumpertz et al., 2011). Additionally, the gut microbiome can stimulate reprogramming of gene expression in the colon (Qin et al., 2018). Fasting-induced adiposity factor (Fiaf; also known as angiopoietin-like protein 4), a circulating lipoprotein lipase inhibitor whose expression is normally selectively suppressed in the gut epithelium by microbiota (Backhed et al., 2007), plays a central role in triglyceride metabolism (Kim et al., 2010) by inhibiting lipoprotein lipase (LPL) production in adipose tissue and modulating fatty acid oxidation. Some specific components of microbiota might suppress Fiaf in the intestinal epithelia and potentially stimulate host weight gain by impairing triglyceride metabolism and promoting fat storage. Polypeptide hormones and other bioactive molecules released by enterochromaffin (EC) cells in the intestine are also involved in regulating food intake (Gribble and Reimann, 2016). Various Toll-like receptors (TLRs) expressed in EC cells recognize different pathogen-associated molecular patterns and alter the release of polypeptide hormones and other bioactive molecules. For example, lipopolysaccharide (LPS) molecules from Gram-negative bacteria and recognized by TLR4 cause secretion of cholecystokinin (CCK) through a mechanism dependent upon MyD88 and protein kinase C (Bogunovic et al., 2007; Palazzo et al., 2007). The altered intestinal barrier and subsequent translocation of bacteria or bacterial products is now regarded as an important mechanism associated with obesity. Exposure of cultured intestinal epithelial cells to commensal or probiotic microbial species results in upregulation and increased phosphorylation of key tight-junction proteins (Ewaschuk et al., 2008; Anderson et al., 2010). Additionally, some bacterial products play an important role in regulating the intestinal barrier, with associated SCFAs capable of differentially regulating prostaglandin production in myofibroblasts, thereby stimulating mucin-2 expression in intestinal epithelial cells (Willemsen et al., 2003). Obesity is related to the generation of low-grade, chronic inflammation (Lumeng and Saltiel, 2011), and gut-derived antigens are considered potential triggers for this activity. Furthermore, dysbiosis of microbiota can influence the innate and adaptive immune systems of the host via microbial cell components and metabolite signals.
Microbiota has effects beyond local tissue, with adipose tissue considered a primary target. Obesity is characterized as a massive expansion of adipose tissue, and growing evidence suggests that gut microbiota contribute to metabolic disorders through an axis of communication with adipose tissue. LPS has been identified as a triggering factor for insulin resistance in adipose tissue. In the trans-cellular pathway, LPS is actively transported into the cell in proportion to the fat content of the chime, followed by transfer to other lipoproteins by translocases. LPS-rich lipoproteins are absorbed by especially large adipocytes exhibiting high metabolic activity (Hersoug et al., 2016). Additionally, SCFAs produced by gut microbiota also participate in insulin-mediated fat accumulation in adipocytes through activation of the SCFA receptors G-protein coupled receptor (GPR)43 and GPR41 in adipocytes, which subsequently inhibits lipolysis and encourages adipocyte differentiation (Kimura et al., 2013). Intriguingly, MicroPET-CT results showed that microbiota depletion leads to increased glucose disposal primarily in inguinal subcutaneous adipose tissue and perigonadal visceral adipose tissue (Suarez-Zamorano et al., 2015), thereby stimulating energy expenditure through thermogenesis. This process was largely dependent upon eosinophils and the type 2 cytokines interleukin (IL)-4, IL-13, and IL-5 through alternative activation of M2 macrophages. Specific metabolic effects of some genes in adipocytes are also largely dependent upon altered microbiota composition. A recent study demonstrated that specific deletion of the endocannabinoid system synthesizing enzyme in adipocytes (NAPE-PLD) induced obesity and altered the browning program, with these changes partly mediated by a shift in gut-microbiota composition. These findings support those from a previous study showing that FMT was also capable of partially transferring a phenotype to GF mice (Geurts et al., 2015).
The liver is continually exposed to gut-derived signals, including those originating from bacterial components and products, through the receipt of ~70% of the blood supply from the portal vein, which enables direct venous outflow from the intestines. Alteration of gut commensal bacteria has consistently been associated with increased risk of obesity related liver disease [e.g., nonalcoholic fatty liver disease (NAFLD)], with a dysbiotic microbiome frequently observed among obese individuals with NAFLD (Turnbaugh et al., 2009). NAFLD severity is associated with gut dysbiosis and a shift in the metabolic function of gut microbiota, with Bacteroides abundance independently associated with nonalcoholic steatohepatitis (NASH), and Ruminococcus abundance associated with significant fibrosis (Boursier et al., 2016). GF mice colonized with intestinal bacteria from HFD mice develop NAFLD and had display hepatic lipid levels similar to those of donor mice, thereby implicating the gut microbiome in hepatic lipid accumulation (Le Roy et al., 2013).
Multiple lines of evidence link dysbiosis to obesity related liver disease. NAFLD presents with intestinal-bacterial overgrowth and enhanced intestinal permeability. Following bacterial generation of LPS, NF-κB is stimulated to recruit inflammatory cells, thereby promoting inflammation and fibrosis in advanced NAFLD (Elsharkawy and Mann, 2007). LPS also activates the NLRP3 infammasome via TLR4 and TLR9, which play an important role in fibrosis development in NAFLD (Wree et al., 2014). In addition to direct interactions associated with gut-derived bacterial signals, certain metabolites also play a role in NAFLD pathophysiology. Gut microbiota has profound effects on bile-acid metabolism by promoting deconjugation, dehydrogenation and dehydroxylation of primary bile acids. Additionally, alteration of the gut microbiome leads to changes in the bile-acid pool, which affects the farnesoid X receptor (FXR) nuclear antagonist involved in the regulation of bile acid, as well as lipid and glucose metabolism (Li et al., 2013), and could cause metabolic dysfunction, including obesity and insulin resistance. SCFAs lower hepatic fatty acid synthase activity and increase hepatic lipid oxidation, with this shift associated with increased phosphorylation and activation of adenosine monophosphate-activated protein kinase (AMPK) and its downstream target acetyl-CoA carboxylase (den Besten et al., 2015). Fiaf is also involved in the mechanism linking the microbiome to NAFLD, where dysbiotic microbiota inhibits Fiaf secretion from intestinal cells and leads to activation of LPL, carbohydrate-responsive element binding protein, (ChREBP) and sterol regulatory element-binding protein 1(SREBP-1), and subsequent triglyceride accumulation in the liver (Backhed et al., 2004). Ethanol is another bacterial product involved in NAFLD progression, with blood ethanol levels statistically significantly increased in patients with NASH (Zhu et al., 2013) and possibly related to a higher abundance of alcohol-producing Proteobacteria. Trimethylamine N-oxide (TMAO) is a small, colorless amine oxide generated from choline by gut-microbial metabolism, and its accumulation reduces bile-acid-synthetic enzymes (Cyp7a1 and Cyp27a1) and bile-acid transporters (Oatp1, Oatp4, Mrp2 and Ntcp) in the liver (Koeth et al., 2013). Additionally, patients with NAFLD have a higher level of Erysipelotrichia, which are linked to choline metabolism (Spencer et al., 2011). Therefore, dysbiosis in obesity is likely to impact metabolic homeostasis.
Similarly, the central nervous system receives constant neural and chemical input from the gut and is responsible for integrating this information and generating appropriate food-reward signaling to maintain homeostasis (Fetissov, 2017). Bacteria and their metabolites might target the brain directly via vagal stimulation or indirectly through immune-neuroendocrine mechanisms (Torres-Fuentes et al., 2017a). The vagal nerve transmits information from enteral content to the nucleus tractus solitaries, where the information is then distributed to the hypothalamus, which regulates appetite, food intake and energy balance. Activation of the vagus nerve is partly dependent upon the secretion of chemical signals, such as gut peptide YY (PYY), glucagon-like peptide 1 (GLP-1) and CCK, by enteroendocrine cells. Additionally, several bacterial strains can modify gut-hormone secretion (Balakumar et al., 2016), which can also be released into circulation and thereby affect appetite and satiety via hypothalamic neuroendocrine pathways. This effect is at least partly dependent upon microbiota-derived metabolites. For example, lactate is the preferred substrate for neurons and contributes to postprandial satiety. Moreover, lactate is capable of being abundantly produced in the gut by Lactobacilli, Enterobacteriaceae and Bifidobacteria (Silberbauer et al., 2000). SCFAs not only serve as an important energy source, but also act as chemical messengers or signaling molecules through their ability to increase proglucagon and pro-PYY gene expression to increase plasma GLP-1 and PYY levels and either inhibit ghrelin secretion (Nohr et al., 2013) or regulate appetite by releasing it into circulation. However, the reported results specific to this activity are inconsistent. For example, acetate, the main SFCA secreted by intestinal bacteria, is taken up by the brain and plays a direct role in suppressing appetite via central hypothalamic mechanisms (Frost et al., 2014). Another study reported that increased production of acetate by altered gut microbiota leads to activation of the parasympathetic nervous system accompanied by increased ghrelin secretion, hyperphagia and obesity (Perry et al., 2016). Furthermore, gut bacteria can also affect the central control of appetite by producing neuroactive metabolites, including serotonin and γ-aminobutyric acid, because these neurotransmitters are involved in the normal regulation of energy balance. Additionally, gut microbiota is associated with inflammation via LPS, which leads to activation of immune cells (B cells or dendritic cells) and cytokine production (Torres-Fuentes et al., 2017b).
Overall, two broad, but not mutually exclusive, mechanistic categories exist for the effects of microbiota on metabolic disorders: 1) direct interaction of gut microbiota with local tissue and 2) indirect interaction with distant organs through metabolic signals. It is tempting to speculate that the effects of microbiota on metabolism-related organs, whether capable of modulating inflammatory responses or regulating active molecular signals, are fundamental elements in the process of obesity, which would provide an environment factor as the cause of the complex pathology of obesity. There is compelling evidence supporting modulation of microbiota to treat obesity and related disorders.
Dietary intake appears to be a major regulator of the structure and function of gut microbiota. Results show that carbohydrate restriction and diets rich in fiber and vegetables are associated with health benefits due in part to microbial changes (Cotillard et al., 2013; Mardinoglu et al., 2018). Administration of prebiotics, probiotics and synbiotics have long been proposed as ways of modifying metabolic disorders, which are largely dependent upon altered microbiota composition. Multi-strain probiotic supplementation can reduce liver transaminases, tumor necrosis factor-α level and insulin resistance (Sepideh et al., 2016). Additionally, probiotic Lactobacillus rhamnosus GG is effective in the prevention of hepatic steatosis and injury partly through modulation of hepatic AMPK activation (Zhang et al., 2015), and probiotic strain Bifidobacterium animalis subsp. Lactis 420 supplementation reduces bacterial translocation of Gram-negative bacteria from the Enterobacteriaceae group to normalize adipose-tissue inflammation (Amar et al., 2011). Interventions with prebiotics can also modulate gut microbiota and significantly reduce body weight, percent body fat, and desire for high-calorie foods, as well as improve insulin sensitivity, low-grade chronic inflammation and lipid metabolism (Dewulf et al., 2013; Hume et al., 2017; Nicolucci et al., 2017). In addition to its effect on peripheral organs, prebiotic supplementation also improves appetite control in children with obesity (Hume et al., 2017).
A rather harsh method of modulating microbial composition is FMT, which can alter the entire microbial community. FMT is a way to normalize the composition and functionality of gut microbiota by transferring an infusion of a fecal suspension from a healthy individual to the gastrointestinal tract of another person. This method has now become widely accepted as a highly successful rescue treatment for recurrent Clostridium difficile infection (Drekonja et al., 2015). Related data concerning FMT as a treatment for obesity and related metabolic disorders in humans are relatively sparse. Transplanting fecal matter from lean donors into obese or individuals with metabolic syndromes was recently examined. Although the results indicated no significant decrease in BMI at 6-weeks post-transplantation, there was a significant increase in insulin sensitivity (Vrieze et al., 2012; Kootte et al., 2017). Additionally, loss of microbial diversity is common in patients with obesity, and gut-microbial diversity was increased significantly after FMT from a lean donor. Notably in this case, the number of butyrate-producing bacteria was increased; however, whether enhanced diversity or changes in specific bacterial species contribute to the effect of FMT remains unknown.
CONCLUSION
Considering the key role of gut microbiota in host metabolism, mechanistic investigations of microbiota modulation have demonstrated its restorative potential for both gut-microbiota composition and functionality. Therefore, such modulation represents a promising strategy for compositional variations and a potential therapeutic target for the treatment of obesity and other metabolic diseases. However, there remains considerable controversy regarding the precise role of gut microbiota in obesity, and more interventional clinical trials are critical for continued progress.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 81730016) and National Clinical Research Center for Digestive Diseases, Xi’an, China (grant No. 2015 BAI13B07).
COMPLIANCE WITH ETHICS GUIDELINES
Sun Lijuan, Ma Lanjing, Ma Yubo, Zhang Faming, Zhao Changhai, and Nie Yongzhan declare that they have no conflicts of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
ABBREVIATIONS
- ACC
acetyl CoA carboxylase
- AMPK
adenosine monophosphate activated protein kinase
- BMI
body mass index
- CCK
cholecystokinin
- ChREBP
carbohydrate responsive element binding protein
- CNS
central nervous system
- EC
enterochromaffin
- FAS
fatty acid synthase
- Fiaf
fasting-induced adiposity factor
- FMT
fecal microbiota transplantation
- FXR
farnesoid X receptor
- GABA
γ-aminobutyric acid
- GF
germ free
- GI
gastrointestinal
- GLP-1
glucagon like peptide 1
- GPR
G-protein coupled receptor
- HFD
high-fat diet
- LPL
inhibiting lipoprotein lipase
- LPS
lipopolysaccharides
- NAFLD
non-alcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NTS
nucleus tractus solitaries
- PYY
peptide YY
- SCFA
short-chain fatty acids
- SREBP-1
sterol regulatory element-binding protein 1
- TLRs
Toll-like receptors
References
- Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. Embo Mol Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RC, Cookson AL, McNabb WC, Kelly WJ, Roy NC. Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol Lett. 2010;309:184–192. doi: 10.1111/j.1574-6968.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R, Raghavan S, Soundarajan A, Grover S, Batish VK, et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur J Nutr. 2016;57:279–295. doi: 10.1007/s00394-016-1317-7. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Dave SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–G1783. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A, MacDonald R, Rutks I, Wilt TJ. Fecal microbiota transplantation for clostridium difficile infection: a systematic review. Ann Intern Med. 2015;162:630–638. doi: 10.7326/M14-2693. [DOI] [PubMed] [Google Scholar]
- Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van LM, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol. 2017;13:11–25. doi: 10.1038/nrendo.2016.150. [DOI] [PubMed] [Google Scholar]
- Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science. 2016;354:69–73. doi: 10.1126/science.aaf5094. [DOI] [PubMed] [Google Scholar]
- Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts L, Everard A, Van Hul M, Essaghir A, Duparc T, Matamoros S, Plovier H, Castel J, Denis RG, Bergiers M, et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat Commun. 2015;6:6495. doi: 10.1038/ncomms7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- Hersoug LG, Moller P, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2016;17:297–312. doi: 10.1111/obr.12370. [DOI] [PubMed] [Google Scholar]
- Hume MP, Nicolucci AC, Reimer RA. Prebiotic supplementation improves appetite control in children with overweight and obesity: a randomized controlled trial. Am J Clin Nutr. 2017;105:790–799. doi: 10.3945/ajcn.116.140947. [DOI] [PubMed] [Google Scholar]
- Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- Kim HK, Youn BS, Shin MS, Namkoong C, Park KH, Baik JH, Kim JB, Park JY, Lee KU, Kim YB, et al. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 2010;59:2772–2780. doi: 10.2337/db10-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26:611–619. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Rasanen SM, Lee S, Mancina RM, Bergentall M, Pietilainen KH, et al. An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metab. 2018;27:559–571. doi: 10.1016/j.cmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr Rev. 2017;39:133–153. doi: 10.1210/er.2017-00192. [DOI] [PubMed] [Google Scholar]
- Nicolucci AC, Hume MP, Martinez I, Mayengbam S, Walter J, Reimer RA. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153:711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- Palazzo M, Balsari A, Rossini A, Selleri S, Calcaterra C, Gariboldi S, Zanobbio L, Arnaboldi F, Shirai YF, Serrao G, et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol. 2007;178:4296–4303. doi: 10.4049/jimmunol.178.7.4296. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Roberts JD, Grimm SA, Lih FB, Deterding LJ, Li R, Chrysovergis K, Wade PA. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018;19:7. doi: 10.1186/s13059-018-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- Sepideh A, Karim P, Hossein A, Leila R, Hamdollah M, Mohammad EG, Mojtaba S, Mohammad S, Ghader G, Seyed MA. Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr. 2016;35:500–505. doi: 10.1080/07315724.2015.1031355. [DOI] [PubMed] [Google Scholar]
- Silberbauer CJ, Surina-Baumgartner DM, Arnold M, Langhans W. Prandial lactate infusion inhibits spontaneous feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R646–R653. doi: 10.1152/ajpregu.2000.278.3.R646. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–1501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2:747–756. doi: 10.1016/S2468-1253(17)30147-4. [DOI] [PubMed] [Google Scholar]
- Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2:747–756. doi: 10.1016/S2468-1253(17)30147-4. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wree A, McGeough MD, Pena CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med. 2014;92:1069–1082. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang C, Wang C, Zhao H, Zhao C, Chen Y, Wang Y, McClain C, Feng W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J Nutr Biochem. 2015;26:337–344. doi: 10.1016/j.jnutbio.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]


