Abstract
Synaptic plasticity is important for maintaining normal neuronal activity and proper neuronal functioning in the nervous system. It is crucial for regulating synaptic transmission or electrical signal transduction to neuronal networks, for sharing essential information among neurons, and for maintaining homeostasis in the body. Moreover, changes in synaptic or neural plasticity are associated with many neuropsychiatric conditions, such as schizophrenia (SCZ), bipolar disorder (BP), major depressive disorder (MDD), and Alzheimer's disease (AD). The improper maintenance of neural plasticity causes incorrect neurotransmitter transmission, which can also cause neuropsychiatric conditions. Gas neurotransmitters (gasotransmitters), such as hydrogen sulfide (H2S), nitric oxide (NO), and carbon monoxide (CO), play roles in maintaining synaptic plasticity and in helping to restore such plasticity in the neuronal architecture in the central nervous system (CNS). Indeed, the upregulation or downregulation of these gasotransmitters may cause neuropsychiatric conditions, and their amelioration may restore synaptic plasticity and proper neuronal functioning and thereby improve such conditions. Understanding the specific molecular mechanisms underpinning these effects can help identify ways to treat these neuropsychiatric conditions.
1. Introduction
The polish psychologist Konorski (1948) first used the term “synaptic plasticity” to describe consistent and activity-dependent changes in synaptic strength [1]. Synaptic plasticity is an experience-dependent change in synaptic strength [2]. Changes in synaptic strength are essential for information storage during memory formation [3], and recent work has revealed that synaptic plasticity also plays roles in other adaptive responses, including mood stability, drug addiction, and chronic pain [4]. The mechanisms underpinning synaptic plasticity are broadly linked to long-term memory. Synapse modifications are commonly monitored by two important phenomena: long-term potentiation (LTP) and long-term depression (LTD), which cause an increase or a reduction in synaptic strength, respectively. LTP and LTD also have roles in memory and learning [1]. Neurotransmitters are the chemical messengers that activate, amplify, and harmonize signals between neurons and other cells in the body. Neuronal functions rely on a balance between the number of relevant excitatory and inhibitory processes, which may happen individually or concomitantly [5].
The gas neurotransmitters (gasotransmitters) in our body include hydrogen sulfide (H2S), nitric oxide (NO), and carbon monoxide (CO); they play essential roles in normal physiology and under pathological conditions. H2S is a member of the gasotransmitter family that is associated with the maintenance of neuronal plasticity, excitability, and the central nervous system (CNS) [6]. N-Methyl-D-aspartate (NMDA) receptors are targets of H2S in the brain; H2S potentiates the activity of NMDA receptors and facilitates the induction of hippocampal LTP [7]. Hence, a recent study demonstrated that H2S could reduce NMDA receptor-mediated currents in pyramidal neurons of the Cornu Ammonis (CA3) region of neonatal hippocampal slices [6]. NO is a ubiquitous signaling molecule in the brain as well as in other organs in the body, and many reviews have described its role in retrograde signaling [8], cellular function, synaptic plasticity [9], development, excitotoxicity, blood flow, and mental health [10]. NO inhibits the activity of NMDA receptors and thereby reduces the effects of glutamate and induces changes in neural transmission. A reduction in NMDA receptor (NMDAR) expression is associated with the change in synaptic plasticity driven by the age-related conditions in sensory input, demonstrating age-related impairment in the function of the NMDAR/NO signaling pathway in the CNS [11]. Physiologically, CO is generated by two heme oxygenases, hemeoxygenase-1 (HO-1) and hemeoxygenase-2 (HO-2), which catalyze the catabolism of heme groups [12]. HO-2 is concentrated in hippocampal pyramidal cells; therefore, CO might be a candidate retrograde messenger for LTP as the HO inhibitor zinc protoporphyrin IX (Znpp-9) blocks the induction of LTP in hippocampal slices [13].
In this review, we will briefly describe the role of synaptic plasticity in normal neuronal functioning or homeostasis and examine how alterations in neural plasticity hamper the release and signaling of neurotransmitters, such as H2S, NO, and CO, to cause neuropsychiatric conditions, such as major depressive disorder (MDD), schizophrenia (SCZ), bipolar disorder (BD), and Alzheimer's disease (AD). We will also address how the upregulation or downregulation of these gasotransmitters affects disease progression. Finally, we will discuss therapeutic options and how, by understanding the pathways through which alterations in neural plasticity cause disorders, we can target the responsible molecules to prevent these neuropsychiatric conditions.
2. Synaptic Plasticity and Its Neurobiology
Synaptic plasticity in the mature nervous system includes structural and morphological modifications, such as dendritic spine growth and synaptogenesis [14]. These modifications are the cellular response to the changes in neuronal activity that are thought to be responsible for learning and memory [15]. The mitochondria present in axonal terminals and the dendrites of neurons play important roles in synaptic activity [16].
Various neurotransmitter receptors are functionally linked with protein kinases as well as other G-proteins that modulate cascades of molecules which in turn maintain essential cellular functions [17]. As an example, the mitogen-activated protein kinase- (MAPK-) related pathway activates transcription factors associated with learning, memory, and cell proliferation as well as apoptosis. This pathway intricates extracellular stimuli via the phosphorylation of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and P38 as well as other kinases. Similarly, the MAPK-involved pathway and 3′-5′-cyclic adenosine monophosphate- (cAMP-) associated pathway are also jointed to activate neurotransmitter receptors as well as modulate cellular functions via the activation of protein kinase A (PKA), exchange protein stimulated by cAMP (EPAC), and other molecules [18]. Modifications of the MAPK-and c-AMP-related signaling pathways may affect intracellular Ca2+ levels, neurotransmitter receptors, transcription factors, and the cross link between signaling pathways as well as other biological functions which is essential for neuroplasticity [19].
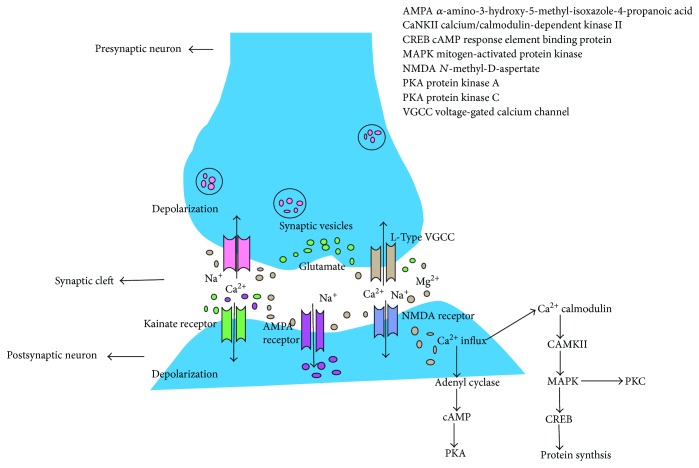
Structurally, synaptic plasticity involves the insertion into or the removal of α-amino-3-hydroxy-5-methyl-isoxazole-4-propanoic acid (AMPA) receptors from the postsynaptic membrane and the enlargement or shrinkage of the dendritic spines where most excitatory synapses (~90%) are located [20]. Functionally, synaptic plasticity is regarded as the LTP or LTD of synaptic strength, demonstrating changes in conductance via AMPA receptors (AMPARs) in the postsynaptic membrane. During the period of plasticity, NMDAR activation allows calcium ions (Ca2+) to cross the postsynaptic membrane and initiate intracellular signaling cascades (Figure 1). These cascades trigger gene transcription, AMPAR trafficking via action dynamics, reorganization of the cytoskeleton, and enlargement or elimination of dendritic spines. The integrity of the synaptic structure, AMPAR trafficking, and dendritic spine dynamics are all pivotal for generating lasting synaptic plasticity changes (Figure 1) [20].
Figure 1.
Transmission of signals through the synaptic junctions. Signals or impulses at the presynaptic terminal trigger the release of glutamate that binds to glutamate receptors at the postsynaptic membrane. Activation of α-amino-3-hydroxy-5-methyl-isoxazole-4-propanoic acid (AMPA) as well as kainate receptors which subsequently transport sodium ions that trigger postsynaptic depolarization. As membrane potential changes, it initiates the release of magnesium ions which blocks N-methyl-D-aspartate (NMDA) receptors. Influx of calcium via NMDA channels sets off a chain of events which establish long-term potentiation. Kainate receptors at the presynaptic end also seem to facilitate synaptic transmission at particular synapses by accumulating neurotransmitter release.
On the neurobiological level, learning and memory depend on regulated signaling processes at synapses as well as synaptic communication between neurons and other cellular partners. Molecular-level plasticity can be driven by increased expression of plasticity-related genes, such as brain-derived neurotrophic factor (BDNF), calcium/calmodulin kinase II (CaMKII), and cyclic AMP (cAMP) response element binding (CREB) protein, as well as by enhanced surface expression of glutamatergic AMPARs and NMDARs. The neurotrophin BDNF and its signaling partners are the main regulators of synaptic plasticity, a biological process that regulates synaptic strength via neuronal activity [21]. Different neuromodulatory factors affect neuronal plasticity such as BDNF which may serve as a real mediator rather than simply a modulator of synaptic plasticity and synaptic communication [21]. Moreover, BDNF and neurotransmitter signaling cascades can work together in close temporal association to induce immediate and guided effects on synaptic plasticity [22]. However, more attention has been given to BDNF because specifically interfering with BDNF-related signaling is a key strategy for initiating neuronal and functionally restorative treatments for neurological and psychiatric disorders [23].
AMPARs and NMDARs are the receptors that synergize at postsynaptic terminals to facilitate different forms of synaptic plasticity (Figure 1). Constant activation of AMPARs by a series of impulses arriving at presynaptic terminals leads to depolarization of the presynaptic membrane, which removes the magnesium ions (Mg2+) that are obstructed at NMDARs [24]. Hence, consistent with the Hebb hypothesis, the simultaneous excitation of pre- and postsynaptic neurons expedites the gating of NMDA channels and strengthens the synapse. This is a crucial feature of NMDA channels that is specifically associated with synaptic plasticity and its high permeability to calcium. Consequently, the second messenger calcium modulates a battery of signaling pathways and the responses that collectively elicit synaptic modification [25]. NMDARs are also involved in synaptic plasticity, but the situation is far more complex, as many forms of LTP are imparted by diverse inputs in various neurons. One intriguing case is that of activity-dependent synaptic plasticity, which is stimulated by presynaptic NMDA channels. In the lateral nucleus of the amygdala, neuronal activity induces a form of LTP that requires NMDARs but is independent of postsynaptic activity [26]. These observations suggest that NMDA functions, which are critical for learning and memory, are not limited to postsynaptic terminals [25].
Voltage-gated calcium channels (VGCCs) play roles in signal transduction between neurons as well as in various forms of synaptic plasticity. Interestingly, patients with AD express higher levels of L-type VGCC in the hippocampus compared with control subjects [27]. Activity-dependent neuroplastic mechanisms in the hippocampus that are fundamental to learning and memory, such as LTP, can be altered by the preceding synaptic activity. The concept of neuroplasticity has been implicated in various neurological and psychiatric diseases and conditions, including AD, depression, SCZ, aging, epilepsy, neurodevelopmental disorders, metabolic disorders, and neuroinflammation, such as multiple sclerosis (MS) [28].
In summary, various receptors and ion channels regulate synaptic plasticity, proper neural functioning, and neural homeostasis.
3. Gasotransmitters (H2S, NO, and CO) in the Nervous System
Three main gasotransmitters that play crucial physiological roles in the body were discovered recently: H2S, CO, and NO. These gasotransmitters also have pathologic functions [29].
3.1. H2S
H2S is an essential signaling molecule with many homeostatic functions, such as neurotransmission and neuromodulation; it is also associated with learning, memory, and nociception [29]. In vivo, five enzymes are associated with H2S synthesis: cystathionine β-synthase (CBS), 3-mercaptopyruvate sulfurtransferase (3-MST), cystathionine γ-lyase (CSE), cysteine aminotransferase (CAT), and D-amino acid oxidase (DAAO) [30]. CBS is thought to be the major H2S-producing enzyme in the brain [31].
Novel signaling molecules linked to polysulfide (H2Sn) such as hydrogen persulfide and trisulfide (H2S2 and H2S3) help maintain neuronal transmission, vascular tone, cytoprotection, inflammation, and oxygen-sensing. A recent study reported that H2S2, H2S3, and H2S are generated by 3-MST [32]. H2S2 and H2S3 are also produced via an interaction between H2S and NO. H2Sn performs additional physiological functions, such as stimulating transient receptor potential ankyrin 1 (TRPA1) channels to impart Ca2+ influx in astrocytes [33, 34] and dorsal root ganglion (DRG) neurons [35]. Additionally, H2S2 along with H2S3 shields neuronal cells from oxidative as well as carbonyl stresses through exerting reduced synthesis of glutathione, which is dependent on the nuclear factor erythroid 2-related factor 2 (Nrf2) [36]. Hylin and Wood demonstrated that cysteine residues in proteins can be persulfurated in the presence of 3-mercaptopyruvate (3-MP), a substrate of 3-MST [37]. Another potential mechanism of persulfuration involves H2S2 and H2S3 generated by 3-MST, which promptly interact with free cysteine and glutathione (GSH) to generate cysteine-persulfide (Cys-SSH) and glutathione-persulfide (GSSH) and also react with the cysteine residues in proteins to produce persulfurated proteins. Alternatively, 3-MST can transfer sulfur from 3-MP to cysteine, GSH, H2S, and cysteine residues to generate Cys-SSH, GSSH, H2S2, and persulfurated proteins. It is possible that these pathways proceed together to generate persulfurated species [38].
H2S accelerates the initiation of hippocampal LTP, which is a synaptic model of memory development, by increasing the function of NMDARs [7]. CBS is expressed in the brain, and the neuronal activity of H2S stimulates the flow of Ca2+ between astrocytes and neurons to adjust synaptic function [39]. The responses of astrocytes to H2S are suppressed by wide-spectrum transient receptor potential (TRP) channel blockers, lanthanide ions (La3+), gadolinium ion (Gd3+), and ruthenium red (RR). In a 2013 study, Kimura et al. showed that polysulfide induces Ca2+ inflow by stimulating transitory receptor potential TRPA1 channels in rat astrocytes [33]. The optimum activity was imparted at 0.5 μM, which is 1/130 of the concentration of H2S needed to obtain feedback of similar magnitude. Additionally, TRPA1-specific agonists, allyl isothiocyanate, and cinnamaldehyde induced Ca2+ inflow, whereas responses to polysulfides were suppressed by the TRAP1-specific inhibitors HC-030031 and AP-18 as well as by TRAP1-specific small interfering RNA (siRNA). This study demonstrated that polysulfides are required for the H2S-derived signaling molecules that activate TRP channels in the brain [33].
Kimura demonstrated that exogenous H2S expedites the induction of hippocampal LTP by increasing NMDAR activity [40]. For example, an H2S donor enhanced NMDAR-mediated currents in the entorhinal cortex and the potentiating effect of exogenous H2S on NMDAR-dependent LTP has been revealed that physiological role of endogenous sulfhydration in plasticity [41]. Another recent study identified a crucial role for activity-dependent sulfhydration in D-serine-dependent synaptic plasticity. Specifically, neuronal activity facilitated H2S production and sulfhydrated serine racemase (SR) formation, and use of an H2S donor enhanced hippocampal D-serine availability, expedited hippocampal LTP via a D-serine-dependent pathway, and slowed age-related LTP impairment [41]. In this study, H2S levels and SR sulfhydration were reduced significantly in aged rats. As H2S is an important reducing agent, these changes restored D-serine levels in the hippocampus of aged rats and replenished the deficits in D-serine-dependent plasticity. Additionally, endogenous H2S signals protected against the age-associated impairment of synaptic plasticity [41]. The results of this study suggest that H2S-linked sulfhydration plays an essential role in D-serine-dependent synaptic plasticity, probably by regulating SR activation. Therefore, therapies that involve inhaled H2S or compounds that moderately raise brain H2S levels may be effective for the treatment of age-associated memory impairment [41].
3.2. NO
NO is regarded as a chemical transmitter which has essential functions in the mammalian central as well as peripheral nervous system [42]. NO is a gaseous molecule that can passively cross cell membranes via diffusion. It is generated by the conversion of the amino acid L-arginine into L-citrulline via the enzyme NO synthase (NOS) and inducible NOS (iNOS). Constitutive nitric oxide synthase (cNOS), or type I, is the neuronal NOS (nNOS), and it is expressed at high levels in the brain, especially in the cortex [43]. Additionally, type III or endothelial NOS (eNOS) is expressed in the endothelium. cNOS generates low levels of NO (nM range) for a short duration (seconds–minutes) under regular physiological conditions because it needs to bind to calmodulin, which occurs only while local calcium levels are increased [43].
NO is the second mediator that can activate NMDARs, which are a subtype of glutamatergic receptors [44]. NMDARs are related to the NO system. NMDAR activation persistently enhances the activity of neuronal nitric oxide synthase (nNOS) in the neuronal cytoplasm. It then catalyzes the generation of endogenous NO from L-arginine followed by the enhanced release of NO from neurons [45, 46]. Activation of these receptors by glutamate stimulates the calcium influx into cells and the generation of NO by NOS, which rapidly stimulates guanylate cyclase and increases cyclic guanosine monophosphate (cGMP) synthesis [47]. The concentration of NO reflects glutamatergic neurotransmission [48]. Hence, other glutamate receptors, such as AMPA, can also produce NO; this pathway modulates the release of glutamate and dopamine. AMPARs are important ion channels that have four subunits that operate like NMDARs, such as Glu1–4 or GluA-D [49]. Nevertheless, AMPAR trafficking, expression, and S-nitrosylation activity are maintained by NO. An ATPase named N-ethylmaleimide-sensitive factor (NSF) is enriched in neurons which binds with GluR2, stabilizing or recycling AMPARs with postsynaptic membranes [50]. Physiologically, synaptic NSF is S-nitrosylated by endogenous, neuronally derived NO in the mouse brain. Activation of NMDAR increases the binding of NSF to GluR2, as well as the surface insertion of GluR2. Together, these studies revealed a NO-sensitive pathway involving NMDARs and AMPARs. In particular, NMDARs stimulate NO generation, which enhances NSF S-nitrosylation, stimulates its association with GluR2, and increases the surface expression of GluR2-containing AMPARs. However, the direct S-nitrosylation activity of AMPARs has not been studied [50]. Additionally, NO is associated with the storage, uptake, and release of mediators, such as acetylcholine, noradrenaline, gamma amino butyric acid (GABA), taurine, and a glycine [51]. NO can stimulate its own extrasynaptic receptors, which are located some distance from sites of NO synthesis. In addition, NO is associated with the process of development of the nervous system [8]. For example, nNOS-containing neurons actively participate in the rostral path of neuroblast migration, which involves new synaptic connections and influences neurogenesis [52]. Astrocyte migration is also regulated by the release of NO under the actions of iNOS. NO is also recognized as critical for the formation of synapses and the growth of nerve fibers [53].
3.3. CO
CO is a new gaseous neuromodulatory agent that functions as a neurotransmitter or neuromodulator [54]. CO is produced during heme metabolism by HO-1 and HO-2; HO-1 is an inducible enzyme, and HO-2 is constitutively expressed. HO is the enzyme responsible for CO synthesis in vivo; it catalyzes the metabolism of heme to biliverdin, free iron, and CO [55]. There are three distinct HO isoforms: HO-1, HO-2, and HO-3. Of these, HO-1 and HO-2 are the most studied and best known [56] and are expressed in many tissues, including neural tissue [57]. The CO generated from heme by HO stimulates soluble guanylate cyclase activity, which promotes an increase in cGMP in neurons as well as cardiovascular functions [58]. CO may also have involved in biological activities via alternative pathways, such as the activation of cyclooxygenase, which participates in fever generation by acting on the CNS [54]. In the CNS, the CO/heme oxygenase axis plays a vital role in processes associated with cytoprotection, vasomodulation, neuroinflammation, cell death, metabolism, and cellular redox responses [59]. CO was first recognized as a neurotransmitter by Verma et al. [60]. Their research led to broad investigations into CO, heme oxygenase, and the exogenous administration of CO as a method of imparting neuroprotection and regulating tissue homeostasis in response to pathophysiological conditions, such as cerebral ischemia, cerebrovasodilation, and neurodegenerative diseases [61]. In neurons, CO-induced cGMP generation helps protect from cell death, and NO signaling is associated with the anti-inflammatory effects of CO in microglia [62].
Semiquantitative cytokine profiling of cell lysates and conditioned culture medium demonstrated increased vascular endothelial growth factor (VEGF) levels in CO-treated cultures (cell lysates) compared with controls. This is consistent with other experiments describing that CO increases VEGF levels in astrocytes and cardiomyocytes [63]. Surprisingly, a decrease in neurotrophin-3 and an increase in neurotrophin-4 levels were found in lysates from cells treated with CO. Although no studies have assessed the effects of CO treatment on neurotrophin-3 and neurotrophin-4, both neurotrophins are associated with neuronal growth and synapse formation, maturation, and plasticity. Additionally, neurotrophin-3 is expressed in neural stem cells (NSCs), and it stimulates neuronal differentiation and survival [62].
In conclusion, various gasotransmitters, such as H2S, NO, and CO, have potential roles in maintaining synaptic plasticity in the nervous system.
4. Role of Gasotransmitters in Neuropsychiatric Disease
4.1. MDD
MDD is a lifelong catastrophic mental disorder with high rates of morbidity and mortality [64]. The lifelong chronic–recurrent persistence of MDD is associated with very high economic and social burdens [65]. It is expected to be the second leading cause of disability worldwide by the World Health Organization (WHO) by 2020 [66]. The two gasotransmitters such as H2S and NO are found to have some functions in MDD.
4.1.1. MDD and H2S
H2S is a toxic gas characterized by the smell of rotten eggs. Physiological concentrations of H2S selectively increase NMDAR-induced responses as well as the advantageous induction of LTP [7]. One study demonstrated that H2S can help maintain amygdala-dependent emotional memory by increasing the function of GluN2B-expressing NMDARs in the amygdala of rats [67]. Pathophysiological concentrations (200 pM) of sodium hydrogen sulfate (NaHS), an H2S donor, stimulates seizure-like events in rats in vivo and in vitro, which may be due to increased neuronal excitation [68]. Previous studies indicated that H2S might improve depressive and anxiety-related behaviors in nonstressed rats and mice, but the effects of H2S on MDD animal models and the potential mechanism behind these effects are unknown [69]. To understand the actions and underlying mechanisms of H2S related to depressive-like behavior, a recent study (intraperitoneally) injected the H2S donor NaHS or administered inhaled H2S in a chronic unpredictable mild stress (CUMS) model. The role of the mechanistic target of rapamycin (mTOR) signaling pathway and glutamate receptors (Figure 2) in the antidepressant effects of H2S was evaluated [70]. The results indicated that a deficiency in endogenous H2S in the hippocampus is responsible for the abnormal behaviors associated with CUMS, whereas enhancing hippocampal H2S levels by the administration of NaHS or inhalation of H2S could correct the depressive-like behaviors of rats within a few hours. This suggests that H2S could function as a rapid-onset antidepressant. Additionally, H2S could counteract the loss of dendritic spines in the hippocampus that is associated with CUMS [70].
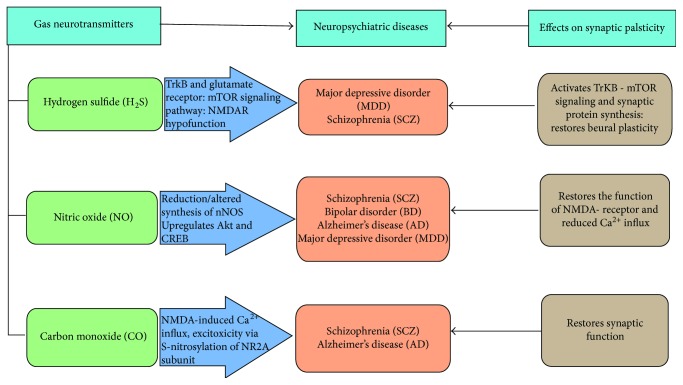
Figure 2.
Role of gas neurotransmitters in neuropsychiatric diseases. The three gas neurotransmitters such as hydrogen sulfide (H2S), nitric oxide (NO), and carbon dioxide (CO) have a role in neuropsychiatric conditions such as major depressive disorder (MDD), schizophrenia (SCZ), bipolar disorder (BD), and Alzheimer's disease (AD) to maintain proper synaptic plasticity as well as neural homeostasis. H2S has a role in tropomyosin receptor kinase B (TrKB) and glutamate as well as mechanistic targets of rapamycin (mTOR) signaling pathways, and it activates the TrKB-mTOR signaling pathway as well as synaptic protein in MDD. NO has a role in the regulation of altered synthesis of nNOS as well as upregulates Akt and cyclic AMP (cAMP) response element binding (CREB) protein which restores function in N-methyl-D aspartate (NMDA) receptor and reduces Ca2+ influx in schizophrenia, MDD, and AD. CO has a role in NMDA-induced calcium ion (Ca2+) influx or excitotoxicity via S-nitrosylation of antiglutamate receptor NMDAR2A (NR2A) subunit and restores synaptic function in AD and SCZ. However, these gas neurotransmitters work on various ways to maintain or restore synaptic plasticity in these neuropsychiatric diseases.
BDNF induces traditional antidepressant actions, and BDNF deletion in the hippocampus weakens antidepressant behavioral responses [71]. Some studies demonstrated that H2S reversed the decrease in tropomyosin receptor kinase B (TrKB) receptors induced by CUMS, demonstrating the pivotal role of neurotrophic signaling in the antidepressant effects of H2S. These findings are consistent with observations that H2S exerted neuroprotective effects against formaldehyde-induced toxicity in PC12 cells via the BDNF–TrKB pathway [72]. This suggests that the synaptogenesis induced by glutamate receptor activation requires the release of BDNF to activate TrKB-mTOR signaling and synaptic protein synthesis (Figure 2). In another study, it was unclear whether the peripheral effects of H2S played a role in the antidepressant responses; thus, additional studies are needed [70]. Nevertheless, this study also demonstrated that the acute application of H2S, via either the H2S donor NaHS or H2S gas inhalation, induced robust antidepressant effects that were mediated by activation of the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway followed by the enhanced synthesis of synaptic proteins containing postsynaptic density protein 95 (PSD95) and synaptophysin. H2S also increases the levels of TrKB receptors, which further increases the activity of the GluR1 and GluR2 subunits of AMPARs. An improved understanding of the roles of H2S could provide insight into potential therapeutic interventions for depression [70].
Ketamine is a noncompetitive blocker of NMDAR that also stimulates the mTOR signaling pathway and subsequently increases the synthesis of the proteins involved in synapses to induce fast-acting antidepressant effects [73]. A study on ketamine-induced antidepressant effects provided an opportunity to explore the ability of new antidepressants with rapid-acting effects to provide sustained relief and fewer side effects. Various studies have demonstrated a link between H2S and the mTOR signaling pathway. A recent study demonstrated that H2S could decrease smoking-induced autophagic cell death by activating mTOR [74]. A novel H2S-releasing molecule GYY4137 (water-soluble, slow-releasing H2S donor) likely protected against high glucose-induced cytotoxicity by activating the mTOR signaling pathway in H9C2 (embryonic cardiomyocyte cell line) cells [75]. Additionally, H2S ameliorated hepatic ischemia/reperfusion injury by stimulating phosphorylation of the pyruvate dehydrogenase kinase 1 (PDK-1)/Akt (protein kinase B)/mTOR/70 kDa ribosomal protein S6 kinase (p70S6K) axis [76]. The effects of H2S on mTOR activation are consistent with the mechanism of rapid-onset antidepressants [70].
H2S is a gasotransmitter that activates the TrKB or mTOR signaling pathways to exert antidepressant effects that are indirectly associated with synaptic protein synthesis or restoration of synaptic plasticity in MDD.
4.1.2. MDD and NO
NO is a highly diffusible and reactive molecule that is synthesized and released with the help of NOSs during the conversion of arginine into citrulline, generating NO in the process [77]. NO mediates the effects of various neurotransmitters, such as norepinephrine, serotonin, glutamate, and dopamine, and thereby plays an essential role in the neurobiology of major depression. Modified NO levels in various brain regions, cerebrospinal fluid (CSF), blood, and exhaled gas have been reported in depression [78]. A meta-analysis revealed disorders in neurooxidative pathways in major depression [79]. Major depression is associated with nitrosative stress, as marked by elevated iNOS function and nitration, as well as by protein nitrosylation [80]. In depression, both neurooxidative and neuronitrative pathways may cause neuroprogression, such as the neuronal dysfunctions caused by oxidative pathways following enhanced neurotoxicity and cytotoxicity, disorders in synaptic plasticity, and reduced neuroprotection [81].
Postmortem studies of patients with MDD revealed reduced neuronal NO synthase levels in the locus coeruleus and a lower number of density of NO synthase-immunoreactive neurons in the hypothalamic nuclei of patients compared with healthy controls [82]. When peripheral NO levels were measured in MDD, some studies reported increased levels [83], whereas other studies found no change [84]. In medication-free depression, various studies found reduced NO levels [85]. In MDD, NO levels were reduced in drug-free patients experiencing depressive episodes in one study [85], but they were either increased or unchanged in another study [84]. Lu et al. demonstrated that NO levels were much higher in MDD patients but then decreased after antidepressant treatment. In the same study, the levels of amino acids, such as citrulline and arginine, were estimated as an index of NO synthesis [78]. In another study, elevated plasma NO levels were reported in male rat models of chronic and unpredictable stress as well as in first-episode MDD patients [86]. The plasma levels of NO metabolites, such as nitrite and nitrate, which reflect plasma NO levels, are also increased in depression [84]. One study described that elevated plasma NO levels in melancholic MDD patients are persistent [78]. This is consistent with previous reports, which also found increased NO plasma levels in MDD patients [87]. Additionally, the elevated NO plasma levels may be associated with suicide attempts in these patients. Modified glutamatergic and decreased GABAergic activity and NO neurotransmission were reported in various brain systems in depression, and this may have a critical effect on the neuronal functions associated with stress responses and mood maintenance [78].
NO is an essential gasotransmitter for neuronal homeostasis, and its upregulation is linked with MDD found in some studies above. Maintaining physiological concentrations of NO could be an effective therapeutic option in MDD.
4.2. SCZ
SCZ is a complex psychiatric illness caused by dysregulation of multiple brain neurotransmitter systems, such as those involving dopamine, glutamate, GABA, serotonin, and acetylcholine. Hence, modifications to these neurotransmitter systems [88] have led to hypotheses centering on the expression and functions of neurotransmitter receptors as critical elements of the pathophysiology of this condition; assimilation of signaling mediated by various neurotransmitter receptors is a pivotal step in achieving the functional interactions of receptor activation [89]. Subsequently, modifications of signal integration pathways may have a role to the pathophysiology of SCZ [88]. Gasotransmitters such as H2S, NO, and CO have some roles in SCZ.
4.2.1. SCZ and NO
NO is associated with synaptic plasticity, neural plasticity, and cognition [90]. It bolsters the survival and differentiation of neurons as well as exhibits long-lasting events by maintaining transcription factors and altering gene expression. Lower concentrations of NO induce neuroprotective effects and support physiological signaling events, leading to neurotransmission and vasodilatation. In contrast, higher concentrations promote inflammatory effects and are neurotoxic [91]. It was hypothesized that NO could act as a retrograde messenger at synapses, transmitting signals from target neurons back to the synapses and maintaining synaptic plasticity. These same characteristics also allow NO to signal to any local compartment and to cells with defective synaptic activity and NOS expression [92]. Recent evidence revealed roles for NO and related molecules in the pathogenesis of SCZ. Changes in various effects of NO in CNS development may result in neurodevelopmental changes involved in SCZ [92]. NO is associated with many processes in the brain, such as the maintenance of synaptic plasticity, the release of mediators, and the development of nervous tissue [93].
Russian scientists Averbukh et al. (1966) and Bulba et al. (1968) first hypothesized that NO was involved in the onset of SCZ [94]. Studies reporting elevated levels of NO in the postmortem brain tissue [95] and plasma [96] of patients with SCZ also support a link between NOS activity and SCZ. The amount of nNOS differs in patients with SCZ and healthy controls [97]; yet, this issue is conflicted. The nNOS levels in the cortex of the cerebelli of patients with SCZ did not differ from the levels found in those of healthy controls in a study performed by Doyle and Slater in 1995 [98]. In another study, enhanced NO synthase activity was detected in Purkinje cells and the dentate nucleus of patients with SCZ but not with depression [99]. However, data regarding the presence of NOS in the neocortex are inconsistent [100]. Although the upregulated expression of nNOS was reported in the prefrontal cortex in SCZ [100], contrasting data have also been published. Striking data were carried out in the period of investigations of neurons of hypothalamus. The downregulation of nNOS-containing neurons was reported in the periventricular nucleus of patients with SCZ and affective disorders [101]. It was reported that NO in the hypothalamus maintains the synthesis and release of the hormones that maintain the hypothalamic–pituitary–adrenal system (HPAS), including oxytocin, vasopressin, and corticoliberin. The altered production and release of these peptides leads to hyperactivation of HPAS in patients with SCZ [102].
NO levels have also been measured in biological fluids from SCZ patients. The level of NOS and its metabolites in the blood of patients with SCZ and depression has been assessed in many studies, and the results are conflicting [92]. A meta-analysis performed by Maia-de-Oliveira et al. found no significant difference in the NO levels of patients with SCZ and healthy controls. However, higher levels of NO were found in patients treated with antipsychotics, highlighting the influence of these drugs on the metabolism of NOS [103]. Therefore, the enhanced formation of NO does not seem to be caused by NMDARs. This suggests that AMPARs likely play an important role, especially as they are expressed at high levels in patients with SCZ [104]. The subsequent release of NO results in disturbed synaptogenesis and synaptic remodeling as well as in synaptic membrane modification [105].
Antipsychotics modify NO metabolism in the brain. For example, haloperidol suppresses nNOS activity [106]. Hence, the long-term administration of this drug results in nNOS hyperactivity in the striatum of rats [107]. The authors of this study demonstrated that late modification of nNOS activity in the neostriatum during antipsychotic treatment plays an important role in the pathogenesis of late dyskinesia. It is worth repeating that nNOS activity is higher in the plasma of patients with SCZ receiving antipsychotics compared with healthy controls [103]. These studies call into question the influence of nNOS activity in the brains of patients with SCZ [108]. The effects of antipsychotics on other NOS isoforms have also been studied. Clozapine can prevent iNOS activity and reduce microglial inflammation and NO levels in the brain [109]. The effects of antipsychotics on NO metabolism restore the normal function of NMDARs [92].
In summary, SCZ is a neuropsychiatric disorder in which normal synaptic plasticity is hampered. NO plays important roles in maintaining synaptic functions and synaptic plasticity in account of maintaining proper neuronal functioning.
4.2.2. SCZ, H2S, and CO
CBS-derived H2S is needed for amygdalar synaptic plasticity and fear conditioning in rats. In particular, inhibiting the function of amygdalar CBS prevented activity-stimulated H2S production, blocked LTP initiation, and altered cued fear memory in rats [110]. Treatment with an H2S donor corrected the LTP and memory impairments caused via CBS inhibition. This CBS inhibition was related to the maintenance of NMDAR function, as the NMDAR-supported synaptic response was lower when CBS was inhibited and the use of a H2S donor increased the amplitude of the NMDAR EPSPs (5-enolpyruvylshikimate-3-phosphate) to a level comparable to those of the normal controls. This suggests that H2S homeostasis in the brain is critical for the generation of synaptic plasticity and memory. S-Adenosylmethionine (SAM) stimulates CBS activity; it combines with the regulatory C-terminal domain of CBS to activate the generation of endogenous H2S. Nevertheless, the mechanisms by which CBS inhibition alters amygdalar synaptic plasticity and memory require further investigation. Activation of NMDAR modulates synaptic plasticity, learning, and memory, and NMDAR hypofunction (Figure 2) was linked to cognitive deficits in aging as well as other psychiatric disorders, such as SCZ [110].
One study showed that prenatal exposure to CO leads to a variety of neurological effects. Lower concentrations of CO lead to a variety of neurobehavioral disorders in rat offspring. Prenatal CO exposure also hampers various neurotransmitters in the growing brains of male rats; low concentrations altered the mesolimbic dopaminergic function and sexual behavior [111]. Changes in cerebellar catecholamines linked these changes to deficits in motor test performance, learning, and memory, as determined by the reduced total GABA content in the cerebelli of 10-day-old rats exposed to CO prenatally. This suggests that GABAergic neurons may have a specific role in CO toxicity. Another study demonstrated that GABAergic neurons may be specifically vulnerable to CO toxicity. Therefore, GABA signaling is modified in neurological disorders, such as SCZ [111]. HO-1 expression in SCZ can be increased by oxidative and inflammatory stimuli [112]. The selective overexpression of HO-1 in the astrocytes of transgenic mice resulted in oxidative stress, lower neuronal reelin content, increased dopamine and serotonin concentrations in the basal ganglia, decreased D1 receptor binding in the nucleus acumens, and altered hippocampal cytoarchitectures. These pathological changes were related to enhanced co-motor activity and reduced proton pump inhibitors but did not affect anxiety or motor balance [113].
H2S and CO have a potential role in neuronal homeostasis, and maintaining proper amounts of these gasotransmitters is a crucial factor in case of SCZ.
4.3. BD
BD is a severe neuropsychiatric condition that results in repeated episodes of mania, which are pathologically energized states characterized by poor judgment, euphoria, irritability, and in depressive episodes, which are characterized by dispiriting moods, decreased energy, volitional states, and decreased cognitive capacity [114]. The gasotransmitter NO is related to the BD which is briefly discussed below.
4.3.1. BD and NO
Modified NO signaling, which directly affects neurotransmitter release and synaptic plasticity cascades, has been demonstrated in BD. Lithium maintains NO levels in preclinical models. However, the effects of lithium ion NO levels have not been studied in humans [115]. Upregulated NO levels were reported during various mood states [116], particularly depressive episodes [117]. The NO pathway is particularly important in neuropsychiatric disorders. Altered NO levels affect neurotransmitter release [90] and synaptic plasticity [118]. High concentrations of NO have dose-dependent neurotoxic effects, whereas physiological concentrations play neuromodulatory and neuroprotective roles [91]. The neuroprotective effects of NO include reducing Ca2+ influx and subsequent cell death (Figure 2) [119]. Additionally, NO upregulates the expression of the neuroprotective proteins Akt and CREB (Figure 2) [120] and the potent antioxidant bilirubin [121].
NO effects are persistent with neuroprotective as well as neurotrophic roles of lithium [122]. Lithium is the standard and first-line treatment option for BD [123]. Its mechanism of action is complex and involves multiple intracellular signaling pathways. Various animal studies have revealed that lithium maintains central and peripheral NO levels [124] and significantly increases NO levels in BD depression after 6 weeks of treatment. However, there was no marked difference in NO levels between unmedicated BD patients and matched healthy controls [122]. These experiments suggest that NO levels may be maintained by lithium in humans. Along these lines, an increasing body of preclinical evidence suggests that lithium can directly target NO signaling [124]. For example, lithium upregulates NOS messenger RNA (mRNA) expression in glial cultured cells [125], the hypothalamus [126], and the hippocampus [127] and also increases cortical NO metabolites in rodents. Other preclinical studies showed that lithium reduces NO metabolites [128] in rat neural tissues [129]. The upregulated NO levels were not associated with clinical improvement, increasing the possibility that the effects of lithium ion NO may be an epiphenomenon or an intermediate pathway for the antidepressant effect [115].
In a recent study, the plasma NO levels in BD patients did not differ from those of healthy controls [115]. In terms of mood disorders, studies on NO have yielded mixed results. At the same time, several studies reported enhanced NO levels in BD [116], and another study that analyzed NO levels during a depressive episode in BD [117] showed elevated NO metabolite levels in subjects with BD receiving multiple medications that can influence NO [103]. The fact that the sample contained drug-free patients with a short duration of illness means that it is possible that NO plays a more important role in BD late in the course of the illness, after patients have been exposed to chronic insults such as repetitive episodes, medications, and comorbidities. Additionally, the number of previous mood episodes was positively correlated with NO levels in BD [130]. These studies may support a crucial role for NO signaling in the trophic and neuroprotective effects of lithium in BD and other neuropsychiatric disorders [115].
In conclusion, the gasotransmitter NO plays a pivotal role in maintaining neural plasticity and proper neuronal functioning in the service of preserving the activity of the CNS in BD.
4.4. AD
AD is characterized by the loss of neurons and synapses in the hippocampus, cerebral cortex, and subcortical regions, as well as the formation of amyloid beta (Aβ) plaques and neurofibrillary lesions. The main protein component of plaques is amyloid-β, which is derived from the proteolytic cleavage of amyloid precursor protein (APP). Mutations associated with early-onset of familial AD increase Aβ production. Aβ isolated directly from human AD brains caused impaired synaptic plasticity and memory in rodents [131]. Synaptic activity and chronic sleep restriction increase the amount of Aβ in brain and intestinal fluid, as well as plaque formation in APP transgenic mice [132]. AD is related with some gasotransmitters such as CO and NO, which is discussed briefly in the following subsections.
4.4.1. AD and CO
As discussed above, mammalian tissues express two isoforms of heme oxygenase: HO-1 and HO-2. The third isoform, HO-3, is a retrotransposition of the HO-2 gene and is only found in rats [133]. The basal expression of HO-1 in the normal brain is restricted to small groups of scattered neurons and neuroglia [134], whereas HO-2 is more broadly expressed across the neuraxis [60]. HO-1 is a 32 kDa protein that catalyzes the breakdown of heme to free iron, CO, and biliverdin. In “stressed” astroglia, HO-1 hyperactivity stimulates mitochondrial iron sequestration and macroautophagy, which may be responsible for the pathological iron accumulation and bioenergetic failure observed in AD as well as in other neurodevelopmental conditions. The expression of glial HO-1 may also affect neuroplasticity and cell survival by modulating the brain sterol metabolism and the proteasomal deterioration of neurotoxic proteins [135].
HO-1 immunoreactivity in glia increases progressively as aging progresses in the normal human brain [136]. HO-1 deteriorates as neural tissue senesces, which may be responsible for the biogenesis of the corpora amylacea and glycoprotein-rich inclusions generally encountered in aging mammalian cells [137]. The number of glial fibrillary acid protein- (GFAP-) positive astrocytes that express HO-1 is increased significantly in the hippocampus and cerebral cortex of patients with AD compared with age-matched, nondemented controls. An excessive increase in glial HO-1 levels is already apparent in the brains of subjects with mild cognitive impairment (MCI), which is a common precursor or indication of incipient AD [138]. In the temporal cortex of patients with MCI, the number of astrocytes with immunoreactivity for HO-1 is related to the degree of neurofibrillary pathology and also the reductions in scores on tests of global cognition, episodic memory, semantic memory, and working memory. Similarly, HO-1 expression in astroglia is associated with lower scores for global cognition, perceptual speed, and semantic memory.
Elevated CO is found in the above studies in AD, and regulating physiological levels of CO could be a therapeutic option in case of AD.
4.4.2. AD and NO
Deficits in synaptic plasticity are increasingly recognized as causes of memory loss in AD [139]. However, the early mechanisms driving synaptic pathophysiology are poorly understood. Short-term plasticity and long-term plasticity are calcium-dependent processes that can be altered by second messengers, such as NO. NO is produced by NOS via NMDAR-mediated calcium entry [140]. NO signaling is involved in neurodegenerative diseases via the formation of reactive nitrogen species and cGMP signaling cascades [141]. NO also has neuroprotective effects, as shown in AD mouse models in which it reduces cell loss and tau pathology [142]. In AD models, NO can be altered via various mechanisms. For example, the NMDAR-mediated calcium entry that activates NOS is augmented by abnormal ryanodine receptor- (RyR-) mediated calcium-induced calcium release [143]. NOS protein levels and RyR levels are also increased in both AD mouse models and human AD brains [144]. In presynaptic AD mice, these conditions that amplify NO levels occur alongside exaggerated hippocampal synaptic depression. These deficits occur when homeostasis is challenged, such as in the presence of reduced RyR-calcium release. Although the hippocampal network and cognitive performance appear normal, they clearly are not [145].
In one study, the primary site of NO regulation in 3×-Tg-AD mice was the presynaptic terminals, where it increased evoked and spontaneous vesicle release, as determined by PPF assays and spontaneous vesicle-release properties [146]. In this study, NO could increase transmitter release via cGMP signaling as well as the S-nitrosylation of synaptic proteins, which increases the presynaptic binding of syntaxin to VAMP and SNAPZS [147]. NO also alters the magnitude of vesicular release by converting reserve pool vesicles into easily releasable vesicles [148]. NO can also increase RyR channel opening, possibly via S-nitrosylation. The opposing interactions between enhanced RyR-calcium signaling and increased nNOS expression in AD neurons can sustain enhanced NO production or synthesis and also increase presynaptic gain. Postsynaptically, the neuroprotective characteristics of NO curb the excessive NMDAR-induced calcium influx and excitotoxicity by causing S-nitrosylation of the antiglutamate receptor NMDAR2A (NR2A) subunit (Figure 2). At the same time, the S-nitrosylation of caspase-3, -8, and -9 decreases apoptosis. The enhanced nNOS levels and NO activity in AD brains may have a neuroprotective role, as demonstrated by the selectively spared NOS-positive neurons in AD. Hence, constant increases in NO have harmful effects, such as oxidative stress, the fragmentation loss of synaptic functioning, and apoptosis [141].
In summary, AD is a major neuropsychiatric disorder, and the regulation of NO is essential for maintaining proper neuronal functioning and synaptic plasticity in the CNS during AD.
5. Future Directions
Gasotransmitters are the essential molecules that help regulate synaptic and neuroplasticity in the CNS. Three gas neurotransmitters, H2S, NO, and CO, were discussed briefly. These gasotransmitters are related to neuropsychiatric conditions. For example, H2S downregulation was involved with the progression of MDD in one study, but the molecular mechanisms by which reduced H2S levels lead to MDD need to be studied further [69, 70]. In terms of other gasotransmitters, higher NO levels were reported in the postmortem brains of SCZ patients [103]; thus, downregulation or modified regulation could be a therapeutic option for treating SCZ. NO levels are also altered in BD patients, and this could facilitate the identification of therapies to treat these neuropsychiatric conditions. Additionally, increased levels of HO-1 or CO were observed in AD patients, which highlights how gasotransmitters may be involved with neuropsychiatric conditions and how regulating these gasotransmitters could help treat these disorders.
6. Conclusion
Maintaining synaptic plasticity is crucial for regulating neuronal health and homeostasis. Neuronal functioning declines over time or under stressful conditions whereas many reactive species can lead to different neuropsychiatric conditions, such as SCZ, MDD, BD, and AD. Several gasotransmitters, such as H2S, NO, and CO, balance synaptic plasticity when the normal condition is altered directly or indirectly. However, as the mechanisms or pathways through which they act are poorly understood, further studies are needed. Because the up- or downregulation of these gasotransmitters is responsible for causing the pathological conditions that lead to neuropsychiatric diseases, the normalization of their levels could exert protective effects. Hence, ensuring that the levels of these gasotransmitters are appropriate could help in the treatment of neuropsychiatric conditions. Moreover, improvements in our understanding of these pathways may lead to the identification of new therapeutic options for these neuropsychiatric conditions.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2018R1A2B6001123).
Abbreviations
- AD:
Alzheimer's disease
- AMPA:
α-Amino-3-hydroxy-5-methyl-isoxazole-4-propanoic acid
- AMPARs:
α-Amino-3-hydroxy-5-methyl-isoxazole-4-propanoic acid receptors
- Aβ:
Amyloid beta
- APP:
Amyloid precursor protein
- BD:
Bipolar disorder
- BDNF:
Brain derivative neurotrophic factor
- CA3:
Cornu Ammonis 3
- CaMKII:
Calcium/calmodulin-dependent kinase II
- CREB:
Cyclic AMP response element binding protein
- CO:
Carbon monoxide
- Ca2+:
Calcium ion
- CBS:
Cystathionine β-synthase
- CSE:
Cystathionine γ-lyase
- CAT:
Cysteine aminotransferase
- cGMP:
Cyclic guanosine monophosphate
- cAMP:
Cyclic adenosine monophosphate
- CUMS:
Chronic unpredictable mild stress
- DAAO:
D-Amino acid oxidase
- DRG:
Dorsal root ganglion
- ERK:
Extracellular signal-regulated kinase
- EPAC:
Exchange protein stimulated by cAMP
- GABA:
Gamma amino butyric acid
- GFAP:
Glial fibrillary acid protein
- GSH:
Glutathione
- GSSH:
Glutathione persulfide
- H2Sn:
Polysulfide
- H2S:
Hydrogen sulfide
- HO-1:
Hemeoxygenase-1
- HO-2:
Hemeoxygenase-2
- HO-3:
Hemeoxygenase-3
- HPAS:
Hypothalamic–pituitary–adrenal system
- JNK:
C-Jun N-terminal kinase
- LTP:
Long-term potentiation
- LTD:
Long-term depression
- MDD:
Major depressive disorder
- MAPK:
Mitogen-activated protein kinases
- 3-MST:
3-Mercaptopyruvate sulfurtransferase
- mTOR:
Mechanistic target of rapamycin
- mRNA:
Messenger ribonucleic acid
- MCI:
Mild cognitive impairment
- NMDA:
N-Methyl-D-aspartate
- NMDARs:
N-Methyl-D-aspartate receptors
- NO:
Nitric oxide
- NOS:
Nitric oxide synthase
- NaHS:
Sodium hydrogen sulfate
- NSCs:
Neural stem cells
- Nrf2:
Nuclear factor erythroid 2-related factor 2
- PKA:
Protein kinase A
- RyR:
Ryanodine receptor
- SCZ:
Schizophrenia
- SSRIs:
Serotonin reuptake inhibitors
- SAM:
S-Adenosylmethionine
- siRNA:
Small interfering RNA
- TrkB:
Tropomyosin receptor kinase B
- TRPA1:
Transient receptor potential ankyrin 1
- VGCC:
Voltage-gated calcium channels
- VEGF:
Vascular endothelial growth factor
- WHO:
World Health Organization.
Conflicts of Interest
The authors reported no potential conflict of interests.
References
- 1.Kumar A. Long-term potentiation at CA3–CA1 hippocampal synapses with special emphasis on aging, disease, and stress. 2011;3:1–20. doi: 10.3389/fnagi.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss T. V. P., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Bramham C. R., Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Malenka R. C., Bear M. F. LTP and LTD: an embarrassment of riches. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Rico E. P., Rosemberg D. B., Seibt K. J., Capiotti K. M., Da Silva R. S., Bonan C. D. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. 2011;33(6):608–617. doi: 10.1016/j.ntt.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Yakovlev A. V., Kurmasheva E. D., Giniatullin R., Khalilov I., Sitdikova G. F. Hydrogen sulfide inhibits giant depolarizing potentials and abolishes epileptiform activity of neonatal rat hippocampal slices. 2017;340:153–165. doi: 10.1016/j.neuroscience.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 7.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardingham N., Dachtler J., Fox K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. 2013;7:1–19. doi: 10.3389/fncel.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hölscher C. Nitric oxide, the enigmatic neuronal messenger: its role in synaptic plasticity. 1997;20(7):298–303. doi: 10.1016/S0166-2236(97)01065-5. [DOI] [PubMed] [Google Scholar]
- 10.Steinert J. R., Chernova T., Forsythe I. D. Nitric oxide signaling in brain function, dysfunction, and dementia. 2010;16(4):435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- 11.Jung J., Na C., Huh Y. Alterations in nitric oxide synthase in the aged CNS. 2012;2012:7. doi: 10.1155/2012/718976.718976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poss K. D., Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. 1997;94(20):10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens C. F., Wang Y. Reversal of long-term potentiation by inhibitors of haem oxygenase. 1993;364(6433):147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- 14.Mattson M. P. Mitochondrial regulation of neuronal plasticity. 2007;32(4-5):707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- 15.Carlisle H. J., Kennedy M. B. Spine architecture and synaptic plasticity. 2005;28(4):182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Todorova V., Blokland A. Mitochondria and synaptic plasticity in the mature and aging nervous system. 2017;15(1):166–173. doi: 10.2174/1570159x14666160414111821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauceri D., Gardoni F., Marcello E., Di Luca M. Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. 2007;100(4):1032–1046. doi: 10.1111/j.1471-4159.2006.04267.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng X., Ji Z., Tsalkova T., Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. 2008;40(7):651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichenberg A. A. The assessment of neuropsychological functioning in schizophrenia. 2010;12(3):383–392. doi: 10.31887/DCNS.2010.12.3/areichenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho V. M., Lee J.-A., Martin K. C. The cell biology of synaptic plasticity. 2011;334(6056):623–628. doi: 10.1126/science.1209236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park H., Poo M. Neurotrophin regulation of neural circuit development and function. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 22.Sasi M., Vignoli B., Canossa M., Blum R. Neurobiology of local and intercellular BDNF signaling. 2017;469(5-6):593–610. doi: 10.1007/s00424-017-1964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu B., Nagappan G., Guan X., Nathan P. J., Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. 2013;14(6):401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 24.Daw N. W., Stein P. S. G., Fox K. The role of NMDA receptors in information processing. 1993;16(1):207–222. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- 25.Voglis G., Tavernarakis N. The role of synaptic ion channels in synaptic plasticity. 2006;7(11):1104–1110. doi: 10.1038/sj.embor.7400830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humeau Y., Shaban H., Bissière S., Lüthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. 2003;426(6968):841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- 27.Coon A. L., Wallace D. R., Mactutus C. F., Booze R. M. L-type calcium channels in the hippocampus and cerebellum of Alzheimer’s disease brain tissue. 1999;20(6):597–603. doi: 10.1016/S0197-4580(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 28.Novkovic T., Mittmann T., Manahan-Vaughan D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. 2015;25(1):1–15. doi: 10.1002/hipo.22342. [DOI] [PubMed] [Google Scholar]
- 29.Shefa U., Yeo S. G., Kim M. S., et al. Role of gasotransmitters in oxidative stresses, neuroinflammation, and neuronal repair. 2017;2017:15. doi: 10.1155/2017/1689341.1689341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibuya N., Koike S., Tanaka M., et al. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. 2013;4:p. 1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 31.Lee M., Schwab C., Yu S., McGeer E., McGeer P. L. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. 2009;30(10):1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Vandiver M. S., Paul B. D., Xu R., et al. Sulfhydration mediates neuroprotective actions of parkin. 2013;4:p. 1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura Y., Mikami Y., Osumi K., Tsugane M., Oka J., Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. 2013;27(6):2451–2457. doi: 10.1096/fj.12-226415. [DOI] [PubMed] [Google Scholar]
- 34.Koike S., Kawamura K., Kimura Y., Shibuya N., Kimura H., Ogasawara Y. Analysis of endogenous H2S and H2Sn in mouse brain by high-performance liquid chromatography with fluorescence and tandem mass spectrometric detection. 2017;113:355–362. doi: 10.1016/j.freeradbiomed.2017.10.346. [DOI] [PubMed] [Google Scholar]
- 35.Hatakeyama Y., Takahashi K., Tominaga M., Kimura H., Ohta T. Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. 2015;11(1):p. 24. doi: 10.1186/s12990-015-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura Y., Toyofuku Y., Koike S., et al. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. 2015;5(1, article 14774) doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hylin J. W., Wood J. L. Enzymatic formation of polysulfides from mercaptopyruvate. 1959;234(8):2141–2144. [PubMed] [Google Scholar]
- 38.Kimura Y., Koike S., Shibuya N., Lefer D., Ogasawara Y., Kimura H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. 2017;7(1, article 10459) doi: 10.1038/s41598-017-11004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai Y., Tsugane M., Oka J.-I., Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. 2004;18(3):557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- 40.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. 2000;267(1):129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- 41.Li Y.-L., Wu P.-F., Chen J.-G., et al. Activity-dependent sulfhydration signal controls N-methyl-D-aspartate subtype glutamate receptor-dependent synaptic plasticity via increasing d-serine availability. 2017;27(7):398–414. doi: 10.1089/ars.2016.6936. [DOI] [PubMed] [Google Scholar]
- 42.Garthwaite J. From synaptically localized to volume transmission by nitric oxide. 2016;594(1):9–18. doi: 10.1113/JP270297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prickaerts J., Van Goethem N. P., Gulisano W., Argyrousi E. K., Palmeri A., Puzzo D. Physiological and pathological processes of synaptic plasticity and memory in drug discovery: do not forget the dose-response curve. 2017;817:59–70. doi: 10.1016/j.ejphar.2017.05.058. [DOI] [PubMed] [Google Scholar]
- 44.Hoque K. E., Indorkar R. P., Sammut S., West A. R. Impact of dopamine–glutamate interactions on striatal neuronal nitric oxide synthase activity. 2010;207(4):571–581. doi: 10.1007/s00213-009-1687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. 1988;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 46.Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. 1992;8(1):3–11. doi: 10.1016/0896-6273(92)90104-L. [DOI] [PubMed] [Google Scholar]
- 47.Szabadits E., Cserép C., Szőnyi A., et al. NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. 2011;31(16):5893–5904. doi: 10.1523/JNEUROSCI.5938-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolluru G. K., Shen X., Bir S. C., Kevil C. G. Hydrogen sulfide chemical biology: pathophysiological roles and detection. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dingledine R., Borges K., Bowie D., Traynelis S. F. The glutamate receptor ion channels. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 50.Wang J. Q., Chu X.-P., Guo M.-L., et al. Modulation of ionotropic glutamate receptors and acid-sensing ion channels by nitric oxide. 2012;3:1–6. doi: 10.3389/fphys.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan B. H., Wong P. T. H., Bian J. S. Hydrogen sulfide: a novel signaling molecule in the central nervous system. 2010;56(1):3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Blasko J., Fabianova K., Martoncikova M., Sopkova D., Racekova E. Immunohistochemical evidence for the presence of synaptic connections of nitrergic neurons in the rat rostral migratory stream. 2013;33(6):753–757. doi: 10.1007/s10571-013-9956-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooke R. M., Mistry R., Challiss R. A. J., Straub V. A. Nitric oxide synthesis and cGMP production is important for neurite growth and synapse remodeling after axotomy. 2013;33(13):5626–5637. doi: 10.1523/JNEUROSCI.3659-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steiner A. A., Colombari E., Branco L. G. S. Carbon monoxide as a novel mediator of the febrile response in the central nervous system. 1999;277(2):R499–R507. doi: 10.1152/ajpregu.1999.277.2.r499. [DOI] [PubMed] [Google Scholar]
- 55.Maines M. D. Carbon monoxide: an emerging regulator of cGMP in the brain. 1993;4(5):389–397. doi: 10.1006/mcne.1993.1049. [DOI] [PubMed] [Google Scholar]
- 56.Ewing J. F., Maines M. D. In situ hybridization and immunohistochemical localization of heme oxygenase-2 mRNA and protein in normal rat brain: differential distribution of isozyme 1 and 2. 1992;3(6):559–570. doi: 10.1016/1044-7431(92)90068-D. [DOI] [PubMed] [Google Scholar]
- 57.Marks G. S., Brien J. F., Nakatsu K., McLaughlin B. E. Does carbon monoxide have a physiological function? 1991;12(5):185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- 58.Morita T., Perrella M. A., Lee M.-E., Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. 1995;92(5):1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Queiroga C. S. F., Vercelli A., Vieira H. L. A. Carbon monoxide and the CNS: challenges and achievements. 2015;172(6):1533–1545. doi: 10.1111/bph.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. 1993;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 61.Hung S.-Y., Liou H.-C., Kang K.-H., Wu R.-M., Wen C.-C., Fu W.-M. Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. 2008;74(6):1564–1575. doi: 10.1124/mol.108.048611. [DOI] [PubMed] [Google Scholar]
- 62.Dreyer-Andersen N., Almeida A. S., Jensen P., et al. Intermittent, low dose carbon monoxide exposure enhances survival and dopaminergic differentiation of human neural stem cells. 2018;13(1, article e0191207) doi: 10.1371/journal.pone.0191207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi Y. K., Kim C.-K., Lee H., et al. Carbon monoxide promotes VEGF expression by increasing HIF-1α protein level via two distinct mechanisms, translational activation and stabilization of HIF-1α protein. 2010;285(42):32116–32125. doi: 10.1074/jbc.M110.131284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kessler R. C., Berglund P., Demler O., et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 65.Miret M., Ayuso-Mateos J. L., Sanchez-Moreno J., Vieta E. Depressive disorders and suicide: epidemiology, risk factors, and burden. 2013;37(10):2372–2374. doi: 10.1016/j.neubiorev.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Murray C. J. L., Lopez A. D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 67.Wang C.-M., Yang Y.-J., Zhang J.-T., et al. Regulation of emotional memory by hydrogen sulfide: role of GluN2B-containing NMDA receptor in the amygdala. 2015;132(1):124–134. doi: 10.1111/jnc.12961. [DOI] [PubMed] [Google Scholar]
- 68.Luo Y., Wu P. F., Zhou J., et al. Aggravation of seizure-like events by hydrogen sulfide: involvement of multiple targets that control neuronal excitability. 2014;20(5):411–419. doi: 10.1111/cns.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen W.-L., Xie B., Zhang C., et al. Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in behavioral models of depression and anxiety. 2013;24(7):590–597. doi: 10.1097/FBP.0b013e3283654258. [DOI] [PubMed] [Google Scholar]
- 70.Hou X.-Y., Hu Z.-L., Zhang D.-Z., et al. Rapid antidepressant effect of hydrogen sulfide: evidence for activation of mTORC1-TrkB-AMPA receptor pathways. 2017;27(8):472–488. doi: 10.1089/ars.2016.6737. [DOI] [PubMed] [Google Scholar]
- 71.Monteggia L. M., Barrot M., Powell C. M., et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang J.-M., Zhou C.-F., Gao S.-L., et al. BDNF-TrkB pathway mediates neuroprotection of hydrogen sulfide against formaldehyde-induced toxicity to PC12 cells. 2015;10(3, article e0119478) doi: 10.1371/journal.pone.0119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li N., Lee B., Liu R.-J., et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X., An G., Chen J. Hydrogen sulfide improves left ventricular function in smoking rats via regulation of apoptosis and autophagy. 2014;19(6):998–1005. doi: 10.1007/s10495-014-0978-z. [DOI] [PubMed] [Google Scholar]
- 75.Wei W., Hu X., Zhuang X., Liao L., Li W. GYY4137, a novel hydrogen sulfide-releasing molecule, likely protects against high glucose-induced cytotoxicity by activation of the AMPK/mTOR signal pathway in H9c2 cells. 2014;389(1-2):249–256. doi: 10.1007/s11010-013-1946-6. [DOI] [PubMed] [Google Scholar]
- 76.Shimada S., Fukai M., Wakayama K., et al. Hydrogen sulfide augments survival signals in warm ischemia and reperfusion of the mouse liver. 2015;45(7):892–903. doi: 10.1007/s00595-014-1064-4. [DOI] [PubMed] [Google Scholar]
- 77.Griffith O. W., Stuehr D. J. Nitric oxide synthases: properties and catalytic mechanism. 1995;57(1):707–734. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 78.Lu Y.-R., Zhang Y., Rao Y.-B., et al. The changes in and relationship between plasma nitric oxide and corticotropin-releasing hormone in patients with major depressive disorder. 2018;45(1):10–15. doi: 10.1111/1440-1681.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu T., Zhong S., Liao X., et al. A meta-analysis of oxidative stress markers in depression. 2015;10(10, article e0138904) doi: 10.1371/journal.pone.0138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gałecki P., Gałecka E., Maes M., et al. The expression of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients with recurrent depressive disorder. 2012;138(3):360–366. doi: 10.1016/j.jad.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 81.Kanchanatawan B., Tangwongchai S., Sughondhabhirom A., et al. Add-on treatment with curcumin has antidepressive effects in Thai patients with major depression: results of a randomized double-blind placebo-controlled study. 2018;33(3):621–633. doi: 10.1007/s12640-017-9860-4. [DOI] [PubMed] [Google Scholar]
- 82.Bernstein H. G., Heinemann A., Krell D., et al. Hypothalamic nitric oxide synthase in affective disorder: focus on the suprachiasmatic nucleus. 2005;51(3):279–284. [PubMed] [Google Scholar]
- 83.Suzuki E., Yagi G., Nakaki T., Kanba S., Asai M. Elevated plasma nitrate levels in depressive states. 2001;63(1–3):221–224. doi: 10.1016/S0165-0327(00)00164-6. [DOI] [PubMed] [Google Scholar]
- 84.Kim Y.-K., Paik J.-W., Lee S.-W., Yoon D., Han C., Lee B.-H. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. 2006;30(6):1091–1096. doi: 10.1016/j.pnpbp.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 85.García R. G., Zarruk J. G., Barrera C., et al. Plasma nitrate levels and flow-mediated vasodilation in untreated major depression. 2011;73(4):344–349. doi: 10.1097/PSY.0b013e31821566cf. [DOI] [PubMed] [Google Scholar]
- 86.Lu Y.-R., Fu X. Y., Shi L. G., et al. Decreased plasma neuroactive amino acids and increased nitric oxide levels in melancholic major depressive disorder. 2014;14(1):p. 123. doi: 10.1186/1471-244X-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao S.-F., Lu Y.-R., Shi L.-G., et al. Nitric oxide synthase and nitric oxide alterations in chronically stressed rats: a model for nitric oxide in major depressive disorder. 2014;47:136–140. doi: 10.1016/j.psyneuen.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Funk A. J., McCullumsmith R. E., Haroutunian V., Meador-Woodruff J. H. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. 2012;37(4):896–905. doi: 10.1038/npp.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volk D. W., Eggan S. M., Lewis D. A. Alterations in metabotropic glutamate receptor 1α and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. 2010;167(12):1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prast H., Philippu A. Nitric oxide as modulator of neuronal function. 2001;64(1):51–68. doi: 10.1016/S0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 91.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D. A., Giuffrida Stella A. M. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 92.Nasyrova R. F., Ivashchenko D. V., Ivanov M. V., Neznanov N. G. Role of nitric oxide and related molecules in schizophrenia pathogenesis: biochemical, genetic and clinical aspects. 2015;6:1–16. doi: 10.3389/fphys.2015.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gibbs S. M. Regulation of neuronal proliferation and differentiation by nitric oxide. 2003;27(2):107–120. doi: 10.1385/MN:27:2:107. [DOI] [PubMed] [Google Scholar]
- 94.Averbukh M. L., Kas'ko A. F., Nikolenko E. S., Rybas I. I. On the diagnostic significance of Black’s reaction in psychiatric patients. 1965;5:289–291. [PubMed] [Google Scholar]
- 95.Yao J. K., Leonard S., Reddy R. D. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. 2004;30(4):923–934. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- 96.Zhang M., Zhao Z., He L., Wan C. A meta-analysis of oxidative stress markers in schizophrenia. 2010;53(1):112–124. doi: 10.1007/s11427-010-0013-8. [DOI] [PubMed] [Google Scholar]
- 97.Akyol O., Zoroglu S., Armutcu F., Sahin S., Gurel A. Nitric oxide as a physiopathological factor in neuropsychiatric disorders. 2004;18(3):377–390. [PubMed] [Google Scholar]
- 98.Doyle C. A., Slater P. Application of [3H] l-NG-nitro-arginine labelling to measure cerebellar nitric oxide synthase in patients with schizophrenia. 1995;202(1-2):49–52. doi: 10.1016/0304-3940(95)12196-X. [DOI] [PubMed] [Google Scholar]
- 99.Bhattacharjee S., Lukiw W. J. Alzheimer’s disease and the microbiome. 2013;7:1–4. doi: 10.3389/fncel.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baba H., Suzuki T., Arai H., Emson P. C. Expression of nNOS and soluble guanylate cyclase in schizophrenic brain. 2004;15(4):677–680. doi: 10.1097/00001756-200403220-00020. [DOI] [PubMed] [Google Scholar]
- 101.Bernstein H.-G., Stanarius A., Baumann B., et al. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. 1998;83(3):867–875. doi: 10.1016/S0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- 102.Ryan M. C. M., Sharifi N., Condren R., Thakore J. H. Evidence of basal pituitary–adrenal overactivity in first episode, drug naïve patients with schizophrenia. 2004;29(8):1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 103.Maia-de-Oliveira J. P., Trzesniak C., Oliveira I. R., et al. Nitric oxide plasma/serum levels in patients with schizophrenia: a systematic review and meta-analysis. 2012;34:149–162. doi: 10.1016/j.rbp.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Tanda K., Nishi A., Matsuo N., et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. 2009;2(1):p. 19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sunico C. R., Portillo F., González-Forero D., Moreno-López B. Nitric oxide-directed synaptic remodeling in the adult mammal CNS. 2005;25(6):1448–1458. doi: 10.1523/JNEUROSCI.4600-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang X. R., Wang Y. X., Zhang Z. J., Li L., Reynolds G. P. The effect of chronic antipsychotic drug on hypothalamic expression of neural nitric oxide synthase and dopamine D2 receptor in the male rat. 2012;7(4, article e33247) doi: 10.1371/journal.pone.0033247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lau Y.-S., Petroske E., Meredith G. E., Wang J. Q. Elevated neuronal nitric oxide synthase expression in chronic haloperidol-treated rats. 2003;45(7):986–994. doi: 10.1016/S0028-3908(03)00314-9. [DOI] [PubMed] [Google Scholar]
- 108.Tarazi F. I., Zhang K., Baldessarini R. J. Long-term effects of newer antipsychotic drugs on neuronal nitric oxide synthase in rat brain. 2002;7(4):297–300. doi: 10.1016/S1089-8603(02)00126-X. [DOI] [PubMed] [Google Scholar]
- 109.Ribeiro B. M. M., do Carmo M. R. S., Freire R. S., et al. Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: reversal by clozapine. 2013;151(1–3):12–19. doi: 10.1016/j.schres.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 110.Chen H. B., Wu W. N., Wang W., et al. Cystathionine-β-synthase-derived hydrogen sulfide is required for amygdalar long-term potentiation and cued fear memory in rats. 2017;155:16–23. doi: 10.1016/j.pbb.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 111.Trentini J. F., O’Neill J. T., Poluch S., Juliano S. L. Prenatal carbon monoxide impairs migration of interneurons into the cerebral cortex. 2016;53:31–44. doi: 10.1016/j.neuro.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Prabakaran S., Swatton J. E., Ryan M. M., et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. 2004;9(7):684–697. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- 113.Song X.-Q., Lv L.-X., Li W.-Q., Hao Y.-H., Zhao J.-P. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. 2009;65(6):481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 114.Murray C. J. L., Lopez A. D. Evidence-based health policy--lessons from the global burden of disease study. 1996;274(5288):740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 115.de Sousa R. T., Zanetti M. V., Busatto G. F., et al. Lithium increases nitric oxide levels in subjects with bipolar disorder during depressive episodes. 2014;55:96–100. doi: 10.1016/j.jpsychires.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andreazza A. C., Kauer-Sant'Anna M., Frey B. N., et al. Oxidative stress markers in bipolar disorder: a meta-analysis. 2008;111(2-3):135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 117.Selek S., Savas H. A., Gergerlioglu H. S., Bulbul F., Uz E., Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. 2008;107(1–3):89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Bon C. L. M., Garthwaite J. On the role of nitric oxide in hippocampal long-term potentiation. 2003;23(5):1941–1948. doi: 10.1523/JNEUROSCI.23-05-01941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu L., Stamler J. S. NO: an inhibitor of cell death. 1999;6(10):937–942. doi: 10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- 120.Riccio A., Alvania R. S., Lonze B. E., et al. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. 2006;21(2):283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 121.Sergent O., Griffon B., Morel I., et al. Effect of nitric oxide on iron-mediated oxidative stress in primary rat hepatocyte culture. 1997;25(1):122–127. doi: 10.1002/hep.510250123. [DOI] [PubMed] [Google Scholar]
- 122.de Sousa R. T., van de Bilt M. T., Diniz B. S., et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. 2011;494(1):54–56. doi: 10.1016/j.neulet.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 123.Bschor T., Bauer M. Efficacy and mechanisms of action of lithium augmentation in refractory major depression. 2006;12(23):2985–2992. doi: 10.2174/138161206777947650. [DOI] [PubMed] [Google Scholar]
- 124.Ghasemi M., Dehpour A. R. The NMDA receptor/nitric oxide pathway: a target for the therapeutic and toxic effects of lithium. 2011;32(7):420–434. doi: 10.1016/j.tips.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 125.Feinstein D. L. Potentiation of astroglial nitric oxide synthase type-2 expression by lithium chloride. 1998;71(2):883–886. doi: 10.1046/j.1471-4159.1998.71020883.x. [DOI] [PubMed] [Google Scholar]
- 126.Anai H., Ueta Y., Serino R., Nomura M., Nakashima Y., Yamashita H. Activation of hypothalamic neuronal nitric oxide synthase in lithium-induced diabetes insipidus rats. 2001;26(2):109–120. doi: 10.1016/S0306-4530(00)00030-5. [DOI] [PubMed] [Google Scholar]
- 127.Bagetta G., Corasaniti M. T., Melino G., Paoletti A. M., Finazziagro A., Nistico G. Lithium and tacrine increase the expression of nitric oxide synthase mRNA in the hippocampus of rat. 1993;197(3):1132–1139. doi: 10.1006/bbrc.1993.2595. [DOI] [PubMed] [Google Scholar]
- 128.Harvey B. H., Carstens M. E., Taljaard J. J. F. Evidence that lithium induces a glutamatergic: nitric oxide-mediated response in rat brain. 1994;19(4):469–474. doi: 10.1007/BF00967326. [DOI] [PubMed] [Google Scholar]
- 129.Bhalla P., Singla N., Dhawan D. K. Potential of lithium to reduce aluminium-induced cytotoxic effects in rat brain. 2010;23(2):197–206. doi: 10.1007/s10534-009-9278-4. [DOI] [PubMed] [Google Scholar]
- 130.Savas H. A., Gergerlioglu H. S., Armutcu F., et al. Elevated serum nitric oxide and superoxide dismutase in euthymic bipolar patients: impact of past episodes. 2006;7(1):51–55. doi: 10.1080/15622970510029993. [DOI] [PubMed] [Google Scholar]
- 131.Van Spronsen M., Hoogenraad C. C. Synapse pathology in psychiatric and neurologic disease. 2010;10(3):207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kang J. E., Lim M. M., Bateman R. J., et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scapagnini G., D’Agata V., Calabrese V., et al. Gene expression profiles of heme oxygenase isoforms in the rat brain. 2002;954(1):51–59. doi: 10.1016/S0006-8993(02)03338-3. [DOI] [PubMed] [Google Scholar]
- 134.Barañano D. E., Snyder S. H. Neural roles for heme oxygenase: contrasts to nitric oxide synthase. 2001;98(20):10996–11002. doi: 10.1073/pnas.191351298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schipper H. M., Song W. A heme oxygenase-1 transducer model of degenerative and developmental brain disorders. 2015;16(12):5400–5419. doi: 10.3390/ijms16035400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hirose W., Ikematsu K., Tsuda R. Age-associated increases in heme oxygenase-1 and ferritin immunoreactivity in the autopsied brain. 2003;5:S360–S366. doi: 10.1016/S1344-6223(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 137.Song W., Zukor H., Liberman A., et al. Astroglial heme oxygenase-1 and the origin of corpora amylacea in aging and degenerating neural tissues. 2014;254:78–89. doi: 10.1016/j.expneurol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schipper H. M., Song W., Zukor H., Hascalovici J. R., Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. 2009;110(2):469–485. doi: 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- 139.Nisticò R., Cavallucci V., Piccinin S., et al. Insulin receptor β-subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. 2012;14(4):262–269. doi: 10.1007/s12017-012-8184-z. [DOI] [PubMed] [Google Scholar]
- 140.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. 2008;27(11):2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao Q.-F., Yu J.-T., Tan L. S-Nitrosylation in Alzheimer’s disease. 2015;51(1):268–280. doi: 10.1007/s12035-014-8672-2. [DOI] [PubMed] [Google Scholar]
- 142.Wilcock D. M., Lewis M. R., van Nostrand W. E., et al. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. 2008;28(7):1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goussakov I., Miller M. B., Stutzmann G. E. NMDA-mediated Ca2+ influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. 2010;30(36):12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shilling D., Muller M., Takano H., et al. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. 2014;34(20):6910–6923. doi: 10.1523/JNEUROSCI.5441-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chakroborty S., Stutzmann G. E. Early calcium dysregulation in Alzheimer’s disease: setting the stage for synaptic dysfunction. 2011;54(8):752–762. doi: 10.1007/s11427-011-4205-7. [DOI] [PubMed] [Google Scholar]
- 146.Chakroborty S., Kim J., Schneider C., West A. R., Stutzmann G. E. Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer’s disease mice. 2015;35(17):6893–6902. doi: 10.1523/JNEUROSCI.4002-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palmer Z. J., Duncan R. R., Johnson J. R., et al. S-Nitrosylation of syntaxin 1 at Cys145 is a regulatory switch controlling Munc18-1 binding. 2008;413(3):479–491. doi: 10.1042/BJ20080069. [DOI] [PubMed] [Google Scholar]
- 148.Ratnayaka A., Marra V., Bush D., Burden J. J., Branco T., Staras K. Recruitment of resting vesicles into recycling pools supports NMDA receptor-dependent synaptic potentiation in cultured hippocampal neurons. 2012;590(7):1585–1597. doi: 10.1113/jphysiol.2011.226688. [DOI] [PMC free article] [PubMed] [Google Scholar]