Abstract
Precision medicine is an approach to disease prevention and treatment that takes into account genetic variability and environmental and lifestyle influences that are unique to each patient. It facilitates stratification of patient populations that vary in their susceptibility to disease and response to therapy. Shared databases and the implementation of new technology systems designed to advance the integration of this information will enable health care providers to more accurately predict and customize prevention and treatment strategies for patients. Although precision medicine has had a limited impact in most areas of medicine, it has been shown to be an increasingly successful approach to cancer therapy. Despite early promising results targeting aberrant signaling pathways or inhibitors designed to block tumor-driven processes such as angiogenesis, limited success emphasizes the need to discover new biomarkers and treatment targets that are more reliable in predicting response to therapy and result in better health outcomes. Recent successes in the use of immunity-inducing antibodies have stimulated increased interest in the use of precision immunotherapy of head and neck squamous cell carcinoma. Using next-generation sequencing, the precise profiling of tumor-infiltrating lymphocytes has great promise to identify hypoimmunogenic cancer that would benefit from a rationally designed combinatorial approach. Continued interrogation of tumors will reveal new actionable targets with increasing therapeutic efficacy and fulfill the promise of precision therapy of head and neck cancer.
Keywords: head and neck neoplasms, genomics, biomarkers, immunotherapy, diagnosis, molecular targeted therapy
Precision Medicine and Precision Therapy
Precision medicine is an emerging approach to disease prevention and treatment that takes into account genetic, proteomic, transcriptomic, and metabolomic variability, as well as environment and lifestyle influences that are unique to each patient (Snyderman et al. 2016). Using shared databases and bioinformatics to facilitate the integration of this information, precision medicine will enable health care providers to more accurately predict and customize prevention and treatment strategies for patients. Although to date precision medicine has had a limited impact in most areas of medicine, it has been shown to be an increasingly successful approach in the treatment of cancer (Garraway et al. 2013; Collins and Varmus 2015; Mes et al. 2016). As progress is made in integrating large-scale biological databases and with continued advances in the biosciences and technology, the promise of precision health and medicine will be fully realized (Ritchie et al. 2015; Prawira et al. 2017). In this concise review, we describe the current and evolving strategies in precision therapy of head and neck squamous cell carcinoma (HNSCC). The therapeutic arm of precision medicine is precision therapy or targeted therapy. It is a strategy that uses the unique genomic and biological characteristics of a tumor to identify treatment-specific targets (https://www.cancer.gov/about-cancer/treatment/types/precision-medicine).
Cancer Genomics and Precision Therapy of HNSCC
Cancer is fundamentally a genomic disease. There are 2 broad categories of cancer mutations. Acquired or somatic mutations occur throughout an individual’s life and are the most common cause of what is described as “sporadic” cancers. Tobacco, alcohol consumption, and human papillomavirus (HPV) infection are the most common cause of head and neck cancer. Germline mutations, on the other hand, are inherited mutations, passed on from one generation to the next. These types of mutations are far less common, accounting for 5% to 10% of cancers. It is common for most cancers to contain multiple mutated oncogenes and tumor suppressor genes (Brower 2011; Garraway et al. 2013).
Next-generation sequencing has enabled researchers to characterize the mutational landscape of many tumors, including HNSCC, helping investigators identify previously known mutations and novel mutations (Stransky et al. 2011; Razzouk 2014). The revelation of these new potentially actionable therapeutic targets may shed light on new avenues of therapy and also reveal new insights into mechanism of tumor progression. Currently, for some cancers, genetic testing is used to assess the carrier status of patients with heritable or acquired genetic mutations that are associated with an increased risk for developing cancer (Zavras et al. 2012; Eccles et al. 2016; Peters et al. 2017). For breast and ovary, this has led to the adoption of more aggressive strategies to either prevent or minimize disease onset and severity (Gail et al. 1989; Wacholder et al. 2010). In regard to oral and HNSCC, retrospective studies have shown that loss of heterozygosity at chromosome 9p and 3p is important in risk assessment of oral precancer (Jordan et al. 2012; Zhang et al. 2012). Also, gene expression profiling of premalignant oral leukoplakias has revealed insights into the identity of genetic lesions that predispose to the development of oral cancer (Saintigny et al. 2011). As precision technology continues to evolve, the ability to accurately predict the onset and severity of head and neck cancers will improve (Wang et al. 2015).
While the genetic mutations encountered in HNSCC are primarily somatic in nature, germline mutations and chromosomal aberrations with loss-of-function mutations of tumor suppressor genes have been found in dysplasias and families with multiple instances of HNSCC (Vinarsky et al. 2009; Stransky et al. 2011; Tanaka et al. 2012; Razzouk 2014; Scheckenbach et al. 2014). Somatic mutations in HNSCC are numerous. Some of the more commonly encountered mutations affecting key growth regulatory and survival pathways are TP53, PI3K, epidermal growth factor receptor (EGFR), p16, and NOTCH1 (Leemans et al. 2011; Bednarek et al. 2016; Giefing et al. 2016). Their expression is altered in almost all of HNSCC. EGFR is overexpressed at high levels in HNSCC and, when encountered, is associated with poor prognosis (Pomerantz and Grandis 2003). Somatic mutations in the tyrosine kinase domain of EGFR are rare, however (Loeffler-Ragg et al. 2006).
HPV-associated HNSCC is a biologically distinct entity (Slebos et al. 2006; Stransky et al. 2011). Approximately 90% of HPV-associated oropharyngeal cancers (oral cavity, larynx, and hypopharynx) can be attributed to high-risk HPV type 16 (HPV16) (Kreimer et al. 2005). Unlike HNSCC caused by tobacco and alcohol, the mechanisms of neoplastic transformation by HPV are different. HPV16 contains 2 oncogenes, E6 and E7, which inactivate p53 and the retinoblastoma gene (Rb) respectively. The E6 and E7 oncoproteins of HPV16 complex with ubiquitin ligases to facilitate degradation of p53 and retinoblastoma proteins, respectively (Chung and Gillison 2009). Both of these genes play a critical role in growth regulation and, when inactivated, result in dysregulation of the cell cycle and enhanced cell proliferation (Zhang et al. 1999; Wiest et al. 2002; Stephen et al. 2013).
The identification of tumor-specific genomic alterations has fundamentally changed the field of cancer therapeutics, leading to the development of new target-specific therapies. Indeed, these “targeted therapies” act on specific mutations identified as suspected “drivers” of cancer progression. New genomic and proteomic strategies have also made it possible to classify tumors according to their mutation status or other molecular alterations, rather than relying on histology or tissue of origin. This molecular typing of tumors has helped to identify new druggable targets (Giefing et al. 2016; Moreira et al. 2017). A targeted cancer therapy that is gaining significant momentum is immunotherapy (Bauman et al. 2017). Many newly developed therapies based on immune checkpoint inhibitors can induce an immune response and tumor regression by blocking immunosuppression (Rosenberg 2014; Ferris et al. 2016). The potential of biomarkers in precision medicine and of immunotherapy in HNSCC will be discussed in some detail in this review.
Biomarker Targets for Precision Medicine in HNSCC
Precision medicine is selection of therapy that is most suited for the individual patient. Currently, tumor stage and location, rather than tumor biology, are the basis of treatment selection in HNSCC (Weiss and Hayes 2014). However, different tumors at the same stage may respond differently to the same treatment.
Targeted therapy against a specific molecule that has a crucial role in tumor progression has been approved for use in HNSCC. The efficacy of targeted therapy would likely be enhanced by use in appropriately selected patients (i.e., in the context of personalized medicine). Of emerging interest are genomic, proteomic, transcriptomic, and metabolomic markers of a tumor that will facilitate treatment selection. Prognostic biomarkers provide information about the likely outcome of a cancer relative to disease progression, recurrence, or death and are independent of treatment (Ballman 2015). In contrast, predictive biomarkers provide information about the likely outcome of a treatment in patients who do or do not have the biomarker (Ballman 2015). This section will primarily focus on biomarkers that enhance selection of conventional treatments and targeted therapy and will reference their prognostic value if relevant.
The heterogeneity of HNSCC provides a challenge to treatment selection by tumor stage, which is informed by clinical and imaging information (Mena et al. 2017). To overcome this hurdle, molecular biomarkers are intensely investigated with the intent of identifying objective parameters (a “biomarker signature”) for selection of the most appropriate treatment. The ultimate goal is to improve patient survival and reduce morbidity.
While tobacco, alcohol, and HPV16 are etiologic factors for HNSCC, HPV-positive and HPV-negative HNSCC are distinct entities (Chaturvedi et al. 2016). Survival rates are better for patients with HPV-positive than HPV-negative oropharyngeal squamous cell carcinoma (Chaturvedi et al. 2016). Among patients with HPV-positive HNSCC, those who had never smoked had better survival than those who had smoked, and both groups did better than patients with HPV-negative tumors (Chaturvedi et al. 2016). HPV16 can be detected by in situ hybridization for genomic DNA or immunostaining for p16 (Lingen et al. 2013). Although HPV status is the only clinically relevant prognostic marker for oropharyngeal HNSCC, it is not a predictive biomarker. In contrast to oropharyngeal HNSCC, HPV16 is not a major risk factor for oral cavity squamous cell carcinoma; in fact, only 5.9% of these lesions exhibited positivity for high-risk HPV E6/E7 (Lingen et al. 2013). Therefore, HPV status is neither a prognostic nor a predictive biomarker for oral cavity HNSCC.
Overexpression of EGFR, observed in >90% HNSCCs, is correlated with poor clinical outcome, including poor overall and disease-free survival and high locoregional recurrence (Ang et al. 2002). However, its association with response to treatment is inconsistent (Vermorken et al. 2007).
p53 is a tumor suppressor gene that is mutated in one-third to two-thirds of HNSCC, leading to disruption of the DNA damage response and impaired growth control (Klein and Grandis 2010). This is associated with reduced survival time and resistance to radiation and chemotherapy (Zhou et al. 2016). Recent studies showed that patients with HNSCC exhibiting high-risk p53 mutations had more cisplatin resistance and poorer survival than those with low-risk mutations (Zhou et al. 2016).
In a recent small study on recurrent/metastatic HPV-positive HNSCC, the molecular profile was similar to HPV-negative tumors (Morris et al. 2017). These included more TP53 mutations, whole-genome duplication, and 3p deletion than HPV-positive primary tumors. Although these findings may be relevant to targeted therapy and de-escalation of chemoradiation, confirmation is required in a larger cohort (Morris et al. 2017).
E-cadherin, a component of the adherens junction, is linked to the cytoskeleton via β catenin. Suppression of E-cadherin is associated with reduced disease-free survival. These and other epithelial to mesenchymal transition biomarkers were reviewed previously (Scanlon et al. 2013). Other proteins such as Bcl2 (B-cell lymphoma 2) and DNA repair proteins have been linked to treatment outcomes (Nix et al. 2005; Mehra et al. 2013). Although several proteins, including Bcl2 and DNA repair proteins, have been linked to treatment response, given the heterogeneity of HNSCC, it is likely that a panel of biomarkers will ultimately facilitate treatment selection.
Companion diagnostics is a term used for biomarkers that allow targeted therapy to be used in the context of precision medicine (Mankoff et al. 2016). These may be broadly grouped into predictive and response biomarkers. Predictive biomarkers are linked to a specific targeted therapy and are often the target or a related molecule (Mankoff et al. 2016). Response biomarkers, also termed pharmacodynamics biomarkers, reflect the response to treatment (Mankoff et al. 2016). Molecular imaging may provide opportunities for predictive and response biomarkers (Mankoff et al. 2016).
Targeted therapy approved for use in HNSCC is the EGFR inhibitor, cetuximab (Saba et al. 2017). Cetuximab competitively antagonizes binding of epidermal growth factor (EGF) and transforming growth factor α (TGFα) to EGFR, thereby inhibiting downstream signaling cascades (Saba et al. 2017). In a randomized trial comparing radiation plus cetuximab with radiation alone, the combination treatment was more effective in locoregional control of HNSCC and reduced patient mortality (Bonner et al. 2006).
Recently, immunotherapeutic agents were approved for the treatment of HNSCC (Ferris et al. 2016; Lemery et al. 2017). The immunotherapeutic agents target programmed cell death 1 (PD1), thereby interrupting interaction with its ligand (PD-L1). PD1/PDL1 induces a signaling cascade that suppresses T cells, thereby allowing HNSCC to evade immunosurveillance (Prasad and Kaestner 2017). Currently, these therapies are not used in the context of precision medicine in HNSCC. Given the extensive molecular profiling of HNSCC, it is likely that companion diagnostics will be developed to facilitate selection of patients who will be most responsive to targeted therapy and immunotherapy.
Targeting the PI3K/AKT/mTOR and Angiogenesis Signaling Pathways
Signaling pathways are often dysregulated in HNSCC. The PI3K/AKT/mTOR pathway is frequently dysregulated in HNSCC and as such is potentially a valuable therapeutic target (Nathan et al. 2007; Simpson et al. 2015; Van Waes and Musbahi 2017). The PI3K/AKT/mTOR signaling pathway plays a vital role in a number of physiological process, including cell growth, survival, and metabolism (Simpson et al. 2015; Van Waes and Musbahi 2017). Mutations in PI3KCA and PTEN oncogenes, downstream components on this pathway, activate mTOR. This enhances cell growth, survival, and tumor progression, features that are common to most human malignancies, including HNSCC (Agrawal et al. 2011; Stransky et al. 2011; Dorsey and Agulnik 2013). Also, genetic alterations in PI3K have been linked to progression of oral dysplasias to carcinomas and in general are associated with a poor prognosis. Inhibition of mTOR by rapamycin and second-generation inhibitors temsirolimus and everolimus has shown antitumor activity in xenograft models (Amornphimoltham et al. 2005; Nathan et al. 2007). However, phase I studies of PI3K-mTOR inhibitors demonstrated enhanced toxicity, a narrow therapeutic window, and eventual tumor progression upon cessation of treatment (Mohan et al. 2015; Vander Broek et al. 2015). The clinical response rate in phase II trials has been disappointing (Geiger et al. 2016) Despite the success of preclinical models, the response to PI3K/AKT/mTOR inhibitors remains controversial (Simpson et al. 2015; Van Waes and Musbahi 2017).
Angiogenesis has long been proposed as a therapeutic target for a wide variety of tumors (Mineta et al. 2000; Vassilakopoulou et al. 2015). The principal antiangiogenic target to date has been vascular endothelial growth factor (VEGF) and downstream VEGF-mediated processes that regulate endothelial cell survival and therapeutic resistance. For example, bevacizumab, a humanized VEGF monoclonal antibody, inhibits angiogenesis and facilitates the delivery of chemotherapeutic agents by inducing vascular normalization (Jain 2001). This is a process by which antiangiogenic therapy restores the balance between pro- and antiangiogenic signaling, thereby inducing a more structurally and functionally normal vasculature. This maturation of microvessels at the tumor site is believed to explain why combining antiangiogenic agents with chemoradiation increases the delivery of anticancer drugs to the tumor site (Guo et al. 2003). The effectiveness of VEGF/VEGF receptor (VEGFR) agents such as bevacizumab, sunitinib, or sorafenib, when used as single agents to treat HNSCC, has been limited and in some cases discontinued due to life-threatening side effects (Machiels et al. 2010; Williamson et al. 2010; Salama et al. 2011). When bevacizumab was used in combination with chemoradiation, it was associated with enhanced tumor regression, reduced the probability of recurrence, and enhanced the response to standard chemotherapy and radiotherapy. In 2 studies in which bevacizumab was combined with 5-fluorouracil, hydroxyurea, and radiotherapy, the addition of bevacizumab was associated with a median survival time of 10.3 mo, and for reirradiated patients with recurrent nonmetastatic disease, the 2-y cumulative incidence of disease death rate was 51% (Seiwert et al. 2008). While these results suggest antiangiogenic agents may be a valuable adjunct to conventional chemoradiation of HNSCC, their long-term therapeutic value remains uncertain.
Precision Immunotherapy of HNSCC
The recent renewed enthusiasm in cancer immunotherapy largely stems from the clinical success of therapeutic monoclonal antibodies (mAbs). These mAbs can be generally classified into 2 categories targeting the induction and maintenance phases of antitumor immunity. The first category of immunity-inducing mAbs targets tumor antigens or the costimulatory pathways to expand the effector cell repertoire and enhance immune killing. But activated effector cells frequently enter into an exhaustion state due to the highly immunosuppressive tumor microenvironment. This state of exhaustion is, at least in part, mediated by the inhibitory immune checkpoint receptor (ICR) signaling. The second category of mAbs inhibits ICRs and maintains effective immune killing (Li et al. 2015; Kansy et al. 2017). As we garner new knowledge from the completed and emerging clinical trials, HNSCC patient responses to these mAbs are found to be highly variable, which entails carefully tailored immunogenomics-informed treatment protocols.
Immunotherapeutic mAbs in Ongoing HNSCC Clinical Trials
In 2016, the Food and Drug Administration (FDA) approved 2 mAbs targeting an ICR (PD-1), pembrolizumab and nivolumab, to manage recurrent/metastatic (R/M) HNSCC. In an initial phase Ib trial (KEYNOTE-012), pembrolizumab was well tolerated in patients with R/M HNSCC, with an overall 18% response rate (Seiwert et al. 2016). In a more recent phase Ib trial (KEYNOTE-028), pembrolizumab demonstrated an objective response rate of 25.9% in patients with PD-L1–positive nasopharyngeal carcinoma (Hsu et al. 2017). A phase III trial of nivolumab (CheckMate 141) revealed a 13.1% response rate among patients with R/M HNSCC (Ferris et al. 2016). As most effector CD8-positive T cells are dysfunctional in the tumor microenvironment with expression of markers for functional exhaustion such as high levels of PD-1 (Kansy et al. 2017), targeting ICR is a promising approach to maintain robust antitumor immunity. But patient response to ICR inhibitors likely requires an existing tumor-specific T-cell repertoire. Hence, a combination of ICR blockade with mAbs, which activate effector T cells and improve the induction phase of antitumor immunity, is being explored in the clinics. These mAbs largely target the costimulatory molecules on immune cells, such as CD137, OX40, and CD357, to amplify effector cell function. A combination of costimulatory pathway activation and ICR inhibition has been proposed in new HNSCC clinical trials, which are discussed in depth in a recent review (Bauman et al. 2017). In addition, an EGFR-targeted mAb, cetuximab, also shows promises in inducing antitumor effector function. It binds to EGFR and activates natural killer (NK) cells through antibody-dependent cell-mediated cytotoxicity (ADCC). Enhanced NK function leads to increased release of interferon (IFN)–γ, promoting the TH1/Tc1 skewing of tumor-specific T cells (Srivastava et al. 2013). Notably, cetuximab was recently shown to induce endoplasmic reticulum (ER) stress in HNSCC (Lei et al. 2016), and ER stress potently stimulates immunogenic cell death, which further enhances immune killing (Pozzi et al. 2016). Hence, cetuximab improves the induction of immunity by engaging both cancer cells and tumor-infiltrating effectors. Among the sophisticated combinatorial strategies, protocol selection depends on the assessment of basal antitumor immunity. But a consistent approach to quantitate cancer immunogenicity is still lacking in the current pathology practice, representing a major knowledge gap in precision immunotherapy.
Experimental Immunotherapeutic Strategies
To exploit the full potential of ICR blockade, immune-priming strategies are pivotal to expand the tumor-specific T-cell repertoire. Stimulating costimulatory pathways in immune cells using mAbs is a promising method to nonspecifically augment basal host immunity. Although evidence is still scarce, this strategy likely requires preexisting infiltration of immune cells or a T-cell–inflamed microenvironment. However, most patients with HNSCC have a poor infiltration of T cells, which may limit this strategy to a group of patients. As a mechanism of effector T-cell homing to the tumor bed, DNA damage in cancer cells results in increased cytoplasmic exposure to DNA fragments, which activates stimulator of interferon genes (STING)–mediated type I IFN signaling and promotes the chemotactic recruitment of antigen-presenting cells and T cells (Deng et al. 2014; Woo et al. 2014; Corrales et al. 2016), but cancers frequently inhibit this central pathway.
To enhance immune detection and improve T-cell infiltration, several conceptual designs are being tested in preclinical models and clinical trials. 1) It is essential to dissect the regulatory network of STING. Novel cancer inhibitors of STING are potential new targets to enhance cancer immunogenicity. 2) Mutation-associated neoantigens critically control the repertoire of tumor-specific T cells. Higher neoantigen load is associated with superior responses to ICR inhibition (Van Allen et al. 2015). The DNA repair pathway is a major checkpoint against genome instability. Thus, DNA repair-competent tumors likely present features of a cold cancer. Depending on the specific mechanisms of DNA repair in individual tumors, coupling DNA damage-inducing agents with ICR blockade may offer benefits to a subset of HNSCC patients. 3) HNSCC has shown daunting heterogeneity, with variable portions of the tumor made up of cancer stem cells, which are highly tumorigenic and immunosuppressive (Prince et al. 2007; Lee et al. 2016). To overcome cancer stem cell–potentiated resistance to therapy, a targeted approach using a cancer stem cell dendritic cell vaccine yields significant tumor regression and prevents lung metastasis (Hu et al. 2016). Hence, more accurate diagnostic tools to identify tumors with high percentages of cancer stem cells and a cancer stem cell–targeted treatment modality will likely contribute to the expanded pool of responders to immunotherapy. 4) With the development of novel vaccine delivery vehicles and neoantigen prediction pipelines, a personalized anticancer vaccine has proven to be a powerful tool to drive tumor-specific immunity (Sahin et al. 2017). A focused review on anti-HNSCC vaccines in this special issue summarizes the recent advances in the field (Tan et al. 2018) Due to the consistent expression of viral proteins in HPV-positive oropharyngeal cancer, E6 and E7 are ideal targets for the design of cancer antigen-specific vaccines (Tan et al. 2018). But in HPV-negative HNSCC, a major challenge to generate neoantigen-targeted vaccines is the time and cost associated with neoantigen identification and subsequent personalized manufacture. Hence, automated neoantigen prediction pipelines and economic vaccine delivery vehicles will bring a potentially transformative technology to the precision immunotherapy of HNSCC.
Potential Biomarkers for Cold HNSCC
Since the initial approval more than a decade ago, numerous clinical trials have been completed with cetuximab. A systematic review reveals that less than 20% of patients with R/M HNSCC respond to cetuximab (Reeves et al. 2011). Similarly, a phase III clinical trial, CheckMate 141, shows that the HNSCC patient response rates to anti–PD-1 are also below 15% (Ferris et al. 2016). Due to the high cost associated with mAb therapy and the emerging novel combinatorial protocols for poor responders, it is urgent to streamline the diagnostic algorithms and stratify hot and cold HNSCC.
In contrast to the notion that HPV-positive HNSCCs exhibit better response profiles than HPV-negative tumors with chemoradiotherapy, HPV status alone cannot be used to predict patient response to anti–PD-1 (Ferris et al. 2016; Bauman et al. 2017). High expression levels of ICR ligands, such as PD-L1, have been examined as a potential biomarker, and about 50% to 60% of HNSCCs express PD-L1 (Concha-Benavente et al. 2015). In the initial trial (KEYNOTE-012), a tumor was considered PD-L1 positive if the tumor or stroma contained >1% PD-L1–positive cells. PD-L1 positivity was associated with better overall response and progression-free survival (Seiwert et al. 2016). However, PD-L1 staining in cancer cells is patchy (Li et al. 2015), and its immunostaining is prone to sampling error. Notably, PD-L1 expression on tumor cells is dispensable for tumor response to the ICR blockade. In contrast, PD-L1 expression in host myeloid cells plays an essential role in modulating antitumor immunity (Tang et al. 2018). Thus, the use of IHC staining of PD-L1 on cancer cells alone as a biomarker has been challenging. Results from the CheckMate 141 trial suggest that patients with HNSCC can benefit from nivolumab regardless of PD-L1 status, using the same 1% cutoff for PD-L1 positivity (Harrington et al. 2017).
Evidence gleaned from the studies of tumor-infiltrating lymphocytes (TILs) suggests that TIL compositions hold enormous promise for complementing the current TNM staging and identifying cold cancers. The current immunoscore to characterize tumor immune infiltrate relies on immunohistochemistry (IHC)–based enumeration of effector populations such as CD8-positive cytotoxic T lymphocytes and CD45RO-positive memory T cells. Fully appreciating the significant prognostic values of these well-characterized markers, it is also important not to overlook other less predominant yet prognostically significant immune cell subsets. For example, in a recent pan-cancer type microarray gene expression study, plasma cells were unexpectedly found to be the strongest biomarker for a favorable outcome, while neutrophils were inversely correlated with patient survival (Gentles et al. 2015).
With the rapid evolution of next-generation sequencing methods, mapping the TIL landscape based on immune cell signature gene expression has emerged as a highly promising approach to assess the complete immune infiltration profile in tumors. A major challenge associated with gene expression deconvolution is the frequent data contamination by outliers, which are frequently observed in tumor sequencing data sets and inevitably skew the TIL scores. More robust and automated machine learning tools are essential to identify and remove these outliers to fully unleash the power of HNSCC immunogenomics in stratifying patients based on cancer immunogenicity.
Conclusion
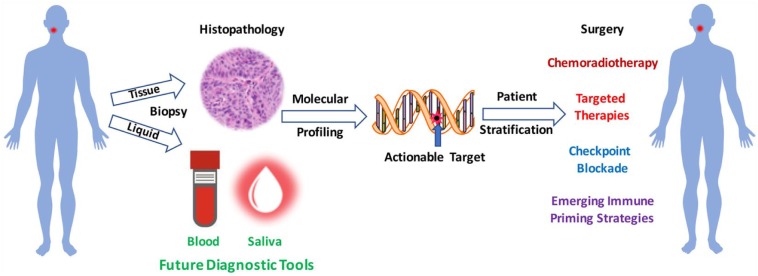
Targeted therapy and checkpoint blockade immunotherapy epitomize new precision treatments for HNSCC. Main challenges lie in better biomarker-assisted patient stratification and protocol selection (Fig.). Resistance to current targeted therapies and immunotherapy typically arises in signaling redundancy and compensatory mechanisms driving immune escape. Thus, efforts in dissecting the molecular circuitry that sustains cancer cell metabolic and proliferation needs, despite targeted blockade, and that induce peripheral immune tolerance hold promise for a more effective portfolio of combination approaches to reduce morbidity and improve outcome.
Figure.
Rational design of combinatorial treatments for head and neck cancer. The goal of precision therapy is to provide therapy that is most suited to the individual’s cancer. In a patient suspected of having cancer, a tissue biopsy is taken to confirm the diagnosis and identify the appropriate treatment strategy. In the future, a sample of blood or saliva containing circulating tumor DNA or other tumor products will be employed in tumor diagnosis. Using molecular profiling, an actionable target is identified to enable the treating team of health care professionals to select a therapeutic approach that is specific for an individual patient’s tumor, eventually leading to reduced morbidities and improved outcomes.
Author Contributions
P.J. Polverini, contributed to conception and design, drafted and critically revised the manuscript; N.J. D’Silva, Y.L. Lei, contributed to conception, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work is supported in part by the Sharon and Larry Daniels Cancer Research Fund (P.J.P.) and National Institutes of Health grants R35 DE027551 (N.J.D.), R01 DE022567 (N.J.D.), R01 DE026728 (Y.L.L.), and R00 DE024173 (Y.L.L.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, et al. 2011. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in notch1. Science. 333(6046):1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, Molinolo AA, Gutkind JS. 2005. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 65(21):9953–9961. [DOI] [PubMed] [Google Scholar]
- Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. 2002. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 62(24):7350–7356. [PubMed] [Google Scholar]
- Ballman KV. 2015. Biomarker: predictive or prognostic? J Clin Oncol. 33(33):3968–3971. [DOI] [PubMed] [Google Scholar]
- Bauman JE, Cohen E, Ferris RL, Adelstein DJ, Brizel DM, Ridge JA, O’Sullivan B, Burtness BA, Butterfield LH, Carson WE, et al. 2017. Immunotherapy of head and neck cancer: emerging clinical trials from a National Cancer Institute head and neck cancer steering committee planning meeting. Cancer. 123(7):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek K, Kiwerska K, Szaumkessel M, Bodnar M, Kostrzewska-Poczekaj M, Marszalek A, Janiszewska J, Bartochowska A, Jackowska J, Wierzbicka M, et al. 2016. Recurrent CDK1 overexpression in laryngeal squamous cell carcinoma. Tumour Biol. 37(8):11115–11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. 2006. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 354(6):567–578. [DOI] [PubMed] [Google Scholar]
- Brower V. 2011. Epigenetics: unravelling the cancer code. Nature. 471(7339):S12–S13. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, D’Souza G, Gillison ML, Katki HA. 2016. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. population. Oral Oncol. 60:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CH, Gillison ML. 2009. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 15(22):6758–6762. [DOI] [PubMed] [Google Scholar]
- Collins FS, Varmus H. 2015. A new initiative on precision medicine. N Engl J Med. 372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. 2015. Identification of the cell-intrinsic and extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res. 76(5):1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, McWhirter SM, Dubensky TW, Jr, Gajewski TF. 2016. The host sting pathway at the interface of cancer and immunity. J Clin Invest. 126(7):2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. 2014. Sting-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 41(5):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey K, Agulnik M. 2013. Promising new molecular targeted therapies in head and neck cancer. Drugs. 73(4):315–325. [DOI] [PubMed] [Google Scholar]
- Eccles DM, Balmana J, Clune J, Ehlken B, Gohlke A, Hirst C, Potter D, Schroeder C, Tyczynski JE, Gomez Garcia EB. 2016. Selecting patients with ovarian cancer for germline BRCA mutation testing: findings from guidelines and a systematic literature review. Adv Ther. 33(2):129–150. [DOI] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. 2016. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. 1989. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 81(24):1879–1886. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Verweij J, Ballman KV. 2013. Precision oncology: an overview. J Clin Oncol. 31(15):1803–1805. [DOI] [PubMed] [Google Scholar]
- Geiger JL, Bauman JE, Gibson MK, Gooding WE, Varadarajan P, Kotsakis A, Martin D, Gutkind JS, Hedberg ML, Grandis JR, et al. 2016. Phase II trial of everolimus in patients with previously treated recurrent or metastatic head and neck squamous cell carcinoma. Head Neck. 38(12):1759–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. 2015. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 21(8):938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giefing M, Wierzbicka M, Szyfter K, Brenner JC, Braakhuis BJ, Brakenhoff RH, Bradford CR, Sorensen JA, Rinaldo A, Rodrigo JP, et al. 2016. Moving towards personalised therapy in head and neck squamous cell carcinoma through analysis of next generation sequencing data. Eur J Cancer. 55:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY. 2003. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 162(4):1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington KJ, Ferris RL, Blumenschein G, Jr, Colevas AD, Fayette J, Licitra L, Kasper S, Even C, Vokes EE, Worden F, et al. 2017. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (Checkmate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 18(8):1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, Mehnert JM, Algazi A, van Brummelen EMJ, Saraf S, et al. 2017. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 35(36):4050–4056. [DOI] [PubMed] [Google Scholar]
- Hu Y, Lu L, Xia Y, Chen X, Chang AE, Hollingsworth RE, Hurt E, Owen J, Moyer JS, Prince ME, et al. 2016. Therapeutic efficacy of cancer stem cell vaccines in the adjuvant setting. Cancer Res. 76(16):4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. 2001. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 7(9):987–989. [DOI] [PubMed] [Google Scholar]
- Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, Jiang B, Wakely P, Xiao W, Gillison ML. 2012. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 36(7):945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansy BA, Concha-Benavente F, Srivastava RM, Jie HB, Shayan G, Lei Y, Moskovitz J, Moy J, Li J, Brandau S, et al. 2017. PD-1 status in CD8(+) T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 77(22):6353–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JD, Grandis JR. 2010. The molecular pathogenesis of head and neck cancer. Cancer Biol Ther. 9(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. 2005. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 14(2):467–475. [DOI] [PubMed] [Google Scholar]
- Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY, Sunwoo JB. 2016. CD44+ cells in head and neck squamous cell carcinoma suppress T-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin Cancer Res. 22(14):3571–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans CR, Braakhuis BJ, Brakenhoff RH. 2011. The molecular biology of head and neck cancer. Nat Rev Cancer. 11(1):9–22. [DOI] [PubMed] [Google Scholar]
- Lei Y, Kansy BA, Li J, Cong L, Liu Y, Trivedi S, Wen H, Ting JP, Ouyang H, Ferris RL. 2016. EGFR-targeted mAb therapy modulates autophagy in head and neck squamous cell carcinoma through NLRX1-TUFM protein complex. Oncogene. 35(36):4698–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery S, Keegan P, Pazdur R. 2017. First FDA approval agnostic of cancer site—when a biomarker defines the indication. N Engl J Med. 377(15):1409-1412. [DOI] [PubMed] [Google Scholar]
- Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, Kane LP, Ferris RL. 2015. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 75(3):508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, Perez-Ordonez B, Jordan RC, Gillison ML. 2013. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 49(1):1–8. [DOI] [PubMed] [Google Scholar]
- Loeffler-Ragg J, Witsch-Baumgartner M, Tzankov A, Hilbe W, Schwentner I, Sprinzl GM, Utermann G, Zwierzina H. 2006. Low incidence of mutations in EGFR kinase domain in Caucasian patients with head and neck squamous cell carcinoma. Eur J Cancer. 42(1):109–111. [DOI] [PubMed] [Google Scholar]
- Machiels JP, Henry S, Zanetta S, Kaminsky MC, Michoux N, Rommel D, Schmitz S, Bompas E, Dillies AF, Faivre S, et al. 2010. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 28(1):21–28. [DOI] [PubMed] [Google Scholar]
- Mankoff DA, Edmonds CE, Farwell MD, Pryma DA. 2016. Development of companion diagnostics. Semin Nucl Med. 46(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R, Zhu F, Yang DH, Cai KQ, Weaver J, Singh MK, Nikonova AS, Golemis EA, Flieder DB, Cooper HS, et al. 2013. Quantification of excision repair cross-complementing group 1 and survival in p16-negative squamous cell head and neck cancers. Clin Cancer Res. 19(23):6633–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena E, Thippsandra S, Yanamadala A, Redy S, Pattanayak P, Subramaniam RM. 2017. Molecular imaging and precision medicine in head and neck cancer. PET Clin. 12(1):7–25. [DOI] [PubMed] [Google Scholar]
- Mes SW, Leemans CR, Brakenhoff RH. 2016. Applications of molecular diagnostics for personalized treatment of head and neck cancer: state of the art. Expert Rev Mol Diagn. 16(2):205–221. [DOI] [PubMed] [Google Scholar]
- Mineta H, Miura K, Ogino T, Takebayashi S, Misawa K, Ueda Y, Suzuki I, Dictor M, Borg A, Wennerberg J. 2000. Prognostic value of vascular endothelial growth factor (VEGF) in head and neck squamous cell carcinomas. Br J Cancer. 83(6):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Vander Broek R, Shah S, Eytan DF, Pierce ML, Carlson SG, Coupar JF, Zhang J, Cheng H, Chen Z, et al. 2015. MEK inhibitor PD-0325901 overcomes resistance to PI3K/mTOR inhibitor PF-5212384 and potentiates antitumor effects in human head and neck squamous cell carcinoma. Clin Cancer Res. 21(17):3946–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira J, Tobias A, O’Brien MP, Agulnik M. 2017. Targeted therapy in head and neck cancer: an update on current clinical developments in epidermal growth factor receptor-targeted therapy and immunotherapies. Drugs. 77(8):843–857. [DOI] [PubMed] [Google Scholar]
- Morris LG, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, Wong RJ, Lee NY, Sherman EJ, Baxi SS, et al. 2017. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform. JAMA Oncol. 3(2):244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan CO, Amirghahari N, Rong X, Giordano T, Sibley D, Nordberg M, Glass J, Agarwal A, Caldito G. 2007. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 67(5):2160–2168. [DOI] [PubMed] [Google Scholar]
- Nix P, Cawkwell L, Patmore H, Greenman J, Stafford N. 2005. Bcl-2 expression predicts radiotherapy failure in laryngeal cancer. Br J Cancer. 92(12):2185–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ML, Garber JE, Tung N. 2017. Managing hereditary breast cancer risk in women with and without ovarian cancer. Gynecol Oncol. 146(1):205–214. [DOI] [PubMed] [Google Scholar]
- Pomerantz RG, Grandis JR. 2003. The role of epidermal growth factor receptor in head and neck squamous cell carcinoma. Curr Oncol Rep. 5(2):140–146. [DOI] [PubMed] [Google Scholar]
- Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, Sigismund S, Ravenda PS, Bonaldi T, Zampino MG, et al. 2016. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med. 22(6):624–631. [DOI] [PubMed] [Google Scholar]
- Prasad V, Kaestner V. 2017. Nivolumab and pembrolizumab: monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin Oncol. 44(2):132–135. [DOI] [PubMed] [Google Scholar]
- Prawira A, Pugh TJ, Stockley TL, Siu LL. 2017. Data resources for the identification and interpretation of actionable mutations by clinicians. Ann Oncol. 28(5):946–957. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. 2007. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 104(3):973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzouk S. 2014. Translational genomics and head and neck cancer: toward precision medicine. Clin Genet. 86(5):412–421. [DOI] [PubMed] [Google Scholar]
- Reeves TD, Hill EG, Armeson KE, Gillespie MB. 2011. Cetuximab therapy for head and neck squamous cell carcinoma: a systematic review of the data. Otolaryngol Head Neck Surg. 144(5):676–684. [DOI] [PubMed] [Google Scholar]
- Ritchie MD, Holzinger ER, Li R, Pendergrass SA, Kim D. 2015. Methods of integrating data to uncover genotype-phenotype interactions. Nat Rev Genet. 16(2):85–97. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. 2014. Decade in review-cancer immunotherapy: entering the mainstream of cancer treatment. Nat Rev Clin Oncol. 11(11):630–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba NF, Mody MD, Tan ES, Gill HS, Rinaldo A, Takes RP, Strojan P, Hartl DM, Vermorken JB, Haigentz M, Jr, et al. 2017. Toxicities of systemic agents in squamous cell carcinoma of the head and neck (SCCHN): a new perspective in the era of immunotherapy. Crit Rev Oncol Hematol. 115:50–58. [DOI] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, et al. 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 547(7662):222–226. [DOI] [PubMed] [Google Scholar]
- Saintigny P, Zhang L, Fan YH, El-Naggar AK, Papadimitrakopoulou VA, Feng L, Lee JJ, Kim ES, Ki Hong W, Mao L. 2011. Gene expression profiling predicts the development of oral cancer. Cancer Prev Res (Phila). 4(2):218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama JK, Haraf DJ, Stenson KM, Blair EA, Witt ME, Williams R, Kunnavakkam R, Cohen EE, Seiwert T, Vokes EE. 2011. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Ann Oncol. 22(10):2304–2309. [DOI] [PubMed] [Google Scholar]
- Scanlon CS, Van Tubergen EA, Inglehart RC, D’Silva NJ. 2013. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 92(2):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheckenbach K, Baldus SE, Balz V, Freund M, Pakropa P, Sproll C, Schafer KL, Wagenmann M, Schipper J, Hanenberg H. 2014. RAD51C—a new human cancer susceptibility gene for sporadic squamous cell carcinoma of the head and neck (HNSCC). Oral Oncol. 50(3):196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et al. 2016. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17(7):956–965. [DOI] [PubMed] [Google Scholar]
- Seiwert TY, Haraf DJ, Cohen EE, Stenson K, Witt ME, Dekker A, Kocherginsky M, Weichselbaum RR, Chen HX, Vokes EE. 2008. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol. 26(10):1732–1741. [DOI] [PubMed] [Google Scholar]
- Simpson DR, Mell LK, Cohen EE. 2015. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 51(4):291–298. [DOI] [PubMed] [Google Scholar]
- Slebos RJ, Yi Y, Ely K, Carter J, Evjen A, Zhang X, Shyr Y, Murphy BM, Cmelak AJ, Burkey BB, et al. 2006. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 12(3 Pt 1):701–709. [DOI] [PubMed] [Google Scholar]
- Snyderman R, Meade C, Drake C. 2016. Value of personalized medicine. JAMA. 315(6):613. [DOI] [PubMed] [Google Scholar]
- Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, Lopez-Albaitero A, Gibson SP, Gooding WE, Ferrone S, et al. 2013. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 19(7):1858–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. 2013. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, et al. 2011. The mutational landscape of head and neck squamous cell carcinoma. Science. 333(6046):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YS, Sansanaphongpricha K, Prince MEP, Sun D, Wolf GT, Lei YL. 2018. Engineering vaccines to reprogram immunity against head and neck cancer. J Dent Res. 97(6):627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Weinel S, Nagy N, O’Driscoll M, Lai-Cheong JE, Kulp-Shorten CL, Knable A, Carpenter G, Fisher SA, Hiragun M, et al. 2012. Germline mutation in ATR in autosomal-dominant oropharyngeal cancer syndrome. Am J Hum Genet. 90(3):511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, et al. 2018. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest. 128(2):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al. 2015. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 350(6257):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes C, Musbahi O. 2017. Genomics and advances towards precision medicine for head and neck squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2(5):310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Broek R, Mohan S, Eytan DF, Chen Z, Van Waes C. 2015. The PI3K/Akt/mTOR axis in head and neck cancer: functions, aberrations, cross-talk, and therapies. Oral Dis. 21(7):815–825. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulou M, Psyrri A, Argiris A. 2015. Targeting angiogenesis in head and neck cancer. Oral Oncol. 51(5):409–415. [DOI] [PubMed] [Google Scholar]
- Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J. 2007. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 25(16):2171–2177. [DOI] [PubMed] [Google Scholar]
- Vinarsky V, Fine RL, Assaad A, Qian Y, Chabot JA, Su GH, Frucht H. 2009. Head and neck squamous cell carcinoma in FAMMM syndrome. Head Neck. 31(11):1524–1527. [DOI] [PubMed] [Google Scholar]
- Wacholder S, Hartge P, Prentice R, Garcia-Closas M, Feigelson HS, Diver WR, Thun MJ, Cox DG, Hankinson SE, Kraft P, et al. 2010. Performance of common genetic variants in breast-cancer risk models. N Engl J Med. 362(11):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, James N, Rettig EM, Guo T, Pickering CR, et al. 2015. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 7(293):293ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Hayes DN. 2014. Classifying squamous cell carcinoma of the head and neck: prognosis, prediction and implications for therapy. Expert Rev Anticancer Ther. 14(2):229–236. [DOI] [PubMed] [Google Scholar]
- Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. 2002. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 21(10):1510–1517. [DOI] [PubMed] [Google Scholar]
- Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, Urba SG. 2010. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol. 28(20):3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, et al. 2014. Sting-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 41(5):830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavras AI, Yoon AJ, Chen MK, Lin CW, Yang SF. 2012. Association between polymorphisms of DNA repair gene ERCC5 and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 114(5):624–629. [DOI] [PubMed] [Google Scholar]
- Zhang HS, Postigo AA, Dean DC. 1999. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFbeta, and contact inhibition. Cell. 97(1):53–61. [DOI] [PubMed] [Google Scholar]
- Zhang L, Poh CF, Williams M, Laronde DM, Berean K, Gardner PJ, Jiang H, Wu L, Lee JJ, Rosin MP. 2012. Loss of heterozygosity (LOH) profiles—validated risk predictors for progression to oral cancer. Cancer Prev Res (Phila). 5(9):1081-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Liu Z, Myers JN. 2016. Tp53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cell Biochem. 117(12):2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]