Abstract
Purpose of Review
Although a fine-grained understanding of the neurobiology of posttraumatic stress disorder (PTSD) is yet to be elucidated, the last two decades have seen a rapid growth in the study of PTSD using neuroimaging techniques. The current review summarizes important findings from functional and structural neuroimaging studies of PTSD, by primarily focusing on their relevance towards an emerging network-based neurobiological model of the disorder.
Recent Findings
PTSD may be characterized by a weakly connected and hypoactive default mode network (DMN) and central executive network (CEN), that are putatively destabilized by an overactive and hyperconnected salience network (SN) – which appears to have a low threshold for perceived saliency, and inefficient DMN-CEN modulation.
Summary
There is considerable evidence for large-scale functional and structural network dysfunction in PTSD. Nevertheless, several limitations and gaps in the literature need to be addressed in future research.
Keywords: posttraumatic stress disorder, default mode network, central executive network, salience network, functional magnetic resonance imaging, structural MRI
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric illness that is characterized by persistent symptoms of re-experiencing, avoidance of trauma-related stimuli, negative thoughts and feelings, and arousal and reactivity, that develops in certain individuals in the aftermath of a traumatic event [1]. Prevalence rates of PTSD are estimated at 6.8% in the general population in the United States [2] and at 23% in US Veterans [3]. A fine-grained understanding of the neurobiology of PTSD is yet to be elucidated. However, the last two decades have seen a rapid growth in the study of PTSD using neuroimaging techniques, that were made possible by considerable advancements in the technology and methods.
Advances in functional neuroimaging methods, such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT), have enabled the exploration of dynamic neural changes involved in the pathophysiology of PTSD. In PET and SPECT, a radioactive tracer is administered to quantify regional brain metabolism or regional cerebral blood flow, providing a measure of neuronal activity. In contrast, fMRI is based on the intrinsic blood-oxygen-level dependent (BOLD) signal and does not require the administration of a tracer. By measuring regional metabolic or blood flow changes, PET and SPECT inherently provide a more direct estimates of neuronal activation; while in fMRI, several techniques have been developed to indirectly quantify this activity. For example, the amplitudes of low-frequency fluctuations (ALFFs) of the BOLD signal can be quantified as a measure of intrinsic regional activity. Several meta-analyses have pooled multi-modal brain activation studies (PET, SPECT, and fMRI) using an activation likelihood estimation (ALE) approach, which may provide a more confirmatory evidence to complement findings from individual studies [4–7]. In addition to activation studies, functional connectivity has been also employed in the study of psychopathology. Functional connectivity usually refers to a functional coupling or association (e.g. Pearson correlation, granger causality) between time series extracted from different voxels or regions-of-interest (ROIs). The structural characteristics of the brain can be assessed using structural MRI (sMRI). To date, the majority of sMRI studies in PTSD have focused on volumetric gray matter (GM) alterations, but a growing body of research includes measures such as cortical thickness, and morphometry. Finally, white matter tract connectivity and integrity can be studied using diffusion MRI (dMRI), which capitalizes on the diffusion of water molecules to create a contrast in the images, therefore identifying membrane-bound fibers (i.e., axons). The constraint of the movement of water molecules along a longitudinal tract can be quantified as fractional anisotropy (FA), where lower values may reflect impaired overall integrity of white matter tracts.
As part of a Special Issue on PTSD, this selective review will summarize important findings from functional and structural neuroimaging studies of PTSD, by primarily focusing on their relevance towards an emerging network-based neurobiological model of the disorder [8]. The readers are referred to [9–13] for complementary and alternative neurobiological models of PTSD, and to [14, 7, 4–6] for more comprehensive systematic reviews and meta-analyses.
Network-Based Neurobiological Model of PTSD
Traditionally, evidence of brain alterations in PTSD have been presented under a fronto-limbic model that includes the amygdala, medial prefrontal cortex (mPFC), and hippocampus as core implicated structures. This model stipulates that an overactive amygdala is responsible for heightened arousal and exaggerated fear, aggravated by loss of top-down inhibition due to a dysfunctional mPFC; the hippocampus integrates into this picture by failing to identify safe or otherwise non-threatening situations thereby contributing to avoidance and re-experiencing (referred to in the hippocampal literature as pattern separation) [15]. Indeed, several lines of evidence from different imaging techniques have corroborated a disruption within these structures [9].
However, it is becoming increasingly clear that the scope of the functional abnormalities requires a broader approach to better capture the complexity of the disorder. Compelling evidence suggests that the brain is organized into functionally distinct brain networks with high intrinsic connectivity—dubbed intrinsic connectivity networks (ICNs) [16]. ICNs are identified using data-driven decomposition methods, and therefore their identification does not require a priori hypotheses [16]. Rather than constraining this review to alterations within discrete structures under the traditional fronto-limbic neurocircuitry model of PTSD, we attempt to present the evidence of functional alterations in the broader framework of large-scale network dysfunction (see Figure 1 & Table 1). Generally, in PTSD, the vast majority of the evidence has fallen under one of three of such networks. They are known as the default mode, central executive (also referred to as frontoparietal control), and the salience (also referred to as ventral attention) networks. Collectively, disruption in these three networks has been dubbed the triple network model by Menon [8].
Figure 1. Network-based neurobiological model of PTSD.
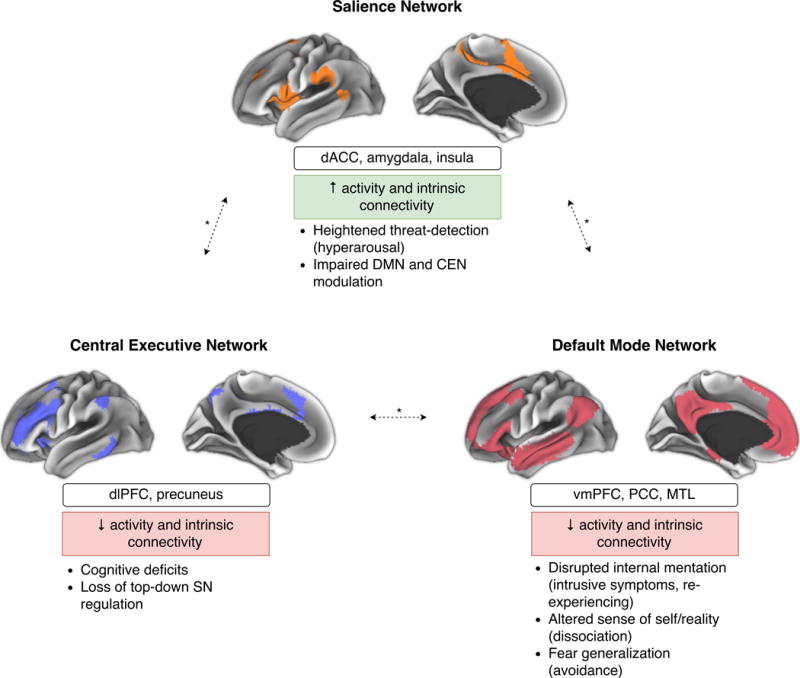
The figure shows the cortical representations of the salience network (SN; orange), default mode network (DMN; red), and central executive network (CEN; blue) in healthy individuals [16]; notable region-of-interests (ROIs) within these networks; the changes in PTSD predicted by the model; and the putative resulting phenotypic abnormalities. Alterations within and between the SN, CEN, and DMN may underlie the psychopathology of PTSD. According to this model, the SN is hyperconnected and hyperactive and has a low threshold for perceived saliency (underlying symptoms of hyperarousal), and is incapable of efficient DMN-CEN modulation (i.e. switching between task-relevant and task-irrelevant behavior); the CEN is a weakly interconnected and hypoactive (paralleling impaired cognition), and is incapable of top-down SN regulation; finally, the DMN is a weakly interconnected and hypoactive resulting in disrupted ability to maintain a calm inner state (intrusive symptoms), altered sense of self/world (dissociation), and fear generalization (avoidance; mediated by the hippocampus). * Denotes altered between-network connectivity.
Table 1.
Summary of neuroimaging findings in PTSD
| Intrinsic Fc | Functional Activity | Between-network Fc | WM | GM | |
|---|---|---|---|---|---|
| DMN | ↓ vmPFC-hippocampus [20], vmPFC-posterior hippocampus [43], PCC-posterior hippocampus [43], PCC-hippocampus [21], PCC-vmPFC [21], overall [23], PCC-vmPFC-MTL [24] | ↓ vmPFC, PCC [5–7, 4] ↓↑ hippocampus [5, 6] ↑ anterior hippocampus [6] |
↑ Increased insula-hippocampus [20], amygdala-vmPFC [61] | ↓ FA in cingulum (vmPFC-PCC) [26–28] ↓ FA in vmPFC-hippocampus [29, 30] ↓ vmPFC [25] |
↓ vmPFC [31], PCC [32], MTL [32–34], hippocampus [35] ↓ anterior hippocampus [38, 39] |
| CEN | ↓ premotor cortex-dlPFC, premotor cortex- parietal cortex/middle frontal gyrus [52], precuneus-CEN [24] | ↓ dlPFC [4, 6] ↑ precuneus [6] |
↑ SN-CEN coupling [62] | ↑ FA in dlPFC [59] | ↑↓ dlPFC [54–57] |
| SN | ↑ amygdala-insula [60, 20], amygdala-dACC [61] ↓ dACC-SN [24] |
↑ amygdala [4–6], dACC [6], anterior insula [4, 6, 7] | – | ↓ FA in insula, cingulum [25–27] | ↓↑ amygdala [65–67] ↓ dACC [68, 14, 31], insula [31, 57, 69] |
Abbreviations — Fc: Functional Connectivity; WM: White Matter; GM: Gray Matter; DMN: Default Mode Network; CEN: Central Executive Network; SN: Salience Network; vmPFC: Ventral Prefrontal Cortex; PCC: Posterior Cingulate Cortex; MTL: Medial Temporal Lobe; FA: Fractional Anisotropy; dlPFC: Dorsolateral Prefrontal Cortex; dACC: Dorsal Anterior Cingulate Cortex.
One of the most robustly identifiable networks is known as the default mode network (DMN). In contrast to most ICNs, the DMN exhibits relative hypoactivity during cognitive and stimulus-driven tasks, and is most active at rest. Indeed, some of the main functions of the DMN are thought to involve self-referential thought and other introspective processes—activities that predominantly occur at rest. Structurally, this network consists of core regions in the posterior cingulate cortex (PCC), ventromedial prefrontal cortex (vmPFC), and medial temporal lobe (MTL; including the hippocampus) [17]. Although ICNs constitute functionally-coupled entities by definition, structures of the DMN seem to additionally exhibit strong structural interconnections, highlighting the evolutionary importance of this network [18]. Given the known manifestations of intrusive symptoms, dissociation, and avoidance in PTSD [1], it is possible that a dysfunction in core structures of the DMN, or functional or structural dysconnectivity between these structures, may mirror these behavioral changes [8].
The central executive network (CEN), is active during cognitively demanding tasks, goal-directed behavior, and cognitive control of emotions, and is centered around the dorsolateral prefrontal cortex (dlPFC), encompassing the middle frontal gyrus, precuneus, and parts of the premotor cortex [8, 16]. A disrupted CEN may contribute to the cognitive and memory deficits, and the loss of top-down emotional control observed in PTSD [8].
The salience network (SN) is involved in the detection of salient internal and external stimuli. Core structures that are part of the SN are the amygdala, insula, and dorsal anterior cingulate cortex (dACC) [8]. Within the SN, based on the perceived threat level, the anterior insula is thought to arbitrate/modulate the dynamics between CEN and DMN (switching between task-relevant and task-irrelevant behavior), and effective connectivity analyses have inferred its causal control over these two networks in the normal brain [8, 19]. Consequently, aberrancy in the SN may alter these threat-detection functions, and could underlie behaviors such as hyperarousal [8].
Evidence from Structural and Functional Neuroimaging Studies
Default Mode Network
Resting-state ROI-to-ROI fMRI connectivity studies have demonstrated a decreased coupling between known structures of the DMN in PTSD, such as the vmPFC-hippocampus [20], PCC-hippocampus [21, 22], and PCC-vmPFC [21], often correlating with symptom severity. DMN functional dysconnectivity has also been replicated using approaches other than ROI-to-ROI connectivity. Using a more comprehensive representation of the DMN and graph theoretical analysis, one study showed a decrease in overall connectivity strength in the PTSD group [23]. Another study made use of signal decomposition methods and found evidence of decreased affinity of the PCC to the rest of the DMN during an autobiographical memory task [24]. With regard to structural connectivity in PTSD, reduced FA has been described within the white matter of the vmPFC region [25], in the white matter linking vmPFC to PCC (i.e., cingulum bundle) [26–28], and in the white matter linking the vmPFC to the hippocampus [29, 30]. These findings suggest that the structural changes may at least partly contribute to the alterations observed at the functional level. Structurally, localized GM reductions (i.e., volume or thickness) have been described in the vmPFC [31], PCC [32], MTL [32–34], and hippocampus [35]. Functional imaging data here shows vmPFC and PCC hypoactivity across a range of symptom provocation and cognitive-emotional studies [5, 6], and during resting-state (although less consistently) [7, 4].
With regard to functional activity in the hippocampus, the bulk of the evidence has shown increased activation in PTSD, though a number of studies have reported hypoactivation as well [5, 6]. The hippocampus is a functionally and structurally heterogeneous brain region and performs several functions. A particularly relevant division is along its long axis. The anterior part of the hippocampus is implicated in stress response, emotion-related memory, and pattern completion, mediated via strong connections to the amygdala [36, 37]; while the posterior portion is thought to be more involved in spatial functions, and pattern separation, and is anchored in the DMN-proper [37, 17]. Focused structural (shape and volumetric) alterations in the anterior hippocampus have been described in the literature [38, 39], and in specific hippocampal subfields (e.g., cornu amonis 3, and dentate gyrus) [9, 40]. A pattern of functional dysconnectivity between anterior hippocampus and the rest of the brain is also emerging [41]. Moreover, while the majority of ROI activation studies did not make the distinction between anterior vs. posterior hippocampus, the hippocampal activation cluster that was identified in a whole-brain meta-analysis in PTSD was located anteriorly [6]. Here, hyperactivity could be a reflection of intrusive recollections and a disruption in the extinction process [42].
Fewer studies have reported on the posterior hippocampus. In a resting-state connectivity study, the posterior (but not anterior) hippocampus was found to have decreased connectivity to other regions primarily in the DMN (PCC, vmPFC, precuneus) in the PTSD group [43]. Another study found a loss of differentiation in anterior vs. posterior functional connectivity profile in the PTSD group compared to controls [44]. Here, future ROI activation studies that make the distinction between the different parts of the hippocampus will be helpful in informing whether there is a differential activation pattern.
Response to therapy has been linked to normalization of DMN abnormalities in some studies. For example, a trial of paroxetine in patients with PTSD showed an association between increased hippocampal volume post-treatment and overall symptom improvement [45]. Similarly, the response to psychotherapy has been linked to normalization in structural changes in the rostral ACC [46–48], hippocampus [49, 50], and PCC [32]. However, it remains unclear whether different types of therapy are associated with specific changes. To date, response to both cognitive behavioral therapy and prolonged exposure therapy has been linked to rostral ACC [51, 46] and hippocampal [49, 51] GM increases. Conversely, an increase in the GM of the PCC may be more specific to response to eye movement desensitization and reprocessing therapy [32].
Central Executive Network
Although the CEN remains generally under-investigated in PTSD, there is evidence of weaker functional connectivity within the CEN. A decreased connectivity between the premotor cortex and dlPFC (middle frontal gyrus) was associated with increasing trauma exposure, while the reduced connectivity between premotor cortex and parietal cortex/middle frontal gyrus was associated with increased PTSD symptom severity [52]. In another fMRI connectivity study involving an autobiographical memory task, the CEN in PTSD patients showed decreased ability to recruit the precuneus [24]—another structure of the CEN. Connectivity between the CEN and the DMN also appears to be crucial, as increased dlPFC to PCC resting-state connectivity has been linked to response to mindfulness-based exposure therapy [53].
In PTSD, the dlPFC shows hypoactivity at rest [4], and across several cognitive-emotional tasks [6]. In contrast, other regions such as the precuneus may be hyperactive under emotional tasks [6]. These findings have been paralleled in the sMRI literature with evidence showing reduced dlPFC thickness in PTSD in cross-sectional studies [54–57]. In a longitudinal study of trauma victims, increased dlPFC thickness was found approximately one year after trauma [58]. Moreover, greater dlPFC thickness initially was linked to later symptom reduction, earlier improvement, and gradual cortical thickness normalization later in the course (the cohort remained largely treatment-naïve throughout the study) [58]. The discrepancy between the cross-sectional and longitudinal studies could be due to assessment at different time points, and highlights the relevance of studying PTSD chronicity. Congruent with the findings from the longitudinal study, white matter FA in the dlPFC appears to be increased in PTSD [59].
Taken together, these findings suggest that an increased emotional-modulation burden on the CEN immediately after trauma could lead to compensatory increase in dlPFC cortical thickness and white matter FA. Functionally, the CEN may be invested in modulating outside ICNs (e.g., SN; see Salience Network) in order to dampen/compensate for trauma-induced changes in these networks (e.g., SN/amygdala hyperactivity). The outwards investment of the CEN comes at the expense of decreased within-CEN functional connectivity and hypoactivity, which could manifest as cognitive and attentional symptoms. With recovery from PTSD, less demand on the CEN induces normalization (i.e., reduction) in dlPFC thickness. This is consistent with the known role of the CEN plays in top-down control [42].
Salience Network
Increased functional coupling, or ROI-to-ROI connectivity between paired regions of the SN, such as amygdala-insula [60, 20], and amygdala-dACC [61] has been generally described in PTSD at rest. In contrast, decreased connectivity between the dACC and the rest of the SN was reported during an autobiographical memory task [24]. Increased insula-hippocampus [20] and amygdala-vmPFC [61] connectivity at rest have been described and represent increased SN-DMN connectivity. Furthermore, during an autobiographical memory task, PTSD was associated with an aberrant recruitment of the amygdala into the DMN (rather than the SN) [24]. Finally, increased SN to CEN coupling was also evident in PTSD under a threat processing paradigm [62]. Within PTSD, individuals with the dissociative-subtype (PTSD+DS) appear to exhibit increased amygdala-dlPFC and amygdala-PCC connectivity compared to individuals with non-dissociative PTSD (PTSD-DS) [63].
One of the most replicable findings in functional studies of PTSD has been increased amygdala activity. This finding has been captured both at rest, and across different task paradigms [4–6]. The dACC, unlike the ventral or dorsolateral regions of the PFC, is also hyperactive in PTSD [6]. This is not surprising given the affiliation of the dACC to the SN (in contrast the vmPFC is part of the DMN, and the dlPFC is part of the CEN) [8]. In the insula, there have been some mixed results with regard to its activity in PTSD [5]. Yet, a pattern is emerging where the anterior part show increased activity at rest and under various emotional and cognitive paradigms, while the posterior insula appears to be hypoactive [4, 6, 7]. This is better understood in the context of evidence that implicate the anterior insula in functions such as the arbitration between the CEN and DMN, and the regulation of physiological changes during social-emotional tasks (anteroventrally; via connections to the amygdala) and cognitive-executive tasks (anterodorsally) [64, 19]. In contrast, posterior portions of the insula predominantly involve somatomotor functions [64]. Structurally, the integrity of core elements of the SN has been shown to be altered in PTSD. This is characterized by GM alterations in the amygdala [65–67], GM reductions in the dACC [68, 14, 31]; and abnormalities in the insula. The latter is evident by decreased GM [31], cortical thickness [57, 69], and FA in the white matter [25], as well as by increased betweenness centrality [69]—a measure of the importance of a particular node in the network.
Increased functional coupling and hyperactivity within the SN at rest may indicate a state of “primed” saliency. Weakened connectivity between the dACC and the rest of the SN becomes evident during tasks (e.g., autobiographical recall), potentially indicating a disrupted top-down inhibition under challenge. Exaggerated SN function could disrupt its ICN arbitration role, which is evident by increased SN-DMN and SN-CEN connectivity. Increased SN-CEN connectivity could alternatively be reflecting an augmented compensatory CEN top-down function aimed at dampening SN hyperactivity (though the two possibilities are not mutually exclusive). Furthermore, increased SN-CEN functional dysconnectivity in PTSD+DS vs. PTSD-DS may potentially represent emotional overmodulation in dissociative PTSD [70].
A summary of the evidence for the network-based model has been provided in Table 1.
Conclusions and Limitations
Overall, there is considerable evidence for large-scale network dysfunction in PTSD. Altered dynamics within, and between the DMN, CEN, and SN are potentially capable of accounting for the varying and heterogeneous endophenotypes of the disorder. Generally, PTSD may be characterized by a weakly interconnected and hypoactive DMN, putatively destabilized by an overactive and hyperconnected SN. The latter appears to have a low threshold for saliency, and to be incapable of efficient DMN-CEN modulation. Abnormalities within the CEN may underlie some of the cognitive, executive, and emotional regulatory dysfunctions in PTSD. An enhanced CEN to DMN connectivity, which has been linked to treatment response, may be an acquired resilience or bypass mechanism by which trauma-exposed individuals adapt to/overcome a specific circuit or nodal aberrancy.
The chain of events and the causal interactions between these networks has not been studied in PTSD, and may be crucial in forming a more complete understanding of the mechanism behind the disorder. Furthermore, it is unclear if structural changes underlie the functional abnormalities, or whether the chronicity of the functional abnormalities induces long-term structural changes [71]. Longitudinal multi-modal studies will be crucial in informing such questions. Several limitations in neuroimaging studies limit the interpretability and generalizability of the findings. Within the same imaging modality, different first-level processing pipelines result in considerable heterogeneity, and to date, no consensus has been reached for a “golden standard” that balances between sensitivity to detect subtle changes on one hand, and improved resistance to noise/artifact on the other (e.g., test-retest performance). Since the majority of the functional connectivity literature in PTSD consists of ROI-to-ROI studies, we had to approximate ICN-level changes from discrete ROI-to-ROI observations. It is important to note that single ROIs oftentimes do not represent the entirety of the ICNs in question (e.g., using dlPFC to PCC connectivity as a surrogate for CEN-DMN connectivity may be providing an incomplete picture). A more refined approach could entail using validated brain-parcellation atlases, combined with parcel-to-ICN affiliation information, and graph theory tools to study whole-ICN connectivity [23, 72]. Other approaches that are also devoid of this limitation include the use of decomposition methods (e.g., independent component analysis) to compare the spatial extent of different ICNs (in this context, the interpretability of “reduced spatial extent of the DMN” would be analogous to “decreased connectivity within the DMN”) [24]. Both of these approaches may provide essential information, complementing the findings from ROI-to-ROI connectivity studies. Task paradigms during imaging acquisition, which range from resting-state to symptom provocation, are a powerful way to probe various cognitive-emotional processes. Nonetheless, they may hinder generalizability of the findings and their replication across studies. This shortcoming could be addressed in the future by developing standardized “stress tests” of psychopathology, which preferably would consist of a battery of tasks aimed at highlighting changes within and across psychiatric disorders.
Although not specifically a shortcoming of imaging techniques, the choice of the comparator group (i.e., matched healthy vs. trauma-exposed control) has a number of implications that have not been addressed in this manuscript. Unifying the sample under a traumatic event may serve as a common ground to selectively probe dysfunctional trauma-induced changes. However, trauma-exposed individuals who do not develop PTSD may be a particularly resilient group with pre-exposure protective factors. They may also be characterized by post-trauma resilience-conferring behavioral and brain changes. This may affect the interpretability of results and may increase the prospect of confounding factors.
For example, one study found that increased ACC volume may be a trait-like marker for a person’s ability to recover from trauma because it predicts improvement of PTSD symptoms but does not change during or after treatment [47]. An investigation of trauma survivors who had not developed PTSD in the eight years following the event found significantly greater cortical thickness in several areas of the temporal lobe compared to controls, suggesting that hypertrophy of the cortex may be either a premorbid protective factor, or a resilience-related development following trauma [73]. As previously mentioned under Central Executive Network, a longitudinal study of victims of a subway arson attack showed that trauma-exposed victims with PTSD had significantly increased cortical thickness in the dlPFC compared to trauma-naïve controls approximately 1 year following the trauma. Further, the larger this cortical thickness increase, the greater the ensuing reduction of PTSD symptoms [58]. Collectively, the data suggests that the dorsolateral PFC may not only increase in response to trauma and/or PTSD, but that this may be an adaptive structural alteration which, could promote healing after a traumatic event. As the field continues to investigate the etiology and resultant pathophysiology of PTSD, additional possible protective factors may be identified.
Another clinically-relevant question is whether the brain abnormality findings represent a premorbid vulnerability to develop PTSD, or are instead a consequence of the disorder (covered in detail in the following reviews [74, 75]). This growing body of evidence suggests that some regions (such as the hippocampus) may at least partially predispose vulnerability for PTSD [76–78], while others (like portions of the ACC) may be a consequence of the neurotoxic effects of chronic stress in those who develop PTSD following a trauma [79]. In general, current imaging-derived neurobiological models of PTSD are largely based on cross-sectional studies that lack multiple assessments over time within a longitudinal experimental design. Furthermore, participants in imaging studies that focus on PTSD related to childhood trauma are usually adults whose brains have matured. Any comprehensive model of PTSD-related structural and functional brain abnormalities should also include developmental data obtained on traumatized children, since childhood trauma and PTSD appear to manifest differently in the pediatric brain [80] (See review by Herringa elsewhere in this Special Issue [81]).
While the triple network model provides a framework to understand psychopathology, it is unlikely to account for all PTSD abnormalities. Thus, it is important for the field to continue pursuing data-driven exploratory methods. For example, one structure that did not received much attention in PTSD but has been repeatedly implicated by model-free whole-brain meta-analyses, is the cerebellum [4, 7, 5]. Although there has been a recent increased interest in this structure [82, 83], the specific role of the cerebellum in stress-related psychopathology is yet to be fully elucidated.
Acknowledgments
The authors would like to thank the US Department of Veterans Affairs National Center for PTSD, the NIMH, and The Brain and Behavior Foundation for their support. We would also like to thank our colleagues for their thoughtful conversation while preparing this manuscript.
Funding:
This work was supported by the US Department of Veterans Affairs (DVA) National Center for PTSD, NIH [MH-101498]; Brain and Behavior Foundation Young Investigator Award [NARSAD]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. The sponsors had no role in the preparation, review, or approval of the manuscript.
Footnotes
Declaration of Conflicting Interests
Dr. Abdallah has served as a consultant or on advisory boards for Genentech and Janssen. He also serves as editor for the journal Chronic Stress published by SAGE Publications, Inc. All other authors report that there is no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Dsm-5. American Psychiatric Association. Amer Psychiatric Pub Incorporated; 2013. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, et al. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans: a meta-analysis. J Anxiety Disord. 2015;31:98–107. doi: 10.1016/j.janxdis.2015.02.003.. [DOI] [PubMed] [Google Scholar]
- 4•.Koch SBJ, Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. ABERRANT RESTING‐STATE BRAIN ACTIVITY IN POSTTRAUMATIC STRESS DISORDER: A META‐ANALYSIS AND SYSTEMATIC REVIEW. Depression and Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. This is a meta-analysis and systematic review of resting-state functional neuroimaging findings in PTSD, and includes connectivity and activation studies. [DOI] [PubMed] [Google Scholar]
- 5.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36(9):2130–42. doi: 10.1016/j.neubiorev.2012.06.003.. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Liu J, Zhang J, Zhan W, Li L, Wu M, et al. Altered resting-state functional activity in posttraumatic stress disorder: A quantitative meta-analysis. Sci Rep. 2016;6:27131. doi: 10.1038/srep27131.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. https://doi.org/10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–87. doi: 10.1038/nrn3339.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Sheynin J, Liberzon I. Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.11.014. This recent review describes PTSD abnormalities in terms of threat-detection, context-processing, and emotion-regulation circuit dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neuroscience Letters. 2017;649:147–55. doi: 10.1016/j.neulet.2016.11.064. https://doi.org/10.1016/j.neulet.2016.11.064. This review focuses on glutamate and GABA neurochemical and receptor imaging in PTSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matosin N, Cruceanu C, Binder EB. Preclinical and Clinical Evidence of DNA Methylation Changes in Response to Trauma and Chronic Stress. Chronic Stress. 2017;1:2470547017710764. doi: 10.1177/2470547017710764.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krystal JH, Abdallah CG, Averill LA, Kelmendi B, Harpaz-Rotem I, Sanacora G, et al. Synaptic Loss and the Pathophysiology of PTSD: Implications for Ketamine as a Prototype Novel Therapeutic. Curr Psychiatry Rep. 2017;19(10):74. doi: 10.1007/s11920-017-0829-z.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2015;232(1):1–33. doi: 10.1016/j.pscychresns.2015.01.002. https://doi.org/10.1016/j.pscychresns.2015.01.002. This is a meta-analysis and systematic review of volumetric structural MRI findings in PTSD. [DOI] [PubMed] [Google Scholar]
- 15.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60(4):376–82. doi: 10.1016/j.biopsych.2006.06.004.. [DOI] [PubMed] [Google Scholar]
- 16.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65. doi: 10.1152/jn.00338.2011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011.. [DOI] [PubMed] [Google Scholar]
- 18.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–62. doi: 10.1016/j.neuron.2010.02.005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chand GB, Dhamala M. Interactions Among the Brain Default-Mode, Salience, and Central-Executive Networks During Perceptual Decision-Making of Moving Dots. Brain Connect. 2016;6(3):249–54. doi: 10.1089/brain.2015.0379.. [DOI] [PubMed] [Google Scholar]
- 20.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74(9):904–11. doi: 10.1097/PSY.0b013e318273bf33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–94. [PMC free article] [PubMed] [Google Scholar]
- 22.Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M. Default Mode Network Subsystems Are Differentially Disrupted in Posttraumatic Stress Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2(4):363–71. doi: 10.1016/j.bpsc.2016.12.006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiki T, Averill C, Wrocklage K, Scott JC, Alexander-Bloch A, Southwick S, et al. 581. The Default Mode Network in Posttraumatic Stress Disorder (PTSD): A Data-Driven Multimodal Approach. Biological Psychiatry. 2017;81(10, Supplement):S235. https://doi.org/10.1016/j.biopsych.2017.02.451. [Google Scholar]
- 24.St Jacques PL, Kragel PA, Rubin DC. Neural networks supporting autobiographical memory retrieval in posttraumatic stress disorder. Cognitive, Affective, & Behavioral Neuroscience. 2013;13(3):554–66. doi: 10.3758/s13415-013-0157-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Wang Z, Ding W, Wan J, Zhuang Z, Zhang Y, et al. Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PLoS One. 2013;8(12):e83473. doi: 10.1371/journal.pone.0083473.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res. 2013;214(3):260–8. doi: 10.1016/j.pscychresns.2013.09.002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennis DM, van Rooij DS, Reijnen MA, Geuze DE. The predictive value of dorsal cingulate activity and fractional anisotropy on long-term PTSD symptom severity. Depress Anxiety. 2017 doi: 10.1002/da.22605. [DOI] [PubMed] [Google Scholar]
- 28.Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, et al. White Matter Integrity in Highly Traumatized Adults With and Without Post-Traumatic Stress Disorder. Neuropsychopharmacology. 2012;37(12):2740–6. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, et al. Structural and Functional Connectivity in Posttraumatic Stress Disorder: Associations with Fkbp5. Depress Anxiety. 2016;33(4):300–7. doi: 10.1002/da.22483.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, et al. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp. 2013;34(11):2808–16. doi: 10.1002/hbm.22100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng L, Jiang J, Jin C, Liu J, Zhao Y, Wang W, et al. Trauma-specific Grey Matter Alterations in PTSD. Sci Rep. 2016;6:33748. doi: 10.1038/srep33748.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nardo D, Hogberg G, Looi JC, Larsson S, Hallstrom T, Pagani M. Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. J Psychiatr Res. 2010;44(7):477–85. doi: 10.1016/j.jpsychires.2009.10.014.. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Li YJ, Luo EP, Lu HB, Yin H. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One. 2012;7(6):e39025. doi: 10.1371/journal.pone.0039025.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66(12):1373–82. doi: 10.1001/archgenpsychiatry.2009.160.. [DOI] [PubMed] [Google Scholar]
- 35.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15(6):798–807. doi: 10.1002/hipo.20102.. [DOI] [PubMed] [Google Scholar]
- 36.Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: A combined anterograde and retrograde tracing study in the rat. The Journal of Comparative Neurology. 2006;496(3):349–68. doi: 10.1002/cne.20919.. [DOI] [PubMed] [Google Scholar]
- 37.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience. 2014;15(10):655–69. doi: 10.1038/nrn3785.. [DOI] [PubMed] [Google Scholar]
- 38.Vythilingam M, Luckenbaugh DA, Lam T, Morgan CA, 3rd, Lipschitz D, Charney DS, et al. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res. 2005;139(2):89–99. doi: 10.1016/j.pscychresns.2005.04.003.. [DOI] [PubMed] [Google Scholar]
- 39.Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, et al. The Association of PTSD Symptom Severity With Localized Hippocampus and Amygdala Abnormalities. Chronic Stress. 2017;1:2470547017724069. doi: 10.1177/2470547017724069.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67(3):296–303. doi: 10.1001/archgenpsychiatry.2009.205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdallah CG, Wrocklage KM, Averill CL, Akiki T, Schweinsburg B, Roy A, et al. Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Transl Psychiatry. 2017;7(2):e1045. doi: 10.1038/tp.2017.12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):169–91. doi: 10.1038/npp.2009.83.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–98. doi: 10.1038/npp.2013.122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR, Neria Y. Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. Journal of Psychiatric Research. 2017;94:15–22. doi: 10.1016/j.jpsychires.2017.06.003. http://dx.doi.org/10.1016/j.jpsychires.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryant RA, Felmingham K, Whitford TJ, Kemp A, Hughes G, Peduto A, et al. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33(2):142–6. [PMC free article] [PubMed] [Google Scholar]
- 47.Dickie EW, Brunet A, Akerib V, Armony JL. Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychol Med. 2013;43(3):645–53. doi: 10.1017/S0033291712001328.. [DOI] [PubMed] [Google Scholar]
- 48.Helpman L, Papini S, Chhetry BT, Shvil E, Rubin M, Sullivan GM, et al. Ptsd Remission after Prolonged Exposure Treatment Is Associated with Anterior Cingulate Cortex Thinning and Volume Reduction. Depress Anxiety. 2016;33(5):384–91. doi: 10.1002/da.22471.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy-Gigi E, Szabo C, Kelemen O, Keri S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol Psychiatry. 2013;74(11):793–800. doi: 10.1016/j.biopsych.2013.05.017.. [DOI] [PubMed] [Google Scholar]
- 50.Rubin M, Shvil E, Papini S, Chhetry BT, Helpman L, Markowitz JC, et al. Greater hippocampal volume is associated with PTSD treatment response. Psychiatry Res. 2016;252:36–9. doi: 10.1016/j.pscychresns.2016.05.001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helpman L, Papini S, Chhetry BT, Shvil E, Rubin M, Sullivan GM, et al. PTSD REMISSION AFTER PROLONGED EXPOSURE TREATMENT IS ASSOCIATED WITH ANTERIOR CINGULATE CORTEX THINNING AND VOLUME REDUCTION. Depression and Anxiety. 2016;33(5):384–91. doi: 10.1002/da.22471.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cisler JM, Steele JS, Smitherman S, Lenow JK, Kilts CD. Neural Processing Correlates of Assaultive Violence Exposure and PTSD Symptoms during Implicit Threat Processing: A Network Level Analysis among Adolescent Girls. Psychiatry research. 2013;214(3) doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, et al. ALTERED DEFAULT MODE NETWORK (DMN) RESTING STATE FUNCTIONAL CONNECTIVITY FOLLOWING A MINDFULNESS-BASED EXPOSURE THERAPY FOR POSTTRAUMATIC STRESS DISORDER (PTSD) IN COMBAT VETERANS OF AFGHANISTAN AND IRAQ. Depression and Anxiety. 2016;33(4):289–99. doi: 10.1002/da.22481.. [DOI] [PubMed] [Google Scholar]
- 54.Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41(3):675–81. doi: 10.1016/j.neuroimage.2008.03.007.. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Wu M, Liao Y, Ouyang L, Du M, Lei D, et al. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neuroscience & Biobehavioral Reviews. 2014;43:163–72. doi: 10.1016/j.neubiorev.2014.04.003.. [DOI] [PubMed] [Google Scholar]
- 56.Mollica RF, Lyoo I, Chernoff MC, Bui HX, Lavelle J, Yoon SJ, et al. Brain Structural Abnormalities and Mental Health Sequelae in South Vietnamese Ex–Political Detainees Who Survived Traumatic Head Injury and Torture. Archives of General Psychiatry. 2009;66(11):1221–32. doi: 10.1001/archgenpsychiatry.2009.127.. [DOI] [PubMed] [Google Scholar]
- 57.Wrocklage KM, Averill LA, Cobb Scott J, Averill CL, Schweinsburg B, Trejo M, et al. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 2017;27(5):515–25. doi: 10.1016/j.euroneuro.2017.02.010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch Gen Psychiatry. 2011;68(7):701–13. doi: 10.1001/archgenpsychiatry.2011.70.. [DOI] [PubMed] [Google Scholar]
- 59.Li L, Lei D, Li L, Huang X, Suo X, Xiao F, et al. White Matter Abnormalities in Post-traumatic Stress Disorder Following a Specific Traumatic Event. EBioMedicine. 2016;4:176–83. doi: 10.1016/j.ebiom.2016.01.012. http://dx.doi.org/10.1016/j.ebiom.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, et al. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MW, McCarthy G, et al. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39(2):351–9. doi: 10.1038/npp.2013.197.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabellino D, Tursich M, Frewen PA, Daniels JK, Densmore M, Theberge J, et al. Intrinsic Connectivity Networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr Scand. 2015;132(5):365–78. doi: 10.1111/acps.12418.. [DOI] [PubMed] [Google Scholar]
- 63.Nicholson AA, Densmore M, Frewen PA, Theberge J, Neufeld RW, McKinnon MC, et al. The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology. 2015;40(10):2317–26. doi: 10.1038/npp.2015.79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, et al. Functional organization of the human anterior insular cortex. Neuroscience Letters. 2009;457(2):66–70. doi: 10.1016/j.neulet.2009.03.101. https://doi.org/10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 65.Pietrzak RH, Averill LA, Abdallah CG, Neumeister A, Krystal JH, Levy I, et al. Amygdala-hippocampal volume and the phenotypic heterogeneity of posttraumatic stress disorder: a cross-sectional study. JAMA Psychiatry. 2015;72(4):396–8. doi: 10.1001/jamapsychiatry.2014.2470.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuo JR, Kaloupek DG, Woodward SH. Amygdala Volume in Combat-Exposed Veterans With and Without Posttraumatic Stress Disorder: A Cross-sectional Study. Archives of General Psychiatry. 2012;69(10):1080–6. doi: 10.1001/archgenpsychiatry.2012.73.. [DOI] [PubMed] [Google Scholar]
- 67.Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of general psychiatry. 2012;69(11) doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, et al. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Research: Neuroimaging. 2009;174(3):210–6. doi: 10.1016/j.pscychresns.2009.06.001. https://doi.org/10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Mueller SG, Ng P, Neylan T, Mackin S, Wolkowitz O, Mellon S, et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res. 2015;234(2):194–201. doi: 10.1016/j.pscychresns.2015.09.006.. [DOI] [PubMed] [Google Scholar]
- 70.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167(6):640–7. doi: 10.1176/appi.ajp.2009.09081168.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdallah CG, Southwick SM, Krystal JH. Neurobiology of posttraumatic stress disorder (PTSD): A path from novel pathophysiology to innovative therapeutics. Neuroscience Letters. 2017;649:130–2. doi: 10.1016/j.neulet.2017.04.046. https://doi.org/10.1016/j.neulet.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 72.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–78. doi: 10.1016/j.neuron.2011.09.006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nilsen AS, Hilland E, Kogstad N, Heir T, Hauff E, Lien L, et al. Right temporal cortical hypertrophy in resilience to trauma: an MRI study. Eur J Psychotraumatol. 2016;7:31314. doi: 10.3402/ejpt.v7.31314.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17(7):337–47. doi: 10.1016/j.tics.2013.05.005.. [DOI] [PubMed] [Google Scholar]
- 75.Robinson BL, Shergill SS. Imaging in posttraumatic stress disorder. Curr Opin Psychiatry. 2011;24(1):29–33. doi: 10.1097/YCO.0b013e3283413519.. [DOI] [PubMed] [Google Scholar]
- 76.Bremner JD. Hypotheses and controversies related to effects of stress on the hippocampus: an argument for stress-induced damage to the hippocampus in patients with posttraumatic stress disorder. Hippocampus. 2001;11(2):75–81. doi: 10.1002/hipo.1023. discussion 2-4. [DOI] [PubMed] [Google Scholar]
- 77.Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am J Psychiatry. 2004;161(12):2194–200. doi: 10.1176/appi.ajp.161.12.2194.. [DOI] [PubMed] [Google Scholar]
- 78.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–7. doi: 10.1038/nn958.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63(6):550–6. doi: 10.1016/j.biopsych.2007.06.022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patriat R, Birn RM, Keding TJ, Herringa RJ. Default-Mode Network Abnormalities in Pediatric Posttraumatic Stress Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(4):319–27. doi: 10.1016/j.jaac.2016.01.010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herringa RJ. Trauma, PTSD, and the Developing Brain. Current Psychiatry Reports. 2017;19(10):69. doi: 10.1007/s11920-017-0825-3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apps R, Strata P. Neuronal circuits for fear and anxiety [mdash] the missing link. Nat Rev Neurosci. 2015;16(10):642. doi: 10.1038/nrn4028. [DOI] [PubMed] [Google Scholar]
- 83.Meabon JS, Huber BR, Cross DJ, Richards TL, Minoshima S, Pagulayan KF, et al. Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci Transl Med. 2016;8(321):321ra6. doi: 10.1126/scitranslmed.aaa9585. [DOI] [PubMed] [Google Scholar]


