Abstract
Intraflagellar transport (IFT) is a bidirectional transport process that occurs along primary cilia and specialized sensory cilia, such as photoreceptor outersegments. Genes coding for various IFT components are associated with ciliopathies. Mutations in IFT172 lead to diseases ranging from isolated retinal degeneration to severe syndromic ciliopathies. In this study, we created a mouse model of IFT172-associated retinal degeneration to investigate the ocular disease mechanism. We found that depletion of IFT172 in rod photoreceptors leads to a rapid degeneration of the retina, with severely reduced electroretinography (ERG) responses by 1 month and complete outer-nuclear layer (ONL) degeneration by 2 months. We investigated molecular mechanisms of degeneration and show that IFT172 protein reduction leads to mislocalization of specific photoreceptor outersegment (OS) proteins (RHO, RP1, IFT139), aberrant light-driven translocation of alpha transducin and altered localization of glioma-associated oncogene family member 1 (GLI1). This mouse model exhibits key features of the retinal phenotype observed in patients with IFT172-associated blindness and can be used for in vivo testing of ciliopathy therapies.
Introduction
Primary cilia are non-motile microtubule-based organelles that are present in the majority of vertebrate cell types. They are involved in cellular signaling that is crucial for the development and functioning of most organs (1). Mutations in genes coding for ciliary proteins lead to ciliopathies, rare genetic disorders that may affect the retina, brain, olfactory epithelium, heart, liver, kidney, bone, gonads and adipose tissues, in isolation or as part of specific syndromes (2–4). Vision loss is a common feature of ciliopathies because the photoreceptor OS is a highly specialized sensory cilium that is critical for phototransduction (5–7).
The assembly and maintenance of the cilium requires intraflagellar transport (IFT), the bidirectional protein trafficking that occurs along the axoneme that is necessary for the growth and maintenance of the cilium (8). IFT is dependent on two large protein complexes: IFT-B is responsible for anterograde transport to the ciliary tip and IFT-A is responsible for retrograde transport and return of materials to the basal region (9). Mutations in many of these IFT components, including IFT172, have been associated with disease (10–21).
IFT172 is a peripheral IFT-B complex protein that is thought to mediate the transition from anterograde to retrograde transport at the tip of the cilium (22–24). Therefore, even though it is associated with the anterograde machinery, its temperature-sensitive mutants in Chlamydomonas display phenotypes typical of retrograde proteins, including elongation of the cilium (24,25). Elongation of the cilium was also observed in cultured fibroblasts obtained from patients with IFT172-associated disease (10). In contrast, node cells of mouse embryos harboring a homozygous p.Leu1564Pro mutation in Ift172 (wim mutants) lacked all cilia (26). Mice homozygous for loss of function Ift172 alleles wim and slb, or the hypomorphic avc1 allele, die in utero or at birth, respectively (26–28), therefore photoreceptor degeneration in a murine Ift172 model has not been described to date. However, homozygous ift172 zebrafish mutants have abnormal photoreceptor development, where photoreceptor cilia fail to extend, in addition to severe multisystemic defects (29,30).
Given IFT172’s role in retinal disease, we created rod photoreceptor-specific Ift172 knock-out mice by crossing mice that have a conditional Ift172 allele (31) with mice that express Cre driven by the Rhodopsin promoter (32). We generated and validated an IFT172-specific antibody, which allowed us to monitor reduction of the IFT172 protein, and to demonstrate that depletion of IFT172 in the mouse retina causes a rapid retinal degeneration. We also showed that decreasing levels of IFT172 lead to aberrant localization of specific proteins.
Results
Targeted disruption of Ift172 in rod photoreceptors leads to IFT172 protein depletion
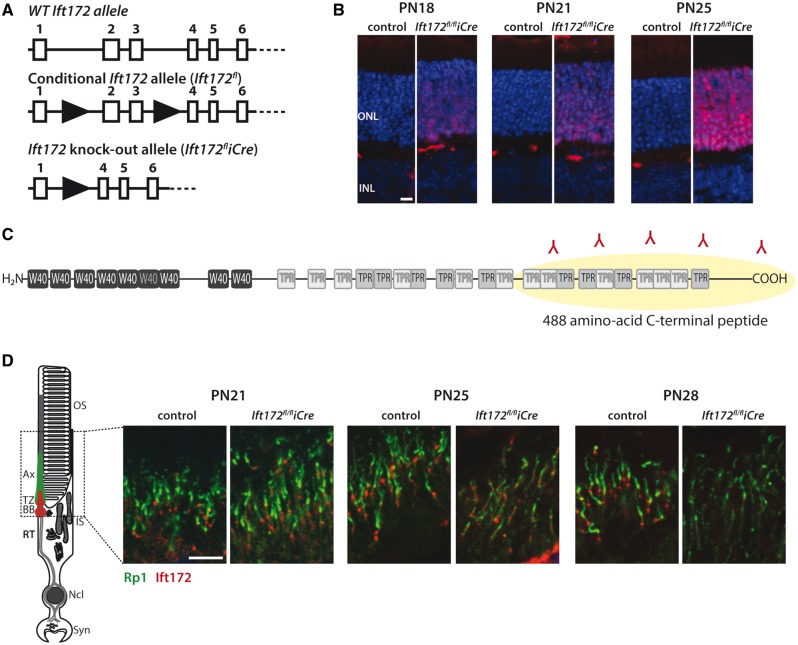
A rod photoreceptor-specific Ift172 knock-out mouse was generated by crossing mice carrying a conditional knock-out allele (Ift172fl) (31) with transgenic mice harboring Cre recombinase under a Rhodopsin promoter (iCre) (32) (Fig. 1A). Expression of iCre in the Ift172 knock-out mouse (Ift172fl/fliCre) was specific to the ONL and expressed in the majority of the rod photoreceptors at postnatal day 25 (PN25) (Fig. 1B). iCre is necessary for generating the Ift172 knock-out allele (Ift172fliCre) and as a consequence for depletion of the IFT172 protein. Therefore, the time course of IFT172 protein depletion was monitored by immunostaining of mouse retinas with a specific anti-IFT172 antibody, developed to the C-terminus of the protein (Fig. 1C). Complete depletion of the IFT172 protein in rod photoreceptors was observed at PN28 (Fig. 1D). Quantitative (q)PCR results on mouse retinas at PN10, PN21 and PN25 corroborate these findings, where changes in Ift172 transcript levels were not significant in Ift172fl/fliCre mice compared with the floxed (Ift172fl/fl) control mice, despite significant levels of iCre transcript present at PN10 (Supplementary Material, Fig. S1).
Figure 1.
Generation of the Ift172fl/fliCre mouse model and of the IFT172 antibody. (A) Schematic representation of the targeted Ift172 allele (Ift172fl/fl) and the knock-out allele (Ift172fl/fliCre). (B) Expression of Cre recombinase (red) in the ONL at postnatal days 18, 21 and 25. Nuclei are counterstained with Hoechst (blue). The scale bar represents 10 μm. (C) Schematic representation of human IFT172 protein, containing repetitive W40 and TPR motifs. A total of 488 carboxyl terminal amino acid residues were chosen for the rabbit polyclonal antibody generation. (D) Staining for IFT172 (red) and RP1 (green) at postnatal days 21, 25 and 28 showing depletion of IFT172 from rods by P28 in Ift172fl/fliCre mice. The scale bar represents 5 μm.
IFT172-deficient mouse retinas degenerate rapidly
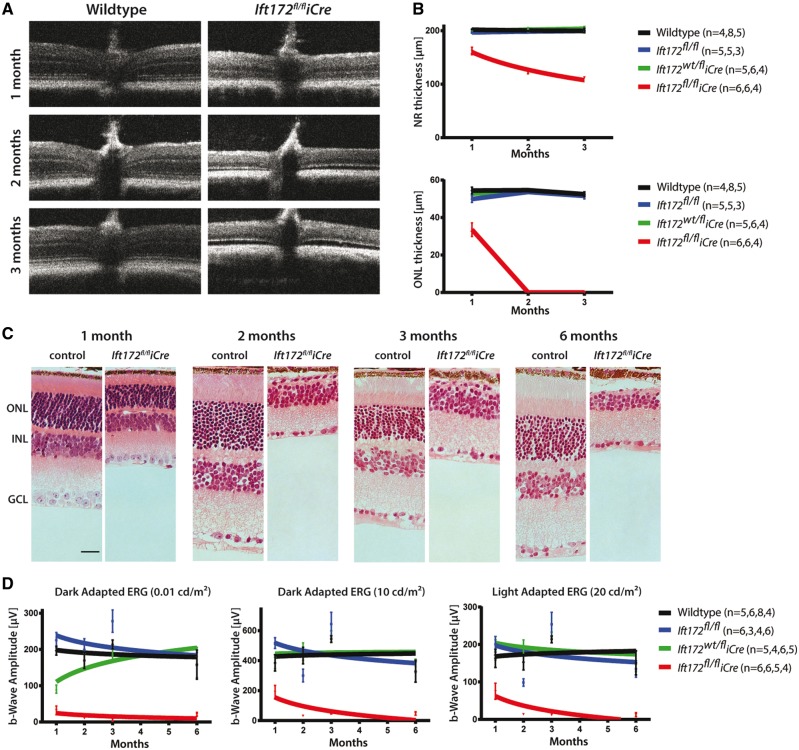
Retinas of the Ift172fl/fliCre mice showed thinning of the ONL at 1 month (38% reduction, adjusted P-value <0.01) compared with other littermate control genotypes (wild-type, Ift172fl/fl and Ift172wt/fliCre) and complete degeneration of the ONL by 2 months, as observed by optical coherence tomography (OCT) (Fig. 2A–C, Supplementary Material, Fig. S2). In addition, a reduction of the OS length was observed at 1 month (Fig. 2C). The heterozygous mice (Ift172wt/fliCre) and the control floxed mice (Ift172fl/fl) did not show significant differences in retinal architecture compared with the wild-type mice (Fig. 2A and B). In contrast to some patients with IFT172-associated disease, retinal cysts were not apparent in the Ift172fl/fliCre mice by OCT (11). ERG testing concordantly demonstrated that at 1 month Ift172fl/fliCre mice showed an 84% reduction of rod-driven b-wave amplitude at 0.01 cd/m2 light stimulus after dark adaptation compared with the wild-type controls (32.3 ±11 µV versus 196.9 ±12 µV, adjusted P-value <0.0001) (Fig. 2D). Mixed rod/cone (10 cd/m2 light stimulus) responses were reduced by half at the same age (191.2 ±44 µV versus 385.2 ±54 µV, adjusted P-value <0.05). The reduction in cone-isolated responses (20 cd/m2 light stimulus after light adaptation) was statistically significant from 2 months of age (13.8 ±2.5 µV versus 152.9 ±20 µV, adjusted P-value <0.001), indicating a secondary cone degeneration (Fig. 2D). By 2 months of age, the ERG was severely affected or undetectable in Ift172fl/fliCre mice across the stimulus conditions (Fig. 2D). Mice homozygous for the floxed allele (Ift172fl/fl) or heterozygous retina-specific knock-out mice (Ift172wt/fliCre) were followed up to 6 months and maintained normal retinal function and structure (Fig. 2D, Supplementary Material, Fig. S2).
Figure 2.
Ift172fl/fliCre mice display rapid retinal degeneration. (A) OCT data obtained at 1, 2 and 3 months of age in Ift172fl/fliCre and wild-type control mice shows diminishing thickness of ONL in the Ift172fl/fliCre mice. (B) Graphical representation of the neural retina and the ONL thickness measured from OCT images in Ift172fl/fliCre and control genotypes demonstrates decreased neural retinal thickness mainly due to thinning and eventual loss of the ONL by 2 months. (C) Representative histology sections from Ift172fl/fliCre and control mice. The scale bar represents 50 μm. (D) b-wave amplitudes from dark-adapted (0.01 and 10 cd/m2 light stimulus) and light-adapted (20 cd/m2 light stimulus) ERGs performed on Ift172fl/fliCre and control genotypes. The number of mice used in each ERG and OCT experimental condition is indicated in brackets in the order of time points taken (i.e. 1, 2, 3 and 6 months).
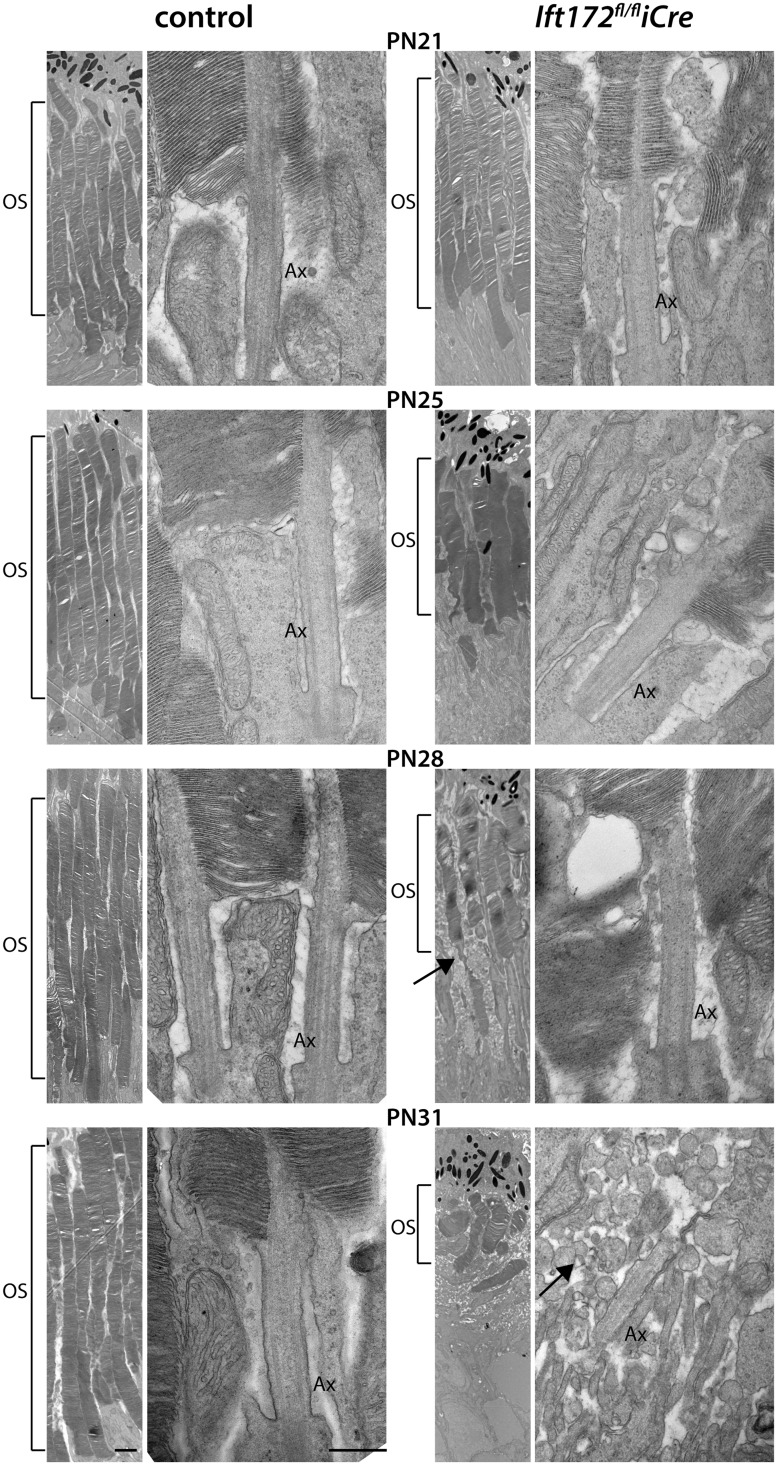
Ultrastructural analyses of the retina in the Ift172fl/fliCre mice showed no signs of photoreceptor degeneration at PN21 (Fig. 3). However, at PN25, shortening of the OS was observed, and at PN28, OS disc disorganization and accumulation of extracellular debris was seen (Fig. 3). At these stages, no changes in axoneme length were observed. At PN31 the OSs were disorganized and they appeared to have lost the connection with the axoneme and the innersegment (IS) of the photoreceptor cell (Fig. 3). Based on these results, further studies were performed on mice up to PN28.
Figure 3.
TEM data ultrastructure of Ift172fl/fliCre and control mice showing OS shortening starting at PN25, marked with square brackets. Membranous cellular debris at PN28 and PN31 is indicated (black arrows). Intact transition zone axonemes (Ax), connecting the ISs and the OS discs are visible up to PN28. At PN31 where severe photoreceptor degeneration with fragmented cell compartments (OS, Ax, membranous cell debris) is visible. Scale bar on the lower magnification picture represents 2μm and on the higher magnification picture at 500 nm.
Depletion of IFT172 leads to mislocalization of RP1 and IFT139
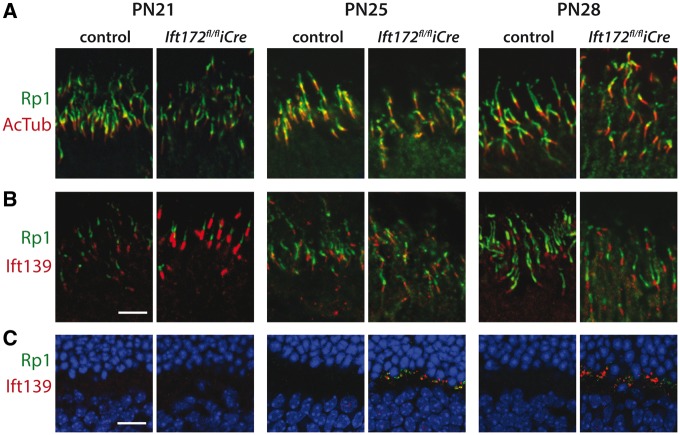
Disruption of the OS structure led us to investigate the localization of the axoneme-associated proteins RP1 and acetylated tubulin (AcTub) (33). AcTub showed no differences in localization between the mutant and the control retinas up to PN28. RP1 staining, however, showed shortening of the signal relative to acTub at PN28 in mutant mice compared with the controls (1.85 ± 0.08 versus 1.09 ± 0.04, P-value <0.0001) (Fig. 4A, Supplementary Material, Fig. S3), and it was observed to mislocalize to the synaptic terminals (Fig. 4B).
Figure 4.
Cilia-associated protein localization. (A) Ciliary axoneme staining with RP1 (green) and AcTub (red) showing shortening of the Rp1 signal relative to AcTub in Ift172fl/fliCre mice compared with controls at PN28 (see also Supplementary Material, Fig. S3). RP1 mislocalization to the IS is visible in Ift172fl/fliCre mice starting at PN25. (B) Staining of IFT139 (red) at the base of the ciliary axoneme, marked with RP1 staining (green). (C) IFT139 (red) and RP1 (green) both mislocalize to the synaptic region in Ift172fl/fliCre mice, but not in controls, starting at PN25. Nuclei in (A), (B) and (C) are counterstained with Hoechst (blue). The scale bars represent 5 μm.
Since IFT172 is thought to be involved in the switching between the anterograde and retrograde transport, mislocalization of the IFT components is expected. For example, before the complete cessation of IFT, accumulation of the IFT particles at the tip of the cilium would be likely, as seen in Chlamydomonas (24,25). We therefore tested localization of two components of the retrograde IFT (IFT43 and IFT139) and a Bbsome protein (BBS9). Of these three, only IFT139 showed mislocalization at the synaptic terminals of rods, however no changes in location at the axoneme were seen (Fig. 4B, Supplementary Material, Fig. S4).
Mislocalization of photoreceptor outersegment proteins in Ift172fl/fliCre mice
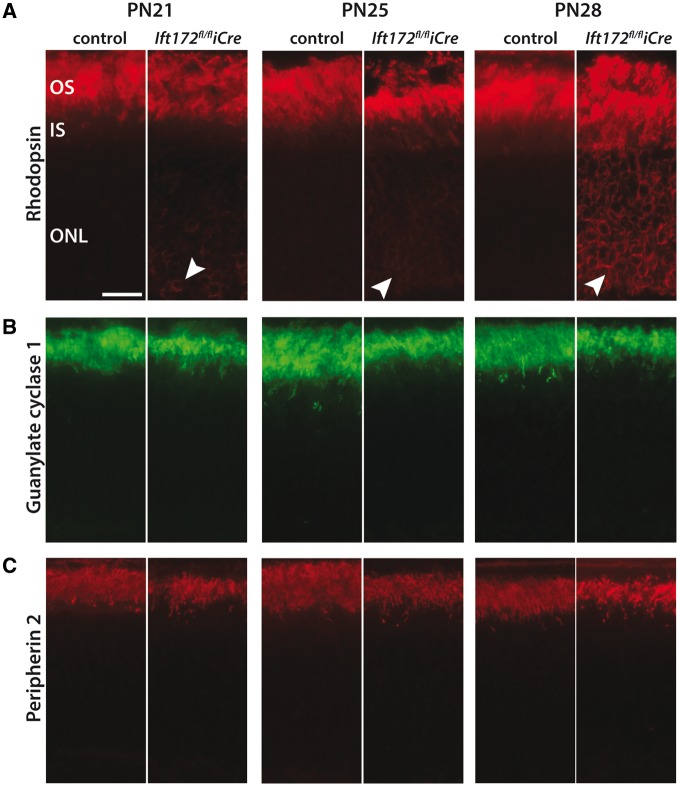
Depletion of a component of the IFT machinery is expected to affect IFT overall and thus alter trafficking of the OS proteins. Localization of four different OS proteins was investigated: two phototransduction proteins dependent on IFT [rhodopsin (RHO) and guanylate cyclase-1 (GC-1)] (34–36), one protein supporting disc structure [peripherin (PRPH2)] (37) and one phototransduction protein independent of IFT (transducin) (38,39). Early signs of RHO mislocalization became apparent at PN21 and PN25, where the protein was detected around the nuclei. This pattern became stronger at PN28 (Fig. 5). Depletion of IFT172, however, had no visible effect on localization of GC-1 and PRPH2 (Fig. 5).
Figure 5.
OS protein localizations. (A) RHO (red) mislocalization to the ONL (white arrowheads) is visible at PN21 and progresses over time in Ift172fl/fliCre mice compared with Ift172fl/fl controls. (B and C). Guanyl cyclase 1 (green) and Peripherin2 (red) localize appropriately to the OS at all tested time points in both Ift172fl/fliCre mice and Ift172fl/fl controls. The scale bar represents 10 μm.
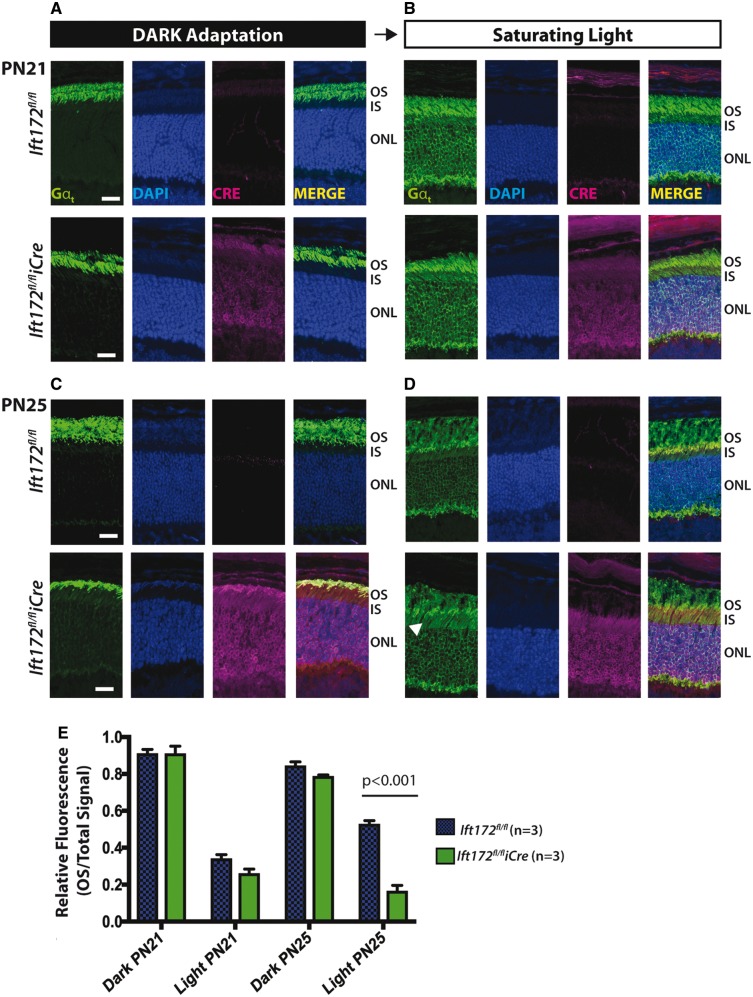
Localization of transducin in rods is light dependent, where in the darkness, transducin is predominantly present in the OSs and upon bright light stimulation it is translocated to the IS (40,41). This light-driven translocation is an adaptation of rods to be able to respond to different light conditions and it is widely believed to occur by diffusion (38,39,42–44). To establish if depletion of IFT172 had an effect on the translocation of the alpha subunit of transducin (Gαt), we tested the localization of this protein in dark-adapted and in photobleached retinas from Ift172fl/fliCre and Ift172fl/fl control littermates at PN21 and PN25 (Fig. 6). These two time points were chosen because the mice show only early signs of retinal degeneration in which alteration of the photoreceptor structure and early signs of RHO mislocalization (Figs 3 and 5) are mild or absent. At PN21, the location of Gαt followed the expected pattern, with the protein detected only in the OSs of the dark-adapted photoreceptors and distributed throughout the photoreceptors in the light-adapted tissue (Fig. 6A and B). At PN25, however, Gαt is detectable in the cell bodies of the dark-adapted photoreceptors of the Ift172fl/fliCre to a greater extent than in the Ift172fl/fl control mice, however after Bonferroni–Sidak mutlicomparison corrections, this difference was not statistically significant (Fig. 6C and E). In the light, more Gαt protein was seen in the IS of the Ift172fl/fliCre than the control mice (Fig. 6D). Quantification of the relative fluorescence signal in the OSs compared with the total fluorescence of the retina, revealed that the OS Gαt content is lower in the mutant mice compared with the controls by 36.3% (P = 0.0003) (Fig. 6E).
Figure 6.
Transducin translocation kinetics are affected in the absence of IFT172. (A) Retina cryosections from dark-adapted mice at PN21 stained for transducin-Gαt (green), nuclei (Hoechst, blue) and Cre (magenta). Transducin is predominantly localized in rod OS region in Ift172fl/fliCre (bottom panel) and the littermate Ift172fl/flcontrols (top panel). (B) Retina cryosections from light-adapted mice at PN21 stained with transducin-Gαt (green), DAPI (blue) and Cre (magenta). In both Ift172fl/fliCre (bottom panel) and littermate Ift172fl/flcontrols (top panel), transducin is translocated to rod IS and inner retinal region. (C) Retina cryosections from dark-adapted mice at PN25 showing predominant transducin-Gαt localization to rod OS, in both genotypes. In addition, presence of transducin-Gαt in the IS of the Ift172fl/fliCre mice but not the controls was observed. (D) Retina cryosections from light-adapted mice at PN25 showing significantly higher transducin-Gαt staining in the IS of Ift172fl/fliCre mice compared to Ift172fl/fl littermate controls (white arrowhead). (E) Relative fluorescence intensity of the OS signal in relation to the total retina signal plotted for Ift172fl/fliCre and Ift172fl/fl control in dark and light conditions at PN21 and PN25. Three mice were used in each genotyope, condition and time point. The scale bars represent 10 μm. Abbreviations: OS, outersegment; IS, innersegment; ONL, outer nuclear layer.
IFT172-deficient mouse retinas show early mislocalization of Hedgehog signaling pathway protein GLI1
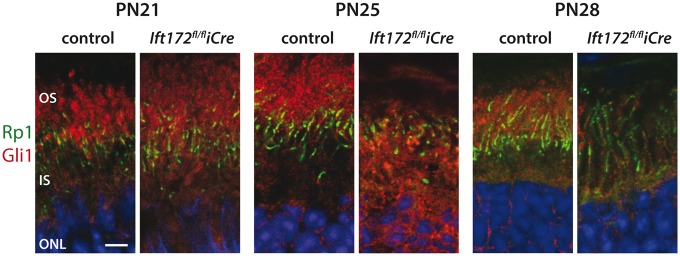
Primary cilia that modulate developmental signaling events, such as Hedgehog (Hh) signaling and all three Hedgehog signaling proteins (Sonic, Desert and Indian), have been shown to be expressed by the retinal ganglion cells or the retinal pigment epithelium (RPE) in the developing and adult murine eye (45). Moreover, depletion of IFT172 has been shown to affect expression of Hh components in the developing brain (28). We therefore investigated the role of Hh signaling in the degenerating photoreceptors due to abnormal IFT. Using immunofluorescence analyses, we investigated the localization of smoothened (SMO) and the glioma-associated oncogene (GLI) family members 1, 2 and 3 (46,47). Of the four proteins, only GLI1 showed differing localization in the Ift172fl/fliCre retinas compared with the control mice. In control mice, GLI1 was predominantly detected in the OSs, while in the Ift172fl/fliCre mice GLI1 localized to the ISs at PN25 and PN28. At PN28, the mutant photoreceptors had a substantially reduced expression of GLI1 as well (Fig. 7). SMO localized to the base of the photoreceptor cilia in the control and the mutant mice, GLI2 showed a diffuse IS staining and GLI3 expression was detected in the inner nuclear layer (INL) and ganglion cells but not in the photoreceptors (Supplementary Material, Fig. S5).
Figure 7.
GLI1 localization. Photoreceptor localization of GLI1 (red) at PN21–28. GLI1 mislocalization within the photoreceptor IS in Ift172fl/fliCre mice, as compared with the mainly OS localization of GLI1 in control mice is visible at PN25. By PN28, GLI1 photoreceptor expression appears largely diminished in Ift172fl/fliCre mice, compared with controls. RP1 (green) marks the transition zone and nuclei are counterstained with Hoechst (blue). The scale bar represents 5 μm.
Discussion
The present study describes a targeted retina-specific Ift172 knock-out mouse, which was generated to model retinal disease caused by mutations in IFT172 (10,11,21,48). The Rho-iCre-mediated disruption of Ift172 resulted in a rapid retinal degeneration occurring within days of the IFT172 protein depletion. The mutant Ift172 mice showed severely reduced retinal thickness and ERG responses by 1 month and absent photoreceptor cells by 2 months. Ultrastructural analyses revealed shortened OSs by PN25 and degenerated photoreceptors by PN31. The molecular mechanisms present in the degenerating retina included mislocalization of specific OS proteins (RHO, RP1, IFT139), altered localization of the GLI1 transcription factor and aberrant light-driven translocation of transducin.
Validating the retina-specific Ift172 knock-out mouse involved precise timing of the Ift172 gene disruption and subsequent protein depletion. Cre recombinase under control of the Rho promoter (iCre) showed increasing expression through to PN25, which led to undetectable levels of the IFT172 protein by PN28. However, the first signs of photoreceptor OS shortening were seen before the complete IFT172 depletion, at PN25 and within 6 days, by PN31, the OSs were fully degenerated. The degeneration occurs therefore faster than the normal 10-day cycle of the OS regeneration in mice (49), which implies an additional function of IFT in the photoreceptors besides the transport of the OS building proteins. Of note, a uniform iCre recombinase protein expression was observed later than previously reported (32). Here, a uniform presence of iCre in the ONL was observed at PN25. This may be due to a lower copy of the transgene caused by numerous mouse crosses and due to chromatin modifications that may affect the transgene expression (50–52). Even though significant levels of the iCre transcript has been observed at PN10 (∼15% of GAPDH, Supplementary Material, Fig. S1), this technique is not informative about how uniform this expression is throughout the ONL. These Cre expression differences, together with the varying half-lives of proteins under study, bring to light the importance of carefully measuring loss of protein expression in conditional knock-out experiments with the use of a specific antibody.
While the clinical presentation of IFT172-associated retinal degeneration is characterized by rod-cone dystrophy, some aspects of this disease are variable. For instance, symptom onset has been reported in young children and in older adolescents, and some of these patients develop macular cysts (10,11,21,48). These differences may be due to the primary disease-causing mutations or genetic modifiers of the disease (10,11,21,48). It is believed that patients with isolated RP and milder forms of ciliopathy (11,21,48) carry at least one hypomorphic allele, since two null alleles are incompatible with life (26,27). After the Cre-mediated excision of exons 2 and 3, however, the Ift172fl/fliCre mice carry two null alleles of Ift172 in the rod photoreceptors, which explains the more rapid degeneration. Rapid degeneration may also be the reason for the lack of retinal cysts in the Ift172fl/fliCre mouse, which either didn’t form or were too small to be detected by the OCT. Nevertheless, key features of IFT172-associated retinal degeneration are represented in the phenotype of the knock-out mouse, making it a suitable in situ model for investigating the mechanisms underlying this disease.
Some selected OS/cilia-related proteins, such as RHO, RP1 and IFT139 showed mislocalization, which may indicate a general protein trafficking disturbance in the cell. The first symptom of the failing IFT machinery that was observed in the Ift172fl/fliCre mice was RHO mislocalization at PN21. Accumulation of RHO in rod cell bodies has been reported in other murine models, where anterograde complex B (Ift88) and retrograde complex A (Ift140) genes were perturbed (35,53). The results obtained in this study add further evidence supporting IFT’s role in opsin transport. In the present study we have not detected mislocalization of two other cilia markers tested (IFT43 or BBS9). One of the reasons for this fact could be due to differences in the abundance of these proteins compared with RHO, RP1 and IFT139 and also with the sensitivity of the antibody reagents. Additional studies would have to be performed to fully explain why certain and not all of the cilia-associated proteins show mislocalization in the retina degenerating due to the Ift172 depletion.
Localization of two other OS proteins, GC1 and PRPH2, was not altered in the mutant mice up to PN28 (Fig. 5). PRPH2, is a structural protein, necessary for the OS disc rim formation (42). Previous studies have indicated that PRPH2 and RHO utilize separate methods of transport to reach the OS, which is in agreement with our findings (37,42,54). The lack of GC1 mislocalization was, however, surprising since it requires RHO for trafficking to the OSs (54). One of the reasons for this result could be a low abundance of the GC1 protein and the low sensitivity of the immunohistochemical assay. Noteworthy, GC1 accumulation was also absent in the rod cell bodies of the Rho−/− retinas (54).
Unexpectedly, decreasing levels of IFT172-affected light-driven translocation of alpha transducin at PN25. Specifically, transducin showed increased localization within the IS and around the photoreceptor nuclei after light adaptation (Fig. 6). It is thought that light-mediated translocation of transducin and the reciprocal translocation of arrestin occur via diffusion and thus are IFT independent (38–44). Therefore, it is not clear how perturbed IFT can alter protein diffusion through the transition zone. It is plausible that at the PN25 time point, the structure of the axoneme starts to disintegrate, forming a barrier against diffusion. A similar conclusion has been drawn from studies in mouse retinal explants, where drug-mediated disruption of actin filaments and microtubules led to impaired distribution of transducin and arrestin during the dark adaptation (55). Involvement of cytoskeleton in light-mediated protein translocation was also observed in Xenopus laevis (56,57). Another possible explanation is that the transducin mislocalization observed in our model is secondary to the impaired trafficking of RHO. Current evidence suggests that light-activated RHO and intact disc membranes are needed to act as ‘sinks’ for arrestin/transducin transport (43,58,59). Therefore it is plausible that perturbations in RHO transport would lead to secondary mislocalization of transducin. The timeline of this theory also matches our findings of RHO mislocalization at PN21, preceding transducin mislocalization detectable at PN25.
Numerous studies have shown the importance of ciliogenesis and IFT on Hh signaling during development; however, little is known about how the Hh signaling pathway functions in cilia after development and during degeneration (26,60–64). Generally, activation of the Hh pathway in vertebrate cells involves binding of the ligand (Sonic, Desert, or Indian Hedgehog) by the patched (PTCH1) receptor present at the ciliary membrane and subsequent activation and translocation of SMO to the cilium. SMO subsequently blocks GLI repressors (mostly GLI3) and triggers GLI transcriptional activators (mostly GLI2 and GLI1), which translocate from the cilium to the nucleus (63,64). Double-mutant mouse experiments have demonstrated that IFT acts downstream of the Hh receptor PTCH1 and SMO, but upstream of the GLI effectors (26,61,63,64). We therefore used immunofluorescence analyses to determine the localization of key Hh pathway components in the degenerating retina. Lack of differences in localization of SMO, GLI2 and GLI3 indicate that Hh pathway was not activated in the degenerating retina of the Ift172fl/fliCre mice. Immunofluorescence analysis of GLI1, however, gave contrary results, as this protein translocated from the cilium to the ISs and the peri-nuclear region of the degenerating photoreceptors. Functional differences between GLI1 and GLI2/GLI3 transcription factors have been noted before. For example in contrast to GLI2 and GLI3, GLI1 lacks a repressor domain and homozygous Gli1 knock-out mice develop normally (46,65). GLI1 has also been reported to be activated in a Hh independent fashion (66), which is likely the case in the present study. Currently, the role of GLI1 in developed photoreceptors is unknown. Whether the observed translocation of GLI1 from the OS has a protective function or if it enhances photoreceptor degeneration remains to be determined. Further research will be necessary to fully answer the question about its role in normal and degenerating retinas.
The results of this study highlight the crucial nature of transition from anterograde to retrograde IFT within the rod photoreceptors and expand our understanding of IFT’s role in protein trafficking within these cells. A profound degeneration of the OSs occurred within just a matter of days after IFT172 was depleted, which was shown by a thorough characterization of the degeneration between PN21 and PN28. Phenotypic and molecular results from this time-course analysis indicate that the hypomorphic condition present in patients with IFT172-associated retinal degeneration is most accurately modeled at PN25, when depletion of some but not all of the IFT172 protein leads to the first signs of photoreceptor degeneration. This model is therefore useful to understand the IFT-associated disease and for the development of potential treatments for patients with these diseases.
Materials and Methods
Ethics statement
The in-vivo experiments performed on mice were performed according to protocols approved by the Animal Care Committee from the Massachusetts Eye and Ear Infirmary. All procedures were performed to minimize suffering in accordance with the animal care rules in our institution in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Mouse Husbandry and Genotyping
Mice were housed in the animal facility of the Schepens Eye Research Institute, provided a standard rodent diet and housed in standard 12 h alternating light/dark cycles.
Genomic DNA was isolated from ear biopsies (Allele-In-One reagent, Allele Biotechnology, San Diego, CA, USA). Two genotyping PCR reactions (Hot FirePol DNA polymerase, Solis Biodyne, Tartu, Estonia) were performed for the Ift172 allele (F: 5′-GAAGAGTTGGGTGTAAGAAATGC-3′ and R: 5′-CTGGAGCTACATCAAAGACAG-3′) and the iCre allele (F: 5′-TCAGTGCCTGGAGTTGCGCTGTGG-3′ and R: 5′-CACAGACAGGAGCATCTTCCAG-3′). Both PCR reactions were performed in 2.0 mM MgCl2 with the thermocycling protocol as follows: 95°C for 15 min; 35 cycles of 94°C for 30 s, 60°C for iCre/62°C for Ift172 for 30 s; 72°C for 1 min; final extension: 72°C for 5 min. Gel electrophoresis was performed on the PCR products. The Ift172 floxed allele produced a 380 bp band, whereas the wild-type ift172 allele produced a 176 bp band. Presence of the iCre allele produced a 200 bp band.
Electroretinography (ERG)
Full-field ERGs were performed on mice at 1, 2, 3 and 6 months of age to assess rod and cone photoreceptor function (67). Mice were dark adapted overnight, then approximately 20 min before procedure they were anesthetized by intraperitoneal injection of ketamine and xylazine diluted in sterile saline, and the eyes were dilated by topical application of 1% tropicamide. ERG was recorded simultaneously from both eyes in response to 4 ms broadband stimuli with the use of gold ring electrodes (Diagnosys, Lowell, MA) and the ColorDome Ganzfeld system (Diagnosys), as reported before (68).
In dark-adapted mice, rod-driven ERGs were recorded in response to a 0.01 cd·s/m2 light stimulus (10 flashes at 0.2 Hz), and mixed rod/cone ERGs were recorded in response to a 10 cd·s/m2 light stimulus (three flashes at 0.03 Hz). Next, mice were light-adapted by exposure to a steady, rod-suppressing background light (30 cd/m2) for 10 min. This background light remained during the acquisition of cone-driven ERGs that were recorded in response to a 20 cd·s/m2 light stimulus (20 flashes at 0.5 Hz). The magnitude of the ERG b-wave was measured as the absolute voltage change from the trough of the a-wave (or from the voltage measured at the expected implicit time of that trough, should the a-wave be undetectable) to the b-wave peak (68).
Non-linear (semilog) regression was used to fit the averaged data of mice in each condition for the different time points (GraphPad Prism 7, San Diego, CA, USA). The statistical significance of the results was assessed with two-way ANOVA and post-hoc t-test with Bonferroni–Sidak correction for multiple comparisons (GraphPad Prism 7).
Optical coherence tomography (OCT)
OCT was performed on mice at 1, 2 and 3 months of age. After anesthesia and pupil dilation (as above), cross-sectional retinal images were acquired with the InVivoVue OCT system (Bioptogen, Morrisville, NC, USA). A rectangular and radial OCT volume centered on the optic nerve head was captured for both eyes. A B-scan located approximately 200 μm temporal and nasal of the ventral optic nerve head was selected for measurement. Retinal thickness was measured as the distance between the nerve fiber layer and the hyporeflective boundary between the RPE and choroid. The statistical significance of the results was assessed with two-way ANOVA and post-hoc t-test with Bonferroni–Sidak correction for multiple comparisons (GraphPad Prism 7).
Histology
The mice at 1, 2, 3 and 6 months of age were sacrificed by transcardial perfusion [4% paraformaldehyde (PFA), approximately 30 ml per mouse]. After perfusion, each eye was enucleated and placed in 4% PFA in PBS overnight for post-fixation. After dehydration with graded ethanol solutions, the eyes were embedded in glycol methacrylate (Technovit 7100, Heraeas Kulzer GmBH, Wehrheim, Germany) and 3 μm thick sections were cut at the level of the optic nerve head (LKB Historange microtome). The sections were stained with hematoxylin and eosin and imaged by bright field microscopy (Eclipse Ti, Nikon, Tokyo, Japan).
Immunofluorescence
The pups at ages PN18–PN31 were sacrificed by CO2 asphyxiation and the eyes were enucleated. Since many of the cilia antibodies are incompatible with tissue fixation, one eye was fresh frozen and one fixed. For the non-fixed tissue, the eye was immediately embedded (OCT reagent, Tissue-Tek, Fisher Scientific, USA) within molds and snap frozen using 200 proof ethanol over dry ice. For the fixed tissues, the cornea and the lens were dissected and the eyes were placed in 4% PFA for 1 h post-fixation, and the eyecups were cryopreserved [30% sucrose in phosphate-buffered saline buffer (PBS), overnight at 4°C] (Sigma-Aldrich, USA) and embedded (OCT reagent). The eyes were cryosectioned into 10 µm slices and then processed using the following procedure: blocking and permeabilization [0.05% Triton X-100, 1% bovine serium albumin (BSA) in PBS, 1 h at RT]; primary antibody staining (dilutions listed below, 0.3% Triton X-100 in PBS, overnight at 4°C); incubation of the secondary antibodies conjugated with a fluorophore (Alexa Fluor 488, AlexaFluor 555 and AlexaFluor 647) (Thermo Fisher, Waltham, MA, USA) (1: 500 dilution, 0.3% Triton X-100 in PBS, 2 h at RT). The nuclei were counterstained with Hoechst after the secondary antibody incubation (1: 1000 dilution in PBS for 15 min at RT). In between the steps the retina sections were washed four times with PBS. The slides were mounted with coverslips with Fluoromount medium (Electron Microscopy Sciences, Hatfield, PA, USA). The images were acquired in a sequential manner, with pictures taken every 0.5µm in the z-plane using confocal microscopy (SP5, Leica Microsystems, USA). Figure 5 images were taken with a fluorescent microscope (Eclipse Ti, Nikon, Tokyo, Japan). Rp1 and acTub signal length has been quantified on the confocal images using a freehand line tool and a length measurement option in an image processing software (ImageJ, National Institute of Mental Health, MD, USA). Unpaired t-test was used to assess the statistical significance of measurements between conditions (GraphPad Prism).
Primary antibodies used on 4% PFA fixed tissues were: mouse-anti-Cre (1: 500, Millipore, MAB3120); mouse-anti-RHO (1: 1000, Millipore, MAB5356); mouse-anti-Transducin (1: 100, BD BioSciences, 610589). Primary antibodies used on non-fixed tissue after brief postfixation on the slide (1% PFA in PBS for 15 min prior to staining) were: guinea pig-anti-GLI2 (1: 1000, kind gift from Dr Eggenschwiler); rabbit-anti-GLI3 (1: 200, Thermo Fisher, PS5-28029); mouse-anti-AcTub (1: 200, Sigma-Aldrich, T6793—100 ul); rabbit-anti-IFT139 (1: 500, custom). Primary antibodies used with non-fixed tissue were rabbit-anti-IFT43 (1: 100, Proteintech, 24338—1-AP); mouse-anti-PRPH2 (1: 15, kind gift from Dr. Molday); rabbit-anti-GLI1 (1: 200, abcam, ab92611); rabbit-anti-SMO (1: 1000, kind gift from Dr Anderson); rabbit-anti-BBS9 (1: 500, Sigma-Aldrich, HPA021289—100UL); mouse-anti-Gc1 (1: 1000, kind gift from Dr Baer); chicken-anti-RP1 (1: 1000, custom); rabbit-anti-IFT172 (1: 1000, custom).
Ift172 antibody development
A custom affinity-purified rabbit anti-IFT172 polyclonal antibody was generated through a comercial provider (SC1676 PolyExpress Premium Service, GenScript USA Piscataway, NJ, USA). For rabbit immunizations, a 488 amino acid peptide from the C terminus of the human IFT172 protein was chosen: EEYEREATKKGARGVEGFVEQARHWEQAGEYSRAVDCYLKVRDSGNSGLAEKCWMKAAELSIKFLPPQRNMEVVLAVGPQLIGIGKHSAAAELYLNLDLVKEAIDAFIEGEEWNKAKRVAKELDPRYEDYVDQHYKEFLKNQGKVDSLVGVDVIAALDLYVEQGQWDKCIETATKQNYKILHKYVALYATHLIREGSSAQALALYVQHGAPANPQNFNIYKRIFTDMVSSPGTNCAEAYHSWADLRDVLFNLCENLVKSSEANSPAHEEFKTMLLIAHYYATRSAAQSVKQLETVAARLSVSLLRHTQLLPVDKAFYEAGIAAKAVGWDNMAFIFLNRFLDLTDAIEEGTLDGLDHSDFQDTDIPFEVPLPAKQHVPEAEREEVRDWVLTVSMDQRLEQVLPRDERGAYEASLVAASTGVRALPCLITGYPILRNKIEFKRPGKAANKDNWNKFLMAIKTSHSPVCQDVLKFISQWCGGLPSTSFSFQ.
RNA extraction and quantitative real-time (q)PCR
The pups at ages PN10–PN25 were sacrificed by CO2 asphyxiation, the eyes were enucleated and the retinas dissected and immediately frozen in N2(aq.). The RNA was extracted with a commercially available kit, with an on-column DNA digestion option (RNAeasy, Qiagen, Gernamtown, MD). After performing the reverse transcription (SuperScript II, Thermo Fisher Scientific) using oligo-dT primers, 10 ng of cDNA was used in each qPCR reaction (Fast SYBR Green Master Mix, Thermo Fisher). Real-time qPCR was performed in the appropriate thermocycler (Mx3005P, Agilent Technologies). The following primers were used for the amplifications: GAPDH (reference gene, F: 5′-CTTCACCACCATGGAGAAGG-3′ and R: 5′-GGTTCACACCCATCACAAAC-3′); IFT172 (F: 5′-CAGTTGAAGCACCTGAGGAC-3′ and R: 5′-TCGGTCCACTGTGCAGACAG-3′), RHO (F: 5′-CCACAGGCTGTAATCTCGAG-3′ and R: 5′-CGAAGCGGAAGTTGCTCATC-3′), iCRE (same as genotyping primers). The data was normalized by the GAPDH expression using the delta-CT method (RHO and iCRE) and further normalized to the floxed control mice using the delta-delta-CT method (IFT172). The data was analyzed with a statistical software (GraphPad Prism 7), applying two-way ANOVA and post-hoc t-test with Bonferroni–Sidak correction for multiple comparisons.
Transmission electron microscopy (TEM) materials and methods
Mice were sacrificed by transcardial perfusion with half strength Karnovsky’s fixative (2% formaldehyde + 2.5% glutaraldehyde, in 0.1 M sodium cacodylate buffer, pH 7.4; Electron Microscopy Sciences, Hatfield, PA, USA). The eyes were enucleated and after cornea and lens dissection, the eye cups were post-fixed with half strength Karnovsky’s fixative for a minimum of 24 h under refrigeration. Next, samples were rinsed with 0.1 M sodium cacodylate buffer, post-fixed with 2% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1.5 h, en bloc stained with 2% aqueous uranyl acetate for 30 min, then dehydrated with graded ethyl alcohol solutions, transitioned with propylene oxide and resin infiltrated in tEPON-812 epoxy resin (Tousimis, Rockville, MD, USA) utilizing an automated EM tissue processor (Lynx 2, Electron Microscopy Sciences, Hatfield, PA, USA). The processed samples were oriented into tEPON-812 epoxy resin and polymerized in a 60°C oven. Semi-thin sections were cut at 1 µm thickness through the mid-equatorial plane traversing the optic nerve and stained with 1% toluidine blue in 1% sodium tetraborate aqueous solution for assessment by light microscopy. Ultrathin sections (80 nm) were cut from each sample block (EM UC7 ultramicrotome, Leica Microsystems, Buffalo Grove, IL, USA) and collected onto either 2 × 1 mm, single slot formvar carbon-coated or 200 mesh uncoated copper grids and air dried. The ultrathin sections on grids were stained with aqueous 2.5% aqueous gadolinium triacetate hydrate and Sato’s lead citrate stains using a modified Hiraoka grid staining system. Ultrathin sections were imaged using a transmission electron microscope (Tecnai G2 Spirit, FEI, Hillsboro, OR) at 80 kV and images acquired with a digital camera at 2000 × 2000 pixel and 16-bit resolution (AMT XR41 CCD camera, Advanced Microscopy Techniques, Woburn, MA).
Transducin translocation experiment
In this experiment, the mice were distributed in two different groups. For each group, three Ift172fl/fliCre mice and littermate Ift172fl/fl control mice were used. Group 1: The mice were dark-adapted overnight, euthanized in darkness with CO2 and their eyes were enucleated under dark conditions, fixed (4% PFA in PBS) and prepared for cryosectioning as described in previous section. Group 2: The pupils of mice were dilated as described above in ERG section, and mice were exposed to saturating light conditions (1400 lux) for 20 min. CO2 was used to euthanize the mice, and their eyes were enucleated and prepared from dryosectioning as before. The eyes from different conditions were sectioned in one block and they were immonolabeled with mouse-anti-Transducin antibody (1: 100, BD BioSciences, 610589) on the same slide. The images were taken with confocal microscopy (SP5, Leica Microsystems, USA). ImageJ software was used to analyze signal intensities, where signal from the OS was divided by the total signal form the whole retina. The analysis conditions were kept exact for test and control mice. Two-way ANOVA and a post-hoc multiple t-test with Bonferroni–Sidak correction were used for statistical analysis (Graphpad, Prism 7 software).
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
The authors thank Erin Zampaglione and Rosanno Butcher for their technical assistance.
Conflict of Interest statement. None declared.
Funding
This work was supported by grants from the Fight for Sight (2016 International Retinal Research Foundation Grant-In-Aid Award to K.M.B.), Research to Prevent Blindness Medical Student Research Fellowship (P.R.G.), National Eye Institute [EY012910 (E.A.P.) and P30EY014104 (MEEI core support), P30EYE003790 (SERI core support)], the Foundation Fighting Blindness (USA, E.A.P.).
References
- 1. Goetz S.C., Anderson K.V. (2010) The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet., 11, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mockel A., Perdomo Y., Stutzmann F., Letsch J., Marion V., Dollfus H. (2011) Retinal dystrophy in Bardet-Biedl syndrome and related syndromic ciliopathies. Prog. Retin. Eye Res., 30, 258–274. [DOI] [PubMed] [Google Scholar]
- 3. Cortés C.R., Metzis V., Wicking C. (2015) Unmasking the ciliopathies: craniofacial defects and the primary cilium. Interdiscip. Rev. Dev. Biol., 4, 637–653. [DOI] [PubMed] [Google Scholar]
- 4. Waters A.M., Beales P.L. (2011) Ciliopathies: an expanding disease spectrum. Pediatr. Nephrol., 26, 1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramamurthy V., Cayouette M. (2009) Development and disease of the photoreceptor cilium. Clin. Genet., 76, 137–145. [DOI] [PubMed] [Google Scholar]
- 6. Rachel R.A., Li T., Swaroop A. (2012) Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia, 1, 22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bujakowska K.M., Liu Q., Pierce E.A. (2017) Photoreceptor cilia and retinal ciliopathies. Cold Spring Harb. Perspect. Biol., 9, a028274. 10.1101/cshperspect.a028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pedersen L.B., Rosenbaum J.L. (2008) Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol., 85, 23–61. [DOI] [PubMed] [Google Scholar]
- 9. Williamson S.M., Silva D.A., Richey E., Qin H. (2012) Probing the role of IFT particle complex A and B in flagellar entry and exit of IFT-dynein in Chlamydomonas. Protoplasma, 249, 851–856. [DOI] [PubMed] [Google Scholar]
- 10. Halbritter J., Bizet A.A., Schmidts M., Porath J.D., Braun D.A., Gee H.Y., McInerney-Leo A.M., Krug P., Filhol E., Davis E.E.. et al. (2013) Defects in the IFT-B component IFT172 cause Jeune and Mainzer-Saldino syndromes in humans. Am. J. Hum. Genet., 93, 915–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bujakowska K.M., Zhang Q., Siemiatkowska A.M., Liu Q., Place E., Falk M.J., Consugar M., Lancelot M.-E., Antonio A., Lonjou C.. et al. (2015) Mutations in IFT172 cause isolated retinal degeneration and Bardet-Biedl syndrome. Hum. Mol. Genet., 24, 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis E.E., Zhang Q., Liu Q., Diplas B.H., Davey L.M., Hartley J., Stoetzel C., Szymanska K., Ramaswami G., Logan C.V.. et al. (2011) TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat. Genet., 43, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coussa R.G., Otto E.A., Gee H.-Y., Arthurs P., Ren H., Lopez I., Keser V., Fu Q., Faingold R., Khan A.. et al. (2013) WDR19: an ancient, retrograde, intraflagellar ciliary protein is mutated in autosomal recessive retinitis pigmentosa and in Senior-Loken syndrome. Clin. Genet., 84, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arts H.H., Bongers E.M.H.F., Mans D.A., van Beersum S.E.C., Oud M.M., Bolat E., Spruijt L., Cornelissen E.A.M., Schuurs-Hoeijmakers J.H.M., de Leeuw N.. et al. (2011) C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J. Med. Genet., 48, 390–395. [DOI] [PubMed] [Google Scholar]
- 15. Bredrup C., Saunier S., Oud M.M., Fiskerstrand T., Hoischen A., Brackman D., Leh S.M., Midtbø M., Filhol E., Bole-Feysot C.. et al. (2011) Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am. J. Hum. Genet., 89, 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huber C., Cormier-Daire V. (2012) Ciliary disorder of the skeleton. Am. J. Med. Genet. Part C Semin. Med. Genet., 160C, 165–174. [DOI] [PubMed] [Google Scholar]
- 17. Aldahmesh M.A., Li Y., Alhashem A., Anazi S., Alkuraya H., Hashem M., Awaji A.A., Sogaty S., Alkharashi A., Alzahrani S.. et al. (2014) IFT27, encoding a small GTPase component of IFT particles, is mutated in a consanguineous family with Bardet-Biedl syndrome. Hum. Mol. Genet., 23, 3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Girisha K.M., Shukla A., Trujillano D., Bhavani G.S., Hebbar M., Kadavigere R., Rolfs A. (2016) A homozygous nonsense variant in IFT52 is associated with a human skeletal ciliopathy. Clin. Genet., 10.1111/cge.12762. [DOI] [PubMed] [Google Scholar]
- 19. Walczak-Sztulpa J., Eggenschwiler J., Osborn D., Brown D.A., Emma F., Klingenberg C., Hennekam R.C., Torre G., Garshasbi M., Tzschach A.. et al. (2010) Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am. J. Hum. Genet., 86, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bizet A.A., Becker-Heck A., Ryan R., Weber K., Filhol E., Krug P., Halbritter J., Delous M., Lasbennes M.-C., Linghu B.. et al. (2015) Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat. Commun., 6, 8666.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaefer E., Stoetzel C., Scheidecker S., Geoffroy V., Prasad M.K., Redin C., Missotte I., Lacombe D., Mandel J.-L., Muller J.. et al. (2016) Identification of a novel mutation confirms the implication of IFT172 (BBS20) in Bardet–Biedl syndrome. J. Hum. Genet., 10.1038/jhg.2015.162. [DOI] [PubMed] [Google Scholar]
- 22. Lucker B.F., Behal R.H., Qin H., Siron L.C., Taggart W.D., Rosenbaum J.L., Cole D.G. (2005) Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J. Biol. Chem., 280, 27688–27696. [DOI] [PubMed] [Google Scholar]
- 23. Taschner M., Bhogaraju S., Lorentzen E. (2012) Architecture and function of IFT complex proteins in ciliogenesis. Differentiation, 83, S12–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pedersen L.B., Miller M.S., Geimer S., Leitch J.M., Rosenbaum J.L., Cole D.G. (2005) Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr. Biol., 15, 262–266. [DOI] [PubMed] [Google Scholar]
- 25. Iomini C., Babaev-Khaimov V., Sassaroli M., Piperno G. (2001) Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol., 153, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huangfu D., Liu A., Rakeman A.S., Murcia N.S., Niswander L., Anderson K.V. (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature, 426, 83–87. [DOI] [PubMed] [Google Scholar]
- 27. Friedland-Little J.M., Hoffmann A.D., Ocbina P.J.R., Peterson M.A., Bosman J.D., Chen Y., Cheng S.Y., Anderson K.V., Moskowitz I.P. (2011) A novel murine allele of intraflagellar transport protein 172 causes a syndrome including VACTERL-like features with hydrocephalus. Hum. Mol. Genet., 20, 3725–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gorivodsky M., Mukhopadhyay M., Wilsch-Braeuninger M., Phillips M., Teufel A., Kim C., Malik N., Huttner W., Westphal H. (2009) Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev. Biol., 325, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lunt S.C., Haynes T., Perkins B.D. (2009) Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect Hedgehog signaling. Dev. Dyn., 238, 1744–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sukumaran S., Perkins B.D. (2009) Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Res., 49, 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Howard P., Howard T., Maurer R. (2010) Generation of mice with a conditional allele for Ift172. Transgenic Res., 19, 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li S., Chen D., Sauvé Y., McCandless J., Chen Y.-J., Chen C.-K. (2005) Rhodopsin-iCre transgenic mouse line for Cre-mediated rod-specific gene targeting. Genesis, 41, 73–80. [DOI] [PubMed] [Google Scholar]
- 33. Liu Q., Lyubarsky A., Skalet J.H., Pugh E.N., Pierce E.A. (2003) RP1 is required for the correct stacking of outer segment discs. Investig. Ophthalmol. Vis. Sci., 44, 4171–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marszalek J.R., Liu X., Roberts E. a., Chui D., Marth J.D., Williams D.S., Goldstein L.S. (2000) Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell, 102, 175–187. [DOI] [PubMed] [Google Scholar]
- 35. Pazour G.J., Baker S.A., Deane J. a., Cole D.G., Dickert B.L., Rosenbaum J.L., Witman G.B., Besharse J.C. (2002) The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol., 157, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Insinna C., Besharse J. (2008) Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev. Dyn., 237, 1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee E.S., Burnside B., Flannery J.G. (2006) Characterization of peripherin/rds and Rom-1 transport in rod photoreceptors of transgenic and knockout animals. Investig. Ophthalmol. Vis. Sci., 47, 2150–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calvert P.D., Strissel K.J., Schiesser W.E., Pugh E.N., Arshavsky V.Y. (2006) Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol., 16, 560–568. [DOI] [PubMed] [Google Scholar]
- 39. Calvert P.D., Schiesser W.E., Pugh E.N. (2010) Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J. Gen. Physiol., 135, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Broekhuyse R.M., Tolhuizen E.F., Janssen A.P., Winkens H.J. (1985) Light induced shift and binding of S-antigen in retinal rods. Curr. Eye Res., 4, 613–618. [DOI] [PubMed] [Google Scholar]
- 41. Philp N.J., Chang W., Long K. (1987) Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett., 225, 127–132. [DOI] [PubMed] [Google Scholar]
- 42. Pearring J.N., Salinas R.Y., Baker S.A., Arshavsky V.Y. (2013) Protein sorting, targeting and trafficking in photoreceptor cells. Prog. Retin. Eye Res., 36, 24–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slepak V.Z., Hurley J.B. (2007) Mechanism of light-induced translocation of arrestin and transducin in photoreceptors: interaction-restricted diffusion. IUBMB Life, 60, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nair K.S., Hanson S.M., Mendez A., Gurevich E.V., Kennedy M.J., Shestopalov V.I., Vishnivetskiy S.A., Chen J., Hurley J.B., Gurevich V.V.. et al. (2005) Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron, 46, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wallace V.A. (2008) Proliferative and cell fate effects of Hedgehog signaling in the vertebrate retina. Brain Res., 1192, 61–75. [DOI] [PubMed] [Google Scholar]
- 46. Hui C., Angers S. (2011) Gli proteins in development and disease. Annu. Rev. Cell Dev. Biol., 27, 513–537. [DOI] [PubMed] [Google Scholar]
- 47. Ocbina P.J.R., Eggenschwiler J.T., Moskowitz I., Anderson K.V. (2011) Complex interactions between genes controlling trafficking in primary cilia. Nat. Genet., 43, 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lucas-Herald A.K., Kinning E., Iida A., Wang Z., Miyake N., Ikegawa S., McNeilly J., Ahmed S.F. (2015) A Case of functional growth hormone deficiency and early growth retardation in a child with IFT172 mutations. J. Clin. Endocrinol. Metab., 100, 1221–1224. [DOI] [PubMed] [Google Scholar]
- 49. Young R., (1983) The ninth Frederick H. Verhoeff lecture. The life history of retinal cells. Trans. Am. Ophthalmol. Soc., 81, 193–228. [PMC free article] [PubMed] [Google Scholar]
- 50. Montoliu L., Chávez S., Vidal M. (2000) Variegation associated with lacZ in transgenic animals: a warning note. Transgenic Res., 9, 237–239. [DOI] [PubMed] [Google Scholar]
- 51. Li Q., Emery D.W., Han H., Sun J., Yu M., Stamatoyannopoulos G. (2005) Differences of globin transgene expression in stably transfected cell lines and transgenic mice. Blood, 105, 3346–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vooijs M., Jonkers J., Berns A. (2001) A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep., 2, 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crouse J.A., Lopes V.S., Sanagustin J.T., Keady B.T., Williams D.S., Pazour G.J. (2014) Distinct functions for IFT140 and IFT20 in opsin transport. Cytoskeleton, 71, 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pearring J.N., Spencer W.J., Lieu E.C., Arshavsky V.Y. (2015) Guanylate cyclase 1 relies on rhodopsin for intracellular stability and ciliary trafficking. Elife, 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reidel B., Goldmann T., Giessl A., Wolfrum U. (2008) The translocation of signaling molecules in dark adapting mammalian rod photoreceptor cells is dependent on the cytoskeleton. Cell Motil. Cytoskeleton, 65, 785–800. [DOI] [PubMed] [Google Scholar]
- 56. McGinnis J.F., Matsumoto B., Whelan J.P., Cao W. (2002) Cytoskeleton participation in subcellular trafficking of signal transduction proteins in rod photoreceptor cells. J. Neurosci. Res., 67, 290–297. [DOI] [PubMed] [Google Scholar]
- 57. Peterson J.J., Orisme W., Fellows J., McDowell J.H., Shelamer C.L., Dugger D.R., Smith W.C. (2005) A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Investig. Ophthalmol. Vis. Sci., 46, 3988–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Artemyev N.O. (2008) Light-dependent compartmentalization of transducin in rod photoreceptors. Mol. Neurobiol., 37, 44–51. [DOI] [PubMed] [Google Scholar]
- 59. Kerov V., Artemyev N.O. (2011) Diffusion and light-dependent compartmentalization of transducin. Mol. Cell. Neurosci., 46, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weatherbee S.D., Niswander L.A., Anderson K.V. (2009) A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum. Mol. Genet., 18, 4565–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huangfu D., Anderson K.V. (2005) Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. U. S. A., 102, 11325–11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. He M., Agbu S., Anderson K.V. (2016) Microtubule motors drive Hedgehog signaling in primary cilia. Trends Cell Biol., xx, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bangs F., Anderson K.V. (2017) Primary cilia and mammalian Hedgehog signaling. Cold Spring Harb. Perspect. Biol., 9, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eggenschwiler J.T., Anderson K.V. (2007) Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol., 23, 345–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park H.L., Bai C., Platt K.A., Matise M.P., Beeghly A., Hui C.C., Nakashima M., Joyner A.L. (2000) Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development, 127, 1593–1605. [DOI] [PubMed] [Google Scholar]
- 66. Nolan-Stevaux O., Lau J., Truitt M.L., Chu G.C., Hebrok M., Fernández-Zapico M.E., Hanahan D. (2009) GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev., 23, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marmor M.F., Fulton A.B., Holder G.E., Miyake Y., Brigell M., Bach M. (2009) ISCEV Standard for full-field clinical electroretinography (2008 update). Doc. Ophthalmol., 118, 69–77. [DOI] [PubMed] [Google Scholar]
- 68. Greenwald S.H., Charette J.R., Staniszewska M., Shi L.Y., Brown S.D.M., Stone L., Liu Q., Hicks W.L., Collin G.B., Bowl M.R.. et al. (2016) Mouse models of NMNAT1-Leber congenital amaurosis (LCA9) recapitulate key features of the human disease. Am. J. Pathol., 186, 1925–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.