Abstract
Background
There is a gap in research focused on gender-based differences in non-referral populations with celiac disease.
Aims
The aim of this study was to estimate those differences in a unique population-based cohort of patients with celiac disease with respect to 1) presenting symptoms, 2) associated autoimmune disorders, and 3) survival.
Methods
Clinical data were systematically abstracted from the electronic medical record of a population-based incident cohort of patients with celiac disease. Logistic regression was used to assess the strength of the association of presenting symptoms and gender. Survival differences between genders were evaluated with Cox regression.
Results
We included 282 patients (females 65%, median age 39 years old) diagnosed between 1990 and 2015. The female to male ratio was 1.85:1. Men and women presented similarly. Women were more likely to present with constipation (OR 2.33; 95% CI: 1.06–5.12; p=0.035). Anemia and abdominal distention or bloating were more frequently seen in women, but not on a statistically significant level. Overall autoimmune diseases were equally prevalent (31.6%) in males (30.2%) and females (32.2%) (p = 0.74). Hypothyroidism predominated in women. Age-adjusted survival was lower among men than women (HR 3.00; 95% CI 1.26–7.21, p=0.014), but not more so than in the general population. Cancer was the most common cause of death and there were two possible celiac disease-related deaths.
Conclusions
This study showed that men and women are more alike than unalike when it comes to celiac disease presentation and prevalence of concurrent autoimmune disease.
Keywords: Sprue, Sex, Gender, Population, Autoimmune Conditions
Introduction
Celiac disease (CD) is a systemic immune-mediated illness characterized by enteropathy after exposure to gluten [1]. It affects between 0.5%–1% of the United States and European population [2,3,4]. There is a female predominance of diagnosed disease (2:1 to 10:1) [5,6,7]. CD can affect women’s health by decreasing bone mineral density and fertility, increasing risk of concurrent autoimmune disease, and negatively impacting quality of life [8,9,10,11]. Gender-related differences in clinical presentation of CD have been suggested [12,13,14]. Women are more likely to experience abdominal pain and iron deficiency anemia [13,14,15]. Elevations in liver enzymes, weight loss, and the presence of dermatitis herpetiformis appear more common in men [15]. Women reportedly struggle more with persistent symptoms and poorer health-related quality of life even while adhering to a gluten-free diet [11].
The aim of this study was to estimate gender-based differences in a unique population-based cohort of patients with CD with respect to 1) presenting symptoms, 2) anthropomorphic measurements, 3) associated autoimmune disorders, 4) concurrent illnesses including depression and osteoporosis, 5) laboratory studies, and 6) survival.
Methods
This population-based incidence cohort included individuals living in Olmsted County, Minnesota with CD. Patients diagnosed between 1990 and 2015 were included. The Rochester Epidemiology Project (REP) was used to build the cohort along with Mayo Clinic’s Celiac Registry [16,17]. Using REP allows for comprehensive data from this Midwestern community as it links records from both major healthcare providers in the community: Mayo Clinic and Olmsted Medical Center [18]. It combines clinical documentation from Mayo Clinic and its hospitals (St Mary’s and Methodist) with that of the Olmsted Medical Group and its affiliated hospital to create a population-based fund of data [18].
Cases of diagnosed CD were identified based on inpatient and outpatient medical records, ICD-9 coding for celiac disease (579.0) or dermatitis herpetiformis (694.0), serology, and histopathology [17]. Patients were identified using the REP medical index, the Celiac Registry established by previous studies, and review of patient charts [16,17]. The infrastructure of the REP allowed us to systematically abstract data from the electronic medical record of those with a diagnosis of CD in this defined geographic area. Baseline characteristics, mode of clinical presentation (classical, non-classical, silent), severity of disease, specific presenting symptoms, family history of celiac disease, serological status, prescription for gluten-free diet, and biopsy results described by the histologic patterns in the modified Marsh classification were examined [19,20]. Symptoms were abstracted from charts using patient description of symptoms documented in the history of present illness as the chief complaint and physician diagnosis documentation in the impression, report and plan. Celiac disease was defined as biopsy with villous atrophy or increased intraepithelial lymphocytes per the modified Marsh criteria and a documented response (symptom improvement) to a gluten-free diet [19]. In the absence of biopsy, CD was diagnosed if there were two positive serology tests – specifically, tissue transglutaminase and endomysial antibodies – along with a documented response to a gluten-free diet. Patients were also included in the cohort if they were given a clinical diagnosis based on symptoms of malabsorption and had a documented response to a gluten-free diet. This last set of patients encompasses a small number of individuals who did not have a biopsy due to already following a gluten-free diet or high risk of pursuing a biopsy secondary to comorbid medical conditions or old age.
Using the Oslo criteria, classical CD was defined as presenting with malabsorption: steatorrhea, diarrhea, or weight loss [1]. Severe celiac disease was defined as the concomitant occurrence of weight loss and diarrhea. Non-classical CD was defined as patients without signs of malabsorption [1]. In order to be non-classical the patients had to have some other symptom documented in the notes such as constipation, abdominal pain, flatulence, nausea, vomiting, ascites, edema, anorexia, anemia, aphthous ulcers, or another item from a list of symptoms. Individual symptoms were defined by patient description and physician interpretation without the use of formal metrics. Asymptomatic CD was defined by a lack of symptoms, but serology and histology consistent with a diagnosis of CD [1].
Laboratory values and serology were extracted up to 2 years before and 1 month after diagnosis with CD.
Height, weight, and body mass index (BMI) were compared to an age- and gender-matched control population.
The study group was analyzed for other autoimmune disorders such as diabetes mellitus type 1, hypothyroidism, dermatitis herpetiformis, and other conditions.
Frequency of depression was investigated by reviewing clinical documentation for a diagnosis of major depressive disorder or depression. Frequency of osteoporosis was measured by clinical documentation and review of bone mineral density scan results when available.
Follow up data on all-cause mortality was collected along with cause of death if known.
Statistical Analysis
Categorical data were presented as count (percentage) and analyzed using Chi-square and Fisher’s exact test where appropriate [21,22]. Logistic regression was used to assess the strength of the association of presenting symptoms and gender [23]. The Kaplan-Meier product limit method was used to estimate survival rates and compare the cohort to the expected survival of the Minnesota white population [24]. Cox regression was used to compare the survival between genders while controlling for age [23]. Statistical significance was defined with an alpha level of .05 without adjusting for multiple comparisons. Statistical analyses were performed using SAS statistical programming language (Version 9.4 SAS Institute Inc.; Cary, North Carolina, USA).
Ethical Issues
This study was approved by the institutional review boards of the Mayo Clinic Foundation (11-005678) and Olmsted Medical Center (046-OMC-11). Patients who declined research authorization were excluded.
Results
Patients
We included 282 incident cases of CD. The cohort was predominantly female (n=183, 65%). Mean age was 46 with a range of 18 to 84.9 years old at diagnosis. Women were diagnosed younger than men, on average at 44.5 years compared to 48.9 years (p=0.030). The vast majority of the study group was Caucasian (93.6%) (Table 1).
Table 1.
Baseline Characteristics of Cohort of Celiac Patients
| 1.M (N=121) |
2.F (N=227) |
Total (N=348) |
p value | |
|---|---|---|---|---|
|
| ||||
| Age | 0.051 | |||
|
| ||||
| N | 121 | 227 | 348 | |
|
| ||||
| Mean (SD) | 41.8 (20.8) | 37.7 (20.1) | 39.1 (20.5) | |
|
| ||||
| Range | (1.2–80.8) | (1.2–84.9) | (1.2–84.9) | |
|
| ||||
| Race | 0.872 | |||
|
| ||||
| White | 112 (92.6%) | 214 (94.3%) | 326 (93.7%) | |
|
| ||||
| Other | 2 (1.7%) | 2 (0.9%) | 4 (1.1%) | |
|
| ||||
| Asian | 1 (0.8%) | 2 (0.9%) | 3 (0.9%) | |
|
| ||||
| Unknown | 6 (5.0%) | 9 (4.0%) | 15 (4.3%) | |
|
| ||||
| Family History | 32 (26.4%) | 61 (26.9%) | 93 (26.7%) | 0.933 |
|
| ||||
| Clinical Presentation: | 0.882 | |||
| - Classical | − 52 (43%) | − 102 (44.9%) | − 154 (44.3%) | |
| - Asymptomatic | − 6 (5%) | − 13 (5.7%) | − 19 (5.5%) | |
| - Non-classical | − 63 (52.1%) | − 112 (49.3%) | − 175 (50.3%) | |
|
| ||||
| Severe Celiac Disease | 11 (9.1%) | 25 (11%) | 36 (10.3%) | 0.583 |
|
| ||||
| Histologic Findings: | 0.072 | |||
| - None | − 10 (10.4%) | − 6 (3.4%) | − 16 (4.6%) | |
| - Partial | − 50 (52.1%) | − 96 (54.9%) | − 146 (42.0%) | |
| - Subtotal | − 6 (6.3%) | − 6 (3.4%) | − 12 (3.4%) | |
| - Total | − 30 (31.3%) | − 67 (38.3%) | − 97 (27.9%) | |
| - Missing | − 25 | − 52 | − 77 | |
|
| ||||
| Prescribed Gluten-Free Diet | 112 (94.1%) | 217 (96.4%) | 329 (94.5%) | 0.602 |
|
| ||||
| TTG - IgA | 0.953 | |||
|
| ||||
| 0.Negative | 9 (9.9%) | 17 (10.1%) | 26 (7.4%) | |
|
| ||||
| 1 .Positive | 82 (90.1%) | 151 (89.9%) | 233 (67%) | |
|
| ||||
| Missing | 30 | 59 | 89 (25.6%) | |
|
| ||||
| TTG - IgG | 0.793 | |||
|
| ||||
| 0.Negative | 32 (62.7%) | 52 (60.5%) | 84 (24.1%) | |
|
| ||||
| 1 .Positive | 19 (37.3%) | 34 (39.5%) | 53 (15.2%) | |
|
| ||||
| Missing | 70 | 141 | 211 (60.7%) | |
|
| ||||
| EMA | 0.402 | |||
|
| ||||
| 0.Negative | 13 (22.4%) | 31 (30.4%) | ||
|
| ||||
| 1 .Positive | 44 (75.9%) | 67 (65.7%) | ||
|
| ||||
| 2.Indeterminate | 1 (1.7%) | 4 (3.9%) | ||
|
| ||||
| Missing | 51 | 108 | ||
|
| ||||
| Unknown | 12 (0.0%) | 17 (0.0%) | ||
Kruskal Wallis
Fisher Exact
Chi-Square
Clinical Presentation
Non-classical presentation of celiac disease was the most common mode of presentation (n=139; 49.3%) and was similar between genders (females, 48.6%; males, 50.5%; p=0.92). Asymptomatic presentation was seen in about 5.7% of patients equally common in the genders. Severe celiac disease was seen in 12% of females and 9.1% of males (p=0.45). On histologic samples, the amount of villous atrophy was similar between genders. Over 90% of patients were prescribed a gluten-free diet (females, 96.1%; males, 92.9%; p=0.47). The remaining patients did not have the diet recommendations documented by the physician or were already documented as following a gluten-free diet.
Symptoms
Abdominal pain was the most common presenting symptom seen in 112 (39.7%) subjects. Constipation was seen more often in women (OR 2.33; 95% CI 1.06–5.12; p=0.035). Gender differences were not observed with respect to other presenting symptoms as other confidence intervals overlapped 1. Approximately 37% of women and 25% of men were anemic (OR 1.71; 95% CI 0.99–2.95; p=0.053), which was the third most common presenting symptom after abdominal pain and diarrhea respectively (Figure 1).
Figure 1. Gender-Specific Prevalence of Symptoms.
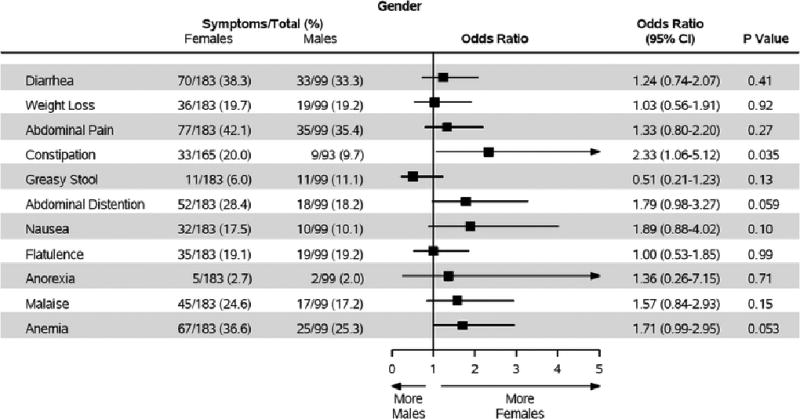
Compares the odds ratios of different symptoms of presentation of celiac disease between men and women.
Laboratory and Serology
Serology results were similar between genders. A significant amount of data was missing. Tissue transglutaminase (TTG) IgA was the most commonly performed test on 204 (72%) subjects and was often positive (females, 90.8%; males, 89.0%; p=0.68). TTG IgG positivity was around 31% (females 32.8%; males 18.6%; p=0.64) in those who had measurements (n=106, 37.6%). Endomysial antibody was measured in 124 (44%) patients with similar positive results between genders (females, 66.3%; males 77.3%; p=0.47) (Table 1).
Low ferritin was more frequent in women (females, 56.3%; males, 35.5%; p=0.009). Otherwise, vitamin B12, folate, iron, and ALT levels were comparable.
Anthropomorphic Measurements
Women were shorter and weighed less than men with an overall Body Mass Index (BMI) that was lower. After adjusting for controls using the National Health and Nutrition Examination Survey (NHANES) white population, there was not a significant difference in height, weight, or BMI in the celiac population compared to controls (Table 2).
Table 2.
Anthropometric Measurements at Diagnosis
| Median Values | Unadjusted | Adjusted (NHANES controls) Z- scores |
||||
|---|---|---|---|---|---|---|
| Females | Males | P-value | Females | Males | P-value | |
| Weight (kg) | 61.3 | 78.5 | <0.0001 | −0.2 | 0.0 | 0.09 |
| Height (cm) | 162.8 | 177.8 | <0.0001 | 0.3 | 0.3 | 0.97 |
| BMI | 22.5 | 25.2 | 0.01 | −0.3 | −0.1 | 0.36 |
Wilcoxon
Autoimmune Diseases
Autoimmune disease was seen in 89 (31.6%) subjects. The presence of at least one concurrent autoimmune disease was similar between genders (females, 32.2%; males, 30.3%; p=0.74). The most common autoimmune disease seen in 14.5% of subjects was hypothyroidism (females, 17.5%; males, 9.1%; p=.061). Twelve (4.3%) subjects had two, and two (0.7%) subjects had three concurrent autoimmune diseases. There were no significant differences between men and women (Figure 2).
Figure 2. Odds Ratios for Concurrent Autoimmune Disease.
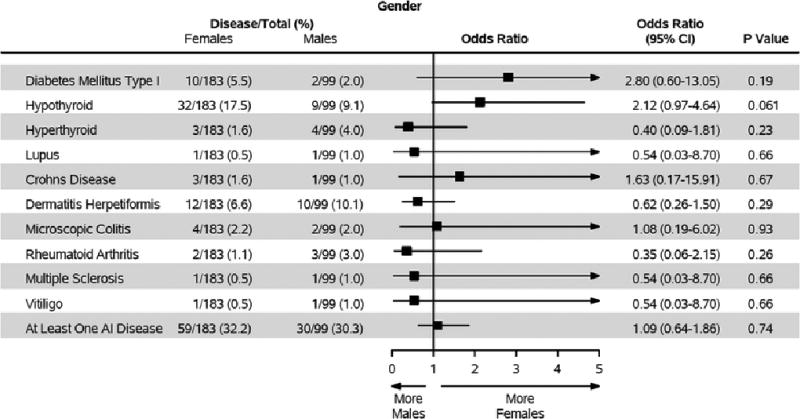
Compares the odds ratios of different associated autoimmune conditions co-occurring with celiac disease between men and women.
Depression and Osteoporosis
Depression was diagnosed in 60 (21.3%) subjects (females, 24.0%; males, 16.2%; p=0.12), with 12 of the 60 cases discovered after CD diagnosis. There was no difference between men and women (before diagnosis p=0.23, after diagnosis p=0.55). To validate the physician diagnosis of depression, medication lists were reviewed. The vast majority of patients (54 of 60) were on medical therapy such as a selective serotonin reuptake inhibitor (SSRI). Numerous patients were also referred for counseling or visited with specialists in psychiatry per the medical record.
Osteoporosis was found in 34 (12.1%) subjects and osteopenia in 7 (2.5%) subjects. A total of 101 of the 183 women were tested before or within a year of diagnosis and 47 of the 99 men. Amongst women >50 years old in the study, the rate of osteoporosis was 35.5% compared to 15.6% of men >50 years old. The mean Z-score for women <50 was 0.0, women >50 was −0.6, men <50 was −0.4 and men >50 was −0.8.
Mortality
Twenty-three subjects expired during follow-up, 12 males and 11 females. Using the reverse Kaplan-Meier method, the median follow-up time was 8.80 years (95% CI 7.60–9.60). Survival was lower among men than women (unadjusted HR 2.54; 95% CI 1.09–5.88; p=0.285; age adjusted HR 3.00; 95% CI 1.26–7.21; p=0.014) which is consistent with the general population (Figure 3). These survival rates were within the expected range for the Minnesota white population used as a control (study population was 94% Caucasian) (Figure 4). Cancer was the most common cause of death documented in seven individuals. Types of cancer included lung (multiple patients), pancreatic, prostate, T-cell non-Hodgkin lymphoma, and small bowel adenocarcinoma. Vascular disease including myocardial infarction, cerebrovascular accident and others accounted for five deaths. Dementia was responsible for three deaths and other, such as infection or respiratory failure, for eight deaths. There were two possible celiac disease-related deaths (lymphoma and small bowel adenocarcinoma), one in each gender group.
Figure 3. Kaplan-Meier Curve for Mortality.
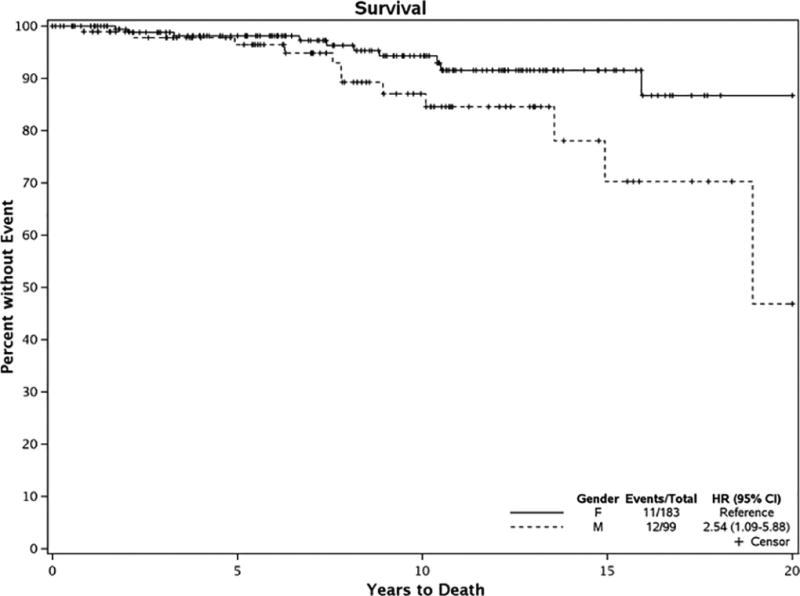
Is a Kaplan-Meier curve comparing survival of men and women in the cohort of patients with diagnosed celiac disease.
Figure 4. Kaplan-Meier Curves Comparison.
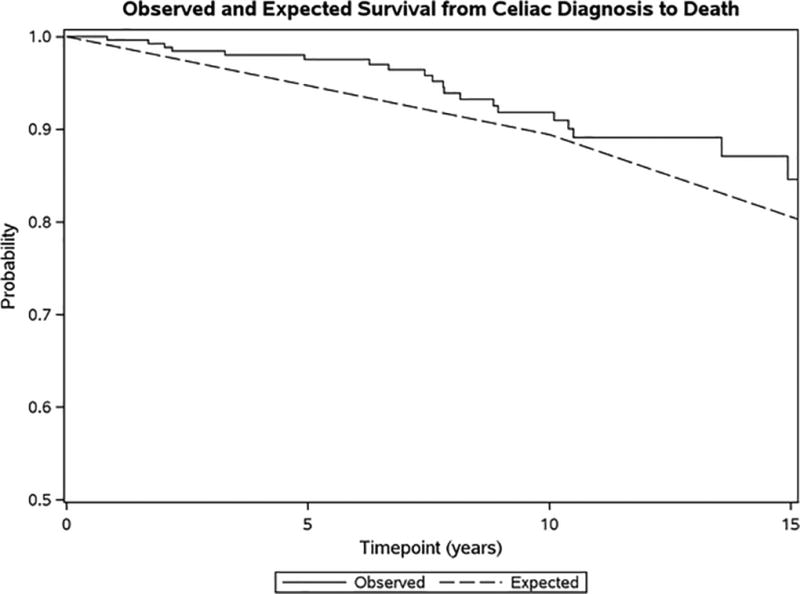
Compares observed versus expected survival in the cohort of patients with diagnosed celiac disease compared to controls (Minnesota white population).
Discussion
The principal findings of this population-based study include: 1) women are diagnosed more frequently and at an earlier age, 2) women are more likely to present with constipation, 3) women were more likely to have a low ferritin before diagnosis, 4) other autoimmune diseases were common in both genders, 5) rates of osteoporosis and depression in CD were much higher than the national average for both men and women, and 6) mortality in males was higher than in females, but not in excess compared to the general population.
This study confirms the female predominance in diagnosed CD as 65% of the sample was female (1.85:1 ratio). Women were diagnosed at a younger age, approximately 4 years earlier than their male counterparts. This could represent a difference in the natural history of CD or women seeking medical care earlier in their course of illness.
Women presented with constipation more often, which is theoretically an unusual finding in a traditionally diarrheal illness. Previous studies have also reported constipation as a frequent symptom of CD [25,26]. In an Italian study it was found in 13% of celiac patients, which is similar to our results of 9% of men and 20% of women [25]. Studies on patients with chronic constipation show high rates of CD in both adults and children [26,27,28]. This finding in our study may be confounded by patient reporting of symptoms leading to recall bias as well as other disorders such as pelvic floor dysfunction and irritable bowel syndrome constipation type which predominate in women. Additionally, physiologic differences in pelvic floor anatomy and other factors such as hormones like estrogen and progesterone may factor into the frequency with which constipation is seen in women. Only 36.5% of patients listed diarrhea as their presenting symptom; this proportion is lower than in previous studies (54–85%) [29,30]. This data suggests an increase in awareness of non-classical CD presentations [16]. Abdominal pain was the most frequent symptom seen in both genders.
The ferritin deficiency observed in women could be multifactorial. Women who are menstruating are more prone to iron deficiency and this data could represent a spurious decrease in ferritin levels in the CD population. Over 50% of women had low ferritin and anemia was observed in 36.6% of these women as a main symptom. A previous study indicates that the odds of CD in people with iron deficiency is 28-fold compared to controls [31]. Post-menopausal women and men of any age with low ferritin, a surrogate for iron deficiency, could benefit from CD testing.
The anthropomorphic measurements may suggest that CD diagnosed during adult life may not affect height and weight, as these metrics were similar between the incident cohort and controls.
Uncovering 31.6% of patients with concurrent autoimmune disease is consistent with prior studies showing an increased prevalence of autoimmune conditions in patients with CD [32,33]. This percentage is significantly higher than previous studies and the general population where estimated prevalence is about 3.2% [34]. This study supports the association of CD with an increased risk for concurrent autoimmune diseases. This relationship should be kept in mind during the management of patients with CD in order to diagnose and treat potential coexisting conditions in a timely fashion. The mechanism underlying this increased risk is poorly understood but potentially is related to environmental triggers and shared genetic susceptibility [35].
An increased risk for depression and osteoporosis were observed in this cohort [36]. These numbers indicate that mental and bone health are important components of follow up. Mood assessments should be considered a key aspect of the clinical visit (e.g. PHQ-9). A National Institutes of Health study that looked at 12-month prevalence for major depressive episodes in American adults showed that 6.7% of all adults were clinically depressed and approximately 8.2% of all females and 4.8% of all males [36]. Our findings were about three times the national average within each gender group, consistent with previous CD studies [37,38]. Special consideration should be given to prevention of osteoporosis in younger patients with CD and aggressive treatment of the condition in older patients. The bone health of men appears to be more profoundly impacted according to z-scores, however men were tested less frequently (41% males <50, 48% females <50, 56% males >50, 69% females >50). The national average for women >50 years of age with osteoporosis is 16%, we found a rate of 35.5% [39]. The national average for men is 4%, while our study revealed 15.6% of men >50 with osteoporosis [39]. For women with CD the rate of osteoporosis is greater than twice and for men the rate is almost four times the national average.
Mortality in patients with CD, while different between men and women, was not outside of the expected range for the Minnesota Caucasian population. Previous studies showed modest increases in mortality with CD [40,41]. Other studies showed no major excess mortality compared to the general population [42]. Those patients with minor symptoms had lesser mortality and those patients with poor compliance with the gluten-free diet had increased mortality [43]. It is possible that death rates are affected by treatment with a gluten-free diet. This hypothesis identifies a future direction of research on this cohort which would be monitoring their compliance with a gluten-free diet and the relationship between strictness and mortality.
Limitations of the study include that the majority of study participants were non-Hispanic whites meaning results may not be generalizable to other populations. The sample size of this population was relatively small at 282 patients and therefore may have been underpowered to detect certain associations. Patient descriptions and physician interpretations were used for the presenting symptoms as opposed to formal criteria which could introduce bias. For example, constipation was defined by the physician documentation in the note and did not rely on objective criteria such as Bristol stool form, Rome criteria, or even an informal practical definition of <3 bowel movements per week and subjective sensation of straining – the symptom was listed based on physician judgment. It was certainly helpful and supported the diagnosis when physicians quantified the number of bowel movements in their documentation, but this was not always the case. We were also unable to validate the degree of constipation by reviewing the medication list for laxatives as these are often purchased over the counter and do not appear in the patient’s medical record. Other limitations are related to the retrospective design. Missing laboratory values can also threaten data integrity. Strengths of the study include its population-based nature which limits referral bias. Additionally, this study offers a robust bank of data and the medical record was directly reviewed for clinical details which provides a thorough snapshot of the patient’s CD.
In conclusion, this study seems to indicate more similar qualities between genders than differences when it comes to celiac disease; perhaps we are more alike than unalike.
Acknowledgments
Grant Support: NIH R01-DK57892 (JAM). This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Joseph A. Murray (JAM): grant support from National Institutes of Health, Alvine Pharmaceuticals, and Alba Therapeutics; ongoing support from Oberkotter Foundation and Broad Medical Research Program at CCFA; serves on advisory board of Celimmune, LLC; previous consultant to BioLineRx, GlaxoSmithKline (GSK), Genentech, and Glenmark Pharmaceuticals Ltd; and current consultant to ImunnosanT, Institute for Protein Design (PvP Biologics), Takeda Pharmaceutical Company, Ltd., Innovate Biopharmaceuticals, Inc., and Intrexon.
Footnotes
Conflict of Interest Disclosures: Other authors have no disclosures.
References
- 1.Ludvigsson JF, Leffler DA, Bai J, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013 Jan;62(1):43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012 Oct;107(10):1538–44. doi: 10.1038/ajg.2012.219. [DOI] [PubMed] [Google Scholar]
- 3.Llorente-Alonso MJ, Fernández-Acenero MJ, Sebastián M. Gluten intolerance: sex and age-related features. Can J Gastroenterol. 2006 Nov;20(11):719–22. doi: 10.1155/2006/470273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gujral N, Freeman HJ, Thomson AB. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18(42):6036–6059. doi: 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivarsson A, Persson LA, Juto P, et al. High prevalence of undiagnosed coeliac disease in adults: a Swedish population-based study. J Intern Med. 1999;245:63–68. doi: 10.1046/j.1365-2796.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 6.Gomez JC, Selvaggio GS, Viola M, et al. Prevalence of celiac disease in Argentina: screening of an adult population in the La Plata area. Am J Gastroenterol. 2001;96:2700–2704. doi: 10.1111/j.1572-0241.2001.04124.x. [DOI] [PubMed] [Google Scholar]
- 7.Rutz R, Ritzler E, Fierz W, et al. Prevalence of asymptomatic celiac disease in adolescents of eastern Switzerland. Swiss Med Wkly. 2002;132:43–47. doi: 10.4414/smw.2002.09793. [DOI] [PubMed] [Google Scholar]
- 8.Zanchetta MB, et al. Significant bone microarchitecture impairment in premenopausal women with active celiac disease. Bone. 2015;76:149–157. doi: 10.1016/j.bone.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Arora S, Lal S, et al. Celiac Disease in Women With Infertility: A Meta-Analysis. J Clin Gastroenterol. 2016 Jan;50(1):33–9. doi: 10.1097/MCG.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 10.Cojocaru M, Cojocaru I, Silosi I. Multiple Autoimmune Syndrome. Maedica. 2010 Apr;5(2):132–134. [PMC free article] [PubMed] [Google Scholar]
- 11.Hallert C, Sandlund O, Broqvist M. Perceptions of health-related quality of life of men and women living with coeliac disease. Scand J Caring Sci. 2003 Sep;17(3):301–7. doi: 10.1046/j.1471-6712.2003.00228.x. [DOI] [PubMed] [Google Scholar]
- 12.Bai D, Brar P, Holleran S, et al. Effect of gender on the manifestations of celiac disease: evidence for greater malabsorption in men. Scand J Gastroenterol. 2005;40:183–7. doi: 10.1080/00365520510011498. [DOI] [PubMed] [Google Scholar]
- 13.Ciacci C, Cirillo M, Sollazzo R, et al. Gender and clinical presentation in adult celiac disease. Scand J Gastroenterol. 1995;30:1077–81. doi: 10.3109/00365529509101610. [DOI] [PubMed] [Google Scholar]
- 14.Rubio-Tapia A, Jansson-Knodell CL, Rahim MW, et al. Influence of gender on the clinical presentation and associated diseases in adults with celiac disease. Gac Med Mex. 2016 Oct;152(Suppl 2):38–46. Spanish. [PubMed] [Google Scholar]
- 15.Bardella MT, Fredella C, Saladino V, et al. Gluten intolerance: gender- and age-related differences in symptoms. Scand J Gastroenterol. 2005;40:15–9. doi: 10.1080/00365520410008169. [DOI] [PubMed] [Google Scholar]
- 16.Murray JA, Van Dyke C, Plevak MF, et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003 Jan;1(1):19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Rubio-Tapia A, Van Dyke C, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol. 2013 May;108(5):818–24. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996 Mar;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 19.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992 Jan;102(1):330–54. [PubMed] [Google Scholar]
- 20.Oberhuber G. Histopathology of celiac disease. Biomed Pharmacother. 2000;54:368–72. doi: 10.1016/S0753-3322(01)80003-2. [DOI] [PubMed] [Google Scholar]
- 21.Fleiss JL. Statistical Methods for Rates and Proportions. 2. New York, NY: John Wiley & Sons; 1981. [Google Scholar]
- 22.Altman DG. Practical Statistics for Medical Research. London, England: Chapman & Hall; 1991. [Google Scholar]
- 23.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34(2):187–220. [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 25.Volta U, Caio G, Stanghellini V, et al. The changing clinical profile of celiac disease: a 15-year experience (1998–2012) in an Italian referral center. BMC Gastroenterology. 2014;14(194):1–8. doi: 10.1186/s12876-014-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang HJ, Facio L, Iantorno G, et al. Increased prevalence of celiac disease and positive markers of gluten sensitivity in patients with chronic constipation. Gastroeneterology. 2011 May;140(5):S-444. [Google Scholar]
- 27.Sadjadei N, Hosseinmardy S, Hakimzadeh M, et al. Anti-TTG among children with chronic functional constipation unresponsive to 6 weeks of treatment of constipation. Arq Gastroenterol. 2017;54(3):197–200. doi: 10.1590/S0004-2803.201700000-22. [DOI] [PubMed] [Google Scholar]
- 28.Pelleboer RA, Janssen RL, Deckers-Kocken JM, et al. Celiac disease is overrepresented in patients with constipation. J Pediatr (Rio J) 2012;88(2):173–6. doi: 10.2223/JPED.2155. [DOI] [PubMed] [Google Scholar]
- 29.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126–31. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 30.Talley NJ, Valdovinos M, Petterson TM, et al. Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol. 1994;89:843–6. [PubMed] [Google Scholar]
- 31.Murray JA, McLachlan S, Adams PC, et al. Association between celiac disease and iron deficiency in Caucasians, but not non-Caucasians. Clin Gastroenterol Hepatol. 2013 Jul;11(7):808–14. doi: 10.1016/j.cgh.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosnes J, Cellier C, Viola S, et al. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hepatol. 2008;6:753–8. doi: 10.1016/j.cgh.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–43. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 35.Rubio-Tapia A, Murray JA. Celiac disease beyond the gut. Clin Gastroenterol Hepatol. 2008;6:722–3. doi: 10.1016/j.cgh.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 (HHS Publication No. SMA 15-4927, NSDUH Series H-50) [Google Scholar]
- 37.Ludvigsson JF, Reutfors J, Osby U, et al. Coeliac disease and risk of mood disorders--a general population-based cohort study. J Affect Disord. 2007;99:117–26. doi: 10.1016/j.jad.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Ciacci C, Iavarone A, Mazzacca G, et al. Depressive symptoms in adult coeliac disease. Scand J Gastroenterol. 1998 Mar;33(3):247–50. doi: 10.1080/00365529850170801. [DOI] [PubMed] [Google Scholar]
- 39.Looker AC, Borrud LG, Dawson-Hughes B, et al. Osteoporosis or Low Bone Mass at the Femur Neck or Lumbar Spine in Older Adults: United States, 2005–2008. NCHS Data Brief. 2012 Apr;93:1–8. [PubMed] [Google Scholar]
- 40.Peters U, Askling J, Gridley G, et al. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163(13):1566–1572. doi: 10.1001/archinte.163.13.1566. [DOI] [PubMed] [Google Scholar]
- 41.Ludvigsson JF, Montgomery SM, Ekbom A, et al. Small-Intestinal Histopathology and Mortality Risk in Celiac Disease. JAMA. 2009;302(11):1171–1178. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 42.Abdul Sultan A, Crooks CJ, West J, et al. Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis. Gut. 2015 Aug;64(8):1220–6. doi: 10.1136/gutjnl-2014-308285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrao G, Corazza GR, et al. Mortality in patients with celiac disease and their relatives: a cohort study. The Lancet. 2001;358(9279):356–361. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]


