Attention-Deficit Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder characterized by a constellation of symptoms that includes developmentally inappropriate inattention, impulsivity, and hyperactivity, with symptom onset during childhood (American Psychiatric Association, 2013). ADHD is one of the most common neurodevelopmental disorders, with the prevalence estimated to be between 2.5 and 11 percent in adults and children (American Psychiatric Association, 2013; Fayyad et al., 2007; Visser et al., 2014; Willcutt, 2012). Individuals with ADHD typically experience impairments that affect work, school, and relationships (Able, Johnston, Adler, & Swindle, 2007; de Graaf et al., 2008; Hinshaw, 2002; Wehmeier, Schacht, & Barkley, 2010). Additionally, ADHD can be a stressor to families because children with ADHD tend to be more difficult to parent and are more likely to be injured (Hinshaw, 2002). Finally, ADHD is a societal burden -- the annual societal cost of ADHD was estimated to be $143 - $266 billion (Doshi et al., 2012). The large burden to individuals, families, and society has led to a burgeoning field of research regarding risk factors for ADHD.
Potential Mechanisms Linking Birth Weight and ADHD
Given the developmental nature of ADHD, much research has focused on early life risk factors that may result in adverse neurodevelopmental sequela and the manifestation of symptoms of ADHD, with birth weight emerging as one of the most robust early life risk factors for ADHD. The Developmental Origins of Health and Disease (DOHaD), an important theory related to such research, proposed that a developing fetus may undergo “fetal programming” (prenatal modifications to help the developing fetus adapt to perturbations in the prenatal environment), but such modifications may ultimately leave the fetus ill-equipped for the perinatal environment, resulting in chronic health conditions (Barker, 1998). Originally, DOHaD was advanced to link early development adversity with cardiovascular conditions, but research predicated on DOHaD has expanded to examine developmental origins of psychological symptoms and disorders.
Genetic variation is one of the first factors affecting a developing fetus’s ability to survive, both in-utero and after birth. Therefore, it has been proposed that a common genetic liability may account for the association between birth weight and ADHD. Additionally, twin studies investigating the genetic and environmental contributions to ADHD and birth weight have consistently demonstrated that both traits have a high heritability (70% and 50%, respectively; Derks, Hudziak, & Boomsma, 2009; Faraone et al., 2005; Lunde, Melve, Gjessing, Skjaerven, & Irgens, 2007; Nikolas & Burt, 2010). A study utilizing longitudinal data from the Netherlands Twin Registry examined the extent to which birth weight exerts a causal influence on attention problems by testing the association between birth weight and attention problems in monozygotic twin pairs, dizygotic twin pairs, and unrelated pairs (randomly selected unrelated individuals) discordant for birth weight (Groen-Blokhuis, Middeldorp, van Beijsterveldt, & Boomsma, 2011). Interestingly, the negative association between birth weight and attention problems was stronger in the twin with lower birth weight, and this finding was consistent across monozygotic, dizygotic, and unrelated pairs, indicating genetic factors are likely not driving the association between birth weight and ADHD. Similarly, a twin study utilizing population-level data from Sweden came to the same conclusion, as the associations between birth weight and ADHD symptom ratings were similar for monozygotic and dizygotic twins (Pettersson et al., 2015). Thus, a common genetic liability does not appear to explain the association between ADHD and birth weight, although future work should continue to examine potential mediation or moderation of the association by genetic variations.
Another potential mechanism linking birth weight and ADHD is catch-up growth, or the weight gained in the first years of life. Individuals born low birth weight often receive special nutritional formulas to help them reach normal weight within the first one to two years of life. Despite the positive effects conferred by the nutritional formulas, empirical work has found that rapid catch-up growth is associated with increased risk for diabetes and hypertension and lower IQ (Estourgie-van Burk et al., 2009; Huxley, Shiell, & Law, 2000; Ong, 2007). To investigate this further, Groen-Blokhuis et al. (2011) examined the effect of catch-up growth in twin pairs, as well as unrelated pairs, and found that catch-up growth was not associated with attention problems. However, additional research is required to replicate this finding.
Additionally, a recent review (Smith, Schmidt-Kastner, McGeary, Kaczorowski, & Knopik, 2016) postulated an etiological pathway linking birth weight and ADHD via prenatal ischemia-hypoxia (IH), an insufficient supply of blood and oxygen in utero. As reviewed in the article, prenatal IH is associated with lower birth weight (Henriksen & Clausen, 2002; Kinzler & Vintzileos, 2008) and an increased risk for ADHD (Getahun et al., 2013; Owens & Hinshaw, 2013). Further, prenatal IH is thought to result in epigenetic changes and altered gene expression in the brain as a means of promoting tissue survival (Watson, Watson, McCann, & Baugh, 2010; Wu, Sun, & Li, 2013), but these epigenetic changes may also confer risk for neurodevelopmental problems later in life (Mueller & Bale, 2008; Schmidt-Kastner, van Os, Esquivel, Steinbusch, & Rutten, 2012).
Although the etiological pathways described above may prove fruitful, it is also important to consider confounding factors associated with birth weight and ADHD, and to determine the extent to which the association between birth weight and ADHD may vary by such factors. Clarifying the degree to which confounds may contribute to their association will lead to a better understanding of the relationship between birth weight and ADHD, and indicate which pathways are more likely.
Factors Associated with Birth Weight and ADHD
Several other prenatal risk factors have been identified as risk factors for both low birth weight and ADHD (Silva, Colvin, Hagemann, & Bower, 2014; Sucksdorff et al., 2015; Wiggs, Elmore, Nigg, & Nikolas, 2016). Unfortunately, many of these correlated risk factors are not controlled for in a number of studies, and questions remain regarding specificity of the effect of birth weight, as opposed to other correlated risk factors, on the development of ADHD symptoms.
Gestational age at birth is highly correlated with birth weight, with earlier gestational age correlated with lower birth weight (Kitchen, 1968; Olsen, Groveman, Lawson, Clark, & Zemel, 2010). Several studies have focused on gestational age at birth instead of birth weight as a risk factor for ADHD, and meta-analyses indicate there is indeed a negative association between gestational age and ADHD symptoms (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009; Bhutta, Cleves, Casey, Cradock, & Anand, 2002). In light of these findings, some have suggested that birth weight is merely a proxy for gestational age and does not exert an independent effect on ADHD symptoms.
Similar to gestational age, prenatal exposure to tobacco also has been associated with both birth weight and the development of ADHD (Linnet et al., 2005; Mick, Biederman, Faraone, Sayer, & Kleinman, 2002; Milberger, Biederman, Faraone, Chen, & Jones, 1996; Milberger, Biederman, Faraone, & Jones, 1998), resulting in contentious debate about the harm prenatal smoking may cause not only during the newborn period, but also throughout childhood and adulthood. Thus, given the possibility that prenatal smoking is a causal risk factor for both low birth weight and ADHD, it is possible that the association between birth weight and ADHD symptoms is partially, if not fully, accounted for by exposure to prenatal smoking.
Finally, two related factors, the geographic region of the study and the race of study participants, are often correlated with socio-economic status (Costello, Keeler, & Angold, 2001; National Center for Education Statistics, 2007; World Health Organization, 2009), which is in turn associated with access to prenatal care, birth weight, and risk for development of ADHD (Fiscella, Franks, M.R., & Clancy, 2008; Gray, Edwards, Schultz, & Miranda, 2014; Larsson, Sariaslan, Langstrom, D’Onofrio, & Lichtenstein, 2014; Silal, Penn-Kekana, Harris, Birch, & McIntyre, 2012). Individuals of lower SES typically have reduced access to adequate prenatal care and prenatal nutrition, which increases the likelihood that they will have a child born low birth weight (Gortmaker, 1979; Moore, Origel, Key, & Resnik, 1986; Quick, Greenlick, & Roghmann, 1981; Showstack, Budetti, & Minkler, 1984). Further, inadequate prenatal nutrition has been linked to neurodevelopmental disorders (Susser, Hoek, & Brown, 1998).
In addition to considering factors associated with birth weight and ADHD, it is also important to consider the extent to which the correlation between ADHD and birth weight may vary based on sex, given the large sex difference in the prevalence of ADHD (Akinbami, Xiang, Pastor, & Reuben, 2011; Visser et al., 2014). Previous studies have rarely examined sex as a moderator of the association between birth weight and ADHD symptoms, and the few studies that have examined sex as a moderator have produced mixed results (Momany et al., 2016; Murray et al., 2015). A recent review by Martel (2013) proposed that Sexual Selection Theory could explain the sex difference observed in ADHD. Sexual Selection Theory argues that males have an increased risk of developing ADHD after exposure to early life risk factors such as low birth weight (please see Martel (2013) for a review of sexual selection theory).
Methodological Considerations in Studies of Birth Weight and ADHD
In addition to the factors that may conceptually account (or partially account) for the association between birth weight and ADHD, there are several methodological factors worth considering as well. First, a variety of measures have been used to assess ADHD symptoms (e.g., questionnaire, diagnostic interview), and these measures vary by both method of administration and diagnostic validity (Gordon et al., 2006; Lahey & Willcutt, 2002; Pelham, Fabiano, & Massetti, 2005). Similarly, previous work has indicated the validity of ADHD symptom ratings varies by the informant used (e.g., parent, teacher, self; Erhardt, Epstein, Conners, Parker, & Sitarenios, 1999; Martel, Schimmack, Nikolas, & Nigg, 2015; Powe et al., 1998; Van Voorhees, Hardy, & Kollins, 2011). Methodological variation also exists in the ascertainment of birth weight (i.e. hospital record, parental report, self-report). Retrospective parental reports have been demonstrated to be reliable, even up to 30 years after birth (Catov et al., 2006; Lumey, Stein, & Ravelli, 1994), but retrospective self-reports have not been found to be as reliable (Troy et al., 1996). Age of study participants may also affect the estimate of the association between birth weight and ADHD symptoms since previous work has suggested that individuals manifesting primarily hyperactive-impulsive symptoms or less severe symptoms of ADHD may no longer experience impairment in adulthood (American Psychiatric Association, 2013; Hart, Lahey, Loeber, Applegate, & Frick, 1995; Lara et al., 2009; Leopold et al., 2016; Willcutt, 2012).
Finally, several different study designs have been employed to investigate birth weight and ADHD symptoms. Some studies dichotomize individuals based on birth weight, other studies dichotomize individuals based on ADHD status, and still other studies examine one or both variables as continuous measures. Furthermore, studies that dichotomize individuals based on birth weight or ADHD symptoms often use stringent cut-offs, such as birth weight less than 1500g. Given the influence of sampling from extreme ends of the birth weight and ADHD symptom spectrums may have on the association, it is important to consider the participant recruitment method (e.g., based on birth weight, based on ADHD diagnosis, etc.) when quantifying the association across studies. Similarly, the mean birth weight of the sample provides information about the composition of the sample with regard to birth weight (i.e. primarily participants born low birth weight), and allows for the examination of the association between birth weight and ADHD symptoms across the distribution of birth weight.
The Current Study
As discussed, numerous studies have suggested that there is an association between birth weight and ADHD symptoms. However, a systematic quantification of the association between birth weight and ADHD symptoms is warranted given the variability in effect sizes and study designs, the lack of studies controlling for risk factors correlated with birth weight and ADHD, and the paucity of studies examining sex as a moderator of the association. It should be noted that a meta-analysis examining the association between birth weight and ADHD has been conducted previously (Aarnoudse-Moens et al., 2009), and findings of this study indicated infants born very low birth weight and/or very preterm were more likely to experience attention problems compared to infants born normal birth weight or at term. However, the previous meta-analysis focused solely on the association among very low birth weight and/or very preterm child. Thus, the purpose of the current meta-analysis is to quantify the magnitude of the overall association between birth weight and ADHD across the birth weight and ADHD symptomology spectrums, and to determine whether there are moderators of this association. It is hypothesized that there will be a small, but significant, association between birth weight and ADHD symptoms, such that lower birth weight is associated with greater ADHD symptoms. Further, because previous literature has indicated there is a dose-response relationship between birth weight and ADHD symptoms, it is hypothesized that the association will be moderated by the mean birth weight of the sample, such that samples with a lower mean birth weight have a larger (more negative) effect size. Additionally, given the recent theoretical argument that males may be particularly susceptible to early life risk factors that confer risk for ADHD (Martel, 2013), it is hypothesized that the association will be moderated by sex, with samples that have a higher percentage of males exhibiting larger effect sizes. Finally, given previous empirical evidence that birth weight is a robust risk factor for ADHD, it is hypothesized that the association will not differ when including variables correlated with birth weight and ADHD as moderators (i.e., gestational age and prenatal tobacco exposure).
METHOD
Sample of Studies
Studies were identified for the current meta-analyses by conducting searches through May 2016 in the Medline (PubMed), PsychInfo, and ProQuest Dissertation and Theses databases. The search terms “birth weight” and “birthweight” were used in pairwise combination with the terms “attention deficit hyperactivity disorder”, “attention deficit disorder”, “hyperkinetic disorder”, “hyperkinesis”, “attention problems”, “inattention”, “impulsivity”, and “hyperactivity.” The terms could appear anywhere in the text for the searches conducted in the PubMed and Psychinfo databases, but the terms were required to be keywords for the ProQuest Disseration and Theses database. To further identify eligible studies, a backward search was conducted from the reference list of a previous meta-analysis that examined neurobehavioral outcomes in extremely low birth weight infants (Aarnoudse-Moens et al., 2009).
Inclusion criteria
Studies had to meet the following criteria to be included in the meta-analysis: (1) studies were empirical and published in the English language in a peer-reviewed journal or were a component of a dissertation or thesis (literature reviews, meta-analyses, and case studies were not included), (2) studies included a measure of birth weight, (3) studies included a measure of ADHD symptoms or diagnosis (parent report of previous diagnosis, diagnosis from a medical record, diagnostic interview, or questionnaire assessing ADHD symptoms) and (4) studies examined the relationship between birth weight and ADHD in human subjects.
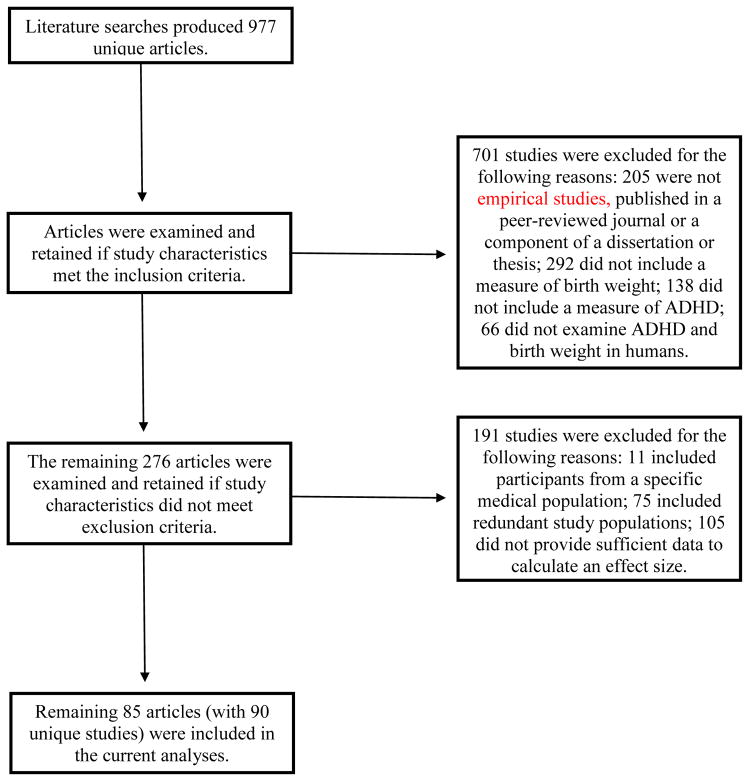
The literature search produced 977 unique studies. Article titles and abstracts were initially examined for inclusion in the meta-analysis by the main coder (AMM). Manuscripts were reviewed if it was unclear whether the study met inclusion criteria based on the article title and abstract. Of the 977 unique studies identified, 701 studies did not meet inclusion criteria per the main coder (see Figure 1). An independent rater (JMK) examined 10 percent of the identified studies to determine study inclusion with adequate reliability (κ = .65, percentage agreement = 87%). Any disagreements were identified by both coders and discussed; in all cases, agreement was reached among discussion.
Figure 1.
Flow chart of studies excluded from current analyses.
Additionally, studies were excluded if the participants in the study were part of a specific medical population (e.g., fetal alcohol syndrome, velocardiofacial syndrome), if the study population had already been reported on in another study (the study with the largest sample size was retained), or if the study did not provide sufficient data to calculate an effect size. Several study population characteristics were used to ensure study populations did not overlap, and these were: the dates of recruitment, cohort name if applicable (e.g., TRAILS study), and the city and country from which participants were recruited. If a study did not provide sufficient data to calculate an effect size, the corresponding author was contacted to obtain this information (21% response rate). This resulted in 94 unique effect sizes (N = 4,645,482) in the current meta-analysis. Of the studies included, five studies (n = 1,580) were unpublished dissertations or theses and, therefore, have not undergone formal peer-review (Adger-Antonikowski, 2009; Duris, 2002; Fazio, 2008; Johnson-Cramer, 1999; Williams, 2010).
Management of non-independent samples
Studies had non-independent data for several reasons. First, studies often had more than one measure of ADHD symptoms (e.g., self-report of symptoms and clinician diagnosis). Second, studies had non-independent data if multiple informants were included in the study. Finally, some studies were longitudinal and therefore included data on ADHD symptoms at multiple time points, resulting in non-independent data. For several of the studies with non-independent samples, one of the measures or one of the time points in the longitudinal studies had a larger sample size. In these cases, these data were used to calculate the effect size. If the sample size was the same for multiple time points and the same type of measure (e.g. two questionnaires) was used at each time point, an average effect size was calculated. However, if the sample size was the same at multiple time points, but different types of measures (e.g. parental questionnaire and interview) were used at each time point, the effect size from the time point that used the measure with the highest diagnostic validity was used. Hence, DSM-based diagnostic interviews or clinical diagnoses were utilized over informant and self-report questionnaires and informant report measures were utilized over self-report measures (Erhardt et al., 1999; Powe et al., 1998; Van Voorhees et al., 2011). Finally, some studies included data on two informant-report measures (i.e., teacher and parent or mother and father) with the same sample size. In these cases, an average effect size was calculated (for procedures see Borenstein, 2009).
Coding Procedures
Variables coded included the following (see moderator variables section below for additional information): mean gestational age of the sample, percent exposed to prenatal tobacco, country, race (i.e. percent Caucasian, African American, Asian, and Latino), sex (percentage of sample that is male), ADHD measurement method, informant who provided ratings of ADHD symptoms, mean age at assessment of ADHD symptoms, birth weight source, sample type, mean birth weight of the sample, and test statistics. For each study, an effect size r (Pearson’s correlation) was calculated for the association between birth weight and ADHD symptoms. Effect sizes calculated from unadjusted statistics were used when possible; however statistics adjusted for various covariates were included if unadjusted statistics were not reported.
All studies were initially coded by the main coder (AMM), who also developed the coding procedure. This rater trained the independent rater (JMK) on coding procedures, and the independent rater coded 10 percent of the included studies (effect size estimates, sample sizes, and the twelve moderator variables) with adequate reliability (κ = 0.6 1, percentage agreement = 70 100%). The raters reviewed coding discrepancies and were able to reach consensus among discussion.
Moderator Variables
Several variables were examined as moderators of the association between ADHD and birth weight. The following moderators were analyzed as continuous variables: percent of participants in a study exposed to any prenatal tobacco, percent of the sample that was male, and the percent of the sample that identified as Caucasian, African American, Asian, and Latino. Importantly, race was analyzed within countries, as opposed to across all countries, because of the different meanings race may have in different countries.
Several variables were examined as categorical moderators: geographic region, ADHD measurement method, informant of ADHD symptoms, sample type, and method of birth weight ascertainment. Geographic region was coded as the continent from which the participants were recruited, as the number of studies from any one country was too small for analysis. ADHD measurement method was classified as “medical record” if the study used ADHD diagnostic status as indicated in a medical or school record, “ interview” if the study employed structured or semi-structured diagnostic interviews with the participant or with a parent, “questionnaire” if the study used questionnaire measures filled out by a parent, a teacher, or the participant, and “multiple methods” if the study utilized more than one measurement method (e.g., questionnaire and interview) to assess ADHD symptoms (see Supplemental Table 1 for specific measures used in the included studies). Informant of ADHD symptoms was classified as “clinician rated” if a clinician (e.g., psychologist, psychiatrist, etc.) determined ADHD status or the study utilized diagnostic status from a medical or school record, whereas studies utilizing parental reports, teacher reports, or self-reports of ADHD symptoms were classified as “parent rated,” “teacher rated,” and “self rated,” respectively. Studies that used ratings from multiple informants (e.g., parent and teacher) to determine ADHD status were classified as “multi-person rated.” Sample type was coded as “birth weight based” if studies compared individuals born low birth weight to individuals born normal birth weight, “ADHD based” if studies dichotomized individuals based on ADHD diagnostic status, “community based” if participants were recruited from the community, and “population based” if the study utilized large databases containing medical information on all individuals born in a particular country, county, or city during a specified time period. Finally, studies that extracted birth weight data from medical records were categorized as “medical record,” studies relying on retrospective parental report or self-report were categorized as “parental report” or “self-report,” respectively, and studies that used more than one method to ascertain birth weight (i.e., medical record and parental report) were categorized as “multiple methods.”
Mean gestational age of the sample, the mean birth weight of the sample, and mean participant age at ADHD assessment were analyzed as continuous and categorical variables. Studies were categorized as term (>=37 weeks), moderate preterm (28 weeks <gestational age <37 weeks), and very preterm (<=28 weeks; World Health Organization, 1992). Similarly, the mean birth weight of the sample was categorized into normal birth weight (>=2500g), low normal birth weight (1500g>birth weight>2500g), and very low birth weight (<=1500g; World Health Organization, 1992). Finally, age at ADHD assessment was binned into four groups for categorical analysis: 3–5.9 years, 6–8.9 years, 9–12.9 years, and 13–20 years.
Analytic Strategy
Analyses were conducted using the metafor package in R (Viechtbauer, 2010). First, analyses were conducted to examine potential publication bias. Then, analyses were employed to identify studies that were potential outliers. Following these analyses, average effect sizes were calculated for all 94 studies (including unpublished studies) included in the current meta-analysis and for the 89 studies that were published. Additionally, average effect sizes were calculated for the following subsets: studies that reported effect sizes adjusted for various covariates, all studies that reported unadjusted effect sizes (unpublished dissertations and theses included), and studies that reported unadjusted effect sizes and had undergone formal peer-review (unpublished dissertations and theses excluded). None of the adjusted studies were unpublished, and thus separate analyses with and without unpublished studies were not necessary for the adjusted studies. Finally, the twelve moderators detailed above were examined.
Publication Bias
The trim-and-fill method (Duval & Tweedie, 2000) was used to evaluate the impact of publication bias and to address the “file drawer problem” (Rosenthal, 1979). This method provides an estimate of the number of missing studies, as well as an adjusted effect size that includes the filled studies. Additionally, a test of funnel plot asymmetry was conducted using Egger’s Test of the Intercept (Egger, Davey Smith, Schneider, & Minder, 1997) which provided a statistical test of the relation between sample size and effect size.
Outlier Analyses
Several analyses were employed in an attempt to detect outliers that may be affecting the results, but a study was only excluded from analysis if it was an outlier in all three of the outlier analyses. First, standardized residuals for each study were derived and any study with a standardized residual with an absolute value greater than three was considered an outlier (Viechtbauer & Cheung, 2010). The second method was a visual examination of a forest plot from a leave-one-out analysis, with outliers indicated by the effect size deviating a significant amount from the other effect sizes when a particular study is omitted (Viechtbauer, 2010; Viechtbauer & Cheung, 2010). The third method was to generate a forest plot of increasing effect sizes. Any study that had a confidence interval that did not overlap with other study confidence intervals was considered an outlier.
Meta-analytic strategy
Average effect sizes were calculated using the ‘rma’ function, which fit meta-analytic random and mixed effects models, and the method was specified to be DerSimonian-Laird (DerSimonian & Laird, 1986). A test for residual heterogeneity was also conducted by the ‘rma’ function, resulting in a Q-statistic (Cochran, 1954), which tested whether the variability in the observed effect size is larger than expected based on the sampling variability. A significant test suggested that the true outcomes were heterogeneous, warranting the examination of moderators. Additionally, the I2 statistic, which quantified the percentage of variation representing true heterogeneity as opposed to sampling error, was calculated and included, as I2 is less sensitive to the number of studies included in the analysis (Higgins & Thompson, 2002; Higgins, Thompson, Deeks, & Altman, 2003).
Moderator Analyses
Studies that only provided statistics adjusted for covariates were not included in moderator analyses. To examine the effect of moderators, mixed-effect models were employed, with continuous and categorical moderators specified using the ‘mods’ argument (categorical moderators used the ‘factor’ function within the ‘mods’ argument). Continuous moderators were tested using meta-regression techniques and categorical moderators were tested one at a time, similar to an ANOVA. Once moderators were included in the model, a Qm-test was utilized to test whether variability in the observed outcomes was significantly accounted for by variability in the moderators. The amount of variance in effect sizes accounted for by each moderator (R2) was estimated. Finally, moderator analyses were conducted with and without the unpublished studies included to determine the extent to which the unpublished studies may be influencing the results.
RESULTS
Study information, including sample size and data on moderator variables for studies with unadjusted effect sizes is presented in Appendix 1. Sample size and covariates included in analysis for studies with adjusted effect sizes are presented in Appendix 2.
Appendix 1. Unadjusted studies included in the meta-analysis.
| Study | N Birth Weight (g) |
Sample Type |
Geographic Region |
Measure Type |
Informant | Birth Weight Source |
Age (years) |
Birth Weight (g) | Male (%) |
Caucasian (%) |
Non-Caucasian (%) |
Gestational Age (weeks) |
Tobacco (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adger-Antonikowski, 2009 | 68 | BW | N. America | Questionnaire | Parent | Parent report | 4.3 | 1497 | 41 | 83 | 10 | 34 | --- |
| Anderson et al., 2011 | 362 | BW | Australia/NZ | Questionnaire | Parent | Medical record | 8.1 | 2111 | 53 | --- | --- | 33 | --- |
| Anderson et al., 2003 | 498 | BW | Australia/NZ | Questionnaire | Parent | Medical record | 8.8 | 2014 | 47 | --- | --- | 32 | --- |
| Andreias et al., 2010 | 359 | BW | N. America | Questionnaire | Parent | Medical record | 8 | 2031 | 38 | --- | --- | --- | --- |
| Asbury et al., 2006 | 938 | Community | Europe | Questionnaire | Teacher | Parent report | 7 | --- | 100 | --- | --- | --- | --- |
| Asbury et al., 2006 | 1104 | Community | Europe | Questionnaire | Teacher | Parent report | 7 | --- | 0 | --- | --- | --- | --- |
| Beverly et al., 2008 | 45 | Community | N. America | Questionnaire | Parent | Medical record | 11 | --- | 44 | --- | --- | --- | --- |
| Bora et al., 2011 | 212 | BW | Australia/NZ | --- | Multiple | Medical record | 6 | 2343 | 53 | --- | --- | 34 | --- |
| Botting et al., 1997 | 284 | BW | Europe | Interview | Parent | Medical record | 12 | --- | 65 | --- | --- | --- | --- |
| Boulet et al., 2011 | 87,578 | Population | N. America | Medical record | Parent | Parent report | --- | --- | 51 | 78 | 15 | --- | --- |
| Breeman et al., 2016 | 488 | BW | Europe | Interview | Parent | Parent report | 7 | 2275 | 50 | --- | --- | --- | --- |
| Breslau et al., 1996 | 414 | BW | N. America | --- | Multiple | Medical record | 6 | --- | 44 | 23 | 77 | --- | 35 |
| Breslau et al., 1996 | 409 | BW | N. America | --- | Multiple | Medical record | 6 | --- | 53 | 92 | 8 | --- | 27 |
| Chu et al., 2012 | 407 | ADHD | Asia | Medical record | Clinician | Medical record | 9 | 3186 | 79 | --- | --- | 38 | --- |
| Class et al., 2014 | 3,292,773 | Population | Europe | Medical record | Clinical | Medical record | --- | --- | 52 | --- | --- | 40 | --- |
| Conrad, Richman, Lindgren, & Nopoulos, 2010 | 104 | BW | N. America | --- | Multiple | Medical record | 11.5 | 2365 | 51 | 88 | --- | --- | --- |
| Crea et al., 2014 | 449 | Community | N. America | Questionnaire | Parent | Parent report | 16.7 | 3161 | 51 | 55 | 32 | --- | --- |
| Davis, Burns, Snyder, & Robinson, 2007 | 94 | BW | N. America | Questionnaire | Parent | Medical record | 5.3 | 1010 | 44 | 70 | 20 | 28 | --- |
| de Zeeuw et al., 2012 | 88 | ADHD | Europe | Interview | Parent | Parent report | 10 | 3493 | 86 | --- | --- | 40 | 17 |
| Duris, 2002 | 117 | BW | N. America | Medical record | Clinician | Parent report | 8 | 2316 | 51 | 52 | 48 | 35 | --- |
| Elgen et al., 2013 | 268 | BW | Europe | Interview | --- | --- | 19 | --- | --- | --- | --- | --- | --- |
| Emond et al., 2008 | 164 | BW | S. America | --- | Multiple | Medical record | 8.2 | 2774 | --- | --- | --- | 39 | --- |
| Fazio, 2012 | 588 | ADHD | N. America | Interview | Parent | --- | 8.5 | --- | 70 | 90 | --- | --- | 26 |
| Frederick, 2012 | 50 | Community | N. America | Questionnaire | --- | --- | --- | 3500 | 100 | --- | --- | --- | --- |
| Galera et al., 2011 | 1665 | Community | N. America | Questionnaire | Parent | Medical record | --- | --- | 51 | --- | --- | --- | --- |
| Groen-Blokhuis et al., 2011 | 6631 | Population | Europe | Questionnaire | Parent | Parent report | 3 | --- | 0 | --- | --- | --- | --- |
| Groen-Blokhuis et al., 2011 | 6661 | Population | Europe | Questionnaire | Parent | Parent report | 3 | --- | 100 | --- | --- | --- | --- |
| Grunau et al., 2004 | 84 | BW | N. America | Questionnaire | Parent | --- | 17.5 | 1748 | 60 | --- | --- | 31 | --- |
| Hack et al., 2004 | 207 | BW | N. America | Questionnaire | Parent | Medical record | 20 | --- | 100 | --- | --- | --- | --- |
| Hack et al., 2004 | 235 | BW | N. America | Questionnaire | Parent | Medical record | 20 | --- | 0 | --- | --- | --- | --- |
| Halmoy, Klungsoyr, Skjaerven, & Haavik, 2012 | 1,150,705 | ADHD | Europe | Medical record | Clinician | Medical record | --- | --- | 51 | --- | --- | --- | --- |
| Hanc et al., 2015 | 615 | ADHD | Europe | Medical record | Clinician | Medical record | 10.5 | 3383 | 100 | --- | --- | --- | --- |
| Hanke et al., 2003 | 136 | BW | Europe | Questionnaire | Parent | Medical Record | 6.2 | 45 | --- | --- | --- | --- | |
| Hatch, Healey, & Halperin, 2014 | 197 | Community | N. America | --- | Multiple | Parent report | 4.3 | 3288 | 74 | 40 | 42 | --- | --- |
| Huang et al., 2012 | 84 | BW | Asia | Questionnaire | Parent | --- | --- | --- | 59 | --- | --- | --- | --- |
| Hultman et al., 2007 | 1055 | Population | Europe | Questionnaire | Parent | Medical record | 8.5 | --- | 100 | --- | --- | --- | --- |
| Hultman et al., 2007 | 1066 | Population | Europe | Questionnaire | Parent | Medical record | 8.5 | --- | 0 | --- | --- | --- | --- |
| Indredavik et al., 2010 | 146 | BW | Europe | Interview | Parent | Medical record | 14 | 2753 | 48 | --- | --- | 35 | --- |
| Jaspers et al., 2013 | 1664 | ADHD | Europe | Questionnaire | Parent | Medical record | --- | --- | 48 | --- | --- | --- | 31 |
| Johnson-Kramer, 1999 | 36 | BW | N. America | Medical record | Clinician | Medical record | 10.4 | 1787 | 100 | --- | --- | --- | --- |
| Kadziela-Olech & Piotrowska-Jastrzebska, 2005 | 100 | ADHD | Europe | Questionnaire | Parent | Parent report | 7.4 | --- | 85 | --- | --- | --- | --- |
| Knopik et al., 2005 | 5,872 | Population | N. America | Interview | Parent | Parent report | 14.4 | --- | 0 | 87 | --- | --- | 37 |
| Kreppner, O’Connor, & Rutter, 2001 | 214 | Community | Europe | --- | Multiple | Medical record | 6 | --- | 50 | --- | --- | --- | --- |
| Lahti et al., 2006 | 267 | Community | Europe | Questionnaire | Parent | Medical record | --- | 3573 | 46 | --- | --- | --- | 9 |
| Langley et al., 2007 | 356 | ADHD | Europe | Interview | Parent | Parent report | 9.2 | 3286 | 90 | --- | --- | --- | 46 |
| Linnet et al., 2005 | 4,105 | Population | Europe | Medical record | Clinician | --- | --- | --- | --- | --- | --- | --- | 36 |
| McCormick, Gortmaker, & Sobol, 1990 | 10,522 | Population | N. America | Questionnaire | Parent | Parent report | --- | --- | --- | --- | --- | --- | --- |
| McGrath et al., 2005 | 184 | BW | N. America | Questionnaire | Parent | Medical record | 4 | 1742 | 50 | 88 | 12 | 32 | --- |
| Melchior et al., 2015 | 1,113 | Community | Europe | Questionnaire | Parent | Medical record | 5 | --- | 53 | --- | --- | --- | 21 |
| Mick et al., 2002 | 483 | ADHD | N. America | Interview | Parent | Parent report | 11.7 | --- | 53 | 96 | --- | --- | 14 |
| Minde, Webb, & Sykes, 1968 | 112 | ADHD | N. America | Medical record | Clinician | Medical record | 9.8 | 3250 | 89 | --- | --- | --- | --- |
| Mitchell, Aman, Turbott, & Manku, 1987 | 97 | ADHD | Australia/NZ | --- | Multiple | Parent report | 8.9 | 3236 | 85 | --- | --- | --- | --- |
| Momany et al., 2016 | 773 | ADHD | N. America | --- | Multiple | Parent report | 12.3 | 3398 | 56 | 74 | 16 | --- | 10 |
| Morales, Polizzi, Sulliotti, Mascolino, & Perricone, 2013 | 120 | BW | Europe | Questionnaire | Parent | Parent report | 5.2 | 2463 | 46 | --- | --- | 36 | --- |
| Murray et al., 2015 | 3,748 | Population | S. America | Questionnaire | Parent | Medical record | 4 | --- | 52 | --- | --- | --- | 27 |
| Nosarti, Allin, Frangou, Rifkin, & Murray, 2005 | 116 | BW | Europe | Questionnaire | Parent | Medical record | 15 | 2300 | 55 | --- | --- | 34 | --- |
| O’Callaghan & Harvey, 1997 | 87 | BW | Australia/NZ | --- | Multiple | Medical record | 10.7 | 860 | 31 | --- | --- | 27 | --- |
| Offord, Sullivan, Allen, & Abrams, 1979 | 63 | ADHD | N. America | Interview | Parent | --- | 12.5 | 3315 | 100 | --- | --- | --- | --- |
| Parker, Collett, Speltz, & Werler, 2016 | 489 | Community | N. America | Questionnaire | Multiple | Parent report | 7 | 3429 | 49 | 75 | 24 | 39 | 16 |
| Perkinson-Gloor et al., 2015 | 105 | BW | Europe | Questionnaire | Parent | --- | 8.3 | 2235 | 66 | --- | --- | 34 | 7 |
| Razza et al., 2016 | 751 | Community | N. America | Questionnaire | Teacher | Medical record | 6 | --- | 48 | --- | --- | --- | --- |
| Rice et al., 2010 | 779 | Community | --- | Questionnaire | Parent | --- | 6.7 | 3074 | --- | --- | --- | --- | 5 |
| Rickards, Kelly, Doyle, & Callanan, 2001 | 161 | BW | Australia/NZ | Questionnaire | Teacher | Medical record | 14 | 1740 | 56 | --- | --- | 32 | --- |
| Ross, Lipper, & Auld, 1992 | 168 | BW | N. America | Medical record | Clinician | --- | 7.5 | --- | 52 | 51 | 45 | --- | --- |
| Sasaluxnanon & Kaewpornsawan, 2005 | 241 | ADHD | Asia | --- | Multiple | Parent report | 8.8 | --- | 88 | --- | --- | --- | --- |
| Sato et al., 2004 | 80 | BW | Asia | Medical record | Clinician | Medical record | 5 | 1812 | --- | --- | --- | --- | --- |
| Schmitz et al., 2006 | 200 | ADHD | S. America | Interview | Parent | Parent report | 11.8 | 3305 | 68 | --- | --- | --- | 24 |
| Sciberras, Ukoumunne, & Efron, 2011 | 4,464 | Community | Australia/NZ | Questionnaire | Parent | Parent report | 6.8 | --- | 51 | --- | --- | --- | --- |
| Scott et al., 2012 | 259 | BW | N. America | Interview | Parent | Parent report | 6 | 1917 | 46 | 40 | 60 | --- | --- |
| Sengupta et al., 2006 | 191 | ADHD | N. America | Medical record | Clinician | Parent report | 9 | --- | 87 | --- | --- | --- | 51 |
| Simonds & Aston, 1980 | 106 | BW | N. America | Interview | Parent | Medical record | --- | --- | --- | --- | --- | --- | --- |
| Smith et al., 2014 | 398 | ADHD | --- | Questionnaire | Parent | --- | 10.7 | 3389 | 83 | --- | --- | 40 | --- |
| St. Sauver et al., 2004 | 5,631 | Population | N. America | Medical record | Clinician | Medical record | --- | --- | 52 | 98 | --- | --- | --- |
| Strang-Karlsson et al., 2008 | 334 | BW | Europe | Questionnaire | --- | Medical record | 22.5 | 2396 | 41 | 100 | 0 | 35 | 18 |
| Sullivan, Msall, & Miller, 2012 | 180 | BW | N. America | Medical record | Clinician | Medical record | 17.1 | 1805 | 46 | --- | --- | 33 | --- |
| Sykes et al., 1997 | 260 | BW | Europe | Questionnaire | Teacher | Medical record | 7.4 | --- | 0 | --- | --- | --- | --- |
| Sykes et al., 1997 | 182 | BW | Europe | Questionnaire | Teacher | Medical record | 7.4 | --- | 100 | --- | --- | --- | --- |
| Szatmari, Saigal, Rosenbaum, & Campbell, 1993 | 274 | BW | N. America | --- | Multiple | Parent report | 8 | 2163 | 46 | --- | --- | --- | --- |
| Taylor, Klein, Minich, & Hack, 2000 | 164 | BW | N. America | Medical record | Clinician | Medical record | 11.1 | --- | 32 | 55 | --- | --- | --- |
| Van den Bergh & Marcoen, 2004 | 72 | Community | Europe | --- | Multiple | Medical record | 8.5 | 3301 | 53 | --- | --- | --- | --- |
| Van Hus, Potharst, Jeukens-Visser, Kok, & Van Wassenear-Leemhuis, 2014 | 165 | BW | Europe | --- | Multiple | Medical record | 5.2 | 2285 | 45 | --- | --- | 35 | --- |
| Wagner, Schmidt, Lemery-Chalfant, Leavitt, & Goldsmith, 2009 | 748 | Community | N. America | --- | Parent | Medical record | 8.1 | 2503 | 51 | --- | --- | 36 | --- |
| Wiles et al., 2006 | 5,333 | Community | Europe | Questionnaire | Parent | Medical record | 6.8 | 3470 | --- | --- | --- | --- | --- |
| Williams, 2010 | 771 | BW | N. America | Questionnaire | Parent | Medical record | --- | 2292 | 48 | 72 | --- | --- | 29 |
| Yang, Chen, Yen, & Chen, 2015 | 61 | BW | Asia | Interview | Parent | Medical record | 13.4 | 1208 | 52 | --- | --- | 30 | --- |
Appendix 2. Adjusted studies included in the meta-analysis.
| Study | N | Covariates included in estimate of effect size |
|---|---|---|
| Gatzke-Kopp & Beauchaine, 2007 | 117 | Household income, maternal and paternal antisocial symptoms, gestational age, maternal substance abuse & maternal smoke exposure |
| Heinonen et al., 2011 | 893 | Sex |
| Jackson & Beaver, 2015 | 1,266 | Age, sex, race, maternal disengagement, & genetic markers |
| Kelly, Nazroo, McMunn, Boreham, & Marmot, 2001 | 5,181 | Age, sex, birth weight, social class, single parenthood, & smoke exposure |
| Langley et al., 2010 | 1261 | Age & medication use |
| Nadeau, Tessier, Boivin, Lefebvre, & Robaey, 2003 | 162 | Family adversity |
| Pettersson et al., 2015 | 21,775 | Sex & gestational age |
| van Mil et al., 2015 | 6,015 | Sex & age |
Publication Bias and Outlier Analyses
Results of the trim-and-fill analysis examining whether effect sizes were missing from the left side of the mean effect size estimated two effect sizes to be missing. However, the trim-and-fill analysis examining whether effect sizes were missing from the right side of the mean effect size did not indicate any studies to be missing on the right. Egger’s Test of the Intercept did not show evidence of publication bias in the current analyses (z = −1.19, p = .23), and the estimate of the effect size after adjusting for studies missing on the left resulted in a small change in the effect size (change in r = 0.0036). Thus, the degree of publication bias detected in the trim-and-fill analysis of the left side is likely not influencing the estimate of the effect size substantially. The only study found to be an outlier in all three outlier analyses and excluded from subsequent analyses was the study by Astbury and colleagues (1985).
Birth Weight and ADHD
A summary of the effect size estimates and 95% confidence intervals for the overall effects for all studies, adjusted studies only, and unadjusted studies only are presented in Table 1.
Table 1.
Predicted overall effects with 95% confidence intervals from random effects analyses of birth weight and ADHD symptoms
| k | N | r̄ | 95% CI | I2 | |
|---|---|---|---|---|---|
| All Studies | 93 | 4,645,482 | −.15*** | −.16, −.13 | 98.9 |
| All Published Studies | 88 | 4,643,902 | −.15*** | −.17, −.13 | 99.0 |
| Unadjusted Studies | 85 | 4,609,947 | −.14*** | −.16, −.12 | 98.7 |
| Unadjusted Published Studies | 80 | 4,608,367 | −.14*** | −.16, −.12 | 98.8 |
| Adjusted Studies | 8 | 35,535 | −.18* | −.35, −.01 | 99.5 |
Note.
p < .05,
p < .01,
p < .001. CI=confidence interval. Adjusted studies include studies that only reported statistics that had been adjusted for covariates. Unadjusted studies include studies that reported statistics without any adjustment for covariates.
Adjusted and unadjusted studies
The analysis examining the association between birth weight and ADHD symptoms in 93 studies (including unpublished studies) revealed a small, albeit significant, overall meta-analytic effect of r = −.15 (95% CI: [−.16, −.13]), indicating lower birth weight was associated with increased ADHD symptoms. Significant heterogeneity was detected (Q (92) = 8673.9.0, p < .0001 and I2 = 98.9) indicating examination of moderators of the association was warranted. Exclusion of unpublished studies did not significantly alter the overall meta-analytic effect (r = −.15 (95% CI: [−.17, −.13]), and significant heterogeneity was detected (Q (87) = 8646.8, p < .0001 and I2 = 99.0).
Unadjusted studies
The analysis examining the association between birth weight and ADHD symptoms in studies that reported statistics unadjusted for covariates also found a small, but significant, overall meta-analytic effect. This analysis included 85 studies and revealed a meta-analytic effect of r = −.14 (95% CI: [−.16, −.12]), again indicating lower birth weight was associated with increased ADHD symptoms. Significant heterogeneity was still detected when examining only unadjusted studies (Q (84) = 6598.0, p<.0001 and I2 = 98.7). Results remained the same after excluding the five unpublished studies (r = −.14 (95% CI: [−.16, −.12]) and significant heterogeneity remained (Q (79) = 6571.2, p < .0001 and I2 = 98.8).
Adjusted studies
Similar to the analyses for all studies and unadjusted studies only, the analysis examining the association between birth weight and ADHD symptoms in studies that reported statistics adjusted for covariates resulted a significant overall effect of r = −.18 (95% CI: [−.35, −.01]). Eight studies were included in the analysis and significant heterogeneity was detected in the analysis (Q (7) = 1286.4, p < .0001 and I2 = 99.5).
Moderators of the Association Between Birth Weight and ADHD
Each class within a categorical moderator was required to have at least three studies to be included in analyses. Moderator analyses used effect sizes from studies that reported unadjusted statistics. Thus a total of 85 studies were potentially included in analyses of moderators, although the number of studies for any given moderator varied based on availability of relevant data. The results from the categorical and continuous moderator analyses with all unadjusted studies (unpublished included) are presented in Tables 2 and 3, respectively. The results from the categorical and continuous moderator analyses with unpublished studies excluded are presented in Supplementary Tables 1 and 2, respectively. Given that the overall meta-analytic effect size was the same with and without the unpublished studies, and that the moderator analyses resulted in similar results regardless of inclusion of unpublished studies, the moderator analyses discussed below included k = 94 studies (studies from all effect sizes were unadjusted for covariates, k = 89 published and 5 unpublished).
Table 2.
Predicted effects with 95% confidence intervals from mixed effects analyses for categorical moderators including unpublished studies
| k | N | r̄ | 95% CI | QM | p | R2 | |
|---|---|---|---|---|---|---|---|
| Gestational Age | 0.2 | .91 | .00 | ||||
| Very preterm | 4 | 326 | −.15 | −.43, .13 | |||
| Moderate preterm | 15 | 3,516 | −.16 | −.24, −.08 | |||
| Term | 6 | 3,299,845 | −.13 | −.23, −.03 | |||
| Geographic Region | 34.7 | <.0001 | .22 | ||||
| South America | 3 | 4,112 | −.04 | −.07, −.01 | |||
| Asia | 5 | 873 | −.27 | −.49, −.06 | |||
| Australia & New Zealand | 7 | 5,881 | −.13 | −.21, −.05 | |||
| Europe | 31 | 4,477,464 | −.13 | −.16, −.11 | |||
| North America | 37 | 120,440 | −.15 | −.18, −.11 | |||
| ADHD measure | 35.5 | <.0001 | .11 | ||||
| Medical record | 15 | 4,542,862 | −.14 | −.17, −.10 | |||
| Interview | 14 | 9,262 | −.21 | −.29, −.13 | |||
| Questionnaire | 50 | 55,665 | −.11 | −.13, −.08 | |||
| Multiple methods | 6 | 2,158 | −.25 | −.43, −.07 | |||
| Informant | 9.9 | .04 | .24 | ||||
| Parent | 47 | 146,703 | −.13 | −.15, −.10 | |||
| Clinician | 14 | 4,455,284 | −.15 | −.18, −.11 | |||
| Teacher | 6 | 3,396 | −.13 | −.19, −.08 | |||
| Multiple | 15 | 3,912 | −.18 | −.29, −.07 | |||
| Self | 3 | 652 | −.13 | −.44, −.17 | |||
| Age | 0.2 | .98 | .00 | ||||
| 3–5.9 years | 13 | 20,071 | −.14 | −.19, −.08 | |||
| 6–8.9 years | 30 | 21,436 | −.16 | −.21, −.11 | |||
| 9–12.9 years | 17 | 4,406 | −.15 | −.23, −.07 | |||
| 13–20 years | 12 | 8,113 | −.16 | −.24 −.08 | |||
| Birth Weight | |||||||
| Ascertainment | 0.5 | .49 | .19 | ||||
| Parental report | 26 | 128,760 | −.15 | −.18, −.12 | |||
| Medical or birth record | 48 | 4,474,495 | −.13 | −.15, −.10 | |||
| Sample Type | 43.4 | <.0001 | .05 | ||||
| ADHD based | 16 | 6,376 | −.18 | −.27, −.08 | |||
| Birth Weight based | 40 | 8,546 | −.20 | −.24, −.15 | |||
| Community based | 17 | 18,678 | −.06 | −.09, −.04 | |||
| Population based | 12 | 4,576,347 | −.09 | −.12, −.06 | |||
| Birth Weight | 5.6 | .06 | .20 | ||||
| Very low birth weight | 4 | 310 | −.09 | −.30, .11 | |||
| Low birth weight | 21 | 5,009 | −.20 | −.28, −.13 | |||
| Normal birth weight | 21 | 11,803 | −.11 | −.16, −.06 | |||
Note. CI=confidence interval. P-values derived from tests of heterogeneity examining the amount of variability explained by each moderator variable.
Table 3.
Predicted effects with 95% confidence intervals from mixed effects analyses for continuous moderators with unpublished studies included
| k | N | Intercept | b | SE | Qm | p | R2 | |
|---|---|---|---|---|---|---|---|---|
| Birth weight (g) | 46 | 17,122 | −.28 | <.0001 | <.0001 | 2.9 | .09 | .23 |
| Caucasian | 20 | 104,812 | −.30 | .0024 | .0011 | 4.7 | .03 | .28 |
| African American | 13 | 91,199 | −.006 | −.0055 | .0010 | 25.7 | <.001 | .67 |
| Asian | 9 | 2,704 | −.188 | .0130 | .0067 | 3.7 | .05 | .14 |
| Latino | 8 | 2,445 | −.145 | .0071 | .0063 | 1.3 | .26 | .00 |
| Gestational age (wks) | 25 | 3,298,161 | −.12 | −.0010 | .0089 | <.1 | .91 | .00 |
| Prenatal tobacco | 20 | 22,749 | −.05 | −.0017 | .0021 | 0.6 | .43 | .04 |
| Sex | 77 | 4,588,590 | −.12 | −.0004 | .0003 | 1.3 | .25 | .00 |
| Age at assessment (yrs) | 72 | 54,026 | −.15 | −.0006 | .0033 | <.1 | .86 | .00 |
Note: p-values derived from tests of heterogeneity examining the amount of variability explained by each moderator variable. Caucasian and non-Caucasian refers to the percentage of the sample that is Caucasian and non-Caucasian, respectively. Prenatal tobacco is the percentage of the sample that was exposed to prenatal tobacco. Sex is the percentage of the sample that is male.
Gestational age
Raw data on gestational age at birth was provided for 25 of the studies and ranged between 27 and 40 weeks. Gestational age was not found to significantly moderate the association between birth weight and ADHD symptoms when it was examined as a continuous (QM (1) < .1, p = .91, R2=.00) or categorical variable (QM (2) = .2, p = .91, R2 = .00).
Prenatal tobacco exposure
The percentage of participants exposed to any maternal prenatal tobacco use was calculated for 20 studies and ranged from 5 to 55 percent. Effect sizes were not found to differ based on exposure to maternal prenatal tobacco use (QM (1) = 0.6, p = .43, R2=.04).
Geographic region
Two studies were excluded from analysis as these studies reported results for samples recruited from two different continents. The remaining 83 unadjusted studies included three studies from South America, five studies from Asia, seven studies from Australia & New Zealand, 31 studies from Europe, and 37 studies from North America. Results indicated significant differences in effect sizes by geographic region (QM (4) = 34.7, p < .0001, R2 = .22). Effect size estimates for each continent were as follows: r = −.04 (−.07, −.01) for South America, r = −.28 (−.49, −.06) for Asia, r = −.13 (−.21, −.05) for Australia & New Zealand, r = −.13 (−.16, −.11) for Europe, and r = −.15 (−.18, −.11) for North America.
Race
The United States was the only country with enough studies to examine race within country as previously discussed. Thus, race was examined within the 20 studies conducted in the United States. Effect sizes varied systematically by the percentage of the sample that was Caucasian (QM (1) = 4.7, p = .03, R2 = .28) and African American (QM (1) = 25.7, p < .001, R2 = .67). Notably, the magnitude of the association between birth weight and ADHD symptoms was found to be smaller (less negative) as the percentage of Caucasian participants in a sample increased. Additionally, the magnitude of the association between birth weight and ADHD symptoms was found to be larger (more negative) as the percentage of African American participants in a sample increased. Finally, the percentages of the samples that were Asian (QM (1) = 3.7, p = .05, R2 = .14) and Latino (QM (1) = 1.3, p = .26, R2 = .00) were not found to be significant moderators.
Sex
The majority of studies (k=77) reported raw data on the percentage of participants who were males and ranged from 0 to 100 percent. Significant heterogeneity was not explained by the percentage of the participants who were male (QM (1) = 1.3, p = .25, R2 = .00).
ADHD measurement method
The type of ADHD measure used to assess ADHD symptoms was classified into four categories: interview (k=14), multiple methods (k=6), medical record (k=15), and questionnaire (k=50). Analyses revealed effect sizes differed according to the type of ADHD measure (QM (3) = 35.5, p < .0001) and approximately 11% of the heterogeneity was accounted for by the variability associated with the type of ADHD measure used (R2 = .11). The effect size estimates for samples utilizing medical records, interviews, questionnaires, and multiple methods were r = −.14 (−.17, −.10), r = −.21 (−.29, −.13), r = −.11 (−.13, −.08), and r = −.25 (−.43, −.07) respectively.
Informant
Five categories were used to classify the individual rating the ADHD symptoms or participants: parent (k = 47), clinician (k = 14), teacher (k = 6), self (k = 3) or multiple informants (k = 15). Of the studies including ratings from multiple people, 14 of the studies included ratings from a parent and a teacher and the remaining study had ratings from a parent, teacher, and clinician. The individual who rated participant ADHD symptoms accounted for significant heterogeneity in effect sizes (QM (4) = 9.9, p = .04, R2 = .24). Effect sizes were r = −.13 (−.15, −.10) for parental ratings, r = −.15 (−.18, −.11) for clinician ratings, r = −.13 (−.19, −.08) for teacher ratings, r = −.13 (−.44, −.17) for self-ratings, and r = −.18 (−.29, −.07) for ratings from multiple people.
Age at assessment
Seventy-two studies provided raw data on the age of participants at the time of assessment of ADHD symptoms. Participant age was not found to explain significant heterogeneity in the current analysis when examined as a continuous (QM (1) = 0.3, p = .86, R2 = .00) or categorical moderator (QM (3) = 0.2, p = .98, R2 = .00).
Birth weight ascertainment
Four studies did not provide information on the method used to ascertain birth weight of participants. Additionally, studies that used multiple methods to ascertain birth weight were excluded, as these studies often used one method (i.e., medical record extraction) for some participants and a different method (i.e., parental report) for other participants. Finally, studies utilizing self-reported birth weight were excluded because only two studies used this method. In the end, 74 studies were included in the analysis; 26 studies that utilized retrospective parental report and 48 studies that extracted birth weight from medical records. Effect sizes did not vary systematically by the method used to ascertain birth weight (QM (1) = 0.5, p = .49, R2 = .19).
Sample type
As detailed in the methods section, studies were categorized into one of four sample types: ADHD based studies (k=16), birth weight based studies (k=40), community studies (k=17), or population studies (k=12). Results indicated that the association between birth weight and ADHD symptoms did vary significantly by sample type (QM (3) = 43.4, p < .0001, R2 = .05). Estimates of effect sizes by sample type were as follows: r = −.18 (−.27, −.08) for ADHD based samples, r = −.20 (−.24, −.15) for birth weight based samples, r = −.06 (−.09, −.04) for community based samples, and r = −.09 (−.12, −.06) for population based samples.
Birth weight
Forty-six studies provided data on participant’s birth weight, allowing for calculation of the mean birth weight of the sample. Again, we examined mean birth weight of the sample as a moderator to determine if effect sizes systematically across the spectrum of reported birth weights. The mean birth weight of the sample did not account for a significant amount of heterogeneity when examined as a continuous (QM (1) = 2.9, p = .09, R2 = .23 or categorical (QM (2) = 5.6, p = .06, R2 = .20) variable.
DISCUSSION
The current study aimed to quantitatively investigate the association between birth weight and ADHD symptoms, and to test whether the magnitude of this association varied depending on a variety of factors. Results indicate that there is a small, but significant, negative association between birth weight and ADHD, such that lower birth weight is associated with increased symptoms of ADHD. Further, sample type, geographic region of the sample, Caucasian and African American race (in the United States), informant ratings of ADHD symptoms, and measurement method used to assess ADHD symptoms all accounted for significant heterogeneity in the association between birth weight and ADHD symptoms.
Previous research investigating the association between birth weight and ADHD symptoms has resulted in mixed findings, with some studies reporting a positive association such that lower birth weight was associated with fewer symptoms of ADHD (e.g., Duris, 2002; Johnson-Cramer, 1999), and other studies reporting a negative association (e.g., Indredavik et al., 2010; Sasaluxnanon & Kaewpornsawan, 2005). Further, the effect sizes in previous studies varied greatly given the diverse populations from which participants were recruited (e.g., Anderson, Doyle, & Victorian Infant Collaborative Study, 2003; Asbury, Dunn, & Plomin, 2006; Beverly, McGuinness, & Blanton, 2008; Class, Rickert, Larsson, Lichtenstein, & D’Onofrio, 2014; Groen-Blokhuis et al., 2011) and the variety of study designs that were utilized to examine the association between birth weight and ADHD (e.g., Frederick, 2012; Hack et al., 2004; Hultman et al., 2007; Kreppner, O’Connor, & Rutter, 2001; Linnet et al., 2005). The current findings therefore extend the literature by combining effect sizes from a variety of samples to provide an overall effect size regardless of recruitment method or sample population. The largest effect sizes were found for studies that recruited participants based on birth weight or on ADHD diagnostic status. These studies typically recruited individuals from the extreme ends of the ADHD and birth weight spectrums, such that individuals with sub-threshold ADHD symptoms were not included in the studies, or individuals were required to be born very low birth weight (<1500g) or extremely low birth weight (<1000g) as opposed to just low birth weight (<2500g). Thus, this finding suggests that the effect size may increase as the extremity of low birth weight or ADHD symptoms increases. Interestingly, previous research has indicated there is indeed a dose-response relationship between birth weight and ADHD symptoms, with individuals born at increasingly lower birth weights at increasingly higher risks of developing ADHD (D’Onofrio et al., 2013). However, mean birth weight of the sample was not a significant moderator of the association. This may be due to insufficient power since many studies did not provide mean birth weight of the sample. Additionally, it may be that studies recruiting from the extreme end of the birth weight studies vary along a number of confounding factors (e.g., neonatal morbidities), which in turn may affect the estimate of the effect size between birth weight and ADHD. As is discussed later, continued investigation regarding the effects of such confounding factors is important for an enhanced understanding of the relationship between birth weight and ADHD.
The geographic region from which participants were recruited and Caucasian and African American race (among United States samples) were found to significantly contribute to the heterogeneity in the overall effect size. Notably, socio-economic status and access to adequate prenatal care, which are associated with birth weight and ADHD (Gray et al., 2014; Larsson et al., 2014; Silal et al., 2012), vary greatly by geographic region and ethnicity, and could potentially explain the different effect sizes observed for different geographic regions and ethnicity. Still, the result of the geographic region analysis is likely limited to North American and European samples because very few published papers included samples that were recruited from the other geographic regions (Asia, South America, Australia/New Zealand). Furthermore, there may have been insufficient power to detect moderation by Asian and Latino race/ethnicity given the small number of studies reporting the percentage of the sample that identified as Asian or Latino.
Studies that used multiple informants (i.e. parent and teacher) of ADHD symptoms or that used multiple measurement methods had significantly larger effect sizes than those that used other informants (i.e. parent, teacher, self, or clinician) or single measurement methods (i.e. questionnaire or medical record). Notably, the use of multiple informants and multiple measurement methods has been demonstrated to have the highest validity in ADHD assessment (Erhardt et al., 1999; Gualtieri & Johnson, 2005; Powe et al., 1998; Van Voorhees et al., 2011). Thus, studies that used methods with higher diagnostic validity may have been better able to differentiate between individuals with ADHD and controls. Lastly, gestational age at birth, birth weight, prenatal tobacco exposure, sex, age at assessment, and birth weight ascertainment were not found to account for heterogeneity in the association between birth weight and ADHD symptoms.
Limitations
There are some limitations to the current research. First, some of the categorical variables had a small number of studies included in certain classes (e.g., geographic region, rater of ADHD symptoms), which may have resulted in less accurate estimates of effect sizes for the categorical classes with fewer studies. Similarly, the continuous variables included as moderators varied in the number of studies reporting data, with some variables having few studies reporting usable data, and this may have resulted in decreased power to detect significant effects. Additionally, given the different meaning and implications race may have depending on country, future investigations would benefit from examining the effect of race in countries other than the United States.
Although the current analyses did examine moderation of effect sizes by variables associated with birth weight and ADHD symptoms (i.e., prenatal tobacco exposure, gestational age), there are several other factors that have been shown to be associated with birth weight and/or ADHD symptoms that could not be included in the current analyses due to lack of reported data. For instance, maternal medical status (e.g., maternal age at birth, gestational diabetes, maternal BMI, maternal depression) and prenatal exposure to other substances (e.g., recreational drugs, alcohol) are potential moderators of interest, but studies did not report sufficient data on these variables to include them in the analyses.
A final limitation of the current meta-analysis is that the majority of included studies examined the association between birth weight and ADHD symptoms in children and adolescents. Although age at assessment was not found to be a significant moderator of effect sizes, only three studies examined the association in adult participants, limiting the ability to conclude whether the effect of birth weight on symptoms of ADHD remains in adults. Importantly, the decision-making that was used to handle non-independent data from longitudinal studies may have biased the results, as studies with larger sample sizes (and therefore typically younger participant ages) were included. For instance, five studies conducted when participants were 18 years or older were excluded because the participants were part of a longitudinal study that examined birth weight and ADHD symptoms at an earlier age. Excluding such studies may have resulted in a decreased ability to detect variation in effect sizes across mean participant age.
Implications
The findings of the current study have important considerations for theory, methodology, and clinical practice. First, in line with the Developmental Origin of Health and Disease hypothesis, present findings confirm a negative association between an early life risk factor, low birth weight, and the development of ADHD symptoms such that being born at a lower birth weight is associated with risk for adverse neurodevelopmental sequela. Additionally, the findings of the present study indicate the association does not vary depending on factors correlated with birth weight and symptoms of ADHD, such as gestational age at birth and prenatal tobacco exposure. Although the current analyses identified differences in effect sizes based on several variables, substantial heterogeneity remained after accounting for these variables, indicating other factors not examined in the current analyses may be contributing to variation in effect sizes. Several early life risk factors (e.g. maternal medical status, prenatal alcohol exposure) could not be examined in the current study, as data on these variables was largely unreported. From a methodological standpoint, future research examining early life risk factors of ADHD should examine the numerous other variables that are associated with low birth weight and ADHD, as these other variables may account for some of the heterogeneity that is unexplained in current analyses.
Finally, clinicians monitoring the growth and development of children born low birth weight should also monitor for the development of ADHD symptoms. Although individuals born low birth weight are not guaranteed to develop symptoms of ADHD, they are at a higher risk. By monitoring individuals at higher risk of developing ADHD, detection of symptoms may occur earlier in development, allowing for clinical intervention to be implemented before impairment due to symptoms becomes severe. Ultimately, it is in the best interest of the individual to identify ADHD symptoms and provide intervention as soon as possible in order to reduce impairments in school, work, and relationships.
Future Directions
As discussed previously, future work should focus on disentangling the multiple correlated risk factors shown to be associated with ADHD. Birth weight has emerged as one of the most robust risk factors, but it is also one of the most researched risk factors due to the ease with which researchers can ascertain this information from medical records or parents. However, individuals who are born low birth weight, particularly those born very or extremely low birth weight, are at risk for a number of prenatal and neonatal comorbidities, and they are often exposed to a variety of neonatal interventions. Indeed, a better understanding of the biological mechanisms underlying the association between birth weight and ADHD may be garnered by investigating the interaction among various prenatal and neonatal risk factors. Moreover, a better understanding of the biological mechanisms contributing to low birth weight and ADHD could potentially lead to the identification of protective factors that may buffer against the development of ADHD symptoms in individuals born low birth weight. Additionally, prior work (Morgan, Loo, & Lee, 2016; Wiggs et al., 2016) has found neuropsychological mechanisms to be significant mediators of the association between birth weight and ADHD symptoms. As such, future research may benefit from examining the extent to which prenatal and neonatal risk factors commonly suffered by those born low birth weight are associated with the neuropsychological mediators of the association between birth weight and ADHD symptoms.
Additionally, although gestational age and prenatal tobacco exposure may not have an effect on the magnitude of the association between birth weight and ADHD, they may still be important risk factors for the development of ADHD. Research efforts examining early life risk factors for ADHD should continue to consider the contribution of these risk factors to the development of ADHD, and future studies investigating the association between birth weight and ADHD symptoms should consider including these factors as covariates in analyses. Furthermore, future research should examine other variables previously identified as predictors of birth weight (e.g., prenatal exposure to other substances, gestational diabetes; Knopik et al., 2005; Linnet et al., 2003; Ornoy, 2005; Ornoy, Ratzon, Greenbaum, Wolf, & Dulitzky, 2001) as potential moderators of the association between birth weight and ADHD.
Finally, the results of the current moderation analyses indicate that sampling from extreme samples results in a larger effect size, suggesting there is a nonlinear relationship between birth weight and ADHD symptoms. However, a nonlinear association would be more conclusively demonstrated via the use of continuous measures of birth weight and ADHD symptoms. A recent study by Momany and colleagues (2016) tested such a nonlinear association using continuous measurements, and found the nonlinear association to be significant in predicting hyperactivity-impulsivity. However, few, if any, other studies have examined the nonlinear association between birth weight and ADHD using continuous measures. Therefore, future research should attempt to replicate this finding utilizing continuous measures of birth weight and ADHD symptoms.
Conclusion
The current study provided a meta-analytic estimate of the association between birth weight and symptoms of ADHD. Findings indicate birth weight appears to be a robustly associated with ADHD symptoms, such that lower birth weight is associated with increased ADHD symptoms. Interestingly, variation in the association is partially explained by sample type, geographic region, African American and Caucasian race, the informant of ADHD symptoms, and ADHD measurement method. However, substantial unexplained variation in the association between birth weight and ADHD symptoms remains, indicating future work should focus on the correlates of birth weight that may also be contributing to the development of ADHD symptoms.
Supplementary Material
Acknowledgments
Funding: Allison Momany and Jacyln Kamradt were supported by NIHGMS grant T32 GM108540.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
Asterisks (*) indicate studies included in the meta-analysis.
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Able SL, Johnston JA, Adler LA, Swindle RW. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol Med. 2007;37(1):97–107. doi: 10.1017/S0033291706008713. [DOI] [PubMed] [Google Scholar]
- *.Adger-Antonikowski A. A functionalist perspective of language ability and behavioral synchrony in the development of emotion regulation. 70. ProQuest Information & Learning; US: 2009. [Google Scholar]
- Akinbami LJ, Xiang L, Pastor PN, Reuben CA. NCHS Data Brief. Vol. 70. Hyattsville, MD: National Center for Health Statistics; 2011. Attention Deficit Hyperactivity Disorder Among Children Aged 5–7 Years in the United States, 1998–2009. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- *.Anderson P, Doyle LW Victorian Infant Collaborative Study G. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- *.Anderson PJ, De Luca CR, Hutchinson E, Spencer-Smith MM, Roberts G, Doyle LW. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol. 2011;36(1):57–73. doi: 10.1080/87565641.2011.540538. [DOI] [PubMed] [Google Scholar]
- *.Andreias L, Borawski E, Schluchter M, Taylor HG, Klein N, Hack M. Neighborhood influences on the academic achievement of extremely low birth weight children. J Pediatr Psychol. 2010;35(3):275–283. doi: 10.1093/jpepsy/jsp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Asbury K, Dunn JF, Plomin R. Birthweight-discordance and differences in early parenting relate to monozygotic twin differences in behaviour problems and academic achievement at age 7. Dev Sci. 2006;9(2):F22–F31. doi: 10.1111/j.1467-7687.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- *.Astbury J, Orgill AA, Bajuk B, Yu VY. Neonatal and neurodevelopmental significance of behaviour in very low birthweight children. Early Hum Dev. 1985;11(2):113–121. doi: 10.1016/0378-3782(85)90098-2. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies, and health in later life. 2. Edinburgh; New York: Churchill Livingstone; 1998. [Google Scholar]
- *.Beverly BL, McGuinness TM, Blanton DJ. Communication and academic challenges in early adolescence for children who have been adopted from the former Soviet Union. Lang Speech Hear Serv Sch. 2008;39(3):303–313. doi: 10.1044/0161-1461(2008/029). [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- *.Bora S, Pritchard VE, Moor S, Austin NC, Woodward LJ. Emotional and behavioural adjustment of children born very preterm at early school age. J Paediatr Child Health. 2011;47(12):863–869. doi: 10.1111/j.1440-1754.2011.02105.x. [DOI] [PubMed] [Google Scholar]
- *.Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38(8):931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- *.Boulet SL, Schieve LA, Boyle CA. Birth weight and health and developmental outcomes in US children, 1997–2005. Matern Child Health J. 2011;15(7):836–844. doi: 10.1007/s10995-009-0538-2. [DOI] [PubMed] [Google Scholar]
- *.Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D. Attention problems in very preterm children from childhood to adulthood: The Bavarian Longitudinal Study. Journal of Child Psychology and Psychiatry. 2016;57(2):132–140. doi: 10.1111/jcpp.12456. [DOI] [PubMed] [Google Scholar]
- *.Breslau N, Brown GG, Deldotto JE, Kumar S, Ezhuthachan S, Andreski P, Hufnagle KG. Psychiatric sequelae of low birth weight at 6 years of age. J Abnorm Child Psychol. 1996;24(3):385–400. doi: 10.1007/BF01441637. [DOI] [PubMed] [Google Scholar]
- Catov JM, Newman AB, Kelsey SF, Roberts JM, Sutton-Tyrrell KC, Garcia M, … Ness RB. Accuracy and reliability of maternal recall of infant birth weight among older women. Ann Epidemiol. 2006;16(6):429–431. doi: 10.1016/j.annepidem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- *.Chu SM, Tsai MH, Hwang FM, Hsu JF, Huang HR, Huang YS. The relationship between attention deficit hyperactivity disorder and premature infants in Taiwanese: a case control study. BMC Psychiatry. 2012;12:85. doi: 10.1186/1471-244x-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Class QA, Rickert ME, Larsson H, Lichtenstein P, D’Onofrio BM. Fetal growth and psychiatric and socioeconomic problems: population-based sibling comparison. Br J Psychiatry. 2014;205(5):355–361. doi: 10.1192/bjp.bp.113.143693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- *.Conrad AL, Richman L, Lindgren S, Nopoulos P. Biological and environmental predictors of behavioral sequelae in children born preterm. Pediatrics. 2010;125(1):e83–e89. doi: 10.1542/peds.2009-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Keeler GP, Angold A. Poverty, race/ethnicity, and psychiatric disorder: a study of rural children. Am J Public Health. 2001;91(9):1494–1498. doi: 10.2105/ajph.91.9.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Crea TM, Chan K, Barth RP. Family environment and attention-deficit/hyperactivity disorder in adopted children: associations with family cohesion and adaptability. Child Care Health Dev. 2014;40(6):853–862. doi: 10.1111/cch.12112. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry. 2013;70(11):1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Davis DW, Burns B, Snyder E, Robinson J. Attention problems in very low birth weight preschoolers: are new screening measures needed for this special population? J Child Adolesc Psychiatr Nurs. 2007;20(2):74–85. doi: 10.1111/j.1744-6171.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- de Graaf R, Kessler RC, Fayyad J, ten Have M, Alonso J, Angermeyer M, … Posada-Villa J. The prevalence and effects of adult attention-deficit/hyperactivity disorder (ADHD) on the performance of workers: results from the WHO World Mental Health Survey Initiative. Occup Environ Med. 2008;65(12):835–842. doi: 10.1136/oem.2007.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.de Zeeuw P, van Belle J, van Dijk S, Weusten J, Koeleman B, Janson E, … Durston S. Imaging gene and environmental effects on cerebellum in Attention-Deficit/Hyperactivity Disorder and typical development. Neuroimage Clin. 2012;2:103–110. doi: 10.1016/j.nicl.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks EM, Hudziak JJ, Boomsma DI. Genetics of ADHD, hyperactivity, and attention probelms. In: Kim Y, editor. Handbook of Behavior Genetics. New York: Springer; 2009. pp. 361–378. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, … Neumann PJ. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51(10):990–1002. e1002. doi: 10.1016/j.jaac.2012.07.008. [DOI] [PubMed] [Google Scholar]
- *.Duris MG. The incidence of attention deficit hyperactivity disorder among children born at low birth weight. 63. ProQuest Information & Learning; US: 2002. [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgen IB, Holsten F, Odberg MD. Psychiatric disorders in low birthweight young adults. Prevalence and association with assessments at 11 years. Eur Psychiatry. 2013;28(7):393–396. doi: 10.1016/j.eurpsy.2012.06.002. [DOI] [PubMed] [Google Scholar]
- *.Emond AM, Lira PI, Lima MC, Grantham-McGregor SM, Ashworth A. Development and behaviour of low-birthweight term infants at 8 years in northeast Brazil: a longitudinal study. Acta Paediatr. 2006;95(10):1249–1257. doi: 10.1080/08035250600615127. [DOI] [PubMed] [Google Scholar]
- Erhardt D, Epstein JN, Conners CK, Parker JDA, Sitarenios G. Self-ratings of ADHD sypmtoms in adults II: Reliability, validity, and diagnostic sensitivity. Journal of Attention Disorders. 1999;3(3):153–158. [Google Scholar]
- Estourgie-van Burk GF, Bartels M, Hoekstra RA, Polderman TJ, Delemarre-van de Waal HA, Boomsma DI. A twin study of cognitive costs of low birth weight and catch-up growth. J Pediatr. 2009;154(1):29–32. doi: 10.1016/j.jpeds.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, … Jin R. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402–409. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- *.Fazio LT. Impact of pregnancy, delivery, and infancy complications on attention-deficit hyperactivity disorder. 69. ProQuest Information & Learning; US: 2008. [Google Scholar]
- Fiscella K, Franks P, MRG, Clancy CM. Inequality in quality: Addressing socioeconomic, racial, and ethnic disparities in health care. Journal of the American Medical Association. 2008;283:2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- *.Frederick MJ. Birth weight predicts scores on the ADHD Self-Report Scale and attitudes towards casual sex in college men: A short-term life history strategy? Evolutionary Psychology. 2012;10(2):342–351. doi: 10.1177/147470491201000212. [DOI] [PubMed] [Google Scholar]
- *.Galera C, Cote SM, Bouvard MP, Pingault JB, Melchior M, Michel G, … Tremblay RE. Early risk factors for hyperactivity-impulsivity and inattention trajectories from age 17 months to 8 years. Arch Gen Psychiatry. 2011;68(12):1267–1275. doi: 10.1001/archgenpsychiatry.2011.138. [DOI] [PubMed] [Google Scholar]
- *.Gatzke-Kopp LM, Beauchaine TP. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry Hum Dev. 2007;38(4):255–269. doi: 10.1007/s10578-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun D, Rhoads GG, Demissie K, Lu SE, Quinn VP, MJF, … Jacobsen SJ. In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics. 2013;131(1):e53–e61. doi: 10.1542/peds.2012-1298. [DOI] [PubMed] [Google Scholar]
- Gordon M, Antshel K, Faraone S, Barkley R, Lewandowski L, Hudziak JJ, … Cunningham C. Symptoms versus impairment: the case for respecting DSM-IV’s Criterion D. J Atten Disord. 2006;9(3):465–475. doi: 10.1177/1087054705283881. [DOI] [PubMed] [Google Scholar]
- Gortmaker SL. The effects of prenatal care upon the health of the newborn. Am J Public Health. 1979;69(7):653–660. doi: 10.2105/ajph.69.7.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SC, Edwards SE, Schultz BD, Miranda ML. Assessing the impact of race, social factors and air pollution on birth outcomes: a population-based study. Environ Health. 2014;13(1):4. doi: 10.1186/1476-069X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Groen-Blokhuis MM, Middeldorp CM, van Beijsterveldt CEM, Boomsma DI. Evidence for a causal association of low birth weight and attention problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(12):1247–1254. doi: 10.1016/j.jaac.2011.09.007. [DOI] [PubMed] [Google Scholar]
- *.Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of extremely low birth weight (< or =800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics. 2004;114(6):e725–732. doi: 10.1542/peds.2004-0932. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG. ADHD: Is Objective Diagnosis Possible? Psychiatry (Edgmont) 2005;2(11):44–53. [PMC free article] [PubMed] [Google Scholar]
- *.Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor HG, Flannery D, … Borawski E. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 2004;114(4):932–940. doi: 10.1542/peds.2003-1017-L. [DOI] [PubMed] [Google Scholar]
- *.Halmøy A, Klungsøyr K, Skjærven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71(5):474–481. doi: 10.1016/j.biopsych.2011.11.013. [DOI] [PubMed] [Google Scholar]
- *.Hanc T, Slopien A, Wolanczyk T, Dmitrzak-Weglarz M, Szwed A, Czapla Z, … Cieslik J. ADHD and overweight in boys: cross-sectional study with birth weight as a controlled factor. Eur Child Adolesc Psychiatry. 2015;24(1):41–53. doi: 10.1007/s00787-014-0531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hanke C, Lohaus A, Gawrilow C, Hartke I, Kohler B, Leonhardt A. Preschool development of very low birth weight children born 1994–1995. Eur J Pediatr. 2003;162(3):159–164. doi: 10.1007/s00431-002-1127-1. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ. Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J Abnorm Child Psychol. 1995;23(6):729–749. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- *.Hatch B, Healey DM, Halperin JM. Associations between birth weight and attention−deficit/hyperactivity disorder symptom severity: Indirect effects via primary neuropsychological functions. Journal of Child Psychology and Psychiatry. 2014;55(4):384–392. doi: 10.1111/jcpp.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Heinonen K, Raikkonen K, Pesonen AK, Andersson S, Kajantie E, Eriksson JG, … Lano A. Trajectories of growth and symptoms of attention-deficit/hyperactivity disorder in children: a longitudinal study. BMC Pediatr. 2011;11:84. doi: 10.1186/1471-2431-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritace versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81(2):112–114. doi: 10.1034/j.1600-0412.2002.810204.x. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SP. Is ADHD an impairing condition in childhood and adolescence? In: Jensen PS, Cooper JR, editors. Attention deficit hyperactivity disorder: State of the science-best practices. Kingston, NJ, US: Civic Research Institute; 2002. [Google Scholar]
- *.Huang JH, Huang HL, Chen HL, Lin LC, Tseng HI, Kao TJ. Inattention and development of toddlers born in preterm and with low birth weight. Kaohsiung J Med Sci. 2012;28(7):390–396. doi: 10.1016/j.kjms.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hultman CM, Torrång A, Tuvblad C, Cnattingius S, Larsson JO, Lichtenstein P. Birth Weight and Attention-Deficit/Hyperactivity Symptoms in Childhood and Early Adolescence: A Prospective Swedish Twin Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(3):370–377. doi: 10.1097/01.chi.0000246059.62706.22. [DOI] [PubMed] [Google Scholar]
- Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catchup growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- *.Indredavik MS, Vik T, Evensen KA, Skranes J, Taraldsen G, Brubakk AM. Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J Dev Behav Pediatr. 2010;31(4):286–294. doi: 10.1097/DBP.0b013e3181d7b1d3. [DOI] [PubMed] [Google Scholar]
- *.Jackson DB, Beaver KM. Sibling differences in low birth weight, dopaminergic polymorphisms, and ADHD symptomatology: Evidence of GxE. Psychiatry Res. 2015;226(2–3):467–473. doi: 10.1016/j.psychres.2015.01.025. [DOI] [PubMed] [Google Scholar]
- *.Jaspers M, de Winter AF, Buitelaar JK, Verhulst FC, Reijneveld SA, Hartman CA. Early childhood assessments of community pediatric professionals predict autism spectrum and attention deficit hyperactivity problems. J Abnorm Child Psychol. 2013;41(1):71–80. doi: 10.1007/s10802-012-9653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johnson-Cramer NL. Assessment of school-aged children with comorbidity of attention deficit hyperactivity disorder and low birth weight classifications. 59. ProQuest Information & Learning; US: 1999. [Google Scholar]
- *.Kadziela-Olech H, Piotrowska-Jastrzebska J. The duration of breastfeeding and attention deficit hyperactivity disorder. Rocz Akad Med Bialymst. 2005;50:302–306. [PubMed] [Google Scholar]
- *.Kelly YJ, Nazroo JY, McMunn A, Boreham R, Marmot M. Birthweight and behavioural problems in children: a modifiable effect? Int J Epidemiol. 2001;30(1):88–94. doi: 10.1093/ije/30.1.88. [DOI] [PubMed] [Google Scholar]
- Kinzler W, Vintzileos A. Fetal growth restriction: a modern apporach. Curr Opin Obstet Gynecol. 2008;20(2):125. doi: 10.1097/GCO.0b013e3282f7320a. [DOI] [PubMed] [Google Scholar]
- Kitchen WH. The relationship between birth weight and gestational age in an australian hospital population. J Paediatr Child Health. 1968;4(1):29–37. [Google Scholar]
- *.Knopik VS, Sparrow EP, Madden PAF, Bucholz KK, Hudziak JJ, Reich W, … Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: A female twin study. Psychol Med. 2005;35(5):625–635. doi: 10.1017/S0033291704004155. [DOI] [PubMed] [Google Scholar]
- *.Kreppner JM, O’Connor TG, Rutter M. Can inattention/overactivity be an institutional deprivation syndrome? J Abnorm Child Psychol. 2001;29(6):513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Willcutt EG. Validity of the diagnosis and dimensions of attention-deficit hyperactivity disorder. In: Jensen PS, Cooper JR, editors. Attention deficit hyperactivity disorder: State of the science-best practices. Kingston, NJ: Civic Research Institute; 2002. pp. 1–23. [Google Scholar]
- *.Lahti J, Räikkönen K, Kajantie E, Heinonen K, Pesonen AK, Järvenpää AL, Strandberg T. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. Journal of Child Psychology and Psychiatry. 2006;47(11):1167–1174. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- *.Langley K, Fowler T, Ford T, Thapar AK, van den Bree M, Harold G, … Thapar A. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2010;196(3):235–240. doi: 10.1192/bjp.bp.109.066274. [DOI] [PubMed] [Google Scholar]
- *.Langley K, Holmans PA, van den Bree MBM, Thapar A. Effects of low birth weight, maternal smoking in pregnancy and social class on the phenotypic manifestation of Attention Deficit Hyperactivity Disorder and associated antisocial behaviour: Investigation in a clinical sample. BMC Psychiatry. 2007;7 doi: 10.1186/1471-244X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara C, Fayyad J, de Graaf R, Kessler RC, Aguilar-Gaxiola S, Angermeyer M, … Sampson N. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry. 2009;65(1):46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Sariaslan A, Langstrom N, D’Onofrio B, Lichtenstein P. Family income in early childhood and subsequent attention deficit/hyperactivity disorder: a quasi-experimental study. J Child Psychol Psychiatry. 2014;55(5):428–435. doi: 10.1111/jcpp.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DR, Christopher ME, Burns GL, Becker SP, Olson RK, Willcutt EG. Attention-deficit/hyperactivity disorder and sluggish cognitive tempo throughout childhood: temporal invariance and stability from preschool through ninth grade. J Child Psychol Psychiatry. 2016 doi: 10.1111/jcpp.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, … Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160(6):1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- *.Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E, Henriksen TB. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116(2):462–467. doi: 10.1542/peds.2004-2054. [DOI] [PubMed] [Google Scholar]
- Lumey LH, Stein AD, Ravelli AC. Maternal recall of birthweights of adult children: validation by hospital and well baby clinic records. Int J Epidemiol. 1994;23(5):1006–1012. doi: 10.1093/ije/23.5.1006. [DOI] [PubMed] [Google Scholar]
- Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165(7):734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- Martel MM. Sexual selection and sex differences in the prevalence of childhood externalizing and adolescent internalizing disorders. Psychol Bull. 2013;139(6):1221–1259. doi: 10.1037/a0032247. [DOI] [PubMed] [Google Scholar]
- Martel MM, Schimmack U, Nikolas M, Nigg JT. Integration of symptom ratings from multiple informants in ADHD diagnosis: a psychometric model with clinical utility. Psychol Assess. 2015;27(3):1060–1071. doi: 10.1037/pas0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McCormick MC, Gortmaker SL, Sobol AM. Very low birth weight children: behavior problems and school difficulty in a national sample. J Pediatr. 1990;117(5):687–693. doi: 10.1016/s0022-3476(05)83322-0. [DOI] [PubMed] [Google Scholar]
- *.McGrath MM, Sullivan M, Devin J, Fontes-Murphy M, Barcelos S, DePalma JL, Faraone S. Early Precursors Of Low Attention And Hyperactivity In A Preterm Sample At Age Four. Issues Compr Pediatr Nurs. 2005;28(1):1–15. doi: 10.1080/01460860590913945. [DOI] [PubMed] [Google Scholar]
- *.Melchior M, Hersi R, van der Waerden J, Larroque B, Saurel-Cubizolles MJ, Chollet A, Galera C. Maternal tobacco smoking in pregnancy and children’s socio-emotional development at age 5: The EDEN mother-child birth cohort study. Eur Psychiatry. 2015;30(5):562–568. doi: 10.1016/j.eurpsy.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41(4):378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- *.Mick E, Biederman J, Prince J, Fischer MJ, Faraone SV. Impact of low birth weight on attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics. 2002;23(1):16–22. doi: 10.1097/00004703-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry. 1996;153(9):1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998;27(3):352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- *.Minde K, Webb G, Sykes D. Studies on the hyperactive child. VI. Prenatal and paranatal factors associated with hyperactivity. Developmental Medicine and Child Neurology. 1968;10(3):355–363. doi: 10.1111/j.1469-8749.1968.tb02897.x. [DOI] [PubMed] [Google Scholar]
- *.Mitchell EA, Aman MG, Turbott SH, Manku M. Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clin Pediatr (Phila) 1987;26(8):406–411. doi: 10.1177/000992288702600805. [DOI] [PubMed] [Google Scholar]
- *.Momany AM, Kamradt JM, Ullsperger JM, Elmore AL, Nigg J, Nikolas M. Sex moderates the impact of birth weight on childhood externalizing psychopathology. J Abnorm Psychol. 2016 doi: 10.1037/abn0000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TR, Origel W, Key TC, Resnik R. The perinatal and economic impact of prenatal care in a low-socioeconomic population. Am J Obstet Gynecol. 1986;154(1):29–33. doi: 10.1016/0002-9378(86)90387-x. [DOI] [PubMed] [Google Scholar]
- *.Morales MR, Polizzi C, Sulliotti G, Mascolino C, Perricone G. Early precursors of low attention and hyperactivity in moderately and very preterm children at preschool age. Pediatr Rep. 2013;5(4):e18. doi: 10.4081/pr.2013.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JE, Loo SK, Lee SS. Neurocognitive Functioning Mediates the Prospective Association of Birth Weight With Youth ADHD Symptoms. J Clin Child Adolesc Psychol. 2016:1–10. doi: 10.1080/15374416.2016.1183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Murray E, Matijasevich A, Santos IS, Barros AJD, Anselmi L, Barros FC, Stein A. Sex differences in the association between foetal growth and child attention at age four: Specific vulnerability of girls. Journal of Child Psychology and Psychiatry. 2015;56(12):1380–1388. doi: 10.1111/jcpp.12422. [DOI] [PubMed] [Google Scholar]
- *.Nadeau L, Tessier R, Boivin M, Lefebvre F, Robaey P. Extremely Premature and Very Low Birthweight Infants: A Double Hazard Population? Social Development. 2003;12(2):235–248. doi: 10.1111/1467-9507.00231. [DOI] [Google Scholar]
- National Center for Education Statistics. Status and trends in the education of racial and ethnic minorities. 2007 Retrieved from http://nces.ed.gov/pubs2007/minoritytrends/
- Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. J Abnorm Psychol. 2010;119(1):1–17. doi: 10.1037/a0018010. [DOI] [PubMed] [Google Scholar]
- *.Nosarti C, Allin MP, Frangou S, Rifkin L, Murray RM. Hyperactivity in Adolescents Born Very Preterm Is Associated with Decreased Caudate Volume. Biol Psychiatry. 2005;57(6):661–666. doi: 10.1016/j.biopsych.2004.12.003. [DOI] [PubMed] [Google Scholar]
- *.O’Callaghan MJ, Harvey JM. Biological predictors and co-morbidity of attention deficit and hyperactivity disorder in extremely low birthweight infants at school. J Paediatr Child Health. 1997;33(6):491–496. doi: 10.1111/j.1440-1754.1997.tb01657.x. [DOI] [PubMed] [Google Scholar]
- *.Offord DR, Sullivan K, Allen N, Abrams N. Delinquency and hyperactivity. Journal of Nervous and Mental Disease. 1979;167(12):734–741. doi: 10.1097/00005053-197912000-00003. [DOI] [PubMed] [Google Scholar]
- Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- Ong KK. Catch-up growth in small for gestational age babies: good or bad? Curr Opin Endocrinol Diabetes Obes. 2007;14(1):30–34. doi: 10.1097/MED.0b013e328013da6c. [DOI] [PubMed] [Google Scholar]
- Ornoy A. Growth and neurodevelopmental outcome of children born to mothers with pregestational and gestational diabetes. Pediatr Endocrinol Rev. 2005;3(2):104–113. [PubMed] [Google Scholar]
- Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab. 2001;14(Suppl 1):681–689. doi: 10.1515/jpem.2001.14.s1.681. [DOI] [PubMed] [Google Scholar]
- Owens EB, Hinshaw SP. Perinatal problems and psychiatric comorbidity among children with ADHD. J Clin Child Adolesc Psychol. 2013;42(6):762–768. doi: 10.1080/15374416.2013.785359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Parker SE, Collett BR, Speltz ML, Werler MM. Prenatal smoking and childhood behavior problems: is the association mediated by birth weight? J Dev Orig Health Dis. 2016:1–9. doi: 10.1017/s2040174416000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J Clin Child Adolesc Psychol. 2005;34(3):449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- *.Perkinson-Gloor N, Hagmann-von Arx P, Brand S, Holsboer-Trachsler E, Grob A, Weber P, Lemola S. The role of sleep and the hypothalamic-pituitary-adrenal axis for behavioral and emotional problems in very preterm children during middle childhood. J Psychiatr Res. 2015;60:141–147. doi: 10.1016/j.jpsychires.2014.10.005. [DOI] [PubMed] [Google Scholar]
- *.Pettersson E, Sjolander A, Almqvist C, Anckarsater H, D’Onofrio BM, Lichtenstein P, Larsson H. Birth weight as an independent predictor of ADHD symptoms: a within-twin pair analysis. J Child Psychol Psychiatry. 2015;56(4):453–459. doi: 10.1111/jcpp.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe TJ, Doherty BJ, Panichelli-Mindel SM, Karustis JL, Eiraldi RB, Anastopoulos AD, DuPaul GJ. The predictive validity of parent and teacher reports of ADHD symptoms. Journal of Psychopathology and Behavioral Assessment. 1998;20(1):57–81. [Google Scholar]
- Quick JD, Greenlick MR, Roghmann KJ. Prenatal care and pregnancy outcome in an HMO and general population: a multivariate cohort analysis. Am J Public Health. 1981;71(4):381–390. doi: 10.2105/ajph.71.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Razza RA, Martin A, Brooks-Gunn J. Links between motor control and classroom behaviors: Moderation by low birth weight. Journal of Child and Family Studies. 2016 doi: 10.1007/s10826-016-0410-0. No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: Disentangling environmental and inherited influences. Psychol Med. 2010;40(2):335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rickards AL, Kelly EA, Doyle LW, Callanan C. Cognition, academic progress, behavior and self-concept at 14 years of very low birth weight children. J Dev Behav Pediatr. 2001;22(1):11–18. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- *.Ross G, Lipper E, Auld PA. Hand preference, prematurity and developmental outcome at school age. Neuropsychologia. 1992;30(5):483–494. doi: 10.1016/0028-3932(92)90095-4. [DOI] [PubMed] [Google Scholar]
- *.Sasaluxnanon C, Kaewpornsawan T. Risk factor of birth weight below 2,500 grams and attention deficit hyperactivity disorder in Thai children. J Med Assoc Thai. 2005;88(11):1514–1518. [PubMed] [Google Scholar]
- *.Sato M, Aotani H, Hattori R, Funato M. Behavioral outcome including attention deficit hyperactivity disorder/hyperactivity disorder and minor neurological signs in perinatal high-risk newborns at 4–6 years of age with relation to risk factors. Pediatr Int. 2004;46(3):346–352. doi: 10.1111/j.1442-200x.2004.01883.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP. An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry. 2012;17(12):1194–1205. doi: 10.1038/mp.2011.183. [DOI] [PubMed] [Google Scholar]
- *.Schmitz M, Denardin D, Laufer Silva T, Pianca T, Hutz MH, Faraone S, Rohde LA. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1338–1345. doi: 10.1097/s0890-8567(09)61916-x. [DOI] [PubMed] [Google Scholar]
- *.Sciberras E, Ukoumunne OC, Efron D. Predictors of parent-reported attention-deficit/hyperactivity disorder in children aged 6–7 years: a national longitudinal study. J Abnorm Child Psychol. 2011;39(7):1025–1034. doi: 10.1007/s10802-011-9504-8. [DOI] [PubMed] [Google Scholar]
- *.Scott MN, Taylor HG, Fristad MA, Klein N, Espy KA, Minich N, Hack M. Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. Journal of Developmental and Behavioral Pediatrics. 2012;33(3):202–213. doi: 10.1097/DBP.0b013e3182475287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sengupta SM, Grizenko N, Schmitz N, Schwartz G, Ben Amor L, Bellingham J, … Joober R. COMT Val108/158Met gene variant, birth weight, and conduct disorder in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1363–1369. doi: 10.1097/01.chi.0000251212.44491.46. [DOI] [PubMed] [Google Scholar]
- Showstack JA, Budetti PP, Minkler D. Factors associated with birthweight: an exploration of the roles of prenatal care and length of gestation. Am J Public Health. 1984;74(9):1003–1008. doi: 10.2105/ajph.74.9.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silal SP, Penn-Kekana L, Harris B, Birch S, McIntyre D. Exploring inequalities in access to and use of maternal health services in South Africa. BMC Health Serv Res. 2012;12:120. doi: 10.1186/1472-6963-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;133(1):e14–e22. doi: 10.1542/peds.2013-1434. [DOI] [PubMed] [Google Scholar]
- *.Simonds JF, Aston LA. Preterm birth, low birth weight, and hyperkinetic behavior in children. South Med J. 1980;73(9):1237–1238. doi: 10.1097/00007611-198009000-00020. [DOI] [PubMed] [Google Scholar]
- *.Smith TF, Anastopoulos AD, Garrett ME, Arias−Vasquez A, Franke B, Oades RD, … Ashley−Koch A. Angiogenic, neurotrophic, and inflammatory system SNPs moderate the association between birth weight and ADHD symptom severity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2014;165(8):691–704. doi: 10.1002/ajmg.b.32275. [DOI] [PubMed] [Google Scholar]
- Smith TF, Schmidt-Kastner R, McGeary JE, Kaczorowski JA, Knopik VS. Pre- and perinatal ischemia-hypoxia, the ischemia-hypoxia response pathway, and ADHD risk. Behav Genet. 2016;46(3):467–477. doi: 10.1007/s10519-016-9784-4. [DOI] [PubMed] [Google Scholar]
- *.St Sauver JL, Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Early life risk factors for attention-deficit/hyperactivity disorder: a population-based cohort study. Mayo Clin Proc. 2004;79(9):1124–1131. [PubMed] [Google Scholar]
- *.Strang-Karlsson S, Räikkönen K, Pesonen AK, Kajantie E, Paavonen EJ, Lahti J, … Andersson S. Very low birth weight and behavioral symptoms of attention deficit hyperactivity disorder in young adulthood: The Helsinki study of very-low-birth-weight adults. Am J Psychiatry. 2008;165(10):1345–1353. doi: 10.1176/appi.ajp.2008.08010085. [DOI] [PubMed] [Google Scholar]
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, Sourander A. Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/Hyperactivity Disorder. Pediatrics. 2015;136(3):e599–608. doi: 10.1542/peds.2015-1043. [DOI] [PubMed] [Google Scholar]
- *.Sullivan MC, Msall ME, Miller RJ. 17-year outcome of preterm infants with diverse neonatal morbidities: Part 1--Impact on physical, neurological, and psychological health status. J Spec Pediatr Nurs. 2012;17(3):226–241. doi: 10.1111/j.1744-6155.2012.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine. Am J Epidemiol. 1998;147(3):213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- *.Sykes DH, Hoy EA, Bill JM, McClure BG, Halliday HL, Reid MM. Behavioural adjustment in school of very low birthweight children. J Child Psychol Psychiatry. 1997;38(3):315–325. doi: 10.1111/j.1469-7610.1997.tb01516.x. [DOI] [PubMed] [Google Scholar]
- *.Szatmari P, Saigal S, Rosenbaum P, Campbell D. Psychopathology and adaptive functioning among extremely low birthweight children at eight years of age. Dev Psychopathol. 1993;5(3):345–357. doi: 10.1017/S0954579400004454. [DOI] [Google Scholar]
- *.Taylor HG, Klein N, Minich NM, Hack M. Middle-school-age outcomes in children with very low birthweight. Child Dev. 2000;71(6):1495–1511. doi: 10.1111/1467-8624.00242. [DOI] [PubMed] [Google Scholar]
- Troy LM, Michels KB, Hunter DJ, Spiegelman D, Manson JE, Colditz GA, … Willett WC. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int J Epidemiol. 1996;25(1):122–127. doi: 10.1093/ije/25.1.122. [DOI] [PubMed] [Google Scholar]
- *.Van den Bergh BRH, Marcoen A. High Antenatal Maternal Anxiety Is Related to ADHD Symptoms, Externalizing Problems, and Anxiety in 8- and 9-Year-Olds. Child Dev. 2004;75(4):1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- *.Van Hus JW, Potharst ES, Jeukens-Visser M, Kok JH, Van Wassenaer-Leemhuis AG. Motor impairment in very preterm-born children: links with other developmental deficits at 5 years of age. Developmental Medicine and Child Neurology. 2014;56(6):587–594. doi: 10.1111/dmcn.12295. [DOI] [PubMed] [Google Scholar]
- *.van Mil NH, Steegers-Theunissen RP, Motazedi E, Jansen PW, Jaddoe VW, Steegers EA, … Tiemeier H. Low and high birth weight and the risk of child attention problems. J Pediatr. 2015;166(4):862–869. e861–863. doi: 10.1016/j.jpeds.2014.12.075. [DOI] [PubMed] [Google Scholar]
- Van Voorhees EE, Hardy KK, Kollins SH. Reliability and Validity of Self- and Other-Ratings of Symptoms of ADHD in Adults. Journal of Attention Disorders. 2011;15(3):224–234. doi: 10.1177/1087054709356163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36(3):1–48. [Google Scholar]
- Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, … Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34–46. e32. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wagner AI, Schmidt NL, Lemery-Chalfant K, Leavitt LA, Goldsmith HH. The limited effects of obstetrical and neonatal complications on conduct and attention-deficit hyperactivity disorder symptoms in middle childhood. Journal of Developmental and Behavioral Pediatrics. 2009;30(3):217–225. doi: 10.1097/DBP.0b013e3181a7ee98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JA, Watson CJ, McCann A, Baugh J. Epigenetics, the epicenter of the hypoxic response. Epigenetics. 2010;5(4):293–296. doi: 10.4161/epi.5.4.11684. [DOI] [PubMed] [Google Scholar]
- Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209–217. doi: 10.1016/j.jadohealth.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Wiggs K, Elmore AL, Nigg JT, Nikolas MA. Pre- and Perinatal Risk for Attention-Deficit Hyperactivity Disorder: Does Neuropsychological Weakness Explain the Link? J Abnorm Child Psychol. 2016 doi: 10.1007/s10802-016-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wiles NJ, Peters TJ, Heron J, Gunnell D, Emond A, Lewis G. Fetal growth and childhood behavioral problems: results from the ALSPAC cohort. Am J Epidemiol. 2006;163(9):829–837. doi: 10.1093/aje/kwj108. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Williams NA. Short and long-term effects of birth weight and neonatal medical complications on children’s emotional and behavioral outcomes. 70. ProQuest Information & Learning; US: 2010. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) Geneva: WHO; 1992. [Google Scholar]
- World Health Organization. Demographic and socioeconomic statistics 2009 [Google Scholar]
- Wu X, Sun J, Li L. Chronic cerebrovascular hypoperfusion affects global DNA methylation and histone acetylation in rat brain. Neurosci Bull. 2013;29(6):685–692. doi: 10.1007/s12264-013-1345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Yang P, Chen YH, Yen CF, Chen HL. Psychiatric diagnoses, emotional-behavioral symptoms and functional outcomes in adolescents born preterm with very low birth weights. Child Psychiatry Hum Dev. 2015;46(3):358–366. doi: 10.1007/s10578-014-0475-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.